Abstract
Vascular stiffening in the pulmonary arterial bed is increasingly recognized as an early disease marker and contributor to right ventricular workload in pulmonary hypertension. Changes in pulmonary artery stiffness throughout the pulmonary vascular tree lead to physiologic alterations in pressure and flow characteristics that may contribute to disease progression. These findings have led to a greater focus on the potential contributions of extracellular matrix remodeling and mechanical signaling to pulmonary hypertension pathogenesis. Several recent studies have demonstrated that the cellular response to vascular stiffness includes upregulation of signaling pathways that precipitate further vascular remodeling, a process known as mechanobiological feedback. The extracellular matrix modifiers, mechanosensors, and mechanotransducers responsible for this process have become increasingly well-recognized. In this review, we discuss the impact of vascular stiffening on pulmonary hypertension morbidity and mortality, evidence in favor of mechanobiological feedback in pulmonary hypertension pathogenesis, and the major contributors to mechanical signaling in the pulmonary vasculature.
Keywords: vascular stiffness, pulmonary arterial stiffness, pulmonary hypertension, mechanotransduction, cellular mechanosensors, YAP/TAZ, matrix stiffness, vascular remodeling
Introduction
Pulmonary Hypertension is characterized by progressive pulmonary vascular remodeling that leads to exertional dyspnea, severe hypoxemia, and ultimately to right heart failure. The most severe form of the disease, PAH, initially and primarily affects the pulmonary arterial tree, and is associated with a 5-year survival of 61% (Farber et al., 2015). Other forms of PH result from other cardiac or pulmonary pathologies, but can progress to severe arterial remodeling (Rabinovitch et al., 1979; Wilkinson et al., 1988; Tuder et al., 2007; Kulik, 2014). For PH with significant vascular remodeling, treatment options are currently limited to pulmonary vasodilator therapies. These can lead to modest improvements in exercise capacity, but remain unable to reverse the disease process or significantly prolong survival (Fredenburgh et al., 2009; McLaughlin et al., 2009; Sutendra and Michelakis, 2014; Farber et al., 2015). These limitations have motivated extensive research to disrupt or reverse vascular remodeling.
Pulmonary arterial stiffness is increasingly appreciated as an important marker and predictor of disease severity and poor functional status in PH (Wang and Chesler, 2011; Schafer et al., 2016; Thenappan et al., 2016). Multiple lines of evidence indicate that increased vascular stiffness occurs prior to detectable changes in characteristic hemodynamic parameters, such as increased PVR (Pellegrini et al., 2014; Dragu et al., 2015; Ploegstra et al., 2018). Unlike in the systemic circulation, both proximal and distal vessels contribute to PA compliance, and current research has found evidence for early stiffening throughout the pulmonary vascular tree. Multiple processes may contribute to this vascular stiffening in vivo, including vascular smooth muscle tone, arrangement and structure of arterial laminae, local composition of extracellular matrix, and alterations in arterial geometry (thickness, diameter, and branching pattern).
This review will briefly address our current understanding of the clinical impact of pulmonary vascular stiffness. We will then focus on evidence for PA stiffening as a critical early driver of vascular remodeling – a process known as mechanobiological feedback. A close examination of the molecular mechanisms by which vascular cells promote, sense, and pathologically respond to stiffening is critical for our understanding of this process. Therapeutic targeting of key mechanotransduction mediators offers the potential to disrupt mechanobiological feedback and prevent or reverse pathologic vascular remodeling.
Clinical Impact of Vascular Stiffening in PH
Increased vascular stiffness in the systemic circulation is strongly associated with incident systemic hypertension, and has been convincingly shown to precede the development of hypertension in multiple cohorts (Mitchell et al., 2004; Kaess et al., 2012; Weisbrod et al., 2013). Furthermore, arterial stiffness is a predictor of cardiovascular mortality (Boutouyrie et al., 2002; Wang et al., 2010; Ben-Shlomo et al., 2014). Loss of compliance in the large arteries results in increased pulse pressure, pulsatile afterload, and flow pulsatility, which have been shown to impact left ventricular (LV) remodeling (Kelly et al., 1992; Kaess et al., 2016; Bell et al., 2017) and promote microvascular damage in distal vascular beds of the brain and kidney (Hashimoto and Ito, 2011; Cooper and Mitchell, 2016; Pase et al., 2016; Tsao et al., 2016; Huang et al., 2017). Similarly, recent evidence from both patients and disease models has led to a growing appreciation for the impact of vascular stiffening in the pulmonary circulation as well (Wang and Chesler, 2011; Schafer et al., 2016; Thenappan et al., 2016).
Several groups have examined associations between mortality and non-invasive measures of proximal pulmonary vascular stiffness. Measurement techniques have varied across groups, and include intravascular ultrasound (Ploegstra et al., 2018) or MRI (Gan et al., 2007) to measure proximal arterial luminal area change (and calculate PA distensibility), as well as more global measures of vascular system capacitance [right ventricular (RV) stroke volume/pulse pressure] via cardiac catheterization or echocardiography (Mahapatra et al., 2006a,b; Pellegrini et al., 2014; Al-Naamani et al., 2015). These measurements of vascular stiffness have been strongly associated with mortality in pediatric PAH patients (Ploegstra et al., 2018), idiopathic PAH cohorts (Mahapatra et al., 2006a,b; Gan et al., 2007), scleroderma-associated PH (Campo et al., 2010), and PH in the setting of heart failure (Pellegrini et al., 2014; Al-Naamani et al., 2015; Dragu et al., 2015). Several studies demonstrated that stiffness measures were better predictors of mortality than the more typically measured resistance indices (PVR, PVR index, transpulmonary gradient) (Mahapatra et al., 2006b; Pellegrini et al., 2014; Al-Naamani et al., 2015; Dragu et al., 2015; Ploegstra et al., 2018), and particularly demonstrated prognostic utility in patients with normal PVR (Pellegrini et al., 2014; Dragu et al., 2015; Ploegstra et al., 2018).
Beyond its utility as a marker of mortality, PA stiffness may better predict early disease and functional status in PH than PVR and other traditional clinical parameters (Hunter et al., 2008; Sanz et al., 2009; Kang et al., 2011; Lau et al., 2016; Malhotra et al., 2016). One group measured vascular impedance (a global measurement of opposition to pulsatile flow that incorporates resistance and arterial stiffness) using pulse-wave Doppler ultrasound and right heart catheterization in a cohort of patients with PAH (Hunter et al., 2008). Impedance was a better predictor of worsening clinical status than PVR index alone in PAH patients, and particularly improved predictions of mild clinical worsening. Kang et al. (2011) used same-day MRI measurements with right heart catheterization to calculate proximal PA distensibility, and demonstrated that this was a better predictor of poor functional status (6MWD < 400 m) than PVR index or pulmonary capacitance in a small PAH cohort. Another set of investigators demonstrated that these MRI-derived measures of PA stiffness correlate well with hemodynamic severity and were sensitive to abnormal pulmonary vascular response to exercise that may represent early disease (Sanz et al., 2009). More recently, global PA distensibility was calculated during cardiopulmonary exercise testing using the Linehan distensible vessel model (Linehan et al., 1992; Malhotra et al., 2016). In a mixed cohort of patients with heart failure and PAH, arterial distensibility was a good independent predictor of peak oxygen consumption in a multivariable analysis that included resting hemodynamics (Malhotra et al., 2016). Lau et al. (2016) used a similar strategy to demonstrate decreased global arterial distensibility in patients with normal resting hemodynamics who had known (thromboembolic or biopsy-proven) PH or who subsequently developed PH during follow-up.
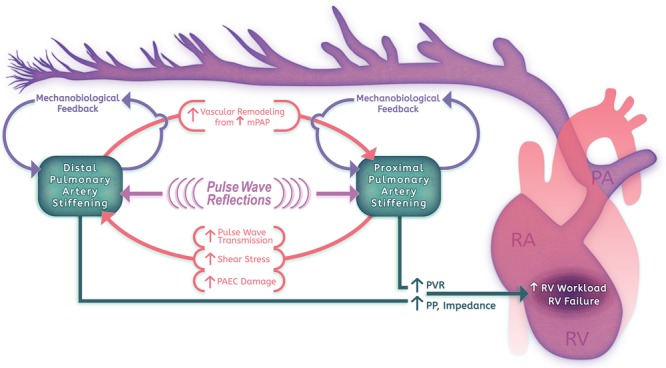
The identification of early changes in PA stiffness and of strong associations between stiffness and PH morbidity has validated PA distensibility as a marker for early disease. This has motivated further investigation into whether vascular stiffening plays a key role in PH pathogenesis and progression (Figure 1). Physiologically, there is strong evidence that arterial stiffening contributes significantly to RV afterload and RV failure. Standard measurements of RV afterload using PVR account for only the steady-flow component of RV work, however, arterial stiffness is the critical determinate of RV pulsatile afterload (Tan W. et al., 2014; Bloodworth et al., 2015). In the pulmonary circulation, pulsatile afterload contributes approximately 23% to the workload of the RV, and has not been found to change through the course of disease (Saouti et al., 2010a). Unlike vascular resistance, which is localized primarily to small arteries and arterioles, pulsatile afterload reflects the status of vessels throughout the pulmonary vascular tree (Saouti et al., 2010a; Wang and Chesler, 2011). Proximal large artery stiffness has been comparatively well studied, but only contributes 15–25% of the total oscillatory work; direct measurement of the distal vasculature has been difficult to obtain (Bloodworth et al., 2015). Through disease modeling and advanced imaging approaches, measures of PA stiffness are increasingly being used to evaluate RV function and outcomes. These pioneering physiological studies suggest that evaluating PH without taking into account vascular stiffness is incomplete; incorporating stiffness measures allows a more complete analysis of RV workload, improves outcome prediction, and will likely be useful for monitoring response to therapy.
FIGURE 1.
Pulmonary arterial stiffening in PAH pathogenesis.
One important physiologic relationship that has emerged from evaluations of global pulmonary artery stiffness is that PVR and PA capacitance (a measure inversely related to stiffness) have an inverse hyperbolic relationship (Lankhaar et al., 2008; Saouti et al., 2009). In particular, Lankhaar et al. (2008) demonstrated that this relationship holds true in patients with and without PH and that current treatments do not greatly alter this coupling. Clinically, this inverse relationship means that large measurable changes in compliance are manifested by relatively small changes in resistance in early disease, and that large measurable changes in resistance yield only small changes in compliance in later disease (Lankhaar et al., 2008; Saouti et al., 2010b). These authors suggest that this physiology in large part explains why measures of vascular stiffness are excellent markers for early PH (Wang and Chesler, 2011; Thenappan et al., 2016).
Increasing evidence from disease models also implicates a critical role for PA stiffening in a positive feedback cycle of pathologic vascular remodeling. Distal vasculopathy can promote increased mean pressures, resulting in large vessel wall thickening, vessel dilation, and extensive alterations in ECM content (Rabinovitch et al., 1979; Birukova et al., 2013). Stiffening in the proximal vasculature can amplify pulse wave transmission to the distal vessels, resulting in shear stress, inflammation, and smooth muscle cell (SMC) remodeling behaviors (Li et al., 2009; Scott et al., 2013). Finally, vascular stiffening itself may promote local remodeling through alterations in gene expression and cellular behaviors in response to the local mechanical microenvironment – a process we have termed mechanobiological feedback (Bertero et al., 2015; Liu et al., 2016; Dieffenbach et al., 2017). We will discuss the impact of both proximal and distal PA stiffening on vascular remodeling in further detail below.
Proximal PA Stiffening and Its Consequences on Vascular Remodeling
Stiffness of proximal pulmonary arteries is determined in large part by the composition and structure of the ECM proteins comprising large portions of the vessel wall. These proteins include proteoglycans and glycoproteins, but elastin and collagen predominate (Tsamis et al., 2013). Elastin is a resilient, flexible fiber that allows repetitive stretching of the vessel wall, whereas collagen provides strength but is much less deformable, limiting arterial compliance and preventing damage to the vessel (Tsamis et al., 2013; Schafer et al., 2016). As a result, in healthy tissues at low strains the more compliant mechanical properties of elastin predominate, with collagen carrying loads only under high-strain conditions (Hunter et al., 2011). Remodeling of these arteries during PH leads to vessel dilation, inflammatory cell accumulation, degradation of elastin, and collagen accumulation (Lammers et al., 2008). Examination of mice with variable vascular elastin expression shows a gradual increase in mPAP that inversely correlates with elastin levels (Shifren et al., 2008). Specifically, ex vivo biaxial strain testing and accompanying two-photon microscopy in explanted human tissues demonstrated severe elastin fragmentation and collagen accumulation in PH that correlated well with collagen-predominant (stiff) mechanics even under low-strain conditions (Rogers et al., 2013). Work in the chronic hypoxia neonatal calf model has also shown that the elastin layer can stiffen to allow adaptation to higher pressures, but this may primarily be a response observed early in life when elastin synthesis is present at much higher levels (Lammers et al., 2008; Schafer et al., 2016).
For many years, the standard dogma has been that proximal vessel stiffening is a relatively late disease manifestation, however, studies in animal models increasingly demonstrate that proximal stiffening occurs relatively early after exposure to pathologic stimulation (Tan W. et al., 2014). Seminal work by Myrick and Reid suggested early increases in proximal pulmonary artery stiffness in rats in the setting of hypoxia, with an increase in artery diameter of more than 50% after 3 days of exposure (Meyrick and Reid, 1979b). These investigations later showed a doubling in the thickness of the elastic lamina after 10 days of exposure, with collagen and elastin contributing proportionally (Meyrick and Reid, 1980). In the same rat hypoxia model, the Riley laboratory group found increased collagen synthesis by 3 days and a twofold increase in hydroxyproline wall content within 5 days of hypoxia exposure that correlated with a doubling in stiffness of the PA bed, as measured by pressure-volume loops (Poiani et al., 1990; Tozzi et al., 1994). Kobs et al. (2005) more recently performed ex vivo testing of the passive mechanical properties of mouse PAs in the hypoxia model at relatively early time points (1–2 weeks of hypoxia). They found a significant (∼25%) increase in effective elastic modulus and decreased compliance of proximal mouse PAs that correlated histologically with proportional thickening of both collagen and elastin elements of the arterial wall (Kobs et al., 2005).
Work by the Rabinovitch laboratory has demonstrated early internal elastic lamina disruption and increases in elastolytic activity in the monocrotaline (MCT) rat model of PH within the first few days after injection, and weeks before the development of hemodynamic changes (Todorovich-Hunter et al., 1992). Serine elastase appears to be responsible for this degradation, and its upregulation is associated with later development of neointimal lesions (Kim et al., 2011). Furthermore, disruption of this early change in stiffness via serine elastase inhibition is beneficial in preventing or reversing elevated PA pressures and RV hypertrophy. This has been convincingly shown in multiple disease models, including hypoxia, MCT, S100A4, and sugen-hypoxia models of PH (Maruyama et al., 1991; Ye and Rabinovitch, 1991; Cowan et al., 2000a; Zaidi et al., 2002; Kim et al., 2011; Nickel et al., 2015), although recent studies suggest an independent effect on bone morphogenic protein signaling that may also contribute (Nickel et al., 2015). Collagen remodeling has also been found to be important in this early time period, as mice with impaired ability to degrade collagen fail to improve proximal vascular stiffening and tend to have higher PA pressures during recovery (Ooi et al., 2010).
A large body of recent work by Stenmark, Tan, and colleagues has elucidated a putative mechanism by which early proximal pulmonary vascular stiffening can impact PH progression via induction of high pulsatile flow in the distal vasculature (Li et al., 2009, 2013; Scott et al., 2013; Tan W. et al., 2014; Tan Y. et al., 2014). In the pulmonary circulation, proximal pulmonary arteries are 10–20-fold more compliant compared to systemic arteries (Lammers et al., 2008), but smaller vessels also contribute significantly (∼80%) to the total compliance of the pulmonary vascular tree (Saouti et al., 2010b). Nevertheless, the large and medium-sized elastic pulmonary arteries of the upstream vasculature expand passively during systole and recoil during diastole, serving to convert pulsatile flow to a more constant flow in distal vessels while also dampening pressure variations throughout the cardiac cycle (Tan W. et al., 2014). Constant, low-pulsatility laminar flow is physiologic for vascular endothelium, resulting in high levels of NO production, endothelial barrier integrity, and low expression of adhesion and procoagulatory molecules. Experimental disturbance of flows from this state in vivo lead to compensatory vascular remodeling that functions to maintain shear stresses near physiologic levels, but may also contribute to pathological processes such as atherosclerosis (Chiu and Chien, 2011). During PH progression, stiffer proximal vessels will distend less with each stroke volume and therefore transmit greater flow and pressure pulsatility to the more distal vasculature (Scott et al., 2013; Su et al., 2013).
To study the effects of increased flow transmission from stiffened proximal arteries, these investigators used an in vitro system that simulated high flow pulsatility compared to low flow pulsatility with the same mean flow stresses (simulating the same static pressures/mPAP) on human PAECs. Compared to normal flow pulsatility, high flow pulsatility led to attenuated release of the pulmonary vasodilators NO and prostaglandin-F1α (PGF1α) and enhanced release of the potent vasoconstrictor endothelin (Li et al., 2009). Over short timeframes, this change in pulsatility induced expression of inflammatory leukocyte adhesion molecules (such as intercellular adhesion molecule, endothelial leukocyte adhesion molecule, and monocyte chemo-attractant protein-1) and led to endothelial cell proliferation (Li et al., 2013). Using a co-culture system of PAECs and PASMCs, these authors demonstrated that high-pulsatility flow induced PASMC hypertrophic responses, including expression of the contractile proteins α-smooth muscle actin (αSMA) and smooth muscle myosin heavy chain, without affecting proliferation (Scott et al., 2013). Treatment with a variety of vasodilators, including ACE inhibitors and endothelin antagonists, could prevent this effect. The putative mechanosensing mechanisms underlying endothelial responses to shear stress will be discussed below. These in vitro models suggest that proximal vascular stiffening may induce or exacerbate distal vascular vasoconstriction, inflammation, and remodeling, providing a key contribution to the progression of disease.
Distal and Microvascular PA Stiffening and Mechanobiological Feedback
Muscularization of normally non-muscularized “intra-acinar” pulmonary arteries was recognized as an early finding (day 3 of exposure) by Meyrick and Reid in their initial studies of hypoxia- and Crotolaria-induced PH in rats (Meyrick and Reid, 1979a,b). This neomuscularization notably occurs before proliferation, and was found to represent hypertrophy and metaplasia of precursor SMCs not normally visible by light microscopy (Hislop and Reid, 1976; Meyrick et al., 1978). Recent lineage tracing experiments by the Greif lab in the mouse hypoxia model have meticulously identified these SMC progenitors, which arise from the border region between muscular and non-muscular arteries and express both αSMA and the undifferentiated mesenchymal marker platelet derived growth factor receptor-β (Sheikh et al., 2014, 2015). Early in the development of hypoxia-induced PH, individual progenitor cells migrate distally, dedifferentiate, and clonally expand to enable muscularization in the distal arteries by days 5–10 post-hypoxia (Sheikh et al., 2015).
Our recent work has added to the field by directly characterizing the stiffness of the pulmonary vasculature locally at the micron scale in models of PH and human PAH. To do so, we developed a methodology to characterize the local elastic properties of the lung using atomic force microscopy (AFM) (Liu F. et al., 2010; Liu et al., 2015; Liu and Tschumperlin, 2011). Optimal measurements use large spherical tips (1–2.5 μm radius) on thick (20–50 μm) sections with sufficient force (50 pN) to interrogate vessel mechanical properties (Sicard et al., 2017). Although this method requires unfixed tissue, limiting some applications, it allows for unparalleled spatial resolution to measure local tissue stiffening in even the smallest vessels (Liu and Tschumperlin, 2011). Examination of human tissue has allowed us to determine the range of medial stiffness relevant to disease (Liu et al., 2016), and interestingly demonstrated a normal ∼2-fold increase in PA stiffness with aging (Sicard et al., 2018). These observations raise the possibility that some forms of PH may be related to an accelerated aging phenotype or represent genetic/epigenetic dysregulation of the normal age-related process.
Using this AFM approach, we examined the spatial and temporal distribution of PA stiffening in both the MCT and sugen-hypoxia rat models of PH (Liu et al., 2016). We found that there is an early (7 day) 1.5–2-fold increase in pulmonary artery stiffening in distal vessels (<100 μm in diameter) in both the MCT and sugen-hypoxia models that subsequently progresses to an approximately sixfold (0.6 kPa to 3.6–4 kPa) increase in vascular stiffness in later stage disease (Liu et al., 2016). Wall thickening detectable by light microscopy and more proximal arterial medial stiffening (vessel diameter 100–300 μm) occurs subsequent to this distal stiffening, followed by changes in PA pressures and frank RVH. Taken together, our results suggest that medial PA stiffening arises early in distal vessels and progresses proximally in these models, preceding detectable hemodynamic changes and frank RV dysfunction. Notably, our methodology examines local-scale changes in stiffness in the vessel media of intrapulmonary vessels, so we would not have detected early changes in elastic laminae of the large arteries discussed above. Current evidence indicates that both proximal and distal vascular remodeling are likely important to disease pathogenesis, but their relative contributions and the direction of propagation require further study, particularly in human disease.
These changes in the local mechanical environment in early PH suggest a potential role for PA stiffness in the progression of disease. From a physiologic standpoint, smaller vessels contribute significantly to total arterial compliance as well as to vascular resistance; thus, increased stiffness and muscularization will impact both static and oscillatory RV workload (Saouti et al., 2010b). Due to pulse-wave reflection and proximal artery dilation in response to mean pressure changes, there will be increased circumferential stress on proximal vessels, contributing to inflammation and vascular remodeling in these vessels (Figure 1; Wang and Chesler, 2011).
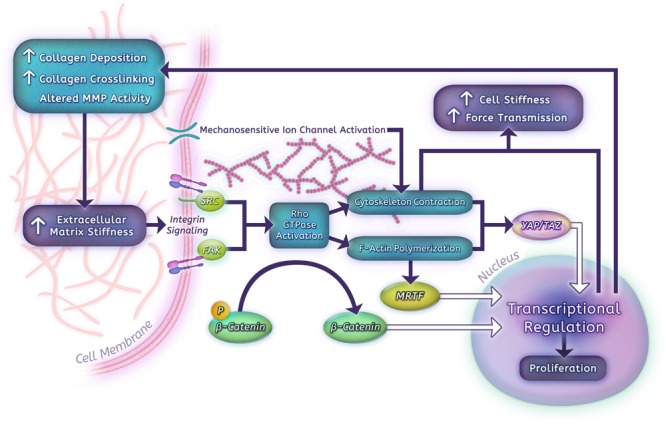
Beyond physiologic principles, the mechanical micro environment is being increasingly recognized for its critical role in regulating key cellular processes during organogenesis (Mammoto and Ingber, 2010) and in the development of pathology (Georges et al., 2007; Klein et al., 2009; Liu F. et al., 2010; Liu et al., 2015, 2016; Gulino-Debrac, 2013; Perepelyuk et al., 2013; Pickup et al., 2014; Huveneers et al., 2015; Tschumperlin, 2015). Pathogenic vascular stiffness has been found to precede the development of systemic hypertension (Kaess et al., 2012), and leads to microvascular endothelial and SMC dysfunction (Huveneers et al., 2015). We have extensively examined the response of pulmonary vascular cells to pathologic matrix stiffness in vitro using discrete stiffness polyacrylamide hydrogels (Liu F. et al., 2010; Liu et al., 2016; Dieffenbach et al., 2017), and have found that stiffness induces changes that can drive further vascular remodeling – a process we call mechanobiological feedback.
Human PASMCs and PAECs grown on hydrogels that span the range from normal parenchyma to highly remodeled vessels (shear modulus 0.1–25.6 kPa) show a robust proliferative response to matrix stiffness (Liu et al., 2016). In PASMCs, contractile force generation, as assessed by traction force microscopy, is likewise highly stiffness dependent (Dieffenbach et al., 2017), which may in part be due to remodeling of the actin cytoskeleton. These remodeling phenotypes correlate with increased expression of matrix proteins, such as collagen I, fibronectin, and LOX, a key collagen cross-linking enzyme (Liu et al., 2016; Dieffenbach et al., 2017). Additional downstream consequences of arterial stiffness include increased fibrotic changes and ECM modifications via a miR-130/301-mediated mechanism and stiffness-dependent alterations in glutaminolysis that impact proliferation and migration of vascular cells (Bertero et al., 2015, 2016). We have also found that stiffness-induced activation of pulmonary vascular cells is at least in part dependent on downregulation of cyclooxygenase-2 (COX-2) expression and COX-2-dependent prostanoid production (Fredenburgh et al., 2008; Liu F. et al., 2010; Liu et al., 2016; Dieffenbach et al., 2017). Importantly, suppression of matrix stiffening or stiffness-induced signaling using a LOX inhibitor (β-aminopropionitrile) or a long-acting prostanoid (iloprost) at the time of early PA stiffening prevented distal PA stiffening and later hemodynamic consequences of PH in vivo, demonstrating the therapeutic potential of disrupting mechanobiological feedback (Bertero et al., 2015; Liu et al., 2016). Over the past several years, a number of molecules have emerged as likely mediators of mechanobiologic feedback in the pulmonary vascular system, and may make promising future therapeutic targets to disrupt PH development and progression (Figure 2).
FIGURE 2.
Mechanisms of mechanobiological feedback.
Key Molecular Pathways Involved in Mechanobiological Feedback
Non-structural Determinants of Matrix Stiffness
Vascular stiffness ultimately arises from a combination of factors, including increased ECM deposition, matrix stiffening, vascular cell proliferation/hypertrophy, and active smooth muscle contraction. The major structural ECM components and their role in large vessel stiffness were discussed previously, however, matrix composition and mechanical properties are also altered by enzymatic activity that is closely regulated at the cellular level (Figure 2).
As detailed above, serine elastase production is elevated in several models of PH (Maruyama et al., 1991; Ye and Rabinovitch, 1991; Cowan et al., 2000a; Zaidi et al., 2002; Kim et al., 2011; Nickel et al., 2015), and its expression has been found to correlate with development of neointimal lesions in one model (Kim et al., 2011). In addition to shifting workload to less compliant collagen fibers (Schafer et al., 2016), elastin fragmentation within the medial layer can directly promote a variety of SMC remodeling behaviors, including phenotype switching, migration, and proliferation (Chelladurai et al., 2012). Mechanistically, this occurs through liberation of matrix bound SMC mitogens such as fibroblast growth factor (Thompson and Rabinovitch, 1996) and exposure of fibronectin-derived peptides and previously hidden integrin binding sites (Kafienah et al., 1998). Inhibition of serine elastases can lead to regression of progressive medial PA hypertrophy in culture ex vivo (Cowan et al., 2000b), and is also associated with decreased PA remodeling in disease models (Maruyama et al., 1991; Ye and Rabinovitch, 1991; Cowan et al., 2000a; Zaidi et al., 2002; Kim et al., 2011; Nickel et al., 2015). Recent studies by the Stenmark and Harrison groups have also shown inflammatory activation of macrophages by elastin fragments (Schafer et al., 2016). Pidkovka and colleagues, in particular, reported upregulation of the elastase matrix metalloproteinase 12 (MMP12) by mechanical stretch in vitro and MMP12-dependent aortic macrophage accumulation and subsequent vascular stiffening in vivo (Luft, 2012).
MMP12 is one of a large family of MMPs and their endogenous inhibitors (tissue inhibitors of MMPs, or TIMPs) that likely play a role in altering the vascular mechanical microenvironment. MMP2 and MMP9 are metalloproteinases with diverse ECM cleavage functions which were initially identified by their ability to degrade collagen IV of the basement membrane; they are also referred to as gelatinases based on their efficient cleavage of denatured collagen I. Elevated MMP2 expression and collagen IV degradation are seen in PH neointimal lesions (Matsui et al., 2002), and gelatinase levels are elevated in both serum and PASMCs derived from patients with PH (Benisty et al., 2005; Cantini-Salignac et al., 2006; Chelladurai et al., 2012). MMP2 and MMP9 activity are also increased in MCT and hypoxia-induced PH models (Frisdal et al., 2001; Schermuly et al., 2004; George and D’Armiento, 2011). Disruption and turnover of the basement membrane, like the internal elastic lamina, is therefore likely important to the development of intimal and medial hypertrophy. Indeed, gelatinase inhibition can disrupt PA medial hypertrophy ex vivo (Cowan et al., 2000b). Much remains to be understood about the drivers and mechanical impact of these basement membrane alterations.
MMPs also have extensive non-matrix secondary effects via cytokine processing and release from the ECM that likely contribute to the complexity of their regulation and their role in disease (Chelladurai et al., 2012). On a more global level, changes in matrix altering activity through MMP inhibition have been shown to have a mixed phenotype in PH (Chelladurai et al., 2012). While adenoviral-induced overexpression of TIMP1 in the lung is protective in the pro-inflammatory MCT PH model (Vieillard-Baron et al., 2003), both viral-mediated TIMP1 induction and MMP inhibition with doxycycline lead to increased vascular remodeling in the setting of chronic hypoxia (Vieillard-Baron et al., 2000). It is likely that initial collagen breakdown and ECM turnover contribute to PASMC proliferation and vascular remodeling through growth factor release and mechanotransduction, followed by increased collagen deposition and vascular stiffening (Bloodworth et al., 2015). Further study is greatly needed to clarify this process.
Lysyl oxidases are copper-dependent amine oxidase enzymes responsible for covalent cross-linking of collagen and elastin fibers, a process that imparts structural stability. Recently, Nave et al. (2014) identified upregulation of LOX family members (LOX, LOXL1, LOXL2, LOXL3, LOXL4) in multiple forms of human PH, including within plexiform and concentric vascular lesions. Expression of LOX, LOXL1, and LOXL2 was upregulated in human PASMCs in response to hypoxia, and increased in both the MCT rat model and the hypoxia mouse model of PH. In the hypoxia model, inhibition of LOX attenuated small artery muscularization, reduced vascular wall thickness and elastin accumulation, and decreased total collagen accumulation and cross-linking (Nave et al., 2014). We and the Chan laboratory have expanded on these findings, demonstrating that stiff matrix could induce LOX expression in PAAFs, as well as increase fibrillar collagen isoforms and collagen expression (Bertero et al., 2015). In particular, LOX upregulation was a key component of a complex mechanobiological positive feedback loop along with mechanotransductors YAP/TAZ and MiR 130/301 (see below) that led to progressive ECM stiffening in vivo. We verified the impact of LOX inhibition on hypoxia-induced PH, and tied it directly to inhibition of this mechanotransduction circuit. More importantly, we closely examined the impact of LOX inhibition in the MCT PH model, and demonstrated decreased collagen deposition and remodeling, reduced medial thickening, and directly decreased arterial stiffness as assessed by AFM (Bertero et al., 2015). These findings correlated with improvement in RV systolic pressures and RVH, as well as reduced activity of downstream mechanotransduction effectors as indicated above. The impressive impact of LOX inhibition in the MCT and hypoxia models illustrates successful disruption of mechanobiological feedback, and serves as a useful demonstration of the potential power of this approach.
Transglutaminase-2 (TG2) is a calcium-dependent crosslinking enzyme that is known to remodel collagen I and other components of the ECM, resulting in increased matrix stability and tissue rigidity (Eckert et al., 2014). In the systemic circulation, abolition of endothelial NO synthase (eNOS) activity upregulated matrix TG2 expression and crosslinking, and led to increased aortic and carotid stiffening (Jung et al., 2013). In PH, the Fanburg laboratory has shown that TG2 activity is upregulated in multiple rodent disease models, as well as in the serum of patients with PAH (Wei et al., 2012; DiRaimondo et al., 2014). In human PASMCs, TG2 was regulated both by mechanosensitive calcium channels (TRPV4, see below) and by hypoxia-inducible factor 1α, and was required for hypoxia-induced PASMC hyperproliferation (Penumatsa et al., 2014). Finally, TG2 inhibitor studies in the mouse sugen-hypoxia model demonstrated TG2-dependent upregulation of fibrotic markers in the lung and RV (Penumatsa et al., 2017), and also showed attenuated PA pressure elevations with TG2 inhibitor treatment (DiRaimondo et al., 2014). These studies indicate TG2-mediated ECM crosslinking also contributes to matrix stiffness in PH development.
Mechanosensors
All adherent cells derive critical signals from their interactions with the ECM and their mechanical microenvironment in order to regulate cell shape, survival, proliferation, and other phenotypes. Mechanical disruption can be converted to chemical signaling directly via mechanosensitive ion channels. In endothelial cells, a number of mechanosensitive channels, including the TRPV4 and PIEZO1 calcium channels and the inwardly rectifying potassium (Kir) channels, are responsible for shear-flow induced vasodilation through activation of eNOS (Hartmannsgruber et al., 2007; Mendoza et al., 2010; Wang et al., 2016; Ahn et al., 2017). Although not normally subject to blood shear flow, PASMCs are exposed to transmural interstitial flows proportionally to transmural pressure differences, and can also have direct exposure to luminal shear flow in the setting of PH-induced endothelial injury (Makino et al., 2011; Shi and Tarbell, 2011). Calcium signaling subsequently leads to pulmonary vasoconstriction (via activation of myosin light chain kinase) and proliferation (through activation of calmodulin kinase and pro-growth transcription factors) (Kuhr et al., 2012). Song et al. (2014) found that three known mechanosensitive ion channels, TRPV4, TRPM7, and TRPC6 were significantly upregulated in idiopathic PAH PASMCs compared to cells from non-diseased controls. This upregulation correlated with greatly increased cytoplasmic calcium in response to shear stress that could be partially blocked with TRPM7 and TRPV4 channel inhibitors. Yang X.R. et al. (2012) found that TRPV4 was upregulated in the setting of hypoxia in rats, and correlated with increased vascular tone in endothelium-denuded small PAs in this model. Furthermore, TRPV4 knockout mice demonstrated delayed and attenuated vascular remodeling in response to hypoxia, further supporting that these channels play a role in disease pathogenesis (Yang X.R. et al., 2012).
The transmission of mechanical information from the ECM to the cell cytoskeleton and signal transduction machinery is mediated primarily through integrin-based adhesions (Tschumperlin, 2011; Tschumperlin et al., 2018). Integrins, comprising heterodimers of α and β subunits, are transmembrane proteins that recognize specific matrix polypeptides, such as the arginine-glycine-aspartic acid (RGD) sequence in fibronectin. Integrin subunits have only small cytoplasmic domains, but serve as scaffolds for recruiting a variety of cell-signaling machinery into adhesion complexes. Activated integrins are connected to the actomyosin system structurally through interactions with linking proteins such as talin and vinculin (Sun et al., 2016). This machinery, often referred to as a “molecular clutch” allows cells to both transmit forces to the ECM and translate forces into molecular signaling (Case and Waterman, 2015). The inner workings of this machinery are just beginning to be understood. For example, some specific integrin heterodimers display catch-bond properties, allowing longer binding time under increased loading (Kong et al., 2009). Recently, Elosegui-Artola et al. (2016) demonstrated that the force-regulated unfolding of talin competes with integrin unbinding to generate a “rigidity threshold” for downstream mechanical activation in response to force transmission. In the setting of higher rigidity (above 5 kPa in their system), talin unfolding tends to occur before catch-bond release, exposing vinculin binding sites, activating adhesion complexes, and stimulating mechanotransduction signals (YAP nuclear translocation, see below). These processes likely occur through multiple mechanisms, including recruitment of additional integrins by talin and reinforcement of the mechanical clutch by vinculin-actin binding (Saltel et al., 2009; Hirata et al., 2014; Elosegui-Artola et al., 2016). This fine-tuned machinery allows for assembly of cell-matrix adhesion complexes that can respond rapidly to changes in applied forces (Tschumperlin et al., 2018).
As some of the most important mechanosensors, integrins likely have an extensive impact on mechanical signaling during the development of PH. In PASMCs, integrins are differentially regulated in PH disease models, with augmentation of downstream signaling (Umesh et al., 2011). In particular, α1, αv, and α8 signaling are increased in both MCT and chronic hypoxia, whereas α5 and β1 are decreased in chronic hypoxia and α5 and β3 are decreased in the MCT model (Umesh et al., 2011). Chronic vasoconstriction in the setting of worsening disease causes an integrin-dependent reorganization of the actin cytoskeleton favoring increased integrin contacts and cellular stiffening, and allows for an adaptive reduced engagement of the active contractile apparatus (Martinez-Lemus et al., 2004; Sehgel et al., 2013). In endothelial cells, the cytoskeleton is dominated by F-actin stress fibers that attach to integrin-based focal adhesions, and this organization is highly affected by vascular stiffness (Sun et al., 2016). Integrin signaling is involved in and enhances mechanoactivation of ion channels (Huveneers et al., 2015). TRPV4 and integrins play a synergistic role in a positive feedback loop driving endothelial cell alignment in response to cyclic stretch on flexible substrates (Thodeti et al., 2009). Integrin signaling is also known to drive extracellular transforming growth factor-β (TGFβ) activation through mechanically mediated release from its matrix-bound latent complex (Buscemi et al., 2011; Shi et al., 2011). Although high pulsatile flow has been found to increase TGFβ signaling (Scott et al., 2013) and altered TGFβ superfamily signaling is one of the hallmarks of familial and idiopathic PAH (Eickelberg and Morty, 2007; Aschner and Downey, 2016), direct mechanical activation of TGFβ has not yet been well studied in pulmonary vascular cells.
Cells also derive information regarding their mechanical microenvironment through cell-cell contact in the form of intercellular adhesions. The most studied of these cell-cell mechanotransducers are cadherin-based adhesions formed by membrane-spanning proteins of the cadherin family on adjacent cells (Tschumperlin et al., 2018). Several elegant experiments examining cell shape and cytoskeletal responses in cell monolayers have demonstrated conclusively that stiffening of the ECM, as well as internal or external tension forces, lead to remodeling of cell-cell junctions, changes in cell shape, and altered cell migration (Liu Z. et al., 2010; Krishnan et al., 2011; Huveneers et al., 2012). The increase in endothelial contractility and traction force development in stiff matrix via these mechanisms leads to enhanced responsiveness to angiogenic or inflammatory permeability factors during PH progression (Liu Z. et al., 2010; Huynh et al., 2011; Krishnan et al., 2011). In addition to investigations of effects of vascular stiffness, the mechanism of cell-cell response to luminal shear flow has been particularly well-studied in endothelial cells. Acute onset of shear stress or mechanical force leads to engagement of platelet endothelial cell adhesion molecule-1 (PECAM-1), along with vascular endothelial cadherin as an adaptor protein, leading to activation of downstream mechanotransductors (in particular Src family kinases) and upregulation of phosphoinositide 3-kinase and eNOS to regulate vascular tone (Osawa et al., 1997; Tzima et al., 2005; Conway et al., 2013). Fluid shear stress-mediated activation of PECAM-1 also triggers cytoskeletal remodeling in endothelial cells via interaction with integrins and downstream Rho GTPases (Tzima et al., 2001; Goldfinger et al., 2008; Collins et al., 2012). Cell stretch also can activate endothelial proliferation in a VE-cadherin-dependent fashion, likely via the downstream mechanotransductors YAP and TAZ (Neto et al., 2018). Thus, cell-cell adhesions contribute extensively to mechanical and biochemical signaling in ways that are highly relevant to endothelial homeostasis and endothelial injury.
Notable Cytosolic Mechanotransducers
Downstream of cell-matrix and cell-cell adhesion molecules, a large network of biochemical and cytoskeletal-interacting pathways contribute to the transduction of mechanical signals, however, a few key molecules have emerged as critical (Figure 2). For instance, the Src family of tyrosine kinases and FAK are both recruited to focal adhesions, modulate integrin-cytoskeletal links, and regulate each other’s activity (Tschumperlin, 2011; Bloodworth et al., 2015). In particular, Src activity has been shown to increase FAK expression at the cell surface and lead to greater force transmission in vascular smooth muscle; conversely, inhibition of FAK decreases Src activity in PASMCs (Paulin et al., 2014; Bloodworth et al., 2015).
Interestingly, Src activity is elevated in rodent models of PH, and tyrosine kinases with strong Src family inhibitory properties demonstrate improved reversal of experimental PH with treatment (Pullamsetti et al., 2012). Src phosphorylation of p130Cas, a stretch-responsive protein that exposes Src substrate domains under tension, has been shown to mediate mechanotransduction via multiple partners (Zamir and Geiger, 2001; Sawada et al., 2006). Furthermore, p130Cas has been found to be elevated in the serum, distal pulmonary arteries, and cultured PAECs and PASMCs from PAH patients, as well as in hypoxia and MCT models, and serves to amplify pro-remodeling receptor tyrosine kinase activity in the setting of progressive disease (Tu et al., 2012).
Similar to Src, FAK localizes to focal adhesions, self-activates tyrosine phosphorylation, and interacts with numerous components in response to mechanical strain (Fluck et al., 1999; Lee et al., 2000; Wang J.G. et al., 2001; Provenzano et al., 2009). Studies with FAK inhibitors have shown that FAK activation is required for mechanosensing in migration of both fibroblasts (Wang H.B. et al., 2001) and PASMCS (Paulin et al., 2014), and that FAK inhibition can abrogate PH development in the MCT rat model (Paulin et al., 2014).
Mechanical cues downstream of integrin signaling can influence the regulation of small Rho GTPases, including RhoA, Rac1, and CDC42, to alter the dynamics of the actin cytoskeleton and influence cell migration and proliferation. Rho GTPases are activated through recruitment of guanine nucleotide exchange factors (Birukova et al., 2006). Although these factors are often recruited via calcium signaling (Pardo-Pastor et al., 2018) and/or activation of FAK and Src family kinases (Guilluy et al., 2011; Bae et al., 2014; Pardo-Pastor et al., 2018), the exact regulatory mechanisms are integrin and cell-context dependent, and in many cases have not been fully worked out (Hoon et al., 2016). Activated Rho GTPases all promote actin assembly. RhoA is more involved in stress fiber formation, and Rac1 and CDC42 in filopodia and lamellipodia formation, respectively (Hoon et al., 2016).
RhoA is required for focal adhesion assembly and operates within focal adhesion complexes to regulate the dynamics and contractile activity of the actin cytoskeleton (Ridley and Hall, 1992). RhoA and its best-known downstream effector, RhoA-associated protein kinase (ROCK), are stimulated by cell stiffness (Klein et al., 2009), and also contribute to increased intracellular stiffness through stress fiber development and stimulation of actomyosin contraction (Maekawa et al., 1999; Riento and Ridley, 2003; Wang et al., 2009). RhoA and ROCK have been studied extensively in cardiovascular diseases, and have been found to be involved in the development of PH in disease models and in human disease. In endothelial cells, ROCK activity downregulates eNOS (Takemoto et al., 2002), upregulates inflammatory markers, and is responsible for cytoskeletal responses to shear stress (Li et al., 1999; Tzima et al., 2001; Katoh et al., 2008; Conway et al., 2013). ROCK activity is increased in both the media and the intima of pulmonary arteries (Shimizu et al., 2013) and also in cultured PASMCs derived from patients with idiopathic PAH (Guilluy et al., 2009). Treatment with the ROCK inhibitor fasudil suppresses both MCT and hypoxia-induced PH in rodents (Abe et al., 2004, 2006). In PH patients, fasudil has been found to decrease PVR after short-term use, and has had modest success in early clinical trials (Zhang and Wu, 2017). Furthermore, vascular smooth muscle-specific ROCK2 knockout mice were protected from hypoxia-induced PH, decreasing the likelihood of any off-target explanation for fasudil’s success (Shimizu et al., 2013).
Proteomics-based efforts have assiduously identified more than 500 additional individual proteins that are associated closely with cell-matrix adhesion complexes and facilitate cellular interactions with the surrounding mechanical environment (Kuo et al., 2011; Schiller et al., 2011). The overwhelming complexity of the interactions that occur due to mechanical perturbations has led to greater focus on pathways that integrate this information to drive transcriptional programs in response to environmental changes (Tschumperlin et al., 2018).
Transcriptional Mechanotransducers
Gene activation in response to mechanical signaling requires conveying mechanical information to the cell nucleus. While many transcription factors can play a role in this process, a small number of central coordinators of mechanical signaling appear to convey critical mechanical signals via nuclear translocation (Figure 2). β-catenin is a well-known transcriptional coactivator that is normally targeted for degradation in the cytosol via phosphorylation by glycogen synthase kinase-3β (GSK3β), the core member of the β-catenin destruction complex. β-catenin activation canonically occurs via wingless/integrase (Wnt) signaling pathways, and leads to β-catenin-dependent activation of processes involved in proliferation, survival, and migration in many cell types. In epithelial cells, mechanical stretch and tissue stiffness lead to activation of β-catenin downstream of RhoA and ROCK activation (Heise et al., 2011; Samuel et al., 2011). Although stiffness-induced activation has not been well studied in PH, enhanced canonical (β-catenin-activating) Wnt signaling was associated with multiple cell types derived from PAH patients in a large non-biased screen examining gene expression signatures associated with PAH (West et al., 2014). Enhanced Wnt signaling was also found in a separate screen for targets of miRNAs elevated in end-stage PAH (Wu et al., 2016). Pulmonary artery resistance vessels from patients with idiopathic PAH demonstrated endothelial upregulation of β-catenin in idiopathic PAH vessels that correlated with upregulation of RhoA, ROCK, and Rac1 as assessed by immunohistochemistry and qPCR (Laumanns et al., 2009). Finally, downregulation of β-catenin reduces hypoxia-induced PASMC proliferation and PA remodeling in hypoxia models of PH (Yu et al., 2013; Alapati et al., 2014; Jin et al., 2015). These data together indicate a potential role for β-catenin activation downstream of stiffness-induced vascular remodeling in PH.
Nuclear factor-kappa B (NFκB) is a transcription factor most well-known for its involvement in inflammatory and immune responses, including flow-mediated inflammatory responses in endothelial cells (Davis et al., 2004; Orr et al., 2008; Petzold et al., 2009). The Stenmark laboratory group have shown that NFκB activation and subsequent upregulation of inflammatory markers occurs early and continuously in the setting of high-pulsatility flow (Li et al., 2013). Furthermore, this activation is dependent upon cell polarity changes and structural reorganization via actin and microtubule remodeling (Li et al., 2013). NFκB activation is also known to be sensitive to calcium influx, and can be activated by mechanical stretch-induced calcium currents via the TRPC3 channel in fibroblasts (Ishise et al., 2015). In endothelial cells, non-receptor activated currents through TRPC3 can lead to NFκB activation and expression of inflammatory cell adhesion molecules (Smedlund et al., 2010). Inhibition of NFκB genetically in the lung reduced endothelial apoptosis, endothelial-mesenchymal transition, and development of PH in the MCT model. Although further study on mechanical signaling via NFκB is needed, the current studies indicate a role for NFκB in inflammatory and endothelial cell responses to alterations in the mechanical microenvironment.
One critical link between stiffness-mediated cytoskeletal remodeling and gene expression are the MRTFs. The MRTFs form stable complexes with monomeric soluble actin (G-actin), leading to the sequestration of these complexes in the cytoplasm. Polymerization of G-actin into F-actin filaments and stress fibers in the setting of increased mechanical stress or migration liberates these factors to translocate to the nucleus, where they act as cofactors for serum response factor (Tschumperlin et al., 2018). Activity of MRTFs regulates the expression of multiple gene products central to contractile machinery in myofibroblasts (Small et al., 2010; Huang et al., 2012; Haak et al., 2014; Scharenberg et al., 2014), and is a downstream effector of profibrotic signaling by matrix stiffness (Huang et al., 2012) and Rho/ROCK pathways in this setting (Esnault et al., 2014). As stiffness-driven activators of pro-fibrotic and contractile activity, MRTFs are therefore critical components of a profibrotic mechanobiological feedback loop. In endothelial cells, MRTF-A has been shown to mediate inflammatory marker production downstream of RhoA activity in response to exposure to oxidized LDL (Fang et al., 2011). In vascular smooth muscle, MRTF-A was likewise involved in inflammatory mediator activation downstream of endothelin-1 activity (Yang Y. et al., 2014). Inhibition of MRTF-A in the pulmonary vasculature using shRNA injection significantly attenuated the development of hypoxic PH in rats (Yuan et al., 2014). This improvement correlated with reduced expression of inflammatory chemokines in the lung, ECM protein production by PASMCs, and collagen deposition in the PA beds (Yuan et al., 2014). Whether MRTF contributes significantly to mechanobiological feedback in PH via these or other mechanisms has yet to be fully elucidated.
YAP and TAZ are closely related transcriptional modifiers that have recently emerged as powerful effectors of mechanical signaling (Dupont et al., 2011; Halder et al., 2012; Aragona et al., 2013; Dupont, 2016). YAP/TAZ subcellular localization is tightly linked to ECM stiffness, with stiff matrix driving nuclear localization and activation, and soft matrix promoting cytoplasmic retention. Manipulation of YAP/TAZ levels can mimic changes in matrix stiffness with regard to proliferation, apoptosis, and cell differentiation, and can drive lung fibrosis (Dupont et al., 2011; Aragona et al., 2013; Liu et al., 2015).
Recent investigations into the complex signaling that regulates YAP/TAZ activity have only now begun to make this process clear. As members of the Hippo pathway, YAP and TAZ are biochemically regulated by phosphorylation by the serine-threonine kinases LATS1 and LATS2, with phosphorylation leading to cytoplasmic sequestration or degradation (Halder et al., 2012). However, a large variety of mechanical cues, including ECM rigidity, strain, shear stress, and adhesive area, facilitate YAP nuclear localization (Dupont et al., 2011; Wada et al., 2011; Aragona et al., 2013; Chaudhuri et al., 2016; Das et al., 2016; Nakajima et al., 2017) in a fashion potentially independent of YAP phosphorylation status (Dupont et al., 2011; Das et al., 2016; Elosegui-Artola et al., 2017). Inhibitor studies have demonstrated that this mechanical regulation requires cytoskeletal integrity (Dupont et al., 2011; Das et al., 2016), as well as actomyosin contractility (Dupont et al., 2011; Valon et al., 2017), and is inhibited by actin capping and severing proteins (Aragona et al., 2013).
YAP activation is also dependent on sufficient force-loading of integrins to allow talin unfolding and reinforcement of cell-matrix adhesions after integrin-ligand binding (Elosegui-Artola et al., 2016). Based upon these mechanical stimuli, it is not surprising that YAP nuclear localization is impaired by inhibition of FAK (Kim and Gumbiner, 2015; Lachowski et al., 2018), Src (Kim and Gumbiner, 2015), and Rho/ROCK signaling (Dupont et al., 2011; Yu et al., 2012). Most recently, two different reports demonstrated that YAP nuclear localization requires intact stress and strain transmission to the nucleus itself through integrity of the LINC complex (Driscoll et al., 2015; Elosegui-Artola et al., 2017). Nuclear strain transfer is notably dependent only on a patent actin cytoskeleton independent of microtubules and intermediate filaments, providing an explanation for the observation that YAP/TAZ nuclear localization is not impaired by microtubule depolymerization (Dupont et al., 2011). Elosegui-Artola et al. (2017) expanded on this finding by demonstrating that nuclear force application using AFM is sufficient to drive YAP nuclear translocation on soft matrix or stiff substrates with cytoskeletal disruption. Further examination using nuclear transport inhibitors and osmotic perturbations of nuclear shape demonstrated that rigidity-induced nuclear flattening led to increased cytosolic nuclear pore exposure and increased YAP nuclear import (Elosegui-Artola et al., 2017). This elegant series of experiments demonstrates that YAP and TAZ mechanotransduction is a direct readout of force transmission from the matrix through the actin cytoskeleton to the nucleus in the form of stress fiber-mediated nuclear flattening.
Upon nuclear translocation, YAP and TAZ associate with a number of promoter-specific transcription factors to drive mechanical signaling. The most notable and canonical of these factors are members of the transcriptional enhancer activator domain (TEAD) family, whose downstream products drive cellular proliferation and survival (Halder et al., 2012). However, there is mounting evidence for considerable cross-talk between YAP and TAZ and other key mechanotransduction pathways. Specifically, YAP and TAZ were found to be cofactors for SMAD signaling in the nucleus and involved in nuclear cross-talk with MRTFs (Varelas et al., 2008, 2010b; Speight et al., 2016; Yu et al., 2016; Kim et al., 2017), both of which have impacts on mechanical and TGF-β signaling. Additional YAP/TAZ interactions with both TGF-β/SMAD and β-catenin signaling are complex, involving some degree of mutual cooperativity in nuclear transcription but mutual sequestration and degradation in the cytoplasm (Varelas et al., 2010a; Heallen et al., 2011; Azzolin et al., 2012, 2014). In general, these cross-talk mechanisms appear to allow significant synergy in the setting of co-activation or co-inhibition while preventing pathologic feed-forward loops in the setting of mixed signaling.
Recent studies have revealed important roles for YAP and TAZ in vascular pathology and PH development. In vascular smooth muscle, YAP is induced after injury and promotes cellular migration, proliferation, and neointimal formation (Wang et al., 2012; Kimura et al., 2016). In PH, we and the Chan laboratory have found that YAP/TAZ upregulation in response to matrix stiffness promotes collagen deposition and cross-linking in PAAFs via miRNA-130/301 induction (Bertero et al., 2015). ECM remodeled by cells over-expressing YAP or miRNA-130/301 induced proliferation of PAECs, PASMCs, and PAAFs, demonstrating the potential for a positive feedback loop in response to ECM stiffening. Kudryashova et al. (2016) also identified an ECM-associated upregulation of YAP expression that was maintained by PASMCs derived from idiopathic PAH patients compared to controls. This fibronectin-associated elevation in YAP activity was linked to enhanced proliferation and survival via integrin signaling (Kudryashova et al., 2016). In PAECs, YAP/TAZ signaling is required for stiffness-associated increases in glutaminolysis; inhibition of either YAP/TAZ (via verteporfin) or glutaminolysis led to improvements in vascular stiffness, RV pressures, and RVH in the MCT rat model of PH (Bertero et al., 2016). Finally, we have recently examined PASMC response to matrix stiffening, and have found that YAP/TAZ signaling is a critical regulator of stiffness-associated remodeling behaviors (Dieffenbach et al., 2017). Mechanoactivation of YAP and TAZ promotes proliferation, LOX activity, enhanced contractility, and increased migration, all of which lead to further arterial stiffening and feedback amplification of remodeling. Mechanistically, this mechanobiological feedback is driven by YAP/TAZ-mediated suppression of COX-2 expression and prostaglandin signaling, which normally help maintain pulmonary vascular homeostasis (Dieffenbach et al., 2017). Genetic COX-2 deficiency alone in mice leads to PASMC remodeling and more severe hypoxia-induced PH (Fredenburgh et al., 2008, 2009), whereas early treatment with a long-acting prostacyclin analog can attenuate stiffness-induced remodeling behaviors and MCT-induced PH (Liu et al., 2016). Despite very different approaches to the investigation of pulmonary vascular remodeling, these studies each found a central role for YAP/TAZ activation in the process, highlighting the important role of mechanical signaling in PH development.
Alternative Mechanical Effectors
Recent studies of the nuclear lamina have identified several key mechanosensing proteins that connect the nucleus to the actin cytoskeleton (now identified as the LINC complex) (Zhen et al., 2002; Padmakumar et al., 2004, 2005; Poh et al., 2012). Beyond their potential role in mechanical alterations in nuclear transport (as with YAP/TAZ, above) (Elosegui-Artola et al., 2017), these structural components can alter chromatin organization and impact gene transcription in response to substrate stiffness (Janmey et al., 2013; Swift et al., 2013; Alam et al., 2016). Genetic defects in these structural proteins lead to mesenchymal dysfunction and organ fibrosis, including cardiac fibrosis, but have not been linked to PH (Peng et al., 2006).
Like most significant cellular inputs, mechanical forces are likely to induce epigenetic changes in chromatin remodeling via DNA methylation and histone modifications; however, this process is relatively understudied. Some recent examinations have found that matrix stiffness and mechanical inputs can alter chromatin organization (Downing et al., 2013; Crowder et al., 2016; Le et al., 2016) and increase histone acetylation (Valenzuela-Fernandez et al., 2008; Li et al., 2011). These epigenetic changes can persist over time and through cell divisions, and may play a key role in long term changes in response to more transient alterations in the mechanical environment, a term called “molecular memory” (Yang C. et al., 2014; Heo et al., 2015). Further study of these mechanisms will allow a more complete understanding of mechanical signaling.
Finally, the rapidly expanding field of microRNA (miR) biology has led to the examination of miR regulation by mechanical signaling. A recent elegant investigation by Frith et al. (2018) screened for miRs upregulated by stiffness and/or RhoA signaling in mesenchymal stromal cells (MSCs). They verified two miRs (miR-100-5p and miR-143-3p) that biased MSCs further toward differentiation pathways associated with stiff ECM, demonstrating positive mechanobiological feedback via miR activity (Frith et al., 2018). In the endothelium, a large number of “mechano-miRs” have been found to respond to alterations in shear stress, and have been implicated in subsequent inflammatory signaling, apoptosis, and NO production (Kumar et al., 2014). In PH, we and the Chan group identified miR21, miR27a, and YAP/TAZ-dependent miR130/301 complex upregulation in response to ECM stiffness (Bertero et al., 2015). miR21 has been extensively studied in PH and found to have pleiotropic effects, with miR21 knockout mice displaying increased PH severity while miR21 inhibition leads to reduced vascular remodeling after hypoxia (Parikh et al., 2012; Yang S. et al., 2012; Iannone et al., 2014; White et al., 2014). Inhibition of the miR130/301 complex, however, decreased LOX production, fibrillar collagen deposition, and YAP nuclear translocation in cultured fibroblasts and the pulmonary vasculature in vivo, demonstrating disruption of mechanobiological feedback (Bertero et al., 2015). This translated to improved pulmonary pressures and RVH as well as decreased vascular stiffening in experimental PH models (Bertero et al., 2015).
Naturally, given the numerous miRs and their vast networks of targets, there are many miRs that have been found to modulate mechanical signaling pathways, including ECM components (Nanoudis et al., 2017), FAK (Eskildsen et al., 2011), β-catenin (Wu et al., 2016), and YAP (Xu et al., 2015). The role for these miRs in the regulation of mechanical signaling in PH, as well as their therapeutic potential, warrants further study (Negi and Chan, 2017). In particular, miRs are pleiotropic and often broadly expressed, so additional focus on the relative safety and efficacy of miR targeting of these mechanical pathways will be required.
Concluding Remarks
Over the past several years, the increasing evidence for early development of PA stiffening and its contribution to RV workload in PH has led to an increased focus on arterial stiffness changes in the pathogenesis and potential early treatment of disease. Arterial stiffening has been found to occur fairly early throughout the pulmonary vasculature, affecting both large elastic arteries and small distal vessels. The downstream effects of this vascular stiffening include alterations in flow characteristics and changes in ECM composition that predispose to inflammation and continued pathologic vascular remodeling. The molecular mechanosensors that facilitate this process are now beginning to be understood, and their links to downstream signaling that locally reinforce matrix stiffening and force transmission delineated. Multiple recent studies have highlighted this process during PH development, and in so doing have demonstrated a potential therapeutic effect of disrupting this feedback loop. Further study of mechanobiological feedback in PH will be needed to more thoroughly interrogate these pathways and ultimately identify optimal targets for future therapy.
Author Contributions
Review conception and design was by PD, LF, and DT. The figures were illustrated by MM. The manuscript was drafted by PD and LF and was approved by all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- α-SMA
α-smooth muscle actin, an actin monomer upregulated in smooth muscle, myofibroblasts, and endothelial cells undergoing mesenchymal transition
- AFM
atomic force microscopy – a method for measurements of local mechanical stiffness at the microscopic level
- COX-2
Cyclooxygenase 2, the key enzyme for production of multiple prostaglandin lipid mediators
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase, a cellular enzyme responsible for the production of nitric oxide in endothelial cells
- FAK
focal adhesion kinase, a mechanotransducer upregulated by integrin signaling
- GSK3β
Glycogen synthase kinase 3-β, the core member of the β-catenin destruction complex
- kPa
Kilopascal, SI units for pressure and stiffness
- LINC
linker of nucleoskeleton and cytoskeleton complex, a complex of proteins along the nuclear membrane that facilitates interactions between the nucleus and actin cytoskeleton
- LOX
lysyl oxidase, a collagen cross-linking enzyme
- LV
left ventricle
- MCT
monocrotaline, a compound that induces PH in a rodent model
- miRs
Micro-ribonucleic acid oligonucleotides that bind to messenger RNA and regulate gene expression
- MMPs
matrix metalloproteinases, enzymes responsible for enzymatic cleavage of ECM components
- mPAP
mean pulmonary artery pressure, the average pressure measured in the proximal pulmonary arteries during catheterization
- MRI
magnetic resonance imaging
- MRTFs
Myocardin-related transcription factors, transcription factors activated by actin polymerization and known to be important mechnotransducers and activators of serum response factor
- NFκB
Nuclear factor-κB, a transcription factor involved in inflammatory and immune responses, as well as responses to flow stresses in endothelial cells
- NO
Nitric oxide, an exogenously produced vasodilatory signaling molecule
- PA
pulmonary artery
- PAAFs
pulmonary artery adventitial fibroblasts
- PAECs
pulmonary artery endothelial cells
- PAH
pulmonary arterial hypertension, Group 1 PH specifically arising from pathology in the pulmonary arterial tree
- PASMCs
pulmonary artery smooth muscle cells
- PECAM
platelet endothelial cell adhesion molecule, a cell-cell adhesion molecule expressed on endothelial cells
- PGF1α
Prostaglandin-F1α, an enzymatic product of COX-2 activity
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance, defined as the ratio between the fall in pressure across the pulmonary arterial tree and the cardiac output
- Rho GTPases
the Rho family of guanine trinucleotide phosphate hydrolyzing enzymes, known regulators of actin dynamics. The three canonical members are Ras-homolog gene family, member a (RhoA), Ras-related C3 botulinum toxin substrate 1 (Rac1), and Cell division control protein 42 homolog (CDC42)
- ROCK
RhoA-associated kinase, a well-known downstream effector of RhoA
- RV
right ventricle
- RVH
right ventricular hypertrophy, enlargement of the RV free wall associated with PH progression
- SMADs
similar to mothers against decapentaplegic, these intracellular proteins transduce signals from TGFβ superfamily members and act as transcription factors
- SMC
smooth muscle cell
- TAZ
Transcriptional co-activator with PDZ-binding motif, a transcriptional modifier and nuclear mechanotransducer
- TEADs
TEA Domain proteins are the primary nuclear binding partners of YAP and TAZ
- TG2
Transglutaminase-2, a calcium dependent cross-linking enzyme that remodels collagen I and other ECM components
- TGFβ
A cytokine belonging to a superfamily of signaling proteins; TGFβ has frequently been associated with inflammation and fibrosis
- TIMPs
tissue inhibitors of matrix metalloproteinases, secreted proteins that endogenously inhibit MMP activity
- TRPs
Transient receptor potential, a group of ion channels located on the plasma membrane, some of which (TRPV4, TRPC3, TRPC6, TRPM7) are mechanosensitive
- Wnts
Wingless/integrase, a family of signaling molecules and associated receptors
- YAP
yes-associated protein, a transcriptional modifier and mechanotransducer
Footnotes
Funding. This work was supported by grants from the NIH including F32 HL131228 (PD), R01 HL114839 (DT, LF), and R01 HL137366 (DT, LF) as well as an American Heart Association Grant-in-Aid (LF).
References
- Abe K., Shimokawa H., Morikawa K., Uwatoku T., Oi K., Matsumoto Y., et al. (2004). Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ. Res. 94 385–393. 10.1161/01.RES.0000111804.34509.94 [DOI] [PubMed] [Google Scholar]
- Abe K., Tawara S., Oi K., Hizume T., Uwatoku T., Fukumoto Y., et al. (2006). Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J. Cardiovasc. Pharmacol. 48 280–285. 10.1097/01.fjc.0000248244.64430.4a [DOI] [PubMed] [Google Scholar]
- Ahn S. J., Fancher I. S., Bian J. T., Zhang C. X., Schwab S., Gaffin R., et al. (2017). Inwardly rectifying K+ channels are major contributors to flow-induced vasodilatation in resistance arteries. J. Physiol. 595 2339–2364. 10.1113/JP273255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S. G., Zhang Q., Prasad N., Li Y., Chamala S., Kuchibhotla R., et al. (2016). The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 6:38063. 10.1038/srep38063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapati D., Rong M., Chen S., Hehre D., Hummler S. C., Wu S. (2014). Inhibition of beta-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am. J. Respir. Cell Mol. Biol. 51 104–113. 10.1165/rcmb.2013-0346OC [DOI] [PubMed] [Google Scholar]
- Al-Naamani N., Preston I. R., Paulus J. K., Hill N. S., Roberts K. E. (2015). Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 3 467–474. 10.1016/j.jchf.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., et al. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154 1047–1059. 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Aschner Y., Downey G. P. (2016). Transforming growth factor-beta: master regulator of the respiratory system in health and disease. Am. J. Respir. Cell Mol. Biol. 54 647–655. 10.1165/rcmb.2015-0391TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., et al. (2014). YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158 157–170. 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., et al. (2012). Role of TAZ as mediator of Wnt signaling. Cell 151 1443–1456. 10.1016/j.cell.2012.11.027 [DOI] [PubMed] [Google Scholar]
- Bae Y. H., Mui K. L., Hsu B. Y., Liu S. L., Cretu A., Razinia Z., et al. (2014). A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal. 7:ra57. 10.1126/scisignal.2004838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell V., Mccabe E. L., Larson M. G., Rong J., Merz A. A., Osypiuk E., et al. (2017). Relations between aortic stiffness and left ventricular mechanical function in the community. J. Am. Heart Assoc. 6:e004903. 10.1161/JAHA.116.004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benisty J. I., Folkman J., Zurakowski D., Louis G., Rich S., Langleben D., et al. (2005). Matrix metalloproteinases in the urine of patients with pulmonary arterial hypertension. Chest 128(Suppl. 6):572S. 10.1378/chest.128.6_suppl.572S [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Spears M., Boustred C., May M., Anderson S. G., Benjamin E. J., et al. (2014). Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 63 636–646. 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero T., Cottrill K. A., Lu Y., Haeger C. M., Dieffenbach P., Annis S., et al. (2015). Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep. 13 1016–1032. 10.1016/j.celrep.2015.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero T., Oldham W. M., Cottrill K. A., Pisano S., Vanderpool R. R., Yu Q., et al. (2016). Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Invest. 126 3313–3335. 10.1172/JCI86387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova A. A., Adyshev D., Gorshkov B., Bokoch G. M., Birukov K. G., Verin A. D. (2006). GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 290 L540–L548. 10.1152/ajplung.00259.2005 [DOI] [PubMed] [Google Scholar]
- Birukova A. A., Tian X., Cokic I., Beckham Y., Gardel M. L., Birukov K. G. (2013). Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc. Res. 87 50–57. 10.1016/j.mvr.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodworth N. C., West J. D., Merryman W. D. (2015). Microvessel mechanobiology in pulmonary arterial hypertension: cause and effect. Hypertension 65 483–489. 10.1161/HYPERTENSIONAHA.114.04652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutouyrie P., Tropeano A. I., Asmar R., Gautier I., Benetos A., Lacolley P., et al. (2002). Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39 10–15. 10.1161/hy0102.099031 [DOI] [PubMed] [Google Scholar]
- Buscemi L., Ramonet D., Klingberg F., Formey A., Smith-Clerc J., Meister J. J., et al. (2011). The single-molecule mechanics of the latent TGF-beta1 complex. Curr. Biol. 21 2046–2054. 10.1016/j.cub.2011.11.037 [DOI] [PubMed] [Google Scholar]
- Campo A., Mathai S. C., Le Pavec J., Zaiman A. L., Hummers L. K., Boyce D., et al. (2010). Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 182 252–260. 10.1164/rccm.200912-1820OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini-Salignac C., Lartaud I., Schrijen F., Atkinson J., Chabot F. (2006). Metalloproteinase-9 in circulating monocytes in pulmonary hypertension. Fundam. Clin. Pharmacol. 20 405–410. 10.1111/j.1472-8206.2006.00417.x [DOI] [PubMed] [Google Scholar]
- Case L. B., Waterman C. M. (2015). Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 17 955–963. 10.1038/ncb3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S. A., Weaver J. C., et al. (2016). Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15 326–334. 10.1038/nmat4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelladurai P., Seeger W., Pullamsetti S. S. (2012). Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur. Respir. J. 40 766–782. 10.1183/09031936.00209911 [DOI] [PubMed] [Google Scholar]
- Chiu J. J., Chien S. (2011). Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91 327–387. 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C., Guilluy C., Welch C., O’brien E. T., Hahn K., Superfine R., et al. (2012). Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr. Biol. 22 2087–2094. 10.1016/j.cub.2012.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D. E., Breckenridge M. T., Hinde E., Gratton E., Chen C. S., Schwartz M. A. (2013). Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23 1024–1030. 10.1016/j.cub.2013.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper L. L., Mitchell G. F. (2016). Aortic stiffness, cerebrovascular dysfunction, and memory. Pulse 4 69–77. 10.1159/000448176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. N., Heilbut A., Humpl T., Lam C., Ito S., Rabinovitch M. (2000a). Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat. Med. 6 698–702. [DOI] [PubMed] [Google Scholar]
- Cowan K. N., Jones P. L., Rabinovitch M. (2000b). Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J. Clin. Invest. 105 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder S. W., Leonardo V., Whittaker T., Papathanasiou P., Stevens M. M. (2016). Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell 18 39–52. 10.1016/j.stem.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Fischer R. S., Pan D., Waterman C. M. (2016). YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, myosin II- and phospho-YAP-independent pathway during extracellular matrix mechanosensing. J. Biol. Chem. 291 6096–6110. 10.1074/jbc.M115.708313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. E., Grumbach I. M., Fukai T., Cutchins A., Harrison D. G. (2004). Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J. Biol. Chem. 279 163–168. 10.1074/jbc.M307528200 [DOI] [PubMed] [Google Scholar]
- Dieffenbach P. B., Mallarino Haeger C., Coronata A. M. F., Choi K. M., Varelas X., Tschumperlin D. J., et al. (2017). Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 313 L628–L647. 10.1152/ajplung.00173.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRaimondo T. R., Klock C., Warburton R., Herrera Z., Penumatsa K., Toksoz D., et al. (2014). Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem. Biol. 9 266–275. 10.1021/cb4006408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing T. L., Soto J., Morez C., Houssin T., Fritz A., Yuan F., et al. (2013). Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 12 1154–1162. 10.1038/nmat3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragu R., Rispler S., Habib M., Sholy H., Hammerman H., Galie N., et al. (2015). Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur. J. Heart Fail. 17 74–80. 10.1002/ejhf.192 [DOI] [PubMed] [Google Scholar]
- Driscoll T. P., Cosgrove B. D., Heo S. J., Shurden Z. E., Mauck R. L. (2015). Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J. 108 2783–2793. 10.1016/j.bpj.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S. (2016). Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 343 42–53. 10.1016/j.yexcr.2015.10.034 [DOI] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474 179–183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Kaartinen M. T., Nurminskaya M., Belkin A. M., Colak G., Johnson G. V., et al. (2014). Transglutaminase regulation of cell function. Physiol. Rev. 94 383–417. 10.1152/physrev.00019.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelberg O., Morty R. E. (2007). Transforming growth factor beta/bone morphogenic protein signaling in pulmonary arterial hypertension: remodeling revisited. Trends Cardiovasc. Med. 17 263–269. 10.1016/j.tcm.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Elosegui-Artola A., Andreu I., Beedle A. E. M., Lezamiz A., Uroz M., Kosmalska A. J., et al. (2017). Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171 1397.e14–1410.e14. 10.1016/j.cell.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Elosegui-Artola A., Oria R., Chen Y., Kosmalska A., Perez-Gonzalez C., Castro N., et al. (2016). Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 18 540–548. 10.1038/ncb3336 [DOI] [PubMed] [Google Scholar]
- Eskildsen T., Taipaleenmaki H., Stenvang J., Abdallah B. M., Ditzel N., Nossent A. Y., et al. (2011). MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. U.S.A. 108 6139–6144. 10.1073/pnas.1016758108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault C., Stewart A., Gualdrini F., East P., Horswell S., Matthews N., et al. (2014). Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 28 943–958. 10.1101/gad.239327.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Yang Y., Yuan Z., Gao Y., Zhou J., Chen Q., et al. (2011). Myocardin-related transcription factor A mediates OxLDL-induced endothelial injury. Circ. Res. 108 797–807. 10.1161/CIRCRESAHA.111.240655 [DOI] [PubMed] [Google Scholar]
- Farber H. W., Miller D. P., Poms A. D., Badesch D. B., Frost A. E., Muros-Le Rouzic E., et al. (2015). Five-year outcomes of patients enrolled in the reveal registry. Chest 148 1043–1054. 10.1378/chest.15-0300 [DOI] [PubMed] [Google Scholar]
- Fluck M., Carson J. A., Gordon S. E., Ziemiecki A., Booth F. W. (1999). Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am. J. Physiol. 277 C152–C162. 10.1152/ajpcell.1999.277.1.C152 [DOI] [PubMed] [Google Scholar]
- Fredenburgh L. E., Liang O. D., Macias A. A., Polte T. R., Liu X., Riascos D. F., et al. (2008). Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation 117 2114–2122. 10.1161/CIRCULATIONAHA.107.716241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredenburgh L. E., Ma J., Perrella M. A. (2009). Cyclooxygenase-2 inhibition and hypoxia-induced pulmonary hypertension: effects on pulmonary vascular remodeling and contractility. Trends Cardiovasc. Med. 19 31–37. 10.1016/j.tcm.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisdal E., Gest V., Vieillard-Baron A., Levame M., Lepetit H., Eddahibi S., et al. (2001). Gelatinase expression in pulmonary arteries during experimental pulmonary hypertension. Eur. Respir. J. 18 838–845. 10.1183/09031936.01.00084601 [DOI] [PubMed] [Google Scholar]
- Frith J. E., Kusuma G. D., Carthew J., Li F., Cloonan N., Gomez G. A., et al. (2018). Mechanically-sensitive miRNAs bias human mesenchymal stem cell fate via mTOR signalling. Nat. Commun. 9:257. 10.1038/s41467-017-02486-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan C. T., Lankhaar J. W., Westerhof N., Marcus J. T., Becker A., Twisk J. W., et al. (2007). Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132 1906–1912. 10.1378/chest.07-1246 [DOI] [PubMed] [Google Scholar]
- George J., D’Armiento J. (2011). Transgenic expression of human matrix metalloproteinase-9 augments monocrotaline-induced pulmonary arterial hypertension in mice. J. Hypertens. 29 299–308. 10.1097/HJH.0b013e328340a0e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges P. C., Hui J. J., Gombos Z., Mccormick M. E., Wang A. Y., Uemura M., et al. (2007). Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 293 G1147–G1154. 10.1152/ajpgi.00032.2007 [DOI] [PubMed] [Google Scholar]
- Goldfinger L. E., Tzima E., Stockton R., Kiosses W. B., Kinbara K., Tkachenko E., et al. (2008). Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ. Res. 103 177–185. 10.1161/CIRCRESAHA.108.176354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C., Eddahibi S., Agard C., Guignabert C., Izikki M., Tu L., et al. (2009). RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am. J. Respir. Crit. Care Med. 179 1151–1158. 10.1164/rccm.200805-691OC [DOI] [PubMed] [Google Scholar]
- Guilluy C., Swaminathan V., Garcia-Mata R., O’brien E. T., Superfine R., Burridge K. (2011). The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13 722–727. 10.1038/ncb2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino-Debrac D. (2013). Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 1:e24180. 10.4161/tisb.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak A. J., Tsou P. S., Amin M. A., Ruth J. H., Campbell P., Fox D. A., et al. (2014). Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor-regulated gene transcription prevent fibrosis in a murine model of skin injury. J. Pharmacol. Exp. Ther. 349 480–486. 10.1124/jpet.114.213520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G., Dupont S., Piccolo S. (2012). Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13 591–600. 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- Hartmannsgruber V., Heyken W. T., Kacik M., Kaistha A., Grgic I., Harteneck C., et al. (2007). Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2:e827. 10.1371/journal.pone.0000827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto J., Ito S. (2011). Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58 839–846. 10.1161/HYPERTENSIONAHA.111.177469 [DOI] [PubMed] [Google Scholar]
- Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., et al. (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332 458–461. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise R. L., Stober V., Cheluvaraju C., Hollingsworth J. W., Garantziotis S. (2011). Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J. Biol. Chem. 286 17435–17444. 10.1074/jbc.M110.137273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S. J., Thorpe S. D., Driscoll T. P., Duncan R. L., Lee D. A., Mauck R. L. (2015). Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci. Rep. 5:16895. 10.1038/srep16895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Tatsumi H., Lim C. T., Sokabe M. (2014). Force-dependent vinculin binding to talin in live cells: a crucial step in anchoring the actin cytoskeleton to focal adhesions. Am. J. Physiol. Cell Physiol. 306 C607–C620. 10.1152/ajpcell.00122.2013 [DOI] [PubMed] [Google Scholar]
- Hislop A., Reid L. (1976). New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br. J. Exp. Pathol. 57 542–554. [PMC free article] [PubMed] [Google Scholar]
- Hoon J. L., Tan M. H., Koh C. G. (2016). The regulation of cellular responses to mechanical cues by Rho GTPases. Cells 5:E17. 10.3390/cells5020017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Foster M. C., Mitchell G. F., Andresdottir M. B., Eiriksdottir G., Gudmundsdottir H., et al. (2017). Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol. Dial. Transplant. 32 677–684. 10.1093/ndt/gfw050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yang N., Fiore V. F., Barker T. H., Sun Y., Morris S. W., et al. (2012). Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 47 340–348. 10.1165/rcmb.2012-0050OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. S., Lammers S. R., Shandas R. (2011). Pulmonary vascular stiffness: measurement, modeling, and implications in normal and hypertensive pulmonary circulations. Compr. Physiol. 1 1413–1435. 10.1002/cphy.c100005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. S., Lee P. F., Lanning C. J., Ivy D. D., Kirby K. S., Claussen L. R., et al. (2008). Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am. Heart. J. 155 166–174. 10.1016/j.ahj.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S., Daemen M. J., Hordijk P. L. (2015). Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ. Res. 116 895–908. 10.1161/CIRCRESAHA.116.305720 [DOI] [PubMed] [Google Scholar]
- Huveneers S., Oldenburg J., Spanjaard E., Van Der Krogt G., Grigoriev I., Akhmanova A., et al. (2012). Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 196 641–652. 10.1083/jcb.201108120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J., Nishimura N., Rana K., Peloquin J. M., Califano J. P., Montague C. R., et al. (2011). Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 3:112ra122. 10.1126/scitranslmed.3002761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone L., Zhao L., Dubois O., Duluc L., Rhodes C. J., Wharton J., et al. (2014). miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem. J. 462 103–112. 10.1042/BJ20140486 [DOI] [PubMed] [Google Scholar]
- Ishise H., Larson B., Hirata Y., Fujiwara T., Nishimoto S., Kubo T., et al. (2015). Hypertrophic scar contracture is mediated by the TRPC3 mechanical force transducer via NFkB activation. Sci. Rep. 5:11620. 10.1038/srep11620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Wells R. G., Assoian R. K., Mcculloch C. A. (2013). From tissue mechanics to transcription factors. Differentiation 86 112–120. 10.1016/j.diff.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Wang W., Chai S., Liu J., Yang T., Wang J. (2015). Wnt5a attenuates hypoxia-induced pulmonary arteriolar remodeling and right ventricular hypertrophy in mice. Exp. Biol. Med. 240 1742–1751. 10.1177/1535370215584889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. M., Jandu S., Steppan J., Belkin A., An S. S., Pak A., et al. (2013). Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice. Am. J. Physiol. Heart Circ. Physiol. 305 H803–H810. 10.1152/ajpheart.00103.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess B. M., Rong J., Larson M. G., Hamburg N. M., Vita J. A., Cheng S., et al. (2016). Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham heart study. J. Am. Heart Assoc. 5:e002693. 10.1161/JAHA.115.002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess B. M., Rong J., Larson M. G., Hamburg N. M., Vita J. A., Levy D., et al. (2012). Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308 875–881. 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafienah W., Buttle D. J., Burnett D., Hollander A. P. (1998). Cleavage of native type I collagen by human neutrophil elastase. Biochem. J. 330(Pt 2) 897–902. 10.1042/bj3300897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. W., Chang H. J., Kim Y. J., Choi B. W., Lee H. S., Yang W. I., et al. (2011). Cardiac magnetic resonance imaging-derived pulmonary artery distensibility index correlates with pulmonary artery stiffness and predicts functional capacity in patients with pulmonary arterial hypertension. Circ. J. 75 2244–2251. 10.1253/circj.CJ-10-1310 [DOI] [PubMed] [Google Scholar]
- Katoh K., Kano Y., Ookawara S. (2008). Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cells in situ. Vasc. Health Risk Manag. 4 1273–1282. 10.2147/VHRM.S3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. P., Tunin R., Kass D. A. (1992). Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ. Res. 71 490–502. 10.1161/01.RES.71.3.490 [DOI] [PubMed] [Google Scholar]
- Kim N. G., Gumbiner B. M. (2015). Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J. Cell Biol. 210 503–515. 10.1083/jcb.201501025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Hwang D., Lee D., Kim J. H., Kim S. Y., Lim D. S. (2017). MRTF potentiates TEAD-YAP transcriptional activity causing metastasis. EMBO J. 36 520–535. 10.15252/embj.201695137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Haghighat L., Spiekerkoetter E., Sawada H., Alvira C. M., Wang L., et al. (2011). Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am. J. Pathol. 179 1560–1572. 10.1016/j.ajpath.2011.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T. E., Duggirala A., Smith M. C., White S., Sala-Newby G. B., Newby A. C., et al. (2016). The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J. Mol. Cell Cardiol. 90 1–10. 10.1016/j.yjmcc.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E. A., Yin L., Kothapalli D., Castagnino P., Byfield F. J., Xu T., et al. (2009). Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr. Biol. 19 1511–1518. 10.1016/j.cub.2009.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobs R. W., Muvarak N. E., Eickhoff J. C., Chesler N. C. (2005). Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 288 H1209–H1217. 10.1152/ajpheart.01129.2003 [DOI] [PubMed] [Google Scholar]
- Kong F., Garcia A. J., Mould A. P., Humphries M. J., Zhu C. (2009). Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 185 1275–1284. 10.1083/jcb.200810002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R., Klumpers D. D., Park C. Y., Rajendran K., Trepat X., Van Bezu J., et al. (2011). Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am. J. Physiol. Cell Physiol. 300 C146–C154. 10.1152/ajpcell.00195.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashova T. V., Goncharov D. A., Pena A., Kelly N., Vanderpool R., Baust J., et al. (2016). HIPPO-integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 194 866–877. 10.1164/rccm.201510-2003OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhr F. K., Smith K. A., Song M. Y., Levitan I., Yuan J. X. (2012). New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am. J. Physiol. Heart Circ. Physiol. 302 H1546–H1562. 10.1152/ajpheart.00944.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik T. J. (2014). Pulmonary hypertension caused by pulmonary venous hypertension. Pulm. Circ. 4 581–595. 10.1086/678471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kim C. W., Simmons R. D., Jo H. (2014). Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 34 2206–2216. 10.1161/ATVBAHA.114.303425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. C., Han X., Hsiao C. T., Yates J. R., III, Waterman C. M. (2011). Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13 383–393. 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowski D., Cortes E., Robinson B., Rice A., Rombouts K., Del Rio Hernandez A. E. (2018). FAK controls the mechanical activation of YAP, a transcriptional regulator required for durotaxis. FASEB J. 32 1099–1107. 10.1096/fj.201700721R [DOI] [PubMed] [Google Scholar]
- Lammers S. R., Kao P. H., Qi H. J., Hunter K., Lanning C., Albietz J., et al. (2008). Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am. J. Physiol. Heart Circ. Physiol. 295 H1451–H1459. 10.1152/ajpheart.00127.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankhaar J. W., Westerhof N., Faes T. J., Gan C. T., Marques K. M., Boonstra A., et al. (2008). Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur. Heart J. 29 1688–1695. 10.1093/eurheartj/ehn103 [DOI] [PubMed] [Google Scholar]
- Lau E. M., Chemla D., Godinas L., Zhu K., Sitbon O., Savale L., et al. (2016). Loss of vascular distensibility during exercise is an early hemodynamic marker of pulmonary vascular disease. Chest 149 353–361. 10.1378/chest.15-0125 [DOI] [PubMed] [Google Scholar]
- Laumanns I. P., Fink L., Wilhelm J., Wolff J. C., Mitnacht-Kraus R., Graef-Hoechst S., et al. (2009). The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 40 683–691. 10.1165/rcmb.2008-0153OC [DOI] [PubMed] [Google Scholar]
- Le H. Q., Ghatak S., Yeung C. Y., Tellkamp F., Gunschmann C., Dieterich C., et al. (2016). Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18 864–875. 10.1038/ncb3387 [DOI] [PubMed] [Google Scholar]
- Lee H. S., Millward-Sadler S. J., Wright M. O., Nuki G., Salter D. M. (2000). Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J. Bone Miner. Res. 15 1501–1509. 10.1359/jbmr.2000.15.8.1501 [DOI] [PubMed] [Google Scholar]
- Li M., Scott D. E., Shandas R., Stenmark K. R., Tan W. (2009). High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann. Biomed. Eng. 37 1082–1092. 10.1007/s10439-009-9684-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tan Y., Stenmark K. R., Tan W. (2013). High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-kappaB. Cardiovasc. Eng. Technol. 4 26–38. 10.1007/s13239-012-0115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chen B. P., Azuma N., Hu Y. L., Wu S. Z., Sumpio B. E., et al. (1999). Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J. Clin. Invest. 103 1141–1150. 10.1172/JCI5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chu J. S., Kurpinski K., Li X., Bautista D. M., Yang L., et al. (2011). Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys. J. 100 1902–1909. 10.1016/j.bpj.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan J. H., Haworth S. T., Nelin L. D., Krenz G. S., Dawson C. A. (1992). A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J. Appl. Physiol. 73 987–994. 10.1152/jappl.1992.73.3.987 [DOI] [PubMed] [Google Scholar]
- Liu F., Haeger C. M., Dieffenbach P. B., Sicard D., Chrobak I., Coronata A. M., et al. (2016). Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 1:e86987. 10.1172/jci.insight.86987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Lagares D., Choi K. M., Stopfer L., Marinkovic A., Vrbanac V., et al. (2015). Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308 L344–L357. 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Mih J. D., Shea B. S., Kho A. T., Sharif A. S., Tager A. M., et al. (2010). Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190 693–706. 10.1083/jcb.201004082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Tan J. L., Cohen D. M., Yang M. T., Sniadecki N. J., Ruiz S. A., et al. (2010). Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. U.S.A. 107 9944–9949. 10.1073/pnas.0914547107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Tschumperlin D. J. (2011). Micro-mechanical characterization of lung tissue using atomic force microscopy. J. Vis. Exp. 54:2911. 10.3791/2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F. C. (2012). Molecular mechanisms of arterial stiffness: new insights. J. Am. Soc. Hypertens. 6 436–438. 10.1016/j.jash.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., et al. (1999). Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285 895–898. 10.1126/science.285.5429.895 [DOI] [PubMed] [Google Scholar]
- Mahapatra S., Nishimura R. A., Oh J. K., Mcgoon M. D. (2006a). The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J. Am. Soc. Echocardiogr. 19 1045–1050. [DOI] [PubMed] [Google Scholar]
- Mahapatra S., Nishimura R. A., Sorajja P., Cha S., Mcgoon M. D. (2006b). Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J. Am. Coll. Cardiol. 47 799–803. 10.1016/j.jacc.2005.09.054 [DOI] [PubMed] [Google Scholar]
- Makino A., Firth A. L., Yuan J. X. (2011). Endothelial and smooth muscle cell ion channels in pulmonary vasoconstriction and vascular remodeling. Compr. Physiol. 1 1555–1602. 10.1002/cphy.c100023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Dhakal B. P., Eisman A. S., Pappagianopoulos P. P., Dress A., Weiner R. B., et al. (2016). Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ. Heart Fail. 9:e003011. 10.1161/CIRCHEARTFAILURE.115.003011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T., Ingber D. E. (2010). Mechanical control of tissue and organ development. Development 137 1407–1420. 10.1242/dev.024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lemus L. A., Hill M. A., Bolz S. S., Pohl U., Meininger G. A. (2004). Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. FASEB J. 18 708–710. 10.1096/fj.03-0634fje [DOI] [PubMed] [Google Scholar]
- Maruyama K., Ye C. L., Woo M., Venkatacharya H., Lines L. D., Silver M. M., et al. (1991). Chronic hypoxic pulmonary hypertension in rats and increased elastolytic activity. Am. J. Physiol. 261 H1716–H1726. 10.1152/ajpheart.1991.261.6.H1716 [DOI] [PubMed] [Google Scholar]
- Matsui K., Takano Y., Yu Z. X., Hi J. E., Stetler-Stevenson W. G., Travis W. D., et al. (2002). Immunohistochemical study of endothelin-1 and matrix metalloproteinases in plexogenic pulmonary arteriopathy. Pathol. Res. Pract. 198 403–412. 10.1078/0344-0338-00273 [DOI] [PubMed] [Google Scholar]
- McLaughlin V. V., Archer S. L., Badesch D. B., Barst R. J., Farber H. W., Lindner J. R., et al. (2009). ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation task force on expert consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Coll. Cardiol. 53 1573–1619. 10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Fang J., Gutterman D. D., Wilcox D. A., Bubolz A. H., Li R., et al. (2010). TRPV4-mediated endothelial Ca2+influx and vasodilation in response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 298 H466–H476. 10.1152/ajpheart.00854.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Hislop A., Reid L. (1978). Pulmonary arteries of the normal rat: the thick walled oblique muscle segment. J. Anat. 125 209–221. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. (1979a). Development of pulmonary arterial changes in rats fed Crotalaria spectabilis. Am. J. Pathol. 94 37–50. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. (1979b). Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am. J. Pathol. 96 51–70. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. (1980). Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am. J. Pathol. 100 151–178. [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Parise H., Benjamin E. J., Larson M. G., Keyes M. J., Vita J. A., et al. (2004). Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43 1239–1245. 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- Nakajima H., Yamamoto K., Agarwala S., Terai K., Fukui H., Fukuhara S., et al. (2017). Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev. Cell 40 523.e6–536.e6. 10.1016/j.devcel.2017.02.019 [DOI] [PubMed] [Google Scholar]
- Nanoudis S., Pikilidou M., Yavropoulou M., Zebekakis P. (2017). The role of micrornas in arterial stiffness and arterial calcification. an update and review of the literature. Front. Genet. 8:209. 10.3389/fgene.2017.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave A. H., Mizikova I., Niess G., Steenbock H., Reichenberger F., Talavera M. L., et al. (2014). Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 34 1446–1458. 10.1161/ATVBAHA.114.303534 [DOI] [PubMed] [Google Scholar]
- Negi V., Chan S. Y. (2017). Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2:e91327. 10.1172/jci.insight.91327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto F., Klaus-Bergmann A., Ong Y. T., Alt S., Vion A. C., Szymborska A., et al. (2018). YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. eLife 7:e31037. 10.7554/eLife.31037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel N. P., Spiekerkoetter E., Gu M., Li C. G., Li H., Kaschwich M., et al. (2015). Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am. J. Respir. Crit. Care Med. 191 1273–1286. 10.1164/rccm.201412-2291OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi C. Y., Wang Z., Tabima D. M., Eickhoff J. C., Chesler N. C. (2010). The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 299 H1823–H1831. 10.1152/ajpheart.00493.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. W., Hahn C., Blackman B. R., Schwartz M. A. (2008). p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ. Res. 103 671–679. 10.1161/CIRCRESAHA.108.182097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M., Masuda M., Harada N., Lopes R. B., Fujiwara K. (1997). Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 72 229–237. [PubMed] [Google Scholar]
- Padmakumar V. C., Abraham S., Braune S., Noegel A. A., Tunggal B., Karakesisoglou I., et al. (2004). Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp. Cell Res. 295 330–339. 10.1016/j.yexcr.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Padmakumar V. C., Libotte T., Lu W., Zaim H., Abraham S., Noegel A. A., et al. (2005). The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 118 3419–3430. 10.1242/jcs.02471 [DOI] [PubMed] [Google Scholar]
- Pardo-Pastor C., Rubio-Moscardo F., Vogel-Gonzalez M., Serra S. A., Afthinos A., Mrkonjic S., et al. (2018). Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. U.S.A. 115 1925–1930. 10.1073/pnas.1718177115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V. N., Jin R. C., Rabello S., Gulbahce N., White K., Hale A., et al. (2012). MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 125 1520–1532. 10.1161/CIRCULATIONAHA.111.060269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase M. P., Beiser A., Himali J. J., Tsao C., Satizabal C. L., Vasan R. S., et al. (2016). Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47 2256–2261. 10.1161/STROKEAHA.116.013508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin R., Meloche J., Courboulin A., Lambert C., Haromy A., Courchesne A., et al. (2014). Targeting cell motility in pulmonary arterial hypertension. Eur. Respir. J. 43 531–544. 10.1183/09031936.00181312 [DOI] [PubMed] [Google Scholar]
- Pellegrini P., Rossi A., Pasotti M., Raineri C., Cicoira M., Bonapace S., et al. (2014). Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 145 1064–1070. 10.1378/chest.13-1510 [DOI] [PubMed] [Google Scholar]
- Peng X., Kraus M. S., Wei H., Shen T. L., Pariaut R., Alcaraz A., et al. (2006). Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J. Clin. Invest. 116 217–227. 10.1172/JCI24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penumatsa K. C., Toksoz D., Warburton R. R., Hilmer A. J., Liu T., Khosla C., et al. (2014). Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 307 L576–L585. 10.1152/ajplung.00162.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penumatsa K. C., Toksoz D., Warburton R. R., Kharnaf M., Preston I. R., Kapur N. K., et al. (2017). Transglutaminase 2 in pulmonary and cardiac tissue remodeling in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 313 L752–L762. 10.1152/ajplung.00170.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perepelyuk M., Terajima M., Wang A. Y., Georges P. C., Janmey P. A., Yamauchi M., et al. (2013). Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am. J. Physiol. Gastrointest. Liver Physiol. 304 G605–G614. 10.1152/ajpgi.00222.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold T., Orr A. W., Hahn C., Jhaveri K. A., Parsons J. T., Schwartz M. A. (2009). Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am. J. Physiol. Cell Physiol. 297 C814–C822. 10.1152/ajpcell.00226.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup M. W., Mouw J. K., Weaver V. M. (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15 1243–1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegstra M. J., Brokelman J. G. M., Roos-Hesselink J. W., Douwes J. M., Van Osch-Gevers L. M., Hoendermis E. S., et al. (2018). Pulmonary arterial stiffness indices assessed by intravascular ultrasound in children with early pulmonary vascular disease: prediction of advanced disease and mortality during 20-year follow-up. Eur. Heart J. Cardiovasc. Imaging 19 216–224. 10.1093/ehjci/jex015 [DOI] [PubMed] [Google Scholar]
- Poh Y. C., Shevtsov S. P., Chowdhury F., Wu D. C., Na S., Dundr M., et al. (2012). Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat. Commun. 3:866. 10.1038/ncomms1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiani G. J., Tozzi C. A., Yohn S. E., Pierce R. A., Belsky S. A., Berg R. A., et al. (1990). Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ. Res. 66 968–978. 10.1161/01.RES.66.4.968 [DOI] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Keely P. J. (2009). Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28 4326–4343. 10.1038/onc.2009.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullamsetti S. S., Berghausen E. M., Dabral S., Tretyn A., Butrous E., Savai R., et al. (2012). Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 32 1354–1365. 10.1161/ATVBAHA.112.248500 [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Gamble W., Nadas A. S., Miettinen O. S., Reid L. (1979). Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am. J. Physiol. 236 H818–H827. 10.1152/ajpheart.1979.236.6.H818 [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70 389–399. 10.1016/0092-8674(92)90163-7 [DOI] [PubMed] [Google Scholar]
- Riento K., Ridley A. J. (2003). Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4 446–456. 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- Rogers N. M., Yao M., Sembrat J., George M. P., Knupp H., Ross M., et al. (2013). Cellular, pharmacological, and biophysical evaluation of explanted lungs from a patient with sickle cell disease and severe pulmonary arterial hypertension. Pulm. Circ. 3 936–951. 10.1086/674754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltel F., Mortier E., Hytonen V. P., Jacquier M. C., Zimmermann P., Vogel V., et al. (2009). New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control beta3-integrin clustering. J. Cell Biol. 187 715–731. 10.1083/jcb.200908134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M. S., Lopez J. I., Mcghee E. J., Croft D. R., Strachan D., Timpson P., et al. (2011). Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19 776–791. 10.1016/j.ccr.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J., Kariisa M., Dellegrottaglie S., Prat-Gonzalez S., Garcia M. J., Fuster V., et al. (2009). Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc. Imaging 2 286–295. 10.1016/j.jcmg.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Saouti N., Westerhof N., Helderman F., Marcus J. T., Boonstra A., Postmus P. E., et al. (2010a). Right ventricular oscillatory power is a constant fraction of total power irrespective of pulmonary artery pressure. Am. J. Respir. Crit. Care Med. 182 1315–1320. 10.1164/rccm.200910-1643OC [DOI] [PubMed] [Google Scholar]
- Saouti N., Westerhof N., Postmus P. E., Vonk-Noordegraaf A. (2010b). The arterial load in pulmonary hypertension. Eur. Respir. Rev. 19 197–203. 10.1183/09059180.00002210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saouti N., Westerhof N., Helderman F., Marcus J. T., Stergiopulos N., Westerhof B. E., et al. (2009). RC time constant of single lung equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 297 H2154–H2160. 10.1152/ajpheart.00694.2009 [DOI] [PubMed] [Google Scholar]
- Sawada Y., Tamada M., Dubin-Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., et al. (2006). Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127 1015–1026. 10.1016/j.cell.2006.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M., Myers C., Brown R. D., Frid M. G., Tan W., Hunter K., et al. (2016). Pulmonary arterial stiffness: toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr. Hypertens. Rep. 18:4. 10.1007/s11906-015-0609-2 [DOI] [PubMed] [Google Scholar]
- Scharenberg M. A., Pippenger B. E., Sack R., Zingg D., Ferralli J., Schenk S., et al. (2014). TGF-beta-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J. Cell Sci. 127 1079–1091. 10.1242/jcs.142075 [DOI] [PubMed] [Google Scholar]
- Schermuly R. T., Kreisselmeier K. P., Ghofrani H. A., Samidurai A., Pullamsetti S., Weissmann N., et al. (2004). Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ. Res. 94 1101–1108. 10.1161/01.RES.0000126050.41296.8E [DOI] [PubMed] [Google Scholar]
- Schiller H. B., Friedel C. C., Boulegue C., Fassler R. (2011). Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12 259–266. 10.1038/embor.2011.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D., Tan Y., Shandas R., Stenmark K. R., Tan W. (2013). High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 304 L70–L81. 10.1152/ajplung.00342.2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sehgel N. L., Zhu Y., Sun Z., Trzeciakowski J. P., Hong Z., Hunter W. C., et al. (2013). Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am. J. Physiol. Heart Circ. Physiol. 305 H1281–H1287. 10.1152/ajpheart.00232.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A. Q., Lighthouse J. K., Greif D. M. (2014). Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep. 6 809–817. 10.1016/j.celrep.2014.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A. Q., Misra A., Rosas I. O., Adams R. H., Greif D. M. (2015). Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci. Transl. Med. 7:308ra159. 10.1126/scitranslmed.aaa9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., et al. (2011). Latent TGF-beta structure and activation. Nature 474 343–349. 10.1038/nature10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z. D., Tarbell J. M. (2011). Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann. Biomed. Eng. 39 1608–1619. 10.1007/s10439-011-0309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifren A., Durmowicz A. G., Knutsen R. H., Faury G., Mecham R. P. (2008). Elastin insufficiency predisposes to elevated pulmonary circulatory pressures through changes in elastic artery structure. J. Appl. Physiol. 105 1610–1619. 10.1152/japplphysiol.90563.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Fukumoto Y., Tanaka S., Satoh K., Ikeda S., Shimokawa H. (2013). Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice. Arterioscler. Thromb. Vasc. Biol. 33 2780–2791. 10.1161/ATVBAHA.113.301357 [DOI] [PubMed] [Google Scholar]
- Sicard D., Fredenburgh L. E., Tschumperlin D. J. (2017). Measured pulmonary arterial tissue stiffness is highly sensitive to AFM indenter dimensions. J. Mech. Behav. Biomed. Mater. 74 118–127. 10.1016/j.jmbbm.2017.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard D., Haak A. J., Choi K. M., Craig A. R., Fredenburgh L. E., Tschumperlin D. J. (2018). Aging and anatomical variations in lung tissue stiffness. Am. J. Physiol. Lung Cell. Mol. Physiol. 314 L946–L955. 10.1152/ajplung.00415.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. M., Thatcher J. E., Sutherland L. B., Kinoshita H., Gerard R. D., Richardson J. A., et al. (2010). Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 107 294–304. 10.1161/CIRCRESAHA.110.223172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedlund K., Tano J. Y., Vazquez G. (2010). The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ. Res. 106 1479–1488. 10.1161/CIRCRESAHA.109.213314 [DOI] [PubMed] [Google Scholar]
- Song S., Yamamura A., Yamamura H., Ayon R. J., Smith K. A., Tang H., et al. (2014). Flow shear stress enhances intracellular Ca2+signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am. J. Physiol. Cell Physiol. 307 C373–C383. 10.1152/ajpcell.00115.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight P., Kofler M., Szaszi K., Kapus A. (2016). Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFbeta-regulated Smad3. Nat. Commun. 7:11642. 10.1038/ncomms11642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Tan W., Shandas R., Hunter K. S. (2013). Influence of distal resistance and proximal stiffness on hemodynamics and RV afterload in progression and treatments of pulmonary hypertension: a computational study with validation using animal models. Comput. Math. Methods Med. 2013:618326. 10.1155/2013/618326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Guo S. S., Fassler R. (2016). Integrin-mediated mechanotransduction. J. Cell Biol. 215 445–456. 10.1083/jcb.201609037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G., Michelakis E. D. (2014). The metabolic basis of pulmonary arterial hypertension. Cell Metab. 19 558–573. 10.1016/j.cmet.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Swift J., Ivanovska I. L., Buxboim A., Harada T., Dingal P. C., Pinter J., et al. (2013). Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341:1240104. 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M., Sun J., Hiroki J., Shimokawa H., Liao J. K. (2002). Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 106 57–62. 10.1161/01.CIR.0000020682.73694.AB [DOI] [PubMed] [Google Scholar]
- Tan W., Madhavan K., Hunter K. S., Park D., Stenmark K. R. (2014). Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm. Circ. 4 560–580. 10.1086/677370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y., Tseng P. O., Wang D., Zhang H., Hunter K., Hertzberg J., et al. (2014). Stiffening-induced high pulsatility flow activates endothelial inflammation via a TLR2/NF-kappaB pathway. PLoS One 9:e102195. 10.1371/journal.pone.0102195 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thenappan T., Prins K. W., Pritzker M. R., Scandurra J., Volmers K., Weir E. K. (2016). The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann. Am. Thorac. Soc. 13 276–284. 10.1513/AnnalsATS.201509-599FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti C. K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A. L., et al. (2009). TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104 1123–1130. 10.1161/CIRCRESAHA.108.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K., Rabinovitch M. (1996). Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J. Cell. Physiol. 166 495–505. [DOI] [PubMed] [Google Scholar]
- Todorovich-Hunter L., Dodo H., Ye C., Mccready L., Keeley F. W., Rabinovitch M. (1992). Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am. Rev. Respir. Dis. 146 213–223. 10.1164/ajrccm/146.1.213 [DOI] [PubMed] [Google Scholar]
- Tozzi C. A., Christiansen D. L., Poiani G. J., Riley D. J. (1994). Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am. J. Respir. Crit. Care Med. 149 1317–1326. 10.1164/ajrccm.149.5.8173773 [DOI] [PubMed] [Google Scholar]
- Tsamis A., Krawiec J. T., Vorp D. A. (2013). Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J. R. Soc. Interface 10:20121004. 10.1098/rsif.2012.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C. W., Himali J. J., Beiser A. S., Larson M. G., Decarli C., Vasan R. S., et al. (2016). Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 86 619–626. 10.1212/WNL.0000000000002368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumperlin D. J. (2011). Mechanotransduction. Compr. Physiol. 1 1057–1073. 10.1002/cphy.c100016 [DOI] [PubMed] [Google Scholar]
- Tschumperlin D. J. (2015). Matrix, mesenchyme, and mechanotransduction. Ann. Am. Thorac. Soc. 12(Suppl. 1) S24–S29. 10.1513/AnnalsATS.201407-320MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumperlin D. J., Ligresti G., Hilscher M. B., Shah V. H. (2018). Mechanosensing and fibrosis. J. Clin. Invest. 128 74–84. 10.1172/JCI93561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L., De Man F. S., Girerd B., Huertas A., Chaumais M. C., Lecerf F., et al. (2012). A critical role for p130Cas in the progression of pulmonary hypertension in humans and rodents. Am. J. Respir. Crit. Care Med. 186 666–676. 10.1164/rccm.201202-0309OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder R. M., Marecki J. C., Richter A., Fijalkowska I., Flores S. (2007). Pathology of pulmonary hypertension. Clin. Chest Med. 28 23–42 vii. 10.1016/j.ccm.2006.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., Del Pozo M. A., Shattil S. J., Chien S., Schwartz M. A. (2001). Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20 4639–4647. 10.1093/emboj/20.17.4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., et al. (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437 426–431. 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- Umesh A., Paudel O., Cao Y. N., Myers A. C., Sham J. S. (2011). Alteration of pulmonary artery integrin levels in chronic hypoxia and monocrotaline-induced pulmonary hypertension. J. Vasc. Res. 48 525–537. 10.1159/000329593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A., Cabrero J. R., Serrador J. M., Sanchez-Madrid F. (2008). HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 18 291–297. 10.1016/j.tcb.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Valon L., Marin-Llaurado A., Wyatt T., Charras G., Trepat X. (2017). Optogenetic control of cellular forces and mechanotransduction. Nat. Commun. 8:14396. 10.1038/ncomms14396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X., Miller B. W., Sopko R., Song S., Gregorieff A., Fellouse F. A., et al. (2010a). The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 18 579–591. 10.1016/j.devcel.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B. G., et al. (2010b). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell 19 831–844. 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Varelas X., Sakuma R., Samavarchi-Tehrani P., Peerani R., Rao B. M., Dembowy J., et al. (2008). TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10 837–848. 10.1038/ncb1748 [DOI] [PubMed] [Google Scholar]
- Vieillard-Baron A., Frisdal E., Eddahibi S., Deprez I., Baker A. H., Newby A. C., et al. (2000). Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer or doxycycline aggravates pulmonary hypertension in rats. Circ. Res. 87 418–425. 10.1161/01.RES.87.5.418 [DOI] [PubMed] [Google Scholar]
- Vieillard-Baron A., Frisdal E., Raffestin B., Baker A. H., Eddahibi S., Adnot S., et al. (2003). Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer limits monocrotaline-induced pulmonary vascular remodeling in rats. Hum. Gene Ther. 14 861–869. 10.1089/104303403765701150 [DOI] [PubMed] [Google Scholar]
- Wada K., Itoga K., Okano T., Yonemura S., Sasaki H. (2011). Hippo pathway regulation by cell morphology and stress fibers. Development 138 3907–3914. 10.1242/dev.070987 [DOI] [PubMed] [Google Scholar]
- Wang H. B., Dembo M., Hanks S. K., Wang Y. (2001). Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. U.S.A. 98 11295–11300. 10.1073/pnas.201201198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. G., Miyazu M., Matsushita E., Sokabe M., Naruse K. (2001). Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem. Biophys. Res. Commun. 288 356–361. 10.1006/bbrc.2001.5775 [DOI] [PubMed] [Google Scholar]
- Wang K. L., Cheng H. M., Sung S. H., Chuang S. Y., Li C. H., Spurgeon H. A., et al. (2010). Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 55 799–805. 10.1161/HYPERTENSIONAHA.109.139964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Chennupati R., Kaur H., Iring A., Wettschureck N., Offermanns S. (2016). Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Invest. 126 4527–4536. 10.1172/JCI87343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu G., Gao X., Wang Y., Zhang W., Harmon E. Y., et al. (2012). The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler. Thromb. Vasc. Biol. 32 2662–2669. 10.1161/ATVBAHA.112.254730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zheng X. R., Riddick N., Bryden M., Baur W., Zhang X., et al. (2009). ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ. Res. 104 531–540. 10.1161/CIRCRESAHA.108.188524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Chesler N. C. (2011). Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm. Circ. 1 212–223. 10.4103/2045-8932.83453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Warburton R. R., Preston I. R., Roberts K. E., Comhair S. A., Erzurum S. C., et al. (2012). Serotonylated fibronectin is elevated in pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 302 L1273–L1279. 10.1152/ajplung.00082.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod R. M., Shiang T., Al Sayah L., Fry J. L., Bajpai S., Reinhart-King C. A., et al. (2013). Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 62 1105–1110. 10.1161/HYPERTENSIONAHA.113.01744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. D., Austin E. D., Gaskill C., Marriott S., Baskir R., Bilousova G., et al. (2014). Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am. J. Physiol. Cell Physiol. 307 C415–C430. 10.1152/ajpcell.00057.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Dempsie Y., Caruso P., Wallace E., Mcdonald R. A., Stevens H., et al. (2014). Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 64 185–194. 10.1161/HYPERTENSIONAHA.113.03037 [DOI] [PubMed] [Google Scholar]
- Wilkinson M., Langhorne C. A., Heath D., Barer G. R., Howard P. (1988). A pathophysiological study of 10 cases of hypoxic cor pulmonale. Q. J. Med. 66 65–85. [PubMed] [Google Scholar]
- Wu D., Talbot C. C., Jr., Liu Q., Jing Z. C., Damico R. L., Tuder R., et al. (2016). Identifying microRNAs targeting Wnt/beta-catenin pathway in end-stage idiopathic pulmonary arterial hypertension. J. Mol. Med. 94 875–885. 10.1007/s00109-016-1426-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Ahmed A. S., Kang X., Hu G., Liu F., Zhang W., et al. (2015). MicroRNA-15b/16 attenuates vascular neointima formation by promoting the contractile phenotype of vascular smooth muscle through targeting YAP. Arterioscler. Thromb. Vasc. Biol. 35 2145–2152. 10.1161/ATVBAHA.115.305748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Tibbitt M. W., Basta L., Anseth K. S. (2014). Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13 645–652. 10.1038/nmat3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cheng X., Tian W., Zhou B., Wu X., Xu H., et al. (2014). MRTF-A steers an epigenetic complex to activate endothelin-induced pro-inflammatory transcription in vascular smooth muscle cells. Nucleic Acids Res. 42 10460–10472. 10.1093/nar/gku776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Banerjee S., Freitas A., Cui H., Xie N., Abraham E., et al. (2012). miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 302 L521–L529. 10.1152/ajplung.00316.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. R., Lin A. H., Hughes J. M., Flavahan N. A., Cao Y. N., Liedtke W., et al. (2012). Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 302 L555–L568. 10.1152/ajplung.00005.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C. L., Rabinovitch M. (1991). Inhibition of elastolysis by SC-37698 reduces development and progression of monocrotaline pulmonary hypertension. Am. J. Physiol. 261 H1255–H1267. 10.1152/ajpheart.1991.261.4.H1255 [DOI] [PubMed] [Google Scholar]
- Yu F. X., Zhao B., Panupinthu N., Jewell J. L., Lian I., Wang L. H., et al. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150 780–791. 10.1016/j.cell.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu O. M., Miyamoto S., Brown J. H. (2016). Myocardin-Related Transcription factor a and yes-associated protein exert dual control in G protein-coupled receptor- and rhoa-mediated transcriptional regulation and cell proliferation. Mol. Cell. Biol. 36 39–49. 10.1128/MCB.00772-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M., Wang L., Li J. F., Liu J., Li J., Wang W., et al. (2013). Wnt5a inhibits hypoxia-induced pulmonary arterial smooth muscle cell proliferation by downregulation of beta-catenin. Am. J. Physiol. Lung Cell. Mol. Physiol. 304 L103–L111. 10.1152/ajplung.00070.2012 [DOI] [PubMed] [Google Scholar]
- Yuan Z., Chen J., Chen D., Xu G., Xia M., Xu Y., et al. (2014). Megakaryocytic leukemia 1 (MKL1) regulates hypoxia induced pulmonary hypertension in rats. PLoS One 9:e83895. 10.1371/journal.pone.0083895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S. H., You X. M., Ciura S., Husain M., Rabinovitch M. (2002). Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105 516–521. 10.1161/hc0402.102866 [DOI] [PubMed] [Google Scholar]
- Zamir E., Geiger B. (2001). Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114 3583–3590. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wu S. (2017). Effects of fasudil on pulmonary hypertension in clinical practice. Pulm. Pharmacol. Ther. 46 54–63. 10.1016/j.pupt.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Zhen Y. Y., Libotte T., Munck M., Noegel A. A., Korenbaum E. (2002). NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J. Cell Sci. 115 3207–3222. [DOI] [PubMed] [Google Scholar]


