Abstract
Diabetes mellitus is the most common cause of Charcot neuropathy affecting foot and ankle. Acute Charcot foot (CF) presents with a red and swollen foot in contrast to the painless deformed one of chronic CF. Enhanced osteoclastogenesis plays a central role in the pathogenesis of acute CF. Many studies have shown elevated levels of bone turnover markers in patients with acute CF confirming it. These findings have led clinicians to use anti-resorptive agents [bisphosphonates (BP), calcitonin, and denosumab] along with immobilization and offloading in acute CF patients. The maximum evidence among all anti-resorptive agents is available for BPs, although its quality is low. Pamidronate has been shown to reduce the markers of activity of CF like raised skin temperature, pain, edema, and bone turnover markers in the majority of studies. Intravenous BPs are known to cause acute phase reactions leading to flu-like illness following their first infusion, which can be ameliorated by oral acetaminophen. Alendronate is the only oral BP used in these patients. It needs to be taken on an empty stomach with a full glass of water to avoid esophagitis. The side-effects and contraindications to BPs should be kept in mind while treating acute CF patients with them.
Keywords: Charcot foot, Diabetes mellitus, Charcot neuroarthropathy, Bisphosphonates, Pamidronate
Core tip: Bisphosphonate is an attractive treatment option for acute Charcot foot. This is based on the fact that increased osteoclastic activity plays a central role in the pathogenesis of acute Charcot foot. Among bisphosphonates, the maximum evidence in the literature is available for pamidronate. It has been shown to reduce the markers of Charcot foot activity, like raised skin temperature, pain, and edema. However, the quality of evidence is low. They should be used along with immobilization and offloading. The side effects of bisphosphonates and their contra-indications for use should be kept in mind while treating these patients with them.
INTRODUCTION
Charcot foot (CF), also quoted as Charcot neuroarthropathy (CN), derives its name from Jean-Martin Charcot, who in 1868 first described neuroarthropathic changes in patients with tabes dorsalis[1]. It was not until 68 years later, in 1936, that William Riley Jordan first established the association between diabetes mellitus (DM) and painless neuropathic arthropathy of ankle[2]. It is a rare and devastating condition leading to the destruction of bone and joints and culminating in fractures, dislocations, deformities, and amputation of the foot in neglected cases. Virtually any condition that causes neuropathy can lead to CF, such as DM, syphilis, leprosy, spinal cord injury, meningomyelocele, syringomyelia, chronic alcoholism, and a host of other conditions like psoriasis, sarcoidosis, rheumatoid arthritis, human immunodeficiency virus, and Parkinson’s disease. Currently, the world is witnessing an exponential rise in the prevalence of DM and its complications. According to the World Health Organization 2016 report, around 422 million people are living with DM[3]. This has made DM the most common cause of CN affecting foot and ankle. The incidence of CF in diabetic patients ranges between 0.1% and 7.5%[4].
Today, the pathophysiology of CF is still a bone of contention even after one and half centuries since its first description. Conventional theories for it include the neurovascular theory postulated by Charcot himself and the neurotraumatic theory proposed by Volkmann and Virchow[5]. Peripheral sensorimotor neuropathy along with autonomic dysfunction is the essential factor for the development of CN. Usually a trivial trauma in the insensate foot kicks off the inflammatory cascade. Not all neuropathic patients, however, develop CF.
Recent advancements in the understanding of the pathophysiology of CF has shed light on factors like inflammatory cytokines and their interaction with receptor activator of nuclear factor kappa-B (RANK), its ligand (RANKL), and osteoprotegerin (OPG)[6]. Long-standing hyperglycemia, with its complications ranging from neuropathy to formation of advanced glycation end products (AGEs)[7] and protein kinase C (PKC) activation[8], is the major culprit. Calcitonin gene-related peptide (CGRP)[9], Wnt/beta-catenin pathway[10], and OPG gene polymorphisms[11] are new players in the field. Interaction between RANKL, nuclear factor kappa-B (NF-κB), and pro-inflammatory cytokines like tumor necrosis factor alpha (TNF-α), Interleukin-1β (IL-1β), and interleukin-6 (IL-6) lead to localized osteolysis that destroys bone structure[12]. Moreover, DM patients have lower 1,25(OH)2D3 levels, leading to poor mineralization of bone[13]. Lower calcium levels can stimulate parathyroid hormone, thus contributing to bone resorption and osteopenia[14].
Studies have shown increased levels of osteoclastic resorption markers, such as serum carboxyterminal telopeptide of type 1 collagen (1CTP), in patients with CF[15]. Immobilization and avoidance of physical stress by complete offloading with the help of total contact cast (TCC) is the mainstay in the management of CF[16]. However, because of increased osteoclastic activity, the bone destruction continues unabated. Lower limb osteopenia seen in patients with CN along with increased bone resorptive markers make anti-resorptive agents like bisphosphonates (BPs), calcitonin, and denosumab reasonable treatment options, at least for adjuvant purposes. BPs are pyrophosphate (PP) analogs that have been in medical use for around half a century. First generation BPs like etidronate and clodronate have non-nitrogen containing side chains, whereas second and third generation ones like pamidronate, alendronate, ibandronate, risedronate, and zoledronate have nitrogen containing side chains. Nitrogen containing BPs are much more potent than the first generation ones and work by inhibiting farnesyl PP (FPP) synthase in the mevalonate pathway, which is crucial for function and survival of osteoclasts[17]. In this study, we reviewed the available literature on the use of BPs in patients with acute CF.
CLINICAL PRESENTATION
Clinically, CF can present either in acute or chronic stage, and its features vary according to the stage of presentation. A high index of suspicion is required to diagnose CF in its early stage. Acute CF presents with a red and swollen foot, which is warmer than the contralateral normal foot. Patients may have mild to moderate pain or discomfort at this stage, which is much less when compared to those with a similar degree of inflammation without neuropathy[16,18]. Skin temperature difference of ≥ 4° Fahrenheit (or 2° Celsius) between affected and the normal foot indicates active CF[19]. This can be measured using an infrared thermometer at the maximum point of deformity on the affected foot and at the same point on the normal foot. It is also helpful in monitoring the course of CF. Peripheral pedal pulses are typically bounding because of underlying autonomic neuropathy. Clinical presentation at this stage mimics those of deep vein thrombosis, acute gout, and cellulitis, and the diagnostic dilemma is compounded by the inability of radiographs to detect and differentiate these abnormalities. Magnetic resonance imaging can be helpful at an early stage of disease[20]. If treatment is not provided at this stage, it leads to further destruction of bone resulting in irreversible damage. Chronic CF is characterized by resolution of inflammation and establishment of residual deformity. Rocker bottom deformity is the classic abnormality that arises due to collapse of plantar arch in mid foot[21]. This results in abnormal high pressure areas on the weight bearing sites of the plantar surface, making it prone to ulceration[22].
PATHOGENESIS OF ACUTE CHARCOT FOOT
Conventional theories
Two age-old theories pertaining to the pathogenesis of CF that are still pertinent include neurovascular theory and neurotraumatic theory. Neurovascular theory[1] suggests that damage to trophic or vasomotor nerves secondary to the underlying condition results in failure of vasoregulation, causing opening of arteriovenous shunts. This leads to the increased supply of blood to the bone, resulting in greater flux of monocytes and osteoclasts and culminating in bone resorption. Other factors, like peripheral vascular disease, are expected to co-exist with diabetic neuropathy. This leads to decreased blood flow to lower limbs, which can act as a protective factor against CF[23]. This probably explains why CF affects only a fraction of DM patients with neuropathy.
On the other hand, Volkmann and Virchow in their neurotraumatic theory suggested that trauma to the insensate foot leads to CN[5]. Repeated microtrauma in a patient with sensory neuropathy leads to bone destruction and deformity. Though both feet of susceptible patients have the propensity to develop CF, only the one exposed to recurrent trauma develops CF. This provides some ground for the pathogenesis of unilateral CF in the background of generalized neuropathy. However, it has been found to be bilateral in 9% to 39% of cases[24]. With the passage of time, we have now come to know that CF results from the combination of these processes. Autonomic neuropathy weakens the bone because of increased blood supply, whereas sensory neuropathy causes loss of protective sensation leading to unperceived recurrent trauma to the abnormal bone. Muscle weakness due to motor neuropathy adds fuel to the fire, leading to joint instability and abnormal plantar pressures[25]. These progress later to bone fracture and dislocation in foot and ankle.
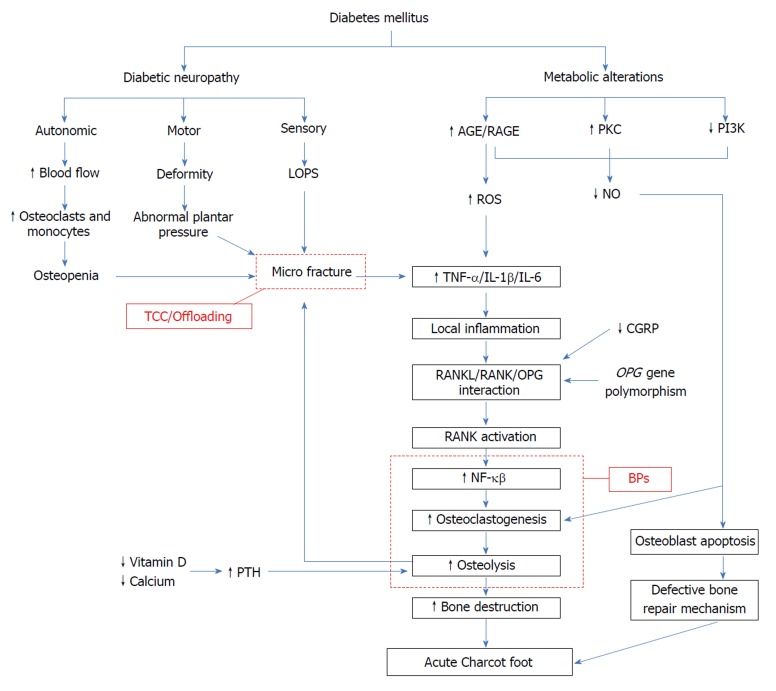
Other factors that play a role in the pathogenesis of CF are: (1) inflammatory cytokines; (2) AGEs; and (3) neuropeptides and inorganic molecules. These mediators finally stimulate osteoclastogenesis, leading to bone loss via RANKL/OPG pathway (Figure 1).
Figure 1.
Pathogenesis leading to acute Charcot foot. AGE: Advanced glycation end products; RAGE: Receptor of AGE; PKC: Protein kinase C; PI3K: Phosphatidylinositol 3 kinase; LOPS: Loss of pain sensation; ROS: Reactive oxygen species; NO: Nitric oxide; TNF-α: Tumor necrosis factor-alpha; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; TCC: Total contact cast; CGRP: Calcitonin gene related peptide; NF-κβ: Nuclear factor κβ; RANKL: Receptor activator of NF-κβ ligand; OPG: Osteoprotegerin; BP: Bisphosphonates; PTH: Parathyroid hormone.
Role of inflammatory cytokines
In addition to his neurovascular theory, Charcot recognized inflammation as one of the contributors to CN. Christensen et al[26] in their study showed that hyperemia during an acute attack of CF was most likely secondary to the inflammation rather than sympathetic neuropathy. Thus, it is unabated inflammation in the background of neuropathy that results in the imbalance between osteoclasts and osteoblasts leading to bone resorption. This pro-inflammatory state can be triggered by repeated microtrauma. Hyperglycemia in DM can lead to increased PKC activity and formation of AGEs along with decreased phosphatidylinositol 3 kinase activity[8]. This, in turn, results in an excessive production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. This storm of pro-inflammatory cytokines disturbs osteoclast-osteoblast homeostasis. Baumhauer et al[12] histologically examined 20 tissue biopsy specimens obtained from patients with CF. Immunohistochemical study of each of these biopsies showed positivity for IL-1, IL-6, and TNF-α. This was conclusive of stimulation of osteoclastic progenitor cells, leading to osteoclastogenesis by the cytokines present in the background during acute and reparative stages of CF. But inflammatory cytokines alone do not directly account for the increased osteoclastogenesis. Jeffcoate et al[27] suggested that inflammatory cytokines lead to increased osteoclastogenesis via increased expression of NF-κB. This results in bone destruction, which again potentiates the inflammatory response thus culminating in a vicious cycle[27]. Increased cytokines lead to increased activity of RANKL that in turn activates RANK, which is expressed on osteoclast precursors. Increased RANK stimulates intracellular pathways, leading to increased formation of NF-κB. NF-κB stimulates differentiation of osteoclast premature cells to mature osteoclasts, culminating in increased osteoclastic activity. Simultaneously, NF-κB up regulates expression of OPG, a decoy receptor for RANKL, which effectively antagonizes its activity[28]. Ndip et al[29] in their study showed that patients with CN have elevated RANKL/OPG ratio and illustrated that abnormal RANKL/OPG signaling plays a crucial role in increased osteoclastic bone resorption. Another bone regulating pathway involving Wnt/β-catenin has been speculated to have some role in bone remodeling in patients with CF[10]. To date, the RANKL/OPG pathway defect remains the most accepted theory.
Role of hyperglycemia
Glycation of collagen occurs normally with aging[30]. Hyperglycemia accelerates this process of non-enzymatic glycation, leading to the formation of Amadori products. These products combine with amino groups on other protein molecules, ending up in formation of the AGEs, which are known to play a major role in various complications of diabetes[31]. AGEs cause irreversible posttranslational modification of proteins, thus rendering them defective. Binding of AGEs to their receptor (RAGE) stimulates nicotinamide adenine dinucleotide phosphate oxidase[32], resulting in the production of reactive oxygen species and subsequently increased expression of NF-κB[33]. Katayama et al[34] elucidated the effects of AGE-modified collagen on differentiation and function of the osteoblastic cell in vitro and suggested that the same changes may lead to osteopenia in diabetic patients. AGEs prevent differentiation of human mesenchymal stem cells[35]. They stimulate apoptosis of osteoblasts through mitogen activated protein kinase and cytosolic apoptotic pathways that are independent of NF-κB activation[36]. AGEs also cause endothelial dysfunction by extinguishing nitric oxide (NO) activity[37]. Soluble RAGE (sRAGE) is a C-terminal splice variant of RAGE and has been shown to be cytoprotective against AGE[38]. Witzke and colleagues in their cross-sectional study concluded that patients with CN had lower levels of sRAGE compared to healthy controls and diabetic patients without CN[7]. They also demonstrated a positive correlation between sRAGE levels and calcaneal bone stiffness suggesting that sRAGE has a protective effect against bone resorption and loss of sRAGE defense may be one of the factors leading to CN. Thus, AGEs lead to increased osteoclastogenesis via the RANKL/NF-κB pathway and decreased bone formation by their action on osteoblasts through multiple pathways.
Role of neuropeptides and inorganic molecules
Research has shown that feedback mechanisms are abnormal in patients with DM, leading to increased expression of RANKL. One such mechanism involves CGRP secreted from the healthy neurons. It antagonizes RANKL expression by increasing the release of anti-inflammatory cytokines like IL-10[14]. This leads to inhibition of osteoclastogenesis. The release of CGRP is reduced in peripheral and autonomic neuropathy, leading to continuous unchecked RANKL activity[39]. NO is an inorganic molecule that plays a role in CN. AGEs, along with increased PKC expression and decreased phosphatidylinositol 3 kinase activity, results in decreased production of NO. Studies have shown that decreased NO levels can stimulate osteoclastogenesis, thereby leading to bone resorption[40]. Endothelial NO synthase (eNOS) also regulates osteoblast proliferation and function[41,42]. eNOS knockout animals have been shown to develop osteoporosis secondary to defective bone formation[42]. Both these molecules were studied by La Fontaine and colleagues in their study[9]. They performed immunohistological analysis of bone specimens from three groups of patients with DM: group 1 included healthy patients without neuropathy, group 2 included those with neuropathy, and group 3 included those with CN stage II or III. They observed decreased levels of CGRP in patients in groups 2 and 3 when compared to group 1. They also found a statistically significant difference in the levels of eNOS, with highest levels in healthy DM patients without neuropathy (group 1) and lowest levels in DM patients with CN (group 3).
ROLE OF ANTIRESORPTIVE THERAPY IN ACUTE CHARCOT FOOT
Increased osteoclastic activity is the essence of pathogenesis leading to CF. Many studies have shown elevated levels of bone turnover markers (BTMs) in patients with acute CF pointing towards this fact.
Gough et al[15] compared BTMs between four groups of patients: acute CF, chronic CF, diabetic controls, and non-diabetic controls. They concluded that levels of serum 1CTP were significantly elevated in patients with acute CF as compared to the other three groups (P < 0.0001). Jostel et al[43] in their review mentioned similar results with urinary cross linked N-telopeptides of type 1 collagen, pointing towards accelerated collagen breakdown in these patients. However, levels of serum procollagen type I carboxy-terminal propeptide did not show intergroup differences.
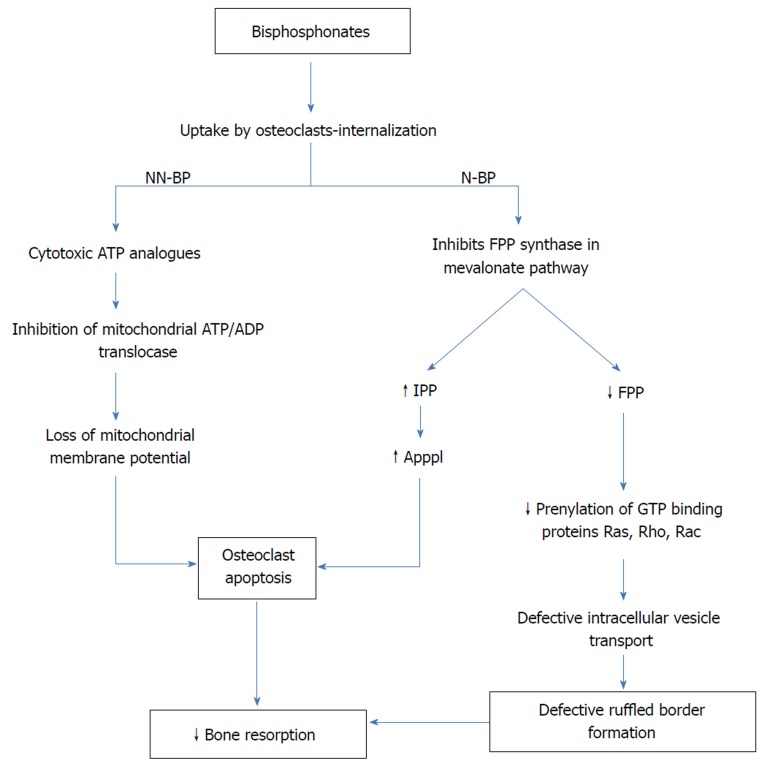
These findings have forced researchers to use anti-resorptive agents along with traditional immobilization in acute CF patients. To date, agents like BPs have been used in multiple studies (discussed later). BPs are the principal agents in the pharmacological armamentarium against diseases, where the osteoblast-osteoclast imbalance is the underlying pathology. They are analogues of inorganic PP binding to hydroxyapatite crystals, which have extremely high affinity for bone mineral. They get deposited in mineralized bone matrix and are released at the time of bone resorption. This high affinity for bone mineral and resultant uptake by activated osteoclasts at the time of resorption ensures its toxic accumulation only in osteoclasts. First generation non-nitrogen containing BPs are metabolized to cytotoxic adenosine triphosphate analogues by osteoclasts. Intracellular deposition of these toxic non-hydrolyzable analogues causes apoptosis of osteoclasts[44]. Unlike their predecessors, second and third generation BPs like alendronate, pamidronate, ibandronate, risedronate, and zoledronate have nitrogen side chain bound to the central carbon, which magnifies their potency manifolds. The mechanism by which nitrogen containing BPs impacts osteoclast activity and survival differs from that of the first generation BPs. After getting internalized, they inhibit FPP synthase, a key enzyme in the mevalonate pathway, which is responsible for production of cholesterol and isoprenoid lipids[45]. As a result, isoprenylation of guanosine triphosphate binding proteins like Ras, Rho, and Rac is inhibited[46]. These signaling proteins are important for the regulation of cell survival, proliferation, and cytoskeletal organization. Of particular importance among these is inhibition of protein prenylation and Ras signaling within osteoclasts, resulting in defective intracellular vesicle transport[47]. Thus, osteoclasts fail to form ruffled borders, which are necessary for resorption of bone. In addition to this, FPP synthase inhibition leads to an increase in isopentenyl diphosphonate, which is further metabolized to triphosphoric acid 1-adenosin-5’-yl ester 3-[3-methylbut-3-enyl] ester, also known as ApppI. Intracellular accumulation of this ATP analogue leads to apoptosis of osteoclasts (Figure 2). Potency of BPs is decided based on the inhibition of FPP synthase activity. In this respect, zoledronate is the most potent BP followed by risedronate, ibandronate, alendronate, and pamidronate with decreasing potency[17]. Moreover, in animal studies, BPs have shown to possess anti-nociceptive effects that can contribute to pain relief in patients with acute CF[48,49].
Figure 2.
Molecular mechanisms of bisphosphonates. NN-BP: Non-nitrogen containing bisphosphonates; N-BP: Nitrogen containing bisphosphonates; FPP: Farnesyl pyrophosphate; IPP: Isopentenyl diphosphonate; ApppI: Triphosphoric acid 1-adenosin-5’-yl ester 3-[3-methylbut-3-enyl]ester.
Other anti-resorptive agents like calcitonin and denosumab have been successfully used in past. Calcitonin is a polypeptide secreted from parafollicular C cells of the thyroid. It inhibits bone resorption by its direct action on the osteoclast calcitonin receptor[50]. Its quick action leads to loss of ruffled border of osteoclasts and decreased number of osteoclasts. It inhibits cytoplasmic motility and generates pseudopodial retraction in osteoclasts[51]. It prevents the production and release of tartrate-resistant acid phosphatase by osteoclasts[52]. It has also been shown that calcitonin may inhibit apoptosis of osteocytes and osteoblasts[53]. To evaluate the effects of calcitonin on disease activity, Bem et al[54] conducted a randomized controlled trial on the effectiveness of intranasal salmon calcitonin 200 IU daily in 32 diabetic patients with acute CF. One group received intra-nasal salmon calcitonin 200 IU daily and calcium supplementation, while the other got only calcium supplements. All patients were offloaded using removable devices. Skin temperature and BTMs (measured monthly for first 3 mo and then at 6 mo) were used for monitoring the course of treatment. Nine patients with renal insufficiency, i.e., serum creatinine > 120 µmol/L, were also included. Skin temperature reduced significantly at 3 mo without much inter-group difference. Significant reduction was noted in levels of 1CTP in the treatment group at 3 mo as compared to control group (P < 0.01). A similar trend was observed for bone-specific alkaline phosphatase (ALP) at 3 mo (P < 0.05), but the intergroup difference disappeared at 6 mo. The authors concluded that intranasal calcitonin not only reduces bone resorption and prevents progression of acute CF but also can be effective in patients with renal insufficiency. Calcitonin also has analgesic action mediated through central as well as peripheral mechanisms[55,56].
As RANKL activation plays a major role in the pathogenesis of acute CF, its inhibition can be an attractive treatment option. Denosumab is a fully human monoclonal antibody that targets RANKL. It prevents interaction between RANKL and its receptor RANK. This leads to inhibition of RANKL, which in turn prevents differentiation of osteoclast precursors to mature multinucleated osteoclasts. The basic difference between BPs and denosumab is that the former act after getting internalized, while the latter works in an extracellular environment[57]. It has been shown to reduce osteoporosis-related fracture[58]. Taking cues from this work, Busch-Westbroek et al[59] performed an observational study to evaluate effects of denosumab in patients with acute CF. Patients seen between 2012 and 2014 were included as controls, and those from 2014 to 2016 were subjected to single subcutaneous injection of 60 mg denosumab. All the patients from 2012 to 2016 were immobilized using TCC and were supplemented with calcium and vitamin D. Fracture resolution time, as judged on radiographs and time to clinical cessation based on usage of TCC, were compared between the two groups. Both parameters were significantly shorter in the group receiving denosumab (P < 0.01). TCC was used until resolution of edema, and skin temperature difference between both feet decreased to less than 2°C in this study.
EVIDENCE OF BISPHOSPHONATE USE IN ACUTE CHARCOT FOOT
Case reports and case series
In 1994, Selby et al[60] first reported use of intravenous (IV) pamidronate in six diabetic patients with acute CF. Patients were treated with infusion of 30 mg of pamidronate followed by five infusions of 60 mg every 2 wk. Skin temperature, as a marker of disease activity, was monitored by an infrared thermometer. All patients reported marked improvement in their mobility and reduction in pain and swelling. Skin temperature difference between the affected and normal foot reduced from 3.4 ± 0.7 °C to 1.0 ± 0.5 °C (P = 0.05). Serum ALP, which was used as BTM, was also significantly reduced (by 25 ± 3%, P < 0.001).
In 1999, Young MJ[61] reported two diabetic patients with CF who were treated with IV infusion of 30 mg of pamidronate followed by two infusions of 60 mg every 2 wk along with immobilization measures. In both patients, skin temperature difference normalized (i.e., < 2 °C), edema and pain subsided after 3 mo, and there was no deformity in the lower limbs.
In 2002, Yu et al[62] reported a case of recurrent CF in a 55-year-old diabetic patient. He was treated with serial Jones compression bandages followed by non-weight bearing brace with a removable pneumatic walker along with three IV infusions of pamidronate each 2 wk apart. The patient improved clinically, swelling disappeared, and he resumed full weight bearing with an ankle-foot orthosis.
In 2002, Pakarinen et al[63] retrospectively studied 36 CF in 32 diabetic patients. Eighteen cases received IV pamidronate 30 to 60 mg once a wk for 6 wk. They did not find any difference in casting time between patients who received pamidronate and those who did not (11 wk vs 13 wk). There was no information regarding criteria used for removal of casts or the indication for BP use in a particular patient. This along with non-uniformity in the timing of cast usage make it difficult to analyze the results of this study.
In 2007, Moreno et al[64] prospectively analyzed the efficacy of pamidronate over 12 mo in four diabetic patients with acute CF. Treatment protocol comprised of three IV infusions of pamidronate each 2 mo apart. The dose used was 60 mg in patients with weight < 70 kilograms and 90 mg in patients with weight > 70 kilograms. Clinical examination, radiographs, and urine BTMs were done before and 12 mo after treatment in all patients. All patients exhibited significant clinical improvement. Urinary BTMs showed a statistically significant reduction. All patients had radiological improvement.
In 2008, Naqvi et al[65] reported three diabetic patients with acute CF. First patient, a 54-year-old female was treated with three IV infusions of 90 mg of pamidronate every 2 mo. After the first infusion, the patient had marked clinical improvement in swelling, pain, erythema, and warmth. Following the second infusion, she was able to bear weight on her foot, and after the last infusion she was ambulant without the walker. The second patient was a 49-year-old African-American female, who was treated with single IV infusion of 60 mg pamidronate along with walking cast and physiotherapy. At 6 and 9 mo follow-ups, signs of inflammation disappeared. Although the natural arch of the foot was lost, she was able to walk with a boot. The third patient was an 82-year-old white woman, who was treated with a single 90 mg of pamidronate infusion. This led to significant clinical improvement, and she was able to walk with the boot at 4 wk of follow-up. One year later, she had no symptoms and was able to walk normally.
In 2002, Rajbhandari et al[23] in their review revealed their anecdotal experience in patients with acute CF. They noted significant symptom relief in these patients with two IV infusions of 90 mg pamidronate.
Observational studies
In 2004, Anderson et al[66] retrospectively evaluated 33 patients of acute CF who were diagnosed between October 1997 and January 2001. These patients were divided in two study groups - group 1 comprising of 18 patients who received IV pamidronate (60 to 90 mg) and group 2 comprising of 15 patients who did not receive any BPs. Both groups received standard immobilization measures. Finally, after excluding five patients each from groups 1 and 2 due to either lack of consent for treatment or bilateral CF or association with some other bone disease or infection, 13 patients from group 1 and 10 patients from group 2 were analyzed. In group 1 patients, limb temperature decreased by 2.8 °F at 48 h and 7.4 °F at 2 wk, whereas group 2 showed no reduction in temperature at 48 h and a reduction of 2.3 °F at 2 wk. The same trend was seen in serum ALP, which plummeted by 53% at 2 wk in group 1 and showed a meager reduction of 9% in group 2. Thus, this study demonstrated a statistically significant reduction in skin temperature and serum ALP in patients treated with pamidronate.
In the largest web-based observational study published in 2012, Game et al[67] surveyed 288 diabetic patients with acute CF from 76 centers across the United Kingdom and Ireland. At baseline, 35% of the subjects were offloaded with the non-removable devices, while 50% were offloaded using the removable devices. Only 25% of patients received IV BPs, and around 20% received oral BPs. Follow-up data regarding resolution was available in 219 patients. The resolution was defined as a time-point when the patient starts walking in either normal or orthotic footwear. For those who received BPs, median resolution time was significantly longer than patients not receiving it (12 mo vs 10 mo, P = 0.005). Resolution time was significantly more in both groups as compared to other studies. One of the reasons for this can be the definition of resolution used, which required the patient to be ambulant. Regarding prolongation of resolution time with BPs, the authors have speculated the following possible explanations: first, BPs may have been used only in patients who had more severe CF or in non-responders to offloading alone. Second, BPs must have been used only if the non-removable device was unavailable. It is very tough to infer much from the results provided by this study. No data were provided regarding skin temperature or any BTMs or the type of BP used.
In 2013, Bharath et al[68] were the first to compare the effects of two BPs in a prospective randomized comparative study of 45 type 2 diabetic patients with acute CF. Patients were randomized into two groups, Z and A receiving a single IV infusion of 5 mg zoledronate (diluted in 100 mL normal saline over 30 min) and oral alendronate 70 mg once weekly, respectively, until resolution of the disease. The complete clinical resolution of the disease process was defined as attainment of a temperature difference of < 1° F between two feet on two different occasions. Patients with serum creatinine ≥ 3 mg/dL or with a history of BP exposure were excluded. For patients in group Z, if serum creatinine was ≥ 2 mg/dL, the dose of zoledronate was reduced to 2.5 mg. Patients in both the groups were offloaded using TCC. Forty patients completed the study (five patients withdrew), and 30 achieved complete resolution (16 patients in group Z and fourteen in group A). Five patients in each group achieved partial clinical resolution. The mean number of days required for complete healing process was around 122 d in both the groups.
Randomized controlled trials
All randomized controlled trials (RCTs) related to the use of BPs in acute CF are described in Table 1. In 2001, Jude et al[69] reported a 12 mo double-blind randomized placebo-controlled trial including 39 diabetic patients with acute CF. At baseline, 21 patients received single 90 mg infusion of pamidronate over 4 h, whereas 18 patients received normal saline (placebo). All patients received standard care of foot immobilization. For the first 3 mo, all patients were followed up at 2-weekly intervals and thereafter at 6, 9, and 12 mo. At each visit, patients were assessed for clinical symptoms, and skin temperature was measured with an infrared thermometer. BTMs like bone-specific ALP and urinary dehydroxypyridinoline were measured at each visit. Skin temperature reduced significantly in both the groups with pamidronate group showing a greater reduction at 4 wk. It dipped further during the study period with no intergroup difference on subsequent visits. Both groups demonstrated symptom score improvement at 3 mo. Following this, the score remained unchanged in the control group over the next 12 mo, whereas the pamidronate group registered further improvement (P < 0.01). Bone-specific ALP showed a significant reduction in the pamidronate group when compared to placebo (P < 0.03) at 4 wk, and this was maintained for at least 12 wk. A similar trend was observed in urinary dehydroxypyridinoline at 4 wk (P < 0.01). Both the BTMs gradually increased towards baseline at 12 mo.
Table 1.
Table of randomized controlled trials on bisphosphonates in acute Charcot foot
| Ref. | BP | Duration (mo) | Jadad score | Subjects |
Outcomes |
|||
| Skin temp | Symptom score | BTM | Others | |||||
| Jude et al[69] | Pamidronate | 12 | 5 | T: n = 21; 90 mg single IV infusion P: n =18; single IV infusion of NS | Significant reduction in both groups; more in T group at 4 wk (P < 0.01) | Significant improvement in T group from 3 – 12 mo (P < 0.01) | BSALP: Significantly greater reduction in T group till 12 wk (P < 0.03) uDPD crosslinks: Significant reduction in T group at 4 wk (P < 0.01) | |
| Pitocco et al[70] | Alendronate | 6 | 1 | T: n = 11; 70 mg once a week orally C: n = 9; no pharmacological treatment | Significant reduction in both groups | Signification reduction in T group at 6 mo (P < 0.05) | 1CTP and uHP: Significant reduction in T Group (P < 0.05) BSALP: Greater reduction in T group (P = 0.06) | |
| Pakarinen et al[71] | Zoledronate | 12 | 4 | T: n = 20; 3 IV infusion of 4 mg at one monthly interval P: n = 19; placebo | Median immobilization time: Significantly greater in T group (P = 0.02) | |||
BP: Bisphosphonate; T: Treatment group; P: Placebo group; C: Control group; IV: Intravenous; BTM: Bone turnover marker; BSALP: Bone specific alkaline phosphatase; 1CTP: Carboxyterminal telopeptide of type 1 collagen; uDPD: Urinary dehydroxypyridinoline; uHP: Urinary hydroxypronline.
In an observer blinded RCT, Pitocco et al[70] studied the efficacy of alendronate in patients with acute CF. Eleven patients included in study group received alendronate 70 mg orally once a week while nine patients in control group received no pharmacological treatment. All patients were followed up for 6 mo and were offloaded using a TCC boot for the first 2 mo, which was followed by a pneumatic walker in the subsequent 4 mo. BTMs like serum 1CTP, serum bone ALP, and urinary hydroxyproline were measured at baseline and at 6 mo of follow up. All these markers showed a significant reduction in the alendronate-treated group when compared to control group (P < 0.05), except for bone ALP (P = 0.06). Dual-energy x-ray absorptiometry done at baseline and at 6 mo showed statistically significant improvement in bone mineral density of total foot (P < 0.05) and distal phalanxes (P < 0.01) in the alendronate group. Visual analogue scale score for pain improved significantly in the treatment group, with no improvement in the control group (P < 0.05). Skin temperature reduced significantly in both groups at 6 mo.
In 2011, Pakarinen et al[71] first studied the effect of zoledronate in 39 diabetic subjects with acute CF in a double-blind randomized placebo-controlled trial. Patients were randomly assigned into two groups receiving three IV infusions of either 4 mg zoledronate or a placebo at 1-mo intervals. Patients with previous BP exposure or severe renal insufficiency were excluded. All patients were initially treated with a non-weight bearing cast and were allowed partial weight bearing when the clinical signs of active CF process subsided. Complete weight bearing was allowed only when the temperature difference between the two feet was less than 1°C for at least last 30 d with no evidence of edema or erythema. All patients were evaluated at baseline, at 2 to 4 wk intervals for the first 3 mo and then at 6, 9, and 12 mo. Finally, 35 patients who completed 12 mo follow-up were analyzed. The final endpoint of this study was median immobilization time, which was significantly longer in the zoledronate treated group as compared to the placebo group (27 wk vs 20 wk, P = 0.02). No information was given regarding BTMs or radiological findings at any point of time. During 12 mo follow-up, one patient relapsed in each group.
DISCUSSION
The main aims of treatment in acute CF are to relieve the patient of symptoms and to avoid complications, such as deformity and ulceration, thus preventing the progression to chronic CF. Immobilization and offloading are the most important components of this treatment. Avoidance of repetitive microtrauma leads to the resolution of edema and swelling. Casting should be continued until the skin temperature difference between the two limbs becomes less than 2°C[16]. However, the basic pathogenesis in CF revolves around osteolysis, which leads to subsequent bone destruction, and immobilization does not address this directly. This creates space for the adjuvant therapy that can inhibit osteolysis and hence bone resorption. BPs, calcitonin, and denosumab are the anti-resorptive agents used to date in these patients. Among BPs, maximum evidence in the literature is available for pamidronate[72]. In a majority of case reports and series, pamidronate was shown to reduce the markers of activity of CF, like skin temperature, pain, edema, and BTMs. In the first RCT assessing the response of BP in acute CF, Jude et al[69] confirmed the beneficial effects of pamidronate in patients with acute CF. This RCT was of high methodological quality, as it was a double-blind, placebo controlled, multi-center study with proper mention about randomization process and statistical analysis. In a retrospective case-control study, Anderson et al[66] reported significant reduction in skin temperature and serum ALP in the pamidronate treated group. However, in a case series by Pakarinen et al[63], no difference was found for casting times when pamidronate was used along with conventional measures. Among other BPs, alendronate in a RCT was shown to reduce pain and BTMs significantly in acute Charcot neuroarthropathy[70]. Additionally, zoledronate, the most potent third generation BP, was surprisingly shown to prolong immobilization times of patients with acute CF[71]. The limitations of this particular RCT were its underpowered nature (due its small sample size) and the discrepancy in the immobilization times. The latest randomized comparative study evaluating the effects of zoledronate and alendronate concluded that both medications had the same response in terms of clinical resolution time and scintigraphic changes. When cost was taken into account, however, alendronate was much less expensive than zoledronate[68].
None of these studies have ventured into the effect of BP on long-term outcome measures like avoidance of ulcerations, deformities, and amputation. The evidence from the available studies is limited because of the non-uniformity in the agent used and heterogeneity in outcome measures. Most studies, except one of Jude et al[69], have methodological flaws like open randomization, lack of blinding, and statistically small sample size. In fact, only Jude et al[69] reported the power analysis.
CLINICAL IMPLICATION
Oral alendronate and IV pamidronate have been efficacious in relieving symptoms and controlling disease activity in patients with acute CF. Oral BPs need to be taken on an empty stomach and with a full glass of water (at least 240 mL) to avoid getting it stuck in the esophagus. The patient should remain in erect posture for at least 30-60 min. Oral BPs have very poor bioavailability, with < 1% of the drug being absorbed from gastrointestinal tract[73]. BP should be taken in the fasting state with avoidance of any food for 30-60 min after taking to prevent its absorption from decreasing further. Retained gastric contents in patients with gastroparesis may also hamper absorption. Contraindications to oral BPs include an inability to follow this strict protocol, any active esophageal pathology like achalasia, varices, or stricture, or any malabsorption disorder like celiac disease, Crohn’s disease, or post gastric bypass surgery[74]. For patients who cannot tolerate oral BPs, IV BPs can be an alternate option. IV BPs are known to cause acute phase reactions leading to flu-like illness in around 10%-30% of patients receiving their first infusion[74]. This can be taken care of by oral acetaminophen.
Vitamin D deficiency, which is common in the diabetic population, should be treated before giving BPs. In patients with renal insufficiency, caution should be exercised while using BPs, especially if glomerular filtration rate < 30-35 mL/min[74]. This is particularly true when given by rapid IV infusion, as it can aggravate or lead to renal dysfunction. Intranasal calcitonin can be an attractive option to treat acute CF in this group of patients. Moreover, BPs like zoledronate[75] and alendronate[76] have been linked with the occurrence of atrial fibrillation. HORIZON Pivotal Fracture Trial has shown a statistically significant increase in the incidence of serious atrial fibrillation in patients treated with zoledronate[75]. However, a large population-based study has refuted these findings[77].
As CN usually develops in diabetic patients with disease duration of more than 10 years, they are also expected to have gastroparesis, nephropathy, coronary artery disease, and various other complications. The above side-effects and contra-indications should be kept in mind while treating such patients with BPs.
CONCLUSION
The meteoric rise in the prevalence of DM has made it the most common cause of CN affecting foot and ankle. In a majority of the studies related to use of BPs in acute CF, pamidronate has been shown to reduce the markers of Charcot activity like skin temperature, pain, edema, and BTMs, but the quality of evidence is weak. Therefore, BPs can be considered as an adjuvant treatment option for acute CF.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Manuscript source: Invited Manuscript
Peer-review started: March 29, 2018
First decision: April 24, 2018
Article in press: June 14, 2018
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beltowski J, Klimontov VV, Serhiyenko VA S- Editor: Wang JL L- Editor: Filipodia E- Editor: Tan WW
Contributor Information
Harsh Durgia, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Jayaprakash Sahoo, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India. jppgi@yahoo.com.
Sadishkumar Kamalanathan, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Rajan Palui, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Kalyani Sridharan, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Henith Raj, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
References
- 1.Charcot JM. Sur quelques arthropathies qui paraiise d´ependre d’une l´esion du cerveau ou de la mouelle ´epini`ere. Arch Physiol Norm Pathol 1868; 1: 161–178 [Google Scholar]
- 2.Jordan WR. Neuritic manifestations in diabetes mellitus. Arch Intern Med. 1936;57:307–366. [Google Scholar]
- 3.World Health Organization. Global report on diabetes 2016. Available from: http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=C87606F0229CFE671C7D3B12FD2581BB?sequence=1.
- 4.Chisholm KA, Gilchrist JM. The Charcot joint: a modern neurologic perspective. J Clin Neuromuscul Dis. 2011;13:1–13. doi: 10.1097/CND.0b013e3181c6f55b. [DOI] [PubMed] [Google Scholar]
- 5.Ramanujam CL, Zgonis T. The Diabetic Charcot Foot from 1936 to 2016: Eighty Years Later and Still Growing. Clin Podiatr Med Surg. 2017;34:1–8. doi: 10.1016/j.cpm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Mabilleau G, Petrova NL, Edmonds ME, Sabokbar A. Increased osteoclastic activity in acute Charcot’s osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia. 2008;51:1035–1040. doi: 10.1007/s00125-008-0992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witzke KA, Vinik AI, Grant LM, Grant WP, Parson HK, Pittenger GL, Burcus N. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. 2011;34:1617–1621. doi: 10.2337/dc10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao HM, Diao JY, Liang XJ, Zhang F, Hao DJ. Pathogenesis and potential relative risk factors of diabetic neuropathic osteoarthropathy. J Orthop Surg Res. 2017;12:142. doi: 10.1186/s13018-017-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Fontaine J, Harkless LB, Sylvia VL, Carnes D, Heim-Hall J, Jude E. Levels of endothelial nitric oxide synthase and calcitonin gene-related peptide in the Charcot foot: a pilot study. J Foot Ankle Surg. 2008;47:424–429. doi: 10.1053/j.jfas.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Folestad A, Ålund M, Asteberg S, Fowelin J, Aurell Y, Göthlin J, Cassuto J. Role of Wnt/β-catenin and RANKL/OPG in bone healing of diabetic Charcot arthropathy patients. Acta Orthop. 2015;86:415–425. doi: 10.3109/17453674.2015.1033606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitocco D, Zelano G, Gioffrè G, Di Stasio E, Zaccardi F, Martini F, Musella T, Scavone G, Galli M, Caputo S, et al. Association between osteoprotegerin G1181C and T245G polymorphisms and diabetic charcot neuroarthropathy: a case-control study. Diabetes Care. 2009;32:1694–1697. doi: 10.2337/dc09-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumhauer JF, O’Keefe RJ, Schon LC, Pinzur MS. Cytokine-induced osteoclastic bone resorption in charcot arthropathy: an immunohistochemical study. Foot Ankle Int. 2006;27:797–800. doi: 10.1177/107110070602701007. [DOI] [PubMed] [Google Scholar]
- 13.Blakytny R, Spraul M, Jude EB. Review: The diabetic bone: a cellular and molecular perspective. Int J Low Extrem Wounds. 2011;10:16–32. doi: 10.1177/1534734611400256. [DOI] [PubMed] [Google Scholar]
- 14.Kaynak G, Birsel O, Güven MF, Oğüt T. An overview of the Charcot foot pathophysiology. Diabet Foot Ankle. 2013;4:21117. doi: 10.3402/dfa.v4i0.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough A, Abraha H, Li F, Purewal TS, Foster AV, Watkins PJ, Moniz C, Edmonds ME. Measurement of markers of osteoclast and osteoblast activity in patients with acute and chronic diabetic Charcot neuroarthropathy. Diabet Med. 1997;14:527–531. doi: 10.1002/(SICI)1096-9136(199707)14:7<527::AID-DIA404>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, Hartemann A, Game F, Jeffcoate W, Jirkovska A, et al. The Charcot foot in diabetes. Diabetes Care. 2011;34:2123–2129. doi: 10.2337/dc11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JR. Bisphosphonates: preclinical review. Oncologist. 2004;9 Suppl 4:3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:357–363. doi: 10.1002/(SICI)1096-9136(199705)14:5<357::AID-DIA341>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong DG, Lavery LA. Monitoring healing of acute Charcot’s arthropathy with infrared dermal thermometry. J Rehabil Res Dev. 1997;34:317–321. [PubMed] [Google Scholar]
- 20.Schlossbauer T, Mioc T, Sommerey S, Kessler SB, Reiser MF, Pfeifer KJ. Magnetic resonance imaging in early stage charcot arthropathy: correlation of imaging findings and clinical symptoms. Eur J Med Res. 2008;13:409–414. [PubMed] [Google Scholar]
- 21.Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg. 1999;38:34–40. doi: 10.1016/s1067-2516(99)80086-6. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe L, Stess RM, Graf PM. Dynamic pressure analysis of the diabetic charcot foot. J Am Podiatr Med Assoc. 1991;81:281–287. doi: 10.7547/87507315-81-6-281. [DOI] [PubMed] [Google Scholar]
- 23.Rajbhandari SM, Jenkins RC, Davies C, Tesfaye S. Charcot neuroarthropathy in diabetes mellitus. Diabetologia. 2002;45:1085–1096. doi: 10.1007/s00125-002-0885-7. [DOI] [PubMed] [Google Scholar]
- 24.Kucera T, Shaikh HH, Sponer P. Charcot Neuropathic Arthropathy of the Foot: A Literature Review and Single-Center Experience. J Diabetes Res. 2016;2016:3207043. doi: 10.1155/2016/3207043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee L, Blume PA, Sumpio B. Charcot joint disease in diabetes mellitus. Ann Vasc Surg. 2003;17:571–580. doi: 10.1007/s10016-003-0039-5. [DOI] [PubMed] [Google Scholar]
- 26.Christensen TM, Simonsen L, Holstein PE, Svendsen OL, Bülow J. Sympathetic neuropathy in diabetes mellitus patients does not elicit Charcot osteoarthropathy. J Diabetes Complications. 2011;25:320–324. doi: 10.1016/j.jdiacomp.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Jeffcoate WJ, Game F, Cavanagh PR. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet. 2005;366:2058–2061. doi: 10.1016/S0140-6736(05)67029-8. [DOI] [PubMed] [Google Scholar]
- 28.Jeffcoate WJ. Charcot neuro-osteoarthropathy. Diabetes Metab Res Rev. 2008;24 Suppl 1:S62–S65. doi: 10.1002/dmrr.837. [DOI] [PubMed] [Google Scholar]
- 29.Ndip A, Williams A, Jude EB, Serracino-Inglott F, Richardson S, Smyth JV, Boulton AJ, Alexander MY. The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes. 2011;60:2187–2196. doi: 10.2337/db10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest. 1981;67:1630–1635. doi: 10.1172/JCI110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43:836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 32.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 33.Mascarenhas JV, Jude EB. The Charcot foot as a complication of diabetic neuropathy. Curr Diab Rep. 2014;14:561. doi: 10.1007/s11892-014-0561-6. [DOI] [PubMed] [Google Scholar]
- 34.Katayama Y, Akatsu T, Yamamoto M, Kugai N, Nagata N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res. 1996;11:931–937. doi: 10.1002/jbmr.5650110709. [DOI] [PubMed] [Google Scholar]
- 35.Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 36.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–353. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson SA, Burns PR. The pathogenesis of Charcot neuroarthropathy: current concepts. Diabet Foot Ankle. 2012;3:12236. doi: 10.3402/dfa.v3i0.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collin-Osdoby P, Rothe L, Bekker S, Anderson F, Osdoby P. Decreased nitric oxide levels stimulate osteoclastogenesis and bone resorption both in vitro and in vivo on the chick chorioallantoic membrane in association with neoangiogenesis. J Bone Miner Res. 2000;15:474–488. doi: 10.1359/jbmr.2000.15.3.474. [DOI] [PubMed] [Google Scholar]
- 41.Riancho JA, Salas E, Zarrabeitia MT, Olmos JM, Amado JA, Fernández-Luna JL, González-Macías J. Expression and functional role of nitric oxide synthase in osteoblast-like cells. J Bone Miner Res. 1995;10:439–446. doi: 10.1002/jbmr.5650100315. [DOI] [PubMed] [Google Scholar]
- 42.van’t Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001;103:255–261. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jostel A, Jude EB. Medical treatment of Charcot neuroosteoarthropathy. Clin Podiatr Med Surg. 2008;25:63–69, vi-vii. doi: 10.1016/j.cpm.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RG, Oppermann U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A. 2006;103:7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 47.Alakangas A, Selander K, Mulari M, Halleen J, Lehenkari P, Mönkkönen J, Salo J, Väänänen K. Alendronate disturbs vesicular trafficking in osteoclasts. Calcif Tissue Int. 2002;70:40–47. doi: 10.1007/s002230010047. [DOI] [PubMed] [Google Scholar]
- 48.Bonabello A, Galmozzi MR, Bruzzese T, Zara GP. Analgesic effect of bisphosphonates in mice. Pain. 2001;91:269–275. doi: 10.1016/S0304-3959(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Guo TZ, Hou S, Wei T, Li WW, Shi X, Clark JD, Kingery WS. Bisphosphonates Inhibit Pain, Bone Loss, and Inflammation in a Rat Tibia Fracture Model of Complex Regional Pain Syndrome. Anesth Analg. 2016;123:1033–1045. doi: 10.1213/ANE.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masi L, Brandi ML. Calcitonin and calcitonin receptors. Clin Cases Miner Bone Metab. 2007;4:117–122. [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidi M, Inzerillo AM, Moonga BS, Bevis PJ, Huang CL. Forty years of calcitonin--where are we now? A tribute to the work of Iain Macintyre, FRS. Bone. 2002;30:655–663. doi: 10.1016/s8756-3282(02)00688-9. [DOI] [PubMed] [Google Scholar]
- 52.Yumita S, Nicholson GC, Rowe DJ, Kent GN, Martin TJ. Biphasic effect of calcitonin on tartrate-resistant acid phosphatase activity in isolated rat osteoclasts. J Bone Miner Res. 1991;6:591–597. doi: 10.1002/jbmr.5650060610. [DOI] [PubMed] [Google Scholar]
- 53.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bem R, Jirkovská A, Fejfarová V, Skibová J, Jude EB. Intranasal calcitonin in the treatment of acute Charcot neuroosteoarthropathy: a randomized controlled trial. Diabetes Care. 2006;29:1392–1394. doi: 10.2337/dc06-0376. [DOI] [PubMed] [Google Scholar]
- 55.Ito A, Yoshimura M. Mechanisms of the analgesic effect of calcitonin on chronic pain by alteration of receptor or channel expression. Mol Pain. 2017;13:1744806917720316. doi: 10.1177/1744806917720316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azria M. Possible mechanisms of the analgesic action of calcitonin. Bone. 2002;30:80S–83S. doi: 10.1016/s8756-3282(02)00701-9. [DOI] [PubMed] [Google Scholar]
- 57.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 58.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 59.Busch-Westbroek TE, Delpeut K, Balm R, Bus SA, Schepers T, Peters EJ, Smithuis FF, Maas M, Nieuwdorp M. Effect of Single Dose of RANKL Antibody Treatment on Acute Charcot Neuro-osteoarthropathy of the Foot. Diabetes Care. 2018;41:e21–e22. doi: 10.2337/dc17-1517. [DOI] [PubMed] [Google Scholar]
- 60.Selby PL, Young MJ, Boulton AJ. Bisphosphonates: a new treatment for diabetic Charcot neuroarthropathy? Diabet Med. 1994;11:28–31. doi: 10.1111/j.1464-5491.1994.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 61.Young MJ. The management of neurogenic arthropathy: a tale of two charcots. Diabetes Metab Res Rev. 1999;15:59–64. doi: 10.1002/(sici)1520-7560(199901/02)15:1<59::aid-dmrr4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 62.Yu GV, Hudson JR. Evaluation and treatment of stage 0 Charcot’s neuroarthropathy of the foot and ankle. J Am Podiatr Med Assoc. 2002;92:210–220. doi: 10.7547/87507315-92-4-210. [DOI] [PubMed] [Google Scholar]
- 63.Pakarinen TK, Laine HJ, Honkonen SE, Peltonen J, Oksala H, Lahtela J. Charcot arthropathy of the diabetic foot. Current concepts and review of 36 cases. Scand J Surg. 2002;91:195–201. doi: 10.1177/145749690209100212. [DOI] [PubMed] [Google Scholar]
- 64.Moreno M, Gratacós J, Casado E, Galisteo C, Orellana C, Larrosa M. [Usefulness of Pamidronate in the Treatment of Charcot’s Arthropathy] Reumatol Clin. 2007;3:257–261. doi: 10.1016/S1699-258X(07)73700-2. [DOI] [PubMed] [Google Scholar]
- 65.Naqvi A, Cuchacovich R, Saketkoo L, Espinoza LR. Acute Charcot arthropathy successfully treated with pamidronate: long-term follow-up. Am J Med Sci. 2008;335:145–148. doi: 10.1097/MAJ.0b013e3180a5e957. [DOI] [PubMed] [Google Scholar]
- 66.Anderson JJ, Woelffer KE, Holtzman JJ, Jacobs AM. Bisphosphonates for the treatment of Charcot neuroarthropathy. J Foot Ankle Surg. 2004;43:285–289. doi: 10.1053/j.jfas.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Game FL, Catlow R, Jones GR, Edmonds ME, Jude EB, Rayman G, Jeffcoate WJ. Audit of acute Charcot’s disease in the UK: the CDUK study. Diabetologia. 2012;55:32–35. doi: 10.1007/s00125-011-2354-7. [DOI] [PubMed] [Google Scholar]
- 68.Bharath R, Bal A, Sundaram S, Unnikrishnan AG, Praveen VP, Bhavani N, Nair V, Jayakumar RV, Kumar H. A comparative study of zoledronic acid and once weekly Alendronate in the management of acute Charcot arthropathy of foot in patients with diabetes mellitus. Indian J Endocrinol Metab. 2013;17:110–116. doi: 10.4103/2230-8210.107818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jude EB, Selby PL, Burgess J, Lilleystone P, Mawer EB, Page SR, Donohoe M, Foster AV, Edmonds ME, Boulton AJ. Bisphosphonates in the treatment of Charcot neuroarthropathy: a double-blind randomised controlled trial. Diabetologia. 2001;44:2032–2037. doi: 10.1007/s001250100008. [DOI] [PubMed] [Google Scholar]
- 70.Pitocco D, Ruotolo V, Caputo S, Mancini L, Collina CM, Manto A, Caradonna P, Ghirlanda G. Six-month treatment with alendronate in acute Charcot neuroarthropathy: a randomized controlled trial. Diabetes Care. 2005;28:1214–1215. doi: 10.2337/diacare.28.5.1214. [DOI] [PubMed] [Google Scholar]
- 71.Pakarinen TK, Laine HJ, Mäenpää H, Mattila P, Lahtela J. The effect of zoledronic acid on the clinical resolution of Charcot neuroarthropathy: a pilot randomized controlled trial. Diabetes Care. 2011;34:1514–1516. doi: 10.2337/dc11-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richard JL, Almasri M, Schuldiner S. Treatment of acute Charcot foot with bisphosphonates: a systematic review of the literature. Diabetologia. 2012;55:1258–1264. doi: 10.1007/s00125-012-2507-3. [DOI] [PubMed] [Google Scholar]
- 73.Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Freeman A, Quan H, Lasseter KC, Mucklow JC, Porras AG. Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther. 1995;58:288–298. doi: 10.1016/0009-9236(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 74.Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocr Pract. 2016;22:1–42. doi: 10.4158/EP161435.GL. [DOI] [PubMed] [Google Scholar]
- 75.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 76.Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831. doi: 10.1001/archinte.168.8.826. [DOI] [PubMed] [Google Scholar]
- 77.Sørensen HT, Christensen S, Mehnert F, Pedersen L, Chapurlat RD, Cummings SR, Baron JA. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population based case-control study. BMJ. 2008;336:813–816. doi: 10.1136/bmj.39507.551644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]