Abstract
Background
Antibiotic-associated colitis caused by Clostridium difficile (C. difficile) is the most common cause of hospital-acquired diarrhea. The pathogenesis of C. difficile colitis is mediated by bacterial toxins. C. difficile infection (CDI) severity may be determined by the fecal level of these toxins.
Objective
The objective of this article is to determine whether fecal C. difficile toxin (CDT) levels are associated with disease severity and prognosis.
Methods
A cross-sectional study of patients admitted with CDI in a tertiary center between 2011 and 2015 was conducted. Fecal CDT levels were determined by quantitative ELISA. Severe CDI was defined as a leukocyte count of > 15 × 103 cells/μl, creatinine levels that deteriorated by > 1.5 times the baseline level, or albumin levels < 3 g/dl.
Results
Seventy-three patients were recruited for this study. Patients with severe CDI (n = 47) had significantly higher toxin levels compared to patients with mild to moderate CDI (n = 26) (651 ng/ml (IQR 138–3200) versus 164 ng/ml (IQR 55.2–400.1), respectively; p = 0.001). A high toxin level (>2500 ng/ml) was associated with an increased mortality rate (odds ratio 11.8; 95% confidence interval 2.5–56).
Conclusions
The fecal CDT level is associated with disease severity and mortality rate. Measuring CDT levels may be an objective and accurate way to define the severity of CDI.
Keywords: Infectious diarrhea, infectious colitis, antibiotics, Clostridium difficile
Key summary
Clostridium difficile infection is the most common cause of antibiotic-associated diarrhea.
It is a major cause of morbidity and mortality among affected patients.
Fecal Clostridium difficile toxin (CDT) level correlates with disease severity and confers an increased risk of 30-day mortality.
Measuring fecal CDT level may allow for better risk stratification and aid in determining patient prognosis.
Introduction
Antibiotic-associated colitis caused by Clostridium difficile (C. difficile) is the most common cause of hospital-acquired diarrhea. The number of cases doubled from 2000 to 2009, and it is a major cause of morbidity and mortality within the elderly hospitalized patient population in the United States.1 C. difficile infection (CDI) is mediated by toxins that cause colon injury and inflammation.2 The widespread usage of antibiotic therapy, which disrupts the intestinal microbial flora and allows colonization of C. difficile, is well recognized as the major risk factor in the spread of this disease,3 along with multiple additional risk factors.4 It is not entirely clear what determines disease severity, and increasing age and the use of exacerbating antibiotics have been purported to play a role.5,6 C. difficile toxin (CDT) levels may also affect disease severity.2 This is supported by the observation that patients with negative toxin immunoassay but with positive polymerase chain reaction for the toxin genes have milder disease compared to patients with a measurable toxin level by immunoassay.7,8 It is therefore plausible that a quantitative analysis of fecal toxin level may reflect disease severity in patients with immunoassay-positive results. Indeed, it has previously been shown that CDT levels correlate with abdominal pain and diarrhea frequency,9 but no correlation between CDT levels and accepted disease severity parameters10 or patient prognosis has been previously demonstrated.
Our study aimed to investigate whether fecal CDT levels correlate with standard and objective measures of disease severity, and whether fecal CDT levels can predict patient outcome.
Materials and methods
Study population
Stool samples were collected, upon suspicion of CDI, as part of the routine investigation of diarrheal disease in patients admitted at the Tel Aviv Medical Center during the years 2011–2015. Patients were included if they suffered from diarrhea and tested positive for C. difficile with an immunoassay test (C. Diff Quik Check®, Tech-Lab, USA) and if there was sufficient quantity of frozen fecal samples (>50 mg of stool) for toxin analysis. Epidemiological, clinical, and laboratory data were collected for all patients. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee, the Helsinki committee, approval number 0528-10-TLV (date of approval: January 18, 2011).
Definitions
Disease severity was defined according to the guidelines of the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA)10 as follows: mild to moderate disease = leukocytosis <15,000 cells/μl and creatinine <1.5 times the premorbid level; severe disease = leukocytosis >15,000 cells/μl or serum creatinine >1.5 times the premorbid level. Severe disease was also defined as a serum albumin level of <3 g/dl at the time of active infection.11 The Charlson comorbidity index was used to assess comorbidities.12
Fecal toxin level
C. difficile-positive fecal samples stored at –80℃ were thawed to room temperature in order to determine the fecal toxin level. The fecal matter was thawed only at the time of analysis in order to minimize the natural degradation of the CDTs.13 Fifty mg of feces was homogenized with 200 μl of diluent buffer and centrifuged (5000 × g for 10 min) to remove particulate matter.
Test procedure
The fecal toxin level was quantified according to the manufacturer’s instructions (C. Diff Tox A/B II™, Tech-Lab, USA), and 96-well plates were used. Two wells were used as negative control (containing diluent buffer), one well as positive control (supplied with the kit), then predetermined standards with known toxin concentration (ng/ml) (Calbiochem, Merck Millipore, USA) and diluted specimen (supernatant). The optical density (OD) was measured at a dual wavelength of 450 nm/620 nm on a microplate enzyme-linked immunosorbent assay reader.
Interpretation of results
A standard curve was created for the toxin concentration by plotting the mean absorbance (OD) against a known toxin concentration (ng/ml) within the linear range in order to quantify the assay results. Samples with ODs outside the linear range were diluted and multiplied by the dilution factor.
Statistics
All data were summarized and displayed as the mean ± standard deviation (SD) for normally distributed continuous variables, as median interquartile range (IQR) for non-normally distributed continuous variables, and as the number of patients plus the percentage in each group for categorical variables. The independent t-test, Mann–Whitney test and chi-square statistics were used for comparisons between study groups for demographic variables, clinical characteristics, and health disease status. The Spearman correlation was used to assess correlations between disease severity as defined by leukocyte count, deterioration in creatinine and serum albumin levels and fecal CDT levels. A multivariate logistic regression was performed to investigate the relationship between mortality and a set of prognostic factors (toxin levels, Charlson comorbidity index scores, and leukocyte counts), adjusting for several potential confounders, such as current chemotherapy treatment, gender, inflammatory bowel disease (IBD), and use of steroids, proton pump inhibitors (PPIs), and antibiotics. The level of significance used for all analyses was two tailed and set at p < 0.05. The SPSS statistical package (Version 22, IBM Inc, Chicago, IL, USA) was used for all statistical analyses.
Results
A total of 73 patients (mean age 69 ± 17.7 years, 60% females, n = 44) who had diarrhea and tested positive for CDT by an immunoassay for C. difficile antigen and for CDT toxin (Tech-Lab, USA) were included in the study.
CDT toxin level as a marker of disease severity
Patients were classified as having mild to moderate or severe disease according to their leukocyte counts or deterioration in the baseline creatinine or serum albumin levels. Table 1 details the demographic, clinical, and disease status characteristics of the groups. The mild-to-moderate group consisted of 26 patients (36%) and the severe group consisted of 47 patients (64%).
Table 1.
Demographic and clinical details of the patient groups divided according to leukocyte count > 15 × 103 cells/μl or deterioration in creatinine of more than 1.5 × baseline or serum albumin < 3g/dl and a combination of these factors.
| Characteristic | Severity of Clostridium
difficile (CD) infection defined by
clinical marker in patients with CD fecal toxin
levels |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocytes |
Deterioration in creatinine |
Albumin |
Combined |
|||||||||
| Mild/ Moderate | Severe | p value | Mild/ Moderate | Severe | p value | Mild/ Moderate | Severe | p value | Mild/ Moderate | Severe | p value | |
| Total patients in group | 40 | 33 | 62 | 9 | 33 | 34 | 26 | 47 | ||||
| Female, no. (%) | 28 (70) | 16 (48) | 0.09 | 38 (61) | 4 (44) | 0.27 | 20 (66) | 21 (62) | 0.56 | 18 (69) | 26 | 0.32 |
| Age (years), mean ± SD | 68 ± 18 | 69 ± 16 | 0.8 | 68 ± 18 | 69 ± 19 | 0.89 | 67 ± 19 | 74 ± 13 | 0.11 | 67 ± 21 | 70 ± 15 | 0.57 |
| Charlson comorbidity index score, median (IQR) | 5 (4–7) | 6 (3–7.5) | 0.8 | 6 (3–7) | 5 (3.5–9.5) | 0.7 | 7 (5–8) | 4 (0–7) | 0.001 | 6 (4–7) | 5 (3–7.2) | 0.3 |
| PUD, no. (%) | 0 (0) | 1 (3) | 0.45 | 0 (0) | 1 (11) | 0.87 | 0 (0) | 1 (3) | 0.5 | 0 (0) | 1 (2) | 1 |
| Diabetes, no. (%) | 13 (33) | 7 (21) | 0.34 | 18 (29) | 2 (22) | 0.51 | 7 (21) | 10 (29) | 0.46 | 7 (27) | 13 (28) | 0.4 |
| CVS dx, no. (%) | 18 (45) | 11 (33) | 0.22 | 23 (37) | 5 (56) | 0.24 | 12 (36) | 15 (44) | 0.35 | 9 (35) | 20 (43) | 0.6 |
| Anemia, no. (%) | 7 (17.5) | 7 (21) | 0.46 | 13 (21) | 1 (11) | 0.43 | 5 (15) | 8 (24) | 0.29 | 3 (12) | 11 (23) | 0.35 |
| Malignancy, no. (%) | 9 (22.5) | 10 (30) | 0.312 | 17 (27) | 2 (22) | 0.55 | 5 (15) | 13 (38) | 0.03 | 4 (15) | 15 (32) | 0.17 |
| IBD, no. (%) | 3 (7.5) | 3 (9) | 0.57 | 5 (8) | 1 (11) | 0.57 | 4 (12) | 2 (6) | 0.32 | 3 (12) | 3 (6) | 0.66 |
| Renal failure, no. (%) | 8 (20) | 8 (24) | 0.44 | 13 (21) | 3 (33) | 0.34 | 6 (18) | 10 (29) | 0.21 | 5 (19) | 11 (23) | 0.77 |
| Dialysis, no. (%) | 3 (8) | 2 (6) | 0.59 | 3 (5) | 2 (22) | 0.12 | 2 (6) | 3 (9) | 0.51 | 1 (4) | 4 (9) | 0.65 |
| Neutropenia, no. (%) | 1 (3) | 1 (3) | 0.7 | 1 (2) | 1 (11) | 0.24 | 0 (0) | 2 (6) | 0.25 | 1 (4) | 1 (2) | 1 |
| Antibiotic usage, no. (%) | 30 (75) | 26 (79) | 0.65 | 47 (76) | 7 (77) | 0.09 | 23 (70) | 28 (82) | 0.2 | 17 (65) | 39 (83) | 0.12 |
| Steroid usage, no. (%) | 9 (23) | 6 (18) | 0.44 | 15 (24) | 0 (0) | 0.1 | 5 (15) | 10 (29) | 0.13 | 5 (19) | 10 (21) | 1 |
| PPI usage, no. (%) | 25 (63) | 22 (67) | 0.45 | 40 (65) | 5 (55) | 0.43 | 20 (66) | 25 (74) | 0.19 | 15 (58) | 32 (68) | 0.45 |
| Chemotherapy, no. (%) | 2 (5) | 6 (18) | 0.08 | 8 (13) | 0 (0) | 0.32 | 4 (12) | 4 (12) | 0.62 | 2 (8) | 6 (13) | 0.7 |
| Death within 30 days, no. (%) | 3 (7.5) | 10 (30) | 0.02 | 11 (18) | 2 (22) | 0.66 | 6 (18) | 6 (18) | 0.52 | 2 (8) | 11 (23) | 0.11 |
| Fecal toxin level (ng/ml), median (IQR) | 167 (55.6–475) | 2174.3 (157.2–4209) | <0.001 | 267.8 (73–855) | 970.8 (120–3839) | 0.21 | 291.7 (78–880) | 248.4 (120–2197.6) | 0.8 | 164 (55.2–400.1) | 651 (138–3200) | 0.001 |
SD: standard deviation; PUD: peptic ulcer disease; CVS: cardiovascular; dx: diagnosis; IBD: inflammatory bowel disease; PPI: peptic ulcer disease; IQR: interquartile range.
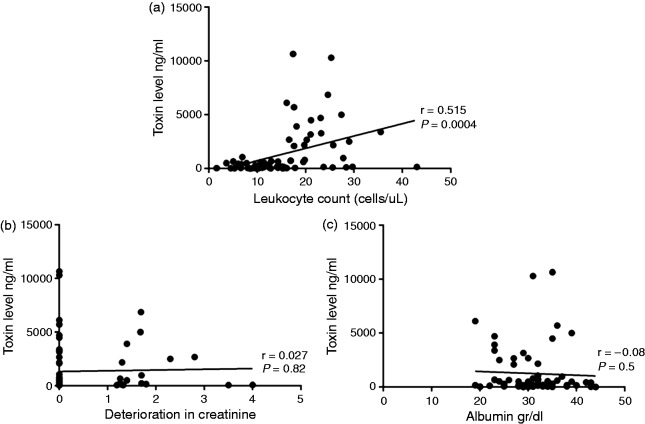
The fecal CDT levels were significantly higher in patients who were classified as having severe disease than those with mild-to-moderate disease (651 ng/ml (IQR 138–3200) versus 164 ng/ml (IQR 55.2–400.1), respectively; p = 0.001) (Figure 1(a)).
Figure 1.
Correlation plots detailing the relationship between fecal CD toxin level and markers of disease severity A) leucocyte count (>15,000 cells/uL), B) deterioration in creatinine level (>1.5×baseline) and C) serum albumin (<3 gr/dl).
We next examined the association between the CDT fecal levels and each of the disease severity subgroups, defined by either leukocyte count or deterioration in baseline creatinine or serum albumin level. Interestingly, the fecal CDT level was significantly higher in severe disease compared to mild-to-moderate disease only when defined by the leukocyte count (i.e., > 15 × 103 cells/μl) (2174.3 ng/ml (IQR 157.5–4209) vs 167 ng/ml (IQR 55.6–475), respectively; p < 0.0001). Furthermore, the fecal CDT level showed significant correlation with the leukocyte count (r = 0.515, p < 0.001) but not with deterioration in the creatinine or serum albumin levels (Figure 1). A receiver operating characteristic curve analysis revealed that a fecal CDT level of 1575 ng/ml reflected a specificity of 100% and a sensitivity of 45% for severe disease (as defined by leukocyte count) (Figure 2).
Figure 2.
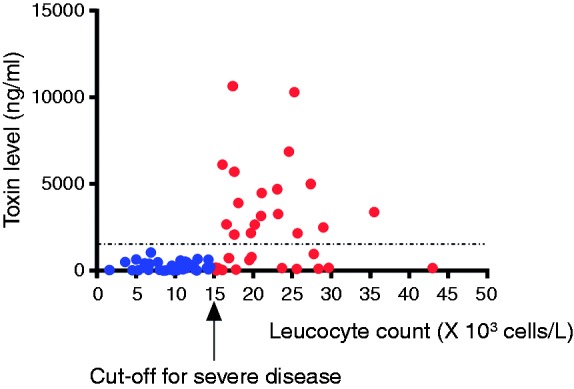
Scatter plot showing that a toxin level of 1575 ng/ml was associated with 100 % specificity for severe disease (as defined by leucocyte count).
Increased fecal CDT level is associated with mortality
Thirteen (17.8%) patients died within 30 days of CDI diagnosis. There were no significant demographic or clinical differences between those who died and those who survived except for increased chemotherapy exposure among the former patients (p = 0.004) (Supplementary table 1). Fecal CDT levels were significantly increased among patients who died within 30 days post-diagnosis compared to those who survived (2676.3 ng/ml (IQR 200.6–5202) versus 182.3 ng/ml (IQR 69.7–672.3), respectively; p = 0.008). These results are further highlighted by a Kaplan–Meier survival chart that shows a significantly decreased survival in those patients with a higher toxin level (p = 0.0006) (Figures 2 and 3). Moreover, in patients with fecal CDT level > 2500 ng/ml, mortality rate was significantly higher (p = 0.003) (Figure 4) and was associated with a death rate of 47% (n = 7) within 30 days of CDI diagnosis.
Figure 3.
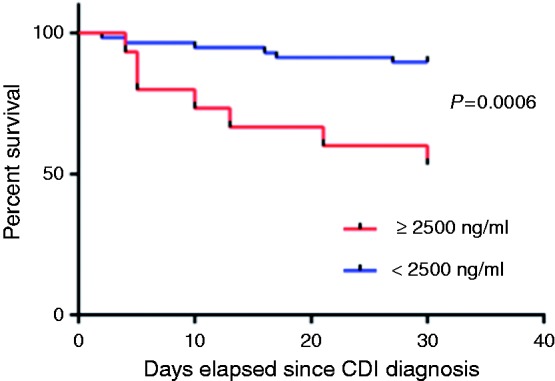
Kaplan-Meier plot showing the significant difference in 30 day mortality between patients with a CD toxin level above 2500 ng/ml and those with a CD toxin level below this cut off.
Figure 4.
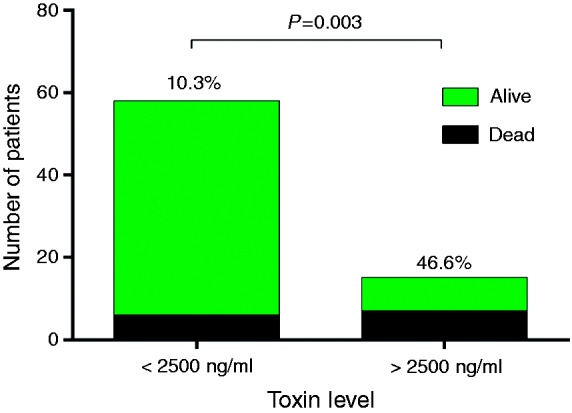
Death rate was significantly higher in patients with a toxin level of greater than 2500 ng/ml.
The fecal CDT level was a predictor of mortality (p = 0.008) in a multivariate logistic regression analysis when adjusted for Charlson comorbidity index scores, sex, IBD, and usage of PPIs, antibiotics, and steroids. Patients with a fecal CDT level of > 2500 ng/ml had an odds ratio (OR) of 11.8 (95% confidence interval (CI) 2.5–56) of dying within 30 days of CDI diagnosis, in contrast to a nonsignificant OR of 1.08 (95% CI 0.99–1.18) in patients with a leukocyte count > 15 × 103 cells/μl (Table 2). Patients who received chemotherapy (n = 8) had a significantly greater mortality risk (OR = 15.4, 95% CI 2.5–97.8) when adjusted for these same variables. Interestingly, the CDT level in the latter patient group was significantly higher compared to patients with no chemotherapy exposure (4702.9 ng/ml (IQR 164.7–6119.2) versus 240.2 ng/ml (IQR 79–745), respectively; p = 0.03), indicating a synergistic effect between these two factors. Indeed, 100% (n = 4) patients with both fecal CDT levels above 2500 ng/ml and a recent chemotherapy treatment died as opposed to a mortality rate of 15% (n = 9) for those with either one or none of those two factors (n = 69, p = 0.001). When patients who received chemotherapy were excluded from the analysis, there was still a trend toward higher fecal CDT levels in those who died compared to those who survived (660 ng/ml (IQR 191–3130) versus 182 ng/ml (IQR 68–667), respectively; p = 0.06).
Table 2.
Adjusted odds ratios for 30-day mortality by clinical feature.
| Predictive variables | Adjusted for Charlson comorbidity index score, IBD, antibiotic use, PPI use, steroid use, and sex |
|---|---|
| Toxin level > 2500 ng/ml (95% CI) | 11.8 (2.5–56.1) |
| Chemotherapy treatment (95% CI) | 15.4 (2.4–98.7) |
| Leukocyte count > 15 × 103 cells/μl (95% CI) | 1.08 (0.93–1.18) |
IBD: inflammatory bowel disease; PPI: proton pump inhibitor; 95% CI: 95% confidence interval.
Discussion
The results of this cross-sectional study revealed an association between the fecal CDT level and CDI disease severity and prognosis. We also found that the fecal CDT level is a good predictor of mortality. Two earlier studies had investigated the association between fecal CDT level and disease severity,9,14 but neither looked into its relation with the standard measures of disease severity (i.e., leukocyte count and a deterioration of creatinine or serum albumin levels) nor with mortality.
In this study, fecal CDT level was significantly higher in patients with severe disease compared to those with mild to moderate disease. This was even more apparent when disease severity was defined solely by an increased leukocyte count (>15,000 cells/μl). Furthermore, the fecal CDT level correlated directly to the leukocyte count, further supporting the notion that the fecal CDT level is important in determining disease severity. Importantly, there were no differences in age, sex, comorbidities, chemotherapy treatment, or in steroid, antibiotic, or PPI use between the groups.
A previous investigation of the relationship between the fecal CDT level and disease severity found a trend toward higher fecal CDT levels in patients with severe disease.9 That study, however, defined severity in clinical terms (i.e., number of stools per day, abdominal pain, and fever) not routinely used to define disease severity. The current study is the first to describe the association between fecal CDT level and CDI severity based on an objective and accepted definition of disease severity.10,11,15 Using this definition avoids the difficulties in quantifying the number of stools per day and the subjective manner of describing abdominal pain.
Currently, accepted measures of disease severity are a high leukocyte count, deterioration in serum creatinine levels, and low serum albumin levels.10 There are inherent caveats using these measures in particular patient groups such as those with neutropenia or with numerous sources of a raised leukocyte count, patients with chronic kidney disease and chronically ill patients with a low albumin level at baseline. These factors may lead to either over- or underestimation of CDI severity. In such patients other measures of disease severity are required and fecal CDT levels may assist in risk-stratifying these patients. In the current study, the fecal CDT level could diagnose severe CDI with 100% specificity. As such, the measuring of fecal CDT levels may help in decision making with regard to implementing more intensive treatment regimens in complicated patients.
An important finding is a positive association between fecal CDT levels and mortality. It is striking the fecal toxin level upon CDI diagnosis was significantly higher than among patients who died compared to those who survived. Patients with a fecal CDT level of > 2500 ng/ml had an OR risk of 11.8 (95% CI 2.5–56) of dying within 30 days of CDI diagnosis after adjustment for potential confounders. It was previously shown that risk factors for complicated CDI and mortality included age, comorbidities, serum albumin level, leukocytosis, increased serum creatinine levels, and a C. difficile ribotype.16 A direct relationship between the fecal CDT level and mortality has not been described before and it suggests that measuring fecal CDT levels may provide a means for good risk stratification in CDI patients and perhaps should complement the other determinants of severity, that is, leukocyte count, deterioration in creatinine levels, and low serum albumin level.
It is noteworthy that chemotherapy was also associated with mortality in this setting. During the study, eight patients were being actively treated with chemotherapy, and five of them died within 30 days of CDI and the fecal toxin level was > 2500 ng/mg in four of those five. This may be indicative of a strong synergistic effect of chemotherapy and high toxin levels on CDI mortality. While chemotherapy is a risk factor for CDI,17,18 it had not been associated with increased CDI severity or associated mortality.5 Despite the small numbers of patients in our current study, the high mortality rate in this patient population should promote further studies to determine whether this result is reproducible and, if so, whether more aggressive treatment of CDI can reduce the mortality rate. It is not clear whether an increased toxin level in this group of patients is a marker of immunosuppression caused by chemotherapy and unrelated to the increased death rate or whether it is the cause of the increased death rate in these patients. It was noted that when patients receiving chemotherapy were excluded, there was still a trend toward higher toxin levels in patients who died, supporting the importance of the CDT level in the pathogenesis and prognosis of these patients. Prospective studies are required to provide better answers to this question.
All the patients in our cohort were admitted to a tertiary care hospital, which uses acceptable treatment protocols19 that include early initiation of antibiotic therapy (metronidazole, vancomycin, or a combination) shortly after CDI diagnosis (Supplementary table 2). Nevertheless, the mortality rates of CDI were high and current measurements of disease severity were not predictive of mortality, thus highlighting the need for a better and more objective definition of disease severity and prognosis. Admission fecal CDT levels of > 2500 ng/ml were associated with a high mortality rate, and they should guide the clinician to initiate more aggressive and more effective treatment methods, such as early initiation of vancomycin, a combination of metronidazole and vancomycin, or early fecal microbial transplantation. Furthermore, these results also provide a rationale for the investigation of treatments that directly target fecal CDT as described in numerous recent studies.20–22
This study has several limitations that bear mention. There was a lack of an association analysis of toxin level and clinical severity indices that were not included owing to the unreliability clinical data collection, particularly the number of bowel movements per day, as this relies on patient memory/awareness and/or accurate determination by the medical staff. This unreliability in data collection also made determining the therapeutic efficacy and clinical improvement difficult to assess and thus the end point of 30-day mortality alone was used to determine outcome. In addition, although we know that the most common strain of C. difficile in our hospital is the hr-02 strain,23 it would be an important addition to the data to perform a strain analysis, given that certain strains (e.g., ribotype 078) are associated with more severe disease.24
Conclusion
This study shows that C. difficile fecal toxin level is associated with increased disease severity and further, very high levels of fecal toxin may predict poorer outcomes and increased short-term mortality in these patients. Fecal CDT level provides a more objective and direct measure of CDI severity than the currently used methods especially considering the multiple comorbidities affecting this patient cohort. Perhaps, using fecal CDT level as a risk stratification tool, more timely and aggressive therapy can be initiated. This study also provides further insight into the pathophysiology of CDI. Finally, although the results of this study are promising, further studies surrounding the prognostic value of C. difficile fecal toxin levels with regards to morbidity, mortality, and relapse rates are required.
Supplementary Material
Acknowledgment
The authors thank Esther Eshkol for her assistance with the professional English editing of this manuscript.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the Tel Aviv Medical Center’s human research committee and the Helsinki committee (approval number 0528-10-TLV; date of approval: 18 January 2011).
Informed consent
Patients with laboratory confirmed CDI were asked to sign an informed consent in order to be included in the study.
References
- 1.Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 2012; 55(Suppl 2): S65–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahida YR, Makh S, Hyde S, et al. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: Induction of interleukin 8 production and apoptosis after cell detachment. Gut 1996; 38: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012; 67: 742–748. [DOI] [PubMed] [Google Scholar]
- 4.Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am J Gastroenterol 2012; 107: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 5.Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis 2009; 15: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manek K, Williams V, Callery S, et al. Reducing the risk of severe complications among patients with Clostridium difficile infection. Can J Gastroenterol 2011; 25: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoldaş Ö, Altındiş M, Cufalı D, et al. A diagnostic algorithm for the detection of Clostridium difficile-associated diarrhea. Balkan Med J 2016; 33: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175: 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akerlund T, Svenungsson B, Lagergren A, et al. Correlation of disease severity with fecal toxin levels in patients with Clostridium difficile-associated diarrhea and distribution of PCR ribotypes and toxin yields in vitro of corresponding isolates. J Clin Microbiol 2006; 44: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31: 431–455. [DOI] [PubMed] [Google Scholar]
- 11.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–498. quiz 499. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 13.Freeman J, Wilcox MH. The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces. J Clin Pathol 2003; 56: 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdon DW, George RH, Mogg GA, et al. Faecal toxin and severity of antibiotic-associated pseudomembranous colitis. J Clin Pathol 1981; 34: 548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iv EC, Iii EC, Johnson DA. Clinical update for the diagnosis and treatment of Clostridium difficile infection. World J Gastrointest Pharmacol Ther 2014; 5: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou Chakra CN, Pepin J, Sirard S, et al. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: A systematic review. PloS One 2014; 9: e98400–e98400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain A, Aptaker L, Spriggs DR, et al. Gastrointestinal toxicity and Clostridium difficile diarrhea in patients treated with paclitaxel-containing chemotherapy regimens. Gynecol Oncol 1998; 71: 104–107. [DOI] [PubMed] [Google Scholar]
- 18.Nielson H, Daugaard G, Tvede M, et al. High prevalence of Clostridium difficile diarrhoea during intensive chemotherapy for germ cell cancer. Br J Cancer 1992; 66: 666–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen NA, Ben Ami R, Guzner-Gur H, et al. Fecal microbiota transplantation for Clostridium difficile-associated diarrhea. Isr Med Assoc J 2015; 17: 510–514. [PubMed] [Google Scholar]
- 20.Bender KO, Garland M, Ferreyra JA, et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci Transl Med 2015; 7: 306ra148–306ra148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito G, Nobile N, Gigli S, et al. Rifaximin improves Clostridium difficile toxin A-induced toxicity in Caco-2 cells by the PXR-dependent TLR4/MyD88/NF-κB pathway. Front Pharmacol 2016; 7: 120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigli S, Seguella L, Pesce M, et al. Cannabidiol restores intestinal barrier dysfunction and inhibits the apoptotic process induced by Clostridium difficile toxin A in Caco-2 cells. United European Gastroenterol J 2017; 5: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler A, Miller-Roll T, Bradenstein R, et al. A national survey of the molecular epidemiology of Clostridium difficile in Israel: The dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis 2015; 83: 21–24. [DOI] [PubMed] [Google Scholar]
- 24.Walker AS, Eyre DW, Wyllie DH, et al. Relationship between bacterial strain type, host biomarkers, and mortality in Clostridium difficile infection. Clin Infect Dis 2013; 56: 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.