Binding of the HIV envelope glycoprotein (Env) to cellular CD4 and chemokine receptors triggers conformational changes in Env that mediate virus entry, but premature triggering of Env conformational changes leads to virus inactivation. Currently, we have a limited understanding of the network of residues that regulate Env conformational changes. Here, we identify residues in HR1 of gp41 that modulate conformational changes in response to gp120 binding to CD4 and show that the mutations in HR1 and HR2 that confer resistance to fusion inhibitors are associated with gp120 mutations in different regions of Env that confer a more open conformation. These findings contribute to our understanding of the regulation of Env conformational changes and efforts to design new entry inhibitors and stable Env vaccine immunogens.
KEYWORDS: fusion inhibitor, fusion, HIV-1 entry, resistance, gp41, gp120, conformational changes, envelope glycoprotein
ABSTRACT
Entry of human immunodeficiency virus type 1 (HIV-1) into host cells is mediated by conformational changes in the envelope glycoprotein (Env) that are triggered by Env binding to cellular CD4 and chemokine receptors. These conformational changes involve the opening of the gp120 surface subunit, exposure of the fusion peptide in the gp41 transmembrane subunit, and refolding of the gp41 N- and C-terminal heptad repeat regions (HR1 and HR2) first into an extended prehairpin intermediate and then into a compact 6-helix bundle (6HB) that facilitates fusion between viral and host cell membranes. Previously, we reported that Envs resistant to HR1 peptide fusion inhibitors acquired key resistance mutations in either HR1 or HR2 that increased 6HB stability. Here, we identify residues in HR1 that contribute not only to fusion inhibitor resistance and 6HB stability but also to reduced reactivity to CD4-induced conformational changes that lead to 6HB formation. While all Envs show increased neutralization sensitivity to mimetic CD4 (mCD4), Envs with either the E560K or Q577R HR1 mutation reduced conformational reactivity to CD4 that resisted viral inactivation and triggering to the 6HB. Using a panel of monoclonal antibodies (mAbs), we further determined that Envs from both HR1 and HR2 resistance pathways exhibit a relaxed trimer conformation due to gp120 adaptive mutations in different regions of Env that segregate by resistance pathway. These findings highlight regions of cross talk between gp120 and gp41 and identify HR1 residues that play important roles in regulating CD4-induced conformational changes in Env.
IMPORTANCE Binding of the HIV envelope glycoprotein (Env) to cellular CD4 and chemokine receptors triggers conformational changes in Env that mediate virus entry, but premature triggering of Env conformational changes leads to virus inactivation. Currently, we have a limited understanding of the network of residues that regulate Env conformational changes. Here, we identify residues in HR1 of gp41 that modulate conformational changes in response to gp120 binding to CD4 and show that the mutations in HR1 and HR2 that confer resistance to fusion inhibitors are associated with gp120 mutations in different regions of Env that confer a more open conformation. These findings contribute to our understanding of the regulation of Env conformational changes and efforts to design new entry inhibitors and stable Env vaccine immunogens.
INTRODUCTION
The HIV-1 envelope glycoprotein (Env) mediates receptor binding and fusion of the virus with host cell membranes. Env is translated as the gp160 polyprotein that is subsequently cleaved by a cellular furin-like protease to the gp120 surface (SU) and gp41 transmembrane (TM) subunits. gp120 and gp41 associate as a non-covalently linked dimer, three of which assemble into a trimer of dimers forming the functional Env spike on the virion and infected cell surface. gp120 binding to both the CD4 primary cellular receptor (1, 2) and either the CXCR4 or CCR5 chemokine coreceptor (3–6) triggers a series of conformational changes that release the membrane fusion function of gp41.
The native Env trimer exists in a metastable state prior to interactions with receptors. In the native conformation, Env predominantly occupies a closed structure, in which the gp120 variable loops and extensive surface glycosylation shield much of the Env core (7–10). Receptor binding opens gp120 to expose the coreceptor binding site (11–13). Receptor and coreceptor binding leads to further conformational changes that enable two heptad repeat (HR) regions in the ectodomain of gp41 to self-assemble into a stable, hairpin-like, six-helix bundle (6HB) structure, the formation of which facilitates membrane fusion (14–17). In the native Env trimer, the C-terminal half of the N-terminal HR (HR1) in gp41 forms an α-helix arranged as a trimeric coiled coil with the other two gp41 protomers of the trimer (18). The N-terminal half of HR1 contains another short helix, with a loop region connecting the two HR1 helical domains. At the extreme N terminus of gp41, the hydrophobic fusion peptide (FP) is partially buried in the HR1 coiled coil. Receptor activation releases gp120 constraints on gp41 so that the entire HR1 region can extend into a single, long, trimeric coiled coil that repositions the FP for insertion into host cell membranes (19–21). This extended Env conformation is often referred to as the prehairpin intermediate (PHI). Resolution of the PHI into the final, stable 6HB draws the membranes together and drives fusion through a series of conformational changes that are poorly understood.
To gain insights into receptor activation of Env and fusion-intermediate conformations, we previously generated a large panel of Envs that are resistant to peptide fusion inhibitors corresponding to the highly conserved HR1 region of gp41 (22–25). According to the dominant negative mode of inhibition, HR1 peptides as monomers or dimers can bind to gp41 HR1, forming a peptide-gp41 coiled coil that traps the PHI (26, 27). In addition, HR1 peptides can oligomerize and bind to gp41 HR2, forming a peptide-gp41 6HB that can also trap the PHI (24, 28). In these resistance cultures, we consistently found that HIV-1 escapes inhibition by HR1 peptide fusion inhibitors through one of two genetic pathways, each defined by a key resistance mutation in HR1 or HR2 (22, 23). Curiously, each pathway was accompanied by a different pattern of gp120 mutations. In the HR1 pathway (pathway 1), mutations in gp120 tended to emerge in residues in the first and second variable regions (V1/V2) and in the vicinity of the CD4 binding site (CD4bs) (23). In the HR2 pathway (pathway 2), gp120 mutations tended to emerge in residues in the third variable region (V3), in the vicinity of the chemokine receptor binding site. These two distinct patterns of associated gp120 and gp41 mutations suggested that they were involved in functional networks controlling Env-mediated virus entry.
In this study, we characterized the functional and structural effects of combinations of Env mutations coselected in our resistance cultures to identify gp120 and gp41 residues that cooperate to regulate receptor use and Env conformational changes. Using a panel of neutralizing and conformation-specific antibodies (Abs), we found that both pathway 1 and pathway 2 Envs evolved relaxed or more-open trimer conformations in their native state, although they did so via different patterns of mutations. Notably, Envs from both pathways showed increased sensitivity to neutralization by a CD4 mimetic compared to the parental wild type, but Envs with mutations in HR1 showed reduced CD4-induced conformational changes in gp41. These studies provide new insights into the regulation of Env conformational changes and suggest ways to modify Env conformations.
RESULTS
gp120 and gp41 mutations influence sensitivity to mCD4 inhibition.
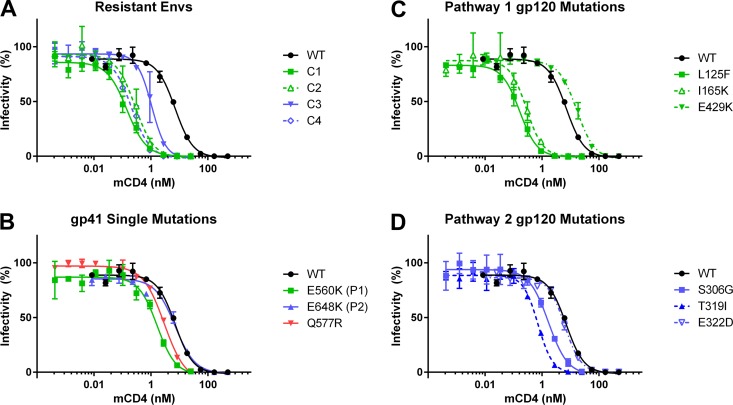
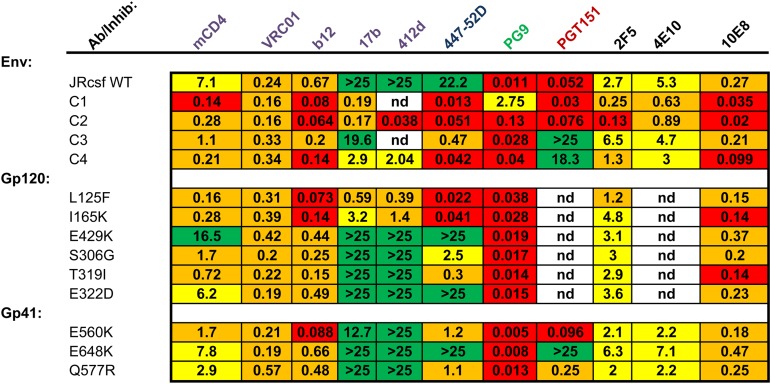
Previously, we isolated four Envs (Table 1) from HIV-1 JRCSF cultures selected for resistance to a 44-mer peptide corresponding to gp41 HR1 (N44) that segregated into two genetic pathways, defined by either the E560K founder resistance mutation in HR1 (pathway 1) or the E648K founder resistance mutation in HR2 (pathway 2) (23). We reported that these peptide-resistant Envs (Table 1) were more sensitive to neutralization by soluble CD4 (sCD4) than the parental, wild-type (WT) Env. Here, we further investigated the contribution of individual mutations in the peptide-resistant Envs to receptor-induced inactivation of Env using the CD4 mimetic M48-U1 (mCD4) (29). As seen with sCD4 in our previous studies (22, 23), all peptide-resistant Envs showed enhanced sensitivity to mCD4 (Fig. 1A). Three of four peptide-resistant Envs (from culture 1 [C1], C2, and C4) had a 25- to 50-fold increase in sensitivity to mCD4, while C3 Env had a 6.5-fold increase in mCD4 sensitivity, likely reflecting fewer mutations in this Env (T319I and E648K) than in the other peptide-resistant Envs with multiple mutations in gp120 and gp41 (Table 1). Mutations in gp41 had only a modest effect on mCD4 sensitivity (Fig. 1B), as seen previously for sCD4 (22, 23). The E560K and Q577R HR1 mutations each increased mCD4 neutralization approximately 4-fold and 2.5-fold, respectively. In contrast, the E648K HR2 mutation conferred little effect.
TABLE 1.
Mutations in Env clones from HR1 peptide resistance cultures
| Pathway | Culture (Env clone) | gp120 mutation(s) | gp41 mutation(s)a |
||
|---|---|---|---|---|---|
| HR1 | HR2 | Other | |||
| 1 | C1 | L125F, I165K | N554K, E560K, V580L | T641I | |
| C2 | L125F, E429K | E560K, Q577R | T641I | ||
| 2 | C3 | T319I | E648K | ||
| C4 | S306G, T319I, E322D | Q577R | T641I, E648K | D620N, V833I | |
Founder mutations are marked in bold.
FIG 1.
Mutations alter sensitivity to inhibition by mCD4. Shown are data for sCD4 mimetic M48-U1 inhibition of pseudoviruses bearing WT Env or culture 1 to culture 4 [C1 to C4] N44-resistant Envs (A), Envs with gp41 mutations (B), Envs with pathway 1 gp120 mutations (C), and Envs with pathway 2 gp120 mutations (D). Data points are the averages of results from at least two independent experiments, each conducted in duplicate. Error bars show standard errors of the means (SEM).
Single gp120 mutations also revealed differences between the pathways (Fig. 1C and D). In Envs from pathway 1, L125F and I165K mutations in V1/V2 increased mCD4 sensitivity by ∼1.5 log units, but the E429K mutation had the opposite effect, conferring approximately 2-fold resistance to mCD4 neutralization (Fig. 1C). This mutation occurred only in the presence of other mutations that dominated the phenotype with enhanced mCD4 sensitivity (Table 1 and Fig. 1A). In contrast, Envs with S306G and/or T319I mutations in V3 from pathway 2 had a more modest effect on mCD4 sensitivity, increasing sensitivity 4- and 10-fold, respectively (Fig. 1D). The E322D mutation in V3 had no effect. Together, these findings suggest that the E648K mutation may constrain the evolution of CD4 sensitivity, while Envs with the E560K mutation in HR1 are able to tolerate single gp120 mutations that confer large increases in CD4 sensitivity.
HR1 modulates mCD4-mediated virus entry.
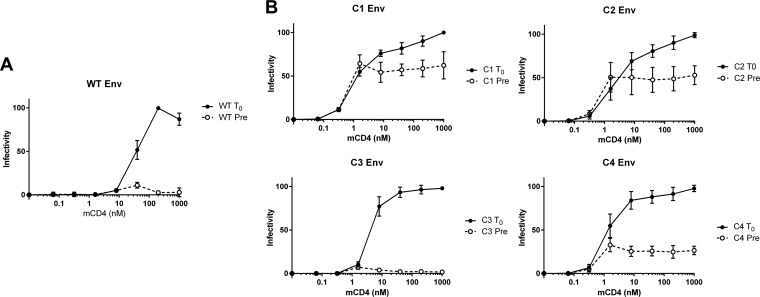
Because the gp120 and gp41 mutations conferred different sensitivities to mCD4, we investigated the effect of these mutations on Env-mediated virus entry. The addition of sCD4 can render HIV-1 competent for fusion for a brief period, but longer incubations can irreversibly inactivate virus, presumably by triggering irreversible conformational changes in Env to fusion-incompetent conformations that may involve the formation of the gp41 6HB. We assessed the contributions of various mutations to CD4-induced conformational changes (Env reactivity) using mCD4 and CCR5+ target cells that lacked CD4 (Fig. 2). Following spinoculation onto target cells, pseudoviruses bearing WT Env treated with mCD4 resulted in infectivity only at relatively high concentrations of mCD4 (WT at time zero [T0]) (Fig. 2A), with half-maximal infectivity at approximately 30 nM mCD4. In contrast, pretreatment with mCD4 for 60 min neutralized infectivity at all mCD4 concentrations (WT Pre) (Fig. 2A). Shortening the mCD4 preincubation time to as little as 15 min still resulted in complete inactivation (data not shown), demonstrating that mCD4 rapidly inactivates pseudoviruses with WT Env.
FIG 2.
mCD4-mediated entry of pseudoviruses into CD4− CCR5+ cells. Shown are data for the infectivity of pseudoviruses bearing WT (A) and C1 to C4 (B) Envs spinoculated onto CD4− CCR5+ 293T target cells under the conditions of mCD4 added following spinoculation (T0) and mCD4 preincubated with pseudoviruses for 1 h at 37°C prior to spinoculation (Pre). Infectivity was normalized to the maximum fusion observed for each pseudovirus. Data points are the averages of results from at least two independent experiments, each conducted in duplicate. Error bars show SEM.
Envs from all peptide-resistant cultures (C1 to C4) mediated virus entry in CD4− CCR5+ target cells at significantly lower concentrations of mCD4 than for the WT (T0) (Fig. 2B), achieving half-maximal infectivity at between 0.75 and 2 nM mCD4. Envs from pathway 1 reacted similarly to Envs from pathway 2. Remarkably, pseudoviruses with C1, C2, and C4 Envs maintained significant infectivity following mCD4 preincubation (Pre) (Fig. 2B). Pseudoviruses bearing C1 and C2 Envs retained approximately 50% infectivity following 60-min preincubations with up to 1,000 nM mCD4. The pathway 2 Env responses were variable. Pseudoviruses with C4 Env retained ∼25% infectivity after 60 min, while pseudoviruses with C3 Env were neutralized similarly to WT Env. C3 Env has only two mutations, which are shared with C4 Env, indicating that additional mutations in C4 Env contributed to the maintenance of fusion competence. Because pathway 1 and 2 Envs have little overlap in mutations, these results suggest that either different combinations of gp120 and gp41 mutations or a limited set of overlapping mutations preserved fusion competence after prolonged pretreatment with mCD4.
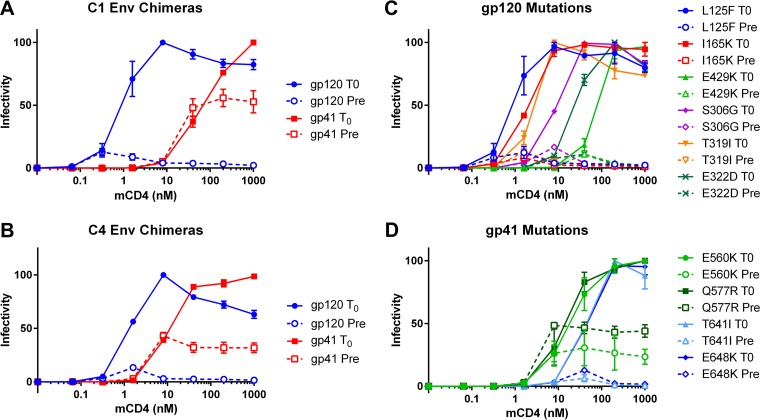
To investigate residues responsible for the different responses to mCD4, we first studied mCD4 pretreatments using chimeric Envs with mutations from the C1 or C4 Envs in either gp120 or gp41, paired with WT sequences in gp41 or gp120, respectively (Fig. 3A and B). Pseudoviruses with the gp120 mutations from C1 Env infected target cells with low levels of mCD4 (50% fusion at ∼0.6 nM mCD4) but lost infectivity after mCD4 preincubation (Fig. 3A, blue solid and dashed curves, respectively). In contrast, pseudoviruses with gp41 mutations from C1 Env infected target cells only with high mCD4 levels, similar to the WT (50% fusion at ∼30 nM mCD4), but retained infectivity after 60-min mCD4 preincubations (Fig. 3A, red solid and dashed curves, respectively). Chimeric Envs with mutations from the C4 Env (Fig. 3B) also showed that gp41 mutations were responsible for the preservation of fusion competence after prolonged treatment with mCD4.
FIG 3.
HR1 mutations reduce inactivation of pseudoviruses pretreated with mCD4. Shown are data for the infectivity of pseudoviruses bearing Envs with C1 (A), C4 (B), gp120 (C), and gp41 (D) mutations spinoculated onto CD4− CCR5+ cells under the conditions described in the legend of Fig. 2, with mCD4 added following spinoculation (T0) and mCD4 preincubated with pseudoviruses for 1 h at 37°C prior to spinoculation (Pre). Data points are the averages of results from at least two independent experiments, each conducted in duplicate. Error bars show SEM.
A subset of single gp120 mutations from both pathways conferred infectivity with lower concentrations of mCD4 (Fig. 3C). Mutations L125F and I165K from pathway 1 Envs and T319I from pathway 2 Envs accounted for most of the increased infectivity using low concentrations of mCD4. The S306G mutation had a more modest effect, lowering the concentration of 50% infectivity to ∼10 nM mCD4, while the E322D and E429K mutations failed to lower the mCD4 requirements. Single gp41 mutations revealed a different pattern (Fig. 3D). The E560K mutation allowed 50% infectivity at a modest mCD4 concentration (∼10 nM mCD4) and ∼25% infectivity following mCD4 preincubation. The Q577R mutation also allowed infectivity at lower mCD4 concentrations and ∼50% retention of infectivity after mCD4 pretreatment. In contrast, the E648K mutation did not lower the mCD4 concentrations needed for infectivity or confer retention of infectivity following mCD4 preincubation. Similarly, T641I behaved like WT Env. These results show that HR1 residues 560 and 577 are responsible for the retention of fusion activity following mCD4 exposure and therefore regulate CD4-mediated triggering (Env reactivity) of Env to a fusion-incompetent conformation.
gp41 HR1 resistance mutations reduce mCD4-induced refolding of gp41 to the 6HB.
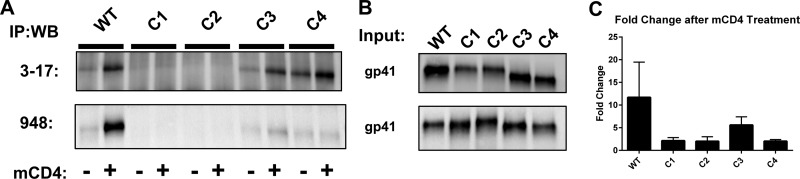
To investigate whether Env inactivation by mCD4 was due to a refolding of gp41 to the 6HB, we assessed the binding of a 6HB-specific monoclonal antibody (mAb) (3-17) to mCD4-treated Envs. This mAb, raised in mice against a 6HB recombinant protein (see Materials and Methods), exhibits an ∼900-fold binding preference for the 6HB over individual HR1 or HR2 peptides (Table 2). The level of immunoprecipitation of pseudoviruses bearing WT Env with the 3-17 mAb was low (5.5% ± 2.5% of the input) in the absence of mCD4 but increased nearly 7-fold (37% ± 1.2% of input) after mCD4 treatment (Fig. 4). In contrast, the level of immunoprecipitation of pseudoviruses bearing C1 and C2 Envs was barely above the background (<5%) after mCD4 treatment (Fig. 4A and C). Pseudoviruses with Envs containing E648K were more similar to WT pseudoviruses. Pseudoviruses with C3 Env showed a 5-fold increase of 6HB formation (Fig. 4C), while pseudoviruses with C4 Env showed significant immunoprecipitation of gp41 prior to mCD4 treatment (28%) and an ∼2-fold increase after treatment. The latter finding is consistent with C4 Env conferring intermediate sensitivity to mCD4 inactivation (Fig. 2B) and suggests that the E648K mutation in HR2 may interfere with the ability of the Q577R mutation to reduce the conformational reactivity of gp41. Finally, we confirmed these results with a polyclonal rabbit serum (948) that was previously shown to preferentially bind CD4-activated Env (30) (Table 2 and Fig. 4A and B) to rule out the possibility that C1 to C4 mutations specifically affected the 3-17 mAb epitope. We conclude that the resistance mutations in HR1 make Env less prone to CD4-induced triggering to the 6HB.
TABLE 2.
ELISA binding of antibodies to gp41 peptides
| Antibody or serum | ELISA binding titera |
||
|---|---|---|---|
| IZN36 + C34 (6HB) | IZN36 | C34 | |
| 3-17 | 0.027 μg/ml | 24.1 μg/ml | UD |
| 948 | 1:30,300 | 1:1,180 | 1:4,680 |
ELISA binding for 3-17 is expressed as the Ab concentration at which half-maximal binding occurred (EC50). ELISA binding for serum 948 is expressed as the serum dilution at which half-maximal binding occurred (EC50). UD, binding undetectable at any tested Ab concentration.
FIG 4.
Detection of gp41 6HB after mCD4 treatment. (A) Immunoprecipitation (IP) of pseudoviruses bearing WT and C1 to C4 Envs in the presence (+) or absence (−) of mCD4 with the anti-6HB monoclonal antibody (3-17) (top) or polyclonal rabbit serum (948) (bottom) and analyzed by reducing SDS-PAGE followed by Western blotting (WB) with a gp41 monoclonal antibody (2F5). (B) One-fifth of the input used in immunoprecipitations analyzed in the same Western blot. (C) Quantitation of fold changes in gp41 expression from immunoprecipitation with MAb 3-17. Bars show averages of data from two independent immunoprecipitation experiment with SEM.
Peptide-resistant Envs reveal distinct genetic pathways that confer relaxed conformations of native Env.
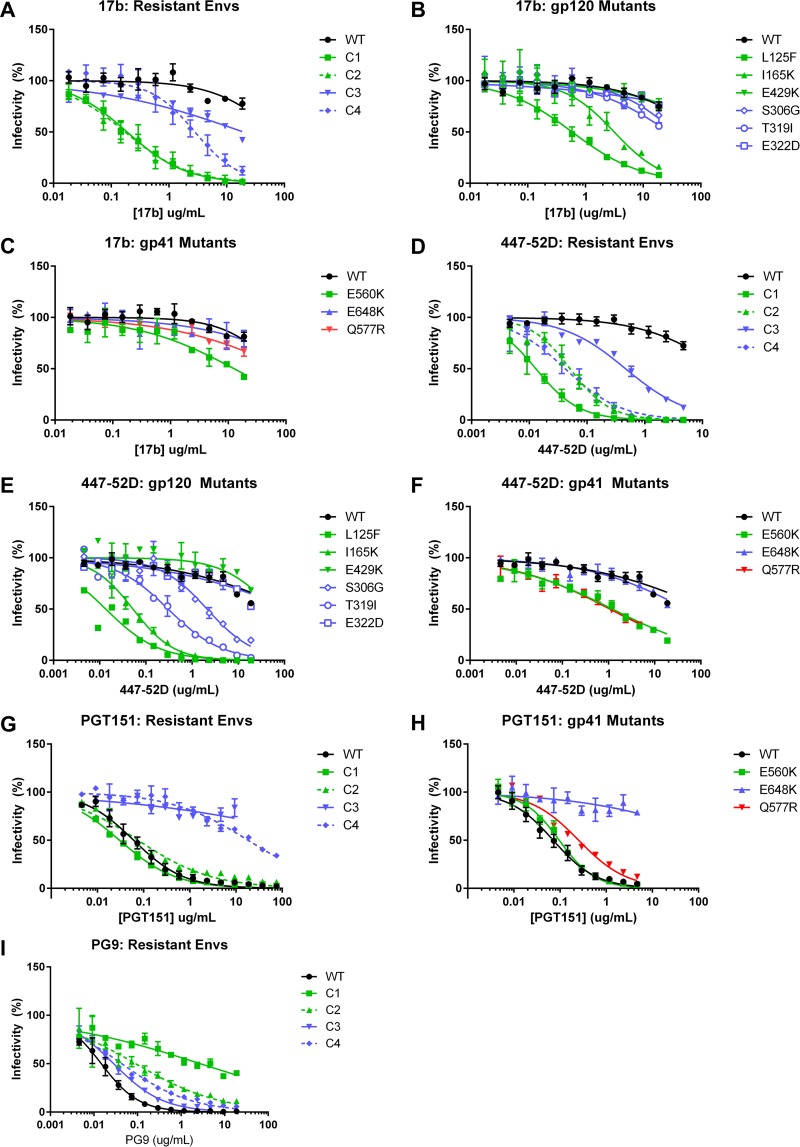
The changes in mCD4 sensitivity and conformational reactivity conferred by mutations in the peptide-resistant Envs led us to further investigate how the mutations altered Env structure and neutralization. We tested the neutralization sensitivity of our peptide-resistant Envs, as well as Envs with individual mutations, against a large panel of mAbs to assess the exposure of specific epitopes (Fig. 5 and Table 2). For the 17b mAb that targets a CD4-induced (CD4i) epitope, WT and C3 Envs were resistant to neutralization (Fig. 5A), but both C1 and C2 Envs had 50% inhibitory concentrations (IC50s) of 0.2 μg/ml, and C4 Env was moderately sensitive, with an IC50 of 2.9 μg/ml. Single gp120 mutations conferred various effects. Envs with L125F or I165K mutations displayed significant sensitivity to the 17b mAb, with IC50s of 0.6 μg/ml and 3.2 μg/ml, respectively. In contrast, Env with the E429K (C2) mutation and Envs with T319I (C3), S306G, and E322D (C4) mutations remained resistant (Fig. 5B). Only when T319I, S306G, and E322D were found in combination (Env C4) did Envs show moderate sensitivity to the 17b mAb (Fig. 5A and B). The E560K mutation in HR1 conferred a modest increase in sensitivity, with an IC50 of 13 μg/ml (Fig. 5C), but other gp41 mutations had no effect. The CD4i 412d mAb showed a pattern similar to that of the 17b mAb (Table 3). Another CD4i mAb, 447-52D, which targets a distinct CD4i epitope in the V3 loop (31, 32), neutralized all peptide-resistant Envs (Fig. 5D). Unlike mAb 17b, g120 mutations S306G and T319I (pathway 2), in addition to L125F and I165K, conferred sensitivity to the 447-52D mAb (Fig. 5E). These residues lie within the base of the V3 loop recognized by 447-52D and individually likely affect the V3 structure locally, without globally inducing a more open CD4i-type conformation. gp41 mutations E560K and Q577R showed a modest increase in 447-52D sensitivity (Fig. 5F). Overall, exposure of CD4i epitopes was often associated with sensitivity to mCD4. Pathway 1 Envs were more sensitive to 17b and 412d, and sensitivity could be largely attributed to specific changes (L125F and I165K) in gp120. Pathway 2 Envs were less sensitive to 17b and 412d, and sensitivity appears to be the result of an accumulation of changes rather than the result of specific changes, as no single pathway 2 mutation had an effect on 17b neutralization.
FIG 5.
Effect of mutations on neutralization by various monoclonal antibodies. Shown are data for neutralization of pseudoviruses bearing Envs with the indicated mutations by 17b (A to C), 447-52D (D to F), PGT151 (G and H), and PG9 (I). Data points are the averages of results from at least two independent experiments, each conducted in duplicate. Error bars show SEM.
TABLE 3.
Neutralization sensitivity of HIV-1 Env pseudovirusa
IC50 values for each MAb are presented as micrograms per milliliter. Red, IC50 of <0.15 μg/ml; orange, 0.15 μg/ml ≤ IC50 < 2 μg/ml; yellow, 2 μg/ml ≤ IC50 < 15 μg/ml; green, IC50 of ≥15 μg/ml. nd, not determined.
Next, we investigated whether the mutations conferred changes in sensitivity to broadly neutralizing antibodies (bnAbs) representing each of the five known neutralizing sites in Env (Fig. 5 and Table 3). In contrast to mCD4, mutations in the peptide-resistant Envs did not affect neutralization of the CD4 binding site (CD4bs) VRC01 bnAb, consistent with its ability to bind multiple trimer conformations and consequently neutralize a broad range of viruses. The similar potencies of VRC01 bnAb against the pseudoviruses in this panel also indicate that there are not large variations in mature Env incorporation in the pseudoviruses. However, the peptide-resistant Envs from both pathways showed 3- to 10-fold increases in sensitivity to the less potent and conformationally dependent CD4bs b12 mAb. HR1 mutation E560K and multiple gp120 mutations (L125F, I165K, and T319I) contributed to neutralization sensitivity, indicating that these residues affect the accessibility of the CD4bs. Using the gp120/gp41 interface PGT151 bnAb (Fig. 5G and H), we found that both C3 and C4 Envs (pathway 2) exhibited remarkable resistance to PGT151, while C1 and C2 Envs retained WT levels of sensitivity (Fig. 5G). Single mutations demonstrated that PGT151 resistance mapped to the E648K resistance mutation in HR2 (Fig. 5H). This finding suggests that E648K leads to structural changes of the gp41 base in the protomer interaction site.
Compared to WT Env, all peptide-resistant Envs were less easily neutralized by the PG9 bnAb (Fig. 5I), which targets V1/V2 at the apex of the native Env trimer. C1 and C2 Envs tended to be more resistant than C3 and C4 Envs. Reduced sensitivity to neutralization by the PG9 bnAb appeared to be due to cumulative gp120 mutations rather than one key mutation (Table 3). Because the PG9 bnAb binds the native Env trimer in a closed conformation, our findings indicate that many different gp120 mutations can relax the native Env conformation so that it can dynamically sample conformations that are more open.
Most of the peptide-resistant Envs showed moderate increases in MPER antibody sensitivity, with the exception of C3. Pathway 1 gp120 mutation L125F showed a modest increase in 2F5 sensitivity, and both mutations I165K and T319I showed ∼2-fold increases in sensitivity to 10E8. Single mutations alone were unable to account for the increase in MPER antibody sensitivity seen in C1, C2, and C4 Envs. Single mutations in gp41 resulted in an even more modest effect on MPER antibody sensitivity; the E560K and Q577R mutations in HR1 slightly increased sensitivity to the 2F5 mAb compared to the WT, while the E648K mutation in HR2 resulted in modest resistance to neutralization by the 2F5 mAb. Residue 648 is close to the MPER but has not been reported to be part of the 2F5 epitope. MPER mAbs 4E10 and 10E8 generally showed patterns of changes in sensitivity similar to that of 2F5, despite different baseline neutralization levels (Table 3).
Taken together, these antibody neutralization results indicate that the peptide-resistant Envs evolved relaxed Env conformations but did so via different genetic pathways. Envs from both pathways have trimers that were more sensitive to mCD4 neutralization. For pathway 1 Envs, key changes in gp120, including L125F or I165K, along with the E560K mutation in HR1, conferred substantial mCD4 sensitivity. For pathway 2 Envs, mCD4 sensitivity was associated with an accumulation of changes in V3 that appeared to have an additive effect on mCD4 sensitivity. Envs from both pathways also had exposed CD4i epitopes, demonstrated by 17b, 412D, and 447-52D mAb neutralization. Finally, compared to WT Env, Envs from both resistance pathways were less sensitive to the PG9 mAb, which targets V1V2 at the trimer apex. However, pathway 1 Envs were more resistant to the PG9 mAb than pathway 2 Envs. These distinct patterns of gp120 mutations associated with HR1 and HR2 resistance mutations suggest long-range functional interactions between these gp41 HR1 and HR2 mutations and specific regions in gp120. In particular, pathway 2 Envs with the founder E648K resistance mutation in HR2 appear to disfavor V1/V2 mutations that have large effects on mCD4 sensitivity.
DISCUSSION
We describe two patterns of functional interactions between gp120 and gp41 residues in a panel of Envs resistant to peptide fusion inhibitors: one involves the gp41 E560K resistance mutation in HR1 along with mutations in V1/V2 and the hydrophobic core of gp120, and the other involves the gp41 E648K resistance mutation in HR2 along with mutations in V3. While both patterns of gp120 mutations conferred enhanced sensitivity to mCD4 inhibition and relaxed gp120 conformations, the HR1 and HR2 mutations conferred different degrees of CD4-induced conformational reactivity in Env. The E648K HR2 mutation maintained wild-type sensitivity to mCD4 inhibition and mCD4-induced triggering to the 6HB, but the E560K and Q577R HR1 mutations each increased sensitivity to mCD4 inhibition and reduced mCD4-induced triggering to the 6HB (Fig. 2 and 4). These distinct pathways provide new insights into different ways in which the Env trimer can sample more open conformations to facilitate interactions with CD4 while regulating receptor-induced conformational changes.
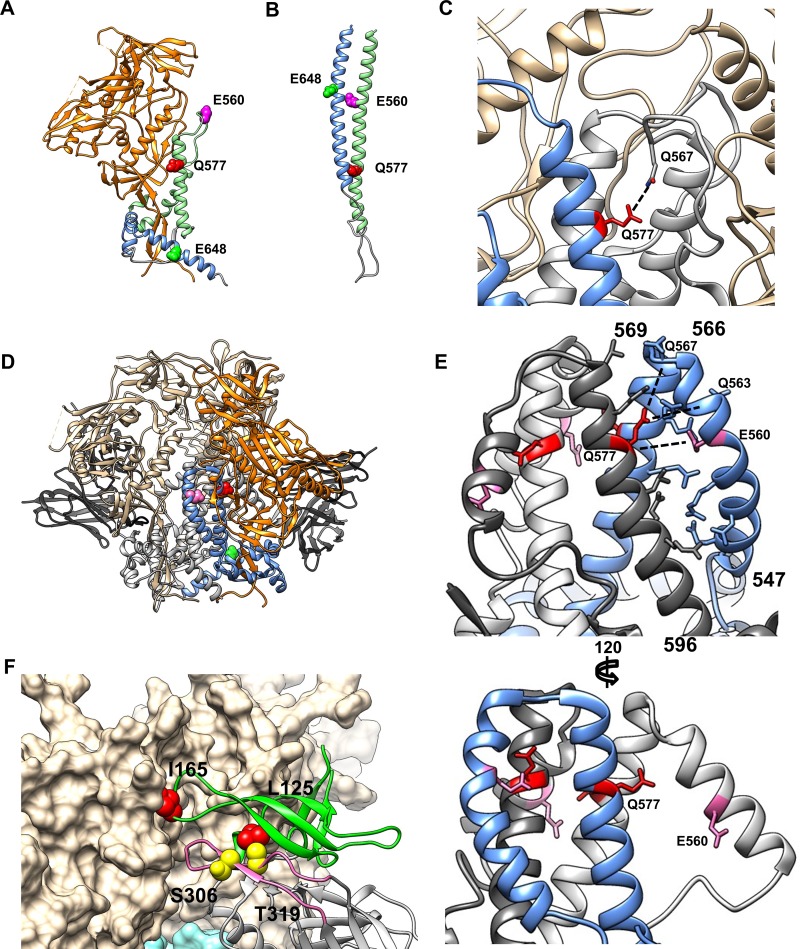
The finding of reduced gp41 conformational reactivity to CD4 conferred by the HR1 mutations initially seemed at odds with our previous findings that the E560K and Q577R HR1 resistance mutations stabilize the 6HB (Fig. 6B) (22, 23, 33). This paradox may be reconciled if the HR1 resistance mutations also affect earlier conformational states of Env. Several recent high-resolution structures reported to represent native-like HIV-1 Env trimers provide clues to potential stabilizing interactions involving the HR1 gp41 resistance mutations. Although most early structures derived from BG505 SOSIP soluble trimers lack resolution of portions of gp41, including HR1 and HR2 (9, 18), residue 577 in the “e” position of the central HR1 coiled coil is resolved in most structures (Fig. 6A, Q577 in red). In the postfusion 6HB structure, residues in the “e” position of the heptad interact with the HR2 helix (Fig. 6B) (34, 35). However, in the prefusion trimer (e.g., PDB accession numbers 5CEZ and 5FUU), Q577 points away from the trimer axis, where it can potentially participate in polar interactions with HR1 residues 552 to 569 in an adjacent protomer (36, 37). These residues reside in a flexible loop in a few trimer structures (e.g., PDB accession number 5CEZ) but are unresolved in others (e.g., PDB accession numbers 4TVP and 4NCO). This poorly resolved region contains the key pathway 1 HR1 E560K mutation as well as Gln at position 567 in the JRCSF strain that is within H-bond range of residue 577 from the adjacent gp41 protomer in the BG505 SOSIP structure (PDB accession number 5CEZ) (Fig. 6C). Thus, the Q577R mutation may allow the formation of an additional interaction(s) between adjacent gp41 protomers that may favor the native conformation of gp41. Interestingly, the Q577R mutation also emerged in resistance to HR2 peptide fusion inhibitors (38).
FIG 6.
Model of gp41 and gp120 interactions. (A) gp120-gp41 monomer from the BG505 SOSIP trimer structure (PDB accession number 5CEZ). gp120 is shown in orange, gp41 HR1 is in light green, and gp41 HR2 is in blue. Key gp41 positions E560, Q577, and E648 are shown in magenta, red, and bright green, respectively. (B) gp41 monomer from the postfusion 6HB structure (PDB accession number 1IF3), colored as described above for panel A. (C) Closeup of the N-HR1 region from the SOSIP trimer structure under PDB accession number 5CEZ. One protomer of gp41 is shown in blue, and the others are in gray; gp120 is in beige. Residue Q577 is highlighted in red, and the potential interaction with Q567 from an adjacent gp41 monomer is indicated. (D) JRFL trimer structure (PDB accession number 5FUU), stabilized by two PGT151 Fabs. A single gp120/gp41 protomer is colored as described above for panel A; the remaining gp120/gp41 protomers are shown in beige and gray. PGT151 Fabs are in black. (E) Closeup of the N-HR1 region of gp41 from the structure reported under PDB accession number 5FUU. Each gp41 protomer is colored separately; the unliganded protomer is shown in blue, and the PGT151-liganded protomers are shown in white and charcoal. (Top) Side view of the unliganded (blue) protomer showing ladder-like packing between the N-HR1 and C-HR1 regions. (Bottom) Side view of a PGT151 liganded protomer showing extension of N-HR1 up and away from the C-HR1 coiled coil. (F) Model of key V1/V2 and V3 gp120 mutations in trimer structure. One protomer of gp120 (PDB accession number 4TVP) is shown as a ribbon diagram with colored domains: V1/V2 is in green, the V3 loop is in pink, and the remainder is in gray. The other two gp120 protomers are shown in beige space-fill. Key V1/V2 residues L125 and I165 are highlighted in red. Key V3 residues S306 and T319 are highlighted in yellow.
An alternative view of the N-terminal half of HR1 comes from the JRFL Env structure (PDB accession number 5FUU) that shows the trimer binding to two PGT151 mAbs, resulting in an asymmetric trimer (Fig. 6D) (36). Each gp41 protomer reveals a different position and conformation of the N-terminal portion of HR1 (Fig. 6E). In Fig. 6E, the unliganded protomer is highlighted in blue, and both protomers bound to mAb PGT151 are shades of gray. While the C-terminal HR1 coiled coil that forms the basis of the Env trimer axis is relatively unperturbed, there is asymmetry of the surrounding regions of gp41. In the unliganded protomer of gp41, N-terminal HR1 residues 547 to 566 form a curved α-helix that packs tightly into the groove formed by the C-terminal HR1 trimeric coiled coil (residues 569 to 596). This groove is lined by the residues occupying “g” and “c” heptad positions of the same protomer and the “b” and “e” heptad positions from its clockwise adjacent protomer. This positions the N-terminal HR1 residues H564, E560, R557, N554, and Q550 in range to directly interact with the C-terminal HR1 coiled coil of the same and adjacent protomers (Fig. 6E, top). Both the E560K and Q577R mutations conferred partial resistance to mCD4-mediated irreversible inactivation (Fig. 3D), indicating that these residues play a key role in Env conformational reactivity to CD4. Modeling suggests that E560K could allow an additional H-bond to the backbone of residue 577 in the adjacent gp41 protomer. Together, the E560K and Q577R mutations could allow multiple additional H-bonds between the N- and C-terminal halves of HR1, between E560K and Q577R and between Q577R and the backbones of Q563 and Q567 in adjacent protomers (Fig. 6E, top), potentially stabilizing gp41 in this tightly packed HR1 conformation.
The other gp41 protomers that are bound to PGT151 display the N-terminal HR1 residues as a straight α-helix, tilted away from the trimer axis and toward the same protomer's gp120 domain (Fig. 6E, bottom) (36). In this conformation, not only is the position of the fusion peptide more exposed, but the stabilizing interactions between the N- and C-terminal HR1 helices described above are not possible. This alternate conformation requiring stabilization by the PGT151 bnAb may represent an intermediate on the pathway toward HR1 extension and formation of the extended gp41 prehairpin intermediate. Further characterization of the native conformation of the Env trimer, particularly gp41, will be necessary to properly order early conformational changes (39). Altogether, our modeling suggests that the decrease in mCD4-mediated triggering of Env to the 6HB conformation in the peptide-resistant Envs may be due to stabilization of the native gp41 conformation via intra- and interprotomer interactions by HR1.
An alternative or additional explanation for reduced mCD4-induced triggering to the 6HB could be that the mutations result in less-efficient contacts between gp120 and gp41 that fail to efficiently transduce receptor-induced conformational changes in the gp120 subunit to the gp41 subunit. The ambiguities in the various gp41 HR1 structures make determining the precise effects of mutations E560K and Q577R difficult at this time. Nonetheless, our functional data indicate that in addition to stabilizing the gp41 postfusion 6HB, these HR1 mutations also reduce native trimer reactivity to CD4-induced Env conformational changes. The finding that the E648K HR2 resistance mutation, which also increases the stability of the 6HB, does not change in CD4-induced conformational reactivity (Fig. 3D) confirms the importance of the HR1-mediated interactions in regulating Env conformational reactivity. We note that residue 560 is immediately adjacent to residue 559 that was previously identified as contributing to the stabilization of the engineered SOSIP trimer. Unlike SOSIP mutation I559P (39), the E560K and Q577R mutations maintain Env fusion activity and function, which allowed us to directly test their effects on Env reactivity to CD4. Altogether, these results highlight the important role that the regulation of the interactions between the N-terminal and C-terminal halves of HR1 has on the conformational triggering of gp41.
In addition to the gp41 mutations, each fusion inhibitor resistance pathway was associated with a distinct set of gp120 changes (Table 1) involving mutations L125F, I165K, and E429K in V1/V2 and the gp120 core (pathway 1) or mutations S306G, T319I, and E322D in V3 (pathway 2). gp120 mutations in both pathways were responsible, either alone or in combination, for increasing sensitivity to many neutralizing antibodies and mCD4. We modeled these residues in the closed, native-like Env trimer structures (Fig. 6F) (PDB accession number 5CEZ) (37) and a recent structure of the open, liganded trimer bound to sCD4 and the 17b mAb (PDB accession number 5VN3) (40). Despite the coevolution of mutations in gp120 and gp41 that affected mCD4 sensitivity and gp41 conformational reactivity, we note that in neither pathway were changes observed in topographical layers 1 to 3 of the gp120 inner domain, previously identified as being key determinants of regulating gp120-gp41 interactions and reactivity to CD4 and the coreceptor (41). Instead, the changes occurred entirely in regions of gp120 that appear to be important for receptor/coreceptor interactions and the maintenance of a closed trimer structure.
Pathway 1 mutations L125F and I165K are located in the V1/V2 region of native gp120 (Fig. 6F) (PDB accession number 4TVP) (18). Residue I165 is in close proximity to the interface between the adjacent gp120 protomer in the native trimer (37). In the liganded structure, the interfaces between gp120 protomers are broken as each gp120 structure opens and extends outwards. Residues L125 and E429 wind up in or lining the binding surface of CD4, while I165 falls into an extended unresolved portion of the V1/V2 loop (40). We found that L125F and I165K mutations strongly influenced sensitivity to mCD4 and CD4bs antibody neutralization. Their position in the structure suggests that they contribute to V1/V2-V3 and gp120 interprotomer interactions, with the mutations allowing the premature opening of the Env trimer apex, confirmed by the loss of sensitivity to neutralization by the PG9 mAb (Fig. 5I and Table 3). Mutation L125F was previously reported to be an important determinant of intraprotomer interactions between V1/V2 and V3 in strain JRFL, allowing the exposure of both V2 and V3 neutralizing epitopes (42). Those studies also identified residue I184 as a potential interprotomer V1/V2 interaction partner with residue 165, consistent with our work identifying I165 as a key determinant of trimer opening in JRCSF Env. In contrast, mutation of neither L125 nor I184 resulted in broad exposure of CD4i epitopes in JRFL Env (42, 43), but L125F and I165K result in CD4i epitope exposure in the context of JRCSF Env. These results suggest that these related strains respond to V1/V2 alterations differently, emphasizing the complexity and potential strain dependence of the coordinated interactions that stabilize the native trimer.
Pathway 2 S306, T319I, and E322D mutations work differently than the pathway 1 mutations. In isolation, these changes had only modest effects on neutralization by most mAbs and a CD4 mimetic (Fig. 5 and Table 3). The exception is the large increase in sensitivity to the 447-52D mAb conferred by the S306G and T319I mutations (Fig. 5E). S306 and T319 are at either side of the V3 base recognized by 447-52D (31) (Fig. 6F, yellow residues), suggesting that these mutations result in a limited exposure/relaxation of V3 alone that may enhance exposure to the coreceptor binding site. This possibility is supported by the position of these residues in the liganded trimer, where S306 is located directly in the 17b binding site, while T319 and E322 are within the unresolved portion of the V3 loop (40). These conclusions are consistent with previous work showing that it is possible to trigger V3 loop exposure independently of V1/V2 exposure and that V1/V2 likely acts as a clamp restraining the unliganded trimer in the closed state (42–49).
None of the gp120 mutations that we describe altered irreversible inactivation by mCD4 treatment (Fig. 3C). Rather, the gp120 mutations lowered the concentration of mCD4 required for mediating fusion without rendering them CD4 independent, consistent with their contribution to a more open or relaxed gp120 trimer conformation. Pathway 1 mutations L125F and I165K and pathway 2 mutation T319I had the greatest effect (Fig. 3C) and correlated well with sensitivity to mCD4 neutralization (Fig. 1C and D). This finding suggests that the increase in neutralization sensitivity is largely due to increased binding and affinity for CD4 rather than irreversible inactivation by conformational triggering of gp41.
The metastable Env trimer has been reported to sample distinct conformational states: state 1 (closed, native), state 2 (relaxed, intermediate), and state 3 (open) (47, 50–52). Primary HIV-1 Envs predominantly occupy state 1 until CD4 engagement, which shifts the trimer toward states 2 and 3 and triggers the cascade of conformational changes leading to fusion. Our findings highlight ways in which Env can alter coupling between gp120 and gp41 conformational changes. The L125F and I165K mutations in V1/V2 and the S306G and T319I mutations together in V3 conferred relaxed trimer conformations that appear to correspond to shifts from state 1 to state 2 or 3. Consistent with this suggestion, these changes also reduce the concentration of CD4 required to mediate fusion.
Our antibody studies indicate that pathway 1 and 2 Envs exist in nonidentical conformations. This suggests that either (i) pathway 1 and 2 Envs are in different states or (ii) each state may contain multiple quasiequivalent conformations sampled by Env, rather than a single, defined, intermediate conformation along a pathway from native “closed” to liganded “open” trimers. The HR1 E560K and Q577R mutations reduce CD4-induced triggering of gp41 and may compensate for the gp120 mutations that facilitate CD4 binding. The coevolution of HR1 mutations that reduce conformational triggering with gp120 mutations that open gp120 suggests that the virus balances efficient receptor use and Env reactivity through coordinated changes in distant domains of both gp120 and gp41, a phenomenon that has been observed previously (49, 53, 54). This observation highlights potential flexibility in gp120 and gp41 interactions that may argue for an expansion of the 3-state model to allow for a greater degree of movement between gp120 and gp41 subunits as well as between trimer protomers as observed by fluorescence resonance energy transfer (FRET) (55).
In summary, our analysis of genetic pathways of HR1 peptide fusion inhibitor resistance provides new insights into Env conformational changes that mediate virus entry. HR1 E560K and Q577K mutations confer not only resistance to fusion inhibitors but also significant resistance to CD4-mediated inactivation via reduced conformational triggering of gp41 to the 6HB. Interprotomer gp41-gp41 interactions in HR1 may contribute to this reduced conformational reactivity. Correspondingly, Envs with the E560K mutation tolerate gp120 mutations that relax the native trimer through the release of V1/V2, which facilitates binding to CD4. In contrast, Envs with the E648K mutation have only modest resistance to CD4-mediated conformational triggering to the 6-HB. Because HR2 resistance mutations do not significantly alter Env reactivity to CD4, gp120 mutations that have a more localized effect on V3 and coreceptor binding site accessibility appear to be favored over individual mutations that dramatically shift CD4bs accessibility and opening of the crown of the Env trimer. These results provide new insights into the regulation of Env conformational changes and suggest potential ways to alter the coupling of trimer opening and receptor binding with gp41 conformational changes. Residues 560 and 577, identified here as having a novel role in stabilizing a prefusion conformation of gp41, may also prove useful for engineering Env immunogens that resist conformational flipping to postfusion Env conformations.
MATERIALS AND METHODS
Cell culture.
293T and U87 cells expressing CD4 and CCR5 (U87-CD4-R5) were provided by Dan Littman (New York University, NY). 293 cells were passaged in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, l-glutamine, 1 mM HEPES, and nonessential amino acids (DMEM+). U87-CD4-R5 cells were passaged in DMEM+ with the addition of 300 μg/ml G418 sulfate and 1 μg/ml puromycin.
Plasmids and constructs.
Env expression vector pCMV/R, Env-deficient HIV genome plasmid pCMVΔ8.2, and luciferase reporter plasmid pHR′-Luc were kindly provided by Gary Nabel (National Institutes of Health, Bethesda, MD). The wild-type JRCSF Env expression plasmid (pVRC JRCSF WT) was created as described previously (22, 23, 33), by inserting the Env gene into pCMV/R via NotI and EcoRV restriction sites. Expression plasmids for N44-resistant culture Envs (C1 to C4) were cloned in the same manner. Expression plasmids for gp120 and gp41 single mutants were generated from pVRC JRCSF WT with the QuikChange II site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). Site-directed mutagenesis and the lack of secondary mutations were confirmed by sequencing the full-length Env gene from recovered clones.
Pseudovirus generation.
Approximately 3 × 106 293T cells were seeded onto a 10-cm dish 18 h prior to transfection. Transfection mixtures were comprised of 0.25 μg pVRC Env and 3.0 μg each of pCMVΔ8.2 and pHR′CMV-Luc, and optionally 1 μg furin expression plasmid, combined with Fugene 6 (Promega, Madison, WI) according to the manufacturer's protocols. Fresh medium was exchanged at 18 to 24 h posttransfection. Pseudoviruses were harvested from culture supernatants at 48 h posttransfection, filtered through a 0.45-μm-pore-size low-binding filter, and immediately stored at −80°C. Pseudovirus lysates were checked by Western blotting using gp41 and p24 antibodies to ensure that levels of Env incorporation into the pseudoviruses were not more than 4-fold different from those for pseudoviruses with WT Env.
Infectivity and neutralization assays.
U87-CD4-R5 cells were seeded onto white 96-well plates at a concentration of 1.7 × 105 cells/ml, 100 μl per well, 24 h prior to infection. Serial 2-fold dilutions of pseudoviruses in medium plus 8 μg/ml Polybrene were plated with positive (WT JRCSF) and negative (mock) controls and incubated at 37°C. At 48 h postinfection, cells were lysed with cell culture lysis reagent, and luciferase activity was assayed with luciferase assay system kit reagents (Promega, Madison, WI) according to the manufacturer's instructions.
For neutralization assays, a standard infectious dose (100,000 relative light units [RLU]) of each pseudovirus was mixed with 2-fold serial dilutions of mAbs or 4-fold serial dilutions of sCD4/mimetic CD4 M48-U1, incubated at 37°C for 1 h, before inoculating target cells and scoring for infectivity as described above. Neutralization dose-response curves were calculated by nonlinear regression using Prism 6 (GraphPad Software Inc., La Jolla, CA). IC50s represent the averages of data from at least two independent experiments, conducted in duplicate. mAbs VRC01 (56), PG9 (57), PGT151 (58), 2F5 (59), 4E10 (59), 17b (60), 412d (61), and 447-52D (62) were kind gifts of Peter Kwong (NIH VRC, Bethesda, MD). mAbs b12 (63) and 10E8 (64) were obtained from the NIH AIDS reagent program. CD4 mimetic M48-U1 was the kind gift of Loïc Martin (Commissariat a l'Energie Atomique, Gif-sur-Yvette, France).
mCD4-mediated entry.
293T cells were transfected with the pcCCR5 expression plasmid (293T-transR5) (3, 65) and seeded onto 96-well plates. Pseudoviruses were spinoculated onto 293T-transR5 cells at 2,000 × g for 1 h at 37°C before the addition of medium containing serial dilutions of mCD4 (T0). Plates were incubated for 48 h at 37°C and then lysed and assayed for luciferase activity as described above. To determine the degree of CD4-mediated inactivation, pseudoviruses were preincubated for 1 h at 37°C with serial dilutions of mCD4 prior to spinoculation onto 293T-transR5 cells (Pre), before measuring infectivity. Infectivity versus the mCD4 concentration was plotted for each treatment and normalized against the maximum infectivity seen in the T0 treatment for each pseudovirus.
Isolation and characterization of 6HB mAb.
A gp41 recombinant protein, JRCSF N34-C28 (amino acid sequence SGIVQQQNNLLRAIEAQQHMLQLTVWGIKQLQARSGGRGGWMEWEKEIENYTNTIYTLIEESQIQQEK), designed to mimic the 6HB by linking 34 residues in HR1 to 28 residues in HR2 with a flexible linker (SGGRGG), was produced in Escherichia coli (GenScript, NJ). JRCSF N34-C28 was used as an immunogen in BALB/c mice to raise a 6HB-specific mAb (Envigo, Madison, WI). Hybridoma supernatants were screened by an enzyme-linked immunosorbent assay (ELISA) for binding to the immunogen, an HR1 peptide stabilized as a trimeric coiled coil (IZN36 peptide; amino acid sequence IKKEIEAIKKEQEAIKKKIEAIEKEISGIVQQQNNLLRAIEAQQHLLQLTVWGIKQLQARIL), an HR2 peptide (C34 peptide; amino acid sequence WMEWEKEIENYTNTIYTLIEESQIQQEKNEQELL), and a mixture of the IZN36 and C34 peptides preincubated in solution for 30 min at 37°C to allow the formation of the 6HB. MAb 3-17 was selected for binding with high affinity in ELISAs to the immunizing antigen and a mixture of IZN36 and C34 peptides but only weakly to IZN36 or C34 peptides alone.
ELISAs were carried out on Immulon 2HB plates coated overnight with gp41 peptides, IZN36, C34, or a mixture of IZN36 and C34. Serial 5-fold dilutions of MAb 3-17 or rabbit serum 948 were applied to ELISA plates after blocking in 5% nonfat dry milk powder solution (NFDM) and incubated for 1 h at 37°C. After primary incubation, ELISA plates were washed four times in TBST (150 mM NaCl, 20 mM Tris [pH 8.0], 0.05% Tween 20) wash buffer. Secondary anti-mouse–horseradish peroxide (HRP) (for 3-17) or anti-rabbit–HRP (for serum 948) Ab was added at a 1:5,000 dilution in blocking buffer, and the mixture was incubated for 30 min at room temperature (RT). Plates were washed four times after secondary Ab incubation and then developed with the SureBlue peroxidase substrate (SeraCare). Reactions were stopped by the addition of 1.0 M sulfuric acid after 15 to 30 min, and the optical density (OD) at 450 nm was recorded on a Opsys MR microplate reader (Dynex). ELISA binding curves and 50% effective concentration (EC50) values were calculated by nonlinear regression using Prism 6 (GraphPad Software). Reported values include average values of data from at least two independent experiments, performed in duplicate.
Immunoprecipitation and Western blotting.
Pseudoviruses were pretreated with or without 1,000 nM mCD4 prior to incubation with 50 μg MAb 3-17 or isotype control antibody for 60 min at 37°C. Pseudoviruses were then pelleted at 20,000 × g for 2 h at 4°C and resuspended overnight in NP-40 lysis buffer (NP-40 LB) (50 mM Tris [pH 8.0], 150 mM NaCl, 1% NP-40) at 4°C. Following resuspension, the viral lysate was incubated with protein G Dynabeads (Thermo Fisher) and equilibrated in NP-40 LB for 30 min at room temperature, prior to washing four times in NP-40 LB and resuspension in 2× Laemmli sample buffer (Bio-Rad). Samples were analyzed by SDS-PAGE under nonreducing conditions and transferred to a 0.2-μm nitrocellulose membrane. The blot was probed with anti-gp41 MAb 2F5 (1 μg/ml in TBST with 1% NFDM), washed four times, incubated with goat anti-human HRP-conjugated secondary Ab (1:5,000 in TBST), and washed an additional four times before development with the LumiGLO Reserve chemiluminescent substrate (KPL). Images were captured with a G:Box gel imaging system (Syngene), and band intensities were evaluated from raw image files by densitometry using ImageJ software.
ACKNOWLEDGMENTS
We thank Ira Berkower and Konstantin Virnik (U.S. Food and Drug Administration, Silver Spring, MD) for critical readings of the manuscript.
This work was supported by institutional funds from the U.S. Food and Drug Administration.
REFERENCES
- 1.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 2.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 3.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135–1148. doi: 10.1016/S0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Broder CC, Kennedy PE, Berger EA. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 7.Kwon YD, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang GY, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O'Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GB, Stuckey J, Thomas PV, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schon A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, et al. 2015. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol 22:522–531. doi: 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJ, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JL, Subramaniam S. 2010. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog 6:e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, Gerard C, Sodroski J. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 13.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 14.Melikyan GB. 2011. Membrane fusion mediated by human immunodeficiency virus envelope glycoprotein. Curr Top Membr 68:81–106. doi: 10.1016/B978-0-12-385891-7.00004-0. [DOI] [PubMed] [Google Scholar]
- 15.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 16.Lu M, Blacklow SC, Kim PS. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol 2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 17.Chan DC, Fass D, Berger JM, Kim PS. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273. doi: 10.1016/S0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 18.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuta RA, Wild CT, Weng Y, Weiss CD. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol 5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 20.He Y, Vassell R, Zaitseva M, Nguyen N, Yang Z, Weng Y, Weiss CD. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J Virol 77:1666–1671. doi: 10.1128/JVI.77.3.1666-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshiba T, Chan DC. 2003. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J Biol Chem 278:7573–7579. doi: 10.1074/jbc.M211154200. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang M, Wang W, De Feo CJ, Vassell R, Weiss CD. 2012. Trimeric, coiled-coil extension on peptide fusion inhibitor of HIV-1 influences selection of resistance pathways. J Biol Chem 287:8297–8309. doi: 10.1074/jbc.M111.324483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, De Feo CJ, Zhuang M, Vassell R, Weiss CD. 2011. Selection with a peptide fusion inhibitor corresponding to the first heptad repeat of HIV-1 gp41 identifies two genetic pathways conferring cross-resistance to peptide fusion inhibitors corresponding to the first and second heptad repeats (HR1 and HR2) of gp41. J Virol 85:12929–12938. doi: 10.1128/JVI.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert DM, Kim PS. 2001. Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc Natl Acad Sci U S A 98:11187–11192. doi: 10.1073/pnas.201392898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Feo CJ, Weiss CD. 2012. Escape from human immunodeficiency virus type 1 (HIV-1) entry inhibitors. Viruses 4:3859–3911. doi: 10.3390/v4123859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci U S A 89:10537–10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng Y, Weiss CD. 1998. Mutational analysis of residues in the coiled-coil domain of human immunodeficiency virus type 1 transmembrane protein gp41. J Virol 72:9676–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Root MJ, Kay MS, Kim PS. 2001. Protein design of an HIV-1 entry inhibitor. Science 291:884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 29.Martin G, Burke B, Thai R, Dey AK, Combes O, Ramos OH, Heyd B, Geonnotti AR, Montefiori DC, Kan E, Lian Y, Sun Y, Abache T, Ulmer JB, Madaoui H, Guerois R, Barnett SW, Srivastava IK, Kessler P, Martin L. 2011. Stabilization of HIV-1 envelope in the CD4-bound conformation through specific cross-linking of a CD4 mimetic. J Biol Chem 286:21706–21716. doi: 10.1074/jbc.M111.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Rosny E, Vassell R, Wingfield PT, Wild CT, Weiss CD. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J Virol 75:8859–8863. doi: 10.1128/JVI.75.18.8859-8863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhillon AK, Stanfield RL, Gorny MK, Williams C, Zolla-Pazner S, Wilson IA. 2008. Structure determination of an anti-HIV-1 Fab 447-52D-peptide complex from an epitaxially twinned data set. Acta Crystallogr D Biol Crystallogr 64:792–802. doi: 10.1107/S0907444908013978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller PM, Arnold BA, Shaw AR, Tolman RL, Van Middlesworth F, Bondy S, Rusiecki VK, Koenig S, Zolla-Pazner S, Conard P, Emini EA, Conley AJ. 1993. Identification of HIV vaccine candidate peptides by screening random phage epitope libraries. Virology 193:709–716. doi: 10.1006/viro.1993.1179. [DOI] [PubMed] [Google Scholar]
- 33.De Feo CJ, Wang W, Hsieh ML, Zhuang M, Vassell R, Weiss CD. 2014. Resistance to N-peptide fusion inhibitors correlates with thermodynamic stability of the gp41 six-helix bundle but not HIV entry kinetics. Retrovirology 11:86. doi: 10.1186/s12977-014-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caffrey M. 2001. Model for the structure of the HIV gp41 ectodomain: insight into the intermolecular interactions of the gp41 loop. Biochim Biophys Acta 1536:116–122. doi: 10.1016/S0925-4439(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 35.Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM. 1998. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J 17:4572–4584. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garces F, Lee JH, de Val N, de la Pena AT, Kong L, Puchades C, Hua Y, Stanfield RL, Burton DR, Moore JP, Sanders RW, Ward AB, Wilson IA. 2015. Affinity maturation of a potent family of HIV antibodies is primarily focused on accommodating or avoiding glycans. Immunity 43:1053–1063. doi: 10.1016/j.immuni.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggink D, Bontjer I, Langedijk JP, Berkhout B, Sanders RW. 2011. Resistance of human immunodeficiency virus type 1 to a third-generation fusion inhibitor requires multiple mutations in gp41 and is accompanied by a dramatic loss of gp41 function. J Virol 85:10785–10797. doi: 10.1128/JVI.05331-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alsahafi N, Debbeche O, Sodroski J, Finzi A. 2015. Effects of the I559P gp41 change on the conformation and function of the human immunodeficiency virus (HIV-1) membrane envelope glycoprotein trimer. PLoS One 10:e0122111. doi: 10.1371/journal.pone.0122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozorowski G, Pallesen J, de Val N, Lyumkis D, Cottrell CA, Torres JL, Copps J, Stanfield RL, Cupo A, Pugach P, Moore JP, Wilson IA, Ward AB. 2017. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547:360–363. doi: 10.1038/nature23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finzi A, Xiang SH, Pacheco B, Wang L, Haight J, Kassa A, Danek B, Pancera M, Kwong PD, Sodroski J. 2010. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell 37:656–667. doi: 10.1016/j.molcel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell RLR, Totrov M, Itri V, Liu X, Fox A, Zolla-Pazner S. 2017. Plasticity and epitope exposure of the HIV-1 envelope trimer. J Virol 91:e00410-17. doi: 10.1128/JVI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zolla-Pazner S, Cohen SS, Boyd D, Kong XP, Seaman M, Nussenzweig M, Klein F, Overbaugh J, Totrov M. 2015. Structure/function studies involving the V3 region of the HIV-1 envelope delineate multiple factors that affect neutralization sensitivity. J Virol 90:636–649. doi: 10.1128/JVI.01645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cimbro R, Gallant TR, Dolan MA, Guzzo C, Zhang P, Lin Y, Miao H, Van Ryk D, Arthos J, Gorshkova I, Brown PH, Hurt DE, Lusso P. 2014. Tyrosine sulfation in the second variable loop (V2) of HIV-1 gp120 stabilizes V2-V3 interaction and modulates neutralization sensitivity. Proc Natl Acad Sci U S A 111:3152–3157. doi: 10.1073/pnas.1314718111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Totrov M. 2014. Estimated secondary structure propensities within V1/V2 region of HIV gp120 are an important global antibody neutralization sensitivity determinant. PLoS One 9:e94002. doi: 10.1371/journal.pone.0094002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Cohen AA, Galimidi RP, Gristick HB, Jensen GJ, Bjorkman PJ. 2016. Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop. Proc Natl Acad Sci U S A 113:E7151–E7158. doi: 10.1073/pnas.1615939113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herschhorn A, Ma X, Gu C, Ventura JD, Castillo-Menendez L, Melillo B, Terry DS, Smith AB III, Blanchard SC, Munro JB, Mothes W, Finzi A, Sodroski J. 2016. Release of gp120 restraints leads to an entry-competent intermediate state of the HIV-1 envelope glycoproteins. mBio 7:e01598-16. doi: 10.1128/mBio.01598-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolchinsky P, Kiprilov E, Bartley P, Rubinstein R, Sodroski J. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J Virol 75:3435–3443. doi: 10.1128/JVI.75.7.3435-3443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herschhorn A, Gu C, Moraca F, Ma X, Farrell M, Smith AB III, Pancera M, Kwong PD, Schon A, Freire E, Abrams C, Blanchard SC, Mothes W, Sodroski JG. 2017. The beta20-beta21 of gp120 is a regulatory switch for HIV-1 Env conformational transitions. Nat Commun 8:1049. doi: 10.1038/s41467-017-01119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munro JB, Mothes W. 2015. Structure and dynamics of the native HIV-1 Env trimer. J Virol 89:5752–5755. doi: 10.1128/JVI.03187-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB III, Kwong PD, Blanchard SC, Mothes W. 2014. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346:759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guttman M, Cupo A, Julien JP, Sanders RW, Wilson IA, Moore JP, Lee KK. 2015. Antibody potency relates to the ability to recognize the closed, pre-fusion form of HIV Env. Nat Commun 6:6144. doi: 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pacheco B, Alsahafi N, Debbeche O, Prevost J, Ding S, Chapleau JP, Herschhorn A, Madani N, Princiotto A, Melillo B, Gu C, Zeng X, Mao Y, Smith AB III, Sodroski J, Finzi A. 2017. Residues in the gp41 ectodomain regulate HIV-1 envelope glycoprotein conformational transitions induced by gp120-directed inhibitors. J Virol 91:e02219-16. doi: 10.1128/JVI.02219-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espy N, Pacheco B, Sodroski J. 2017. Adaptation of HIV-1 to cells with low expression of the CCR5 coreceptor. Virology 508:90–107. doi: 10.1016/j.virol.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma X, Lu M, Gorman J, Terry DS, Hong X, Zhou Z, Zhao H, Altman RB, Arthos J, Blanchard SC, Kwong PD, Munro JB, Mothes W. 2018. HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. Elife 7:e34271. doi: 10.7554/eLife.34271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang CH, McBride R, von Bredow B, Shivatare SS, Wu CY, Chan-Hui PY, Liu Y, Feizi T, Zwick MB, Koff WC, Seaman MS, Swiderek K, Moore JP, Evans D, Paulson JC, Wong CH, Ward AB, Wilson IA, Sanders RW, Poignard P, Burton DR. 2014. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity 40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, Katinger H. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses 10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 60.He Y, D'Agostino P, Pinter A. 2003. Analysis of the immunogenic properties of a single-chain polypeptide analogue of the HIV-1 gp120-CD4 complex in transgenic mice that produce human immunoglobulins. Vaccine 21:4421–4429. doi: 10.1016/S0264-410X(03)00451-1. [DOI] [PubMed] [Google Scholar]
- 61.Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol 150:635–643. [PubMed] [Google Scholar]
- 62.Buchbinder A, Karwowska S, Gorny MK, Burda ST, Zolla-Pazner S. 1992. Synergy between human monoclonal antibodies to HIV extends their effective biologic activity against homologous and divergent strains. AIDS Res Hum Retroviruses 8:425–427. doi: 10.1089/aid.1992.8.425. [DOI] [PubMed] [Google Scholar]
- 63.Abacioglu YH, Fouts TR, Laman JD, Claassen E, Pincus SH, Moore JP, Roby CA, Kamin-Lewis R, Lewis GK. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retroviruses 10:371–381. doi: 10.1089/aid.1994.10.371. [DOI] [PubMed] [Google Scholar]
- 64.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morgenstern JP, Land H. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res 18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]