Abstract
Bladder cancer is a heterogeneous disease that poses unique challenges to the treating clinician. It can be limited to a relatively indolent papillary tumor with low potential for progression beyond this stage to muscle-invasive disease prone to distant metastasis. The former is best treated as conservatively as possible, whereas the latter requires aggressive surgical intervention with adjuvant therapies in order to provide the best clinical outcomes. Risk stratification traditionally uses clinicopathologic features of the disease to provide prognostic information that assists in choosing the best therapy for each individual patient. For bladder cancer, this informs decisions regarding the type of intravesical therapy that is most appropriate for non-muscle-invasive disease or whether or not to administer neoadjuvant chemotherapy prior to radical cystectomy. More recently, tumor genetic sequencing data have been married to clinical outcomes data to add further sophistication and personalization. In the next generation of risk classification, we are likely to see the inclusion of molecular subtyping with specific treatment considerations based on a tumor’s mutational profile.
Keywords: Bladder cancer, bladder cancer genetics, personalized medicine, risk stratification, urothelial carcinoma
Introduction
Bladder cancer ranks among the most common non-cutaneous malignancies in the United States (4 th for men and 11 th for women) and worldwide (6 th for men and 16 th for women) 1– 3. Though it is commonly referred to by the generic term “bladder cancer”, urothelial neoplasms represent a broad spectrum of disease with vastly different treatment pathways from routine bladder surveillance to intravesical therapy to radical surgery with chemotherapy. Staging is broadly divided into two major categories—non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC)—which is perhaps the most important determining factor when choosing an initial therapy. Tumor stage is classified according to the Tumor-Node-Metastasis staging system developed by a collaboration of the American Joint Commission on Cancer and Union for International Cancer Control, now in its 8 th edition which went into effect in January 2018 4. Tumors categorized as NMIBC include carcinoma in situ (CIS), papillary (Ta), or invasive into or beyond the lamina propria (T1). Once the tumor invades the muscularis propria (T2) or beyond (T3 and T4), it is considered MIBC. Somewhat confusingly, these have been referred to as “superficial” and “invasive” disease, respectively, though that practice has been abandoned more recently for the sake of clarity. The tumor grade, describing the aggressiveness of tumor cells based on microscopic appearance, is also very important for the prognosis of bladder cancer. The 2016 World Health Organization classification system for urothelial carcinoma is divided into a binary system of high and low grade, though this is mainly applicable to Ta tumors, since nearly all (≥95%) disease ≥T1 is high grade and CIS is high grade by definition 5.
Diagnosis
A complete clinical assessment for bladder cancer includes history, physical exam, imaging of the upper urinary tracts, and cystoscopy. Cystoscopy is the cornerstone in the diagnosis and treatment of bladder cancer, being used in virtually all patients at some point throughout the course of their disease. Traditionally, this is carried out using a standard light source (“white light”) to illuminate an endoscopic lens with the subsequent image transmitted via a camera head to a monitor. Directly visualized tumors are then biopsied, fulgurated, and/or resected to provide staging information that forms the basis for widely different treatments (i.e. surveillance versus radical cystectomy). Low-grade, papillary tumors not invading into the lamina propria (Ta) are reliably eradicated in a single setting; however, more advanced disease (high-grade and/or T1) is often incompletely resected. Studies have found residual disease to be present in 40–78% of re-transurethral resection (TUR) specimens after original diagnosis of high-grade Ta or any T1, with an upgrading rate to muscle invasion of 2% and 14%, respectively 6– 9. This fact has led to the recommendation by leading urologic organizations that all patients with T1 tumors should undergo a repeat resection within 6 weeks of the original procedure to confirm tumor stage and ensure maximal removal of any residual disease, as this improves response to intravesical therapy 10, 11. Recent technological advances, referred to as photodynamic aids (i.e. Blue Light® cystoscopy and narrow band imaging), promise improved detection over traditional white light cystoscopy alone, theoretically allowing for a more complete endoscopic tumor removal and more accurate risk assessment 12– 19.
The urothelium is not limited to the bladder and urethra; it also extends into the ureters and renal collecting system. The main purpose of imaging in the diagnostic evaluation of bladder cancer is to assess the upper urinary tracts for malignancy and for staging local and distant extent of disease. Computed tomographic urography, which uses intravenous contrast with delayed image acquisition (10–15 minutes) to allow for urinary excretion, has the highest diagnostic value for the detection of upper tract malignancy with a sensitivity of 67–100% and a specificity of 93–99% 20– 23. Magnetic resonance imaging is a viable alternative in patients with iodinated contrast allergy with a diagnostic accuracy of 84–92%, though it is more time intensive and still requires a contrast agent (gadolinium) and, therefore, is a poor choice in patients with significantly impaired renal function 24. Ultrasonography plus retrograde pyelography at the time of cystoscopy can be used if cross-sectional imaging is otherwise contraindicated, but this is suboptimal for assessing disease extent and upper tract involvement, so it is reserved for special circumstances. Upper tract evaluation is recommended as part of initial diagnostic work-up by the American Urological Association (AUA) and European Association of Urology (EAU) despite the very low likelihood of finding synchronous upper tract tumor at the time of NMIBC diagnosis (1.5%); however, certain features like multifocality, trigonal location, and CIS increase the risk (7.5%) 10, 11, 25, 26. A more nuanced approach to long-term surveillance is favored over repeat imaging, being applied only to patients with high-risk tumors, and is our first example of a risk-adapted approach to bladder cancer.
Risk stratification in bladder cancer
Not all bladder cancers are created equal and, therefore, risk stratification is an important tool for achieving optimal patient outcomes while avoiding overtreatment. Formal classification systems exist for NMIBC given the wide variability in possible treatment options (surveillance to radical cystectomy), but a one-size-fits-all approach to MIBC is no longer appropriate either.
Non-muscle-invasive bladder cancer
The risk tables from the European Organization for Research and Treatment of Cancer (EORTC) and scoring system from the Spanish Urological Club for Oncological Treatment (CUETO) for NMIBC classify patients into low, intermediate, or high risk for recurrence and progression to muscle invasion based on factors including grade, stage, tumor size, multifocality, variant histology, lymphovascular invasion, and prior therapy 10, 11, 27, 28. Validation studies based on these tools have shown consistent overestimation of recurrence and progression rates among the high-risk group, likely owing to the suboptimal administration of intravesical therapy seen in the developmental cohorts 29– 32. Though there is now widespread acceptance of induction intravesical immunotherapy (bacillus Calmette-Guérin [BCG]) plus maintenance therapy for 1 to 3 years based on the results of the randomized Southwest Oncology Group (SWOG) protocol, patients from EORTC and CUETO were largely treated with intravesical chemotherapies (mitomycin C, epirubicin, thiotepa, etc.) or a lack of appropriate maintenance BCG 33. Despite the limitations of both aforementioned studies, they form the foundation for the risk stratification systems used by the EAU and AUA to help guide treatment decisions ( Table 1) 10, 11, 34.
Table 1. Risk stratification of non-muscle-invasive bladder cancer 10, 11.
| Risk category | EAU definition 9 | EAU recommendations | AUA definition 10 | AUA recommendations |
|---|---|---|---|---|
| Low | • Primary,
• Solitary (<3 cm), • Low-grade/G1, and • Ta |
• Single immediate post-TUR
instillation of chemotherapy |
• Solitary <3 cm,
• Low-grade, and • Ta |
• Single immediate post-TUR
instillation of chemotherapy |
| Intermediate | • Any disease not fitting
low- or high-risk criteria |
• Single immediate post-TUR
instillation of chemotherapy, and either • Induction chemotherapy for 1 year, or • Induction BCG with 1 year of maintenance therapy |
• Low-grade Ta
recurrence <1 year • Solitary low-grade Ta >3 cm, • Multifocal low-grade Ta, • High-grade Ta ≤3 cm, or • Low-grade T1 |
• Single immediate post-TUR
instillation of chemotherapy, and either • Induction chemotherapy with or without maintenance, or • Induction BCG with maintenance |
| High | • Any T1,
or
• High-grade/G3, or • CIS present, or • Multiple, recurrent, large (>3 cm), papillary (Ta), low-grade/G1 or G2 tumors |
• Single immediate post-TUR
instillation of chemotherapy, and either • Induction BCG with 1–3 years of maintenance therapy, or • Immediate radical cystectomy |
• High-grade T1
• Recurrent high-grade Ta • High-grade Ta >3 cm • CIS • Any high-grade failing BCG • Variant histology • LVI • High-grade prostatic urethral involvement |
• Induction BCG with
maintenance therapy, or • Immediate radical cystectomy for highest risk features (with LVI, variant histology, T1 with CIS, persistent T1 on re-TUR) |
AUA, American Urological Association; BCG, bacillus Calmette-Guérin; CIS, carcinoma in situ; EAU, European Association of Urology; LVI, lymphovascular invasion; TUR, transurethral resection
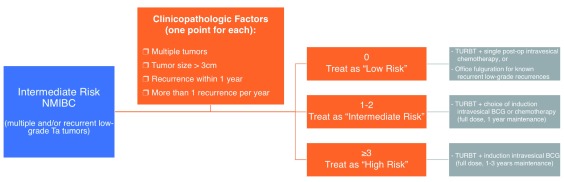
There is general agreement among urological organizations that a more-conservative approach to treatment (cystoscopic resection and single-dose intravesical chemotherapy) is warranted for low-risk tumors (solitary, low-grade, papillary), while the high-risk group (multifocal high-grade, CIS, or any T1) should be managed aggressively through the use of intravesical immunotherapy, and even radical cystectomy in some cases 10, 11. This leaves a broad middle ground for intermediate-risk disease, representing a spectrum ranging from a small, recurrent low-grade papillary tumor to large treatment-resistant low-grade tumors to small high-grade lesions. In order to more effectively tailor an appropriate treatment regimen for these patients, further substratification of intermediate-risk bladder cancer has been proposed by an international consortium of bladder cancer experts using a simple scoring system based on four main tumor features ( Figure 1) 35.
Figure 1. Proposed substratification of intermediate-risk non-muscle-invasive bladder cancer (NMIBC) based on the recommendations of the International Bladder Cancer Group 35.
BCG, bacillus Calmette-Guérin; TURBT, transurethral resection of bladder tumor.
Muscle-invasive bladder cancer
MIBC is often treated as a single disease entity with only one acceptable therapy in the form of neoadjuvant chemotherapy followed by radical cystectomy with urinary diversion and lymphadenectomy 36. However, risk assessment can be applied before or after definitive therapy to identify which patients require the most aggressive approach, specifically those that include adjuvant treatments with significant added toxicity. For example, the decision to delay radical cystectomy so that neoadjuvant chemotherapy can be administered for a small—5% absolute improvement in overall survival at 5 years—but statistically significant benefit can be more precisely applied only to patients with the highest chance of seeing a benefit 37. This topic will be re-addressed in more detail in a later section. Although lacking consensus recommendations for risk grouping from any major urological societies, the stratification and personalization of MIBC therapy is on the verge of a significant paradigm shift supported by a growing body of genomic data.
Pathologic subtypes
The most common histology of bladder cancer is urothelial carcinoma (~90%) followed by squamous, adenocarcinoma, micropapillary, small cell, and other rare tumors 38. Not all urothelial carcinoma exists in pure form; instead divergent differentiation may occur within the tumor exhibiting different morphology and labeled as “variant histology” 5. Of these variants, several are worth noting, as certain subtypes are known to be associated with a more aggressive clinical course. When taken as a group, variant histology is associated with more advanced stage at diagnosis and may not be amenable to standard treatment algorithms, even when adjusting for stage. For instance, plasmacytoid is particularly aggressive with up to 90% diagnosed with extension outside of the bladder (≥T3) and poor responsiveness to cisplatinum-based adjuvant chemotherapy following radical cystectomy 39– 41. Early cystectomy appears to offer survival advantage for micropapillary disease, even for clinical stage T1, because of a high rate of pathologic upstaging and node positivity 42– 46. Squamous differentiation, the most recognized subtype, and glandular are often reported on pathology reports; however, clinical outcomes are no different than those for conventional urothelial carcinoma 47, 48. These should also be recognized as very different to pure squamous cell carcinoma and adenocarcinoma of the bladder, which are distinct histologic entities lacking urothelial components 49, 50. Other less-common variants worth mentioning include nested, lymphoepithelioma-like, and small cell, the latter being morphologically similar to the lung cancer version and sharing the same aggressive clinical course 51, 52.
Molecular subtyping
MIBC ranks among the most highly mutated cancers, with frequencies similar to non-small cell lung cancers and head and neck squamous cell carcinoma, resulting in a heterogeneous mutational profile involving numerous cellular pathways 53– 56. Investigators from around the world have taken on the task of genetically characterizing MIBC samples, resulting in the publication of several major molecular classification systems (the Cancer Genome Atlas [TCGA], Lund, University of North Carolina, MD Anderson, etc.) 55, 57– 59. Each analysis yielded groups of tumors enriched with certain mutations that shared common features with other carcinomas, specifically the breast cancer “basal” and “luminal” subtypes, which led to similarities in the naming schemes. Broadly speaking, basal tumors tend to exhibit a more aggressive phenotype than do luminal tumors and are more prone to metastasis at the time of diagnosis 55– 60. The major publications on this topic have each included a classification system with more-or-less overlap in the mutational profile—i.e. basal-like (UNC), UroB (Lund), Cluster III (TCGA), etc.—that correlates with clinical outcomes. An effort to achieve consensus definitions for all subtypes is underway, thus far producing the Basal-Squamous-like group (BASQ) with elevated KRT5/6 and KRT14 expression but low FOXA1 and GATA3 expression 61. By using the information from cohorts like TCGA, several researchers have sought to define the prognostic significance of these genetic mutations through the creation of molecular subtypes with correlation with clinical outcomes 56– 58, 62, 63.
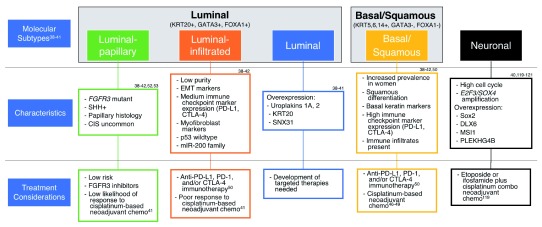
The association of molecular subtypes with response to bladder cancer therapy is certain to help guide treatment in the near future: for instance, which patients are most likely to benefit from neoadjuvant chemotherapy prior to surgery or response to immunotherapy over conventional chemotherapy 56, 57, 64. Systemic therapy for bladder cancer can then be more carefully tailored to the individual patient using information obtained from genetic sequencing to select the best candidates for a given treatment regimen ( Figure 2). For example, tumors exhibiting mutations in genes associated with DNA damage repair (i.e. ERCC2, ERBB2, ATM, and RB1) or those grouped into the basal/squamous subtype display a greater degree of cisplatinum sensitivity as demonstrated by more favorable clinical outcomes among these patients 56, 57, 65– 68. The luminal-papillary and luminal-infiltrated subtypes, on the other hand, show poor response to neoadjuvant chemotherapy; however, tumors with upregulation of the immune checkpoint markers (luminal-infiltrated and basal/squamous) may benefit from treatment with anti-PD-L1, PD-1, and/or CTLA-4 immunotherapy 56, 69.
Figure 2. Molecular subtypes of muscle-invasive bladder cancer with associated clinicopathologic and genomic characteristics.
Proposed treatment considerations are listed for each subtype based on available clinical data 53– 57, 63, 64, 66– 69. CIS, carcinoma in situ; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; DLX6, distal-less homeobox 6; E2F3, E2F transcription factor 3; EMT, epithelial to mesenchymal transition; FGFR3, fibroblast growth factor receptor 3; FOX, forkhead box; GATA, GATA-binding protein; KRT, keratin; miR-200, microRNA 200; msi1, Musashi homolog 1; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PLEKHG4B, pleckstrin homology and RhoGEF domain containing G4B; SHH, sonic hedgehog; SNX31, sorting nexin 31; SOX, SRY-box.
The clinical impact of NMIBC is just as significant as that of MIBC, though maybe not as immediate a threat to survival, especially when taking into account rates of progression reaching as high as 50–60%. A considerable amount of information is available on molecular subtyping for NMIBC that is helping researchers hone in on the most important mutations in the evolution of bladder cancer 59, 70, 71. Low-grade bladder tumors would appear to be genetically distinct from high-grade and MIBC, demonstrating highly conserved genetic mutations of FGFR3, PIK3CA, STAG2, and/or the RTK/RAS/RAF pathway 72– 75. High-grade NMIBC, on the other hand, is more like muscle-invasive disease, exhibiting alteration of DNA damage repair genes ( ERBB2), cell cycle regulators ( p53, RB1, MDM2, and CDKN2A), and chromatin-modifying genes ( KDM6A and ARID1A) 75. Molecular subtyping based on transcriptome analysis of 460 NMIBC and 16 MIBC samples by Hedegaard et al. identified three predominant classes of NMIBC, of which class two showed clustering of high-grade and CIS pathology with an increased risk of clinical progression, indicating that this may be a signature warranting more aggressive, definitive therapy upfront 71. The TERT promoter is worth specific attention owing to similar frequency throughout all tumor stages and grades, regardless of tumor aggressiveness or molecular subtype, and likely represents an early event in the development of urothelial neoplasms 76– 78.
Personalized approach to bladder cancer therapy
Intravesical therapies
Instillation of chemotherapy immediately (within 24 hours) following TUR has been proposed as a method of reducing bladder cancer recurrences, specifically for low- and intermediate-risk disease, and is recommended by both the AUA and the EAU guidelines for NMIBC 10, 11. It is hypothesized to work via two main methods: (1) residual tumor that has not been fully resected will be killed and (2) elimination of free-floating cancerous cells dislodged during resection that may adhere to the urothelium and proliferate in a region separate from the primary 79, 80. The most well-studied agents include mitomycin C, doxorubicin, and epirubicin, all of which have been found to reduce recurrences (HR 0.40–0.65) without impacting progression or survival outcomes 81– 89. This is probably because of the preponderance of low-risk tumors included in the study cohorts, which are already at very low risk of progression to begin with. According to a recent, large meta-analysis, immediate post-resection instillation would appear to be most effective for small (<3 cm), low-grade, papillary tumors with a baseline recurrence rate of >1 year 89. Not all urologists support this mode of treatment owing to the small but catastrophic possibility of chemotherapy leaking into the perivesical space via missed bladder perforation, in some cases resulting in end-stage bladder fibrosis, for the minimal clinical benefit of prolonging time between recurrences of relatively indolent tumors 90, 91. Though the overwhelming majority of available literature may offer support for this viewpoint, Bosscheiter et al. published a prospective, randomized trial of immediate post-operative mitomycin C versus instillation 2 weeks later among more than 2,000 patients across all risk categories, finding an overall reduction in 3-year recurrence (27% versus 36%) and progression (2.7% versus 5.5%) 92. Interestingly, on subgroup analysis, only the intermediate- and high-risk patients—who also received an adjuvant 6- to 12-week course of intravesical mitomycin C—were found to benefit from the immediate post-operative dose.
Induction intravesical therapy refers to a period of weekly instillations of chemotherapy (i.e. mitomycin C, epirubicin, gemcitabine, etc.) or immunotherapy (i.e. BCG) following a complete TUR for NMIBC. At this point, TUR followed by a 6-week course of BCG plus 1 to 3 years of appropriate (3-weekly instillations based on SWOG protocol) maintenance therapy is the first-line option for high-risk disease with a proven improvement in disease recurrence and progression 10, 11, 93– 96. Intermediate-risk bladder cancer, on the other hand, can be successfully treated with either chemotherapy or immunotherapy; however, BCG must be followed by at least 1 year of maintenance in order to maintain an advantage in recurrence over mitomycin C 94, 97. Furthermore, while only BCG has shown improvement in progression for these patients, the potential side effects should be considered when selecting a treatment strategy 97– 99.
Patients who fail an adequate induction course of intravesical therapy, particularly those receiving BCG who are at highest risk for disease progression, pose a therapeutic dilemma: remove the bladder immediately to prevent muscle invasion and possible metastasis or continue local treatment with further instillations. It is preferable to avoid the morbidity and impact on quality of life associated with radical cystectomy; however, there are currently few alternatives in this setting. The lack of a standardized definition of BCG failure, recognizing that not all forms share the same prognosis, has hindered research into this area. To address these shortcomings, an international panel published guidance for clinicians and researchers to aid in creating more uniformity when designing trials and reporting on this group of patients, as well as to differentiate those with poorest prognosis (BCG unresponsive) who may not benefit from further BCG therapy ( Table 2) 100. The AUA recommends enrolment in clinical trials for patients who have demonstrated BCG unresponsiveness but are unwilling or unable to undergo a radical cystectomy 11. Several such trials have offered promising results, with most reporting short-term reduction in recurrence of approximately 30–50%, but longer follow-up is associated with sharp declines in responsiveness, and, therefore, there is insufficient evidence to support any single approach, and radical cystectomy remains the gold standard in this population 94, 101– 107.
Table 2. A classification system for bacillus Calmette-Guérin (BCG) failures 100.
| Type of failure | Description |
|---|---|
| BCG refractory | Persistence of high-grade disease at 6 months (3 months for T1
high grade) following adequate BCG treatment |
| BCG relapsing | Recurrence of high-grade disease following adequate BCG
treatment with a disease-free period of 6 months |
| BCG unresponsive | Includes BCG refractory and BCG relapses within 6 months (12 months
for carcinoma in situ patients) |
| BCG intolerant | Persistence of high-grade disease in a patient who is unable to
tolerate induction BCG secondary to toxicity |
Early cystectomy for non-muscle-invasive bladder cancer
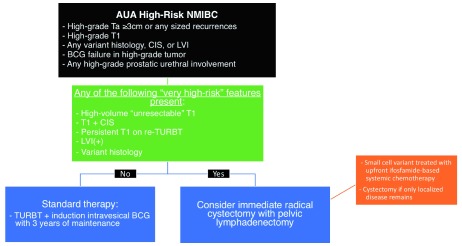
Immediate radical cystectomy may be the best treatment option for selected patients with certain adverse “very high-risk” factors found at the time of diagnosis of NMIBC ( Figure 3). High-volume T1, persistent high-grade T1 on re-TUR, lymphovascular invasion, certain variant histologies (i.e. micropapillary, plasmacytoid, nested), or concomitant CIS have all been associated with increased risk of progression and are best managed with surgery 108– 113. A group from the United Kingdom has designed a prospective, randomized controlled trial, with end of accrual set for March 2018, comparing immediate cystectomy against BCG induction with maintenance therapy for a “very high-risk” population of NMIBC patients 114. Notably excluded from their cohort are any patients with variant histology.
Figure 3. Proposed decision model for immediate radical cystectomy in patients with “very high-risk” non-muscle-invasive bladder cancer (NMIBC) 11, 125– 129.
AUA, American Urological Association; BCG, bacillus Calmette-Guérin; CIS, carcinoma in situ; LVI, lymphovascular invasion; TURBT, transurethral resection of bladder tumor.
Radiotherapy
Trimodal bladder-preserving therapy involves complete endoscopic resection of all visible tumor followed by neoadjuvant chemotherapy and definitive whole-bladder external beam radiotherapy. The use of this strategy is not widespread in the United States, but prospective European cohorts have demonstrated comparable disease-specific outcomes when compared to contemporary radical cystectomy cohorts 115. Limited evidence regarding optimal patient selection has identified increased expression of MRE11, a protein involved in the cellular response to radiation damage, as a potential prognostic marker for improved cancer-specific survival following radiotherapy 116, 117.
Timing of chemotherapy for muscle-invasive bladder cancer
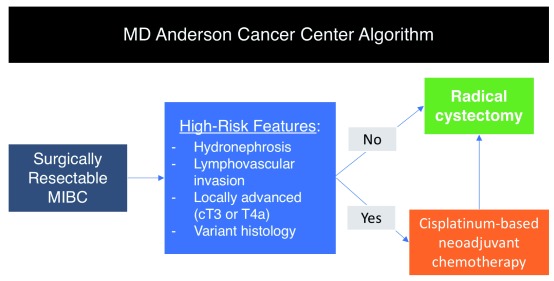
Muscle invasion at diagnosis of bladder cancer is a poor prognostic indicator best treated with neoadjuvant cisplatinum-based multi-agent chemotherapy followed by radical cystectomy according to evidence from several phase III clinical trials 118– 121. Disease status on final pathology is strongly correlated with oncologic outcomes, and neoadjuvant therapy leads to significant downstaging, including rates of complete response in the range of 30–40% compared to only 15% with surgery alone 119. However, this still leaves a large portion of patients who will not derive benefit, instead undergoing unnecessary toxic therapy while at the same time delaying surgery. Risk stratification can be useful in this setting by selecting those patients with the highest risk (pre-operative T3b–T4, hydroureteronephrosis, lymphovascular invasion, and specific histologic variants) for poor outcomes after cystectomy and only administering neoadjuvant chemotherapy to this subgroup ( Figure 4) 122, 123. Likewise, post-operative chemotherapy (i.e. adjuvant) can be administered in patients with proven pathologic predictors of developing metastasis, though evidence to support its use is lacking. One study (EORTC 30994) was able to show an improvement in progression-free survival; however, there was no statistically significant impact on overall survival 124.
Figure 4. Current MD Anderson Cancer Center algorithm for determining which patients should receive neoadjuvant chemotherapy versus immediate radical cystectomy.
Inclusion of molecular markers for further risk stratification is pending clinical validation 37. MIBC, muscle-invasive bladder cancer.
Conclusions
The appropriate management of bladder cancer patients relies heavily upon risk stratification to personalize the therapy for the patient. Currently, clinicopathologic features are the cornerstone of the most widely used risk assessment tools with ample room for improvement. Advances in genetic sequencing of tumors has led to classification systems based on the mutational profile of each individual, offering prognostic information. As these data expand, it is feasible that in the near future this could be integrated to provide guidance that is truly “next generation”.
Abbreviations
AUA, American Urological Association; BCG, bacillus Calmette-Guérin; CIS, carcinoma in situ; CUETO, Spanish Urological Club for Oncological Treatment; EAU, European Association of Urology; EORTC, European Organization for Research and Treatment of Cancer; MIBC, muscle-invasive bladder cancer; NMIBC, non-muscle-invasive bladder cancer; SWOG, Southwest Oncology Group; TCGA, the Cancer Genome Atlas; TUR, transurethral resection
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Michael O Koch, Department of Urology, Indiana University, Indianapolis, Indiana, USA
Francisco X Real, Epithelial Carcinogenesis Group, Cancer Cell Biology Programme, Spanish National Cancer Research Centre, Madrid, Spain
Robert D Siemens, Department of Urology, Kingston General Hospital Research Institute, Kingston, Ontario, Canada
Rodolfo Montironi, School of Medicine, United Hospitals, Institute of Pathological Anatomy and Histopathology, Polytechnic University of the Marche Region (Ancona), Ancona, Italy
Funding Statement
AMK is supported by the Wayne B. Duddlesten Professorship and a Floyd Family Foundation Grant.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved]
References
- 1. Burger M, Catto JW, Dalbagni G, et al. : Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–41. 10.1016/j.eururo.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 4. American Joint Committee on Cancer: AJCC cancer staging manual. Eighth edition. Amin MB, Edge SB, ed. Switzerland: Springer;2017. Reference Source [Google Scholar]
- 5. Humphrey PA, Moch H, Cubilla AL, et al. : The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70(1):106–19. 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 6. Divrik RT, Yildirim U, Zorlu F, et al. : The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol. 2006;175(5):1641–4. 10.1016/S0022-5347(05)01002-5 [DOI] [PubMed] [Google Scholar]
- 7. Gendy R, Delprado W, Brenner P, et al. : Repeat transurethral resection for non-muscle-invasive bladder cancer: a contemporary series. BJU Int. 2016;117 Suppl 4:54–9. 10.1111/bju.13265 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Lazica DA, Roth S, Brandt AS, et al. : Second transurethral resection after Ta high-grade bladder tumor: a 4.5-year period at a single university center. Urol Int. 2014;92(2):131–5. 10.1159/000353089 [DOI] [PubMed] [Google Scholar]
- 9. Cumberbatch MGK, Foerster B, Catto JWF, et al. : Repeat Transurethral Resection in Non-muscle-invasive Bladder Cancer: A Systematic Review. Eur Urol. 2018;73(6):925–33. 10.1016/j.eururo.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 10. Babjuk M, Böhle A, Burger M, et al. : EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71(3):447–61. 10.1016/j.eururo.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 11. Chang SS, Boorjian SA, Chou R, et al. : Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196(4):1021–9. 10.1016/j.juro.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 12. Burger M, Grossman HB, Droller M, et al. : Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013;64(5):846–54. 10.1016/j.eururo.2013.03.059 [DOI] [PubMed] [Google Scholar]
- 13. Chang TC, Marcq G, Kiss B, et al. : Image-Guided Transurethral Resection of Bladder Tumors - Current Practice and Future Outlooks. Bladder Cancer. 2017;3(3):149–59. 10.3233/BLC-170119 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Denzinger S, Burger M, Walter B, et al. : Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology. 2007;69(4):675–9. 10.1016/j.urology.2006.12.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Herr HH: Narrow band imaging cystoscopy. Urol Oncol. 2011;29(4):353–7. 10.1016/j.urolonc.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 16. Kang W, Cui Z, Chen Q, et al. : Narrow band imaging-assisted transurethral resection reduces the recurrence risk of non-muscle invasive bladder cancer: A systematic review and meta-analysis. Oncotarget. 2017;8(14):23880–90. 10.18632/oncotarget.13054 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Lee JY, Cho KS, Kang DH, et al. : A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence vs hexylaminolevulinate fluorescence vs narrow band imaging. BMC Cancer. 2015;15:566. 10.1186/s12885-015-1571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rink M, Babjuk M, Catto JW, et al. : Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur Urol. 2013;64(4):624–38. 10.1016/j.eururo.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 19. Rolevich AI, Zhegalik AG, Mokhort AA, et al. : Results of a prospective randomized study assessing the efficacy of fluorescent cystoscopy-assisted transurethral resection and single instillation of doxorubicin in patients with non-muscle-invasive bladder cancer. World J Urol. 2017;35(5):745–52. 10.1007/s00345-016-1927-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Chlapoutakis K, Theocharopoulos N, Yarmenitis S, et al. : Performance of computed tomographic urography in diagnosis of upper urinary tract urothelial carcinoma, in patients presenting with hematuria: Systematic review and meta-analysis. Eur J Radiol. 2010;73(2):334–8. 10.1016/j.ejrad.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 21. Cowan NC, Turney BW, Taylor NJ, et al. : Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99(6):1363–70. 10.1111/j.1464-410X.2007.06766.x [DOI] [PubMed] [Google Scholar]
- 22. Froemming A, Potretzke T, Takahashi N, et al. : Upper tract urothelial cancer. Eur J Radiol. 2018;98:50–60. 10.1016/j.ejrad.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 23. Rouprêt M, Babjuk M, Compérat E, et al. : European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol. 2018;73(1):111–22. 10.1016/j.eururo.2017.07.036 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Takahashi N, Glockner JF, Hartman RP, et al. : Gadolinium enhanced magnetic resonance urography for upper urinary tract malignancy. J Urol. 2010;183(4):1330–65. 10.1016/j.juro.2009.12.031 [DOI] [PubMed] [Google Scholar]
- 25. Palou J, Rodríguez-Rubio F, Huguet J, et al. : Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. J Urol. 2005;174(3):859–61; discussion 861. 10.1097/01.ju.0000169424.79702.6d [DOI] [PubMed] [Google Scholar]
- 26. Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al. : Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol. 2000;164(4):1183–7. 10.1016/S0022-5347(05)67137-6 [DOI] [PubMed] [Google Scholar]
- 27. Fernandez-Gomez J, Madero R, Solsona E, et al. : Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182(5):2195–203. 10.1016/j.juro.2009.07.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. : Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–5; discussion 475–7. 10.1016/j.eururo.2005.12.031 [DOI] [PubMed] [Google Scholar]
- 29. Fernandez-Gomez J, Madero R, Solsona E, et al. : The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: external validation of the EORTC risk tables. Eur Urol. 2011;60(3):423–30. 10.1016/j.eururo.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 30. Hernández V, De La Peña E, Martin MD, et al. : External validation and applicability of the EORTC risk tables for non-muscle-invasive bladder cancer. World J Urol. 2011;29(4):409–14. 10.1007/s00345-010-0635-2 [DOI] [PubMed] [Google Scholar]
- 31. Seo KW, Kim BH, Park CH, et al. : The efficacy of the EORTC scoring system and risk tables for the prediction of recurrence and progression of non-muscle-invasive bladder cancer after intravesical bacillus calmette-guerin instillation. Korean J Urol. 2010;51(3):165–70. 10.4111/kju.2010.51.3.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xylinas E, Kent M, Kluth L, et al. : Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer. 2013;109(6):1460–6. 10.1038/bjc.2013.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamm DL, Blumenstein BA, Crissman JD, et al. : Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124–9. 10.1016/S0022-5347(05)67707-5 [DOI] [PubMed] [Google Scholar]
- 34. van Kessel KEM, van der Keur KA, Dyrskjøt L, et al. : Molecular Markers Increase Precision of the European Association of Urology Non-Muscle-Invasive Bladder Cancer Progression Risk Groups. Clin Cancer Res. 2018;24(7):1586–93. 10.1158/1078-0432.CCR-17-2719 [DOI] [PubMed] [Google Scholar]
- 35. Kamat AM, Witjes JA, Brausi M, et al. : Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014;192(2):305–15. 10.1016/j.juro.2014.02.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang SS, Bochner BH, Chou R, et al. : Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198(3):552–9. 10.1016/j.juro.2017.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karam JA, Kamat AM: Optimal timing of chemotherapy and cystectomy. F1000 Med Rep. 2010;2: pii: 48. 10.3410/M2-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hansel DE, Amin MB, Comperat E, et al. : A contemporary update on pathology standards for bladder cancer: transurethral resection and radical cystectomy specimens. Eur Urol. 2013;63(2):321–32. 10.1016/j.eururo.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 39. Kaimakliotis HZ, Monn MF, Cary KC, et al. : Plasmacytoid variant urothelial bladder cancer: is it time to update the treatment paradigm? Urol Oncol. 2014;32(6):833–8. 10.1016/j.urolonc.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 40. Keck B, Stoehr R, Wach S, et al. : The plasmacytoid carcinoma of the bladder--rare variant of aggressive urothelial carcinoma. Int J Cancer. 2011;129(2):346–54. 10.1002/ijc.25700 [DOI] [PubMed] [Google Scholar]
- 41. Keck B, Wach S, Stoehr R, et al. : Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer. 2013;13:71. 10.1186/1471-2407-13-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernández MI, Williams SB, Willis DL, et al. : Clinical risk stratification in patients with surgically resectable micropapillary bladder cancer. BJU Int. 2017;119(5):684–91. 10.1111/bju.13689 [DOI] [PubMed] [Google Scholar]
- 43. Kamat AM, Dinney CP, Gee JR, et al. : Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110(1):62–7. 10.1002/cncr.22756 [DOI] [PubMed] [Google Scholar]
- 44. Meeks JJ, Taylor JM, Matsushita K, et al. : Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int. 2013;111(8):E325–30. 10.1111/j.1464-410X.2012.11751.x [DOI] [PubMed] [Google Scholar]
- 45. Sui W, Matulay JT, James MB, et al. : Micropapillary Bladder Cancer: Insights from the National Cancer Database. Bladder Cancer. 2016;2(4):415–23. 10.3233/BLC-160066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vetterlein MW, Wankowicz SAM, Seisen T, et al. : Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer. 2017;123(22):4346–55. 10.1002/cncr.30907 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Amin MB: Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22 Suppl 2:S96–S118. 10.1038/modpathol.2009.26 [DOI] [PubMed] [Google Scholar]
- 48. Mitra AP, Bartsch CC, Bartsch G, Jr, et al. : Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol. 2014;32(2):117–27. 10.1016/j.urolonc.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 49. Abdollah F, Sun M, Jeldres C, et al. : Survival after radical cystectomy of non-bilharzial squamous cell carcinoma vs urothelial carcinoma: a competing-risks analysis. BJU Int. 2012;109(4):564–9. 10.1111/j.1464-410X.2011.10357.x [DOI] [PubMed] [Google Scholar]
- 50. Zaghloul MS, Nouh A, Nazmy M, et al. : Long-term results of primary adenocarcinoma of the urinary bladder: a report on 192 patients. Urol Oncol. 2006;24(1):13–20. 10.1016/j.urolonc.2005.05.027 [DOI] [PubMed] [Google Scholar]
- 51. Warrick JI: Clinical Significance of Histologic Variants of Bladder Cancer. J Natl Compr Canc Netw. 2017;15(10):1268–74. 10.6004/jnccn.2017.7027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Lynch SP, Shen Y, Kamat A, et al. : Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013;64(2):307–13. 10.1016/j.eururo.2012.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kandoth C, McLellan MD, Vandin F, et al. : Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Yap KL, Kiyotani K, Tamura K, et al. : Whole-exome sequencing of muscle-invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clin Cancer Res. 2014;20(24):6605–17. 10.1158/1078-0432.CCR-14-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cancer Genome Atlas Research Network: Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. 10.1038/nature12965 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Robertson AG, Kim J, Al-Ahmadie H, et al. : Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171(3):540–556.e25. 10.1016/j.cell.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Choi W, Porten S, Kim S, et al. : Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. 10.1016/j.ccr.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Damrauer JS, Hoadley KA, Chism DD, et al. : Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111(8):3110–5. 10.1073/pnas.1318376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindgren D, Frigyesi A, Gudjonsson S, et al. : Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res. 2010;70(9):3463–72. 10.1158/0008-5472.CAN-09-4213 [DOI] [PubMed] [Google Scholar]
- 60. Kamat AM, Hahn NM, Efstathiou JA, et al. : Bladder cancer. Lancet. 2016;388(10061):2796–810. 10.1016/S0140-6736(16)30512-8 [DOI] [PubMed] [Google Scholar]
- 61. Lerner SP, McConkey DJ, Hoadley KA, et al. : Bladder Cancer Molecular Taxonomy: Summary from a Consensus Meeting. Bladder Cancer. 2016;2(1):37–47. 10.3233/BLC-150037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rebouissou S, Bernard-Pierrot I, de Reyniès A, et al. : EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med. 2014;6(244):244ra91. 10.1126/scitranslmed.3008970 [DOI] [PubMed] [Google Scholar]
- 63. Sjödahl G, Eriksson P, Liedberg F, et al. : Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J Pathol. 2017;242(1):113–25. 10.1002/path.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Seiler R, Ashab HAD, Erho N, et al. : Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol. 2017;72(4):544–54. 10.1016/j.eururo.2017.03.030 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. : ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur Urol. 2016;69(3):384–8. 10.1016/j.eururo.2015.01.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Liu D, Plimack ER, Hoffman-Censits J, et al. : Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-Invasive Urothelial Bladder Carcinoma. JAMA Oncol. 2016;2(8):1094–6. 10.1001/jamaoncol.2016.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Plimack ER, Dunbrack RL, Brennan TA, et al. : Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol. 2015;68(6):959–67. 10.1016/j.eururo.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. van Allen EM, Mouw KW, Kim P, et al. : Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4(10):1140–53. 10.1158/2159-8290.CD-14-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Ren R, Tyryshkin K, Graham CH, et al. : Comprehensive immune transcriptomic analysis in bladder cancer reveals subtype specific immune gene expression patterns of prognostic relevance. Oncotarget. 2017;8(41):70982–1001. 10.18632/oncotarget.20237 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Hurst CD, Alder O, Platt FM, et al. : Genomic Subtypes of Non-invasive Bladder Cancer with Distinct Metabolic Profile and Female Gender Bias in KDM6A Mutation Frequency. Cancer Cell. 2017;32(5):701–715.e7. 10.1016/j.ccell.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hedegaard J, Lamy P, Nordentoft I, et al. : Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell. 2016;30(1):27–42. 10.1016/j.ccell.2016.05.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Al Hussain TO, Akhtar M: Molecular basis of urinary bladder cancer. Adv Anat Pathol. 2013;20(1):53–60. 10.1097/PAP.0b013e31827bd0ec [DOI] [PubMed] [Google Scholar]
- 73. Billerey C, Chopin D, Aubriot-Lorton MH, et al. : Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158(6):1955–9. 10.1016/S0002-9440(10)64665-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu X, Zhang W, Geng D, et al. : Clinical significance of fibroblast growth factor receptor-3 mutations in bladder cancer: a systematic review and meta-analysis. Genet Mol Res. 2014;13(1):1109–20. 10.4238/2014.February.20.12 [DOI] [PubMed] [Google Scholar]
- 75. Pietzak EJ, Bagrodia A, Cha EK, et al. : Next-generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur Urol. 2017;72(6):952–9. 10.1016/j.eururo.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Allory Y, Beukers W, Sagrera A, et al. : Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65(2):360–6. 10.1016/j.eururo.2013.08.052 [DOI] [PubMed] [Google Scholar]
- 77. Cheng L, Davidson DD, Wang M, et al. : Telomerase reverse transcriptase (TERT) promoter mutation analysis of benign, malignant and reactive urothelial lesions reveals a subpopulation of inverted papilloma with immortalizing genetic change. Histopathology. 2016;69(1):107–13. 10.1111/his.12920 [DOI] [PubMed] [Google Scholar]
- 78. Wang CC, Huang CY, Jhuang YL, et al. : Biological significance of TERT promoter mutation in papillary urothelial neoplasm of low malignant potential. Histopathology. 2018;72(5):795–803. 10.1111/his.13441 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Kaasinen E, Rintala E, Hellström P, et al. : Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. Eur Urol. 2002;42(2):167–74. 10.1016/S0302-2838(02)00260-9 [DOI] [PubMed] [Google Scholar]
- 80. Pode D, Alon Y, Horowitz AT, et al. : The mechanism of human bladder tumor implantation in an in vitro model. J Urol. 1986;136(2):482–6. 10.1016/S0022-5347(17)44926-3 [DOI] [PubMed] [Google Scholar]
- 81. Barghi MR, Rahmani MR, Hosseini Moghaddam SM, et al. : Immediate intravesical instillation of mitomycin C after transurethral resection of bladder tumor in patients with low-risk superficial transitional cell carcinoma of bladder. Urol J. 2006;3(4):220–4. 10.22037/uj.v3i4.166 [DOI] [PubMed] [Google Scholar]
- 82. Berrum-Svennung I, Granfors T, Jahnson S, et al. : A single instillation of epirubicin after transurethral resection of bladder tumors prevents only small recurrences. J Urol. 2008;179(1):101–5; discussion 105–6. 10.1016/j.juro.2007.08.166 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. De Nunzio C, Carbone A, Albisinni S, et al. : Long-term experience with early single mitomycin C instillations in patients with low-risk non-muscle-invasive bladder cancer: prospective, single-centre randomised trial. World J Urol. 2011;29(4):517–21. 10.1007/s00345-011-0691-2 [DOI] [PubMed] [Google Scholar]
- 84. Gudjónsson S, Adell L, Merdasa F, et al. : Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur Urol. 2009;55(4):773–80. 10.1016/j.eururo.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 85. Kang M, Jeong CW, Kwak C, et al. : Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs. Oncotarget. 2016;7(29):45479–88. 10.18632/oncotarget.9991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Perlis N, Zlotta AR, Beyene J, et al. : Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol. 2013;64(3):421–30. 10.1016/j.eururo.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 87. Solsona E, Iborra I, Ricós JV, et al. : Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999;161(4):1120–3. 10.1016/S0022-5347(01)61606-9 [DOI] [PubMed] [Google Scholar]
- 88. Sylvester RJ, Oosterlinck W: An immediate instillation after transurethral resection of bladder tumor in non-muscle-invasive bladder cancer: has the evidence changed? Eur Urol. 2009;56(1):43–5. 10.1016/j.eururo.2009.03.074 [DOI] [PubMed] [Google Scholar]
- 89. Sylvester RJ, Oosterlinck W, Holmang S, et al. : Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur Urol. 2016;69(2):231–44. 10.1016/j.eururo.2015.05.050 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Elmamoun MH, Christmas TJ, Woodhouse CR: Destruction of the bladder by single dose Mitomycin C for low-stage transitional cell carcinoma (TCC)--avoidance, recognition, management and consent. BJU Int. 2014;113(5b):E34–8. 10.1111/bju.12340 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Oddens JR, van der Meijden AP, Sylvester R: One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol. 2004;46(3):336–8. 10.1016/j.eururo.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 92. Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, et al. : Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur Urol. 2018;73(2):226–32. 10.1016/j.eururo.2017.06.038 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Böhle A, Jocham D, Bock PR: Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90–5. 10.1016/S0022-5347(05)64043-8 [DOI] [PubMed] [Google Scholar]
- 94. Malmström PU, Sylvester RJ, Crawford DE, et al. : An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–56. 10.1016/j.eururo.2009.04.038 [DOI] [PubMed] [Google Scholar]
- 95. Shelley MD, Mason MD, Kynaston H: Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev. 2010;36(3):195–205. 10.1016/j.ctrv.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 96. Shelley MD, Wilt TJ, Court J, et al. : Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int. 2004;93(4):485–90. 10.1111/j.1464-410X.2003.04655.x [DOI] [PubMed] [Google Scholar]
- 97. Chou R, Selph S, Buckley DI, et al. : Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J Urol. 2017;197(5):1189–99. 10.1016/j.juro.2016.12.090 [DOI] [PubMed] [Google Scholar]
- 98. Brausi M, Oddens J, Sylvester R, et al. : Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65(1):69–76. 10.1016/j.eururo.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 99. Böhle A, Bock PR: Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63(4):682–6; discussion 686–7. 10.1016/j.urology.2003.11.049 [DOI] [PubMed] [Google Scholar]
- 100. Kamat AM, Sylvester RJ, Böhle A, et al. : Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016;34(16):1935–44. 10.1200/JCO.2015.64.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Barlow LJ, Benson MC: Experience with newer intravesical chemotherapy for high-risk non-muscle-invasive bladder cancer. Curr Urol Rep. 2013;14(2):65–70. 10.1007/s11934-013-0312-2 [DOI] [PubMed] [Google Scholar]
- 102. Di Lorenzo G, Perdonà S, Damiano R, et al. : Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116(8):1893–900. 10.1002/cncr.24914 [DOI] [PubMed] [Google Scholar]
- 103. McKiernan JM, Holder DD, Ghandour RA, et al. : Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guérin treatment failure. J Urol. 2014;192(6):1633–8. 10.1016/j.juro.2014.06.084 [DOI] [PubMed] [Google Scholar]
- 104. Porten SP, Leapman MS, Greene KL: Intravesical chemotherapy in non-muscle-invasive bladder cancer. Indian J Urol. 2015;31(4):297–303. 10.4103/0970-1591.166446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shelley MD, Jones G, Cleves A, et al. : Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review. BJU Int. 2012;109(4):496–505. 10.1111/j.1464-410X.2011.10880.x [DOI] [PubMed] [Google Scholar]
- 106. Steinberg G, Bahnson R, Brosman S, et al. : Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163(3):761–7. 10.1016/S0022-5347(05)67799-3 [DOI] [PubMed] [Google Scholar]
- 107. Sternberg IA, Dalbagni G, Chen LY, et al. : Intravesical gemcitabine for high risk, nonmuscle invasive bladder cancer after bacillus Calmette-Guérin treatment failure. J Urol. 2013;190(5):1686–91. 10.1016/j.juro.2013.04.120 [DOI] [PubMed] [Google Scholar]
- 108. Mathieu R, Lucca I, Rouprêt M, et al. : The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat Rev Urol. 2016;13(8):471–9. 10.1038/nrurol.2016.126 [DOI] [PubMed] [Google Scholar]
- 109. Martin-Doyle W, Leow JJ, Orsola A, et al. : Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol. 2015;33(6):643–50. 10.1200/JCO.2014.57.6967 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Willis DL, Fernandez MI, Dickstein RJ, et al. : Clinical outcomes of cT1 micropapillary bladder cancer. J Urol. 2015;193(4):1129–34. 10.1016/j.juro.2014.09.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Segal R, Yafi FA, Brimo F, et al. : Prognostic factors and outcome in patients with T1 high-grade bladder cancer: can we identify patients for early cystectomy? BJU Int. 2012;109(7):1026–30. 10.1111/j.1464-410X.2011.10462.x [DOI] [PubMed] [Google Scholar]
- 112. Mally AD, Tin AL, Lee JK, et al. : Clinical Outcomes of Patients With T1 Nested Variant of Urothelial Carcinoma Compared to Pure Urothelial Carcinoma of the Bladder. Clin Genitourin Cancer. 2017; pii: S1558-7673(17)30199-4. 10.1016/j.clgc.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ark JT, Keegan KA, Barocas DA, et al. : Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int. 2014;113(6):894–9. 10.1111/bju.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Oughton JB, Poad H, Twiddy M, et al. : Radical cystectomy (bladder removal) against intravesical BCG immunotherapy for high-risk non-muscle invasive bladder cancer (BRAVO): a protocol for a randomised controlled feasibility study. BMJ Open. 2017;7(8):e017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mak RH, Hunt D, Shipley WU, et al. : Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32(34):3801–9. 10.1200/JCO.2014.57.5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Choudhury A, Nelson LD, Teo MT, et al. : MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70(18):7017–26. 10.1158/0008-5472.CAN-10-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Laurberg JR, Brems-Eskildsen AS, Nordentoft I, et al. : Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int. 2012;110(11 Pt C):E1228–36. 10.1111/j.1464-410X.2012.11564.x [DOI] [PubMed] [Google Scholar]
- 118. Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 119. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5; discussion 205–6. 10.1016/j.eururo.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 120. Yuh BE, Ruel N, Wilson TG, et al. : Pooled analysis of clinical outcomes with neoadjuvant cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer. J Urol. 2013;189(5):1682–6. 10.1016/j.juro.2012.10.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. : International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–7. 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Culp SH, Dickstein RJ, Grossman HB, et al. : Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol. 2014;191(1):40–7. 10.1016/j.juro.2013.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Moschini M, Soria F, Klatte T, et al. : Validation of Preoperative Risk Grouping of the Selection of Patients Most Likely to Benefit From Neoadjuvant Chemotherapy Before Radical Cystectomy. Clin Genitourin Cancer. 2017;15(2):e267–e273. 10.1016/j.clgc.2016.07.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 124. Sternberg CN, Skoneczna I, Kerst JM, et al. : Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(1):76–86. 10.1016/S1470-2045(14)71160-X [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 125. Dong F, Shen Y, Gao F, et al. : Nomograms to Predict Individual Prognosis of Patients with Primary Small Cell Carcinoma of the Bladder. J Cancer. 2018;9(7):1152–64. 10.7150/jca.23344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kouba EJ, Cheng L: Understanding the Genetic Landscape of Small Cell Carcinoma of the Urinary Bladder and Implications for Diagnosis, Prognosis, and Treatment: A Review. JAMA Oncol. 2017;3(11):1570–8. 10.1001/jamaoncol.2016.7013 [DOI] [PubMed] [Google Scholar]
- 127. Millis SZ, Bryant D, Basu G, et al. : Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clin Genitourin Cancer. 2015;13(1):e37–49. 10.1016/j.clgc.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 128. Zheng X, Liu D, Fallon JT, et al. : Distinct genetic alterations in small cell carcinoma from different anatomic sites. Exp Hematol Oncol. 2015;4:2. 10.1186/2162-3619-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Siefker-Radtke AO, Kamat AM, Grossman HB, et al. : Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27(16):2592–7. 10.1200/JCO.2008.19.0256 [DOI] [PMC free article] [PubMed] [Google Scholar]