Abstract
Objective
Reactive oxygen species regulate canonical Wnt signaling. However, the role of the redox regulatory protein p66Shc in the canonical Wnt pathway is not known. We investigated if p66Shc is essential for canonical Wnt signaling in the endothelium and determined if the canonical Wnt pathway induces vascular endothelial dysfunction via p66Shc-mediated oxidative stress.
Approach and results
The canonical Wnt ligand Wnt3a induced phosphorylation (activation) of p66Shc in endothelial cells. Wnt3a-stimulated dephosphorylation of β-catenin, and β-catenin-dependent transcription, was inhibited by knockdown of p66Shc. Exogenous hydrogen peroxide (H2O2)-induced β-catenin dephosphorylation was also mediated by p66Shc. Moreover, p66Shc overexpression dephosphorylated β-catenin, and increased β-catenin-dependent transcription, independent of Wnt3a ligand. P66Shc-induced β-catenin dephosphorylation was inhibited by antioxidants N-acetyl cysteine and catalase. Wnt3a upregulated endothelial NADPH Oxidase-4 (NOX-4), and β-catenin dephosphorylation was suppressed by knocking down NOX-4 and by antioxidants. Wnt3a increased H2O2 levels in endothelial cells and impaired endothelium-dependent vasorelaxation in mouse aortas, both of which were rescued by p66Shc knockdown. P66Shc knockdown also inhibited adhesion of monocytes to Wnt3a-stimulated endothelial cells. Further, constitutively active β-catenin expression in the endothelium increased vascular reactive oxygen species and impaired endothelium-dependent vasorelaxation. In vivo, high-fat diet feeding-induced endothelial dysfunction in mice was associated with increased endothelial Wnt3a, dephosphorylated β-catenin, and phosphorylated p66Shc. High-fat diet-induced dephosphorylation of endothelial β-catenin was diminished in mice in which p66Shc was knocked down.
Conclusion
p66Shc plays a vital part in canonical Wnt signaling in the endothelium, and mediates Wnt3a-stimulated endothelial oxidative stress and dysfunction
Introduction
Wnt/β-catenin (canonical) signaling is an evolutionarily conserved pathway which plays an important physiological role in proliferation, differentiation, and cell fate speciation1–3. Canonical Wnt signaling leads to dephosphorylation and stabilization of β-catenin which then associates with T-cell factor (TCF)-lymphoid-enhancer binding factor (LEF) family of transcription factors and regulates expression of Wnt target genes2. Wnt/β-catenin signaling is involved in multitude of cellular responses in different organ systems, and therefore deregulation of signaling is associated with a host of diseases and syndromes, ranging from schizophrenia to cancer to osteoporosis2.
Animal4 and human5, 6 studies show an association between deregulation of Wnt/β-catenin signaling and vascular diseases. Moreover, many Wnt ligands and signaling components are expressed in vascular endothelial cells7, 8. It is also noteworthy that Wnt/β-catenin signaling is augmented in models of aging9 and is upregulated in aged human arteries10. In addition, the reactive oxygen species hydrogen peroxide promotes stabilization of β-catenin11, suggesting a link between oxidative stress and canonical Wnt signaling in aged tissues.
P66Shc belongs to the shcA family of adaptor proteins which share a common (Src homology-2) SH2 domain, a (collagen homology-1) CH1 region and phosphotyrosine binding domain12. P66Shc also possesses a unique amino-terminal CH2 domain. Phosphorylation of serine 36 in the CH2 domain occurs in response to variety of stimuli, including UV rays, H2O2 treatment, and growth factor receptor activation. p66Shc, upon activation further increases intracellular ROS by promoting its generation and inhibiting the expression of antioxidant enzymes13- thus, it acts as a sensor as well as amplifier of oxidative stress. Genetic ablation studies suggest an important role for p66Shc in the regulation of fat accumulation14, endothelial dysfunction15–19, and atherosclerosis20–22.
Both canonical Wnt signaling and p66Shc have been linked to vascular pathology, but crosstalk between the two remains unknown. In the present study, we tested the hypothesis that Wnt/β-catenin signaling mediates endothelial dysfunction through p66Shc.
Material and Methods
Material and methods are available in the online-only Supplement. Briefly, vascular reactivity studies were performed in isolated aortas as previously described23. H2O2 produced by cells was measured in conditioned medium using the Amplex Red probe, as previously described24.
Results
In order to study canonical Wnt signaling in the endothelium, we first verified its existence in endothelial cells using Wnt3a, the prototypical ligand for activation of the canonical pathway. Recombinant Wnt3a stimulated β-catenin dephosphorylation and expression in bovine aortic endothelial cells (BAEC) (Fig 1a). Conditioned medium containing Wnt3a also stimulated total and dephosphorylated (active) β-catenin in BAEC (Supplemental Fig I). In human umbilical vein endothelial cells (HUVEC) as well, Wnt3a conditioned medium and recombinant Wnt3a stimulated dephosphorylation and accumulation of β-catenin in a dose- and time-dependent manner (Fig 1b and Supplemental Fig IIa–c). Wnt3a also stimulated β-catenin-dependent transcriptional response mediated by T-cell factor/Leukemia enhancing factor (TCF/LEF) in BAEC (Fig 1c). Thus, the canonical Wnt signaling machinery is operative in endothelial cells from different vascular beds.
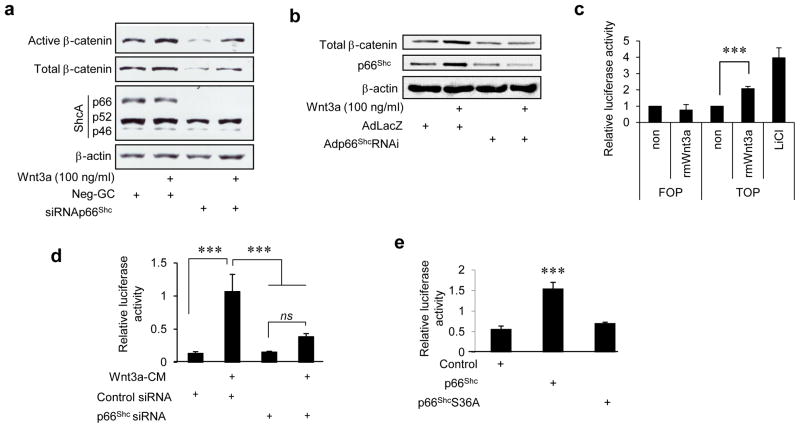
Fig. 1. P66Shc is required for canonical Wnt signaling in endothelial cells.
a) siRNA-mediated knockdown of p66Shc in BAEC inhibits Wnt3a-stimulated dephosphorylation and increase of β-catenin. b) shRNA-mediated knockdown of p66Shc in HUVEC suppresses Wnt3a-stimulated increase of β-catenin. Adp66shcRNAi: adenovirus expressing p66shc shRNA; AdlacZ: control adenovirus encoding E. Coli LacZ. c) Wnt3a stimulates β-catenin-mediated transcription in BAEC measured by TOP-Flash (TOP) luciferase reporter plasmid. Mutated FOP-Flash (FOP) reporter was used as a negative control. Lithium chloride (LiCl) was used as a positive control (n=3). d) Wnt3a-stimulated TOP-Flash luciferase activity in HEK 293 cells is suppressed by knocking down p66Shc (n=3). e) Expression of p66Shc, but not the redox deficient p66Shc S36A, induces β-catenin-mediated transcription (TOP-Flash luciferase activity) in HEK 293 cells (n=3). All values are shown as mean ± SEM. *** P < 0.001 vs. indicated group. ns = not significant. Immunoblots are representative of three experiments.
We then asked if p66Shc is required for canonical Wnt signaling. Knockdown of p66Shc inhibited Wnt3a-stimulated dephosphorylation and accumulation of β-catenin in BAEC and HUVEC (Fig 1a and 1b). Similarly, β-catenin-dependent transcription was dependent on p66Shc (Fig 1d). Moreover, overexpression of p66Shc increased β-catenin-dependent transcription, independent of Wnt3a ligand (Fig 1e). Thus, p66Shc is required for canonical Wnt signaling in endothelial cells and is sufficient to stimulate the canonical Wnt pathway.
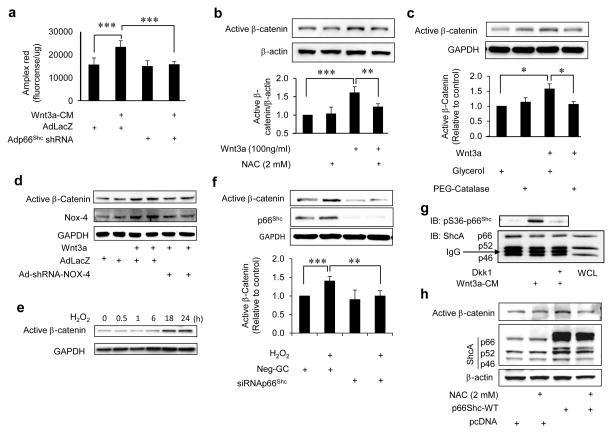
P66Shc plays a central role in regulating the redox status of cells and tissues. Therefore, we questioned if canonical Wnt signaling is dependent on p66Shc-regulated reactive oxygen species. To answer this, we first determined if canonical Wnt signaling is associated with an increase in reactive oxygen species in endothelial cells. Wnt3a led to a significant increase in hydrogen peroxide (H2O2) levels in endothelial cells (Fig 2a). Moreover, H2O2 increase by Wnt3a was abrogated by shRNA-mediated knockdown of p66Shc (Fig 2a). In addition, suppressing oxidative stress with cell-permeable anti-oxidants N-acetyl cysteine (NAC) and PEG-catalase prevented Wnt3a-induced dephosphorylation of β-catenin (Fig 2b & c). We then investigated the involvement of NADPH oxidase-4 (NOX-4), the principal NOX expressed in endothelial cells, in canonical Wnt signaling in endothelial cells. Wnt3a upregulated NOX-4 expression and knockdown of NOX-4 with Ad-shRNA-NOX-4 abrogated Wnt3a-induced dephosphorylation of β-catenin (Fig. 2d). Further, H2O2 alone induced dephosphorylation of β-catenin, an effect which was abrogated by siRNA-mediated knockdown of p66Shc (Fig. 2e & f). These findings underscore the p66Shc-mediated redox-dependent nature of canonical Wnt signaling in endothelial cells, and highlight the role of NOX-4 in this signaling.
Fig. 2. P66Shc-regulated reactive oxygen species mediate canonical Wnt signaling in endothelial cells.
a) Wnt3a conditioned medium (Wnt3a-CM)-induced H2O2 in HUVEC is inhibited by shRNA-mediated knockdown of p66Shc (n=3). Adp66shcRNAi: adenovirus expressing p66shc shRNA; AdlacZ: control adenovirus encoding E. Coli LacZ. b) Wnt3a-induced dephosphorylation of β-catenin in HUVEC is suppressed by the anti-oxidant NAC. Densitometry of active β-catenin/β-actin is shown (n=3). c) Wnt3a-induced dephosphorylation of β-catenin in HUVEC is suppressed by the anti-oxidant PEG-Catalase. Densitometry of active β-catenin/GAPDH is shown (n=3). Glycerol (vehicle) was used as control. d) NOX-4 is upregulated by Wnt3a in HUVEC, and Wnt3a-stimulated dephosphorylation of β-catenin is suppressed by shRNA-mediated down-regulation of NOX-4 (n=3). Ad-shRNA-NOX4: adenovirus expressing NOX-4 shRNA. AdLacZ was used as control. e) H2O2 dephosphorylates β-catenin in HUVEC. f) siRNA-mediated downregulation of p66Shc suppresses H2O2-induced dephosphorylation of β-catenin in HUVEC (n = 4). g) Wnt3a-CM stimulates phosphorylation of p66Shc on serine 36, which is blocked by Dkk1. WCL: whole cell lysate. h) p66Shc-induced dephosphorylation of β-catenin in HUVEC is inhibited by NAC. All data is representative of at least three independent experiments and values are shown as mean ± SEM. ** P < 0.01, *** P < 0.001 vs. indicated group. Immunoblots are representative of 3–4 experiments.
Phosphorylation of p66Shc on serine 36 is essential for its pro-oxidative function12. Because p66Shc mediates H2O2 stimulated by Wnt3a, we inquired if p66Shc is phosphorylated on serine 36 in response to Wnt3a. Wnt3a conditioned medium and recombinant Wnt3a induced rapid serine 36 phosphorylation of p66Shc in endothelial cells (Fig 2g and Supplemental Fig IId). Inhibition of Wnt signaling with the extra-cellular Wnt ligand antagonist Dickkopf-1 (Dkk1) suppressed Wnt3a-stimulated phosphorylation of p66shc (Fig 2g). Moreover, non-phosphorylatable p66Shc (S36A), which is incapable of promoting oxidative stress, did not increase β-catenin-dependent transcription (Fig 1e). In addition, dephosphorylation of β-catenin induced by p66Shc was blunted by the anti-oxidant NAC (Fig 2h). Taken together, these findings further support a role for p66Shc-mediated redox mechanisms in canonical Wnt signaling.
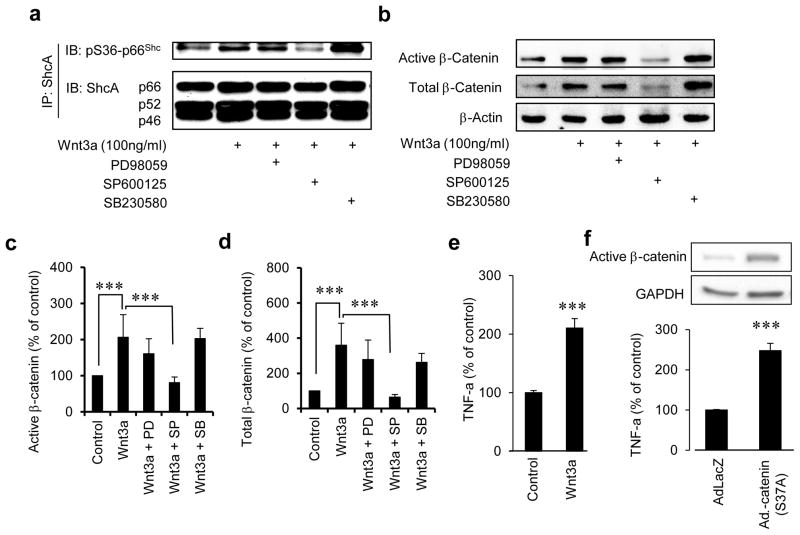
Since several kinases are known to induce phosphorylation of p66Shc on serine 36, we sought to identify the kinase responsible for Wnt3a-induced p66Shc phosphorylation. HUVEC were pre-incubated with specific kinase inhibitors and the effect of Wnt3a on Ser36 phosphorylation of p66Shc was examined. The c-jun N-terminal kinase (JNK) inhibitor SP600125 inhibited Ser36 phosphorylation of p66Shc, while inhibition of mitogen-activated kinase kinase (MEK) with PD98059, or p38MAPK with SB203580 did not (Fig. 3a). Moreover, inhibition of JNK, but not p38MAPK or MEK, decreased active and total β-catenin (Fig. 3b–d). These results show that JNK is the principal kinase responsible for phosphorylation of p66Shc and dephosphorylation of β-catenin in endothelial cells in response to Wnt3a.
Fig. 3. JNK phosphorylates p66Shc in response to Wnt3a.
a) Wnt3a-induced serine 36 phosphorylation of p66Shc in HUVEC is inhibited by the JNK inhibitor SP600125, but not by the MEK inhibitor PD98059, or the p38MAPK inhibitor SB203580. b–d) Wnt3a-induced dephosphorylation and increase of β-catenin in HUVEC is inhibited by the JNK inhibitor SP600125, but not by the MEK inhibitor PD98059, or the p38MAPK inhibitor SB203580. Densitometry of active (dephosphorylated) β-catenin/β-actin and total β-catenin/β-actin is shown (n=3). e) Wnt3a induces expression of tumor necrosis factor-α (TNFα) in HUVEC (n =3). F) Constitutively active non-phosphorylatable form of β-catenin [β-catenin (S37A)] upregulates TNFα in HUVECs (n =3). Adβ-catenin (S37A): adenovirus expressing β-catenin (S37A). All values are shown as mean ± SEM. *** P < 0.001, vs. indicated group. WCL; Whole cell lysate, PD; PD98059, SP; SP600125, SB; SB203580. Immunoblots are representative of three experiments.
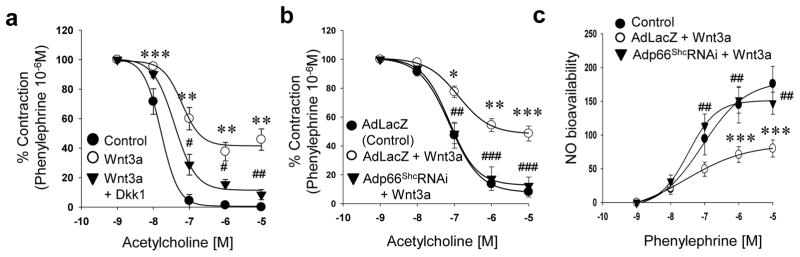
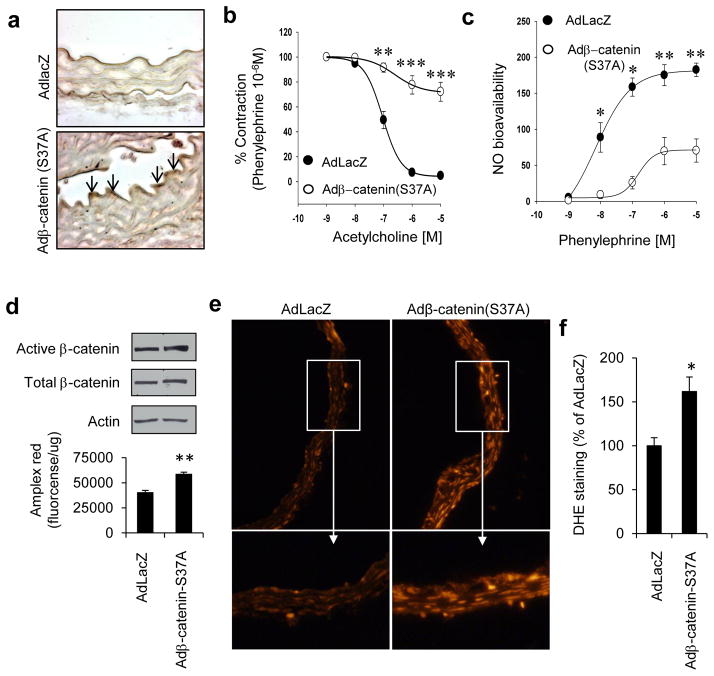
To explore the physiological relevance of Wnt signaling to vascular function, we first determined if canonical Wnt signaling impairs endothelium-dependent vasorelaxation. Incubation of mouse aortas with Wnt3a led to a significant decrease in acetylcholine-stimulated endothelium-dependent vasorelaxation and nitric oxide bioavailability, without affecting sodium nitroprusside-stimulated endothelium-independent vasorelaxation (Fig 4a & c, Supplemental Fig IIIa–b). This impairment of endothelium-dependent vasorelaxation was rescued when aortas were pre-incubated with the Wnt ligand antagonist Dkk1 (Fig 4a). We also investigated if endothelial dysfunction induced by canonical Wnt signaling is mediated by p66Shc. Wnt3a-induced decrease in endothelium-dependent vasorelaxation and NO bioavailability was rescued by shRNA-mediated knockdown of p66Shc in mouse aortas (Fig 4b & c). We further determined the effect of Wnt signaling on vascular function, independent of Wnt ligand. An adenovirus was used to express non-phosphorylatable active β-catenin (S37A) in the endothelium (Fig 5a), thus constitutively activating Wnt signaling independent of Wnt ligand. Expression of β-catenin (S37A) resulted in impairment of endothelium-dependent vasorelaxation (Fig 5b), and a decrease in vascular NO bioavailability (Fig 5c), but did not affect endothelium-independent vasorelaxation (Supplemental Fig IIIc). β-catenin (S37A) expression also increased ROS, both in endothelial cells (Fig 5d), and in the whole vessel (Fig 5e & f). These findings suggest that ROS, in addition to leading to β-catenin dephosphorylation, are also upregulated downstream of β-catenin. To seek out a potential mechanism for β-catenin-induced ROS, we examined the expression of tumor necrosis factor-α (TNFα), a target gene of canonical Wnt signaling in other cell types and experimental models25–27, and one which is well-known to promote endothelial dysfunction28. Wnt3a, as well as active β-catenin (S37A), led to expression of TNFα in endothelial cells (Fig. 3e & f). Taken together, these data show that both ligand-dependent and ligand-independent canonical Wnt signaling impairs endothelium-dependent vasorelaxation, the former via p66Shc.
Fig. 4. Canonical Wnt signaling impairs endothelium-dependent vascular relaxation via p66Shc.
a) Wnt3a decreases acetylcholine-induced endothelium-dependent vasorelaxation of mouse aortas, which is reversed by Dkk1 (n = 4 – 12 aortic rings from three mice). shRNA-mediated knockdown of p66Shc with Adp66ShcRNAi in mouse aortas rescues Wnt3a-induced decrease in b) endothelium-dependent vasorelaxation, and c) bioavailable NO. (n = 5 – 9 aortic rings from three mice). AdLacZ was used as control. All values are shown as Mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. Control, # P < 0.05, ## P < 0.01, ### P < 0.001 vs. Wnt3a.
Fig. 5. Active β-catenin induces endothelial and vascular oxidative stress, and impairs endothelium-dependent vasorelaxation.
Expression of active (non-phosphorylatable) β-catenin (S37A) in endothelium of mouse aortas (a) decreases b) endothelium-dependent vasorelaxation, and c) NO bioavailability (n = 4 – 12 aortic rings from three mice). AdLacZ was used as control. d) β-catenin (S37A) induces H2O2 (Amplex red fluorescence) in HUVEC. e–f) β-catenin (S37A) induces ROS (DHE fluorescence) in mouse aortas. Representative photomicrographs and immunoblots are shown. Adβ-catenin (S37A): adenovirus expressing β-catenin (S37A). All values are shown as Mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. AdLacZ.
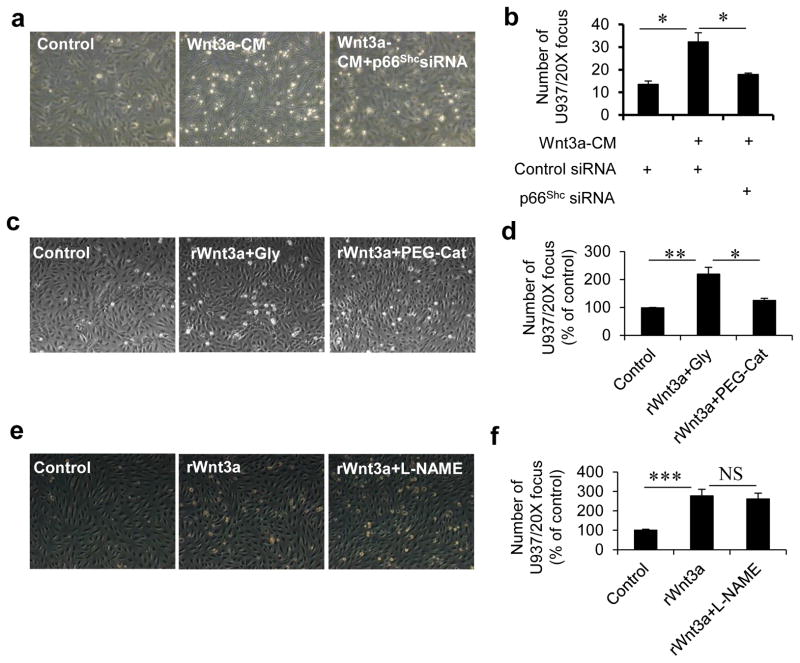
Impairment of endothelium-dependent vasorelaxation is just one manifestation of endothelial dysfunction. We measured an additional readout of endothelial dysfunction: adhesion of leukocytes. Wnt3a increased adhesion of U937 monocytic cells to endothelial cells which was suppressed by knocking down p66Shc (Fig 6a & b) and by the antioxidant PEG-catalase (Fig 6c & d). However, Wnt3a-induced increase of monocyte adhesion was not affected by inhibition of nitric oxide synthase with L-NAME (Fig 6e & f). These data show that canonical Wnt signaling also contributes to the inflammatory milieu of a dysfunctional endothelium, leading to adhesion of leukocytes.
Fig. 6. Canonical Wnt signaling stimulates adhesion of monocytes to endothelial cells via p66Shc and ROS.
a) Representative photographs showing Wnt3a-CM-stimulated adhesion of U937 monocytic cells to HUVEC, which is rescued by siRNA-mediated knockdown of p66Shc (n=3). b) Quantification of adherent monocytes in (a). c) Representative photographs showing effect of catalase on Wnt3a-stimulated adhesion of monocytes to HUVEC (n=3). Glycerol vehicle (Gly) was used as control. d) Quantification of adherent monocytes in (c). e) Representative photographs showing effect of NOS inhibitor L-NAME on Wnt3a-stimulated adhesion of monocytes to HUVEC (n=3). f) Quantification of adherent monocytes in (e). All the values are shown as Mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001, NS=not significant. rWnt3a: recombinant Wnt3a.
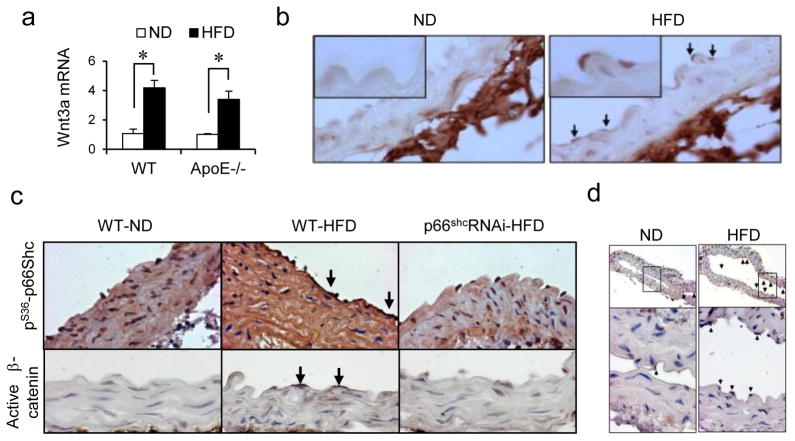
To explore the potential role of the Wnt-p66Shc axis in an in vivo model of endothelial dysfunction, we chose a high-fat diet feeding-stimulated mouse model of hypercholesterolemia in which p66Shc is known to contribute to vascular oxidative stress22. High-fat diet feeding of wild-type mice for 16 weeks impaired endothelium-dependent vasorelaxation and NO bioavailability (Supplemental Fig IV), and increased vascular and endothelial Wnt3a expression (Fig 7a & b). A similar increase in vascular Wnt3a was observed in ApoE−/− mice on a high-fat diet (Fig 7a). High-fat diet feeding also stimulated dephosphorylation of β-catenin (Fig 7c) and expression of c-myc, a target gene of β-catenin-mediated transcription (Fig 7d), in the endothelium. This signifies activation of the canonical Wnt pathway in the endothelium with high-fat diet feeding. Moreover, high-fat diet feeding stimulated serine 36 phosphorylation of p66Shc in the endothelium as well as the media (Fig 7c). To determine the contribution of p66Shc to canonical Wnt signaling in the intact vasculature, we also subjected mice expressing a p66Shc shRNA transgene (p66ShcRNAi) to high-fat diet feeding. In these mice, which are protected from vascular fatty streak formation (Supplemental Fig V), dephosphorylation of β-catenin, as well as phosphorylation of p66Shc, was significantly decreased (Fig 7c), illustrating an important role for p66Shc in high-fat diet-stimulated canonical Wnt signaling in the vasculature.
Fig. 7. High-fat diet activates endothelial canonical Wnt signaling via p66Shc.
(a) High-fat diet (HFD) feeding increases Wnt3a mRNA in whole aorta of wild-type C57Bl/6 (WT) and ApoE−/− mice (n = 3). (b) Representative photomicrographs showing increased immunostaining for Wnt3a in aortic endothelium of WT HFD-fed mice. ND=normal diet. c) High-fat diet feeding stimulates dephosphorylation of vascular β-catenin and serine 36 phosphorylation of p66Shc. Dephosphorylation of endothelial β-catenin is suppressed in mice transgenic for p66Shc shRNA (p66ShcRNAi). d) High-fat diet feeding increases endothelial expression of β-catenin target gene c-myc in WT mice. All the values are shown as Mean ± SEM. * P < 0.05 vs. indicated group.
Discussion
Emerging evidence suggests that both oxidative and nitrosative stress plays a part in Wnt signaling. NADPH oxidase 1 (NOX1) modulates Wnt signaling in progenitor cells of the colon29; NOX1-mediated ROS lead to dissociation of the Wnt signaling mediator Dvl from nucleoredoxin, thus promoting Wnt signaling30; nitrosative stress is important in Wnt activation in diabetic nephropathy31; and Wnt signaling is associated with oxidative stress in retinal pigment epithelium27, and diabetic retinopathy32. Given the role of specific NOXs in endothelial dysfunction33, it is not surprising that NOX-4 is involved in canonical Wnt signaling in endothelial cells. However, in certain tissues, oxidative stress has also been shown to inhibit canonical Wnt signaling. Pro-osteogenic Wnt signaling in bone is suppressed by oxidative stress34. Thus, the role of ROS in promoting or inhibiting Wnt signaling may be cell and tissue-specific.
Although the role of Wnt signaling in endothelium-dependent vasorelaxation has not been previously examined, Wnt signaling has been associated with vasculopathies and vascular inflammation. Specific Wnt ligands promote vascular smooth muscle cell proliferation and are upregulated in the neo-intima of injured vessels35. In addition, activity of the Wnt co-receptors Fizzled4 and Lrp5 is upregulated in neovascularization associated with oxygen-induced proliferative retinopathy36, and activation of the canonical Wnt pathway in monocytes with Wnt3a leads to monocyte adhesion to endothelial cells but decreased trans-endothelial migration37. Furthermore, Wnt signaling may also play a part in the pathogenesis of pulmonary hypertension, as there is exaggerated expression of β-catenin target genes PDGF receptor and axin in smooth muscle cell overgrowth of pulmonary arterial hypertension lesions38. In contrast to a role for Wnt signaling in promoting vascular disease, there is equal evidence that, in the proper context, it is vital for vascular homeostasis. A loss of function mutation in human Lrp6 is associated with early coronary artery disease5, 6, and Lrp6 knockout mice on an ApoE−/− background develop accelerated atherosclerosis in response to high-fat diet4. In addition, Dkk1, a Wnt inhibitor, is upregulated in atherosclerosis and promotes inflammatory interaction between platelets and endothelial cells39. These contrasting effects of Wnt signaling on the vasculature may be partly explained by the fact that, though promoting vascular smooth muscle cell proliferation and endothelial dysfunction through direct effects, Wnt signaling is nevertheless important for systemic glucose and lipid regulation5, 40.
Our data demonstrate that JNK is principally responsible for Wnt3a-stimulated p66Shc phosphorylation. JNK participates in both canonical and non-canonical Wnt signaling41, 42, and studies suggest that activation of non-canonical signaling antagonizes canonical Wnt signaling43, 44. Wnt3a is generally considered a typical ligand for canonical Wnt signaling but it has been shown to activate non-canonical signaling as well45, 46. Moreover, Wnt3a can activate JNK and JNK mediates nuclear localization of β-catenin42. Thus, there is significant crosstalk between canonical and non-canonical signaling and some promiscuity of the mediators responsible for these two forms of Wnt signaling. Although we did not directly examine other readouts of non-canonical signaling, given that JNK mediates Wnt3a-stimulated p66Shc phosphorylation, and is also involved in non-canonical signaling, it would not be surprising if p66Shc plays some part in the non-canonical Wnt pathway in the endothelium.
High-fat diet-feeding activates the tumor suppressor p5347, and p53 impairs endothelium-dependent vasorelaxation15, 48. In addition, endothelial p66Shc is transcriptionally upregulated by p5315. Although our data do not shed light on the role of p53 in hypercholesterolemia-stimulated Wnt signaling in the vasculature, it is noteworthy that p53 is upregulated by Wnt/β-catenin signaling and retards Wnt3a-stimulated proliferation and differentiation of mesenchymal progenitor cells49. P53 also feeds back to suppress Wnt signaling through microRNA-34-induced downregulation of genes of the Wnt pathway50, suggesting that its role, if any, in canonical Wnt signaling in the endothelium may be complex.
ROS have myriad effects on cellular phenotypes. Our data suggest that in endothelial cells, ROS are important upstream mediators, but may also act as downstream effectors of canonical Wnt signaling. This dual role of ROS may be mutually re-enforcing, with p66Shc at the center of this relationship (Supplemental Fig. VI). Suppression of β-catenin expression and β-catenin-mediated transcription by antioxidants indicates an essential role for ROS in upstream transduction of canonical Wnt signaling in endothelial cells. In addition, ROS induced by active β-catenin in endothelial cells and the vasculature, independent of Wnt ligand, suggests that ROS also function as downstream effectors of endothelial dysfunction triggered by canonical Wnt signaling. In this regard, endothelial TNFα upregulated through β-catenin-mediated transcription may be one effector which leads to endothelial dysfunction in a paracrine manner. Other Wnt target genes associated with endothelial dysfunction, such as endothelin-151, may be similarly regulated, and function in a similar manner, in the vasculature. The role of p66Shc in transducing ROS-induced Wnt signaling invokes the additional possibility that external oxidative stresses could impair endothelial function via p66Shc-mediated activation of the canonical Wnt pathway.
In conclusion, our findings provide evidence for a novel and direct effect of canonical Wnt signaling on endothelial function, and identify p66Shc as an important player in endothelial dysfunction induced by canonical Wnt signaling. In addition, concomitant activation of canonical Wnt signaling and phosphorylation of p66Shc in the endothelium with high-fat diet, together with the requirement of p66Shc in high-fat diet-stimulated endothelial β-catenin dephosphorylation, suggests that the Wnt-p66Shc axis is important in vascular oxidative stress and endothelial dysfunction of hypercholesterolemia.
Supplementary Material
Acknowledgments
We thank Jian-xing Ma for the Adβ-catenin (S37A), Toren Finkel for the p66Shc plasmid constructs, and Randall Moon for the TOP-FLASH and FOP-FLASH reporter plasmids.
Sources of Funding
The University of Iowa Professorship in Cardiovascular Medicine, R01 HL070929, R01 HL094959, and R21 HL098892.
Non-standard Abbreviations and Acronyms
- Ach
acetylcholine
- BAECs
bovine aortic endothelial cells
- DKK-1
dickkopf-1
- Dvl
disheveled
- Fz
Frizzled receptor
- HEK
human embryonic kidney
- HFD
high-fat diet
- HUVECs
human umbilical vein endothelial cells
- JNK
c-jun N terminal kinase
- L-NAME
N-nitro-L-arginine methyl ester
- LRP-5/6
LDL-receptor-related protein-5/6
- MEK
mitogen activated protein kinase kinase
- NAC
N-acetyl cysteine
- ND
normal diet
- NO
nitric oxide
- PE
phenylephrine
- SNP
sodium nitroprusside
- TCF/LEF
T -cell factor/lymphoid-enhancer binding factor
- Wnt3a-CM
Wnt3a-conditioned media
- NOX-4
NADPH oxidase-4
Footnotes
Conflicts of interest
None declared.
References
- 1.Cadigan KM, Peifer M. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harbor perspectives in biology. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Developmental cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein e. J Biol Chem. 2003;278:11331–11336. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- 5.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. Lrp6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarzani R, Salvi F, Bordicchia M, Guerra F, Battistoni I, Pagliariccio G, Carbonari L, Dessi-Fulgheri P, Rappelli A. Carotid artery atherosclerosis in hypertensive patients with a functional ldl receptor-related protein 6 gene variant. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2011;21:150–156. doi: 10.1016/j.numecd.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of wnt signaling. Developmental dynamics: an official publication of the American Association of Anatomists. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 8.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a wnt-responsive signal transduction pathway in primary endothelial cells. Biochemical and biophysical research communications. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 10.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, Lompre AM, Nadaud S. The wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011;10:220–232. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 11.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 12.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 13.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 14.Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M. P66shc-generated oxidative signal promotes fat accumulation. J Biol Chem. 2008;283:34283–34293. doi: 10.1074/jbc.M804362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CS, Jung SB, Naqvi A, Hoffman TA, DeRicco J, Yamamori T, Cole MP, Jeon BH, Irani K. P53 impairs endothelium-dependent vasomotor function through transcriptional upregulation of p66shc. Circ Res. 2008;103:1441–1450. doi: 10.1161/CIRCRESAHA.108.181644. [DOI] [PubMed] [Google Scholar]
- 16.Kim CS, Kim YR, Naqvi A, Kumar S, Hoffman TA, Jung SB, Kumar A, Jeon BH, McNamara DM, Irani K. Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovasc Res. 2011;92:466–475. doi: 10.1093/cvr/cvr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YR, Kim CS, Naqvi A, Kumar A, Kumar S, Hoffman TA, Irani K. Epigenetic upregulation of p66shc mediates low-density lipoprotein cholesterol-induced endothelial cell dysfunction. Am J Physiol Heart Circ Physiol. 2012;303:H189–196. doi: 10.1152/ajpheart.01218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, Jung SB, Kim CS, Irani K. Sirt1 deacetylates ape1 and regulates cellular base excision repair. Nucleic Acids Res. 2010;38:832–845. doi: 10.1093/nar/gkp1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, Zhang R, Cai H, Liu DP, Liang CC. Repression of p66shc expression by sirt1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Padura I, de Nigris F, Migliaccio E, Mansueto G, Minardi S, Rienzo M, Lerman LO, Stendardo M, Giorgio M, De Rosa G, Pelicci PG, Napoli C. P66shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein e knockout mice. Endothelium: journal of endothelial cell research. 2008;15:276–287. doi: 10.1080/10623320802487791. [DOI] [PubMed] [Google Scholar]
- 21.Franzeck FC, Hof D, Spescha RD, Hasun M, Akhmedov A, Steffel J, Shi Y, Cosentino F, Tanner FC, von Eckardstein A, Maier W, Luscher TF, Wyss CA, Camici GG. Expression of the aging gene p66shc is increased in peripheral blood monocytes of patients with acute coronary syndrome but not with stable coronary artery disease. Atherosclerosis. 2012;220:282–286. doi: 10.1016/j.atherosclerosis.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, Berkowitz DE, Irani K. Apurinic/apyrimidinic endonuclease 1 regulates endothelial no production and vascular tone. Circ Res. 2004;95:902–910. doi: 10.1161/01.RES.0000146947.84294.4c. [DOI] [PubMed] [Google Scholar]
- 24.Khanday FA, Santhanam L, Kasuno K, Yamamori T, Naqvi A, Dericco J, Bugayenko A, Mattagajasingh I, Disanza A, Scita G, Irani K. Sos-mediated activation of rac1 by p66shc. The Journal of cell biology. 2006;172:817–822. doi: 10.1083/jcb.200506001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiyama A, Yokoyama K, Nukaga T, Sakai D, Mochida J. A complex interaction between wnt signaling and tnf-alpha in nucleus pulposus cells. Arthritis research & therapy. 2013;15:R189. doi: 10.1186/ar4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. Wnt signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123:2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical wnt pathway in age-related macular degeneration. Investigative ophthalmology & visual science. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spillmann F, Van Linthout S, Miteva K, Lorenz M, Stangl V, Schultheiss HP, Tschope C. Lxr agonism improves tnf-alpha-induced endothelial dysfunction in the absence of its cholesterol-modulating effects. Atherosclerosis. 2014;232:1–9. doi: 10.1016/j.atherosclerosis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, Skurnik D, Grodet A, Fay M, Biard D, Lesuffleur T, Deffert C, Moreau R, Groyer A, Krause KH, Daniel F, Ogier-Denis E. Nadph oxidase 1 modulates wnt and notch1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, Kamata T. A crucial role for nox 1 in redox-dependent regulation of wnt-beta-catenin signaling. FASEB J. 2012;26:2049–2059. doi: 10.1096/fj.11-196360. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Li J, Cheng R, Chen Y, Lee K, Hu Y, Yi J, Liu Z, Ma JX. Nitrosative stress plays an important role in wnt pathway activation in diabetic retinopathy. Antioxidants & redox signaling. 2013;18:1141–1153. doi: 10.1089/ars.2012.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou T, Zhou KK, Lee K, Gao G, Lyons TJ, Kowluru R, Ma JX. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type mmtv integration site (wnt) pathway in a rat model of diabetic retinopathy. Diabetologia. 2011;54:459–468. doi: 10.1007/s00125-010-1943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of nadph oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/beta-catenin signaling induces vsmc proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124:1871–1881. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tickenbrock L, Schwable J, Strey A, Sargin B, Hehn S, Baas M, Choudhary C, Gerke V, Berdel WE, Muller-Tidow C, Serve H. Wnt signaling regulates transendothelial migration of monocytes. Journal of leukocyte biology. 2006;79:1306–1313. doi: 10.1189/jlb.0905539. [DOI] [PubMed] [Google Scholar]
- 38.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin c/pdgfr pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 40.Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, Sotoudeh M, Malekzadeh R, Sherwin RS, Mani A. Lrp6 enhances glucose metabolism by promoting tcf7l2-dependent insulin receptor expression and igf receptor stabilization in humans. Cell metabolism. 2013;17:197–209. doi: 10.1016/j.cmet.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu W, Chen L, Kassem M. Activation of non-canonical wnt/jnk pathway by wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochemical and biophysical research communications. 2011;413:98–104. doi: 10.1016/j.bbrc.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao G, Tao Q, Kofron M, Chen JS, Schloemer A, Davis RJ, Hsieh JC, Wylie C, Heasman J, Kuan CY. Jun nh2-terminal kinase (jnk) prevents nuclear beta-catenin accumulation and regulates axis formation in xenopus embryos. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16313–16318. doi: 10.1073/pnas.0602557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huelsken J, Behrens J. The wnt signalling pathway. Journal of cell science. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 45.Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical wnt signaling through g protein-linked pkcdelta activation promotes bone formation. Developmental cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samarzija I, Sini P, Schlange T, Macdonald G, Hynes NE. Wnt3a regulates proliferation and migration of huvec via canonical and non-canonical wnt signaling pathways. Biochemical and biophysical research communications. 2009;386:449–454. doi: 10.1016/j.bbrc.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 47.Xu S, Jiang B, Hou X, Shi C, Bachschmid MM, Zang M, Verbeuren TJ, Cohen RA. High-fat diet increases and the polyphenol, s17834, decreases acetylation of the sirtuin-1-dependent lysine-382 on p53 and apoptotic signaling in atherosclerotic lesion-prone aortic endothelium of normal mice. Journal of cardiovascular pharmacology. 2011;58:263–271. doi: 10.1097/FJC.0b013e3182239eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Kim CS, Hoffman TA, Naqvi A, Dericco J, Jung SB, Lin Z, Jain MK, Irani K. P53 impairs endothelial function by transcriptionally repressing kruppel-like factor 2. Arterioscler Thromb Vasc Biol. 2011;31:133–141. doi: 10.1161/ATVBAHA.110.215061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng X, Yang L, Chang H, Dai G, Wang F, Duan X, Guo L, Zhang Y, Chen G. Wnt/beta-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PloS one. 2014;9:e97283. doi: 10.1371/journal.pone.0097283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim NH, Kim HS, Kim NG, Lee I, Choi HS, Li XY, Kang SE, Cha SY, Ryu JK, Na JM, Park C, Kim K, Lee S, Gumbiner BM, Yook JI, Weiss SJ. P53 and microrna-34 are suppressors of canonical wnt signaling. Science signaling. 2011;4:ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim TH, Xiong H, Zhang Z, Ren B. Beta-catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005;24:597–604. doi: 10.1038/sj.onc.1208237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.