Abstract
STUDY QUESTION
Are all components of the peroxiredoxins (PRDXs) system important to control the levels of reactive oxygen species (ROS) to maintain viability and DNA integrity in spermatozoa?
SUMMARY ANSWER
PRDX6 is the primary player of the PRDXs system for maintaining viability and DNA integrity in human spermatozoa.
WHAT IS KNOWN ALREADY
Mammalian spermatozoa are sensitive to high levels of ROS and PRDXs are antioxidant enzymes proven to control the levels of ROS generated during sperm capacitation to avoid oxidative damage in the spermatozoon. Low amounts of PRDXs are associated with male infertility. The absence of PRDX6 promotes sperm oxidative damage and infertility in mice.
STUDY DESIGN, SIZE, DURATION
Semen samples were obtained over a period of one year from a cohort of 20 healthy non-smoking volunteers aged 22–30 years old.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Sperm from healthy donors was incubated for 2 h in the absence or presence of inhibitors for the 2-Cys PRDXs system (peroxidase, reactivation system and NADPH-enzymes suppliers) or the 1-Cys PRDX system (peroxidase and calcium independent-phospholipase A2 (Ca2+-iPLA2) activity). Sperm viability, DNA oxidation, ROS levels, mitochondrial membrane potential and 4-hydroxynonenal production were determined by flow cytometry.
MAIN RESULTS AND THE ROLE OF CHANCE
We observed a significant decrease in viable cells due to inhibitors of the 2-Cys PRDXs, PRDX6 Ca2+-iPLA2 activity or the PRDX reactivation system compared to controls (P ≤ 0.05). PRDX6 Ca2+-iPLA2 activity inhibition had the strongest detrimental effect on sperm viability and DNA oxidation compared to controls (P ≤ 0.05). The 2-Cys PRDXs did not compensate for the inhibition of PRDX6 peroxidase and Ca2+-iPLA2 activities.
LARGE SCALE DATA
Not applicable.
LIMITATIONS, REASONS FOR CAUTION
Players of the reactivation systems may differ among mammalian species.
WIDER IMPLICATIONS OF THE FINDINGS
The Ca2+-iPLA2 activity of PRDX6 is the most important and first line of defense against oxidative stress in human spermatozoa. Peroxynitrite is scavenged mainly by the PRDX6 peroxidase activity. These findings can help to design new diagnostic tools and therapies for male infertility.
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by The Canadian Institutes of Health Research (MOP 133661 to C.O.), and by RI MUHC—Desjardins Studentship in Child Health Research awarded to M.C.F. The authors have nothing to disclose.
Keywords: 1-Cys peroxiredoxin, 2-Cys peroxiredoxins, peroxiredoxin 6, oxidative stress, DNA oxidation, sperm viability, reactive oxygen species
Introduction
Human spermatozoa are extremely sensitive to oxidative stress, a condition resulting of excessive production of reactive oxygen species (ROS) and/or a decrease in the antioxidant defense system (Gagnon et al., 1991). The oxidative stress can cause an increase in lipid and DNA oxidation and a decrease in sperm motility, mitochondrial activity and viability, leading to infertility (Aitken and Baker, 2006; Koppers et al., 2008). However, ROS in low amounts are also beneficial molecules involved in cell signaling and the capacitation process for acquired fertilizing ability (O’Flaherty et al., 2006a). Therefore, it is necessary that the presence of an antioxidant enzyme system in the spermatozoon will balance the levels of ROS to ensure its normal performance. Our laboratory is a pioneer in characterizing a family of antioxidant enzymes called Peroxiredoxins (PRDXs) that are present in all subcellular compartments of human spermatozoa and can scavenge a wide variety of ROS (O’Flaherty, 2014a,b). They can be classified into two groups according to the cysteine (Cys) residues present in their active site: 2-Cys PRDXs, including PRDX1 to 5, and 1-Cys PRDX, represented by PRDX6 (Rhee et al., 2005). Interestingly, only PRDX6 has peroxidase, calcium-independent phospholipase A2 (Ca2+iPLA2) and lysophospholipid acyltransferase (LPCAT) activities (Chen et al., 2000; Fisher, 2017). This isoform provides a complete system for the repair of peroxidized cell membranes. PRDX6 can reduce peroxidized cell membrane phospholipids by using peroxidase activity and replace the oxidized sn-2 fatty acyl group through hydrolysis/re-acylation by using PLA2 and LPCAT activities (Fisher, 2017). Moreover, PRDXs have proven to be important modulators in redox signaling (Wood et al., 2003a,b) and to have protective effects emphasized by the fact that infertile men have significantly lower levels of PRDXs in semen and higher thiol oxidation, particularly of PRDX6, affecting enzymatic activity (Gong et al., 2012).
The presence of Cys residues in the active site of 2-Cys PRDXs makes them a direct target for H2O2 and other ROS such as tert-butyl hydroperoxide and peroxynitrite (ONOO−) and leads to thiol oxidation that will inactivate PRDXs by forming a disulfide bond between the two Cys (O’Flaherty and de Souza, 2011; O’Flaherty, 2014a, b; Liu and O’Flaherty, 2017). In somatic cells, it is necessary for the activity of the thioredoxin- (TRX) thioredoxin reductase (TRD) system (Wood et al., 2003a,b) to reduce the disulfide groups formed and therefore reactivate 2-Cys PRDXs. Moreover, the TRX/TRD system requires reducing equivalents in the form of NADPH to accomplish its biological role. In human spermatozoa, G6PDH is the known provider of NAPDH (Sarkar et al., 1977) but in bull spermatozoa, the NADPH supply is accomplished by the NADP-dependent isocitrate dehydrogenase (NADP-ICDH) due to the lack of G6PDH (O’Flaherty et al., 2006b). Another potential provider of NADPH, yet to be confirmed is the malic enzyme (ME), since in-vitro studies have detected its activity in human spermatozoa and other species (Mounib, 1974; Niedzwiecka et al., 2017).
In the case of PRDX6 with 1 Cys residue in its active site, the interaction with ROS produces a sulfenic acid group that has to be reduced, involving the glutathione S-transferases pi (GSTpi) and glutathione (GSH) (Zhou et al., 2013). Since human spermatozoa are highly sensitive to increased levels of ROS, it is possible that this sensitivity could be due to alteration of one or more players of the PRDXs antioxidant system. Therefore, we aim to determine the impact of inhibiting PRDXs and the different players involved in their reactivation on the production of different ROS and on viability, DNA oxidation and mitochondrial function in human spermatozoa.
Materials and Methods
Materials
All probes used in this study (calcein AM, Sytox Blue, MitoSOX-Red, JC-1, H2DCFDA, PI) were purchased from Life Technologies (Burlington, Ontario, Canada) as well as the secondary antibody, goat anti-mouse FITC-conjugated antibody. Percoll was obtained from GE Healthcare (Baie d’Urfe, QC, Canada). The mouse monoclonal to 4-hydroxynonenal (HNE J-2) and the mouse monoclonal DNA/RNA Damage (FITC) (clone 15A3) were purchased from Abcam (Toronto, Ontario, Canada) and the 8-hydroxy-2′-deoxyguanosine were from Sigma-Aldrich (Milwaukee, WI, USA). For the inhibitors used in this study, MJ33, bromopyruvic acid, ezatiostat and S-hexylgluthathione were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Conoidin A, auranofin, dehydroepiandrosterone (DHEA), oxalomalic acid and ethacrynic acid were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Sperm sample preparation
Semen samples were obtained from 20 non-smoking healthy volunteers (22–30 years old) after three days of sexual abstinence. This study was approved by Ethics Committee of the McGill University Health Centre as we previously described (Lee et al., 2017) and followed the suggested guidelines for human semen studies (Sanchez-Pozo et al., 2013; Bjordahl et al., 2016). Donors who participated in this study were from our cohort of healthy donors, recruited at the Royal Victoria Hospital, Montreal, Quebec, Canada. The exclusion criteria were the presence of illness or whether the taking of any medication. After collection, semen samples were incubated at 37°C for 30 min to induce liquefaction and sperm motility was analyzed using a computer-assisted semen analysis system (CASA) (Sperm vision HR software v1.01, Penetrating Innovations, Ingersoll, Ontario, Canada) (Suppl. Table S1). Samples were centrifuged on a 4-layer Percoll gradient (95–65% to 40–20%) to select the highly motile spermatozoa. This procedure has been used to select highly motile spermatozoa without increasing ROS levels (Iwasaki and Gagnon, 1992). Spermatozoa were recovered from the 95% layer and the 65–95% interface and concentration was determined using a Neubauer chamber. Samples were then diluted to 50 × 106 cell/ml in Biggers, Whitten and Whittingham (BWW, pH 7.8) medium (Biggers et al., 1971) and used for experimentation. For all experiments, 1 million cells were incubated for 2 h at 37°C with or without the inhibitors described in Table I. We then determine the impact of these inhibitors on sperm viability, ROS production and DNA oxidation as described below.
Table I.
Description of the inhibitors used in this study to inhibit the PRDXs systems.
| Inhibitor | Chemical name | Target | References |
|---|---|---|---|
| Conoidin A | 2,3-bis(bromomethyl)-quinoxaline 1,4-dioxide | 2-Cys PRDXs | Liu et al. (2010), Brizuela et al. (2014), Ryu et al. (2017) |
| Auranofin | S-triethylphosphinegold(I)-2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranoside | Thioredoxin reductase | Gromer et al. (1998), Rigobello et al. (2005), Cox et al. (2008), Hwang-Bo et al. (2017) |
| DHEA | Dehydroepiandrosterone | Glucose-6-phosphate dehydrogenase | Raineri and Levy (1970), Heffner and Milam (1990), Miraglia et al. (2010) |
| Oxalomalic Acid | 3-Carboxy-3-deoxy-2-pentulosaric acid | NADP-dependent isocitrate dehydrogenase | O’Flaherty et al. (2006b), Breininger et al. (2017) |
| 3BP | 3 Bromopyruvate | Malic enzyme | Chang and Hsu (1973), Satterlee and Hsu (1991), Singh et al. (2008) |
| Ezatiostat | (2R)-L-γ-glutamyl-S-(phenylmethyl)-L-cysteinyl-2-phenyl-glycine-1,3-diethyl ester | Glutathione S-transferasePi | Liu et al. (2014), Mahadevan and Sutton (2015), Crawford and Weerapana (2016) |
| S-HexylGluthathione | (2S)-2-Amino-5-[[(2R)-1-(carboxymethylamino)-3-hexylsulfanyl-1-oxopropan-2-yl]amino]-5-oxopentanoic acid | Mitochondrial membrane-bound glutathione transferase(s) (mtMGST1). | Erhardt and Dirr (1996), Gopalakrishnan et al. (1998), Ulziikhishig et al. (2010) |
| Ethacrynic acid | (2,3-Dichloro-4-(2-methylene-1-oxobutyl)phenoxy)acetic acid | Gluthathione adduct | Ploemen et al. (1993), Gopalakrishnan et al. (1998) |
| MJ33 | 1-Hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol lithium | PRDX6 Ca2+-iPLA2 activity | Fisher et al. (1992), Moawad et al. (2017) |
Sperm viability/cytotoxicity
We determined cell viability/cytotoxicity based on the simultaneous determination of live and dead cells with two probes Calcein AM and Sytox Blue that measure intracellular esterase activity and plasma membrane integrity, respectively. Briefly, spermatozoa were incubated at 37°C for 2 h in BWW with or without the inhibitors of the PRDXs system described in Table I and Calcein was added at a final concentration of 2 μM for 15 min. Then, spermatozoa were washed and resuspended in isotonic HEPES-balanced saline (HBS) containing 0.2 μM Sytox Blue in order to quantify the percentage of viable and dead cells by MACSQuant Analyzer flow cytometer (Miltenyi Biotec, Inc., Auburn, CA, USA), by gating the sperm population according to the side and forward scatter density plot. Three different populations were distinguished based on intracellular esterase activity (FITC+) and plasma membrane integrity (VioBlue−): live (FITC+/VioBlue−), dying (FITC+/VioBlue+) and dead (FITC−/VioBlue+) cells. Representative scatterplots with or without the specific inhibitors of the PRDXs system are shown in Supplementary Fig. S1. A minimum of 10 000 events were analyzed.
Sperm DNA oxidation
In order to determine sperm DNA oxidation by assessing the level of 8-hydroxy, 2-deoxyguanosine (8OHdG), 1 million cells were incubated for 2 h at 37°C with or without the inhibitors described in Table I. In another set of experiments, we incubated spermatozoa with or without the mentioned inhibitors for 90 min at 37°C and then added 1 mM H2O2 for an additional 30 min. The positive control was made by incubating spermatozoa with 4 mM H2O2 under the same incubation conditions. The levels of 8OHdG were determined as previously described (Cambi et al., 2013). After treatments, spermatozoa were fixed, washed and incubated with 2 mM DTT in PBS for 45 min at 37°C, to allow the chromatin to decondense. After a wash, cells were incubated with the anti-8OHdG FITC-conjugated (1:1000) antibody in 0.1% Triton X-100 and sodium citrate for 30 min at 37°C in the dark. Then, samples were washed and stained with propidium iodide (PI) to quantify the percentages of FITC positive cells out of the PI-stained cells with the B1 and B3 channels of the 488 nm argon laser using the MACSQuant Analyzer flow cytometry. The specificity of the antibody was confirmed by incubating spermatozoa with the anti-8OHdG antibody, pre-incubated with 8OHdG at 1000-fold with respect to the antibody as previously described (Cambi et al., 2013).
ROS production
Superoxide anion (O2•−) production was measured using MitoSOX-Red, a lipid-soluble cation that selectively targets mitochondria as previously described (Koppers et al., 2008). It is rapidly oxidized by O2•− only and fluoresces red upon binding to nucleic acid. After the treatment with the specific inhibitors, spermatozoa were incubated with MitoSOX-Red and calcein together for 15 min at 37°C at a final concentration of 2 μM for each probe. Then spermatozoa were washed and resuspended in 100 μL of HBS and analyzed by flow cytometry as described above. The positive control for MitoSOX-Red labeling was prepared by incubating a sperm aliquot with 40 μM of Antimycin A for 2 h at 37°C. Data were analyzed as the percentage of live cells producing mitochondrial O2•−.
The dichlorodihydrofluorescein diacetate (H2DCFDA) has high relative reactivity with hydroxyl (•OH) radicals, ONOO− and peroxyl radicals (ROO•) but not with O2•− or H2O2 (Setsukinai et al., 2003). After treatment with the inhibitors, spermatozoa were incubated for 30 min at 37°C in the presence of 10 μM H2DCFDA in PBS and 0.2 μM Sytox blue as a viability indicator. The positive control was also prepared by incubating a sperm aliquot with 100 μM of ferrous perchlorate (II) and 1 mM of H2O2 under the same incubation conditions and according to manufacturer’s protocol.
In some experiments, ONOO– levels were increased by treating spermatozoa with 40 μM of Antimycin A and 1 mM of Spermine NONOate with or without the inhibitor of the 2-Cys PRDXs and PRDX6 system (Table I). The ROS levels produced were measured using H2DCFDA (highly reactive with ONOO−) as described above. Data were analyzed as the mean fluorescence of live cells relative to the negative control.
Mitochondrial membrane potential
The assessment of the mitochondrial membrane potential (MMP) was done using the cationic carbocyanine dye JC-1, according to previous reports (Espinoza et al., 2009). Briefly, 1 million cells were incubated for 2 h with the different inhibitors of the PRDXs system and stained with 2 μM JC-1 for 15 min at 37°C. Afterwards, the cells were washed and stained with 0.2 μM Sytox Blue to exclude the dead cells when analyzed using flow cytometry. When the MMP is high, JC-1 forms J-aggregates inside the mitochondria and emits red fluorescence detected by the B2 channel of the 488 nm argon laser, while in low MMP state, it will remain in the monomer form and emit green fluorescence that is detected by the B1 channel. Results were expressed as the ratio in red/green fluorescence intensity, so mitochondrial depolarization is indicated as a decrease in this ratio.
4-Hydroxynonenal production
An aliquot of the same sample used to assess MitoSox-Red and JC-1 was taken for the detection of 4HNE after 2 h of incubation with or without conoidin A or MJ33, inhibitors of the 2-Cys PRDXs or the Ca2+iPLA2 activity of PRDX6, respectively. Following fixation with 2% Paraformaldehyde for 15 min, spermatozoa were permeabilized with 0.1% Triton X-100 and sodium citrate and incubated with the anti-4HNE antibody (1:50) overnight at 4°C. The next day, samples were washed with 0.1% Triton X-100 PBS and incubated for 1 h at 37°C with the goat anti-mouse FITC-conjugated antibody (1:50). After washing with PBS, the percentages of FITC positive cells were quantified on the MACSQuant Analyzer flow cytometry.
Determination of lipid peroxidation
Lipid peroxidation levels were determined by flow cytometry using a BODIPY 581/591 C11 probe as we previously described (Lee et al., 2017; Moawad et al., 2017). Briefly, spermatozoa were incubated in BWW medium with or without the inhibitor of 2-Cys PRDXs (conoidin A) for 2 h, then washed and incubated with 5 μM BODIPY 581/591 C11 in HBSS for 30 min at 37°C in dark. A positive control was prepared under the same conditions with 40 μM ferrous sulfate (FeSO4). A minimum of 10 000 events were analyzed for each sample using a MACSQuant Analyzer flow cytometer (Miltenyi Biotec, Inc., Auburn, CA, USA). Data are represented as the percentages of cells having a positive BODIPY C11 signal.
Statistical analysis
All data were presented as mean ± SEM. Normal distribution of data was confirmed using Shapiro–Wilk or Lilliefors tests by the Sigma Systat 13 software. Differences with a P-value of ≤0.05 were considered as significant. Statistical differences between groups were determined using ANOVA and Bonferroni or Tukey test, Mann Whitney test or Student’s t-test, followed by Bonferroni’s correction, as appropriate, using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA) and Sigma Systat 13 (Systat Software Inc., San Jose, CA, USA). Correlations and regression analyses were done after transformation of raw data as Log (1 + x).
Results
The inhibition of specific players of the PRDXs system decreases sperm viability or induces cytotoxicity
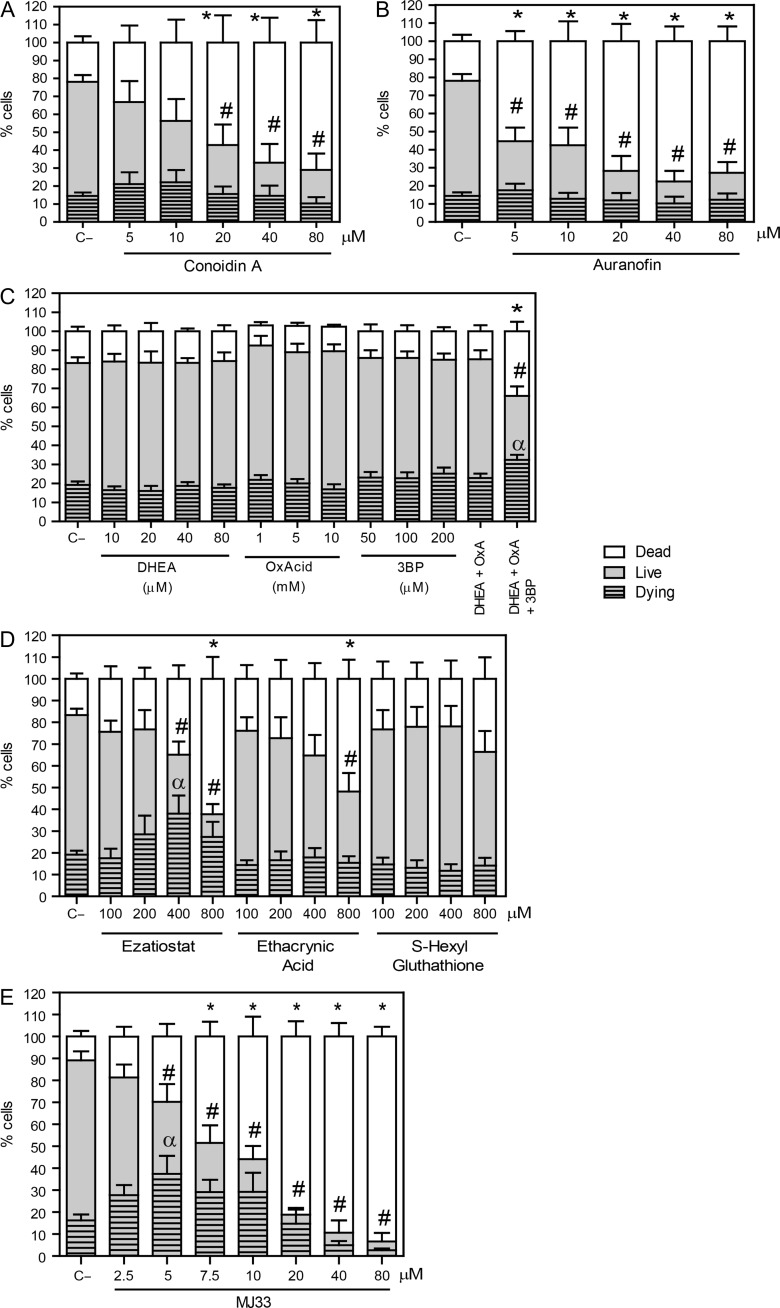
The inhibition of the 2-Cys PRDXs by conoidin A produced a decrease in live spermatozoa compared to control. The dead population significantly increased at concentration equal or higher than 20 μM conoidin A. The percentages of dying spermatozoa were similar in all conoidin A-treated samples compared to the control (Fig. 1A). We found similar findings when auranofin was present in the incubation medium (Fig. 1B), confirming the need of thioredoxin reductase to reactivate the 2-Cys PRDXs and the importance of the TRX/TRD system to reactivate 2-Cys PRDXS to maintain sperm viability. Moreover, we found that only the combination of all three inhibitors for the NADPH-suppliers enzymes had a negative impact on sperm viability by promoting the increase of dying and dead spermatozoa, suggesting that there is a compensatory mechanism when one or two enzymes are inactive (Fig. 1C).
Figure 1.
Inhibition of different players in the PRDXs decreases viability and promotes cytotoxicity. Spermatozoa were incubated in BWW medium at 37°C for 2 h with different concentrations of (A) conoidin A (2-Cys PRDXs inhibitor); (B) auranofin (TRD inhibitor); (C) DHEA, oxalomalic acid or 3 bromopyruvate (inhibitors of G6PDH, NADP-ICDH, or malic enzyme); (D) ezatiostat, ethacryinic acid and S-hexylglutathione (inhibitors of the reactivation system of the PRDX6 peroxidase activity) or (E) MJ33 (inhibitor for the PRDX6 Ca2+-iPLA2 activity). After inhibitors were washed out, spermatozoa were labeled with 2 μM Calcein-AM and 0.2 μM Sytox Blue to assess the percentages of live, dead and dying cells by MACSQuant Analyzer flow cytometry. Results are presented as the mean ± SEM of five to nine measurements performed with sperm samples from different donors. An *, # or α indicate a value significantly (P ≤ 0.05) lower than the control without inhibitors (C−) in the dead, live or dying sperm populations, respectively (ANOVA and Bonferroni post hoc test).
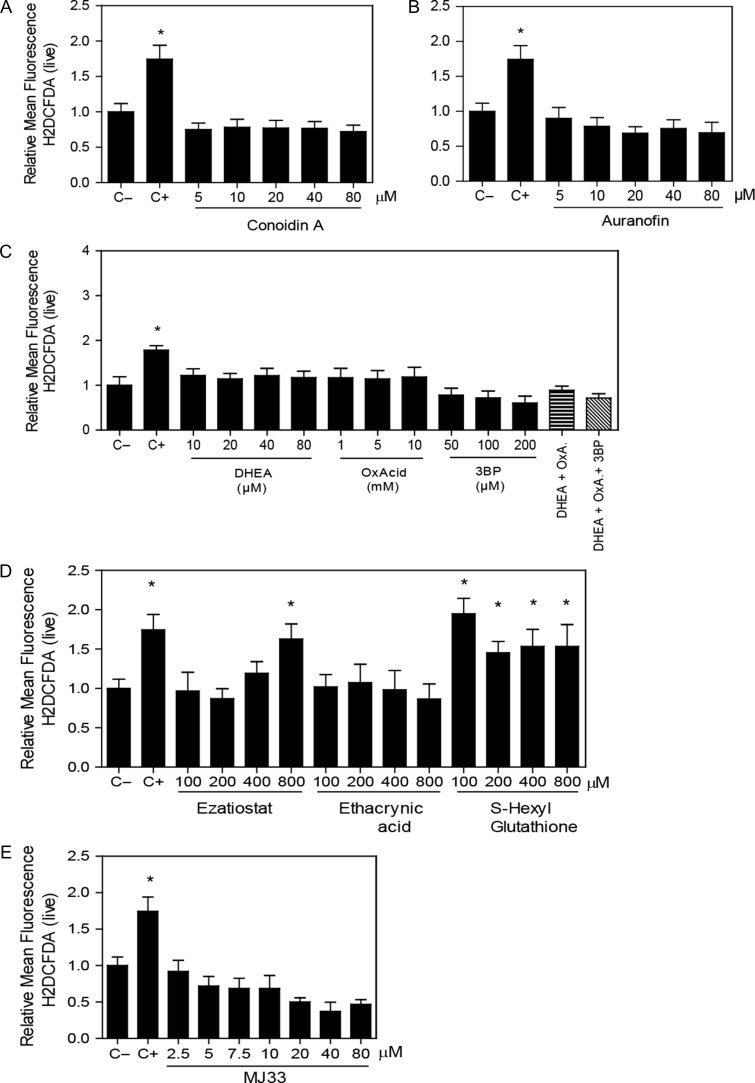
When we inhibited the reactivation system of the PRDX6 peroxidase activity, we found that only ezatiostat and ethacrynic acid were able to decrease sperm viability, the former having an increase in the population of dying spermatozoa (Fig. 1D). However, the major inhibitory effect was seen with MJ33 promoting a significant dose-dependent decrease in the percentage of viable cells, with differences in the live and dying sperm populations at 5 μM compared to the untreated control (Fig. 1E).
The inhibition of the PRDX6 Ca2+-iPLA2 activity has a high impact on sperm DNA oxidation
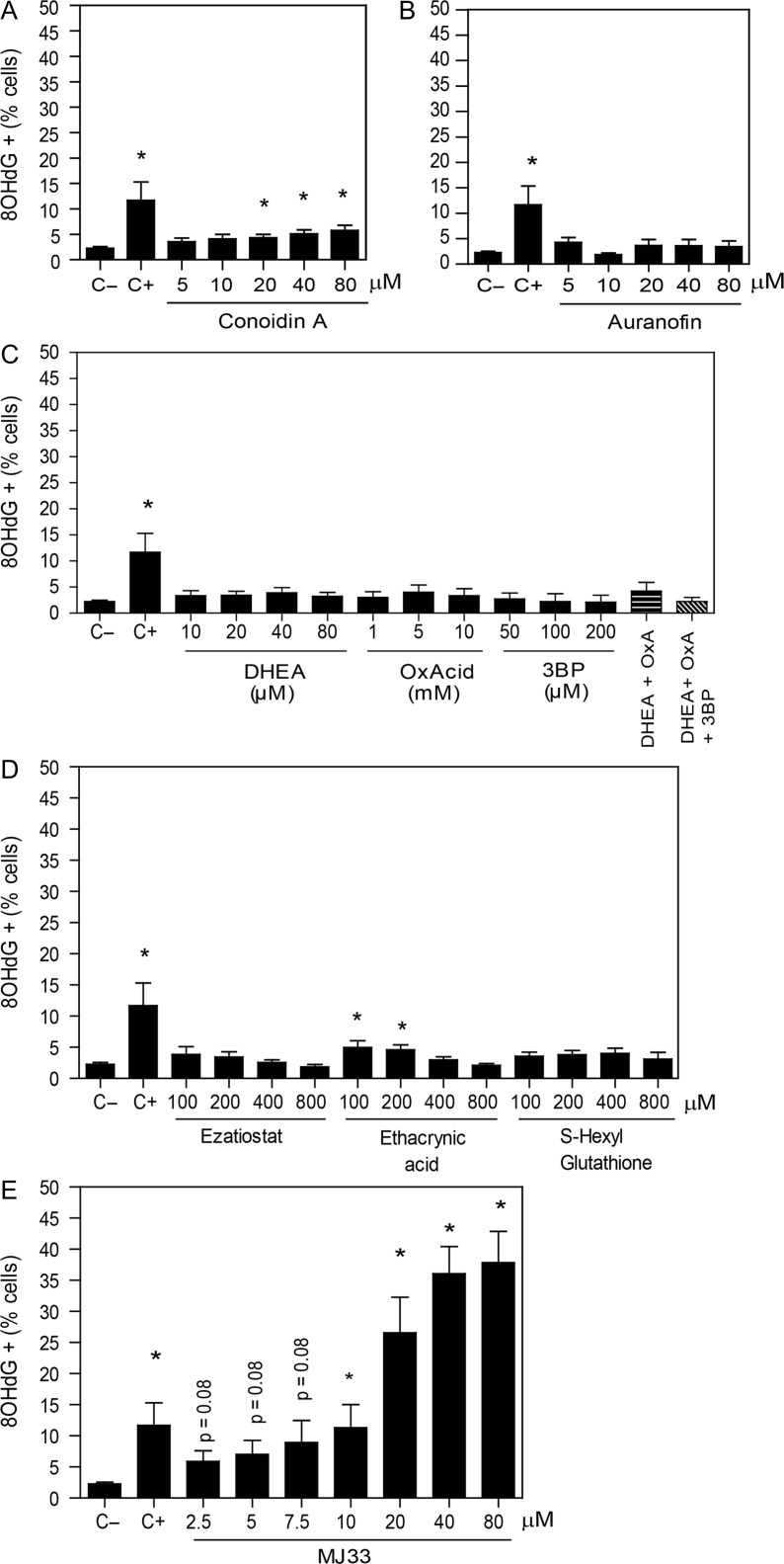
There was a slight dose-dependent increase in the percentage of spermatozoa with high levels of 8OHdG when the 2-Cys PRDXs were inhibited with conoidin A compared to controls (Fig. 2A). Moreover, no changes in sperm DNA oxidation were observed when the TRD activity was inhibited by auranofin (Fig. 2B) or when the inhibitors of enzymes that produce NADPH were used (Fig. 2C).
Figure 2.
DNA damage is increased by the inhibition of the Ca2+-iPLA2 activity of PRDX6. After 2 h of incubation with different concentrations of the inhibitors of (A) the 2-Cys PRDXs, (B) the TRX/TRD system, (C) the NADPH-suppliers’ enzymes, (D) the reactivation system of the PRDX6 peroxidase activity or (E) the Ca2+-iPLA2 activity of PRDX6, spermatozoa were fixed, permeabilized and incubated with anti-8OHdG antibody overnight. The positive control (C+) was provided by incubating spermatozoa with 4 mM H2O2 under the same conditions. The percentage of labeled cells was analyzed by MACSQuant Analyzer flow cytometry and results are presented as the mean ± SEM of seven measurements performed with sperm samples from different donors. *A value significantly (P ≤ 0.05) different from the control without inhibitors (C−) (ANOVA and Bonferroni post hoc test).
We observed a slight but significant increase in sperm DNA oxidation by the inhibition of the reactivation of the PRDX6 peroxidase activity when ethacrynic acid was used (Fig. 2D). Of note, the highest values of 8OHdG levels were observed by the inhibition of the Ca2+-iPLA2 activity of PRDX6 with MJ33 (Fig. 2E).
The addition of 1 mM H2O2 together with the maximum dose used for each inhibitor, shown in Fig. 2, promoted an increase in the percentages of cells with DNA damage in H2O2-treated spermatozoa compared to their respective controls (Supplementary Fig. S2). However, only the treatment with MJ33 or auranofin promoted oxidative damage in H2O2-treated sperm to a level significantly greater than that generated by 80 μM MJ33 alone. Interestingly, the levels of DNA oxidation promoted by 1 mM H2O2 when the 2-Cys PRDXs were inhibited were like those of spermatozoa incubated with MJ33 alone.
MJ33 promotes the increase of superoxide production and the loss of membrane potential in sperm mitochondria
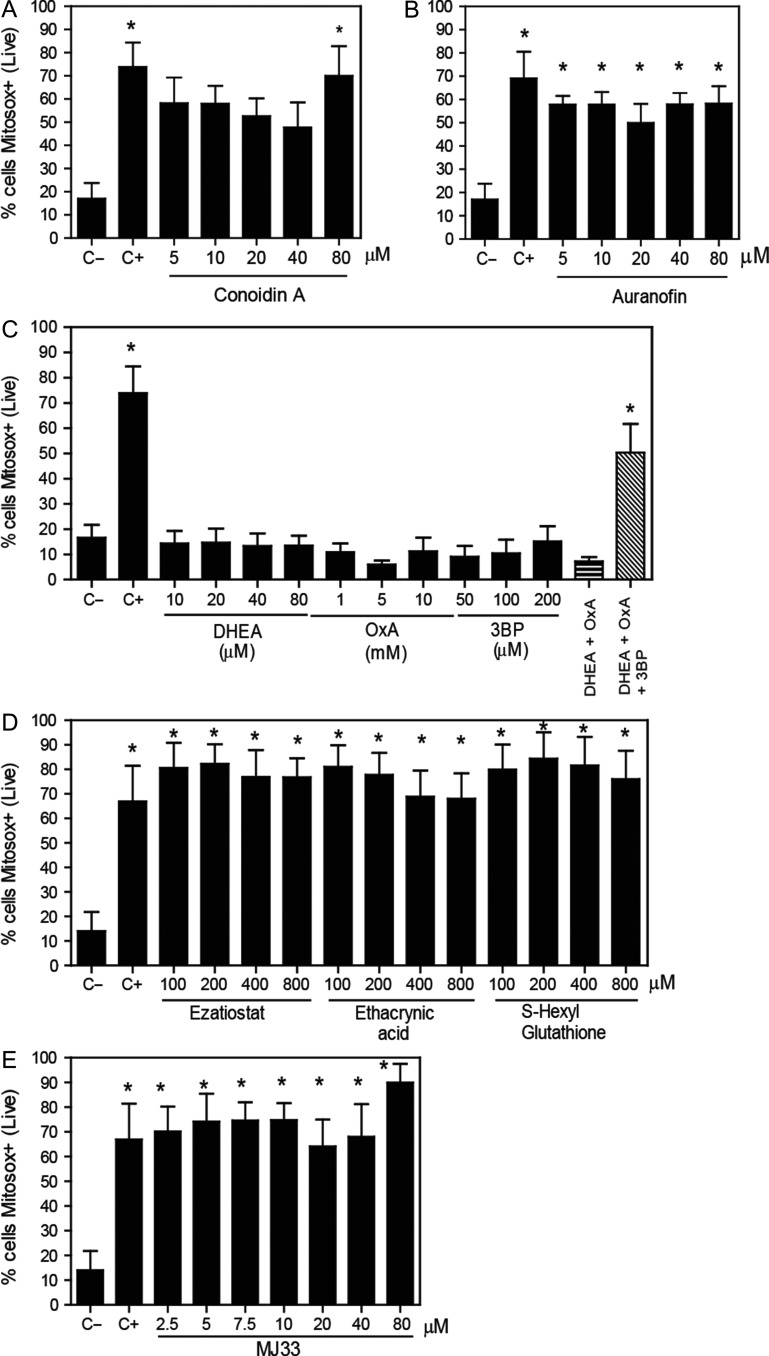
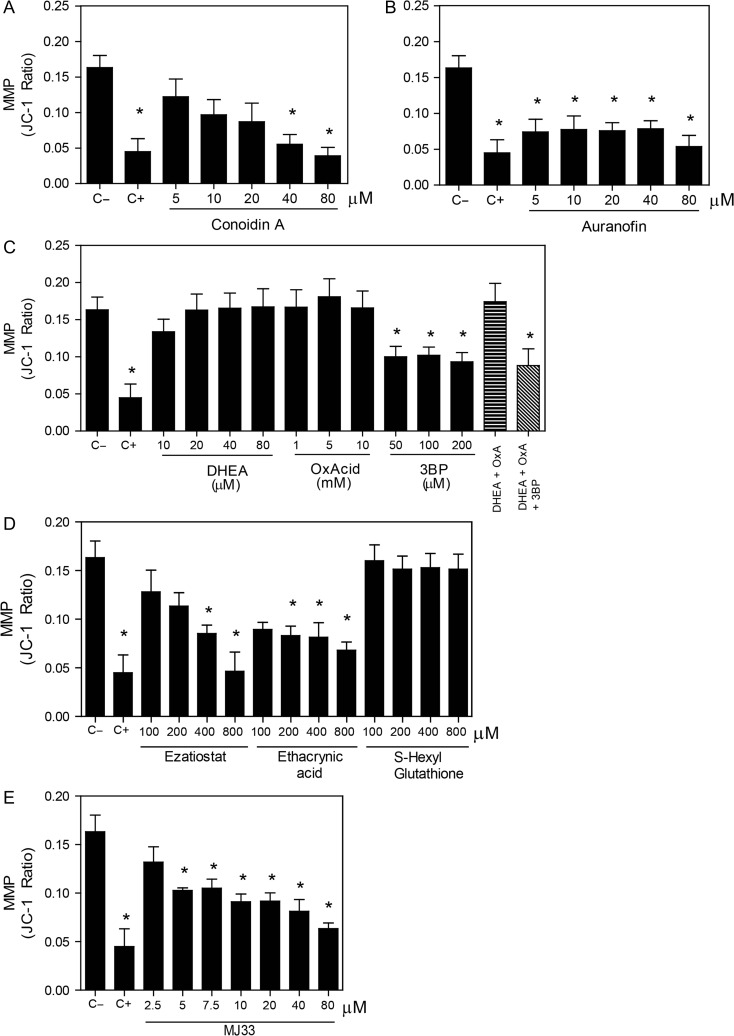
We found that the inhibition of the 2-Cys PRDXs by conoidin A, only at the highest dose used, increased the levels of mitochondrial O2•− (Fig. 3A). In addition, we observed a decrease in the MMP when conoidin A were present at concentrations equal or higher to 40 μM in the incubation medium (Fig. 4A). The inhibition of TRD by auranofin resulted in an increase of mitochondrial O2•− levels (Fig. 3B) and loss of the MMP (Fig. 4B). Of note, we found that only the combination of all three inhibitors of the NADPH-suppliers enzymes increased the levels of mitochondrial O2•− (Fig. 3C). This O2•− increase concurs with the reduction of sperm viability (Fig. 1C). Moreover, the inhibition of the malic enzyme, but not of the others NADPH suppliers, significantly decreased the MMP (Fig. 4C).
Figure 3.
Mitochondrial superoxide production levels are increased by the inhibition of PRDX6. After 2 h of incubation with different concentrations of the inhibitors of (A) the 2-Cys PRDXs, (B) the TRX/TRD system, (C) the NADPH-suppliers enzymes, (D) the reactivation system of the PRDX6 peroxidase activity or (E) the PRDX6 Ca2+-iPLA2 activity, spermatozoa were washed and incubated with 2 μM MitoSOX-RED and Calcein-AM. The positive control (C+) was provided by incubating spermatozoa with 40 μM antimycin A under the same conditions. The percentages of live cells producing superoxide were analyzed by MACSQuant Analyzer flow cytometry. Results are presented as the mean ± SEM of five to eight measurements performed with sperm samples from different donors. *A value significantly (P ≤ 0.05) different from the control without inhibitors (C−) (ANOVA and Bonferroni post hoc test).
Figure 4.
Mitochondrial membrane potential is impaired by the inhibition of the PRDX6. After 2 h of incubation with different concentrations of the inhibitors of (A) the 2-Cys PRDXs, (B) the TRX/TRD system, (C) the NADPH-suppliers enzymes, (D) the reactivation system of the PRDX6 peroxidase activity or (E) the Ca2+-iPLA2 activity of PRDX6, spermatozoa were washed and incubated with 2 μM JC-1 and 0.2 μM Sytox Blue. The positive control (C+) was provided by incubating spermatozoa with 40 μM of Antimycin A under the same conditions. Only live cells were analyzed, and data is express as the ratio in red/green fluorescence intensity indicating depolarization when this ratio is decreased. Results are presented as the mean ± SEM of five measurements performed with sperm samples from different donors. *A value significantly (P ≤ 0.05) different from the control without inhibitors (C−) (ANOVA and Bonferroni post hoc test).
On the other hand, the inhibition of the PRDX6 peroxidase activity showed high levels of mitochondrial O2•− production when all inhibitors were used (Fig. 3D). However, only ezatiostat and ethacrynic acid significantly decreased the MMP (Fig. 4D). These results are in accordance with the fact that S-hexylglutathione did not impair sperm viability (Fig. 1D). Moreover, MJ33 at all concentrations used promoted high levels of mitochondrial O2•− production (Fig. 3E) and MMP loss (Fig. 4E).
PRDX6 Ca2+-iPLA2 activity inhibition generates high levels of 4HNE in spermatozoa
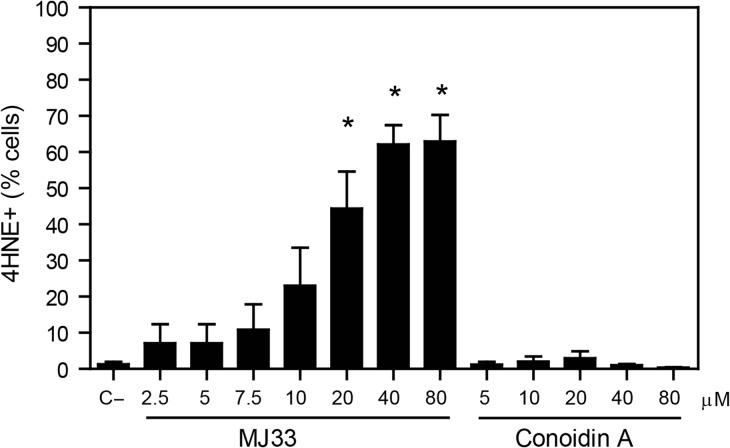
We found that from all the inhibitors used, only MJ33 significantly increased the levels of 4HNE after 2 h of incubation compared to untreated control (Fig. 5). Moreover, the treatment with ezatiostat at the concentration that impairs sperm viability (800 μM) also promoted an increase in endogenous levels of 4HNE in spermatozoa compared to controls (6.2 ± 1.1% and 3.5 ± 0.6%, respectively; n = 5, t-test, P ≤ 0.05). Of note, conoidin A did not generate detectable levels of lipid peroxidation (detected by BODIPY C11 fluoresce) during 2 h of incubation (data not shown).
Figure 5.
Endogenous 4HNE production is highly increased when the inhibition of PRDX6 Ca2+-iPLA2 activity. After 2 h of incubation with different concentrations of MJ33 or conoidin A, inhibitors of the Ca2+-iPLA2 activity of PRDX6 and the 2-Cys PRDXs, respectively, spermatozoa were fixed, permeabilized and incubated with anti-4HNE antibody overnight. The percentage of labeled cells were analyzed by MACSQuant Analyzer flow cytometry and results are presented as the mean ± SEM of four measurements performed with sperm samples from different donors. *A value significantly (P ≤ 0.05) different from the control without inhibitors (C−) (ANOVA and Bonferroni post hoc test).
The levels of 4HNE correlated with the levels of mitochondrial O2•− (Pearson’s coefficient r = 0.63; P = 0.001; n = 24) and with the loss of MMP (Pearson’s coefficient r = −0.81; P = 0.00001; n = 24) only when Ca2+-iPLA2 activity of PRDX6 was inhibited. Moreover, linear regression analyses revealed that the loss of MMP depended on the levels of 4HNE (R2 = 0.66; P = 0.00001; n = 24) and on the O2•− production (R2 = 0.69; P = 0.00001; n = 24) generated in spermatozoa incubated in the presence of MJ33 in the incubation medium.
Percentage of H2DCFDA-labeled spermatozoa increased only by the inhibition of the PRDX6 system
The inhibition of any of the 2-Cys PRDX system players (Fig. 6A–C) or the use of MJ33 (Fig. 6E) did not modify the levels of ROS, detected using H2DCFDA fluorescence. Ezatiostat and S-hexylgluthathione significantly increased the levels of ROS (indicated by the percentage of H2DCFDA-labeled spermatozoa) compared to non-treated controls (Fig. 6D).
Figure 6.
Inhibition of the PRDX6 system increases ROS levels. Spermatozoa were incubated for 2 h with different concentrations of the inhibitors of (A) the 2-Cys PRDXs, (B) the TRX/TRD system, (C) the NADPH-suppliers enzymes, (D) the reactivation system of the PRDX6 peroxidase activity or (E) the Ca2+-iPLA2 activity of PRDX6, then washed and added 10 μM H2DCFDA for 30 min at 37°C. Control positive (C+) was done by incubating spermatozoa with 100 μM of ferrous perchlorate (II) and 1 mM of H2O2 under the same conditions. The mean fluorescence of label cells was analyzed by MACSQuant Analyzer flow cytometry and results are presented relative to control without inhibitors (C−) as the mean ± SEM of six measurements performed with sperm samples from different donors. *A value significantly (P ≤ 0.05) different from the control (C−) (ANOVA and Bonferroni post hoc test).
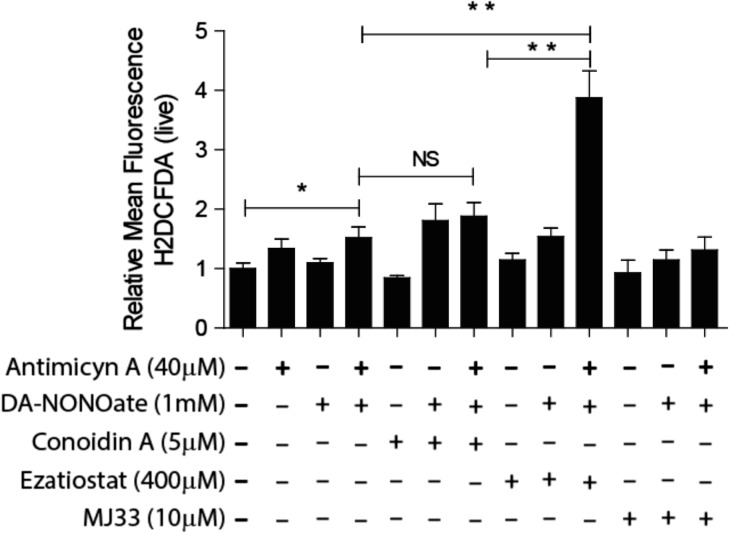
Since in somatic cells, all PRDXs are capable of scavenging ONOO− (Wood et al., 2003a,b; O’Flaherty, 2014b), formed by the combination of O2•− and nitric oxide (NO•), we determined whether the inhibition of PRDXs will increase the levels of this ROS generated by incubating spermatozoa with antimycin A (which increases the levels of O2•− by inhibiting the electron transport at complex III of the respiratory chain in mitochondria) and DA-NONOate (a NO• donor). We found a significant increase of the levels of H2DCFDA relative intensity in spermatozoa treated with the combination of antimycin A and DA-NONOate when the peroxidase activity of PRDX6 was blocked by ezatiostat compared to untreated controls (Fig. 7). This increase was not seen when the 2-Cys PRDXs or the Ca2+-iPLA2 activity of PRDX6 was inhibited.
Figure 7.
Peroxynitrite anion (ONOO−) is scavenged by the peroxidase activity of PRDX6. Spermatozoa were incubated for 2 h with the inhibitor of the 2-Cys PRDXs (5 μM conoidin A), or the GSTpi inhibitor (400 μM ezatiostat) or the Ca2+-iPLA2 activity of PRDX6 (10 μM MJ33) with or without the addition of 40 μM antimycin A or 1 mM DaNonoate. Then, the spermatozoa were washed and incubated with 10 μM H2DCFDA for 30 min at 37°C. The mean fluorescence of labeled cells was analyzed by MACSQuant Analyzer flow cytometry, and results are presented relative to control without inhibitors (C−) as the mean ± SEM of five measurements performed with sperm samples from different donors. *A P ≤ 0.05 value and **a P ≤ 0.01 value (ANOVA and Bonferroni post hoc test). NS: not significant.
Discussion
In this study, we demonstrated that the Ca2+-iPLA2 and peroxidase activities of PRDX6 are the primary player in the antioxidant defense of human spermatozoa since their inhibition promotes an increase in ROS production, leading to an increase in lipid peroxidation, disruption of mitochondrial membrane potential and DNA oxidation. Ultimately, the generated oxidative stress leads to cell death. In fact, the inhibition of the 2-Cys PRDXs did not produce a great increase in ROS production and was unable to increase 4HNE levels. Only when spermatozoa were challenged with H2O2 in the presence of conoidin A, the DNA oxidation increased to similar levels to those generated in spermatozoa treated with MJ33 (Supplementary Fig. S2). Altogether, these findings suggest that PRDX6 is sufficient to overcome the oxidative stress in human spermatozoa.
During ROS removal, 2-Cys PRDXs become inactive by the thiol oxidation of their active site and they are re-activated by the TRX/TRDX/NADH system (Wood et al., 2003a,b). Thus, the decrease on sperm viability, concomitantly with the increase in mitochondrial O2•− and loss of MMP, by auranofin indicates the necessity for active TRD to restore 2-Cys PRDXs activity. This inhibitory effect of auranofin can also be explained by the fact that TRD can reduce oxidized glutathione which could favor the reactivation of PRDX6 peroxidase activity (Sun et al., 2001). Since the GSH content in mammalian spermatozoa is very limited (Li, 1975), the inhibition of TRD could also deplete the spermatozoon of reduced GSH needed for the reactivation of the PRDX6 peroxidase activity, thus producing strong oxidative stress and the impairment of sperm viability. Altogether, these findings highlight the need for 2-Cys PRDXs and their reactivation system (TRX/TRD/NADPH) and suggest the need for TRD to maintain the PRDX6 peroxidase activity to ensure sperm viability.
The required NADPH for the TRX/TRD system is provided by G6PDH in human spermatozoa (Sarkar et al., 1977). Since in bull spermatozoa, G6PDH is absent and NADPH is supplied by NADP-ICDH (O’Flaherty et al., 2006b), we explore whether other reductases such as NADP-ICDH or ME are important to supply NADPH for human spermatozoa. We observed that the inhibition of these reductases increased the levels of O2•− and promoted a loss of MMP that lead to an impairment of sperm viability in a similar fashion to that when conoidin A or auranofin were used. Interestingly, a compensatory mechanism is put in place by other reductases when one of them is inhibited to ensure enough NADPH for the reductive functions of the TRX/TRD system. Altogether, these results suggest that G6PDH, NADP-ICDH and ME are needed to maintain sperm viability in human spermatozoa. Of note, the present study is the first to report the participation of NADP-ICDH and ME in protecting human spermatozoa against oxidative stress.
In somatic cells, the PRDX6 peroxidase activity requires GSTpi and GSH for its reactivation after ROS removal (Chen et al.,, 2000; Zhou et al., 2013). The impairment of sperm viability due to ezatiostat suggests the need for GSTpi to ensure viable spermatozoa. Of note, S-hexylglutathione, an inhibitor of mtGST1, which inhibited capacitation in goat spermatozoa and reduced rat germ cell survival (Gopalakrishnan et al., 1998; Rao and Shaha, 2000), increased the levels of mitochondrial O2•− production without decreasing MMP (Figs 3D and 4D), suggesting that mtGST1 participates in, but it is not essential for, maintaining low levels of mitochondrial ROS in human spermatozoa.
The inhibition of the PRDXs system players differentially promoted the increase in endogenous ROS in human spermatozoa. None of the inhibitors of the 2-Cys PRDXs system promoted high levels of ROS. A deeper investigation revealed that ezatiostat and S-hexylglutathione, increased the ROS levels (indicated by H2DCFDA fluorescence) in treated spermatozoa compared to the untreated control (Fig. 6D). Peroxynitrite (produced by combination of NO• with O2•−) can trigger apoptosis-like changes when the levels are high in human spermatozoa (Aitken, 2011). The observation that ezatiostat was the only inhibitor capable of increasing the levels of ONOO− in antimycin A/DANONOate-treated spermatozoa compared to the appropriate control suggests that the PRDX6 peroxidase activity is the primary scavenger mechanism to remove ONOO– in human spermatozoa.
We deliberately did not mention the production of high H2O2 levels in this study because H2DCFDA has a very low sensitivity for detecting this ROS (Kalyanaraman et al., 2012). However, H2O2 is important in inducing oxidative damage to the spermatozoon (Aitken and Baker, 2006; Morielli and O’Flaherty, 2015). Since the entire 2-Cys PRDXs and PRDX6 react with H2O2 (Wood et al., 2003a,b; Perkins et al., 2015), it is expected that the inhibition of the entire PRDX system would also raise the H2O2 levels in human spermatozoa. The spontaneous or enzymatic dismutation of the mitochondrial O2•− will generate high levels of H2O2. Both types of ROS, in the presence of iron, are responsible for the high levels of lipid peroxidation that we observed in this study (Aitken et al., 1993; Storey, 1997).
The PRDX6 Ca2+-iPLA2 activity is crucial for repair of peroxidized cell membranes in lung epithelium (Fisher, 2017). The absence of PRDX6 or the inhibition of its Ca2+-iPLA2 activity increases the levels of lipid peroxidation associated with a failure to acquire fertilizing ability in mouse and human spermatozoa (Lee et al., 2017; Moawad et al., 2017). Here, we demonstrated that MJ33-treated spermatozoa display a significant increase in endogenous ROS and 4HNE production that leads to a decrease in viability and MMP and high levels of DNA oxidation compared to untreated controls. Although the inhibition of the 2-Cys PRDXs by conoidin A or of the PRDX6 peroxidase activity by ethacrynic acid also generated increased levels of mitochondrial O2•−, the oxidative damage of sperm DNA was markedly higher when the Ca2+-iPLA2 activity of PRDX6 was inhibited compared to those levels observed in spermatozoa treated with inhibitors of 2-Cys PRDXs or the peroxidase activity of PRDX6 (Fig. 2). Altogether, these results indicate the necessity for PRDX6 Ca2+-iPLA2 activity to protect the paternal genome against oxidative damage. The fact that only the treatment with MJ33 or ezatiostat and not with conoidin A lead to the production of endogenous 4HNE (Fig. 5), a toxic product of lipid peroxidation, reinforces the essential role of PRDX6, through its Ca2+-iPLA2 and peroxidase activities, to protect human spermatozoa against lipid peroxidation.
4HNE is mutagenic and reacts with all four DNA bases, with guanosine being the most affected (Zhong and Yin, 2015). The treatment of human spermatozoa with exogenous 4HNE resulted in an increase of mitochondrial O2•− production which leads to DNA oxidation (Aitken et al., 2012). We found that the levels of 4HNE were significantly higher in MJ33- or ezatiostat-treated spermatozoa compared to untreated spermatozoa or those incubated with the other inhibitors used in this study. Peroxynitrite is capable of triggering lipid peroxidation in an iron-free fashion (Radi et al., 1991). The increased 4HNE levels obtained by inhibiting the PRDX6 peroxidase activity with ezatiostat, and the fact that the mitochondrial O2•− production and MMP depended on the levels of 4HNE, suggest participation of ONOO− in the production of lipid peroxidation of human spermatozoa. The inhibition of PRDX6 Ca2+-iPLA2 or peroxidase activities also lead to increased levels of mitochondrial O2•− and loss of MMP. Therefore, we can suggest that these inhibitory treatments triggered the disruption of the normal sperm mitochondrial activity, generating oxidative stress that will promote lipid peroxidation and the subsequent formation of 4HNE. This hydroxynonenal is capable of binding and inhibiting the succinate dehydrogenase, present in the mitochondrial complex II, and thus elevate mitochondrial ROS in human spermatozoa (Aitken et al., 2012). The inhibition of PRDX6 Ca2+-iPLA2 and peroxidase activities promote oxidative stress that will lead to the increases in 8OHdG and 4HNE levels. Both 8OHdG and 4HNE are capable of generating mutations of DNA (Kino and Sugiyama, 2000; Zhong and Yin, 2015). The oxidative stress and high levels of 8OHdG have been associated with infertility in men (Aitken and Baker, 2006). Thus, our findings suggest the primary role of PRDX6 to protect the paternal genome against oxidative damage-dependent mutations.
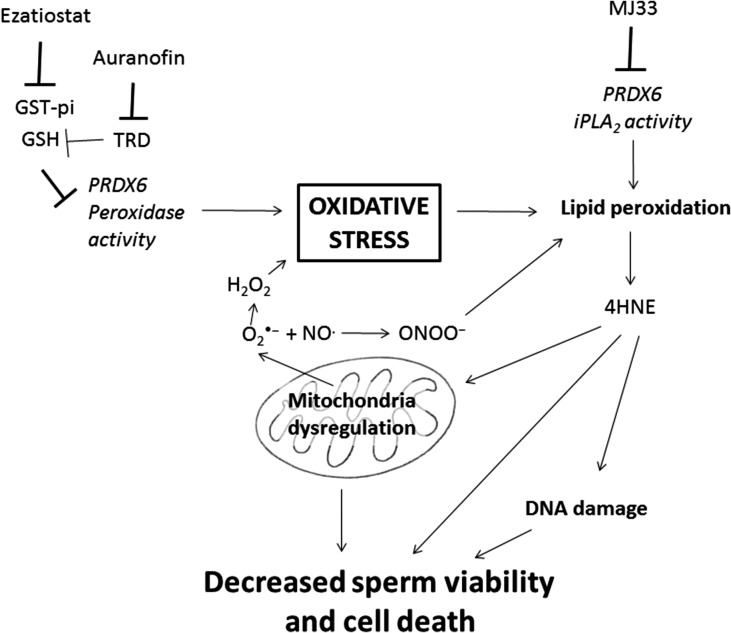
Our results demonstrated for the first time, a major role of PRDX6 peroxidase, and especially Ca2+-iPLA2 activities, in preventing the toxic effects of oxidative stress on viability and DNA integrity in human spermatozoa (Fig. 8). Moreover, we showed that 2-Cys PRDXs are also necessary for protection against oxidative damage of spermatozoa, but they are not able to prevent oxidative stress when the PRDX6 Ca2+-iPLA2 activity is impaired. PRDXs, and particularly PRDX6, are present in low amounts and highly oxidized (therefore inactive) in spermatozoa from infertile men that have high levels of lipid peroxidation and DNA damage (Gong et al., 2012). The findings present in this study further support the need for active PRDXs and particularly PRDX6 to protect spermatozoa against oxidative stress an ensure fertilizing ability. Moreover, the lack of protection by 2-Cys PRDXs is evident in mouse Prdx6−/− spermatozoa which display high levels of oxidative damage and which are unable to fertilize oocytes under in-vivo or in-vitro conditions (Moawad et al., 2017; Ozkosem et al., 2015; 2016). In conclusion, PRDX6 is the primary antioxidant defense mechanism for protecting the paternal genome and maintaining the viability of human spermatozoa.
Figure 8.
Schematic model of action of PRDX6 to assure sperm viability and DNA integrity. The inhibition of PRDX6 Ca2+-iPLA2 by MJ33 or the indirect blockage of its peroxidase activity targeting GST-pi with ezatiostat or TRD with Auranofin, can promote oxidative stress by increasing lipid peroxidation and mitochondria dysregulation. This will lead to an increase in O2•− that together with NO• will produce ONOO– capable of generating lipid peroxidation. As a result, endogenous 4HNE and 8OHdG levels will increase, affecting DNA integrity and ultimately decreasing viability.
Supplementary Material
Acknowledgements
We thank the volunteers who participated in this study.
Authors’ roles
M.C.F. carried out the experiments, contributed with the design of the study, analyzed the data and wrote the article. C.O. conceived and designed the study, analyzed the data and wrote the article.
Funding
The Canadian Institutes of Health Research (MOP 133661 to C.O.). C.O is a Chercheur Boursier Junior 2 scholar from the Fonds de recherche Santé Québec. M.C.F was awarded the H. Grenville Smith fellowship by the Montreal General Hospital Foundation, Montreal Children’s Hospital Foundation, M.C.F was awarded the H. Grenville Smith fellowship by the Montreal General Hospital, Children's Hospital and The Cedars Cancer Foundations. MC is recipient of the Research Institute-McGill University Health Centre-Desjardings Studentship in Child Health Research.
Conflict of interest
None declared.
References
- Aitken RJ. The capacitation-apoptosis highway: oxysterols and mammalian sperm function. Biol Reprod 2011. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol 2006;250:66–69. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil 1993;98:257–265. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem 2012;287:33048–33060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittngham DG. The culture of mouse embryos in vitro In: Daniel JC (ed). Methods in Mammalian Embryology. San Francisco, USA: Freeman, 1971, 86–116. [Google Scholar]
- Bjordahl J, Barratt C, Mortimer D, Jouannet P. How to count sperm properly: checklist for acceptability of studies based on human semen analysis. Hum Reprod 2016;31:227–232. [DOI] [PubMed] [Google Scholar]
- Breininger E, Dubois D, Pereyra VE, Rodriguez PC, Satorre MM, Cetica PD. Participation of phosphofructokinase, malate dehydrogenase and isocitrate dehydrogenase in capacitation and acrosome reaction of boar spermatozoa. Reprod Domest Anim 2017;52:731–740. [DOI] [PubMed] [Google Scholar]
- Brizuela M, Huang HM, Smith C, Burgio G, Foote SJ, McMorran BJ. Treatment of erythrocytes with the 2-Cys peroxiredoxin inhibitor, conoidin A, prevents the growth of Plasmodium falciparum and enhances parasite sensitivity to chloroquine. PLoS One 2014;9:e92411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi M, Tamburrino L, Marchiani S, Olivito B, Azzari C, Forti G, Baldi E, Muratori M. Development of a specific method to evaluate 8-hydroxy, 2-deoxyguanosine in sperm nuclei: relationship with semen quality in a cohort of 94 subjects. Reproduction 2013;145:227–235. [DOI] [PubMed] [Google Scholar]
- Chang G-G, Hsu RY. The substrate analog bromopyruvate as a substrate, an inhibitor and an alkylating agent of malic enzyme of pigeon liver. Biochem Biophys Res Commun 1973;55:580–587. [DOI] [PubMed] [Google Scholar]
- Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 2000;275:28421–28427. [DOI] [PubMed] [Google Scholar]
- Cox AG, Brown KK, Arner ES, Hampton MB. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem Pharmacol 2008;76:1097–1109. [DOI] [PubMed] [Google Scholar]
- Crawford LA, Weerapana E. A tyrosine-reactive irreversible inhibitor for glutathione S-transferase Pi (GSTP1). Mol Biosyst 2016;12:1768–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt J, Dirr H. Effect of glutathione, glutathione sulphonate and S-hexylglutathione on the conformational stability of class pi glutathione S-transferase. FEBS Lett 1996;391:313–316. [DOI] [PubMed] [Google Scholar]
- Espinoza JA, Paasch U, Villegas JV. Mitochondrial membrane potential disruption pattern in human sperm. Hum Reprod 2009;24:2079–2085. [DOI] [PubMed] [Google Scholar]
- Fisher AB. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch Biochem Biophys 2017;617:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Chander A, Jain M. A competitive inhibitor of phospholipase A2 decreases surfactant phosphatidylcholine degradation by the rat lung. Biochem J 1992;288:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon C, Iwasaki A, de Lamirande E, Kovalski N. Reactive oxygen species and human spermatozoa. Ann N Y Acad Sci 1991;637:436–444. [DOI] [PubMed] [Google Scholar]
- Gong S, San Gabriel MC, Zini A, Chan P, O’Flaherty C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J Androl 2012;33:1342–1351. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan B, Aravinda S, Pawshe CH, Totey SM, Nagpal S, Salunke DM, Shaha C. Studies on glutathione S-transferases important for sperm function: evidence of catalytic activity-independent functions. Biochem J 1998;329:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromer S, Arscott LD, Williams CH Jr., Schirmer RH, Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J Biol Chem 1998;273:20096–20101. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Milam M. Inhibition of rabbit lung glucose-6-phosphate dehydrogenase by dehydroepiandrosterone augments oxidant injury. Am J Respir Cell Mol Biol 1990;2:257–261. [DOI] [PubMed] [Google Scholar]
- Hwang-Bo H, Jeong JW, Han MH, Park C, Hong SH, Kim GY, Moon SK, Cheong J, Kim WJ, Yoo YH et al. Auranofin, an inhibitor of thioredoxin reductase, induces apoptosis in hepatocellular carcinoma Hep3B cells by generation of reactive oxygen species. Gen Physiol Biophys 2017;36:117–128. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992;57:409–416. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 2012;52:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino K, Sugiyama H. GC→CG transversion mutation might be caused by 8-oxoguanine oxidation product. Nucleic Acids Symp Ser 2000:139–140. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 2008;93:3199–3207. [DOI] [PubMed] [Google Scholar]
- Lee D, Moawad AR, Morielli T, Fernandez MC, O’Flaherty C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol Hum Reprod 2017;23:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK. The glutathione and thiol content of mammalian spermatozoa and seminal plasma. Biol Reprod 1975;12:641–646. [DOI] [PubMed] [Google Scholar]
- Liu X, An BH, Kim MJ, Park JH, Kang YS, Chang M. Human glutathione S-transferase P1-1 functions as an estrogen receptor alpha signaling modulator. Biochem Biophys Res Commun 2014;452:840–844. [DOI] [PubMed] [Google Scholar]
- Liu G, Botting CH, Evans KM, Walton JA, Xu G, Slawin AM, Westwood NJ. Optimisation of conoidin A, a peroxiredoxin inhibitor. ChemMedChem 2010;5:41–45. [DOI] [PubMed] [Google Scholar]
- Liu Y, O’Flaherty C. In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian J Androl 2017;19:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan D, Sutton GR. Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes. Expert Opin Investig Drugs 2015;24:725–733. [DOI] [PubMed] [Google Scholar]
- Miraglia E, Lussiana C, Viarisio D, Racca C, Cipriani A, Gazzano E, Bosia A, Revelli A, Ghigo D. The pentose phosphate pathway plays an essential role in supporting human sperm capacitation. Fertil Steril 2010;93:2437–2440. [DOI] [PubMed] [Google Scholar]
- Moawad AR, Fernandez MC, Scarlata E, Dodia C, Feinstein SI, Fisher AB, O’Flaherty C. Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Sci Rep 2017;7:12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morielli T, O’Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015;149:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounib MS. NAD- and NADP-malic enzymes in spermatozoa of mammals and fish. FEBS Lett 1974;48:79–84. [DOI] [PubMed] [Google Scholar]
- Niedzwiecka N, Gronczewska J, Skorkowski EF. NAD-preferring malic enzyme: localization, regulation and its potential role in herring (Clupea harengus) sperm cells. Fish Physiol Biochem 2017;43:351–360. [DOI] [PubMed] [Google Scholar]
- Ozkosem B, Feinstein SI, Fisher AB, O’Flaherty C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol 2015;5:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkosem B, Feinstein SI, Fisher AB, O’Flaherty C. Absence of peroxiredoxin 6 amplifies the effect of oxidant stress on mobility and SCSA/CMA3 defined chromatin quality and impairs fertilizing ability of mouse spermatozoa. Biol Reprod 2016;94:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty C. Peroxiredoxins: hidden players in the antioxidant defence of human spermatozoa. Basic Clin Androl 2014. a;24:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty C. The enzymatic antioxidant system of human spermatozoa. Adv Androl 2014. b;2014:1–15. [Google Scholar]
- O’Flaherty C, Beorlegui N, Beconi MT. Heparin- and superoxide anion-dependent capacitation of cryopreserved bovine spermatozoa: requirement of dehydrogenases and protein kinases. Free Radic Res 2006. b;40:427–432. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med 2006. a;40:1045–1055. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Souza AR. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod 2011;84:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci 2015;40:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemen JH, van Ommen B, Bogaards JJ, van Bladeren PJ. Ethacrynic acid and its glutathione conjugate as inhibitors of glutathione S-transferases. Xenobiotica 1993;23:913–923. [DOI] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991;288:481–487. [DOI] [PubMed] [Google Scholar]
- Raineri R, Levy HR. On the specificity of steroid interaction with mammary glucose 6-phosphate dehydrogenase. Biochemistry 1970;9:2233–2243. [DOI] [PubMed] [Google Scholar]
- Rao AV, Shaha C. Role of glutathione S-transferases in oxidative stress-induced male germ cell apoptosis. Free Radic Biol Med 2000;29:1015–1027. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 2005;38:1543–1552. [DOI] [PubMed] [Google Scholar]
- Rigobello MP, Folda A, Baldoin MC, Scutari G, Bindoli A. Effect of Auranofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase. Free Radic Res 2005;39:687–695. [DOI] [PubMed] [Google Scholar]
- Ryu DY, Kim KU, Kwon WS, Rahman MS, Khatun A, Pang MG. Peroxiredoxin activity is a major landmark of male fertility. Sci Rep 2017;7:17174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pozo MC, Mendiola J, Serrano M, Mozas J, Bjorndahl L, Menkveld R, Lewis SE, Mortimer D, Jorgensen N, Barratt CL et al. Proposal of guidelines for the appraisal of SEMen QUAlity studies (SEMQUA). Hum Reprod 2013;28:10–21. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Nelson AJ, Jones OW. Glucose-6-phosphate dehydrogenase (G6PD) activity of human sperm. J Med Genet 1977;14:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlee J, Hsu RY. Duck liver malic enzyme: sequence of a tryptic peptide containing the cysteine residue labeled by the substrate analog bromopyruvate. Biochim Biophys Acta 1991;1079:247–252. [DOI] [PubMed] [Google Scholar]
- Setsukinai K-I, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. Journal of Biological Chemistry 2003;278:3170–3175. [DOI] [PubMed] [Google Scholar]
- Singh R, Lemire J, Mailloux RJ, Appanna VD. A novel strategy involved in [corrected] anti-oxidative defense: the conversion of NADH into NADPH by a metabolic network. PLoS One 2008;3:e2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod 1997;3:203–213. [DOI] [PubMed] [Google Scholar]
- Sun QA, Kirnarsky L, Sherman S, Gladyshev VN. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc Natl Acad Sci U S A 2001;98:3673–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulziikhishig E, Lee KK, Hossain QS, Higa Y, Imaizumi N, Aniya Y. Inhibition of mitochondrial membrane bound-glutathione transferase by mitochondrial permeability transition inhibitors including cyclosporin A. Life Sci 2010;86:726–732. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003. a;300:650–653. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 2003. b;28:32–40. [DOI] [PubMed] [Google Scholar]
- Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biology 2015;4:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lien YC, Shuvaeva T, DeBolt K, Feinstein SI, Fisher AB. Functional interaction of glutathione S-transferase pi and peroxiredoxin 6 in intact cells. Int J Biochem Cell Biol 2013;45:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.