Abstract
Objective
We investigated different dimensions of subjective cognitive decline (SCD) to determine which was the best prognostic risk factor for incident mild cognitive impairment (MCI) among cognitively unimpaired participants.
Methods
We included 1,167 cognitively unimpaired participants, aged 70 to 95 years, from the Mayo Clinic Study of Aging based on 2 concurrent SCD scales (part of the Blessed memory test and the 39-item Everyday Cognition [ECog] scale, which included a validated 12-item derivative) and a single question assessing worry about cognitive decline. We evaluated multiple ways to dichotomize scores. In continuous models, we compared average scores on 4 ECog domains and multidomain (39- and 12-item) ECog scores. Cox proportional hazards models were used to assess the association between each measure and risk of MCI in models adjusted for objective memory performance, depression, anxiety, sex, APOE ε4 carriership, and medical comorbidities.
Results
It was possible to select a substantial group of participants (14%) at increased risk of incident MCI based on combined baseline endorsement of any consistent SCD on the ECog (any item scored ≥3; 12-item ECog hazard ratio [HR] 2.17 [95% confidence interval 1.51–3.13]) and worry (HR 1.79 [1.24–2.58]) in an adjusted model combining these dimensions. In continuous models, all ECog domains and the multidomain scores were associated with risk of MCI with a small advantage for multidomain SCD (12-item ECog HR 2.13 [1.36–3.35] per point increase in average score). Information provided by the informant performed comparable to self-perceived SCD.
Conclusion
Prognostic value of SCD for incident MCI improves when both consistency of SCD and associated worry are evaluated.
Subjective cognitive decline (SCD) is defined as self-perceived cognitive decline among cognitively normal individuals.1 Several studies have suggested that SCD may be associated with an increased risk of incident mild cognitive impairment (MCI) or dementia due to Alzheimer disease (AD),2–5 and with AD biomarkers.6,7 Other studies suggest that SCD may be more associated with nonneurodegenerative causes, such as depressive symptoms, anxiety, certain personality traits, or failing physical health.8–10
One factor that could influence both the frequency and utility of SCD is the way in which SCD is measured.11 The Subjective Cognitive Decline Initiative (SCD-I) Working Group recently published an evaluation of SCD measures used in 19 cohort studies around the world.12 Despite considerable heterogeneity between studies, several aspects of SCD have been proposed to increase the likelihood of underlying AD. These aspects were summarized in a concept called SCD plus.1 For example, underlying AD is thought to be more likely if a person experiences subjective decline in memory, if concern is associated with SCD, and if an informant confirms cognitive decline. The value of these aspects remains to be validated, and it is possible that additional dimensions of SCD are relevant for optimal case finding.11,13
A more precise definition of SCD as a risk factor for MCI will likely have the most immediate consequences for inclusion in therapeutic trials in the preclinical stage of AD, but it also serves an important, more general goal. If there is more clarity about the aspects of SCD that should be deemed alarming, care can be tailored accordingly. Therefore, we examined the relationship between multiple measures of SCD and risk of MCI. These measures included several ways to ascertain severity, a comparison between different SCD domains and between participant- and informant-based ratings in a randomly selected sample from the general population.
Methods
Participants
The Mayo Clinic Study of Aging (MCSA) is a population-based study of cognitive aging that was established in Olmsted County, MN, in October 2004. Details of the study design and conduct of the study are reported elsewhere.14,15 Briefly, all MCSA participants undergo a clinical and cognitive assessment every 15 months. A consensus panel reviewed performances and clinical impressions of all participants. Participants were diagnosed as being cognitively unimpaired (CU) or having MCI based on the clinical assessments and a neuropsychological testing battery. CU participants are cognitively and functionally normal. A diagnosis of MCI was based on published criteria.16 These criteria are implemented clinically, evaluating decline in cognition by history and abnormal cognitive performance for that individual, while daily function is preserved (assessed using the Clinical Dementia Rating or Functional Activities Questionnaire).17,18 There are no fixed cutoff scores or algorithms that define MCI; clinical judgment of the consensus panel is most important in establishing the diagnosis. Each visit is judged blinded to all prior visits. Patients were included in the current study if they were CU, completed both questionnaires for SCD described below, and had at least one follow-up visit thereafter.
Standard protocol approvals, registrations, and patient consents
The study protocols were approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. All subjects provided signed informed consent to participate in the study.
Assessment of SCD
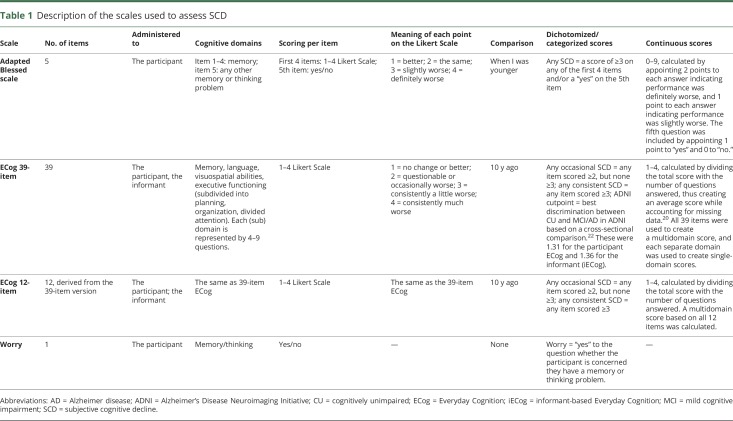
Two questionnaires and one stand-alone question were used to measure SCD. These are described in detail in table 1. From MCSA inception in 2004, participants completed the first 5 questions of the Blessed memory test at each visit.19 Scoring of these questions was described in a previous publication.7 Beginning in 2010, the Everyday Cognition (ECog) scale was also used to evaluate SCD at each visit.20 This scale has a full 39-item version and a shorter 12-item version, which is a validated derivative of the larger scale.21 It is important to note that we used several ways to categorize scores on the adapted Blessed and ECog scales. The adapted Blessed scale was dichotomized at endorsing any SCD (table 1). The published scoring method for the ECog scale depends on calculating a continuous score between 1 and 4, based on an algorithm in which the total score is divided by the total number of answered questions, thus creating an average score, while accounting for missing data.20 We included a previously published cutpoint for this average score on the 39-item ECog scale based on data from the Alzheimer's Disease Neuroimaging Initiative (ADNI).22 We chose to evaluate the cutpoints from this publication that proved to best discriminate CU from MCI/AD cross-sectionally. These were 1.31 for the participants and 1.36 for the informants (personal communication from Dr. Farias dd, March 1, 2017).22 A potential downside of using this average ECog score to create a cutpoint is that many small complaints may also add up to a total score above the cutpoint, while one severe complaint may be more relevant than many small complaints. Based on the hypothesis that the severity of SCD might be more important than the number of complaints, we additionally categorized the 39- and 12-item ECog scales based on having any occasional SCD (any item scored ≥2, but none ≥3) and on having any consistent SCD (any score of ≥3). Lastly, we assessed worry about cognitive decline using a single question (“Are you concerned you have a memory or thinking problem?”), which was administered before starting the ECog.
Table 1.
Description of the scales used to assess SCD
Assessment of comorbidity
The presence of medical comorbidities was assessed using the Charlson Comorbidity Index.23 Depressive symptoms and anxiety were assessed using the Beck Depression Inventory and the Beck Anxiety Inventory, respectively.24,25
Neuropsychological testing
The neuropsychological evaluation is described in detail elsewhere.14 Nine tests covering 4 cognitive domains are assessed each visit. For the current study, we used the memory-specific z scores, because this was the domain most strongly associated with incident MCI (data not shown). The memory z score consists of a combination of normalized scores of 3 tests for delayed recall (Logical Memory II, Visual Reproduction II, and Auditory Verbal Learning Test).26,27
Statistical methods
The first visit at which both the adapted Blessed and the ECog scales were administered was defined as the baseline visit for each included participant. Demographics were compared using Kruskal-Wallis tests or χ2 tests as appropriate. We used Cox proportional hazards models with age as the time scale to assess the association between the different measures of SCD and risk of MCI. The first model was univariable and included only the SCD measure of interest (unadjusted models: n = 1,166 for 39-item ECog, 12-item ECog, and the adapted Blessed scale; n = 1,160–1,165 for ECog domains; n = 1,110 for worry). The second, multivariable, model was adjusted for objective memory performance (delayed recall z score), depression, anxiety, sex, APOE ε4 carriership, and physical comorbidities measured using the Charlson index (n = 1,140 for 39-item ECog, 12-item ECog, and the adapted Blessed scale; n = 1,134–1,139 for domain-specific ECog scores; n = 1,085 for worry). In a final model, we combined 2 dimensions of SCD: the SCD measure most strongly associated with risk of MCI based on either of the questionnaires and worry. We examined the additive value of these measures as well as interactions between the two. For illustrative purposes, a Kaplan-Meier curve was created for this model. Results are presented as hazard ratio (HR) (95% confidence interval).
Data availability
Data will be shared by request from a qualified investigator in accordance with the MCSA data-sharing protocol.
Results
Baseline characteristics
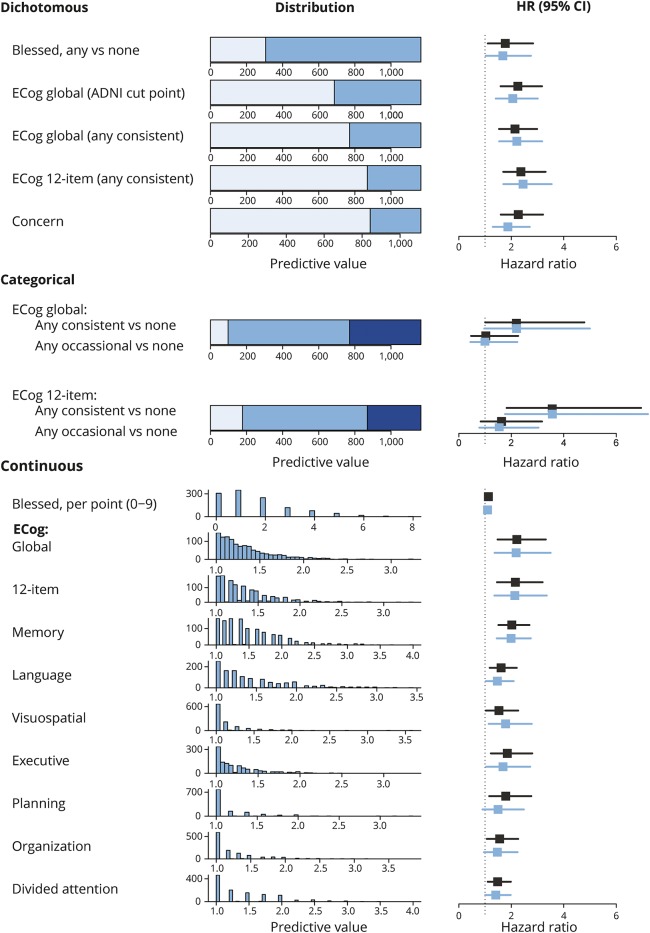
There were 1,167 participants; 585 (50.2%) were female. The median age of the sample was 79.0 years (interquartile range 75.3–83.6). Details of the baseline demographic data are displayed in table 2. During a median follow-up of 3.9 years (interquartile range 2.6–4.2), 143 participants (12%) developed MCI. Distributions of the different ECog subscales are shown in figure 1. Overall, 860 participants (73.8%) endorsed any SCD on the adapted Blessed scale. On the 39-item ECog scale, 673 participants (57.7%) endorsed any occasional SCD (any item ≥2, none ≥3) and 395 (33.9%) endorsed any consistent SCD (any item ≥3). Of 1,110 participants who answered the concern question, 267 (24.1%) indicated they were worried about memory/thinking problems. Most participants and informants who endorse any complaints score between 1 and 2 on the continuous ECog scale (indicating average scores between no complaints [1] and occasional complaints [2]). A small percentage of participants scored 2 or higher on the continuous scale (between 11.6% for memory SCD and 1.4% for visuospatial SCD).
Table 2.
Baseline characteristics
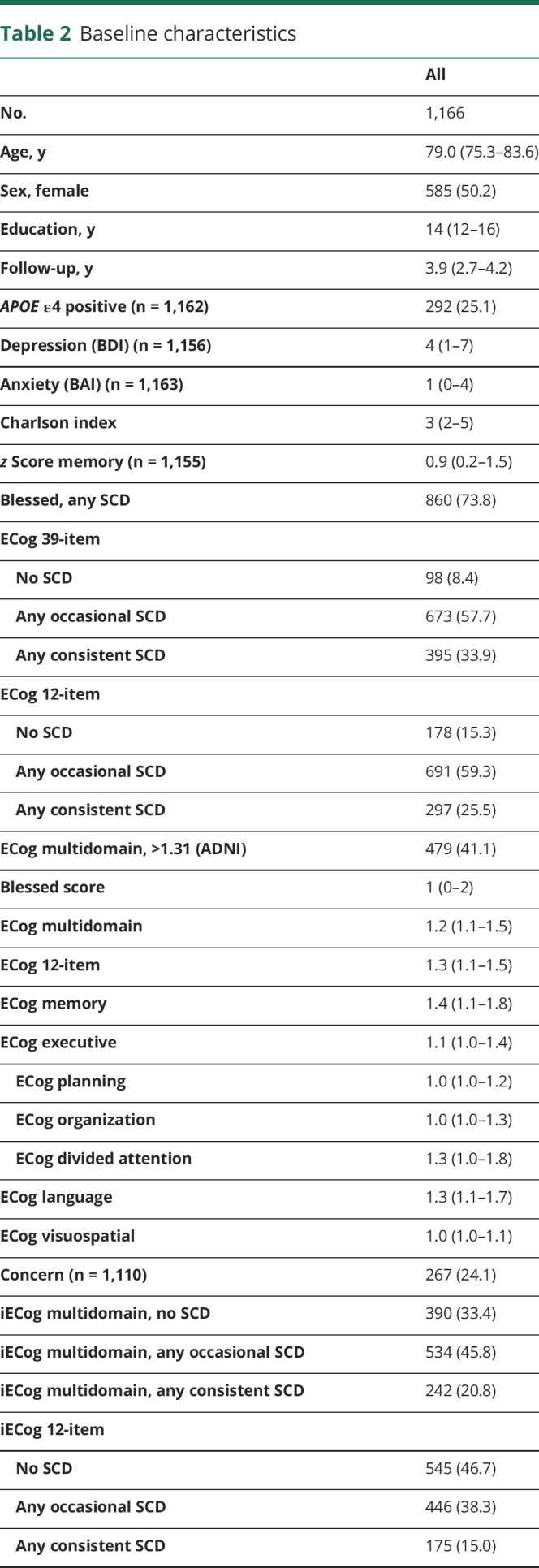
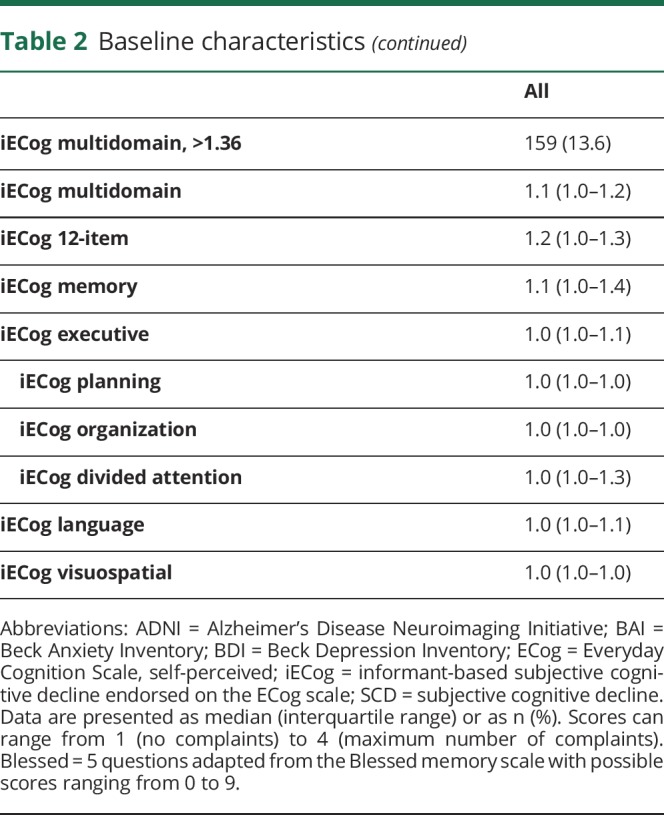
Figure 1. Distribution of self-reported SCD measures and their association with risk of mild cognitive impairment.
Distribution and predictive value of different SCD measures. Unadjusted HRs are depicted in black, adjusted HRs in blue. Dichotomous models: light blue indicates a score below the cutpoint and intermediate blue a score above the cutpoint. Categorical models: light blue indicates no SCD, intermediate blue any occasional SCD (but no consistent SCD), and dark blue any consistent SCD. Continuous models: the histograms either depict the number of participants endorsing any number of points on the Blessed questionnaire or any average score on the ECog domains. HRs in these models represent the risk increase associated with a 1-point increase. ADNI = Alzheimer's Disease Neuroimaging Initiative; CI = confidence interval; ECog = Everyday Cognition; HR = hazard ratio; SCD = subjective cognitive decline.
Correlations between different SCD scales
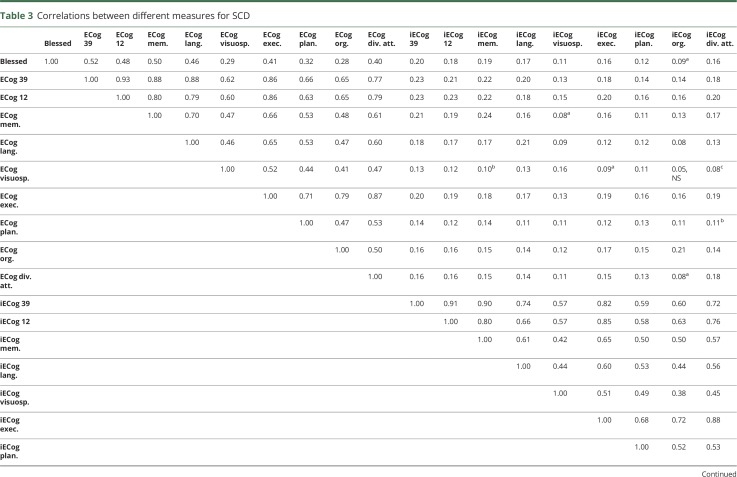
Spearman correlations between the different scales are presented in table 3. The full 39-item ECog scale was highly correlated to the 12-item ECog version (ρ = 0.94, p < 0.0001 for ECog; ρ = 0.92, p < 0.0001 for informant-based ECog [iECog]). The adapted Blessed scale was similarly correlated with the ECog memory domain (ρ = 0.50, p < 0.0001) and the multidomain ECog scores (ρ for 39-item ECog = 0.52, p < 0.0001). There were moderate to large correlations between the various ECog domains. However, the informant-based and participant-based ECog 39-item scores showed smaller correlations (ρ = 0.23, p < 0.0001).
Table 3.
Correlations between different measures for SCD
Relationship between the SCD scales and risk of MCI
We used Cox proportional hazards models to examine the association between the self-reported SCD measures and the risk of incident MCI (figure 1, numerical representation available from Dryad [table 1]: doi.org/10.5061/dryad.643387c). The adapted Blessed scale, the 39- and 12-item versions of the ECog, and their dichotomized derivatives were used to assess multiple cognitive domains. When these scales were assessed individually, the strengths of the association between each test and risk of MCI were similar. HRs ranged from 2.06 (1.54–3.18, p = 0.0002) for the ADNI-based cutpoint to 2.45 (1.70–3.54, p < 0.0001) for endorsing any consistent SCD (any response ≥3 compared to all responses <3) on the 12-item ECog after adjustment for objective memory performance, depression, anxiety, sex, APOE ε4 carriership, and physical comorbidities. Endorsing any SCD on the Blessed scale was also associated with risk of incident MCI, but the strength of the association was less than that of dichotomized scores on the ECog scale. In models examining a categorical variable of SCD (no SCD, occasional SCD, or consistent SCD) compared to no SCD, occasional SCD was not associated with an increased risk of MCI, but consistent SCD was. Continuous scores on the 39-item and 12-item ECog scales also predicted incident MCI. For example, a 1-point increase in average ECog score on the 12-item ECog scale increased the yearly risk of incident MCI 2.13 times (95% confidence interval 1.36–3.50). Of note, an average score of ≥2 was only endorsed by 3.6% of all participants. Endorsing any consistent SCD was much more common (33.9%) and carried a similar relative risk. The continuous score on the adapted Blessed scale was not associated with risk of MCI in adjusted models.
Comparison among SCD domain scores
Next, we used separate Cox proportional hazards models to evaluate the association between each domain-specific average SCD score measured using the 39-item ECog scale (memory, language, executive functioning, visuospatial functioning) and risk of MCI. Higher average scores within each SCD domain were associated with an increased risk of MCI in the adjusted models (figure 1, data available from Dryad [table 2]: doi.org/10.5061/dryad.643387c). Memory SCD performed slightly better than the other domains (adjusted HR 1.99 [1.45–2.74]). Twelve percent of the population attained or exceeded this risk of incident MCI, because an average score of 2 on the memory domain corresponded to the 88th percentile.
Worry about cognitive abilities and risk of MCI
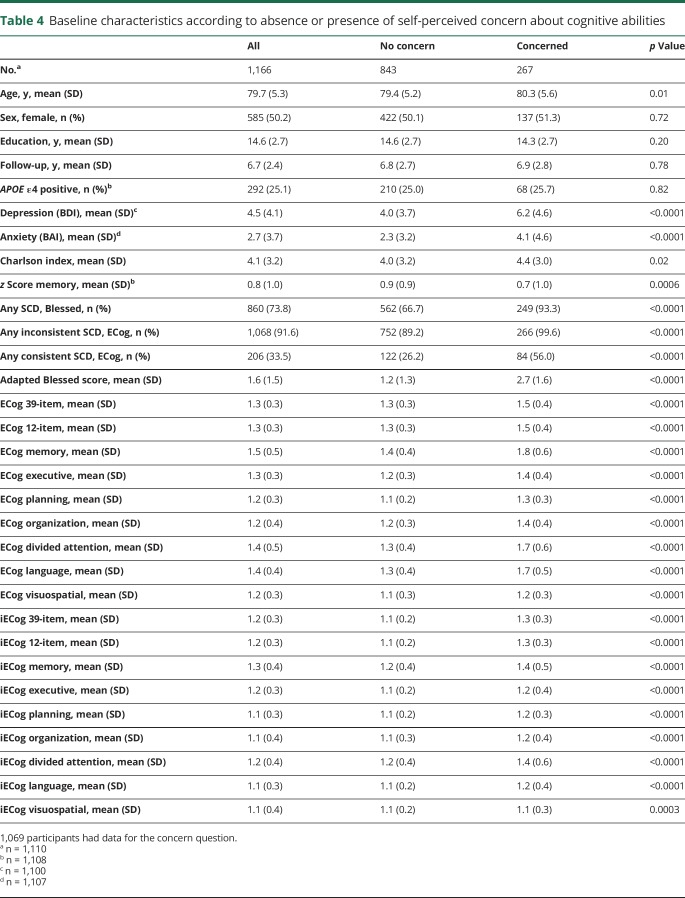
Differences between participants who reported worry about memory/thinking problems and those who did not are presented in table 4. In adjusted models, self-reported worry was associated with a 1.87-fold (1.30–2.70, p = 0.0008) increased risk of MCI.
Table 4.
Baseline characteristics according to absence or presence of self-perceived concern about cognitive abilities
SCD endorsed by the informant-based SCD
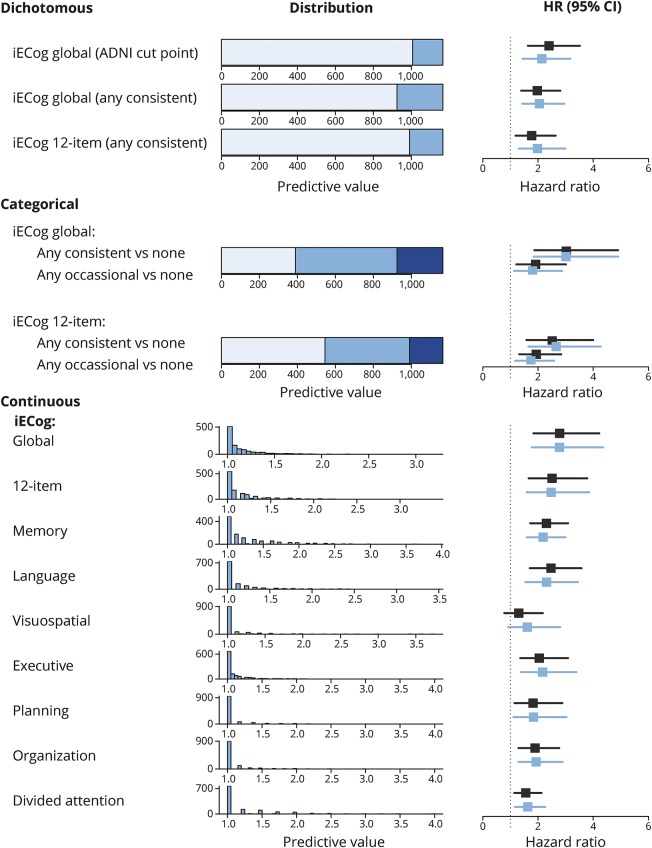
Informants reported any occasional cognitive decline on the 39-item iECog scale in 776 of 1,166 cases (66.6%) (figure 2) and consistent informant-based SCD (iSCD) in 242 cases (20.8%). All multidomain iECog measures and each iECog domain predicted incident MCI in adjusted models except for iSCD in the visuospatial domain. With estimated HRs ranging from 1.98 to 2.14, all dichotomized models performed very similarly. In contrast to results for occasional self-reported SCD, occasional iSCD was also associated with risk of MCI (12-item ECog vs no iSCD HR 1.74 [1.13–2.87], p = 0.006). Consistent iSCD further increased that risk (12-item ECog vs no iSCD HR 2.66 [1.65–4.27], p < 0.0001). Using average scores, multidomain iSCD was most strongly associated with risk of MCI (39-item iECog HR 2.78 [1.77–4.36], p < 0.001) with an average score of 2 corresponding to the 98th percentile. Memory iSCD, language iSCD, and executive iSCD were similarly associated with risk of MCI.
Figure 2. Distribution of informant-based SCD measures and their association with incident mild cognitive impairment.
Distribution and predictive value of different SCD measures. Unadjusted HRs are depicted in black, adjusted HRs in blue. Dichotomous models: light blue indicates a score below the cutpoint and intermediate blue a score above the cutpoint. Categorical models: light blue indicates no SCD, intermediate blue any occasional SCD (but no consistent SCD), and dark blue any consistent SCD. Continuous models: the histograms either depict the number of participants endorsing any number of points on the Blessed questionnaire or any average score on the ECog domains. HRs in these models represent the risk increase associated with a 1-point increase. ADNI = Alzheimer's Disease Neuroimaging Initiative; CI = confidence interval; HR = hazard ratio; iECog = informant-based Everyday Cognition; SCD = subjective cognitive decline.
Combining worry and severity of complaints
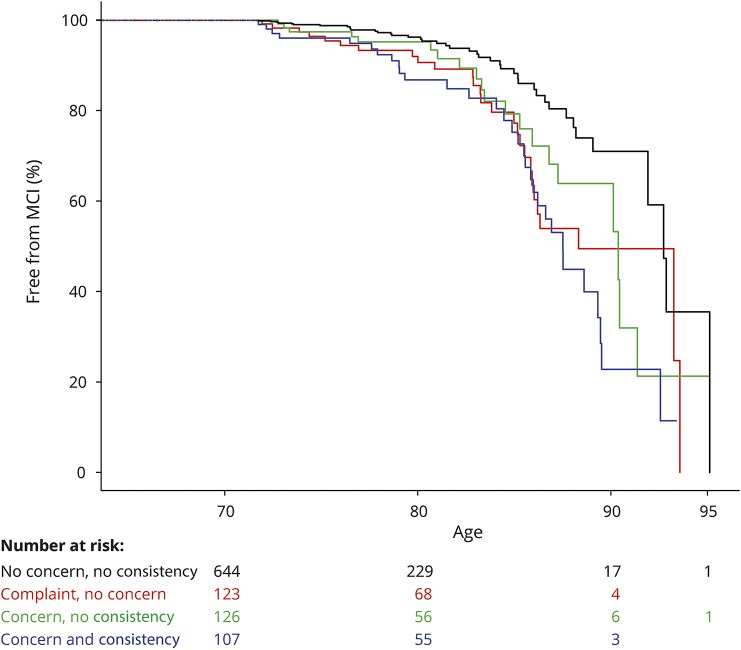
In our final analyses, we evaluated whether worry and severity of self-perceived SCD were independently associated with risk of MCI. We intended to run this analysis with only the best-performing measure of SCD severity. Because the strength of the associations was so similar across the ECog scores, we also considered the practicality of the test (the shortest possible questionnaire). Based on these criteria, we combined any consistent SCD vs no consistent SCD on the 12-item ECog scale (any response ≥3 vs all responses <3) and the concern question (figure 3). We found no interaction between worry and consistent SCD (p = 0.22 in unadjusted models). In fully adjusted additive models, having any consistent SCD increased risk of incident MCI 2.17 times (HR 2.17 [1.51–3.13], p < 0.0001) and being worried about memory/thinking problems increased risk of incident MCI 1.79 times (HR 1.79 [1.24–2.58], p = 0.002).
Figure 3. Kaplan-Meier curve combining consistency of complaints on the 12-item ECog and concern.
Figure with separate lines for combined presence of consistent complaints (denoted by consistency) and worry with age as time scale. Below the figure are numbers entering each time interval. Because participants enter and leave the model based on their age at first ECog and subsequent incident MCI or censorship, numbers at 80, 90, and 95 years old are not derivatives of numbers at 70 years old. ECog = Everyday Cognition; MCI = mild cognitive impairment.
Discussion
This study compares the associations between multiple SCD measures and risk of incident MCI in a randomly selected population-based sample. It provides evidence that SCD as measured using the ECog is a robust construct. Not only memory SCD but also SCD regarding language, visuospatial functions, and executive functions were associated with incident MCI after adjustment for objective memory performance, depression, anxiety, sex, APOE ε4 carriership, and physical comorbidities. In fact, scores combining all SCD domains seemed to perform marginally better than the memory domain alone. If one aims to select a large group of at-risk individuals based on self-reported SCD, the combination of having any consistent SCD on the 12-item ECog and feeling worried about cognitive problems may be the preferred method. Each of these features independently increased risk of incident MCI approximately 2 times, while also having a relatively high frequency in our population.
Consistent with prior evidence, we observed that nearly all elderly experience at least occasional SCD.28,29 However, most population-based studies reported a prevalence of SCD between 12.3% and 57% in similar age groups.3,30–33 These numbers are more akin to the prevalence we found when using any consistent SCD (33.9%) or worry (24.1%) as cutpoints. The differences between studies and those within our own study may be attributable to the various self-report measures used. Most population-based studies used only one question to ascertain SCD,30,32–34 and questions are usually restricted to memory problems.3,29,30,32–34 These methodologic differences may also influence interpretation of results. The presence of SCD was sometimes interpreted as not meaningful, because it was found to be very common. Our results illustrate that, although endorsing any SCD on a multiquestion questionnaire is very common and is not associated with incident MCI, several less common aspects of SCD, such as consistency of the complaint and worry, seem to be very relevant, because they are more closely related to cognitive decline.
Knowing how often certain complaints are endorsed is very useful when interpreting our results: although endorsing at least one consistent complaint was common, endorsing an average of consistent complaints rarely happened. Because HRs of the continuous models indicate a yearly risk increase per point increase in average ECog score, it is important to note that only a small proportion of the population attain or exceed the risks resulting from these models. This caveat is also important to note when interpreting results of a recent study from the University of California that evaluated the association between average ECog scores and incident MCI in a volunteer sample.35 Results were very similar to ours, but average ECog scores were slightly higher than in our study. The study from the University of California did not implement a cutpoint for an at-risk SCD state. Three others did, however.2,5,36 Each of these defined SCD based on a certain cutpoint on a nominal or continuous scale. We show that, compared to a cutpoint on a continuous scale, reporting any consistent SCD works as well when defining a clinical “at-risk state.” This is important, because endorsing any consistent SCD may be more generalizable to clinical practice than a score on a questionnaire.
We ascertained the value of several SCD plus criteria.1 One of these has gained much attention over the past years: the value of worry about cognitive problems. Data from the AgeCoDe study (German Study on Ageing, Cognition, and Dementia) have illustrated that worry is associated with an increased risk of incident dementia above the mere presence of any memory problem.30 Our finding that worry about memory/thinking problems is present in 24% of the population and is associated with risk of MCI in fully adjusted models lends further support to this concept. We add to existing data by providing evidence that consistent SCD and worry are independent predictors of incident MCI.
In addition, the SCD plus criteria propose that memory SCD increases likelihood of underlying AD more than SCD concerning other cognitive domains.1 Our findings are in line with this proposal in the sense that memory is indeed the best predictor of incident MCI when domain-specific SCD scores are compared. However, our results also illustrate the importance of taking all complaints into account, even in an elderly population. Similar to the study from the University of California,35 multidomain SCD performed as well or slightly better than memory alone. A likely reason for this finding is that, while a memory-predominant phenotype of AD is most common, nonmemory phenotypes are found in a substantial number of patients in different dementia cohorts.37 If SCD is a relevant precursor of deficits later in the disease, ascertaining memory SCD without considering other complaints may be too restrictive. In addition, MCI is a syndrome with a biologically heterogeneous background, which could also account for the relevance of part of the nonmemory complaints.
Self-report of consistent SCD at a single point in time is not part of the SCD plus criteria. Based on our results, we propose that it may be one of SCD's most relevant aspects. It is easily ascertained, both on a questionnaire and in daily clinical practice, and its presence increases risk of incident MCI independently of associated worry. Data from the AgeCoDe study have suggested that repeatedly endorsing SCD assessed by a single question over multiple visits in a longitudinal study has a similar effect on risk of clinical progression.38 This can be viewed as another way of evaluating whether someone's SCD is consistent and may therefore be considered congruent with our results.
We found an important difference between iSCD (SCD endorsed by the informant) and self-perceived SCD: informants noticed cognitive decline in the participant less often than participants did, but when noted, occasional iSCD predicted incident MCI, while occasional self-perceived SCD did not. Compared to no SCD, the HRs associated with consistent SCD were very similar in both groups. Overall, continuous measures for SCD seemed to perform equally well in participants and informants, perhaps with a slight overall advantage for the informants. Prior evidence regarding the comparison between iSCD and SCD in CU has been inconsistent. In 2 longitudinal studies, iSCD proved more useful than SCD to predict AD dementia,5,39 but in the Sydney Memory and Ageing study, neither predicted MCI in CU, although iSCD was related to cognition 4 years later in a combined CU/MCI group.40 In ADNI, iSCD was a better indicator of preclinical AD than SCD,41 although other comparative studies found neither iSCD nor SCD was associated with preclinical AD in CU.22,42 Our results indicate different aspects of SCD may be of more value in informants than in participants. This may be reflected in the inconsistency of prior evidence.
An important strength of the current study is that we were able to determine the relationship between SCD and risk of MCI in a sample selected randomly from the general population. We used models that were adjusted for a broad range of possible confounders. SCD has been shown to be associated with depressive symptoms, certain personality traits, anxiety, and physical complaints.9 However, even after adjusting for these factors in our analyses, the association between SCD and risk of MCI remained. Another important confounder included in our study is objective cognitive performance. Associations between SCD and objective performance have been shown to be small at best.43 Part of the proposed value of SCD lies in the hypothesis that SCD can indicate cognitive decline before it becomes obvious on standardized tests. A recent study has shown decline on cognitive testing actually precedes SCD,44 but SCD did precede change in Mini-Mental State Examination scores in the Rotterdam study.45 Analysis of the ADNI dataset has shown that the prognosis of SCD without worse objective cognitive performance was similar to CU without SCD.36 Our results indicate that SCD does add to objective test performance. Limitations include that we could not ascertain every aspect of the SCD plus criteria. For example, others have suggested that a comparison between the participant and others of the same age group might be more predictive than a comparison between earlier periods in time, but we were unable to ascertain this. In addition, the SCD plus criteria mention several biological factors, which we did not include in the current study, because we decided to focus on the clinical characteristics of cognitive decline instead of the combination between SCD and biological factors.
Glossary
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- AgeCoDe study
German Study on Ageing, Cognition, and Dementia
- CU
cognitively unimpaired
- ECog
Everyday Cognition
- HR
hazard ratio
- iECog
informant-based Everyday Cognition
- iSCD
informant-based subjective cognitive decline
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- SCD
subjective cognitive decline
Footnotes
Editorial, page 153
Author contributions
Dr. van Harten: study concept and design, interpretation of results, writing of the manuscript. Dr. Mielke: study concept and design, manuscript revision. D.M. Swenson-Dravis: study concept and design, data acquisition, manuscript revision. C.E. Hagen: data analysis and interpretation, manuscript revision. K.K. Edwards: data analysis and interpretation. Dr. Roberts: study concept and design, manuscript revision. Dr. Geda: study concept and design, manuscript revision. Dr. Knopman: study concept and design, data acquisition, manuscript revision. Dr. Petersen: study concept and design, data acquisition, manuscript revision, study supervision.
Study funding
Funded by NIA U01 AG006786, P50 AG016574, GHR Foundation, and Mayo Foundation.
Disclosure
A. van Harten reports no disclosures relevant to the manuscript. M. Mielke served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc. She receives research support from the NIH (R01 AG49704, P50 AG44170, U01 AG06786, RF1 AG55151), Department of Defense (W81XWH-15-1), and unrestricted research grants from Biogen, Roche, and Lundbeck. D. Swenson-Dravis, C. Hagen, and K. Edwards report no disclosures relevant to the manuscript. R. Roberts receives research funding from the NIH, Roche, and Biogen. Y. Geda serves as a consultant to Lundbeck and receives research support from Roche Inc. D. Knopman serves on a data safety monitoring board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the Alzheimer's Disease Cooperative Study; and receives research support from the NIH. R. Petersen is a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., Genentech, Inc., and Axovant Science. Go to Neurology.org/N for full disclosures.
References
- 1.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley RF, Maruff P, Ames D, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement 2016;12:796–804. [DOI] [PubMed] [Google Scholar]
- 3.Chary E, Amieva H, Pérès K, Orgogozo JM, Dartigues JF, Jacqmin-Gadda H. Short- versus long-term prediction of dementia among subjects with low and high educational levels. Alzheimers Dement 2013;9:562–571. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand 2014;130:439–451. [DOI] [PubMed] [Google Scholar]
- 5.Caselli RJ, Chen K, Locke DEC, et al. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement 2014;10:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrotin A, La Joie R, de La Sayette V, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement 2017;13:550–560. [DOI] [PubMed] [Google Scholar]
- 7.Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 2012;79:1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedro MC, Mercedes MP, Ramón LH, Borja MR. Subjective memory complaints in elderly: relationship with health status, multimorbidity, medications, and use of services in a population-based study. Int Psychogeriatr 2016;28:1903–1916. [DOI] [PubMed] [Google Scholar]
- 9.Hill NL, Mogle J, Wion R, et al. Subjective cognitive impairment and affective symptoms: a systematic review. Gerontologist 2016;56:e109–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snitz BE, Weissfeld LA, Cohen AD, et al. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry 2015;23:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement 2017;13:296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis 2015;48(suppl 1):S63–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Joie R, Perrotin A, Egret S, et al. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: association with medical help seeking, cognitive deficits, and β-amyloid imaging. Alzheimers Dement 2016;5:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology 2010;75:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 19.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797–811. [DOI] [PubMed] [Google Scholar]
- 20.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 2008;22:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaszewski Farias S, Mungas D, Harvey DJ, Simmons A, Reed BR, Decarli C. The measurement of everyday cognition: development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement 2011;7:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rueda AD, Lau KM, Saito N, et al. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer's disease. Alzheimers Dement 2015;11:1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 25.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 26.Wechsler DA. Manual for the Wechsler Memory Scale–Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 27.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychol 1992;6:83–104. [Google Scholar]
- 28.Krell-Roesch J, Woodruff BK, Acosta JI, et al. APOE ε4 genotype and the risk for subjective cognitive impairment in elderly persons. J Neuropsychiatry Clin Neurosci 2015;27:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2010;18:701–710. [DOI] [PubMed] [Google Scholar]
- 30.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67:414–422. [DOI] [PubMed] [Google Scholar]
- 31.Luck T, Luppa M, Matschinger H, Jessen F, Angermeyer MC, Riedel-Heller SG. Incident subjective memory complaints and the risk of subsequent dementia. Acta Psychiatr Scand 2015;131:290–296. [DOI] [PubMed] [Google Scholar]
- 32.Dik MG, Jonker C, Comijs HC, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology 2001;57:2217–2222. [DOI] [PubMed] [Google Scholar]
- 33.van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MMB. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimers Dement 2007;3:92–97. [DOI] [PubMed] [Google Scholar]
- 34.Luck T, Roehr S, Jessen F, Villringer A, Angermeyer MC, Riedel-Heller SG. Mortality in individuals with subjective cognitive decline: results of the Leipzig Longitudinal Study of the Aged (LEILA75+). J Alzheimers Dis 2015;48(suppl 1):S33–S42. [DOI] [PubMed] [Google Scholar]
- 35.Farias ST, Lau K, Harvey D, Denny KG, Barba C, Mefford AN. Early functional limitations in cognitively normal older adults predict diagnostic conversion to mild cognitive impairment. J Am Geriatr Soc 2017;65:1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledo JB, Bjerke M, Chen K, et al. Memory, executive, and multidomain subtle cognitive impairment: clinical and biomarker findings. Neurology 2015;85:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheltens NME, Tijms BM, Koene T, et al. Cognitive subtypes of probable Alzheimer's disease robustly identified in four cohorts. Alzheimers Dement 2017;13:1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfsgruber S, Kleineidam L, Wagner M, et al. Differential risk of incident Alzheimer's disease dementia in stable versus unstable patterns of subjective cognitive decline. J Alzheimers Dis 2016;54:1135–1146. [DOI] [PubMed] [Google Scholar]
- 39.Rabin LA, Wang C, Katz MJ, Derby CA, Buschke H, Lipton RB. Predicting Alzheimer's disease: neuropsychological tests, self-reports, and informant reports of cognitive difficulties. J Am Geriatr Soc 2012;60:1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slavin MJ, Sachdev PS, Kochan NA, et al. Predicting cognitive, functional, and diagnostic change over 4 years using baseline subjective cognitive complaints in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2015;23:906–914. [DOI] [PubMed] [Google Scholar]
- 41.Valech N, Mollica MA, Olives J, et al. Informants' perception of subjective cognitive decline helps to discriminate preclinical Alzheimer's disease from normal aging. J Alzheimers Dis 2015;48(suppl 1):S87–S98. [DOI] [PubMed] [Google Scholar]
- 42.Hollands S, Lim YY, Buckley R, et al. Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimers Dis 2015;43:677–686. [DOI] [PubMed] [Google Scholar]
- 43.Burmester B, Leathem J, Merrick P. Subjective cognitive complaints and objective cognitive function in aging: a systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev 2016;26:376–393. [DOI] [PubMed] [Google Scholar]
- 44.Koppara A, Wagner M, Lange C, et al. Cognitive performance before and after the onset of subjective cognitive decline in old age. Alzheimers Dement 2015;1:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verlinden VJA, van der Geest JN, de Bruijn RFAG, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement 2016;12:144–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared by request from a qualified investigator in accordance with the MCSA data-sharing protocol.