Abstract
Physical activity—a lifestyle factor that is associated with immune function, neuroprotection, and energy metabolism—modulates the cellular and molecular processes in the brain that are vital for emotional and cognitive health, collective mechanisms that can go awry in depression. Physical activity optimizes the stress response, neurotransmitter level and function (e.g., serotonergic, noradrenergic, dopaminergic, and glutamatergic), myokine production (e.g., interleukin-6), transcription factor levels and correlates [e.g., peroxisome proliferator-activated receptor C coactivator-1α [PGC-1α], mitochondrial density, nitric oxide pathway activity, Ca2+ signaling, reactive oxygen specie production, and AMP-activated protein kinase [AMPK] activity], kynurenine metabolites, glucose regulation, astrocytic health, and growth factors (e.g., brain-derived neurotrophic factor). Dysregulation of these interrelated processes can effectuate depression, a chronic mental illness that affects millions of individuals worldwide. Although the biogenic amine model has provided some clinical utility in understanding chronic depression, a need remains to better understand the interrelated mechanisms that contribute to immune dysfunction and the means by which various therapeutics mitigate them. Fortunately, convergent evidence suggests that physical activity improves emotional and cognitive function in persons with depression, particularly in those with comorbid inflammation. Accordingly, the aims of this review are to (1) underscore the link between inflammatory correlates and depression, (2) explicate immuno-neuroendocrine foundations, (3) elucidate evidence of neurotransmitter and cytokine crosstalk in depressive pathobiology, (4) determine the immunomodulatory effects of physical activity in depression, (5) examine protocols used to effectuate the positive effects of physical activity in depression, and (6) highlight implications for clinicians and scientists. It is our contention that a deeper understanding of the mechanisms by which inflammation contributes to the pathobiology of depression will translate to novel and more effective treatments, particularly by identifying relevant patient populations that can benefit from immune-based therapies within the context of personalized medicine.
Keywords: immune, stress, depression, physical activity, neuroprotection, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, growth factors, glutamate
Introduction
Depression is a pervasive health problem that includes emotional, psychomotor, cognitive, and biorhythmic disturbances (Kessler et al., 2005), symptoms that are associated with a 20-fold increase in the risk of suicide (Lépine and Briley, 2011). Current estimates suggest that close to 300 million persons are affected worldwide (Ferrari et al., 2013a), making depression the leading cause of disability as measured by disability-adjusted life years (Reddy, 2010). In addition to the incredible personal toll, the direct and indirect costs of treating depression are staggering. Spending on depression-related costs is $83.1 billion annually in the United States (Greenberg et al., 2003).
Initial progress toward understanding the pathobiology of depression was made following the serendipitous discovery that amine modulation effectuated disturbances in mood (Ghasemi et al., 2017), a finding that suggested that depression was caused by deficits in monoamine function. Accordingly, the majority of therapies for treating depression were derived to target the monoaminergic system. Notwithstanding, extant therapeutics exert a slow pace of action (3–5 weeks), have extensive side effects, and fail to provide full symptom relief in a significant proportion of persons treated (Paul and Skolnick, 2003; Trivedi, 2006). These limitations implicated other factors in depression and prompted stakeholders to diversify their search for mechanisms, biomarkers, and treatments.
Among the alternative mechanisms and therapeutics that have garnered increased attention are immune mechanisms that promote the body's natural response to protect against injury, infection, and emotional stress. The brain regulates central and peripheral immune processes via modulation of neurotransmitters (e.g., serotonergic, noradrenergic, dopaminergic, and glutamatergic), endocrine hormones, and cytokines (nonstructural proteins that are secreted by distinct cell populations and that exert variable effects at multiple levels of the central nervous system, e.g., neuroendocrine, autonomic, and behavioral) (Besedovsky and del Rey, 1992; Niciu et al., 2014; Ghasemi et al., 2017), processes that can go awry in the case of depression.
Indeed, a bevy of research indicates a link between inflammation and depression. Preclinical study demonstrates that stress paradigms (e.g., chronic unpredictable stress, learned helplessness, social defeat, and social isolation) induce pro-inflammatory cytokines centrally and peripherally (Steptoe et al., 2001; Bartolomucci et al., 2003; Grippo et al., 2005; Chourbaji et al., 2006; Audet et al., 2011; Gómez-Lázaro et al., 2011; Moller et al., 2013), changes that correlate with depressive-like symptoms (Kenis and Maes, 2002; Tuglu et al., 2003; Basterzi et al., 2005; Tsao et al., 2006; Dantzer et al., 2008; Miller et al., 2009; Elgarf et al., 2014; Lu et al., 2017) but can be mitigated following antidepressant administration (Dantzer et al., 2008; Guo et al., 2009; Miller et al., 2009; Elgarf et al., 2014; Lu et al., 2017). Others have shown that genetically modified rodents with impaired pro-inflammatory immune signaling fail to exhibit depressive-like behaviors that are induced in wild-type mice following chronic mild stress (Goshen et al., 2008; Brüning et al., 2015). Agents that induce inflammation (e.g., recombinant cytokines) in humans recapitulate symptoms of depression, effects that are circumvented with selective serotonin reuptake inhibitor (SSRI) administration (Hauser et al., 2002). In persons with autoimmune or inflammatory disorders, tumor necrosis factor (TNF) antagonists and certain nonsteroidal anti-inflammatory agents (Brunello et al., 2006; Müller et al., 2006; Tyring et al., 2006; Krishnan et al., 2007; Soczynska et al., 2009; Menter et al., 2010; Fond et al., 2014; Köhler et al., 2014; Abbott et al., 2015) exert antidepressant effects. In the general population, clinical evidence suggests an increased tendency for pro-inflammatory markers in persons with depression (increased interleukin [IL]-6, TNF-α, and CRP) relative to controls (Laske et al., 2008; Steiner et al., 2012), a trend that normalizes following response to antidepressants (Myint et al., 2005). Others have shown that administration of anti-inflammatory agents in combination with antidepressants accelerates and enhances treatment response in a subset of persons with depression (Mendlewicz et al., 2006; Müller et al., 2006; Nery et al., 2008; Akhondzadeh et al., 2009; Abbasi et al., 2012; Raison et al., 2013). Certain polymorphisms in genes associated with inflammation are associated with the risk for the development of mood disorders and treatment response (Bufalino et al., 2013; Michopoulos et al., 2015). Together, these findings implicate inflammation in a subset of persons with depression who likely exhibit unique variations in pathobiology and clinical presentation.
Fascinatingly, parallel evidence demonstrates that a lack of physical activity (PA) promotes the accumulation of visceral fat, adipose infiltration by proinflammatory immune cells, persistent low-grade inflammation (Ouchi et al., 2011), and, thereby, an increased risk for depression (Leonard, 2007). Conversely, adequate levels of persistent PA exert positive immunomodulatory (Hamer and Steptoe, 2007; Walsh et al., 2011) and antidepressant effects (Cooney et al., 2013; Schuch et al., 2016), even in persons who did not remit with conventional antidepressant treatment (Trivedi et al., 2011). Some reports suggest that PA outcomes compare favorably to antidepressant and cognitive behavioral therapy in mild to moderate depression (Mead et al., 2009). The therapeutic effect of long-term PA on depression likely includes the optimization of neurotransmitter level and function, hormone regulation, muscle-derived protein (e.g., peroxisome proliferator-activated receptor C coactivator-1α [Pgc-1α] and IL-6), and neurotrophic factors (Phillips, 2017a). Within contracting skeletal muscles, PA elicits intermittent elevations of IL-6 (Pedersen et al., 2001; Pedersen and Fischer, 2007), which then induces the synthesis of IL-10 and inhibits the release of TNF-α (Schindler et al., 1990; Apostolopoulos et al., 2014; Silverman and Deuster, 2014). Upon release into the local and systemic circulation, IL-10 promotes an anti-inflammatory milieu in the periphery. In the long-term, PA appears to lower levels of proinflammatory cytokines by altering visceral fat mass (de Lemos et al., 2012; Sell et al., 2012) and Toll-like receptors (TLRs) (Lambert et al., 1985; Francaux, 2009; Gleeson et al., 2011; Sell et al., 2012; Drummond et al., 2013), changes that may be particularly beneficial to persons with comorbid depression and metabolic disorders given that activation of TLRs contribute to the development of insulin resistance (Francaux, 2009; Liang et al., 2013). Consideration of its inexpensive low-risk profile and ease of implementation (Barbour et al., 2007) has increasingly led to the suggestion that PA can be deployed as a therapeutic strategy to reduce the degree of depressive symptoms (Cooney et al., 2013; Pemberton and Fuller Tyszkiewicz, 2016) in mild to moderate depression (Carek et al., 2011; Stanton and Reaburn, 2014; Kvam et al., 2016; Schuch et al., 2016) in all age groups (Abu-Omar et al., 2004; Motl et al., 2004).
Because it is important that clinicians and scientists understand the means by which PA can exert immunomodulatory effects in depression from both a self- and patient-education perspective, the aims of this review are to (1) underscore the link between inflammatory correlates and depression, (2) explicate immuno-neuroendocrine foundations, (3) elucidate evidence of monoaminergic and cytokine crosstalk in depressive pathobiology, (4) articulate the immunomodulatory mechanisms and pathways that confer the benefits of PA in depression, (5) examine protocols used to effectuate the benefits of PA in depression, and (6) highlight implications for clinicians and scientists. It is our contention that a deeper understanding of the mechanisms by which inflammation contributes to the pathobiology of depression will translate to novel and more effective treatments, particularly by identifying relevant patient populations that can benefit from immune-based therapies within the context of personalized medicine.
Immuno-neuroendocrine foundations
Within the context of homeostatic challenge, stressors initiate behavioral and immune responses so as to favor vigilance and protection against injury and immune challenge in lieu of explorative activities. The hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system coordinate these activities following activation by endogenous or exogenous stressors (Tsagarakis et al., 1989; Besedovsky and Rey, 2007). Specifically, exposure to psychological and physiological stressors activates the paraventricular nucleus of the hypothalamus. Activation of the paraventricular nucleus results in the release of corticotropin-releasing hormone (CRH), which then stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary. This, in turn, induces cortisol and catecholamine secretion from the adrenals (Figure 1). Initially, the HPA and sympathetic nervous system function to increase cortisol and catecholamine release during challenge to coordinate the fight-or-flight response. These stress hormones inhibit excess production of proinflammatory cytokines (e.g., IL-12, TNF-α, and interferon [IFN]-γ) in healthy individuals with optimal regulatory capacity, while simultaneously increasing production of anti-inflammatory cytokines (e.g., IL-10 and IL-4) (Elenkov and Chrousos, 1999). Concomitantly, cortisol exerts inhibitory effects upon the hypothalamus and pituitary (Crosby and Bains, 2012) through medial prefrontal cortex (mPFC) receptors (Hill et al., 2011a,b) and reduces stress-induced over excitability of the amygdala (Gray et al., 2015) in conditions of health. Temporal and sequential regulation of the immune response is paramount as failure in negative feedback effectuates persistent hypersecretion of proinflammatory cytokines, which can then induce central neuroinflammation (Leonard, 2001; Lacy and Stow, 2011) via active transport mechanisms at the circumventricular organs, binding to blood vessel receptors, or by retrograde transport by the vagus nerve (Maier and Watkins, 2003). By accessing the brain via the aforementioned mechanisms, systemic inflammation can activate resident microglia in the brain (McCusker and Kelley, 2013) following peripheral immune challenge (D'Mello et al., 2009) and promote depression in vulnerable individuals.
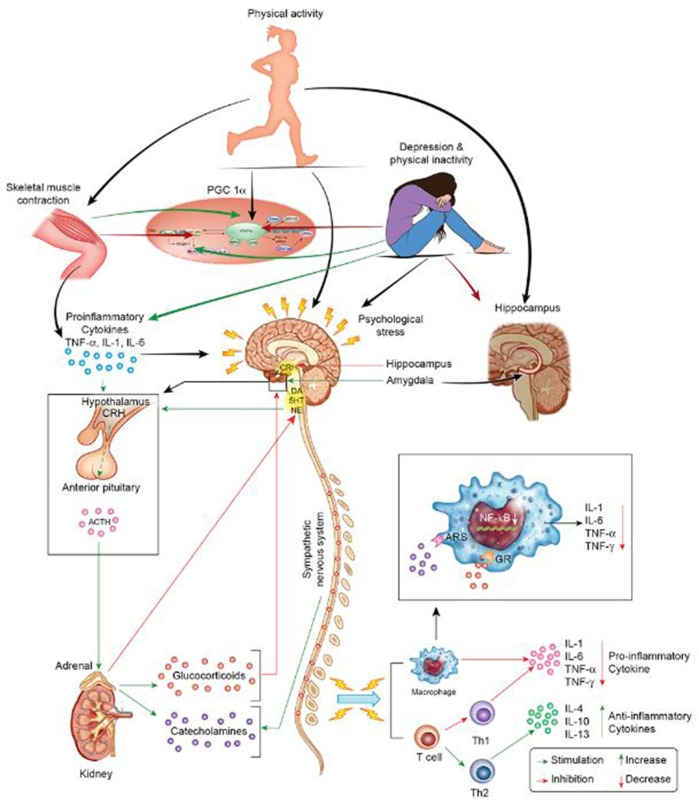
Figure 1.
Stress, inflammation, and depression. The HPA and sympathetic nervous systems regulate the response to stressors, i.e., cytokines, psychological stress, and PA. The systemic response to stress is initiated via CRH secretion by the hypothalamus. CRH stimulates the pituitary to secrete ACTH into systemic circulation. In turn, ACTH secretion stimulates the adrenals to release catecholamines and glucocorticoids, factors that collectively induce pro- or anti-inflammatory cytokine release. Negative feedback mechanisms limit the process of inflammation in times of health. Conversely, persistent stress leads to dysregulation of the HPA with resultant endocrine disturbances in states of disease, e.g., depression. Stress-related disturbances in neuroendocrine hormones are problematic as they disrupt immune modulation and lead to a pro-inflammatory state. By acting as an intermittent stressor, PA exerts its' central and peripheral neuroprotective effects via several avenues. During PA, muscle contractions induce the release of myokines. These factors increase the expression of PGC-1α and decrease the expression of pro-inflammatory cytokines at the molecular level. Moreover, PA directly modulates neurotransmitter level and function (e.g., noradrenergic function), which is important promoting a pro- or anti-inflammatory milieu. Finally, PA increases hippocampal neurotrophic factor levels (e.g., BDNF) to promote hippocampal health and, thereby, promotes stress hormone regulation (e.g., cortisol regulation).
Undoubtedly, persistent systemic inflammation alters the function and expression of glucocorticoid receptors in the HPA axis, changes that impair negative feedback mechanisms (Karanth et al., 1997) at the level of the hypothalamus and anterior pituitary (Besedovsky and Rey, 2007). Chronic stress results in lower diurnal cortisol secretion as well as a blunted stress response (Peeters et al., 2003), which is problematic for immune modulation given the anti-inflammatory characteristics of catecholamines (e.g., noradrenaline (Bergmann et al., 1999) and cortisol (Cupps and Fauci, 1982). Whereas basal levels of noradrenaline promote an anti-inflammatory milieu (Bergmann et al., 1999), depletion of noradrenaline promotes a proinflammatory milieu, an effect that can be blocked by the beta adrenergic receptor agonist isoproterenol (Madrigal et al., 2005). Additionally, disruption of the catecholamine response is deleterious to the brain given that noradrenaline modulates the neuroprotective effects of astrocytes via trophic factor release (Junker et al., 2002). Much evidence clearly demonstrates that HPA dysregulation and prolonged inflammation contribute to depressive pathobiology (Pariante and Miller, 2001).
Thus, on the one hand the immune system defends against endogenous and exogenous stressors. On the other hand, it acts as a regulatory system that is in continual communication with the nervous and the endocrine systems via reciprocal communication mediated by cytokines, hormones, and neurotransmitters (Besedovsky and Rey, 2007). Imbalances among these mediators induce chronic disease conditions such as depression. Some persons with depression exhibit activated cell-mediated immunity with a T helper (Th)1-style response and elevated levels of IFN-γ (Maes et al., 1990; Maes, 2011), whereas others exhibit a distinct subtype wherein a Th2-style response predominates (Fornaro et al., 2013), possibly reflective of different stressor profiles, disease points, or genetic contributions.
At the molecular level, pro-inflammatory cytokine interactions with their cognate receptors initiate signaling events that promote a feedforward inflammatory process if left unchecked. IkB proteins typically sequester inactive transcription factors in the cytoplasm in unstimulated cells. Yet in states of low-grade inflammation, persistent receptor stimulation by cytokines and TLR agonists triggers intracellular signaling events that activate IkB kinase (IKK) activity and induce the dissociation of the IkB protein complex and, in turn, promote IkB degradation. The resultant release of nuclear factor κB (NFkB) dimers (e.g., p50/p60) permits their translocation to the nucleus and binding to cognate DNA sites that then regulates transcription of inflammatory genes and antioxidant defense (Baldwin, 1996).
Within the context of metabolic disorders, it has become increasingly evident that the presence of persistent pro-inflammatory cytokines plays a vital role in certain depressive phenotypes. The global prevalence of depression is approximately 5% in the general population; and yet, this figure approximates 20% or more in persons with obesity, diabetes, and coronary artery disease (Ferrari et al., 2013b), subsets of the population that may be particularly resistant to conventional antidepressant therapy (Raison et al., 2013; Felger et al., 2016; Haroon et al., 2016). The low-grade inflammation that results in these persons derives in part from macrophages and T-cells infiltration of adipocytes in white adipose tissue, liver, and skeletal muscle. This infiltration elicits a state of persistent secretion of proinflammatory cytokines, including TNF-α, IL-1, and IL-6 and a reduction in anti-inflammatory cytokines (e.g., adiponectin) (Hotamisligil, 2006; Kanda et al., 2006; Pedersen, 2009; Ouchi et al., 2011). The proinflammatory cytokines IL-1, IL-6, and TNF-α are thought to play a primal role in the neurotransmitter and neuroendocrine changes that occur in depression given their central role in sickness behavior. Undoubtedly, the presence of pro-inflammatory cytokines markedly affects neurotransmission within regulatory brain circuits related to emotions and induces hormonal changes commensurate with those observed following stress (Gadek-Michalska et al., 2013).
Neurotransmitter and cytokine interactions in depression
Heretofore, we have established how depression is associated with peripheral and central inflammation and, thereby, how anti-inflammatory agents mitigate symptoms. Now we review evidence that inflammatory cytokines alter the level and function of key neurotransmitters that are relevant to depression neurobiology. We also explicate how cytokines activate the kynurenine pathway, lower tryptophan levels, and produce metabolites that modulate dopamine and glutamate function. We then shown how anti-inflammatory therapeutics (pharmacotherapy and PA) can optimize monoamine neurotransmitter level and function by modulating the synthesis, metabolism, and release of serotonin, noradrenaline, dopamine, and glutamate (Phillips, 2017a). Via these mechanisms, inflammation and therapeutics that mitigate depression may dramatically alter its pathobiology by directly impinging on the levels and function of key neurotransmitters that regulate depression circuit function and integrity and, ultimately, the affected individual's emotional and cognitive health.
Serotonergic interactions
The majority of central serotonin-synthesizing neurons within the brain derive from the raphe nuclei, which are located near the midline of the brainstem (Das et al., 2014). The raphe sends more than 500,000 terminals to the cortical and limbic system (Cowen, 1991). This immense number of connections enable the raphe to modulate mood (Canli and Lesch, 2007), appetite (Blundell, 1984), arousal (Dubovsky, 1994), impulsivity (Dubovsky, 1994), aggression (Passamonti et al., 2012), and the sleep-wake cycle (Monti, 2011). Serotonin is synthesized from dietary tryptophan and translocated to the central nervous system, a rate-limiting step in serotonin synthesis. The fact that the serotonergic dorsal raphe is juxtaposed to the cerebral aqueduct suggests a vulnerability to inflammation. Indeed, evidence demonstrates that proinflammatory cytokines alter the functional status of the serotonergic system in the raphe and beyond in a manner similar to that seen in depression, providing a mechanistic explanation for the serotonergic abnormalities and associated symptoms seen in persons with depression.
Direct evidence that cytokines contribute to serotonergic dysfunction in depression pathobiology derives from data from electrophysiological, neurochemical, genetic, behavioral models, as well as from translational investigations. Intracellular recordings performed in a guinea-pig brain stem slice preparation demonstrated that IL-1β decreased spontaneous firing rates of serotonergic neurons by 50%, an effect that was reversible with washout (Manfridi et al., 2003). A parallel investigation in rodents showed that IL-1β inhibited the firing of dorsal raphe serotonergic neurons by enhancement of GABA-ergic inhibitory tone (Brambilla et al., 2007). These electrophysiological findings are congruent with the idea that IL-1 promotes non-rapid eye movement sleep by inhibiting the spontaneous firing of wake-active serotonergic neurons in the dorsal raphe nucleus (Brambilla et al., 2007). By corollary, the latter findings suggest that IL-1 alterations may contribute to alterations in arousal in depression, particularly to disturbances in stage III and IV sleep (Jones et al., 1987). Other work demonstrates that peripheral immune challenge alters the release and metabolism of central serotonin across brain regions (Dunn, 1992; Palazzolo and Quadri, 1992; Cho et al., 1999), changes that alter transporter activity. Serotonin transporters are responsible for transporting serotonin from the synaptic cleft to the presynaptic neurons to terminate signaling. As a high-affinity transporter, serotonin transporters maintain low extracellular serotonin levels in the synapse to prevent overstimulation of receptors and ensure responsiveness. Within this context, it has been determined that chronic exposure to proinflammatory cytokines alters serotonin transporter activity in a regionally specific manner and, thereby, modulates serotonin levels in the nerve terminal (Haase and Brown, 2015). Some preclinical evidence shows that stress-induced activation of the 5-HT2a serotonin receptor decreased hippocampal brain-derived neurotrophic factor (BDNF) levels (Vaidya et al., 1999).
Fortunately, several lines of work suggest that chronic PA modulates the serotonergic system to mitigate depressive symptoms in persons with inflammation. Translational studies demonstrate that plasma-free tryptophan is increased following PA (Davis et al., 1992; Melancon et al., 2012) by catecholamine-induced elevations in lipolysis and non-esterified fatty acids that displace albumin-bound tryptophan (Horowitz and Klein, 2000). Elevations in free tryptophan levels in the periphery increase tryptophan availability to the brain and, in turn, enhance serotonin synthesis (Chaouloff et al., 1986).
It seems logical that serotonin levels are more readily maintained with chronic PA given that it reduces proinflammatory markers (e.g., IFN-γ and TNF-α) and increases anti-inflammatory markers (e.g., IL-6 and IL-10) (Petersen and Pedersen, 1985; Smith et al., 1999; Panagiotakos et al., 2005; Kohut et al., 2006; Liu et al., 2013). For example, persons with depression exhibit a decrease in the pro-inflammatory cytokine TNF-α after submax exercise along with an increase in anti-inflammatory IL-4 (Hallberg et al., 2010). Within adipose tissue, PA limits proinflammatory cytokine secretion and inhibits macrophage infiltration phenotypic switching (from pro- to anti-inflammatory) of macrophages (Kawanishi et al., 2010). Within immune cells and skeletal muscles, PA decreases TLR4 and TLR2 expression (Gleeson, 1985; Lambert et al., 1985; Francaux, 2009), changes that decrease the inflammatory capacity of leukocytes and may alter whole-body chronic inflammation (Gleeson et al., 2006). Studies of human peripheral blood following PA demonstrate a reduction in circulating proinflammatory monocytes (Timmerman et al., 2008) and an increase in circulating regulatory T cells (Yeh et al., 2006).
Undoubtedly, the ability of PA to bias the immune system toward an anti-inflammatory state is significant for the serotonergic system because this state downregulates IDO activity and shifts the ratio of kynurenine metabolites toward neuroprotective kynurenic acid and away from neurotoxic quinolinic acid (Ito et al., 2003; Kiank et al., 2010). Moreover, the reduction of pro-inflammatory cytokines by PA reduces serotonin uptake by transporters to increase serotonin in the nerve terminal (Mössner et al., 2001). Finally, the ability of PA to optimize levels of tryptophan and serotonin exerts positive effects on BDNF and neurogenesis in the prefrontal cortex and hippocampus, underscoring the interrelationship between these two signaling systems (Mattson et al., 2004; Ernst et al., 2006; Esch and Stefano, 2010).
Noradrenergic interactions
Noradrenergic neurons are a vital component of the central “stress circuitry” that induces “fight or flight” behavior, fear, and anger. Noradrenergic synthesizing neurons are primarily located in the locus coeruleus (LC), a brainstem structure within close proximity to the fourth ventricle (Phillips et al., 2016). The LC sends extensive projections to a number of brain regions including the thalamus, frontal and entorhinal cortices, basal lateral amygdala, and hippocampus (Loughlin et al., 1986). The LC's vast arborization and divergence of collaterals permit widespread synaptic and extrasynaptic release of neurotransmitters and neuropeptides throughout the central neuraxis onto neuronal and non-neuronal cells (Fornai et al., 2007, 2011), enabling the LC to exert a significant modulatory effect on behavior and HPA axis secretion. The latter occurs via dense noradrenergic projections from the LC to corticotropin-releasing hormones in the paraventricular nucleus of the hypothalamus, enabling psychological stress and immune challenges to activate the hypothalamus and trigger glucocorticoid release (Gadek-Michalska et al., 2013). Robust evidence demonstrates that proinflammatory cytokines alter the functional status of the noradrenergic system in the LC and beyond, providing a mechanistic explanation for the noradrenergic abnormalities and associated symptoms seen in persons with depression.
Direct evidence that cytokines can contribute to noradrenergic dysfunction derives from electrophysiological, neurochemical, genetic, behavioral models, and from translational studies. Microinjection of IL-1 into the LC region increased firing activity of LC neurons in the rat brain, an effect that was blocked by an IL-1 antagonist. Intraperitoneal injection of a low dose of lipopolysaccharide increased LC firing activity, an effect that lasted 3 weeks after injection (Borsody and Weiss, 2002). IL-2 and IFN-α administration altered LC electrical activity (De Sarro et al., 1990; Nisticò and De Sarro, 1991; Nisticò, 1993). The ability of inflammation to increase LC activity is significant because a common characteristic of effective antidepressants is their ability to decrease LC neuronal activity. Moreover, LC activity is highly correlated with arousal: higher rates of neuronal firing occur during the awake state, whereas complete LC neuronal inhibition occurs during rapid eye movement sleep (Hobson et al., 1974; Aston-Jones and Bloom, 1981), suggesting that alterations in firing patterns in depression may contribute to the sleep disturbances seen in depression. Juxtaposed with the electrophysiological studies are neurochemical investigations that showed that a common response to cytokine release involved an increased noradrenaline metabolism in multiple brain regions (Dunn et al., 1989), but with a preferential activation of the ventral component of the system, which derived from the nucleus tractus solitarius and LC (Dunn, 1988). Lipopolysaccharides IL-1, IL-2, and IFN-α potently activate the noradrenergic system across brain regions (De Sarro et al., 1990; Dunn, 1992; Smagin et al., 1996), a change that is not surprising because sustained stress increases noradrenergic requirements. Pharmacological manipulations that increase noradrenergic action and duration at the synapse (e.g., noradrenergic reuptake inhibitors) elevate mood and attention, mitigating the effects of stress-mediated noradrenergic depletion.
Outside the brain, noradrenaline can modulate autonomic sympathetic postganglionic fibers via primary and secondary lymphoid organs (bone marrow and thymus versus spleen and lymph nodes, respectively) during immune challenge. These actions are accomplished via direct activation of β2-adrenergic receptors that are present on Th1 cells, but not on Th 2 cells (Sanders et al., 1997). Purportedly, the anti-inflammatory effects of β2-adrenergic receptors activation stem from the inhibition of Th1 pro-inflammatory cytokines (e.g., IFN-γ, IL-12, TNF-α) or stimulation of Th2 anti-inflammatory cytokines (IL-10, IL-6, or TGF-β) (van der Poll et al., 1996; Elenkov et al., 2000). It has been shown that noradrenaline suppresses IL-12 production in a dose-dependent fashion and at physiological concentrations, whereas it dose-dependently increases the production of IL-10, effects that are blocked completely by propranolol, a β-adrenergic receptor antagonist (Elenkov et al., 1996). These findings suggest that immune balance is regulated via peripheral end-effectors of the stress system and that chronic dysregulation may bias the system toward a pro-inflammatory status (Elenkov et al., 1996).
Several lines of evidence suggest that PA modulates the noradrenergic system directly and indirectly to mitigate depressive symptoms in persons with inflammation. Within minutes, PA activates the sympathetic nervous system in an activity-dependent manner to modulate the secretion of the adrenal hormone adrenaline. Similarly, within minutes PA activates release of ACTH from the hypothalamus. These intermittent hormonal responses are vital for an anti-inflammatory milieu because intermittent elevations in adrenaline (Bergmann et al., 1999) and cortisol exert anti-inflammatory effects (Cupps and Fauci, 1982). Most studies of PA have reported a higher adrenaline response post exercise in endurance trained persons as compared to untrained controls (Zouhal et al., 2008). Other work has shown that running elevates cortisol levels in saliva and plasma in healthy persons (Duclos et al., 1998; Labsy et al., 2013) when performed at 60% of VO2max (maximum capacity of oxygen uptake) (Labsy et al., 2013). The intermittent nature of PA also contributes to a proportional increase in inactivation of the active steroid (cortisol) into the inert steroid (cortisone). The ability of PA to optimize catecholamine and cortisol levels is paramount in persons with comorbid depression and inflammation because these hormones modulate immune function and yet may be blunted as a consequence of chronic stress.
Within the brain, PA induces the neuronal adaptation that is requisite for mitigating stressful stimuli in varied ways across brain regions. Preclinical study demonstrates that 6 weeks of wheel running reduces LC firing following stress (Greenwood et al., 2003; Greenwood and Fleshner, 2008). Underlying these adaptive effects (Greenwood and Fleshner, 2008) is the upregulation of galanin in the LC, which induces a hyperpolarization of noradrenergic neurons and, thereby, inhibits excessive noradrenaline release (Seutin et al., 1989; Pieribone et al., 1995; Reiss et al., 2009; Murray et al., 2010) in some brain regions. The latter changes are essential for reducing noradrenaline levels in the amygdala to limit anxiety behavior (Sciolino and Holmes, 2012). Recapitulating these findings in humans, it has been shown that galanin increases in plasma after acute episodes of PA (Legakis et al., 2000). Conversely, long-term PA increases noradrenaline levels in the hippocampus to improve cognitive outcomes (Sarbadhikari and Saha, 2006), a finding that may have important implications for microglia and astrocytes in this brain region. The maintenance of basal levels of noradrenaline is important for inhibiting the release of the proinflammatory cytokines by microglia (Feinstein et al., 2002; Mori et al., 2002) and stimulating astrocytes to release trophic factors (e.g., BDNF) for neuroprotection (Junker et al., 2002). Prospective randomized controlled trials have demonstrated that hippocampal volumes increase following long-term aerobic PA (i.e., 1–2 years) (Erickson et al., 2011; Rosano et al., 2017).
Dopaminergic interactions
The majority of dopaminergic neurons are found in the ventral tegmental area (VTA) of the midbrain, the substantia nigra pars compacta, and the arcuate nucleus of hypothalamus. The dopaminergic neurons of these areas project to different brain structures through the mesocortical (with neurons originating in VTA and transporting dopamine to the amygdala, hippocampus, septum, and prefrontal cortex), mesolimbic (with neurons originating in the VTA and transporting dopamine to the nucleus accumbens through the amygdala and hippocampus), and nigrostriatal pathways (with neurons originating in the substantia nigra and transporting dopamine to the hippocampus and dorsal striatum that is comprised of the caudate nucleus and putamen) (Prasad and Pasterkamp, 2009). The diverse origins and ramifications of these pathways explain the varied effects produced by dopaminergic activation (Cho et al., 2006). Whereas optimal signaling in the mesolimbic pathway induces feelings of enjoyment and reinforcement following exposure to pleasurable stimuli (e.g., food, sex, and drugs) and associated contexts (Maas et al., 1997), optimal signaling in the mesocortical is vital for concentration and working memory. In the nigrostriatal system, signaling modulates motoric (planning and execution) and cognitive responses. In contrast, decrements in dopaminergic neurotransmission can effectuate symptoms of impaired ability to experience pleasure (anhedonia), motivation, executive function, and motricity in persons with depression (Nestler and Carlezon, 2006; Tye et al., 2013), a cluster of symptoms that traditional SSRIs often fail to assuage (Dunlop and Nemeroff, 2007; Trivedi et al., 2008). Knowledge that proinflammatory cytokines alter the functional status of the dopaminergic system in a similar manner to that seen in depression (reduces ventral striatal activity to reward cues) suggests a mechanistic explanation for their co-occurrence of inflammation in a distinct subset of persons who are clinically depressed.
Direct evidence that cytokines induce dopaminergic dysfunction derives from data from neurochemical, behavioral, electrophysiological, genetic, and human clinical studies. For instance, it has been shown that both peripheral and central administration of inflammatory agents alter dopamine levels in the brain (Miller et al., 2009), particularly in the striatum (Kamata et al., 2000; Mauriño et al., 2010). Interestingly, some evidence suggests cytokine-specific alterations across brain regions: IL-1 and IL-2 administration increased dopamine turnover in the prefrontal cortex, whereas IL-6 increased turnover in the hippocampus and prefrontal cortex (Zalcman et al., 1994). The effects of cytokine challenge may also be concentration and time dependent. Low concentration of IL-2 administered to mesencephalic cell cultures increased dopamine release, whereas higher concentrations had no effect (Alonso et al., 1993). A parallel in vivo micro dialysis study showed that acute treatment of monkeys with IFN-α increased dopamine release in the striatum, whereas chronic treatment with IFN-α decreased dopamine release. Notably, the decreased dopamine that occurred in the striatum after chronic treatment was correlated with reduced effort-based sucrose consumption (Felger et al., 2013b), an effect that was mitigated by levodopa administration via reverse in vivo microdialysis, suggesting that inflammatory cytokines reduce the availability of dopamine precursors without affecting end-product synthesis or vesicular packaging or release (Felger et al., 2015). Other work demonstrated that immune challenge effectuated decreased intracranial self-stimulation of lateral hypothalamus (Borowski et al., 1998). One of the structures affected by intracranial self-stimulation is the medial forebrain bundle which contains ascending dopaminergic projections from the VTA to the nucleus accumbens (mesolimbic pathway) and passes through the lateral hypothalamus (You et al., 2001; Nestler and Carlezon, 2006). Under basal conditions, the dopaminergic neurons in the VTA area oscillate between low-frequency regular action potentials (tonic activity) and bursts of action potentials (phasic activity patterns) (Schultz, 2002). Transient increases in phasic firing are thought to occur with exposure to unexpected rewards or aversive stimuli, thereby encoding a “reward prediction error” and reinforcing certain behaviors (Tsai et al., 2009). Notably, inflammatory stimuli decrease rodent responding for rewarding electrical stimulation in the lateral hypothalamus (Anisman et al., 1998; Borowski et al., 1998), a change that likely reflects anhedonia secondary to a loss of reward function. Translating this work to humans, it has been shown that volunteers exposed to low-dose polysaccharide exhibited reduced ventral striatal activity to monetary reward cues, a change that correlated with increased depressive symptoms (Eisenberger et al., 2010). It appears that there is a rate of long burst-like spike activity that is requisite for VTA dopaminergic neurons to release sufficient levels of dopamine to promote feelings of reward (Yadid and Friedman, 2008), a phenomenon that may be deleteriously altered in depression and inflammation. Indeed, administration of desipramine to Flinders-sensitive line rats (an animal model of depression) increased the rate of long-bursting, high-spike activity in the VTA in a manner similar to that seen in controls (Yadid and Friedman, 2008). Additionally, integrated behavioral, pharmacological, optogenetic, and electrophysiological methods used by Tye et al. (2013) to assess freely moving rodents showed that inhibition or excitation of dopaminergic neurons in the VTA immediately and bi-directionally modulated (induced or relieved) depressive-like symptoms effectuated by chronic mild stress.
One of the implicated mechanisms by which inflammation can alter dopaminergic signaling is via modulation of tetrahydrobiopterin (BH4), a cofactor that is essential for the degradation of amino acid phenylalanine and biosynthesis of dopamine. Chronic immune challenge correlates with reduced dopamine synthesis (Neurauter et al., 2008). In fact, patients treated with IFN-α demonstrate reduced BH4 function in the brain (Felger et al., 2013a). By corollary, persons subjected to dietary depletion of dopamine (via a tryptophan-depleting beverage) exhibited blunted activation of the ventral striatum during reward anticipation activities (Bjork et al., 2014), recapitulating the effects of immune challenge (Eisenberger et al., 2010). Further attesting to the effects of inflammation and dopaminergic function is recent work in a subgroup of clinically depressed individuals that showed that infliximab (a highly selective TNF-α antagonist) effectuated a strong antidepressant effect (with greatest effects seen in area of motivation), but only in patients with elevated CRP at baseline (Raison et al., 2013).
Interestingly, most studies suggest that PA increases dopamine levels in several brain regions (Brown et al., 1979; de Castro and Duncan, 1985; Dishman, 1997; Meeusen et al., 1997; Soares et al., 1999). These effects putatively stem from the ability of PA to alter metabolism (de Castro and Duncan, 1985; Chaouloff et al., 1986) via modulation of calcium levels (Goffer et al., 2013; Morris and Berk, 2015) and calcium/calmodulin-dependent activation of tyrosine hydroxylase (Greenwood et al., 2011) and mitigate BH4 depletion by inhibiting iNOS induction (Kitagami et al., 2003). Greenwood et al. (2011) demonstrated that young adult male Fischer rats that participated in voluntary wheel—running for 6 weeks exhibited a conditioned place preference for the wheel as well as increased ΔFosB/FosB immunoreactivity in the nucleus accumbens, increased tyrosine hydroxylase mRNA levels in the VTA, and compensatory down-regulation of D2 receptor mRNA in the nucleus accumbens; these findings suggest that (1) long-term voluntary PA is rewarding and alters gene transcription in mesolimbic reward neurocircuitry (Greenwood et al., 2011), (2) post-exercise increases in serum Ca2+ may activate tyrosine hydroxylase enzyme and dopamine synthesis, and (3) PA may reverse inflammation-induced disruptions in dopaminergic transmission in the nucleus accumbens and ventral striatum in models of depression. Another study showed that wheel running increases tyrosine hydroxylase mRNA in the LC (Droste et al., 2006) and substantia nigra (Foley and Fleshner, 2008). Receptor-binding studies suggest that 9 weeks of voluntary PA induced hypersensitivity to dopamine release (Gilliam et al., 1984; MacRae et al., 1987). In line with the aforementioned, it has been suggested that voluntary wheel running alters behavior because the activity is intrinsically rewarding and affects neuroplasticity in the mesolimbic reward pathway (Greenwood et al., 2011). Thereby, PA could serve as a feed-forward mechanism and further increase PA, a phenomenon that would reduce inflammation and metabolic disease in the long- term (Waters et al., 2008).
Glutamatergic interactions
Excitatory glutamatergic neurotransmission provides a basis for communication in the forebrain, cortex, hippocampus, caudate nuclei, thalamic nuclei, and cerebellar nuclei (Paul and Skolnick, 2003). Once released into the synaptic cleft, glutamate acts upon pre- and post-synaptic ionotropic N-methyl-d-aspartate (NMDA) glutamate receptors (NMDARs) in brain regions that modulate monoaminergic activity, emotionality, learning, and behavior (Ghasemi et al., 2014, 2017). NMDARs are tetrameric structures comprised of 7 subunits, including an obligatory GluN1 subunit along with various combinations of GluN2 and GluN3 subunits that differ according to anatomical distribution, developmental profile, and functional activity. Multiple binding sites exist on NMDARs, including those for glycine (D-serine), Mg2+, and other polyamines.
Some evidence suggests that the fronto-limbic glial alterations that occur in depression (Rajkowska and Miguel-Hidalgo, 2007) and comorbid inflammation are the result of an imbalance between the quinolinic acid and kynurenic acid arms of the pathway. Cytokine activation of the kynurenine pathway induces the breakdown of kynurenine into either quinolinic acid or kynurenic acid. The two end- products have diametrically opposing functions that can contribute to neurodegenerative or neuroprotective processes in the brain. Quinolinic acid is a neurotoxic endogenous NMDA receptor agonist, whereas kynurenic acid is a neuroprotective endogenous NMDA receptor antagonist. Within the brain, quinolinic acid is exclusively produced in microglial cells, intimating that microglial activation by proinflammatory cytokines may bias quinolinic acid production and facilitate NMDA agonism (Myint et al., 2007; McNally et al., 2008) along with astrocytic activation and apoptosis (Guillemin et al., 2005). The loss of astrocytes is particularly problematic because they uptake synaptic glutamate to prevent neuronal excitotoxicity, provide metabolic support to neurons via lactate production, produce neuroprotective mediators, and defend against oxidative stress. Quinolinic acid agonism of extrasynaptic NMDARs also inhibits the activity of cAMP response element binding (CREB) protein to block induction of BDNF gene expression (Hardingham et al., 2002). Interestingly, the NMDA antagonist ketamine decreased lipopolysaccharide-induced TNF-α production in astrocyte and microglial cultures (Shibakawa et al., 2005). In the hippocampus, ketamine down-regulated pro-inflammatory cytokines (Wang et al., 2015), an effect that might reduce depression-related hippocampal atrophy and preserve HPA axis feedback (Chen and Guillemin, 2009; Leonard and Maes, 2012). Memantine, another NMDA antagonist, has been shown to mitigate lipopolysaccharide-induced neuroinflammation and restore behaviorally- induced gene expression and spatial learning in the rat (Rosi et al., 2006). Together, these studies suggest that inflammation-induced astrocytic pathology may play an important role in depression via the production of pathogenic substances and loss of normal function. By corollary, strategies that normalize astrocytic function may improve neuronal health and decrease microglial activation.
Notably, evidence suggests that PA positively modulates the glutamatergic system in states of depression and inflammation. PA increases glutamate turnover and prevents excitotoxicity (Jia et al., 2009; Herbst and Holloway, 2016) by improving calcium regulation (Sutoo and Akiyama, 1996). PA also exerts a neuroprotective effect on the brain by modulating glial function. Recently it was shown that rodents exposed to long-term PA (5 days per week × 4 weeks) demonstrate increased BDNF synthesis and release in the dentate gyrus along with altered orientation and morphology of astrocytes (Fahimi et al., 2017). The latter findings suggest the antidepressant effects of aerobic PA may stem in part from (1) PA-induced changes in astrocytic projection length and density that enhance glutamate clearance from the synapse to mitigate glutamate excitotoxicity and (2) astrocytic production of neuroprotective mediators. Others have shown that PA induces an increase in astrocyte cell body area and number of contacts between astrocytic endfeet and blood vessels in the hippocampus, medial prefrontal cortex, and orbitofrontal cortex (Brockett et al., 2015). Since astrocytic endfeet express glucose transporters (Iadecola and Nedergaard, 2007), it seems plausible that PA-induced upregulation of astrocytic endfeet contact with blood vessels may serve as a means to respond to intense energy demand (van Hall et al., 2009), an adaptation that may be particularly important during inflammation and depression. Finally, it has been shown that PA reduced age-related microglial proliferation rate in aged mice 1.5-fold as well as the number of activated microglia 1.8-fold, suggesting that PA reduced inflammatory molecules that stimulate microglia proliferation (Kohman et al., 2012).
At the nexus of antidepressant efficacy and PA: PGC-1α
The nuanced relationship between PA and immune function is complex and incompletely understood, particularly in depression. On the one hand, extreme PA results in inflammation and immunosuppression. On the other hand, moderate PA promotes an anti-inflammatory environment (Gleeson, 1985). At the nexus of PA and immune interactions is a regulator of adaptation: the muscle-derived protein peroxisome proliferator-activated receptor C coactivator-1α (PGC-1α). PA upregulates skeletal expression of PGC-1α (Irrcher et al., 2003; Russell et al., 2003), which is important because this factor controls pro-inflammatory gene expression in muscle partly via inhibition of the NF-κB pathway. The NF-κB pathway contributes to cytokine production and cell survival (Eisele et al., 2013). A reification of the aforementioned concept can be seen in persons with comorbid depression and Type-2 diabetes.
Persons with Type-2 diabetes exhibit persistently elevated basal levels of inflammatory cytokines, which contribute to a state of insulin resistance (Hotamisligil, 2006). Low-grade inflammation putatively results when macrophages inundate white adipose tissue, liver, and skeletal muscle and elicit the persistent secretion of several types of “adipokines,” including the proinflammatory cytokines TNF-α, IL-1, IL-6, and monocyte chemoattractant protein-1 (MCP1) (Skurk et al., 2007). Interestingly, muscle tissue levels of TNF-α and IL-6 negatively correlate with PGC-1α levels in healthy and glucose-intolerant models (Handschin et al., 2007). Therefore, it seems plausible that the reciprocal regulation of PGC-1α and NF-κB is the molecular pivot in skeletal muscle that determines the balance between the trained anti-inflammatory environment endemic to conditions of health and the atrophic pro-inflammatory conditions endemic to states of disease (Figure 2). To better understand this pivot, a closer examination of IL-6 becomes warranted.
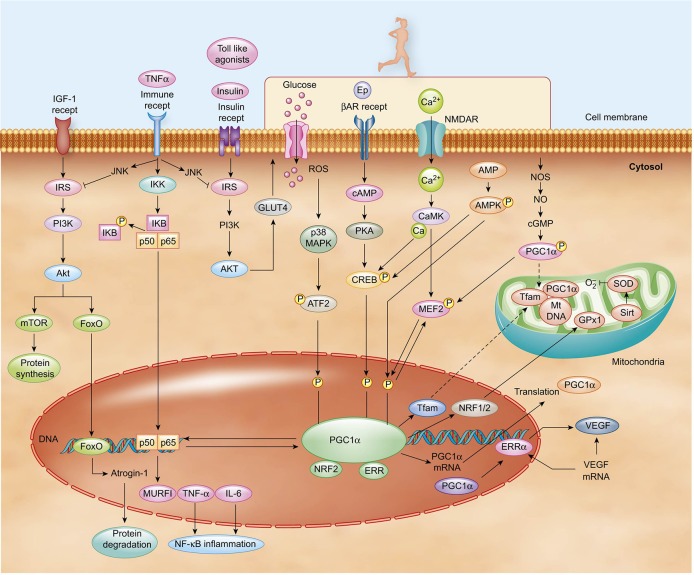
Figure 2.
PA induces the upregulation of PGC-1α expression via multiple signaling pathways. Included among the pathway inputs are contributions from β-adrenergic receptor signaling, Ca2+, AMPK, ROS, and NO. Cytosolic PGC-1α protein translocates to the nucleus and mitochondria once activated. Various transcription factors can modulate metabolic processes, including MEF2, FoxO, ATF, and CREB. In turn, the factors are impinged upon by a multiplicity of signaling pathways. For instance, PA and cytokines activate p38 MAPK, which then induces the activation of MEF2 and ATF2. Insulin activates AKT, which then inhibits FoxO. PGC-1α and NFkB family p60 subunits reciprocally modulate one another to regulate inflammatory pathways.
IL-6 is produced in skeletal muscle and adipose tissue, with adipose tissue contributing 10% to 35% of the body's basal circulating IL-6 level, a percentage that increases alongside rising body fat composition (Mohamed-Ali et al., 1997; Fried et al., 1998; Pedersen and Febbraio, 2012). Chronically elevated baseline IL-6 plasma levels are associated with obesity, insulin resistance, and Type-2 diabetes (Kern et al., 2001; Duncan et al., 2003; Dandona et al., 2004). Obesity-related elevations in IL-6 appear to help fuel the process of low-grade inflammation that accompanies obesity in a feedback response designed to offset energy excess. Bolstering the latter notion is evidence that PA increases IL-6 mRNA expression (Ostrowski et al., 1998; Starkie et al., 2001) in a manner that is contraction and duration dependent (Steensberg et al., 2000), suggesting that IL-6 signals the liver to increase glucose output to regulate blood glucose concentration during times of energy need (Steensberg et al., 2000). Other work shows that IL-6 can increase up to 100-fold with prolonged PA (Fischer, 2006), a trend that was attenuated with carbohydrate ingestion during PA (Nehlsen-Cannarella et al., 1985; Starkie et al., 2001) and pre-exercise glycogen depletion (50%) (Steensberg et al., 2001). Others showed that cytokines can induce white fat browning in peripheral tissue to promote energy expenditure (Lee et al., 2013; Petruzzelli et al., 2014). Furthermore, the induction of cytokine release by NFκB p65 in fat tissue induces energy expenditure in mice (Tang et al., 2010; Jiao et al., 2012). Notably, the chronic IL-6 and TNF-α secretion that results from obesity induces suppressor of cytokine signaling proteins (SOCS) 1 and 2. The net effect is a decrease in insulin-induced activation of insulin receptor substrate (IRS) with a reduction in the metabolic effects of insulin (Tanti et al., 2012) and failed skeletal muscle regeneration and atrophy (Coletti et al., 2005) via mechanisms that likely involve upregulation of TLRs (Lambert et al., 1985; Francaux, 2009; Gleeson et al., 2011; Drummond et al., 2013). With time, the hyperinsulinaemic response results in a decline in secretory capacity of β-cells that are responsible for insulin secretion. Conversely, administration of salicylate or blocking of IKK kinase reversed obesity and diet-induced insulin resistance (Gao et al., 2003; de Alvaro et al., 2004). Similarly, exercise improves insulin sensitivity and glucose uptake in muscle (Wojtaszewski et al., 2000, 2003; Sakamoto et al., 2004). Implicated mechanisms include increased phosphorylation of insulin receptor substrate (IRS) (Weigert et al., 2006).
Paradoxically, endurance-trained athletes exhibit increased levels of intramuscular triglycerides and yet are highly insulin sensitive (Goodpaster et al., 2001). Yet unlike sedentary individuals with comorbid depression and Type-2 diabetes, endurance-trained athletes appear to exhibit a higher mitochondrial density and mitochondrial enzyme capacity, which enhances oxidative phosphorylation and reduces the degree of insulin-sensitizing metabolic byproducts (Attie and Kendziorski, 2003; Mootha et al., 2003; Patti et al., 2003; Tarnopolsky et al., 2007). Moreover, the exercise-induced IL-6 profile in athletes differs from that with chronic inflammation. Whereas IL-6 is released from contracting muscle fibers following flux in Ca2+ and glycogen in exercising athletes (Pedersen, 2009), it appears to be primarily elicited from TLRs in persons with inflammation. Together, the aforementioned evidence suggests that an acute elevation in IL-6 exemplifies an attempt to mitigate energy crises during times of deprivation or excess, but that chronic inflammation promotes sickness behaviors, muscle wasting, and insulin resistance. Undoubtedly, the maintenance of metabolic equilibrium during inflammation involves PGC-1α, the master regulator of energy expenditure and mitochondrial biogenesis.
PGC-1α co-localizes to mitochondria-rich tissues, including skeletal muscle, liver, and brain. Transgenic studies of PGC-1α in rodents suggest the factor modulates local and systemic inflammation, including levels of TNF-α and IL-6 (Handschin and Spiegelman, 2008; Handschin, 2009; Arnold et al., 2011). The ability of PGC-1α to respond to changing metabolic needs during inflammation stems from its ability to selectively bind transcription factors, particularly peroxisome proliferator-activated receptor (PPAR)γ (Puigserver and Spiegelman, 2003), PPARα (Vega et al., 2000), estrogen-related α (ERRα) (Huss et al., 2002), forkhead box O (FoxO) (Puigserver et al., 2003), hepatocyte nuclear factor 4α (HNF4α) (Yoon et al., 2001), and nuclear respiratory factors (NRFs) (Wu et al., 1999). These coregulators affect biological responses that enable cells modulate mitochondrial biogenesis, cellular respiration rates, and energy substrate uptake and utilization—changes that are particularly important for contractile and metabolic adaptations in skeletal muscle (Puigserver and Spiegelman, 2003; Wende et al., 2007; Scarpulla, 2008). For example, PGC-1α coactivation of NRF-1,2 elicits the expression of nuclear-encoded mitochondrial proteins and mitochondrial transcription factor A (Tfam) to stimulate mitochondrial DNA replication and transcription (Kelly et al., 2003; Puigserver and Spiegelman, 2003; Lin et al., 2005). Cell culture studies of myoblasts show that overexpression PGC-1α effectuates an upregulation in respiratory subunit mRNAs, cytochrome c oxidase subunit 4 (COXIV) protein levels, and steady-state levels of mitochondrial DNA (Wu et al., 1999) in an adaptation to facilitate increased oxygen utilization. PGC-1α activity is regulated after PA via translational modifications that include phosphorylation (Jäger et al., 2007), deacetylation (Cantó et al., 2009), and sumoylation (Rytinki and Palvimo, 2008), changes that enhance expression of target genes and PGC-1α itself. Several pathways appear to contribute to these exercise-related modifications, including Ca2+/calmodulin, AMPK, p38/MAPK, and nitric oxide (NO) pathways.
PGC-1α activity is partially regulated by muscle-induced Ca2+ changes and their downstream signaling pathways. PA induces Ca2+ signaling via calmodulin-dependent protein kinase IV (CaMKIV) and calcineurin A, changes that activate myocyte enhancer factor (MEF) 2 (which is important for glucose transport) and impinge upon PGC-1α transcription (Handschin et al., 2003). Interestingly, transgenic mice overexpressing calcineurin A in skeletal muscle exhibit increased slow twitch myofibers, glucose transporter type 4 (GLUT4), mitochondrial enzymes, and PGC-1α (Naya et al., 2000; Ryder et al., 2003), suggesting that PGC-1α may have an insulin-sensitizing role. Corroborating the latter notion are studies showing a negative correlation between muscle PGC-1α levels and mitochondrial activity in insulin resistance and diabetes (Attie and Kendziorski, 2003; Mootha et al., 2003; Patti et al., 2003). In an alternate signaling path, CaMKIV activates cAMP response element (CRE) binding (CREB) protein to augment PGC-1α transcription in various tissues (Herzig et al., 2001; Wu et al., 2002; Handschin et al., 2003) and, in an autoregulatory manner, activates MEF2C and MEF2D (Michael et al., 2001; Lin et al., 2005). Another factor that upregulates PGC-1α during PA is p38MAPK via activation of transcription factor 2 (ATF2) (Cao et al., 2004). Also, aerobic PA-induced Ca2+ release upregulates PGC-1α activity and initiates its translocation to the nucleus, where it interacts with LRP130 to inhibit transcriptional activity of FoxO, which suppresses muscle protein degradation and atrophy (Vechetti-Junior et al., 2016). Via these mechanisms, aerobic PA and its induction of PGC-1α influences muscle fiber type composition, modulates GLUT4 gene expression (Michael et al., 2001), and promotes protein synthesis in muscle cells.
PA also generates reactive oxygen species (ROS), which induces inflammatory cytokine production in skeletal muscle (Ji, 2008), an effect that can be mitigated by the upregulation of mitochondrial ROS-detoxifying enzymes via PGC-1α (St-Pierre et al., 2003; Valle et al., 2005). Deficits in PGC-1α secondary to disuse may promote an inflammatory state that attenuates early benefits of exercise, particularly in those with comorbid depression and chronic inflammation. Yet restoration of PA effectuates a reduction in the ubiquitin-proteasome actions of atrogin-1 and Murf-1, proteins that are involved in atrophy under catabolic conditions (Dupont-Versteegden et al., 1985; Haddad et al., 1985; Okamoto et al., 2011; Suetta et al., 2012), via upregulation of PGC-1α, a metabolic change that promotes muscle recovery by inhibiting the FoxO pathway, possibly by involvement of LRP130 (Vechetti-Junior et al., 2016).
PA also induces changes in AMP-activated protein kinase (AMPK), an energy sensor that becomes active when the AMP/ATP ratio is high (Jørgensen et al., 2005; Pedersen and Febbraio, 2012). Activated AMPK enhances mitochondrial biogenesis and function, for which PGC-1α plays an essential role in activation (Jäger et al., 2007). The up-regulation of PGC-1α putatively occurs following direct phosphorylation by activated AMPK (Jäger et al., 2007). Then, activated PGC-1α may exert significant impact on mitochondrial signal transduction by up-regulating the expression of ERRα, nuclear respiratory factor (NRF)-1, and NRF-2 (Ye et al., 2016), which is important for antioxidant defense (Asghar et al., 2007). Other work suggests that PGC-1α is requisite for the upregulation of skeletal muscle VEGF expression, an effect that is AMPK- mediated (Leick et al., 2009). In addition to its importance in muscle physiology, the AMPK pathway may be particularly important for central neurons that possess small energy reserves (Ronnett and Aja, 2008) as suggested by concomitant AMPK activation in the rodent hippocampus and antidepressant-like effects following ketamine administration (Xu et al., 2013). Via these complex pathways, PGC-1α mediates many known beneficial effects of PA in skeletal muscle physiology and immune function.
Additionally linking PGC-1α with depression is recent groundbreaking preclinical work that demonstrated that exercise-induced augmentation of PGC-1α directly influenced mood by altering the kynurenine pathway via immune-dependent mechanisms (Agudelo et al., 2014). Initially this work proved onerous because PGC-1α is expressed in a variety of systems throughout the body making it difficult to disentangle whether the effects of PA originated from central or peripheral mechanisms. To tackle the problem, Agudelo and colleagues used mice that were genetically modified to produce excessive levels of PGC-1α in type-II skeletal muscle fibers and exposed them to chronic stress in an attempt to induce depressive-like symptoms (Agudelo et al., 2014). They found that mice overexpressing PGC-1α were far more resistant to depressive symptoms in comparison to mice with normal levels of PGC-1α. The researchers then attempted to induce depressive-like symptoms in mice that were genetically engineered to produce lower levels of PGC-1α in their skeletal muscles. This time, after a significant amount of stress, the low PGC-1α mice appeared to “lose hope,” as evidenced by their decreased survival efforts during forced swimming (an indicator of depression), behaviors that were inflammation-dependent (Agudelo et al., 2014; Phillips and Salehi, 2016). Importantly, PGC-1α overexpression effectuated an increased production of kynurenine aminotransferase (KAT), an enzyme that converts kynurenine into kynurenic acid, a substance that cannot pass from the blood to the brain. The conversion of kynurenine into kynurenic acid has tremendous translational potential given that high levels of kynurenine are found in persons with mental illness and rodents administered kynurenine display depressive behavior. Fascinatingly, recent human studies show that aerobic PA increases skeletal muscle KAT levels and, thereby, shifts kynurenine metabolism in the periphery toward kynurenic acid (Schlittler et al., 2016). Altogether, these results suggest that PA induces the release of “hope molecules” from the skeletal muscles of rodents to influence mood disorder symptoms.
To date, much of the aforementioned work has not been extended to large-scale patient populations with comorbid depression and inflammation. Nevertheless, the work provides a strong theoretical basis for the idea that PA can modulate PGC-1α, increase mitochondrial density, alter muscle fiber type, mitigate inflammation, and reduce depressive symptoms, particularly in persons with comorbid depression and diabetes. Undoubtedly, the ability of PA to optimize insulin control would exert significant peripheral and central effects. Acute aerobic PA significantly increases muscle glucose uptake via insulin-dependent mechanisms for 1 h after cessation, and increased glucose uptake persists 12–48 h following prolonged activity via insulin-independent mechanisms (Magkos et al., 2008). Also, improvements in insulin signaling may persist for 24 h when the intensity is increased to near-maximal effort intermittently during trainings of shorter duration (20 min) (Manders et al., 2010; Gillen et al., 2012), with some evidence suggesting that those with the highest baseline insulin resistance yield the greatest effects early in disease progression (Dubé et al., 2012). High-intensity interval training robustly enhances skeletal muscle oxidative capacity and insulin sensitivity in adults with Type-2 diabetes (Cochran et al., 2014; Jelleyman et al., 2015). Similarly, resistance training enhances insulin action (Bacchi et al., 2013), and some evidence suggests that a combination of endurance and resistance exercise effectuates greater improvements (Sigal et al., 2007). Improved insulin sensitivity is paramount as insulin signaling regulates mitochondrial function, energy homeostasis, circuit structure and function (via transmitter receptor trafficking), and plasticity (via alterations in synapse density) (Chiu et al., 2008), effects that may be particularly important in the aging hippocampus (Zhao et al., 2008; De Felice et al., 2009) given its importance for HPA regulation.
So, the question arises as to whether there is currently enough evidence to support the deployment of PA to positively influence depressive symptoms in clinical populations. To answer this important question, Cooney and colleagues conducted a meta-analysis of randomized trials that were published up to March 2013 in which exercise (defined according to American College of Sports Medicine criteria) was compared to standard treatment, no treatment or a placebo treatment, pharmacological treatment, psychological treatment, or other active treatment in adults (aged 18 and over) with depression (Cooney et al., 2013). Thirty-nine studies with a total of 2,326 participants were included in the review. The authors reported that aerobic exercise produced effects comparable to treatment by either antidepressants or psychotherapy. Another meta-analytic study by Silveira and colleagues demonstrated that aerobic PA moderately reduced the signs of depression, with populations over 60 years of age and those with mild depression deriving the greatest response (Silveira et al., 2013). Notwithstanding, there is currently little evidence to indicate which modality of PA is optimal (aerobic, strengthening, flexibility, or combinations). Stanton and Reaburn tried to determine optimal parameters for using PA to treat depression (e.g., frequency, intensity, duration, and type of exercise). All five randomized controlled studies meeting inclusion criteria were aerobic in nature (walking on treadmill or outdoors, cycling on a stationary bike, or training on an elliptical machine) (Stanton and Reaburn, 2014). Positive evidence was found that aerobic PA of moderate intensity, undertaken 3 times weekly, was effective in treating depression, with the ultimate recommendation for duration being a minimum of 9 weeks (Stanton and Reaburn, 2014).
Given evidence that it may be more difficult for persons with comorbid depression and inflammation to benefit from conventional antidepressants, it seems likely that associating pharmacological and nonpharmacological interventions that reduce inflammation may enhance treatment response in persons with comorbid depression and inflammation. Bolstering this notion is evidence that inflammatory cytokines may cancel mechanisms requisite for antidepressant efficacy by increasing monoamine transporter activity, reducing monoamine precursors, reducing enzyme cofactors necessary for monoamine synthesis, activating NF-κB, and reducing glutamate transporters. Fornaro et al. (2013) reported that non-responders to duloxetine exhibited increased levels of proinflammatory cytokine levels in comparison to early-responders. Yoshimura et al. (2009) showed that antidepressant efficacy was contingent upon the restoration of pro- and anti-inflammatory balance and lowering of baseline IL-6 levels (Yoshimura et al., 2009). Post-hoc analysis of clinical trial results by Raison et al. (2013) demonstrated that persons with treatment-resistant depression and high baseline CRP (>5 mg/L) exhibited a higher rate of treatment response (62 vs. 33%) when administered infliximab as compared to a placebo-treated group. Conversely, persons with low CRP (<5 mg/L) levels who were administered placebo experienced a greater reduction in depressive symptoms in comparison to those administered infliximab, a finding that argues against administration of anti-inflammatory agents in cases of depression without apparent inflammation (Raison et al., 2013).
So the question arises as to what can be expected when combining antidepressants with PA: an enhanced effect, a lower antidepressant dose, a higher rate of responders, a decreased rate of relapse, or a reduction in the delay of action? To answer this question, Carneiro et al. (2015) administered pharmacotherapy plus 16 weeks of supervised structured aerobic exercise training program to women with clinical depression in a randomized clinical trial, finding that aerobic exercise was an effective adjuvant to pharmacological therapy (Carneiro et al., 2015). Helgadóttir et al. (2017) assessed outcomes for four interventions: treatment as usual, light intensity exercise, moderate intensity exercise, and vigorous exercise; while all groups experienced decrements in depressive symptoms, persons in light exercise group reported greater symptom relief at 12-month follow-up (Helgadóttir et al., 2017). Siqueira et al. (2016) reported that a 4-week (4x/week) add-on aerobic exercise program significantly decreased the need to render higher doses of sertraline as compared to sertraline monotherapy (Siqueira et al., 2016). Kerling et al. (2015) demonstrated that treatment response was more frequent in persons assigned to an add-on exercise group in comparison to treatment as usual (Kerling et al., 2015). Babyak et al. (2000) assessed the effects of a 4-month course of aerobic exercise, sertraline therapy, or a combination of exercise and sertraline in persons with depression, finding that remitted persons in the exercise group exhibited significantly lower relapse rates than subjects in the medication group (Babyak et al., 2000). Preclinical work suggests that both voluntary PA and antidepressant therapies induce changes in neuroplasticity substrates in a similar time course (Russo-Neustadt et al., 1999), although it remains to be determined whether PA-induced reductions in inflammation could alter the time course in those with comorbid depression and high basal levels of inflammation.
The precise activity parameters that need to be deployed to mitigate depression and comorbid inflammation need to be determined in future work. Some translational work suggests that moderate PA may be an optimal intensity of PA for the promotion of mental health by decreasing TNF-α (Paolucci et al., 2018). Clearly, PA prescriptions are needed that take into account the basal levels of inflammation and response to stress (neuroendocrine and immune status) during intervention. The end goal would be to deploy various forms of PA to intermittently stimulate the immune response so that the levels of pro-inflammatory mediators and stress hormones are optimized. Doing so may require different modalities, depending upon personal factors.
Conclusions and future directions
Undoubtedly, psychiatric illness is defined by a constellation of different symptoms that can be influenced by multiple neural processes and circuits. The heterogeneity of presentations complicates the precise targeting of dysfunction and, by corollary, therapeutics that target those impairments. This conundrum is patently apparent in the management of depression, wherein a growing body of work suggests that a specific subtype of depression with comorbid chronic inflammation exists. Further research that aims to characterize the relationship between inflammation and depression is warranted as it may yield novel treatments for this subgroup that has been shown to be resistant to conventional antidepressant pharmacotherapy. Biomarker identification efforts will be enhanced by methods that triangulate protein and genetic analysis with neuroimaging and behavioral analyses. Ultimately, these studies should be used to identify the immune, endocrine, and neurotransmitter responses in depressive subtypes so that optimal treatments, both pharmacological and nonpharmacological, can be identified and tailored to select patient populations. Clearly, large-scale, multiple-site clinical investigations that study the relationship between PA and depression are needed. Longitudinal studies will be required to evaluate the short- and long-term benefits of combination therapies. The effects of PGC-1α on phenotypic traits—such as adiposity, lean mass, and fasting glucose—and the way that they could be modulated by genetic background (ethnicity) are not precisely understood. Studies that disentangle the relationship between PA, cognitive engagement, diet, social factors, and stress are desperately needed to determine the independent and additive protective effects that each factor exerts (Phillips et al., 2014, 2015; Phillips, 2017b,c), particularly how these factors affect cognitive and emotional function at the synaptic and circuit level (Das et al., 2015). Finally, strategies for overcoming the core symptoms of depression and comorbid health problems are needed so that PA prescriptions can be personalized and adherence maximized. Personalized prescriptions are particularly germane to the topic of depression as the condition is associated with increased morbidity and mortality (Kiecolt-Glaser and Glaser, 2002), and activation of the inflammatory response in persons with depression may engender different treatment responses to various activity regimens. These studies are vital to work at the frontiers of neuroscience that seeks to enable novel application of PA to health and disease and provide a personalized approach to intervention.
Author contributions
CP developed the concept for the study. CP and AF were responsible for manuscript writing and editing.
Conflict of interest statement
AF reports a consulting relationship with Silverberry Genomix, but acknowledges the company did not have a role in this paper. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BH4
tetrahydrobiopterin
- BDNF
brain-derived neurotrophic factor
- CRH
corticotropin-releasing hormone
- CREB
cAMP response element-binding protein
- CRP
c-reactive protein
- ERRα
estrogen-related α
- FoxO
forkhead box O
- GLUT4
glucose transporter type 4
- HPA
hypothalamic–pituitary–adrenal
- IDO, indolamine 2
3-dioxygenase
- IFN
interferon
- IL
interleukin
- IRS
insulin receptor substrate
- KAT
kynurenine aminotransferase
- KMO
kynurenine 3-monooxygenase
- LC
locus coeruleus
- MAPK
mitogen-activated protein kinase
- MEF
myocyte enhancer factor
- MHPG
3-methoxy-4-hydroxypheny-glycol
- NFkB
nuclear factor κB
- NRF
nuclear respiratory factor
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- SOCS
suppressor of cytokine signaling proteins
- PA
physical activity
- Pgc-1α
peroxisome proliferator-activated receptor C coactivator-1α
- SSRI
selective serotonin reuptake inhibitor
- Th
T helper
- TNF
tumor necrosis factor
- TDO, tryptophan 2
3-dioxygenase
- TLRs
Toll-like receptors
- VTA
ventral tegmental area.
References
- Abbasi S. H., Hosseini F., Modabbernia A., Ashrafi M., Akhondzadeh S. (2012). Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J. Affect. Disord. 141, 308–314. 10.1016/j.jad.2012.03.033 [DOI] [PubMed] [Google Scholar]
- Abbott R., Whear R., Nikolaou V., Bethel A., Coon J. T., Stein K., et al. (2015). Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J. Psychosom. Res. 79, 175–184. 10.1016/j.jpsychores.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Abu-Omar K., Rutten A., Lehtinen V. (2004). Mental health and physical activity in the European Union Soz Praventivmed 49, 301–309. 10.1007/s00038-004-3109-8 [DOI] [PubMed] [Google Scholar]
- Agudelo L. Z., Femenia T., Orhan F., Porsmyr-Palmertz M., Goiny M., Martinez-Redondo V., et al. (2014). Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45. 10.1016/j.cell.2014.07.051 [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S., Jafari S., Raisi F., Nasehi A. A., Ghoreishi A., Salehi B., et al. (2009). Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety 26, 607–611. 10.1002/da.20589 [DOI] [PubMed] [Google Scholar]
- Alonso R., Chaudieu I., Diorio J., Krishnamurthy A., Quirion R., Boksa P. (1993). Interleukin-2 modulates evoked release of [3H]dopamine in rat cultured mesencephalic cells. J. Neurochem. 61, 1284–1290. 10.1111/j.1471-4159.1993.tb13620.x [DOI] [PubMed] [Google Scholar]
- Anisman H., Kokkinidis L., Borowski T., Merali Z. (1998). Differential effects of interleukin (IL)-1beta, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res. 779, 177–187. 10.1016/S0006-8993(97)01114-1 [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V., Borkoles E., Polman R., Stojanovska L. (2014). Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 6, 1145–1157. 10.2217/imt.14.76 [DOI] [PubMed] [Google Scholar]
- Arnold A. S., Egger A., Handschin C. (2011). PGC-1alpha and myokines in the aging muscle-a mini-review. Gerontology 57, 37–43. 10.1159/000281883 [DOI] [PubMed] [Google Scholar]
- Asghar M., George L., Lokhandwala M. F. (2007). Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am. J. Physiol. Renal Physiol. 293, F914–F919. 10.1152/ajprenal.00272.2007 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F. E. (1981). Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886. 10.1523/JNEUROSCI.01-08-00876.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie A. D., Kendziorski C. M. (2003). PGC-1alpha at the crossroads of type 2 diabetes. Nat. Genet. 34, 244–245. 10.1038/ng0703-244 [DOI] [PubMed] [Google Scholar]
- Audet M. C., Jacobson-Pick S., Wann B. P., Anisman H. (2011). Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav. Immun. 25, 1197–1205. 10.1016/j.bbi.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Babyak M., Blumenthal J. A., Herman S., Khatri P., Doraiswamy M., Moore K., et al. (2000). Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom. Med. 62, 633–638. 10.1097/00006842-200009000-00006 [DOI] [PubMed] [Google Scholar]
- Bacchi E., Negri C., Targher G., Faccioli N., Lanza M., Zoppini G., et al. (2013). Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology 58, 1287–1295. 10.1002/hep.26393 [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr. (1996). The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14, 649–683. 10.1146/annurev.immunol.14.1.649 [DOI] [PubMed] [Google Scholar]
- Barbour K. A., Edenfield T. M., Blumenthal J. A. (2007). Exercise as a treatment for depression and other psychiatric disorders: a review. J. Cardiopulm. Rehabil. Prev. 27, 359–367. 10.1097/01.HCR.0000300262.69645.95 [DOI] [PubMed] [Google Scholar]
- Bartolomucci A., Palanza P., Sacerdote P., Ceresini G., Chirieleison A., Panerai A. E., et al. (2003). Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 28, 540–558. 10.1016/S0306-4530(02)00039-2 [DOI] [PubMed] [Google Scholar]
- Basterzi A. D., Aydemir C., Kisa C., Aksaray S., Tuzer V., Yazici K., et al. (2005). IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. 20, 473–476. 10.1002/hup.717 [DOI] [PubMed] [Google Scholar]
- Bergmann M., Gornikiewicz A., Sautner T., Waldmann E., Weber T., Mittlbock M., et al. (1999). Attenuation of catecholamine-induced immunosuppression in whole blood from patients with sepsis. Shock 12, 421–427. 10.1097/00024382-199912000-00002 [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A. (1992). Immune-neuroendocrine circuits: integrative role of cytokines. Front. Neuroendocrinol. 13, 61–94. [PubMed] [Google Scholar]
- Besedovsky H. O., Rey A. D. (2007). Physiology of psychoneuroimmunology: a personal view. Brain Behav. Immun. 21, 34–44. 10.1016/j.bbi.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Bjork J. M., Grant S. J., Chen G., Hommer D. W. (2014). Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology 39, 595–604. 10.1038/npp.2013.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J. E. (1984). Serotonin and appetite. Neuropharmacology 23, 1537–1551. 10.1016/0028-3908(84)90098-4 [DOI] [PubMed] [Google Scholar]
- Borowski T., Kokkinidis L., Merali Z., Anisman H. (1998). Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport 9, 3797–3802. 10.1097/00001756-199812010-00006 [DOI] [PubMed] [Google Scholar]
- Borsody M. K., Weiss J. M. (2002). Peripheral endotoxin causes long-lasting changes in locus coeruleus activity via IL-1 in the brain. Acta Neuropsychiatr. 14, 303–321. 10.1034/j.1601-5215.2002.140605.x [DOI] [PubMed] [Google Scholar]
- Brambilla D., Franciosi S., Opp M. R., Imeri L. (2007). Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur. J. Neurosci. 26, 1862–1869. 10.1111/j.1460-9568.2007.05796.x [DOI] [PubMed] [Google Scholar]
- Brockett A. T., LaMarca E. A., Gould E. (2015). Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS ONE 10:e0124859. 10.1371/journal.pone.0124859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. S., Payne T., Kim C., Moore G., Krebs P., Martin W. (1979). Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 46, 19–23. 10.1152/jappl.1979.46.1.19 [DOI] [PubMed] [Google Scholar]
- Brunello N., Alboni S., Capone G., Benatti C., Blom J. M., Tascedda F., et al. (2006). Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int. Clin. Psychopharmacol. 21, 219–225. 10.1097/00004850-200607000-00004 [DOI] [PubMed] [Google Scholar]
- Brüning C. A., Martini F., Soares S. M., Savegnago L., Sampaio T. B., Nogueira C. W. (2015). Depressive-like behavior induced by tumor necrosis factor-alpha is attenuated by m-trifluoromethyl-diphenyl diselenide in mice. J. Psychiatr. Res. 66–67, 75–83. 10.1016/j.jpsychires.2015.04.019 [DOI] [PubMed] [Google Scholar]
- Bufalino C., Hepgul N., Aguglia E., Pariante C. M. (2013). The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav. Immun. 31, 31–47. 10.1016/j.bbi.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Canli T., Lesch K. P. (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109. 10.1038/nn1964 [DOI] [PubMed] [Google Scholar]
- Cantó C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Daniel K. W., Robidoux J., Puigserver P., Medvedev A. V., Bai X., et al. (2004). p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell Biol. 24, 3057–3067. 10.1128/MCB.24.7.3057-3067.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carek P. J., Laibstain S. E., Carek S. M. (2011). Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 41, 15–28. 10.2190/PM.41.1.c [DOI] [PubMed] [Google Scholar]
- Carneiro L. S., Fonseca A. M., Vieira-Coelho M. A., Mota M. P., Vasconcelos-Raposo J. (2015). Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: a randomized clinical trial. J. Psychiatr. Res. 71, 48–55. 10.1016/j.jpsychires.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Chaouloff F., Laude D., Guezennec Y., Elghozi J. L. (1986). Motor activity increases tryptophan, 5-hydroxyindoleacetic acid, and homovanillic acid in ventricular cerebrospinal fluid of the conscious rat. J. Neurochem. 46, 1313–1316. 10.1111/j.1471-4159.1986.tb00656.x [DOI] [PubMed] [Google Scholar]
- Chen Y., Guillemin G. J. (2009). Kynurenine pathway metabolites in humans: disease and healthy states. Int. J. Tryptophan. Res. 2, 1–19. 10.4137/IJTR.S2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. L., Chen C. M., Cline H. T. (2008). Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58, 708–719. 10.1016/j.neuron.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B. P., Song D. Y., Sugama S., Shin D. H., Shimizu Y., Kim S. S., et al. (2006). Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia 53, 92–102. 10.1002/glia.20265 [DOI] [PubMed] [Google Scholar]
- Cho L., Tsunoda M., Sharma R. P. (1999). Effects of endotoxin and tumor necrosis factor alpha on regional brain neurotransmitters in mice. Nat. Toxins 7, 187–95. [DOI] [PubMed] [Google Scholar]
- Chourbaji S., Urani A., Inta I., Sanchis-Segura C., Brandwein C., Zink M., et al. (2006). IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 23, 587–594. 10.1016/j.nbd.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Cochran A. J., Percival M. E., Tricarico S., Little J. P., Cermak N., Gillen J. B., et al. (2014). Intermittent and continuous high-intensity exercise training induce similar acute but different chronic muscle adaptations. Exp. Physiol. 99, 782–791. 10.1113/expphysiol.2013.077453 [DOI] [PubMed] [Google Scholar]
- Coletti D., Moresi V., Adamo S., Molinaro M., Sassoon D. (2005). Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43, 120–128. 10.1002/gene.20160 [DOI] [PubMed] [Google Scholar]
- Cooney G. M., Dwan K., Greig C. A., Lawlor D. A., Rimer J., Waugh F. R., et al. (2013). Exercise for depression. Cochrane Database Syst. Rev. CD 004366. 10.1002/14651858.CD004366.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen P. J. (1991). Serotonin receptor subtypes: implications for psychopharmacology. Br. J. Psychiatry 7–14. [PubMed] [Google Scholar]
- Crosby K. M., Bains J. S. (2012). The intricate link between glucocorticoids and endocannabinoids at stress-relevant synapses in the hypothalamus. Neuroscience 204, 31–37. 10.1016/j.neuroscience.2011.11.049 [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. (1982). Corticosteroid-mediated immunoregulation in man. Immunol. Rev. 65, 133–155. 10.1111/j.1600-065X.1982.tb00431.x [DOI] [PubMed] [Google Scholar]
- Dandona P., Aljada A., Bandyopadhyay A. (2004). Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25, 4–7. 10.1016/j.it.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D., Phillips C., Hsieh W., Sumanth K., Dang V., Salehi A. (2014). Neurotransmitter-based strategies for the treatment of cognitive dysfunction in Down syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 140–148. 10.1016/j.pnpbp.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Das D., Phillips C., Lin B., Mojabi F., Akif Baktir M., Dang V., et al. (2015). Assessment of dendritic arborization in the dentate gyrus of the hippocampal region in mice. J. Vis. Exp. 97:e52371 10.3791/52371 [DOI] [Google Scholar]
- Davis J. M., Bailey S. P., Woods J. A., Galiano F. J., Hamilton M. T., Bartoli W. P. (1992). Effects of carbohydrate feedings on plasma free tryptophan and branched-chain amino acids during prolonged cycling. Eur. J. Appl. Physiol. Occup. Physiol. 65, 513–519. 10.1007/BF00602357 [DOI] [PubMed] [Google Scholar]
- de Alvaro C., Teruel T., Hernandez R., Lorenzo M. (2004). Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279, 17070–17078. 10.1074/jbc.M312021200 [DOI] [PubMed] [Google Scholar]
- de Castro J. M., Duncan G. (1985). Operantly conditioned running: effects on brain catecholamine concentrations and receptor densities in the rat. Pharmacol. Biochem. Behav. 23, 495–500. 10.1016/0091-3057(85)90407-1 [DOI] [PubMed] [Google Scholar]
- De Felice F. G., Vieira M. N., Bomfim T. R., Decker H., Velasco P. T., Lambert M. P., et al. (2009). Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. U.S.A. 106, 1971–1976. 10.1073/pnas.0809158106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos E. T., Oliveira J., Pinheiro J. P., Reis F. (2012). Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid. Med. Cell. Longev. 2012:741545. 10.1155/2012/741545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarro G. B., Masuda Y., Ascioti C., Audino M. G., Nistico G. (1990). Behavioural and ECoG spectrum changes induced by intracerebral infusion of interferons and interleukin 2 in rats are antagonized by naloxone. Neuropharmacology 29, 167–179. 10.1016/0028-3908(90)90057-X [DOI] [PubMed] [Google Scholar]
- Dishman R. K. (1997). Brain monoamines, exercise, and behavioral stress: animal models. Med. Sci. Sports Exerc. 29, 63–74. 10.1097/00005768-199701000-00010 [DOI] [PubMed] [Google Scholar]
- D'Mello C., Le T., Swain M. G. (2009). Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102. 10.1523/JNEUROSCI.3567-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste S. K., Schweizer M. C., Ulbricht S., Reul J. M. (2006). Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J. Neuroendocrinol. 18, 915–925. 10.1111/j.1365-2826.2006.01489.x [DOI] [PubMed] [Google Scholar]
- Drummond M. J., Timmerman K. L., Markofski M. M., Walker D. K., Dickinson J. M., Jamaluddin M., et al. (2013). Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R216–R223. 10.1152/ajpregu.00072.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé J. J., Allison K. F., Rousson V., Goodpaster B. H., Amati F. (2012). Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med. Sci. Sports Exerc. 44, 793–799. 10.1249/MSS.0b013e31823f679f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky S. L. (1994). Beyond the serotonin reuptake inhibitors: rationales for the development of new serotonergic agents. J. Clin. Psychiatry 55(Suppl.), 34–44. [PubMed] [Google Scholar]
- Duclos M., Corcuff J. B., Arsac L., Moreau-Gaudry F., Rashedi M., Roger P., et al. (1998). Corticotroph axis sensitivity after exercise in endurance-trained athletes. Clin. Endocrinol. 48, 493–501. 10.1046/j.1365-2265.1998.00334.x [DOI] [PubMed] [Google Scholar]
- Duncan B. B., Schmidt M. I., Pankow J. S., Ballantyne C. M., Couper D., Vigo A., et al. (2003). Atherosclerosis risk in communities, low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52, 1799–1805. 10.2337/diabetes.52.7.1799 [DOI] [PubMed] [Google Scholar]
- Dunlop B. W., Nemeroff C. B. (2007). The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337. 10.1001/archpsyc.64.3.327 [DOI] [PubMed] [Google Scholar]
- Dunn A. J. (1988). Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 43, 429–435. 10.1016/0024-3205(88)90522-X [DOI] [PubMed] [Google Scholar]
- Dunn A. J. (1992). Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J. Pharmacol. Exp. Ther. 261, 964–969. [PubMed] [Google Scholar]
- Dunn A. J., Powell M. L., Meitin C., Small P. A., Jr. (1989). Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol. Behav. 45, 591–594. 10.1016/0031-9384(89)90078-4 [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden E. E., Fluckey J. D., Knox M., Gaddy D., Peterson C. A. (1985). Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J. Appl. Physiol. 101, 202–212. [DOI] [PubMed] [Google Scholar]
- Eisele P. S., Salatino S., Sobek J., Hottiger M. O., Handschin C. (2013). The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J. Biol. Chem. 288, 2246–2260. 10.1074/jbc.M112.375253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N. I., Berkman E. T., Inagaki T. K., Rameson L. T., Mashal N. M., Irwin M. R. (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I. J., Chrousos G. P. (1999). Stress hormones Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metab. 10, 359–368. 10.1016/S1043-2760(99)00188-5 [DOI] [PubMed] [Google Scholar]
- Elenkov I. J., Papanicolaou D. A., Wilder R. L., Chrousos G. P. (1996). Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc. Assoc. Am. Physicians 108, 374–381. [PubMed] [Google Scholar]
- Elenkov I. J., Wilder R. L., Chrousos G. P., Vizi E. S. (2000). The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 595–638. [PubMed] [Google Scholar]
- Elgarf A. S., Aboul-Fotouh S., Abd-Alkhalek H. A., El Tabbal M., Hassan A. N., Kassim S. K., et al. (2014). Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline. Pharmacol. Biochem. Behav. 126, 152–162. 10.1016/j.pbb.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108, 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst C., Olson A. K., Pinel J. P., Lam R. W., Christie B. R. (2006). Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J. Psychiatry Neurosci. 31, 84–92. [PMC free article] [PubMed] [Google Scholar]
- Esch T., Stefano G. B. (2010). The neurobiology of stress management. Neuro Endocrinol. Lett. 31, 19–39. [PubMed] [Google Scholar]
- Fahimi A., Baktir M. A., Moghadam S., Mojabi F. S., Sumanth K., McNerney M. W., et al. (2017). Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 222, 1797–1808. 10.1007/s00429-016-1308-8 [DOI] [PubMed] [Google Scholar]
- Feinstein D. L., Heneka M. T., Gavrilyuk V., Dello Russo C., Weinberg G., Galea E. (2002). Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 41, 357–365. 10.1016/S0197-0186(02)00049-9 [DOI] [PubMed] [Google Scholar]
- Felger J. C., Hernandez C. R., Miller A. H. (2015). Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int. J. Neuropsychopharmacol. 18:pyu084. 10.1093/ijnp/pyu084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J. C., Li L., Marvar P. J., Woolwine B. J., Harrison D. G., Raison C. L., et al. (2013a). Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 31, 153–160. 10.1016/j.bbi.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J. C., Li Z., Haroon E., Woolwine B. J., Jung M. Y., Hu X., et al. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365. 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J. C., Mun J., Kimmel H. L., Nye J. A., Drake D. F., Hernandez C. R., et al. (2013b). Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38, 2179–2187. 10.1038/npp.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A. J., Charlson F. J., Norman R. E., Patten S. B., Freedman G., Murray C. J., et al. (2013a). Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 10:e1001547. 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A. J., Somerville A. J., Baxter A. J., Norman R., Patten S. B., Vos T., et al. (2013b). Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol. Med. 43, 471–481. 10.1017/S0033291712001511 [DOI] [PubMed] [Google Scholar]
- Fischer C. P. (2006). Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12, 6–33. [PubMed] [Google Scholar]
- Foley T. E., Fleshner M. (2008). Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 10, 67–80. 10.1007/s12017-008-8032-3 [DOI] [PubMed] [Google Scholar]
- Fond G., Hamdani N., Kapczinski F., Boukouaci W., Drancourt N., Dargel A., et al. (2014). Effectiveness and tolerance of anti-inflammatory drugs' add-on therapy in major mental disorders: a systematic qualitative review. Acta Psychiatr. Scand. 129, 163–179. 10.1111/acps.12211 [DOI] [PubMed] [Google Scholar]
- Fornai F., di Poggio A. B., Pellegrini A., Ruggieri S., Paparelli A. (2007). Noradrenaline in Parkinson's disease: from disease progression to current therapeutics. Curr. Med. Chem. 14, 2330–2334. 10.2174/092986707781745550 [DOI] [PubMed] [Google Scholar]
- Fornai F., Ruffoli R., Giorgi F. S., Paparelli A. (2011). The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur. J. Neurosci. 33, 2169–2178. 10.1111/j.1460-9568.2011.07707.x [DOI] [PubMed] [Google Scholar]
- Fornaro M., Rocchi G., Escelsior A., Contini P., Martino M. (2013). Might different cytokine trends in depressed patients receiving duloxetine indicate differential biological backgrounds. J. Affect. Disord. 145, 300–307. 10.1016/j.jad.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Francaux M. (2009). Toll-like receptor signalling induced by endurance exercise. Appl. Physiol. Nutr. Metab. 34, 454–458. 10.1139/H09-036 [DOI] [PubMed] [Google Scholar]
- Fried S. K., Bunkin D. A., Greenberg A. S. (1998). Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83, 847–850. 10.1210/jc.83.3.847 [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A., Tadeusz J., Rachwalska P., Bugajski J. (2013). Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol. Rep. 65, 1655–1662. 10.1016/S1734-1140(13)71527-5 [DOI] [PubMed] [Google Scholar]
- Gao Z., Zuberi A., Quon M. J., Dong Z., Ye J. (2003). Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J. Biol. Chem. 278, 24944–24950. 10.1074/jbc.M300423200 [DOI] [PubMed] [Google Scholar]
- Ghasemi M., Phillips C., Fahimi A., McNerney M. W., Salehi A. (2017). Mechanisms of action and clinical efficacy of NMDA receptor modulators in mood disorders. Neurosci. Biobehav. Rev. 80, 555–572. 10.1016/j.neubiorev.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Ghasemi M., Phillips C., Trillo L., De Miguel Z., Das D., Salehi A. (2014). The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci. Biobehav. Rev. 47, 336–358. 10.1016/j.neubiorev.2014.08.017 [DOI] [PubMed] [Google Scholar]
- Gillen J. B., Little J. P., Punthakee Z., Tarnopolsky M. A., Riddell M. C., Gibala M. J. (2012). Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 14, 575–577. 10.1111/j.1463-1326.2012.01564.x [DOI] [PubMed] [Google Scholar]
- Gilliam P. E., Spirduso W. W., Martin T. P., Walters T. J., Wilcox R. E., Farrar R. P. (1984). The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol. Biochem. Behav. 20, 863–867. 10.1016/0091-3057(84)90008-X [DOI] [PubMed] [Google Scholar]
- Gleeson M. (1985). Immune function in sport and exercise. J. Appl. Physiol. 103, 693–699. 10.1152/japplphysiol.00008.2007 [DOI] [PubMed] [Google Scholar]
- Gleeson M., Bishop N. C., Stensel D. J., Lindley M. R., Mastana S. S., Nimmo M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615. 10.1038/nri3041 [DOI] [PubMed] [Google Scholar]
- Gleeson M., McFarlin B., Flynn M. (2006). Exercise and toll-like receptors. Exerc. Immunol. Rev. 12, 34–53. [PubMed] [Google Scholar]
- Goffer Y., Xu D., Eberle S. E., D'Amour J., Lee M., Tukey D., et al. (2013). Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J. Neurosci. 33, 19034–19044. 10.1523/JNEUROSCI.2454-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Lázaro E., Arregi A., Beitia G., Vegas O., Azpiroz A., Garmendia L. (2011). Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress 14, 537–548. 10.3109/10253890.2011.562939 [DOI] [PubMed] [Google Scholar]
- Goodpaster B. H., He J., Watkins S., Kelley D. E. (2001). Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86, 5755–5761. 10.1210/jcem.86.12.8075 [DOI] [PubMed] [Google Scholar]
- Goshen I., Kreisel T., Ben-Menachem-Zidon O., Licht T., Weidenfeld J., Ben-Hur T., et al. (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728. 10.1038/sj.mp.4002055 [DOI] [PubMed] [Google Scholar]
- Gray J. M., Vecchiarelli H. A., Morena M., Lee T. T., Hermanson D. J., Kim A. B., et al. (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 35, 3879–3892. 10.1523/JNEUROSCI.2737-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. E., Kessler R. C., Birnbaum H. G., Leong S. A., Lowe S. W., Berglund P. A., et al. (2003). The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry 64, 1465–1475. 10.4088/JCP.v64n1211 [DOI] [PubMed] [Google Scholar]
- Greenwood B. N., Fleshner M. (2008). Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 10, 81–98. 10.1007/s12017-008-8029-y [DOI] [PubMed] [Google Scholar]
- Greenwood B. N., Foley T. E., Le T. V., Strong P. V., Loughridge A. B., Day H. E., et al. (2011). Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 217, 354–362. 10.1016/j.bbr.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. N., Kennedy S., Smith T. P., Campeau S., Day H. E., Fleshner M. (2003). Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 120, 269–281. 10.1016/S0306-4522(03)00047-2 [DOI] [PubMed] [Google Scholar]
- Grippo A. J., Sullivan N. R., Damjanoska K. J., Crane J. W., Carrasco G. A., Shi J., et al. (2005). Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology 179, 769–780. 10.1007/s00213-004-2103-4 [DOI] [PubMed] [Google Scholar]
- Guillemin G. J., Wang L., Brew B. J. (2005). Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J. Neuroinflammation 2:16. 10.1186/1742-2094-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. Y., Li C. Y., Ruan Y. P., Sun M., Qi X. L., Zhao B. S., et al. (2009). Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur. J. Pharmacol. 612, 54–60. 10.1016/j.ejphar.2009.03.076 [DOI] [PubMed] [Google Scholar]
- Haase J., Brown E. (2015). Integrating the monoamine, neurotrophin and cytokine hypotheses of depression–a central role for the serotonin transporter? Pharmacol. Ther. 147, 1–11. 10.1016/j.pharmthera.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Haddad F., Adams G. R., Bodell P. W., Baldwin K. M. (1985). Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. 100, 433–441. 10.1152/japplphysiol.01203.2005 [DOI] [PubMed] [Google Scholar]
- Hallberg L., Janelidze S., Engstrom G., Wisen A. G., Westrin A., Brundin L. (2010). Exercise-induced release of cytokines in patients with major depressive disorder. J. Affect. Disord. 126, 262–267. 10.1016/j.jad.2010.02.133 [DOI] [PubMed] [Google Scholar]
- Hamer M., Steptoe A. (2007). Association between physical fitness, parasympathetic control, and proinflammatory responses to mental stress. Psychosom. Med. 69, 660–666. 10.1097/PSY.0b013e318148c4c0 [DOI] [PubMed] [Google Scholar]
- Handschin C. (2009). Peroxisome proliferator-activated receptor-gamma coactivator-1alpha in muscle links metabolism to inflammation. Clin. Exp. Pharmacol. Physiol. 36, 1139–1143. 10.1111/j.1440-1681.2009.05275.x [DOI] [PubMed] [Google Scholar]
- Handschin C., Choi C. S., Chin S., Kim S., Kawamori D., Kurpad A. J., et al. (2007). Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J. Clin. Invest. 117, 3463–3474. 10.1172/JCI31785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C., Rhee J., Lin J., Tarr P. T., Spiegelman B. M. (2003). An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 7111–7116. 10.1073/pnas.1232352100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C., Spiegelman B. M. (2008). The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454, 463–469. 10.1038/nature07206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G. E., Fukunaga Y., Bading H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414. 10.1038/nn835 [DOI] [PubMed] [Google Scholar]
- Haroon E., Fleischer C. C., Felger J. C., Chen X., Woolwine B. J., Patel T., et al. (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry 21, 1351–1357. 10.1038/mp.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P., Khosla J., Aurora H., Laurin J., Kling M. A., Hill J., et al. (2002). A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol. Psychiatry 7, 942–947. 10.1038/sj.mp.4001119 [DOI] [PubMed] [Google Scholar]
- Helgadóttir B., Forsell Y., Hallgren M., Moller J., Ekblom O. (2017). Long-term effects of exercise at different intensity levels on depression: a randomized controlled trial. Prev. Med. 105, 37–46. 10.1016/j.ypmed.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Herbst E. A., Holloway G. P. (2016). Exercise increases mitochondrial glutamate oxidation in the mouse cerebral cortex. Appl. Physiol. Nutr. Metab. 41, 799–801. 10.1139/apnm-2016-0033 [DOI] [PubMed] [Google Scholar]
- Herzig S., Long F., Jhala U. S., Hedrick S., Quinn R., Bauer A., et al. (2001). CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183. 10.1038/35093131 [DOI] [PubMed] [Google Scholar]
- Hill M. N., Hillard C. J., McEwen B. S. (2011a). Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb. Cortex 21, 2056–2064. 10.1093/cercor/bhq280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. N., McLaughlin R. J., Pan B., Fitzgerald M. L., Roberts C. J., Lee T. T., et al. (2011b). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 31, 10506–10515. 10.1523/JNEUROSCI.0496-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J. A., McCarley R. W., Freedman R., Pivik R. T. (1974). Time course of discharge rate changes by cat pontine brain stem neurons during sleep cycle. J. Neurophysiol. 37, 1297–1309. 10.1152/jn.1974.37.6.1297 [DOI] [PubMed] [Google Scholar]
- Horowitz J. F., Klein S. (2000). Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 72, 558S–563S. 10.1093/ajcn/72.2.558S [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Huss J. M., Kopp R. P., Kelly D. P. (2002). Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J. Biol. Chem. 277, 40265–40274. 10.1074/jbc.M206324200 [DOI] [PubMed] [Google Scholar]
- Iadecola C., Nedergaard M. (2007). Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376. 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Irrcher I., Adhihetty P. J., Joseph A. M., Ljubicic V., Hood D. A. (2003). Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 33, 783–793. 10.2165/00007256-200333110-00001 [DOI] [PubMed] [Google Scholar]
- Ito Y., Yonekura R., Maruta K., Koike T., Nakagami Y., Shibata K., et al. (2003). Tryptophan metabolism was accelerated by exercise in rat. Adv. Exp. Med. Biol. 527, 531–535. 10.1007/978-1-4615-0135-0_61 [DOI] [PubMed] [Google Scholar]
- Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. (2007). AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U.S.A. 104, 12017–12022. 10.1073/pnas.0705070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelleyman C., Yates T., O'Donovan G., Gray L. J., King J. A., Khunti K., et al. (2015). The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes. Rev. 16, 942–961. 10.1111/obr.12317 [DOI] [PubMed] [Google Scholar]
- Ji L. L. (2008). Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic. Biol. Med. 44, 142–152. 10.1016/j.freeradbiomed.2007.02.031 [DOI] [PubMed] [Google Scholar]
- Jia J., Hu Y. S., Wu Y., Liu G., Yu H. X., Zheng Q. P., et al. (2009). Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci. 84, 505–511. 10.1016/j.lfs.2009.01.015 [DOI] [PubMed] [Google Scholar]
- Jiao P., Feng B., Ma J., Nie Y., Paul E., Li Y., et al. (2012). Constitutive activation of IKKbeta in adipose tissue prevents diet-induced obesity in mice. Endocrinology 153, 154–165. 10.1210/en.2011-1346 [DOI] [PubMed] [Google Scholar]
- Jones D., Gershon S., Sitaram N., Keshavan M. (1987). Sleep and depression. Psychopathology 20(Suppl. 1), 20–31. 10.1159/000284520 [DOI] [PubMed] [Google Scholar]
- Jørgensen S. B., Wojtaszewski J. F., Viollet B., Andreelli F., Birk J. B., Hellsten Y., et al. (2005). Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 19, 1146–1148. 10.1096/fj.04-3144fje [DOI] [PubMed] [Google Scholar]
- Junker V., Becker A., Huhne R., Zembatov M., Ravati A., Culmsee C., et al. (2002). Stimulation of beta-adrenoceptors activates astrocytes and provides neuroprotection. Eur. J. Pharmacol. 446, 25–36. 10.1016/S0014-2999(02)01814-9 [DOI] [PubMed] [Google Scholar]
- Kamata M., Higuchi H., Yoshimoto M., Yoshida K., Shimizu T. (2000). Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur. Neuropsychopharmacol. 10, 129–132. 10.1016/S0924-977X(99)00067-X [DOI] [PubMed] [Google Scholar]
- Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., et al. (2006). MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505. 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S., Linthorst A. C., Stalla G. K., Barden N., Holsboer F., Reul J. M. (1997). Hypothalamic-pituitary-adrenocortical axis changes in a transgenic mouse with impaired glucocorticoid receptor function. Endocrinology 138, 3476–3485. 10.1210/endo.138.8.5331 [DOI] [PubMed] [Google Scholar]
- Kawanishi N., Yano H., Yokogawa Y., Suzuki K. (2010). Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 16, 105–118. [PubMed] [Google Scholar]
- Kelly A., Vereker E., Nolan Y., Brady M., Barry C., Loscher C. E., et al. (2003). Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J. Biol. Chem. 278, 19453–19462. 10.1074/jbc.M301938200 [DOI] [PubMed] [Google Scholar]
- Kenis G., Maes M. (2002). Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 5, 401–412. 10.1017/S1461145702003164 [DOI] [PubMed] [Google Scholar]
- Kerling A., Tegtbur U., Gutzlaff E., Kuck M., Borchert L., Ates Z., et al. (2015). Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J. Affect. Disord. 177, 1–6. 10.1016/j.jad.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Kern P. A., Ranganathan S., Li C., Wood L., Ranganathan G. (2001). Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 280, E745–E751. 10.1152/ajpendo.2001.280.5.E745 [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Chiu W. T., Demler O., Merikangas K. R., Walters E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiank C., Zeden J. P., Drude S., Domanska G., Fusch G., Otten W., et al. (2010). Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE 5:e11825. 10.1371/journal.pone.0011825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Glaser R. (2002). Depression and immune function: central pathways to morbidity and mortality. J. Psychosom. Res. 53, 873–876. 10.1016/S0022-3999(02)00309-4 [DOI] [PubMed] [Google Scholar]
- Kitagami T., Yamada K., Miura H., Hashimoto R., Nabeshima T., Ohta T. (2003). Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 978, 104–114. 10.1016/S0006-8993(03)02776-8 [DOI] [PubMed] [Google Scholar]
- Köhler O., Benros M. E., Nordentoft M., Farkouh M. E., Iyengar R. L., Mors O., et al. (2014). Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391. 10.1001/jamapsychiatry.2014.1611 [DOI] [PubMed] [Google Scholar]
- Kohman R. A., DeYoung E. K., Bhattacharya T. K., Peterson L. N., Rhodes J. S. (2012). Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav. Immun. 26, 803–810. 10.1016/j.bbi.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut M. L., McCann D. A., Russell D. W., Konopka D. N., Cunnick J. E., Franke W. D., et al. (2006). Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 20, 201–209. 10.1016/j.bbi.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Krishnan R., Cella D., Leonardi C., Papp K., Gottlieb A. B., Dunn M., et al. (2007). Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br. J. Dermatol. 157, 1275–1277. 10.1111/j.1365-2133.2007.08205.x [DOI] [PubMed] [Google Scholar]
- Kvam S., Kleppe C. L., Nordhus I. H., Hovland A. (2016). Exercise as a treatment for depression: a meta-analysis. J. Affect. Disord. 202, 67–86. 10.1016/j.jad.2016.03.063 [DOI] [PubMed] [Google Scholar]
- Labsy Z., Prieur F., Le Panse B., Do M. C., Gagey O., Lasne F., et al. (2013). The diurnal patterns of cortisol and dehydroepiandrosterone in relation to intense aerobic exercise in recreationally trained soccer players. Stress 16, 261–265. 10.3109/10253890.2012.707259 [DOI] [PubMed] [Google Scholar]
- Lacy P., Stow J. L. (2011). Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood 118, 9–18. 10.1182/blood-2010-08-265892 [DOI] [PubMed] [Google Scholar]
- Lambert C. P., Wright N. R., Finck B. N., Villareal D. T. (1985). Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 105, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C., Zank M., Klein R., Stransky E., Batra A., Buchkremer G., et al. (2008). Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res. 158, 83–86. 10.1016/j.psychres.2006.04.023 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Petkova A. P., Granneman J. G. (2013). Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367. 10.1016/j.cmet.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis I. N., Mantzouridis T., Saramantis A., Phenekos C., Tzioras C., Mountokalakis T. (2000). Human galanin secretion is increased upon normal exercise test in middle-age individuals. Endocr. Res. 26, 357–364. 10.3109/07435800009066173 [DOI] [PubMed] [Google Scholar]
- Leick L., Hellsten Y., Fentz J., Lyngby S. S., Wojtaszewski J. F., Hidalgo J., et al. (2009). PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am. J. Physiol. Endocrinol. Metab. 297, E92–E103. 10.1152/ajpendo.00076.2009 [DOI] [PubMed] [Google Scholar]
- Leonard B. E. (2001). The immune system, depression and the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 767–780. 10.1016/S0278-5846(01)00155-5 [DOI] [PubMed] [Google Scholar]
- Leonard B. E. (2007). Inflammation, depression and dementia: are they connected? Neurochem. Res. 32, 1749–1756. 10.1007/s11064-007-9385-y [DOI] [PubMed] [Google Scholar]
- Leonard B., Maes M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 36, 764–785. 10.1016/j.neubiorev.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Lépine J. P., Briley M. (2011). The increasing burden of depression. Neuropsychiatr. Dis. Treat. 7, 3–7. 10.2147/NDT.S19617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Hussey S. E., Sanchez-Avila A., Tantiwong P., Musi N. (2013). Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS ONE 8:e63983. 10.1371/journal.pone.0063983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Handschin C., Spiegelman B. M. (2005). Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370. 10.1016/j.cmet.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Liu W., Sheng H., Xu Y., Liu Y., Lu J., Ni X. (2013). Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav. Brain Res. 242, 110–116. 10.1016/j.bbr.2012.12.041 [DOI] [PubMed] [Google Scholar]
- Loughlin S. E., Foote S. L., Grzanna R. (1986). Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience 18, 307–319. 10.1016/0306-4522(86)90156-9 [DOI] [PubMed] [Google Scholar]
- Lu Y., Ho C. S., Liu X., Chua A. N., Wang W., McIntyre R. S., et al. (2017). Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 12:e0186700. 10.1371/journal.pone.0186700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas J. W., Bowden C. L., Miller A. L., Javors M. A., Funderburg L. G., Berman N., et al. (1997). Schizophrenia, psychosis, and cerebral spinal fluid homovanillic acid concentrations. Schizophr. Bull. 23, 147–154. 10.1093/schbul/23.1.147 [DOI] [PubMed] [Google Scholar]
- MacRae P. G., Spirduso W. W., Cartee G. D., Farrar R. P., Wilcox R. E. (1987). Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci. Lett. 79, 138–144. 10.1016/0304-3940(87)90686-0 [DOI] [PubMed] [Google Scholar]
- Madrigal J. L., Feinstein D. L., Dello Russo C. (2005). Norepinephrine protects cortical neurons against microglial-induced cell death. J. Neurosci. Res. 81, 390–396. 10.1002/jnr.20481 [DOI] [PubMed] [Google Scholar]
- Maes M. (2011). Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 664–675. 10.1016/j.pnpbp.2010.06.014 [DOI] [PubMed] [Google Scholar]
- Maes M., Bosmans E., Suy E., Vandervorst C., De Jonckheere C., Raus J. (1990). Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology 24, 115–120. 10.1159/000119472 [DOI] [PubMed] [Google Scholar]
- Magkos F., Tsekouras Y., Kavouras S. A., Mittendorfer B., Sidossis L. S. (2008). Improved insulin sensitivity after a single bout of exercise is curvilinearly related to exercise energy expenditure. Clin. Sci. 114, 59–64. 10.1042/CS20070134 [DOI] [PubMed] [Google Scholar]
- Maier S. F., Watkins L. R. (2003). Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav. Immun. 17(Suppl. 1), S125–S131. 10.1016/S0889-1591(02)00079-X [DOI] [PubMed] [Google Scholar]
- Manders R. J., Van Dijk J. W., van Loon L. J. (2010). Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med. Sci. Sports Exerc. 42, 219–225. 10.1249/MSS.0b013e3181b3b16d [DOI] [PubMed] [Google Scholar]
- Manfridi A., Brambilla D., Bianchi S., Mariotti M., Opp M. R., Imeri L. (2003). Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur. J. Neurosci. 18, 1041–1049. 10.1046/j.1460-9568.2003.02836.x [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Maudsley S., Martin B. (2004). BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 27, 589–594. 10.1016/j.tins.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Mauriño R., Machado A., Santiago M. (2010). Effect of in vivo striatal perfusion of lipopolysaccharide on dopamine metabolites. Neurosci. Lett. 475, 121–123. 10.1016/j.neulet.2010.03.050 [DOI] [PubMed] [Google Scholar]
- McCusker R. H., Kelley K. W. (2013). Immune-neural connections: how the immune system's response to infectious agents influences behavior. J. Exp. Biol. 216, 84–98. 10.1242/jeb.073411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L., Bhagwagar Z., Hannestad J. (2008). Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 13, 501–510. 10.1017/S1092852900016734 [DOI] [PubMed] [Google Scholar]
- Mead G. E., Morley W., Campbell P., Greig C. A., McMurdo M., Lawlor D. A. (2009). Exercise for depression. Cochrane Database Syst. Rev. CD004366. 10.1002/14651858.CD004366.pub4 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Smolders I., Sarre S., de Meirleir K., Keizer H., Serneels M., et al. (1997). Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol. Scand. 159, 335–341. 10.1046/j.1365-201X.1997.00118.x [DOI] [PubMed] [Google Scholar]
- Melancon M. O., Lorrain D., Dionne I. J. (2012). Exercise increases tryptophan availability to the brain in older men age 57-70 years. Med. Sci. Sports Exerc. 44, 881–887. 10.1249/MSS.0b013e31823ede8e [DOI] [PubMed] [Google Scholar]
- Mendlewicz J., Kriwin P., Oswald P., Souery D., Alboni S., Brunello N. (2006). Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int. Clin. Psychopharmacol. 21, 227–231. 10.1097/00004850-200607000-00005 [DOI] [PubMed] [Google Scholar]
- Menter A., Augustin M., Signorovitch J., Yu A. P., Wu E. Q., Gupta S. R., et al. (2010). The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J. Am. Acad. Dermatol. 62, 812–818. 10.1016/j.jaad.2009.07.022 [DOI] [PubMed] [Google Scholar]
- Michael L. F., Wu Z., Cheatham R. B., Puigserver P., Adelmant G., Lehman J. J., et al. (2001). Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. U.S.A. 98, 3820–3825. 10.1073/pnas.061035098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Rothbaum A. O., Jovanovic T., Almli L. M., Bradley B., Rothbaum B. O., et al. (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 172, 353–362. 10.1176/appi.ajp.2014.14020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. H., Maletic V., Raison C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V., Goodrick S., Rawesh A., Katz D. R., Miles J. M., Yudkin J. S., et al. (1997). Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200. 10.1210/jcem.82.12.4450 [DOI] [PubMed] [Google Scholar]
- Moller M., Du Preez J. L., Viljoen F. P., Berk M., Emsley R., Harvey B. H. (2013). Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav. Immun. 30, 156–167. 10.1016/j.bbi.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Monti J. M. (2011). Serotonin control of sleep-wake behavior. Sleep Med. Rev. 15, 269–281. 10.1016/j.smrv.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273. 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- Mori K., Ozaki E., Zhang B., Yang L., Yokoyama A., Takeda I., et al. (2002). Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 43, 1026–1034. 10.1016/S0028-3908(02)00211-3 [DOI] [PubMed] [Google Scholar]
- Morris G., Berk M. (2015). The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 13:68. 10.1186/s12916-015-0310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mössner R., Daniel S., Schmitt A., Albert D., Lesch K. P. (2001). Modulation of serotonin transporter function by interleukin-4. Life Sci. 68, 873–880. 10.1016/S0024-3205(00)00992-9 [DOI] [PubMed] [Google Scholar]
- Motl R. W., Birnbaum A. S., Kubik M. Y., Dishman R. K. (2004). Naturally occurring changes in physical activity are inversely related to depressive symptoms during early adolescence. Psychosom. Med. 66, 336–342. 10.1097/00006842-200405000-00008 [DOI] [PubMed] [Google Scholar]
- Müller N., Schwarz M. J., Dehning S., Douhe A., Cerovecki A., Goldstein-Muller B., et al. (2006). The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684. 10.1038/sj.mp.4001805 [DOI] [PubMed] [Google Scholar]
- Murray P. S., Groves J. L., Pettett B. J., Britton S. L., Koch L. G., Dishman R. K., et al. (2010). Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides 31, 2264–2268. 10.1016/j.peptides.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint A. M., Kim Y. K., Verkerk R., Scharpe S., Steinbusch H., Leonard B. (2007). Kynurenine pathway in major depression: evidence of impaired neuroprotection. J. Affect. Disord. 98, 143–151. 10.1016/j.jad.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Myint A. M., Leonard B. E., Steinbusch H. W., Kim Y. K. (2005). Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 88, 167–173. 10.1016/j.jad.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Naya F. J., Mercer B., Shelton J., Richardson J. A., Williams R. S., Olson E. N. (2000). Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275, 4545–4548. 10.1074/jbc.275.7.4545 [DOI] [PubMed] [Google Scholar]
- Nehlsen-Cannarella S. L., Fagoaga O. R., Nieman D. C., Henson D. A., Butterworth D. E., Schmitt R. L., et al. (1985). Carbohydrate and the cytokine response to 2.5 h of running. J. Appl. Physiol. 82, 1662–1667. 10.1152/jappl.1997.82.5.1662 [DOI] [PubMed] [Google Scholar]
- Nery F. G., Monkul E. S., Hatch J. P., Fonseca M., Zunta-Soares G. B., Frey B. N. (2008). Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum. Psychopharmacol. 23, 87–94. 10.1002/hup.912 [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Carlezon W. A., Jr. (2006). The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159. 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Neurauter G., Schrocksnadel K., Scholl-Burgi S., Sperner-Unterweger B., Schubert C., Ledochowski M., et al. (2008). Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug. Metab. 9, 622–627. 10.2174/138920008785821738 [DOI] [PubMed] [Google Scholar]
- Niciu M. J., Henter I. D., Sanacora G., Zarate C. A., Jr. (2014). Glial abnormalities in substance use disorders and depression: does shared glutamatergic dysfunction contribute to comorbidity? World J. Biol. Psychiatry 15, 2–16. 10.3109/15622975.2013.829585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò G. (1993). Communications among central nervous system, neuroendocrine and immune systems: interleukin-2. Prog. Neurobiol. 40, 463–475. 10.1016/0301-0082(93)90018-N [DOI] [PubMed] [Google Scholar]
- Nisticò G., De Sarro G. (1991). Behavioral and electrocortical spectrum power effects after microinfusion of lymphokines in several areas of the rat brain. Ann. NY Acad. Sci. 621, 119–134. 10.1111/j.1749-6632.1991.tb16974.x [DOI] [PubMed] [Google Scholar]
- Okamoto T., Torii S., Machida S. (2011). Differential gene expression of muscle-specific ubiquitin ligase MAFbx/Atrogin-1 and MuRF1 in response to immobilization-induced atrophy of slow-twitch and fast-twitch muscles. J. Physiol. Sci. 61, 537–546. 10.1007/s12576-011-0175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K., Rohde T., Zacho M., Asp S., Pedersen B. K. (1998). Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 508(Pt 3), 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011). Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97. 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo D. L., Quadri S. K. (1992). Interleukin-1 inhibits serotonin release from the hypothalamus in vitro. Life Sci. 51, 1797–1802. 10.1016/0024-3205(92)90050-Y [DOI] [PubMed] [Google Scholar]
- Panagiotakos D. B., Pitsavos C., Chrysohoou C., Kavouras S., Stefanadis C., Study A. (2005). The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev. Med. 40, 432–437. 10.1016/j.ypmed.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Paolucci E. M., Loukov D. D., Bowdish M. E., Heisz J. J. (2018). Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 133, 79–84. 10.1016/j.biopsycho.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Pariante C. M., Miller A. H. (2001). Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry 49, 391–404. 10.1016/S0006-3223(00)01088-X [DOI] [PubMed] [Google Scholar]
- Passamonti L., Crockett M. J., Apergis-Schoute A. M., Clark L., Rowe J. B., Calder A. J., et al. (2012). Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol. Psychiatry 71, 36–43. 10.1016/j.biopsych.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti M. E., Butte A. J., Crunkhorn S., Cusi K., Berria R., Kashyap S., et al. (2003). Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U.S.A. 100, 8466–8471. 10.1073/pnas.1032913100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul I. A., Skolnick P. (2003). Glutamate and depression: clinical and preclinical studies. Ann. N.Y. Acad. Sci. 1003, 250–272. 10.1196/annals.1300.016 [DOI] [PubMed] [Google Scholar]
- Pedersen B. K. (2009). Adolph distinguished lecture: muscle as an endocrine organ, IL-6 and other myokines. J. Appl. Physiol. 107, 1006–1014. 10.1152/japplphysiol.00734.2009 [DOI] [PubMed] [Google Scholar]
- Pedersen B. K., Febbraio M. A. (2012). Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465. 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- Pedersen B. K., Fischer C. P. (2007). Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol. Sci. 28, 152–156. 10.1016/j.tips.2007.02.002 [DOI] [PubMed] [Google Scholar]
- Pedersen B. K., Steensberg A., Schjerling P. (2001). Exercise and interleukin-6. Curr. Opin. Hematol. 8, 137–141. 10.1097/00062752-200105000-00002 [DOI] [PubMed] [Google Scholar]
- Peeters F., Nicholson N. A., Berkhof J. (2003). Cortisol responses to daily events in major depressive disorder. Psychosom. Med. 65, 836–841. 10.1097/01.PSY.0000088594.17747.2E [DOI] [PubMed] [Google Scholar]
- Pemberton R., Fuller Tyszkiewicz M. D. (2016). Factors contributing to depressive mood states in everyday life: a systematic review. J. Affect. Disord. 200, 103–110. 10.1016/j.jad.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Petersen A. M., Pedersen B. K. (1985). The anti-inflammatory effect of exercise. J. Appl. Physiol. 98, 1154–1162. 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- Petruzzelli M., Schweiger M., Schreiber R., Campos-Olivas R., Tsoli M., Allen J., et al. (2014). A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447. 10.1016/j.cmet.2014.06.011 [DOI] [PubMed] [Google Scholar]
- Phillips C. (2017a). Physical activity modulates common neuroplasticity substrates in major, depressive and bipolar disorder. Neural Plast. 2017:7014146. 10.1155/2017/7014146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. (2017b). Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017:7260130. 10.1155/2017/7260130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. (2017c). Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017:3589271. 10.1155/2017/3589271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C., Baktir M. A., Das D., Lin B., Salehi A. (2015). The link between physical activity and cognitive dysfunction in Alzheimer disease. Phys. Ther. 95, 1046–1060. 10.2522/ptj.20140212 [DOI] [PubMed] [Google Scholar]
- Phillips C., Baktir M. A., Srivatsan M., Salehi A. (2014). Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front. Cell Neurosci. 8:170. 10.3389/fncel.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C., Fahimi A., Das D., Mojabi F. S., Ponnusamy R., Salehi A. (2016). Noradrenergic system in down syndrome and Alzheimer's disease a target for therapy. Curr. Alzheimer Res. 13, 68–83. 10.2174/1567205012666150921095924 [DOI] [PubMed] [Google Scholar]
- Phillips C., Salehi A. (2016). A special regenerative rehabilitation and genomics letter: is there a “Hope” molecule? Phys. Ther. 96, 581–583. 10.2522/ptj.2016.96.4.581 [DOI] [PubMed] [Google Scholar]
- Pieribone V. A., Xu Z. Q., Zhang X., Grillner S., Bartfai T., Hokfelt T. (1995). Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience 64, 861–874. 10.1016/0306-4522(94)00450-J [DOI] [PubMed] [Google Scholar]
- Prasad A. A., Pasterkamp R. J. (2009). Axon guidance in the dopamine system. Adv. Exp. Med. Biol. 651, 91–100. 10.1007/978-1-4419-0322-8_9 [DOI] [PubMed] [Google Scholar]
- Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., et al. (2003). Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423, 550–555. 10.1038/nature01667 [DOI] [PubMed] [Google Scholar]
- Puigserver P., Spiegelman B. M. (2003). Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24, 78–90. 10.1210/er.2002-0012 [DOI] [PubMed] [Google Scholar]
- Raison C. L., Rutherford R. E., Woolwine B. J., Shuo C., Schettler P., Drake D. F., et al. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J. J. (2007). Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 6, 219–233. 10.2174/187152707780619326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. S. (2010). Depression: the disorder and the burden. Indian J. Psychol. Med. 32, 1–2. 10.4103/0253-7176.70510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss J. I., Dishman R. K., Boyd H. E., Robinson J. K., Holmes P. V. (2009). Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 1266, 54–63. 10.1016/j.brainres.2009.02.030 [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Aja S. (2008). AMP-activated protein kinase in the brain. Int. J. Obes. 32(Suppl. 4), S42–S48. 10.1038/ijo.2008.122 [DOI] [PubMed] [Google Scholar]
- Rosano C., Guralnik J., Pahor M., Glynn N. W., Newman A. B., Ibrahim T. S., et al. (2017). Hippocampal response to a 24-Month physical activity intervention in sedentary older adults. Am. J. Geriatr. Psychiatry 25, 209–217. 10.1016/j.jagp.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S., Vazdarjanova A., Ramirez-Amaya V., Worley P. F., Barnes C. A., Wenk G. L. (2006). Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience 142, 1303–1315. 10.1016/j.neuroscience.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Russell A. P., Feilchenfeldt J., Schreiber S., Praz M., Crettenand A., Gobelet C., et al. (2003). Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52, 2874–2881. 10.2337/diabetes.52.12.2874 [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A., Beard R. C., Cotman C. W. (1999). Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 21, 679–682. 10.1016/S0893-133X(99)00059-7 [DOI] [PubMed] [Google Scholar]
- Ryder J. W., Bassel-Duby R., Olson E. N., Zierath J. R. (2003). Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J. Biol. Chem. 278, 44298–44304. 10.1074/jbc.M304510200 [DOI] [PubMed] [Google Scholar]
- Rytinki M. M., Palvimo J. J. (2008). SUMOylation modulates the transcription repressor function of RIP140. J. Biol. Chem. 283, 11586–11595. 10.1074/jbc.M709359200 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Arnolds D. E., Ekberg I., Thorell A., Goodyear L. J. (2004). Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem. Biophys. Res. Commun. 319, 419–425. 10.1016/j.bbrc.2004.05.020 [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Baker R. A., Ramer-Quinn D. S., Kasprowicz D. J., Fuchs B. A., Street N. E. (1997). Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J. Immunol. 158, 4200–4210. [PubMed] [Google Scholar]
- Sarbadhikari S. N., Saha A. K. (2006). Moderate exercise and chronic stress produce counteractive effects on different areas of the brain by acting through various neurotransmitter receptor subtypes: a hypothesis. Theor. Biol. Med. Model. 3:33. 10.1186/1742-4682-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C. (2008). Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638. 10.1152/physrev.00025.2007 [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. (1990). Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75, 40–47. [PubMed] [Google Scholar]
- Schlittler M., Goiny M., Agudelo L. Z., Venckunas T., Brazaitis M., Skurvydas A., et al. (2016). Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell. Physiol. 310, C836–C840. 10.1152/ajpcell.00053.2016 [DOI] [PubMed] [Google Scholar]
- Schuch F. B., Vancampfort D., Richards J., Rosenbaum S., Ward P. B., Stubbs B. (2016). Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 77, 42–51. 10.1016/j.jpsychires.2016.02.023 [DOI] [PubMed] [Google Scholar]
- Schultz W. (2002). Getting formal with dopamine and reward. Neuron 36, 241–263. 10.1016/S0896-6273(02)00967-4 [DOI] [PubMed] [Google Scholar]
- Sciolino N. R., Holmes P. V. (2012). Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 36, 1965–1984. 10.1016/j.neubiorev.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H., Habich C., Eckel J. (2012). Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 8, 709–716. 10.1038/nrendo.2012.114 [DOI] [PubMed] [Google Scholar]
- Seutin V., Verbanck P., Massotte L., Dresse A. (1989). Galanin decreases the activity of locus coeruleus neurons in vitro. Eur. J. Pharmacol. 164, 373–376. 10.1016/0014-2999(89)90481-0 [DOI] [PubMed] [Google Scholar]
- Shibakawa Y. S., Sasaki Y., Goshima Y., Echigo N., Kamiya Y., Kurahashi K., et al. (2005). Effects of ketamine and propofol on inflammatory responses of primary glial cell cultures stimulated with lipopolysaccharide. Br. J. Anaesth. 95, 803–810. 10.1093/bja/aei256 [DOI] [PubMed] [Google Scholar]
- Sigal R. J., Kenny G. P., Boule N. G., Wells G. A., Prud'homme Fortier M., et al. (2007). Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann. Intern. Med. 147, 357–369. 10.7326/0003-4819-147-6-200709180-00005 [DOI] [PubMed] [Google Scholar]
- Silveira H., Moraes H., Oliveira N., Coutinho E. S., Laks J., Deslandes A. (2013). Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology 67, 61–68. 10.1159/000345160 [DOI] [PubMed] [Google Scholar]
- Silverman M. N., Deuster P. A. (2014). Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 4:20140040. 10.1098/rsfs.2014.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira C. C., Valiengo L. L., Carvalho A. F., Santos-Silva P. R., Missio G., de Sousa R. T., et al. (2016). Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PLoS ONE 11:e0154195. 10.1371/journal.pone.0154195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk T., Alberti-Huber C., Herder C., Hauner H. (2007). Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 92, 1023–1033. 10.1210/jc.2006-1055 [DOI] [PubMed] [Google Scholar]
- Smagin G. N., Swiergiel A. H., Dunn A. J. (1996). Peripheral administration of interleukin-1 increases extracellular concentrations of norepinephrine in rat hypothalamus: comparison with plasma corticosterone. Psychoneuroendocrinology 21, 83–93. 10.1016/0306-4530(95)00019-4 [DOI] [PubMed] [Google Scholar]
- Smith J. K., Dykes R., Douglas J. E., Krishnaswamy G., Berk S. (1999). Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281, 1722–1727. 10.1001/jama.281.18.1722 [DOI] [PubMed] [Google Scholar]
- Soares J., Holmes P. V., Renner K. J., Edwards G. L., Bunnell B. N., Dishman R. K. (1999). Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav. Neurosci. 113, 558–566. 10.1037/0735-7044.113.3.558 [DOI] [PubMed] [Google Scholar]
- Soczynska J. K., Kennedy S. H., Goldstein B. I., Lachowski A., Woldeyohannes H. O., McIntyre R. S. (2009). The effect of tumor necrosis factor antagonists on mood and mental health-associated quality of life: novel hypothesis-driven treatments for bipolar depression? Neurotoxicology 30, 497–521. 10.1016/j.neuro.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Stanton R., Reaburn P. (2014). Exercise and the treatment of depression: a review of the exercise program variables. J. Sci. Med. Sport 17, 177–182. 10.1016/j.jsams.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Starkie R. L., Arkinstall M. J., Koukoulas I., Hawley J. A., Febbraio M. A. (2001). Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 533, 585–591. 10.1111/j.1469-7793.2001.0585a.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A., Febbraio M. A., Osada T., Schjerling P., van Hall G., Saltin B., et al. (2001). Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 537, 633–639. 10.1111/j.1469-7793.2001.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A., van Hall G., Osada T., Sacchetti M., Saltin B., Klarlund Pedersen B. (2000). Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 529(Pt 1), 237–242. 10.1111/j.1469-7793.2000.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Bogerts B., Sarnyai Z., Walter M., Gos T., Bernstein H. G., et al. (2012). Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J. Biol. Psychiatry 13, 482–492. 10.3109/15622975.2011.583941 [DOI] [PubMed] [Google Scholar]
- Steptoe A., Willemsen G., Owen N., Flower L., Mohamed-Ali V. (2001). Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin. Sci. 101, 185–192. 10.1042/cs1010185 [DOI] [PubMed] [Google Scholar]
- St-Pierre J., Lin J., Krauss S., Tarr P. T., Yang R., Newgard C. B., et al. (2003). Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J. Biol. Chem. 278, 26597–26603. 10.1074/jbc.M301850200 [DOI] [PubMed] [Google Scholar]
- Suetta C., Frandsen U., Jensen L., Jensen M. M., Jespersen J. G., Hvid L. G., et al. (2012). Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS ONE 7:e51238. 10.1371/journal.pone.0051238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoo D. E., Akiyama K. (1996). The mechanism by which exercise modifies brain function. Physiol. Behav. 60, 177–181. 10.1016/0031-9384(96)00011-X [DOI] [PubMed] [Google Scholar]
- Tang T., Zhang J., Yin J., Staszkiewicz J., Gawronska-Kozak B., Jung D. Y., et al. (2010). Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J. Biol. Chem. 285, 4637–4644. 10.1074/jbc.M109.068007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti J. F., Ceppo F., Jager J., Berthou F. (2012). Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 3:181. 10.3389/fendo.2012.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnopolsky M. A., Rennie C. D., Robertshaw H. A., Fedak-Tarnopolsky S. N., Devries M. C., Hamadeh M. J. (2007). Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1271–R1278. 10.1152/ajpregu.00472.2006 [DOI] [PubMed] [Google Scholar]
- Timmerman K. L., Flynn M. G., Coen P. M., Markofski M. M., Pence B. D. (2008). Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 84, 1271–1278. 10.1189/jlb.0408244 [DOI] [PubMed] [Google Scholar]
- Trivedi M. H. (2006). Major depressive disorder: remission of associated symptoms. J. Clin. Psychiatry 67(Suppl. 6), 27–32. [PubMed] [Google Scholar]
- Trivedi M. H., Greer T. L., Church T. S., Carmody T. J., Grannemann B. D., Galper D. I., et al. (2011). Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J. Clin. Psychiatry 72, 677–684. 10.4088/JCP.10m06743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi M. H., Hollander E., Nutt D., Blier P. (2008). Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J. Clin. Psychiatry 69, 246–258. 10.4088/JCP.v69n0211 [DOI] [PubMed] [Google Scholar]
- Tsagarakis S., Gillies G., Rees L. H., Besser M., Grossman A. (1989). Interleukin-1 directly stimulates the release of corticotrophin releasing factor from rat hypothalamus. Neuroendocrinology 49, 98–101. 10.1159/000125096 [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Zhang F., Adamantidis A., Stuber G. D., Bonci A., de Lecea L., et al. (2009). Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084. 10.1126/science.1168878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C. W., Lin Y. S., Chen C. C., Bai C. H., Wu S. R. (2006). Cytokines and serotonin transporter in patients with major depression. Prog Neuropsychopharmacol. Biol. Psychiatry 30, 899–905. 10.1016/j.pnpbp.2006.01.029 [DOI] [PubMed] [Google Scholar]
- Tuglu C., Kara S. H., Caliyurt O., Vardar E., Abay E. (2003). Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 170, 429–433. 10.1007/s00213-003-1566-z [DOI] [PubMed] [Google Scholar]
- Tye K. M., Mirzabekov J. J., Warden M. R., Ferenczi E. A., Tsai H. C., Finkelstein J., et al. (2013). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541. 10.1038/nature11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A., et al. (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35. 10.1016/S0140-6736(05)67763-X [DOI] [PubMed] [Google Scholar]
- Vaidya V. A., Terwilliger R. M., Duman R. S. (1999). Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett. 262, 1–4. 10.1016/S0304-3940(99)00006-3 [DOI] [PubMed] [Google Scholar]
- Valle I., Alvarez-Barrientos A., Arza E., Lamas S., Monsalve M. (2005). PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66, 562–573. 10.1016/j.cardiores.2005.01.026 [DOI] [PubMed] [Google Scholar]
- van der Poll T., Coyle S. M., Barbosa K., Braxton C. C., Lowry S. F. (1996). Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J. Clin. Invest. 97, 713–719. 10.1172/JCI118469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G., Stromstad M., Rasmussen P., Jans O., Zaar M., Gam C., et al. (2009). Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 29, 1121–1129. 10.1038/jcbfm.2009.35 [DOI] [PubMed] [Google Scholar]
- Vechetti-Junior I. J., Bertaglia R. S., Fernandez G. J., de Paula T. G., de Souza R. W., Moraes L. N., et al. (2016). Aerobic exercise recovers disuse-induced atrophy through the stimulus of the LRP130/PGC-1alpha complex in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 71, 601–609. 10.1093/gerona/glv064 [DOI] [PubMed] [Google Scholar]
- Vega R. B., Huss J. M., Kelly D. P. (2000). The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell Biol. 20, 1868–1876. 10.1128/MCB.20.5.1868-1876.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N. P., Gleeson M., Pyne D. B., Nieman D. C., Dhabhar F. S., Shephard R. J., et al. (2011). Position statement. Part two: maintaining immune health. Exerc. Immunol. Rev. 17, 64–103. [PubMed] [Google Scholar]
- Wang N., Yu H. Y., Shen X. F., Gao Z. Q., Yang C., Yang J. J., et al. (2015). The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups J. Med. Sci. 120, 241–248. 10.3109/03009734.2015.1060281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters R. P., Renner K. J., Pringle R. B., Summers C. H., Britton S. L., Koch L. G., et al. (2008). Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol. Behav. 93, 1044–1054. 10.1016/j.physbeh.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert C., Hennige A. M., Lehmann R., Brodbeck K., Baumgartner F., Schauble M., et al. (2006). Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J. Biol. Chem. 281, 7060–7067. 10.1074/jbc.M509782200 [DOI] [PubMed] [Google Scholar]
- Wende A. R., Schaeffer P. J., Parker G. J., Zechner C., Han D. H., Chen M. M., et al. (2007). A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 282, 36642–36651. 10.1074/jbc.M707006200 [DOI] [PubMed] [Google Scholar]
- Wojtaszewski J. F., Hansen B. F., Gade K. B., Markuns J. F., Goodyear L. J., Richter E. A. (2000). Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49, 325–331. 10.2337/diabetes.49.3.325 [DOI] [PubMed] [Google Scholar]
- Wojtaszewski J. F., Jorgensen S. B., Frosig C., MacDonald C., Birk J. B., Richter E. A. (2003). Insulin signalling: effects of prior exercise. Acta Physiol. Scand. 178, 321–328. 10.1046/j.1365-201X.2003.01151.x [DOI] [PubMed] [Google Scholar]
- Wu H., Kanatous S. B., Thurmond F. A., Gallardo T., Isotani E., Bassel-Duby R., et al. (2002). Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296, 349–352. 10.1126/science.1071163 [DOI] [PubMed] [Google Scholar]
- Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., et al. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124. 10.1016/S0092-8674(00)80611-X [DOI] [PubMed] [Google Scholar]
- Xu S. X., Zhou Z. Q., Li X. M., Ji M. H., Zhang G. F., Yang J. J. (2013). The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behav. Brain Res. 253, 305–309. 10.1016/j.bbr.2013.07.032 [DOI] [PubMed] [Google Scholar]
- Yadid G., Friedman A. (2008). Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog. Brain Res. 172, 265–286. 10.1016/S0079-6123(08)00913-8 [DOI] [PubMed] [Google Scholar]
- Ye Q., Huang W., Li D., Si E., Wang J., Wang Y., et al. (2016). Overexpression of PGC-1alpha influences mitochondrial signal transduction of dopaminergic neurons. Mol. Neurobiol. 53, 3756–3770. 10.1007/s12035-015-9299-7 [DOI] [PubMed] [Google Scholar]
- Yeh S. H., Chuang H., Lin L. W., Hsiao C. Y., Eng H. L. (2006). Regular tai chi chuan exercise enhances functional mobility and CD4CD25 regulatory T cells. Br. J. Sports Med. 40, 239–243. 10.1136/bjsm.2005.022095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., et al. (2001). Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138. 10.1038/35093050 [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Hori H., Ikenouchi-Sugita A., Umene-Nakano W., Ueda N., Nakamura J. (2009). Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 722–726. 10.1016/j.pnpbp.2009.03.020 [DOI] [PubMed] [Google Scholar]
- You Z. B., Chen Y. Q., Wise R. A. (2001). Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience 107, 629–639. 10.1016/S0306-4522(01)00379-7 [DOI] [PubMed] [Google Scholar]
- Zalcman S., Green-Johnson J. M., Murray L., Nance D. M., Dyck D., Anisman H., et al. (1994). Cytokine-specific central monoamine alterations induced by interleukin-1,−2 and−6. Brain Res. 643, 40–49. 10.1016/0006-8993(94)90006-X [DOI] [PubMed] [Google Scholar]
- Zhao W. Q., De Felice F. G., Fernandez S., Chen H., Lambert M. P., Quon M. J., et al. (2008). Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 22, 246–260. 10.1096/fj.06-7703com [DOI] [PubMed] [Google Scholar]
- Zouhal H., Jacob C., Delamarche P., Gratas-Delamarche A. (2008). Catecholamines and the effects of exercise, training and gender. Sports Med. 38, 401–423. 10.2165/00007256-200838050-00004 [DOI] [PubMed] [Google Scholar]