Abstract
Background/Aim: A common finding in cancer cells is the overexpression of histone deacetylases (HDACs), leading to altered expression and activity of numerous proteins involved in carcinogenesis. Considering that leptin can modulate the levels of HDACs, we hypothesised that leptin receptor antagonists can alter HDAC expression. Materials and Methods: HDAC expression in cells exposed to leptin and leptin receptor antagonists (SHLA and Lan2) were evaluated in ovarian epithelial (OVCAR-3, CaOV3) and folliculoma (COV434, KGN) cells. Results: Higher HDAC expression was found in epithelial compared to folliculoma cells. Leptin increased class I and II HDACs only in OVCAR-3 cells, and SHLA was more potent then Lan-2. In folliculoma cells, leptin only increased class II HDAC expression, Lan-2 was more potent than SHLA in the COV434 and neither antagonist affected the KGN cells. Conclusion: SHLA and Lan2 eliminate the negative effects of leptin on HDAC expression in a cell-type-dependent manner. This is the first report testing leptin receptor blockers as HDAC inhibitors in ovarian cancer cells.
Keywords: Ovarian cancer, leptin, leptin receptors blocker, HDACs expression
Epigenetic changes such as post-translational histone modifications can play an important role in cancer development. Two groups of enzymes are responsible for the acetylation pattern of histones: histone acetylases (HATs) and histone deacetylases (HDACs). The predominance of HDACs over HATs led to repression of the transcription of several genes responsible for carcinogenesis suppression. In addition, HDACs are also responsible for the deacetylation of non-histone proteins which regulate cell-cycle progression, proliferation and apoptosis (1). Mammalian HDACs are divided into four classes. HDACs 1, 2, 3 and 8 constitute class I. HDACs 4-7, 9 and 10 form class II. Class III HDACs are NAD+-dependent enzymes also known as Sirtuins (SIRTs) (2). HDAC11, the only member of Class IV HDACs, combines the features of class I and II (3).
A common finding in cancer cells is the overexpression of HDACs and their increased activity, leading to the altered expression and activity of numerous proteins involved in carcinogenesis. Although the knowledge of ovarian cancer is growing, there is still no proper therapy for the prevention and treatment of this type of cancer (4). There is a growing body of evidence showing that the expression of class I HDACs is increased in ovarian carcinomas (5-7) and this is suspected not only of playing a significant role in carcinogenesis, but also of contributing toward resistance to chemotherapeutic agents used for treating ovarian cancer. Jin et al. (5) showed that HDACs from class I are overexpressed in ovarian cancer samples versus samples obtained from healthy individuals. Hayashi et al. (8) revealed that among the many tissue samples obtained from ovarian cancer patients, HDAC1 and HDAC2 are mainly involved in cancer cell proliferation, while HDAC3 is connected with cell migration processes. Inhibiting HDAC3 results in the inhibition of cancer cell migration. In addition, HDAC expression is increased among patients with ovarian cancer that is resistant to platinum-based chemotherapy (9). Also, HDAC4 (9) and HDAC6 (10) are involved in ovarian cancer resistance and progression. Lapinska et al. (11) showed that combination therapies with HDAC inhibitors may be effective against different types of ovarian cancers.
Obesity is a risk factor for several different types of cancer, significantly promoting cancer incidence, progression, poor prognosis and resistance to anti-cancer therapies. The increased risk of ovarian cancer, both epithelial and folliculoma, are correlated with obesity; elevated leptin secretion and higher leptin receptor expression has been described in ovarian cancer versus non-cancer cells (12,13). Although data pointing to the relationship between HDAC and leptin are scarce, it has been reported that HDAC5 expression is regulated by dietary lipids and leptin and hypothalamic HDAC5 is an important component of leptin signalling (14). One of the histone deacetylase inhibitors, Valproic acid, can decrease leptin mRNA in adipocytes, thereby altering leptin homeostasis (15). Leptin can affect the level of HDACs in pancreatic adenocarcinoma tumorspheres, and, via the modulation of HDACs, leptin can induce pancreatic adenocarcinoma progression and chemoresistance (16). Elevated HDAC1 and HDAC2 expression was noted after leptin administration in rats and correlated with a decrease in sperm quality (17). Sheykhani et al. (18) have shown that leptin can reverse the apoptotic effect of trichostatin A (an inhibitor of first and second class HDACs) on buffalo oocytes.
In our previously published data, we demonstrated that the negative effect of leptin on the proliferation of several epithelial and folliculoma ovarian cancer cells can be partially or completely reversed by leptin receptor antagonist treatment via interactions with cell cycle protein expression (12,13). A number of leptin receptor antagonists have been synthesised for therapeutic use, with several completing pre-clinical testing (19) indicating their possible use in anticancer therapy. HDAC inhibitors seem to be promising anti-cancer drugs, particularly in combination with other anti-cancer drugs and/or radiotherapy.
The question arises whether the leptin receptor blockers may also have a positive impact on HDACs. As a model for this study, we used the epithelial ovarian cancer cell lines, OVCAR-3 and CaOV-3, and folliculoma cell lines KGN and COV434. First, we determined the basal and leptin-stimulated expression of HDAC classes I and II and then the effect of leptin receptor antagonists on the expression of HDAC genes and proteins. Additionally, we evaluated the action on HDAC activity.
Materials and Methods
Materials. Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) was obtained from Gibco by Thermo Fisher Scientific (Waltham, MA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), RPMI-1640, foetal bovine serum (FBS, heat inactivated), penicillin and streptomycin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Leptin was obtained from Sigma Chemical Co. Leptin receptor antagonists (SHLA, Lan1 and Lan2) were obtained from Protein Laboratories Rehovot (PLR) Ltd. (Rehovot, Israel).
Cell culture. OVCAR-3 (established from the malignant ascites of a patient with progressive adenocarcinoma of the ovary after combination chemotherapy with cyclophosphamide) and CaOV-3 (a primary ovarian cancer cell line), were obtained from the American Type Culture Collection (Manassas, VA, USA). COV434 cells were obtained from the Sigma Chemical Co. (St. Louis, MO, USA). The biological characteristics of this cell line have been previously described (20). KGN cells were obtained from Masatoshi Nomura and Hajime Nawata, Kyushu University, Japan (21). CaOV-3 cells were routinely cultured in DMEM with 10% FBS, and OVCAR-3 in RPMI-1640 supplemented with 20% FBS. COV434 cells were routinely cultured in DMEM + 2 mM Glutamine + 10% FBS. KGN cells were routinely cultured in DMEM/F-12 + 10% FBS. Cells were grown in 75 cm2 tissue culture dishes (Nunc, Denmark) in a 37˚C incubator with a humidified mixture of 5% CO2:95% air.
qPCR analysis. Basal HDAC gene expression and the expression of HDACs genes under the influence of leptin and leptin antagonists was determined by qPCR. Cells were seeded into 96-well culture plates at a density of 1.5×104 cells/well (CaOV-3), 1.5×103 cells/well (OVCAR-3), 8×103 cells/well (COV434) and 1.5×104 cells/well (KGN) taking into consideration the size of cells and the population doubling time. The next day, the medium was changed and cells were treated with leptin at a dose of 40 ng/ml alone or with SHLA and leptin. Doses of leptin were chosen based on the literature and our previously published paper (12,13,22). Total RNA isolation and cDNA synthesis was performed using the TaqMan Gene Expression Cell-to-CT Kit (Applied Biosystems, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol. Amplifications were performed using the StepOnePlus system (Applied Biosystems) and the TaqMan starters (Hs02621185_s1, Hs00231032_m1, Hs00187320_m1, Hs00195 814_m1, Hs00608366_m1, Hs00195869_m1, Hs00248789_m1, Hs00206843_m1 (Cat. #4453320) in combination with the TaqMan Gene Expression Master Mix (Applied Biosystems), in accordance with the manufacturer’s instructions. The relative expression of genes was normalised against the endogenous reference gene GAPDH (Human GAPD Endogenous Control, number 4333764F) (ΔCq) and converted to relative expression using the 2-ΔΔCq method. The results are expressed as relative values (RQ).
Western blot analysis. Cells were plated into 24-well plates at a density of 2×105 (CaOV-3), 3.5×104 (OVCAR-3 cells), 4×104 (COV434 cells) and 6×104 (KGN cells) and allowed to attach overnight. On the following day, the media were changed and cells were treated with 40 μg/ml leptin alone or in combination with 1,000 μg/mL SHLA or Lan2. To examine HDAC protein expression, cells were incubated for 48 h. After incubation, cells were washed with ice-cold PBS and lysed with Laemmli lysis buffer (Sigma Chemical Co.). The lysed cells were then scraped, transferred to microtubes and stored at –70˚C until analysis.
Equal amounts of protein (50 μg) from each treatment group were separated by SDS-PAGE and transferred to PVDF membranes using the Bio-Rad Mini-Protean 3 apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA). Blots were incubated overnight with primary antibodies specific to HDAC5, HDAC6 and HDAC7 (#20458S, #7558S, #33418S Cell Signaling Technology Inc., Beverly, MA, USA) at a 1:1,000 dilution. After incubation with the primary antibody, membranes were washed three times with 0.1% Tween-20 in 0.02 M TBS buffer and incubated for 1 h with an anti-rabbit horseradish peroxidase-conjugated secondary antibody (#7074 Cell Signaling Technology Inc.; dilution 1:2,000).
β-Actin was used as an internal loading control; membranes were incubated overnight with primary antibodies specific to β-actin (A5316, Sigma Chemical Co.; dilution 1:2000) and for 1 h with a horseradish peroxidase-conjugated secondary antibody (P0447 DAKO, Glostrup, Denmark; dilution 1:5,000).
Immunopositive bands were visualised using Western Blotting Luminol Reagent (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and ChemiDoc™ XRS+System (Bio-Rad Laboratories Inc.). Individual protein levels were normalised to β-actin controls and the ratio of protein to β-actin was normalised to 1 in the untreated control group.
Histone deacetylase activity assay. HDAC activity was measured using the In Situ HDAC Activity Fluorometric Assay Kit (EPI003, Sigma Chemical Co.). The amounts of fluorescent product were measured using a fluorescence microplate reader (FLx800 Bio-Tek Instruments, Winooski, VT, USA) at an excitation wavelength of 368 nm and an emission wavelength 442 nm. All samples were run in quadruplicate at the same assay.
Statistical analysis. Data were expressed as mean±SEM from the four independent experiments performed in triplicate. Statistical analyses were performed using GraphPad Prism 5. Data were analysed using a two-way analysis of variance (ANOVA) followed by a Tukey’s honestly significant difference (HSD) multiple range test. A value of p<0.05 was considered statistically significant.
Results
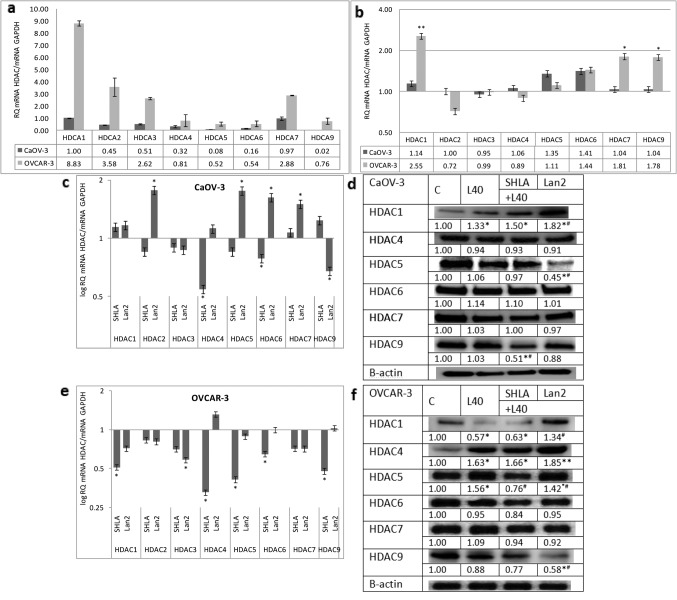
Effect of leptin and leptin receptor antagonists on HDAC gene and protein expression in ovarian epithelial cancer cells. In the OVCAR-3 cell line, the expression of all investigated HDACs was significantly higher than in CaOV-3 cells, with the highest expression noted for HDACs 1, 2, 3 and 7 (Figure 1a). Leptin increased the expression of the HDAC 1, 7 and 9 genes (Figure 1b). From the used blockers, SHLA not only reversed the stimulatory effect of leptin on the HDAC 1 and 9 genes and proteins, but also decreased the expression of the HDAC 4 gene, the HDAC 5 gene and protein and the HDAC6 protein. Lan-2 had no effect on HDAC gene expression, but a strong inhibitory effect was noted on the expression of the HDAC9 protein (Figure 1c-d).
Figure 1. Basal gene expression of histone deacetylases (a). Effect of leptin (b) and leptin receptor antagonists on HDAC gene and protein expression in OVCAR-3 (c, d), and HDAC gene and protein expression in CaOV-3 (e, f) cancer cell lines. Basal mRNA values were evaluated by qPCR after 24 h of cell culture and all the results were normalised to HDAC1 expression in CaOV-3 with a value equal to 1. All values marked with *p<0.05 and **p<0.01 are significantly different from the control values. Values are mean±SEM. All values marked with *p<0.05 are significantly different from the control. All values marked with #p<0.05 are significantly different from leptin-stimulated cells.
In CaOV-3 cells, leptin did not have a statistically significant effect on the expression of any of the investigated HDAC genes (Figure 1b). From the investigated HDACs, SHLA decreased the expression of the HDAC4 gene, but not the protein, and HDAC9 protein expression. Lan-2 increased the expression of the HDAC 2, 5, 6 and 7 genes (Figure 1e). An inhibitory effect on protein expression was observed only in the case of HDAC 5 (Figure 1f).
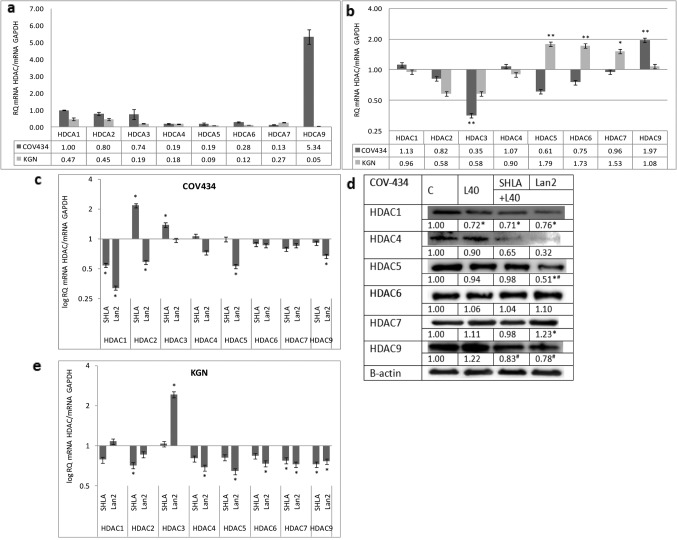
Effect of leptin and leptin receptor antagonists on HDAC gene and protein expression in ovarian granulosa cancer cells. In the COV434 cancer cell line, representing a juvenile form of granulosa cancer, the highest expression of HDAC9 was noted. For other investigated HDAC genes, the expression of HDAC 1, 2 and 3 was four- to seven-fold higher than that of HDAC 4, 5, 6 and 7. The low expression of all investigated HDACs was observed in KGN cells representing the adult form of ovarian granulosa cancer (Figure 2a). In COV434 cells, leptin increased HDAC9 and had an inhibitory effect on HDAC3, while it increased the expression of HDAC 5, 6 and 7 in KGN cells (Figure 2b). SHLA decreased HDAC1 gene expression and the expression of HDAC 1, 4 and 9 proteins. Lan2 was most potent antagonist, decreasing the expression of not only the HDAC1 but also the HDAC5 genes and proteins, as well as the HDAC4 and 9 protein expression (Figure 2c and d). In KGN cells, leptin increased HDAC 4, 5 and 6 gene expression (Figure 2b). Both of the antagonists used had no effect on the investigated HDAC gene expression (Figure 2e).
Figure 2. Basal expression of histone deacetylase genes (a). Effect of leptin (b) and leptin receptor antagonists on HDAC gene and protein expression in COV434 cell lines (c, d) and gene expression in KGN cells (e). Basal and leptin-stimulated mRNA values was evaluated by qPCR after 24 h of cell culture and the results were normalised to HDAC1 expression in COV434 cells with a value equal to 1. All values marked with *p<0.05 and **p<0.01 are significantly different from control values. Values are mean±SEM. HDAC densitometry results were normalised to β-actin loading control to obtain band ratios. All values marked with #p<0.05 are significantly different from the values of leptin at 40 ng/mL.
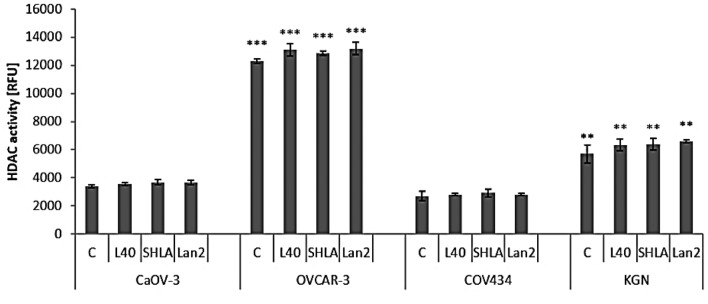
Effect of leptin and leptin receptor antagonists on histone deacetylase activity. In epithelial cell lines, the basal activity of HDAC was higher in OVCAR-3 malignant progressive adenocarcinoma, than in a primary ovarian cancer cell line (CaOV-3). In folliculoma cell lines, the basal activity of HDACs was 2-fold higher in adult (KGN) than in juvenile (COV434) forms. In all of the tested ovarian cancer cell lines, neither leptin nor leptin receptor antagonists changed HDAC activity (Figure 3).
Figure 3. Effect of leptin at a dose of 40 ng/mL and leptin receptor antagonists (SHLA and Lan2) on total histone deacetylase activity. Each point represents the mean±SEM of three independent experiments of four replicates per treatment group. Statistically significant different values are indicated with different letters; the same letters indicating no significant differences, with a<b<c.
Discussion
The elevated expression of many HDAC class proteins has been described in 60-90% of ovarian tumours and many histone- and non-histone-mediated alterations have been described that alter the balance in favour of cellular growth and survival (23).
The presented data clearly showed significantly higher HDAC gene expression in epithelial than in granulosa cells. Moreover, they showed that in the chemoresistant OVCAR-3 cell line, the expression of all investigated HDACs was significantly higher than in primary CaOV-3 cells, with the highest expression noted for HDACs 1, 2, 3 and 7. In granulosa tumour cells, lower HDAC expression was observed in the adult form than in the juvenile form, with the highest expression of HDAC9 found in COV434 cells. Except for the high expression of HDAC7 in OVCAR-3 and HDAC9 in COV434 from class II, in all cases, the expression of HDACs from I class was higher than from class II. The majority of studies showed the enhanced expression of class I HDACs in solid human tumours and in locally advanced, dedifferentiated, strongly proliferating tumours. There is a growing body of evidence that the expression of class I HDACs is increased in ovarian carcinomas (4), and this is suspected not only of playing a significant role in carcinogenesis, but also of contributing towards resistance to chemotherapeutic agents used for treating ovarian cancer (23). This is in agreement with our observation that the highest expression was observed in OVCAR-3 cells.
In COV434, representing the juvenile form, the expression of HDACs 1, 2 and 3 was higher than that of other HDACs. These findings are in agreement with other studies indicating higher expression of histone deacetylases from group I, not only in epithelial ovarian cancer, but also in other cancer types (24-29). HDAC9 has a key role in the development and differentiation of many types of cells, including regulatory T cells. Systemic autoimmune diseases such as lupus, diabetes, and rheumatoid arthritis have dysfunctional effector T cells; this suggests that HDAC9 may act as an epigenetic switch in effector T cell-mediated systemic autoimmunity (30).
The possible action of leptin on HDAC expression in the ovary and in ovarian cancer is not known. However, it has been suggested that hypothalamic HDAC5 activity is a regulator of leptin signalling, which adapts food intake and body weight to our dietary environment, and that the overexpression of HDAC5 can increase leptin sensitivity (14).
Recently published data by Tchio et al. (31) indicated that leptin can only directly act on HDAC4 and HDAC5. However, leptin can indirectly affect other HDACs by influencing microRNAs; leptin can increase miR21 (oncogenic microRNA21), which is able to increase HDAC3 expression, and the combined action of these factors leads to cancer cell proliferation (31). Our study is one of the first to show the cancer cell type-dependent action of leptin on HDAC expression. In epithelial cancer cells, leptin increased HDAC1 expression (from class I) and HDAC7 and HDAC9 gene expression (from class II) only in OVCAR-3 cells. In granulosa tumour cells, leptin increased gene expression only in the class II HDACs 2, 6 and 7, in KGN and HDAC9 in COV434. HDAC6 can be used as a marker for cancer prognosis. In multiple myeloma cells, blocking the expression of HDAC6 can cause apoptosis. In breast cancer MCF-7 cells, HDAC6 can lead to metastasis by up-regulating cell motility (32).
The results of the presented data showed a cell type-dependent action of leptin and leptin receptor antagonists on HDAC expression. In epithelial OVCAR-3 cells, SHLA was more potent then Lan-2; SHLA reversed the stimulatory effect of leptin on HDAC1 and 9, and additionally decreased the protein expression of HDACs 5, 6 and 9. In CaOV-3, characterised by low HDAC gene expression and no action of leptin on HDAC expression, SHLA decreased HDAC4 while Lan2 had no effect on gene expression, but decreased HDAC9 protein expression. Shen et al. (33) showed that epithelial ovarian cancer tissues in stage III/IV had higher HDAC4 expression than in stage I/II. They suggest that the accumulation of HDAC4 induced by fibrillar collagen matrices in the nucleus via the co-localisation of PP1α leads to the repression of p21 mRNA/protein and in turn promotes the proliferation and migration of epithelial ovarian cancer cells. In granulosa tumour cells, Lan-2 seems to be the most potent inhibitor. The reverse stimulatory effect of leptin on HDAC9 and inhibitory effect on HDAC3, and also decreased the expression of HDAC1 and 5 in COV434 cells, while SHLA decreased only HDAC1 gene expression. Kabra et al. (14) identified histone deacetylase 5 (HDAC5) as a regulator of leptin signalling and organismal energy balance. HDAC5 directly regulates STAT3 localisation and transcriptional activity via reciprocal STAT3 deacetylation at Lys685 and phosphorylation at Tyr705. In thte KGN cell line, characterised by very low HDACs expression, no effect of either antagonist on HDAC gene expression was noted, so actions on protein expression have not been evaluated. This is in agreement with Ropero et al. (34), who showed that the treatment of tumours expressing low levels of HDACs might be ineffective because it has been shown that HDAC-negative tumours caused by the mutation of HDAC genes are resistant to HDAC inhibitors.
Various studies carried out on cell lines derived from gynaecological malignancies, such as ovarian, breast and endometrial cancer, have demonstrated that HDAC inhibitors can inhibit tumour cell growth by suppressing proliferation and stimulating apoptosis (23,35). Many known HDAC inhibitors are used in clinics, supporting conventional therapies, including for the treatment of ovarian cancer (36). The pharmacological inhibition of HDAC1, which is associated with chemoresistance in ovarian cancer cells, can increase tumour cell sensitivity to immune cells (37). Nebbioso et al. (38) were able to decrease the expression of leptin receptor genes in adipocytes using selective inhibitors against HDACs from the second class.
Several HDAC inhibitors are used in clinics or clinical trials. Although many are non-selective and block the activity of all HDACs, some are selective against class I or II HDACs. FK228, a small HDAC inhibitor against HDACs from Class I, was demonstrated to be the most potent with regard to a reduction in ovarian cancer cell growth (39). Vorinostat and trichostatin are well known clinically-used HDACi that enhance the cytotoxicity of DNA-targeting drugs (i.e. cisplatin and doxorubicin) (40). Studies from our laboratory have also shown that therapy with HDAC inhibitors such as valproic acid and standard anti-ovarian cancer drugs could be promising treatments (41). PXD101 was shown to act as a single-agent anti-tumour drug in A2780 xenografts, and its effects were enhanced when combined with carboplatin (42). Also, in other cancers such as renal cell carcinoma, HDACs synergise an anti-proliferative effect of chemotherapeutic drugs (43). Another HDAC inhibitor, belinostat, seems to be synergistic with carboplatin and paclitaxel; such a combination was better tolerated than a single therapy (44). Also important during treatment, normal cells are relatively resistant to the effects of HDAC inhibitors (45). The multi-potent role of HDAC inhibitors makes them promising factors in ovarian cancer treatment.
In conclusion, this study showed that leptin can impact on the expression of HDAC genes and proteins in certain ovarian cancer cells. We also suggest that both SHLA and Lan-2, selective leptin receptor antagonists, could exhibit some benefits in ovarian cancer treatment, because they are able to decrease the levels of HDACs.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by K/ZDS/006310, DS/MND/WBiNoZ/ IZ/15/2016, Jagiellonian University in Kraków, Poland.
References
- 1.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 2.Vancurova I, Uddin MM, Zou Y, Vancura A. Combination therapies targeting HDAC and IKK in solid tumours. Trends Pharmacol Sci. 2017;39:295–306. doi: 10.1016/j.tips.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voelter-Mahlknecht S, Ho AD, Mahlknecht U. Chromosomal organisation and localisation of the novel class IV human histone deacetylase 11 gene. Int J Mol Med. 2015;16:589–598. [PubMed] [Google Scholar]
- 4.Doherty JA, Peres LC, Wang C, Way GP, Greene CS, Schildkraut JM. Challenges and opportunities in studying the epidemiology of ovarian cancer subtypes. Curr Epidemiol Rep. 2017;4:211–220. doi: 10.1007/s40471-017-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin KL, Pak JH, Park JY, Choi WH, Lee JY, Kim JH, Nam JH. Expression profile of histone deacetylases 1, 2 and 3 in ovarian cancer tissues. J Gynecol Oncol. 2008;19:185–90. doi: 10.3802/jgo.2008.19.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weichert W, Denkert C, Noske A, Darb-Esfahani S, Dietel M, Kalloger SE, Huntsman DG, Köbel M. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. 2008;10:1021–1027. doi: 10.1593/neo.08474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;13:14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi A, Horiuchi A, Kikuchi N, Hayashi T, Fuseya C, Suzuki A, Konishi I, Shiozawa T. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with down-regulation of E-cadherin. Int J Cancer. 2010;127:1332–1346. doi: 10.1002/ijc.25151. [DOI] [PubMed] [Google Scholar]
- 9.Stronach EA, Alfraidi A, Rama N, Datler C, Studd JB, Agarwal R, Guney TG, Gourley C, Hennessy BT, Mills GB, Mai A, Brown R, Dina R, Gabra H. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 2011;71:4412–4422. doi: 10.1158/0008-5472.CAN-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haakenson J, Zhang X. HDAC6 and ovarian cancer. Int J Mol Sci. 2013;14:9514–9535. doi: 10.3390/ijms14059514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapinska K, Housman G, Byler S, Heerboth S, Willbanks A, Oza A, Sarkar S. The effects of histone deacetylase inhibitor and calpain inhibitor combination therapies on ovarian cancer cells. Anticancer Res. 2016;36:5731–5742. doi: 10.21873/anticanres.11156. [DOI] [PubMed] [Google Scholar]
- 12.Fiedor E, Gregoraszczuk EŁ. The molecular mechanism of action of superactive human leptin antagonist (SHLA) and quadruple leptin mutein Lan-2 on human ovarian epithelial cell lines. Cancer Chemother Pharmacol. 2016;78:611–622. doi: 10.1007/s00280-016-3113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiedor E, Gregoraszczuk EL. Superactive human leptin antagonist (SHLA), triple Lan1 and quadruple Lan2 leptin mutein as a promising treatment for human folliculoma. Cancer Chemother Pharmacol. 2017;80:815–827. doi: 10.1007/s00280-017-3423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabra DG, Pfuhlmann K, García-Cáceres C, Schriever SC, CasqueroGarcía V, Kebede AF, Fuente-Martin E, Trivedi C, Heppner K, Uhlenhaut NH, Legutko B, Kabra UD, Gao Y, Yi CX, Quarta C, Clemmensen C, Finan B, Müller TD, Meyer CW, Paez-Pereda M, Stemmer K, Woods SC, Perez-Tilve D, Schneider R, Olson EN, Tschöp MH, Pfluger PT. Hypothalamic leptin action is mediated by histone deacetylase 5. Nat Commun. 2016;7:10782. doi: 10.1038/ncomms10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagace DC, McLeod RS, Nachtigal MW. Valproic acid inhibits leptin secretion and reduces leptin messenger ribonucleic acid levels in adipocytes. Endocrinology. 2004;145:5493–5503. doi: 10.1210/en.2004-0877. [DOI] [PubMed] [Google Scholar]
- 16.Tchio CM, Harbuzariu A, Harmon T, Beech D, Gonzalez-Perez R. Leptin modulation of PCSC, HDAC, and microRNA in pancreatic adenocarcinoma. Cancer Res. 2016;76:1901. [Google Scholar]
- 17.Almabhouh FA, Osman K, Ibrahim SF, Gupalo S, Gnanou J, Ibrahim E, Singh HJ. Melatonin ameliorates the adverse effects of leptin on sperm. Asian J Androl. 2017;19:647–654. doi: 10.4103/1008-682X.183379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafiei Sheykhani HR, Batavani RA, Najafi GR. Protective effect of leptin on induced apoptosis with trichostatin A on buffalo oocytes. Vet Res Forum. 2016;7:99–104. [PMC free article] [PubMed] [Google Scholar]
- 19.Gertler A, Solomon G. Leptin-activity blockers: development and potential use in experimental biology and medicine. Can J Physiol Pharmacol. 2013;91:873–882. doi: 10.1139/cjpp-2013-0012. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. Characterisation of an immortalized human granulosa cell line (COV434) Mol Hum Reprod. 2000;6:146–153. doi: 10.1093/molehr/6.2.146. [DOI] [PubMed] [Google Scholar]
- 21.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumour cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2011;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 22.Owecki M, Nikisch E, Miczke A, Pupek-Musialik D, Sowiński J. Leptin, soluble leptin receptors, free leptin index, and their relationship with insulin resistance and BMI: high normal BMI is the threshold for serum leptin increase in humans. Horm Metab Res. 2010;42:585–589. doi: 10.1055/s-0030-1253422. [DOI] [PubMed] [Google Scholar]
- 23.Singh BN, Zhou H, Li J, Tipton T, Wang B, Shao G, Gilbert EN, Li Q, Jang SW. Preclinical studies on histone deacetylase inhibitors and therapeutic reagents for endometrial and ovarian cancers. Future Oncol. 2011;7:1415–1428. doi: 10.2217/fon.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Kim SN, Park YS, Kim NH, Han JW, Lee HY, Kim YK. HDAC inhibitors down-regulate MRP2 expression in multidrug resistant cancer cells: Implication for chemosensitisation. Int J Oncol. 2011;38:807–812. doi: 10.3892/ijo.2010.879. [DOI] [PubMed] [Google Scholar]
- 26.Krusche CA, Wülfing P, Kersting C, Vloet A, Böcker W, Kiesel L, Beier HM, Alfer J. Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005;90:15–23. doi: 10.1007/s10549-004-1668-2. [DOI] [PubMed] [Google Scholar]
- 27.Song J, Noh JH, Lee JH, Eun JW, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW. Increased expression of histone deacetylase 2 is found in human gastric cancer. APMIS. 2005;113:264–268. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H, Moriyama S, Nakashima Y, Kobayashi Y, Kiriyama M, Fukai I, Yamakawa Y, Fujii Y. Histone deacetylase 1 mRNA expression in lung cancer. Lung Cancer. 2004;46:171–178. doi: 10.1016/j.lungcan.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Göttlicher M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5:455–463. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 30.Yan K, Cao Q, Reilly CM, Young NL, Garcia BA, Mishra N. Histone deacetylase 9 deficiency protects against effector T cell-mediated systemic autoimmunity. J Biol Chem. 2011;286:28833–28843. doi: 10.1074/jbc.M111.233932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchio CM, Harbuzariu A, Gonzalez-Perez RR. Histone deacetylases, microRNA and leptin crosstalk in pancreatic cancer. World J Clin Oncol. 2017;8:178–189. doi: 10.5306/wjco.v8.i3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen YF, Wei AM, Kou Q, Zhu QY, Zhang L. Histone deacetylase 4 increases progressive epithelial ovarian cancer cells via repression of p21 on fibrillar collagen matrices. Oncol Rep. 2016;35:948–954. doi: 10.3892/or.2015.4423. [DOI] [PubMed] [Google Scholar]
- 34.Ropero S, Fraga MF, Ballestar E, Hamelin R, Yamamoto H, Boix-Chornet M, Caballero R, Alaminos M, Setien F, Paz MF, Herranz M, Palacios J, Arango D, Orntoft TF, Aaltonen LA, Schwartz S Jr., Esteller M. A truncating mutation of HDAC2 in human cancers confers resistance to histone deacetylase inhibition. Nat Genet. 2006;38:566–569. doi: 10.1038/ng1773. [DOI] [PubMed] [Google Scholar]
- 35.Damaskos C, Garmpis N, Valsami S, Kontos M, Spartalis E, Kalampokas T, Kalampokas E, Athanasiou A, Moris D, Daskalopoulou A, Davakis S, Tsourouflis G, Kontzoglou K, Perrea D, Nikiteas N, Dimitroulis D. Histone deacetylase inhibitors: An attractive therapeutic strategy against breast cancer. Anticancer Res. 2017;37:35–46. doi: 10.21873/anticanres.11286. [DOI] [PubMed] [Google Scholar]
- 36.Cooper AL, Greenberg VL, Lancaster PS, van Nagell JR Jr., Zimmer SG, Modesitt SC. In vitro and in vivo histone deacetylase inhibitor therapy with suberoylanilide hydroxamic acid (SAHA) and paclitaxel in ovarian cancer. Gynecol Oncol. 2007;104:596–601. doi: 10.1016/j.ygyno.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Cacan E. Histone deacetylase-1-mediated suppression of FAS in chemoresistant ovarian cancer cells. Anticancer Res. 2016;36:2819–2826. [PubMed] [Google Scholar]
- 38.Nebbioso A, Dell’Aversana C, Bugge A, Sarno R, Valente S, Rotili D, Manzo F, Teti D, Mandrup S, Ciana P, Maggi A, Mai A, Gronemeyer H, Altucci L. HDACs class II-selective inhibition alters nuclear receptor-dependent differentiation. J Mol Endocrinol. 2010;45:219–28. doi: 10.1677/JME-10-0043. [DOI] [PubMed] [Google Scholar]
- 39.Wilson AJ, Holson E, Wagner F, Zhang YL, Fass DM, Haggarty SJ, Bhaskara S, Hiebert SW, Schreiber SL, Khabele D. The DNA damage mark pH2AX differentiates the cytotoxic effects of small molecule HDAC inhibitors in ovarian cancer cells. Cancer Biol Ther. 2011;12:484–93. doi: 10.4161/cbt.12.6.15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–300. [PubMed] [Google Scholar]
- 41.Kwiecińska P, Wróbel A, Taubøll E, Gregoraszczuk EŁ. Valproic acid, but not levetiracetam, selectively decreases HDAC7 and HDAC2 expression in human ovarian cancer cells. Toxicol Lett. 2014;224:225–32. doi: 10.1016/j.toxlet.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 43.Kim MJ, Kim DE, Jeong IG, Choi J, Jang S, Lee JH, Ro S, Hwang JJ, Kim CS. HDAC inhibitors synergize anti-proliferative effect of sorafenib in renal cell carcinoma cells. Anticancer Res. 2012;32:3161–3168. [PubMed] [Google Scholar]
- 44.Dizon DS, Damstrup L, Finkler NJ, Lassen U, Celano P, Glasspool R, Crowley E, Lichenstein HS, Knoblach P, Penson RT. Phase II activity of belinostat (PXD-101), carboplatin, and paclitaxel in women with previously treated ovarian cancer. Int J Gynecol Cancer. 2012;22:979–986. doi: 10.1097/IGC.0b013e31825736fd. [DOI] [PubMed] [Google Scholar]
- 45.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–99. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]