 Depriving microorganisms of bioavailable iron is a promising strategy for new anti-infective agents.
Depriving microorganisms of bioavailable iron is a promising strategy for new anti-infective agents.
Abstract
Depriving microorganisms of bioavailable iron is a promising strategy for new anti-infective agents. The new, highly water-soluble, low molecular weight co-polymer DIBI was developed to selectively bind iron(iii) ions as a tris chelate and acts as a standalone anti-infective. Minimum inhibitory concentration (MIC) studies show DIBI is effective against representative reference strains for Gram-positive and Gram-negative bacteria S. aureus and A. baumannii, and the fungus C. albicans. Compared to the small molecule iron chelators, deferiprone and deferoxamine, DIBI outclassed these by factors of 100 to 1000 for inhibition of initial growth. DIBI and a series of related co-polymers (Mw of 2–9 kDa) were synthesized via reversible addition–fragmentation chain transfer (RAFT) polymerization of a chelating 3-hydroxypyridin-4-one (HPO) methacrylamide monomer and N-vinylpyrrolidone (NVP). Full incorporation of the HPO monomer into the co-polymers from the reaction solution was determined by 1H NMR spectroscopy and ranged from 4.6 to 25.6 mol%. UV-vis spectroscopy showed that all the HPO in DIBI binds readily to iron(iii) in a tris chelate mode to the maximum theoretical iron(iii) binding capacity of the co-polymer. Chemical characterization including single crystal X-ray diffraction analyses of the O-benzyl protected and the functional HPO monomer are discussed. By design, DIBI is highly water soluble; the highest mass fraction in water tested was 70% w/w, without the need of organic co-solvents.
Introduction
The widespread development of antibiotic resistance over recent decades has become a global public health concern.1–4 Less than six months after the first identification in China of the mcr-1 gene resistant to colistin, an antibiotic drug class considered of last resort, a patient in the United States was reportedly infected.5 We have reached the age in which the current suite of antibiotics is no longer a viable and effective solution in combatting antibiotic resistant infection. In addition, the lack of new types of anti-infectives under development6,7 to fight this threat has prompted researchers worldwide to explore new strategies to combat infections.8,9
One approach to combatting antibiotic resistance is through limiting iron availability to microbes to restrict their growth and help facilitate infection clearance.10 In prokaryotic and eukaryotic genera iron has several irreplaceable physiological roles within enzymes needed for DNA synthesis and repair, and thus restricting bioavailable iron limits cell growth.11 The innate immune response in humans against invading microbial pathogens includes sequestration of iron intracellularly to limit its availability to the microorganisms.12 To counter this and other defense mechanisms, pathogenic microbes produce receptor/uptake systems to directly access the body's iron carriers such as transferrin or produce small, high affinity iron-chelators, siderophores, to harvest iron from their environment.13,14 These microbial chelators have a wide range of chemical structures and present one or more types of binding sites, e.g. hydroxamate, catecholate and citrate. The structure and distribution of binding sites in microbial chelators afford high specificity towards iron(iii) ions, commonly presenting binding constants in the range of Kcomplex of 1020–30, but capable of reaching much higher values such as that for enterobactin. Enterobactin, a tricatecholate chelator, is produced by E. coli and can bind an iron(iii) ion with a binding constant of Kcomplex of 1050, the highest value recorded for a microbial siderophore.15
A potential strategy to deprive microbes of iron is to use other iron(iii) chelators to out-compete microbial siderophores and acquisition systems in vivo. Deferoxamine (DFO, Kcomplex 1031) and deferiprone (DFP) are two examples of iron(iii) chelators approved by the FDA and EMA for the treatment of iron overload disorders in humans (Fig. 1).16–18 DFO is a hexadentate hydroxamate-based chelator of bacterial origin that has been shown to facilitate iron uptake by a wide spectrum of bacteria, hence limiting its suitability as an anti-infective agent candidate to deprive bacteria of iron.19 DFP is a fully synthetic HPO chelator that has been used to treat thalassemia major for over two decades. This bidentate chelator forms octahedral tris complexes with iron(iii) and has a binding constant of Kcomplex of 1036.20–22 The large complexation constant puts DFP at par with naturally occurring microbial siderophores, and its clinical applications for the treatment of iron overload conditions make this chelator a very interesting candidate to out-compete microbial siderophores for iron. In vitro studies have shown that some bacteria are inhibited by DFP but at very high chelator concentrations.23,24 Other small iron chelators that incorporate the HPO binding moieties have been synthesized with the aim of competing against bacterial siderophores; however, among the several iron chelators reported, only a few candidates yielded moderate to low inhibitory activity against test microorganisms.25–29
Fig. 1. FDA and EMA-approved iron chelators for iron-overload disorders.
If iron sequestration from microbes is to become a feasible clinical strategy for the treatment of infections the chelators used for this purpose must have a high affinity for iron(iii) ions, sequester iron in a way that is not suitable for microbial uptake, and provide higher anti-microbial activity than the currently employed clinical iron chelators. Additionally, such chelators need to be water-soluble, possess low toxicity to humans, and have a simple synthetic preparation to be economically viable.
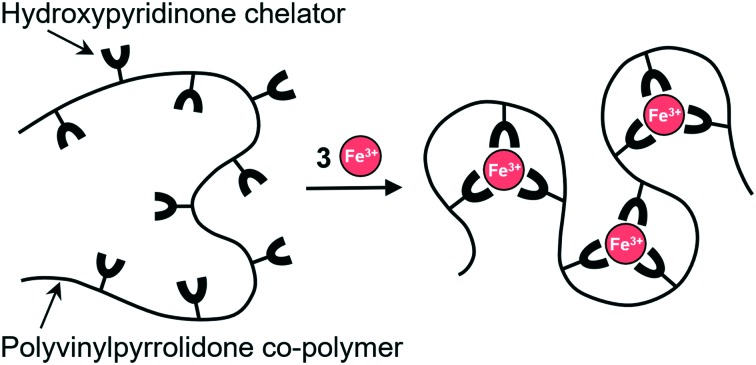
We have developed a different approach to capture iron(iii) by using polymers containing iron binding sites rather than small ligands.30 Ideally, such an iron-binding polymer should have a high selectivity and capacity for iron and be structurally unrecognizable as a suitable source of iron by microbes. Due to their size, high molecular weight polymers would offer low toxicity relative to low molecular weight molecules as they would not be readily taken up by cells.31–33 Transport of iron complexes by microbes is reduced, if not stopped completely, thus limiting their growth while anti-infective drugs and host clearance defense mechanisms take effect. Another aspect is that folding of a polymeric chelator around an iron(iii) ion would create a larger spatial barrier between the metal centres and bacterial siderophores on the exterior of the folded polymer chain. Although iron-binding polymers have great potential, the approaches published so far are fraught with difficulties such as complicated, multi-step synthesis, low yields, and often require co-solvents to improve solubility in water.25,34–36
The work in our group focuses on developing iron-binding polymeric platforms with antimicrobial activity that follows a streamlined, cost effective and scalable approach. In previous publications we showed the efficacy of an iron-binding polymer DIBI against microorganisms and cancer cells29,37–39 and its lack of toxicity in murine models.40,41 The synthesis, basic characterization, and broad-based antimicrobial activity of DIBI, a highly water-soluble, HPO containing polyvinylpyrrolidone co-polymer is described herein with additional detail as provided in a previous patent disclosure.30
Results and discussion
Iron-binding monomer in DIBI
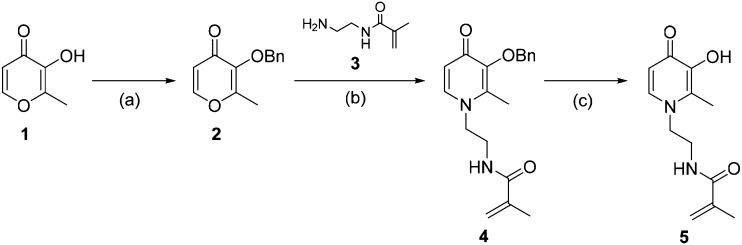
To maximize biocompatibility and confer iron-binding capability to DIBI we chose the HPO chelator motif. We derivatized the hydroxypyridinone ring by introducing an N-ethyl-2-methylacrylamide tether to enable olefin polymerization, resulting in the monomer N-[2-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl)ethyl]methacrylamide (5) (Scheme 1). The synthesis of 5 was a modified procedure from that previously reported by Feng et al.27 that uses a methacrylamide and circumvented the installation of ethylenediamine before the addition of an acrolein group (see ESI†). The first step in the synthetic route of 5 is the formation of 3-benzyloxy-2-methyl-4-pyrone (2) using benzyl chloride (BnCl) to protect the hydroxyl group. The next step is the addition of N-(2-aminoethyl)-2-methyl-2-propeneamide (3) under basic aqueous conditions to generate the pyridin-4-one ring system of 4, followed by the removal of the benzyl protecting group in concentrated hydrochloric acid affording 5 as a beige-coloured, crystalline powder.
Scheme 1. Synthesis of 5 from maltol (1). (a) 1.1 equiv. BnCl, 1.05 equiv. NaOH, MeOH, 85 °C, 2 h; (b) MeOH : H2O (1 : 1), 2 N NaOH, 95 °C, 2 h; (c) conc. HCl, r.t. overnight.
The molecular structure of the intermediate N-[2-(3-(benzyloxy)-2-methyl-4-oxopyridin-1(4H)-yl)ethyl]methacrylamide (4) was obtained by X-ray crystallography (Fig. S1†). Single crystals of 4 were obtained by slow evaporation of an aqueous solution at room temperature. 4 crystallized in the space group P212121 presenting four molecules related by symmetry in the unit cell. The X-ray crystal structure shows the benzyl group and the N-ethyl-2-methacrylamide tether to the nitrogen site of the HPO ring. The only hydrogen bonding interactions present in the crystal structure are between the amide N–H bonds and adjacent HPO carbonyls that propagate along the crystallographic axis b of the unit cell (Fig. S2†).
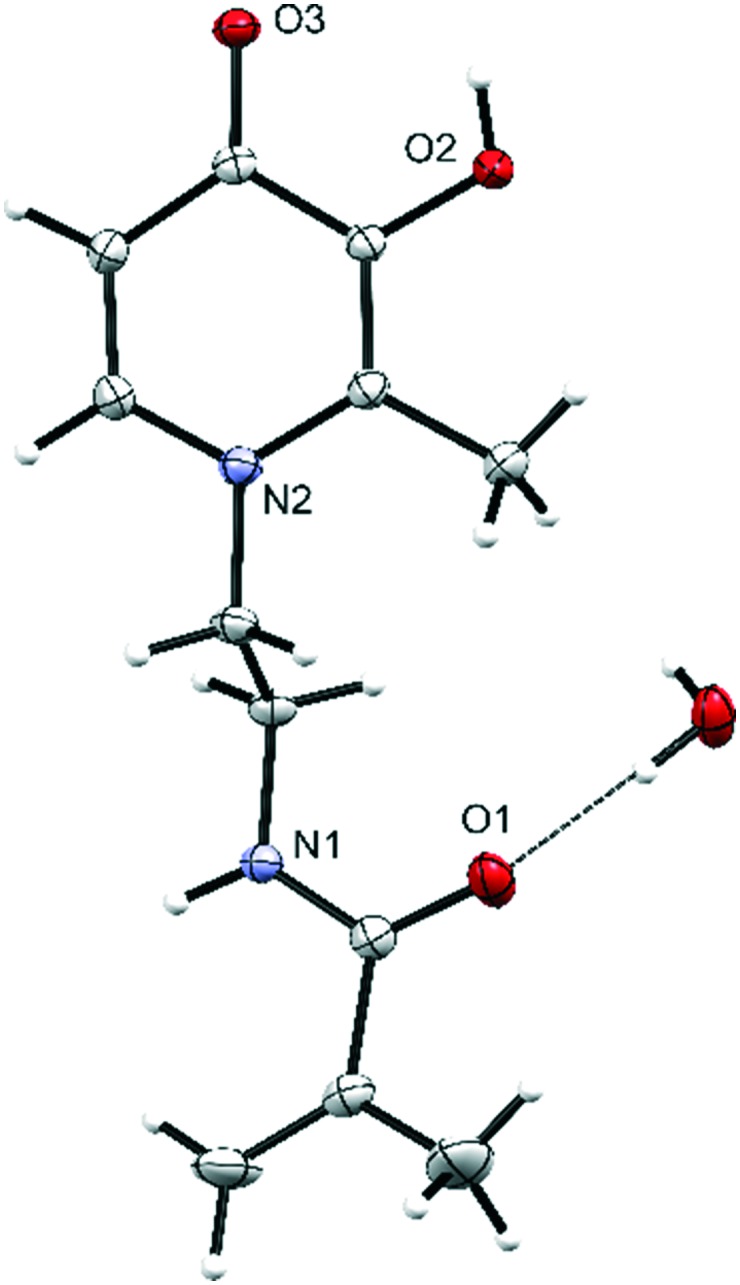
The molecular structure of the functional monomer N-[2-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl)ethyl]-2-methylprop-2-enamide (5) was also obtained by X-ray crystallography (Fig. 2). Single crystals were obtained by slow evaporation in RPMI culture medium at 4 °C. The molecular structure shows the free monomer with the carbonyl and hydroxyl group accessible for iron coordination. The water molecule is hydrogen bonding between three molecules of 5via the carbonyl groups and NH bond of the acrolein portion of 5. The hydrogen bonding network of 5 is propagated by pairs of carbonyl and hydroxyl substituents of the ring (Fig. S3†).
Fig. 2. Molecular structure of 5. Displacement ellipsoids for non-H atoms are shown at 30% probability level and H-atoms are represented by circles of arbitrary size.
The UV-vis characterization of 5, dissolved in water, showed a band at 282 nm and two bands in the range 200–240 nm (Fig. S4†). The signal at longer wavelengths corresponds to the π–π* transition of HPO ring, whereas the absorption bands at shorter wavelengths correspond to the overlapped contribution of the HPO ring and the methacrolein moiety.
Novel family of polyvinylpyrrolidone co-polymers
A family of co-polymers was conceptually designed as simple, siderophore-inspired biocompatible polymers with high water solubility. Polyvinylpyrrolidone (PVP) was chosen as the main structural component for our iron binding co-polymers, as it is highly soluble in water, and well known in the medical field having been used as a plasma volume expander and as an excipient ingredient in many pharmaceutical preparations.42 The polymerization of co-polymers was carried out with varying ratios of 5 and commercially available NVP via RAFT in mild aqueous conditions (Scheme 2 and Table 1). The total monomer-to-RAFT agent ratio was held constant at a ratio of 50 : 1.
Scheme 2. Synthesis of DIBI (Mn: 7.5 kDa, Mw: 9 kDa, PDI: 1.2); TBHP = tert-butyl hydroperoxide, TMEDA = N,N,N′,N′-tetramethylethylenediamine, and RAFT-agent = 2-[(ethoxymethanethioyl)sulfanyl]-2-methylpropanoic acid.
Table 1. Characterization data of a series of co-polymers with varying ratio 5 : NVP a .
| Co-polymer | 5 : NVP in reaction solution | Content of 5 in polymer b (mol%) | Yield | M w c | PDI d |
| a | 1 : 20 | 4.6 | 88% | 3656 | 1.7 |
| b | 1 : 15 | 9.5 | 80% | 4799 | 1.9 |
| c | 1 : 10 | 15.3 | 85% | 3430 | 1.6 |
| d | 1 : 5 | 25.6 | 55% | 2298 | 1.4 |
aTotal monomer content of 50 equivalents relative to remainder of reagents.
bMeasured using 1H NMR spectroscopy (see ESI).
cRelative molecular weight.
dPolydispersity index calculated as Mw/Mn.
The results in Table 1 show that the polymer content of monomer 5 can be controlled by changing the ratio 5 : NVP in the reaction solution. The highest mol% content of 5 gave the lowest isolated yield of 55% that indicates difficulty to incorporate amounts higher than 15 mol% of 5. The RAFT mediated polymerization was found to provide a relatively narrow molecular weight distribution of the co-polymer products with the average Mw of the compositions being somewhat similar at the different 5 : NVP conditions (see ESI†). Based on these results it appeared that a 5 : NVP ratio of between 1 : 10 and 1 : 15 provided the best overall 5 content (10–15 mol%) while retaining high product yields and a PDI below 2.0.
The evolved target polymer DIBI: synthesis and characterization
We decided to further develop a co-polymer containing 15 mol% of 5 to maximize both iron binding capacity and polymerization yield. Our lead compound DIBI was obtained following the above synthesis as an off-white powder in 85% yield. After 2 hours of reaction time the full incorporation of 5 from the reaction solution into DIBI was determined by following the suppression of the CH acrolein signals using 1H NMR spectroscopy (see ESI†). The structure of DIBI comprises 15 mol% of 5, with PVP accounting for the rest of the co-polymer. GPC characterization for DIBI determined a Mw of 9 kDa with a polydispersity index of 1.2 (see ESI†). The good correlation between refractive index and UV-vis profiles indicated a homogeneous composition of 5 across the distribution of polymer molecular weights. By design, DIBI is extremely water-soluble; aqueous solutions of DIBI of 20% w/w were routinely used in our work. The highest mass fraction in water tested was 70% w/w, without using organic solvents (e.g. DMSO) to improve polymer solubility. In comparison, DFP and DFO (mesylate salt) have a maximum water solubility of 1.4% w/w and 5% w/w, respectively.
The UV-vis spectrum of DIBI in water shows the expected HPO band with maximum at 282 nm, as observed in 5, and a group of overlapped signals below 230 nm formed by PVP and amide signals of 5 (Fig. S5†). The extinction coefficient of DIBI is about 9.5 times that of 5 that correlates an average of 9.5 moles 5 per mol of DIBI. The mol% of 5 in DIBI was also determined in MOPS buffer at pH 7.42 by interpolating the intensity of the HPO signal of DIBI in a standard curve of 5 in the same conditions. The results indicated an average content of 13 mol% or 24 mass% of 5 in DIBI, in good agreement with the mol% content of 5 determined by 1H NMR spectroscopy (see ESI†).
Iron-binding capacity of DIBI
The iron-binding activity of DIBI is provided by its platform supported HPO in 5 which are widely distributed within the linear structure of DIBI. The bidentate nature of the chelating monomer 5 led us to expect the formation of octahedral tris complexes with iron(iii), as seen in other HPO coordination complexes.36,43–45 Thus, based on NMR and UV-vis characterization the theoretical chelating capacity of a 122 mM solution of DIBI would be equivalent to 1120 μM of 5 and 373 μM of iron(iii) for the expected DIBI–Fe complex. The electronic spectra obtained from the batch titration of DIBI solutions iron(iii) nitrate showed a metal-to-ligand charge transfer (MLCT) band at 460 nm, indicative of the formation of the iron(iii) complex with DIBI (Fig. 3). Plotting the absorption maxima at 460 nm against iron(iii) concentration gave a plateau at 371 μM iron(iii). This indicated that all binding sites of 5 within DIBI are available for the tris-chelation of iron, resulting in a total iron-binding capacity of 338 μmol of iron per gram of DIBI. No precipitate was visible during the iron titration experiments, and the coloured films obtained upon solution evaporation re-dissolved readily following weeks of being dry.
Fig. 3. UV-vis spectra showing the MLCT band for the iron(iii) titration of 122 mM DIBI at pH 7.42 in MOPS. Inset: Plot of absorbance maxima at 460 nm vs. iron concentration for the iron(iii) titration.
Antimicrobial activity of the family of co-polymers
Select co-polymers presented in Table 1 with relative molecular weights in the range 2–4 kDa were tested using MIC to assess their activity against the growth of S. aureus in RPMI, shown in Table 2. The co-polymers demonstrated extremely low MIC values for S. aureus compared to the free monomer 5 and the clinical iron chelator DFP. This is strong evidence of a structural enhancement of antibacterial activity by the incorporation of 5 into a polymer. The results also indicated lower MICs were achieved by increasing the amount of 5 incorporated into the co-polymer. The control experiment for PVP homopolymer (10 kDa Mw) showed no growth inhibition even at very high concentrations. RPMI was chosen as the assay medium as it is more representative of the host environment compared to other media choices with regard to its iron(iii) content (approx. 0.5 μM).
Table 2. Minimum inhibitory concentrations for S. aureus (ATCC 43300) at 48 h for co-polymers, PVP, 5 and DFP in RPMI.
| Co-polymer | Content of 5 in polymer a (mol%) | MIC |
||
| μg mL–1 | μM b | μMFe c | ||
| a | 9.5 | 8 | 0.21 | 0.07 |
| b | 15.3 | 4 | 0.13 | 0.04 |
| c | 25.6 | 2 | 0.15 | 0.05 |
| PVP | 0 | 256 000 | 25 600 | N/A |
| 5 | N/A | 125 | 530 | 176 |
| DFP | N/A | 40 | 288 | 96 |
aMeasured using 1H NMR spectroscopy (see ESI).
bMolarity expressed as equivalent concentration of 5 based on the mol% of 5 in each polymer.
cMIC value normalized for maximum amount of iron bound by each chelator, assuming octahedral coordination with the following (ligand : Fe) ratios: co-polymer (3 : 1), 5 (3 : 1), DFP (3 : 1). The ratio for co-polymers is expressed according to the tris-chelate complexes of 5.
Antimicrobial activity of DIBI and other medical iron(iii) chelators
Table 3 shows the MIC for DIBI, DFP and DFO against ATCC reference strains of S. aureus and A. baumannii and C. albicans. These opportunistic pathogens were chosen as representative Gram-positive/negative bacteria and yeast, respectively. S. aureus and A. baumannii have been highlighted recently by the WHO as priority targets for the development of new antibacterial treatments because they pose a serious threat to human health in community and clinical settings.1C. albicans is one of the most common fungal yeast opportunistic pathogens in hospital-acquired infections due to their rapid growth on medical devices or on human tissue.46
Table 3. Minimum inhibitory concentrations at 48 h for DFO, DFP and DIBI in RPMI.
| Microorganism | MIC |
||||||||
| DIBI |
DFP |
DFO |
|||||||
| μg mL–1 | μM b | μMFe c | μg mL–1 | μM | μMFe c | μg mL–1 | μM | μMFe c | |
| S. aureus ATCC 43300 a | 4 | 4.06 | 1.35 | 40 | 288 | 96 | >1280 | >1949 | >1949 |
| A. baumannii ATCC 19606 | 2 | 2.03 | 0.67 | 80 | 575 | 192 | >1280 | >1949 | >1949 |
| C. albicans ATCC SC5314 | 2 | 2.03 | 0.67 | 160 | 1150 | 383 | >1280 | >1949 | >1949 |
aMethicillin-resistant Staphylococcus aureus (MRSA).
bConcentration of 5 based on 24 mol% content in DIBI.
cMIC value normalized for maximum amount of iron bound by each chelator, assuming octahedral coordination with the following (ligand : Fe) ratios: DFO (1 : 1), DFP (3 : 1), DIBI (3 : 1). The ratio for DIBI is expressed according to the tris-chelate complexes of 5.
Table 3 shows the measured MIC values for DIBI, DFP and DFO in different units to compare them to anti-infectives (μg mL–1) and other chelators (μM) in the literature. The values expressed in μMFe are normalized based on the iron(iii) complex stoichiometry for each chelator, assuming the formation of the octahedral complexes for each ligand. For DIBI the μMFe values are expressed as the concentration of 5 based on 24% mass content.
The MIC results obtained indicated that DIBI inhibited all three classes of microbes displaying very low MICs.47 The MIC values of DIBI were 2 and 3 orders of magnitude lower than the small molecule iron chelators, which require concentrations in the mg mL–1 range to achieve even modest antimicrobial activity. DFP was more effective at inhibiting bacterial growth over that of fungal yeast C. albicans, whereas DFO was non-inhibitory for all organisms at the maximum concentration tested. Control experiments using commercially available PVP (Mw 10 kDa) showed no antimicrobial effect.
Consistent with our results, it has been reported that the presence of DFO, a bacterial siderophore itself, in culture media can improve microbial growth in vitro while DFP can be mildly inhibitory.19,23,48 However, the internalization of large molecular size polymers or polymer-bound iron by bacteria is less likely because specialized, active transport mechanisms are needed over 600 Da.14
We hypothesize that the enhanced antimicrobial efficacy shown by DIBI can be attributed to its polymer structure. Given the structural similarities between binding sites in DFP and 5, it is reasonable to consider that the complexation constant for 5 would be, at best, of the same magnitude as for DFP. This leads to the possibility that having 5 tethered and dispersed throughout one polymer backbone results in enhanced iron-binding due to intramolecular proximity of neighbouring 5 binding moieties. Therefore, the resulting local concentration of 5 within the polymer platform would be effectively higher than for the free monomers at an equivalent concentration in solution. An additional benefit of having a polymeric structure is the potential for spatial hindrance, which may allow DIBI to sequester iron(iii) ions away from smaller siderophores.
Conclusions
The novel water-soluble co-polymer DIBI displays low MICs in vitro against the bacteria S. aureus and A. baumannii, and the fungus C. albicans. Compared to the low molecular weight clinically used iron chelators, DFO and DFP, DIBI displayed 100 to 1000 times lower MICs against three classes of microbes. DIBI is one of a series of co-polymers reported that were synthesized from the monomers 5 and NVP using RAFT polymerization. The antibacterial activity of DIBI and related co-polymers stems from the amount of HPO-based iron(iii)-binding moieties of 5 present in their composition, which can be easily controlled based on the 5 : NVP feed ratio for the co-polymerization. It is hypothesized that the enhanced antimicrobial activity of DIBI compared to 5 is due to the inability of microbes to internalize the polymer and to the intramolecular proximity of neighbouring 5 binding moieties. The possibility of synergistic effects between DIBI and antibiotics against microbial pathogens is currently under study in our lab.41
Conflicts of interest
BEH has a beneficial interest in Chelation Partners Inc. and MTCA, RGB and DSA are employees of this company. None of the other authors have competing interests in relation to the work presented.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the financial support for this work from the Industrial Research Assistance Program of NRC and Atlantic Canada Opportunities Agency, Natural Sciences and Engineering Research Council, Productivity and Business Skills Initiative. The authors would also like to acknowledge Cape Breton University and the Verschuren Centre for access to equipment. The assistance of Maria Parquet with MIC determinations is also acknowledged.
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1812776 and 1830658. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8md00192h
References
- World Health Organization, WHO publishes list of bacteria for which new antibiotics are urgently needed, Available from: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed December 2017).
- U.S. Department of Agriculture, Proactive Efforts by U.S. Federal Agencies Enable Early Detection of New Antibiotic Resistance, Available from: https://www.usda.gov/media/blog/2016/05/26/proactive-efforts-us-federal-agencies-enable-early-detection-new-antibiotic (accessed December 2017).
- Centers for Disease Control and Prevention, Notes from the Field: Pan-Resistant New Delhi Metallo-Beta-Lactamase-Producing Klebsiella pneumoniae — Washoe County, Nevada, 2016, Available from: https://www.cdc.gov/mmwr/volumes/66/wr/mm6601a7.htm (accessed December 2017). [DOI] [PMC free article] [PubMed]
- The Guardian, Without action on antibiotics, medicine will return to the dark ages, Available from: https://www.theguardian.com/commentisfree/2017/may/19/antibiotics-medicine-dark-ages-overprescribing (accessed December 2017).
- McGann P., Snesrud E., Maybank R., Corey B., Ong A. C., Clifford R., Hinkle M., Whitman T., Lesho E., Schaecher K. E. Antimicrob. Agents Chemother. 2016;60(7):4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Page M. G. P. J. Pharmacokinet. Pharmacodyn. 2017;44:113–132. doi: 10.1007/s10928-017-9506-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Antibacterial agents in clinical development, Available from: http://www.who.int/medicines/areas/rational_use/antibacterial_agents_clinical_development/en/ (accessed December 2017).
- Consoli G. M. L., Granata G., Picciotto R., Blanco A. R., Geraci C., Marino A., Nostro A. MedChemComm. 2018;9:160–164. doi: 10.1039/c7md00527j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. Nat. Rev. Drug Discovery. 2015;14:821–832. doi: 10.1038/nrd4675. [DOI] [PubMed] [Google Scholar]
- Ballouche M., Cornelis P., Baysse C. Recent Pat. Anti-Infect. Drug Discovery. 2009;4:190–205. doi: 10.2174/157489109789318514. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Richards T., Srai S. K. S. Front. Pharmacol. 2014;5:1–8. doi: 10.3389/fphar.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassat J. E., Skaar E. P. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R., Saha N., Donofrio R. S., Bestervelt L. L. J. Basic Microbiol. 2013;53:303–317. doi: 10.1002/jobm.201100552. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Kong X. Nat. Prod. Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- Raymond K. N., Dertz E. A., Kim S. S. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Metallomics. 2010;2:732–740. doi: 10.1039/c0mt00023j. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J. Analyst. 1995;120:845–851. doi: 10.1039/an9952000845. [DOI] [PubMed] [Google Scholar]
- Liu Z. D., Hider R. C. Med. Res. Rev. 2002;22:26–64. doi: 10.1002/med.1027. [DOI] [PubMed] [Google Scholar]
- Lesic B., Foulon J., Carniel E. Antimicrob. Agents Chemother. 2002;46:1741–1745. doi: 10.1128/AAC.46.6.1741-1745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurchi V. M., Crisponi G., Pivetta T., Donatoni M., Remelli M. J. Inorg. Biochem. 2008;102:684–692. doi: 10.1016/j.jinorgbio.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Charalambous J., Dodd A., McPartlin M., Matondo S. O. C., Pathirana N. D., Powell H. R. Polyhedron. 1988;7:2235–2237. [Google Scholar]
- Singh S., Epemolu R. O., Dobbin P. S., Tilbrook G. S., Ellis B. L., Damani L. A., Hider R. C. Drug Metab. Dispos. 1992;20:256–261. [PubMed] [Google Scholar]
- Thompson M. G., Corey B. W., Si Y., Craft D. W., Zurawski D. V. Antimicrob. Agents Chemother. 2012;56:5419–5421. doi: 10.1128/AAC.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.-M., Shin S.-H. J. Korean Med. Sci. 2009;24:289–295. doi: 10.3346/jkms.2009.24.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.-J., Kong X.-L., Li J.-P., Ma Y.-M., Hider R. C., Zhou T. MedChemComm. 2015;6:1620–1625. [Google Scholar]
- Zhou Y. J., Liu M. S., Osamah A. R., Kong X. L., Alsam S., Battah S., Xie Y. Y., Hider R. C., Zhou T. Eur. J. Med. Chem. 2015;94:8–21. doi: 10.1016/j.ejmech.2015.02.050. [DOI] [PubMed] [Google Scholar]
- Feng M. H., van der Does L., Bantjes A. J. Med. Chem. 1993;36:2822–2827. doi: 10.1021/jm00071a013. [DOI] [PubMed] [Google Scholar]
- Moniz T., Silva D., Silva T., Gomes M. S., Rangel M. MedChemComm. 2015;6:2194–2203. [Google Scholar]
- Workman D. G., Hunter M., Dover L. G., Tétard D. J. Inorg. Biochem. 2016;160:49–58. doi: 10.1016/j.jinorgbio.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Holbein B. E., Feng M., Huber A. L. and Kidby D. K., WIPO Pat., 2012167368A1, 2012. [Google Scholar]
- Zhou T., Winkelmann G., Dai Z.-Y., Hider R. C. J. Pharm. Pharmacol. 2011;63:893–903. doi: 10.1111/j.2042-7158.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- Hamilton J. L., Kizhakkedathu J. N. Mol. Cell. Ther. 2015;3:1–15. doi: 10.1186/s40591-015-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhal P. K., Polomoscanik S. C., Avila L. Z., Holmes-Farley S. R., Miller R. J. Adv. Drug Delivery Rev. 2009;61:1121–1130. doi: 10.1016/j.addr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Li J., Olaleye E. D., Kong X., Zhou T., Ma Y., Jurach J., Al Rugaie O., Hider R. C., Zhang G., Alsam S., Abbate V. Polymer. 2016;87:64–72. [Google Scholar]
- El-Gendy N., Qian J., Eshelman K., Rivera M., Berkland C. Biomacromolecules. 2015;16:1480–1488. doi: 10.1021/bm5016392. [DOI] [PubMed] [Google Scholar]
- Zhou T., Neubert H., Liu D. Y., Liu Z. D., Ma Y. M., Kong X. L., Luo W., Mark S., Hider R. C. J. Med. Chem. 2006;49:4171–4182. doi: 10.1021/jm0600949. [DOI] [PubMed] [Google Scholar]
- Holbein B. E., Mira de Orduña R. FEMS Microbiol. Lett. 2010;307:19–24. doi: 10.1111/j.1574-6968.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- Islam S., Jarosch S., Zhou J., Parquet M. del C., Toguri J. T., Colp P., Holbein B. E., Lehmann C. J. Surg. Res. 2016;200:266–273. doi: 10.1016/j.jss.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Thorburn T., Aali M., Kostek L., LeTourneau-Paci C., Colp P., Zhou J., Holbein B., Hoskin D., Lehmann C. Clin. Hemorheol. Microcirc. 2017;67:241–250. doi: 10.3233/CH-179205. [DOI] [PubMed] [Google Scholar]
- Arora N., Caldwell A., Wafa K., Szczesniak A., Caldwell M., Al-Banna N., Sharawy N., Islam S., Zhou J., Holbein B. E., Kelly M., Lehmann C. Clin. Hemorheol. Microcirc. 2018;69(1–2):153–164. doi: 10.3233/CH-189109. [DOI] [PubMed] [Google Scholar]
- Savage K. A., Parquet M. C., Allan D. S., Davidson R. J., Holbein B. E., Lilly E. A., Fidel Jr. P. L., Antimicrob. Agents Chemother.10.1128/AAC.02576-17 , , in press . [Google Scholar]
- Bühler V., Polyvinylpyrrolidone Excipients for Pharmaceuticals - Povidone, Springer-Verlag Berlin Heidelberg, New York, 2005. [Google Scholar]
- Merkofer M., Kissner R., Hider R. C., Koppenol W. H. Helv. Chim. Acta. 2004;87:3021–3034. [Google Scholar]
- Rai B. L., Dekhordi L. S., Khodr H., Jin Y., Liu Z., Hider R. C. J. Med. Chem. 1998;41:3347–3359. doi: 10.1021/jm9707784. [DOI] [PubMed] [Google Scholar]
- Grazina R., Gano L., Sebestík J., Amelia M. S. J. Inorg. Biochem. 2009;103:262–273. doi: 10.1016/j.jinorgbio.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Kojic E. M., Darouiche R. O. Clin. Microbiol. Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Harbeck R. J., McCarter Y. S., Ortez J. H., Rankin I. D., Sautter R. L., Sharp S. E. and Spiegel C. A., Manual of antimicrobial susceptibility testing, American Society for Microbiology, Washington, 2005. [Google Scholar]
- Chan G. C.-F., Chan S., Ho P.-L., Ha S.-Y. Hemoglobin. 2009;33:352–360. doi: 10.3109/03630260903211888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.