Abstract
Background:
Female reproductive tract development is sensitive to the endocrine-disrupting potential of environmental estrogens. Early-life exposure to the dietary phytoestrogen genistein impairs fertility and persistently alters the transcriptome in the oviduct and uterus of rodents. Glucocorticoid signaling, which has recently been shown to be essential for normal fertility in the female mouse uterus, is antagonized by genistein.
Objective:
Our goal was to determine whether early-life exposure to genistein disrupts glucocorticoid signaling in the mouse uterus, which may contribute to infertility.
Methods:
Female C57Bl/6 mice were exposed to either genistein, estradiol, or vehicle (corn oil) on postnatal days 1–5 (PND1–5), and then treated with the synthetic glucocorticoid dexamethasone (Dex: ) or vehicle (saline) on PND5, at weaning on PND21, or as adults on PND56 following adrenalectomy and ovariectomy to evaluate glucocorticoid responsiveness. Uteri were isolated following treatment for gene expression or chromatin immunoprecipitation.
Results:
Neonatal exposure to genistein altered the uterine transcriptome of adult mice and caused substantial changes to the transcriptional response to glucocorticoids. Although expression of the glucocorticoid receptor was not affected, genistein exposure disrupted glucocorticoid receptor recruitment to specific regulatory sites in target genes. Many genes involved in chromatin remodeling were dysregulated in genistein-exposed mice, suggesting that epigenetic reprograming may contribute to the altered glucocorticoid response of the uterus following early-life exposure to genistein. These changes affected the biological activity of glucocorticoids within the uterus, as glucocorticoids antagonized the proliferative effects of estradiol in the uterus of control mice but not genistein-exposed mice.
Conclusions:
Our findings suggest that disruption of glucocorticoid signaling due to early-life exposure to environmental estrogens may in part render the uterus unable to support implantation. https://doi.org/10.1289/EHP1575
Introduction
Environmentally derived compounds with estrogenic structures are recognized endocrine disruptors. The female reproductive tract is particularly sensitive to the effects of such compounds, so much so that toxicologists use the uterotrophic assay to screen for health risk (O’Connor et al. 1996). Exposure to these environmental estrogens, which are present in household and cosmetic products, pesticides and herbicides, food additives, groundwater, plastics, and plants, can impair reproductive function in a number of species. For this reason, the effects of soy consumption on human health have increasingly been the subject of much debate. Soy contains high levels of isoflavones, a class of phytoestrogens that can mimic endogenous estradiol () activity by binding to estrogen receptors (ERs) (Choi et al. 2008). The endocrine-disrupting properties of these compounds present a potential threat to fertility and reproduction in mammals (Caserta et al. 2008).
Although isoflavones have well-described health benefits in cancer (Mohamed et al. 2017; Spagnuolo et al. 2015), the mechanisms underlying these benefits also result in adverse effects on the proliferative nature of the estrogen-sensitive endometrium (Plaza-Parrochia et al. 2017). Genistein is the most abundant of the soybean isoflavones, accounting for approximately 50% of the total soybean isoflavone content (Murphy et al. 2002). Reproductive disturbances have been reported in a number of species fed soy as a significant portion of their diet, including rats, mice, rabbits, sheep, cattle, and cheetahs (Bennetts et al. 1946; Carter et al. 1955; Kendall et al. 1950; Setchell et al. 1987; Thain 1966). A randomized study described an increased incidence of endometrial hyperplasia in women receiving soy supplements long term (Unfer et al. 2004). Serum genistein levels in women consuming a nonvegetarian diet fall within a range of , whereas levels are reported to be between in vegetarians and likely higher in those consuming soy supplements (Elorinne et al. 2016; Peeters et al. 2007).
Early-life exposures to exogenous compounds that mimic the activity of endogenous hormones have the potential to permanently alter developing organs and tissues. Therefore, developmental exposure to genistein is of particular concern given that about 12% of formula-fed infants in the United States are fed soy-based formula during their first year of life (Rossen et al. 2016). Serum genistein levels in these infants occur in the range of , which is several-fold higher than serum levels experienced in adults (vegetarian or nonvegetarian diet) and the dose reported to compete with for estrogen receptor binding (Cao et al. 2009; Rossen et al. 2016; Wang et al. 1996). The reported serum concentrations in infants fed soy formula also overlap with the concentration range shown in rodents to produce persistent adverse reproductive effects (approximately serum genistein) (Doerge et al. 2002). In rodents, neonatal genistein exposure results in significant disruptions to the structure and function of the female reproductive tract that manifest in adults (Jefferson et al. 2002; Newbold et al. 2001). Adult female rodents exposed to genistein as neonates exhibit sub- to complete infertility, resulting from altered estrous cyclicity, disrupted development of the oviduct, and an insufficient uterine environment (Awoniyi et al. 1998; Carter et al. 1955; Jefferson et al. 2009, 2012; Nagao et al. 2001). Global gene analysis of the adult female oviduct following neonatal genistein exposure revealed substantial changes to basal gene expression, as well as the transcriptional response to pregnancy (Jefferson et al. 2011, 2012). Interestingly, marked changes in immune response genes were reported following neonatal genistein exposure, some specific to the onset of pregnancy. Neonatal exposure to diethylstilbestrol (DES), a potent synthetic estrogen, also causes changes to female reproductive tract gene expression (Newbold et al. 2007). Neonatal DES exposure temporarily altered the expression of many chromatin-modifying proteins and persistently altered epigenetic marks in specific genomic regulatory regions in the adult mouse uterus (Jefferson et al. 2013). Soy-formula-fed girls demonstrate site-specific differences in DNA methylation in vaginal epithelial cells compared with girls fed cow formula (Harlid et al. 2017). These studies suggest that the mechanism of infertility following neonatal estrogen exposure during the developmental period is altered transcriptional activity in the adult female reproductive tract.
Classically, glucocorticoids are considered the primary mediators of pro- and anti-inflammatory actions within the immune response (Busillo and Cidlowski 2013). Glucocorticoids exert their effects through the glucocorticoid receptor (GR), a member of the nuclear receptor superfamily of transcription factors (Baxter and Tomkins 1970). Elevated levels of glucocorticoids due to stress or exogenous administration have deleterious effects on the reproductive tract, impacting the hypothalamus, pituitary, and gonads (Whirledge and Cidlowski 2017). Glucocorticoids regulate uterine biology through antagonizing the biological effects of , as well as independently coordinating uterine gene expression (Rhen et al. 2003). During pregnancy, glucocorticoids are thought to regulate many key processes, including the maternal immune response to pregnancy (Whirledge and Cidlowski 2017). In vitro studies have shown that glucocorticoids regulate thousands of genes within human uterine cell types, including genes involved in the inflammatory response that are also commonly regulated by (Whirledge et al. 2012, 2013). In a mouse model, disruption of uterine GR signaling results in a profound subfertile phenotype, characterized by reduced blastocyst implantation, defects in stromal cell decidualization, dysregulation of immune response genes, and altered immune cell recruitment during early pregnancy (SD Whirledge et al. 2015).
When coadministered with glucocorticoids, genistein disrupts GR-dependent transcription of target genes in human uterine endometrial cells in vitro (S Whirledge et al. 2015). These data, in addition to the overlap in reproductive and immune-modulatory functions of genistein and glucocorticoids, suggest that genistein-induced infertility may in part be driven by antagonism of glucocorticoid signaling. To better understand the contribution of glucocorticoid signaling to genistein-induced infertility, we investigated the long-term effects of early-life genistein exposure on glucocorticoid action in the uterus by using a mouse model of subcutaneous neonatal genistein exposure. Glucocorticoid-induced gene expression in the uterus was evaluated in the early postnatal period, prior to puberty, and in adult female mice that were adrenalectomized and ovariectomized postpuberty.
Materials and Methods
Reagents
Dexamethasone (Dex; , 17, 21-triol-3, 20-dione; ) by thin layer chromatography (TLC) and (; ) were purchased from Steraloids. Genistein (4′,5,7-trihydroxyisoflavone; ) was purchased from Sigma Aldrich.
Animal Experiment Study Design
Timed pregnant C57Bl/6 mice were obtained from Charles River at 14.5 d following the presence of a vaginal plug, where the detection of the vaginal plug was considered day 0.5 of pregnancy. Pregnant mice were individually housed, maintained on a controlled 12:12 h light-dark cycle (lights on 0700–1900 hours), and provided ad libitum access to water and food. All mice were provided irradiated Teklad global soy protein-free extruded rodent diet (2920X). Isoflavone levels were measured in this diet to be , compared with commercial diets that contain approximately (Naaz et al. 2003). Litters were randomly assigned to experimental groups, and pups were injected subcutaneously on PND1–5 with either vehicle (corn oil) or genistein ( dissolved in corn oil). The dose of genistein chosen for animal injections produces circulating serum concentrations in mice that correspond to the serum genistein concentration of infants fed soy-based infant formulas () (Cao et al. 2009; Doerge et al. 2002). For female pups, injected subcutaneously results in a maximum serum genistein concentration of with a half-life of 19 h (Doerge et al. 2002). The mouse and human estrogen receptor display relatively similar binding affinities for genistein, and respectively, and genistein has a reported half maximal inhibitory concentration () of for the estrogen receptor (Matthews et al. 2000; Wang et al. 1996). On the last day of control or genistein exposure (PND5), a subset of female mice pooled from several litters was randomly assigned to receive either vehicle (saline) or Dex ( dissolved in saline) by subcutaneous injection. The dose of Dex chosen for experiments has been validated to produce a robust transcriptional response in the mouse uterus and falls within the range of doses employed clinically in obstetrics (Boomsma et al. 2012; SD Whirledge et al. 2015). Tissues were harvested after 4 h, immediately snap-frozen with liquid nitrogen, and stored at until RNA extraction. The remaining pups were weaned on PND21 and litters from the same treatment groups were collectively housed at five females per cage. At weaning, a subset of female mice from pooled litters was randomly assigned to receive vehicle (saline) or Dex ( dissolved in saline) by intraperitoneal injection, and tissues were harvested after 4 h to isolate mRNA. Tissue collected for RNA extraction was snap-frozen with liquid nitrogen and stored at until RNA extraction was performed.
In previous studies employing the postnatal genistein exposure regimen, adult female mice were found to lack a regular estrous cycle (Jefferson et al. 2002). Moreover, variations in endogenous glucocorticoids among mice contribute to heterogeneity in basal gene expression (see Figure S1). In order to evaluate the uterine response to glucocorticoids in adult females without confounding effects of hormonal fluctuations from irregular cycles and endogenous glucocorticoids, the remaining mice underwent bilateral adrenalectomy (ADX) and ovariectomy (OVX) on PND42 to remove endogenous hormones. ADX/OVX animals were maintained on the same diet and provided 0.9% saline water during the 14-d recovery period.
To determine whether neonatal exposure to other estrogens could impact the uterine response to glucocorticoids, a subset of pups were exposed to dissolved in corn oil by subcutaneous injection on PND1–5. The dose of was selected to produce in vivo concentrations that model serum concentrations of during the estrous cycle and a robust uterotrophic response (Modder et al. 2004; Walmer et al. 1992) (see Figure S2). Adult mice underwent OVX/ADX on PND42 as previously described and were maintained on the same diet and provided 0.9% saline water during a 14-d recovery period.
On PND56, female mice from all treatment groups received either vehicle (saline), Dex ( dissolved in saline), ( dissolved in ethanol and diluted in saline), or by intraperitoneal injection. Uteri were collected 1.5 h later for chromatin immunoprecipitation and immunofluorescence, 4 h later for gene expression analysis, or 24 h later for cell proliferation. Tissue collected for RNA extraction was snap-frozen with liquid nitrogen and stored at until RNA extraction was performed. All animal procedures complied with the National Institutes of Health directives for the care and use of laboratory animals. All animals were treated humanely with regard to the alleviation of suffering. These studies were approved by the Institutional Animal Care and Use committees at the National Institute of Environmental Health Sciences.
Quantitative Real-Time PCR
Total RNA was extracted from the whole mouse uterus using the Qiagen RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol with the deoxyribonuclease (DNase) treatment performed on the column. RNA purity and yield was assessed be evaluating the A260/A280 and concentration using the NanoDrop One Spectrophotometer (ThermoFisher Scientific, Waltham, MA). One hundred nanograms total RNA was used to synthesize cDNA using the One-Step RT-PCR Universal Master Mix reagent (ThermoFisher Scientific). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the CFX Connect or CFX384 thermocycler (BioRad) using predesigned primer–probe sets (see Table S1) (ThermoFisher Scientific) in a reaction volume. The thermocycling parameters for each reaction were for 30 min, for 10 min, followed by 40 cycles of for 15 s and for 60 s. Each gene primer–probe set was evaluated in technical duplicates from a standard curve and normalized to that of the reference gene peptidylprolyl isomerase B (PPIB), which was unaffected by treatment. Data are reported as fold change, and analyzed by ANOVA or Student’s t-test using three to five biological replicates per treatment group.
Microarray Analysis
Gene expression analysis was conducted with three independent biological replicates using Agilent Whole Mouse Genome 4x44 multiplex format oligo arrays (product number G4122F; design ID 014868; Agilent Technologies) following the Agilent one-color microarray-based gene expression analysis protocol. The array is formatted to contain 43,379 gene probes. Starting with of total RNA, cyanine 3 (Cy3)-labeled cRNA was produced according to the manufacturer’s protocol. For each sample, of Cy3-labeled cRNAs were fragmented and hybridized for 17 h in a rotating hybridization oven. Slides were washed and then scanned with an Agilent Scanner. Data were obtained using the Agilent Feature Extraction software (version 9.5), using the one-color defaults for all parameters. The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. Preliminary analyses were performed with OmicSoft Array Studio software (version 7.0). In accordance with Minimum Information About a Microarray Experiment (MIAME) guidelines, the raw microarray data will be available in the Gene Expression Omnibus repository at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107788).
Differential gene expression was analyzed using the Partek Genomics Suite (version 6.6; (Partek Inc.). Significant changes in gene expression were defined based on an analysis of variance (ANOVA) p-value of and a false discovery rate of . The lists of significantly regulated probes were visually sorted by Venn diagram (http://bioinformatics.lu/venn.php). Heat maps were generated in Partek Genomics Suite and provided hierarchical clustering dendrograms. The statistically significant probes were also analyzed with Ingenuity Pathway Analysis (IPA; Ingenuity Systems) to evaluate their functional annotation. Gene set enrichment was determined by IPA using Fisher’s exact test (). Significantly regulated genes were also analyzed by gene ontology using Gene Annotation Tool to Help Explain Relationships (GATHER) (Chang and Nevins 2006). Gene ontology GATHER analysis used a Bayes factor cutoff of 5.0.
Corticosterone Assay
Blood samples from experimental animals (11–12 animals per experimental group) were obtained by submandibular vein bleed between 0930 and 1100 hours. Submandibular vein bleed was performed in a procedure room, separate from where the experimental mice were housed. Care was taken to minimize the amount of time each mouse was handled, and mice were placed in a new cage separate from cage-mates immediately following the procedure. At the completion of the procedure, mice were returned to their original cage. Corticosterone was measured by the commercially available DetectX Corticosterone Enzyme Immunoassay Kit (Arbor Assays). All samples were run in duplicate. The limit of detection for the assay was determined to be . The sensitivity was determined to be . The intraassay precision was determined to be 6.5–8.1%. The interassay precision was determined to be 9.9–16.4%.
Immunofluorescence
Cryopreserved frozen uteri from three mice per treatment group were sectioned to serial sections and stored at . Prior to immunostaining, slides were thawed at room temperature. Three sections per uterus were stained for histological analysis with antibodies to GR (1:500; Cell Signaling Technologies) or MKi67 (1:400; Cell Signaling Technologies) overnight at 4°C (see Table S2). Sections were then washed with phosphate buffered saline (PBS) and incubated with secondary antibodies (see Table S2). Hoescht 33342 (1:5,000; Molecular Probes) stain was applied prior to mounting to visualize the nucleus. Images were obtained on a Zeiss LSM780 confocal microscope equipped with a (oil) objective and processed using the Zen 2012 software. Epithelial cell proliferation was scored for each image taken by counting the number of Ki67-positive epithelial cells and dividing by the total number of epithelial cells in the image. For each treatment group, three to four images were counted.
Western Blotting
Tissues from control () and genistein-treated () mice were lysed in Tris glycine SDS sample buffer supplemented with 2-mercaptoethanol (BME). Equal amounts of protein from tissue extracts were separated via SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked with 7.5% skim milk in Tris-buffered saline (TBS) and incubated overnight with mouse monoclonal antibodies (1:10,000; Millipore) and rabbit monoclonal anti-GR antibodies (1:1,000; Cell Signaling Technologies) in 5% milk in TBS-Tween (0.1%) (see Table S2). Membranes were washed and incubated with goat anti-rabbit IRDye 680-conjugated secondary antibody and goat antimouse IRDye 800-conjugated secondary antibody (LI-COR Biosciences) for 1 h at room temperature. The Odyssey LI-COR imaging system (LI-COR Biosciences) was used to visualize protein expression. GR protein levels were normalized to and expressed relative to control.
Chromatin Immunoprecipitation
Uteri were pooled together (five per sample) and pulverized by mortar and pestle in liquid nitrogen. Proteins were fixed to chromatin with 1% paraformaldehyde for 10 min at room temperature. Cells were then washed in ice-cold PBS and cross-linking stopped with glycine. Cells were resuspended in cell lysis buffer containing Hepes-KOH pH 8.0, EDTA, NaCl, 10% glycerol, 0.5% NP-40, 0.25% Triton X-100, and protease inhibitors and dounce homogenized. Samples were centrifuged for 10 min at at 4°C and resuspended in shearing buffer containing Tris-HCl pH 8.0, EDTA, NaCl, 1.0% SDS, 0.1% Na deoxycholate, and 1% Triton X-100 with protease inhibitors. Samples were then sonicated using the Fisher Scientific Model 120 Sonic Dismembrator (ThermoFisher Scientific) at 35% for 6 min to obtain 200- to 500-bp fragments as confirmed by agarose gel electrophoresis. Sheared chromatin was precleared with protein A agarose/salmon sperm DNA (Millipore) and immunoprecipitated overnight with monoclonal GR antibody (1:175; Cell Signaling Technologies) or normal rabbit IgG (1:700; Millipore) (see Table S2). The protein–DNA complexes were then precipitated with protein A magnetic beads (BioRad). After washing and elution, cross-links were reversed and DNA was purified using the QiaQuick PCR Purification kit (Qiagen). Immunoprecipitated DNA was quantified from four to five independent pooled samples per treatment group using qRT-PCR with custom-designed primer–probe sets ordered from Integrated DNA Technologies (see Table S3). Relative expression values for each primer were calculated using the method and set relative to control vehicle IgG values. Previously reported glucocorticoid response elements (GREs) in glucocorticoid-induced leucine zipper (Gilz) and Krüppel-like factor 13 (Klf13) were used to design primers (Cruz-Topete et al. 2016). Regions of GR binding in the mouse serum/glucocorticoid regulated kinase 1 (Sgk1) and period circadian clock 1 (Per1) genes have been previously described (Yu et al. 2010). These intronic sequences for Sgk1 (21,713,350–21,713,672) and Per1 (68,910,412–68,910,788) were then searched for GREs using the JASPAR CORE Vertebrata database consensus GRE (ID, MA0113.3) (http://jaspar.genereg.net/) (Mathelier et al. 2016). The identified GRE for the Sgk1 gene was AGAACAnnnTGTTCT, and the identified GRE for the Per1 gene was AGAACAnnnTGTTCC.
Statistical Analysis
The data are presented as of a minimum of three biological replicates. Statistical significance was determined by ANOVA with Tukey’s post hoc analysis (control vs. treatment group exposed to vehicle or Dex) or Student’s t-test (control vs. genistein) using StatTools software. Statistical significance is reported as (*) or (**).
Results
Effect of Neonatal Genistein Exposure on Glucocorticoid-Regulated Gene Expression in the Uterus
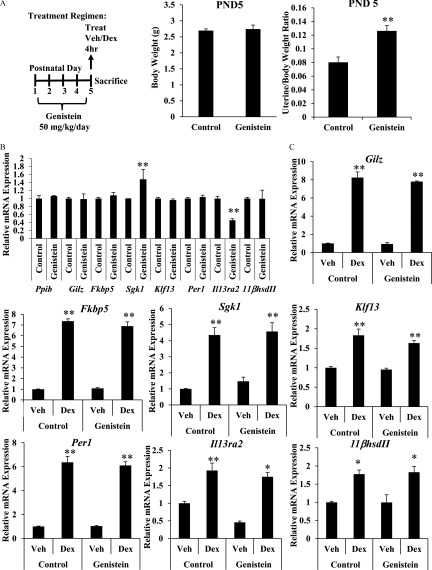
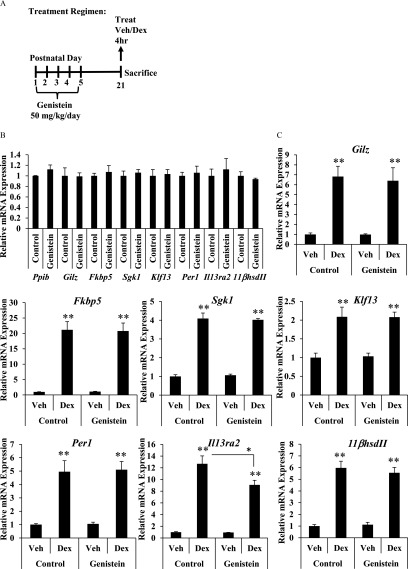
To assess the impact of neonatal genistein exposure on the uterine response to glucocorticoids, female mice were injected subcutaneously on PND1–5 with genistein or corn oil control (Figure 1A). At the completion of the treatment regimen on PND5, a subset of the control and genistein-exposed female mice were treated with the synthetic glucocorticoid Dex () or vehicle (saline) by subcutaneous injection and uteri were collected 4 h post injection. Body and uterine weights were measured and mRNA was isolated from the uterus. Developmental exposure to genistein did not alter body weight, but the estrogenic activity of genistein transiently increased the uterine/body weight ratio at PND5 (Figure 1A). Expression of Gilz, FK506 binding protein 5 (Fkbp5), Sgk1, Klf13, and Per1, and interleukin 13 receptor subunit alpha 2 (Il13ra2) and dehydrogenase II (), classic target genes of GR signaling, was quantified by qRT-PCR. On PND5, genistein exposure did not alter basal mRNA expression of Gilz, Fkbp5, Klf13, or Per1, , or the reference gene peptidylprolyl isomerase B (Ppib) (Figure 1B), although basal expression of Sgk1 was significantly higher and basal expression of Il13ra2 was significantly lower in the genistein-exposed mice. Dex treatment induced all of these genes equivalently in both treatment groups, suggesting that genistein-exposed mice had normal glucocorticoid responsiveness at that time (Figure 1C). Basal expression and glucocorticoid-mediated induction of Ppib, Gilz, Fkbp5, Sgk1, Klf13, Per1, Il13ra2, and were also evaluated at weaning (PND21) in a subset of the control and genistein-exposed female mice treated for 4 h with Dex () or vehicle (saline) (Figure 2A). On PND21, prior genistein exposure did not affect basal expression of Ppib, Gilz, Fkbp5, Sgk1, Klf13, Per1, Il13ra2, or mRNA (Figure 2B). Furthermore, the induction of Gilz, Fkbp5, Sgk1, Klf13, Per1, and by Dex treatment was indistinguishable in the genistein-exposed and control uteri, suggesting that the uterine response to glucocorticoids was not altered prior to puberty (Figure 2C).
Figure 1.
Effect of neonatal genistein exposure on immediate glucocorticoid target gene expression. (A) Schematic representation of dosing schedule and experimental end points. Body and uterine weights (g) were determined on PND5 for control and genistein-treated mice. Data represent mean of . ** determined by Student’s t-test. (B and C) Relative mRNA expression of Ppib, Gilz, Fkbp5, Sgk1, Klf13, Per1, , and Il13ra2 as measured by qRT-PCR 4 h following subcutaneous injection of vehicle or Dex on PND5. (B) Basal gene expression was determined by comparing the vehicle-treated genistein group to vehicle-treated controls. (C) Fold change was determined by reporting values relative to vehicle-treated controls. Values are normalized to the reference gene peptidylprolyl isomerase B (Ppib). Bar graphs show the from 3 to 5 animals. ** as determined by ANOVA with Tukey’s post hoc analysis. Dex, dexamethasone; PND, postnatal day; qRT-PCR, quantitative real-time polymerase chain reaction; Treat, treatment; Veh, vehicle.
Figure 2.
Effect of neonatal genistein exposure on glucocorticoid target gene expression prior to puberty. (A) Schematic representation of dosing schedule and experimental end points. (B and C) Relative mRNA expression of Ppib, Gilz, Fkbp5, Sgk1, Klf13, Per1, , and Il13ra2 as measured by qRT-PCR 4 h following intraperitoneal injection of vehicle or Dex on PND21. (B) Basal gene expression was determined by comparing vehicle-treated genistein group to vehicle-treated controls. (C) Fold change was determined by reporting values relative to vehicle-treated controls. Values are normalized to the reference gene peptidylprolyl isomerase B (Ppib). Bar graphs show the from 4 to 5 animals. ** as determined by ANOVA with Tukey’s post hoc analysis. Dex, dexamethasone; PND, postnatal day; qRT-PCR, quantitative real-time polymerase chain reaction; Treat, treatment; Veh, vehicle.
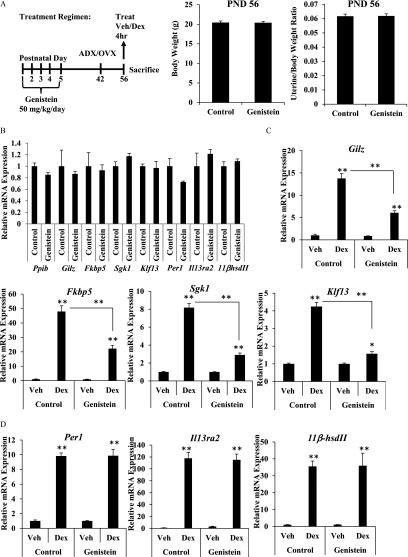
Long-term effects of the neonatal genistein exposure were evaluated in adult female mice that underwent the postnatal genistein dosing regimen (Figure 3A). Adult control and genistein-exposed female mice were adrenalectomized/ovariectomized (ADX/OVX) on PND42 to remove endogenous hormones. Following a 2-wk recovery period, ADX/OVX mice were treated with Dex or vehicle (saline) by intraperitoneal injection and uteri were harvested after 4 h. At this time point, body weight and uterine/body weight ratio were equivalent between treatment groups (Figure 3A). Basal mRNA expression of Ppib, Gilz, Fkbp5, Sgk1, Klf13, Per1, IL13ra2, and was not different in adult mice exposed to genistein on PND1–5 compared with control mice (Figure 3B). However, induction of the glucocorticoid-responsive genes Gilz, Fkbp5, Sgk1, and Klf13 was significantly blunted in adult genistein-exposed mice compared with control mice (Figure 3C). Glucocorticoid-mediated induction of Per1, , and Il13ra2 was not affected by early-life genistein exposure (Figure 3D). These data indicate that glucocorticoid signaling is selectively altered in the mature adult uterus but not the neonatal uterus by early-life exposure to genistein (see Table S4).
Figure 3.
Effect of neonatal genistein exposure on glucocorticoid target gene expression in the adult uterus. (A) Schematic representation of dosing schedule, surgical intervention, and experimental end points. Body and uterine weight (g) was determined on PND56 for control and genistein-treated mice. Data represent mean of . (B and C) Relative mRNA expression of Ppib, Gilz, Fkbp5, Sgk1, Klf13, Per1, , and Il13ra2 as measured by qRT-PCR 4 h following intraperitoneal injection of vehicle or Dex in adult ADV/OVX mice on PND56. (B) Basal expression was determined by comparing the vehicle-treated genistein group to vehicle-treated control group. (C) Fold change was determined by reporting values relative to vehicle-treated controls. Values are normalized to the reference gene Ppib. (D) Per1, , and Il13ra2 mRNA expression was determined by reporting values relative to vehicle-treated controls. Bar graphs show the from 3 to 5 animals. * , ** as determined by ANOVA with Tukey’s post hoc analysis. ADX/OVX, adrenalectomized/ovariectomized; Dex, dexamethasone; PND, postnatal day; qRT-PCR, quantitative real-time polymerase chain reaction; Treat, treatment; Veh, vehicle.
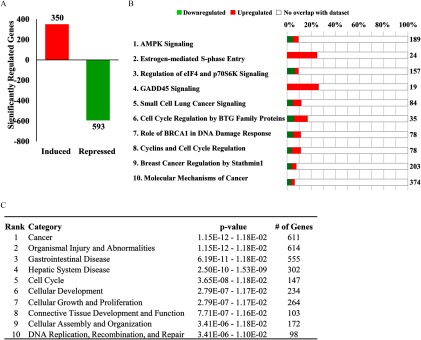
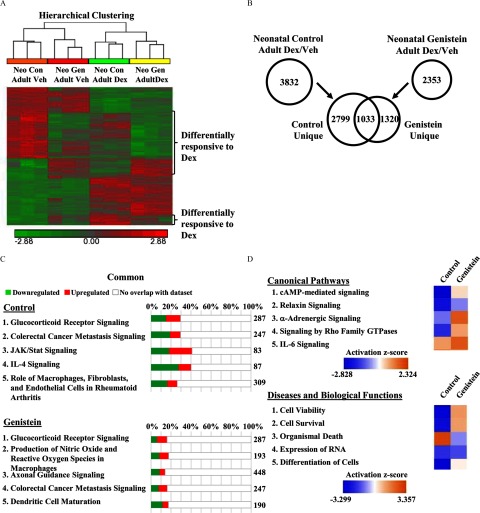
Neonatal genistein exposure caused lasting effects to basal gene expression in the oviduct (Jefferson et al. 2011). In order to determine the long-term effects on basal gene expression in the uterus, whole-genome microarray analysis was performed on uteri from three biological replicates of adult ADX/OVX mice previously exposed to corn-oil control or genistein as neonates (Figure 4). A comparison of the significantly regulated genes identified 943 genes that demonstrated different basal expression levels in the uterus of adult mice exposed to genistein as neonates compared with controls (Figure 4A). Of the significantly regulated genes, 350 genes were induced, whereas 593 genes were repressed. Shown in Table S5 are the top 10 induced and repressed genes determined by fold change. Interestingly, IPA performed on the genes with significantly different basal expression identified many molecular and biological pathways related to regulation of cell cycle and cancer (Figure 4B). Among IPA diseases and biological functions, cancer was the top ranked and cell cycle the fifth ranked annotation most significantly associated with the dysregulated genes (Figure 4C).
Figure 4.
Effect of neonatal genistein exposure on basal gene expression in the uterus. (A) Whole genome microarray analysis was performed on total RNA isolated from the uterus of adult ADX/OVX PND56 mice previously exposed to control or genistein. Three biological replicates were evaluated for each treatment group. The number of probes that were significantly different () between the control and genistein-exposed group were graphed as induced (red) or repressed (green). (B) Significantly different genes were evaluated in IPA software and the top 10 regulated canonical pathways are reported. The total number of genes in the IPA-annotated pathway is listed on the right side of the graph. The total percentage of significantly regulated genes is depicted as the sum of induced and repressed genes. (C) The top 10 diseases and biological functions are listed. The p-value range and number of genes within the listed functions is provided. ADX/OVX, adrenalectomized/ovariectomized; IPA, Ingenuity Pathway Analysis; PND, postnatal day.
Genome-Wide Gene Expression Analysis
Attenuated induction of Gilz, Fkbp5, Sgk1, and Klf13 by glucocorticoids in the uterus of adult mice exposed to genistein during development suggested that the transcriptional response to glucocorticoids was altered, thus whole-genome microarray analysis was performed on uteri from adult control and genistein-exposed mice treated with vehicle or Dex for 4 h. Three biological replicates were evaluated for each treatment group. A heat map of sample replicates and hierarchical clustering analysis of glucocorticoid-responsive genes indicated samples separated into distinct groups based on exposure and treatment (Figure 5A). Glucocorticoids significantly altered the expression of 3,832 genes (1,903 genes induced and 1,929 genes repressed) in the uteri of control mice compared with 2,353 genes (1,335 genes induced and 1,018 genes repressed) in the uteri of genistein-exposed mice (Figure 5B). A comparison of the genes significantly regulated by glucocorticoids in both the control and genistein-exposed groups revealed only 1,033 genes in common, including 77 genes that were differentially regulated by glucocorticoids (see Table S6). Neonatal genistein exposure resulted in the loss of glucocorticoid regulation of 2,799 genes and the unique regulation of 1,320 genes by glucocorticoids.
Figure 5.
Effect of genistein exposure on genome-wide regulation by glucocorticoids. (A) Adult ADX/OVX (PND56) mice, exposed as neonates to control or genistein, were treated with vehicle (saline) or Dex for 4 h. Whole genome microarray analysis was performed on total RNA isolated from the uterus, with three biological replicates evaluated for each treatment group. Heat map and hierarchical clustering of significantly regulated genes (), where red represents induced genes and green represents repressed genes. (B) The number of significantly regulated genes by treatment () were compared between the control and genistein groups by Venn diagram. (C) Significantly regulated genes were annotated with IPA software. The top five regulated canonical pathways are reported for the control and genistein groups. The total number of genes in the IPA-annotated pathway is listed on the right side of the graph. The total percentage of significantly regulated genes is depicted as the sum of induced and repressed genes. (D) Genes uniquely regulated by Dex in the control (2,799) and genistein (1,320) groups. The top five canonical pathways and diseases and biological functions were ranked and assigned an activation z-score (orange indicates induced; blue indicates repressed). ADX/OVX, adrenalectomized/ovariectomized; Con, control; Dex, dexamethasone; IPA, Ingenuity Pathway Analysis; Neo, neonatal; Veh, vehicle.
To identify the molecular and biological pathways associated with the glucocorticoid-regulated uterine transcriptome in control and genistein-exposed mice, the annotation of significantly regulated genes was evaluated in IPA software. IPA identified GR signaling as the top canonical pathway regulated by glucocorticoid treatment in both control and genistein-exposed mice (Figure 5C). Evaluation of this pathway revealed significantly fewer genes regulated in genistein-exposed mice (see Figure S3). All of the top canonical pathways regulated in genistein-exposed mice had a smaller percent of significantly regulated genes than the top five canonical pathways regulated in control mice, reflecting the overall suppression of glucocorticoid signaling following neonatal genistein exposure. For example, the top canonical pathway regulated by glucocorticoids in control mice was GR signaling, where 85 of 287 genes were regulated (29.6%). In genistein-exposed mice, only 46 of 287 genes were regulated by glucocorticoids, representing 16% of the total genes in the pathway. The top predicted diseases and biological functions by IPA analysis and a separate gene ontology analysis revealed similar annotated functions in the control- and genistein-treated mice, though the number of significantly regulated genes in each function was diminished in the genistein-treated mice (see Tables S7–S9).
The genes commonly regulated by glucocorticoids in the control and genistein-exposed mice were compared to determine if early-life exposure to genistein altered the extent or directionality of the uterine transcriptional response to glucocorticoids. Of the 1,033 genes, 13 genes were regulated in the opposite direction (anticorrelated) (see Table S10), and a change in the magnitude of induction or repression greater than 2-fold was found for 64 genes. A comparison analysis of the genes common to the control and genistein-exposed mice indicated the predicted activation of the top pathways and functions was analogous between treatment groups, indicating that the differential regulation of common genes did not affect activation of predicted functions (see Figure S4). Interestingly, a comparison analysis of the top canonical pathways and diseases and biological functions in the uniquely regulated genes (2,799 unique genes in control mice; 1,320 unique genes in genistein-exposed mice) showed numerous differences in the predicted activation, including several pathways/functions that were predicted to have opposing activation scores (Figure 5D). These data indicate that disruption of glucocorticoid signaling by neonatal exposure to genistein leads to the dysregulation of many genes and pathways with potentially important roles in uterine biology.
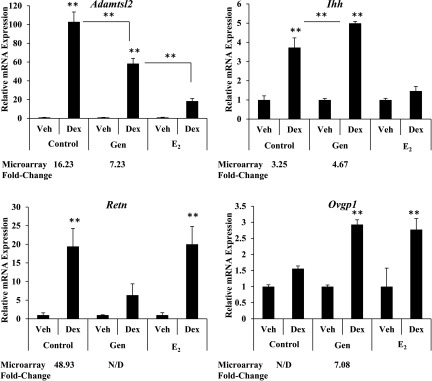
In order to assess whether the altered transcriptional response to glucocorticoids in adult mice following neonatal genistein exposure was specific to genistein or broadly represents early-life exposure to estrogens, subcutaneous injection of dissolved in corn oil was also included as a treatment arm in the PND1–5 injection regimen. Adult ADX/OVX female mice exposed to control, genistein, or treatment on PND1–5 were treated with Dex or vehicle (saline) for 4 h on PND56. The differential expression of select genes identified by the whole-genome microarray analysis was validated in an independent cohort of genistein-exposed mice and compared with mice exposed to (Figure 6). Genes were chosen for analysis based on their differential patterns of glucocorticoid-responsiveness determined by whole-genome microarray analysis. Specifically, genes with changes in the magnitude of induction or exhibiting a novel regulation pattern following neonatal genistein exposure were evaluated. Expression of a disintegrin-like and metallopeptidase with thrombospondin type 1 motif (ADAMTS)-like 2 (Adamtsl2) mRNA was significantly increased in response to glucocorticoid treatment in control mice. The magnitude of induction by glucocorticoids was blunted in genistein-exposed mice and further decreased in mice. Induction of Indian hedgehog (Ihh) mRNA by glucocorticoids was validated in the uterus of control and genistein-exposed mice. However, up-regulation of Ihh was absent following Dex treatment in mice. Microarray results indicated that early-life exposure to genistein impeded glucocorticoid-mediated induction of Resistin (Retn), which was validated in independent samples. Interestingly, early-life exposure to did not prevent the induction of Retn by glucocorticoids. Oviductal glycoprotein 1 (Ovgp1) was uniquely regulated by glucocorticoids following neonatal genistein exposure. exposure produced a comparable effect. These results suggest that the ability of estrogens to specifically alter glucocorticoid signaling in the uterus may be applicable to many other estrogenic compounds.
Figure 6.
Effect of neonatal genistein exposure on glucocorticoid-responsive genes differentially regulated in genistein-exposed mice. Adult ADX/OVX (PND56) mice, exposed as neonates to control, genistein or on PND1–5, were treated with vehicle (saline) or Dex for 4 h. Relative mRNA expression in the uterus of Adamtsl2, Ihh, Retn, and Ovgp1 were measured by qRT-PCR and values were normalized to the reference gene Ppib. Dex treatment for each neonatal exposure group (vehicle, genistein, or ) was set relative to vehicle treatment for that group (i.e., treated with Dex set relative to vehicle). The reported fold change from microarray analysis is listed below the qRT-PCR results. The results represent the mean of . ** according to ANOVA with Turkey’s post hoc analysis. ADX/OVX, adrenalectomized/ovariectomized; Dex, dexamethasone; , estradiol; Gen, genistein; N/D, not determined to be significantly regulated by microarray analysis; PND, postnatal day; qRT-PCR, quantitative real-time polymerase chain reaction; Veh, vehicle.
Effect of Neonatal Genistein Exposure on GR Recruitment to Target Genes
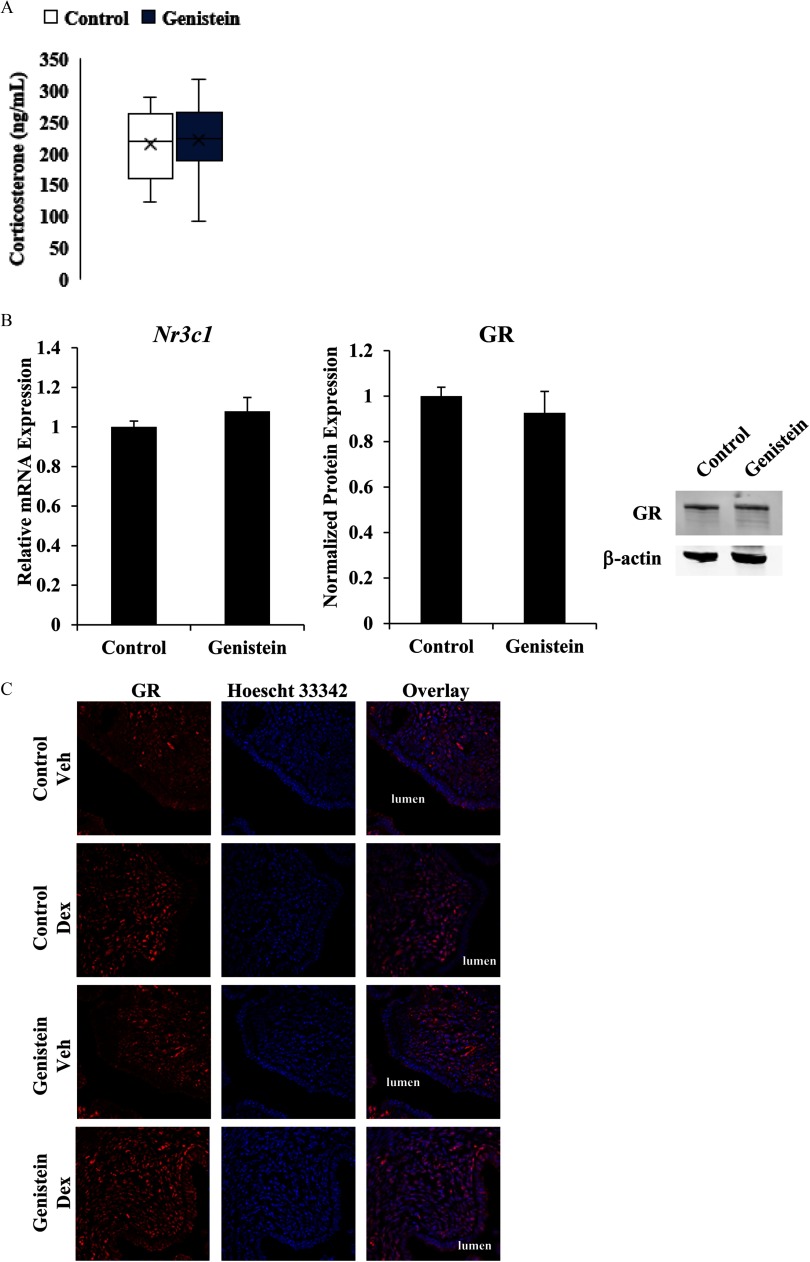
To define the mechanism by which neonatal genistein exposure alters uterine glucocorticoid signaling, we evaluated circulating levels of glucocorticoids, expression of GR, and nuclear translocation of GR in response to ligand. There were no differences in serum levels of corticosteroids in adult control and genistein-exposed mice (Figure 7A). The expression of uterine GR mRNA (Nr3c1) and protein was similar in control and genistein-treated mice, indicating that differences in glucocorticoid responsiveness were not due to insufficient ligand or receptor expression (Figure 7B). The ability of GR to undergo translocation to the nucleus following ligand binding was assessed by immunostaining and confocal microscopy. ADX/OVX adult control and genistein-treated mice were treated with vehicle (saline) or Dex for 1.5 h. Glucocorticoid treatment led to nuclear translocation of GR, which was otherwise localized to the cytoplasm, in luminal epithelial and stromal cells of both control and genistein-treated mice, suggesting that altered gene expression in the genistein-exposed mice was not a consequence of deficient nuclear translocation (Figure 7C). These data demonstrate the presence of an intact glucocorticoid signaling system in the uterus of genistein-exposed mice.
Figure 7.
Effect of neonatal genistein exposure on ligand and GR expression. (A) Serum levels of corticosterone were measured in adult mice with intact ovaries and adrenal glands from serum collected between 0930 and 1130 hours in the morning. Data represent the mean of . (B) Relative mRNA expression of GR (Nr3c1) was measured by qRT-PCR in adult ADX/OVX control and genistein-exposed mice. Expression was normalized to the reference gene Ppib and set relative to expression in control mice. GR protein levels, quantified by western blot analysis, were normalized to levels of the reference protein , which was not altered by treatment group (see Figure S5). GR protein levels were set relative to control mice. The results and image represent the mean of . (C) Representative images of GR expression and localization (red) in luminal epithelial and endometrial stromal cells. Hoescht 33342 was used to visualize nuclei (blue). Images were taken at . ADX/OVX, adrenalectomized/ovariectomized; Dex, dexamethasone; GR, glucocorticoid receptor; qRT-PCR, quantitative real-time polymerase chain reaction; Veh, vehicle.
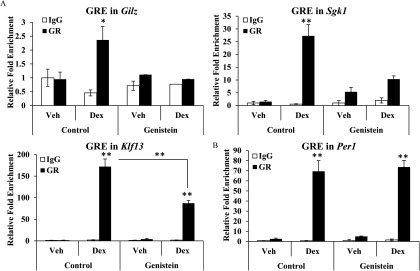
The genomic actions of GR are mediated through a physical interaction with DNA response elements or through associations with chromatin-bound proteins. Chromatin immunoprecipitation (ChIP) assays were performed on the uteri of control and genistein-treated ADX/OVX adults 1.5 h following vehicle or Dex treatment to determine whether altered glucocorticoid signaling following neonatal genistein exposure resulted from dysregulated GR recruitment to regulatory regions of target genes. For this experiment, GR recruitment was evaluated for Gilz, Sgk1, and Klf13, which demonstrated blunted induction of mRNA expression by glucocorticoid treatment following neonatal genistein exposure, and Per1, which did not demonstrate differences in glucocorticoid-induced mRNA expression. A functional GRE has been reported for Gilz and Klf13 (Cruz-Topete et al. 2016), and in silico analysis of the Sgk1 and Per1 genes identified GREs in the introns of these genes with complete homology to the critical nucleotides of the consensus sequence. Similar to our findings of reduced induction of Gilz mRNA in response to glucocorticoids in mice exposed to genistein, recruitment of GR to a previously identified GRE in Gilz was abrogated in the uterus of genistein-exposed mice (Figure 8A). Glucocorticoid receptors were strongly recruited to the Sgk1 intron-3 GRE in uteri from control mice exposed to glucocorticoids (Figure 8A). However, Dex-induced recruitment of GR to the GRE of Sgk1 was weak in genistein-treated mice and did not obtain significance. Activated GRs were recruited to the Klf13 intron-1 GRE in both control and genistein-treated mice. However, recruitment of GR was significantly less in the genistein-treated mice compared with control mice. In contrast to our findings for Gilz, Sgk1, and Klf13, glucocorticoid-induced GR recruitment to the Per1 gene was not affected by neonatal genistein exposure (Figure 8B). These results suggest that neonatal genistein exposure alters GR recruitment to regulatory regions in a gene-specific manner.
Figure 8.
Effect of neonatal genistein exposure on GR recruitment to specific GREs. Adult ADX/OVX PND56 mice, exposed neonatally to control or genistein, were treated with vehicle (saline) or Dex for 1.5 h. ChIP assays were performed with rabbit IgG or rabbit anti-GR antibody. Coimmunoprecipitated DNA was analyzed by qRT-PCR using primers to GREs and plotted relative to input DNA. (A) Relative fold enrichment of GR recruitment to Gilz, Sgk1, and Klf13 as determined by ChIP assays. (B) Relative fold enrichment of GR recruitment to Per1. The results represent the mean of . * , ** as determined by Student’s t-test. ADX/OVX, adrenalectomized/ovariectomized; ChIP, chromatin immunoprecipitation; Dex, dexamethasone; GR, glucocorticoid receptor; GRE, glucocorticoid response element; IgG, immunoglobulin G; PND, postnatal day; Veh, vehicle.
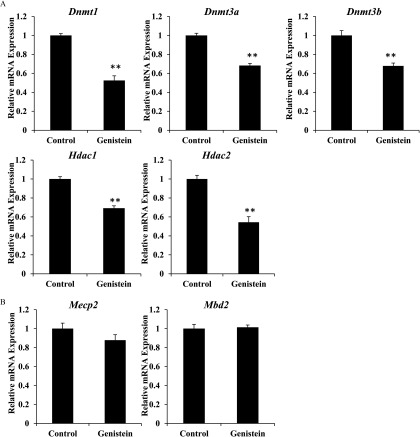
The recruitment of GR to accessible regions of chromatin is partially dependent on the maintenance of an open state by chromatin- and DNA-modifying enzymes (Mundade et al. 2014). mRNA expression of genes involved in DNA and histone modifications was evaluated to explore the underlying mechanism by which neonatal genistein exposure results in changes to the profile of GR recruitment. Compared with control mice, basal mRNA levels of DNA (cytosine-5)-methyltransferases 1 (Dnmt1), 3A (Dnmt3a), and 3B (Dnmt3b) and histone deacetylases 1 (Hdac1) and 2 (Hdac2) were lower in adult ADX/OVX mice exposed to genistein as neonates (Figure 9A). Relative mRNA expression of Methyl-CpG-binding domain protein 2 (Mbd2) and Methyl-CpG-binding protein 2 (Mecp2), transcriptional repressors that bind to methylated DNA, did not significantly change (Figure 9B). These data suggest that neonatal genistein exposure causes long-term effects on the expression of specific genes involved in DNA and histone modifications.
Figure 9.
Effect of neonatal genistein exposure on expression of genes involved in modifying DNA and chromatin. (A) Relative mRNA expression of Dnmt1, Dnmt3a, Dnmt3b, Hdac1, and Hdac2 in adult ADX/OVX mice exposed previously to control or genistein. (B) Relative mRNA expression of Mbd2 and Mecp2 in adult ADX/OVX mice exposed previously to control or genistein. Values were normalized to the reference gene Ppib and set relative to expression in control mice. **. as determined by Student’s t-test. ADX/OVX, adrenalectomized/ovariectomized.
Effect of Neonatal Genistein Exposure on the Functions of Glucocorticoids in the Adult Mouse Uterus
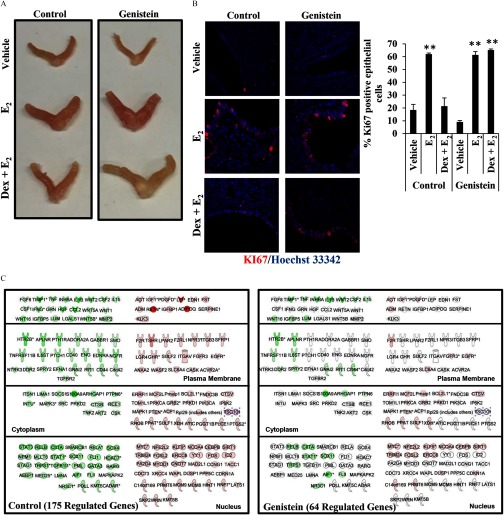
The ability of exogenous glucocorticoids to antagonize the uterotrophic effects of has been well described (Howe et al. 1990; Rabin et al. 1990; Rhen et al. 2003). To evaluate whether changes in glucocorticoid signaling resulting from neonatal genistein exposure disrupted the biological functions of glucocorticoids in the uterus, morphology was evaluated in control and genistein-exposed mice 24 h following vehicle (saline), , or Dex and injection in 3 mice per treatment group (Figure 10A). In control and genistein-exposed mice, treatment induced the classic uterotrophic response, which is characterized by increased uterine weight through water imbibition and cell proliferation (see Figure S2). Glucocorticoid administration with blocked estrogen-induced uterine growth in control mice but was only able to partially restrict estrogen-induced uterine growth in genistein-exposed mice. Cell proliferation is partially responsible for increased uterine weight following exposure (Quarmby and Korach 1984). Uterine sections from control and genistein-exposed mice treated with vehicle, , or were stained for the proliferation marker Ki-67 to assess whether glucocorticoid antagonism of cell proliferation was altered (Figure 10B). significantly increased uterine epithelial cell proliferation in control and genistein-exposed mice compared with vehicle-treated mice. In control mice, glucocorticoids administered with blocked the increase in proliferation. However, the number of Ki67 positive cells was not reduced in the genistein-exposed mice treated with . Because we observed significant differences in the regulation of cell proliferation, we evaluated genes significantly regulated by glucocorticoids in the cellular growth and proliferation pathway by IPA (Figure 10C). In control mice, glucocorticoids regulated 175 genes related to cellular growth and proliferation. In genistein-exposed mice, glucocorticoid regulation of this pathway was limited, where only 64 of 175 genes were significantly regulated. Therefore, the failure of glucocorticoids to antagonize the uterotrophic effects of in genistein-exposed mice are likely the result of altered glucocorticoid signaling.
Figure 10.
Effect of disruption of glucocorticoid signaling on glucocorticoid antagonism of uterine estrogen signaling. (A) Representative images of adult uteri following 24-h treatment with vehicle (saline), , or and Dex. (B) Proliferative marker MKi67 (red) by immunofluorescence in cross-sections from the control and genistein groups following 24-h treatment with vehicle, , or and Dex. Hoescht 33342 staining of the nuclei is shown in blue. Images taken at . The percentage of Ki67 positive cells was determined by the ratio of Ki67 positive epithelial cells/total number of epithelial cells per image. (C) IPA identified genes involved in cellular growth and proliferation as significantly regulated by Dex. Glucocorticoid treatment regulated 175 genes associated with proliferation of connective tissue cells in the uterus of control mice. Green indicates repression and red indicates up-regulation of gene expression. Dex, dexamethasone; , estradiol; IPA, Ingenuity Pathway Analysis.
Discussion
Disruptions to the endocrine system by environmental compounds have varying consequences depending on the timing of exposure. Early-life exposures can result in persistent adverse effects due to the sensitive nature of the developing organs. For example, the long-term consequences of in utero exposure to the xenoestrogen DES are well-documented in humans (Reed and Fenton 2013). Human epidemiological data linking developmental exposure to phytoestrogens and adult pathologies remain limited, but animal models have clearly described lasting effects, some of which parallel the effects resulting from neonatal DES exposure (Adgent et al. 2012; D’Aloisio et al. 2012; Harlid et al. 2017; Nagao et al. 2001; Newbold et al. 2001; Padilla-Banks et al. 2006; Upson et al. 2015). In rodents, disruptions to the reproductive tract following neonatal genistein exposure correspond to alterations in the expression of genes key to the morphology and function of the reproductive tract (Jefferson et al. 2011). Global gene expression has been evaluated in the rodent oviduct following neonatal genistein exposure, but the assessment of the long-term consequences of neonatal genistein exposure in the rodent uterus has been limited to candidate genes (Begum et al. 2006; Jefferson et al. 2012; Tang et al. 2008). The results presented here demonstrate that early-life exposure to genistein at an environmentally relevant dose, which is comparable to exposure in infants consuming soy-based formula, causes substantial genome-wide changes in uterine gene expression and glucocorticoid responsiveness in adult mice (Jefferson and Williams 2011).
Neonatal genistein exposure, at concentrations equivalent in estrogenic activity to DES, resulted in uterine adenocarcinoma in adult mice (Newbold et al. 2001). Our microarray analysis indicated that neonatal genistein exposure altered the transcriptional profile of the adult uterus at baseline, and analysis of these genes suggested a potential mechanism for the observed uterine adenocarcinoma. IPA software ranked canonical pathways related to cell cycle regulation and cancer as the top pathways for the set of genes significantly altered by neonatal genistein exposure. In agreement with the identified canonical pathways, the top regulated disease was cancer. These data support genome-wide transcriptional reprograming as a possible mechanism leading to uterine adenocarcinoma.
Neonatal genistein exposure also renders the uterus unable to support implantation and pregnancy. Uterine glucocorticoid signaling plays an essential role in establishing endometrial receptivity and implantation (SD Whirledge et al. 2015). Moreover, aberrant glucocorticoid signaling driven by stress-induced or exogenous elevations in glucocorticoid levels impairs reproductive success (Whirledge and Cidlowski 2017). In order to understand whether long-term effects on glucocorticoid signaling contribute to the deficient uterine environment, the glucocorticoid-regulated transcriptome was evaluated in the uterus of adult mice previously exposed to vehicle or genistein as neonates. The transcriptional response to glucocorticoids was persistently altered by neonatal exposure to genistein, and this differential pattern of regulation was validated in independent samples for the majority of tested genes. Gene ontology analysis indicated that the uniquely altered genes reflect preferentially activated and repressed canonical pathways and biological functions in the uterus. The pathways and functions activated by those genes uniquely regulated by glucocorticoids in the control group compared with those in the genistein-exposed group suggest long-lasting changes to uterine function may in part be mediated by selectively altered glucocorticoid signaling. For example, the cAMP signal transduction pathway mediates hormonal signals in the endometrium and plays a critical role in endometrial stromal cell decidualization (Brar et al. 1997; Telgmann et al. 1997). The activation status of the cAMP-mediated signaling pathway in response to glucocorticoids was predicted to be reversed in the uterus of adult mice exposed to genistein early in life. Furthermore, the glucocorticoid-mediated activation status of four of the top five diseases and biological functions were also predicted to be inversely correlated in the genistein-exposed mice compared with controls. These differentially responsive functions include cell viability, cell survival, organismal death, and differentiation of cells, which are essential functions critical to uterine remodeling in support of pregnancy. Similar functions were also found to be dysregulated in the subfertile uterine-GR deficient mouse in response to hormone priming for implantation, suggesting that disruption to glucocorticoid regulation of these functions could impair uterine competence (SD Whirledge et al. 2015).
The altered regulation of glucocorticoid target genes could be accounted for by differential recruitment of GR to regulatory sequences upon activation. Indeed, enrichment of GR at GREs within classic glucocorticoid target genes Gilz, Sgk1, and Klf13 was reduced in the uterus of adult female mice following neonatal genistein exposure, which corresponded to blunted mRNA induction of these genes in response to glucocorticoids. Deficient GR transactivation in this limited set of candidate genes may have implications for uterine biology and fertility. Sgk1 null mice have significantly fewer offspring related to spontaneous fetal loss, and lower endometrial SGK1 expression was reported in women with recurrent pregnancy loss (Salker et al. 2011). Although the actions of Gilz and Klf13 in the uterus are still under investigation, early studies suggest the expression of these genes may be important for endometrial function. Targeted reduction of Klf13 mRNA in human endometrial stromal cells decreased the expression of Bmp2, a morphogenic factor essential for stromal cell differentiation in early human pregnancy (Heard et al. 2012). Gilz is expressed in the mouse endometrium during pregnancy and levels distinctly change near parturition (Zhao et al. 2006). The biological significance of this is not clear, but GILZ is an important mediator of inflammation, which is a hallmark of parturition.
Modulation of transcription factor recruitment by genistein has been demonstrated in vitro (Chang et al. 2008; Chen et al. 2013; Li et al. 2008), although these findings are the first to show the persistent effects of genistein on recruitment in vivo. The mechanism leading to altered GR recruitment in a gene-specific manner subsequent to neonatal genistein exposure is likely reprograming of the epigenetic landscape during development, which has been documented for several xenoestrogens (Bromer et al. 2010; Jefferson et al. 2013; Strakovsky et al. 2014). Neonatal exposure to genistein in rodents resulted in site-specific hypo- and hypermethylation and associated changes in gene expression, which was also reported in vaginal epithelial cells from soy-formula-fed infants (Harlid et al. 2017; Strakovsky et al. 2014; Tang et al. 2008). Such site-specific changes to the epigenetic landscape may have contributed to the latent differences in glucocorticoid responsiveness of Gilz, Sgk1, and Klf13 compared with Per1, , and Il13ra2 in the uterus of adult mice following neonatal genistein exposure. Epigenetic changes have been associated with changes in the expression of chromatin-modifying enzymes (Greathouse et al. 2012). We found that mRNA expression of certain DNA methyltransferases and histone deacetylases was repressed in the uterus of adult genistein-exposed mice, possibly promoting global changes to basal gene expression and facilitating altered glucocorticoid responses via changes in the expression of specific co-regulators and/or enzymes that influence GR binding to target genes. In addition to changes to the chromatin environment, other mechanisms of transcriptional regulation, including posttranslational modifications to GR or cofactor availability, may also contribute to altered glucocorticoid signaling following early-life exposure to genistein.
It is notable that genistein can act as both an estrogen receptor ligand and inhibitor of receptor tyrosine kinases (Akiyama et al. 1987). However, neonatal genistein exposure with the estrogen receptor antagonist ICI 182780 blocked the transcriptional and functional consequences in the oviduct, suggesting that the effects of genistein on the female reproductive tract are mediated by its estrogenic activities (Jefferson et al. 2011, 2012). In agreement with this, we found that early-life exposure to also altered glucocorticoid-mediated gene expression in the uterus of adult mice. The expression of Ovgp1 was uniquely induced by Dex following postnatal treatment with genistein or . Early-life exposure to genistein or also diminished the magnitude of induction of Adamtsl2 by Dex. For some genes evaluated, the response to prenatal was unique from that of genistein, suggesting these molecules can have gene-specific effects. Prenatal exposure to the xenoestrogens bisphenol A and DES induce opposite effects on the methylation status of the promoter and intron 1 of homeobox A10, which suggests that xenoestrogens can exert distinct developmental effects potentially related to their estrogenic activity (Bromer et al. 2009, 2010). Moreover, these findings indicate that disrupted uterine glucocorticoid signaling may occur following exposure to a broad range of estrogenic chemicals and expand the implications for these findings.
Conclusion
The data presented herein demonstrate that early-life exposure to genistein disrupts both basal gene expression in the uterus and the transcriptional response to glucocorticoids in adult mice. We found that these differences occurred in a gene-specific manner. Although expression of GR was not altered, GR recruitment to transcriptional response elements was diminished in genes where neonatal genistein exposure blunted the glucocorticoid response. Furthermore, the ability of glucocorticoids to function as biological antagonists to the uterotrophic actions of was blocked by early-life exposure to genistein. Selectively altered glucocorticoid signaling may in part contribute to the subfertile/infertile phenotype following environmental estrogen exposure, though further study is warranted to determine the mechanisms by which disrupted glucocorticoid signaling impairs uterine receptivity.
Supplemental Material
Acknowledgments
We acknowledge K. Gerrish from the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) Molecular Genomics Core and J. Tucker from the NIEHS Fluorescence Microscopy and Imaging Center for their technical support. The authors also thank L. Wyrick from NIEHS Arts and Photography. This research was supported by the Intramural Research Program of the NIH/NIEHS (S.D.W., R.H.O, and J.A.C.) and grants K99ES022983 and R00ES022983 awarded by the NIH/NIEHS (S.D.W.).
References
- Adgent MA, Daniels JL, Rogan WJ, Adair L, Edwards LJ, Westreich D, et al. 2012. Early-life soy exposure and age at menarche. Paediatr Perinat Epidemiol 26(2):163–175, PMID: 22324503, 10.1111/j.1365-3016.2011.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262(12):5592–5595, PMID: 3106339. [PubMed] [Google Scholar]
- Awoniyi CA, Roberts D, Veeramachaneni DN, Hurst BS, Tucker KE, Schlaff WD. 1998. Reproductive sequelae in female rats after in utero and neonatal exposure to the phytoestrogen genistein. Fertil Steril 70(3):440–447, PMID: 9757872. [DOI] [PubMed] [Google Scholar]
- Baxter JD, Tomkins GM. 1970. The relationship between glucocorticoid binding and tyrosine aminotransferase induction in hepatoma tissue culture cells. Proc Natl Acad Sci USA 65(3):709–715, PMID: 4315614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum M, Tashiro H, Katabuchi H, Suzuki A, Kurman RJ, Okamura H. 2006. Neonatal estrogenic exposure suppresses PTEN-related endometrial carcinogenesis in recombinant mice. Lab Invest 86(3):286–296, PMID: 16402032, 10.1038/labinvest.3700380. [DOI] [PubMed] [Google Scholar]
- Bennetts HW, Underwood EJ, Shier FL. 1946. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J 22:2–12, PMID: 21028682. [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Keay SD, Macklon NS. 2012. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev 6:CD005996, PMID: 22696356, 10.1002/14651858.CD005996.pub3. [DOI] [PubMed] [Google Scholar]
- Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. 1997. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 6(3):301–307, PMID: 9368687, 10.1007/BF02820507. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Wu J, Zhou Y, Taylor HS. 2009. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150(7):3376–3382, PMID: 19299448, 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. 2010. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J 24(7):2273–2280, PMID: 20181937, 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Cidlowski JA. 2013. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab 24(3):109–119, PMID: 23312823, 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, et al. 2009. Isoflavones in urine, saliva, and blood of infants: Data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environm Epidemiol 19:223–234, PMID: 18665197, 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter MW, Matrone G, Smart WW Jr. 1955. Effect of genistin on reproduction of the mouse. J Nutr 55(4):639–645, PMID: 14368363. [DOI] [PubMed] [Google Scholar]
- Caserta D, Maranghi L, Mantovani A, Marci R, Maranghi F, Moscarini M. 2008. Impact of endocrine disruptor chemicals in gynaecology. Hum Reprod Update 14(1):59–72, PMID: 18070835, 10.1093/humupd/dmm025. [DOI] [PubMed] [Google Scholar]
- Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, et al. 2008. Estrogen receptors α and β as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol 22(5):1032–1043, PMID: 18258689, 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Nevins JR. 2006. GATHER: a systems approach to interpreting genomic signatures. Bioinformatics 22(23):2926–2933, PMID: 17000751, 10.1093/bioinformatics/btl483. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang S, Zhou T, Huang C, McLaughlin A, Chen G. 2013. Liver X receptor alpha mediated genistein induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1) in Hep G2 cells. Toxicol Appl Pharmacol 268(2):106–112, PMID: 23352501, 10.1016/j.taap.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Ha TY, Ahn JY, Kim SR, Kang KS, Hwang IK, et al. 2008. Estrogenic activities of isoflavones and flavones and their structure-activity relationships. Planta Med 74(1):25–32, PMID: 18095219, 10.1055/s-2007-993760. [DOI] [PubMed] [Google Scholar]
- Cruz-Topete D, He B, Xu X, Cidlowski JA. 2016. Krüppel-like factor 13 is a major mediator of glucocorticoid receptor signaling in cardiomyocytes and protects these cells from DNA damage and death. J Biol Chem 291:(37):19374–19386, PMID: 27451392, 10.1074/jbc.M116.725903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. 2012. Early-life exposures and early-onset uterine leiomyomata in black women in the Sister Study. Environ Health Perspect 120(3):406–412, PMID: 22049383, 10.1289/ehp.1103620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Banks EP, Jefferson WN, Newbold RR. 2002. Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett 184(1):21–27, PMID: 12104044. [DOI] [PubMed] [Google Scholar]
- Elorinne AL, Alfthan G, Erlund I, Kivimäki H, Paju A, Salminen I, et al. 2016. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PloS One 11(2):e0148235, PMID: 26840251, 10.1371/journal.pone.0148235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, et al. 2012. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res 10(4):546–557, PMID: 22504913, 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlid S, Adgent M, Jefferson WN, Panduri V, Umbach DM, Xu Z, et al. 2017. Soy formula and epigenetic modifications: analysis of vaginal epithelial cells from infant girls in the IFED study. Environ Health Perspect 125(3):447–452, PMID: 27539829, 10.1289/EHP428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard ME, Pabona JM, Clayberger C, Krensky AM, Simmen FA, Simmen RC. 2012. The reproductive phenotype of mice null for transcription factor Krüppel-like factor 13 suggests compensatory function of family member Krüppel-like factor 9 in the peri-implantation uterus. Biol Reprod 87(5):115, PMID: 22993382, 10.1095/biolreprod.112.102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe RS, Lee YH, Fischkoff SA, Teuscher C, Lyttle CR. 1990. Glucocorticoid and progestin regulation of eosinophil chemotactic factor and complement C3 in the estrogen-treated rat uterus. Endocrinology 126(6):3193–3199, PMID: 2351113, 10.1210/endo-126-6-3193. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, et al. 2013. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol Endocrinol 27(10):1666–1677, PMID: 24002655, 10.1210/me.2013-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. 2002. Neonatal exposure to genistein induces estrogen receptor (ER)α expression and multioocyte follicles in the maturing mouse ovary: evidence for ERβ-mediated and nonestrogenic actions. Biol Reprod 67(4):1285–1296, PMID: 12297547. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. 2009. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod 80(3):425–431, PMID: 19005167, 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Phelps JY, Cantor AM, Williams CJ. 2012. Neonatal phytoestrogen exposure alters oviduct mucosal immune response to pregnancy and affects preimplantation embryo development in the mouse. Biol Reprod 87(1):10, 1–10, PMID: 22553218, 10.1095/biolreprod.112.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Phelps JY, Gerrish KE, Williams CJ. 2011. Permanent oviduct posteriorization after neonatal exposure to the phytoestrogen genistein. Environ Health Perspect 119(11):1575–1582, PMID: 21810550, 10.1289/ehp.1104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Williams CJ. 2011. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod Toxicol 31(3):272–279, PMID: 20955782, 10.1016/j.reprotox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall KA, Salisbury GW, Vandemark NL. 1950. Sterility in the rabbit associated with soybean hay feeding. J Nutr 42(4):487–500, PMID: 14804155. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. 2008. Regulation of Akt/FOXO3a/GSK-3β/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem 283(41):27707–27716, PMID: 18687691, 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, Lee J, et al. 2016. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 44(D1):D110–D115, PMID: 26531826, 10.1093/nar/gkv1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Celius T, Halgren R, Zacharewski T. 2000. Differential estrogen receptor binding of estrogenic substances: a species comparison. J Steroid Biochem Mol Biol 74(4):223–234, PMID: 11162928. [DOI] [PubMed] [Google Scholar]
- Modder UI, Riggs BL, Spelsberg TC, Fraser DG, Atkinson EJ, Arnold R, et al. 2004. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur J Endocrinol 151(4):503–510, PMID: 15476452. [DOI] [PubMed] [Google Scholar]
- Mohamed SIA, Jantan I, Haque MA. 2017. Naturally occurring immunomodulators with antitumor activity: an insight on their mechanisms of action. Int Immunopharmacol 50:291–304, PMID: 28734166, 10.1016/j.intimp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Mundade R, Ozer HG, Wei H, Prabhu L, Lu T. 2014. Role of ChIP-seq in the discovery of transcription factor binding sites, differential gene regulation mechanism, epigenetic marks and beyond. Cell Cycle 13(18):2847–2852, PMID: 25486472, 10.4161/15384101.2014.949201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PA, Barua K, Hauck CC. 2002. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Analyt Technol Biomed Life Sci 777(1–2):129–138, PMID: 12270206. [DOI] [PubMed] [Google Scholar]
- Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR, Lubahn DB, et al. 2003. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 144(8):3315–3320, PMID: 12865308, 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. 2001. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol 15(4):399–411, PMID: 11489596. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Banks EP, Bullock B, Jefferson WN. 2001. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res 61(11):4325–4328, PMID: 11389053. [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK. 2007. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog 46(9):783–796, PMID: 17394237, 10.1002/mc.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Cook JC, Craven SC, Van Pelt CS, Obourn JD. 1996. An in vivo battery for identifying endocrine modulators that are estrogenic or dopamine regulators. Fundam Appl Toxicol 33:(2):182–195, PMID: 8921337. [PubMed] [Google Scholar]
- Padilla-Banks E, Jefferson WN, Newbold RR. 2006. Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology 147:(10):4871–4882, PMID: 16857750, 10.1210/en.2006-0389. [DOI] [PubMed] [Google Scholar]
- Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, et al. 2007. Variations in plasma phytoestrogen concentrations in European adults. J Nutr 137(5):1294–1300, PMID: 17449595. [DOI] [PubMed] [Google Scholar]
- Plaza-Parrochia F, Romero C, Valladares L, Vega M. 2017. Endometrium and steroids, a pathologic overview. Steroids 126:85–91, PMID: 28827068, 10.1016/j.steroids.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Quarmby VE, Korach KS. 1984. The influence of 17β-estradiol on patterns of cell division in the uterus. Endocrinology 114(3):694–702, PMID: 6697957, 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- Rabin DS, Johnson EO, Brandon DD, Liapi C, Chrousos GP. 1990. Glucocorticoids inhibit estradiol-mediated uterine growth: possible role of the uterine estradiol receptor. Biol Reprod 42(1):74–80, PMID: 2310819. [DOI] [PubMed] [Google Scholar]
- Reed CE, Fenton SE. 2013. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today 99(2):134–146, PMID: 23897597, 10.1002/bdrc.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Grissom S, Afshari C, Cidlowski JA. 2003. Dexamethasone blocks the rapid biological effects of 17β-estradiol in the rat uterus without antagonizing its global genomic actions. FASEB J 17(13):1849–1870, PMID: 14519664, 10.1096/fj.02-1099com. [DOI] [PubMed] [Google Scholar]
- Rossen LM, Simon AE, Herrick KA. 2016. Types of infant formulas consumed in the United States. Clin Pediatr (Phila) 55:278–285, PMID: 26149849, 10.1177/0009922815591881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salker MS, Christian M, Steel JH, Nautiyal J, Lavery S, Trew G, et al. 2011. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med 17(11):1509–1513, PMID: 22001908, 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Gosselin SJ, Welsh MB, Johnston JO, Balistreri WF, Kramer LW, et al. 1987. Dietary estrogens—a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology 93:(2):225–233, PMID: 3297906. [DOI] [PubMed] [Google Scholar]
- Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, et al. 2015. Genistein and cancer: Current status, challenges, and future directions. Adv Nutr 6(4):408–419, PMID: 26178025, 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Lezmi S, Flaws JA, Schantz SL, Pan YX, Helferich WG. 2014. Genistein exposure during the early postnatal period favors the development of obesity in female, but not male rats. Toxicol Sci 138(1):161–174, PMID: 24361872, 10.1093/toxsci/kft331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, et al. 2008. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology 149(12):5922–5931, PMID: 18669593, 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgmann R, Maronde E, Taskén K, Gellersen B. 1997. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138(3):929–937, PMID: 9048592, 10.1210/endo.138.3.5004. [DOI] [PubMed] [Google Scholar]
- Thain RI. 1966. Bovine infertility possibly caused by subterranean clover. Further report and herd histories. Aust Vet J 42(6):199–203, PMID: 6007040. [DOI] [PubMed] [Google Scholar]
- Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. 2004. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fert Steril 82(1):145–148, PMID: 15237003, 10.1016/j.fertnstert.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, Scholes D, Holt VL. 2015. Early-life factors and endometriosis risk. Fert Steril 104(4):964–971.e5, PMID: 26211883, 10.1016/j.fertnstert.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL, Nelson KG. 1992. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology 131(3):1458–1466, PMID: 1505477, 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM. 1996. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 17(2):271–275, PMID: 8625449. [DOI] [PubMed] [Google Scholar]
- Whirledge S, Cidlowski JA. 2017. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab 28(6):399–415, PMID: 28274682, 10.1016/j.tem.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge S, Dixon D, Cidlowski JA. 2012. Glucocorticoids regulate gene expression and repress cellular proliferation in human uterine leiomyoma cells. Horm Cancer 3(3):79–92, PMID: 22311344, 10.1007/s12672-012-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge S, Senbanjo LT, Cidlowski JA. 2015. Genistein disrupts glucocorticoid receptor signaling in human uterine endometrial Ishikawa cells. Environ Health Perspect 123(1):80–87, PMID: 25136773, 10.1289/ehp.1408437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge S, Xu X, Cidlowski JA. 2013. Global gene expression analysis in human uterine epithelial cells defines new targets of glucocorticoid and estradiol antagonism. Biol Reprod 89(3):66, PMID: 23843231, 10.1095/biolreprod.113.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge SD, Oakley RH, Myers PH, Lydon JP, DeMayo F, Cidlowski JA. 2015. Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc Natl Acad Sci USA 112(49):15166–15171, PMID: 26598666, 10.1073/pnas.1508056112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CY, Mayba O, Lee JV, Tran J, Harris C, Speed TP, et al. 2010. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PloS One 5(12):e15188, PMID: 21187916, 10.1371/journal.pone.0015188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Koon D, Bethin KE. 2006. Identification of transcription factors at the site of implantation in the later stages of murine pregnancy. Reproduction 131(3):561–571, PMID: 16514199, 10.1530/rep.1.00874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.