Abstract
Background:
Emerging evidence suggests that perfluoroalkyl substances (PFASs) are endocrine disruptors and may contribute to the etiology of type 2 diabetes (T2D), but this hypothesis needs to be clarified in prospective human studies.
Objectives:
Our objective was to examine the associations between PFAS exposures and subsequent incidence of T2D in the Nurses’ Health Study II (NHSII). In addition, we aimed to evaluate potential demographic and lifestyle determinants of plasma PFAS concentrations.
Methods:
A prospective nested case–control study of T2D was conducted among participants who were free of diabetes, cardiovascular disease, and cancer in 1995–2000 [(): ) of age]. We identified and ascertained 793 incident T2D cases through 2011 () years of follow-up: ). Each case was individually matched to a control (on age, month and fasting status at sample collection, and menopausal status and hormone replacement therapy). Plasma concentrations of five major PFASs, including perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexanesulfonate, perfluorononanoic acid, and perfluorodecanoic acid were measured. Odds ratios (ORs) of T2D by PFAS tertiles were estimated by conditional logistic regression.
Results:
Shorter breastfeeding duration and higher intake of certain foods, such as seafood and popcorn, were significantly associated with higher plasma concentrations of PFASs among controls. After multivariate adjustment for T2D risk factors, including body mass index, family history, physical activity, and other covariates, higher plasma concentrations of PFOS and PFOA were associated with an elevated risk of T2D. Comparing extreme tertiles of PFOS or PFOA, ORs were 1.62 (95% CI: 1.09, 2.41; ) and 1.54 (95% CI: 1.04, 2.28; ), respectively. Other PFASs were not clearly associated with T2D risk.
Conclusions:
Background exposures to PFASs in the late 1990s were associated with higher T2D risk during the following years in a prospective case–control study of women from the NHSII. These findings support a potential diabetogenic effect of PFAS exposures. https://doi.org/10.1289/EHP2619
Introduction
Accumulating evidence documents the important role of endocrine-disrupting chemicals (EDCs) in the pathogenesis of obesity, type 2 diabetes (T2D), and other metabolic conditions (Casals-Casas and Desvergne 2011; Heindel et al. 2015). The EDCs exert their detrimental effects primarily through modulating nuclear receptors, such as estrogen receptors and peroxisome proliferator-activated receptor () (Casals-Casas and Desvergne 2011; Heindel et al. 2015). Although the relationship of EDCs, such as polychlorinated biphenyls, with T2D or obesity have been extensively examined in basic science research and prospective human studies (Casals-Casas and Desvergne 2011; Heindel et al. 2015; Lee et al. 2014; Wu et al. 2013), evidence for perfluoroalkyl substances (PFASs) is limited. Blood concentrations of these ubiquitous environmental pollutants are declining in Americans overall, but they remain detectable in almost the entire population (Calafat et al. 2007; Kato et al. 2011; Liu et al. 2018; Olsen et al. 2017). These chemicals possess endocrine-disrupting properties and are known to activate PPARs in rodents (Ikeda et al. 1985; Kennedy et al. 2004; Lau et al. 2007; Rosen et al. 2008). The mechanistic pathways may also involve activation and disruption of the functions of other regulators (Puigserver et al. 1998; Scharmach et al. 2012; Spiegelman et al. 2000; Vanden Heuvel et al. 2006; Wu et al. 1999) of lipid and glucose metabolism (Hayhurst et al. 2001; Jones et al. 2005; Rhee et al. 2003). Despite the evidence indicating potentially obesogenic and diabetogenic effects of PFASs, most data linking PFAS exposure with T2D are derived from cross-sectional studies, with equivocal findings (Cardenas et al. 2017; Christensen et al. 2016; Conway et al. 2016; Karnes et al. 2014; MacNeil et al. 2009; Su et al. 2016; Zhang et al. 2015).
To address prospective associations between PFASs and T2D risk, we conducted a prospective nested case–control study of U.S. female nurses participating in the Nurses’ Health Study II (NHSII) and examined the hypothesis that higher plasma concentrations of PFASs are associated with a higher T2D risk. We also evaluated potential demographic and lifestyle determinants of PFAS exposures that have been reported in other cohorts of women (Lauritzen et al. 2016; Manzano-Salgado et al. 2016), including age, region of residence, breastfeeding duration, body weight, and diet.
Methods
Study Population
The NHSII is an ongoing prospective cohort study that consists of 116,430 U.S. female nurses enrolled in 1989 when they responded to a questionnaire inquiring about a multitude of variables, including body weight and height, demographics, lifestyle habits, history of chronic diseases and medication use (Zong et al., unpublished data, 2018). Follow-up questionnaires were administered biennially to update the lifestyle and medical history information. A total of 29,611 NHSII participants 32–52 y of age provided blood samples in 1995–2000, which were transmitted to a central biorepository via overnight courier. Upon arrival, the samples were immediately processed and aliquoted into cryotubes, which were stored in the vapor phase of liquid nitrogen freezers at (). Among participants who provided blood samples, a high follow-up rate of has been maintained.
Nested Case–Control Study Design
Pariticipants who provided blood samples and were free of self-reported prevalent diabetes, cardiovascular disease, and cancer at sample collection were eligible for the current investigation. Among these participants, we prospectively identified and confirmed 793 T2D cases (as explained in detail below) during biennial follow-up from blood collection to June 2011 (range: 1–16 y, : ). One control was randomly selected for each case using the risk-set sampling approach (Prentice and Breslow 1978). Cases and controls were individually matched for age at blood sample collection, month of sample collection (March–May, June–August, September–November, and December–February), fasting status (yes/no), ethnicity, menopausal status and hormone replacement therapy (HRT; premenopause, postmenopause and never used HRT, postmenopause and former or current HRT use, or missing). Menopausal status and HRT in cases were matched to controls, as these factors may affect associations for various biomarkers to be examined in the same nested case–control study (Salpeter et al. 2006). To minimize reverse causation bias, T2D cases diagnosed within the first year following the blood sample collection were excluded.
The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard T.H. Chan School of Public Health.
Ascertainment of T2D
If participants reported having a T2D diagnosis in follow-up questionnaires, they were then sent a validated supplemental questionnaire (Manson et al. 1991) to confirm or refute the self-report. We used at least one of the following American Diabetes Association (ADA) 1998 criteria to confirm T2D diagnosis based on self-reported information from a supplemental questionnaire: a) an elevated glucose concentration (fasting plasma glucose , random plasma glucose , or plasma glucose after an oral glucose load) and at least one symptom related to diabetes; b) no symptoms, but elevated plasma glucose concentrations on two separate occasions; or c) treatment with insulin or oral hypoglycemic medication. In our nested case–control study, only confirmed T2D cases were included, in which 30 (3.8%), 647 (81.6%), and 655 (82.6%) met criterion a, b, and c, respectively. In the validation study of T2D diagnosis, 61 of 62 (98.3%) questionnaire-confirmed cases of T2D were reconfirmed after an endocrinologist reviewed the medical records without the information from the supplementary questionnaire (Manson et al. 1991).
Assessment of Diet
A validated semiquantitative food frequency questionnaire (sFFQ) (Willett 1998) was first administered in 1991 and sent to participants quadrennially thereafter to update dietary habits. In the sFFQs, we asked about average consumption frequency of about 130 food items during the past year, with a prespecified serving size for each item. Good validity and reproducibility of the assessments of individual food items, including fruits, vegetables, red meats, seafood, beverages, and popcorn and other grain products, have been demonstrated in validation studies (Feskanich et al. 1993; Giovannucci et al. 1991; Salvini et al. 1989). Nutrient intake was estimated by multiplying the consumption frequency of each relevant food item by its contents of the nutrient and then summing intake of the nutrient across all food items (Wedick et al. 2012). We derived an alternative healthy eating index (AHEI) score to evaluate the overall quality of diet (Chiuve et al. 2012). This score summarizes the consumption of 11 foods and nutrients most predictive of chronic disease risk, including vegetables, fruits, whole grains, sugar-sweetened beverages and fruit juice, nuts and legumes, red and processed meat, trans fats, long-chain n-3 fats, polyunsaturated fats, sodium, and alcohol. A higher score indicates a better diet quality.
Laboratory Measurements
In the current study, plasma concentrations of PFASs were measured using on-line solid phase extraction and liquid chromatography coupled to a triple quadropole mass spectrometer (Haug et al. 2009; Vestergaard et al. 2012), with minor modifications. We quantified five PFASs that have a high detection rate () and can be reliably measured in the general population: perfluorooctane sulfate (PFOS), perfluorooctanoic acid (PFOA), perfluorohexanesulfonate (PFHxS), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA). Concentrations of minor PFASs with a lower detection rate and/or higher laboratory measurement errors [coefficients of variation (CVs) ] are provided in Table S1. Several plasma T2D risk markers were also measured at baseline: hemoglobin A1c (HbA1c), total cholesterol, and triacylglycerol were measured on the Roche P Modular system (Roche Diagnostics), total adiponectin concentrations were measured using an enzyme-linked immunosorbent assay (ALPCO), and fasting insulin was measured by a radioimmunoassay (Linco). The intraassay CVs ranged from 0.7% for insulin to 4.0% for total adiponectin.
To ensure that the laboratory assessments of biomarkers are free of systematic measurement error, we processed and analyzed samples of matched case–control pairs in the same analytical run. Within each batch, matched samples were assayed by the same technician in a random sequence under identical conditions. We placed splits of blinded quality control samples in the batches of the case–control samples to monitor the quality of these assays. The average intraassay CV% were 6.1% for PFOS, 8.7% for PFOA, 9.7% for PFHxS, 12.5% for PFNA, and 14.4% for PFDA. Internal quality assurance data using the NIST SRM1958 showed average interassay CV% of 7.4% for PFOS, 7.2% for PFOA, 4.0% for PFHxS and 6.5% for PFNA. Although no certified value is given for PFDA, this peak shows a CV% of 10.6%.
Within-Person Reproducibility of PFASs
In a pilot study, we measured PFAS concentrations in a random sample of 58 participants who provided two blood specimens collected 1–2 y apart. The study design has been described elsewhere (Kotsopoulos et al. 2010). Samples from the same individuals were handled identically, shipped and processed in the same batch, and placed on the same plate with random position. The lab personnel were blinded to sample status. Intraclass correlation coefficients (ICCs) between two samples were calculated and used to evaluate the reproducibility of the PFAS analyses.
Statistical Methods
We replaced PFAS values below the detection limit () by the detection limit divided by the square root of 2. Given the imprecision of the PFAS measurements, we adjusted the PFAS concentrations for batch effects (Rosner et al. 2008). According to this method, we first ran a linear regression model to regress PFAS concentrations on batch indicator dummy variables. PFAS concentrations were then recalibrated by subtracting the difference between the coefficient of each individual batch and the average of the coefficients of all batches. All analyses were based on recalibrated values of PFASs unless otherwise indicated.
In controls, we calculated Spearman correlation coefficients () to evaluate intercorrelations among PFASs. To examine whether PFAS exposure is associated with insulin resistance, glucose homeostasis, and dyslipidemia that predispose T2D development, we calculated of PFASs with total adiponectin, fasting insulin, HbA1c, and blood lipids. We explored the association of plasma PFAS concentrations with major demographic and lifestyle determinants that have been reported in other cohorts of women (Lauritzen et al. 2016; Manzano-Salgado et al. 2016), including age, states of residence, breastfeeding history, baseline body mass index (BMI), and overall diet quality measured by AHEI, by calculating least-square means of PFAS concentrations by categories of the determinants using a generalized linear regression model. Covariates were primarily derived from the questionnaire administered in 1995 (or questionnaires administered closest to 1995). States of residence were classified as coastal (AL, AK, CA, CT, DE, FL, GA, HI, ME, MD, MA, NH, NJ, NC, OR, RI, SC, VA, and WA), Great Lakes region (IL, IN, MI, MN, NY, OH, PA, and WI), and inland (all other states). Lifetime breastfeeding duration in months was obtained from the 1993 questionnaire using the question “How many months in total (all births combined) did you breast feed?”. To account for additional breastfeeding between 1993 and the blood draw, we further adjusted for number of births during this period using subsequent questionnaires. The multivariate models simultaneously adjusted for age, BMI, breastfeeding history, number of births, states of residence, and AHEI, in addition to matching factors. Matching factors were included to account for potential variations from the matching design. Because of the right-skewed distributions, PFAS values were log transformed (base 10) before the analysis, and transformed back to the original scale for presentation. We also examined individual food items, primarily seafood and popcorn as known dietary sources of PFAS exposure (Jian et al. 2017; Picó et al. 2011; Sinclair et al. 2007; Tittlemier et al. 2007; Trier et al. 2011). Finally, we calculated between plasma PFASs and intakes of all foods collected in the sFFQ for an overview of PFAS–diet associations. Primary food groups included dairy foods, fruits, vegetables, eggs and meats, breads and cereals, beverages, and sweets and baked goods. These analyses were limited to controls to minimize the possibility that preclinical T2D stages of cases affect plasma PFAS concentrations and T2D risk markers. In addition, controls are more representative of the overall source population.
The associations between plasma PFAS concentrations and T2D risk were evaluated using conditional logistic regression. The models were adjusted for the matching factors, and we further controlled for predictors of PFAS concentrations and/or known risk factors of T2D, including family history of diabetes (yes, or no), oral contraceptive use (never used, past user, or current user), breastfeeding duration at blood draw (nulliparous or parous without breastfeeding, , or ) and number of children delivered after 1993 (0, 1, or ), state of residence (coastal, Great Lakes region, or inland), smoking status (never, former, or current), alcohol intake (abstainer, , , or ), metabolic equivalent of task for physical activity (MET-h/wk; , 3–8.9, 9–17.9, 18–26.9, or ), baseline BMI (; , 23.0–24.9, 25.0–29.9, 30.0–34.9, or ), and AHEI score (in quintiles). Participants were categorized into tertiles according to the distribution of PFAS concentrations among controls. p-Values for linear trend were calculated by modeling the median value of each tertile as a continuous variable. In addition, we modeled log-transformed PFAS concentrations as continuous variables. Dose–response relationship of PFASs with T2D risk was evaluated using restricted cubic spline regressions with 3 knots after excluding participants in the lowest 2.5% and highest 2.5% of PFAS concentrations to minimize potential impact of outliers (Durrleman and Simon 1989), and p for nonlinearity was examined with a likelihood ratio test (LRT) comparing the model with linear term only to the model with the linear plus cubic spline terms.
Relevant secondary analyses were performed. We stratified the PFAS–T2D analyses by median time of follow-up (which was 2005) and by the median AHEI (which was 48). Statistical significance of effect modification (p for interaction) was evaluated by LRT, comparing models with and without interaction terms between tertiles of PFAS concentrations and the effect modifiers. Lastly, in a sensitivity analysis, we excluded batches with higher laboratory imprecision (within-batch CV ) and repeated the analysis using measured PFAS concentrations that were not adjusted for batch effects.
All p-values were two-sided. Data were analyzed with the Statistical Analysis Systems software package (version 9.4; SAS Institute, Inc.).
Results
The repeated PFAS measurements in two blood samples collected 1–2 y apart demonstrated high reproducibility: The ICCs were 0.91 for PFOS, 0.90 for PFOA, 0.94 for PFHxS, 0.87 for PFNA, and 0.82 for PFDA. Table 1 shows the characteristics of T2D cases and controls at the study baseline. Apart from matching factors, T2D cases were less likely to live in coastal states and had a high-risk profile, such as a higher BMI; lower physical activity; shorter breastfeeding duration; a poorer AHEI score; higher plasma concentrations of total cholesterol, triglycerides, HbA1c, and fasting insulin; and lower total adiponectin than controls. PFASs were detectable among all participants, with the exception of PFDA, which was not detected in seven cases and five controls. PFOS was the predominant PFAS with the highest average concentrations, whereas PFDA concentrations were the lowest in this population.
Table 1.
Characteristics of type 2 diabetes cases and controls at blood sample collection in the Nurses’ Health Study II.
| Characteristics | Cases () | Controls () | p-Valuea |
|---|---|---|---|
| Age at blood sample collection (y) | 0.97 | ||
| Caucasians (%) | 0.20 | ||
| Yes | 756 (95.3) | 766 (96.6) | |
| No | 37 (4.7) | 27 (3.4) | |
| Fasting at blood draw (%) | 0.21 | ||
| Yes | 522 (69.0) | 559 (71.9) | |
| No | 271 (31.0) | 234 (28.1) | |
| Menopausal status and hormone use (%) | |||
| Premenopause | 538 (67.8) | 538 (67.8) | |
| Postmenopausal, never used | 29 (3.7) | 29 (3.7) | |
| Postmenopausal, current or past user | 122 (15.4) | 122 (15.4) | |
| Missing | 104 (13.1) | 104 (13.1) | |
| States of residenceb | 0.03 | ||
| Coastal | 124 (15.6) | 164 (20.7) | |
| Great Lakes region | 442 (55.7) | 411 (51.8) | |
| Inland | 227 (28.6) | 218 (27.5) | |
| BMI () | |||
| Family history of diabetes (%) | |||
| Yes | 257 (32.4) | 132 (16.7) | |
| No | 536 (67.6) | 661 (83.3) | |
| Physical activity (MET-h/wk) | 0.001 | ||
| Smoking status (%) | |||
| Never smoked | 499 (62.9) | 515 (64.9) | 0.02 |
| Former smoker | 195 (24.6) | 212 (26.7) | |
| Current smoker | 99 (12.5) | 66 (8.3) | |
| Alcohol intake (%) | |||
| Abstainers | 350 (44.1) | 263 (33.3) | |
| 348 (43.9) | 376 (47.4) | ||
| 51 (6.4) | 87 (11.0) | ||
| 44 (5.6) | 67 (8.4) | ||
| Breastfeeding duration through blood draw (%) | 0.06 | ||
| Nulliparous or parous without breastfeeding | 287 (36.2) | 254 (32.0) | |
| 256 (32.3) | 245 (30.9) | ||
| 211 (26.6) | 259 (32.7) | ||
| Missing | 39 (4.9) | 35 (4.4) | |
| Number of births after 1993 | 0.38 | ||
| Use of oral contraceptive (%) | 0.03 | ||
| Current user | 106 (13.4) | 94 (11.9) | |
| Former user | 668 (84.2) | 661 (83.4) | |
| Never used | 19 (2.4) | 38 (4.8) | |
| AHEI score | |||
| Total cholesterol (g/L) | 2.14 (1.89–2.38) | 2.03 (1.80–2.28) | |
| Triglycerides (g/L) | 1.79 (1.26–2.59) | 1.06 (0.75–1.55) | |
| Total adiponectin (ng/mL)c | 4.19 (3.22–5.90) | 7.23 (5.22–9.93) | |
| HbA1c (%)c | 5.81 (5.58–6.13) | 5.41 (5.25–5.57) | |
| Fasting insulin ()c | 13.49 (8.06–19.46) | 6.00 (4.03–8.89) | |
| PFASs (ng/mL) | |||
| PFOS | 35.7 (26.4–48.3) | 33.1 (23.3–46.8) | 0.003 |
| PFOA | 4.96 (3.70–6.67) | 4.57 (3.35–6.16) | |
| PFHxS | 2.15 (1.35–3.79) | 2.01 (1.32–3.51) | 0.16 |
| PFNAa | 0.60 (0.44–0.85) | 0.61 (0.42–0.88) | 0.89 |
| PFDA | 0.13 (0.09–0.19) | 0.16 (0.11–0.23) |
Note: Continuous variables are shown as or median interquartile ranges (IQR), and categorical variables are shown as number (%). AHEI, alternative healthy eating index; BMI, body mass index; HbA1c, hemoglobin A1c; IQR, interquartile range; MET, metabolic equivalent task; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; SD, standard deviation.
p-Value estimates are based on Student’s t-test for variables expressed as , Wilcoxon rank–sum test for variables expressed as median (IQR), or Pearson test for variables expressed as percentages. Data are complete unless otherwise indicated.
Coastal states: AL, AK, CA, CT, DE, FL, GA, HI, ME, MD, MA, NH, NJ, NC, OR, RI, SC, VA, and WA; Great Lakes region states: IL, IN, MI, MN, NY, OH, PA, and WI; and inland states: all other states.
Missing data for cases: for HbA1c, for fasting insulin, and for PFNA; for controls: for total adiponectin, for HbA1c, for fasting insulin, and for PFNA.
In Table 2, we show the PFAS concentrations by age, states of residence, BMI, breastfeeding history, AHEI, and consumptions of seafood and popcorn among controls. After multivariate adjustment for covariates, increasing age correlated with higher PFHxS () but not with other PFASs. Participants living in inland states had higher PFHxS, PFNA, and PFDA concentrations than those living in coastal regions. None of the PFASs was associated with baseline BMI, except for PFDA that was associated with lower BMI. Longer breastfeeding duration was inversely associated with all PFASs except PFDA. Higher AHEI was associated with lower PFOS concentrations. More frequent seafood consumption was associated with higher PFHxS and PFNA concentrations, and higher popcorn intake was linked to higher PFOS concentrations. Correlation coefficients between other individual food items and PFAS concentrations were not significant after Bonferroni correction of p-values (see Table S2).
Table 2.
Plasma PFAS concentrations [median (IQR)] in ng/mL by demographic, lifestyle, and dietary factors among controls.
| Variable | PFOS | PFOA | PFHxS | PFNA | PFDA | |
|---|---|---|---|---|---|---|
| Age at baseline (y) | ||||||
| 105 | 32.9 (29.3–36.8) | 4.37 (3.89–4.92) | 2.06 (1.75–2.44) | 0.60 (0.53–0.67) | 0.16 (0.14–0.18) | |
| 40–50 | 555 | 34.1 (32.5–35.7) | 4.94 (4.71–5.18) | 2.22 (2.07–2.37) | 0.62 (0.59–0.65) | 0.16 (0.15–0.17) |
| 133 | 30.8 (27.7–34.2) | 4.22 (3.78–4.71) | 2.64 (2.26–3.09) | 0.66 (0.59–0.73) | 0.16 (0.14–0.18) | |
| p-Trenda | 0.39 | 0.60 | 0.04 | 0.28 | 0.92 | |
| States of residenceb | ||||||
| Coastal | 164 | 34.9 (32.1–38.0) | 4.62 (4.23–5.05) | 2.09 (1.85–2.37) | 0.66 (0.60–0.72) | 0.18 (0.16–0.20) |
| Lake | 411 | 32.8 (31.1–34.6) | 4.68 (4.43–4.95) | 2.22 (2.05–2.40) | 0.64 (0.61–0.68) | 0.16 (0.15–0.17) |
| p vs. Coastalc | 0.24 | 0.81 | 0.45 | 0.59 | 0.10 | |
| Inland | 218 | 33.1 (30.7–35.7) | 4.93 (4.56–5.33) | 2.49 (2.23–2.78) | 0.56 (0.52–0.61) | 0.14 (0.13–0.16) |
| p vs. Coastalc | 0.37 | 0.29 | 0.04 | 0.008 | 0.002 | |
| BMI at baseline [] | ||||||
| 455 | 32.5 (31.0–34.2) | 4.61 (4.37–4.85) | 2.18 (2.02–2.35) | 0.62 (0.59–0.65) | 0.17 (0.16–0.18) | |
| 25–30 | 211 | 34.5 (32.1–37.1) | 5.08 (4.71–5.49) | 2.40 (2.15–2.67) | 0.64 (0.60–0.70) | 0.16 (0.14–0.17) |
| 127 | 34.2 (31.1–37.6) | 4.65 (4.21–5.14) | 2.35 (2.04–2.70) | 0.60 (0.54–0.66) | 0.14 (0.13–0.16) | |
| p-Trenda | 0.23 | 0.4 | 0.21 | 0.81 | 0.008 | |
| Breastfeeding duration through blood draw (months)d | ||||||
| Nulliparous or parous without breastfeeding | 254 | 34.1 (31.8–36.4) | 5.05 (4.70–5.42) | 2.39 (2.17–2.65) | 0.67 (0.62–0.72) | 0.16 (0.15–0.18) |
| 245 | 35.6 (33.3–38.1) | 4.94 (4.60–5.31) | 2.36 (2.14–2.61) | 0.62 (0.58–0.67) | 0.16 (0.15–0.17) | |
| 259 | 30.7 (28.8–32.8) | 4.33 (4.03–4.64) | 2.01 (1.82–2.22) | 0.56 (0.53–0.61) | 0.15 (0.14–0.16) | |
| p-Trenda | 0.04 | 0.003 | 0.02 | 0.001 | 0.14 | |
| AHEI | ||||||
| Tertile 1, 38.8 (34.8–41.0) | 229 | 35.2 (32.8–37.8) | 4.82 (4.47–5.19) | 2.23 (2.01–2.48) | 0.59 (0.55–0.64) | 0.15 (0.14–0.16) |
| Tertile 2, 48.6 (45.8–49.9) | 255 | 33.8 (31.6–36.1) | 4.67 (4.36–5.01) | 2.40 (2.18–2.66) | 0.62 (0.58–0.67) | 0.16 (0.15–0.17) |
| Tertile 3, 58.4 (54.6–62.8) | 309 | 31.6 (29.7–33.6) | 4.73 (4.44–5.04) | 2.17 (1.98–2.38) | 0.64 (0.60–0.69) | 0.16 (0.15–0.18) |
| p-Trenda | 0.02 | 0.76 | 0.63 | 0.08 | 0.11 | |
| Seafood intake (servings/week) | ||||||
| Tertile 1, 0.47 (0.23–0.70) | 237 | 33.9 (31.6–36.4) | 4.87 (4.52–5.23) | 2.15 (1.94–2.38) | 0.57 (0.53–0.61) | 0.15 (0.14–0.16) |
| Tertile 2, 1.20 (0.97–1.43) | 310 | 32.9 (31.0–35.0) | 4.68 (4.39–4.99) | 2.10 (1.92–2.30) | 0.62 (0.58–0.66) | 0.16 (0.15–0.17) |
| Tertile 3, 2.47 (1.97–3.23) | 246 | 33.3 (31.1–35.6) | 4.68 (4.36–5.03) | 2.61 (2.36–2.88) | 0.67 (0.63–0.72) | 0.17 (0.16–0.18) |
| p-Trenda | 0.72 | 0.47 | 0.009 | 0.002 | 0.03 | |
| Popcorn intake (servings/week) | ||||||
| Tertile 1, 0.23 (0.22–0.47) | 293 | 29.7 (27.9–31.6) | 4.65 (4.36–4.97) | 2.46 (2.24–2.70) | 0.65 (0.60–0.69) | 0.16 (0.15–0.17) |
| Tertile 2, 0.73 (0.73–1.00) | 288 | 32.2 (30.3–34.2) | 4.58 (4.29–4.89) | 2.13 (1.94–2.34) | 0.60 (0.57–0.65) | 0.15 (0.14–0.16) |
| Tertile 3, 2.98 (2.00–3.50) | 212 | 40.9 (38.1–44.0) | 5.09 (4.71–5.49) | 2.18 (1.96–2.44) | 0.61 (0.56–0.66) | 0.16 (0.15–0.18) |
| p-Trenda | 0.11 | 0.08 | 0.24 | 0.96 |
Note: Least-squared means of batched-corrected plasma PFASs were estimated using generalized linear regression model with adjustment of age (in years), ethnicity (white, or others), time of blood draw (March–May, June–August, September–November, and December–February), fasting status (yes, or no), states of residence (coastal, Great Lakes region, or inland), family history of diabetes (yes, or no), menopausal status and postmenopausal hormone use (premenopause, postmenopause and never use hormone, postmenopause and former or current use hormone, or missing), oral contraceptive use (never used, past user, or current user), breastfeeding duration (nulliparous or parous without breastfeeding, , or ), number of children delivered after 1993 (0, 1, or ), BMI (, , 23.0–24.9, 25.0–29.9, 30.0–34.9, or ), and AHEI (alternative health eating index, in quintiles) without self-adjustment. AHEI was not adjusted for when modeling seafood or popcorn intake as predictors. AHEI, alternative healthy eating index; BMI, body mass index; IQR, interquartile range; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
p-Values for trend were calculated by modeling the ordinal values of categories as continuous variables.
Coastal states: AL, AK, CA, CT, DE, FL, GA, HI, ME, MD, MA, NH, NJ, NC, OR, RI, SC, VA, and WA; Great Lakes region states: IL, IN, MI, MN, NY, OH, PA, and WI; and inland states: all other states.
p-Values for between-group differences comparing the Great Lakes region (first) and inland (second) residents with coastal residents.
Missing data: missing because of women who did not remember breastfeeding duration or replied “pass through.”
Among controls, individual PFASs were significantly intercorrelated with one another ( ranging from 0.20 to 0.67; see Table S3). In general, PFAS concentrations were not significantly correlated with total adiponectin, fasting insulin, or HbA1c at baseline. We found significant correlations between certain PFASs and blood lipids, although none of these values was above 0.15.
In crude analyses where only the matching factors were considered in multivariate adjustment, PFOS and PFOA concentrations were significantly associated with a higher T2D risk (Table 3). In contrast, higher concentrations of PFDA were associated with a lower T2D risk in the crude model. After adjustment for other covariates, the associations were slightly attenuated but remained statistically significant for PFOS and PFOA; comparing extreme tertiles, the ORs were 1.62 [95% confidence interval (CI): 1.09, 2.41] and 1.54 (95% CI: 1.04, 2.28), respectively. The association for PFDA was attenuated toward the null after the same adjustment.
Table 3.
Associations between plasma PFAS concentrations and incident type 2 diabetes risk in the Nurses’ Health Study II.
| Exposure | Range (median) (ng/mL) | Cases () | Controls () | Model 1 [OR (95% CI)]a | Model 2 [OR (95% CI)]a |
|---|---|---|---|---|---|
| PFOS | |||||
| Tertile 1 | 6.04–26.3 (19.7) | 199 | 264 | Reference | Reference |
| Tertile 2 | 26.3–41.4 (33.1) | 285 | 265 | 1.50 (1.16, 1.94) | 1.63 (1.25, 2.12) |
| Tertile 3 | 41.4–421 (56.3) | 309 | 264 | 1.57 (1.07, 2.30) | 1.62 (1.09, 2.41) |
| p-Trendb | 0.02 | ||||
| PFOSc | 1.18 (1.06, 1.31) | 1.15 (0.98, 1.35) | |||
| p-Value | 0.002 | 0.08 | |||
| PFOA | |||||
| Tertile 1 | 0.99–3.76 (2.89) | 215 | 264 | Reference | Reference |
| Tertile 2 | 3.76–5.48 (4.57) | 262 | 265 | 1.24 (0.96, 1.60) | 1.27 (0.87, 1.86) |
| Tertile 3 | 5.48–112 (7.36) | 316 | 264 | 1.59 (1.23, 2.05) | 1.54 (1.04, 2.28) |
| p-Trendb | 0.0003 | 0.03 | |||
| PFOSc | 1.22 (1.09, 1.35) | 1.24 (1.06, 1.45) | |||
| p-Value | 0.0004 | 0.009 | |||
| PFHxS | |||||
| Tertile 1 | 0.32–1.49 (1.09) | 240 | 264 | Reference | Reference |
| Tertile 2 | 1.49–2.90 (2.01) | 277 | 265 | 1.09 (0.85, 1.40) | 1.15 (0.79, 1.67) |
| Tertile 3 | 2.91–429 (4.77) | 276 | 264 | 1.14 (0.88, 1.46) | 1.26 (0.86, 1.86) |
| p-Trendb | 0.33 | 0.24 | |||
| PFOSc | 1.08 (0.97, 1.20) | 1.05 (0.89, 1.23) | |||
| p-Value | 0.16 | 0.58 | |||
| PFNA | |||||
| Tertile 1 | 0.09–0.47 (0.37) | 240 | 264 | Reference | Reference |
| Tertile 2 | 0.47–0.77 (0.61) | 301 | 265 | 1.21 (0.94, 1.54) | 1.08 (0.75, 1.56) |
| Tertile 3 | 0.77–7.74 (1.05) | 252 | 264 | 1.00 (0.76, 1.31) | 0.99 (0.67, 1.48) |
| p-Trendb | 0.98 | 0.97 | |||
| PFOSc | 1.03 (0.92, 1.14) | 1.04 (0.89, 1.22) | |||
| p-Value | 0.60 | 0.62 | |||
| PFDA | |||||
| Tertile 1 | 0.01–0.13 (0.09) | 379 | 265 | Reference | Reference |
| Tertile 2 | 0.13–0.20 (0.16) | 247 | 264 | 0.62 (0.49, 0.79) | 0.91 (0.64, 1.32) |
| Tertile 3 | 0.20–1.95 (0.27) | 167 | 264 | 0.43 (0.33, 0.56) | 0.71 (0.48, 1.05) |
| p-Trendb | 0.09 | ||||
| PFOSc | 0.72 (0.65, 0.81) | 0.90 (0.77, 1.04) | |||
| p-Value | 0.16 |
Note: Model 1, conditioned on matching factors, including age, month of sample collection, fasting status, menopausal status and postmenopausal hormone use (premenopause, postmenopause and never use hormone, postmenopause and former or current use hormone, or missing). Model 2, further adjusted for family history of diabetes (yes, or no), oral contraceptive use (never used, past user, or current user), breastfeeding duration at blood draw (nulliparous or parous without breastfeeding, , or ) and number of children delivered after 1993 (0, 1, or ), states of residence (coastal, Great Lakes region, or inland), smoking status (never, former, or current), alcohol intake (abstainer, , , or ), physical activity (MET-h/wk, , 3–8.9, 9–17.9, 18–26.9, or ), baseline BMI (kg/m2, , 23.0–24.9, 25.0–29.9, 30.0–34.9, or ), and AHEI score (in quintiles). AHEI, alternative healthy eating index; BMI, body mass index; CI, confidence interval; MET, metabolic equivalent of task; OR, odds ratio; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Odds ratios according to batch-corrected PFAS tertiles were estimated using conditional logistic regression.
p-Values for trend were calculated by analyzing median PFAS values in each category as a continuous variable.
ORs for a 1-SD increase in -transformed PFASs, which were 0.2337381 for PFOS, 0.250798 for PFOA, 0.3580725 for PFHxS, 0.2636537 for PFNA, 0.2903877 for PFDA.
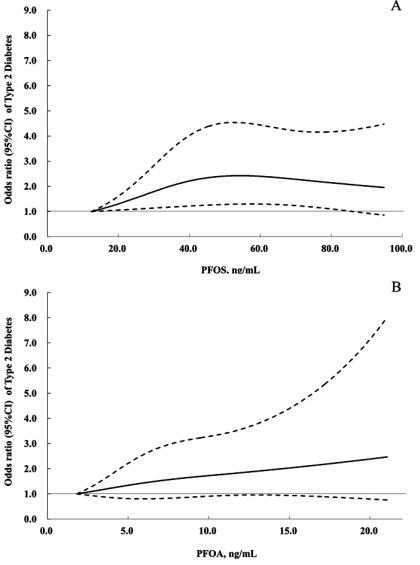
When modeling the associations for each SD increment of log-transformed PFASs, we found significantly higher T2D risk associated with PFOA [ (95% CI: 1.06, 1.45), ]. Dose–response curve (Figure 1A) demonstrated a positive relationship between PFOS concentrations and T2D risk () and a nonsignificant trend towards increased T2D risk at higher PFOA concentrations (Figure 1B).
Figure 1.
Dose–response relationships of PFOS (A) and PFOA (B) concentrations with type 2 diabetes risk in the Nurses’ Health Study II. The analysis was performed using restricted cubic spline regressions with 3 knots, and p for nonlinearity was examined by a likelihood ratio test comparing a model with a linear term only to the model with the linear plus cubic spline terms. Study participants with the lowest 2.5% and highest 2.5% of PFAS concentrations were excluded to minimize potential impact of outliers. Odds ratios (ORs) were estimated using cubic spline regression after adjusting for all covariates listed in the footnote of Table 3. PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid. The solid lines represents ORs, and the dotted lines 95% confidence intervals.
When we restricted the analysis to batches with low CVs (within-batch CV% ), PFOS and PFOA remained associated with higher T2D risk after adjusting for matching variables and confounding factors (Model 2; see Table S4). Stratified analysis did not find significant interaction between time of diabetes diagnosis and baseline PFAS concentrations, nor did we observe interactions between PFASs and AHEI score (see Table S5).
Discussion
In U.S. nurses without known occupational exposure to PFASs, we observed significant associations between higher baseline concentrations of these chemicals, especially PFOS and PFOA, in plasma and an increased T2D risk during follow-up for an average of 6.7 y.
In our analysis, age was not a significant predictor of PFAS concentrations, probably reflecting the fact that blood samples were collected before the peak plasma concentrations, that is, before the production of PFOS and PFOA was phased out by U.S. producers (Miralles-Marco and Harrad 2015). Unlike the lipophilic persistent organic pollutants, such as polychlorinated biphenyls that are significantly correlated with body adiposity (De Roos et al. 2012; Zong et al. 2015), BMI was not a predictor of PFAS concentrations in our study, as was the case among participants in the National Health and Nutrition Examination Survey (Nelson et al. 2010). In contrast, longer breastfeeding duration has been consistently associated with lower PFAS concentrations in women in the current and previous studies (Mondal et al. 2014; Thomsen et al. 2010; Zong et al. 2016). Diet has been thought to be one of the primary exposure routes to PFASs among general populations without occupational exposure history; one study reported as much as 61% of total PFAS exposure could be due to diet (Tittlemier et al. 2007). We also found that intakes of seafood, and popcorn were correlated with PFAS concentrations. Our findings are consistent with those from European countries (Ericson et al. 2008; Halldorsson et al. 2008; Tittlemier et al. 2007). However, due to differences in food production and consumption, data from other populations may not be extrapolated to Americans. Dietary sources of PFASs in the United States are only partly known (Domingo and Nadal 2017), and potential impacts of diet on observed associations between PFAS exposure and health outcomes remain to be elucidated.
The existing literature represents somewhat mixed evidence regarding the associations between PFAS exposure and diabetes risk. The largest cross-sectional investigation was from the C8 Health Project, in which estimated lifetime exposure showed no association with T2D risk or fasting glucose (Karnes et al. 2014), whereas plasma concentrations of PFOA, PFOS, PFNA, and PFHxS were inversely associated with prevalent diabetes (Conway et al. 2016; MacNeil et al. 2009). Conversely, a study among Wisconsin male anglers found that several PFASs were positively associated with prevalent prediabetes and diabetes (Christensen et al. 2016). Among Chinese living in Taiwan, a positive association of serum PFOS was seen with prevalent diabetes; however, PFOA, PFNA, and perfluoroundecanoic acid (PFUnA) showed inverse associations (Su et al. 2016). In terms of prospective evidence, Cardenas et al. (2017) found no significant association between PFASs and diabetes incidence () among 957 participants from the Diabetes Prevention Program trial, which might be explained by the strong effects of lifestyle intervention that could have dwarfed the potential impacts of other risk factors on diabetes risk. The European Youth Heart Study found that plasma PFOA concentrations at 9 y of age were associated with decreased function at adolescence (15 y of age), although not in early adulthood (21 y of age) (Domazet et al. 2016). Development of gestational diabetes was associated with the serum PFOA concentrations in pregnant women, although another study showed less clear associations (Matilla-Santander et al. 2017; Valvi et al. 2017; Zhang et al. 2015). These equivocal findings might be attributed to reverse causation by existing diabetes or prediabetes conditions linked to the cross-sectional study design used in most previous investigations. In addition, the dose–response relationship between PFAS exposures and diabetes incidence at different exposure concentrations is unknown. In the two studies reporting inverse associations of PFASs and T2D risk (Conway et al. 2016; Su et al. 2016), serum PFAS concentrations were much higher than those observed in the current and other previous studies (mean in the C8 Health Study, and mean PFOA, PFNA, and , 3.8, and , respectively, among Chinese adults). For the remaining studies (Cardenas et al. 2017; Christensen et al. 2016; Domazet et al. 2016; Matilla-Santander et al. 2017; Valvi et al. 2017; Zhang et al. 2015), serum concentrations of PFOA and PFOSs were similar to or lower than those in our study. Further studies are needed to elucidate dose–related diabetogenic effects of PFAS exposure, and whether such effects of PFAS exposure may be age and/or sex dependent.
Potential mechanisms underlying associations between PFASs and T2D risk are unclear. Experimental studies have found that PFASs activate (Rosen et al. 2008; Wolf et al. 2012) and (Buhrke et al. 2013; Rosen et al. 2008; Vanden Heuvel et al. 2006), which regulate energy homeostasis, lipid and glucose metabolism, and adipocyte differentiation and function (Berger and Moller 2002). Accumulating evidence has also suggested that PFASs may also interfere with human metabolism through PPAR-independent pathways. For example, PFOA altered expression of proteins in human liver cells that are regulated by hepatocyte nuclear factor (Scharmach et al. 2012), which is a key regulator of lipid metabolism and gluconeogenesis (Hayhurst et al. 2001; Rhee et al. 2003) and is involved in thyroid hormone homeostasis (Ohguchi et al. 2008). Recent evidence from in vitro studies has further demonstrated potentially estrogenic and antiestrogenic effects of PFASs (Henry and Fair 2013; Kraugerud et al. 2011). Furthermore, PFOA increases oxidative stress and mitochondrial dysfunction that lead to apoptosis and cytotoxicity in rat RIN-m5F cells (Suh et al. 2017). These mechanisms may contribute to the effects of subchronic, elevated exposures to PFASs on thyroid hormone homeostasis, liver toxicity, and body weight observed in animal experiments (Lau et al. 2007; Post et al. 2012). More recent studies also reported that perinatal PFOS exposure in rats led to abnormalities of glucose and lipid homeostasis at adulthood (Lv et al. 2013; Wan et al. 2014). In addition, 4-wk treatment of adult mice with PFOA interfered with glucose metabolism and induced insulin hypersensitivity (Yan et al. 2015). Another in vivo study in mice showed that PFOA induced histopathological changes in the pancreas through increasing oxidative stress (Kamendulis et al. 2014). In zebrafish, embryonic exposure to PFOS likewise led to abnormal pancreatic development (Sant et al. 2017). Although these studies explore different outcomes, in concert, they support that PFAS exposure may affect metabolic functions. However, given the apparent between-species differences in pharmacokinetics and tissue distribution of PFASs, as well as functional and structural differences in PPARs (Lau et al. 2007; Rakhshandehroo et al. 2009; Seacat et al. 2002, 2003), caution must be taken when extrapolating data regarding PFASs from animal studies to humans. We did not observe strong correlations between PFAS levels and established diabetes risk markers, such as adiponectin, insulin, or HbA1c, although the cross-sectional nature of these correlations excluded causal inference. Sporadic reports of human cross-sectional studies illustrated a possible link between PFASs exposures and altered thyroid or liver functions (Gallo et al. 2012; Knox et al. 2011; Melzer et al. 2010). Thus, impaired thyroid metabolism or liver functions may also play a role, whereas oxidative stress and estrogenic effects observed in experimental studies are among other possible modes of action that may explain the PFASs–T2D associations. In addition, it is possible that PFASs have stronger effects among individuals at a higher risk of diabetes (e.g., overweight) or during periods of weight change (e.g., growth spurts in childhood and puberty) (Domazet et al. 2016; Timmermann et al. 2014), which warrant investigations in future studies.
The prospective study design, high follow-up rate, good quality of self-reported diet, lifestyle, and medical history in this medically literate study population; multivariate adjustment for T2D risk factors and predictors of PFAS exposures; and rigorous laboratory quality control procedures are among the major strengths of the current study. A few caveats deserve to be considered. First, the generalizability may be limited to U.S. female health professionals and similar groups without an occupational PFAS exposure history. Second, although the homogeneity in regard to the socioeconomic status may render the study population less subject to confounding, we cannot rule out the possibility that the observed associations could be partially due to residual or unmeasured confounding, especially by other pollutants that may be correlated with PFASs. Third, we assessed PFAS concentrations in one single blood sample and thus cannot exclude the possibility that PFAS long-term exposure concentrations might be somewhat misclassified, despite our pilot study demonstrating excellent reproducibility of the PFASs in blood samples collected 1–2 y apart. Such reproducibility is likely due to the long elimination half-lives of PFASs in the human circulation (Olsen et al. 2007) and perhaps also a fairly stable, continuous exposure through diet, drinking water, and consumer products in the population studied. Fourth, we did not measure fasting glucose, thyroid hormones, estrogen metabolites, or other potential mediators that could possibly link PFAS exposure with T2D and therefore cannot determine whether the positive associations may be linked with these potential pathways.
In conclusion, higher baseline plasma concentrations of PFOS and PFOA were associated with an elevated incidence of T2D in a nested case–control study population of U.S. women followed from 1995 to 2011. These associations warrant attention in prospective cohort studies and in mechanistic studies to elucidate the dose–dependent links between PFASs exposure and T2D risk. Although the production of PFOS and PFOA has been voluntarily phased out in the United States, their persistence in the environment and in humans renders them a lasting concern in regard to human metabolic health.
Supplemental Material
Acknowledgments
This study was funded by National Institutes of Health (NIH) research grants CA87969, CA49449, DK58845, DK58785, DK082486, CA50385, CA67262, ES021372, and ES022981. Q.S. is supported by NIH grant HL035464, and P.G., Q.S., and D.V. are supported by NIH grant P42ES 027706.
Reference List
- Berger J, Moller DE. 2002. The mechanisms of action of PPARs. Annu Rev Med 53:409–435, PMID: 11818483, 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Buhrke T, Kibellus A, Lampen A. 2013. In vitro toxicological characterization of perfluorinated carboxylic acids with different carbon chain lengths. Toxicol Lett 218(2):97–104, PMID: 23391484, 10.1016/j.toxlet.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: 18007991, 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, et al. 2017. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the Diabetes Prevention Program trial. Environ Health Perspect 125(10):107001, PMID: 28974480, 10.1289/EHP1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162, PMID: 21054169, 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. 2012. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142(6):1009–1018, PMID: 22513989, 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Thompson BA, Anderson HA. 2016. Perfluoroalkyl substances in older male anglers in Wisconsin. Environ Int 91:312–318, PMID: 27003842, 10.1016/j.envint.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Conway B, Innes KE, Long D. 2016. Perfluoroalkyl substances and beta cell deficient diabetes. J Diabetes Complications 30(6):993–998, PMID: 27311784, 10.1016/j.jdiacomp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos AJ, Ulrich CM, Sjodin A, McTiernan A. 2012. Adiposity, body composition, and weight change in relation to organochlorine pollutant plasma concentrations. J Expo Sci Environ Epidemiol 22(6):617–624, PMID: 22588213, 10.1038/jes.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. 2016. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart Study. Diabetes Care 39(10):1745–1751, PMID: 27489335, 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. 2017. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem 65(3):533–543, PMID: 28052194, 10.1021/acs.jafc.6b04683. [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R. 1989. Flexible regression models with cubic splines. Stat Med 8(5):551–561, PMID: 2657958, 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Ericson I, Martí-Cid R, Nadal M, Van Bavel B, Lindström G, Domingo JL. 2008. Human exposure to perfluorinated chemicals through the diet: intake of perfluorinated compounds in foods from the Catalan (Spain) market. J Agric Food Chem 56(5):1787–1794, PMID: 18251500, 10.1021/jf0732408. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. 1993. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 93(7):790–796, PMID: 8320406, 10.1016/0002-8223(93)91754-E. [DOI] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, et al. 2012. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 120(5):655–660, PMID: 22289616, 10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, et al. 1991. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 133(8):810–817, PMID: 2021148, 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. 2008. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol 42(23):8971–8977, PMID: 19192827, 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. 2009. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A 1216(3):385–393, PMID: 19026423, 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. 2001. Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21(4):1393–1403, PMID: 11158324, 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold R, Schug TT. 2015. Endocrine disruptors and obesity. Nat Rev Endocrinol 11(11):653–661, PMID: 26391979, 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- Henry ND, Fair PA. 2013. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J Appl Toxicol 33(4):265–272, PMID: 21935973, 10.1002/jat.1736. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Aiba K, Fukuda K, Tanaka M. 1985. The induction of peroxisome proliferation in rat liver by perfluorinated fatty acids, metabolically inert derivatives of fatty acids. J Biochem 98(2):475–482, PMID: 4066651, 10.1093/oxfordjournals.jbchem.a135302. [DOI] [PubMed] [Google Scholar]
- Jian JM, Guo Y, Zeng L, Liang-Ying L, Lu X, Wang F, et al. 2017. Global distribution of perfluorochemicals (PFCs) in potential human exposure source–a review. Environ Int 108:51–62, PMID: 28800414, 10.1016/j.envint.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, et al. 2005. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A 102(17):6207–6212, PMID: 15833818, 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamendulis LM, Wu Q, Sandusky GE, Hocevar BA. 2014. Perfluorooctanoic acid exposure triggers oxidative stress in the mouse pancreas. Toxicol Rep 1:513–521, PMID: 28962265, 10.1016/j.toxrep.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnes C, Winquist A, Steenland K. 2014. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ Res 128:78–83, PMID: 24299613, 10.1016/j.envres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: 21469664, 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kennedy GL, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, et al. 2004. The toxicology of perfluorooctanoate. Crit Rev Toxicol 34(4):351–384, PMID: 15328768, 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, et al. 2010. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the nurses’ health studies. Cancer Epidemiol Biomark Prev, 19(4):938–946, PMID: 20332276, 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SS, Jackson T, Frisbee SJ, Javins B, Ducatman AM. 2011. Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J Toxicol Sci 36(4):403–410, PMID: 21804304, 10.2131/jts.36.403. [DOI] [PubMed] [Google Scholar]
- Kraugerud M, Zimmer KE, Ropstad E, Verhaegen S. 2011. Perfluorinated compounds differentially affect steroidogenesis and viability in the human adrenocortical carcinoma (H295R) in vitro cell assay. Toxicol Lett 205(1):62–68, PMID: 21641976, 10.1016/j.toxlet.2011.05.230. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lauritzen HB, Larose TL, Øien T, Odland JØ, van de Bor M, Jacobsen GW, et al. 2016. Factors associated with maternal serum levels of perfluoroalkyl substances and organochlorines: a descriptive study of parous women in Norway and Sweden. PLoS One 11(11):e0166127, PMID: 27824939, 10.1371/journal.pone.0166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR Jr, Vandenberg LN. 2014. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 35(4):557–601, PMID: 24483949, 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Wen LL, Chu PL, Lin CY. 2018. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ Pollut 232:73–79, PMID: 28923343, 10.1016/j.envpol.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, et al. 2013. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol 28(9):532–542, PMID: 23983163, 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- MacNeil J, Steenland NK, Shankar A, Ducatman A. 2009. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA). Environ Res 109(8):997–1003, PMID: 19740462, 10.1016/j.envres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. 1991. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 338(8770):774–778, PMID: 1681160, 10.1016/0140-6736(91)90664-B. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Martinez D, Ibarluzea J, et al. 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: 27132161, 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Matilla-Santander N, Valvi D, Lopez-Espinosa MJ, Manzano-Salgado CB, Ballester F, Ibarluzea J, et al. 2017. Exposure to perfluoroalkyl substances and metabolic outcomes in pregnant women: evidence from the Spanish INMA birth cohorts. Environ Health Perspect 125(11):117004, PMID: 29135438, 10.1289/EHP1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. 2010. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect 118(5):686–692, PMID: 20089479, 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles-Marco A, Harrad S. 2015. Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution. Environ Int 77:148–159, PMID: 25728452, 10.1016/j.envint.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, et al. 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect 122(2):187–192, PMID: 24280536, 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118(2):197–202, PMID: 20123614, 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguchi H, Tanaka T, Uchida A, Magoori K, Kudo H, Kim I, et al. 2008. Hepatocyte nuclear factor 4α contributes to thyroid hormone homeostasis by cooperatively regulating the type 1 iodothyronine deiodinase gene with GATA4 and Krüppel-like transcription factor 9. Mol Cell Biol 28(12):3917–3931, PMID: 18426912, 10.1128/MCB.02154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, et al. 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environ Res 157:87–95, PMID: 28528142, 10.1016/j.envres.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Picó Y, Farré M, Llorca M, Barceló D. 2011. Perfluorinated compounds in food: a global perspective. Crit Rev Food Sci Nutr 51(7):605–625, PMID: 21793724, 10.1080/10408391003721727. [DOI] [PubMed] [Google Scholar]
- Post GB, Cohn PD, Cooper KR. 2012. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 116:93–117, PMID: 22560884, 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Breslow NE. 1978. Retrospective studies and failure time models. Biometrika 65(1):153–158, 10.1093/biomet/65.1.153. [DOI] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839, PMID: 9529258, 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rakhshandehroo M, Hooiveld G, Muller M, Kersten S. 2009. Comparative analysis of gene regulation by the transcription factor PPARα between mouse and human. PLoS One 4(8):e6796, PMID: 19710929, 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, et al. 2003. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc Natl Acad Sci USA 100(7):4012–4017, PMID: 12651943, 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, et al. 2008. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPARα and CAR. Toxicol Sci 103(1):46–56, PMID: 18281256, 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, Falkner B. 2008. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 167(6):653–666, PMID: 18230679, 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. 2006. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 8(5):538–554, PMID: 16918589, 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. 1989. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 18(4):858–867, PMID: 2621022, 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- Sant KE, Jacobs HM, Borofski KA, Moss JB, Timme-Laragy AR. 2017. Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio. Environ Pollut 220(pt B):807–817, PMID: 27810111, 10.1016/j.envpol.2016.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmach E, Buhrke T, Lichtenstein D, Lampen A. 2012. Perfluorooctanoic acid affects the activity of the hepatocyte nuclear factor 4 alpha (HNF4α). Toxicol Lett 212(2):106–112, PMID: 22609092, 10.1016/j.toxlet.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, et al. 2003. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183(1–3):117–131, PMID: 12504346. [DOI] [PubMed] [Google Scholar]
- Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL. 2002. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol Sci 68(1):249–264, PMID: 12075127, 10.1093/toxsci/68.1.249. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB, Kannan K. 2007. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environ Sci Technol 41(4):1180–1185, PMID: 17593716. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Puigserver P, Wu Z. 2000. Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int J Obes Relat Metab Disord 24(suppl 4):S8–S10, PMID: 11126248, 10.1038/sj.ijo.0801492. [DOI] [PubMed] [Google Scholar]
- Su TC, Kuo CC, Hwang JJ, Lien GW, Chen MF, Chen PC. 2016. Serum perfluorinated chemicals, glucose homeostasis and the risk of diabetes in working-aged Taiwanese adults. Environ Int 88:15–22, PMID: 26700417, 10.1016/j.envint.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Suh KS, Choi EM, Kim YJ, Hong SM, Park SY, Rhee SY, et al. 2017. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol Med Rep 15(6):3871–3878, PMID: 28440430, 10.3892/mmr.2017.6452. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Haug LS, Stigum H, Frøshaug M, Broadwell SL, Becher G. 2010. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ Sci Technol 44(24):9550–9556, PMID: 21090747, 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- Timmermann CA, Rossing LI, Grøntved A, Ried-Larsen M, Dalgård C, Andersen LB, et al. 2014. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab 99(4):E608–E614, PMID: 24606078, 10.1210/jc.2013-3460. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao XL, et al. 2007. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J Agric Food Chem 55(8):3203–3210, PMID: 17381114, 10.1021/jf0634045. [DOI] [PubMed] [Google Scholar]
- Trier X, Granby K, Christensen JH. 2011. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int 18(7):1108–1120, PMID: 21327544, 10.1007/s11356-010-0439-3. [DOI] [PubMed] [Google Scholar]
- Valvi D, Oulhote Y, Weihe P, Dalgård C, Bjerve KS, Steuerwald U, et al. 2017. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int 107:205–215, PMID: 28753482, 10.1016/j.envint.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci 92(2):476–489, PMID: 16731579, 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Nielsen F, Andersson AM, Hjollund NH, Grandjean P, Andersen HR, et al. 2012. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod 27(3):873–880, PMID: 22246448, 10.1093/humrep/der450. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Leung PY, Wong CK. 2014. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. PLoS One 9(1):e87137, PMID: 24498028, 10.1371/journal.pone.0087137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, et al. 2012. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 95(4):925–933, PMID: 22357723, 10.3945/ajcn.111.028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC. 1998. Nutritional Epidemiology. 2nd Edition. New York, NY:Oxford University Press. [Google Scholar]
- Wolf CJ, Schmid JE, Lau C, Abbott BD. 2012. Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARα) by perfluoroalkyl acids (PFAAs): further investigation of C4–C12 compounds. Reprod Toxicol 33(4):546–551, PMID: 22107727, 10.1016/j.reprotox.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, et al. 2013. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the Nurses' Health Study and meta-analysis. Environ Health Perspect 121(2):153–161, PMID: 23131992, 10.1289/ehp.1205248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Spiegelman BM. 1999. Transcriptional activation of adipogenesis. Curr Opin Cell Biol 11(6):689–694, PMID: 10600710. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhang H, Zheng F, Sheng N, Guo X, Dai J. 2015. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci Rep 5:11029, PMID: 26066376, 10.1038/srep11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. 2015. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril 103(1):184–189, PMID: 25450302, 10.1016/j.fertnstert.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong G, Grandjean P, Wang X, Sun Q. 2016. Lactation history, serum concentrations of persistent organic pollutants, and maternal risk of diabetes. Environ Res 150:282–288, PMID: 27336232, 10.1016/j.envres.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong G, Grandjean P, Wu H, Sun Q. 2015. Circulating persistent organic pollutants and body fat distribution: evidence from NHANES 1999-2004. Obesity (Silver Spring) 23(9):1903–1910, PMID: 26237202, 10.1002/oby.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.