 Tropomyosin receptor kinases receptor C is expressed at high levels on the surface of tumors from metastatic breast cancer, metastatic melanoma, glioblastoma, and neuroblastoma.
Tropomyosin receptor kinases receptor C is expressed at high levels on the surface of tumors from metastatic breast cancer, metastatic melanoma, glioblastoma, and neuroblastoma.
Abstract
Tropomyosin receptor kinases receptor C is expressed at high levels on the surface of tumors from metastatic breast cancer, metastatic melanoma, glioblastoma, and neuroblastoma. Previous studies have shown synthetic TrkC ligands bearing agents for photodynamic therapy could be used to completely ablate 4T1 metastatic breast tumors and suppress metastatic spread in vivo. Modification of these probes (A in the text) to make them suitable for near infrared optical imaging in vivo would require a substantial increase in molecular mass (and hence increased vulnerability to undesirable absorption, metabolism and immunogenicity effects), or significant changes to the probe design which might compromise binding to TrkC in histochemical studies and on live cells. The research featured here was undertaken to investigate if the second strategy could be achieved without compromising binding to TrkC-expressing tissues. Specifically, an “aza-BODIPY” probe was synthesized to replace a spacer fragment in the original probe A. In the event, this new probe design (1a in the text) binds TrkC+ breast cancer in live cell cultures, in histochemical studies and in an in vivo murine model. Probe 1a binds TrkC+ tissues with good contrast with respect to healthy tissues, and much more strongly than an isomeric, non-TrkC binding, probe (1b) prepared as a negative control.
Introduction
Tropomyosin receptor kinases (Trk's) are cell-surface receptors that bind the neurotrophin growth hormones.1 Docking neurotrophin-3 (NT3) with TrkC, for instance, triggers intracellular phosphorylation then a network of cell signaling processes that lead to cell growth and differentiation.2,3 Over-expression of Trk receptors is associated with several forms of cancer, including neuroblastoma,4–16 glioblastoma,14,17–21 metastatic breast cancer22–28 and metastatic melanoma.29–37 Consequently, functionalized Trk ligands may be prepared as possible imaging agents for cancer.28,38–40 Synthetic TrkB-41 and pan-Trk-binding agents16 functionalized with radionuclei have been explored for imaging with positron emission tomography (PET), but, to the best of our knowledge, no synthetic imaging agents have been reported for selective optical-imaging of TrkC in vivo.
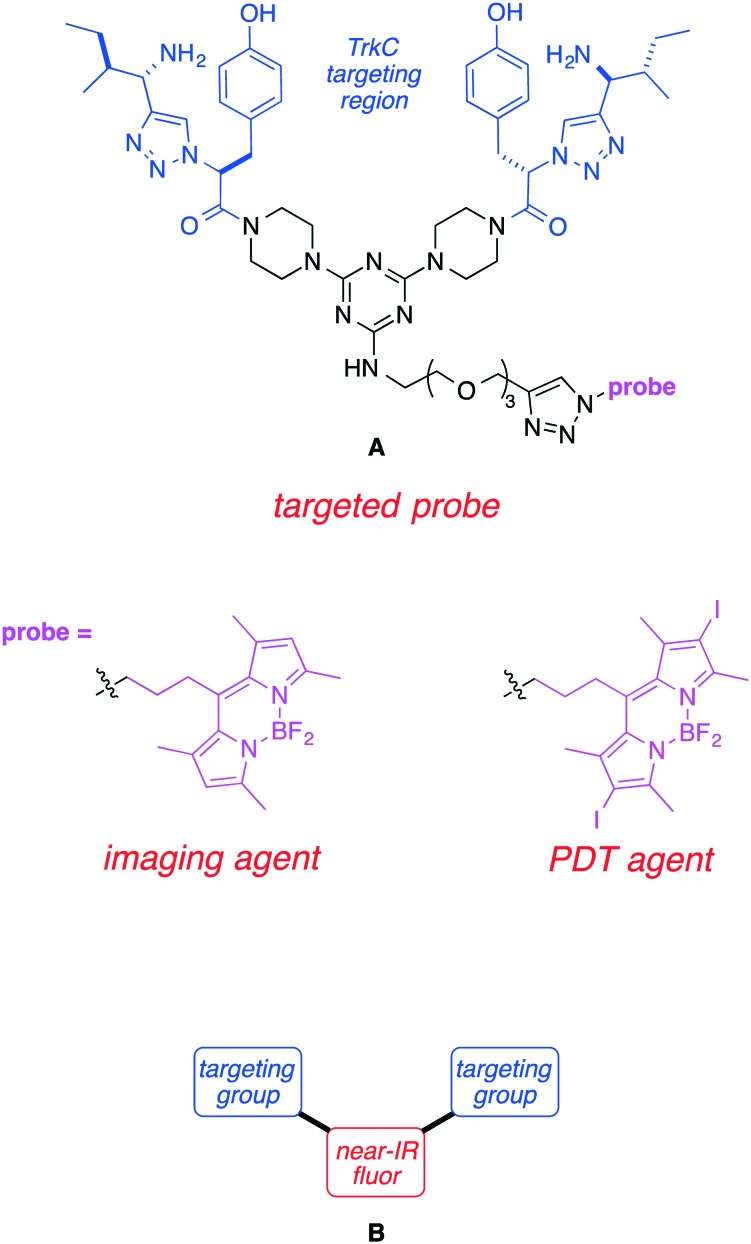
Prior research from these laboratories featured the TrkC-homing ligand in structure A. Probe A was shown to be internalized by TrkC-expressing cells and it could also be used as a staining agent for metastatic breast cancer and melanoma tissue, both of which are associated with TrkC overexpression. Moreover, one 10 mg kg–1 dose of A coupled to a BODIPY-based PDT agent was shown to almost completely ablate a 4T1 primary tumor, and suppress metastatic spread.42
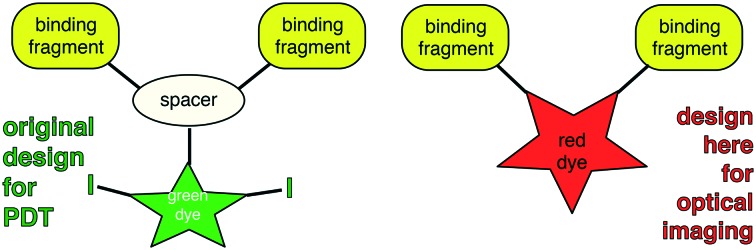
Probe A is not ideal for optical imaging because it absorbs light optimally at a wavelength (around 500 nm) that is too short for efficient penetration of more than a few mm in tissue.43,44 To circumvent this, the most obvious solution would be to prepare similar molecules with a near-IR-absorbing probe directly replacing the BODIPY. However, modifications to fluors to make them near-IR absorbing invariably increase their molecular size; this is undesirable because larger structures tend to be more vulnerable to undesirable absorption, metabolism, and excretion effects. We reasoned that one way to reconcile these opposing design criteria is to replace the black part of structure A with a near-IR fluor giving the generic structures B (Fig. 1). Designs B eliminate the triazine core which serves no purpose other than as a scaffold-spacer. One possibility would be to replace it with an aza-BODIPY dye; these dyes tend to have significantly longer absorption and emission wavelength maxima than their BODIPY analogs.45–47
Fig. 1. First generation TrkC targeting ligand-conjugate A and the design concept in this paper B.
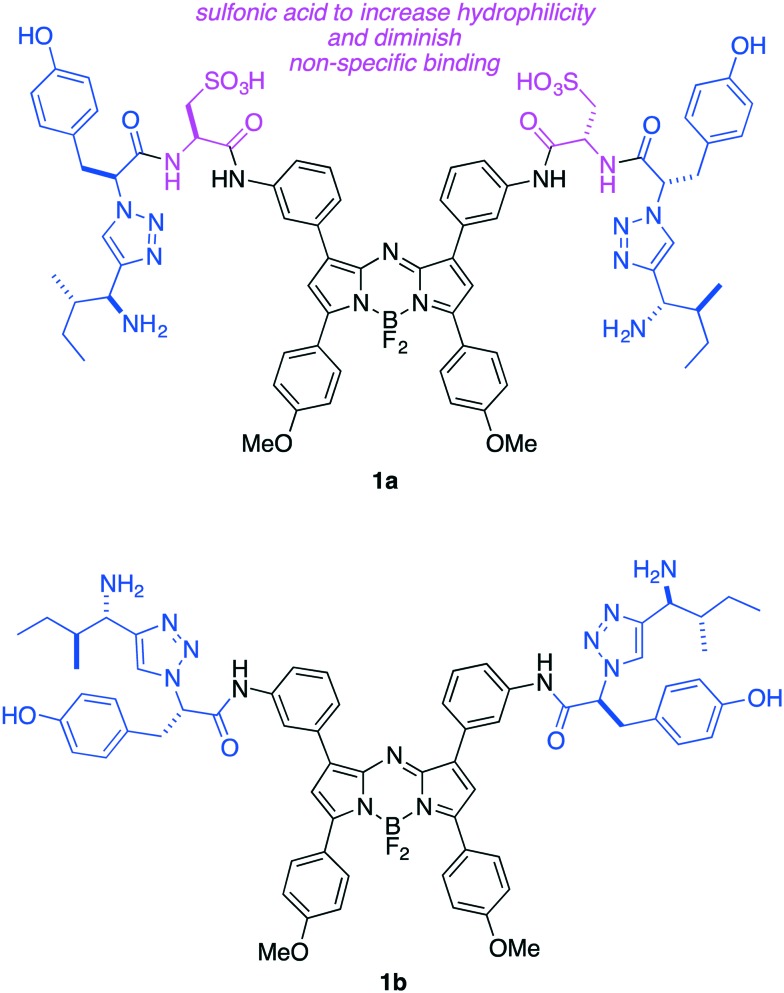
Based on the considerations outlined above, we designed aza-BODPY dyes 1a and b (Fig. 2). Probe 1a was appealing because the sulfonic acid groups would enhance the hydrophilicity of the molecule and their negative charge might reduce non-specific binding to cells. Conversely, 1b has the advantages of smaller size, and more facile synthetic access. We anticipated the best of these two probe candidates, based on solubility and spectroscopic experiments, would be tested for: (i) binding and internalization by TrkC-expressing cells; (ii) staining TrkC-expressing fixed tissue (histochemistry); and, (iii) comparative distribution in healthy mice and ones bearing TrkC-expressing breast cancer.
Fig. 2. Second generation TrkC targeting ligands 1a and 1b featured in this work.
Results and discussion
Probe syntheses
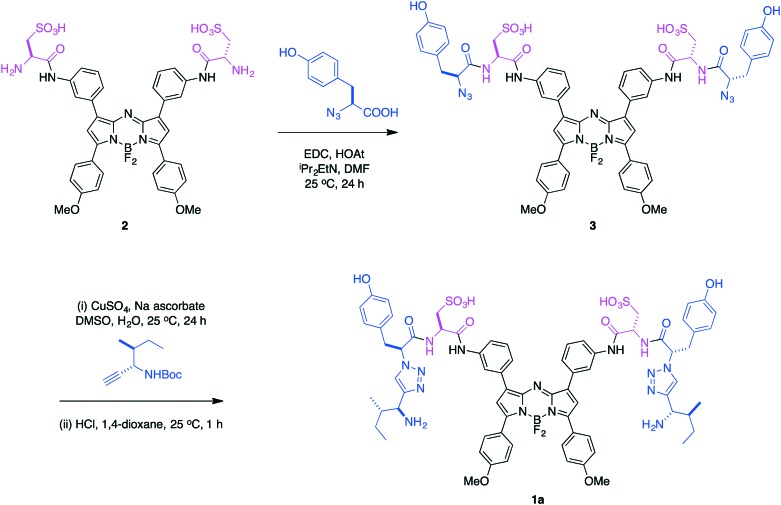
Coupling of the azido acid from l-tyrosine48 with the disulfonated aza-BODIPY 2, prepared as described,49 gave the diazide 3 (Scheme 1). A copper-mediated cycloaddition50,51 of 3 with the alkyne derived from l-isoleucine48 gave the desired product 1a. Probe 1b was prepared via an analogous route, but beginning with a diamine aza-BODIPY precursor to 2; a scheme and full details of that synthesis are given in the ESI.†
Scheme 1. Preparation of TrkC-targeted near IR probe 1a.
Distribution of probes 1a and b in TrkC+ murine breast cancer cells
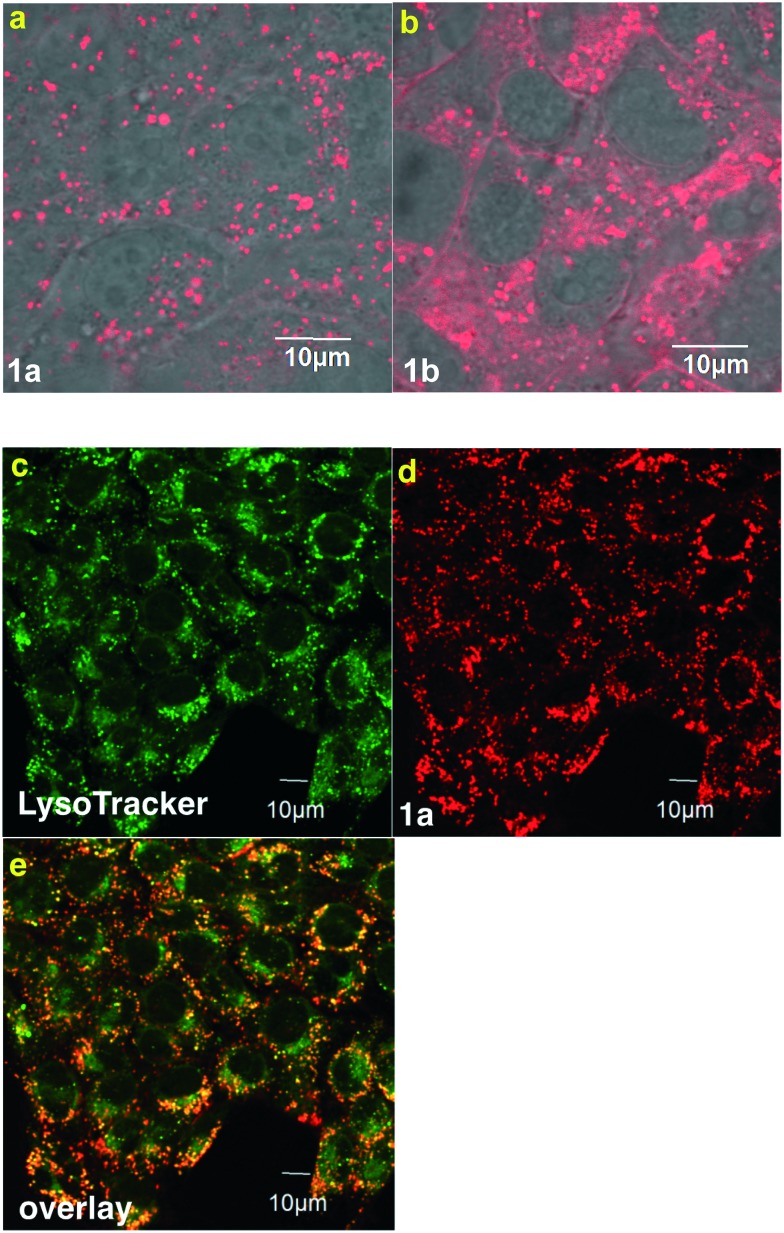
TrkC-expressing, 4T1 murine breast cancer cells were treated with probes 1a and b. Confocal microscopy (Fig. 3a) showed the sulfonated system 1a was efficiently internalized whereas the more lipophilic system 1b had poor solubility and appeared to give significantly more cell-surface binding (Fig. 3b). For both probes, the material that was internalized overlaid with a lysosome tracker, indicating it accumulated in that organelle. These data support the assertion that the sulfonic acid groups of 1a reduce non-specific binding to TrkC+ cells relative to the neutral probe 1b, as expected because of repulsion between the negatively charged sulfonic acids and polar head groups at the cell surface.52 Probe 1a showed no sign of toxicity to 4T1 cells at concentrations up to and including 25 μM (Fig. S1†). All the subsequent work in this research was focused on 1a, and studies of 1b were discontinued, because these data indicate 1a may have better characteristics for non-specific binding.
Fig. 3. Imaging with 4T1 cells. a Probe 1a was internalized; whereas, b much more of 1b accumulated in the cell walls (overlay images of fluorescent probes and bright field imaging). c LysoTracker and probe 1a (d) colocalized as shown in e.
Histochemistry
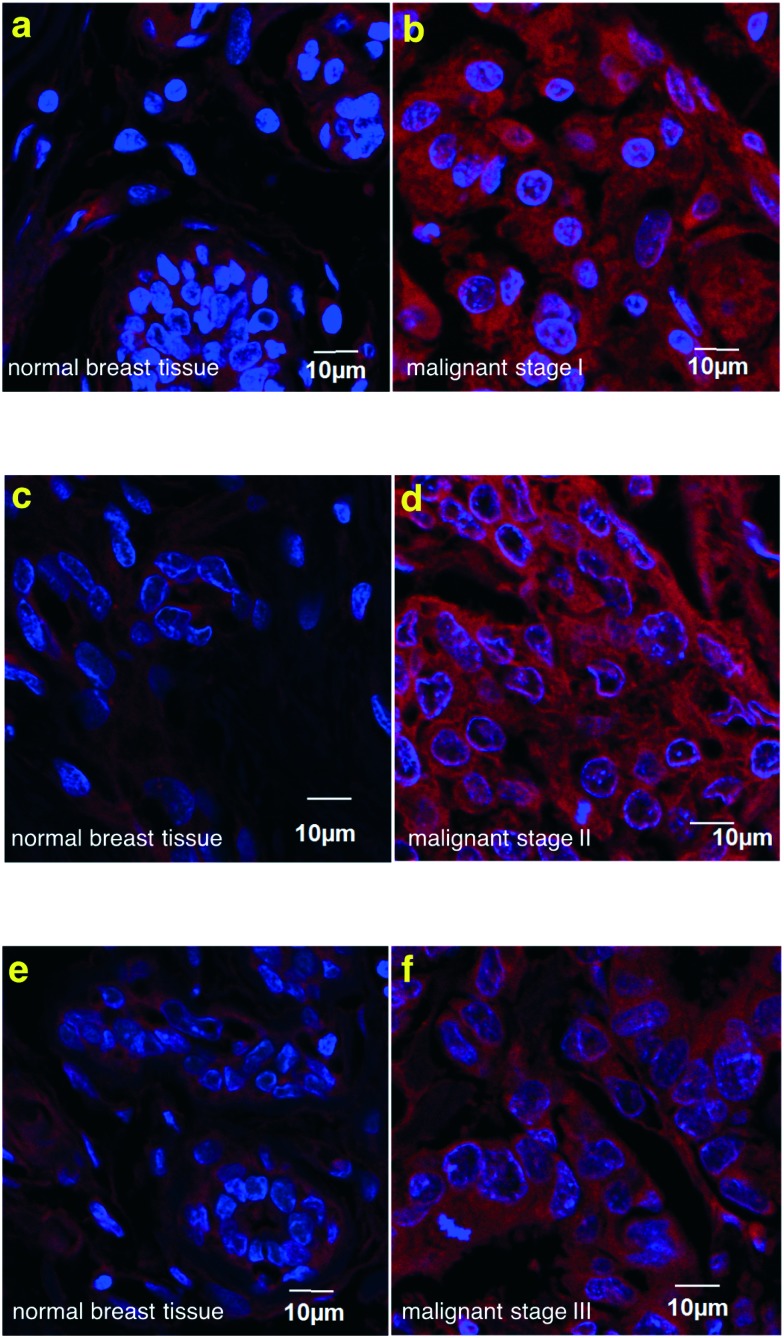
Commercial samples of fixed human tissue from patients with stage 1, 2, and 3 malignant breast cancer were purchased (US Biomax). Probe 1a was used to treat these samples and the staining was compared to that observed for normal breast tissue proximal to the tumor, obtained from the same patient in each case; illustrative data are shown in Fig. 4. In all cases the tumor tissue stained strongly whereas the healthy samples showed much less fluorescence. We were encouraged by the selectivity, but slightly surprised that the healthy tissue showed faint staining. It appears that this is genuine binding to the TrkC receptor in the healthy tissue (rather than non-specific binding or fluorescence leakage into the red channel) because fluorescently labeled TrkC mAb also stained weakly the normal tissue (Fig. S2†). This may mean the tumor environment is causing the surrounding normal tissue to upregulate TrkC expression.
Fig. 4. Histochemistry on human breast tissue array. Probe 1a stained on breast cancer tissue (b, d, f) much brighter than matched adjacent breast tissue control (a, c, e), (example from 3 cases).
Optical imaging with 1ain vivo
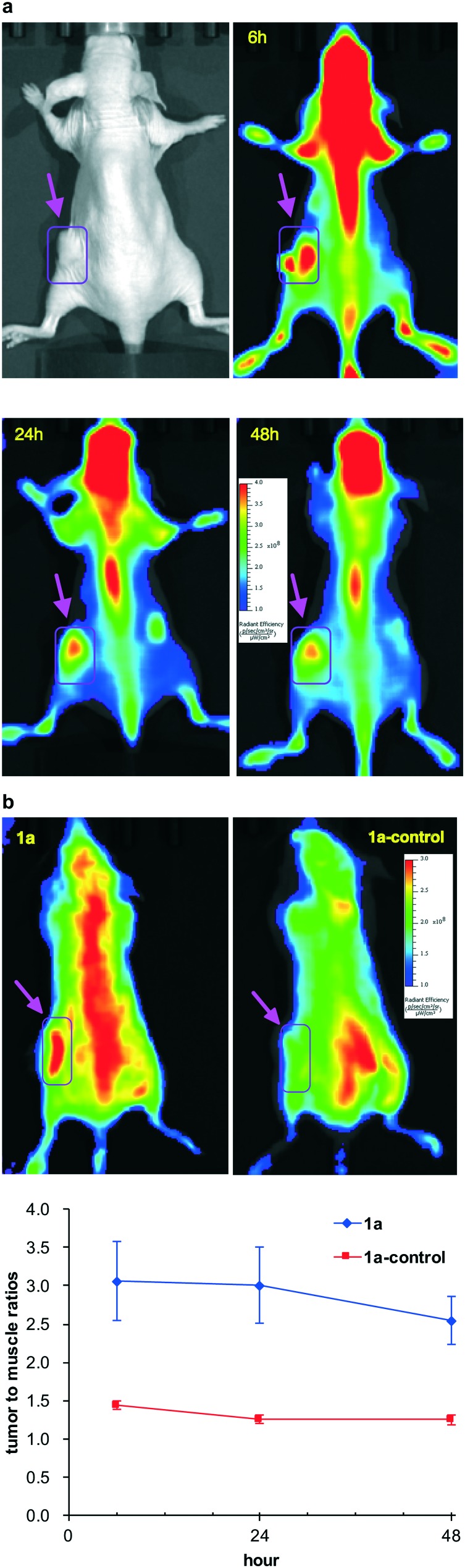
A 4T1 breast cancer xenograft model was used (female BALB/c nu/nu mice, 8–10 mm diameter 4T1 tumors, iv administration) to evaluate 1a as an in vivo optical imaging probe. Fig. 5 showed the NIR fluorescence images of nude mice bearing orthotopic 4T1 tumors after intravenous injection of 10 nmol of 1a. The 4T1 tumors were clearly visualized with good tumor to background contrast at all time points (6, 24 and 48 h). As reported, Trk receptors are a family of tyrosine kinases that regulates synaptic strength and plasticity in the mammalian nervous system. TrkC is ordinarily activated by binding with NT-3 and is expressed by proprioceptive sensory.53 We observed high uptake of 1a in central nervous system (CNS) which correlated with the expression of TrkC receptor.
Fig. 5. a In vivo NIR fluorescence images (at 6 h, 24 h, and 48 h p.i.) of 4T1 tumor-bearing mice injected (iv) with 1a. Arrows indicate the tumors. b Representative NIR fluorescent images of mice injected with 1a (left) or 1a-control compound at 48 h p.i. (right). The arrows indicate the tumors (top); an ROI study of tumor/normal tissue (muscle) ratio of 1a and 1a-control in the imaging groups (n = 3).
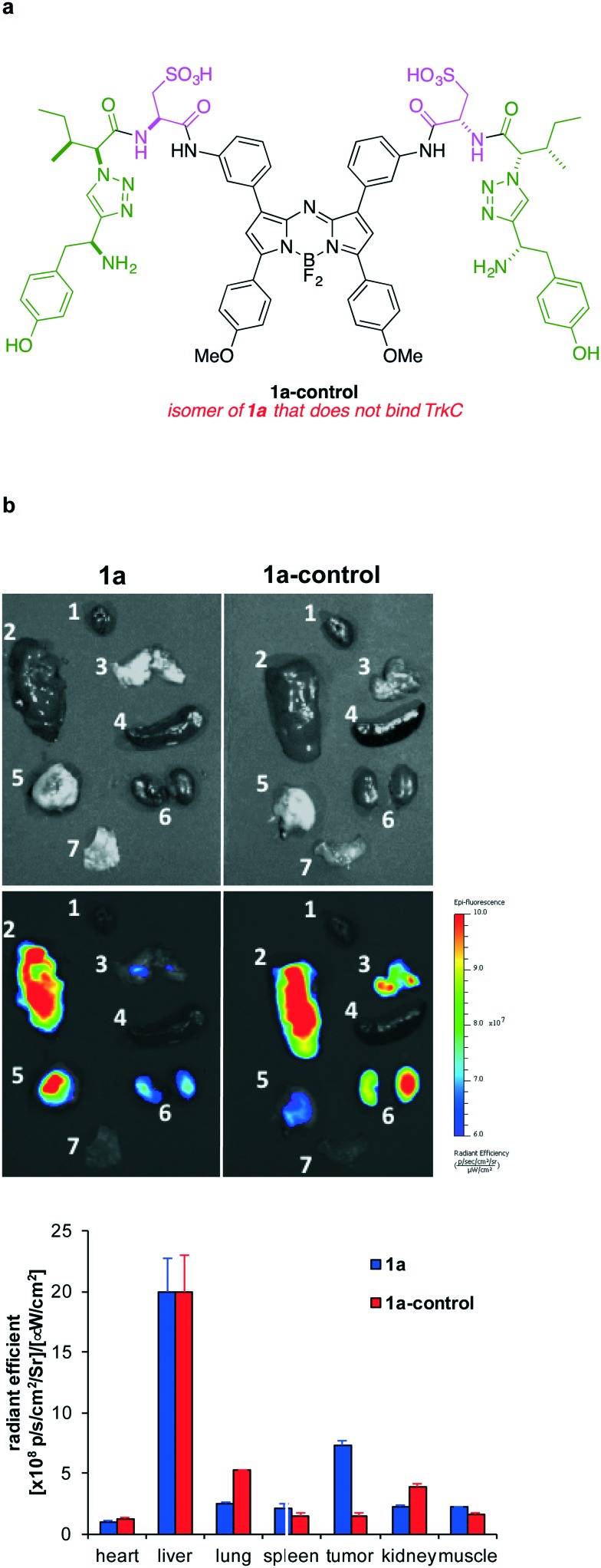
To validate the TrkC targeting specificity of 1a, the scrambled negative control, 1a-control, was prepared (structure in Fig. 6 and Scheme S2†) and tested for in vivo imaging and ex vivo studies (Fig. 5 and 6). 4T1 tumor uptake of the negative control was much lower at all time points comparing to 1a, which confirmed the specific TrkC targeting. As shown in Fig. 5b, the tumor/normal tissue fluorescent signal ratios in the imaging group were 3.06 ± 0.88 at 6 h, 3.01 ± 0.85 at 24 h, and 2.54 ± 0.55 at 48 h p.i., respectively, which is 2–3 times higher than that of the control group (1.44 ± 0.09 at 6 h, 1.26 ± 0.08 at 24 h, and 1.21 ± 0.19 at 48 h p.i.).
Fig. 6. a Structure of 1a-control. b Representative ex vivo fluorescent images of organs (1 heart, 2 liver, 3 lungs, 4 spleen, 5 tumor, 6 kidneys, 7 muscle) collected from the mice injected with 1a and 1a-control at 48 h p.i.; and, quantification of fluorescence in those organs (n = 3).
Ex vivo imaging was performed to acquire the fluorescent images of internal organs for both imaging and control groups as shown in Fig. 6. The tumor fluorescent intensities in the imaging group (7.29 ± 0.67) are significantly higher than in the control group (1.52 ± 0.29), also confirming the specificity of the probe 1a for TrkC. The average radiant efficiency of other organs in control group was similar to that of the imaging group.
Conclusion
Compounds 1a and 1b are sufficiently different to the known probes A that it was not certain that these would also bind TrkC. In the event, 1a was selectively internalized into TrkC expressing cells, and stained human metastatic breast tumor tissues from cancer patients. In vivo, 1a accumulated in a TrkC-expressing (4T1) breast cancer tumor model to a much higher degree than the isomeric control that does not bind TrkC (1a-control). Accumulation of both 1a and 1a-control in the liver was also observed, but this is to be expected for such a lipophilic compound (aza-BODIPY cores are very hydrophobic). The key probe 1a did not accumulate in other organs to any extent that would preclude optical imaging of a primary breast cancer tumor.
Materials and methods
Cell culture and imaging
Murine breast cancer 4T1 cells were obtained from American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) at 37 °C in humidified atmosphere with 5% CO2. Subcellular localization was measured on living 4T1 cells using an Olympus FV1000 Confocal Microscope. Throughout, digital images were captured with a 100×/1.4 oil objective with the following filter sets: for LysoTracker Green: excitation 488 nm; for probes 1a and 1b: excitation 633 nm. Sequential optical sections (Z-stacks) from the basal-to-apical surfaces of the cell were also acquired.
Intracellular localization
4T1 cells were incubated with 1a, 1 μM, for 12 h at 37 °C. After the cells were washed with PBS, LysoTracker Green (Life Technology, 500 nM) was added and the cells were incubated for 30 min at 37 °C. The cells were washed again with PBS before imaging.
Histochemistry
Two slides of human breast cancer tissue microarrays (BRC962) containing 36 cases of breast cancers and 12 cases of normal, reactive and benign tumour tissues of the breast in duplicates were purchased from US Biomax, Inc. 1a and anti-TrkC antibody – N-terminal (ab188592, Abcam U.S.A.) were used as staining materials and the histology protocol was performed according to a published procedure.42 All tissues were visualized using an Olympus FV1000 confocal microscope.
Animal model
Murine breast cancer 4T1 cells were obtained from American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) at 37 °C in humidified atmosphere with 5% CO2. Animal procedures were performed according to a protocol approved by Houston Methodist Research Institute Animal Care and Use Committee. Murine 4T1 cells (5 × 106) were suspended in 100 μL PBS and injected orthotopically into the lower left mammary fat pad of 6 weeks female BALB/c nu/nu mice (Charles River). Mice were subjected to the in vivo imaging studies when tumors reached 8–10 mm in diameter.
In vivo fluorescence imaging and ex vivo biodistribution study
In vivo and ex vivo fluorescence imaging was performed with IVIS200 imaging system, and quantified by Living Imaging software (Xenogen, CA). Excitation and emission filters were set at 640 nm and 680 nm respectively as suggested by the system for image acquisition. For in vivo studies (n = 3), stock solutions (∼1 mM) of 1a and 1a-control conjugate in DMSO was diluted with PBS for injection. Each tumor-bearing mouse was injected intravenously with 10 nmol of 1a. Images were acquired at 6 h, 24 h and 48 h post-injection (p.i.). For control studies (n = 3), mice were injected with 10 nmol of 1a-control following the same procedures described above. Regions of interest (ROI) were drawn over the tumor and adjacent normal tissue to calculate the in vivo tumor/normal tissue ratio. In the ROI analysis, mean fluorescence intensities of the tumor area (tumor) defined as fluorescent radiant efficiency and of the area at the adjacent normal tissue (muscle) were calculated. After the 48 h imaging, mice were sacrificed, and tumors, muscles and major organs (i.e., heart, lung, liver, spleen and kidney) were excised for ex vivo imaging acquisition. ROI analysis and quantification of the fluorescence signals (n = 3) were performed with Living Imaging (Xenogen, CA).
Notes
The authors declare no competing financial interests.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We thank DoD BCRP Breakthrough Award (BC141561), CPRIT (RP150559 and RP170144), The Robert A. Welch Foundation (A-1121), and High Impact Research (HIR (UM.C/625/1/HIR/MOHE/MED/17 & UM.C/625/1/HIR/MOHE/MED/33) from the Ministry of Higher Education, Malaysia, for financial support. The NMR instrumentation at Texas A&M University was supported by a grant from the National Science Foundation (DBI-9970232) and the Texas A&M University System. The Olympus FV1000 confocal microscope acquisition was supported by the Office of the Vice President for Research at Texas A&M University. We thank the preclinical imaging core facility of Houston Methodist Research Institute for imaging support. Procedures for animal housing, maintenance, and euthanization were performed according to the American Veterinary Medical Association guidelines, and the IACUC approval from Methodist Hospital.
Footnotes
†Electronic supplementary information (ESI) available: Scheme and full details of compounds syntheses and characterizations, cell toxicity of 1a and TrkC antibody stained on breast tissues. See DOI: 10.1039/c7md00328e
References
- Patapoutian A., Reichardt L. F. Curr. Opin. Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Chao M. V. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Tessarollo L., Tsoulfas P., Martin-Zanca D., Gilbert D. J., Jenkins N. A., Copeland N. G., Parada L. F. Development. 1993;118:463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- Ryden M., Sehgal R., Dominici C., Schilling F. H., Ibanez C. F., Kogner P. Br. J. Cancer. 1996;74:773–779. doi: 10.1038/bjc.1996.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Nakagawara A., Ikegaki N., Liu X. G., Brodeur G. M. Oncogene. 1996;12:37–41. [PubMed] [Google Scholar]
- Brodeur G. M., Nakagawara A., Yamashiro D. J., Ikegaki N., Liu X. G., Azar C. G., Lee C. P., Evans A. E. J. Neuro-Oncology. 1997;31:49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Liu X. G., Lee C. P., Nakagawara A., Ikegaki N., McGregor L. M., Baylin S. B., Brodeur G. M. Eur. J. Cancer. 1997;33:2054–2057. doi: 10.1016/s0959-8049(97)00309-2. [DOI] [PubMed] [Google Scholar]
- Encinas M., Iglesias M., Llecha N., Comella J. X. J. Neurochem. 1999;73:1409–1421. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- Grotzer M. A., Janss A. J., Fung K. M., Biegel J. A., Sutton L. N., Rorke L. B., Zhao H., Cnaan A., Phillips P. C., Lee V. M. Y., Trojanowski J. Q. J. Clin. Oncol. 2000;18:1027–1035. doi: 10.1200/JCO.2000.18.5.1027. [DOI] [PubMed] [Google Scholar]
- Evangelopoulos M. E., Weis J., Kruttgen A. J. Neuro-Oncology. 2004;66:101–110. doi: 10.1023/b:neon.0000013492.37426.0c. [DOI] [PubMed] [Google Scholar]
- Laneve P., Di Macrotullio L., Gioia U., Fiori M. E., Ferretti E., Gulino A., Bozzoni I., Caffarelli E. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7957–7962. doi: 10.1073/pnas.0700071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Minturn J. E., Ho R., Simpson A. M., Iyer R., Varela C. R., Light J. E., Kolla V., Evans A. E. Clin. Cancer Res. 2009;15:3244–3250. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassili M., Birman E., Schor N. F., Saragovi H. U. Cancer Chemother. Pharmacol. 2010;65:1047–1056. doi: 10.1007/s00280-009-1110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel L., Costa B., Fainzilber M. Dev. Neurobiol. 2010;70:298–303. doi: 10.1002/dneu.20769. [DOI] [PubMed] [Google Scholar]
- Bernard-Gauthier V., Aliaga A., Aliaga A., Boudjemeline M., Hopewell R., Kostikov A., Rosa-Neto P., Thiel A., Schirrmacher R. ACS Chem. Neurosci. 2015;6:260–276. doi: 10.1021/cn500193f. [DOI] [PubMed] [Google Scholar]
- Kumar S., de Vellis J. J. Neurosci. Res. 1996;44:490–498. doi: 10.1002/(SICI)1097-4547(19960601)44:5<490::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hagel C., Hamel W., Muller S., Kluwe L., Westphal M. Acta Neuropathol. 1998;96:357–364. doi: 10.1007/s004010050906. [DOI] [PubMed] [Google Scholar]
- Calatozzolo C., Salmaggi A., Pollo B., Sciacca F. L., Lorenzetti M., Franzini A., Boiardi A., Broggi G., Marras C. Neurol. Sci. 2007;28:304–310. doi: 10.1007/s10072-007-0843-8. [DOI] [PubMed] [Google Scholar]
- Assimakopoulou M., Kondyli M., Gatzounis G., Maraziotis T., Varakis J. BMC Cancer. 2007;7:202–210. doi: 10.1186/1471-2407-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S., Chan Jennifer A., Krishna N., Pisklakova A., Qu X., Fenstermacher David A., Fournier M., Vrionis Frank D., Tran N., Kenchappa Rajappa S., Forsyth Peter A. J. Biol. Chem. 2014;290:3814–3824. doi: 10.1074/jbc.M114.599373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P., Edkins S., Davies H., Greenman C., Cox C., Hunter C., Bignell G., Teague J., Smith R., Stevens C., O'Meara S., Parker A., Tarpey P., Avis T., Barthorpe A., Brackenbury L., Buck G., Butler A., Clements J., Cole J., Dicks E., Edwards K., Forbes S., Gorton M., Gray K., Halliday K., Harrison R., Hills K., Hinton J., Jones D., Kosmidou V., Laman R., Lugg R., Menzies A., Perry J., Petty R., Raine K., Shepherd R., Small A., Solomon H., Stephens Y., Tofts C., Varian J., Webb A., West S., Widaa S., Yates A., Brasseur F., Cooper C. S., Flanagan A. M., Green A., Knowles M., Leung S. Y., Looijenga L. H. J., Malkowicz B., Pierotti M. A., Teh B., Yuen S. T., Nicholson A. G., Lakhani S., Easton D. F., Weber B. L., Stratton M. R., Futreal P. A., Wooster R. Nat. Genet. 2005;37:590–592. doi: 10.1038/ng1571. [DOI] [PubMed] [Google Scholar]
- Ivanov S. V., Panaccione A., Brown B., Guo Y., Moskaluk C. A., Wick M. J., Brown J. L., Ivanova A. V., Issaeva N., El-Naggar A. K., Yarbrough W. G. Oncogene. 2013;32:3698–3710. doi: 10.1038/onc.2012.377. [DOI] [PubMed] [Google Scholar]
- Jin W., Kim G.-M., Kim M.-S., Lim M.-H., Yun C.-H., Jeong J., Nam J.-S., Kim S.-J. Carcinogenesis. 2010;31:1939–1947. doi: 10.1093/carcin/bgq180. [DOI] [PubMed] [Google Scholar]
- Jin W., Yun C., Kwak M. K., Kim T. A., Kim S. J. Oncogene. 2007;26:7684–7691. doi: 10.1038/sj.onc.1210571. [DOI] [PubMed] [Google Scholar]
- Jin W., Yun C., Jeong J., Park Y., Lee H.-D., Kim S.-J. J. Biol. Chem. 2008;283:1391–1400. doi: 10.1074/jbc.M705052200. [DOI] [PubMed] [Google Scholar]
- Blasco-Gutierrez M. J., San Jose-Crespo I. J., Zozaya-Alvarez E., Ramos-Sanchez R., Garcia-Atares N. Cancer Invest. 2007;25:405–410. doi: 10.1080/07357900701206349. [DOI] [PubMed] [Google Scholar]
- Hondermarck H. Cytokine Growth Factor Rev. 2012;23:357–365. doi: 10.1016/j.cytogfr.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Xu X., Tahan S. R., Pasha T. L., Zhang P. J. J. Cutaneous Pathol. 2003;30:318–322. doi: 10.1034/j.1600-0560.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- Marconi A., Terracina M., Fila C., Franchi J., Bonte F., Romagnoli G., Maurelli R., Failla C. M., Dumas M., Pincelli C. J. Invest. Dermatol. 2003;121:1515–1521. doi: 10.1111/j.1523-1747.2003.12624.x. [DOI] [PubMed] [Google Scholar]
- Marchetti D., Murry B., Galjour J., Wilke-Greiter A. J. Cell. Biochem. 2003;88:865–872. doi: 10.1002/jcb.10473. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J. C., Seftor E. A., Kirschmann D. A., Quaranta V., Seftor R. E. B. Ann. N. Y. Acad. Sci. 2003;995:151–161. doi: 10.1111/j.1749-6632.2003.tb03218.x. [DOI] [PubMed] [Google Scholar]
- Marconi A., Panza M. C., Bonnet-Duquennoy M., Lazou K., Kurfurst R., Truzzi F., Lotti R., De Santis G., Dumas M., Bonte F., Pincelli C. Int. J. Cosmet. Sci. 2006;28:255–261. doi: 10.1111/j.1467-2494.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Truzzi F., Marconi A., Lotti R., Dallaglio K., French L. E., Hempstead B. L., Pincelli C. J. Invest. Dermatol. 2008;128:2031–2040. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- Botchkarev V. A., Yaar M., Peters E. M. J., Raychaudhuri S. P., Botchkareva N. V., Marconi A., Raychaudhuri S. K., Paus R., Pincelli C. J. Invest. Dermatol. 2006;126:1719–1727. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- Marchetti D., Denkins Y., Reiland J., Greiter-Wilke A., Galjour J., Murry B., Blust J., Roy M. Pathol. Oncol. Res. 2003;9:147–158. doi: 10.1007/BF03033729. [DOI] [PubMed] [Google Scholar]
- Denkins Y., Reiland J., Roy M., Sinnappah-Kang N. D., Galjour J., Murry B. P., Blust J., Aucoin R., Marchetti D. Neuro-Oncology. 2004;6:154–165. doi: 10.1215/S115285170300067X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi A., Le Anh T., Doebele Robert C. Cancer Discovery. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truzzi F., Marconi A., Pincelli C. Derm.-Endocrinol. 2011;3:32–36. doi: 10.4161/derm.3.1.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle L., Adriaenssens E., Yazidi-Belkoura I. E., Bourhis X. L., Nurcombe V., Hondermarck H. Curr. Cancer Drug Targets. 2004;4:463–470. doi: 10.2174/1568009043332853. [DOI] [PubMed] [Google Scholar]
- Bernard-Gauthier V., Boudjemeline M., Rosa-Neto P., Thiel A., Schirrmacher R. Bioorg. Med. Chem. 2013;21:7816–7829. doi: 10.1016/j.bmc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Kue S. C., Kamkaew A., Lee H. B., Chung L. L., Kiew L. V., Burgess K. Mol. Pharmaceutics. 2015;12:212–222. doi: 10.1021/mp5005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkaew A., Lim Siang H., Lee Hong B., Kiew Lik V., Chung Lip Y., Burgess K. Chem. Soc. Rev. 2012;42:77–88. doi: 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuah S. G., You Y. RSC Adv. 2012;2:11169–11183. [Google Scholar]
- Ziessel R., Goze C., Ulrich G. Synthesis. 2007;6:936–949. [Google Scholar]
- Loudet A. and Burgess K., BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties, Handbook of Porphyrin Science: With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine, ed. K. Kadish, K. Smith and R. Guilard, World Scientific, 2010, p. 203. [Google Scholar]
- Lu H., Mack J., Yang Y., Shen Z. Chem. Soc. Rev. 2014;43:4778–4823. doi: 10.1039/c4cs00030g. [DOI] [PubMed] [Google Scholar]
- Angell Y., Chen D., Brahimi F., Saragovi H. U., Burgess K. J. Am. Chem. Soc. 2008;130:556–565. doi: 10.1021/ja074717z. [DOI] [PubMed] [Google Scholar]
- Kamkaew A., Burgess K. Chem. Commun. 2015;51:10664–10667. doi: 10.1039/c5cc03649f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldal M., Tornoe C. W. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- Wu P., Fokin V. V. Aldrichimica Acta. 2007;40:7–17. [Google Scholar]
- Johnson G. A., Muthukrishnan N., Pellois J.-P. Bioconjugate Chem. 2013;24:114–123. doi: 10.1021/bc3005254. [DOI] [PubMed] [Google Scholar]
- Segal R. A. Annu. Rev. Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.