 We developed a transcylcooctene-based chemical probe for quantitative measurement of intracellular HDAC1/2 occupancy.
We developed a transcylcooctene-based chemical probe for quantitative measurement of intracellular HDAC1/2 occupancy.
Abstract
Histone deacetylases (HDACs) regulate diverse cellular processes, and are promising targets for a number of diseases. Here we describe the design and utilization of a largazole-based chemical probe to quantitatively measure the intracellular occupancy of HDAC1 and HDAC2 by dacinostat. Surprisingly, the probe was unable to enrich HDAC3 despite its nanomolar potency in a biochemical assay, further proving the necessity of cell-based target occupancy assays to understand compound potency in physiologically-relevant settings. This occupancy assay has the potential to aid the development of novel HDAC1/2 inhibitors in drug discovery.
Histone deacetylases (HDACs), which hydrolyse ε-N-acetylated lysine residues of histone and non-histone proteins, play critical roles in the epigenetic and post-transcriptional regulation of numerous genes and proteins. Based on sequence similarity, 18 human HDACs are divided into four classes. Classes I, II and IV are Zn2+-dependent, while class III are NAD+-dependent. HDACs have been reported to be involved in various cellular processes, including cell proliferation, inflammation,1 DNA replication and repair.2 Deregulation of these enzymes is observed in various tumor cells,3 making them potential targets for cancer therapy. A few HDAC inhibitors, such as vorinostat, panobinosat and belinostat, have been approved for the treatment of haematological malignancies and solid tumors.4 Although no compound has yet been approved for non-oncology indications, HDAC inhibitors could be useful for a number of non-cancer disorders. For instance, it has been demonstrated that HDAC1 and HDAC2 are therapeutic targets for sickle cell disease (SCD),5 an autosomal recessive genetic disease caused by a mutation in the β-globin gene that leads to polymerization of deoxygenated haemoglobin, deformation of red blood cells, vaso-occlusion, anaemia, renal failure and shortened life expectancy. Inhibition of HDAC1 and HDAC2 activity induces the expression of γ-globin, the fetal form of β-globin, and prevents haemoglobin polymerization and subsequent disease manifestations.
Proof of target engagement is a key pillar for successful target validation using chemical probes6 and helps to reduce attrition rates in drug discovery.7 Quantitative measurement of target engagement, in conjunction with functional biomarker analysis, can be used to determine efficacious drug concentrations in the clinic. Previously we have shown the importance of studying intracellular target occupancy, which represents a more physiologically-relevant assessment of compound potency.8–10 This may be particularly important for HDACs, since they usually exist in multi-protein repressor complexes within a cellular environment, such as HDAC1 and HDAC2 in Sin3, NuRD (nucleosome remodelling and deacetylation) and CoREST (corepressor for element-1-silencing transcription factor) complexes, and HDAC3 in the NCoR (nuclear receptor corepressor) complex.11 As protein–protein interactions may significantly alter the conformations of protein active sites that subsequently affect ligand binding, IC50 values from biochemical assays may not necessarily represent the actual compound potency in live cells. Photoaffinity probes for HDACs have been described in the literature,12–14 but they can suffer from low protein labelling efficiency and thus may not be suitable for quantitative target engagement studies. A bead-based chemoproteomics approach allowed comprehensive profiling of HDAC inhibitors against HDAC complexes in a human proteome, but is incompatible with live cells.15 Here we rationally designed a novel clickable HDAC chemical probe and then utilized it to quantitatively measure intracellular target engagement of dacinostat in in vitro differentiated human erythroid progenitor (CD71+) cells, a physiologically-relevant cell culture system for γ-globin induction. Together with mRNA data, our target occupancy studies helped determine the drug concentration and target coverage required to achieve a significant induction of γ-globin. This work has the potential to guide the use of HDAC inhibitors in the clinic for the treatment of SCD.7
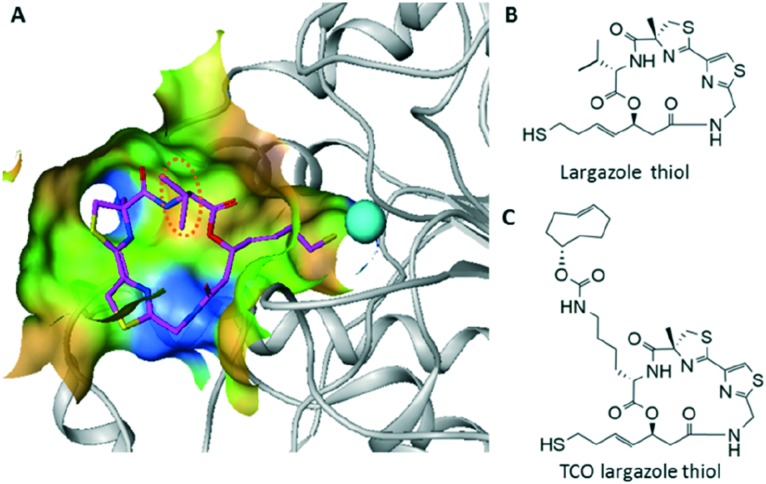
A clickable HDAC probe was designed based on the scaffold of a potent class I HDAC inhibitor, largazole,16,17 which was first isolated from marine cyanobacterium symploca sp.18 The crystal structure of HDAC8 with largazole thiol revealed that the thiol side chain extends deep into the active site to coordinate with Zn2+, and the isopropyl group of the valine residue faces towards the solvent (Fig. 1A and B),19 which may tolerate further modifications without affecting its HDAC activity. Compared to the copper(i)-catalysed azide-alkyne cycloaddition (CuAAC), the inverse electron demand Diels–Alder reaction (IEDDA) between trans-cyclooctenes (TCO) and 1,2,4,5-tetrazines proceeds more rapidly and efficiently,20,21 which is a favourable feature that may reduce or avoid issues caused by dissociation of the drug from its target after cell lysis, and provide a more accurate measurement for subsequent target occupancy studies. Thus, we chose TCO as the click handle and appended it to the valine side chain of largazole thiol, resulting in a TCO largazole thiol probe (Fig. 1C) that would allow subsequent investigations into the enrichment of HDACs from live cells.
Fig. 1. Chemical probe design. (A) Crystal structure of HDAC8 with largazole thiol. (B) Chemical structure of largazole thiol. (C) Chemical structure of TCO largazole thiol.
As shown in Scheme S1,‡ the synthesis of the probe was accomplished through an amine intermediate (J in Scheme S1‡) that was subsequently conjugated with (E)-cyclooct-4-enyl-2,5-dioxo-1-pyrrolidinyl carbonate (I in Scheme S1‡) followed by acid deprotection of the S-trityl group.
We then measured the biochemical activity of the probe against class I HDACs (HDAC1, 2, 3, 8). Despite approximately 2–10 fold loss of activity, TCO largazole thiol exhibits single digit nanomolar IC50s against HDAC1, 2, and 3 (Table 1).
Table 1. Biochemical activity.
| HDAC isoform IC50 (nM) |
||||
| HDAC1 | HDAC2 | HDAC3 | HDAC8 | |
| Largazole thiol a | 0.4 | 0.9 | 0.7 | 102 |
| TCO largazole thiol | 1.9 | 6.6 | 1.1 | 1115 |
aData from ref. 22.
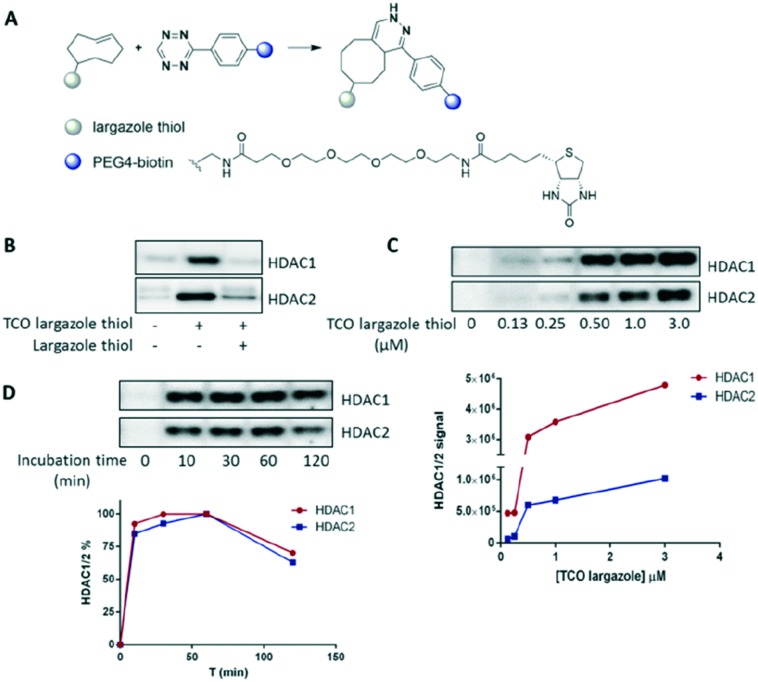
Additionally, although the probe showed modest membrane permeability (Papp = 3.23 × 10–6 cm s–1), we were confident that it would be suitable for subsequent cell-based occupancy assays (we would expect a biotin conjugate of largazole to possess insufficient permeability, thus vindicating the use of the silent reporter click handle). We then sought to test the capability of TCO largazole thiol to enrich HDACs from cells. After probe treatment, human erythroid progenitor cells were lysed and the lysate was then ‘clicked’ with tetrazine-PEG4-biotin (IEDDA, Fig. 2A), enabling affinity capture of HDACs by streptavidin beads. TCO largazole thiol was able to specifically enrich both HDAC1 and HDAC2, as evidenced by the successful competition by largazole thiol (Fig. 2B). However, HDAC3 was not observed after enrichment (data not shown), despite the 1 nM potency of the probe in the biochemical assay. These results suggest that the NCoR complex, or other protein interactors, may prevent the TCO largazole thiol probe from accessing the active site of HDAC3. This observation further proves the necessity of a cellular occupancy assay to assess compound potency.
Fig. 2. Probe assessment. (A) IEDDA between TCO and tetrazine-PEG4-biotin. (B) Enrichment of HDAC1 and HDAC2 from cells using TCO largazole thiol. Optimization of probe concentration (C) and probe incubation time (D) for the occupancy assay.
In further experiments, TCO largazole thiol displayed efficient labelling and achieved satisfactory enrichment of HDAC1 and HDAC2 at a low micromolar concentration (Fig. 2C). Maximum labelling was achieved after a 10 minute incubation with the probe (Fig. 2D), indicative of a rapid intake and engagement of the probe with the target proteins. A longer probe incubation time (>60 minutes) resulted in a slight decrease in labelling, possibly caused by modifications of the probe within the cells, e.g. thiol-disulfide exchange.
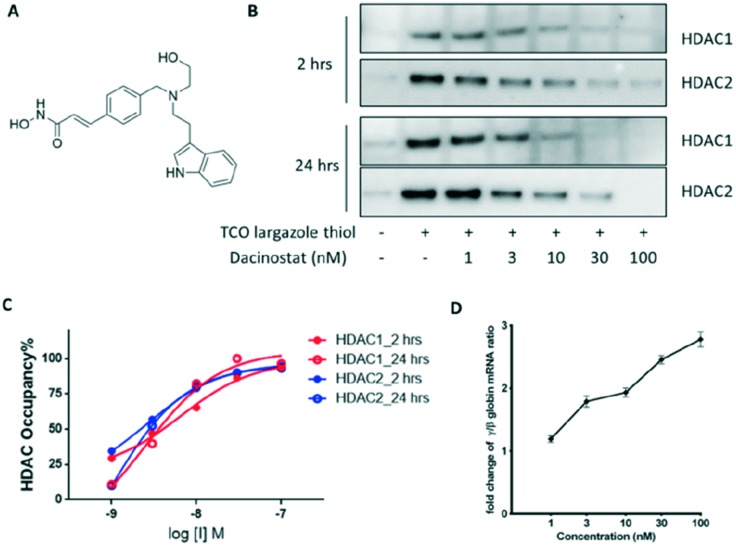
We then attempted to measure HDAC1/2 occupancy by dacinostat (structure shown in Fig. 3A, also known as NVP-LAQ824), a hydroxamate HDAC inhibitor that was previously reported to increase γ-globin expression in a K562 cell line.23 Occupancy curves were generated using the optimised assay conditions (Fig. 3B and C). OC50 values, which represent the drug concentration needed to achieve 50% target coverage, were determined to be approximately 6 nM and 3 nM against HDAC1 and HDAC2, respectively, 2 hours after drug treatment. The potency measured in the occupancy assay is similar to the IC50 values from the biochemical assay (5 nM for both HDAC1 and HDAC2 (ref. 24)). In addition, dacinostat maintained similar occupancy after 24 hours incubation. Lastly, a significant up-regulation of γ-globin (>2-fold) was only observed at 3 nM or higher concentrations of dacinosat (Fig. 3D). Together, these results suggest that at least 40 to 50% occupancy of HDAC1 and HDAC2 is needed to achieve functional pharmacology (pillar 3)6 in human erythroid progenitors.
Fig. 3. Dacinostat treatment in human erythroid progenitor cells. (A) Chemical structure of dacinostat. (B) HDAC1/2 enrichment after 2 and 24 hour treatment. (C) HDAC1/2 occupancy by dacinostat. (D) γ-Globin mRNA analysis.
TCO-tetrazine ligation has been demonstrated to be useful for cell imaging applications, such as protein cellular localization,25,26 and super-resolution imaging of cellular organelles,27 due to the efficiency and selectivity of the click reaction. Recent development of various fluorogenic tetrazines enables no wash, live cell imaging in a rather short time frame.28–30 We are currently investigating the application of TCO largazole thiol for HDAC1/2 imaging in live cells, which could provide valuable information on the protein dynamics of HDAC1/2 during cellular events.
Conclusions
Here we described the design, synthesis and application of a TCO largazole thiol probe to assess intracellular target engagement of dacinostat. Our data provide further understanding of the relationship between HDAC occupancy and γ-globin induction. The occupancy assay would be broadly useful to help establish the ‘three pillars’ for HDAC1/2 inhibitors and aid selection of the proper compound for clinical studies against SCD and other relevant diseases.
Supplementary Material
Footnotes
†All authors are current or former Pfizer employees.
‡Electronic supplementary information (ESI) available: Materials and experimental procedures. See DOI: 10.1039/c6md00633g
References
- Leus N. G., Zwinderman M. R., Dekker F. J. Curr. Opin. Chem. Biol. 2016;33:160–168. doi: 10.1016/j.cbpa.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara S. Cell Cycle. 2015;14:1779–1785. doi: 10.1080/15384101.2015.1042634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. C., Johnstone R. W. J. Clin. Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg K. J., Johnstone R. W. Nat. Rev. Drug Discovery. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- Bradner J. E., Mak R., Tanguturi S. K., Mazitschek R., Haggarty S. J., Ross K., Chang C. Y., Bosco J., West N., Morse E., Lin K., Shen J. P., Kwiatkowski N. P., Gheldof N., Dekker J., DeAngelo D. J., Carr S. A., Schreiber S. L., Golub T. R., Ebert B. L. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12617–12622. doi: 10.1073/pnas.1006774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnage M. E., Chekler E. L., Jones L. H. Nat. Chem. Biol. 2013;9:195–199. doi: 10.1038/nchembio.1197. [DOI] [PubMed] [Google Scholar]
- Morgan P., Van Der Graaf P. H., Arrowsmith J., Feltner D. E., Drummond K. S., Wegner C. D., Street S. D. Drug Discovery Today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Hett E. C., Xu H., Geoghegan K. F., Gopalsamy A., Kyne, Jr. R. E., Menard C. A., Narayanan A., Parikh M. D., Liu S., Roberts L., Robinson R. P., Tones M. A., Jones L. H. ACS Chem. Biol. 2015;10:1094–1098. doi: 10.1021/cb5009475. [DOI] [PubMed] [Google Scholar]
- Jones L. H. Future Med. Chem. 2015;7:2131–2141. doi: 10.4155/fmc.15.100. [DOI] [PubMed] [Google Scholar]
- Xu H., Gopalsamy A., Hett E. C., Salter S., Aulabaugh A., Kyne R. E., Pierce B., Jones L. H. Org. Biomol. Chem. 2016;14:6179–6183. doi: 10.1039/c6ob01078d. [DOI] [PubMed] [Google Scholar]
- Kelly R. D., Cowley S. M. Biochem. Soc. Trans. 2013;41:741–749. doi: 10.1042/BST20130010. [DOI] [PubMed] [Google Scholar]
- Salisbury C. M., Cravatt B. F. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1171–1176. doi: 10.1073/pnas.0608659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury C. M., Cravatt B. F. J. Am. Chem. Soc. 2008;130:2184–2194. doi: 10.1021/ja074138u. [DOI] [PubMed] [Google Scholar]
- Albrow V. E., Grimley R. L., Clulow J., Rose C. R., Sun J., Warmus J. S., Tate E. W., Jones L. H., Storer R. I. Mol. BioSyst. 2016;12:1781–1789. doi: 10.1039/c6mb00109b. [DOI] [PubMed] [Google Scholar]
- Bantscheff M., Hopf C., Savitski M. M., Dittmann A., Grandi P., Michon A. M., Schlegl J., Abraham Y., Becher I., Bergamini G., Boesche M., Delling M., Dumpelfeld B., Eberhard D., Huthmacher C., Mathieson T., Poeckel D., Reader V., Strunk K., Sweetman G., Kruse U., Neubauer G., Ramsden N. G., Drewes G. Nat. Biotechnol. 2011;29:255–265. doi: 10.1038/nbt.1759. [DOI] [PubMed] [Google Scholar]
- Bowers A., West N., Taunton J., Schreiber S. L., Bradner J. E., Williams R. M. J. Am. Chem. Soc. 2008;130:11219–11222. doi: 10.1021/ja8033763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y., Taori K., Kim H., Hong J., Luesch H. J. Am. Chem. Soc. 2008;130:8455–8459. doi: 10.1021/ja8013727. [DOI] [PubMed] [Google Scholar]
- Taori K., Paul V. J., Luesch H. J. Am. Chem. Soc. 2008;130:1806–1807. doi: 10.1021/ja7110064. [DOI] [PubMed] [Google Scholar]
- Cole K. E., Dowling D. P., Boone M. A., Phillips A. J., Christianson D. W. J. Am. Chem. Soc. 2011;133:12474–12477. doi: 10.1021/ja205972n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karver M. R., Weissleder R., Hilderbrand S. A. Bioconjugate Chem. 2011;22:2263–2270. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R., Liu S., Hassink M., Huang C. W., Yap L. P., Park R., Fox J. M., Li Z., Conti P. S. Bioorg. Med. Chem. Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J., Luesch H. Nat. Prod. Rep. 2012;29:449–456. doi: 10.1039/c2np00066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlak M., Fugazza C., Elangovan S., Marini M. G., Marongiu M. F., Moi P., Fraietta I., Cappella P., Barbarani G., Font-Monclus I., Mauri M., Ottolenghi S., Gasparri F., Ronchi A. PLoS One. 2015;10:e0141083. doi: 10.1371/journal.pone.0141083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Jeffers M., Kumar S., Hackett C., Boldog F., Khramtsov N., Qian X., Mills E., Berghs S. C., Carey N., Finn P. W., Collins L. S., Tumber A., Ritchie J. W., Jensen P. B., Lichenstein H. S., Sehested M. Biochem. J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- Yang K. S., Budin G., Reiner T., Vinegoni C., Weissleder R. Angew. Chem., Int. Ed. 2012;51:6598–6603. doi: 10.1002/anie.201200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Yang K. S., Weissleder R. PLoS One. 2013;8:e81275. doi: 10.1371/journal.pone.0081275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R. S., Takakura H., Thompson A. D., Rivera-Molina F., Allgeyer E. S., Bewersdorf J., Toomre D., Schepartz A. Angew. Chem., Int. Ed. 2014;53:10242–10246. doi: 10.1002/anie.201403349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. C., Meimetis L. G., Hilderbrand S. A., Weissleder R. Angew. Chem., Int. Ed. 2013;52:6917–6920. doi: 10.1002/anie.201301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimetis L. G., Carlson J. C., Giedt R. J., Kohler R. H., Weissleder R. Angew. Chem., Int. Ed. 2014;53:7531–7534. doi: 10.1002/anie.201403890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Cisneros B. T., Cole C. M., Devaraj N. K. J. Am. Chem. Soc. 2014;136:17942–17945. doi: 10.1021/ja510839r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.