 In this study, a series of benzimidazole–rhodanine conjugates were designed, synthesized and investigated for their topoisomerase II (Topo II) inhibitory and cytotoxic activities.
In this study, a series of benzimidazole–rhodanine conjugates were designed, synthesized and investigated for their topoisomerase II (Topo II) inhibitory and cytotoxic activities.
Abstract
In this study, a series of benzimidazole–rhodanine conjugates were designed, synthesized and investigated for their topoisomerase II (Topo II) inhibitory and cytotoxic activities. The results from Topo II-mediated pBR322 DNA relaxation and cleavage assays showed that the synthesized compounds might act as Topo II catalytic inhibitors. Certain compounds displayed potent Topo II inhibition at 10 μM. The cytotoxic activities of these compounds against HeLa, A549, Raji, PC-3, MDA-MB-201, and HL-60 cancer cell lines were evaluated. The results indicated that these compounds exhibited strong antiproliferative activity. A good relationship was observed between the Topo II inhibitory potency and the cytotoxicity of these compounds. The structure–activity relationship revealed that the electronic effects, the phenyl group, and the rhodanine moiety were particularly important for the Topo II inhibitory potency and cytotoxicity.
1. Introduction
DNA topoisomerases are important cellular enzymes found in almost all types of living cells. These enzymes mediated the topological adjustments required for DNA replication, transcription, recombination, repair, and chromatin assembly.1–3 These enzymes are important molecular drug targets and inhibitors of these enzymes are widely used as effective anticancer agents. Topoisomerase inhibitors are used in clinical treatment of cancer for more than 30 years. About 50% of chemotherapeutic regimens use at least one drug that targets these enzymes.4 There are two types of topoisomerases in human: type I topoisomerase (Topo I) and type II topoisomerase (Topo II). Topo II has been reported as the specific target of certain most active anticancer drugs such as etoposide, doxorubicin and amsacrine.5 However, Topo II inhibitors have some therapeutic limitations because of their serious side effects during cancer chemotherapy. Thus, development of novel anticancer Topo II inhibitors is necessary for improving cancer treatment.6–10
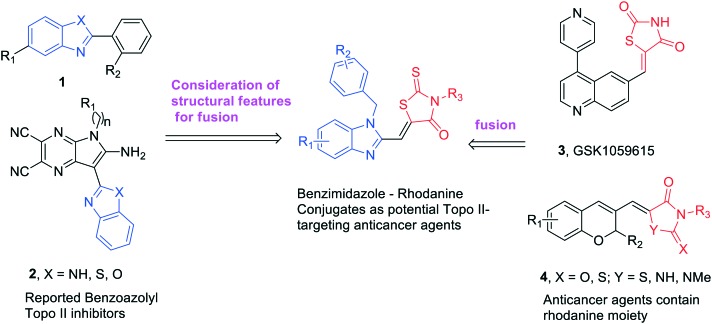
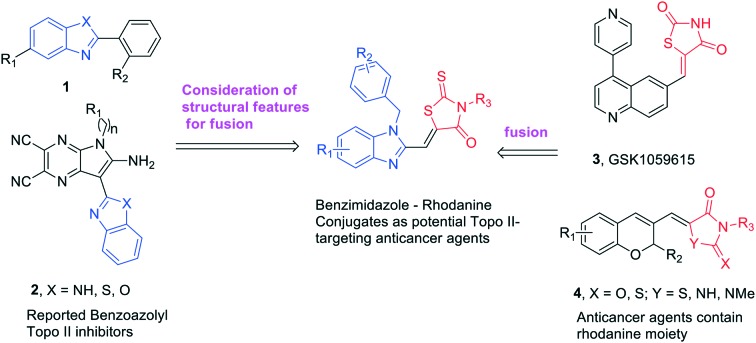
Benzimidazole is found in many clinically useful drugs11 and has a precious scaffold for the preparation of a wide variety of biological active compounds such as antibacterial, anti-inflammatory and anticancer agents.12 Several benzimidazole derivatives were reported as novel Topo II inhibitors (1 and 2, Fig. 1). Structure–activity relationship (SAR) studies showed that the benzimidazole rings as the fused system in the structures are significant for Topo II inhibitory potency and the phenyl group linked to benzimidazole is particularly important.13,14 Mechanism studies and molecular docking revealed that these compounds function as Topo II catalytic inhibitors by blocking the ATP-binding site of the enzyme.15,16
Fig. 1. Design of novel agents as potential Topo II-targeting anticancer agents.
Rhodanine is recognized as a privileged heterocycle in medicinal chemistry.17 The molecules containing the rhodanine moiety are known for their rich pharmacological profile, including antibacterial, anti-parasitic, anti-microbial, anti-tubercular and anticancer.18–21 Rhodanines including their relatively simple derivatives as well as complexes or hybrids/conjugates bearing a non-fused rhodanine core possess good activity against different types of cancer.22–24 For example, commercial GSK1059615 (3, Fig. 1) is a reversible, ATP-competitive, inhibitor of PI3Kα, which shows potential anticancer activity. Compound 4 (Fig. 1) displays potent cytotoxicity toward several cancer cell lines.
Pharmacophore hybridization strategies are often used for the design of new drugs and a certain number of successful examples are reported in drug discovery and development.25–28 We have recently designed and synthesized various Topo II inhibitors through a pharmacophore hybridization strategy and the results demonstrated that these conjugates possessed generally higher cytotoxicity and Topo II inhibitory potency than the individual agents alone.16,20,29 In an effort to discover novel anticancer agents that target Topo II more efficiently, benzimidazole–rhodanine conjugates (Fig. 1) were synthesized in this work and the Topo I and II inhibition, the DNA interaction, and the cytotoxicity properties of these newly synthesized compounds were evaluated. It was found that these compounds displayed significant Topo II inhibitory potency and showed effective cytotoxicity against six human cancer cell lines.
2. Results and discussion
2.1. Chemistry
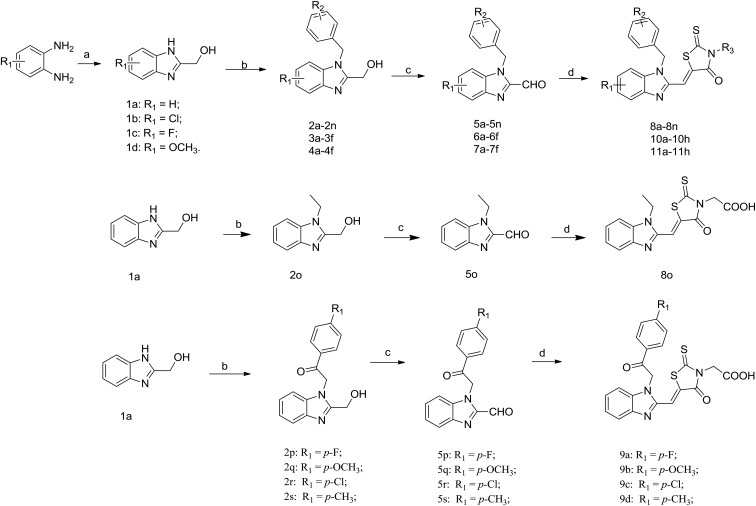
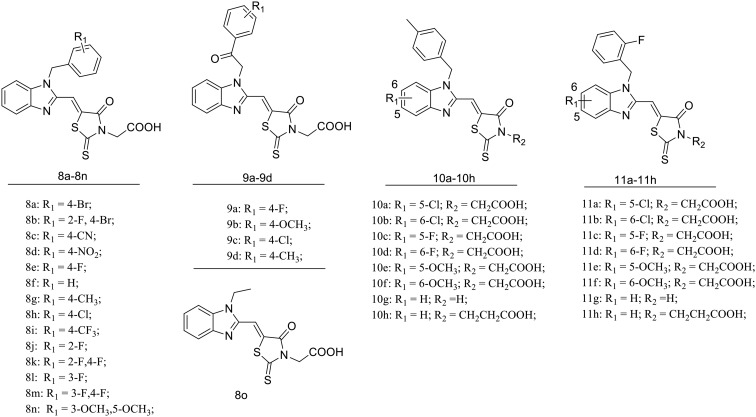
A series of benzimidazole–rhodanine conjugates (8a–8o, 9a–9d, 10a–10h and 11a–11h) were synthesized as outlined in Scheme 1. The synthetic strategy consisted of the benzimidazole and rhodanine moieties and then the condensation of these moieties to provide the target compounds (Fig. 2). Substituted-(benzo[d]-imidazole-2-yl)methanols (1a–1d) were prepared by refluxing substituted-1,2-diaminobenzene with glycolic acid in hydrochloric acid. Substituted-(1-alkyl-1H-benzo[d]imidazole-2yl)methanols (2a–2s, 3a–3f and 4a–4f) were achieved by N-alkylation of 1a–1d with appropriate benzyl bromide, bromoethane or 2-bromo-1-phenylethanone using K2CO3 as the base in 27–88% yield. Substituted-1-alkyl-1H-benzo[d]imidazole-2-carbaldehydes (5a–5s, 6a–6f and 7a–7f) were obtained by oxidation of the corresponding primary alcohols with the Dess–Martin reagent in 36–68% yield.30 The target compounds (8a–8o, 9a–9d, 10a–10h and 11a–11h) were synthesized through the Horner–Wadsworth–Emmons reaction of rhodanine moiety with the prepared aldehydes (5a–5s, 6a–6f and 7a–7f) in 69–91% yield. Since the reaction was not region-selective in the preparation of 3a–3f and 4a–4f, the R1 group could be either in the C5 or C6 position of the benzimidazole scaffold in the process of substitution reaction.31 2D NOESY spectroscopy was performed to determine the isomer of 3a and 3b (Fig. S1 and S2†). The synthesized compounds were characterized by 1H NMR, 13C NMR, and HRMS (ESI), which were in full accordance with their depicted structures and the purities were confirmed by analytical HPLC.
Scheme 1. Synthesis route of the target compounds. Reagents and conditions: (a) glycolic acid, 4 N HCl, 100 °C, 6 h; (b) DMF, appropriate benzyl bromide, bromoethane or 2-bromo-1-phenylethanone, K2CO3, rt, 24 h; (c) DCM, Dess–Martin reagent, 4 °C, 1 h; (d) rhodanine moiety, NaOAc, acetic acid, 110 °C, 4 h.
Fig. 2. Chemical structure of the target compounds.
2.2. Cytotoxicity
The synthesized compounds were evaluated for their cytotoxic activities using the MTT assay against six human cancer cell lines including: the human acute leukemia cell line (HL-60), human cervical cancer cell line (HeLa), human breast cancer cell line (MDA-MB-201), adenocarcinomic human alveolar basal epithelial cancer cell line (A549), human lymphoma cancer cell line (Raji), and human prostate cancer cells line (PC-3). Etoposide and camptothecin were chosen as positive controls. The inhibitory activities (IC50) of the tested compounds are listed in Table 1.
Table 1. Cytotoxic activity and Topo II inhibitory potency of the synthesized compounds.
| Cpd. | IC50
a
(mean ± SD) (μM) |
Topo II inhibition b | |||||
| HeLa | HL-60 | MDA-MB-201 | Raji | PC-3 | A549 | ||
| 8a | 12.94 ± 3.43 | 0.87 ± 0.20 | 8.72 ± 2.44 | 1.19 ± 0.24 | 8.49 ± 2.38 | 9.12 ± 3.17 | + |
| 8b | >50 | 6.42 ± 1.72 | 28.36 ± 5.62 | 11.37 ± 2.20 | 21.12 ± 4.63 | 27.31 ± 5.72 | — |
| 8c | 16.31 ± 4.25 | 7.64 ± 0.89 | 11.24 ± 3.16 | 9.78 ± 3.14 | 14.42 ± 3.25 | 26.58 ± 5.43 | — |
| 8d | 18.57 ± 4.05 | 5.32 ± 1.25 | 7.76 ± 2.25 | 2.51 ± 0.62 | 8.27 ± 2.28 | 11.42 ± 3.16 | — |
| 8e | 13.92 ± 3.47 | 1.42 ± 0.46 | 2.62 ± 0.82 | 1.77 ± 0.34 | 4.86 ± 1.23 | 3.57 ± 1.24 | ++ |
| 8f | 22.66 ± 4.62 | 0.96 ± 0.24 | 1.82 ± 0.57 | 3.86 ± 1.12 | 10.79 ± 2.42 | 8.76 ± 2.68 | + |
| 8g | 1.28 ± 0.23 | 0.54 ± 0.18 | 1.04 ± 0.26 | 0.96 ± 0.31 | 2.64 ± 0.85 | 3.22 ± 1.26 | ++ |
| 8h | 8.72 ± 1.21 | 2.65 ± 0.73 | 5.58 ± 1.25 | 11.42 ± 2.89 | 9.67 ± 2.33 | 17.51 ± 3.69 | ++ |
| 8i | 21.42 ± 5.02 | 3.56 ± 0.94 | 12.46 ± 3.82 | 18.83 ± 4.26 | 22.86 ± 5.61 | 25.28 ± 6.75 | ++ |
| 8j | 0.64 ± 0.12 | 0.21 ± 0.13 | 0.33 ± 0.16 | 1.23 ± 0.35 | 1.86 ± 0.53 | 2.67 ± 0.89 | ++ |
| 8k | 26.12 ± 5.46 | 6.52 ± 1.16 | 11.48 ± 4.28 | 8.96 ± 1.78 | 21.68 ± 5.47 | 12.83 ± 3.56 | — |
| 8l | >50 | 7.21 ± 2.49 | 22.46 ± 5.28 | 13.28 ± 3.46 | 35.71 ± 6.55 | 23.16 ± 6.58 | — |
| 8m | 15.98 ± 3.42 | 2.74 ± 0.74 | 3.76 ± 1.51 | 7.25 ± 2.76 | 5.97 ± 1.69 | 13.42 ± 3.63 | + |
| 8n | 17.69 ± 4.04 | 1.41 ± 0.53 | 1.28 ± 0.53 | 2.11 ± 0.76 | 6.37 ± 1.61 | 5.17 ± 1.52 | ++ |
| 8o | 3.52 ± 0.78 | 2.12 ± 0.23 | 1.88 ± 0.72 | 5.56 ± 1.38 | 3.25 ± 1.12 | 9.44 ± 2.41 | — |
| 9a | 14.79 ± 3.03 | 3.96 ± 0.73 | 6.31 ± 2.18 | 8.24 ± 2.21 | 9.87 ± 2.34 | 12.77 ± 3.60 | ++ |
| 9b | 26.68 ± 6.21 | 5.44 ± 1.03 | 12.86 ± 4.12 | 6.32 ± 1.78 | 8.75 ± 2.24 | 5.15 ± 1.66 | — |
| 9c | 27.26 ± 5.87 | 2.43 ± 0.56 | 1.12 ± 0.56 | 3.18 ± 1.16 | 6.45 ± 1.40 | 9.85 ± 3.11 | ++ |
| 9d | 27.41 ± 5.71 | 15.88 ± 3.26 | 16.85 ± 4.18 | 12.28 ± 3.56 | 8.75 ± 1.79 | 9.46 ± 2.17 | ++ |
| 10a | 21.44 ± 1.96 | 2.72 ± 0.69 | 13.72 ± 4.58 | 20.88 ± 4.52 | 26.71 ± 6.32 | 38.61 ± 5.13 | ++ |
| 10b | 10.96 ± 1.82 | 2.84 ± 0.91 | 3.96 ± 1.27 | 2.79 ± 0.86 | 1.52 ± 0.36 | 9. 97 ± 3.16 | ++ |
| 10c | 2.38 ± 1.22 | 1.17 ± 0.43 | 6.55 ± 2.13 | 3.28 ± 1.37 | 10.24 ± 2.79 | 13.82 ± 4.41 | ++ |
| 10d | 3.92 ± 0.77 | 1.69 ± 0.83 | 3.86 ± 1.35 | 2.44 ± 0.77 | 5.82 ± 1.75 | 9.64 ± 2.79 | ++ |
| 10e | 1.82 ± 0.56 | 0.77 ± 0.25 | 1.22 ± 0.54 | 1.79 ± 0.53 | 3.64 ± 1.08 | 5.68 ± 1.84 | ++ |
| 10f | 6.44 ± 0.98 | 1.92 ± 0.58 | 3.77 ± 1.28 | 10.76 ± 2.67 | 16.17 ± 4.84 | 23.61 ± 4.59 | ++ |
| 10g | 25.25 ± 5.20 | 2.66 ± 0.83 | 6.42 ± 1.22 | 4.48 ± 1.23 | 9.78 ± 2.46 | 5.31 ± 1.66 | + |
| 10h | 11.2 ± 1.82 | 4.14 ± 0.79 | 3.79 ± 1.68 | 6.68 ± 1.28 | 9.31 ± 2.75 | 8.74 ± 2.43 | — |
| 11a | 9.52 ± 1.08 | 0.87 ± 0.23 | 1.67 ± 0.73 | 2.78 ± 0.98 | 3.48 ± 1.62 | 5.64 ± 1.99 | ++ |
| 11b | 7.62 ± 0.93 | 5.23 ± 1.33 | 9.57 ± 3.62 | 8.87 ± 1.89 | 17.31 ± 4.81 | 26.86 ± 5.76 | ++ |
| 11c | >50 | >50 | 22.44 ± 5.38 | 20.42 ± 4.21 | >50 | 32.65 ± 6.48 | — |
| 11d | >50 | 5.23 ± 1.26 | >50 | 22.37 ± 5.28 | >50 | >50 | + |
| 11e | 22.48 ± 2.91 | 3.41 ± 1.03 | 16.92 ± 4.76 | 11.46 ± 3.68 | 24.71 ± 5.88 | 18.35 ± 4.87 | — |
| 11f | 28.13 ± 3.62 | 6.44 ± 1.73 | 22.44 ± 7.68 | 12.35 ± 3.28 | >50 | 36.18 ± 9.45 | ++ |
| 11g | 36.11 ± 7.72 | 8.42 ± 2.13 | 21.33 ± 5.38 | 25.48 ± 5.57 | >50 | >50 | + |
| 11h | 26.52 ± 0.98 | 5.72 ± 1.67 | 9.53 ± 2.71 | 11.27 ± 3.62 | 28.61 ± 5.32 | 18.73 ± 4.28 | — |
| Etoposide | 28.61 ± 3.98 | 0.58 ± 0.11 | 30.28 ± 5.41 | 1.21 ± 0.24 | 13.18 ± 3.65 | 2.46 ± 0.67 | ++ |
| Camptothecin | >50 | 0.42 ± 0.19 | 16.44 ± 4.86 | 3.27 ± 1.21 | 18.24 ± 4.53 | 2.21 ± 0.58 | n.d. c |
aEach assay was performed in quadruplicate with the number of determinations N > 2, and the results are expressed as mean values, where the IC50 means the concentration of drug needed to reduce the cell number to 50%.
bThe relative Topo II inhibitory potencies of the synthesized compounds are presented as follows: —, no detectable activity at 20 μM; +, weak activity at 20 μM; ++, strong activity at 20 μM.
cNot detect.
Most of the compounds displayed significant cytotoxic activities with low micromolar IC50 values. In addition, a good correlation was found between the Topo II inhibitory potency and the cytotoxic activities. Compounds 8g, 8j, 8n, 9a, 9c, 10a, and 10c–10e, which showed strong Topo II inhibitory potency, displayed promising cytotoxic activity against six cancer cell lines. Specially, 8g and 8j, which exhibited excellent Topo II inhibitory activity at 10 μM, showed more potent cytotoxic activity, with IC50 values ranging from 0.54 to 3.22 μM and from 0.21 to 2.67 μM, respectively. Whereas, other compounds, revealed to be poor Topo II inhibitors, showed low or moderate cytotoxic activities. It is worth to mention that HL-60 was revealed to be more sensitive to these compounds, as a lower IC50 value was obtained compared to those of other cancer cell lines (Table 1).
2.3. Topo II inhibition and SAR study
Topo II-mediated DNA relaxation, cleavage of the complex and unwinding assays as well as molecular docking were performed to evaluate the ability of the synthesized compounds to inhibit Topo II and to investigate their mode of action.
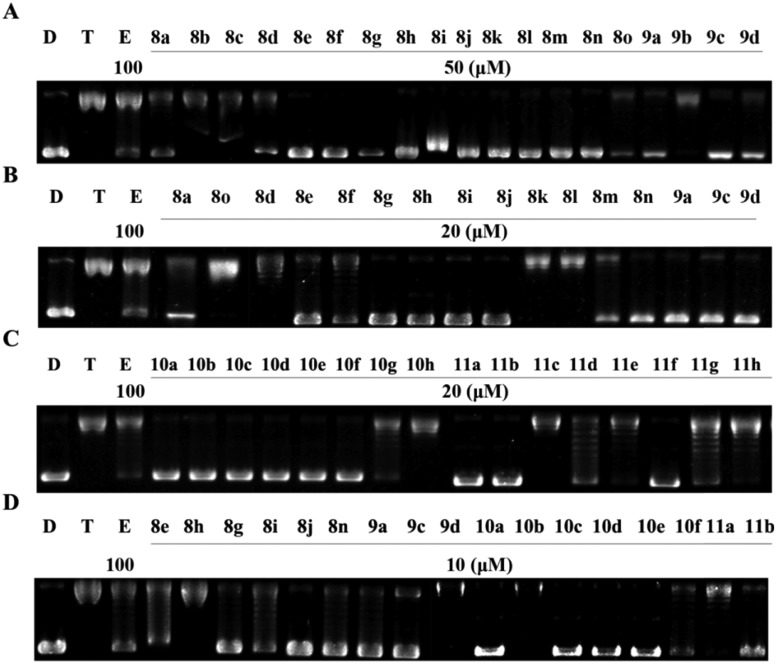
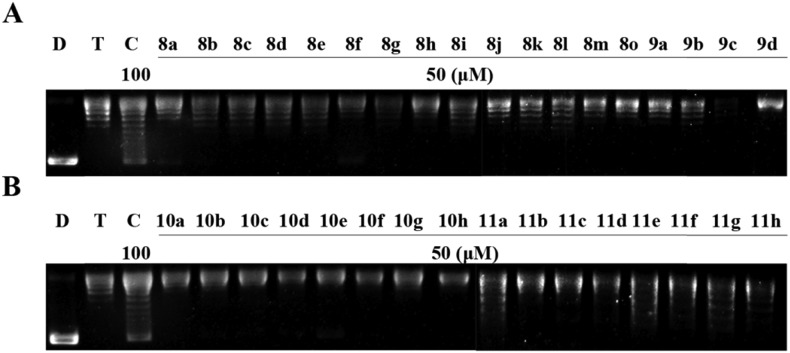
The Topo II-mediated DNA relaxation assay was first employed to determine the capacity of these compounds, using etoposide as the positive control and pBR322 DNA plasmid as the substrate.8 Compounds 8a–8o and 9a–9d (Fig. 2) were first synthesized and used in the assay. As shown in Fig. 3A and B, most of the tested compounds exhibited significant Topo II inhibitory potency at 50 μM and displayed a similar effect with etoposide. Compounds 8g, 8j, 8n, 9a, and 9c showed the best inhibitory potency at 10 μM (Fig. 3D). It was found that the benzyl group is necessary for the activity (8f, Fig. 3A), since its replacement with the ethyl group (8o, Fig. 3A) led to a decrease of inhibitory activity. Moreover, compounds 8g and 8n with electron donating groups showed better Topo II inhibition than those with electron withdrawing groups (8a, 8d, 8e, and 8h, Fig. 3A, B and D). Along with this observation, 8b, 8k, and 8m with two electron withdrawing substitution groups on benzyl showed weaker activity than those with one electron withdrawing substitution group (8j, Fig. 3A, B and D). The results indicated that the benzyl group and the electronic effects of substitutions have significant impact on Topo II inhibitory activity and should be maintained in further structure modification.
Fig. 3. Agarose gel assay for Topo II inhibition by the synthesized compounds. (A–D) Lane D, pBR322 DNA; lane T, pBR322 DNA + Topo II; lane E, pBR322 DNA + Topo II + etoposide (100 μM); other lanes, pBR322 DNA + Topo II + the synthesized compounds.
In order to investigate the effect of the rhodanine-3-acetic acid moiety on the inhibition of Topo II activity, it was changed to rhodanine (10g and 11g) or rhodanine-3-(3-propionic acid) (10h and 11h). It was found that, the acetic acid moiety was crucial, since 10g, 11g, 10h, and 11h did not show better Topo II inhibition at 20 μM (Fig. 3C). These results revealed that the acetic acid group linked to rhodanine was also optimal for the activity.
Next, the benzimidazole scaffold was modified with various substitutions (–F, –Cl, and –OCH3) on the benzene ring. 5 or 6-Monosubstituted isomers can be obtained (10a–10f and 11a–11f) and no obvious difference in Topo II inhibitory potency was observed between the isomers at 50 μM (Fig. 3C). The results showed that 10a, 10c, 10d, and 10e also displayed strong Topo II inhibitory activity even at 10 μM (Fig. 3C and D). However, modified 8j with the same substitutions (11a–11f) led to a decrease of Topo II inhibitory potency (Fig. 3C and D).
A Topo I-mediated DNA relaxation assay was employed to study whether this class of compound also targeted Topo I. The results are presented in Fig. 4. None of the tested compounds exhibited Topo I inhibitory potency even at 50 μM, indicating that these compounds displayed selective inhibition against Topo II.
Fig. 4. Topo I inhibitory activity of the synthesized compounds. (A) and (B) Lane D: pBR322 DNA; lane T: pBR322 DNA + Topo I; lane C: pBR322 DNA + Topo I + camptothecin (100 μM); other lanes: pBR322 DNA + Topo I + the synthesized compounds (50 μM).
As compounds 8g and 8j showed the best Topo II inhibitory and cytotoxic activities, they were selected for the further mechanism studies.
2.4. Compounds 8g and 8j are non-intercalative Topo II catalytic inhibitors
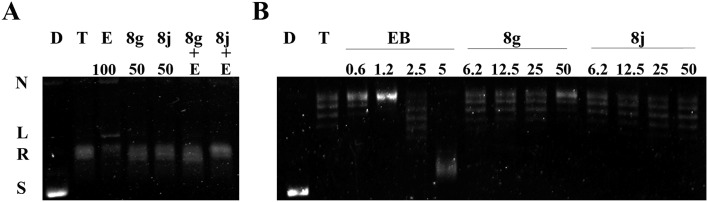
Topo II inhibitors have different mechanism of actions. Topo II poisons stabilize the cleavage complex of DNA and promote the formation of linear DNA, whereas Topo II catalytic inhibitors block the activity of the enzyme to perform catalysis.1–3 In addition, Topo II catalytic inhibitors can antagonize Topo II poison-mediated DNA damage.16 The Topo II-mediated DNA cleavage assay was employed for investigating the mode of action of 8g and 8j. As shown in Fig. 5A, etoposide, a classical Topo II poison, produced the linear DNA, while the linear form of the DNA was not observed when 8g and 8j were used up to 50 μM. In addition, pretreatment with 8g and 8j could reduce the amount of linear DNA trapped by etoposide. These results provide evidence that 8g and 8j act as Topo II catalytic inhibitors.
Fig. 5. (A) Effects of compounds 8g and 8j on Topo II-mediated DNA cleavage complex formation. Lanes 1 and 2: control group of supercoiled pBR322 DNA without or with Topo II; lanes 3–5, effects of etoposide (100 μM) and tested compounds (50 μM) on Topo II with DNA; lanes 6 and 7, pretreatment of the tested compounds (50 μM) antagonizes the etoposide (100 μM)-enhanced DNA cleavage. The positions of supercoiled DNA (S), relaxed DNA (R), linear DNA (L), and nicked DNA (N) are indicated. (B) The unwinding capacity of 8g and 8j. Lane D, pBR322 DNA; lane T, pBR322 DNA + Topo I; other lanes, pBR322 DNA + Topo I + 8g, 8j, or EB at different concentrations.
There are two types of Topo II catalytic inhibitors: DNA intercalators and non-intercalators.5 The Topo I-mediated DNA unwinding assay was carried out to determine whether 8g and 8j function as intercalators. In this assay, the supercoiled DNA, relaxed by excessive Topo I, could be regenerated if the test compound is a DNA intercalator. As shown in Fig. 5B, ethidium bromide (EB), a classic intercalator of DNA, did help the regeneration of the supercoiled DNA at 5 μM, while 8g and 8j didn't even at 50 μM. These findings suggest that 8g and 8j are non-intercalating Topo II catalytic inhibitors.
2.5. Molecular docking
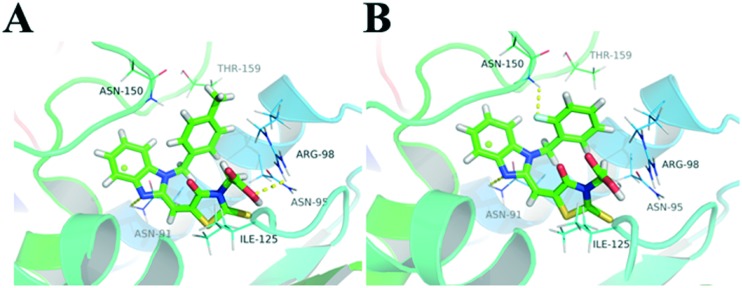
Molecular docking was carried out to identify the potential interactions of these compounds with Topo II by using the Surflex-Dock program incorporated with the SYBYL software package (Tripos. Inc. St. Louis. MO).32,33 We first docked 8g and 8j into various binding pockets in Topo II. The best result was obtained when the compounds were docked into the ATP-binding pocket (PDB code: ; 1ZXM). As shown in Fig. 6, for both compounds, the nitrogen group (N3) of benzimidazole formed a special hydrogen bond with the amino group of the residues Asn91, and the carboxylic group formed a hydrogen bond with the amino groups of Arg98 and Asn95. These hydrogen bonds may provide stability to the enzyme–ligand interactions. The benzimidazole group showed hydrophobic interactions with the hydrophobic part of the enzyme, whereas the phenyl group formed hydrophobic interactions with residues Asn150 and Thr159. These results showed that 8g and 8j are catalytic inhibitors, whose mode of action seems to occupy the ATP-binding pocket of the ATPase domain of Topo II and make favorable interactions with its key residues.
Fig. 6. Schematic representation of the proposed binding modes of 8g and 8j with the catalytic site of the ATPase domain of Topo II (PDB code:; 1ZXM) (A, compound 8g; B, compound 8j).
3. Conclusion
In this study, a series of benzimidazole–rhodanine conjugates were designed and synthesized as potential Topo II-targeting anticancer agents. The results revealed that these compounds displayed strong Topo II inhibitory potency and nine of them, namely, 8g, 8j, 8n, 9a, 9c, 10a, 10c, 10d, and 10e, almost completely suppressed the Topo II activity at 10 μM. The SAR study revealed that the benzyl group and the electronic effects of substitutions as well as the rhodanine moiety have significant impact on Topo II inhibitory potency. Mechanism studies demonstrated that the leading compounds 8g and 8j function as non-intercalative Topo II catalytic inhibitors. The molecular docking analysis suggested that the inhibition mode of 8g and 8j may be through the blocking of the ATP-binding site of the enzyme. These compounds displayed potent cytotoxic activities with low micromolar IC50 values toward six cancer cell lines. Meanwhile, most of the compounds showed a good relationship between their Topo II inhibitory potency and cytotoxicity. In conclusion, the pharmacophore hybridization strategy used in this work on the basis of benzimidazole with a rhodanine moiety represents a feasible way to discover Topo II-targeting anticancer agents. Further work is ongoing to optimize the bioactivity of these compounds.
4. Experimental section
4.1. Chemistry
All reagents were commercially available and purchased from Sigma-Aldrich, TCI, Alfa Aesar, and Aladdin. They were used without further purification. HPLC grade methanol was ordered from Sinopharm (China) and silica gel (200–300 mesh) was purchased from Qingdao Haiyang Chemical Co., Ltd. 1H and 13C NMR spectra were recorded in DMSO-d6 or CDCl3 with a Bruker BioSpin GmbH spectrometer at 400 and 101 MHz, respectively. The chemical shifts are reported in parts per million (ppm) relative to residual CDCl3 (δ = 7.26, 1H; δ = 77.0, 13C) and DMSO-d6 (δ = 2.50, 1H; δ = 39.5, 13C) in the corresponding deuterium agents. High-resolution mass spectra (HRMS) were recorded on Shimadzu LCMS-IT-TO. The melting point (mp) was determined using an SRS-OptiMelt automated melting point instrument without correction. The purities of the synthesized compounds were confirmed by analytical HPLC performed with a dual pump Shimadzu LC-20AB system equipped with an Ultimate XB-C18 column and eluted with methanol/water (80%) at a flow rate of 0.5 mL min–1, and the purities were proved to be higher than 95%. The chemical structure and the general method for the synthesis of the intermediates are listed in the ESI.†
4.2. General method for synthesis of the target compounds (8a–8o, 9a–9d, 10a–10h and 11a–11h)
A mixture of the appropriate aldehydes (5a–5s, 6a–6f and 7a–7f, 0.1 mmol), the rhodanine moiety (0.1 mmol), and NaOAc (0.3 mmol) in acetic acid (6 mL) was heated to 110 °C for 4 h. Then, it was cooled to room temperature and poured into water. The product was then filtered through the suction pump, washed with water/EtOH (1/1, v/v) to remove the excess acetic acid and recrystallized from EtOH.
4.2.1. (Z)-2-(5-((1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8a)
Rhodanine-3-acetic acid and 5a were used as reactants to give 8a. Yellow solid, yield: 73%, mp: 297.1–299.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.45 (s, 1H), 7.97 (s, 1H), 7.85 (d, J = 7.5 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.53 (d, J = 8.1 Hz, 2H), 7.41–7.33 (m, 2H), 7.10 (d, J = 8.0 Hz, 2H), 5.88 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 147.8, 143.6, 136.9, 135.9, 132.3, 130.3, 129.2, 125.5, 124.3, 121.4, 120.6, 116.4, 112.0, 46.1, 45.2. HRMS (ESI): C20H14BrN3O3S2 [M + H]+ 487.9717, found 487.9711.
4.2.2. (Z)-2-(5-((1-(4-Bromo-2-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8b)
Rhodanine-3-acetic acid and 5b were used as reactants to give 8b. Yellow solid, yield: 86%, mp: 283.7–285.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.46 (s, 1H), 8.03 (s, 1H), 7.85 (d, J = 7.4 Hz, 1H), 7.67 (d, J = 7.6 Hz, 1H), 7.60 (d, J = 9.9 Hz, 1H), 7.39–7.33 (m, 3H), 7.05 (t, J = 8.2 Hz, 1H), 5.91 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3 (d, J = 251.0 Hz), 161.6, 159.1, 147.9, 143.5, 135.7, 131.1 (d, J = 4.5 Hz), 130.2, 128.5 (d, J = 3.5 Hz), 125.5, 124.3, 123.7 (d, J = 14.7 Hz), 122.0 (d, J = 9.6 Hz), 120.6, 119.6 (d, J = 24.5 Hz), 116.6, 111.9, 45.2, 41.4 (d, J = 2.6 Hz). HRMS (ESI): calcd for C20H13BrFN3O3S2 [M + H]+ 505.9663, found 505.9658.
4.2.3. (Z)-2-(5-((1-(4-Cyanobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl) acetic acid (8c)
Rhodanine-3-acetic acid and 5c were used as reactants to give 8c. Yellow solid, yield: 91%, mp: 284.6–286.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.47 (s, 1H), 7.96 (s, 1H), 7.87 (d, J = 7.0 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.66 (d, J = 8.0 Hz, 1H), 7.41–7.34 (m, 2H), 7.28 (d, J = 8.0 Hz, 2H), 6.02 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.2, 167.8, 166.3, 147.9, 143.56, 143.0, 135.9, 133.3, 130.5, 127.8, 125.6, 124.4, 120.6, 119.0, 116.2, 111.9, 111.1, 46.3, 45.2. HRMS (ESI): calcd for C20H14FN3O3S2 [M + H]+ 435.0531, found 435.0542.
4.2.4. (Z)-2-(5-((1-(4-Nitrobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8d)
Rhodanine-3-acetic acid and 5d were used as reactants to give 8d. Yellow solid, yield: 79%, mp: 290.5–292.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 8.20 (d, J = 8.4 Hz, 2H), 7.97 (s, 1H), 7.88 (d, J = 6.5 Hz, 1H), 7.67 (d, J = 8.1 Hz, 1H), 7.41–7.33 (m, 4H), 6.08 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.2, 167.7, 166.2, 147.8, 147.5, 144.9, 143.6, 135.8, 130.5, 128.1, 125.6, 124.5, 124.4, 120.7, 116.2, 111.9, 46.2, 45.2. HRMS (ESI): calcd for C20H14N4O5S2 [M + H]+ 455.0439, found 455.0427.
4.2.5. (Z)-2-(5-((1-(4-Fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8e)
Rhodanine-3-acetic acid and 5e were used as reactants to give 8e. Yellow solid, yield: 83%, mp: 287.7–289.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.46 (s, 1H), 7.99 (s, 1H), 7.85 (d, J = 7.6 Hz, 1H), 7.72 (d, J = 7.9 Hz, 1H), 7.41–7.33 (m, 2H), 7.23–7.15 (m, 4H), 5.88 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 162.1 (d, J = 244.0 Hz), 147.7, 143.6, 135.9, 133.6 (d, J = 3.0 Hz), 130.3, 129.2 (d, J = 8.4 Hz), 125.5, 124.3, 120.6, 116.4, 116.1 (d, J = 21.6 Hz), 112.0, 46.0, 45.2. HRMS (ESI): calcd for C21H14N4O3S2 [M + H]+ 428.0508, found 428.0524.
4.2.6. (Z)-2-(5-((1-Benzyl-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8f)
Rhodanine-3-acetic acid and 5f were used as reactants to give 8f. Yellow solid, yield: 77%, mp: 293.4–295.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.45 (s, 1H), 7.97 (s, 1H), 7.85 (d, J = 7.4 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.40–7.32 (m, 4H), 7.29–7.24 (m, 1H), 7.15 (d, J = 7.5 Hz, 2H), 5.89 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 147.8, 143.6, 137.4, 136.0, 130.1, 129.4, 128.2, 127.0, 125.4, 124.2, 120.6, 116.5, 112.0, 46.7, 45.2. HRMS (ESI): calcd for C20H15N3O3S2 [M + H]+ 409.0619, found 409.0627.
4.2.7. (Z)-2-(5-((1-(4-Methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8g)
Rhodanine-3-acetic acid and 5g were used as reactants to give 8g. Yellow solid, yield: 87%, mp: >300 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.47 (s, 1H), 7.95 (s, 1H), 7.84 (d, J = 7.6 Hz, 1H), 7.72 (d, J = 7.8 Hz, 1H), 7.40–7.32 (m, 2H), 7.16–7.10 (m, 2H), 7.08–7.02 (m, 2H), 5.82 (s, 2H), 4.74 (s, 2H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 147.7, 143.6, 137.6, 136.0, 134.4, 130.1, 129.9, 127.0, 125.4, 124.2, 120.5, 116.6, 112.1, 46.5, 45.3, 21.1. HRMS (ESI): calcd for C21H17N3O3S2 [M + H]+ 424.0753, found 424.0739.
4.2.8. (Z)-2-(5-((1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8h)
Rhodanine-3-acetic acid and 5h were used as reactants to give 8h. Yellow solid, yield: 90%, mp: >300 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.46 (s, 1H), 7.97 (s, 1H), 7.85 (d, J = 7.6 Hz, 1H), 7.70 (d, J = 7.7 Hz, 1H), 7.42–7.33 (m, 4H), 7.19–7.15 (m, 2H), 5.89 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 147.8, 143.6, 136.5, 135.9, 132.9, 130.3, 129.4, 128.9, 125.5, 124.3, 120.6, 116.4, 112.0, 46.0, 45.2. HRMS (ESI): calcd for C20H14ClN3O3S2 [M + H]+ 444.0212, found 444.0226.
4.2.9. (Z)-2-(5-(1-(4-Trifluoromethylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-(4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8i)
Rhodanine-3-acetic acid and 5i were used as reactants to give 8i. Yellow solid, yield: 81%, mp: 281.8–283.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 7.98 (s, 1H), 7.87 (d, J = 7.1 Hz, 1H), 7.70 (m, 3H), 7.42–7.35 (m, 2H), 7.32 (d, J = 8.0 Hz, 2H), 6.02 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.7, 166.3, 147.9, 143.6, 142.1, 135.9, 130.5, 128.8 (q, J = 32.0 Hz), 127.7, 126.3, (q, J = 3.7 Hz), 125.6, 124.6 (q, J = 272.0 Hz), 120.6, 116.3, 111.2, 46.2, 45.2. HRMS (ESI): calcd for C21H14F3N3O3S2 [M + H]+ 478.0471, found 478.0462.
4.2.10. (Z)-2-(5-((1-(2-Fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8j)
Rhodanine-3-acetic acid and 5j were used as reactants to give 8j. Yellow solid, yield: 82%, mp: 272.6–274.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 8.03 (s, 1H), 7.84 (d, J = 7.2 Hz, 1H), 7.69 (d, J = 7.3 Hz, 1H), 7.42–7.31 (m, 3H), 7.29–7.20 (m, 1H), 7.19–7.07 (m, 2H), 5.93 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.4, 167.8, 166.3, 160.5 (d, J = 245.7 Hz), 147.9, 143.5, 135.8, 130.7 (d, J = 8.2 Hz), 130.1, 129.6 (d, J = 3.8 Hz), 125.4 (d, J = 2.3 Hz), 125.4, 124.2, 124.0 (d, J = 14.5 Hz), 120.6, 116.7, 116.2 (d, J = 20.9 Hz), 112.0, 45.2, 41.7 (d, J = 3.4 Hz). HRMS (ESI): calcd for C20H14FN3O3S2 [M + H]+ 428.0516, found 428.0523.
4.2.11. (Z)-2-(5-((1-(2,4-Difluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8k)
Rhodanine-3-acetic acid and 5k were used as reactants to give 8k. Yellow solid, yield: 77%, mp: 261.8–263.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.48 (s, 1H), 8.05 (s, 1H), 7.84 (d, J = 7.6 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.44–7.19 (m, 4H), 7.06 (t, J = 8.5 Hz, 1H), 5.90 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.4, 167.8, 166.3, 162.5 (dd, J = 247.2, 12.2 Hz), 160.64 (dd, J = 248.9, 12.5 Hz), 147.8, 143.5, 135.7, 131.1 (dd, J = 10.0, 5.5 Hz), 130.1, 125.4, 124.3, 120.6, 120.5 (dd, J = 14.8, 3.6 Hz), 116.6, 112.4 (dd, J = 21.3, 3.5 Hz), 111.95, 104.9 (t, J = 25.8 Hz), 45.2, 41.3 (d, J = 2.4 Hz). HRMS (ESI): calcd for C20H13F2N3O3S2 [M + H]+ 446.0427, found 446.0438.
4.2.12. (Z)-2-(5-((1-(3-Fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8l)
Rhodanine-3-acetic acid and 5l were used as reactants to give 8l. Yellow solid, yield: 71%, mp: 288.2–290.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.55 (s, 1H), 7.97 (s, 1H), 7.86 (d, J = 7.4 Hz, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.45–7.32 (m, 3H), 7.12 (t, J = 8.5 Hz, 1H), 7.05 (d, J = 9.8 Hz, 1H), 6.90 (d, J = 7.6 Hz, 1H), 5.91 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 162.7 (d, J = 244.7 Hz), 147.8, 143.6, 140.2 (d, J = 7.2 Hz), 135.9, 131.5 (d, J = 8.4 Hz), 130.4, 125.56, 124.3, 122.9 (d, J = 2.6 Hz), 120.6, 116.3, 115.1 (d, J = 20.9 Hz), 114.0 (d, J = 22.1 Hz), 111.9, 46.2 (d, J = 0.6 Hz), 45.2. HRMS (ESI): calcd for C20H14FN3O3S2 [M + H]+ 428.0516, found 428.0522.
4.2.13. (Z)-2-(5-((1-(3,4-Difluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8m)
Rhodanine-3-acetic acid and 5m were used as reactants to give 8m. Yellow solid, yield: 76%, mp: 267.6–269.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.46 (s, 1H), 8.00 (s, 1H), 7.85 (d, J = 7.6 Hz, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.48–7.28 (m, 4H), 6.93 (s, 1H), 5.88 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 149.9 (dd, J = 246.8, 12.8 Hz), 149.4 (dd, J = 246.1, 12.4 Hz), 147.8, 143.6, 135.8, 135.1 (dd, J = 5.4, 3.9 Hz), 130.4, 125.6, 124.3, 123.9 (dd, J = 6.7, 3.5 Hz), 120.6, 118.5 (d, J = 17.4 Hz), 116.6 (d, J = 17.8 Hz), 116.3, 111.9, 45.7, 45.2. HRMS (ESI): calcd for C20H13F2N3O3S2 [M + H]+ 446.0527, found 446.0532.
4.2.14. (Z)-2-(5-((1-(3,5-Dimethoxybenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8n)
Rhodanine-3-acetic acid and 5n were used as reactants to give 8n. Yellow solid, yield: 78%, mp: 259.8–261.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.95 (s, 1H), 7.85 (d, J = 7.5 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.44–7.33 (m, 2H), 6.41 (t, J = 2.1 Hz, 1H), 6.27 (d, J = 2.1 Hz, 2H), 5.79 (s, 2H), 4.74 (s, 2H), 3.66 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.7, 166.3, 161.3, 147.8, 143.5, 139.7, 136.1, 130.1, 125.5, 124.2, 120.6, 116.5, 112.0, 105.4, 99.3, 55.7, 46.6, 45.2. HRMS (ESI): calcd for C22H19N3O5S2 [M + H]+ 470.0882, found 470.0871.
4.2.15. (Z)-2-(5-((1-Ethyl-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (8o)
Rhodanine-3-acetic acid and 5o were used as reactants to give 8o. Yellow solid, yield: 81%, mp: 226.5–227.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.45 (s, 1H), 7.93 (s, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 8.1 Hz, 1H), 7.40 (t, J = 7.2 Hz, 1H), 7.34 (t, J = 7.2 Hz, 1H), 4.76 (s, 2H), 4.60 (q, J = 7.1 Hz, 2H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.4, 167.8, 166.4, 147.0, 143.6, 135.4, 129.7, 125.2, 124.0, 120.5, 116.6, 111.7, 45.2, 38.7, 16.3. HRMS (ESI): calcd for C15H13N3O3S2 [M + H]+ 348.0428, found 348.0422.
4.2.16. (Z)-2-(5-((1-(2-(4-Fluorophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (9a)
Rhodanine-3-acetic acid and 5p were used as reactants to give 9a. Yellow solid, yield: 81%, mp: 247.7–249.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.27–8.21 (m, 2H), 7.97 (s, 1H), 7.89–7.85 (m, 1H), 7.68–7.64 (m, 1H), 7.51 (t, J = 8.7 Hz, 2H), 7.38–7.32 (m, 2H), 6.42 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO) δ 198.5, 192.3, 167.8, 166.3, 166.1(d, J = 253.2 Hz), 148.8, 143.4, 136.5, 132.1 (d, J = 9.7 Hz), 131.6 (d, J = 2.7 Hz), 129.8, 125.2, 124.1, 120.4, 117.4, 116.4 (d, J = 22.1 Hz), 111.89, 50.6, 45.2. HRMS (ESI): calcd for C21H14FN3O4S2 [M + H]+ 456.0429, found 456.0431.
4.2.17. (Z)-2-(5-((1-(2-(4-Methoxyphenyl)-2-oxoethyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (9b)
Rhodanine-3-acetic acid and 5q were used as reactants to give 9b. Yellow solid, yield: 72%, mp: 256.4–258.1 °C. δ 13.36 (s, 1H), 8.12 (d, J = 8.7 Hz, 2H), 7.90 (s, 1H), 7.88–7.82 (m, 1H), 7.67–7.61 (m, 1H), 7.38–7.32 (m, 2H), 7.17 (d, J = 8.7 Hz, 2H), 6.37 (s, 2H), 4.74 (s, 2H), 3.90 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.5, 191.8, 167.8, 166.3, 164.5, 148.8, 143.5, 136.5, 131.4, 129.7, 127.7, 125.2, 124.0, 120.4, 117.4, 114.6, 111.8, 56.2, 50.2, 45.2. HRMS (ESI): calcd for C22H17N3O5S2 [M + H]+ 468.0653, found 468.0641.
4.2.18. (Z)-2-(5-((1-(2-(4-Chlorophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (9c)
Rhodanine-3-acetic acid and 5r were used as reactants to give 9c. Yellow solid, yield: 83%, mp: 256.9–258.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.16 (d, J = 8.5 Hz, 2H), 7.98 (s, 1H), 7.90–7.83 (m, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.69–7.63 (m, 1H), 7.38–7.32 (m, 2H), 6.44 (d, J = 19.5 Hz, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.5, 192.7, 167.8, 166.3, 148.8, 143.4, 139.6, 136.5, 133.5, 130.9, 129.8, 129.4, 125.2, 124.1, 120.4, 117.4, 111.9, 50.6, 45.2. HRMS (ESI): calcd for C22H17ClN3O4S2 [M + H]+ 472.0193, found 472.0172.
4.2.19. (Z)-2-(4-Oxo-5-((1-(2-oxo-2-(p-tolyl)ethyl)-1H-benzo[d]imidazol-2-yl)methylene)-2-thioxothiazolidin-3-yl)acetic acid (9d)
Rhodanine-3-acetic acid and 5s were used as reactants to give 9d. Yellow solid, yield: 76%, mp: 263.1–265.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.06 (d, J = 7.7 Hz, 2H), 7.93 (s, 1H), 7.88–7.84 (m, 1H), 7.67–7.63 (m, 1H), 7.47 (d, J = 7.6 Hz, 2H), 7.40–7.34 (s, 2H), 6.39 (s, 2H), 4.74 (s, 2H), 2.46 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.5, 193.1, 167.8, 166.3, 148.8, 145.3, 143.5, 136.5, 132.3, 129.9, 129.7, 129.1, 125.2, 124.1, 120.4, 117.4, 111.8, 50.4, 45.2, 21.8. HRMS (ESI): calcd for C22H17N3O4S2 [M + H]+ 452.0727, found 452.0731.
4.2.20. (Z)-2-(5-((5-Chloro-1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (10a)
Rhodanine-3-acetic acid and 6a were used as reactants to give 10a. Yellow solid, yield: 84%, mp: 297.9–299.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.48 (s, 1H), 7.95–7.91 (m, 2H), 7.77 (d, J = 8.8 Hz, 1H), 7.42 (dd, J = 8.7, 1.8 Hz, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 5.83 (s, 2H), 4.74 (s, 2H), 2.24 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.0, 167.8, 166.3, 148.8, 142.2, 137.6, 136.7, 134.2, 130.8, 123.0, 129.9, 127.0, 124.7, 121.9, 116.1, 111.9, 46.6, 45.3, 21.1. HRMS (ESI): calcd for C21H16ClN3O3S2 [M + H]+ 458.0334, found 458.0329.
4.2.21. (Z)-2-(5-((6-Chloro-1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (10b)
Rhodanine-3-acetic acid and 6b were used as reactants to give 10b. Yellow solid, yield: 69%, mp: >300 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.48 (s, 1H), 7.94–7.91 (m, 2H), 7.86 (d, J = 8.7 Hz, 1H), 7.37 (dd, J = 8.7, 1.9 Hz, 1H), 7.15 (d, J = 7.9 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 5.83 (s, 2H), 4.74 (s, 2H), 2.25 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.1, 167.8, 166.3, 149.1, 144.1, 137.6, 134.8, 134.2, 131.1, 129.9, 128.7, 127.0, 125.5, 119.8, 116.9, 113.5, 46.7, 45.2, 21.1. HRMS (ESI): calcd for C21H16ClN3O3S2 [M + H]+ 458.0334, found 458.0343.
4.2.22. (Z)-2-(5-((5-Fluoro-1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (10c)
Rhodanine-3-acetic acid and 6c were used as reactants to give 10c. Yellow solid, yield: 77%, mp: 291.5–292.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 7.96 (s, 1H), 7.78–7.74 (m, 1H), 7.68 (dd, J = 9.5, 2.4 Hz, 1H), 7.32–7.24 (m, 1H), 7.15 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 5.85 (s, 2H), 4.76 (s, 2H), 2.25 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.1, 167.8, 166.3, 159.9 (d, J = 238.1 Hz), 149.1, 143.7 (d, J = 13.3 Hz), 137.6, 134.3, 132.8, 130.7, 129.9, 127.0, 116.2, 114.0 (d, J = 26.7 Hz), 113.2 (d, J = 10.5 Hz), 105.7 (d, J = 24.1 Hz), 46.7, 45.3, 21.1. HRMS (ESI): calcd for C21H16FN3O3S2 [M + H]+ 442.0651, found 442.0641.
4.2.23. (Z)-2-(5-((6-Fluoro-1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (10d)
Rhodanine-3-acetic acid and 6d were used as reactants to give 10d. Yellow solid, yield: 73%, mp: >300 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.48 (s, 1H), 7.93 (s, 1H), 7.91–7.87 (m, 1H), 7.70 (dd, J = 9.2, 2.4 Hz, 1H), 7.27–7.21 (m, 1H), 7.16 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 5.82 (s, 2H), 4.75 (s, 2H), 2.26 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.1, 167.8, 166.38, 160.6 (d, J = 241.1 Hz), 148.7 (d, J = 3.2 Hz), 140.2, 137.6, 136.5, 134.2, 129.9, 127.1, 127.0, 121.9 (d, J = 10.4 Hz), 116.3, 112.9 (d, J = 26.0 Hz), 98.5 (d, J = 27.9 Hz), 46.6, 45.2, 21.1. HRMS (ESI): calcd for C21H16FN3O3S2 [M + H]+ 442.0651, found 442.0647.
4.2.24. (Z)-2-(5-((5-Methoxy-1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (10e)
Rhodanine-3-acetic acid and 6e were used as reactants to give 10e. Yellow solid, yield: 85%, mp: 295.3–296.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 7.92 (s, 1H), 7.61 (d, J = 9.0 Hz, 1H), 7.32 (d, J = 2.3 Hz, 1H), 7.13 (d, J = 8.0 Hz, 2H), 7.05–7.00 (m, 3H), 5.78 (s, 2H), 4.73 (s, 2H), 3.83 (s, 3H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.3, 167.8, 166.3, 157.4, 147.5, 144.6, 137.5, 134.5, 130.8, 129.9, 129.1, 127.0, 116.6, 116.5, 112.6, 101.5, 56.1, 46.5, 45.2, 21.1. HRMS (ESI): calcd for C22H19N3O4S2 [M + H]+ 454.0821, found 454.0826.
4.2.25. (Z)-2-(5-((6-Methoxy-1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (10f)
Rhodanine-3-acetic acid and 6f were used as reactants to give 10f. Yellow solid, yield: 84%, mp: 294.2–295.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.43 (s, 1H), 7.84 (s, 1H), 7.73 (d, J = 8.9 Hz, 1H), 7.29 (d, J = 2.2 Hz, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 6.98 (dd, J = 8.9, 2.3 Hz, 1H), 5.79 (s, 2H), 4.72 (s, 2H), 3.82 (s, 3H), 2.24 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.2, 167.8, 166.3, 158.6, 146.8, 138.5, 137.5, 137.2, 134.5, 129.9, 127.8, 127.0, 121.4, 116.7, 115.0, 94.2, 56.3, 46.2, 45.2, 21.1. HRMS (ESI): calcd for C22H19N3O4S2 [M + H]+ 454.0821, found 454.0812.
4.2.26. (Z)-5-((1-(4-Methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-2-thioxothiazolidin-4-one (10g)
Rhodanine and 5g were used as reactants to give 10g. Yellow solid, yield: 86%, mp: >300 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.77 (s, 1H), 7.80 (d, J = 7.5 Hz, 1H), 7.70 (d, J = 6.8 Hz, 2H), 7.38–7.30 (m, 2H), 7.12 (d, J = 7.9 Hz, 2H), 7.03 (d, J = 7.9 Hz, 2H), 5.78 (s, 2H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 200.7, 169.2, 147.8, 143.5, 137.5, 136.0, 134.5, 133.8, 129.9, 127.0, 125.1, 124.0, 120.4, 114.5, 111.9, 46.4, 21.1. HRMS (ESI): calcd for C19H15N3OS2 [M + H]+ 366.0752, found 366.0743.
4.2.27. (Z)-3-(5-((1-(4-Methylbenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)propanoic acid (10h)
Rhodanine-3-propanoic acid and 5g were used as reactants to give 10h. Yellow solid, yield: 92%, mp: 267.1–269.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.49 (s, 1H), 7.86 (s, 1H), 7.82 (d, J = 7.4 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.35 (dq, J = 13.5, 6.6 Hz, 2H), 7.13 (d, J = 7.9 Hz, 2H), 7.03 (d, J = 8.0 Hz, 2H), 5.81 (s, 2H), 4.25–4.20 (m, 2H), 2.67–2.62 (m, 2H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.4, 172.2, 166.7, 147.8, 143.6, 137.5, 136.0, 134.5, 130.8, 129.9, 127.0, 125.3, 124.1, 120.5, 115.5, 112.0, 46.4, 31.4, 21.1. HRMS (ESI): calcd for C22H19N3O3S2 [M + H]+ 438.0934, found 438.0926.
4.2.28. (Z)-2-(5-((5-Chloro-1-(2-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (11a)
Rhodanine-3-acetic acid and 7a were used as reactants to give 11a. Yellow solid, yield: 71%, mp: 296.4–298.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.50 (s, 1H), 8.02 (s, 1H), 7.94 (d, J = 1.8 Hz, 1H), 7.74 (d, J = 8.8 Hz, 1H), 7.42 (dd, J = 8.8, 2.0 Hz, 1H), 7.40–7.34 (m, 1H), 7.27–7.22 (m, 1H), 7.19–7.10 (m, 2H), 5.95 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.1, 167.8, 166.3, 160.49 (d, J = 245.9 Hz), 149.2, 144.1, 134.7, 131.1, 130.8 (d, J = 8.2 Hz), 129.6 (d, J = 3.8 Hz), 128.7, 125.5 (d, J = 3.4 Hz), 123.9 (d, J = 14.5 Hz), 119.8, 116.4, 116.2, 113.5, 45.3, 42.0 (d, J = 3.4 Hz). HRMS (ESI): calcd for C20H13ClFN3O3S2 [M + H]+ 462.0142, found 462.0131.
4.2.29. (Z)-2-(5-((6-Chloro-1-(2-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (11b)
Rhodanine-3-acetic acid and 7b were used as reactants to give 11b. Yellow solid, yield: 76%, mp: 288.7–290.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.48 (s, 1H), 7.98 (s, 1H), 7.90 (d, J = 1.8 Hz, 1H), 7.87 (d, J = 8.7 Hz, 1H), 7.41–7.36 (m, 2H), 7.28–7.22 (m, 1H), 7.20–7.10 (m, 2H), 5.94 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.1, 167.8, 166.3, 160.48 (d, J = 246.0 Hz), 148.9, 142.1, 136.6, 130.8, 129.9, 129.5 (d, J = 3.8 Hz), 125.4 (d, J = 3.3 Hz), 124.4, 123.8 (d, J = 14.6 Hz), 121.9, 116.2, 111.9, 45.3, 41.9 (d, J = 2.9 Hz). HRMS (ESI): calcd for C20H13ClFN3O3S2 [M + H]+ 462.0142, found 462.0137.
4.2.30. (Z)-2-(5-((5-Fluoro-1-(2-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (11c)
Rhodanine-3-acetic acid and 7c were used as reactants to give 11c. Yellow solid, yield: 77%, mp: 292.4–293.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.47 (s, 1H), 8.01 (s, 1H), 7.73 (dd, J = 9.0, 4.7 Hz, 1H), 7.68 (dd, J = 9.5, 2.3 Hz, 1H), 7.39–7.35 (m, 1H), 7.31–7.21 (m, 2H), 7.18–7.10 (m, 2H), 5.94 (s, 2H), 4.75 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.1, 167.8, 166.3, 160.6 (d, J = 240.6 Hz), 160.5 (d, J = 246.0 Hz), 148.8 (d, J = 3.1 Hz), 140.2, 136.4 (d, J = 14.0 Hz), 130.9 (d, J = 8.1 Hz), 123.0, 129.6 (d, J = 3.8 Hz), 125.4 (d, J = 3.4 Hz), 123.7 (d, J = 14.6 Hz), 122.0 (d, J = 10.3 Hz), 116.4, 116.2, 112.9 (d, J = 25.7 Hz), 98.4 (d, J = 28.1 Hz), 45.2, 41.9 (d, J = 2.6 Hz). HRMS (ESI): calcd for C20H13F2N3O3S2 [M + H]+ 446.0471, found 446.0438.
4.2.31. (Z)-2-(5-((6-Fluoro-1-(2-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (11d)
Rhodanine-3-acetic acid and 7d were used as reactants to give 11d. Yellow solid, yield: 83%, mp: 275.4–277.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.46 (s, 1H), 7.97 (s, 1H), 7.90–7.86 (m, 1H), 7.64 (dd, J = 9.2, 2.3 Hz, 1H), 7.39–7.34 (m, 1H), 7.28–7.20 (m, 2H), 7.18–7.11 (m, 2H), 5.91 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.2, 167.8, 166.3, 160.5 (d, J = 245.9 Hz), 159.9 (d, J = 237.9 Hz), 149.3, 143.6 (d, J = 13.3 Hz), 132.6, 130.8 (d, J = 8.4 Hz), 130.6 129.6 (d, J = 3.8 Hz), 125.4 (d, J = 3.3 Hz), 123.8 (d, J = 14.5 Hz), 116.4, 116.2, 113.9 (d, J = 26.6 Hz), 113.1 (d, J = 10.1 Hz), 105.9 (d, J = 24.2 Hz), 45.2, 41.9 (d, J = 3.1 Hz). HRMS (ESI): calcd for C20H13F2N3O3S2 [M + H]+ 446.0451, found 446.0442.
4.2.32. (Z)-2-(5-((1-(2-Fluorobenzyl)-5-methoxy-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (11e)
Rhodanine-3-acetic acid and 7e were used as reactants to give 11e. Yellow solid, yield: 80%, mp: 278.8–280.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.46 (s, 1H), 7.99 (s, 1H), 7.58 (d, J = 9.0 Hz, 1H), 7.38–7.32 (m, 2H), 7.26–7.21 (m, 1H), 7.15 (t, J = 7.4 Hz, 1H), 7.09–7.05 (m, 1H), 7.01 (dd, J = 9.0, 2.3 Hz, 1H), 5.89 (s, 2H), 4.74 (s, 2H), 3.83 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.4, 167.8, 166.4, 160.4 (d, J = 245.8 Hz), 157.4, 147.7, 144.5, 130.7 (d, J = 8.2 Hz), 129.5 (d, J = 3.8 Hz), 129.1, 125.4 (d, J = 3.4 Hz), 124.1 (d, J = 14.5 Hz), 116.7, 116.5, 116.1 (d, J = 20.9 Hz), 112.6, 101.6, 56.1, 45.2, 41.7 (d, J = 3.2 Hz). HRMS (ESI): calcd for C21H16FN3O4S2 [M + H]+ 458.0619, found 458.0623.
4.2.33. (Z)-2-(5-((1-(2-Fluorobenzyl)-6-methoxy-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid (11f)
Rhodanine-3-acetic acid and 7f were used as reactants to give 11f. Yellow solid, yield: 91%, mp: 283.4–285.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H), 7.91 (s, 1H), 7.74 (d, J = 8.9 Hz, 1H), 7.38–7.34 (m, 1H), 7.29–7.23 (m, 2H), 7.15 (t, J = 7.6, 0.8 Hz, 1H), 7.03 (t, J = 7.7, 1.2 Hz, 1H), 6.99 (dd, J = 8.9, 2.3 Hz, 1H), 5.90 (s, 2H), 4.73 (s, 2H), 3.81 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 198.2, 167.8, 166.3, 160.4 (d, J = 245.6 Hz), 158.5, 147.0, 138.5, 137.0, 130.7 (d, J = 8.0 Hz), 128.0, 125.4 (d, J = 3.4 Hz), 124.1 (d, J = 14.6 Hz), 121.4, 116.8, 116.1 (d, J = 20.7 Hz), 114.9, 94.3, 56.2, 45.2, 41.4 (d, J = 3.5 Hz). HRMS (ESI): calcd for C21H16FN3O4S2 [M + H]+ 458.0619, found 458.0626.
4.2.34. (Z)-5-((1-(2-Fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-2-thioxothiazolidin-4-one (11g)
Rhodanine and 5j were used as reactants to give 11g. Yellow solid, yield: 90%, mp: 258.4–260.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.81 (s, 1H), 7.89–7.61 (m, 3H), 7.27 (m, 4H), 7.19–6.98 (m, 2H), 5.88 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 200.7, 169.2, 160.4 (d, J = 245.8 Hz), 148.0, 143.5, 135.8, 133.9, 130.7 (d, J = 8.1 Hz), 129.5 (d, J = 3.6 Hz), 125.8.6, 125.3 (d, J = 3.2 Hz), 124.1 (d, J = 14.5 Hz), 120.4, 116.1 (d, J = 20.9 Hz), 114.6, 111.8, 41.6 (d, J = 3.3 Hz). HRMS (ESI): calcd for C14H12FN3OS2 [M + H]+ 370.0418, found 370.0432.
4.2.35. (Z)-3-(5-((1-(2-Fluorobenzyl)-1H-benzo[d]imidazol-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)propanoic acid (11h)
Rhodanine-3-propanoic and 5j were used as reactants to give 11h. Yellow solid, yield: 86%, mp: 258.4–260.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.51 (s, 1H), 7.94 (s, 1H), 7.85–7.81 (m, 1H), 7.68 (d, J = 7.5 Hz, 1H), 7.39–7.31 (m, 3H), 7.27–7.22 (m, 1H), 7.14 (t, J = 7.4 Hz, 1H), 7.06 (t, J = 7.0 Hz, 1H), 5.92 (s, 2H), 4.26–4.20 (m, 2H), 2.68–2.62 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 198.4, 172.2, 166.7, 160.4 (d, J = 245.9 Hz), 148.0, 143.5, 135.8, 131.00, 130.8 (d, J = 8.2 Hz), 129.6 (d, J = 3.5 Hz), 125.4 (d, J = 7.2 Hz), 124.1, 120.5, 116.1 (d, J = 20.9 Hz), 115.6, 111.9, 41.6, 41.5, 31.4. HRMS (ESI): calcd for C21H16FN3O3S2 [M + H]+ 442.0617, found 442.0623.
4.3. Biological evaluation
4.3.1. Topo I inhibitory activity
In this relaxation assay, pBR322 plasmid (TaKaRa, Kyoto, Japan) was used to determine the effects of the synthesized compounds on DNA relaxation catalyzed by Topo I (TaKaRa, Kyoto, Japan) by using camptothecin as a positive control. The reaction mixture was prepared according to the literature with minor modifications, and incubated at 37 °C for 30 min. The reactions were terminated by the addition of dye solution containing 1% SDS, 0.02% bromophenol blue and 50% glycerol. The mixtures were applied to 1% agarose gel and subjected to electrophoresis for 1.5 h in 1× TAE buffer (40 mM Tris-acetate, 2 mM EDTA). Gels were stained for 30 min in an aqueous solution of Ged Red (0.5 μg ml–1). DNA bands were visualized by transillumination with UV light and then photographed with an Alpha Innotech digital imaging system.6
4.3.2. Topo I-mediated DNA unwinding assay
The relaxed pBR322 DNA plasmid utilized in this unwinding assay was generated by treating pBR322 with Topo I in the reaction buffer (50 mM Tris-HCl, pH 7.5, 50 mM KCl, 10 mM MgCl2, 0.5 mM DTT, 0.1 mM EDTA, and 30 μg ml–1 BSA) prior to the addition of other components. Assay mixtures are composed of 0.1 μg relaxed pBR322 DNA, Topo I (4 U), and the tested compounds in 20 μL Topo I reaction buffer. Following a 10 min incubation of DNA and drugs at room temperature, Topo I (1 U) was added, and the reaction mixtures were incubated at 37 °C for 30 min. An equal volume of phenol chloroform was added to stop the reaction. Aqueous samples (20 μL) were removed from the reaction, and 3 μL of stop solution (0.77% SDS, 77 mmol NaEDTA, pH 8.0) followed by 2 μL of agarose gel loading buffer was added to each sample. Samples were subjected to electrophoresis in 1× TAE buffer (40 mM Tris-acetate, 2 mM EDTA). DNA bands were visualized by transillumination with UV light and then photographed with an Alpha Innotech digital imaging system.8
4.3.3. Topo II inhibitory activity
The relaxation assay was carried out according to the manufacturer's instructions with minor modifications. The reaction mixture containing 200 ng of pBR322 DNA plasmid and 1 U of Topo II (TopoGEN) was incubated in the presence or absence of the synthesized compounds in a final volume of 20 μL in Topo II reaction buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10 mM MgCl2, 2 mM ATP, 0.5 mM DTT, and 30 μg mL–1 BSA). The mixture was incubated for 30 min at 37 °C and was terminated with 6× stop buffer (5 μL per 20 μL reaction volume). The stop buffer contained 5% sarcosyl, 0.02% bromophenol blue, and 25% glycerol. Reaction products were analyzed on a 1% agarose gel by electrophoresis in TAE buffer (40 mM Tris-acetate and 2 mM EDTA) for 1.5 h at 75 V. Gels were stained for 30 min in an aqueous solution of Ged Red (0.5 μg mL–1). DNA bands were visualized through transillumination with UV light and then photographed with an Alpha Innotech digital imaging system.16
4.3.4. Topo II-mediated DNA cleavage reaction assay
In brief, Topo II (6 U), 0.1 μg of pBR322 DNA, and 50 μM synthesized compounds (or etoposide, 100 μM) were employed in a total of 20 μL of Topo II buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10 mM MgCl2, 2 mM ATP, 0.5 mM DTT, and 30 μg mL–1 BSA). After incubating for 6 min at 37 °C to reach the cleavage religation equilibrium, cleavage intermediates were trapped by adding 2 μL of 1% SDS, followed by 2 μL of 250 mM NaEDTA at pH 8.0. Proteinase K was added (2 μL of 0.8 mg mL–1), and the reaction mixtures were incubated for 30 min at 45 °C to digest the Topo II. Samples were mixed with 2 μL of agarose gel loading buffer (30% sucrose, 0.5% bromophenol blue, and 0.5% xylene cyanol FF in 10 mM Tris-HCl, pH 7.9), heated at 72 °C for 2 min, and subjected to electrophoresis in a 1% agarose gel in TAE buffer (40 mM Tris-acetate and 2 mM EDTA) for 1 h at 75 V. Gels were stained for 30 min in an aqueous solution of Ged Red (0.5 μg mL–1) and kept under electrophoresis for 30–45 min at 75 V. Cleavage was monitored by the conversion of negatively supercoiled plasmid to nicked DNA. DNA bands were visualized by UV light and photographed with an Alpha Innotech digital imagingsystem.16
4.3.5. MTT assay
Briefly, the cells were plated at a density of 5000 per well in 96-well microplates and allowed to incubate overnight. The compounds were added to the wells at increasing concentrations (0–50 μM). After 48 h, each well was treated with 20 μL MTT (2.5 mg mL–1) solution, and the cells were further incubated at 37 °C for 4 h. At the end of the incubation, the untransformed MTT was removed, and 100 μL of DMSO was added. The microplates were well shaken to dissolve the formazan dye, and the absorbance at 570 nm was measured using a microplate reader (Bio-Tek).29
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8md00278a
References
- Bailly C. Chem. Rev. 2012;112:3611–3640. doi: 10.1021/cr200325f. [DOI] [PubMed] [Google Scholar]
- Pommier Y. ACS Chem. Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Sun Y., Huang S. N., Nitis J. L. Nat. Rev. Mol. Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capranico G., Marinello J., Chillemi G. J. Med. Chem. 2017;60:2169–2192. doi: 10.1021/acs.jmedchem.6b00966. [DOI] [PubMed] [Google Scholar]
- Nitiss J. L. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou M. S., Zarate M., Ricci F., Damia G., Pieraccini S., Dapiaggi F., Sironi M., Lo Presti L., García-Argaez A. N., Dalla Via L., Passarella D. Eur. J. Med. Chem. 2016;118:79–89. doi: 10.1016/j.ejmech.2016.03.090. [DOI] [PubMed] [Google Scholar]
- Christodoulou M. S., Calogero F., Baumann M., García-Argaez A. N., Pieraccini S., Sironi M., Dapiaggi F., Bucci R., Broggini G., Gazzola S., Liekens S., Silvani A., Lahtela-Kakkonen M., Martinet N., Nonell-Canals A., Santamaría-Navarro E., Baxendale I. R., Dalla Via D., Passarella D. Eur. J. Med. Chem. 2015;92:766–775. doi: 10.1016/j.ejmech.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Yao B. L., Mai Y. W., Chen S. B., Hua H. T., Yao P. F., Ou T. M., Tan J. H., Wang H. G., Li D., Huang S. L., Gu L. Q., Huang Z. S. Eur. J. Med. Chem. 2015;92:540–553. doi: 10.1016/j.ejmech.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Zhuo S. T., Li C. Y., Hu M. H., Chen S. B., Yao P. F., Huang S. L., Ou T. M., Tan J. H., An L. K., Li D., Huang Z. S. Org. Biomol. Chem. 2013;11:3989–4005. doi: 10.1039/c3ob40325d. [DOI] [PubMed] [Google Scholar]
- Ortega J. A., Riccardi L., Minniti E., Borgogno M., Arencibia J. M., Greco M. L., Minarini A., Sissi C., Vivo M. D. J. Med. Chem. 2018;61:1375–1379. doi: 10.1021/acs.jmedchem.7b01388. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., Li S. Q., Liao W. L., Xie Z. G., Wang M. S., Cao Y., Zhang J., Xu Z. G. Tetrahedron. 2015;71:8424–8427. [Google Scholar]
- Akhtar W., Khan M. F., Verma G., Shaquiquzzaman M., Rizvi M. A., Mehdi S. H., Akhter M., Mumtaz-Alam M. Eur. J. Med. Chem. 2017;106:705–753. doi: 10.1016/j.ejmech.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Baviskar A. T., Madaan C., Preet R., Mohapatra P., Jain V., Agarwal A., Guchhait S. K., Kundu C. N., Banerjee U. C., Bharatnam P. V. J. Med. Chem. 2011;54:5013–5030. doi: 10.1021/jm200235u. [DOI] [PubMed] [Google Scholar]
- Baviskar A. T., Amrutkar S. M., Trivedi N., Chaudhary V., Nayak A., Guchhait S. K., Banerjee U. C., Bharatam P. V., Kundu C. N. ACS Med. Chem. Lett. 2015;6:481–485. doi: 10.1021/acsmedchemlett.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshani G., Nayak A., Amrutkar S. M., Das S., Guchhait S. K., Kundu C. N., Banerjee U. C. ACS Med. Chem. Lett. 2016;7:1056–1061. doi: 10.1021/acsmedchemlett.6b00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. H., Zeng P., Chen S. B., Yao P. F., Mai Y. W., Tang J. H., Ou T. M., Huang S. L., Li D., Gu L. Q., Huang Z. S. J. Med. Chem. 2016;59:238–252. doi: 10.1021/acs.jmedchem.5b01284. [DOI] [PubMed] [Google Scholar]
- Mendgen T., Steuer C., Klein C. D. J. Med. Chem. 2012;55:743–753. doi: 10.1021/jm201243p. [DOI] [PubMed] [Google Scholar]
- Tang S. Q., Lee Y. Y. I., Packiaraj D. S., Ho H. K., Chai C. L. L. Chem. Res. Toxicol. 2015;28:2019–2023. doi: 10.1021/acs.chemrestox.5b00247. [DOI] [PubMed] [Google Scholar]
- Kaminskyy D., Kryshchyshyn A., Lesyk R. Expert Opin. Drug Discovery. 2017;12:1233–1252. doi: 10.1080/17460441.2017.1388370. [DOI] [PubMed] [Google Scholar]
- Li P. H., Jiang H., Zhang W. J., Li Y. L., Zhao M. C., Zhou W., Zhang L. Y., Tang Y. D., Dong C. Z., Huang Z. S., Chen H. X., Du Z. Y. Eur. J. Med. Chem. 2018;145:498–510. doi: 10.1016/j.ejmech.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Li C., Liu J. C., Li Y. R., Gou C., Zhang M. L., Liu H. Y., Li X. Z., Zheng C. J., Piao H. R. Bioorg. Med. Chem. Lett. 2015;25:3052–3056. doi: 10.1016/j.bmcl.2015.04.081. [DOI] [PubMed] [Google Scholar]
- Shubedar D. D., Shaikh M. H., Shigate B. B., Nawale L., Sarkar D., Khedar V. M., Khedar V. M., Khan F. A. K., Sangshetti J. N. Eur. J. Med. Chem. 2017;125:385–399. doi: 10.1016/j.ejmech.2016.09.059. [DOI] [PubMed] [Google Scholar]
- Fu H., Hou X., Wang L., Dun Y., Yang X., Fang H. Bioorg. Med. Chem. Lett. 2015;25:5265–5269. doi: 10.1016/j.bmcl.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Azizmohammadi M., Khoobi M., Ramazani A., Emami S., Zarrin A., Firuzi O., Miri R., Shafiee A. Eur. J. Med. Chem. 2013;59:15–22. doi: 10.1016/j.ejmech.2012.10.044. [DOI] [PubMed] [Google Scholar]
- Hueso-Falcón I., Amesty Á., Anaissi-Afonso L., Lorenzo-Castrillejo I., Machín F., Estévez-Braun A. Bioorg. Med. Chem. Lett. 2017;27:484–489. doi: 10.1016/j.bmcl.2016.12.040. [DOI] [PubMed] [Google Scholar]
- Tan S. Y., Sun D. H., Lyu J. K., Sun X., Wu F. S., Li Q., Yang Y. Q., Liu J. X., Wang X., Chen Z., Li H. L., Qian X. H., Xu Y. F. Bioorg. Med. Chem. 2015;23:5672–5680. doi: 10.1016/j.bmc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Sun H., Tawa G., Wallqvist A. Drug Discovery Today. 2012;17:310–324. doi: 10.1016/j.drudis.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu C., Ge H., Song M., Chen J. H., Zhou H., Qi Q., Wang F., Ma X., Yang X., Zhang G., Ding Y., Zhou D., Peng P., Shih C. K., Xu J., Wu F. ACS Med. Chem. Lett. 2014;5:921–926. doi: 10.1021/ml5001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Zhang W. J., Li P. H., Dong C. Z., Zhang K., Chen H. X., Du Z. Y. Bioorg. Med. Chem. Lett. 2018;28:1320–1323. doi: 10.1016/j.bmcl.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Wang R. B., Chen C. S., Zhang X. J., Zhang C. D., Zhong Q., Chen G. L., Zhang Q., Zheng S. L., Wang G. D., Chen Q. H. J. Med. Chem. 2015;58:4713–4726. doi: 10.1021/acs.jmedchem.5b00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. X., Zhang C. J., Deng X. Q., Wang Q., Hou S. P., Liu T. T., Xing X. L., Xiao H. R. Eur. J. Med. Chem. 2012;54:403–412. doi: 10.1016/j.ejmech.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Classen S., Olland S., Berger J. M. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10629–10634. doi: 10.1073/pnas.1832879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Ruthenburg A. J., Bechis S. K., Verdine G. L. J. Biol. Chem. 2005;280:37041–37047. doi: 10.1074/jbc.M506520200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.