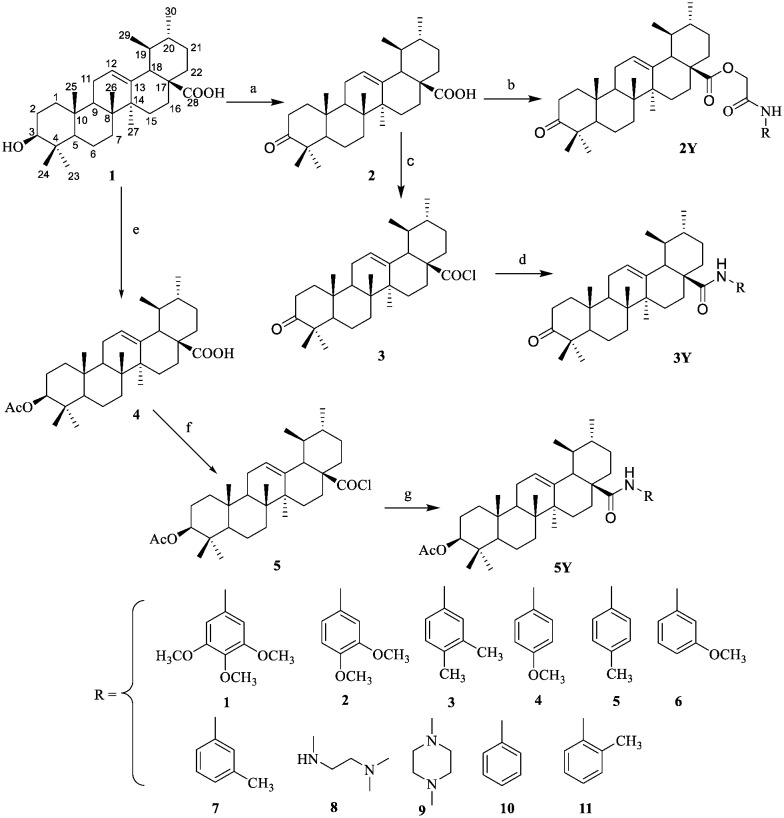
 A series of inhibitors of NF-κB based on ursolic acid (UA) derivatives containing functionalized aniline or amide side chains were synthesized and evaluated for inhibition of NF-κB as well as their antitumor effects.
A series of inhibitors of NF-κB based on ursolic acid (UA) derivatives containing functionalized aniline or amide side chains were synthesized and evaluated for inhibition of NF-κB as well as their antitumor effects.
Abstract
A series of inhibitors of NF-κB based on ursolic acid (UA) derivatives containing functionalized aniline or amide side chains were synthesized and evaluated for inhibition of NF-κB as well as their antitumor effects. These compounds exhibited significant inhibition activity toward NF-κB with IC50 values at micromolar concentrations in the NCI-H460 lung adenocarcinoma cell line. A docking study of the most active compound 5Y8 revealed key interactions between 5Y8 and the active site of NF-κB in which the functionalized amide moiety at the C-28 position and an ester group at the C-3 position were important for improving the activity. In particular, compound 5Y8 appeared to be the most potent compound against the NCI-H460 cell line, and displayed similar efficiency in drug-sensitive versus drug-resistant cancer cell lines, at least partly, by blocking the NF-κB signaling pathway and inducing apoptosis. Mechanistically, compound 5Y8 might trigger the apoptotic signaling pathway. Thus, the rational design of UA derivatives with functionalized aniline or amide side chains offers significant potential for the discovery of a new class of NF-κB inhibitors with the ability to induce apoptosis and reverse multidrug resistance in the NCI-H460 lung adenocarcinoma cell line.
Introduction
Increasing resistance to chemotherapy is tightly linked to aberrant activation of nuclear factor kappa B (NF-κB) signaling implicated both in inflammation and in initiation, promotion, and progression of tumorigenesis.1–3 NF-κB, which is known to be the master regulator of a wide variety of anti-apoptotic proteins, is commonly over-expressed and constitutively activated in different types of hematologic cancers and solid tumors.4 Increasing evidence suggested that the suppression of NF-κB activation may prevent tumor resistance to chemotherapeutic agents, shift the death–survival balance toward apoptosis, and improve the efficacy of current anticancer drugs.5,6 Therefore, design of NF-κB inhibitors is a promising approach to develop new cancer therapeutics and overcome drug resistance.
Triterpenoids are highly multifunctional and have attracted much attention as potential anticancer agents due to their ability to interact with multiple biological targets. The anticancer activities of triterpenoids appear to be mediated, at least in part, by their ability to block NF-κB activation, induce apoptosis, inhibit signal transduction and cell proliferation, suppress angiogenesis, cause mitochondrial dysfunction and modulate MDR genes and proteins.7–11 Recently, the pentacyclic triterpenoid ursolic acid (UA) serving as one of the major effective elements of many traditional Chinese medicines has attracted a lot of interest because of its anticancer properties.12–16 UA and its derivatives could act at various stages of tumor development to inhibit tumor initiation and promotion, as well as to induce tumor cell differentiation and apoptosis.14 Notably, these compounds were found to be potent inhibitors of NF-κB.13,17,18 Moreover, previous studies have reviewed that the modification in position 3 and/or 28 of UA has properties of increasing the inhibition activity of anticancer drugs in various cells.19–21 However, to the best of our knowledge, the derivatives of UA have not been thoroughly explored for their antitumor activity, especially as inhibitors of NF-κB. Thus, in order to find potentially important anticancer drug candidates, further studies need to investigate the mechanism of their antiproliferative activity.
Simultaneously, considerable attention has been also focused on aniline or amide moieties which have been widely used as functional groups in medicinal chemistry. Research has shown that the incorporation of an aniline or amide moiety into pharmaceutical cores could effectively improve the antitumor activity and the cells selectively.22,23 In addition, our previous studies have also demonstrated that aniline derivatives had potential antitumor activity.24 The presence of a hydrophilic residue, however, improved the anticancer activity of the compounds while still retaining their ability to trigger apoptosis. The aniline and amide residues seem to be able to interact quite well with, up to now, a still unknown intracellular target/receptor. Thus, aniline or amide groups were rationally designed and introduced into the UA structure. These congeners were evaluated for their in vitro anticancer activity and TNF-α-induced NF-κB activation against lung carcinoma cell line NCI-H460. The mode of action of a representative compound 5Y8 that blocks the TNF-α-induced NF-κB pathway is also investigated.
Results and discussion
Chemistry
A series of UA derivatives containing amide moieties were synthesized as outlined in Schemes 1 and 2. As shown in Scheme 1, UA (1) was subjected to oxidation using Jones reagent at 0 °C which resulted in the formation of C-3 oxidized derivative 2 in almost quantitative yield. Chloroacetic acid (12) was treated with oxalyl chloride to provide compound 13. Compound 13 was then treated with a variety of substituted phenyl amines to afford compounds 14, as shown in Scheme 2. UA derivatives 2Y were obtained by the condensation of compound 2 with compounds 14 in CH2Cl2 at room temperature. Compound 2 was treated with oxalyl chloride to afford the intermediate 28-acyl chloride 3 and this intermediate was then reacted with a variety of substituted phenyl amines to give compounds 3Y. 3-Acetyl ursolic acid (4) was synthesized by the treatment of ursolic acid 1 with acetic anhydride in dry pyridine in the presence of 4-dimethylaminopyridine (DMAP).25 Compound 4 was treated with oxalyl chloride to provide the intermediate 28-acyl chloride 5, which was highly reactive and was then coupled with a variety of substituted phenyl amines to produce compounds 5Y. All the new compounds were confirmed by spectroscopic methods, including 1H NMR, 13C NMR and high resolution mass spectroscopy.
Scheme 1. General synthetic route to compounds 2Y, 3Y and 5Y. Reagents and conditions: (a) Me2CO, Jones reagent, 0 °C; (b) K2CO3, 14, DMF, r.t; (c) CH2Cl2, (COCl)2, r.t; (d) CH2Cl2, Et3N, aniline, r.t; (e) AC2O, reflux, r.t; (f) CH2Cl2, (COCl)2, r.t; (g) CH2Cl2, Et3N, aniline, r.t.
Scheme 2. General synthetic route to compounds 14. (a) CH2Cl2, (COCl)2, r.t; (b) CH2Cl2, Et3N, aniline, r.t.
In vitro cytotoxic activity
The in vitro cytotoxicity of the synthesized compounds was evaluated by MTT assay in a panel of five human cancer cell lines including human gastric cancer cells (MGC-803), human lung cancer cells (NCI-H460), human liver cancer cells (HepG2), human non-small cell lung cancer cells (A549), and human ovarian cancer cells (T24), with 10-hydroxycamptothecin (HCPT) as the positive control. The results are shown in Table 1.
Table 1. Effect of the title compounds (2Y1–2Y11, 3Y1–3Y10 and 5Y1–5Y10) on the cell viability of different cell lines.
| Compd. | IC50
a
(μM) |
|||||
| MGC-803 | Spca-2 | NCI-H460 | A549 | T24 | HL-7702 | |
| 2Y1 | 38.73 ± 0.58 | 27.94 ± 0.48 | 39.59 ± 0.93 | 35.82 ± 0.79 | 37.84 ± 0.79 | >50 |
| 2Y2 | 38.18 ± 0.71 | 18.85 ± 0.56 | 22.76 ± 0.61 | 35.21 ± 0.60 | 27.70 ± 1.23 | >50 |
| 2Y4 | 27.89 ± 0.52 | 19.44 ± 0.63 | 17.60 ± 0.57 | 24.58 ± 0.83 | 26.23 ± 0.45 | >50 |
| 2Y5 | 16.59 ± 0.91 | 15.59 ± 0.46 | 10.91 ± 0.45 | 13.10 ± 1.46 | 15.69 ± 1.03 | >50 |
| 2Y6 | >50 | >50 | >50 | >50 | >50 | >50 |
| 2Y7 | >50 | >50 | >50 | >50 | >50 | >50 |
| 2Y10 | 16.05 ± 0.51 | 16.28 ± 0.35 | 13.75 ± 1.43 | 14.13 ± 0.55 | 16.04 ± 1.19 | >50 |
| 2Y11 | 16.25 ± 0.77 | 18.74 ± 0.89 | 14.01 ± 1.03 | 16.25 ± 1.15 | 17.81 ± 2.03 | >50 |
| 3Y1 | 40.07 ± 0.62 | 29.88 ± 0.73 | 34.31 ± 1.45 | 41.18 ± 1.34 | 39.83 ± 0.84 | >50 |
| 3Y2 | 32.50 ± 0.56 | 28.42 ± 0.69 | 30.20 ± 0.98 | 38.73 ± 0.92 | 37.33 ± 0.32 | >50 |
| 3Y3 | 21.38 ± 0.87 | 22.49 ± 0.38 | 19.37 ± 1.03 | 20.86 ± 1.26 | 24.56 ± 1.06 | >50 |
| 3Y4 | 29.72 ± 0.74 | 21.08 ± 0.21 | 28.11 ± 1.42 | 39.93 ± 1.10 | 32.48 ± 1.47 | >50 |
| 3Y5 | 18.59 ± 0.58 | 17.30 ± 0.59 | 11.91 ± 1.18 | 17.91 ± 1.29 | 20.05 ± 1.21 | >50 |
| 3Y7 | >50 | >50 | >50 | >50 | >50 | >50 |
| 3Y8 | 7.35 ± 1.03 | 6.91 ± 0.81 | 4.35 ± 0.49 | 6.01 ± 0.75 | 6.35 ± 0.37 | >50 |
| 3Y9 | 25.58 ± 1.09 | 22.43 ± 0.44 | 19.23 ± 1.12 | 23.44 ± 0.95 | 27.25 ± 1.13 | >50 |
| 3Y10 | 15.05 ± 0.60 | 13.50 ± 0.75 | 12.89 ± 2.04 | 16.32 ± 1.21 | 13.57 ± 1.25 | >50 |
| 5Y1 | 37.18 ± 0.56 | 28.69 ± 1.13 | 21.07 ± 1.53 | 36.46 ± 1.24 | 30.53 ± 0.36 | >50 |
| 5Y2 | 27.82 ± 0.88 | 12.88 ± 0.98 | 11.91 ± 0.89 | 22.90 ± 1.16 | 24.73 ± 0.65 | >50 |
| 5Y3 | 15.01 ± 0.92 | 12.25 ± 0.41 | 10.44 ± 0.34 | 12.36 ± 0.86 | 16.61 ± 1.28 | >50 |
| 5Y4 | 25.20 ± 0.13 | 13.65 ± 0.54 | 10.63 ± 1.21 | 31.12 ± 1.17 | 23.77 ± 1.31 | >50 |
| 5Y5 | 13.38 ± 0.48 | 11.23 ± 1.08 | 9.60 ± 1.38 | 10.33 ± 1.13 | 10.57 ± 1.54 | >50 |
| 5Y6 | >50 | >50 | >50 | >50 | >50 | >50 |
| 5Y7 | >50 | >50 | >50 | >50 | >50 | >50 |
| 5Y8 | 7.96 ± 0.69 | 5.38 ± 0.54 | 3.08 ± 0.70 | 4.32 ± 0.98 | 5.51 ± 0.76 | >50 |
| 5Y9 | 21.63 ± 0.73 | 29.16 ± 0.87 | 21.18 ± 0.92 | 24.15 ± 1.17 | 25.42 ± 1.43 | >50 |
| 5Y10 | 14.75 ± 0.65 | 11.65 ± 0.33 | 11.38 ± 1.05 | 12.86 ± 1.01 | 13.06 ± 0.87 | >50 |
| UA | 27.58 ± 1.07 | 30.21 ± 0.58 | 32.17 ± 0.76 | 35.79 ± 0.37 | 29.29 ± 0.80 | >50 |
| HCPT | 6.55 ± 1.71 | 12.63 ± 1.54 | 16.59 ± 1.36 | 2.75 ± 1.22 | 11.83 ± 1.81 | 12.83 ± 0.58 |
aIC50 values are presented as the mean ± SD (standard error of the mean) from five independent experiments.
As shown in Table 1, UA suppressed proliferation of the above five cancer cell lines to different extents (from 27.58 to 35.79 μM). Most of the title compounds (2Y1–2Y11, 3Y1–3Y10 and 5Y1–5Y10) which were modified from UA exhibited a considerable growth inhibitory effect and were active in the micromolar range. Besides, it was important to note that compounds 3Y8 and 5Y8 showed better potent antiproliferative activity than UA (as well as HCPT) against these five cancer cell lines. Evidently, compound 5Y8 displayed the best proliferation inhibition among these compounds, with IC50 values of 7.96 μM, 8.14 μM, 3.08 μM, 4.32 μM and 5.51 μM against MGC-803, HepG2, NCI-H460, A549 and T24 cancer cell lines, respectively. The results suggested that the introduction of aniline or amide moieties at C-28 may improve the antitumor activity of UA.
The SAR study reveals that substitutions on the phenyl ring of the aniline ring at C-28 led to reduction in antiproliferative activity in comparison with the unsubstituted derivatives (2Y10, 3Y10 and 5Y10). Compounds 2Y6, 2Y7, 3Y7, 5Y6 and 5Y7 having 3-methyl and 3-methoxy substituents on the aniline ring at the C-28 position exhibited low activity against all the five cell lines, respectively. Comparing the inhibitory concentrations of the pairs (with similar substitution) 2Y4 and 2Y5, 3Y4 and 3Y5, and 5Y4 and 5Y5 clearly indicates that methyl substitution shows relatively enhanced activity compared to methoxy substitution, as did the addition of a second methyl group at the ortho-position (3Y3, 5Y3). Considering the effects of compounds 3Y1 and 5Y1, the presence of a polymethoxy moiety instead of a phenyl group was unfavorable in this case. Introducing an electron-withdrawing dimethylethylenediamine (3Y8 and 5Y8) proved to be better in inhibiting cell proliferation against all cell lines in comparison with aniline groups. Meanwhile, the introduction of a methyl piperazine group in 3Y9 and 5Y9 led to moderate activity. These results prompted us to conclude that bulky substituents replacing the amide at position C-28 had a negative influence on the growth inhibitory activity. Furthermore, the modification of the acetyl moiety at the C-3 position into a ketone group caused a loss of potency: compound 5Y8 showed a lower cytotoxicity than 3Y8 toward the five human tumor cell lines.
As the selectivity of antitumor agents for cancer cells over non-malignant cells is important to avoid numerous severe side effects, all compounds were tested using a non-cancerous liver cell line (HL-7702). As shown in Table 1, the cytotoxic activities of most compounds against cancer cells were much higher than those against HL-7702 normal cells, making them good candidates as anticancer drugs. In summary, these modifications yielded a small increase in cytotoxic activity compared with HCPT and a low but significant improvement in selectivity of the compounds.
In vitro inhibition of TNF-α-induced NF-κB activation in the NCI-H460 lung adenocarcinoma cell line
In a second step, we evaluated the impact of UA derivatives on TNF-α-induced NF-κB transcriptional activity, which is considered as a relevant therapeutic target in hematological cancer and malignancies26 as well as in lung cancer.27 The human lung adenocarcinoma NCI-H460 cell line transiently co-transfected with NF-κB-luc was used to monitor the effects of lantadene congeners on tumor necrosis factor-alpha (TNF-α)-induced NF-κB activation. The title compounds (except 2Y6, 2Y7, 3Y7, 5Y6 and 5Y7) were evaluated in a dose-dependent manner to determine the concentration needed to inhibit 50% of TNF-α-induced NF-κB activation (IC50). The results of inhibition of TNF-α-induced NF-κB activation in the NCI-H460 lung adenocarcinoma cell line by the tested compounds are shown in Table 2. As shown in Table 2, the newly synthesized UA derivatives are potent NF-κB inhibitors, with IC50 values mostly in the micromolar range. The tested compounds showed improved inhibition of TNF-α-induced NF-κB activation compared to the parent compound UA, supporting the conclusion that the introduction of the functionalized aniline or amide side chains led to an increase in the activity. Notably, the reduction of the C-3 keto group of compounds 2Y and 3Y into the C-3 ester group of compounds 5Y led to an increase in the activity. When the trimethoxyaniline group of compound 5Y1 was replaced with a p-methoxyaniline (compound 5Y5) or a dimethylethylenediamine group (compound 5Y8), the inhibition potency was markedly increased, and the inhibitory activity was increased 7-fold and 41-fold compared with that of 5Y1, respectively. This was consistent with the cytotoxicity assay results and further confirmed the importance of small bulky and strongly electrophilic groups at the C-28 position that played an important role in binding of the compounds to the receptor site.
Table 2. In vitro inhibition of TNF-α-induced NF-κB activation in the NCI-H460 lung adenocarcinoma cell line.
| Compd. | IC50 (μM) | Compd. | IC50 (μM) |
| 2Y1 | >20 | 3Y9 | 15.51 ± 1.02 |
| 2Y2 | >20 | 3Y10 | 7.13 ± 0.44 |
| 2Y4 | 15.96 ± 0.21 | 5Y1 | 14.83 ± 0.38 |
| 2Y5 | 8.04 ± 0.56 | 5Y2 | 10.14 ± 0.34 |
| 2Y10 | 10.32 ± 0.29 | 5Y3 | 4.80 ± 0.47 |
| 2Y11 | 16.24 ± 0.60 | 5Y4 | 3.64 ± 0.52 |
| 3Y1 | >20 | 5Y5 | 2.26 ± 0.48 |
| 3Y2 | >20 | 5Y8 | 0.36 ± 0.08 |
| 3Y3 | 15.17 ± 0.76 | 5Y9 | 4.80 ± 0.55 |
| 3Y4 | >20 | 5Y10 | 2.33 ± 0.17 |
| 3Y5 | 5.43 ± 0.68 | UA | >20 |
| 3Y8 | 0.94 ± 0.27 |
Docking model
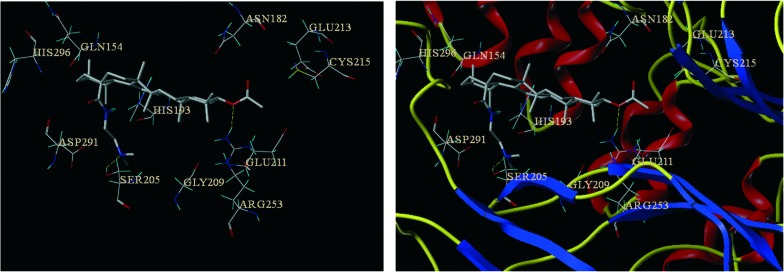
To understand the interactions between the most active compound 5Y8 and the target of interest (NF-κB), we performed molecular docking calculations on the active site of NF-κB (PDB ID: ; 1IKN)28 using SYBYL-X 2.0 software (Fig. 1) and the results are summarized in Table 3. The interaction mode of compound 5Y8 (docking score = 9.56), with the best docking score and interaction in the binding site of the NF-κB receptor, is described and compared with those of other derivatives and is shown in Fig. 1. Some key residues, such as ASN182, SER205, GLY209 and ARG253, as well as hydrogen bonds between the selected compound and the residues are also labeled. As shown in Fig. 1, compound 5Y8 was stabilized predominantly by the hydrophilic or hydrophobic group of GLN154, HIS193, SER205 and ARG253. The nitrogen of the dimethylamine functionality of the C28-amide side chain, which is a primarily important moiety, formed a hydrogen bond with the amino acid residue SER205 of the target protein. In addition, the oxygen of the ester group interacted with amine hydrogens of ARG253 by means of hydrogen bonding, which confirmed that this moiety is also crucial for binding. Apart from hydrogen bonding, the pentacyclic triterpenoid scaffold also showed hydrophobic and van der Waals interactions with GLN154, ASN182, HIS193, GLY209 and HIS296 residues of the target protein, while the C28-amide side chain demonstrated hydrophobic and van der Waals interactions with ASP291 and SER205 residues of the active site.
Fig. 1. Binding mode of compound 5Y8 in the active site of ; 1IKN. Ligands and the important residues for binding interactions are represented by stick and line models. The hydrogen bonds are shown as yellow dotted lines.
Table 3. Docking score for all studied compounds.
| Compd. | Total score | Crash | Polar | D-score | PMF-score | G-score | Chemscore | Cscore |
| 2Y1 | 5.08 | –2.86 | 1.10 | –175.57 | –112.44 | –287.75 | –17.36 | 5 |
| 2Y2 | 6.81 | –2.18 | 1.75 | –129.30 | –45.49 | –214.12 | –21.77 | 4 |
| 2Y4 | 6.75 | –1.59 | 1.03 | –134.51 | –73.40 | –280.51 | –20.03 | 5 |
| 2Y5 | 7.08 | –2.26 | 1.01 | –166.80 | –46.59 | –309.23 | –20.79 | 5 |
| 2Y6 | 4.24 | –4.70 | 1.26 | –152.27 | –95.77 | –254.47 | –16.16 | 4 |
| 2Y7 | 4.40 | –2.03 | 0.81 | –131.76 | –31.45 | –246.82 | –18.30 | 3 |
| 2Y10 | 7.28 | –1.95 | 0.82 | –140.79 | –58.95 | –271.70 | –17.60 | 4 |
| 2Y11 | 6.13 | –2.81 | 1.37 | –145.01 | –20.04 | –270.31 | –16.18 | 3 |
| 3Y1 | 4.83 | –1.77 | 2.21 | –135.54 | –77.55 | –183.19 | –16.81 | 3 |
| 3Y2 | 4.76 | –3.10 | 1.12 | –147.28 | –49.61 | –251.52 | –20.79 | 4 |
| 3Y3 | 6.62 | –2.08 | 1.05 | –130.97 | –18.64 | –258.47 | –21.47 | 4 |
| 3Y4 | 6.86 | –2.00 | 1.00 | –127.72 | –19.14 | –229.53 | –21.42 | 2 |
| 3Y5 | 7.91 | –2.43 | 1.14 | –127.23 | –12.69 | –256.19 | –21.01 | 4 |
| 3Y7 | 4.12 | –3.33 | 0.99 | –109.98 | –54.86 | –218.05 | –15.43 | 2 |
| 3Y8 | 9.28 | –2.66 | 1.00 | –111.99 | –29.50 | –242.11 | –15.54 | 2 |
| 3Y9 | 6.56 | –2.83 | 0.98 | –127.58 | –21.39 | –292.87 | –19.41 | 4 |
| 3Y10 | 7.47 | –1.37 | 0.86 | –120.10 | –10.91 | –237.96 | –24.50 | 4 |
| 5Y1 | 6.90 | –2.93 | 0.87 | –153.32 | –47.13 | –254.76 | –15.29 | 3 |
| 5Y2 | 7.70 | –1.78 | 1.15 | –138.32 | –39.58 | –218.84 | –20.41 | 3 |
| 5Y3 | 7.13 | –3.50 | 2.28 | –121.80 | –18.81 | –209.80 | –23.74 | 3 |
| 5Y4 | 7.72 | –3.54 | 1.01 | –136.19 | –62.07 | –245.72 | –16.15 | 3 |
| 5Y5 | 8.89 | –3.50 | 1.08 | –131.91 | –34.06 | –232.12 | –15.56 | 3 |
| 5Y6 | 4.90 | –2.64 | 1.12 | –128.92 | –31.16 | –235.49 | –19.95 | 2 |
| 5Y7 | 4.75 | –3.14 | 0.77 | –126.24 | –27.07 | –222.16 | 15.34 | 3 |
| 5Y8 | 9.56 | –1.50 | 1.20 | –117.20 | –53.31 | –227.72 | –16.86 | 4 |
| 5Y9 | 5.84 | –1.79 | 0.88 | –127.90 | –36.48 | –251.70 | –14.69 | 3 |
| 5Y10 | 7.78 | –2.30 | 2.28 | –116.73 | –15.37 | –200.69 | –23.06 | 3 |
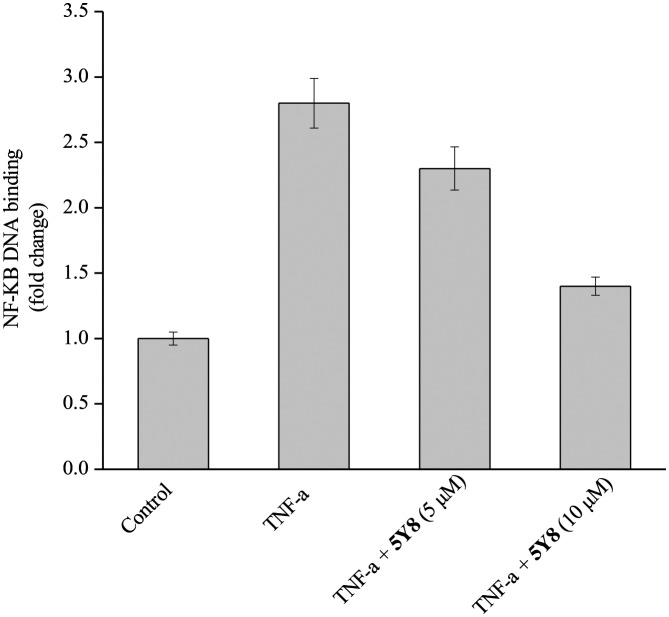
Compound 5Y8 inhibits NF-κB DNA binding
Because of the pivotal role of the transcription factor NF-κB in tumorigenesis and survival,1,3 it was speculated that 5Y8 might mediate its anticancer effects by modulating the NF-κB activation pathway. Therefore, we next investigated the effect of 5Y8 on constitutive NF-κB activation in NCI-H460 cells. Results of ELISA-based DNA binding assay clearly showed that treatment with various concentrations (5 and 10 μM) of 5Y8 significantly suppressed TNF-α-induced NF-κB activation (Fig. 2). 5Y8 reduced the NF-κB DNA binding ability by nearly 2.3-fold as compared with TNF-α and these results indicate that 5Y8 can modulate constitutive NF-κB activation in NCI-H460 cells.
Fig. 2. ELISA-based DNA binding assay to evaluate NF-κB DNA binding ability following compound 5Y8 treatment. The data is expressed as mean ± SD, compared with the untreated control and TNF-α-treated control (p < 0.05).
Effects of selected compounds on drug-resistant cell lines
Drug resistance is a critical therapeutic problem that limits the efficacies of most anticancer drugs for a variety of human cancer cells. According to the above biological results, we further evaluated the sensitivity to the selected compounds of two doxorubicin (DOX)-resistant and nonresistant cancer cell lines (NCI-H460/DOX and HepG2/DOX), with DOX as the positive control. As shown in Table 4, DOX exhibited low activity against the NCI-H460/DOX and HepG2/DOX resistant cell lines. Interestingly, the activity of compounds 3Y8 and 5Y8 was not markedly changed for these two DOX-resistant cancer cell lines compared with the sensitive ones; their IC50 values against the DOX-resistant NCI-H460 and HepG2 cell lines were 6.80–11.38 and 5.20–10.82 μM, respectively. It was significant to observe that compound 5Y8 had a much lower resistance factor (1.68 for the resistant NCI-H460 cell line and 1.33 for the resistant HepG2 cell line). Moreover, except for 2Y10 and 5Y9, the other selected compounds exhibited potent cytotoxicity against parental cells and the corresponding DOX-resistant cells comparable to that of DOX, and had smaller resistance factors, suggesting that these compounds might be useful in the treatment of drug-refractory tumors resistant to DOX.
Table 4. Cytotoxic effects of selected compounds on NCI-H460/DOX and HepG2/DOX cell lines.
| Compd. | IC50
a
(μM) |
|||||
| NCI-H460 | NCI-H460/DOX | Resistant factor | HepG2 | HepG2/DOX | Resistant factor | |
| 2Y5 | 10.91 ± 0.45 | 23.75 ± 1.12 | 2.18 | 20.08 ± 0.79 | 39.68 ± 1.07 | 1.98 |
| 2Y10 | 13.75 ± 1.43 | 26.62 ± 0.54 | 1.94 | 24.18 ± 0.95 | >50 | — |
| 3Y5 | 11.91 ± 1.18 | 20.94 ± 0.87 | 1.76 | 17.61 ± 0.47 | 28.47 ± 1.28 | 1.62 |
| 3Y8 | 4.35 ± 0.49 | 6.80 ± 1.34 | 1.56 | 9.05 ± 0.62 | 11.38 ± 1.41 | 1.26 |
| 3Y10 | 12.89 ± 2.04 | 25.68 ± 1.32 | 1.99 | 18.12 ± 0.79 | 36.68 ± 1.03 | 2.02 |
| 5Y3 | 10.44 ± 0.34 | 18.86 ± 0.99 | 1.81 | 17.37 ± 0.95 | 26.23 ± 0.56 | 1.51 |
| 5Y4 | 10.63 ± 1.21 | 22.56 ± 0.78 | 2.12 | 24.48 ± 1.03 | 47.05 ± 0.78 | 1.92 |
| 5Y5 | 9.60 ± 1.38 | 13.47 ± 1.18 | 1.40 | 12.51 ± 0.97 | 20.41 ± 1.34 | 1.63 |
| 5Y8 | 3.08 ± 0.70 | 5.20 ± 1.04 | 1.68 | 8.14 ± 0.95 | 10.82 ± 1.24 | 1.33 |
| 5Y9 | 21.18 ± 0.92 | 49.72 ± 0.61 | 2.35 | 26.97 ± 1.06 | >50 | — |
| 5Y10 | 11.38 ± 1.05 | 17.24 ± 1.01 | 1.52 | 12.60 ± 0.54 | 27.36 ± 0.47 | 2.17 |
| DOX | 5.18 ± 1.75 | >50 | — | 6.91 ± 0.96 | >50 | — |
aIC50 values are presented as the mean ± SD (standard error of the mean) from five separate experiments.
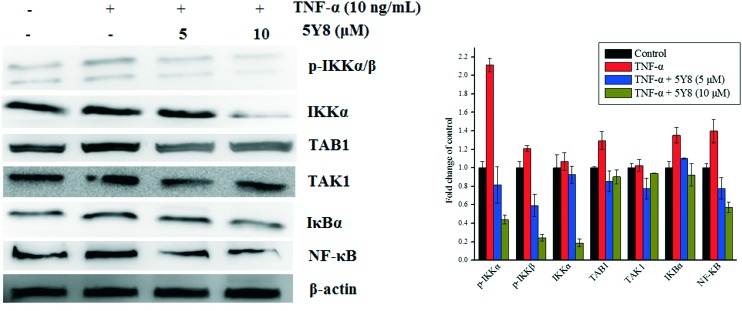
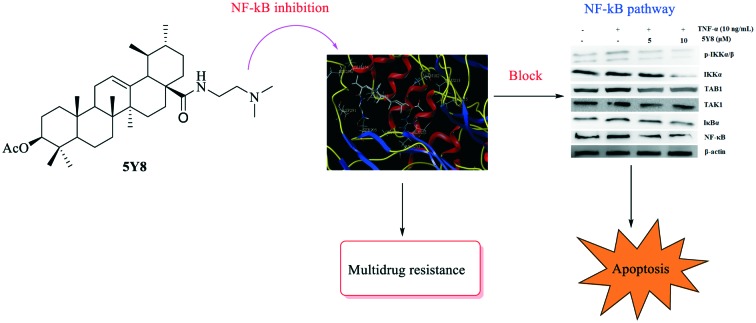
Compound 5Y8 inhibits the TNF-α–TAK1–NF-κB signaling cascade in NCI-H460 cells
As a mediator of the activated NF-κB signaling cascade, TAK1 (TGF-β-activated kinase 1) binds to the adaptor protein, TAB1, and subsequently activates downstream signaling kinases, such as the inhibitors IκB kinase (IKK)α/β, mitogen-activated protein kinase (MAPK), and c-jun NH2-kinase (JNK), and modulates NF-κB-dependent genes.29,30 Because disturbances in TAK1–NF-κB signaling are implicated in many types of cancer progression, inhibition of the TAK1–NF-κB cascade could activate the intrinsic caspase pathway and lead to apoptosis.31 To determine whether TNF-α–TAK1–NF-κB signaling is involved in the compound 5Y8-induced inhibition of NCI-H460 cells, we examined the NF-κB, TAK1 and TAB1 protein expressions in TNF-α-challenged NCI-H460 cells. The effects of compound 5Y8 on the constitutive levels of TAK1, TAB1 and NF-κB in NCI-H460 cells are given in Fig. 3. The increase in the expression of total TAK1, TAB1 and NF-κB stimulated by TNF-α was reduced after treatment of NCI-H460 cells with compound 5Y8, in a concentration-dependent manner. These results suggest that compound 5Y8 blocks the TNF-α-induced interaction of TAB1 with TAK1 and inhibits recruitment of the TAK1/TAB1 complex, thereby mediating the inactivation of TAK1 and downstream signaling.
Fig. 3. Suppression of the NF-κB signaling cascade in compound 5Y8-induced growth inhibition of NCI-H460 cells. The effect of compound 5Y8 on the binding affinity of total TAK1, TAB1, IKKα, p-IKKα/β and NF-κB p65 stimulated with TNF-α. β-Actin was used as loading control. Means ± SD were from three separate experiments, P < 0.05.
In an attempt to investigate whether the inhibition of NF-κB by compound 5Y8 is IKKβ-dependent, we also examined the phosphorylated IKKα/β protein expressions in the TNF-α-challenged NCI-H460 cells. After treatment with TNF-α, the phosphorylated IKKα/β levels were markedly increased and the treatment with compound 5Y8 before TNF-α stimulation reduced the TNF-α-induced IKKα/β phosphorylation and the IKKα expression. Compound 5Y8 inhibited the IKKα/β phosphorylation in a concentration-dependent manner. These data suggest that compound 5Y8 inhibits TNF-α-induced NF-κB activity via impairment of the TAK1/TAB1 complex and inactivation of downstream IKKα/β signaling synchronously.
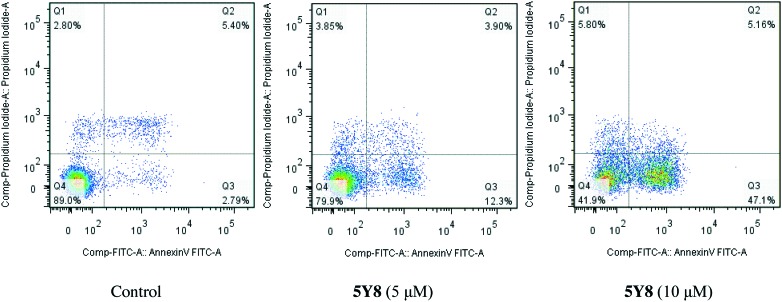
Compound 5Y8 induces apoptosis in NCI-H460 cells
In order to confirm whether the 5Y8-induced reduction in cell viability was responsible for the induction of apoptosis, NCI-H460 cells were co-stained with PI and annexin-V/FITC, and the number of apoptotic cells was estimated by flow cytometry (Fig. 4). Four-quadrant images were observed by flow cytometry analysis: the Q1 area represented damaged cells which appeared during the process of cell collection, the Q2 region showed necrotic cells and later stage apoptotic cells; early apoptotic cells were located in the Q3 area and the Q4 area showed normal cells. A dose-dependent increase in the percentage of apoptotic cells was noted after the cells were treated with compound 5Y8 at the concentrations of 5 μM and 10 μM for 24 h. As shown in Fig. 4, few (8.19%) apoptotic cells were present in the control panel; in contrast, the percentage rose to 16.20% at the concentration of 5 μM after treatment with 5Y8 for 24 h. At the concentration of 10 μM, there was a further increase to 52.26% after treatment with 5Y8. These results clearly confirmed that, compared with the control, compound 5Y8 effectively induced apoptosis in NCI-H460 cells in a dose-dependent manner.
Fig. 4. Annexin V-FITC and PI staining to evaluate apoptosis in NCI-H460 cells following compound 5Y8 treatment. NCI-H460 cells were treated with 5Y8 (5 and 10 μM, for 24 h), incubated with annexin V-FITC and PI and analyzed using flow cytometry.
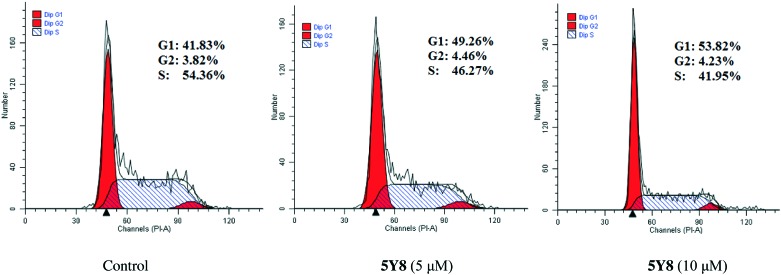
Cell cycle analysis
To determine the possible role of cell cycle arrest in UA amide derivative-induced growth inhibition, NCI-H460 cells were treated with different concentrations of compound 5Y8. Cell cycle distribution was investigated by flow cytometric analysis following staining of DNA with propidium iodide (PI). After treatment with compound 5Y8 at different concentrations for 48 h, it was observed that S phase cells gradually decreased and G2 phase cells did not change significantly, while G1 phase cells compared with the control cells gradually increased (Fig. 5). These results suggest that the target compound 5Y8 mainly arrested NCI-H460 cells in the G1 phase.
Fig. 5. Compound 5Y8-induced cell cycle arrest at the G1/S phase. NCI-H460 cells were treated with 5 or 10 μM compound 5Y8 for 24 h. Cells were fixed, stained with propidium iodide (PI), and assessed by flow cytometry. Untreated cells were used for comparison.
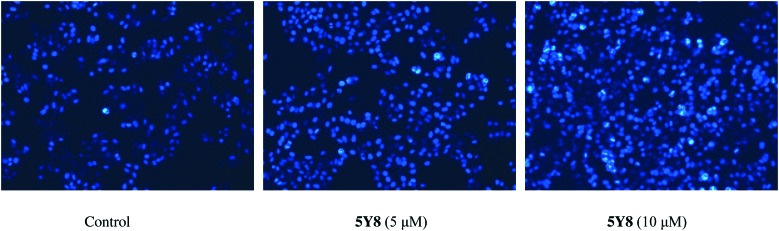
Morphological characterization of NCI-H460 cell apoptosis using Hoechst 33258
In order to further validate cell apoptosis following treatment with compound 5Y8, NCI-H460 cells treated with compound 5Y8 at 5 and 10 μM for 24 h were stained with Hoechst 33258. Our observations showed that control cells exhibited weak blue fluorescence (Control). Following treatment with compound 5Y8, some cells emitted brilliant blue fluorescence, and the nuclei of NCI-H460 cells appeared hyper-condensed (brightly stained). The number of apoptotic nuclei containing condensed chromatin increased significantly when NCI-H460 cells were treated with compound 5Y8 (10 μM) for 24 h (Fig. 6), indicating that apoptosis of NCI-H460 cells was induced by compound 5Y8 in a dose-dependent manner.
Fig. 6. Morphological changes in the nuclei (typical of apoptosis) of cultured NCI-H460 cells induced by compound 5Y8. NCI-H460 cells were treated with 5 and 10 μM 5Y8 for 24 h and stained with Hoechst 33258. Selected fields illustrated the occurrence of apoptotic cells. Cells with condensed chromatin (brightly stained) were defined as apoptotic NCI-H460 cancer cells. Images were acquired using a Nikon Te2000 deconvolution microscope (magnification = 200×).
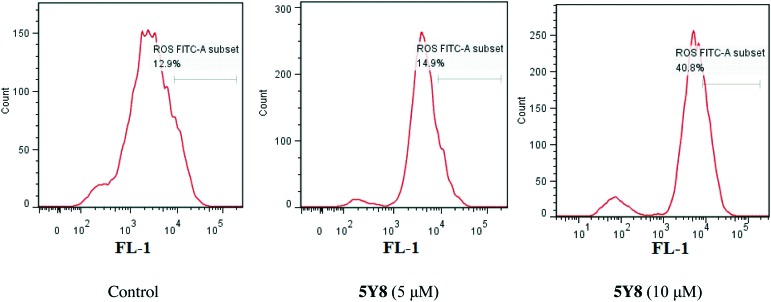
Compound 5Y8 triggers ROS generation
Reactive oxygen species (ROS) are highly harmful elements to cells as they initiate oxidative stress and ultimately cause cellular damage. Excessive ROS generation renders cells vulnerable to apoptosis.32 Some research studies show that NF-κB protects cells from oxidative stress by activating expression of various antioxidant systems.33 To determine whether 5Y8 triggers ROS generation in NCI-H460 cells to induce apoptosis, the ROS level was measured with and without (control) treatment of 5Y8 at 5 μM and 10 μM for 24 h, using the fluorescent probe 2,7-dichlorofluorescein diacetate (DCF-DA) by flow cytometry. As shown in Fig. 7, the results showed that compound 5Y8 induced an increase of the ROS level in NCI-H460 cells. After exposure to 10 μM 5Y8 for 24 h, the ROS level was 40.8%, which is more than three times that of the control. Taken together, these results show that 5Y8 causes oxidative imbalance in NCI-H460 cells. This induction of oxidative burst is a key factor behind the loss of NF-κB activity triggered by compound 5Y8.
Fig. 7. Compound 5Y8 affected the levels of intracellular ROS in NCI-H460 cells. NCI-H460 cells were treated with compound 5Y8 at 5 and 10 μM for 24 h and detected by flow cytometry. The values represent the mean ± SD of three independent experiments (p < 0.05).
Conclusions
A series of UA derivatives with functionalized aniline or amide side chains were designed and synthesized as apoptosis inducers which targeted NF-κB with high selectivity and an optimal inhibition profile. These compounds exhibited significant inhibition activity toward NF-κB in the NCI-H460 lung adenocarcinoma cell line with IC50 values at micromolar concentrations. A docking study of the most active compound 5Y8 revealed key interactions between 5Y8 and the NF-κB receptor in which the small, bulky and strongly electrophilic groups of the functionality side chain at the C-28 position were important for improving the activity. It is noteworthy that further antitumor activity screening revealed that some compounds exhibited better inhibitory activity than the parent compound UA or the commercial anticancer drug HCPT. Moreover, the selected compounds markedly reversed multidrug resistance in DOX-resistant cancer cells. In particular, compound 5Y8 (IC50 = 3.08 ± 0.70 μM) exhibited the best anticancer activity against the NCI-H460 cell line and displayed more potent inhibitory activity than HCPT. Simultaneous molecular mechanism studies suggested that the target compound 5Y8 may inhibit NCI-H460 cells by blocking the NF-κB signaling pathway. Furthermore, cell cycle analysis indicated that compound 5Y8 arrested the NCI-H460 cell line in the G1 phase. Consequently, the rational design of UA derivatives with functionalized aniline or amide side chains offers significant potential for the discovery of a new class of NF-κB inhibitors with the ability to induce apoptosis and reverse multidrug resistance in the NCI-H460 lung adenocarcinoma cell line. The precise mechanism of this action requires further investigation.
Experimental methods
General
Compound 4 was synthesized according to the literature.25 UA with more than 95% purity was purchased from Wu Han Sheng Tian Yu Biotech Co. Ltd. All the chemical reagents and solvents used were of analytical grade. Silica gel (300–400 mesh) used in column chromatography was provided by Tsingtao Marine Chemistry Co. Ltd. 1H NMR and 13C NMR were recorded on a BRUKER AV-500 spectrometer with TMS as an internal standard in CDCl3. Mass spectra were acquired on an FTMS ESI spectrometer.
General procedure for the preparation of compounds 2Y
To a solution of 1 (400 mg, 0.88 mmol) in acetone (9 ml) at 0 °C, 2 drops of Jones reagent were added and the reaction mixture was stirred at room temperature. The reaction was monitored by TLC until its completion in around 2 h. After quenching the reaction with cold water, the crude product was extracted with ethyl acetate (3 × 20 ml). The organic layer was dried over sodium sulfate and purified through column chromatography to give pure compound 2. Compound 2 (1 mmol) and K2CO3 (2 mmol) were added to DMF (15 mL) and stirred at room temperature for 30 min, after which compound 14 (4 mmol) was added and KI (0.5 mmol) was dripped into the mixture. After being stirred for another 12 h, the reaction mixture was poured onto 100 mL of distilled water and partitioned with ethyl acetate (3 × 20 mL). The organic layer was washed with saturated sodium chloride, dried over Na2SO4 and purified via silica gel column chromatography with petroleum ether/ethyl acetate to obtain compound 2Y. Experimental: NMR spectra were recorded on BRUKER AVANCE 400M and 500M NMR spectrometers in CDCl3.
Compound 2Y1
Yield 83.4%; 1H NMR (400 MHz, CDCl3) δ 7.80 (s, 1H), 6.75 (s, 2H), 5.33 (s, 1H), 4.61 (dd, J = 128.7, 15.5 Hz, 2H), 3.83 (s, 9H), 1.09 (s, 3H), 1.05 (s, 3H), 1.00 (s, 3H), 0.96 (d, J = 5.9 Hz, 3H), 0.91 (s, 3H), 0.88 (d, J = 6.3 Hz, 3H), 0.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.4, 176.0, 165.5, 153.5, 153.4, 139.5, 132.8, 132.6, 125.2, 98.7, 98.0, 63.1, 56.2, 56.2, 55.3, 53.2, 48.5, 47.4, 46.7, 42.9, 42.4, 39.5, 39.2, 39.2, 38.9, 36.8, 36.6, 34.1, 32.4, 30.5, 27.9, 26.4, 24.5, 23.6, 23.3, 21.5, 21.1, 19.5, 17.1, 16.9, 14.9. HR-MS (m/z) (ESI): calcd for C41H59NO7 [M – H+]: 677.4292; found: 676.4225.
Compound 2Y2
Yield 92.5%; 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 1H), 6.97–6.92 (m, 1H), 6.82 (s, 2H), 5.34 (s, 1H), 4.84–4.43 (m, 2H), 3.87 (s, 6H), 1.10 (s, 3H), 1.06 (s, 3H), 1.01 (s, 3H), 0.97 (d, J = 6.0 Hz, 3H), 0.93–0.87 (m, 6H), 0.74 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.5, 175.9, 165.5, 149.3, 146.7, 139.5, 125.3, 112.9, 112.4, 105.9, 105.1, 63.1, 56.2, 56.1, 56.0, 55.9, 55.3, 53.2, 48.5, 47.4, 46.7, 42.9, 42.4, 39.5, 39.2, 39.2, 38.9, 36.6, 34.1, 32.4, 30.5, 27.9, 26.5, 24.5, 23.6, 21.5, 21.1, 19.5, 16.9, 14.9. HR-MS (m/z) (ESI): calcd for C40H57NO6 [M + H+]: 647.4186; found: 648.4578.
Compound 2Y4
Yield 91.0%; 1H NMR (400 MHz, CDCl3) δ 7.78 (s, 1H), 7.37 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.8 Hz, 2H), 5.33 (s, 1H), 4.63 (dd, J = 158.0, 15.6 Hz, 2H), 3.79 (s, 3H), 1.10 (s, 3H), 1.06 (s, 3H), 1.01 (s, 3H), 0.97 (d, J = 6.0 Hz, 3H), 0.89 (d, J = 5.8 Hz, 6H), 0.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.5, 175.9, 165.5, 157.1, 139.5, 129.5, 125.3, 122.7, 114.4, 63.0, 55.5, 55.3, 53.2, 48.5, 47.4, 46.7, 42.4, 39.5, 39.2, 38.9, 36.8, 36.6, 34.1, 32.4, 30.5, 27.9, 26.5, 24.5, 23.6, 23.2, 21.5, 21.1, 19.5, 17.1, 16.9, 15.0. HR-MS (m/z) (ESI): calcd for C39H55NO5 [M – H+]: 617.4080; found: 616.4011.
Compound 2Y5
Yield 87.5%; 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 1H), 7.35 (d, J = 8.2 Hz, 2H), 7.15 (d, J = 8.0 Hz, 2H), 5.35 (s, 1H), 4.63 (dd, J = 135.5, 15.6 Hz, 2H), 2.33 (s, 3H), 1.11 (s, 3H), 1.07 (s, 3H), 1.02 (s, 3H), 0.98 (d, J = 6.1 Hz, 3H), 0.90 (d, J = 6.9 Hz, 6H), 0.74 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.6, 175.9, 165.4, 139.3, 134.9, 134.0, 129.7, 129.6, 125.4, 120.8, 120.2, 63.1, 55.3, 53.2, 48.5, 47.4, 46.7, 42.9, 42.4, 39.5, 39.2, 39.1, 38.9, 36.8, 36.6, 34.1, 32.4, 30.5, 27.9, 26.5, 24.5, 23.6, 23.3, 21.5, 21.1, 20.9, 19.5, 17.1, 16.9, 14.9. HR-MS (m/z) (ESI): calcd for C39H55NO4 [M + H+]: 601.4131; found: 602.4559.
Compound 2Y6
Yield 88.6%; 1H NMR (400 MHz, CDCl3) δ 7.84 (s, 1H), 7.23 (d, J = 8.2 Hz, 2H), 6.95 (d, J = 8.0 Hz, 1H), 6.71 (dd, J = 8.3, 1.2 Hz, 1H), 5.35 (s, 1H), 4.63 (dd, J = 120.0, 15.6 Hz, 2H), 3.80 (s, 3H), 1.11 (s, 3H), 1.06 (s, 3H), 1.01 (s, 3H), 0.97 (d, J = 6.0 Hz, 3H), 0.94–0.87 (m, 6H), 0.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.6, 175.9, 165.5, 160.3, 139.2, 137.9, 129.8, 125.4, 112.6, 110.8, 106.5, 63.1, 55.4, 55.3, 53.2, 48.5, 47.4, 46.7, 42.3, 39.5, 39.2, 39.1, 38.9, 36.8, 36.6, 34.1, 32.4, 30.5, 27.9, 26.5, 24.5, 23.6, 23.3, 21.5, 21.1, 19.5, 17.1, 16.9, 15.0. HR-MS (m/z) (ESI): calcd for C39H55NO5 [M + H+]: 617.4080; found: 618.4125.
Compound 2Y7
Yield 82.9%; 1H NMR (400 MHz, CDCl3) δ 7.86 (s, 1H), 7.36 (s, 1H), 7.25 (d, J = 4.9 Hz, 2H), 7.02–6.97 (m, 1H), 5.37 (s, 1H), 4.66 (dd, J = 132.4, 15.6 Hz, 2H), 2.37 (s, 3H), 1.13 (s, 3H), 1.09 (s, 3H), 1.04 (s, 3H), 1.00 (d, J = 6.1 Hz, 3H), 0.93 (d, J = 5.2 Hz, 6H), 0.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.6, 175.9, 165.5, 141.4, 139.3, 139.1, 136.5, 128.9, 126.0, 125.4, 121.4, 117.8, 63.1, 55.3, 53.2, 48.5, 47.4, 46.7, 42.3, 39.5, 39.2, 39.1, 38.9, 36.8, 36.6, 34.1, 32.4, 30.5, 27.9, 26.5, 24.5, 23.6, 23.3, 21.5, 21.1, 19.5, 17.1, 16.9, 15.0. HR-MS (m/z) (ESI): calcd for C39H55NO4 [M + H+]: 601.4131; found: 602.4556.
Compound 2Y10
Yield 85.3%; 1H NMR (400 MHz, DMSO) δ 10.00 (s, 1H), 7.58 (d, J = 6.8 Hz, 2H), 7.33–7.30 (m, 2H), 7.07 (d, J = 7.2 Hz, 1H), 5.21 (s, 1H), 4.59 (q, J = 14.4 Hz, 2H), 1.08 (s, 3H), 1.01 (s, 3H), 0.95 (d, J = 5.5 Hz, 9H), 0.84 (d, J = 6.3 Hz, 3H), 0.74 (s, 3H). 13C NMR (101 MHz, DMSO) δ 216.6, 176.4, 165.9, 139.0, 138.3, 129.3, 129.2, 125.4, 124.3, 119.8, 119.6, 62.7, 54.7, 52.9, 47.9, 47.1, 46.5, 44.0, 42.2, 39.5, 39.0, 38.9, 38.8, 36.6, 36.5, 34.2, 32.5, 30.6, 27.9, 26.8, 24.3, 23.7, 23.5, 21.6, 21.5, 19.6, 17.4, 17.0, 15.3. HR-MS (m/z) (ESI): calcd for C39H53NO4 [M + H+]: 587.3975; found: 588.4146.
Compound 2Y11
Yield 89.3%; 1H NMR (400 MHz, CDCl3) δ 7.81 (s, 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.22 (d, J = 7.6 Hz, 2H), 7.13 (t, J = 7.1 Hz, 1H), 5.30 (s, 1H), 4.68 (dd, J = 94.3, 15.7 Hz, 2H), 2.29 (s, 3H), 1.10 (s, 3H), 1.07 (s, 3H), 1.02 (s, 3H), 0.97 (d, J = 5.9 Hz, 3H), 0.92 (s, 3H), 0.88 (d, J = 6.4 Hz, 3H), 0.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.5, 175.8, 165.5, 138.9, 134.6, 130.7, 129.6, 127.0, 126.0, 125.7, 123.5, 63.3, 55.3, 53.3, 48.6, 47.4, 46.7, 42.3, 39.5, 39.2, 39.1, 38.9, 36.9, 36.6, 34.1, 32.5, 30.5, 27.9, 26.5, 24.5, 23.6, 23.3, 21.5, 21.1, 19.5, 17.9, 17.1, 17.0, 14.8. HR-MS (m/z) (ESI): calcd for C39H55NO4 [M + H+]: 601.4131; found: 602.4557.
General procedure for the preparation of compounds 3Y
Compound 2 (0.14 mmol) added to dry CH2Cl2 was stirred at 0 °C, and oxalyl chloride was dripped into the mixture and stirred at room temperature for 6 h. After the reaction, the solvent and excess oxalyl chloride were evaporated under reduced pressure. Then, anilines (0.56 mmol) were added to the mixture and stirred at room temperature for 4 h. After dilution with ethyl acetate (25 mL), the mixture was washed with water (3 × 20 mL). The organic phase was dried over anhydrous sodium sulfate. Filtration and evaporation of solvent under reduced pressure were carried out. The residue was purified by column chromatography on silica gel and eluted with petroleum ether/ethyl acetate (V : V = 4 : 1) to give a white solid 3Y.
Compound 3Y1
Yield 87.5%; 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 1H), 6.74 (s, 2H), 5.52 (s, 1H), 3.81 (d, J = 14.6 Hz, 9H), 1.14 (s, 3H), 1.07 (s, 3H), 1.01 (s, 6H), 0.98 (s, 3H), 0.92 (d, J = 6.3 Hz, 3H), 0.78 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.4, 176.2, 153.3, 140.5, 134.6, 134.2, 125.8, 97.5, 60.9, 56.1, 55.2, 54.5, 48.7, 47.4, 46.8, 42.8, 39.9, 39.6, 39.3, 39.1, 37.1, 36.6, 34.1, 32.3, 30.9, 27.9, 26.9, 26.5, 25.1, 23.7, 23.2, 21.5, 21.1, 19.5, 17.3, 16.9, 15.3. HR-MS (m/z) (ESI): calcd for C39H57NO5 [M + H+]: 619.4237; found: 620.4292.
Compound 3Y2
Yield 90.4%; 1H NMR (400 MHz, CDCl3) δ 7.60 (s, 1H), 7.41 (d, J = 2.1 Hz, 1H), 6.77 (d, J = 8.6 Hz, 1H), 6.69 (dd, J = 8.6, 2.2 Hz, 1H), 5.50 (s, 1H), 3.85 (d, J = 11.9 Hz, 6H), 1.14 (s, 3H), 1.07 (s, 3H), 1.01 (s, 6H), 0.98 (s, 3H), 0.92 (d, J = 6.4 Hz, 3H), 0.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.5, 176.0, 149.1, 145.7, 140.4, 132.0, 125.8, 111.4, 111.2, 104.8, 56.1, 55.9, 55.2, 54.4, 48.5, 47.4, 46.8, 42.8, 39.9, 39.6, 39.4, 39.1, 37.1, 36.6, 34.1, 32.3, 30.9, 28.0, 26.6, 25.1, 23.7, 23.2, 21.5, 21.2, 19.5, 17.3, 16.8, 15.3. HR-MS (m/z) (ESI): calcd for C38H55NO4 [M + H+]: 589.4131; found: 590.4188.
Compound 3Y3
Yield 91.5%; 1H NMR (400 MHz, DMSO) δ 8.77 (s, 1H), 7.39 (s, 1H), 7.14 (t, J = 7.8 Hz, 1H), 6.89 (t, J = 7.6 Hz, 1H), 6.37–6.27 (m, 1H), 5.33 (s, 1H), 2.26 (s, 3H), 2.15 (s, 3H), 1.09 (s, 3H), 1.00 (s, 3H), 0.95 (d, J = 7.9 Hz, 9H), 0.88 (d, J = 6.3 Hz, 3H), 0.70 (s, 3H). 13C NMR (101 MHz, DMSO) δ 216.6, 175.6, 148.8, 137.8, 129.1, 121.4, 118.0, 117.2, 115.2, 111.8, 54.8, 52.2, 48.1, 47.1, 46.5, 42.2, 39.5, 39.3, 39.0, 38.8, 36.8, 36.6, 34.2, 32.5, 30.8, 27.9, 26.8, 23.9, 23.8, 23.5, 21.7, 21.6, 21.6, 21.5, 19.5, 17.6, 17.0, 15.2. HR-MS (m/z) (ESI): calcd for C38H55NO2 [M – H+]: 557.4233; found: 556.5283.
Compound 3Y4
Yield 86.8%; 1H NMR (400 MHz, CDCl3) δ 7.69 (s, 1H), 7.32 (t, J = 1.9 Hz, 1H), 7.17 (t, J = 8.1 Hz, 1H), 7.05 (t, J = 8.0 Hz, 1H), 6.84 (d, J = 8.0 Hz, 1H), 6.28–6.22 (m, 1H), 5.51 (s, 1H), 3.79 (s, 3H), 1.14 (s, 3H), 1.07 (s, 3H), 1.00 (d, J = 12.6 Hz, 9H), 0.92 (d, J = 6.4 Hz, 3H), 0.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.5, 176.2, 160.2, 139.4, 129.5, 125.9, 110.1, 107.9, 103.9, 101.1, 55.3, 55.2, 55.1, 54.4, 48.7, 47.4, 46.8, 42.8, 39.9, 39.6, 39.4, 39.1, 37.0, 36.6, 34.1, 32.3, 30.9, 27.9, 26.6, 25.1, 23.7, 23.3, 21.5, 21.2, 19.5, 17.3, 16.8, 15.3. HR-MS (m/z) (ESI): calcd for C37H53NO3 [M – H+]: 559.4025; found: 558.3954.
Compound 3Y5
Yield 80.3%; 1H NMR (400 MHz, DMSO) δ 8.75 (s, 1H), 7.42 (d, J = 8.3 Hz, 2H), 7.06 (d, J = 8.2 Hz, 2H), 5.33 (s, 1H), 2.24 (s, 3H), 1.09 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 7.8 Hz, 9H), 0.88 (d, J = 6.3 Hz, 3H), 0.69 (s, 3H). 13C NMR (101 MHz, DMSO) δ 216.5, 175.4, 146.3, 139.1, 137.2, 132.4, 129.1, 125.0, 120.9, 114.6, 54.8, 52.3, 47.9, 47.1, 46.6, 42.7, 39.5, 39.3, 39.0, 38.8, 36.9, 36.6, 34.1, 32.5, 30.9, 27.9, 26.8, 23.9, 23.8, 23.5, 21.6, 21.5, 20.9, 19.5, 17.6, 17.0, 15.2. HR-MS (m/z) (ESI): calcd for C37H53NO2 [M + H+]: 543.4076; found: 544.4129.
Compound 3Y7
Yield 83.6%; 1H NMR (400 MHz, DMSO) δ 8.69 (s, 1H), 7.32 (s, 1H), 7.02 (dd, J = 15.8, 8.1 Hz, 2H), 6.77 (d, J = 8.0 Hz, 1H), 2.15 (s, 3H), 1.08 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 7.0 Hz, 9H), 0.88 (d, J = 6.3 Hz, 3H), 0.69 (s, 3H). 13C NMR (101 MHz, DMSO) δ 216.6, 175.4, 139.1, 136.2, 129.6, 125.0, 122.1, 120.8, 118.4, 117.1, 54.8, 52.2, 48.0, 47.1, 46.5, 42.2, 39.5, 39.3, 39.0, 38.8, 36.9, 36.6, 34.2, 32.5, 30.9, 27.9, 26.8, 23.8, 23.5, 21.6, 21.5, 20.0, 19.9, 19.1, 17.6, 17.0, 15.2. HR-MS (m/z) (ESI): calcd for C37H53NO2 [M + H+]: 543.4076; found: 544.4129.
Compound 3Y8
Yield 82.7%; 1H NMR (400 MHz, CDCl3) δ 6.74 (s, 1H), 5.33 (s, 1H), 2.65 (s, 2H), 2.44 (s, 6H), 1.97 (s, 2H), 1.07 (d, J = 6.0 Hz, 6H), 1.02 (s, 6H), 0.92 (d, J = 5.6 Hz, 3H), 0.86 (d, J = 6.1 Hz, 3H), 0.81 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.7, 178.5, 139.2, 125.6, 57.7, 55.2, 53.5, 47.8, 47.4, 46.8, 44.6, 42.5, 39.7, 39.6, 39.3, 38.9, 37.2, 36.7, 36.1, 34.1, 32.4, 30.9, 27.9, 26.6, 24.6, 23.5, 23.3, 21.5, 21.1, 19.6, 17.1, 16.9, 15.2. HR-MS (m/z) (ESI): calcd for C34H56N2O2 [M + H+]: 524.4342; found: 525.4201.
Compound 3Y9
Yield 84.6%; 1H NMR (400 MHz, DMSO) δ 5.12 (s, 1H), 3.49 (s, 4H), 2.25 (s, 4H), 2.16 (s, 3H), 1.06 (s, 3H), 1.00 (s, 3H), 0.98 (s, 3H), 0.96 (s, 3H), 0.93 (s, 3H), 0.84 (d, J = 6.3 Hz, 3H), 0.73 (s, 3H). 13C NMR (101 MHz, DMSO) δ 216.6, 174.5, 139.2, 124.7, 55.3, 54.8, 48.2, 47.1, 46.6, 46.1, 45.4, 42.2, 40.0, 39.8, 39.6, 39.4, 39.0, 38.7, 36.7, 34.2, 33.9, 32.6, 30.5, 28.2, 26.8, 23.5, 23.3, 21.6, 21.5, 19.6, 17.8, 16.9, 15.3. HR-MS (m/z) (ESI): calcd for C35H56N2O2 [M + H+]: 536.4342; found: 537.4297.
Compound 3Y10
Yield 82.8%; 1H NMR (400 MHz, CDCl3) δ 7.68 (s, 1H), 7.45 (d, J = 8.3 Hz, 2H), 7.29 (t, J = 7.8 Hz, 2H), 6.78–6.66 (m, 1H), 5.51 (t, J = 3.2 Hz, 1H), 1.15 (s, 3H), 1.07 (s, 3H), 1.00 (d, J = 11.4 Hz, 9H), 0.93 (d, J = 6.4 Hz, 3H), 0.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 217.5, 176.2, 140.2, 138.1, 129.3, 128.9, 125.9, 124.0, 119.7, 115.1, 77.4, 77.2, 77.0, 76.7, 55.2, 54.4, 48.7, 47.4, 46.8, 42.8, 39.9, 39.6, 39.4, 39.1, 37.1, 36.6, 34.1, 32.3, 30.9, 27.9, 26.6, 25.1, 23.7, 23.3, 21.5, 21.2, 19.5, 17.3, 16.8, 15.3. HR-MS (m/z) (ESI): calcd for C36H51NO2 [M + H+]: 529.3920; found: 530.3976.
General procedure for the preparation of compounds 5Y
Compound 4 was synthesized according to the literature.25 3-O-Acetylursolic acid 2 (4 mmol) added to dry CH2Cl2 (20 mL) was stirred at 0 °C and oxalyl chloride (12 mmol) was dripped into the mixture and stirred at room temperature for 6 h. The solvent was removed by evaporation under reduced pressure, and dry CH2Cl2 (3 × 5 mL) was added to the residue, concentrated to dryness to give 5. Then, anilines (0.56 mmol) were added to the mixture and stirred at room temperature for 4 h. After dilution with ethyl acetate (25 mL), the mixture was washed with water (3 × 20 mL). The organic phase was dried over anhydrous sodium sulfate. Filtration and evaporation of solvent under reduced pressure were carried out. The residue was purified by column chromatography on silica gel and eluted with petroleum ether/ethyl acetate (V : V = 4 : 1) to give a white solid 5Y.
Compound 5Y1
Yield 88.5%; 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H, –CONH–), 6.73 (s, 2H), 5.49 (s, 1H), 4.47 (dd, J = 10.4, 5.2 Hz, 1H), 3.80 (d, J = 13.3 Hz, 9H), 2.02 (s, 3H), 1.12 (s, 3H), 0.97 (s, 3H), 0.91 (d, J = 8.5 Hz, 6H), 0.83 (d, J = 8.9 Hz, 6H), 0.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.3 (–CONH–), 171.0 (–COO–), 153.2, 140.4, 134.6, 134.2, 126.0, 97.4, 80.8, 60.9, 56.1, 55.3, 54.4, 48.6, 47.4, 42.7, 39.9, 39.6, 39.1, 38.4, 37.7, 37.0, 36.8, 32.7, 30.9, 28.0, 27.9, 25.2, 23.6, 23.5, 23.2, 21.3, 21.2, 18.1, 17.3, 17.0, 16.7, 15.6. HR-MS (m/z) (ESI): calcd for C41H61NO6 [M + H+]: 663.4499; found: 664.4547.
Compound 5Y2
Yield 85.6%; 1H NMR (400 MHz, DMSO) δ 8.68 (s, 1H, –CONH–), 7.21 (d, J = 2.1 Hz, 1H), 7.08 (dd, J = 8.7, 2.1 Hz, 1H), 6.84 (d, J = 8.8 Hz, 1H), 5.30 (s, 1H), 4.40 (dd, J = 11.2, 4.5 Hz, 1H), 3.70 (d, J = 1.9 Hz, 6H), 2.00 (s, 3H), 1.09 (s, 3H), 0.96 (s, 3H), 0.89–0.85 (m, 6H), 0.81 (d, J = 4.8 Hz, 6H), 0.67 (s, 3H). 13C NMR (101 MHz, DMSO) δ 175.2 (–CONH–), 170.5 (–COO–), 148.8, 145.2, 139.1, 133.4, 124.9, 112.8, 112.3, 106.2, 80.4, 56.2, 55.8, 55.0, 52.2, 47.9, 47.3, 42.1, 39.3, 38.8, 38.2, 37.7, 36.9, 32.9, 30.8, 28.3, 27.9, 23.9, 24.0, 23.71, 23.4, 21.6, 21.4, 18.2, 17.6, 17.1, 17.1, 15.6. HR-MS (m/z) (ESI): calcd for C40H59NO5 [M + H+]: 633.4393; found: 634.4429.
Compound 5Y3
Yield 89.0%; 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 1H, –CONH–), 7.34 (s, 1H), 7.08 (d, J = 7.8 Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 5.47 (s, 1H), 4.54–4.43 (m, 1H), 2.21 (d, J = 10.3 Hz, 6H), 2.04 (s, 3H), 1.12 (s, 3H), 0.97 (s, 3H), 0.91 (d, J = 8.8 Hz, 6H), 0.84 (d, J = 10.0 Hz, 6H), 0.71 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.1 (–CONH–), 171.0 (–COO–), 140.1, 137.1, 135.9, 132.2, 129.8, 125.9, 121.1, 117.1, 80.9, 55.3, 54.3, 48.5, 47.5, 42.6, 39.9, 39.6, 39.2, 38.4, 37.7, 37.1, 36.8, 32.7, 30.9, 28.1, 27.9, 25.1, 23.6, 23.3, 21.3, 21.2, 19.9, 19.2, 18.1, 17.3, 16.9, 16.7, 15.6. HR-MS (m/z) (ESI): calcd for C40H59NO3 [M + H+]: 601.4495; found: 602.4549.
Compound 5Y4
Yield 84.7%; 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 1H, –CONH–), 7.35 (d, J = 8.9 Hz, 2H), 6.82 (d, J = 8.9 Hz, 2H), 5.45 (s, 1H), 4.48 (dd, J = 10.0, 5.6 Hz, 1H), 3.76 (s, 3H), 2.03 (s, 3H), 1.12 (s, 3H), 0.97 (s, 3H), 0.91 (d, J = 6.5 Hz, 6H), 0.84 (d, J = 9.2 Hz, 6H), 0.71 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.0 (–CONH–), 171.0 (–COO–), 156.2, 140.2, 131.4, 125.9, 121.4, 114.1, 80.9, 55.4, 55.2, 54.3, 48.4, 47.5, 42.6, 39.9, 39.6, 39.1, 38.4, 37.7, 37.1, 36.8, 32.7, 30.9, 28.1, 27.9, 25.1, 23.5, 23.3, 21.3, 21.2, 18.1, 17.3, 16.9, 16.7, 15.6. HR-MS (m/z) (ESI): calcd for C39H57NO4 [M + H+]: 603.4288; found: 604.4342.
Compound 5Y5
Yield 85.8%; 1H NMR (400 MHz, DMSO) δ 8.73 (s, 1H, –CONH–), 7.41 (d, J = 8.3 Hz, 2H), 7.06 (d, J = 8.3 Hz, 2H), 5.30 (s, 1H), 4.40 (dd, J = 11.2, 4.5 Hz, 1H), 2.24 (s, 3H), 2.00 (s, 3H), 1.08 (s, 3H), 0.95 (d, J = 6.1 Hz, 3H), 0.89–0.85 (m, 6H), 0.80 (d, J = 4.8 Hz, 6H), 0.64 (s, 3H). 13C NMR (101 MHz, DMSO) δ 175.4 (–CONH–), 170.5 (–COO–), 139.1, 137.2, 132.4, 129.1, 125.0, 120.9, 80.4, 55.0, 52.2, 47.9, 47.3, 42.1, 39.3, 38.8, 38.2, 37.7, 36.9, 32.9, 30.8, 28.3, 27.9, 23.9, 23.9, 23.7, 23.4, 21.5, 21.4, 20.9, 18.2, 17.6, 17.1, 15.6. HR-MS (m/z) (ESI): calcd for C39H57NO3 [M + H+]: 587.4338; found: 588.4392.
Compound 5Y6
Yield 82.9%; 1H NMR (400 MHz, CDCl3) δ 7.70 (s, 1H, –CONH–), 7.32 (s, 1H), 7.17 (t, J = 8.1 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.63 (dd, J = 8.2, 1.9 Hz, 1H), 5.49 (s, 1H), 4.49 (dd, J = 10.2, 5.5 Hz, 1H), 3.79 (s, 3H), 2.04 (s, 3H), 1.13 (s, 3H), 0.98 (s, 3H), 0.94–0.89 (m, 6H), 0.84 (d, J = 9.9 Hz, 6H), 0.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.4 (–CONH–), 171.0 (–COO–), 160.1, 140.2, 139.5, 129.5, 126.1, 111.6, 110.1, 105.2, 80.8, 55.3, 55.2, 54.4, 48.7, 47.5, 42.7, 39.9, 39.6, 39.1, 38.4, 37.7, 37.1, 36.8, 32.7, 30.9, 28.1, 27.9, 25.2, 23.6, 23.5, 23.3, 21.3, 21.2, 18.1, 17.3, 16.8, 16.7, 15.6. HR-MS (m/z) (ESI): calcd for C39H57NO4 [M + H+]: 603.4288; found: 604.4342.
Compound 5Y7
Yield 88.2%; 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H, –CONH–), 7.40 (s, 1H), 7.19–7.11 (m, 2H), 6.88 (d, J = 6.6 Hz, 1H), 5.48 (s, 1H), 4.49 (dd, J = 10.1, 5.3 Hz, 1H), 2.31 (s, 3H), 2.03 (s, 3H), 1.13 (s, 3H), 0.98 (s, 3H), 0.94–0.89 (m, 6H), 0.84 (d, J = 10.0 Hz, 6H), 0.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.2 (–CONH–), 171.0 (–COO–), 140.1, 138.78, 138.1, 128.6, 126.0, 124.8, 120.4, 116.7, 80.9, 77.4, 77.3, 77.1, 76.7, 55.3, 54.3, 48.6, 47.5, 42.6, 39.9, 39.6, 39.1, 38.4, 37.7, 37.1, 36.8, 32.7, 30.9, 28.1, 27.9, 25.1, 23.6, 23.6, 23.3, 21.5, 21.3, 21.2, 18.1, 17.3, 16.9, 16.7, 15.6. HR-MS (m/z) (ESI): calcd for C39H57NO3 [M + H+]: 587.4338; found: 588.4400.
Compound 5Y8
Yield 89.7%; 1H NMR (400 MHz, CDCl3) δ 6.61 (s, 1H, –CONH–), 5.29 (s, 1H), 4.47 (dd, J = 9.9, 5.9 Hz, 1H), 3.82 (s, 2H), 2.50 (t, J = 4.8 Hz, 2H), 2.32 (s, 6H), 2.02 (s, 3H), 1.07 (s, 3H), 0.92 (s, 6H), 0.87–0.82 (m, 9H), 0.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 178.3 (–CONH–), 171.0 (–COO–), 139.2, 125.7, 80.9, 57.7, 55.3, 53.7, 47.7, 47.5, 44.9, 42.4, 39.7, 39.6, 39.0, 38.3, 37.7, 37.2, 36.8, 36.4, 32.8, 30.9, 28.1, 27.9, 24.7, 23.5, 23.4, 23.2, 21.3, 21.2, 18.2, 17.2, 17.0, 16.7, 15.5. HR-MS (m/z) (ESI): calcd for C36H60N2O3 [M + H+]: 568.4604; found: 569.4658.
Compound 5Y9
Yield 90.1%; 1H NMR (400 MHz, CDCl3) δ 5.19 (s, 1H), 4.50–4.44 (m, 1H), 3.74 (d, J = 68.0 Hz, 8H), 2.33 (s, 3H), 2.02 (s, 3H), 1.05 (s, 6H), 0.92 (d, J = 4.7 Hz, 6H), 0.83 (d, J = 4.4 Hz, 6H), 0.72 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 175.4 (–CON–), 171.0 (–COO–), 125.2, 81.0, 55.4, 54.8, 48.5, 47.6, 45.6, 44.9, 42.1, 39.4, 38.7, 38.3, 37.7, 36.9, 34.3, 32.9, 30.5, 28.2, 28.1, 23.6, 23.3, 21.3, 21.2, 18.2, 17.4, 16.9, 16.7, 15.5. HR-MS (m/z) (ESI): calcd for C37H60N2O3 [M + H+]: 580.4604; found: 581.4655.
Compound 5Y10
Yield 88.9%; 1H NMR (400 MHz, CDCl3) δ 7.70 (s, 1H, –CONH–), 7.44 (d, J = 7.7 Hz, 2H), 7.29 (d, J = 7.6 Hz, 2H), 7.06 (t, J = 7.4 Hz, 1H), 5.49 (s, 1H), 4.49 (dd, J = 10.2, 5.6 Hz, 1H), 2.03 (s, 3H), 1.13 (s, 3H), 0.98 (s, 3H), 0.94–0.89 (m, 6H), 0.84 (d, J = 10.1 Hz, 6H), 0.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.3 (–CONH–), 171.0 (–COO–), 140.2, 138.2, 128.9, 126.1, 123.9, 119.7, 80.9, 55.2, 54.3, 48.6, 47.5, 42.7, 39.9, 39.6, 39.1, 38.4, 37.7, 37.1, 36.8, 32.7, 30.9, 28.1, 27.9, 25.1, 23.6, 23.5, 23.3, 21.3, 21.2, 18.1, 17.3, 16.9, 16.7, 15.6. HR-MS (m/z) (ESI): calcd for C38H55NO3 [M + H+]: 573.4182; found: 574.4242.
Cytotoxicity assay
The cell lines MGC-803, Spca-2, NCI-H460, A549, T24, HepG2, NCI-H460/DOX, HepG2/DOX and HL-7702 were obtained from the Shanghai Cell Bank in the Chinese Academy of Sciences. The MGC-803, Spca-2, NCI-H460, A549, T24, HepG2, NCI-H460/DOX, HepG2/DOX and HL-7702 cell lines were grown on 96-well microtitre plates at a cell density of 10 × 105 cells per well in DMEM with 10% FBS. DMEM and FBS were obtained from Gibco-Thermo (BRL Co. Ltd., USA). The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2/95% air overnight. Therewith, the cells were exposed to different concentrations of target compounds and HCPT, and incubated for another 48 h. The cells were stained with 10 μl of MTT in the incubator for about 4 h. The medium was thrown away and replaced by 100 mL of DMSO. The O. D. value was read at 570/630 nm using an enzyme labeling instrument.
In vitro inhibition of TNF-α-induced NF-κB activation in NCI-H460 lung cells
NCI-H460 cells were cultured in 12-well plates and transiently co-transfected with 0.2 mg of a pNF-κB-Luc vector (Stratagene, La Jolla, CA) and 0.2 mg of pSV-β-galactosidase dissolved in 3 ml of lipofectamine or lipofectamine 2000 (Invitrogen, Carlsbad, CA) as the internal control. The plasmids were transfected according to the manufacturer's instructions. After 6 h, the medium was changed and cells were cultured for 6 h. The cells were then treated with TNF-α (15 ng ml–1) and test compounds simultaneously for 7 h. The NCI-H460 cells treated with TNF-α alone served as positive controls, while cells without TNF-α treatment served as negative controls. Luciferase activities from these cells were then measured by using the Bright-Glo Luciferase Assay kit from Promega (Madison, WI), following the manufacturer's protocol. The relative NF-κB activities of the cells treated with the test compounds were obtained as the ratio of its luciferase activity to that from the positive controls, both of which have been corrected with background (signals from negative controls) and cell viability. Under these experimental conditions, none of the test compounds induced significant toxicity to NCI-H460 cells (<5% reduction of cell viability). The IC50 of each fraction was determined by fitting the relative NF-κB activity to the drug concentration by using a sigmoidal dose response model of varied slope in GraphPad Prism 6. The IC50 reported herein is the average of at least three replicates.
Molecular docking
To study the binding mode of the inhibitors in the active site of the NF-κB protein, molecular docking was performed using the Surflex-Dock module in SYBYL-X 2.0. The crystal structure of the NF-κB complex was retrieved from the RCSB Protein Data Bank (PDB entry code: 1IKN).28 The ligands were docked in the corresponding protein's binding site by an empirical scoring function and a patented search engine in Surflex-Dock. Before the docking process, the natural ligand was extracted; the water molecules were removed from the crystal structure. Subsequently, the protein was prepared by using the Biopolymer module implemented in Sybyl. The polar hydrogen atoms were added. The automated docking manner was applied in the present work. Other parameters were established by default in the software. Surflex-Dock total scores, which were expressed in –log10 (Kd) units to represent binding affinities, were applied to estimate the ligand–receptor interactions of the newly designed molecules.
Apoptosis analysis
NCI-H460 cells were seeded at a density of 2 × 106 cells per mL of DMEM with 10% FBS on 6-well plates to a final volume of 2 mL. The plates were incubated overnight and then treated with different concentrations of compound 5Y8 for 24 h. Briefly, after treatment with compound 5Y8 for 24 h, the cells were collected and washed with PBS twice, and then resuspended in 1× binding buffer (0.1 M Hepes/NaOH (pH 7.4), 1.4 M NaCl, 25 mM CaCl2) at a concentration of 1 × 106 cells per ml. The cells were subjected to 5 μL of FITC annexin V and 5 μL of propidium iodide (PI) staining using an annexin-V FITC apoptosis kit (BD, Pharmingen) after which 100 μL of the solution was transferred to a 5 mL culture tube and incubated for 30 min at RT (25 °C) in the dark. The apoptosis ratio was quantified by system software (CellQuest; BD Biosciences).
Cell cycle analysis
The NCI-H460 cell line was treated with different concentrations of compound 5Y8. After 48 h of incubation, cells were washed twice with ice-cold PBS, fixed and permeabilized with ice-cold 70% ethanol at –20 °C overnight. The cells were treated with 100 μg ml–1 RNase A at 37 °C for 30 min after washing with ice-cold PBS, and finally stained with 1 mg ml–1 propidium iodide (PI) (BD, Pharmingen) in the dark at 4 °C for 30 min. Analysis was performed with the system software (CellQuest; BD Biosciences).
Hoechst 33258 assay
NCI-H460 cells (2 × 106 cells) were seeded into six-well tissue culture plates and exposed to compound 5Y8 (5 and 10 μM) for 24 h. The cells were fixed in 4% paraformaldehyde for 10 min, and then the medium was discarded. The cells were then washed twice with cold PBS and incubated with 0.5 mL of Hoechst 33258 (Beyotime, China) in the dark for 5 min. After 5 min of incubation, the cells were washed twice with cold PBS and the results were analyzed with a Nikon ECLIPSE TE2000-S fluorescence microscope using 350 nm excitation and 460 nm emission wavelengths.
ROS assay
NCI-H460 cells were seeded into six-well plates and subjected to various treatments. In the following treatment, cells were collected and washed with PBS twice, then resuspended in 10 mM DCFH-DA (Beyotime, Haimen, China) dissolved in cell-free medium at 37 °C for 30 min in the dark, and washed three times with PBS. Cellular fluorescence was quantified by flow cytometry at 485 nm excitation and 538 nm emission.
Western blot
Total cell lysates from cultured NCI-H460 cells after compound 5Y8 treatments as mentioned earlier were obtained by lysing the cells in ice-cold RIPA buffer with protease and phosphatase inhibitors and stored at –20 °C for future use. The protein concentrations were quantified by the Bradford method (BIO-RAD) using a Varioskan multimode instrument (Thermo Fisher Scientific). Equal amounts of protein per lane were applied in 12% SDS polyacrylamide gel for electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences). After the membrane was blocked at room temperature for 2 h in blocking solution, primary antibodies were added and incubated at 4 °C overnight. TAK1, TAB1, IKKα, p-IKKα/β and NF-κB p65 antibodies were purchased from Imgenex, USA. After three TBST washes, the membrane was incubated with the corresponding horseradish peroxidase-labeled secondary antibodies (1 : 2000) (Santa Cruz) at room temperature for 1 h. The membranes were washed with TBST three times for 15 min and the protein blots were detected with a chemiluminescence reagent (Thermo Fisher Scientific Ltd.). The X-ray films were developed with a developer and fixed with a fixer solution.
NF-κB DNA binding assay
To determine NF-κB activation, we performed DNA binding assay using a TransAM NF-κB kit according to the manufacturer's instructions and as previously described.34 Briefly, 20 μg of nuclear proteins was added into a 96-well plate coated with an unlabeled oligonucleotide containing the consensus binding site for NF-κB (5′-GGGACTTTCC-3′) and incubated for 1 h. The wells were washed and incubated with antibodies against the NF-κB p65 subunit. An HRP-conjugated secondary antibody was then applied to detect the bound primary antibody and provided the basis for colorimetric quantification. The enzymatic product was measured at 450 nm using a microplate reader (TECAN Systems).
Statistics
The data were processed by means of the Student's t-test with the significance level P ≤ 0.05 using SPSS.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81260472, 21362002 and 21431001), Special Research Fund for the Doctoral Program of Higher Education (No. 20134504110002), the State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Science and Technology of China (CMEMR2016-B06), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 1107047002).
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00105c
References
- Karin M., Greten F. R. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Vijayalekshmi R. V., Sung B. Clin. Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Sung B. Cancer Discovery. 2011;1:469–671. doi: 10.1158/2159-8290.CD-11-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikarsky E., Porat R. M., Stein I., Abramovitch R., Amit S., Kasem S., Gutkovich-Pyest E., Urieli-Shoval S., Galun E., Ben-Neriah Y. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Li F., Sethi G. Biochim. Biophys. Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Shen H. M., Tergaonkar V. Apoptosis. 2009;14:348–363. doi: 10.1007/s10495-009-0315-0. [DOI] [PubMed] [Google Scholar]
- Suthar S. K., Boon H. L., Sharma M. Eur. J. Med. Chem. 2014;74:135–144. doi: 10.1016/j.ejmech.2013.12.052. [DOI] [PubMed] [Google Scholar]
- Patil K. R., Mohapatra P., Patel H. M., Goyal S. N., Ojha S., Kundu C. N., Patil C. R. PLoS One. 2015;10:e0125709. doi: 10.1371/journal.pone.0125709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Lehtonen M., Suuronen T., Kaarniranta K., Huuskonen J. Cell. Mol. Life Sci. 2008;65:2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. J., Liu L., Huang Z. J., Lai Y. S., Ji H., Peng S. X., Tian J. D., Zhang Y. H. J. Med. Chem. 2013;56:4641–4655. doi: 10.1021/jm400393u. [DOI] [PubMed] [Google Scholar]
- Yan X.-J., Gong L. H., Zheng F. Y., Cheng K. J., Chen Z. S., Shi Z. Drug Discovery Today. 2014;19:482–488. doi: 10.1016/j.drudis.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Shanmugam M. K., Dai X., Kumar A. P., Tan B. K. H., Sethi G., Bishayee A. Biochem. Pharmacol. 2013;85:1579–1587. doi: 10.1016/j.bcp.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Manu K. A., Kuttan G. Int. Immunopharmacol. 2008;8:974–981. doi: 10.1016/j.intimp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Hsu L. Y., Kuo P. O., Lin C. C. Life Sci. 2004;75:2303–2316. doi: 10.1016/j.lfs.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Xavier C. P. R., Lima C. F., Pedro D. F. N., Wilson J. M., Kristiansen K., Pereira-Wilson C. J. Nutr. Biochem. 2013;24:706–712. doi: 10.1016/j.jnutbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Prasad S., Yadav V. R., Sung B., Reuter S., Kannappan R., Deorukhkar A., Diagaradjane P., Wei C., Baladandayuthapani V., Krishnan S., Guha S., Aggarwal B. B. Clin. Cancer Res. 2012;18:4942–4953. doi: 10.1158/1078-0432.CCR-11-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xing D., Chen Q., Chen W. R. Int. J. Cancer. 2010;127:462–473. doi: 10.1002/ijc.25044. [DOI] [PubMed] [Google Scholar]
- Shishodia S., Majumdar S., Banerjee S., Aggarwal B. B. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- Hua S.-X., Huang R.-Z., Ye M.-Y., Pan Y.-M., Yao G.-Y., Zhang Y., Wang H.-S. Eur. J. Med. Chem. 2015;95:435–452. doi: 10.1016/j.ejmech.2015.03.051. [DOI] [PubMed] [Google Scholar]
- Mendes V. I. S., Bartholomeusz G. A., Ayres M., Gandhi V., Salvador J. A. R. Eur. J. Med. Chem. 2016;123:317–331. doi: 10.1016/j.ejmech.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y. Q., Liu D., Cai L. L. Bioorg. Med. Chem. 2009;17:848–854. doi: 10.1016/j.bmc.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Cao D., Liu Y. B., Yan W., Wang C. Y., Bai P., Wang T. J., Tang M. H., Wang X. Y., Yang Z., Ma B. Y., Ma L., Lei L., Wnag F., Xu B. X., Zhou Y. Y., Yang T., Chen L. J. J. Med. Chem. 2016;59:5721–5739. doi: 10.1021/acs.jmedchem.6b00158. [DOI] [PubMed] [Google Scholar]
- Krapf M. K., Wiese M. J. Med. Chem. 2016;59:5449–5461. doi: 10.1021/acs.jmedchem.6b00330. [DOI] [PubMed] [Google Scholar]
- Li J.-F., Huang R.-Z., Yao G.-Y., Ye M.-Y., Wang H.-S., Pan Y.-M., Xiao J.-T. Eur. J. Med. Chem. 2014;86:175–188. doi: 10.1016/j.ejmech.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li J. X., Zhao J. W., Wang S. Z., Pan Y. Bioorg. Med. Chem. Lett. 2005;15:1629–1632. doi: 10.1016/j.bmcl.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Lim K. H., Yang Y., Staudt L. M. Immunol. Rev. 2012;246:359–378. doi: 10.1111/j.1600-065X.2012.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos G. T., Sherrill T. P., Cheng D. S., Scoggins R. M., Han W., Polosukhin V. V., Connelly L., Yull F. E., Fingleton B., Blackwell T. S. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18514–18549. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxford T., Huang D. B., Malek S., Ghosh G. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. Nat. Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shim J. H., Xiao C., Paschal A. E., Bailey S. T., Rao P., Hayden M. S. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglio D., Palakurthi S., Byth K., Vega F., Toader D., Saeh J., Neelapu S. S., Younes A. Blood. 2012;120:347–355. doi: 10.1182/blood-2011-07-369397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Anderson D., Dhawan A. Apoptosis. 2012;17:852–870. doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- Chang L., Hirata H., Karin M. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Shanmugam M. K., Rajendran P., Li F., Nema T., Vali S., Abbasi T., Kapoor S., Sharma A., Kumar A. P., Ho P. C., Hui K. M., Sethi G. J. Mol. Med. 2011;89:713–727. doi: 10.1007/s00109-011-0746-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.