 A practical and efficient protocol for the amination of DNA-conjugated aryl iodide using a novel ligand was developed.
A practical and efficient protocol for the amination of DNA-conjugated aryl iodide using a novel ligand was developed.
Abstract
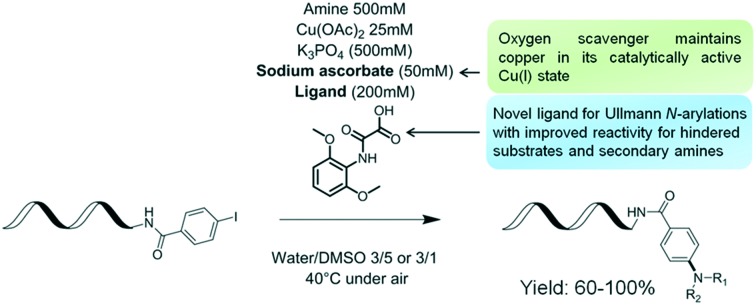
Herein, we describe the development of copper-catalyzed cross-coupling of DNA-conjugated aryl iodides with aliphatic amines. This protocol leverages a novel ligand, 2-((2,6-dimethoxyphenyl)amino)-2-oxoacetic acid, to effect the transformation in aqueous DMSO, under mild conditions and in air, making it an ideal candidate for the synthesis of DNA-encoded libraries.
DNA-encoded libraries (DELs) consist of collections of hundreds of millions to billions synthetic small molecules each conjugated to a unique DNA sequence, or a DNA tag.1,2 These vast collections of synthetic compounds are screened in mixtures by affinity selection processes, making them powerful tools for the discovery of ligands to biological targets.1,3–5 To build these libraries, synthetic small molecules are constructed directly on DNA using split-and-mix combinatorial chemistry protocols often involving several hundreds if not thousands of building blocks.1,6 Therefore, DEL synthesis rests on the development of methodologies for the formation of covalent bonds under conditions compatible with the solubility and stability of the nucleic acid tags,1,7–10 tolerant of a wide substrate scope and practical enough to facilitate miniaturization and parallelization. Thus, “nitrogen-capping” reactions such as acylation, urethanation, sulfonylation and reductive amination have been applied to the synthesis of millions to trillions of DNA-encoded compounds,1,7 among which numerous potent protein ligands have been identified.5 The formation of N-aryl bonds is another nitrogen-derivatization transformation commonly deployed in medicinal chemistry for the generation of bioactive molecules.11–14 It is therefore not surprising that several DNA-encoded library chemistry groups sought to identify amine arylation conditions compatible with the generation of DNA-encoded libraries. One approach to construct N-aryl bonds in the presence of oligonucleotides involves performing nucleophilic aromatic substitution (NAS) using heteroaromatic systems (Scheme 1). While successfully used in DEL synthesis,15–19 it is limited in practice to strongly-activated heteroaromatic systems such as triazine15,16,19 and pyrimidine8 chlorides, or nitro-substituted aromatic fluorides.20
Scheme 1. Current and proposed N-arylation approaches for DEL synthesis. A: 46 equiv. R2–NH2 (200 mM DMA stock), 80 °C, 6 h. B: CuI or Cu(OAc)2 (25 mM), ligand (50–200 mM), Na ascorbate (50 mM), base K3PO4 (500 mM), R4–NH2 (500 mM), DMSO/water 1/1, 5/3 or 1/3, 40 °C.
In order to extend the scope of this transformation to less-activated aromatic cores, we and others21 chose to investigate the copper-catalyzed Ullmann N-arylation of amines with aryl halides22–24 (Scheme 1). Indeed, while this manuscript was in preparation, Lu et al. reported their efforts to optimize palladium- and copper-based N-arylation of DNA-conjugated aryl iodides with primary aliphatic and aromatic amines. While a wide range of such amines was shown to be competent under the reported conditions, cross-coupling reactions involving secondary amines still appeared to pose a significant challenge.21 Spurred by the large numbers of ligands reported to enable such cross-couplings under mild reaction conditions in polar solvents25–27 and, in some cases, in the presence of air28 and water,29 we surmised that a systematic survey of catalytic systems might unlock previously unreachable portions of chemical space.
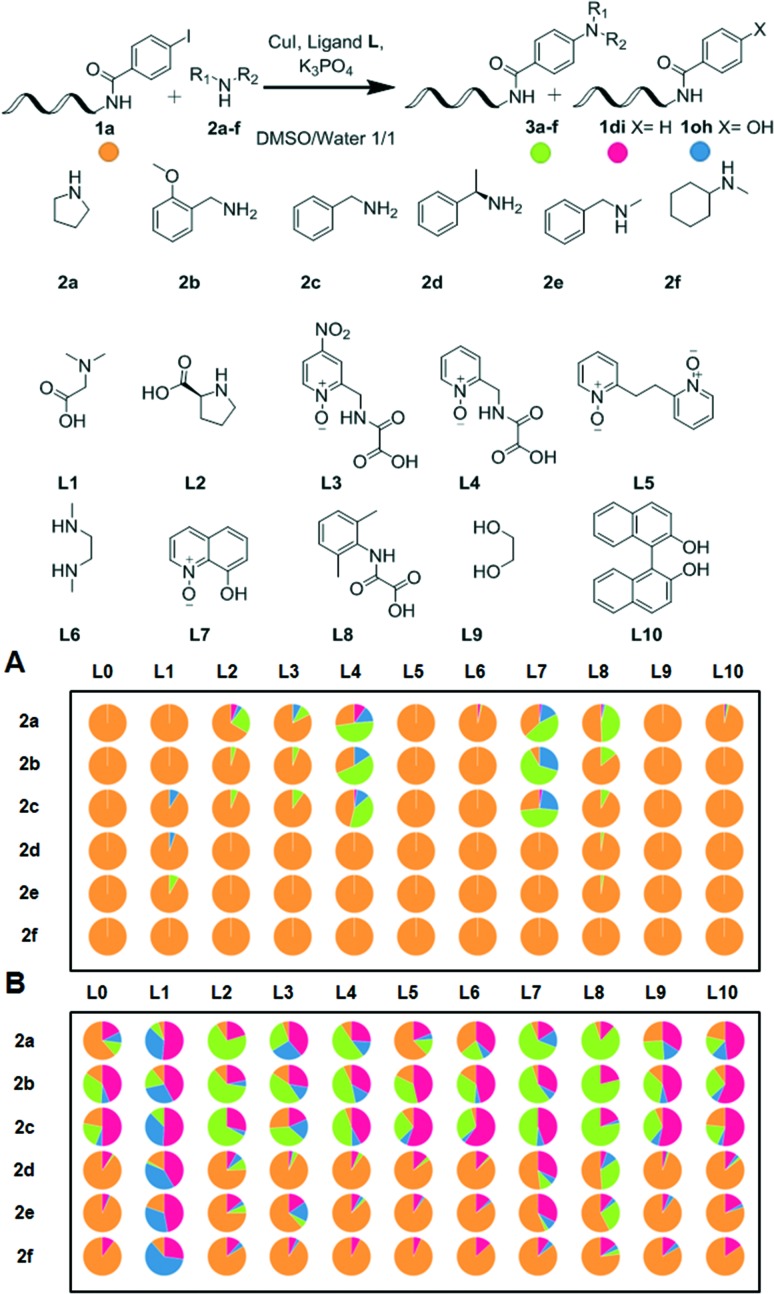
We started with investigating the model on-DNA coupling reaction between DNA-conjugated aryl iodide 1a and a set of amine substrates 2a–f mediated by ligands L1–10 (ref. 26 and 28–34) under reaction conditions compatible with DNA-encoded library synthesis.7,8 Namely, we desired to manipulate building block stock solutions in water-miscible organic co-solvents and in a plate format (Fig. 1A).
Fig. 1. Initial screening of known ligands for the copper-catalyzed coupling of representative amines and model DNA-conjugated aryl iodide 1a. Reaction conditions: A: 1a (1 nmol), 2a–f (500 mM), CuI (25 mM), ligand L (50 mM), K3PO4 (500 mM), DMSO (8 μl), water (8 μl), 40 °C, 3 h; B: 1a (1 nmol), 2a–f (500 mM), CuI (25 mM), ligand L (50 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO (8 μl), water (8 μl), 40 °C, 3 h. Reactions performed in air. Pie chart areas represent conversion to 3a–f (%) as determined by UPLC-TOF.
Among the ligands reported for the N-arylation of aliphatic amines, L7 (ref. 33) (8-hydroxyquinoline 1-oxide) and L8 (ref. 35) (DMPAO: 2-(2,6-dimethylphenylamino)-2-oxoacetic acid) showed moderate to good conversion of 1a with amines 2a, 2b and 2c to the respective products 3a,363b and 3c under aerobic aqueous conditions at 40 °C. Notably, L8 provided the cleanest reaction profile and lowest proportion of side products 1di and 1oh while only limited amounts of DNA degradation could be observed by LC-MS.37 Satisfied with these early, positive results, we did not further investigate oligonucleotide damage at this stage and proceeded with reaction optimization.
Since library synthesis under an inert atmosphere or using degassed solvents can prove cumbersome in practice, we repeated the experiment to investigate the effect of an additional reducing agent introduced to circumvent the risk of aerobic oxidation of the copper catalyst (Fig. 1B). The superior conversions of the starting DNA-conjugate into products and by-products observed in the presence of ascorbic acid provided circumstantial evidence that copper oxidation indeed limited the reactivity of the system. Gratifyingly, when 50 mM aqueous sodium ascorbate solution was added to the reaction (2 eq. compared to CuI), significantly improved conversions to the N-arylated products 3a–c could be observed. Again, ligand L8 led consistently to higher yields of products than any of the other ligands tested L1–10.
Ligand L8 was originally reported as particularly efficient for the N-arylation of hindered secondary aliphatic amines.35 Under our aqueous conditions, it performed better for the N-arylation of hindered substrates 2d, N-methylbenzylamine 2e and N-methylcyclohexylamine 2f (Fig. 1B, entries 2d/L8, 2e/L8 and 2f/L8) than any of the other candidates. Encouraged by this positive trend, we set out to investigate alternative designs of L8.
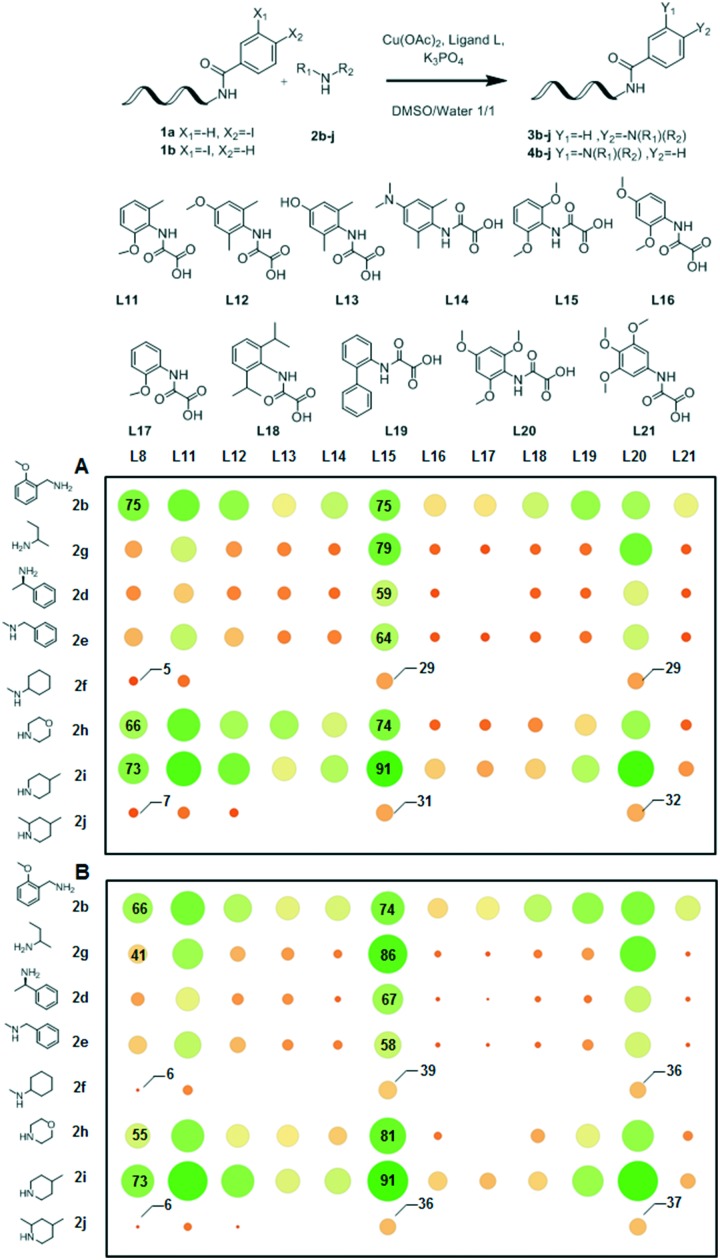
The group of Ma demonstrated the utility of the two methyl groups in L8 and the beneficial effect of introducing electron-donating moieties on structurally-related oxalic diamide ligands.38 Since the steric and electronic properties of the ligand appeared critical to its efficiency in this study, we synthesized 11 analogues of L8 and evaluated them for competency in the amination of DNA-conjugates 1a and 1b with a panel of diverse amines (Fig. 2A and B). We elected to use this particular pair of DNA-conjugates to rule out any electronic effect that could be specific to 1a. For practical reasons, we also replaced copper(i) iodide with copper(ii) acetate, as the latter is not sensitive to oxidation, yielding stable stock solutions in either DMSO or water. In a preliminary experiment, this change did not appear to cause any detrimental effect (shown in Fig. 1B/combination 1a/2b/L8, 78% compared with Fig. 2A/combination 1a/2b/L8, 75% conversion). The results of this screening experiment showed a dramatic influence of the ligand structure on its reactivity. As evidenced by the conversion observed for ligands L8, L12, L13 and L14, the addition of electron-donating moieties to para- did not provide noticeable advantages. Replacing one of the methyl groups in L8 with methoxy (L8 → L11) led to an increase in conversion across the panel of tested amines, while increasing the steric bulk around the oxalic amide (L8 → L18) did not provide any noticeable advantage. Ligands lacking two ortho-substituents (L17, L19 and L21) were generally found to be inferior to L8 despite attempts to vary the electron density on the aromatic ring. Finally, di-ortho-substitution appeared mandatory to obtain efficient ligands: replacing both methyl groups on L8 by two methoxy groups led to the strikingly more efficient ligand L15 and this finding was shown by the matched pair L12 → L20. Notably, using 1b as the substrate, a 6-fold improvement in the product yield could be observed for secondary amine 2f when L15 was used instead of L8 (Fig. 2A: L8–2f: 6%; L15–2f: 39%).
Fig. 2. Structural and electronic effects on ligand efficiency: selected screening results. Reaction conditions: A: 1a (1 nmol), 2b–j (500 mM), Cu(OAc)2 (25 mM), ligand L (50 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO (8 μl), water (8 μl), 40 °C, 3 h. B: 1b (1 nmol), 2b–j (500 mM), Cu(OAc)2 (25 mM), ligand L (50 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO (8 μl), water (8 μl), 40 °C, 3 h. The color and diameter of circles correlate with the conversion to 3b–j (A) or 4b–j (B) determined by UPLC-TOF analysis.
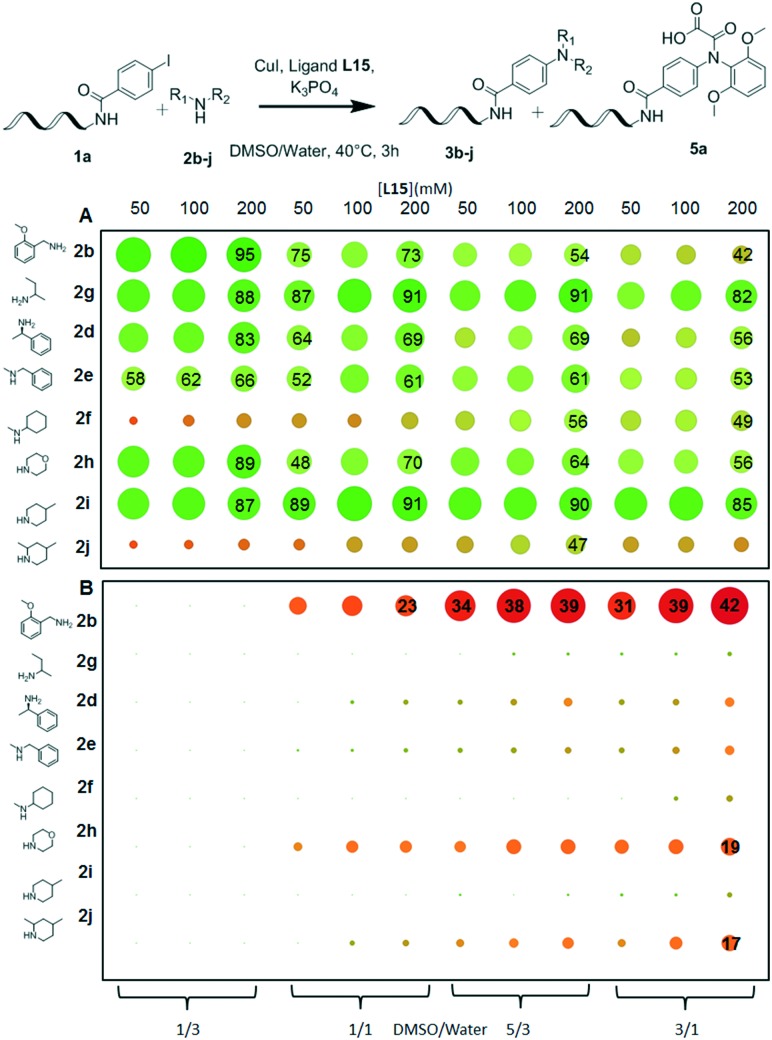
While excellent conversions were already observed for L15 in most cases, the yields were still low to moderate for the most hindered substrates N-methylcyclohexylamine 2f and 2,4-dimethylpiperidine 2j. We therefore went through another round of reaction condition optimization, studying the effect of the concentrations of ligand L15, base, sodium ascorbate and amine on the yield of the N-arylation of 2f with 1b. While varying the concentrations of amine, base and ascorbic acid had marginal effects on conversion, increasing the concentration of ligands to 200 mM did cause a noticeable increase in the formation of N-arylated 2f (data not shown, please see ESI† section VII). We therefore sought to validate this observation in the reaction of DNA-conjugated aryl iodides 1a and 1b39 with our set of diverse test amines. Suspecting that the proportion of DMSO to water might also affect the yield and formation of side-products during the reaction, we introduced this additional parameter in the experimental design (Fig. 3).
Fig. 3. Optimization of the concentration of L15 and co-solvent proportion in a plate format. Reaction conditions: A: 1a (1 nmol), 2b–j (500 mM), Cu(OAc)2 (25 mM), ligand L15 (50–200 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO/water (1/3–3/1 16 μl), 40 °C, 3 h. The color and diameter of circles correlate with the yield of 3b–j determined by UPLC-TOF analysis. B: 1a (1 nmol), 2b–j (500 mM), Cu(OAc)2 (25 mM), ligand L15 (50–200 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO/water (1/3–3/1 16 μl), 40 °C, 3 h. The color and diameter of circles correlate with the yield of 5a determined by UPLC-TOF analysis.
This experiment confirmed that increasing the concentration of ligands had a beneficial effect on the conversion for reactions with hindered substrates 2f and 2j (Fig. 3 and ESI† VI1). In addition, increasing the ligand concentration and DMSO proportion worked synergistically to improve the yield of the reaction with a maximal 56% yield of 3f obtained with a 200 mM concentration of L15 in DMSO/water: 5/3. However, these observations could not be broadly generalized as the proportion of DMSO had a deleterious effect on the conversions observed for primary amines 2b and 2d (Fig. 3A). Indeed, altering the DMSO/water ratio from 1/3 to 3/1 caused 56% reduction in the yield of N-arylation between 1a and 2-methoxybenzylamine 2b (Fig. 3A line 2b).
We hypothesize that the amine and DMSO compete for the coordination of the copper catalyst and as a consequence, an inhibitory effect of this co-solvent is observed for higher ratios. Indeed, using 1a as the substrate in particular, increasing the proportion of DMSO led to the appearance of side-products resulting from unwanted N-arylation of the ligand, with up to 42% of the starting material 1a being converted into 5a when combined with 2-methoxybenzylamine (Fig. 3B and ESI† VI2).
Therefore, while our initial studies were conducted with an equal proportion of DMSO and water in the reaction medium, we found that lowering the proportion of DMSO to DMSO/water: 1/3 (conditions 1) led to a more efficient system for the majority of unhindered primary and secondary amines, while at the same concentration of ligand L15, more hindered substrates like 2f or 2j did benefit from increasing the proportion of co-solvent to a ratio of DMSO/water: 5/3 (conditions 2). With all other parameters being kept equal, the perspective of using different solvent proportions to accommodate specific amine building block subsets during library synthesis was judged acceptable. At this stage of our optimization campaign, we decided to conduct a more thorough analysis of DNA damage caused by conditions 1. In a manner analogous to that described by the group of Paegel,40 model reactions were conducted in the presence of a small amount of amplifiable DNA. Gratifyingly, quantification by qPCR after work-up and comparison with a reference sample indicated that 65% of the amplifiable DNA remained after being subjected to conditions 1. This amount is in line with those observed for reactions we have already deployed in the synthesis of large discovery libraries40 (a full experimental account is provided in the ESI† IX).
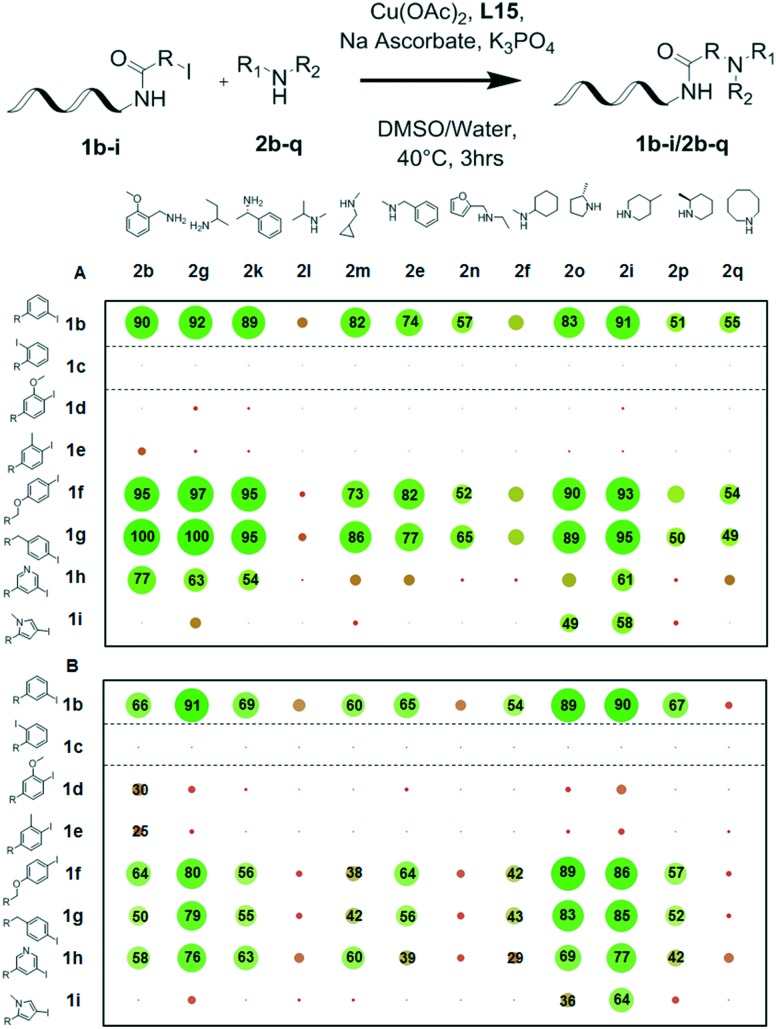
With these results in hand, we proceeded with investigating the scope of conditions 1 and 2 on a combinatorial matrix of 8 DNA-conjugated aryl iodides with 12 different amines (Fig. 4). This experiment confirmed that conditions 1 led to useful yields for the majority of substrate combinations as long as the halogen atom found itself in an unhindered environment. For benzene derivatives, electronic effects on the aromatic ring seemed to have negligible influence on the conversions observed, as most amines gave good to excellent conversions (41–100%) with substrates 1b, 1f, 1g and 1h. Interestingly, the 3-iodopyrrole-derived substrate 1i could only be combined with cyclic secondary amines 2o and 2i, giving 49% and 58% conversion, respectively, while reactions with simpler amines failed to yield useful amounts of coupling products (Fig. 4A). The N-arylation reaction appeared, however, to be very sensitive to steric hindrance. Indeed, little to no conversion was observed with substrates 1c, 1d and 1e having substituents ortho- to the reactive halogen (Fig. 4A). In contrast, it was satisfying to observe that unfavourable combinations like 1d/2b or 1e/2b were still formed in moderate yield (30 and 25% respectively) under conditions 2, when these products were only formed as traces under conditions 1 (Fig. 4B). Interestingly, higher yields were also obtained under conditions 2 for the reactions involving iodopyridine 1h as the substrate (Fig. 4B). Taken together, these results highlighted the nuances of reactivity imparted by both coupling partners: thorough vetting of building blocks prior to library synthesis will be necessary to ensure useful yields of final library products.41 Conditions 2 appeared to be most beneficial to very specific substrate combinations, which may warrant their deployment in the synthesis of focused DNA-encoded libraries. In contrast, conditions 1 appeared to more generally lead to higher product yields, making them our natural choice for the synthesis of larger discovery libraries.
Fig. 4. Scope determination of the N-arylation of substrates 1b–1i with amines 2b–2q. A: Reaction conditions 1: 1b–i (1 nmol), 2b–q (500 mM), Cu(OAc)2 (25 mM), ligand L15 (200 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO/water (1/3 16 μl), 40 °C, 3 h. B: Reaction conditions 2: 1b–i (1 nmol), 2b–q (500 mM), Cu(OAc)2 (25 mM), ligand L15 (200 mM), sodium ascorbate (50 mM), K3PO4 (500 mM), DMSO/water (5/3 16 μl), 40 °C, 3 h.
Conclusions
In conclusion, we have developed a novel ligand and a set of reaction conditions to facilitate the copper-catalyzed cross-coupling of DNA-conjugated aryl iodides with a variety of aliphatic amines, most notably relatively hindered secondary amines. Importantly, the catalytic system operates at low temperature and in air, using an organic co-solvent compatible with the generation and storage of a large number of amine building block stock solutions. These features render our reaction conditions highly amenable to the synthesis of DNA-encoded libraries based on the formation of Csp2–N bonds and complement the existing NAS and N-arylation protocols.21
Conflicts of interest
The authors declare no competing interest.
Supplementary Material
Acknowledgments
The research leading to these results was supported by the NIBR Postdoctoral Program (Y. Ruff). We thank Dr. Christoph Gaul for his help during the preparation of this manuscript. We are also grateful to Mr. Xavier Pellé and Ms. Sandrine Liverneaux for fruitful discussions and their help with the purification of small molecule–oligonucleotide conjugates.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8md00185e
References
- Goodnow Jr R. A., Dumelin C. E., Keefe A. D. Nat. Rev. Drug Discovery. 2017;16:131–147. doi: 10.1038/nrd.2016.213. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Neri D. Drug Discovery Today. 2016;21:1828–1834. doi: 10.1016/j.drudis.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon H., Klika Škopić M., Jung K., Bugain O., Brunschweiger A. ACS Chem. Biol. 2016;11:296–307. doi: 10.1021/acschembio.5b00981. [DOI] [PubMed] [Google Scholar]
- Ottl J., in A Handbook for DNA-Encoded Chemistry, John Wiley & Sons, Inc., 2014, 10.1002/9781118832738.ch14, pp. 319–347. [DOI] [Google Scholar]
- Goodnow R. A. and Davie C. P., in Annu. Rep. Med. Chem., ed. R. A. Goodnow, Academic Press, 2017, vol. 50, pp. 1–15. [Google Scholar]
- Creaser S. P. and Acharya R. A., in A Handbook for DNA-Encoded Chemistry, John Wiley & Sons, Inc., 2014, 10.1002/9781118832738.ch6, pp. 123–151. [DOI] [Google Scholar]
- Luk K.-C. and Satz A. L., in A Handbook for DNA-Encoded Chemistry, John Wiley & Sons, Inc., 2014, 10.1002/9781118832738.ch4, pp. 67–98. [DOI] [Google Scholar]
- Satz A. L., Cai J., Chen Y., Goodnow R., Gruber F., Kowalczyk A., Petersen A., Naderi-Oboodi G., Orzechowski L., Strebel Q. Bioconjugate Chem. 2015;26:1623–1632. doi: 10.1021/acs.bioconjchem.5b00239. [DOI] [PubMed] [Google Scholar]
- McGregor L. M. and Liu D. R., in A Handbook for DNA-Encoded Chemistry, John Wiley & Sons, Inc., 2014, 10.1002/9781118832738.ch17, pp. 377–415. [DOI] [Google Scholar]
- Probst N., Lartia R., Théry O., Alami M., Defrancq E., Messaoudi S. Chem. – Eur. J. 2017;24:1795–1800. doi: 10.1002/chem.201705371. [DOI] [PubMed] [Google Scholar]
- Fischer C., Koenig B. Beilstein J. Org. Chem. 2011;7:59–74. doi: 10.3762/bjoc.7.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley S. D., Jordan A. M. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- Carey J. S., Laffan D., Thomson C., Williams M. T. Org. Biomol. Chem. 2006;4:2337–2347. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- Schneider N., Lowe D. M., Sayle R. A., Tarselli M. A., Landrum G. A. J. Med. Chem. 2016;59:4385–4402. doi: 10.1021/acs.jmedchem.6b00153. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Acharya R. A., Arico-Muendel C. C., Belyanskaya S. L., Benjamin D. R., Carlson N. R., Centrella P. A., Chiu C. H., Creaser S. P., Cuozzo J. W., Davie C. P., Ding Y., Franklin G. J., Franzen K. D., Gefter M. L., Hale S. P., Hansen N. J. V., Israel D. I., Jiang J., Kavarana M. J., Kelley M. S., Kollmann C. S., Li F., Lind K., Mataruse S., Medeiros P. F., Messer J. A., Myers P., O'Keefe H., Oliff M. C., Rise C. E., Satz A. L., Skinner S. R., Svendsen J. L., Tang L., van Vloten K., Wagner R. W., Yao G., Zhao B., Morgan B. A. Nat. Chem. Biol. 2009;5:647–654. doi: 10.1038/nchembio.211. [DOI] [PubMed] [Google Scholar]
- Deng H., O'Keefe H., Davie C. P., Lind K. E., Acharya R. A., Franklin G. J., Larkin J., Matico R., Neeb M., Thompson M. M., Lohr T., Gross J. W., Centrella P. A., O'Donovan G. K., Bedard K. L., van Vloten K., Mataruse S., Skinner S. R., Belyanskaya S. L., Carpenter T. Y., Shearer T. W., Clark M. A., Cuozzo J. W., Arico-Muendel C. C., Morgan B. A. J. Med. Chem. 2012;55:7061–7079. doi: 10.1021/jm300449x. [DOI] [PubMed] [Google Scholar]
- Kollmann C. S., Bai X., Tsai C.-H., Yang H., Lind K. E., Skinner S. R., Zhu Z., Israel D. I., Cuozzo J. W., Morgan B. A., Yuki K., Xie C., Springer T. A., Shimaoka M., Evindar G. Bioorg. Med. Chem. 2014;22:2353–2365. doi: 10.1016/j.bmc.2014.01.050. [DOI] [PubMed] [Google Scholar]
- Arico-Muendel C. C. MedChemComm. 2016;7:1898–1909. [Google Scholar]
- Ding Y., O'Keefe H., DeLorey J. L., Israel D. I., Messer J. A., Chiu C. H., Skinner S. R., Matico R. E., Murray-Thompson M. F., Li F., Clark M. A., Cuozzo J. W., Arico-Muendel C., Morgan B. A. ACS Med. Chem. Lett. 2015;6:888–893. doi: 10.1021/acsmedchemlett.5b00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Zhou J., Sundersingh F. S., Summerfield J., Somers D., Messer J. A., Satz A. L., Ancellin N., Arico-Muendel C. C., Bedard K. L., Beljean A., Belyanskaya S. L., Bingham R., Smith S. E., Boursier E., Carter P., Centrella P. A., Clark M. A., Chung C.-w., Davie C. P., Delorey J. L., Ding Y., Franklin G. J., Grady L. C., Herry K., Hobbs C., Kollmann C. S., Morgan B. A., Kaushansky L. J., Zhou Q. ACS Med. Chem. Lett. 2015;6:919–924. doi: 10.1021/acsmedchemlett.5b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Roberts S. E., Franklin G. J., Davie C. P. MedChemComm. 2017;8:1614–1617. doi: 10.1039/c7md00289k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambiagio C., Marsden S. P., Blacker A. J., McGowan P. C. Chem. Soc. Rev. 2014;43:3525–3550. doi: 10.1039/c3cs60289c. [DOI] [PubMed] [Google Scholar]
- Ley S. V., Thomas A. W. Angew. Chem., Int. Ed. 2003;42:5400–5449. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- Beletskaya I. P., Cheprakov A. V. Coord. Chem. Rev. 2004;248:2337–2364. [Google Scholar]
- Ma D., Cai Q., Zhang H. Org. Lett. 2003;5:2453–2455. doi: 10.1021/ol0346584. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ling J., Zhang Y., Zhang A., Yao Q. Eur. J. Org. Chem. 2015;2015:4153–4161. [Google Scholar]
- Bhunia S., Pawar G. G., Kumar S. V., Jiang Y., Ma D. Angew. Chem., Int. Ed. 2017;56:16136–16179. doi: 10.1002/anie.201701690. [DOI] [PubMed] [Google Scholar]
- Kwong F. Y., Klapars A., Buchwald S. L. Org. Lett. 2002;4:581–584. doi: 10.1021/ol0171867. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Yang B., Zhang A., Yao Q. Org. Biomol. Chem. 2015;13:4101–4114. doi: 10.1039/c5ob00045a. [DOI] [PubMed] [Google Scholar]
- Surry D. S., Buchwald S. L. Chem. Sci. 2010;1:13–31. doi: 10.1039/C0SC00107D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Fu H., Jiang Y., Zhao Y. J. Org. Chem. 2007;72:672–674. doi: 10.1021/jo062060e. [DOI] [PubMed] [Google Scholar]
- Zhang H., Cai Q., Ma D. J. Org. Chem. 2005;70:5164–5173. doi: 10.1021/jo0504464. [DOI] [PubMed] [Google Scholar]
- Yang K., Qiu Y., Li Z., Wang Z., Jiang S. J. Org. Chem. 2011;76:3151–3159. doi: 10.1021/jo1026035. [DOI] [PubMed] [Google Scholar]
- Liang L., Li Z., Zhou X. Org. Lett. 2009;11:3294–3297. doi: 10.1021/ol9010773. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang X., Yao Q., Ma D. Org. Lett. 2012;14:3056–3059. doi: 10.1021/ol301135c. [DOI] [PubMed] [Google Scholar]
- The structure of 3a, 3h and 4h was further confirmed by synthesis using an alternative route (ESI X)
- Representative screening results, DNA degradation side products and product characterization can be found in the ESI section IV
- Zhou W., Fan M., Yin J., Jiang Y., Ma D. J. Am. Chem. Soc. 2015;137:11942–11945. doi: 10.1021/jacs.5b08411. [DOI] [PubMed] [Google Scholar]
- Representative screening results for the substrate 1b can be found in the ESI section VI
- Malone M. L., Paegel B. M. ACS Comb. Sci. 2016;18:182–187. doi: 10.1021/acscombsci.5b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As a rule of thumb, we estimate that a 40% product yield is sufficient to ensure adequate representation of each member of the library, provided that the presence of unknown by-products is limited
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.