Abstract
Focal adhesions are highly dynamic multi-protein complexes found at the cell surface and effectively link the cell's internal cytoskeleton to a complex mixture of macromolecules known as the extracellular matrix and mediate transmission of signals from the extracellular matrix to the nucleus. Zyxin is one of the key focal adhesion proteins and is also found to shuttle in the nucleus. Although the mechanism of shuttling to the nucleus unclear, it moves out from the nucleus through a leucine-rich nuclear export signal sequence. It is known to contribute to fundamental cellular activities such as cell migration, adhesion and proliferation by interacting with a variety of cellular proteins. It is also linked with a number of cancers such as melanoma, hepatocellular carcinoma, oral squamous-cell carcinoma, Ewing sarcoma and prostate cancer. However, in many cases, the precise mechanisms by which the absence or presence of zyxin contributes to cancer progression or suppression is unknown. Thus, more work is required to gain insights into how zyxin modulates cellular functions in relationship to cancer. This review summarises the role of zyxin in cancer, with an emphasis on conflicting roles in prostate cancer.
Keyword: Cancer research
1. Introduction
Cell adhesion to the extracellular matrix (ECM) is essential for the normal functioning of many tissues in humans including the skin, the gut and the kidneys. At the cellular level, cell adhesion regulates a host of activities including cell migration, proliferation and death. A characteristic feature of cancer is that cell adhesion to the ECM is perturbed in such a way that control of normal cell function is lost. Often, cell attachment to the ECM is required for transfer of signals from outside of the cells to inside that direct many functions in cells, including progression through the cell cycle; and cells that detach from the ECM undergo programmed cell death (apoptosis). Cell adhesion to the ECM is mediated primarily through a group of transmembrane receptors that cluster into complex multi-molecular structures known as focal adhesions (FAs), which link the ECM to the actin cytoskeleton.
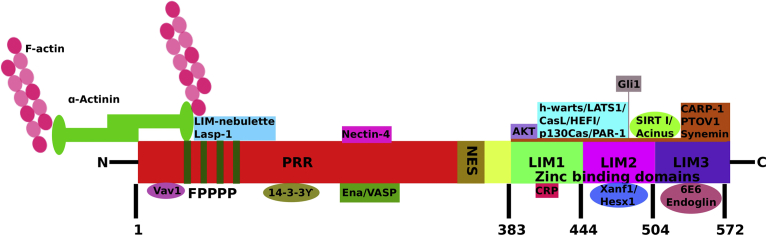
Zyxin, a phosphoprotein, is implicated in actin cytoskeleton assembly and is mainly localized at FAs. It can, however, shuttle between the cytoplasm and nucleus and plays a role in transcription [1]. The mechanisms by which zyxin shuttles between nucleus and cytoplasm are unclear. However, it contains a leucine-rich nuclear export signal sequence (Fig. 1) that helps its exit from the nucleus to the cytoplasm [2]. Leptomycin B was found to block zyxin shuttling from the nucleus and result in nuclear accumulation of zyxin [3]. It is composed of 572 amino acids and has two major segments; an N-terminal domain containing proline-rich sequences and three LIM (Lin11, Isl-1 & Mec-3) domains at the C-terminus, through which it interacts with a variety of proteins. Fig. 1 illustrates the domain architecture of zyxin and some of its known binding partners, that allow zyxin to plays a pivotal role in a host of cellular activities. In addition to zyxin, the Zyxin subfamily also includes Ajuba, LIMD1 (LIM domain-containing protein 1), LPP (Lipoma-preferred partner), TRIP6 (Thyroid receptor-interacting protein 6) and WTIP (Wilms Tumor protein-1 interacting protein) [4].
-
1.
Multiple binding partners of Zyxin and effect on DNA damage/repair.
Fig. 1.
Domain architecture of zyxin and its key binding partners (not drawn to scale). PRR – Proline rich region; FPPPP – Proline rich repeat; NES – Nuclear Export Signal; LIM – Lin-11, Isl-1, and Mec-3M; CRP – Cysteine rich protein and VASP is Vasodilator-stimulated phosphoprotein.
As outlined in Fig. 1, zyxin has been identified as a key interacting partner for a number of molecules, which allows it to execute a plethora of cellular functions. For example, a yeast two-hybrid screen resulted in the identification of the cell-cell adhesion receptor nectin-4 as a zyxin binding partner. Docking of zyxin to nectin-4 receptor is required for the localization of zyxin at the FAs site and the associated strong cell-cell adhesions [5]. Moreover; with depletion of nectin, zyxin fails to localize at the FAs [5]. Furthermore, zyxin was also found to be associate with the homeodomain interacting protein kinase 2 (HIPK2) protein. HIPK2 is a critical regulator of cell fate, in response to genomic damage. For example, in unstressed cells, HIPK2 is maintained at very low amounts. However, during the stress conditions such as UV exposure, irradiation or chemotherapy that leads to severe genomic damage, HIPK2 is activated, followed by phosphorylation of p53 that leads to apoptosis [6]. Zyxin expression was found to be critical for HIPK2 stability as zyxin depletion results in HIPK2 degradation, resulting in inhibition of DNA damage-induced p53 phosphorylation by HIPK2. Thus, zyxin is a critical regulator of DNA damage-induced cell death through regulation of HIPK2 – P53 signaling axis [7].
Zyxin also plays a crucial role in the organization of the actin cytoskeleton [8]. It was demonstrated to interact with Ena/VASP family proteins and recruit them at the FAs [9]. LIM domains are known to act as docking sites for several proteins; and are essential for a variety of cellular functions, including cell differentiation and proliferation. For example, the LIM1-2 domains interact with CasL/HEF1 and p130Cas [10] that further affect cell adhesion and motility. This interaction also has implications in cell spreading, and migration [11] as well as regulation of the Hippo signaling pathway [12].
-
2.
Zyxin and cancer
-
2.1.
Zyxin acts as an oncogene.
Based on the information available in the literature, it is evident that zyxin has Yin and Yang relationship with cancer development. It was found to act as a possible oncogene at some sites promoting cancer development or progression, while it acts as a possible tumour suppressor in other organs protecting from cancer progression. It is not very clear why zyxin has these possible opposing actions; but we know this can be affected by the organ involved, experimental conditions and more importantly by the presence/absence of interacting proteins.
For example, zyxin was linked to melanoma via the Wilms' Tumour protein (WT1) that is associated with melanoma proliferation. WT1 was found to be expressed in malignant melanoma in >80% of cells, while it is not expressed in normal skin or in benign nevi. Silencing WT1 significantly resulted in reduced zyxin expression and reduced melanoma cell proliferation [13]. A more recent study found that peroxisome proliferator activated receptors (PPARs) activation could result in downregulation of WT1 and zyxin, suppressing melanoma cell growth [14]. Another study demonstrated that zyxin was upregulated in melanoma cells compared to melanocytes. Interestingly, the expression of zyxin is directly related to cell spreading and proliferation and inversely related to differentiation. Treatment of melanoma cells with 12-O-tetradecanoylphorbol-13-acetate; which acts in normal cells as a protein kinase C (PKC) activator, results in depletion of PKC in melanoma cells, associated with suppressed Zyxin expression, inhibited cell spreading and proliferation, and promoted differentiation [15].
The expression pattern of zyxin was also studied among cases with hepatocellular carcinoma (HCC). Zyxin over-expression was evident in one-third of cases and was 60 folds higher in cases with multiple tumours. Zyxin over-expression was also found more evident in cases that developed recurrence following hepatectomy [16]. LIM and SH3 protein 1 (LASP-1) has been demonstrated to play an important role in cancer development and progression, through binding with zyxin and affecting actin filament dynamics. LASP-1 expression analysis in 144 HCC cases revealed that LASP-1 over-expression is directly correlated with worsening clinical prognosis and poor overall survival [17]. LASP-1 also expresses in high amounts in breast cancer patients and a study found that transfecting cells with LASP-1 specific siRNA could result in up to 50% suppression of tumour cell proliferation [1]. LASP-1 expression was investigated in a series of 216 ccRCC (clear cell Renal Cell Carcinoma) tissues and correlated with clinical data. LASP-1 expression was significantly higher in ccRCC tissues compared to non-tumour tissue and its upregulation was found to be associated with a larger tumour size and higher tumour stage [18]. LASP-1 was also found to mediate proliferation and migration of ovarian epithelial tumours. A study performed with 26 patients with an ovarian tumour found that LASP-1 was expressed in 54% of malignant tissue, while all benign tissue lacked LASP-1 expression. Interestingly, silencing of LASP-1 leads in depletion of zyxin at FA points and decreases cell migration [19]. Thus, zyxin plays vital roles in breast carcinoma, HCC and ccRCC via its interaction with LASP-1.
A study that included 102 patients with malignant renal tumours and 12 patients with benign renal tumours, to identify serum peptides as a possible aiding diagnostic utility for malignant tumours revealed that zyxin was only found to be over-expressed in malignant renal tumours [20]. A similar study by Ma et al [21]. was performed for breast cancer that presented that zyxin was expressed in 70% of breast cancer tissue but only in 5% normal breast tissues. They even found that higher immunostaining intensity was correlating with histological stage and lymph nodes metastases. Moreover; Trip6 (a member of Zyxin family) overexpression was associated with the proliferation of non-Hodgkin lymphoma and to inversely correlate with survival and time to recurrence [22]. Its high expression levels were also detected in Glioma tissue and the overall survival rate of these patients decreased as the level of Trip6 increased [23]. These data show that Trip6 overexpression promotes poor clinical outcome in a dose-dependent manner.
In addition, zyxin was established as an interacting partner of the Human papilloma virus 6 (HPV6) E6 protein. An HPV infection leads to genital warts as well as penile and cervical carcinoma. Binding of HPV6 E6 protein with zyxin leads to relocalization of zyxin from cytoplasm to nucleus where it acts as a transcriptional activator, promoting warts growth and cell proliferation [24].
-
2.2.
Zyxin acts as a tumour suppressor.
Cell Cycle and Apoptosis Regulator Protein-1 (CARP-1) is a zyxin-binding partner. CARP-1 was originally described as a protein required for tumor cell death in response to retinoids and adriamycin [25]. LIM domains of zyxin were determined to be the critical regions for CARP-1 binding as well as for zyxin's proapoptotic effect [26]. It was also demonstrated that CARP-1 and zyxin cooperate to promote apoptosis [26]. CARP-1 was found to be downregulated in 63% of gastric cancer tissues; of which, low-intensity staining was identified in 81% cases while no patients with gastric cancer had a high-intensity staining for CARP-1 [27]. Another study on zyxin found that it is downregulated in Ewing sarcoma where zyxin is more concentrated in the cytoplasm rather than cell adhesion junctions. Zyxin gene transfer, however, could result in a redistribution of zyxin, leading to reorganization of actin cytoskeleton and decreasing cell motility and growth [28].
Myopodin (Synaptopodin 2) is tumour suppressor gene located at 4q25 on a human chromosome that is deleted in patients with prostate cancer [29]. Zyxin was found to interact directly with myopodin and this interaction was found to be essential for the tumour suppressor effect of myopodin on prostate cancer growth and progression. Deletion of the sequence required for myopodin-zyxin interaction abolished myopodin mediated suppression of cell motility and invasiveness [30]. Another study showed that deletion of myopodin was associated with higher rate of metastases and clinical relapse of prostate cancer, irrespective of Gleason grade, while persistence of myopodin was associated with lower rate of metastases and clinical relapse [31]. Kai et al. [32] demonstrated that the response of PC3 cells to myopodin differ according to the external stimuli. They used conditioned medium (CM) and 10% fetal bovine serum (FBS) to show that both chemotactants can enhance PC3 cell migration, with CM being 5 times more potent than FBS. They found that myopodin suppressed motility of cells in presence of the strong CM stimulus, while it enhanced cell motility in presence of weak FBS stimulus. A further study by the same authors demonstrated that the apparently opposing effects on motility are existing together to provide optimal tumour suppression; as myopodin was found to inhibit motility of the neoplastic acinar cells, while promoting motility of the non-cancerous basal cells maintaining its integrity in resisting cancerous cells invasion [33].
A study from Memorial Sloan Kettering Cancer Center included testicular specimens from patients with a testicular tumour treated between 1985 and 2002 and correlated with survival outcomes. Myopodin was found to exert a possible tumour suppressor role and was an independent predictive marker for overall survival [34]. A more recent study (2015) from the same cancer center found different genetic regions to be associated with testicular cancer disease-free survival. They could build a gene model from these genes including myopodin and that genetic model was found to be an independently associated with survival outcomes [35].
A study on 173 patients with bladder transitional cell carcinoma could identify that a low level of zyxin was significantly associated with higher tumour grade and stage [36]. Zyxin exhibits its suppressor effect in bladder cancer through the β-catenin signaling pathway. β-catenin is a cytoplasmic protein that participates in the assembly of the cell to cell adhesions junctions through binding with zyxin, E-cadherin and moesin. Alteration of physiological balance for β-catenin interaction with zyxin, E-cadherin or moesin could account for the invasiveness potential for bladder cancer [37]. β-catenin was also found to interact with the Wnt signaling pathway resulting in various adverse effects including osteoporosis and cancer progression [38].
To summarize; zyxin, a phosphoprotein, is involved in actin cytoskeleton assembly and is mainly localized at FAs maintaining tissue integrity. It can, however; shuttle between cytoplasm and nucleus, affecting transcription. It has dual oncogenic and tumour suppressor actions. The differential mode of action is exerted based on the organ involved and its interacting protein partners. Zyxin possibly exerts its oncogenic effect through a weakness at the FA points promoting tumour progression and invasiveness, while its tumour suppressor effect may be induced by affecting nuclear transcriptional function. A recent study suggested that zyxin may exert its actions through a mitotic-phosphorylation- dependent manner, with the phosphorylation acting as the trigger for zyxin activity towards promoting cancer growth and proliferation [39]. Most of the zyxin's effect is however mediated through its interactions with other proteins, rather than being a sole independent effect. Future studies are required to decipher the mechanisms by which zyxin exerts differential actions on cancer development. Table 1 illustrates the role of zyxin in different tumours.
Table 1.
Effect of zyxin on different tumours.
| Cancer | Zyxin effect | Possible mechanism |
|---|---|---|
| Melanoma | Oncogene | Directly results in cell spreading and proliferation. Could be through interaction with WT1 |
| Hepatocellular carcinoma | Oncogene | Through binding with LASP-1 affecting actin filament dynamics and resulting in tumour spread. |
| Breast cancer | Oncogene | Directly results in cell spreading and proliferation. Through binding with LASP-1. |
| Clear cell Renal cell carcinoma | Oncogene | Directly results in cell spreading and proliferation. Through binding with LASP-1. |
| Ovarian cancer | Oncogene | Through binding with LASP-1. |
| Non Hodgkin lymphoma | Oncogene | Directly results in cell spreading and proliferation. |
| Glioma | Oncogene | Directly results in cell spreading and proliferation. |
| Penile cancer | Oncogene | Indirect effect through binding with HPV6 E6 protein, enhancing wart growth and cancer development. |
| Cervical cancer | Oncogene | Indirect effect through binding with HPV6 E6 protein, enhancing wart growth and cancer development. |
| Gastric cancer | Suppressor | Through binding with CARP-1, it promotes apoptosis. |
| Ewing sarcoma | Suppressor | Decreases cell motility and growth, by localization in cytoplasm. |
| Prostate cancer | Suppressor | Through interacting with Myopodin, acting as an activator for Myopodin tumour suppressor effect. |
| Testicular cancer | Suppressor | Through interacting with Myopodin. |
| Bladder cancer | Suppressor | Through interacting with β-catenin, interfering with its pathway and suppressing tumour proliferation. |
2. Conclusion
Zyxin is an important protein involved in cell adhesions and affecting nuclear transcription. It is mainly located at FAs but can shuttle to the cytoplasm or the nucleus. It can bind to several proteins to produce specific regulatory actions on cells and according to the interacting protein type and organ involved, the resulting action can be a promoter of cells growth and progression or apoptosis. Zyxin is linked as a promoter for renal cell carcinoma, and tumour suppressor to prostate, bladder and testicular cancer. It interacts with HPV6 and results in wart growth and progression. It plays a variety of biological functions and is an attractive target for the treatment of many cancers, however, the mechanisms underlying these functions are unclear and merit further work. We hope that this simplified review will open new doors for future research to include zyxin as a potential new target for cancer therapy, possibly through activating or suppressing its expression and/or nuclear shuttling.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Trushar R. Patel was supported by the Canada Research Chair program.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Grunewald T.G., Kammerer U., Schulze E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp. Cell Res. 2006;312:974–982. doi: 10.1016/j.yexcr.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Gilmore T.D. Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochim. Biophys. Acta. 2003;1593:115–120. doi: 10.1016/s0167-4889(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 3.Hervy M., Hoffman L., Beckerle M.C. From the membrane to the nucleus and back again: bifunctional focal adhesion proteins. Curr. Opin. Cell Biol. 2006;18:524–532. doi: 10.1016/j.ceb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Beckerle M.C. Zyxin: zinc fingers at sites of cell adhesion. Bioassays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- 5.Gregory Call S., Brereton D., Bullard J.T. A zyxin- nectin interaction facilitates Zyxin localization to cell-cell adhesions. Biochem. Biophys. Res. Commun. 2011;415:485–489. doi: 10.1016/j.bbrc.2011.10.099. [DOI] [PubMed] [Google Scholar]
- 6.Bitomsky N., Hofmann T.G. Apoptosis and autophagy: regulation of apoptosis by DNA damage signalling - roles of p53, p73 and HIPK2. FEBS J. 2009;276:6074–6083. doi: 10.1111/j.1742-4658.2009.07331.x. [DOI] [PubMed] [Google Scholar]
- 7.Crone J., Glas C., Schultheiss K., Moehlenbrink J., Krieghoff-Henning E., Hofmann T.G. Zyxin is a critical regulator of the apoptotic HIPK2-p53 signaling axis. Cancer Res. 2011;71:2350–2359. doi: 10.1158/0008-5472.CAN-10-3486. [DOI] [PubMed] [Google Scholar]
- 8.Han J., Liu G., Profirovic J., Niu J., Voyno-Yasenetskaya T. Zyxin is involved in thrombin signaling via interaction with PAR-1 receptor. FASEB J. 2009;23:4193–4206. doi: 10.1096/fj.09-131862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nix D.A., Fradelizi J., Bockholt S. Targeting of zyxin to sites of actin membrane interaction and to the nucleus. J. Biol. Chem. 2001;276:34759–34767. doi: 10.1074/jbc.M102820200. [DOI] [PubMed] [Google Scholar]
- 10.Yi J., Kloeker S., Jensen C.C. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J. Biol. Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- 11.Drees B.E., Andrews K.M., Beckerle M.C. Molecular dissection of zyxin function reveals its involvement in cell motility. J. Cell Biol. 1999;147:1549–1560. doi: 10.1083/jcb.147.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauskolb C., Pan G., Reddy B.V., Oh H., Irvine K.D. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011 Jun;9(6) doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner N., Panelos J., Massi D., Wagner K.D. The Wilms' tumor suppressor WT1 is associated with melanoma proliferation. Pflugers Arch. 2008;455:839–847. doi: 10.1007/s00424-007-0340-1. [DOI] [PubMed] [Google Scholar]
- 14.Michiels J.F., Perrin C., Leccia N., Massi D., Grimaldi P., Wagner N. PPARbeta activation inhibits melanoma cell proliferation involving repression of the Wilms' tumour suppressor WT1. Pflugers Arch. 2010;459:689–703. doi: 10.1007/s00424-009-0776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Gaag E.J., Leccia M.T., Dekker S.K., Jalbert N.L., Amodeo D.M., Byers H.R. Role of zyxin in differential cell spreading and proliferation of melanoma cells and melanocytes. J. Invest. Dermatol. 2002;118:246–254. doi: 10.1046/j.0022-202x.2001.01657.x. [DOI] [PubMed] [Google Scholar]
- 16.Sy S.M., Lai P.B., Pang E. Novel identification of zyxin upregulations in the motile phenotype of hepatocellular carcinoma. Mod. Pathol. 2006;19:1108–1116. doi: 10.1038/modpathol.3800626. [DOI] [PubMed] [Google Scholar]
- 17.Wang H., Li W., Jin X., Cui S., Zhao L. LIM and SH3 protein 1, a promoter of cell proliferation and migration, is a novel independent prognostic indicator in hepatocellular carcinoma. Eur. J. Cancer. 2013;49:974–983. doi: 10.1016/j.ejca.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Yang F., Zhou X., Du S. LIM and SH3 domain protein 1 (LASP1) overexpression was associated with aggressive phenotype and poor prognosis in clear cell renal cell cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunewald T.G., Kammerer U., Winkler C. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br. J. Cancer. 2007;96:296–305. doi: 10.1038/sj.bjc.6603545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianazza E., Chinello C., Mainini V. Alterations of the serum peptidome in renal cell carcinoma discriminating benign and malignant kidney tumors. J. Proteomics. 2012;76:125–140. doi: 10.1016/j.jprot.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Ma B., Cheng H., Gao R. Zyxin-Siah2-Lats2 axis mediates cooperation between Hippo and TGF-β signalling pathways. Nat. Commun. 2016;7:11123. doi: 10.1038/ncomms11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao X., Xu X., Wu Y. Overexpression of TRIP6 promotes tumor proliferation and reverses cell adhesion-mediated drug resistance (CAM-DR) via regulating nuclear p27(Kip1) expression in non-Hodgkin's lymphoma. Tumour Biol. 2016;37:1369–1378. doi: 10.1007/s13277-015-3939-4. [DOI] [PubMed] [Google Scholar]
- 23.Lin V.T., Lin V.Y., Lai Y.J. TRIP6 regulates p27 KIP1 to promote tumorigenesis. Mol. Cell Biol. 2013;33:1394–1409. doi: 10.1128/MCB.01149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degenhardt Y.Y., Silverstein S. Interaction of zyxin, a focal adhesion protein, with the e6 protein from human papillomavirus type 6 results in its nuclear translocation. J. Virol. 2001;75:11791–11802. doi: 10.1128/JVI.75.23.11791-11802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rishi A.K., Zhang L., Boyanapalli M. Identification and characterization of a cell cycle and apoptosis regulatory protein-1 as a novel mediator of apoptosis signaling by retinoid CD437. J. Biol. Chem. 2003;278:33422–33435. doi: 10.1074/jbc.M303173200. [DOI] [PubMed] [Google Scholar]
- 26.Hervy M., Hoffman L.M., Jensen C.C., Smith M., Beckerle M.C. The LIM protein zyxin binds CARP-1 and promotes apoptosis. Genes Cancer. 2010;1:506–515. doi: 10.1177/1947601910376192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu F., Xue J.X., Hu Y.C. CARP is a potential tumor suppressor in gastric carcinoma and a single-nucleotide polymorphism in CARP gene might increase the risk of gastric carcinoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amsellem V., Kryszke M.H., Hervy M. The actin cytoskeleton-associated protein zyxin acts as a tumor suppressor in Ewing tumor cells. Exp. Cell Res. 2005;304:443–456. doi: 10.1016/j.yexcr.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Lin F., Yu Y.P., Woods J. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am. J. Pathol. 2001;159:1603–1612. doi: 10.1016/S0002-9440(10)63006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y.P., Luo J.H. Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Res. 2006;66:7414–7419. doi: 10.1158/0008-5472.CAN-06-0227. [DOI] [PubMed] [Google Scholar]
- 31.Jing L., Liu L., Yu Y.P. Expression of myopodin induces suppression of tumor growth and metastasis. Am. J. Pathol. 2004;164:1799–1806. doi: 10.1016/S0002-9440(10)63738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kai F., Tanner K., King C., Duncan R. Myopodin isoforms alter the chemokinetic response of PC3 cells in response to different migration stimuli via differential effects on Rho- ROCK signaling pathways. Carcinogenesis. 2012;33:2100–2107. doi: 10.1093/carcin/bgs268. [DOI] [PubMed] [Google Scholar]
- 33.Kai F., Fawcett J.P., Duncan R. Synaptopodin-2 induces assembly of peripheral actin bundles and immature focal adhesions to promote lamellipodia formation and prostate cancer cell migration. Oncotarget. 2015;6:11162–11174. doi: 10.18632/oncotarget.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korkola J.E., Houldsworth J., Feldman D.R. Identification and validation of a gene expression signature that predicts outcome in adult men with germ cell tumors. J. Clin. Oncol. 2009;27:5240–5247. doi: 10.1200/JCO.2008.20.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korkola J.E., Heck S., Olshen A.B. Development and validation of a gene-based model for outcome prediction in germ cell tumors using a combined genomic and expression profiling approach. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Carbayo M., Socci N.D., Charytonowicz E. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–6980. PMID: 12460915. [PubMed] [Google Scholar]
- 37.Shiina H., Igawa M., Urakami S. Alterations of beta- and gamma-catenin in N-butyl-N-(-4-hydroxybutyl)nitrosamine-inducedmurine bladder cancer. Cancer Res. 2001;61:7101–7109. PMID: 11585741. [PubMed] [Google Scholar]
- 38.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J., Zeng Y., Cui L. Zyxin promotes colon cancer tumorigenesis in a mitotic phosphorylation-dependent manner and through CDK8-mediated YAP activation. Proc. Natl. Acad. Sci. U. S. A. 2018 Jul 2 doi: 10.1073/pnas.1800621115. pii: 201800621. [DOI] [PMC free article] [PubMed] [Google Scholar]