Abstract
Development is a process precisely coordinated in both space and time. Spatial precision has been quantified in a number of developmental systems, and such data have contributed significantly to our understanding of, for example, morphogen gradient interpretation. However, comparatively little quantitative analysis has been performed on timing and temporal coordination during development. Here, we use Drosophila to explore the temporal robustness of embryonic development within physiologically normal temperatures. We find that development is temporally very precise across a wide range of temperatures in the three Drosophila species investigated. However, we find temperature dependence in the timing of developmental events. A simple model incorporating history dependence can explain the developmental temporal trajectories. Interestingly, history dependence is temperature-specific, with either effective negative or positive feedback at different temperatures. We also find that embryos are surprisingly robust to shifting temperatures during embryogenesis. We further identify differences between tropical and temperate species, potentially due to different mechanisms regulating temporal development that depend on the local environment. Our data show that Drosophila embryonic development is temporally robust across a wide range of temperatures. This robustness shows interesting species-specific differences that are suggestive of different sensitivity to temperature fluctuations between Drosophila species.
Keywords: temporal robustness, embryonic development, temperature sensitivity, Drosophila
1. Introduction
Multicellular organism development is characterized by the ability to complete morphogenesis with little variation between individuals. In particular, quantitative experiments on patterning processes early in embryogenesis have shed light on this level of reproducibility [1–4]. During early embryonic development, coarse gradients are subsequently refined to reach a pattern resolved at the single-cell level [5]. Remarkably, the spatial precision of patterning often remains unaffected in the face of environmental fluctuations within typical physiological ranges. For example, the fly wing vein patterning operates at the physical limit (i.e. at the single-cell level) and that this limit is robust to a wide temperature range [6]. While much attention has been brought to the level of spatial precision, developmental time precision is comparatively poorly studied. However, events during embryogenesis must be tightly coordinated temporally. In the Drosophila embryo, the time to hatching roughly doubles upon a temperature change from 21°C to 16°C. Therefore, exploring temporal reproducibility is essential to gain insights into how development is coordinated and also how organisms respond to environmental changes.
Endotherm animals maintain relatively constant body temperature, within a degree or two from their optimal temperature. A classic example of the mechanisms in place to maintain constant temperature is the capability of blood vessels to acutely alter their diameter to either promote (dilate) or restrict (constrict) heat release [7]. Past studies have shown that a combination of metabolic and behavioural responses sustains body temperature, such as induced shivering to increase body heat [8]. By contrast, ectotherm animals are unable to regulate their body temperature and, therefore, rely on behavioural responses to maintain their body within physiologically adequate temperatures when exposed to varying environments [8–12]. For example, the genes Painless and Pyrexia are critical for high-temperature nociception in Drosophila larvae [13–15]. In their absence, larvae exhibit latencies in sensing and moving to colder temperatures. The porcelain crab, Petrolisthes, remains under stones during low tides when the temperature may raise over 20°C in 6 h [16]. Kenyan chameleons (Chamaeleo dilepis and Chamaeleo jacksonii) alter their skin coloration to a darker tone in order to effectively absorb early morning sun, allowing them to reach their optimal temperature faster [17]. Furthermore, sex determination of several species of reptiles, including crocodiles and most turtles, is particularly sensitive to temperature [18]. A hotter environment is correlated with increased levels of aromatase, an enzyme converting androgen to oestrogen. Therefore, hot temperatures direct gonad differentiation to the female fate, while colder temperatures induce male fate. As exemplified above, the combination of physiological and behavioural responses is instrumental for the maintenance of organism viability in varying environmental conditions. How temperature affects developmental time has also been widely studied [19–21]. Cross-species analysis has even found evidence for a ‘biological clock’ that links developmental time with temperature and body size [22]. However, despite the importance of precise temporal regulation during development, the quantification of developmental time is considerably less comprehensive than analogous studies of spatial precision.
The ability to respond to environmental changes is unequal throughout the life cycle of any ectotherm animal. While Drosophila larvae and adults clearly exhibit acute behavioural responses to environmental changes [11], the embryonic stage is unable to do so and is, therefore, vulnerable to perturbations [23]. It has been hypothesized that female flies can improve offspring fitness by depositing eggs in thermally favourable locations [24], though this appears unlikely as the temperature at a given time does not reflect the future temperature. In particular, the Drosophila embryo typically experiences at least one day/night cycle with corresponding temperature changes. Given the apparent vulnerability of Drosophila embryos, it is important to understand whether embryos exhibit significant changes in developmental time precision at certain temperatures and how they respond to varying environments.
Here, we began by asking: is Drosophila embryonic development temporally robust at different temperatures? By robust, we mean that the heterochronicity (fluctuations in developmental time) between embryos under equal conditions is of the order of a few per cent (as a percentage of mean time), which is comparable to the robust spatial boundaries defined in the Drosophila embryo. We find that embryonic development of three Drosophila species (D. melanogaster, D. simulans and D. virilis) is temporally robust across a broad range of temperatures, with relative errors comparable to the relative error in spatial positioning of many gene boundaries. Having quantified the temporal robustness of Drosophila embryonic development, we then asked: (i) is development temporally robust to temperature variability; and (ii) does the temporal robustness display temperature-dependent behaviour? A combination of temperature shifts and pulse experiments in D. melanogaster reveals that temporal robustness is sensitive to temperature fluctuations in early embryogenesis, but the temporal error does not increase substantially. Furthermore, we find that the statistical properties of the heterochronicity are temperature dependent, with embryogenesis most temporally robust around intermediate (19–23°C) temperatures. We are able to explain this observation through a simple model that incorporates history dependence of the temporal trajectories through development. Finally, we discuss differences in the temporal robustness between the different Drosophila species. To summarize, our work highlights that the duration of Drosophila embryonic development is highly robust at typical physiological temperatures, but there are important differences in how temperate and tropical species temporally adapt to temperature changes.
2. Material and methods
2.1. Fly stocks
We used in-bred Drosophila melanogaster (D. melanogaster) OregonR, Drosophila simulans (D. simulans) Rakujuen and Drosophila virilis (D. virilis) viri-HUE lines to minimize genetic diversity in our samples. Flies were maintained with standard fly food containing cornflour, dextrose, brewer's yeast, Bacto Agar and 10% Nipagin. Flies were kept at 25°C through all life cycles. Prior to imaging, flies were caged and kept at 25°C. Flies were allowed to lay on an apple juice agar plate (agar, sucrose and apple juice) where the embryos were collected. Only imaged embryos were subjected to different temperatures.
2.2. Sample preparation
Twenty non-dechorionated embryos were aligned on an apple juice agar plate (figure 1a). Embryos were selected at the blastoderm stage and allowed to develop at a precise temperature (in nearly all experiments the temperature fluctuations δT were very small compared to the temperature (δT/T < 0.5%, figure 1b) until hatching in Halocarbon oil 27 to visualize developmental stages. The embryos were imaged on a Nikon SMZ18 stereomicroscope appended with a Julabo GmbH temperature control device. The temporal resolution was 2 min. All experiments were performed on the same microscope set-ups with identical illumination strength. At constant temperatures, temperature shift and fluctuation experiments, embryo survival rate (defined by whether larvae hatched) was greater than 70% (electronic supplementary material, figure S1a–d). Most experiments were repeated at least three times, with a minimum of 35 embryos in each temperature batch (and greater than 70 for most temperatures). We repeated experiments at 16, 21 and 25°C in D. melanogaster over a year apart to check that our results were robust to experimental drift, such as different batches of food and multiple generations later.
Figure 1.
Experimental set-up. (a) Left: Image of 20 D. melanogaster embryos in the microscope set-up. Right: Highlighting the different landmarks used in the paper to analyse temporal development, see also Material and methods and table 1. (b) Measured temperatures compared with the temperature set and corresponding standard deviation.
2.3. Image analysis
The developmental time was scored based on seven developmental landmarks (figure 1a and table 1). We used the cephalic furrow formation as time 0 to set each embryo to a common start time. Furthermore, we scored the time of germband retraction, head involution, midgut broadening, muscle contraction, trachea filling and hatching to span the entire embryogenesis at regular intervals. Cephalic furrow formation is a transient process and constitutes the first event of gastrulation. Invagination of cells occurs on the lateral side of the embryo at about 65% of the embryo length (from the posterior of the embryo). The germband retraction stage is identified when the germband shortens at the dorsal side of the embryo. Head involution occurs midway through embryogenesis, due to internalization of the ectodermal tissue and rearrangement of cells. The midgut broadening landmark is identified by the formation of a triangular shape laterally. Muscular movement is characterized by uncontrolled twitching of muscles. Before hatching, the tracheal tree is filled with air and is visible due to the rapid darkening of the trachea. Finally, we scored the time when larvae hatch from the embryonic case. Landmark identification was done visually and by the same experimenter for all movies. To minimize potential bias, the experimenters taking the measurements and analysing the data were different.
Table 1.
Developmental landmarks used to time Drosophila embryonic development.
| label | stage | description |
|---|---|---|
| 0 | cephalic furrow formation | ingression of cells around 30% embryo length, starting with cells on the lateral sides of the embryo |
| 1 | germband retraction | retraction of germband from dorsal side of embryo, with embryo transitioning from parasegmental to segmental division |
| 2 | head involution | internalization and rearrangements of head segments |
| 3 | midgut broadening | expansion of gut structures |
| 4 | muscle contractions | uncontrolled twitching of muscles |
| 5 | trachea filling | trachea system becoming filled with air |
| 6 | hatching | larvae exits from the embryonic egg shell |
2.4. Statistics
From our experience with spatial patterning and also from observing that larvae often hatch at similar times, we expect the relative temporal error (coefficient of variation, CVτ = s.d./mean) to be small (approx. 2–5%), with a similarly small standard deviation (around 1–2%). We performed a power analysis to estimate the required minimum number of embryos for each condition. To observe a difference between a mean CVτ = 0.04 ± 0.02 and CVτ = 0.05 ± 0.02 with power 0.8 requires n = 34 samples (calculated using t-test). p-Values were calculated (unless otherwise stated) using a two-tailed t-test comparison. In all datasets for D. melanogaster, D. simulans and D. virilis, n > 35. Error on the temporal variation was estimated using bootstrapping, with 100 simulations performed per dataset. For the covariance analysis, we considered the five intermediate landmarks: cephalic furrow formation is used to define a common time 0 in each experiment and the temporal variability in hatching is significantly larger than for the other landmarks. Sample size for each experiment is given in table 2 and electronic supplementary material, figure S1.
Table 2.
CVτ and covariance (Cov) relative to landmark (1) in temporal trajectories in D. melanogaster, D. simulans and D. virilis. For experiments without temperature shift, the p-value is calculated from comparing the mean CVτ and Cov for landmarks 2–5 (i.e. excluding hatching), relative to 21°C. For temperature shift experiments, the mean CVτ and Cov are calculated for landmarks 4–5 (after temperature shift) and compared with the mean CVτ before the shift (landmarks 1 and 2) and Cov of embryos maintained at a constant temperature (at temperature after shift), respectively.
| landmark |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
||||||||||
| species | temperature °C (n in brackets) | CVτ % |
Cov | CVτ % |
Cov | CVτ % |
Cov | CVτ % |
Cov | CVτ % |
Cov | CVτ % |
Cov | p CVτ | p Cov |
| D. melanogaster | 16 (n = 88) | 3.7 | n.a. | 3.0 | 0.48 | 3.2 | 0.29 | 2.5 | 0.39 | 1.8 | 0.42 | 3.3 | 0.34 | 0.67 | 0.046 |
| 21 (n = 80) | 3.7 | n.a. | 2.6 | 0.35 | 2.8 | 0.28 | 2.2 | 0.20 | 2.0 | 0.16 | 2.8 | 0.13 | n.a. | n.a. | |
| 25 (n = 98) | 5.0 | n.a. | 5.6 | 0.76 | 5.8 | 0.69 | 6.1 | 0.69 | 5.6 | 0.72 | 7.1 | 0.72 | <10−4 | <10−4 | |
| 16–21 (n = 78) | 4.1 | n.a. | 2.6 | 0.48 | 1.7 | 0.26 | 2.0 | 0.30 | 1.7 | 0.28 | 2.7 | 0.30 | 0.07 | 0.56 | |
| 21–16 (n = 77) | 3.6 | n.a. | 2.6 | 0.67 | 6.6 | 0.36 | 4.2 | 0.22 | 2.4 | 0.32 | 2.6 | 0.30 | 0.49 | 0.17 | |
| 21–26 (n = 79) | 2.2 | n.a. | 2.2 | 0.13 | 2.5 | 0.36 | 2.8 | 0.24 | 2.9 | 0.26 | 3.5 | 0.23 | 0.05 | <10−4 | |
| 26–21 (n = 70) | 4.6 | n.a. | 5.5 | 0.79 | 6.9 | 0.73 | 5.1 | 0.71 | 4.3 | 0.78 | 4.5 | 0.80 | 0.75 | 2 × 10−4 | |
| D. simulans | 16 (n = 80) | 5.2 | n.a. | 3.7 | 0.54 | 3.6 | 0.32 | 3.0 | 0.31 | 2.9 | 0.55 | 9.4 | 0.47 | 0.08 | 0.89 |
| 21 (n = 54) | 4.2 | n.a. | 3.7 | 0.55 | 4.2 | 0.24 | 4.1 | 0.49 | 4.0 | 0.49 | 6.0 | 0.46 | n.a. | n.a. | |
| 25 (n = 80) | 6.3 | n.a. | 5.3 | 0.27 | 6.8 | 0.27 | 5.9 | 0.67 | 5.7 | 0.25 | 7.3 | 0.23 | 0.04 | 0.065 | |
| D. virilis | 16 (n = 41) | 3.3 | n.a. | 2.6 | 0.59 | 2.7 | 0.51 | 3.3a | 0.42 | 1.3 | 0.26 | 1.3 | 0.40 | 0.97 | 0.15 |
| 21 (n = 36) | 3.4 | n.a. | 2.4 | 0.39 | 2.5 | −0.05 | 7.0a | 0.50 | 1.4 | 0.17 | 2.8 | 0.10 | n.a. | n.a. | |
| 25 (n = 43) | 5.5 | n.a. | 3.6 | 0.22 | 3.2 | −0.02 | 10a | 0.43 | 2.8 | 0.29 | 4.0 | 0.34 | 0.11 | 0.60 | |
aLarge CVτ due to experimental error in identifying muscle twitching in D. virilis, not biological variation.
2.5. Modelling
The simulations were performed in Matlab. The experimentally measured time between landmarks at each temperature was used to determine the corresponding values of λ, the input mean for the Gaussian distribution. We take the standard deviation as λ1/2, since the measured standard deviation from the distributions already incorporates the effects of any effective negative or positive feedback. We use a Gaussian distribution rather than the Erlang distribution (which describes the distribution of time between events in a Poisson process) since we lack sufficient information to reliably parametrize the Erlang distribution. The data for the first time point (corresponding to germband retraction) were distributed as measured experimentally, as there were no previous time course data available—as cephalic furrow is used to define time 0. For subsequent events, the history dependence was implemented as described in the text. For each temperature, 1000 simulations were performed, where random numbers were generated using the Matlab function randn. Fitting of r, the history-dependent parameter, was done to data at 16, 21 and 25°C for each species. We first attempted to use a single value for all three temperatures. For D. simulans, this gave a good fit to the data, but for D. virilis and D. melanogaster this resulted in a poor fit at least at one temperature. For D. melanogaster, r = 0 resulted in a poor fit to the data except at low temperatures (electronic supplementary material, figure S1e). Likewise, using r = 40 min or r = −40 min for all data resulted in a poor fit (electronic supplementary material, figure S1f,g, respectively). Therefore, for D. virilis and D. melanogaster we allowed two values of r, depending on temperature, as outlined in the Results. Note, our approach with the model was not to find the best value of r at each temperature, but to find a minimal range of values for r that can explain as wide a portion of the data as possible. To model the temperature shift experiments, the value of r corresponded to the temperature of the system at each particular landmark. However, we find that for shifts from 26°C, a better fit was achieved with r = 0, not r < 0 after the temperature shift. Finally, the actual time of temperature shift for each embryo is slightly different. The time leading up to and immediately after the temperature shift were both drawn as random Gaussian variables and the relative contribution of each weighted to ensure that the average time of development corresponded to experimental measurements. For D. virilis data for muscle twitching were excluded due to experimental error in determining the onset of such twitching.
3. Results
3.1. Temporal development of Drosophila embryos is robust across a wide temperature range
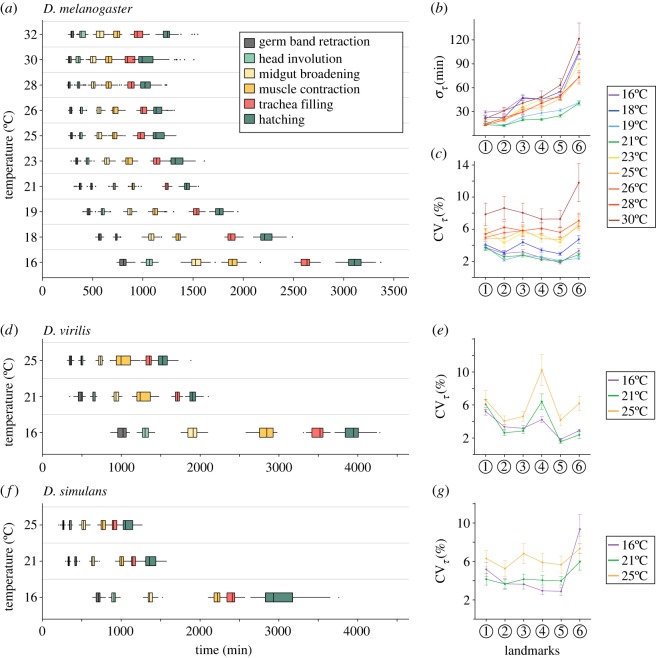
Developmental time in D. melanogaster increases markedly as temperature decreases (figure 2a, [21]). However, the temporal variation, στ, shows a more complicated behaviour. If temporal variability in embryonic development is largely due to random variations, then we expect the error in timing to increase with developmental time. If the temporal development of the embryo is a continuous process with little history dependence or checkpoints, then we expect 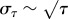 . However, we find that embryos developing in the range 19–21°C typically have reduced variation than at both high (greater than 23°C) and low (less than 19°C) temperatures (figure 2b). At low temperatures, larger στ is unsurprising, due to accumulation of more error from the longer developmental time. The increased temporal error at higher temperatures is surprising given that developmental time is significantly shorter.
. However, we find that embryos developing in the range 19–21°C typically have reduced variation than at both high (greater than 23°C) and low (less than 19°C) temperatures (figure 2b). At low temperatures, larger στ is unsurprising, due to accumulation of more error from the longer developmental time. The increased temporal error at higher temperatures is surprising given that developmental time is significantly shorter.
Figure 2.
Drosophila embryonic development is temporally robust. (a) Distribution of developmental times at each landmark scored for D. melanogaster. (b) Absolute error in developmental time at each landmark for D. melanogaster at different temperatures. (c) Relative error in developmental time at each landmark for D. melanogaster at different temperatures. (d) Distribution of developmental times at each landmark scored for D. virilis. Colour coding as (a). (e) Relative error in developmental time at each landmark for D. virilis at 16, 21 and 25°C. (f) Distribution of developmental times at each landmark scored for D. simulans. Colour coding as (a). (e) Relative error in developmental time at each landmark for D. simulans at 16, 21 and 25°C. All error bars are standard deviation, which in (c,e,g) are estimated by bootstrapping.
As the mean developmental time varies drastically across temperatures, we reason that a more appropriate measure is the coefficient of variation, CVτ = στ/τ. This dimensionless measure enables comparison of the variability in developmental time while accounting for changes in total developmental time with temperature. This is analogous to quantification of the spatial precision of boundaries, where the boundary position is typically scaled by the embryo length. If the temporal variability is dominated by random noise, then we expect 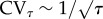 . Remarkably, for all temperatures in the range 16–28°C, CVτ was less than 6% for all landmarks except hatching (figure 2c). At very high temperatures CVτ increases further, with larger embryo-to-embryo variability at 30°C (figure 2c). Indeed, embryos developing at 30°C have a temporal variation around 4–5 times larger than embryos developing at 21°C.
. Remarkably, for all temperatures in the range 16–28°C, CVτ was less than 6% for all landmarks except hatching (figure 2c). At very high temperatures CVτ increases further, with larger embryo-to-embryo variability at 30°C (figure 2c). Indeed, embryos developing at 30°C have a temporal variation around 4–5 times larger than embryos developing at 21°C.
For T < 23°C, we observed a gradual decrease in CVτ during development, qualitatively consistent with 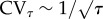 . However, CVτ at higher temperatures was significantly larger than at low temperatures (e.g. p = 0.003, comparing mean CVτ at midgut broadening for embryos developing in range 23–26°C with those below 21°C). Furthermore, CVτ was approximately constant throughout embryonic development for embryos developing above 23°C (figure 2c). Although temporal development is robust at high temperatures, there are clear differences in behaviour in CVτ compared with lower temperatures.
. However, CVτ at higher temperatures was significantly larger than at low temperatures (e.g. p = 0.003, comparing mean CVτ at midgut broadening for embryos developing in range 23–26°C with those below 21°C). Furthermore, CVτ was approximately constant throughout embryonic development for embryos developing above 23°C (figure 2c). Although temporal development is robust at high temperatures, there are clear differences in behaviour in CVτ compared with lower temperatures.
We next explored the temporal robustness of development in two related Drosophila species, D. virilis and D. simulans, which diverged around 40 and 5 Mya from D. melanogaster, respectively (from flybase.org). We chose to focus on temperatures 16, 21 and 25°C as these represent the range of standard laboratory conditions for Drosophila. We selected D. virilis as it develops in more temperate climates and has a significantly longer developmental time than D. melanogaster (figure 2d; electronic supplementary material, figure S2a and movie S1, [21]). As with D. melanogaster, the absolute temporal error is higher at low temperatures (electronic supplementary material, figure S2b). At high temperatures CVτ is smaller for D. virilis (4.9 ± 0.2) compared with D. melanogaster (5.3 ± 0.5, p = 0.05), but CVτ is similar between the two species at low temperature (figure 2e). Therefore, the temporal development of D. virilis is also robust across a wide range of temperatures.
We studied D. simulans as it is a tropical species similar to D. melanogaster, but with faster development (figure 2f; electronic supplementary material, figure S2a and movie S1, [21]). CVτ is around 4%, suggesting the D. simulans development is temporally robust. It is noteworthy though that CVτ is larger in D. simulans than D. melanogaster at all landmarks (excluding hatching) at T = 16°C and T = 21°C (p-value < 10−2 for all conditions). Interestingly, for intermediate temperatures (T = 21°C), CVτ is relatively constant throughout development for D. simulans, in contrast to D. melanogaster where it decreases with developmental time. Again, we see that CVτ at high temperatures is significantly greater than at low temperatures (p-value < 10−3), even though embryo viability is similar (figure 2g and electronic supplementary material, figure S1c).
As with D. melanogaster, both D. virilis and D. simulans have larger absolute temporal errors at 16°C, though the absolute temporal error at 21 and 25°C is surprisingly similar for both species (electronic supplementary material, figure S2b,c). Finally, we tested more systematically the dependence of CVτ on the developmental time. Fitting 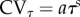 for each species at each temperature, we find that D. virilis has relatively constant s around −0.5 at all temperatures tested (electronic supplementary material, figure S2d). However, for both D. melanogaster and D. simulans, s approaches zero at higher temperatures. Each species is temporally robust except at very high temperatures, but there is clear temperature dependence in the temporal variability.
for each species at each temperature, we find that D. virilis has relatively constant s around −0.5 at all temperatures tested (electronic supplementary material, figure S2d). However, for both D. melanogaster and D. simulans, s approaches zero at higher temperatures. Each species is temporally robust except at very high temperatures, but there is clear temperature dependence in the temporal variability.
3.2. Temporal coordination in varying temperature environments
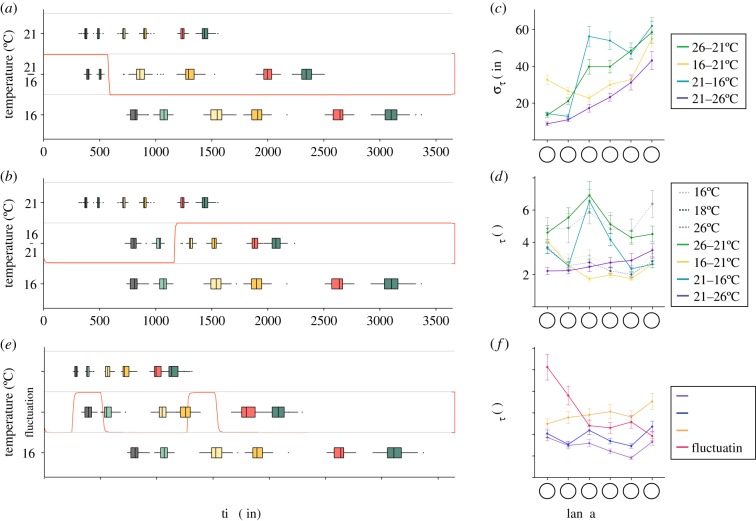
To investigate further the temporal trajectories and how they depend on temperature, we recorded D. melanogaster embryos developing in varying temperature environments. First, we considered shifts of temperature 16°C to/from 21°C (figure 3a,b) and 21°C to/from 26°C (electronic supplementary material, figure S3) after head involution. We chose these temperatures such that the temperature change was ±5°C from 21°C. Clear shifts are observable in the timing error (figure 3c), but these appear largely transient. Looking at CVτ, we see that decreasing or increasing the temperature to or from 21°C after head involution results in acute increase of the relative temporal error, but these are largely reduced by hatching (figure 3d and electronic supplementary material, figure S3). Therefore, the temporal development of D. melanogaster is surprisingly robust to abrupt temperature variations.
Figure 3.
Drosophila melanogaster embryonic development under thermal perturbations. (a,b) Distribution of developmental times at each landmark scored for D. melanogaster under 16°C to/from 21°C temperature shift. Temperature was shifted after head involution and is represented by a red curve. Colour coding as in figure 1a. (c) Absolute error in developmental time at each landmark for D. melanogaster in 16°C to/from 21°C and 21°C to/from 26°C temperature shift experiments. (d) Relative error in developmental time at each landmark for D. melanogaster in 16°C to/from 21°C and 21°C to/from 26°C temperature shift experiments. (e) Distribution of developmental times at each landmark scored for D. melanogaster in temperature pulse experiments. Temperature was shifted at regular interval and is represented by a red curve. Colour coding as in figure 1a. (f) Relative error in developmental time at each landmark for D. melanogaster in pulse experiments. All error bars are standard deviation, which in (d,f) are estimated by bootstrapping.
To further test the temporal robustness of D. melanogaster, we recorded the temporal development of embryos at 16°C applied with two +10°C temperature pulses of 4 h duration during development (figure 3e). This temperature range was chosen as it represents the regime of robust temporal development. Measuring CVτ, we see that the first temperature pulse results in a large temporal perturbation, but this shift is largely negated by midgut broadening. The second pulse results in a much smaller shift in CVτ. After midgut broadening, the average CVτ is between 26 and 18°C (the overall average temperature throughout development) (figure 3f). Therefore, abrupt shifts in temperature have little long-term effect on temporal trajectories.
3.3. The temporal coordination between developmental landmarks is temperature dependent
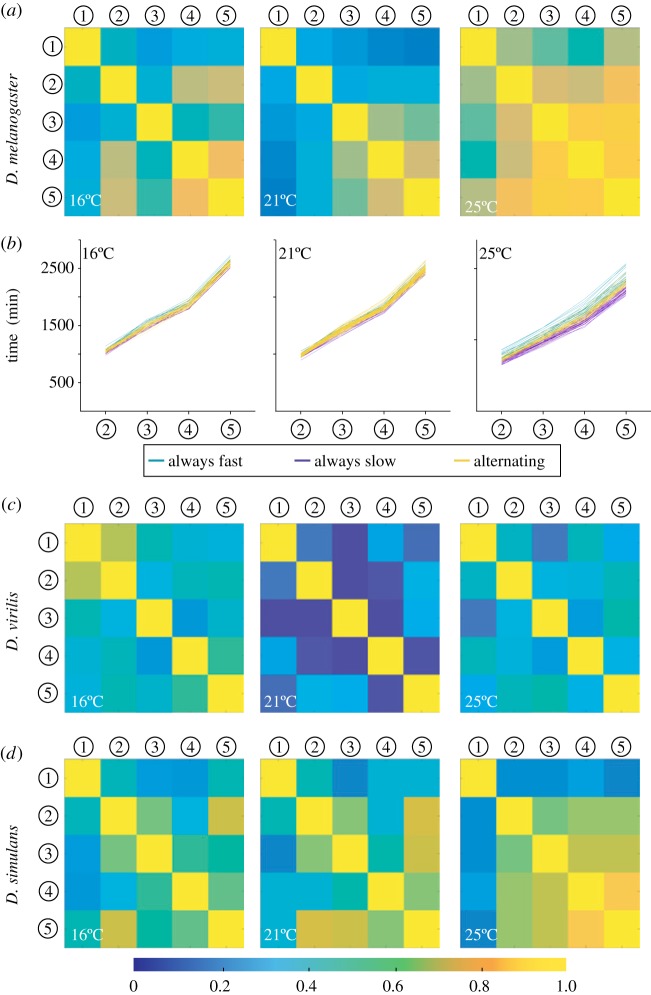
To better understand how such temporal robustness emerges, we investigated how the timing of developmental landmarks depended on the developmental history of the embryo. We performed a covariance analysis across all landmarks for D. melanogaster at constant temperatures to examine how correlated the timings of later landmarks were with earlier events (figure 4a and electronic supplementary material, figure S4a). The covariance is significantly reduced at 21°C compared to both lower and higher temperatures (table 2). These results corroborated with individual time courses, which indicated that embryos tend to continue on the same temporal trajectory throughout development at low and high temperatures: e.g. an embryo that is developing (relatively) fast early on, also develops (relatively) faster later in development (figure 4b).
Figure 4.
Temporal trajectories are history dependent. (a) Covariance between each landmark for D. melanogaster at 16, 21 and 25°C. (b) Individual embryo time courses at 16, 21 and 26°C colour coded by whether an embryo's development is always faster than the mean at that temperature (turquoise), always slower than the mean at that temperature (purple), or whether the trajectory alternates at least once between being faster or slower than the mean (yellow). (c,d) Covariance between each landmark at 16, 21 and 25°C for D. virilis (c) and D. simulans (d).
We also calculated the covariance in developmental times across different landmarks for D. virilis (figure 2c) and D. simulans (figure 2d). For D. virilis, the covariance was typically smaller than for D. melanogaster (table 2). In particular, the covariance at high temperatures is significantly less than that in D. melanogaster (p < 0.01). By contrast, the covariance of D. simulans was very similar to that of D. melanogaster, except for correlations with germband retraction at 25°C.
To test this last observation further, we checked how the proportion of temporal trajectories that were always fast or slow changed if we excluded germband retraction (electronic supplementary material, figure S4b). For D. virilis, little change was observed with 74% and 72% of the temporal trajectories varying about the mean developmental times when beginning from and after cephalic furrow, respectively, at 25°C. For D. simulans, there was a larger change in the proportion of track types with mixed trajectories at 25°C (62% to 49%, p = 0.25), but the shift itself was not significant. However, comparing D. virilis and D. simulans, we see that excluding germband retraction resulted in a significant difference between the proportion of embryos with mixed temporal trajectories in D. simulans and D. virilis (p = 0.03). For D. melanogaster, there was a marked decrease in the proportion of track types with mixed trajectories at 25°C (43% to 27%, p = 0.01). These results indicate that temporal variability early in embryogenesis can impact the rest of embryonic development.
We also performed the covariance analysis for the temperature shift experiments. The shift from 26°C to 21°C resulted in continuing high covariance between developmental landmarks, which explains the large CVτ despite reduction to 21°C (electronic supplementary material, figure S4c). By contrast, embryos initially raised at 21°C but then shifted to 26°C retained a relatively small covariance (electronic supplementary material, figure S4c). For changes to and from 16°C, the covariance behaviour was similar (electronic supplementary material, figure S4c). Therefore, we see that development at a particular temperature during early development affects the heterochronicity of later processes.
3.4. A single correlation parameter can explain the temperature dependence of CVτ
To better understand these observations, we simulated developmental temporal trajectories (see Methods: Simulations). In each simulation, we have five landmarks denoted by i = 1, …, 5 (cephalic furrow defined time 0 and we excluded hatching). The time of occurrence for each landmark is denoted by τi. In each simulation, the developmental time for landmarks was calculated as 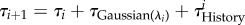 , where
, where 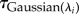 was drawn from a Gaussian distribution with mean
was drawn from a Gaussian distribution with mean 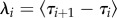 and standard deviation
and standard deviation 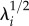 , determined by the experimentally measured time between landmarks i and i + 1.
, determined by the experimentally measured time between landmarks i and i + 1.  represents correlations between the timing of previous landmarks and the subsequent landmark. For simplicity, we take the form
represents correlations between the timing of previous landmarks and the subsequent landmark. For simplicity, we take the form 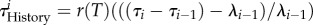 , where r is a (temperature-dependent) constant. If r = 0, there is no history dependence. For r < 0, temporal variations are reduced by, for example, slowing down temporal trajectories that are faster than the mean population development time. For r > 0, temporal trajectories that are faster than the mean population development time are reinforced, increasing the temporal error.
, where r is a (temperature-dependent) constant. If r = 0, there is no history dependence. For r < 0, temporal variations are reduced by, for example, slowing down temporal trajectories that are faster than the mean population development time. For r > 0, temporal trajectories that are faster than the mean population development time are reinforced, increasing the temporal error.
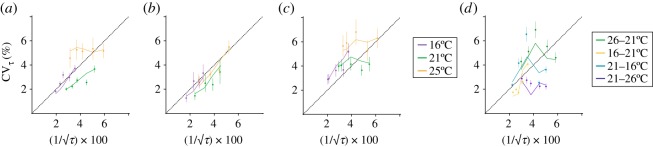
This simple model for embryonic temporal development fits the observed CVτ with a temperature dependent rmel where rmel (T > 23°C) ≈ +40 min, rmel (T < 23°C) ≈−40 min (figure 5a). Fluctuations at high temperatures are dominated by τHistory whereas at low temperatures τGaussian dominates. For D. virilis, we predicted that since development is longer, there is greater potential for feedback to regulate developmental time. Consistent with this, we found rvir (T > 23°C) ≈ −25 min, rvir (T < 23°C) ≈ −70 min for the D. virilis data (figure 5b). For D. simulans, we had the opposite prediction: since developmental time is faster, we expected that any history dependence would amplify, rather than reduce, the temporal variability. Intriguingly, we found that a single parameter rsim = +35 min was able to fit our D. simulans data (figure 5c). Therefore, the different temporal trajectories of the three species can be encapsulated within a single parameter that defines the level of history dependence and whether such history dependence dampens (r < 0) or amplifies (r > 0) temporal fluctuations. For D. melanogaster, the sign of r changes with temperature but for the faster developing D. simulans r is positive and for the slower developing D. virilis r is negative at all temperatures analysed.
Figure 5.
A single parameter defines the level of history dependence. (a–d) Relative temporal error against 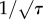 compared with model predictions: (a) D. melanogaster, (b) D. virilis, (c) D. simulans and (d) D. melanogaster with shifted temperature. Dashed line corresponds to
compared with model predictions: (a) D. melanogaster, (b) D. virilis, (c) D. simulans and (d) D. melanogaster with shifted temperature. Dashed line corresponds to 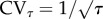 . All error bars are standard deviation, estimated by bootstrapping.
. All error bars are standard deviation, estimated by bootstrapping.
Finally, to further test our phenomenological model for the temporal trajectories we examined the temperature shift experiments. The model can qualitatively replicate our experimental observations using rmel as above, but imposing that rmel = 0 after the temperature shift from 26°C to 21°C (figures 5d). In conclusion, we can qualitatively explain the observed behaviour of CVτ at both high and low temperatures in three species with a single-fitting parameter.
4. Discussion and conclusion
Our results show that Drosophila temporal development is highly robust across three different species and a wide range of temperatures. Interestingly, this level of precision is similar to that of embryonic spatial precision of gene expression boundaries along the anterior–posterior axis in Drosophila [2]. We find that the behaviour of the temporal trajectories is non-trivial, in the sense that the statistical correlations vary both with temperature and between species. It is notable that the tropical species have generally similar correlative behaviour, whereas the temperate D. virilis has distinct covariance.
At high temperatures in D. melanogaster, our simple model can replicate the observed variability with behaviour akin to reinforcement in the temporal trajectories, whereby trajectories that are fast (slow) compared to the mean developmental time are favoured to remain fast (slow) throughout development. At intermediate temperatures, we find behaviour akin to negative feedback, whereby trajectories that are fast (slow) compared to the mean developmental time are unlikely to remain fast (slow) throughout development. This is suggestive of temperature-specific regulation. By contrast, (i) the more rapidly developing D. simulans has behaviour consistent with positive feedback at all temperatures analysed; and (ii) the more slowly developing D. virilis has behaviour consistent with negative feedback at all temperatures analysed. For rapidly developing embryos, a cohort of eggs laid at similar times will hatch close together even with noise in their temporal trajectories. Taking these observations into account, we reasoned that there may be some constraint on the absolute error—i.e. processes may exist to maximize the number of embryos within a cohort that hatch within a particular time window. Therefore, we went back and compared the absolute temporal errors between species. At 25°C all three species had very similar absolute error, i.e. the negative feedback in D. virilis at 25°C is sufficient to compensate for the longer developmental times (electronic supplementary material, figure S5). Conversely, at 16°C, D. virilis typically had larger absolute temporal error than the other species except near hatching. Note this is because the other two species have increased temporal precision, not because of a decrease in D. virilis precision. These results suggest that regulatory mechanisms may exist to control developmental time, and these are tuned to respond to temperature variations. Interestingly, D. melanogaster shows high thermal tolerance in the embryo which is lost in the adult, suggesting that the embryo has active mechanisms to adjust for temperature changes [25] and these may play a role in regulating developmental time.
The assay presented here is a viable platform for understanding how embryos adapt to subtle changes in environment. Past investigations have generally focused on the effect of temperature at larval and adult stages. The differences in embryonic timing between species of Drosophila has been quantified and shown to obey the Arrhenius rule for reaction rates (electronic supplementary material, figure S6a and [21]). Recent work has shown that Drosophila raised at a specific temperature did not select for flies optimal (in terms of fecundity) at that temperature [26]. Rather, flies exposed to temporally varying temperatures displayed increased fecundity across a broad temperature range, outperforming flies maintained at specific temperatures. Temporally variable environments have also been shown to delay reproductive maturation [27,28]. The evolution of adaptability to temperature changes has also been intensively studied [11]. Our study addresses an important gap; understanding the effect of temperature on an immobile, and therefore vulnerable, entity. Evolution has shaped Drosophila embryos to be able to cope with a wide range of temperatures. Strikingly, acute temperature changes across the natural physiological range have a modest effect on the temporal variability. Essentially, our results indicate that Drosophila embryos have the machinery in place to adapt to temperature changes.
We noted interesting differences in the behaviour of tropical and temperate species. To test this observation further, we obtained the original dataset from [21] which covered 11 different Drosophila species. The number of embryos for each species and temperature ranged from 6 to over 70. These embryos were collected in a different environment and under different imaging conditions (e.g. we did not dechorionate the embryos) making direct comparison with our results difficult (electronic supplementary material, figure S6b). Owing to the variable sample sizes, we consider two sets: (i) tropical (D. simulans, D. ananassae, D. seychelia, D. willistoni, D. yakuba, D. erecta); and (ii) non-tropical (D. virilis, D. mojavensis, D. persimilis, D. pseudoobscura). Comparing the change in CVτ between cellularization and trachea filling, we find that tropical species show significantly more variability in CVτ as temperature is varied than non-tropical species (electronic supplementary material, figure S6c,d). This is consistent with our above results and suggests that species that are exposed to wider temperature fluctuations have developed regulatory processes to buffer the effects of such temperature changes on developmental time. However, more detailed species-specific analysis will be required to confirm this observation. Along these lines, a recent observation revealed that the Drosophila βtubulin97EF is upregulated at low temperatures and contributes to stabilize microtubules [29]. This example demonstrates that differential regulation of intracellular components is necessary for acclimation to environmental changes. Furthermore, it would be interesting to generate a profile of miRNAs and small non-coding molecules in general at different temperatures as these molecules are known to buffer noise and to respond to environmental changes [30,31].
The timing noise in single cells has been quantified in a number of systems [32,33]. Work on bacterial cells has shown that changes in temperature alter both the cell growth rate and the time to division equally [34] and this can be explained within a cyclic autocatalytic reaction whereby each element catalyses the next element [35]. In particular, temperature-dependent scaling of cellular time is sufficient to explain experimental observations of bacterial growth response to temperature changes. It will be interesting to extend our simple model to see whether such general physical principles also apply to developing systems.
The careful control of timing during development, for example in the segmentation clock [36,37], and in adults, such as circadian rhythms [38], is essential for life. Yet, heterochronicity has been shown to be crucial for the evolution of new traits. By altering the timing or sequence of developmental effects, new features can emerge, such as increased segment number in snakes [39,40]. Our quantitative results demonstrating a temperature-specific response in the temporal trajectories of development are suggestive of factors regulating the timing of development. In the larvae, a number of hormonal signals have been identified that regulate developmental time [41,42], but currently little is known about mechanisms of temporal regulation in the embryo. Finally, there has been significant work in trying to understand ecological adaptation to changing environments [43], and it will be interesting to quantify the temporal trajectories of development in a continuously fluctuating environment.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Patrice Koehl and Amit Singh for the advice on the statistical analysis.
Data accessibility
This article has no additional data.
Authors' contributions
J.C., C.A. and T.E.S. designed the experiments. J.C. performed the experiments and quantified developmental times. C.A. and T.E.S. analysed the data. T.E.S. performed the simulations. All authors contributed to the writing of the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was funded through a National Research Foundation Singapore Fellowship to T.E.S. (NRF2012NRF-NRFF001-094).
References
- 1.Houchmandzadeh B, Wieschaus E, Leibler S. 2002. Establishment of developmental precision and proportions in the early Drosophila embryo. Nat. Gen. 415, 798–802. ( 10.1038/415798a) [DOI] [PubMed] [Google Scholar]
- 2.Gregor T, Tank DW, Wieschaus EF, Bialek W. 2007. Probing the limits to positional information. Cell 130, 153–164. ( 10.1016/j.cell.2007.05.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manu SS, et al. 2009. Canalization of gene expression in the Drosophila blastoderm by gap gene cross regulation. PLoS Biol. 7, e1000049 ( 10.1371/journal.pbio.1000049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubuis JO, Samanta R, Gregor T. 2013. Accurate measurements of dynamics and reproducibility in small genetic networks. Mol. Sys. Biol. 9, 639 ( 10.1038/msb.2012.72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little SC, Tikhonov M, Gregor T. 2013. Precise developmental gene expression arises from globally stochastic transcriptional activity. Cell 154, 789–800. ( 10.1016/j.cell.2013.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abouchar L, Petkova MD, Steinhardt CR, Gregor T. 2014. Fly wing vein patterns have spatial reproducibility of a single cell. J. R. Soc. Interface 11, 20140443 ( 10.1098/rsif.2014.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowell LB. 1974. Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 54, 75–159. ( 10.1152/physrev.1974.54.1.75) [DOI] [PubMed] [Google Scholar]
- 8.Ivanov KP. 2006. The development of the concepts of homeothermy and thermoregulation. J. Therm. Biol. 31, 24–29. ( 10.1016/j.jtherbio.2005.12.005) [DOI] [Google Scholar]
- 9.Hoffmann AA, Sørensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. ( 10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 10.Kellett M, Hoffmann AA, McKechnie SW. 2005. Hardening capacity in the Drosophila melanogaster species group is constrained by basal thermotolerance. Funct. Ecol. 19, 853–858. ( 10.1111/j.1365-2435.2005.01025.x) [DOI] [Google Scholar]
- 11.Dillon ME, Wang G, Garrity PA, Huey RB. 2009. Thermal preference in Drosophila. J. Therm. Biol. 34, 109–119. ( 10.1016/j.jtherbio.2008.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey WD, Wilson RI, Laurent G, Benzer S. 2003. painless, a Drosophila gene essential for nociception. Cell 113, 261–273. ( 10.1016/S0092-8674(03)00272-1) [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, et al. 2005. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 37, 305–310. ( 10.1038/ng1513) [DOI] [PubMed] [Google Scholar]
- 15.Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, Zhao ZQ, Guo AK. 2006. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 5, 602–613. ( 10.1111/j.1601-183X.2006.00213.x) [DOI] [PubMed] [Google Scholar]
- 16.Stillman JH. 2002. Causes and consequences of thermal tolerance limits in rocky intertidal porcelain crabs, genus Petrolisthes. Integr. Comp. Biol. 42, 790–796. ( 10.1093/icb/42.4.790) [DOI] [PubMed] [Google Scholar]
- 17.Walton BM, Bennett AF. 1993. Temperature-dependent color change in Kenyan chameleons. Physiol. Zool. 66, 270–287. ( 10.1086/physzool.66.2.30163690) [DOI] [Google Scholar]
- 18.Pieau C. 1996. Temperature variation and sex determination in reptiles. Bioessays 18, 19–26. ( 10.1002/bies.950180107) [DOI] [Google Scholar]
- 19.Smith-Gill SJ, Naturalist KBTA. 1979. Predicting amphibian metamorphosis. Am. Nat. 113, 563–585. ( 10.1086/283413) [DOI] [Google Scholar]
- 20.Jarosík V, Kratochvíl L, Honek A, Dixon AFG. 2004. A general rule for the dependence of developmental rate on temperature in ectothermic animals. Proc. R. Soc. Lond. B 271, S219–S221. ( 10.1098/rsbl.2003.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuntz SG, Eisen MB. 2014. Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species. PLoS Genet. 10, e1004293 ( 10.1371/journal.pgen.1004293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. 2002. Effects of size and temperature on developmental time. Nature 417, 70–73. ( 10.1038/417070a) [DOI] [PubMed] [Google Scholar]
- 23.Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. 2005. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature 434, 1134–1138. ( 10.1038/nature03509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stillwell RC, Fox CW. 2005. Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology 86, 924–934. ( 10.1890/04-0547) [DOI] [Google Scholar]
- 25.Lockwood BL, Gupta T, Scavotto R. 2018. Disparate patterns of thermal adaptation between life stages in temperate vs. tropical Drosophila melanogaster. J. Evol. Biol. 31, 323–331. ( 10.1111/jeb.13234) [DOI] [PubMed] [Google Scholar]
- 26.Condon C, Cooper BS, Yeaman S, Angilletta MJ. 2014. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution 68, 720–728. ( 10.1111/evo.12296) [DOI] [PubMed] [Google Scholar]
- 27.Tuljapurkar S. 1990. Delayed reproduction and fitness in variable environments. Proc. Natl Acad. Sci. USA 87, 1139–1143. ( 10.1073/pnas.87.3.1139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rees M, Ellner SP. 2009. Integral projection models for populations in temporally varying environments. Ecol. Monogr. 79, 575–594. ( 10.1890/08-1474.1) [DOI] [Google Scholar]
- 29.Myachina F, Bosshardt F, Bischof J, Kirschmann M, Lehner CF. 2017. Drosophilaβ-Tubulin 97EF is upregulated at low temperature and stabilizes microtubules. Development 144, 4573–4587. ( 10.1242/dev.156109) [DOI] [PubMed] [Google Scholar]
- 30.Herranz H, Cohen SM. 2010. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 24, 1339–1344. ( 10.1101/gad.1937010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung AKL, Sharp PA. 2010. MicroRNA functions in stress responses. Mol. Cell 40, 205–215. ( 10.1016/j.molcel.2010.09.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amir A, Kobiler O, Rokney A, Oppenheim AB, Stavans J. 2007. Noise in timing and precision of gene activities in a genetic cascade. Mol. Sys. Biol. 3, 4558 ( 10.1038/msb4100113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurkovsky E, Nachman I. 2013. Event timing at the single-cell level. Brief. Funct. Genomics 12, 90–98. ( 10.1093/bfgp/els057) [DOI] [PubMed] [Google Scholar]
- 34.Iyer-Biswas S, et al. 2014. Scaling laws governing stochastic growth and division of single bacterial cells. Proc. Natl Acad. Sci. USA 111, 15 912–15 917. ( 10.1073/pnas.1403232111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyer-Biswas S, Crooks GE, Scherer NF, Dinner AR. 2014. Universality in stochastic exponential growth. Phys. Rev. Lett. 113, 185 ( 10.1103/PhysRevLett.113.028101) [DOI] [PubMed] [Google Scholar]
- 36.Lauschke VM, Tsiairis CD, François P, Aulehla A. 2013. Scaling of embryonic patterning based on phase-gradient encoding. Nature 493, 101–105. ( 10.1038/nature11804) [DOI] [PubMed] [Google Scholar]
- 37.Soroldoni D, Jörg DJ, Morelli LG, Richmond DL, Schindelin J, Julicher F, Oates AC. 2014. A Doppler effect in embryonic pattern formation. Science 345, 222–225. ( 10.1126/science.1253089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi JS. 2017. Transcriptional architecture of the mammalian circadian clock. Nat. Gen. 18, 164–179. ( 10.1038/nrg.2016.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquie O. 2008. Control of segment number in vertebrate embryos. Nature 454, 335–339. ( 10.1038/nature07020) [DOI] [PubMed] [Google Scholar]
- 40.Keyte AL, Smith KK. 2014. Heterochrony and developmental timing mechanisms: changing ontogenies in evolution. Semin. Cell Dev. Biol. 34, 99–107. ( 10.1016/j.semcdb.2014.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombani J, Andersen DS, Léopold P. 2012. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582–585. ( 10.1126/science.1216689) [DOI] [PubMed] [Google Scholar]
- 42.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. 2012. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–582. ( 10.1126/science.1216735) [DOI] [PubMed] [Google Scholar]
- 43.Evans MR. 2012. Modelling ecological systems in a changing world. Proc. R. Soc. B 367, 181–190. ( 10.1098/rstb.2011.0172) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.