Abstract
Objectives:
To determine if the large and highly reproducible interindividual differences in arousal intensity and heart rate response to arousal (ΔHR) during non-REM sleep are heritable.
Methods:
Polysomnograms of 55 monozygotic (14 male and 41 female pairs) and 36 dizygotic (15 male and 21 female pairs) same-sex twin pairs were analyzed. Arousals were scored using the 2012 American Academy of Sleep Medicine criteria. Arousal intensity was scaled (between 0 and 9) using an automatic algorithm based on the change in electroencephalogram time and frequency characteristics. The ΔHR was determined at each arousal. We calculated average arousal duration, average arousal intensity, average overall ΔHR, average ΔHR at a given arousal intensity, slope of ΔHR per arousal intensity, and arousal intensity threshold of ΔHR.
Results:
The intraclass correlations among monozygotic and dizygotic twin pairs were 0.663 and 0.146, respectively, for average arousal intensity, and 0.449 and 0, respectively, for arousal intensity threshold of ΔHR controlling for age, sex, and race. These values imply large broad sense heritability (H2) for these traits. This evidence was confirmed by a robust maximum likelihood-based variance components estimation approach, with an additive genetic heritability of 0.64 (95% confidence interval: 0.48 to 0.80) for average arousal intensity and a combined additive and dominance genetic heritability and of 0.46 (0.25 to 0.68) for arousal intensity threshold of ΔHR. Results also suggested significant additive genetic effects for average arousal duration, ΔHR at arousal intensity scale 4 and the overall average ΔHR.
Conclusion:
Genetic factors explain a significant fraction of the phenotypic variability for average arousal intensity and arousal intensity threshold of ΔHR. Results suggest that the duration of arousals and specific average ΔHR values may also be heritable traits.
Clinical trial registration:
Keywords: polysomnogram, monozygotic, dizygotic
Statement of Significance
This study in twins is the first to show that underlying genetic factors significantly influence arousals and the heart rate response to arousals during sleep. Utilizing multiple, complementary techniques for estimating trait heritability in monozygotic and dizygotic twin pairs without sleep disorders, we found that the average arousal intensity and the arousal intensity associated with a heart rate response are moderately to highly heritable traits. These results add further evidence that many sleep characteristics are heritable and therefore influenced by underlying genetic factors. Our results suggest that outcomes related to arousals associated with sleep disorders (e.g., daytime sleepiness and cardiovascular morbidities) may be heritable.
INTRODUCTION
Arousals from sleep are very common in patients with sleep disorders. Arousals from sleep are associated with a transient autonomic reflex activation that increases ventilation, blood pressure, and heart rate (HR).1–5 Frequent arousals from sleep cause sleep fragmentation, which can lead to impaired cognitive function6–9 and may also contribute to chronic hypertension in patients with obstructive sleep apnea (OSA).10–12 Arousals are conventionally scored when an abrupt shift in electroencephalography (EEG) to higher frequencies occurs for at least 3 seconds, preceded by at least 10 seconds of stable sleep.13,14 The visual appearance of cortical arousals meeting this definition varies considerably within and between subjects. Previous studies have reported very little variability in the number of arousals and arousal index from night to night.15–19
We recently developed an automatic scaling algorithm to score the intensity of arousals between 0 (no relative change in EEG characteristic) and 9 (the most intense arousal). Using wavelet transform to quantify the change in EEG time and frequency characteristics due to arousal,20,21 average arousal intensity varies considerably between subjects with sleep disorders.22 Furthermore, a strong correlation exists between intensity of arousal and average HR response to arousal (ΔHR), while the calculated slope of the relationship varies considerably between subjects.22 These interindividual differences in average arousal intensity and ΔHR may indicate different susceptibility to the development of the functional and cardiovascular complications of sleep disorders.
In a recent polysomnogram (PSG) study of adults without sleep disorders, we showed that the average intensity of arousals and ΔHR at a given arousal intensity in non-REM sleep are highly variable across subjects but highly reproducible within subjects.23 To determine if genetic factors contribute to these interindividual differences in arousal characteristics and the resulting increases in heart rate during non-REM sleep, we measured characteristics of spontaneous arousals and ΔHR in 55 monozygotic (MZ) and 36 dizygotic (DZ) twin pairs.
METHODS
PSG Files
The PSG files used in this study were generated in a previous study on heritability of response to sleep deprivation, and the methods of subject recruitment and participant characteristics have been previously detailed.24 Individuals on modafinil, methylphenidate, β-blocker medication, or any medication to promote sleep or relieve pain were excluded from participation. The original data set consisted of 59 monozygotic (MZ) and 41 dizygotic (DZ) same-sex twin pairs (N = 200 total participants). Of these, 9 pairs were removed due to at least one of the twins having either incomplete data (n = 5 pairs) or failing to meet data quality criteria of ≥20 arousals with available ΔHR. Therefore, our final study sample for analysis consisted of 182 participants, made up of 55 monozygotic (mean ± SD age 29.2 ± 6.9 years; 14 male and 41 female pairs) and 36 dizygotic (age 26.2 ± 7.5 years; 15 male and 21 female pairs) twin pairs. Twin zygosity was determined by a DNA-based polymerase chain reaction (PCR) analysis of peripheral blood using 12 highly polymorphic short-tandem repeat loci and amelogenin.25 The original study was approved by the Institutional Review Boards at the University of Pennsylvania and the subcontracted sites, the University of Chicago, and Virginia Commonwealth University. Written-informed consent was obtained from each participant.
Significant sleep pathology was excluded on a PSG study performed at least 2 weeks prior to admission to the Clinical and Translational Research Center.24 All participants were required to have an apnea–hypopnea index and periodic limb movement index <5 events/hour. For 24 hours prior to and during their admission, participants were asked not to smoke or drink alcohol. During the admission, they were given three regular meals and snacks but were not allowed to drink caffeinated beverages. Participants were allowed to sleep ad libitum on days 1 and 2. PSGs were performed on those two nights and the recordings on the second night were used for analysis in the current study. The PSG montage included 4 EEG signals (C3M2, C4M1, O1M2, and O2M1), bilateral electrooculogram, chin muscle electromyogram, and electrocardiogram (lead 1).
The files were scored for conventional sleep variables by the automatic system developed at YRT Limited and validated in an independent multicenter study.26 The automatic scoring followed 2012 AASM guidelines for scoring sleep and arousals.14 The onsets and ends of arousals were identified by an experienced technologist strictly from the EEG without regard to any other signals (e.g., EMG or heart rate).
Arousal Scaling
Automatic scaling of arousal intensity was performed as described in detail previously (Figure 1).22 Briefly, each arousal in non-REM sleep was scaled automatically based on the magnitude of changes (during arousal versus before arousal) in 14 wavelet characteristics derived from each of the two central EEG recordings (C3M2 and C4M1) and assigning a scale from zero (least intense) to 9 (most intense) from the calculated wavelet characteristics and a previously constructed training data set. The wavelet characteristics and the training set used in the present study were identical to the ones used in our earlier study.22 The arousal scale was relative to the population, that is, a given arousal intensity was of the same magnitude for each individual in the population. When the arousal scale differed in the two central electrodes, the higher of the two values was used. No signal other than the EEG was used in this automatic process. For each file, we determined the total number of arousals and the mean and standard deviation (SD) values of arousal intensity scale and arousal duration.
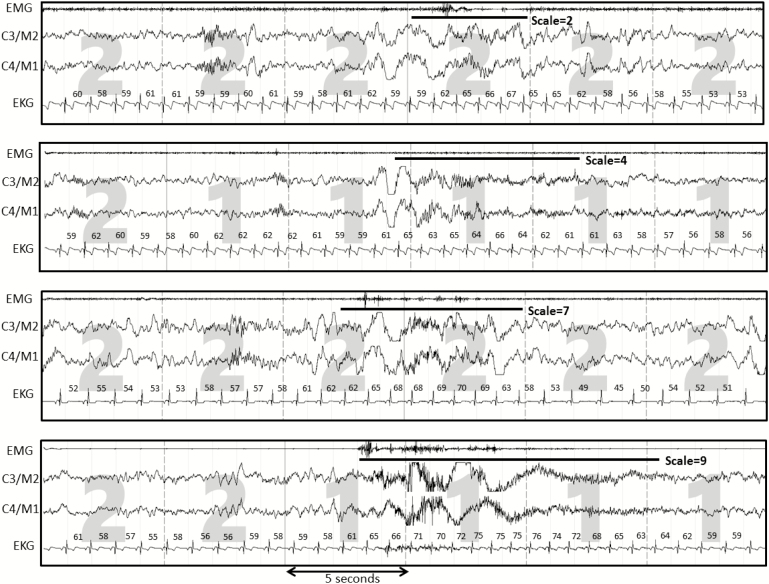
Figure 1.
Examples of arousals with different scales in the same subject. EMG, chin electromyogram; C3/M2 and C4/M1 are electroencephalograms; EKG, electrocardiogram. Numbers above the electrocardiogram represent instantaneous heart rate. Arousal scale is highly sensitive to beta power (as in the lower two panels).
Heart Rate Response to Arousal
The ΔHR was determined as the difference between maximum heart rate during arousal (i.e., from arousal onset to 8 seconds after end of arousal) and maximum baseline heart rate. Beat-by-beat heart rate in the interval 2–12 seconds preceding the arousal was used as baseline heart rate. The 2 seconds preceding arousal were avoided in baseline determination due to the observation that arousal-associated tachycardia may begin up to 2 seconds before arousal.3
As described previously,22 the arousal-associated change in HR incorporates a component that is unrelated to the arousal (short-term spontaneous changes in HR). To minimize the impact of this background noise, as shown in Figure 2, the ΔHR values for all arousals at each arousal intensity scale were averaged, and arousal intensities represented by only one observation were not included in the regression.
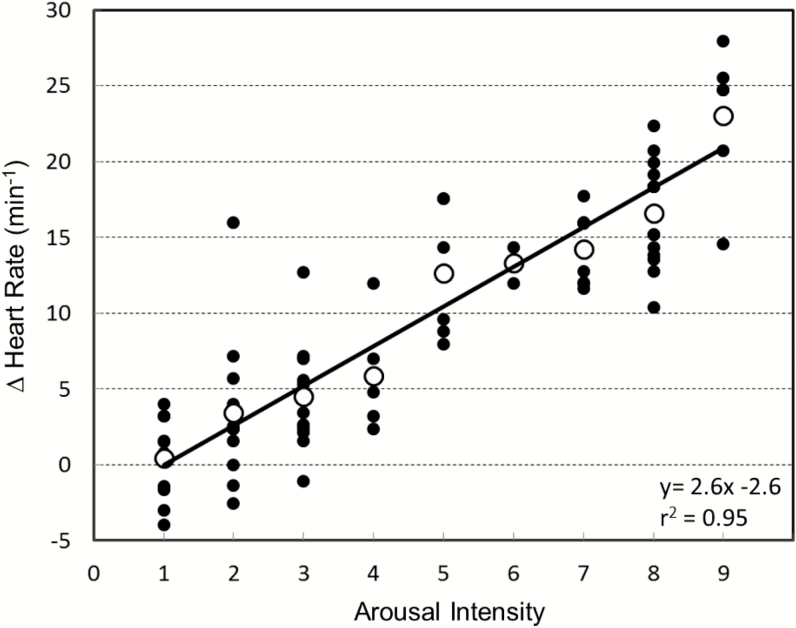
Figure 2.
Change in heart rate with the arousal (ΔHR) at different arousal intensities in a representative subject. Each solid dot is the ΔHR with each arousal and each open dot is the average ΔHR at a given arousal intensity. The equation of best-fitted regression line to the average HR responses is y = 2.6x − 2.6. The arousal intensity threshold for ΔHR is 1.0.
In each subject, we calculated average arousal duration, average arousal intensity, average overall ΔHR, average ΔHR at arousal intensity scales of 4 (ΔHR4), 5 (ΔHR5), and 8 (ΔHR8). To estimate the relationship between arousal intensity scale and average ΔHR values, we derived subject-specific slope of ΔHR per arousal intensity and arousal intensity threshold of ΔHR, that is, the minimum arousal intensity at which a HR response was present, from within-subject linear regression models of average ΔHR at each arousal intensity against arousal intensity score (Figure 2). To ensure reliable estimates of the ΔHR response at given arousal intensities (ΔHR4, ΔHR5, and ΔHR8), values were obtained from subject-specific regression equations, rather than from the average of actual ΔHR values at each scale.
Statistical Analyses
Continuous variables were summarized using means and SD and categorical variables using frequencies and percentages. Demographic and outcome measures were compared between MZ and DZ twins using linear (for continuous) or logistic (for categorical) mixed-model regression, accounting for zygosity differences in covariance. This and all subsequent modeling was performed using SAS Version 9.4 (SAS Institute, Inc, Cary, North Carolina).
We assessed the estimated heritability of the following measures: arousal index; average arousal duration; average arousal intensity; overall average ΔHR; model-derived estimates of slope of ΔHR per arousal intensity; arousal intensity threshold for ΔHR; and ΔHR4, ΔHR5, and ΔHR8.
Heritability of these arousal and heart rate response to arousal characteristics was estimated using three complementary approaches as in our previous study24: (1) classical approaches based on differences in MZ- and DZ-specific intraclass correlation coefficients (ICCs)27; (2) analysis of variance (ANOVA) based on within pair (WP) or among component estimates28,29; and (3) maximum likelihood-based estimation (MLE) of heritability utilizing variance components under specific inheritance model characterizations.30–33 Given the robustness of the MLE approach, heritability estimates from these models were considered primary. Additional details of each method are presented subsequently. For methods (1) and (3), heritability estimates were obtained unadjusted and after controlling for race, age, gender, and age × gender interactions. Given nine traits of interest, statistical significance in the ANOVA and MLE methods was based on a Bonferroni-corrected threshold of p < .0056; a p < .05 was considered suggestive evidence of heritability.
The first approach to estimating heritability was based on the classical approach using ICC statistics.27 Specifically, ICC statistics are calculated within MZ (ICCMZ) and DZ (ICCDZ) twins, separately, as the ratio of between twin and overall variability [σ2B / (σ2W + σ2B)]. Using these ICC statistics, broad-sense heritability (the proportion of phenotypic variance explained by all genetic factors; H2) is calculated as 2∙(ICCMZ – ICCDZ). In addition to the heritability estimate, the proportion of phenotypic variance explained by common environmental effects (C2) on a given trait was calculated as 2∙(ICCDZ) – ICCMZ. Negative estimates of C2 suggest that shared environment does not play a strong role in influencing a given trait, and suggest that an ADE inheritance model (which models additive and dominance genetic effects and individual effects) is appropriate (see below for discussion of inheritance models). Similarly, C2>0 suggests common environment plays a role, and an ACE inheritance model (which models additive genetic, common environment, and individual effects) may be more appropriate.
In addition to this classical approach, we utilized ANOVA-based methods to calculate broad-sense heritability based on zygosity specific within-twin pair (MSWMZ and MSWDZ) and among-twin pair (MSAMZ and MSADZ) mean square (MS) estimates.28,29 Based on these mean squares, two possible estimates of heritability can be obtained. The choice of estimate is based on a comparison of the total phenotype variance between MZ and DZ twin pairs, using a two-sided F-test of the ratio FTV = [(MSADZ + MSWDZ) / (MSAMZ + MSWMZ)] or FTV = [(MSAMZ + MSWMZ) / (MSADZ + MSWDZ)], where the larger sum of WP and among-pair mean squares is the numerator. The first estimate, which is valid when the total phenotypic variance F-test p > .20, is a within pair heritability (H2WP) = [2∙(MSWDZ – MSWMZ)] / [(MSAMZ + MSWDZ + MSADZ + MSWMZ)/4]. Significance of this estimate is based on a one-sided F-test of the ratio FWP = MSWDZ / MSWMZ. The second estimate, which is unbiased when the total phenotypic variance F-test p<0.20, is the among component (AmCo) heritability (H2AmCo) = [(MSAMZ – MSADZ) + (MSWDZ – MSWMZ)] / [(MSAMZ + MSWDZ + MSADZ + MSWMZ)/4]. The H2AmCo estimate is a weighted average of the WP and among-pair mean square heritability estimates, and significance is based on a one-sided F-test of the ratio FAC = (MSAMZ + MSWDZ) / (MSADZ + MSWMZ).
Finally, robust estimates of heritability were calculated using variance components from MLE mixed-effects modeling procedures.30–33 Random effects estimates were utilized to assess different heritability models,31,33 examining specific components of transmission, including additive genetic effects (A), dominance genetic effects (D), common environmental effects (C), and unique individual effects (E). This modeling approach utilizes the same covariance expectations as path analysis or structural equation modeling and leverages similar goodness-of-fit tests to determine the most appropriate inheritance model. Importantly, in the model parameterization utilized here, accurate estimates of heritability can be obtained without restricting variance components to be >0, therefore allowing the opportunity to identify poor model fit utilizing standard likelihood ratio tests.30,31 Specifically, using the estimate of the proportion of phenotypic variance explained by shared environmental factors in the ACE model (denoted h2C) an initial model was selected to represent the inheritance pattern as either ACE (if h2C > 0) or ADE (if h2C ≤ 0).33 Subsequent to initial model selection, goodness-of-fit tests were utilized to determine whether reduced models provided similar model fit, including likelihood ratio tests for nested models (i.e., ACE vs. AE, CE, E or ADE vs. AE, E). Akaike Information Criteria (AIC) statistics were used to compare between nonnested AE and CE models if both suggested better fit than the more complicated ACE model. Component-specific heritability estimates (i.e., proportion of total variance due to A, D, A+D, C or E factors) were then determined based on the variance component estimates in the best-fitting model. Model comparisons and initial variance proportion estimates were obtained using linear mixed models (PROC MIXED in SAS) as described previously.31,33 Estimates of statistical significance and 95% confidence intervals (CIs) for resulting estimates were derived using nonlinear mixed model procedures (PROC NLMIXED in SAS), under the null hypothesis that the true variance proportion (i.e., heritability) is equal to zero.
RESULTS
Study Population
The characteristics of the participants are detailed in Table 1. On average, the population consisted of young adults (age 28.0 ± 7.3 years, body mass index 24.3 ± 4.3 kg/m2), with a majority female (68%) and self-reported Caucasian (78%). There were no statistically significant differences in demographic characteristics between MZ and DZ pairs; MZ twins were 3 years older on average (p =.056). A larger proportion (p = .122) of MZ twins (n = 46 pairs; 83.6%) were Caucasian compared to DZ twins (n = 25 pairs; 69.4%). The remaining DZ twin pairs reported being African American (n = 11 pairs; 30.6%), while MZ twin pairs reported multiple races (n = 6 African American pairs [10.9%], n = 3 Asian/mixed race pairs [5.5%]).
Table 1.
Demographic characteristics of monozygotic and dizygotic twin pairsa.
| Measure | Total (N = 182) | Monozygotic twins (MZ) (n = 110) | Dizygotic twins (DZ) (n = 72) | pb |
|---|---|---|---|---|
| Age, years | 27.99 ± 7.26 | 29.17 ± 6.86 | 26.19 ± 7.54 | .056 |
| Male, n (%) | 58 (31.9%) | 28 (25.5%) | 30 (41.7%) | .114 |
| Caucasian, n (%) | 142 (78.0%) | 92 (83.6%) | 50 (69.4%) | .122 |
| BMI, kg/m2 | 24.26 ± 4.32 | 24.44 ± 4.72 | 23.99 ± 3.63 | .610 |
| CES-D total scorec | 14.93 ± 3.90 | 14.70 ± 3.66 | 15.28 ± 4.26 | .377 |
| ESS total scored | 5.72 ± 3.30 | 5.39 ± 2.83 | 6.23 ± 3.87 | .152 |
| Global PSQI scoree | 3.50 ± 1.87 | 3.49 ± 1.92 | 3.53 ± 1.80 | .902 |
| Total sleep time (min) | 405.0 ± 59.1 | 398.2 ± 58.6 | 415.5 ± 58.7 | .105 |
| Sleep efficiency (%) | 84.55 ± 10.13 | 83.91 ± 10.08 | 85.53 ± 10.21 | .370 |
| Time in NREM sleep (min) | 315.5 ± 44.2 | 310.0 ± 41.6 | 324.0 ± 46.9 | .069 |
| Number of Arousals | 70.26 ± 31.47 | 71.22 ± 30.27 | 68.79 ± 33.39 | .681 |
| AHI (events/hr) | 1.80 ± 1.40 | 1.64 ± 1.41 | 2.03 ± 1.35 | .119 |
| PLMI (events/hr) | 1.92 ± 5.35 | 2.45 ± 6.29 | 1.12 ± 3.34 | .153 |
Abbreviations: MZ, monozygotic; DZ, dizygotic; BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; ESS, Epworth Sleepiness Scale; PLMI, periodic limb movement index; PSQI, Pittsburgh Sleep Quality Index; NREM, non-rapid eye movement sleep.
aResults presented as mean ± SD for continuous measures or n (%) for categorical measures.
b p value from mixed linear or logistic model comparing MZ and DZ groups, controlling for possible correlation within MZ and DZ pairs.
c n = 2 missing values.
d n = 7 missing values.
e n = 5 missing values.
There were no significant differences in sleep, arousal, or ΔHR measures between the MZ and DZ twins (Tables 1 and 2). DZ twins had approximately 14 minutes more NREM sleep on average (p = .069). The mean number of arousals in all subjects was 70.3 ± 31.5, and the average arousal duration was 7.5 ± 0.8 seconds. The study population had moderately intense arousals (mean intensity scale 4.8 ± 0.9) on average, with an arousal intensity threshold of ΔHR of 0.97 ± 0.89 for MZ twins and 0.91 ± 0.91 for DZ twins (p = .702). The average ΔHR was 9.4 ± 3.8 min−1, with DZ twins having a higher average ΔHR compared to MZ twins (10.3 ± 4.2 vs. 8.9 ± 3.5 min-1), although the difference was not statistically significant (p = .066).
Table 2.
Variable summary (mean ± SD) of monozygotic and dizygotic twin pairsa.
| Measure | Total (N = 182) | Monozygotic twins (MZ) (n = 110) | Dizygotic twins (DZ) (n = 72) | Mean (95% CI) Differenceb | pc |
|---|---|---|---|---|---|
| Arousal Index (events/hour) | 12.56 ± 5.72 | 12.84 ± 5.81 | 12.14 ± 5.49 | 0.70 (−1.45 to 2.86) | .519 |
| Average arousal duration (seconds) | 7.51 ± 0.78 | 7.46 ± 0.79 | 7.58 ± 0.76 | −0.12 (−0.39 to 0.16) | .395 |
| Average arousal intensity scale | 4.83 ± 0.85 | 4.77 ± 0.83 | 4.92 ± 0.89 | −0.15 (−0.47 to 0.16) | .333 |
| Slope of ΔHR per arousal intensity | 2.55 ± 0.76 | 2.45 ± 0.69 | 2.70 ± 0.84 | −0.25 (−0.53 to 0.02) | .074 |
| Arousal intensity threshold of ΔHR | 0.95 ± 0.89 | 0.97 ± 0.89 | 0.91 ± 0.91 | 0.06 (−0.25 to 0.37) | .702 |
| Average ΔHR (min−1) | 9.43 ± 3.80 | 8.90 ± 3.45 | 10.25 ± 4.17 | −1.35 (−2.78 to 0.09) | .066 |
| ΔHR at arousal intensity scale 4 (min-1) | 7.49 ± 2.39 | 7.18 ± 2.31 | 7.96 ± 2.44 | −0.78 (−1.68 to 0.12) | .090 |
| ΔHR at arousal intensity scale 5 (min-1) | 10.04 ± 2.87 | 9.63 ± 2.72 | 10.66 ± 3.01 | −1.03 (−2.12 to 0.06) | .063 |
| ΔHR at arousal intensity scale 8 (min-1) | 17.68 ± 4.78 | 16.97 ± 4.38 | 18.76 ± 5.16 | −1.79 (−3.59 to 0.01) | .051 |
Abbreviations: MZ, monozygotic; DZ, dizygotic; ΔHR, heart rate response to arousal; CI, confidence interval.
aResults presented as mean ± SD.
bDifference calculated as MZ value minus DZ value, estimated from mixed linear model.
cp value from mixed linear model comparing MZ and DZ groups, controlling for possible correlation within MZ and DZ pairs.
As a preliminary examination of the relative similarity of traits in MZ and DZ pairs, we compared the WP absolute difference in each phenotype between zygosity groups (Table 3); traits showing greater similarity between MZ pairs compared to DZ pairs are more likely to be strongly influenced by genetic factors. On average, the MZ twin pairs were more similar than DZ twin pairs, with smaller within pair absolute differences for each phenotype. Statistically significant differences were observed for the average arousal intensity (p = .011) and average ΔHR (p = .037), and there was a borderline nonsignificant difference for the arousal intensity threshold of ΔHR (p = .056).
Table 3.
Absolute within pair phenotype differences in monozygotic and dizygotic twin pairs.
| Measure | Absolute within pair differencea | ||
|---|---|---|---|
| MZ twins (N = 110) | DZ twins (N = 72) | pb | |
| Arousal Index | 3.75 ± 3.38 | 3.99 ± 4.47 | .7689 |
| Average arousal duration | 0.64 ± 0.50 | 0.71 ± 0.67 | .5921 |
| Average arousal intensity scale | 0.50 ± 0.42 | 0.86 ± 0.73 | .0114 |
| Slope of ΔHR per arousal intensity | 0.57 ± 0.44 | 0.63 ± 0.48 | .5435 |
| Arousal intensity threshold of ΔHR | 0.67 ± 0.54 | 0.99 ± 0.88 | .0563 |
| Average ΔHR | 2.18 ± 1.86 | 3.13 ± 2.43 | .0370 |
| ΔHR at arousal intensity scale 4 | 1.55 ± 1.31 | 1.98 ± 1.25 | .1226 |
| ΔHR at arousal intensity scale 5 | 1.98 ± 1.44 | 2.27 ± 1.24 | .3220 |
| ΔHR at arousal intensity scale 8 | 3.43 ± 2.46 | 3.55 ± 2.29 | .8259 |
Abbreviations: MZ, monozygotic; DZ, dizygotic; ΔHR, heart rate response to arousal;
aValues represent mean ± standard deviation of the absolute value of the within pair difference in the indicated measure;
bp value from t-test comparing within pair differences between MZ and DZ twin pairs.
Classical Heritability Estimates
Estimates from the classical approach to heritability calculations for the arousal and ΔHR measures (including unadjusted and adjusted ICCs, H2 and C2) are shown in Table 4. Generally, ICC values were larger for MZ twins than DZ twins, suggesting genetic factors explain some proportion of phenotypic variability (i.e., broad-sense heritability). In adjusted analyses, an ADE model was the preferred genetic model for average arousal intensity, arousal intensity threshold of ΔHR, and average ΔHR, suggesting significant contributions of genetic factors, but not shared environment, to the phenotypic variability. The ICCs for MZ and DZ twin pairs were 0.663 and 0.146, respectively, for the average arousal intensity scale, and were 0.449 and 0 for arousal intensity threshold of ΔHR. These values imply large H2 values for both measures (including a value >1 for average arousal intensity), indicating genetic factors explain a large amount of phenotypic variability; the zero ICC for DZ for arousal intensity threshold likely arises from sampling error around a small WP correlation. Moreover, the corresponding large negative values of C2 imply that shared environment does not explain phenotypic variance for these traits. Similarly, the adjusted H2 for average ΔHR was 0.616, with a slightly negative C2. In contrast, a combination of environmental and genetic contributions to phenotypic variance was suggested for all other traits in adjusted analyses. That is, for each measure, there were positive H2 and C2 estimates, suggesting that both genetic and shared environmental factors explain phenotypic variability. Among these traits, the average arousal duration, which showed a preferred ADE model in unadjusted analyses, had the highest H2 estimate of 0.433, and only a small estimated proportion of variable explained by common environment (C2 = 0.032).
Table 4.
Heritability estimatesa of heart rate response to arousal measures in twins.
| Measure | Unadjusted | Adjustedb | ||||||
|---|---|---|---|---|---|---|---|---|
| ICCMZc | ICCDZc | H2d | C2e | ICCMZc | ICCDZc | H2d | C2e | |
| Arousal Index | 0.628 | 0.438 | 0.380 | 0.248 | 0.594 | 0.443 | 0.300 | 0.293 |
| Average arousal duration | 0.473 | 0.204 | 0.538 | −0.065 | 0.465 | 0.249 | 0.433 | 0.032 |
| Average arousal intensity scale | 0.689 | 0.202 | 0.972 | −0.284 | 0.663 | 0.146 | 1.035 | −0.372 |
| Slope of ΔHR per arousal intensity | 0.456 | 0.562 | −0.211 | 0.667 | 0.306 | 0.237 | 0.139 | 0.167 |
| Arousal intensity threshold of ΔHR | 0.533 | 0.000 | 1.065 | −0.533 | 0.449 | 0.000 | 0.899 | −0.449 |
| Average ΔHR | 0.660 | 0.557 | 0.208 | 0.453 | 0.548 | 0.240 | 0.616 | −0.068 |
| ΔHR at arousal intensity scale 4 | 0.621 | 0.547 | 0.148 | 0.472 | 0.529 | 0.342 | 0.372 | 0.156 |
| ΔHR at arousal intensity scale 5 | 0.600 | 0.636 | −0.071 | 0.671 | 0.489 | 0.388 | 0.203 | 0.286 |
| ΔHR at arousal intensity scale 8 | 0.541 | 0.672 | −0.262 | 0.803 | 0.399 | 0.376 | 0.047 | 0.352 |
Abbreviations: ICC, Intraclass Correlation Coefficient; H2, classical broad-sense heritability; C2, estimated proportion of phenotypic variability explained by common environmental factors; PGM, preferred genetic model; ΔHR, heart rate response to arousal.
aNegative values of H2 or common environment (C2) suggest genetic or environment effects are likely not important, respectively, while values greater than 1 suggest strong genetic or environmental effects.
bAdjusted for race, age, sex, and age by sex interaction.
cICC: Intraclass Correlation Coefficient, equal to σ2B / (σ2W + σ2B).
dH2: classical, broad-sense heritability estimate, equal to 2(ICCMZ – ICCDZ).
eC2: Estimated proportion of phenotypic variability explained by common environmental factors, equal to 2(ICCDZ) – ICCMZ. C2 estimates < 0 suggest that an ADE inheritance model may be more appropriate and C2>0 imply an ACE model may better represent the inheritance pattern, where A = additive genetic effects, C = common environmental effects, D = dominant genetic effects, and E = unique individual effects. Thus, ACE models additive genetic, common environment and individual effects, whereas ADE models additive and dominant genetics and individual effects.
ANOVA-Based Heritability Estimates
As a complementary method for estimating unadjusted, broad-sense heritability for each trait, we subjected the arousal and ΔHR measures to heritability analysis using the ANOVA approach.29 As stated earlier, there were no significant differences in mean values between MZ and DZ twins for any of the measures (Table 2). Similarly, we did not observe any differences in the variability of within-twin means between MZ and DZ twins (Table 5). An essential assumption when performing the ANOVA-based heritability analysis is that total phenotype variance is similar for MZ and DZ twins. There was no evidence of statistically significant zygosity differences in total phenotype variance for any of the traits. These results suggest the assumptions of the twin model are valid in the ANOVA context. Suggestive evidence (p < .20) for a difference in total variability was observed for the slope of ΔHR per arousal intensity (p = .065), average ΔHR (p = .074), and ΔHR at arousal intensity scale 8 (p = 0.117; Table 5). Thus, for these measures, the among component heritability estimate from the ANOVA was used, while the within pair ANOVA estimate was used for all other traits. Based on this analysis, both the average arousal intensity scale (H2 = 1.13, p < .001) and arousal intensity threshold of ΔHR (H2 = 1.24, p = .002) showed significant evidence of heritability. Although theoretically the actual value of H2 should be bounded by 1, these results generally support the findings of large genetic contributions to phenotypic variability from the classical approach.
Table 5.
ANOVA-based heritability estimates of heart rate response to arousal measures in MZ and DZ twinsa.
| Measures | Within pair average value | Total variance | Heritability estimate | |||
|---|---|---|---|---|---|---|
| F-testb | T-testc | F-testb | ||||
| p | p | p | H2 | pd | Type | |
| Arousal index | .530 | .519 | .730 | 0.307 | .129 | WP |
| Average arousal duration | .416 | .395 | .798 | 0.460 | .117 | WP |
| Average arousal intensity scale | .535 | .333 | .507 | 1.127 | <.001 | WP |
| Slope of ΔHR per arousal intensity | .124 | .074 | .065 | −0.605 | .898 | AmCo |
| Arousal intensity threshold of ΔHR | .170 | .702 | .831 | 1.237 | .002 | WP |
| Average ΔHR | .293 | .066 | .074 | −0.248 | .695 | AmCo |
| ΔHR at arousal intensity scale 4 | .821 | .090 | .600 | 0.240 | .165 | WP |
| ΔHR at arousal intensity scale 5 | .447 | .063 | .334 | 0.085 | .348 | WP |
| ΔHR at arousal intensity scale 8 | .168 | .051 | .117 | −0.662 | .911 | AmCo |
aHeritability estimates using analysis of variance (ANOVA)-based variance formulas for within pairs (WP) or among component (AmCo) heritability. The WP estimate is most valid when the total variance F-test has a p>.20, and calculated as H2 = [2 • (MSWDZ - MSWMZ)] / [(MSAMZ + MSWDZ + MSADZ + MSWMZ)/4]. The AmCo estimate is valid when the total variance F-test has a p<.20, and calculated H2 = [(MSAMZ - MSADZ) + (MSWDZ - MSWMZ)] / [(MSAMZ + MSWDZ + MSADZ + MSWMZ)/4]28,29.
bF-test p-value comparing variability in indicated measure between MZ and DZ twins.
cT-test p-value comparing within pair average values in indicated measure between MZ and DZ twins.
done-sided p-values based on F-tests of significance for heritability estimate, using F-ratio = MSWDZ / MSWMZ for WP heritability estimates and an F-ratio = (MSAMZ + MSWDZ) / (MSADZ + MSWMZ) for AmCo heritability estimates. Abbreviations: H2: broad-sense heritability, ΔHR: heart rate response to arousal, WP: within pairs heritability estimate, AmCo: among component heritability estimate.
MLE Heritability Models
The arousal and ΔHR measures were used to estimate parameters of alternative genetic transmission models based on MLE techniques (Table 6). As discussed in the Methods, the MLE approach was considered primary and, while resulting heritability estimates varied, this approach clarified the findings from the classical and ANOVA-based methods. The best-fitting model was determined through goodness-of-fit tests, with the initial model chosen based on the estimated h2C effect in the ACE model; an h2C > 0 implies a common environment effect and thus the ACE model, whereas an h2C ≤ 0 implies no common environmental effect and an ADE model. This model-based approach suggested significant additive and dominance genetic effects (ADE model) for the arousal intensity threshold of ΔHR [h2G (95% CI) = 0.46 (0.25 to 0.68), p < .0001]. In addition, significant additive genetic effects (AE model) were observed for average arousal intensity scale [h2G (95% CI) = 0.64 (0.48 to 0.80), p < .0001], average arousal duration [h2G (95% CI) = 0.44 (0.24 to 0.64), p < .0001], average ΔHR [h2G (95% CI) = 0.57 (0.39 to 0.74), p < .0001], and ΔHR4 [h2G (95% CI) = 0.51 (0.34 to 0.69), p < .0001]. Thus, phenotypic variability for each of these traits appears to be clearly influenced by underlying genetic factors. For the remaining measures (arousal index, slope of ΔHR per arousal intensity, ΔHR5, and ΔHR8), there was no evidence for a significant genetic contribution to phenotypic variance in adjusted models, but there was evidence that common environment explained between 32% and 54% of phenotypic variability, depending on the trait.
Table 6.
Maximum likelihood model-based heritability estimates of heart rate response to arousal measures in twinsa.
| Measure | MLE estimates of proportion of variability explained for Genetic (G), Common Environment (C) and Individual (E) factors within best fitting modelb | |||
|---|---|---|---|---|
| Unadjusted | ||||
| Model | h2G (95% CI)c | h2C (95% CI) | h2E (95% CI) | |
| Arousal index | AE | 0.625 (0.482, 0.768) | – | 0.375 (0.232, 0.518) |
| Average arousal duration | AE | 0.457 (0.261, 0.652) | – | 0.543 (0.348, 0.739) |
| Average arousal intensity scale | AE | 0.692 (0.554, 0.830) | – | 0.308 (0.170, 0.446) |
| Slope of ΔHR per arousal intensity | CE | – | 0.513 (0.359, 0.666) | 0.487 (0.334, 0.641) |
| Arousal intensity threshold of ΔHR | ADE | 0.533 (0.349, 0.717) | – | 0.467 (0.283, 0.651) |
| Average ΔHR | AE | 0.723 (0.608, 0.838) | – | 0.277 (0.162, 0.392) |
| ΔHR at arousal intensity scale 4 | CE | – | 0.593 (0.458, 0.728) | 0.407 (0.272, 0.542) |
| ΔHR at arousal intensity scale 5 | CE | – | 0.621 (0.493, 0.749) | 0.379 (0.251, 0.507) |
| ΔHR at arousal intensity scale 8 | CE | – | 0.609 (0.479, 0.740) | 0.391 (0.260, 0.521) |
| Measure | Adjusted† | |||
| Model | h2G (95% CI)c | h2C (95% CI) | h2E (95% CI) | |
| Arousal Index | CE | – | 0.543 (0.396, 0.690) | 0.457 (0.310, 0.604) |
| Average arousal duration | AE | 0.437 (0.236, 0.639) | – | 0.563 (0.361, 0.764) |
| Average arousal intensity scale | AE | 0.641 (0.479, 0.803) | – | 0.359 (0.197, 0.521) |
| Slope of ΔHR per arousal intensity | CE | – | 0.318 (0.131, 0.505) | 0.682 (0.495, 0.869) |
| Arousal intensity threshold of ΔHR | ADE | 0.464 (0.252, 0.677) | – | 0.536 (0.323, 0.748) |
| Average ΔHR | AE | 0.565 (0.390, 0.739) | – | 0.435 (0.261, 0.610) |
| ΔHR at arousal intensity scale 4 | AE | 0.514 (0.336, 0.692) | – | 0.486 (0.308, 0.664) |
| ΔHR at arousal intensity scale 5 | CE | – | 0.445 (0.278, 0.612) | 0.555 (0.399, 0.722) |
| ΔHR at arousal intensity scale 8 | CE | – | 0.412 (0.239, 0.585) | 0.588 (0.415, 0.761) |
Abbreviations: h2G = proportion of phenotypic variability explained by genetic factors; h2C = proportion of phenotypic variability explained by shared environment; h2E = proportion of phenotypic variability explained by individual factors; ΔHR: heart rate response to arousal.
aModel adjusted for race, age, sex, and age × sex interaction.
b“Best Fitting Model” determined through goodness of fit tests, utilizing likelihood ratio tests for nested models (i.e., ACE vs. AE, CE or E and ADE vs. AE or E) and AIC statistics for non-nested models (e.g., CE vs. AE), when both models indicated better fit than the full model. The starting model (ACE or ADE) was chosen based on the estimated common environment effect in the ACE model heritability analyses, such that h2C ≤ 0 implies an ADE model and h2C > 0 implies an ACE model.
c h2G represents to proportion of phenotypic variability explained by genetic factors, based on the best-fitting model. If AE or ACE are chosen as the best fitting model, h2G is equal to the heritability due to additive genetic effects only, whereas h2G is equal to the combination of the additive and dominant genetic effects when ADE is chosen as the best model; Model Descriptions: A = additive genetic effects, C = common environmental effects, D = dominant genetic effects, and E = unique individual effects. Thus, ACE model suggests additive genetic, common environment and individual effects, whereas ADE model suggests additive and dominant genetics and individual effects.
CONCLUSION / DISCUSSION
This study in twins is the first to show that underlying genetic factors significantly influence measures of average arousal intensity and heart rate response to arousal. Utilizing multiple, complementary techniques for estimating trait heritability, we show robust evidence that the average arousal intensity and the arousal intensity threshold for ΔHR are moderately to highly heritable traits. In addition, evidence supports a heritable component for certain arousal characteristics, including the duration of arousals, the overall average ΔHR, and the specific ΔHR at arousal intensity of 4. These results add further evidence that genetic factors explain a significant fraction of the phenotypic variability of many sleep characteristics. Furthermore, our results in adults without sleep disorders on PSG add important evidence to the growing literature that suggests that the variable clinical responses in individuals with sleep disorders may be related to heritable traits. Individuals with a higher average arousal intensity may be more likely to develop daytime sleepiness and cognitive complications related to arousal and a greater HR response to a given arousal intensity may indicate a more labile cardiovascular system that is more susceptible to the development of hypertension.
Of the different ΔHR measures utilized in this study, the arousal intensity threshold for ΔHR and the average arousal intensity showed significant heritability across all 3 complementary methods. In classical analyses, MZ twins showed within-pair concordance in arousal intensity threshold for ΔHR that was significantly higher than that of DZ twin pairs, resulting in a large adjusted H2 estimate of 0.90 (Table 4). Similarly, average arousal intensity showed strong heritability in classical analyses, with an adjusted H2 = 1.04. These results were confirmed using an ANOVA approach (Table 5), in which the arousal intensity threshold of ΔHR and average arousal intensity were the only measures with statistically significant heritability estimates. It should be noted that H2 values above 1.0 should not arise in the case of simple additive genetic transmission and imply that there might be important genetic dominance effects (i.e., an ADE model). Supporting this evidence, the covariate adjusted MLE of specific genetic transmission models (Table 6) found that an additive and dominance genetic (ADE) model was the most appropriate inheritance mode for arousal intensity threshold of ΔHR. Statistical evidence only supported the additive genetic effects (AE) model for average arousal intensity scale; however, the p value selecting the AE model was trending toward the ADE model (p = .095) which may have been chosen in a larger sample. Model estimates were reduced in these methods, compared to the more classical techniques, with a genetic heritability of 0.46 (95% CI: 0.25 to 0.68) for arousal intensity threshold of ΔHR in the ADE model and a heritability of 0.64 (0.48 to 0.80) for average intensity scale in the AE model.
Importantly, the choice of three complementary approaches to heritability facilitates comparisons with earlier literature. In addition, because these methods differ with regard to their assumptions, differences among results reveal possible violations of assumptions, enabling a refinement of models used to obtain estimates. The classical approach’s major advantage is its simplicity in computation. The classical approach is complemented by the ANOVA approach because the ANOVA approach includes assessments of the validity of the twin model as applied to the data at hand. Both of these techniques estimate broad-sense heritability, that is, the proportion of phenotypic variability explained by additive, dominant, and epistatic genetic effects. The MLE approach allows the specification of various genetic transmission models (including narrow-sense heritability [additive only] or additive plus dominance genetic effects) and permits direct computations of confidence intervals and associated p values. Moreover, MLE techniques allow determination of the best inheritance modes using standard likelihood ratio tests. Regardless of differences in the magnitudes of heritability estimates, all three models resulted in the same conclusion for the arousal intensity threshold for ΔHR and the average arousal intensity, that is, that heritability of these traits is high.
While no other measures were found to be heritable across all methods (primarily due to lack of statistical significance in ANOVA-based techniques), several traits had evidence for an additive genetic transmission model in MLE methods. The average ΔHR (h2G = 57%) and ΔHR at intensity scale 4 (h2G = 51%) had AE model-based heritability estimates that were larger than the estimated ADE model-based heritability for arousal intensity threshold of ΔHR. In addition, the average arousal duration showed moderate heritability due to additive genetic effects (h2G = 44%). While results for all measures should be replicated in future studies, including using variance components and other techniques designed for nontwin studies with genome-wide data,34,35 these MLEs provide strong evidence that multiple arousal and heart rate response-related traits are influenced by genetic factors.
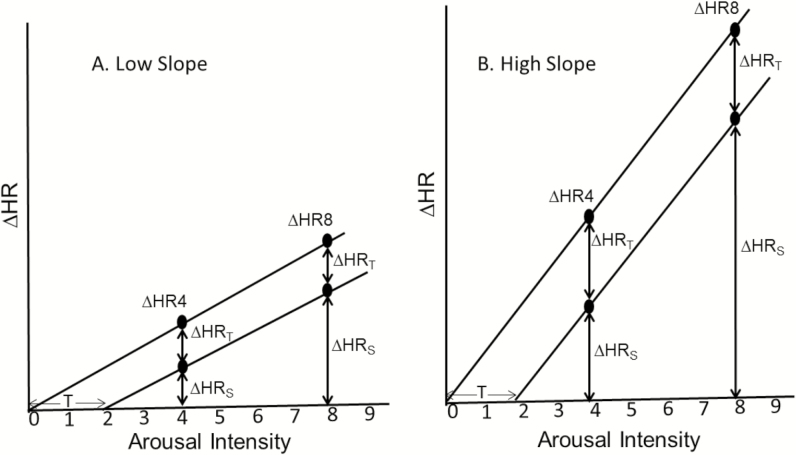
Our previous study found that the HR response to a given arousal intensity is highly reproducible within a subject.23 The current study provides mixed evidence regarding whether these measures are genetically determined. Interestingly, while there was no evidence for a genetic influence on the ΔHR5 or ΔHR8, we did observe heritability for the ΔHR4 (in adjusted models only) and for the average ΔHR across all arousal intensities. These findings can be explained if one considers what determines the HR response to a given arousal intensity. Specifically, ΔHRx = (x-T)*Slope, where x is the arousal intensity and T and Slope are the X intercept and slope of the relation, respectively (Figure 3). The current results show that the threshold (T; minimum arousal intensity at which there is a HR response) is heritable, while the slope of the HR response to arousal intensity beyond this threshold (Slope) is mostly determined by nongenetic factors. Thus, ΔHRx is a function of both heritable and nonheritable components. As shown in Figure 3, the contribution of the genetically determined threshold (T) to ΔHRx (ΔHRT) is constant across all arousal intensities, whereas the contribution of nongenetically determined slope (ΔHRS) increases progressively as arousal intensity increases. Therefore, ΔHRx (ΔHRT + ΔHRS) becomes progressively less affected by the heritable threshold trait and more affected by the nonheritable slope at more intense arousals, resulting in a lower estimate of the proportion of variability in ΔHRx explained by genetic factors at higher values of x.
Figure 3.
Schematic representation of the impact of slope and intercept on ΔHR at specific arousal intensities. (A) A shift in intercept when slope is relatively low. (B) the same shift in intercept when slope is high. ΔHRT, contribution of shift in intercept to ΔHR; ΔHRS, contribution of slope to ΔHR. Note that the contribution of threshold shift (T) to ΔHR (i.e. ΔHRT + ΔHRS) progressively decreases as arousal intensity increases regardless of slope.
It is well established that sleep fragmentation, as judged by arousal frequency, has independent and widespread consequences on cognitive function, daytime sleepiness, endocrine function, ventilatory responses, and mood (see Bonnet et al for review6). Martin et al.36,37 have also found that auditory-induced subthreshold arousals (i.e., where EEG changes do not meet the accepted criteria) also impair next day function although not to the same extent as threshold arousals. Thus, it appears that the clinical consequences of frequent arousals are influenced by the intensity/duration of the EEG changes. Although this needs to be confirmed experimentally, it is reasonable to suggest that, all else being the same, people who are prone to develop more intense arousals are at greater risk of developing complications from sleep fragmentation.
This study was performed in adults without sleep disorders on PSG, and it is not known whether the heritable traits identified in this population are generalizable to individuals with sleep disorders such as OSA. Average arousal intensity in patients with OSA varies widely (3.4–5.9).22 This wide range cannot be explained by differences in event severity, since an even wider range of arousal intensities exists in individuals without sleep disorder.23 It is also a common clinical experience that some patients with OSA having very high arousal frequency have little or no symptoms of excessive somnolence and vice versa.38,39 Although a correlation exists between the arousal index and objective tests of sleepiness in patients with OSA, the correlation is quite weak (r = 0.23); in patients with an arousal index of 40 events/hour, mean sleep onset latency on the multiple sleep latency test ranged from 1 to 16 minutes.40 It is possible that this wide range of sleepiness at the same arousal index may be in part related to the differences in arousal intensity among these patients. Since the current study shows that genetic factors explain a significant fraction of the phenotypic variability in average arousal intensity, it may be that the tendency for some people to be more adversely affected by sleep fragmentation is an inherited trait that determines arousal intensity. Further studies are needed to determine if average arousal intensity influences the change in daytime function as a result of sleep fragmentation.
The average arousal-related increase in HR during sleep is a mathematical function of average arousal intensity, the arousal intensity threshold of ∆HR (X intercept), and the slope of the relation. The current study indicates that average arousal intensity and the arousal intensity threshold of ∆HR are strongly influenced by genetic factors. There is mounting evidence that arousals are an independent risk factor for the development of hypertension.41,42 To the extent that a larger HR response to arousals reflects greater arousal-related autonomic activation, which may in turn increase the risk of hypertension, our results suggest that such risk may be largely determined by genetic factors. Overall, the heritable traits described here represent promising sleep-related phenotypes for future genetic studies.
FUNDING
This study was supported by NIH P50 HL060287, P01 HL094307 and UL1RR024134 from the National Center for Research Resources.
DISCLOSURE STATEMENT
STK receives grant support from Philips-Respironics; AIP is The John L. Miclot Professor of Medicine at the University of Pennsylvania. Funds for this endowment were provided by the Philips Respironics Foundation. AA was an employee of YRT Ltd. MY is the owner of YRT Ltd, a R&D company in Winnipeg, Canada, that develops devices for the diagnosis of sleep disorders, including the technology of arousal scaling.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The Penn Twins Cohort and the MidAtlantic Twins Registry assisted in recruitment of the twin pairs. The contributions of Melissa Fernando, Michele Pliner, Marian Whitlock, and Colleen Crowley to the study are gratefully acknowledged and to Daniel Barrett for assistance in manuscript preparation.
REFERENCES
- 1. Horner RL. Autonomic consequences of arousal from sleep: mechanisms and implications. Sleep. 1996; 19(10 Suppl): S193–S195. [DOI] [PubMed] [Google Scholar]
- 2. Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol (1985). 1995; 79(1): 151–162. [DOI] [PubMed] [Google Scholar]
- 3. Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000; 111(9): 1611–1619. [DOI] [PubMed] [Google Scholar]
- 4. Trinder J, Allen N, Kleiman J, et al. On the nature of cardiovascular activation at an arousal from sleep. Sleep. 2003; 26(5): 543–551. [PubMed] [Google Scholar]
- 5. Trinder J, Padula M, Berlowitz D, et al. Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. J Appl Physiol (1985). 2001; 90(4): 1455–1463. [DOI] [PubMed] [Google Scholar]
- 6. Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med. 2007; 3(2): 133–145. [PubMed] [Google Scholar]
- 7. Colt HG, Haas H, Rich GB. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991; 100(6): 1542–1548. [DOI] [PubMed] [Google Scholar]
- 8. Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep. 2000; 23(Suppl 4: S102–S108. [PubMed] [Google Scholar]
- 9. Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010; 185: 91–103. [DOI] [PubMed] [Google Scholar]
- 10. Davies RJ, Crosby J, Prothero A, Stradling JR. Ambulatory blood pressure and left ventricular hypertrophy in subjects with untreated obstructive sleep apnoea and snoring, compared with matched control subjects, and their response to treatment. Clin Sci (Lond). 1994; 86(4): 417–424. [DOI] [PubMed] [Google Scholar]
- 11. Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000; 283(14): 1829–1836. [DOI] [PubMed] [Google Scholar]
- 12. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342(19): 1378–1384. [DOI] [PubMed] [Google Scholar]
- 13. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992; 15(2): 173–184. [PubMed] [Google Scholar]
- 14. Berry RB, Brooks R, Gemaldo CE, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.0. Darian, IL: American Academy of Sleep Medicine; 2012 [Google Scholar]
- 15. Katz ES, Greene MG, Carson KA, et al. Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr. 2002; 140(5): 589–594. [DOI] [PubMed] [Google Scholar]
- 16. Loredo JS, Clausen JL, Ancoli-Israel S, Dimsdale JE. Night-to-night arousal variability and interscorer reliability of arousal measurements. Sleep. 1999; 22(7): 916–920. [DOI] [PubMed] [Google Scholar]
- 17. Milross MA, Piper AJ, Norman M, et al. Night-to-night variability in sleep in cystic fibrosis. Sleep Med. 2002; 3(3): 213–219. [DOI] [PubMed] [Google Scholar]
- 18. Scholle S, Scholle HC, Kemper A, et al. First night effect in children and adolescents undergoing polysomnography for sleep-disordered breathing. Clin Neurophysiol. 2003; 114(11): 2138–2145. [DOI] [PubMed] [Google Scholar]
- 19. Sforza E, Chapotot F, Pigeau R, Buguet A. Time of night and first night effects on arousal response in healthy adults. Clin Neurophysiol. 2008; 119(7): 1590–1599. [DOI] [PubMed] [Google Scholar]
- 20. Daubechies I. Ten lectures on wavelets. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1992 [Google Scholar]
- 21. Grossmann A, Morlet J. Decomposition of Hardy functions into square integrable wavelets of constant shape. SIAM J Math Anal. 1984; 15(4): 723–736. [Google Scholar]
- 22. Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014; 37(4): 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Azarbarzin A, Ostrowski M, Younes M, et al. Arousal responses during overnight polysomnography and their reproducibility in healthy young adults. Sleep. 2015; 38(8): 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuna ST, Maislin G, Pack FM, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012; 35(9): 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becker A, Busjahn A, Faulhaber HD, et al. Twin zygosity. Automated determination with microsatellites. J Reprod Med. 1997; 42(5): 260–266. [PubMed] [Google Scholar]
- 26. Malhotra A, Younes M, Kuna ST, et al. Performance of an automated polysomnography scoring system versus computer-assisted manual scoring. Sleep. 2013; 36(4): 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falconer DS, Mackay TFC. Introduction to quantitive genetics. 4th ed Harlow, United Kingdom: Addison Wesley Longman; 1996 [Google Scholar]
- 28. Christian JC, Borhani NO, Castelli WP, et al. Plasma cholesterol variation in the National Heart, Lung and Blood Institute Twin Study. Genet Epidemiol. 1987; 4(6): 433–446. [DOI] [PubMed] [Google Scholar]
- 29. Christian JC, Kang KW, Norton JJ., Jr Choice of an estimate of genetic variance from twin data. Am J Hum Genet. 1974; 26(2): 154–161. [PMC free article] [PubMed] [Google Scholar]
- 30. Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet. 2006; 36(2): 331–340. [DOI] [PubMed] [Google Scholar]
- 31. Feng R, Zhou G, Zhang M, Zhang H. Analysis of twin data using SAS. Biometrics. 2009; 65(2): 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McArdle JJ, Prescott CA. Mixed-effects variance components models for biometric family analyses. Behav Genet. 2005; 35(5): 631–652. [DOI] [PubMed] [Google Scholar]
- 33. Rabe-Hesketh S, Skrondal A, Gjessing HK. Biometrical modeling of twin and family data using standard mixed model software. Biometrics. 2008; 64(1): 280–288. [DOI] [PubMed] [Google Scholar]
- 34. Visscher PM, Yang J, Goddard ME. A commentary on ‘common SNPs explain a large proportion of the heritability for human height’ by Yang et al. (2010). Twin Res Hum Genet. 2010; 13(6): 517–524. [DOI] [PubMed] [Google Scholar]
- 35. Yang J, Benyamin B, McEvoy BP, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010; 42(7): 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin SE, Engleman HM, Deary IJ, Douglas NJ. The effect of sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1996; 153(4 Pt 1): 1328–1332. [DOI] [PubMed] [Google Scholar]
- 37. Martin SE, Engleman HM, Kingshott RN, Douglas NJ. Microarousals in patients with sleep apnoea/hypopnoea syndrome. J Sleep Res. 1997; 6(4): 276–280. [DOI] [PubMed] [Google Scholar]
- 38. Lacedonia D, Carpagnano GE, Sabato R, et al. Characterization of obstructive sleep apnea-hypopnea syndrome (OSA) population by means of cluster analysis. J Sleep Res. 2016; 25(6): 724–730. [DOI] [PubMed] [Google Scholar]
- 39. Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014; 44(6): 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997; 155(5): 1596–1601. [DOI] [PubMed] [Google Scholar]
- 41. Lofaso F, Coste A, Gilain L, Harf A, Guilleminault C, Goldenberg F. Sleep fragmentation as a risk factor for hypertension in middle-aged nonapneic snorers. Chest. 1996; 109(4): 896–900. [DOI] [PubMed] [Google Scholar]
- 42. Noda A, Yasuma F, Okada T, Yokota M. Influence of movement arousal on circadian rhythm of blood pressure in obstructive sleep apnea syndrome. J Hypertens. 2000; 18(5): 539–544. [DOI] [PubMed] [Google Scholar]