Abstract
The impact of aging on proprioceptive sensory neurons and intrafusal muscle fibers (IMFs) remains largely unexplored despite the central function these cells play in modulating voluntary movements. Here, we show that proprioceptive sensory neurons undergo deleterious morphological changes in middle age (11- to 13-month-old) and old (15- to 21-month-old) mice. In the extensor digitorum longus and soleus muscles of middle age and old mice, there is a significant increase in the number of Ia afferents with large swellings that fail to properly wrap around IMFs compared with young adult (2- to 4-month-old) mice. Fewer II afferents were also found in the same muscles of middle age and old mice. Although these age-related changes in peripheral nerve endings were accompanied by degeneration of proprioceptive sensory neuron cell bodies in dorsal root ganglia (DRG), the morphology and number of IMFs remained unchanged. Our analysis also revealed normal levels of neurotrophin 3 (NT3) but dysregulated expression of the tyrosine kinase receptor C (TrkC) in aged muscles and DRGs, respectively. These results show that proprioceptive sensory neurons degenerate prior to atrophy of IMFs during aging, and in the presence of the NT3/TrkC signaling axis.
Keywords: Ia/II afferents, Dorsal root ganglia, Muscle spindle, Motor function, Degeneration
Coordinated movements, such as gait, fine voluntary movements, posture, and balance become increasingly more difficult to initiate and properly carry out with aging (1,2). These motor deficits affect the quality of life of elder individuals and increase the incidence of falls (3–5). Motor deficits also contribute to adverse health effects caused by pathophysiological changes associated with aging (6–9). Thus, it is critical to understand when and how specific components of the somatic motor system are impacted during aging in order to slow the erosion of these motor skills. To date, we know that aging results in significant molecular, morphological, and functional changes in α-motor neurons (10–14), extrafusal muscle fibers (15–21), and their neuromuscular junctions (NMJs) (22–28). Although α-motor neurons and extrafusal muscle fibers carry out all voluntary movements, their activation and function are under the control of neurons located throughout the nervous system. Among these, proprioceptive sensory neurons play a critical role in coordinating complex movements by relaying information about the activity of skeletal muscles to α-motor neurons (29,30).
Proprioceptive sensory neurons are located in dorsal root ganglia (DRG) and bifurcate to innervate skeletal muscles and neurons in the central nervous system. Type Ia/II proprioceptive sensory neurons innervate intrafusal muscle fibers (IMFs) to form a complex, referred to as a muscle spindle, that is sensitive to the speed and extent of muscle contraction (30). The centrally projecting branches of Ia/II afferents then relay this information to α-motor neurons to adjust the contraction or relaxation of muscles, a prerequisite for coordinating complex movements. In recent years, we have learned that diseases and injuries cause deleterious structural changes at nerve endings of proprioceptive sensory neurons (31–35). In animals harboring mutant genes that cause amyotrophic lateral sclerosis (ALS) or spinal muscular atrophy, proprioceptive nerve endings in muscle spindles and in the spinal cord degenerate prior to the onset of neurological symptoms (31,32,36). Following severing of peripheral afferents, a permanent reorganization of proprioceptive nerve endings terminating on motor neurons occurs, which has been proposed to contribute to long-lasting motor dysfunction (35,37). Despite these findings and the fact that motor function declines with increasing age, very little is known regarding the impact of aging on proprioceptive sensory neurons (38,39). Likewise, the effect of aging on IMFs is still poorly understood (38–40).
In this paper, we examined proprioceptive sensory neurons and IMFs in aging mice. We demonstrate that type Ia afferents undergo deleterious structural changes in muscle spindles of the extensor digitorum longus (EDL) and soleus muscles in middle age (11- to 13-month-old) and old (15- to 21-month-old) mice compared with young mice (2- to 4-month-old). In older mice, we also found fewer II afferents in muscle spindles and a significant reduction in the number of proprioceptive sensory neuron cell bodies in L3 DRGs. Despite these changes in proprioceptive sensory neurons, IMFs appeared largely spared in middle age and old mice. Additionally, we show that neurotrophin 3 (NT3) and tyrosine kinase receptor C (TrkC) remain expressed at high levels in muscles and DRGs of old mice, indicating that other molecular changes likely contribute to degeneration of these neurons with increasing age.
Materials and Methods
Source of Mice
The following mice were obtained from Jackson laboratories: parvalbumin-Cre (41) (referred to here as PVCre) and Thy1-STOP-YFP line 15 (42) (referred to here as STP-YFP). Thy1-YFP16 (referred to here as YFP (43) mice were a generous gift from Dr. Joshua Sanes. To visualize proprioceptive sensory neurons in the DRG, we mated PVCre and STP-YFP (referred to as PVCre;STP-YFP). Mice were anesthetized using isoflurane and then perfused transcardially with 10mL of 0.1M phosphate-buffered saline (PBS), followed by 25mL of 4% paraformaldehyde (PFA) in 0.1M PBS (pH 7.4). Only male mice were analyzed in this study. All experiments were carried out under NIH guidelines, and animal protocols were approved by the Virginia Tech Institutional Animal Care and Use Committee.
Analysis of Ia/II Afferents and NMJs
Animals expressing YFP (Thy1-YFP) in all neurons were used to visualize nerve endings in skeletal muscles. Whole EDL and soleus muscles were used to analyze sensory nerve endings. The EDL separates into four smaller muscles that attach to the four smaller toes. The three divisions of the EDL that attach to toes 2–4 were used for anatomical analysis of nerve endings. These divisions of the EDL muscle can be readily whole mounted and all afferents can be imaged at high resolution using confocal microscopy. To visualize the NMJ postsynaptic site, muscles were incubated with alexa-555-tagged α-bungarotoxin (Life Technologies, B35451; 1:1,000) in 0.1M PBS for 1 hour at room temperature to label nicotinic acetylcholine receptors (nAChRs). Following incubation, muscles were washed three times with 0.1M PBS and whole mounted using Vectashield (Vector Laboratories, H-1000). To analyze proprioceptive nerve endings and NMJs, maximum intensity projections from confocal z-stacks were created with Zen Black (Zeiss). The following structural features of proprioceptive sensory neurons were compared between age groups in ImageJ software: (a) distance between spirals; the distance in micrometers was measured between each annulospiral wrapping around the belly of each muscle spindle, (b) the width of the nerve; the width of each spiral (coil) wrapping around the belly of each muscle spindle was measured in micrometers, (c) unraveling; less than 10 spirals wrapping around IMFs at the equatorial region, or significantly disorganized annulospirals, and (d) blebbing; large aggregates of YFP near or at afferent nerve terminals. NMJs were analyzed based on innervation from motor neurons, noted by colocalization between YFP and α-bungarotoxin. In all experiments, at least three animals of the same age and gender were examined per age group. At least five nerve endings per muscle were examined. At least 50 NMJs were examined per animal.
Analysis of Intrafusal and Extrafusal Muscle Fibers
Fresh frozen tibialis anterior (TA) muscles were cut in half at the largest diameter and placed in 10mm × 10mm × 5mm Tissue-Tek Cryomolds with Tissue Freezing Medium (Triangle Biomedical Science, Inc.). With a cryostat, 16-μm sections were collected on gelatin-coated slides. After washing three times with 0.1M PBS, the sections were incubated with blocking buffer solution containing 0.1% Triton X-100, 3% bovine serum albumin (BSA), and 5% goat serum diluted in 0.1M PBS for 1 hour. Sections were incubated with primary antibody overnight at 4°C and then washed three times with 0.1M PBS before adding secondary antibody for 2 hours at room temperature. Following secondary antibody incubation, sections were washed three times with 0.1M PBS, incubated with DAPI (4′,6-diamidino-2-phenylindole: Sigma-Aldrich, 28718-90-3; 1:1,000) for 5 minutes and mounted with Vectashield. The following primary antibody was diluted in blocking buffer to label IMFs: myosin heavy chain S46 (DSHB S46; deposited by Dr. Frank E. Stockdale; 1:50). The following primary antibody was diluted in blocking buffer to label extrafusal muscle fibers: laminin (Sigma-Aldrich, L9393; 1:100). We used the following secondary antibodies from Life Technologies diluted 1:1,000 in blocking buffer: Alexa-568 goat anti-mouse IgG1 and Alexa-488 donkey anti-rabbit. Muscle fibers were visualized with Zeiss LSM 700 using maximum intensity projections from confocal z-stacks created with Zen Black (Zeiss). The area of each fiber was analyzed using ImageJ. All IMFs in each section were analyzed. For extrafusal muscle fibers, at least 30 randomly selected fibers were analyzed in each section.
Analysis of Proprioceptive Sensory Axons in the EDL Muscle of Young and Old Mice
Proprioceptive sensory axons were analyzed in whole-mounted EDL muscles following the method used by Vaughan and colleagues (31). Briefly, transgenic mice expressing YFP in all neurons were used to visualize axonal bundles innervating the EDL muscle from 2-, 11-, and 15-month-old mice. We counted sensory axons innervating the second outermost portion of the EDL muscle, which innervates the second toe. Nerve branches primarily composed of proprioceptive sensory axons were identified by tracing axons back from muscle spindles. To examine sensory axons, high resolution confocal images of sensory axons were obtained with a 40× objective and used to generate 2D and 3D images from a z-stack scan. With the default zeiss image file, we used the cut function in Zen Black 2012 image processing (Zeiss) to digitally slice through the 3D plane of the axon bundle and count the number of axons in each bundle.
Analysis of Proprioceptive Sensory Neuron Somata in DRG Located in Lumbar Region 3
Following perfusion, spinal columns were immediately dissected and postfixed in 4% PFA overnight at 4°C. The spinal column was then washed three times with 0.1M PBS and cut at the last rib to separate cervical and thoracic regions from lumbar and sacral regions. The DRG from the lumbar region 3 (L3) was dissected from PVCre;STP-YFP and whole mounted with Vectashield. To visualize whole DRGs and examine the sensory soma, high resolution images were tiled with a Zeiss LSM 700 motorized confocal microscope. The total number of parvalbumin-positive neurons was quantified in ImageJ.
Expression Analysis Using Quantitative PCR
Mice were anesthetized with isoflurane, and the TA muscle and DRGs were immediately removed. Total RNA was extracted using Aurum Total RNA Mini kit (Bio-rad) following manufacturer’s instructions. cDNA was synthesized from 500ng of total RNA using iScript cDNA synthesis kit (Bio-Rad). PCR amplification was performed on the Bio-Rad CFX connect Real-Time System (Bio-Rad) using iTaq Universal SYBR Green Supermix (Bio-Rad). Analysis was done in Bio-Rad CFX Manager 3.1 software with normalized expression (ΔΔCq) relative to control (4 months). Primers used are presented in Table 1.
Table 1.
qPCR Primers
| Primer | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| NT3 | GCCACGGAGATAAGCAAGAA | ACGGATGCCATGGTTACTTC |
| TrkC | GCTCCCTCACCCAATTCTCT | CCAGTACTTCCGTCAGGGTC |
| GAPDH | CCCACTCTTCCACCTTCGATG | GTCCACCACCCTGTTGCTGTAG |
Notes: GAPDH = glyceraldehyde 3-phosphate dehydrogenase; NT3 = neurotrophin 3; TrkC = tyrosine kinase receptor C.
Statistical Analysis
A one-way analysis of variance with Bonferroni post hoc was used to determine statistical significance between treatment groups. Analysis was performed using R statistics.
Results
Ia Proprioceptive Afferents Degenerate in the EDL Muscle of Aging Mice
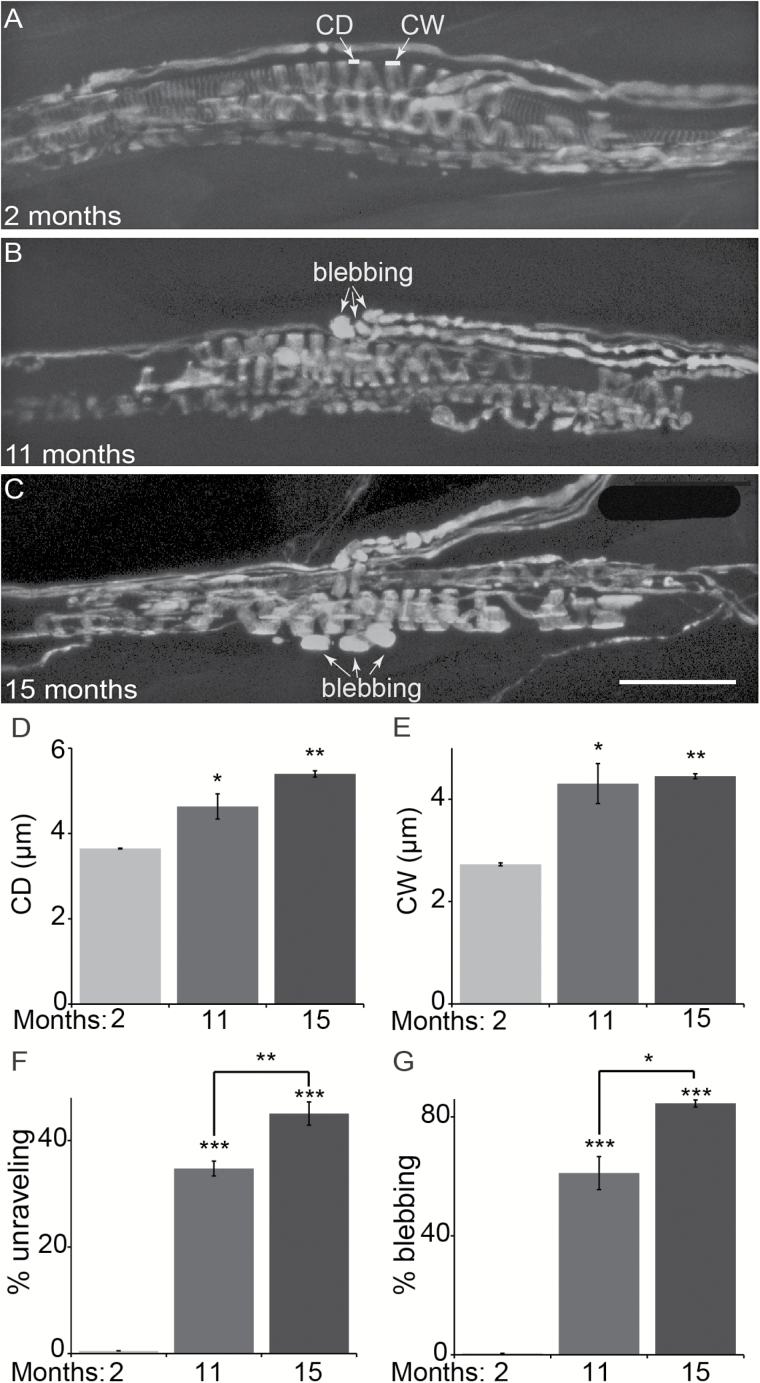
We examined Ia afferents in the EDL and soleus muscles from 2-, 11-, and 15-month-old animals. To visualize Ia afferents, we used mice expressing YFP in all neurons (Thy1-YFP line 16 (43); referred to as YFP). We first analyzed Ia afferents in the EDL muscle because it can be separated into four individual sections and whole mounted from adult mice, making it possible to image Ia afferents in all muscle spindles using light microscopy and at high resolution. We specifically examined proprioceptive sensory afferents in the three divisions of the EDL muscle that attach to toes 2–4. These divisions are thin, making it possible to whole mount them and analyze all sensory afferents using high resolution confocal microscopy imaging. Regardless of age, the three divisions of the EDL muscle together contained on average seven muscle spindles. These afferents were also recently shown to degenerate in the EDL muscle in mice expressing the ALS-causing mutant gene, SOD1G93A (31). To start, we quantified several structural characteristics of Ia afferents because of their reproducible pattern of innervation of muscle spindles (Figure 1A–C). These included (a) the distance between annulospirals’ coils formed as Ia afferents wrap around IMFs (Figure 1A), (b) the coil width (Figure 1A), and (c) the percentage of Ia afferents unraveling from IMFs. In old animals, the distance between coils is significantly larger (5.4±0.07 µm) compared with young animals (3.6±0.01 µm) (Figure 1D). The coil width was also larger in old (4.5±0.05 µm) compared with young adult animals (2.7±0.03 µm) (Figure 1E). These structural changes resulted in an increased number of Ia afferents not properly wrapping around IMFs (herein referred to as unravelling) in old (45% ± 2) compared with young (1% ± 0.8) mice (Figure 1F). Along with these changes, proprioceptive afferents often exhibited large axonal blebs, which are aberrant accumulation of YFP, near or within muscle spindles in aged EDL muscles (Old = 85% ±1.19; Young = 1.5% ± 0.23) (Figure 1B, C, and G). These findings demonstrate that Ia afferents acquire deleterious structural changes in muscle spindles during aging that likely precedes their degeneration as previously demonstrated in aged rats (38).
Figure 1.
Age-related changes in Ia afferents in the extensor digitorum longus (EDL) muscle. Ia afferents were examined in whole-mounted EDL muscles from 2-, 11-, and 15-month-old mice expressing YFP in all peripheral axons (A–C). Coil distance (CD in A; left arrow) and coil width (CW in A; right arrow) were measured in Ia afferents at muscle spindles. In 11- and 15-month-old mice, the coil distance and width were significantly increased compared with 2-month-old mice (D,E). Fewer spirals are found properly wrapping around the equatorial region of intrafusal muscle fibers, defined as unravelling (F), in older mice. Degeneration of the nerve endings was also associated with an increased incidence of axons with large blebs (G) in aged mice. Blebbing is indicated by the arrows in B and C. At least three animals per age group and at least five nerve endings per muscle were analyzed. Scale bar = 50 µm. Error bar = STE. *p < .05, **p < .01, ***p < .001.
Ia Proprioceptive Afferents also Degenerate in the Soleus Muscle in Aging Mice
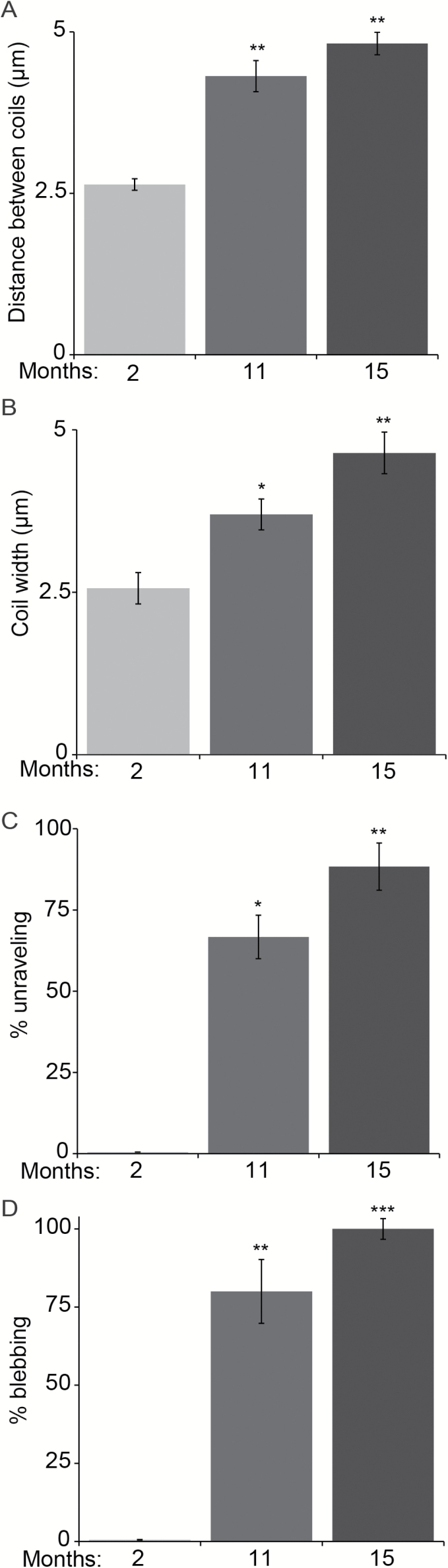
We next asked if aging has a similar effect on Ia afferents in the soleus muscle. In contrast to the EDL, the soleus muscle is primarily composed of slow type (oxidative) muscle fibers and plays a critical function in maintaining posture. We assessed Ia afferents in the soleus muscle from the same mice used to examine the EDL muscle. Regardless of age, we found approximately five muscle spindles in the soleus muscle. However, the majority of Ia afferents exhibited similar age-related structural features as found in the EDL muscle. The distance between coils and the width of each coil increased as animals aged (Figure 2A and B). There were also more Ia afferents unravelling at muscle spindles (Figure 2C). In addition, we found a significant increase in the number of proprioceptive sensory axons with large blebs in aged soleus muscles (Figure 2D).
Figure 2.
Age-related changes in Ia afferents in the soleus muscle. Ia afferents expressing YFP were examined in whole-mounted soleus muscles from the same animals used to analyze extensor digitorum longus muscles in Figure 1. The coil distance (A) and width (B) significantly increased in older soleus muscles. The incidence of Ia afferents unravelling from the equatorial region of intrafusal muscle fibers was also higher in older mice (C). Sensory axons with large blebs were more numerous in old mice (D). At least three animals per age group and at least five nerve endings per muscle were analyzed. Error bar = STE. *p < .05, **p < .01, ***p < .001.
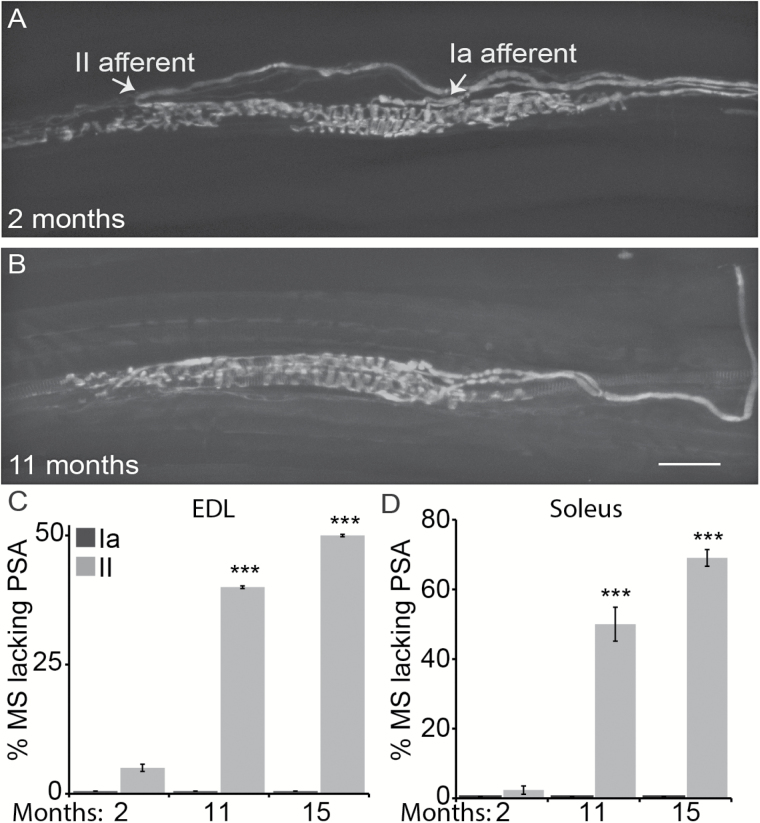
Fewer II Proprioceptive Afferents Innervate Muscles in Middle Age and Old Mice
We analyzed type II afferents in the same muscle spindles innervated by type Ia afferents. Type II afferents innervate IMFs toward their polar region (Figure 3A), where they form irregular flower spray-like structures that are highly variable between spindles. Because the innervation pattern of these nerve ending is highly variable, we chose to count the number of II afferents innervating muscle spindles (Figure 3A and B) rather than carry out a detailed structural analysis in young, middle age, and old mice. In contrast to Ia afferents, fewer II afferents were found innervating muscle spindles in the EDL and soleus muscles of aged compared with young mice (Figure 3C and D). This finding therefore suggests that II afferents are more severely affected than Ia afferents by aging.
Figure 3.
Loss of II afferents in the extensor digitorum longus (EDL) and soleus muscles of middle age and old mice. Proprioceptive sensory nerve endings were visualized using transgenic mice expressing YFP in neurons. Type Ia afferents (A; right arrow) form primary muscle spindles whereas type II afferents (A; left arrow) form secondary muscle spindles. In older mice, there were fewer II afferents innervating muscle spindles (MS) in the EDL (C) and soleus (D) muscles. However, the number of type Ia afferents was unchanged in both muscles in aged mice (C,D). At least three animals were analyzed per age group and at least five nerve endings per muscle. PSA = proprioceptive sensory afferent. Scale bar = 50 µm. Error bar = STE. *p < .05, **p < .01, ***p < .001.
Rates of Ia/II Proprioceptive Afferents Degeneration Differs Between the Soleus and EDL Muscles
During the course of our study, we noticed differences in the rate of aging between sensory afferents innervating the soleus and EDL muscles. Compared with the EDL muscle, there are more Ia afferents unraveling from IMFs with large blebs in the soleus muscle of middle and old age mice (Supplementary Figure 1A and B). The soleus muscle was also innervated by fewer type II afferents compared with EDL muscles in old mice (Supplementary Figure 1C). The difference in the rate of Ia/II afferent degeneration between the soleus and EDL muscles may be due to a variety of factors, including the different functional demands placed on these two muscles and cellular composition (23,44,45).
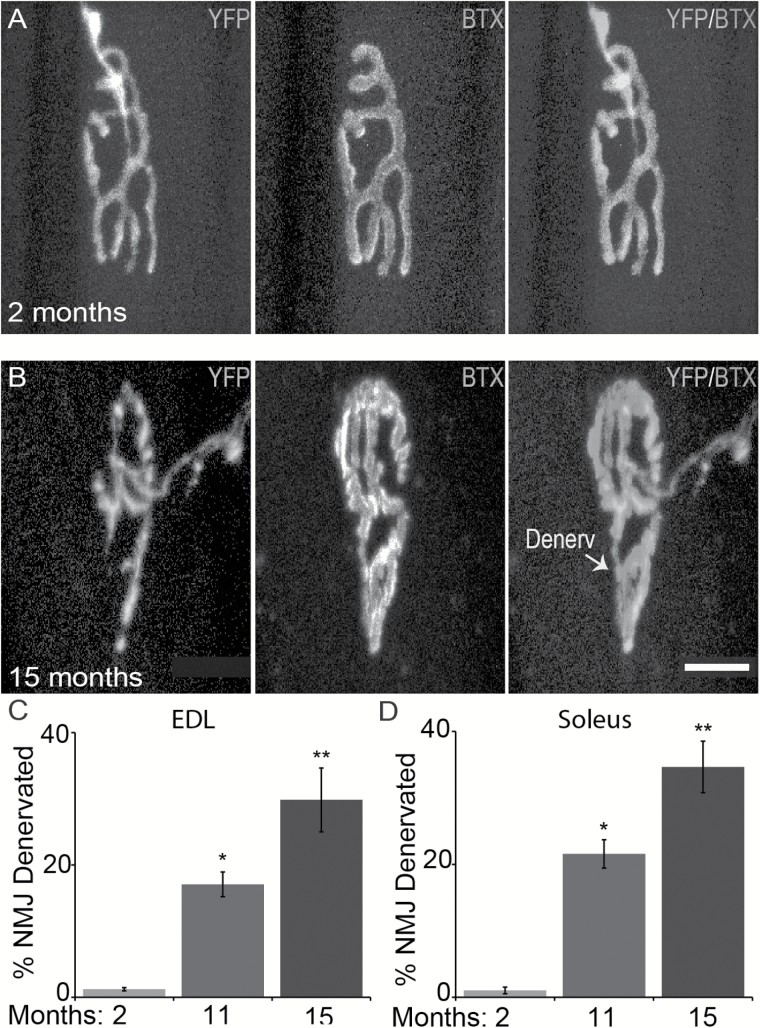
Aging Affects α-Motor Axon Terminals in the Same Muscles Used to Analyze Ia/II Afferents
It is well documented that motor axons begin to degenerate and vacate postsynaptic sites at NMJs starting at 6 months of age (28). We thus examined NMJs in the same muscles used to analyze Ia/II afferents to confirm that the EDL and soleus muscles were undergoing normal age-related changes. Because motor axons were also labeled with YFP, it facilitated our analysis of motor nerve endings at NMJs in young and old mice. To visualize postsynaptic sites on extrafusal muscle fibers, we stained muscles with fluorescently tagged α-bungarotoxin, which labels nAChRs. As previously shown, the incidence of NMJs partially or fully denervated increases with aging in the same EDL (Figure 4A–C) and soleus (Figure 4D) muscles used to examine Ia/II proprioceptive sensory afferents. These findings further demonstrate that peripheral nerve endings critical for motor function degenerate during the normal aging process.
Figure 4.
Age-related changes in α-motor axons parallel those found in sensory afferents. Analysis of α-motor axons (YFP) innervating the postsynaptic site on extrafusal muscle fibers, visualized by using fluorescently tagged α-bungarotoxin (BTX). In the same muscles used to examine sensory afferents, fewer α-motor axons were found fully innervating postsynaptic sites in older mice (A–D). At least three animals per age group and at least 50 NMJs per muscle were analyzed. Scale bar = 20 µm. Error bar = STE. *p < .05, **p < .01, ***p < .001.
IMF Number and Size Are Unchanged in Aged Mice
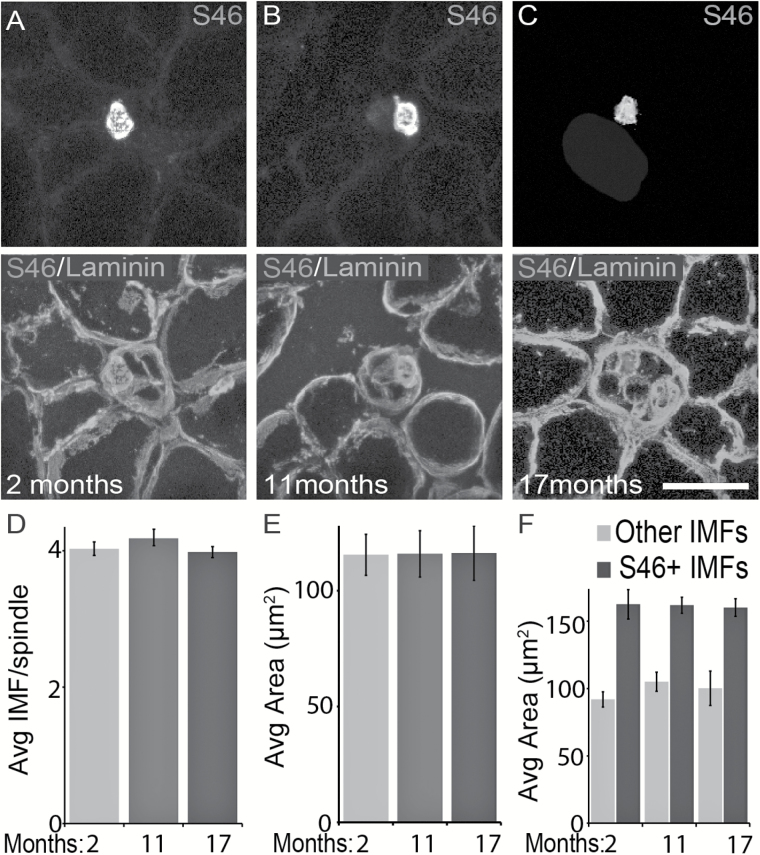
Proprioceptive sensory neurons and their nerve endings depend on IMFs to remain structurally intact and survive. To explore the possibility that age-related changes in IMFs cause degeneration of proprioceptive sensory neurons, we stained 16 µm cross-sections of the TA muscle from young and old mice with the S46 antibody (46–48). This antibody binds to the slow-tonic myosin heavy chain isoform, which is expressed by IMFs (49). As previously shown (49), S46 labeled only one IMF per muscle spindle in the TA muscle from young and old mice (Figure 5A–C). The size of S46-positive muscle fibers suggests that they are in fact nuclear bag1 and bag2 IMFs (Figure 5F). Unlabeled intrafusal fibers residing adjacent to S46-positive fibers were identified using a pan-laminin antibody, which stains the perimeter of all muscle fibers (Figure 5A–C). In the TA muscle of 11- and 17-month-old mice, there is no difference in the number and average size of IMFs within individual muscle spindles compared with 2-month-old mice (Figure 5D and E). Additional analysis showed that the number and size of S46-positive and S46-negative IMFs are unchanged in middle age and old mice (Figure 5F). Although IMFs appear structurally normal in older mice, it remains possible that subcellular and molecular changes in these cells affect Ia/II afferents during aging.
Figure 5.
The number and size of intrafusal muscle fibers (IMFs) are unchanged in older mice. Nuclear bag fibers were stained using an antibody against S46, which binds to slow-tonic myosin heavy chain isoform (A–C). The perimeter of all muscle fibers was labeled with an antibody against laminin (A–C). We examined the tibialis anterior muscle of 2-month (A), 11-month (B), and 17-month-old (C) animals. The number of IMFs per spindle is similar between young and older mice (D). Aging did not affect the size of S46-positive and S46-negative IMFs (E). S46-positive IMFs do not differ between mice of different age groups (F). At least three animals per age group and at least 30 muscle fibers per muscle were analyzed. Scale bar = 50 µm. Error bar = STE. *p < .05, **p < .01, ***p < .001.
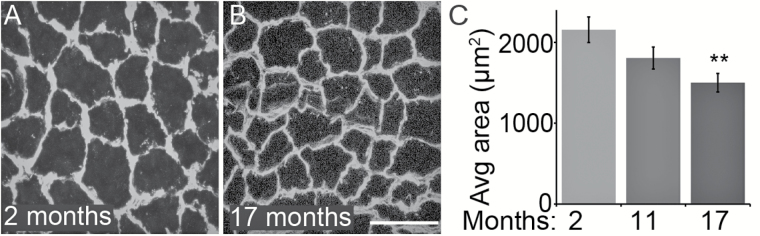
Extrafusal Muscle Fibers’ Size Decreases With Aging
The lack of morphological changes in IMFs prompted us to ask if extrafusal muscle fibers are also unchanged in the same TA muscles of 11- and 17-month-old mice. Laminin staining was used to determine the perimeter of these muscle fibers (Figure 6A and B). We determined the area of 30 extrafusal muscle fibers located throughout the TA muscle per muscle section for all animals used in this study. In contrast to IMFs, we found a significant reduction in the size of extrafusal muscle fibers in the TA of 17-month-old mice (Figure 6C). Thus extrafusal muscles, predominantly fast-type in the TA muscle, undergo age-related morphological changes before IMFs during aging.
Figure 6.
The size of extrafusal muscle fibers decreases with aging. The perimeter of extrafusal muscle fibers in the tibialis anterior muscle was revealed by immunostaining for laminin in young and old mice (A,B). In 17-month-old mice, there is a significant reduction in the size of extrafusal muscle fibers compared with those in 2-month-old mice (C). At least three animals per age group and 30 muscle fibers per animal were analyzed. Scale bar = 50 µm. Error bar = STE. **p < .01.
Loss of Proprioceptive Sensory Axons in Muscles and Cell Bodies in DRGs of Aged Mice
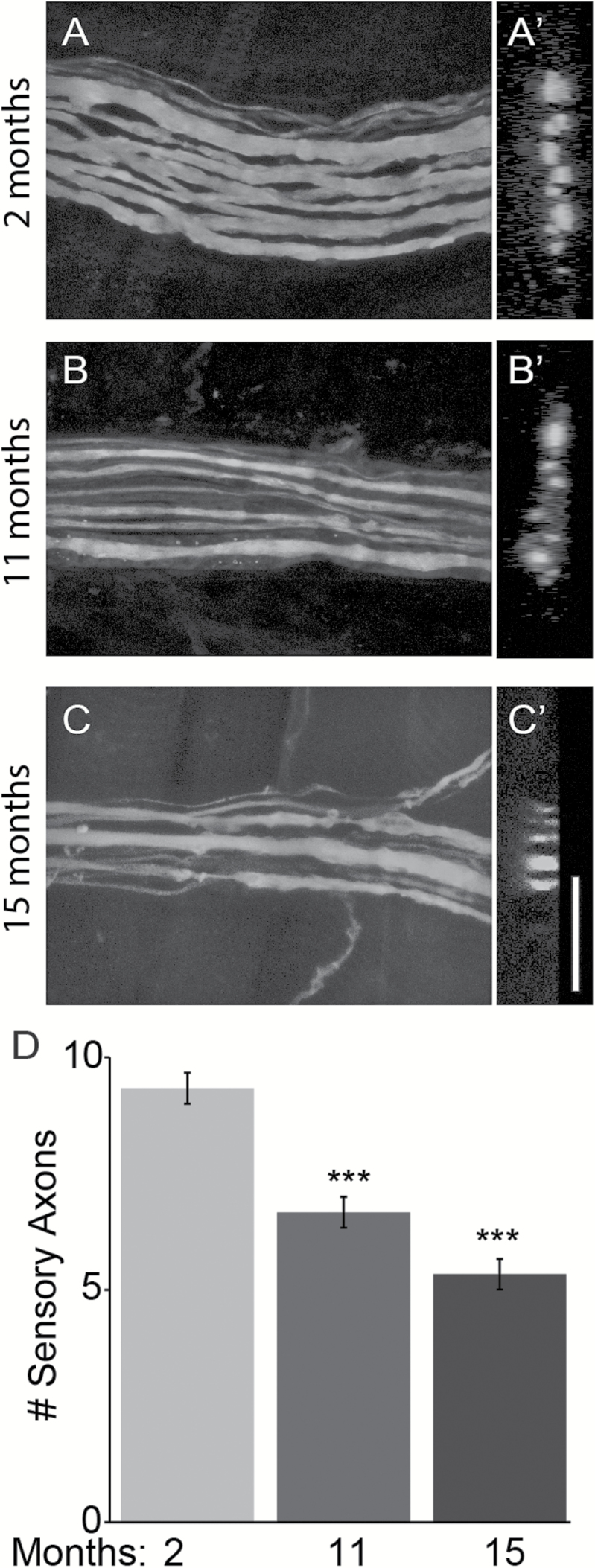
The lack of morphological alterations in IMFs combined with the loss of II afferents from aged muscle spindles suggested that aging directly affects proprioceptive sensory neurons. To explore this possibility, we first counted the number of proprioceptive sensory axons innervating the division of the EDL muscle responsible for controlling the second toe. To clearly trace sensory axons, we imaged afferents innervating spindles located away from NMJs, thus avoiding α-motor axons. To count axons, we obtained high resolution longitudinal images (Figure 7A–C), and used these images to generate 3D optical cross-section slides (Figure 7A′–C′). Irrespective of the image used, we found fewer proprioceptive sensory axons in middle age and old mice compared with young mice (Figure 7A–D). The remaining sensory axons in aged mice often appeared thinner compared with those in young mice (Figure 7A–C).
Figure 7.
Aging causes complete degeneration of sensory axons innervating the extensor digitorum longus (EDL) muscle in middle age and old mice. Sensory axons innervating the portion of the EDL muscle that controls the 2nd digit were analyzed using transgenic mice expressing YFP in neurons (A–C). Axon number was determined by digitally slicing through the 3D image plane (A′–C′). Fewer sensory axons innervating the EDL muscle were found in 11- and 15-month-old mice (D). Aged sensory axons also often exhibited deleterious structural changes that include thinning and large swellings. At least three animals per age group were analyzed. Scale bar = 10 µm. Error bar = STE. ***p < .001.
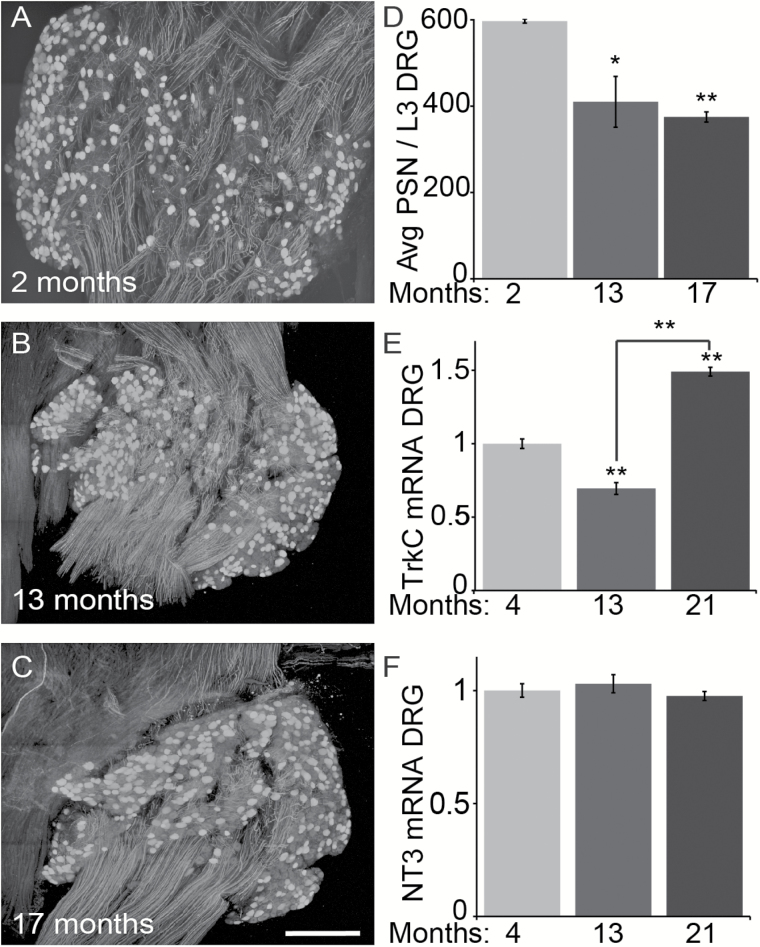
We next asked if the cell body of proprioceptive sensory neurons degenerates with increasing age. We examined the soma of proprioceptive sensory neurons in L3 DRG from 2-, 13-, and 17-month-old mice. To visualize proprioceptive sensory neurons in DRGs, we crossed parvalbumin-Cre (PVCre) and Thy1-STOP-YFP (STP-YFP) mice. Progenies from this mating express YFP in parvalbumin-positive neurons (PVCre;STP-YFP), and thus only proprioceptive sensory neurons were labeled within DRGs (Figure 8A–C). We used these mice to count the number of YFP-positive neurons in L3 DRGs. Compared with 2-month-old mice, we found a significant decrease in proprioceptive sensory neurons in L3 DRGs from 13- and 17-month-old mice, respectively (Figure 8D). These data demonstrate that the axons and cell bodies of proprioceptive sensory degenerate with increasing age, hence explaining the loss of II afferents in muscle spindles of aged mice.
Figure 8.
Fewer proprioceptive sensory neurons are present in middle age and old DRGs. Proprioceptive sensory neurons (PSNs) selectively expressing YFP within DRGs were whole mounted and visualized using confocal microscopy (A–C). Analysis of L3 DRGs revealed that PSNs are significantly reduced in 11- and 17-month-old mice compared with 2-month-old mice (D). Tyrosine kinase receptor C (TrkC) transcripts are reduced in 13-month-old but then significantly increase in 21-month-old DRGs (E) whereas neurotrophin 3 (NT3) transcripts remain unchanged in the tibialis anterior muscle (F) compared with 4-month-old mice. At least three animals per age group were analyzed. Scale bar = 150 µm. Error bar = STE. *p < .05, **p < .01.
Expression of NT3 Is Unchanged and TrkC Is Dysregulated in Aged Mice
Proprioceptive sensory neurons depend on NT3, released by muscle fibers, and activation of TrkC to develop and survive (30). Suggesting that NT3 is not the primary driver of proprioceptive sensory neuron degeneration, we found NT3 transcripts expressed at similar levels in the TA muscle of 13- and 21-month-old mice compared with 4-month-old mice (Figure 8F). In contrast, TrkC expression was dysregulated with increasing age in DRGs. We found that TrkC expressed at lower levels in 13-month-old mice compared with 4-month-old mice (Figure 8E). In stark contrast, TrkC is significantly increased in 21-month-old TA compared with 4- and 13-month-old mice (Figure 8E). The different levels of TrkC in middle age and old DRGs show that proprioceptive sensory neurons undergo intrinsic molecular alterations that initiate or function to fend off pathological changes caused by aging.
Discussion
We show that proprioceptive sensory neurons degenerate as mice age. The majority of Ia afferents fail to maintain their annulospiral structures in the EDL and soleus muscles of 11- and 15-month old mice compared with 2-month-old mice. In these older muscles, there are fewer II sensory afferents innervating muscle spindles but no obvious morphological changes in IMFs. Along with changes in peripheral nerve endings, there are fewer proprioceptive sensory neurons in DRGs located in L3 of 13- and 17-month-old mice. Based on these findings and roles of NT3 and TrkC in the survival and maintenance of these neurons, we explored the possibility that their level is decreased in aged muscles and DRGs, respectively. We show that NT3 is expressed at similar levels in muscles and TrkC is increased in DRGs of aged mice, suggesting that other molecular alterations drive pathological changes in aging proprioceptive sensory neurons.
Impact of Aging on Peripheral Nerves With Critical Roles in Motor Function and Their Muscle Fibers
The data presented in this manuscript expand on published findings demonstrating that peripheral axons, important for voluntary movements, undergo significant changes during aging (38,39,50). However, our study revealed new information regarding the impact of aging on Ia/II afferents and on IMFs. Although the number of Ia afferents is unchanged, despite exhibiting age-related structural changes, there is a significant loss of group II afferents in 11- and 15-month-old mice. We obtained this additional information using a holistic approach to examine the impact of aging on proprioceptive sensory neurons and in their native configuration using confocal microscopy.
The analysis of whole-mounted muscles also allowed us to directly compare Ia and II afferents and to conclude that aging differentially affects these two different sensory afferents. However, our data do not provide information explaining the different rates of aging of Ia and II afferents. Their known roles may instead provide insights regarding their different rates of degeneration during aging (51). Although Ia afferents innervate all IMFs, including dynamic nuclear bag fibers responsible for detecting fast changes in muscle length, II afferents only innervate static nuclear bag and chain IMFs. This difference in innervation pattern means that Ia afferents are more frequently activated compared with II afferents. Thus, it is possible that the increased activity of Ia afferents helps prevent the accumulation of age-related changes, as has been shown for subtypes of α-motor neurons that innervate tonically active muscle fibers (23). It is also plausible that molecules unique to each of these two different proprioceptors may play critical functions in their response to age-related pathophysiological changes (51). Unfortunately, such molecules have not been identified.
IMFs Are Morphologically Normal in Aged Mice
The general morphological characteristics and the number of IMFs were unchanged in older mice. However, it is possible that IMFs already contain subcellular and molecular changes associated with aging in 17-month-old mice, possibilities not addressed in this study. Together with the degeneration of innervated sensory afferents, such changes are likely to result in the atrophy of IMFs in older mice. Supporting this hypothesis, degenerating IMFs have been found in aged rats and humans (39,52).
In the same muscles used to examine IMFs, aging caused a moderate yet significant reduction in the size of extrafusal muscle fibers. There are a number of differences between intrafusal and extrafusal muscle fibers that could underlie their differential susceptibility to aging. These include differences in expression of select myogenic genes and transcription factors (51,53–55). Intrafusal and extrafusal muscle fibers also have distinct functional demands, with extrafusal muscle fibers generating significantly more force compared with IMFs (56). Extrafusal muscle fibers are also solely responsible for generating the force required to carry out all voluntary movements, including lifting heavy objects. Another important difference is that whereas extrafusal fibers directly connect with tendons, IMFs are contained within a sheath of connective tissue that does not attach to tendons (57). Hence, IMFs are likely less affected by age-related changes known to occur in tendons (58), and better insulated from age-related systemic molecular changes.
Loss of Proprioceptive Sensory Neurons in DRGs and Role of NT3/TrkC in Aging
In addition to degeneration of peripheral afferents, we found that proprioceptive sensory neurons degenerate in L3 DRGs of 13- and 17-month-old mice, consistent with the loss of II afferents from muscle spindles. These findings also support published studies showing an overall reduction in sensory neurons in DRGs of old rats and rabbits (59,60). Based on these findings, we hypothesized that reduced levels of NT3 and TrkC underlie the degeneration of proprioceptive sensory neurons in aged mice. NT3 and TrkC are well documented to promote the survival of proprioceptive sensory neurons (30). Muscle fibers secrete NT3 to activate TrkC located at the nerve terminal of proprioceptive sensory afferents. Contrary to our expectations, however, we found no change in levels of NT3 in the TA muscle in middle age and old mice. In contrast, our analysis of TrkC showed that while it is reduced in DRGs of middle age mice, it is significantly increased in DRGs of old mice. Despite the shifting expression of TrkC during aging, it is expressed at high levels, along with NT3, in aging mice indicating that other molecular changes within the NT3/TrkC signaling axis may contribute to degeneration of proprioceptive sensory neurons. For example, aging may disrupt NT3/TrkC endocytosis, retrograde transport to the cell body, and ability to activate appropriate effectors that function to promote the maintenance and survival of proprioceptive sensory neurons. An alternative possibility is that enhanced and unimpeded activation of TrkC could result in degeneration of aging proprioceptive sensory neurons (61). It is also plausible that other molecular alterations not related to NT3/TrkC mediated signaling could cause degeneration of proprioceptive sensory neurons during aging.
Contribution of Proprioceptive Sensory Neurons to Age-Related Motor Deficits
This study provides critical information regarding the potential contribution of proprioceptive sensory neurons to the loss of motor function that occurs with increasing age. Age-related degeneration of proprioceptive sensory neurons likely alters the pattern and rate of α-motor neuron activation, thus compromising the initiation and coordination of simple and complex movements. While this study does not test the impact of age-related proprioceptive degeneration on motor function, published studies have found that balance and coordination deficits occur in aging mice and humans (62,63). If additional studies demonstrate that proprioceptive sensory neurons contribute to age-related motor deficits, therapeutic interventions would need to be designed to slow their degeneration.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (K01NS085071 to G.V.), the National Institute of Aging (R56AG051501 to G.V.) of the National Institutes of Health.
Supplementary Material
References
- 1. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi:10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 2. Sorond FA, Cruz-Almeida Y, Clark DJ, et al. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. J Gerontol A Biol Sci Med Sci. 2015;70:1526–1532. doi:10.1093/gerona/glv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin FC. Falls risk factors: assessment and management to prevent falls and fractures. Can J Aging. 2011;30:33–44. doi:10.1017/S0714980810000747 [DOI] [PubMed] [Google Scholar]
- 4. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. doi:10.1016/j.maturitas.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 5. Soriano TA, DeCherrie L V, Thomas DC. Falls in the community-dwelling older adult: a review for primary-care providers. Clin Interv Aging. 2007;2:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi:10.1056/NEJM199601113340202 [DOI] [PubMed] [Google Scholar]
- 7. Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43:2184–2188. [DOI] [PubMed] [Google Scholar]
- 8. Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47:330–334. [DOI] [PubMed] [Google Scholar]
- 9. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi:10.1136/jnnp.2006.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das MM, Avalos P, Suezaki P, et al. Human neural progenitors differentiate into astrocytes and protect motor neurons in aging rats. Exp Neurol. 2016;280:41–49. doi: 10.1016/j.expneurol.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 11. Rygiel KA, Grady JP, Turnbull DM. Respiratory chain deficiency in aged spinal motor neurons. Neurobiol Aging. 2014;35:2230–2238. doi:10.1016/j.neurobiolaging.2014.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–219. [DOI] [PubMed] [Google Scholar]
- 13. Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. 1997;127(Suppl):1011S–1013S. [DOI] [PubMed] [Google Scholar]
- 14. Kawamura Y, O’Brien P, Okazaki H, Dyck PJ. Lumbar motoneurons of man II: the number and diameter distribution of large- and intermediate-diameter cytons in “motoneuron columns” of spinal cord of man. J Neuropathol Exp Neurol. 1977;36:861–870. [DOI] [PubMed] [Google Scholar]
- 15. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi:10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hepple RT. Sarcopenia—a critical perspective. Sci Aging Knowledge Environ. 2003;2003:pe31 doi:10.1126/sageke.2003.46.pe31 [DOI] [PubMed] [Google Scholar]
- 17. Fiatarone MA, Evans WJ. The etiology and reversibility of muscle dysfunction in the aged. J Gerontol. 1993;48:77–83. [DOI] [PubMed] [Google Scholar]
- 18. Marzetti E, Lees HA, Manini TM, et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS One. 2012;7:e32829 doi:10.1371/journal.pone.0032829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKenzie D, Bua E, McKiernan S, Cao Z, Aiken JM. Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur J Biochem. 2002;269:2010–2015. [DOI] [PubMed] [Google Scholar]
- 20. Weisleder N, Brotto M, Komazaki S, et al. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J Cell Biol. 2006;174:639–645. doi:10.1083/jcb.200604166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosant C, Nagel M-D, Pérot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol. 2007;42:301–308. doi:10.1016/j.exger.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 22. Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11:641–656. [DOI] [PubMed] [Google Scholar]
- 23. Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7:e34640 doi:10.1371/journal.pone.0034640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardasis CA, LaFontaine DM. Aging rat neuromuscular junctions: a morphometric study of cholinesterase-stained whole mounts and ultrastructure. Muscle Nerve. 1987;10:200–213. [DOI] [PubMed] [Google Scholar]
- 25. Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. [DOI] [PubMed] [Google Scholar]
- 26. Punga AR, Ruegg MA. Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases. Curr Opin Pharmacol. 2012;12:340–346. doi:10.1016/j.coph.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 27. Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl. 1997;5:S83–S87. [DOI] [PubMed] [Google Scholar]
- 28. Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. doi:10.1073/pnas.1002220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi:10.1152/physrev.00048.2011 [DOI] [PubMed] [Google Scholar]
- 30. Chen H-H, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 2003;13:96–102. [DOI] [PubMed] [Google Scholar]
- 31. Vaughan SK, Kemp Z, Hatzipetros T, Vieira F, Valdez G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J Comp Neurol. 2015;523:2477–2494. doi:10.1002/cne.23848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mentis GZ, Blivis D, Liu W, et al. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69:453–467. doi:10.1016/j.neuron.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD. SMN is required for sensory-motor circuit function in Drosophila. Cell. 2012;151:427–439. doi:10.1016/j.cell.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sangari S, Iglesias C, El Mendili MM, Benali H, Pradat PF, Marchand-Pauvert V. Impairment of sensory-motor integration at spinal level in amyotrophic lateral sclerosis. Clin Neurophysiol. 2016;127:1968–1977. doi:10.1016/j.clinph.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 35. Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration: I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol. 2011;106:2450–2470. doi:10.1152/jn.01095.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sábado J, Casanovas A, Tarabal O, et al. Accumulation of misfolded SOD1 in dorsal root ganglion degenerating proprioceptive sensory neurons of transgenic mice with amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014:852163 doi:10.1155/2014/852163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration: II. Loss of functional connectivity with motoneurons. J Neurophysiol. 2011;106:2471–2485. doi:10.1152/jn.01097.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim GH, Suzuki S, Kanda K. Age-related physiological and morphological changes of muscle spindles in rats. J Physiol. 2007;582:525–538. doi:10.1113/jphysiol.2007.130120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swash M, Fox KP. The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci. 1972;16:417–432. [DOI] [PubMed] [Google Scholar]
- 40. Winarakwong L, Muramoto T, Soma K, Takano Y. Age-related changes and the possible adaptability of rat jaw muscle spindles: immunohistochemical and fine structural studies. Arch Histol Cytol. 2004;67:227–240. [DOI] [PubMed] [Google Scholar]
- 41. Hippenmeyer S, Vrieseling E, Sigrist M, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159 doi:10.1371/journal.pbio.0030159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chakkalakal J V, Kuang S, Buffelli M, Lichtman JW, Sanes JR. Mouse transgenic lines that selectively label Type I, Type IIA, and Types IIX+B skeletal muscle fibers. Genesis. 2012;50:50–58. doi:10.1002/dvg.20794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. [DOI] [PubMed] [Google Scholar]
- 44. Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967;193:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schiaffino S, Hanzlíková V, Pierobon S. Relations between structure and function in rat skeletal muscle fibers. J Cell Biol. 1970;47:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sokoloff AJ, Li H, Burkholder TJ. Limited expression of slow tonic myosin heavy chain in human cranial muscles. Muscle Nerve. 2007;36:183–189. doi:10.1002/mus.20797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komori T, Gyobu H, Ueno H, Kitamura T, Senba E, Morikawa Y. Expression of kin of irregular chiasm-like 3/mKirre in proprioceptive neurons of the dorsal root ganglia and its interaction with nephrin in muscle spindles. J Comp Neurol. 2008;511:92–108. doi:10.1002/cne.21838 [DOI] [PubMed] [Google Scholar]
- 48. Miller JB, Crow MT, Stockdale FE. Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J Cell Biol. 1985;101:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kucera J, Fan G, Walro J, Copray S, Tessarollo L, Jaenisch R. Neurotrophin-3 and trkC in muscle are non-essential for the development of mouse muscle spindles. Neuroreport. 1998;9:905–909. [DOI] [PubMed] [Google Scholar]
- 50. Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. doi:10.1073/pnas.1002220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi:10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- 52. Desaki J, Nishida N. A further observation of muscle spindles in the extensor digitorum longus muscle of the aged rat. J Electron Microsc. 2010;59:79–86. doi:10.1093/jmicro/dfp038 [DOI] [PubMed] [Google Scholar]
- 53. Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. Proc Natl Acad Sci USA. 2004;101:12218–12223. doi:10.1073/pnas.0404240101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soukup T, Jirmanová I. Regulation of myosin expression in developing and regenerating extrafusal and intrafusal muscle fibers with special emphasis on the role of thyroid hormones. Physiol Res. 2000;49:617–633. [PubMed] [Google Scholar]
- 55. Albert Y, Whitehead J, Eldredge L, Carter J, Gao X, Tourtellotte WG. Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis. J Cell Biol. 2005;169:257–268. doi:10.1083/jcb.200501156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Edman KAP, Radzyukevich T, Kronborg B. Contractile properties of isolated muscle spindles of the frog. J Physiol. 2002;541:905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maier A. Development and regeneration of muscle spindles in mammals and birds. Int J Dev Biol. 1997;41:1–17. [PubMed] [Google Scholar]
- 58. Ovalle WK, Dow PR, Nahirney PC. Structure, distribution and innervation of muscle spindles in avian fast and slow skeletal muscle. J Anat. 1999;194:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: neuron number estimates using the disector method and confocal optical sectioning. J Comp Neurol. 1998;396:211–222. [PubMed] [Google Scholar]
- 60. Pannese E, Sartori P, Martinelli C, Ledda M. Age-related decrease in the overall extent of perikaryal projections in rabbit spinal ganglion neurons. Neurosci Lett. 1998;254:177–179. [DOI] [PubMed] [Google Scholar]
- 61. Nikoletopoulou V, Lickert H, Frade JM, et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467:59–63. doi:10.1038/nature09336 [DOI] [PubMed] [Google Scholar]
- 62. Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:11 doi:10.1186/s13041-016-0191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Justice JN, Carter CS, Beck HJ, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age. 2014;36:583–592. doi:10.1007/s11357-013-9589-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.