Abstract
Prenatal alcohol exposure has been linked to a broad range of developmental deficits, with eyeblink classical conditioning (EBC) among the most sensitive endpoints. This fMRI study compared EBC-related brain activity in 47 children with fetal alcohol syndrome (FAS), partial FAS (PFAS), heavily exposed (HE) non-syndromal children, and healthy controls. All of the children had previously participated in two EBC studies conducted as part of our longitudinal study of fetal alcohol spectrum disorders. Although learning-related behavioral differences were seen in all groups during the scans, controls showed more conditioned responses (CR) than the alcohol-exposed groups. Despite lower conditioning levels relative to controls, the exposed groups exhibited extensive cerebellar activations. Specifically, children with FAS/PFAS showed increased activation of cerebellar lobule VI in session 2, while HE children showed increased activation in session 1. Continuous measures of prenatal alcohol use correlated with learning-related activations in cerebellum and frontal cortices. Only controls showed significant cerebellar activation—CR correlations in the deep nuclei and lateral lobule VI, suggesting that these key regions supporting EBC may be functionally disorganized in alcohol-exposed children. These findings are the first to characterize abnormalities in brain function associated with the behavioral conditioning deficits seen in children with prenatal alcohol exposure.
Keywords: cerebellum, cerebellar volume, fetal alcohol syndrome, gray matter volume, learning, prenatal alcohol exposure, white matter volume
Introduction
Heavy prenatal alcohol exposure produces a wide range of physical, cognitive, and behavioral deficits. Fetal alcohol syndrome (FAS), the most severe of the fetal alcohol spectrum disorders (FASD), is characterized by distinctive craniofacial dysmorphology, small head circumference, and growth retardation (Stratton et al. 1996; Hoyme et al. 2005). Partial FAS (PFAS) is also characterized by facial dysmorphology, together with small head circumference, growth retardation, or cognitive and/or behavioral dysfunction. Other heavily exposed (HE) non-syndromal children lack the distinctive facial anomalies but exhibit similar cognitive impairment as syndromal children (Coles et al. 1997; Mattson et al. 1998; Jacobson et al. 2008; Dodge et al. 2009; Suttie et al. 2013; Lewis et al. 2015). Cognitive deficits are frequently seen in general intelligence, executive functioning, and learning and memory (Mattson and Riley 2011).
One brain region specifically targeted by heavy prenatal alcohol exposure is the cerebellum (Norman et al. 2009), a structure that is critical for eyeblink classical conditioning (EBC) in humans and laboratory animals (Woodruff-Pak 1988; Christian and Thompson 2003; Thurling et al. 2015). Cerebellar and brain stem anomalies were reported in the earliest autopsy studies of heavy prenatal alcohol exposure (Jones and Smith 1973; Clarren and Smith 1978), including cerebellar dysgenesis in 10 of 16 FAS autopsies (Clarren 1986). In EBC, repeated pairings of a neutral conditioned stimulus (CS) and an unconditioned stimulus (US) cause the CS to elicit a conditioned response (CR) in anticipation of the US. The neural circuitry supporting EBC has been mapped out in considerable detail in animal models (Woodruff-Pak 1988; Christian and Thompson 2003). Specifically, contributions from the cerebellar cortex (Yeo et al. 1984, 1985; Yeo and Hardiman 1992), particularly in lateral lobule VI and cerebellar deep nuclei (Lavond et al. 1984; McCormick and Thompson 1984a, 1984b), have been well documented. In addition, EBC has been used to investigate cerebellar development and the ontogeny of learning in rodents and humans (Stanton et al. 1992; Freeman et al. 1995; Stanton et al. 1998; Freeman 2010; Cheng et al. 2014). Heavy exposure to ethanol during the equivalent of the third trimester of pregnancy in humans disrupts EBC in rat weanlings and adults (Stanton and Goodlett 1998), a deficit that is mediated by a dose-dependent cell loss and altered neural activity in the deep cerebellar nuclei (Green et al. 2002a, 2002b; Lindquist et al. 2013).
In a prospective, longitudinal study of children with prenatal alcohol exposure in Cape Town, South Africa, we found a remarkably consistent deficit in EBC at 5 years of age (Jacobson et al. 2008). Not a single child with full FAS met criterion for conditioning, compared with about one-third of the PFAS and HE groups and 75.0% of controls from the same community. These data corroborated a previous small-sample study of alcohol-exposed children (Coffin et al. 2005) and were confirmed in a second 11-year-old Cape Town sample (Jacobson et al. 2011b). Functional magnetic resonance imaging (fMRI) studies have described brain activation during EBC in healthy adults (Ramnani et al. 2000; Knuttinen et al. 2002; Cheng et al. 2008) and children (Cheng et al. 2014). The purpose of this study was to use fMRI to identify which components of the well-characterized EBC neural circuit are disordered in alcohol-exposed children (Jacobson et al. 2008, 2011b). We focus primarily on cerebellar regions known to mediate EBC acquisition in laboratory animals—lobule VI and deep nuclei—and neocortical regions that may be activated to compensate for learning deficits. Regional brain activations were also examined in relation to amount of alcohol consumed during pregnancy.
Materials and Methods
Participants
The sample consisted of 47 children (21 male, 26 female) born to Cape Coloured (mixed ancestry) women in Cape Town, South Africa. Pregnant women were recruited into the Cape Town Longitudinal Cohort between July 1999 and January 2002 from the antenatal clinic of a Midwife Obstetric Unit that serves an economically disadvantaged, predominantly Cape Coloured population (Jacobson et al. 2008). The prevalence of FAS in this community is among the highest in the world (May et al. 2007, 2013). It is a consequence of heavy maternal drinking during pregnancy, due to poor psychosocial circumstances and the traditional dop system, in which farm laborers were paid, in part, with wine. Although the dop system has been outlawed, heavy recreational alcohol consumption persists in certain sectors in urban and rural Cape Coloured communities (Jacobson et al. 2008; May et al. 2013).
Each gravida was interviewed at her first antenatal visit (M = 17.8 weeks gestation, standard deviation (SD) = 6.6) regarding her alcohol consumption at time of recruitment and conception, using a timeline follow-back interview (Jacobson et al. 2002). Any woman averaging at least 1.0 oz absolute alcohol (AA) per day, ≈ 2 standard drinks, or reporting at least two incidents of binge drinking (5 standard drinks/occasion) during the first trimester of pregnancy was invited to participate in the study. Women initiating antenatal care who abstained or drank no more than minimal were also invited to participate as controls. All but one control mother abstained during pregnancy; the single light drinker reported consuming only 2 drinks on 3 occasions during pregnancy. Women <18 years of age and those with diabetes, epilepsy, or cardiac problems requiring treatment were not included. Religiously observant Muslim women were also excluded because their religious practices prohibit alcohol consumption, and they would, therefore, have been disproportionately represented among the controls. Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, multiple births (e.g., twins), and seizures. Informed consent was obtained from each mother at recruitment and at the laboratory visits. Approval for human research was obtained from the Wayne State University and University of Cape Town (UCT) institutional review boards. All women who reported drinking during pregnancy were advised to stop or reduce their intake and referred for treatment, if they were willing.
A research nurse and staff driver transported the mother and child to our laboratory at the UCT Faculty of Health Sciences and on a separate visit to the Cape Universities Brain Imaging Centre (CUBIC), a research-dedicated, child-friendly facility, for the scanning sessions. Each mother was interviewed in the laboratory antenatally and at 1-month postpartum regarding her alcohol and drug use during pregnancy. Interviews were conducted in Afrikaans or English, depending on the mother's preference. Mothers and children were given breakfast, a snack, and lunch at each visit. At the end of the visits, the mother received a small monetary compensation and photograph of her child, and the child was given a small gift.
Alcohol and Drug Use
In the timeline follow-back interview administered at recruitment, the mother was asked about her drinking on a day-by-day basis during a typical 2-week period around time of conception, with recall linked to specific times of day and activities. If her drinking had changed since conception, she was also asked about her drinking during the past 2 weeks and when her drinking had changed. At a subsequent follow-up antenatal visit, the mother was again asked about her drinking during the previous 2 weeks. At 1-month postpartum, she was asked about her drinking during a typical 2-week period during the latter part of pregnancy. Volume was recorded for each type of alcohol beverage consumed each day and converted to oz of AA using multipliers proposed by Bowman et al. (1975) (liquor—0.4, beer—0.04, wine—0.2). Alcohol abuse and/or dependence were diagnosed during pregnancy based on DSM-IV criteria using the alcohol module of the Diagnostic Interview Schedule (Robins et al. 1995) and the AUDADIS-IV (Grant et al. 1995) at 5 years for the 3 mothers for whom it was not assessed in infancy. Mothers were asked how many cigarettes they smoked/day and how many days/week they used marijuana (“dagga”), methaqualone (“mandrax”), cocaine, or any other illicit drugs during pregnancy.
In September 2005, we organized a clinic at which each child was independently examined for growth and FAS anomalies using a standard protocol (Hoyme et al. 2005) by two US-based expert FAS dysmorphologists (HE Hoyme, MD, and LK Robinson, MD). There was substantial agreement between the dysmorphologists on their assessments of all dysmorphic features, including palpebral fissure length and philtrum and vermilion ratings based on the Astley and Clarren (2001) rating scales (r = 0.80, 0.84, and 0.77, respectively). One child who could not attend the clinic was examined by a Cape Town-based expert FASD dysmorphologist (N Khaole), for whom there was substantial inter-examiner agreement on assessment of dysmorphic features with the two US dysmorphologists (see Jacobson et al. 2008). HEH, LKR, SWJ, JLJ, and CDM subsequently conducted a case conference to reach consensus regarding which children met criteria for FAS and PFAS diagnoses. Based on these assessments, children were classified into one of three groups: children with FAS or PFAS (n = 23), HE non-syndromal children (n = 16), and healthy controls (n = 8). All of the children had participated in two previous behavioral EBC assessments at our UCT Child Development Research Laboratory—one at 5.0 years (SD = 0.2) (Jacobson et al. 2008); the other, at 9.6 years (SD = 0.8) (Jacobson et al. 2011b)—both prior to the present EBC neuroimaging assessment at 10.6 years (SD = 0.6). At 5 years two sessions consisting of 50 trials each were administered on the same day; a third session, administered the following day to those children who did not meet criterion of 40% CRs in session 2. The same procedures were followed at 9 years except that 2 more sessions were administered the following day to those children who did not meet the 40% CR criterion in session 2. For a detailed description of the Laboratory methods, see Jacobson et al. (2008, 2011b). In an attempt to limit transfer effects from this past training, the left eye was conditioned in the present study, whereas the right eye was conditioned in the two previous studies (Jacobson et al. 2008, 2011b). In addition, the training context in the scanner was quite different than the laboratory where the previous conditioning sessions were run. This includes the EBC equipment itself, which was commercially available in the previous studies but custom designed for the scanner in the present study. All the children had also participated in other fMRI assessments, but none had previously received conditioning trials in the scanner. Thus, the (altered) conditioning procedure was performed in a very different but nonetheless familiar training environment.
The Wechsler Intelligence Scale for Children, 4th edition (WISC-IV) (Wechsler 2003) was administered at the 10-year visit (Diwadkar et al. 2013). Testing was conducted in Afrikaans or English, depending on the primary language of instruction in the child's school. The WISC-IV was translated by a native Afrikaans-speaking Master's level neuropsychologist (MP), who had extensive experience working with the children in this cohort, and was back-translated by a second fluent Afrikaans speaker (CDM). At the 5-year assessment, we had administered the Junior South African Individual Scale (JSAIS) (Madge et al. 1981), a measure of overall intellectual competence that has been normed for South African children in Afrikaans and English. The 10-year WISC-IV IQ scores for the 47 children in this study were strongly correlated with their 5-year JSAIS scores, r = 0.78, P < 0.001.
To assess attention deficit hyperactivity disorder (ADHD), CDM administered the Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS) to each mother, and each child’s classroom teacher completed the Disruptive Behavior Disorders (DBD) Scale (Pelham et al. 1992). Participants were assigned a DSM-IV ADHD diagnosis following criteria developed in consultation with an expert in ADHD research (J Nigg, PhD). An ADHD classification was assigned if at least 6 of the 9 inattention and/or 6 of the 9 hyperactivity /impulsivity symptoms were endorsed (“pretty much” or “very much true”) by one or more informants, and some impairment was reported by 7 years of age and in two or more settings.
Conditioning Procedure
Behavioral Apparatus
The EBC apparatus was the same one used in our previous study comparing healthy children with adults (Cheng et al. 2014). Stimulus presentation was controlled, and behavioral data were recorded using a laptop computer interfaced to an NI USB-6218 data acquisition module running custom software developed under LabView version 7.1 (National Instruments, Austin, TX). Auditory stimuli were presented through the Siemens MR scanner headphones. A video (Milo and Otis) was projected without its soundtrack through a waveguide in-line with the bore of the magnet onto a rear projection screen positioned behind the bore of the magnet and viewed using an adjustable mirror attached to the single channel head coil. Standard laboratory safety goggles were modified by attaching the end of a polyethylene tube (Nalgene, Rochester, NY), which delivered an air puff to the left eye, and an MRI-compatible infrared sensor, which recorded eyeblinks. Air puff delivery was controlled by a solenoid valve (Asco, Florham Park, NJ), and a fiber-optic probe (RoMack Inc., Williamsburg, VA) measured the reflectance of infrared light from the left eye (Miller et al. 2005; Cheng et al. 2008, 2014). The safety goggles holding the infrared sensor and air nozzle were securely attached (with a Velcro strap) to the child's head to minimize effects of head motion, and the sensor and air nozzle were aimed at the child's left eye. Once the child was fitted with the conditioning goggles and positioned in the magnet, several presentations of the US were delivered to familiarize him/her with the air puff and to check that each puff generated a blink that registered on the computer screen. The examiner monitored the computer output continuously throughout the scanning session to ensure that the air puff nozzle and infrared sensor continued to be properly aimed. The child was asked to lie as still as possible and to watch the video, paying attention to the video while distracting tones and air puffs were presented.
Imaging Apparatus
The children were familiarized with the scanning procedures by first practicing in a mock scanner. The MR scans were acquired on a 3 T Allegra (Siemens, Erlangen, Germany) MRI scanner. High-resolution T1-weighted structural MR images were acquired using a 3D echo planar imaging (EPI) navigated (Tisdall et al. 2012) multiecho MPRAGE sequence (van der Kouwe et al. 2008) that had been optimized for morphometric analyses using FreeSurfer software. Imaging parameters were: FOV: 256 × 256 mm; 128 sagittal slices, TR: 2530 ms; TE: 1.53/3.21/4.89/6.57 ms; TI: 1100 ms; Flip angle: 7°; voxel size: 1.3 × 1.0 × 1.3 mm3. The 3D EPI navigator provided real-time motion tracking and correction (Tisdall et al. 2012), which served to substantially reduce the presence of motion artifacts in the structural imaging data. During the EBC sessions, a T2*-weighted gradient echo, EPI pulse sequence was used to collect 205 whole brain functional volumes sensitive to blood oxygen level dependent (BOLD) contrast (TR 2000 ms, TE 30 ms, 34 interleaved slices, slice thickness 3 mm, gap 1.5 mm, FOV 200 mm × 200 mm, in-plane resolution 3.125 × 3.125 mm2). Total brain, gray matter, white matter, and cerebellar volumes were measured from the MPRAGE images using FreeSurfer (Fischl et al. 2002).
To determine whether there was any evidence of cerebellar dysgenesis, an expert neuroanatomist (CW) examined each of the hand-traced cerebella, using a protocol in which he looked in all 3 sets of planes and examined the midline slice and then looked at the position (i.e., whether they were significantly rotated which they occasionally are), general shape, central white matter in each lobe, laminae of white matter, peduncles, inferior vermis, and flocculonodular lobe (lobule 10).
Presentation of Stimuli
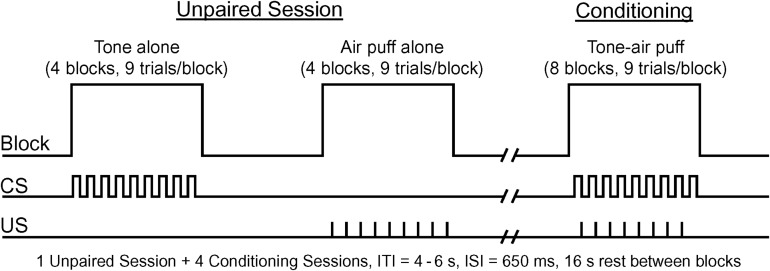
We used a modified version of a conditioning procedure that produced significant learning in children and young adults (Cheng et al. 2008, 2014). The CS tone was a binaural 1000 Hz tone (95 dB), lasting 750 ms that coterminated with a 100 ms corneal air puff to the left eye (10 psi measured at the delivery site). Audiometry assessments confirmed that all children could readily detect a 1000 Hz tone at a minimum of 40 dB. Trials were grouped into blocks: 9 trials/block, 2 s/trial, 4–6 s inter-trial interval (ITI), such that each block lasted 44–48 s. Each session consisted of eight 9-trial blocks. Unpaired trials were presented first, consisting of 4 sets of alternating tone alone and air puff alone blocks (9 stimulus presentations per block). Conditioning blocks consisted of 8 paired CS–US trials, plus a ninth CS-alone probe trial. Blocks were separated by 16 s rest periods (Fig. 1). The variable ITI in the current paradigm (4–6 s) is similar to the ITI we used previously (Cheng et al. 2008, 2014) and was selected to ensure a sufficient number of trials to permit analysis of the fMRI data. These temporal parameters were also selected to provide enough trials to permit conditioning within a limited time period and to ensure participant comfort. We performed 5 functional acquisitions—1 unpaired session and 4 paired conditioning sessions. Each imaging session lasted 6 min, 36 s. Participants were provided a 2-h lunch break between the second and third conditioning sessions.
Figure 1.
Experimental study design. Unpaired session consisted of 4 alternating tone alone and 4 air puff alone blocks. Participants were then administered 4 conditioning sessions, which consisted of 8 blocks (8 CS–US and 1 CS-alone trials/block) of tone-air puff pairings. Blocks ranged from 44 to 48 s (4–6 s ITI) and time between blocks was 16 s (ISI = interstimulus interval).
Data Analysis
The 300-ms time period prior to presentation of the tone served as a baseline for eyeblink responses. Eyeblink response amplitudes during this baseline period were compared with the maximum blink amplitude during the 350-ms preceding the airpuff to determine whether a CR occurred in each trial. The 350-ms pre-US presentation time window was selected to allow for cross study comparisons (Finkbiner and Woodruff-Pak 1991; Jacobson et al. 2008, 2011b; Cheng et al. 2014) and to exclude voluntary and alpha blink responses as CRs (Spence and Ross 1959). To qualify as a CR, the difference between the maximum blink amplitude during this 350-ms time window and the mean response amplitude during the baseline period had to exceed 3 times the standard deviation of the mean during the baseline. Behavioral performance was expressed as the percentage of trials with CRs (% CR). This behavioral measure of learning was examined in a session (unpaired and conditioning sessions 1–4) by group (FAS/PFAS, HE, Controls) repeated measures mixed analysis of variance (ANOVA). We also compared conditioning levels in the scanner to conditioning levels previously collected in our UCT behavioral laboratory following the procedures described in Jacobson et al. (2008) in a location (scanner, laboratory) by group by sessions mixed ANOVA.
Structural and functional imaging preprocessing and statistical analyses were performed with Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included motion correction, slice scan time correction, structural data coregistration, normalization, and spatial smoothing. EPI functional images were realigned and resliced correcting for minor motion artifacts, and structural images were coregistered to the mean motion-corrected functional image for each participant. Whole brain structural and functional data were transformed into standard stereotaxic space according to the Montreal Neurological Institute (MNI) protocol. A Talairach transformation was performed, and these whole brain coordinates are reported in the tables (Talairach and Tournoux 1988; Lancaster et al. 2007). Cerebellar structural and functional data were isolated and normalized into standard stereotaxic space using the spatially unbiased atlas template (SUIT) of the human cerebellum and brain stem (Diedrichsen 2006). Following SUIT and Talairach transformation, voxel dimension was 2 mm3. Functional images were spatially smoothed with a Gaussian filter (full-width half-maximum 5 mm) and temporally high-pass filtered at 128 s. Visual inspection of the EPI functional images was performed to monitor for excessive head movement. No significant group differences were seen on the SPM8 motion parameters (F(2,44) = 1.28, P = 0.29). Given the age range and diagnoses of the children, all structural and functional data were carefully inspected for proper normalization, and every child was found to be acceptable. In a block design analysis, the general linear model was used to estimate individual subject activations, and a random-effects analysis was conducted on all participants. Estimated mean beta weights were used as a measure of brain activation.
Contrasts were designed to examine brain activity changes as a function of associative learning and to account for motor performance of the unconditioned response (conditioning vs. unpaired trials) within each group. Between-group contrasts were also performed to examine learning-related changes as a function of diagnoses (FAS/PFAS and HE relative to controls and FAS/PFAS relative to HE). To control for Type I error, Monte Carlo simulations (Forman et al. 1995) were performed, which indicated that activation clusters of at least 25 (whole brain) or 10 (cerebellum) voxels were significant at a P < 0.01 level. Activation clusters surviving this threshold are reported in tabular form.
In addition, a whole brain regression analysis was performed using the maternal report of alcohol consumption (oz AA/day) during pregnancy as the predictor of interest. The 6 cerebellar regions of interest (ROIs) that emerged from this analysis were similar to those seen in the between-group contrasts. Given the consistency of the cerebellar ROIs associated with AA/day with those associated with diagnostic group, we used the ROIs related to AA/day to test for potential confounds. A 4-mm diameter sphere surrounding the peak activation voxel was used to represent activation within each ROI. Five variables were considered as potential confounders: maternal years of education, smoking during pregnancy, child sex, age at scan, and blood lead level at age 5 years. Any control variable related even weakly (at P < 0.10) to activation in a cerebellar ROI was considered a potential confounder of the effect of alcohol on that ROI. Each ROI was examined in a regression analysis in relation to AA/day and its potential confounders.
The relation of regional brain activations to behavioral performance (% CRs) was examined in 3 a priori ROIs previously reported to be involved during EBC in numerous animal and human studies using multiple techniques and in different species, including humans, rabbits, and rodents. These included lateral lobule VI (Yeo et al. 1984; Ramnani et al. 2000; Cheng et al. 2008, 2014), cerebellar deep nuclei (Lavond et al. 1984; McCormick and Thompson 1984a, 1984b; Lavond et al. 1985; Cheng et al. 2014), and hippocampus (Berger et al. 1976; McCormick and Thompson 1984a; Christian and Thompson 2003; Cheng et al. 2008, 2014). Cerebellar ROIs (bilateral deep nuclei and lobules VI) from the SUIT atlas (Diedrichsen 2006; Diedrichsen et al. 2011) and hippocampal ROIs based on probabilistic maps (Amunts et al. 2005) were used to sample individual subject brain activity in these regions.
Results
Sample Characteristics
Demographic and background characteristics of the children and their mothers are summarized in Table 1. There were no significant group differences in age and sex of the children. Mothers of the children in the exposed groups consumed 0.1–14.8 standard drinks/day. However, as we and others have previously reported, mothers did not drink on a daily basis but concentrated their drinking on the weekends, averaging 2.9–25.2 drinks per occasion. Among the drinkers, 12.8% met DSM-IV criteria for alcohol abuse, 33.3% for alcohol dependence. Only one mother reported using marijuana; one, cocaine; and none, methaqualone (“mandrax”) during pregnancy. A large majority (70.2%) smoked cigarettes, with 27.6% smoking 10 or more cigarettes per day.
Table 1.
Sample characteristics
| Controls | HE | FAS/PFAS | ||
|---|---|---|---|---|
| (n = 8) | (n = 16) | (n = 23) | F or X2 | |
| Maternal/Primary caregiver characteristics | ||||
| Mother's age at delivery | 24.7 (3.3) | 24.7 (5.1) | 30.0 (7.3) | 4.32* |
| Parity | 1.9 (0.8) | 1.4 (0.7) | 3.0 (1.8) | 6.08** |
| Primary caregiver's education (y) | 9.9 (1.4) | 9.7 (1.9) | 7.5 (2.9) | 5.11** |
| Socioeconomic statusa | 26.6 (7.6) | 26.8 (8.5) | 16.7 (7.7) | 9.35*** |
| Primary caregiver's marital status (% married) | 87.5 | 43.8 | 47.8 | 4.62† |
| oz AA/day (across pregnancy) | 0.002 (0.004) | 0.4 (0.5) | 1.2 (1.5) | 5.00* |
| Prenatal cigarettes (number/day) | 1.1 (2.1) | 5.6 (6.4) | 7.8 (6.3) | 3.83* |
| Child characteristics | ||||
| Age at scan | 10.5 (0.7) | 10.8 (0.6) | 10.4 (0.5) | 1.91 |
| Sex (% male) | 37.5 | 43.8 | 47.8 | 0.27 |
| Blood lead concentration (μg/dl) | 8.4 (2.6) | 9.0 (3.8) | 12.2 (6.6) | 2.54† |
| Total gray matter volume (cm3) | 706.6 (26.9) | 730.0 (73.6) | 673.5 (62.0) | 3.96* |
| Cerebellar gray matter volume (cm3) | 105.1 (7.4) | 109.1 (11.7) | 99.4 (11.6) | 3.68* |
| Cerebellar white matter volume (cm3) | 26.3 (2.3) | 26.7 (3.6) | 23.5 (3.7) | 4.65* |
| Attention deficit hyperactivity disorder (%) | 0.0 | 18.8 | 26.1 | 2.61 |
| WISC-IV IQb | 73.5 (11.5) | 76.5 (12.3) | 64.8 (9.7) | 5.78** |
†P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001. Values are Mean (SD) or percent.1 oz AA ≈ 2 standard drinks.
aHollingshead (2011) Four factor index of social status.
bWechsler Intelligence Scales for Children-4th edition.
Severity of FAS diagnosis was related to alcohol use during pregnancy with mothers of children with FAS and PFAS averaging 7–9 drinks/occasion on about 2 days/week (Table 1). There was no significant group difference in incidence of ADHD. As expected, there was a significant between group difference in IQ, with children with FAS or PFAS scoring more poorly than HE non-syndromal (P < 0.05) and controls (P = 0.06). There was no significant difference between the IQ scores of the children with FAS and PFAS. The children with FAS/PFAS had smaller total brain, gray matter, white matter, and cerebellar volumes.
Behavioral Findings
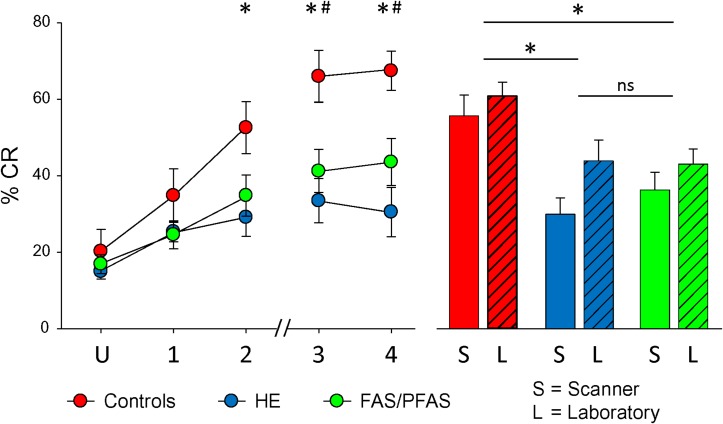
UR amplitude prior to conditioning did not differ across groups (F(2,46) = 0.407, P > 0.667). Mean (+SEs) of peak-UR-amplitude-minus-baseline were 2.36 (+1.15), 2.52 (+1.23), and 2.09 (+0.73) volts for the FAS/PFAS, HE, and Control groups, respectively. Thus, there were no group differences in US processing or motor performance that might explain differences in other measures. Significant changes in percent conditioned eyeblink responses (% CRs) were observed across sessions (Fig. 2, left). A 3 (groups) × 5 (sessions) repeated measures ANOVA showed significant main effects of session (F(4, 172) = 30.19, P < 0.001), group (F(2, 43) = 4.46, P = 0.017), and a session × group interaction (F(8, 172) = 2.57, P = 0.01). Post hoc comparisons (as indicated with * and # in Fig. 2) showed that the control group produced more CRs than the HE group during sessions 2–4 (all ts > 2.72, Ps < 0.02) and more CRs than the FAS/PFAS group during sessions 3–4 (all ts > 2.18, Ps < 0.05). There were no significant group differences during unpaired or conditioning session 1 (all ts < 1.54, Ps > 0.10). The low level (15–20%) of CRs during the unpaired session suggests that there was little “recall” of previous training outside the scanner in this study (see Discussion). Also, the HE group did not differ from the FAS/PFAS group in any of the sessions (all ts < 1.43, Ps > 0.10). The exposure group by session interaction term remained significant after adjustment for total brain volume (F(8, 168) = 3.08, P = 0.003) gray matter volume (F(8, 168) = 2.95, P = 0.004), white matter volume (F(8, 168) = 3.14, P = 0.002), and cerebellar volume, F(8, 168) = 2.74, P = 0.007.
Figure 2.
Left graph shows the development of CRs over time. Relative to the unpaired sessions (U), the control group (red) showed a significantly greater number of CRs in sessions 2–4 and the HE (blue) and FAS/PFAS (green) groups showed significantly greater CRs in sessions 1–4. Between groups analyses revealed that controls conditioned better than the HE group (*) in sessions 2–4 and the FAS/PFAS group (#) in sessions 3–4. Right graph shows average CRs inside the scanner (S) for sessions 1–4 compared with CRs measured in the behavioral laboratory. Collapsing across location, controls conditioned better than the FAS/PFAS and HE groups (*), but the FAS/PFAS group did not differ from the HE group. The hash marks on the x-axis indicate a lunch break.
Within-group ANOVAs showed main effects of session for the control group (F(4,28) = 14.19, P < 0.000003), HE group (F(4,60) = 3.95, P < 0.008), and FAS/PFAS group (F(4,84) = 13.59, P < 0.00000002). In post hoc comparisons, the control group showed more % CRs during conditioning sessions 2–4 relative to the unpaired session (all ts > 3.09, Ps < 0.02). The HE and FAS/PFAS groups both showed more % CRs during conditioning sessions 1–4 relative to the unpaired session (ts > 2.35, Ps < 0.05, and ts > 2.70, Ps < 0.02, respectively). The failure of the control group to show significant conditioning during session 1 may be due to larger variance during unpaired conditioning related to its smaller n.
Repeated measures ANOVA comparing % CRs collected inside the scanner with those collected in our UCT behavioral laboratory a year earlier (Fig. 2, right) revealed a significant main effect of location (F(1,44) = 4.80, P < 0.05) and group (F(2,44) = 4.11, P < 0.05), but no significant location × group interaction (F(2,44) = 0.16, P > 0.20). Learning was generally better in the laboratory than in the scanner. In post hoc comparisons collapsing across locations, the control group produced more CRs than the HE (t(29) = 2.77, P < 0.05) and FAS/PFAS (t(22) = 2.76, P < 0.05) groups, but the HE and FAS/PFAS groups did not differ from each other (t(37) = 0.10, P > 0.20).
Cerebellar Neuroimaging Findings
The examination of each of the hand-traced cerebella indicated that only one child, who had been diagnosed with FAS, showed definite dysmorphology. In the midline slice the prominent ascending lamina in the anterior lobe was abnormal in pattern and rapidly divided into two laminae of similar size adjacent to the midline on both right and left. The left lobe was smaller anteriorly and slightly displaced across the midline and upwards posteriorly. The laminae were abnormal in pattern in the anterior lobe. All parts of the cerebellum were present on both sides. One other child, who had been diagnosed with PFAS, had a variant in the pattern of one prominent lamina, but this may have been an unusual variant as the cerebellum was otherwise normal. All the rest of the cerebella were morphologically normal.
Sessions 1–4 Combined
Contrasts between unpaired and conditioning sessions 1–4 combined revealed learning-related changes in the cerebellum by each of the groups. The control group recruited several regions in the inferior cerebellum, with the largest significant activations found in left lobule VIIb (−14, −51, −59, 231 voxels) and right lobules VIII (22, −57, −41, 826 voxels) and IX (17, −49, −42, 558 voxels). The HE group seemed to rely on superior cerebellar regions, with the largest significant regional activations in left and right lobule VI (−19, −63, −18, 325 and 26, −65, −20, 384 voxels, respectively). Large significant regions of activation in the FAS/PFAS group were found in left Crus I (−33, −86, −27, 968 voxels) and Crus II (−18, −75, −38, 459 voxels), and right lobule IX (1, −64, −50, 1017 voxels). Given the significance of lobule VI in previous studies of EBC, it was not surprising to see this area show significant learning-related activation patterns in all children (control: −38, −52, −28, 63 voxels; HE: −19, −63, −18, 325 voxels; FAS/PFAS: −27, −63, −18, 37 voxels).
Sessions 1 and 2 (Early Learning)
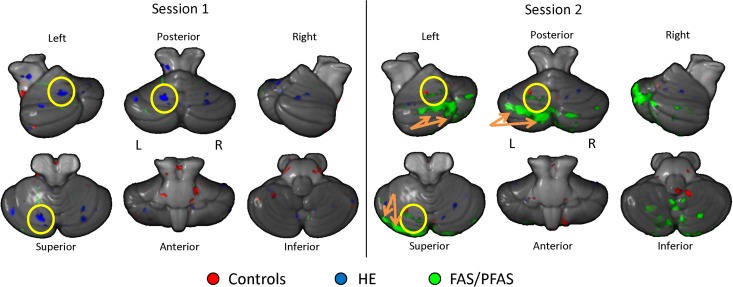
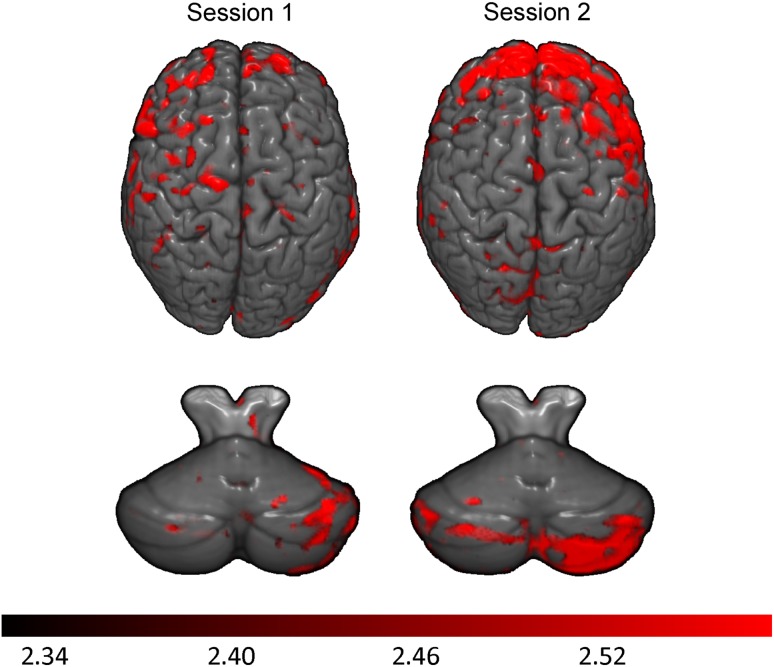
Since a significant increase in % CRs was observed early in training for all groups (Fig. 2), contrasts of brain activation during sessions 1 and 2 relative to the unpaired session were performed to determine areas of activation involved in the initial, active learning of the CS–US relationship. Activated regions within each group for sessions 1 and 2, and cerebellar activation maps are shown in Figure 3. In control children, the total number of activated voxels (red) during conditioning session 1 was 3072, compared with 1996 voxels in session 2. The HE group (blue) activated a total of 2254 cerebellar voxels during session 1 but only 283 voxels during session 2. The FAS/PFAS group (green) activated a total of 1509 voxels during session 1 compared with 14 146 voxels during session 2. The change from session 1 to session 2 illustrates how tissue activation can fluctuate across sessions as a function of diagnosis even when no behavioral change is seen. Further, significantly greater left lobule VI activation was detected during session 1 (but not session 2) for the HE group and during session 2 (but not session 1) for the FAS/PFAS group (highlighted in yellow circles). However, the FAS/PFAS group showed greater bilateral dentate activity (left: −11, −62, −30; 32 voxels; right: 15, −62, −35; 174 voxels) during session 1.
Figure 3.
Learning-related cerebellar regions of activation shown for all 3 groups (red: controls, blue: HE, green: FAS/PFAS). Clusters represent greater activation during conditioning sessions 1 and 2 relative to unpaired sessions. Yellow circles indicate left hemispheric lobule VI activation and orange arrows indicate Crus I and II activity.
Non-Cerebellar Neuroimaging Findings
Sessions 1–4 Combined
Activation patterns from non-cerebellar structures were analyzed to investigate the possibility that the exposed children were recruiting additional regions to supplement cerebellar involvement in learning. The control group showed greater neocortical activation in bilateral frontal regions (left inferior frontal: −43, 25, 4, 58 voxels and right precentral gyrus: 62, −7, 34, 93 voxels) and in the left temporal (−59, −46, 12, 53 voxels) and parietal lobes (−28, −66, 50, 94 voxels). The HE group showed very little activity in non-cerebellar regions, with greater activation detected only in the middle temporal gyrus (−64, 9 −12, 57 voxels), anterior cingulate (−1, 42, −6, 88 voxels), and lentiform nucleus (−25, −26, −3, 64 voxels). The FAS/PFAS group showed increased activation primarily in bilateral frontal regions (left inferior frontal: −60, 22, −4, 245 voxels and right middle frontal: 41, 30, 24, 88 voxels).
Between Group Comparisons
Comparisons were performed between the exposed and control groups to investigate differences in learning-related activation patterns as a function of diagnosis (Table 2) and between the FAS/PFAS and HE groups specifically (Table 3). Despite lower levels of learning relative to the control group, the HE group showed greater left lobule VI activation during sessions 1–4. Cerebellar brain activation patterns during the initial learning sessions (1 and 2) also showed interesting results. Relative to controls, the HE group showed significantly greater bilateral lobule VI activity during session 1, while the FAS/PFAS group showed greater left lobule VI activity during session 2. Finally, there were no changes in activation in the left deep nuclei and lobule VI across sessions within any of the 3 groups.
Table 2.
Brain activation differences between exposed and control groups
| Sessions 1–4 | Session 1 | Session 2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | SPM {Z} | N Vox | Brain region | X | Y | Z | SPM {Z} | N Vox | Brain region | X | Y | Z | SPM {Z} | N Vox | Brain region |
| FAS/PFAS > Controls | |||||||||||||||||
| 4 | −65 | −35 | 2.8 | 35 | Vermis VIII | −64 | 21 | 5 | 3.4 | 283 | L. Inf. Frontal Gyrus | −7 | −69 | −15 | 3 | 169 | L. Lobule VI |
| −39 | 66 | −14 | 3.4 | 110 | L. Mid. Frontal Gyrus | −23 | −72 | −21 | 3 | 114 | L. Lobule VI | ||||||
| −18 | −90 | 1 | 2.9 | 38 | L. Lingual Gyrus | −32 | −82 | −27 | 2.6 | 43 | L. Crus I | ||||||
| 60 | 21 | 20 | 3 | 35 | R. Inf. Frontal Gyrus | −43 | −75 | −27 | 2.7 | 27 | L. Crus I | ||||||
| 27 | 65 | −9 | 3 | 30 | R. Sup. Frontal Gyrus | ||||||||||||
| HE > Controls | |||||||||||||||||
| −2 | −68 | −16 | 3 | 110 | Vermis VI | 30 | −73 | −25 | 2.9 | 188 | R. Lobule VI | ||||||
| −18 | −62 | −18 | 3.1 | 37 | L. Lobule VI | −46 | −73 | −23 | 3.9 | 68 | L. Crus I | None | |||||
| 30 | −73 | −25 | 2.7 | 30 | R. Lobule VI | −31 | −61 | −55 | 2.7 | 68 | L. Lobule VIII | ||||||
| −30 | −69 | −24 | 2.7 | 24 | L. Lobule VI | −2 | −67 | −16 | 3 | 61 | Vermis VI | ||||||
| 12 | −72 | −17 | 2.5 | 11 | R. Lobule VI | 11 | −72 | −15 | 2.9 | 43 | R. Lobule VI | ||||||
| −30 | −62 | −57 | 2.5 | 10 | L. Lobule VI | −37 | −68 | −38 | 3 | 37 | L. Crus II | ||||||
| −29 | −67 | −24 | 2.7 | 20 | L. Lobule VI | ||||||||||||
| 7 | 18 | −28 | 3.2 | 51 | R. Rectal Gyrus | −13 | −74 | −17 | 2.6 | 17 | L. Lobule VI | ||||||
| −8 | 45 | −8 | 2.9 | 33 | L. Ant. Cingulate | −19 | −72 | −18 | 2.7 | 16 | L. Lobule VI | ||||||
| −29 | −83 | −19 | 2.8 | 11 | L. Crus I | ||||||||||||
| 65 | −64 | −1 | 3.8 | 59 | R. Inf. Temporal Gyrus | ||||||||||||
| −54 | 51 | −15 | 3.2 | 53 | L. Mid. Frontal Gyrus | ||||||||||||
| −60 | 12 | −1 | 3.3 | 43 | L. Sup. Temporal Gyrus | ||||||||||||
| −19 | 65 | −8 | 3 | 33 | L. Sup. Frontal Gyrus | ||||||||||||
| −61 | 27 | 35 | 3.2 | 26 | L. Mid. Frontal Gyrus | ||||||||||||
| Controls > FAS/PFAS | |||||||||||||||||
| 0 | −52 | 6 | 3.1 | 24 | Vermis VI, V | −11 | −46 | 11 | 3.2 | 103 | L. Post. Cingulate | 0 | −52 | 6 | 3 | 18 | Vermis IV, V |
| −40 | −76 | −19 | 3.1 | 19 | L. Crus I | ||||||||||||
| −52 | 25 | −21 | 3.2 | 46 | L. Sup. Temporal Gyrus | ||||||||||||
| −11 | −46 | 11 | 2.9 | 89 | L. Post. Cingulate | −64 | −13 | −16 | 3.1 | 28 | L. Inf. Temporal Gyrus | ||||||
| −51 | 5 | −29 | 3 | 36 | L. Mid. Temporal Gyrus | ||||||||||||
| Controls > HE | |||||||||||||||||
| 15 | −51 | −41 | 3.2 | 695 | R. Lobule IX | −37 | −97 | 18 | 3.8 | 77 | L. Mid. Occipital Gyrus | −5 | 38 | 55 | 3.3 | 63 | L. Sup. Frontal Gyrus |
| 29 | −59 | −47 | 2.9 | 63 | R. Louble VIII | −17 | 53 | 2 | 3.5 | 49 | L. Medial Frontal Gyrus | −54 | 21 | −16 | 3.2 | 60 | L. Sup. Temporal Gyrus |
| 53 | −63 | −48 | 3.1 | 57 | R. Crus II | 3 | −15 | 3 | 3.1 | 41 | R. Thalamus | −24 | 5 | 69 | 2.7 | 34 | L. Sup. Frontal Gyrus |
| −18 | −51 | −58 | 2.7 | 43 | L. Lobule VIII | 69 | −39 | 32 | 3.2 | 26 | R. Inf. Parietal Lobule | −22 | −100 | −6 | 3 | 32 | L. Lingual Gyrus |
| 29 | −52 | −61 | 2.5 | 32 | R. Louble VIII | −20 | −49 | 10 | 2.8 | 26 | L. Post. Cingulate | −41 | −23 | −29 | 3 | 25 | L. Inf. Temporal Gyrus |
| 30 | −92 | −13 | 2.9 | 25 | R. Fusiform Gyrus | ||||||||||||
MNI (cerebellar) and Talairach (non-cerebellar) coordinates of activation maxima (Talairach and Tournoux 1988; Schmahmann et al. 2000) in the FAS/PFAS and HE groups relative to controls during conditioning Sessions 1–4, Session 1, and Session 2. Regions listed were thresholded at a minimum cluster size of 10 (cerebellar) and 25 (non-cerebellar) voxels and z-scores of P < 0.01 (corrected).
Table 3.
Brain activation differences between FAS/PFAS and HE groups
| Sessions 1–4 | Session 1 | Session 2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | SPM {Z} | N Vox | Brain region | X | Y | Z | SPM {Z} | N Vox | Brain region | X | Y | Z | SPM {Z} | N Vox | Brain region |
| FAS/PFAS > HE | |||||||||||||||||
| 29 | −64 | −61 | 3.3 | 355 | R. Lobule VIII | 15 | −56 | −40 | 3.1 | 193 | R. Lobule VIII | 21 | −92 | −29 | 2.9 | 866 | R. Crus II |
| 15 | −55 | −41 | 2.6 | 53 | R. Lobule VIII | −19 | −79 | −28 | 2.9 | 110 | L. Crus I | −9 | −92 | −34 | 2.9 | 320 | L. Crus II |
| 27 | −73 | −46 | 2.5 | 30 | R. Lobule VIIb | −18 | −53 | −55 | 2.8 | 70 | L. Lobule VIII | 40 | −77 | −29 | 3.1 | 257 | R. Crus I |
| 55 | −68 | −38 | 2.6 | 22 | R. Crus I | −1 | −45 | −23 | 2.5 | 12 | Vermis I, II | −25 | −68 | −20 | 2.7 | 120 | L. Lobule VI |
| 56 | −59 | −45 | 2.7 | 12 | R. Crus II | 41 | −70 | −47 | 2.5 | 108 | R. Crus II | ||||||
| −69 | 2 | 3 | 3.6 | 61 | L. Sup. Temporal Gyrus | −34 | −86 | −30 | 2.6 | 88 | L. Crus I | ||||||
| −64 | 21 | 7 | 3.3 | 166 | L. Inf. Frontal Gyrus | −64 | 19 | 5 | 3.5 | 45 | L. Inf. Frontal Gyrus | −8 | −86 | −21 | 3.1 | 72 | L. Crus I |
| −39 | −95 | 20 | 3.6 | 32 | L. Mid. Occipital Gyrus | 14 | 63 | −22 | 3.1 | 36 | R. Sup. Frontal Gyrus | 51 | −67 | −47 | 2.9 | 72 | R. Crus II |
| 65 | −23 | 25 | 2.9 | 26 | R. Inf. Parietal Lobule | −58 | 26 | −7 | 3 | 32 | L. Inf. Frontal Gyrus | −19 | −84 | −21 | 2.6 | 70 | L. Crus I |
| 20 | −22 | −24 | 3.2 | 27 | R. Parahipp. Gyrus | −14 | −58 | −54 | 2.5 | 35 | L. Lobule VIII | ||||||
| 54 | −67 | −39 | 2.7 | 16 | R. Crus II | ||||||||||||
| 49 | −74 | −45 | 3.3 | 15 | R. Crus II | ||||||||||||
| 50 | −74 | −44 | 2.6 | 12 | R. Crus II | ||||||||||||
| 14 | −53 | −41 | 2.4 | 11 | R. Lobule IX | ||||||||||||
| −25 | 56 | 33 | 3.2 | 209 | L. Sup. Frontal Gyrus | ||||||||||||
| −48 | 33 | 32 | 2.9 | 197 | L. Mid. Frontal Gyrus | ||||||||||||
| −42 | 38 | 25 | 2.8 | L. Mid. Frontal Gyrus | |||||||||||||
| −33 | 12 | 61 | 3.2 | 175 | L. Mid. Frontal Gyrus | ||||||||||||
| 21 | 41 | 32 | 2.9 | 174 | R. Sup. Frontal Gyrus | ||||||||||||
| 29 | 59 | 18 | 4 | 174 | R. Mid. Frontal Gyrus | ||||||||||||
| −11 | 32 | 59 | 3.3 | 162 | L. Sup. Frontal Gyrus | ||||||||||||
| −35 | 34 | 48 | 3.4 | 159 | L. Mid. Frontal Gyrus | ||||||||||||
| −62 | 31 | 3 | 3.2 | 75 | L. Inf. Frontal Gyrus | ||||||||||||
| 10 | −100 | −16 | 2.9 | 62 | R. Lingual Gyrus | ||||||||||||
| 34 | −95 | −8 | 2.8 | 49 | R. Inf. Occipital Gyrus | ||||||||||||
| 47 | 30 | 28 | 2.8 | 48 | R. Mid. Frontal Gyrus | ||||||||||||
| −14 | 55 | 43 | 2.7 | 42 | L. Sup. Frontal Gyrus | ||||||||||||
| 30 | 20 | 53 | 2.7 | 32 | R. Mid. Frontal Gyrus | ||||||||||||
| −18 | 12 | 72 | 3 | 31 | L. Sup. Frontal Gyrus | ||||||||||||
| 67 | −27 | 25 | 2.7 | 27 | R. Inf. Parietal Lobule | ||||||||||||
| HE > FAS/PFAS | |||||||||||||||||
| 36 | −61 | −23 | 2.9 | 35 | R. Lobule VI | 28 | −73 | −20 | 3 | 227 | R. Lobule VI | −50 | −42 | −36 | 2.9 | 45 | L. Crus I |
| 24 | −54 | −47 | 2.5 | 14 | R. Lobule VIII | −45 | −71 | −22 | 3.7 | 125 | L. Crus I | ||||||
| −49 | −43 | −44 | 2.4 | 13 | L. Crus II | 37 | −61 | −25 | 2.8 | 68 | R. Lobule VI | 35 | −37 | 72 | 3.6 | 75 | R. Postcentral Gyrus |
| −29 | −59 | −58 | 2.6 | 46 | L. Lobule VIII | −4 | 6 | −19 | 3.5 | 61 | L. Medial Frontal Gyrus | ||||||
| −25 | −18 | −3 | 4 | 121 | L. Lentiform Nucleus | 12 | −62 | −56 | 2.7 | 42 | R. Lobule VIII | −23 | 56 | −12 | 3.3 | 55 | L. Sup. Frontal Gyrus |
| 16 | 14 | −25 | 3.1 | 73 | R. Inf. Frontal Gyrus | 23 | −50 | −50 | 2.5 | 38 | R. Lobule VIII | 39 | −69 | 51 | 2.9 | 44 | R. Sup. Parietal Lobule |
| −4 | 43 | −8 | 3.3 | 63 | L. Medial Frontal Gyrus | −17 | −72 | −19 | 2.7 | 25 | L. Lobule VI | −52 | 13 | −30 | 2.8 | 30 | L. Sup. Temporal Gyrus |
| −49 | 13 | −28 | 2.9 | 59 | L. Sup. Temporal Gyrus | −16 | −81 | −17 | 3.2 | 25 | L. Lobule VI | ||||||
| 24 | 24 | −33 | 3.3 | 53 | R. Sup. Temporal Gyrus | ||||||||||||
| 47 | 19 | −17 | 3 | 52 | R. Sup. Temporal Gyrus | −25 | −20 | −3 | 4 | 131 | L. Lentiform Nucleus | ||||||
| 14 | 14 | −25 | 4.1 | 97 | R. Inf. Frontal Gyrus | ||||||||||||
| 18 | 28 | −31 | 3.4 | 48 | R. Orbital Gyrus | ||||||||||||
| −44 | −64 | 3 | 3.2 | 25 | L. Mid. Temporal Gyrus | ||||||||||||
MNI (cerebellar) and Talairach (non-cerebellar) coordinates of activation maxima (Talairach and Tournoux 1988; Schmahmann et al. 2000) for between groups comparisons during conditioning Sessions 1–4, Session 1, and Session 2. Regions listed were thresholded at a minimum cluster size of 10 (cerebellar) and 25 (non-cerebellar) voxels and z-scores of P < 0.01 (corrected).
Activations in Relation to a Continuous Measure of Maternal Alcohol Consumption
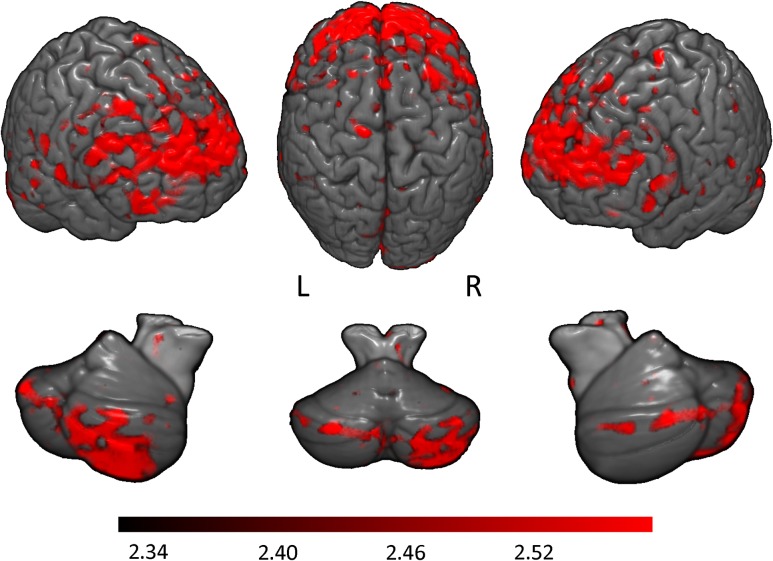
A whole brain regression analysis was performed using the maternal alcohol report (oz AA/day) during pregnancy as a predictor of interest. Greater prenatal alcohol exposure was associated with greater learning-related activations during conditioning sessions 1–4 and during initial learning (sessions 1 and 2) in both the cerebellar and neocortical regions (Fig. 4). Neocortical regions where activity significantly correlated with the continuous measure of exposure included bilateral superior, inferior, and middle frontal gyri, left lingual gyrus, left cuneus, and right inferior parietal lobe. Cerebellar regions where activity correlated with this measure included bilateral Crus I, right lobule VIIb, left lobules VI and IX, and vermis VI. Maps for individual sessions 1 and 2, when behavioral differences began to emerge between the controls and exposed groups (Fig. 2), are also shown (Fig. 5).
Figure 4.
Magnitude of correlation between learning-related activations (conditioning sessions 1–4) and mother's report of alcohol consumption (oz of AA) during pregnancy. Higher maternal alcohol consumption predicted greater activation in these regions. Top row represents neocortical activations; bottom row, cerebellar activations. Color bar indicates magnitude of z-scores.
Figure 5.
Magnitude of correlation between learning-related activations (conditioning sessions 1 and 2) and mother's report of alcohol consumption (oz of AA) during pregnancy. Higher maternal alcohol consumption predicted greater activation in these regions. Top row represents neocortical activations; bottom row, cerebellar activations. Color bar indicates magnitude of z-scores.
Regression analysis was used to examine the degree to which the relation of prenatal alcohol exposure to activation in each of the 6 cerebellar ROIs that emerged from this analysis might be confounded with demographic background or other exposures. These analyses indicate that the relation of AA/day to bilateral Crus 1, right lobule VIIb, and left lobule VI cannot be attributed to confounding with maternal education, smoking during pregnancy, child sex, age at scan, or postnatal lead exposure. However, the effects of AA/day on left lobule IX and vermis VI were no longer significant after controlling for maternal education or smoking during pregnancy.
Relation of Neuroimaging Data to Behavior
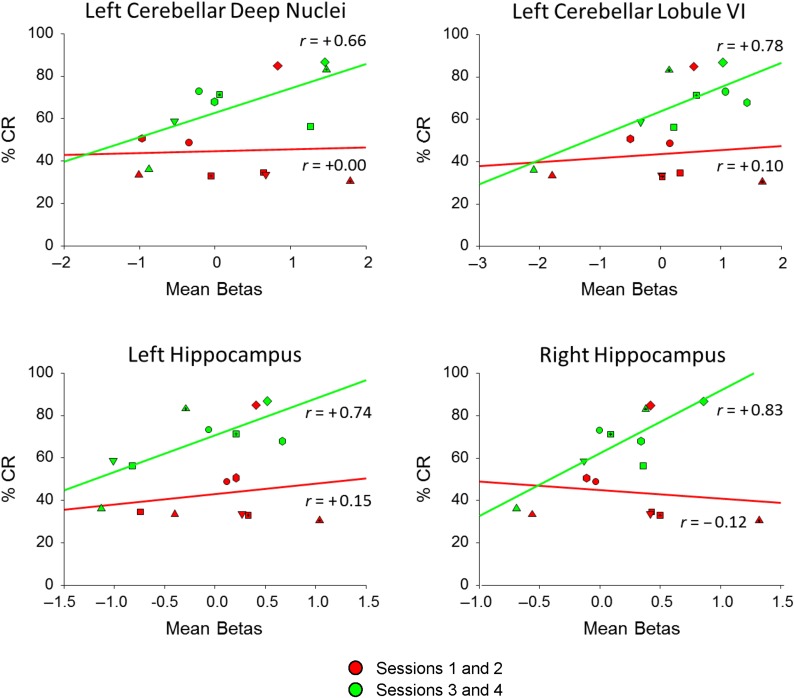
Mean activation within 6 structurally defined ROIs (bilateral cerebellar deep nuclei, lateral lobules VI, and hippocampus) was examined in relation to % CRs. These regions were selected because they have been shown in the animal literature to contribute significantly to the production of CR. The data were averaged to compare the early sessions (1 and 2) data with the late sessions (3 and 4).
The control group showed significant positive correlations between % CRs and activity in several brain structures, whereas the FAS/PFAS and HE groups did not (all Ps > 0.15) (Table 4). For the control group, significant correlations with % CRs were observed for sessions 3 and 4 in left lateral lobule VI, and left and right hippocampus. Scatterplots with regression lines show the positive relation of mean activity in these structures with performance in the control group during the sessions 3 and 4 (Fig. 6).
Table 4.
Relation of brain activation to percent CR
| Sessions | Left | Right | Left | Right | Left | Right | |
|---|---|---|---|---|---|---|---|
| Deep nuclei | Deep nuclei | Lobule VI | Lobule VI | Hippocampus | Hippocampus | ||
| Controls | 1 and 2 | 0.00 | −0.21 | 0.10 | 0.31 | 0.15 | −0.12 |
| 3 and 4 | 0.66† | 0.46 | 0.78* | 0.62 | 0.74* | 0.83* | |
| HE | 1 and 2 | −0.02 | −0.02 | 0.07 | −0.02 | 0.01 | 0.06 |
| 3 and 4 | 0.04 | 0.07 | −0.15 | −0.08 | −0.14 | −0.01 | |
| FAS/PFAS | 1 and 2 | 0.25 | 0.24 | 0.22 | 0.18 | 0.15 | 0.19 |
| 3 and 4 | 0.09 | 0.16 | 0.16 | 0.16 | 0.22 | 0.29 |
Pearson correlation values between mean brain activity during early (Sessions 1 and 2) and late (Sessions 3 and 4) sessions with behavioral performance for these sessions. Brain activation in the FAS/PFAS/HE groups was not significantly correlated with learned behavior (all Ps > 0.17). Brain activity in the control group (lobule VI and hippocampus) was significant correlated during sessions 3 and 4 (bolded). †P < 0.10, *P < 0.05
Figure 6.
Scatterplots with regression lines for the control group showing the relation of regional brain activation to CR. Early (red) session (1 and 2) behavior was not correlated with brain activity while late (green) session (3 and 4) behavior was positively correlated. Unique data plots represent individual subjects.
Discussion
This is the first study to investigate directly how brain activity during human EBC is affected by prenatal alcohol exposure. All of the children were generally familiar with the EBC task as they participated in two previous EBC experiments involving delay conditioning outside the scanner (Jacobson et al. 2008, 2011b). However, evidence for “recall” or positive transfer of this training was limited (see below). It is noteworthy that the behavioral performance data confirm our previous findings linking prenatal alcohol exposure to impaired conditioning in children. Although the alcohol-exposed children were impaired in EBC relative to controls, both the FAS/PFAS and HE groups showed significant learning over multiple sessions. Despite their impaired learning, the exposed children showed extensive cerebellar activations, and our continuous measure of maternal alcohol consumption during pregnancy was associated with learning-related increased activation in both the cerebellar and frontal regions. Patterns of activation in lobule VI, a cerebellar region known to be involved in EBC acquisition, differed between groups, with the HE children showing greater activation during the first conditioning session; FAS/PFAS, during the second. Only healthy controls showed significant correlations between behavioral conditioning (% CRs) and cerebellar activations, providing additional evidence that these regions may be functionally damaged in the exposed groups.
Behavior
The impairment of EBC by prenatal alcohol exposure in this study was similar to that reported in previous studies with these subjects (Jacobson et al. 2008, 2011b). Participation in these previous laboratory EBC experiments did not result in recall of previously trained CRs in the present study. The level of responding during the unpaired session of 15–20% is typical of that seen in human subjects that are naïve (e.g., Cheng et al. 2008) or receive unpaired training (Herbert et al. 2003), including children tested in this scanner (Cheng et al. 2014). This absence of “recall” likely reflects the switch to the eye that was not trained in the earlier studies, major differences in the training context and the time passed (>1 year) since the previous laboratory training sessions. This absence of recall does not allow us to entirely rule out a role for “savings” in the outcome of this study. The controls in the present study showed somewhat better acquisition of CRs relative to naïve participants trained with the same methods in this scanner at about the same age (mean age = 11.5 years; range = 9.3–13.8; Cheng et al. 2014). Whether this difference of about 10% CR production reflects cross-experiment variation or a true savings effect is difficult to determine without additional studies. We also do not know whether alcohol-exposed groups would have shown poorer CR acquisition if they had not been previously trained on EBC.
EBC performance was poorer in the MRI scanner than in the laboratory, presumably due to the change in the environment, including the noise generated by the scanner, sense of confinement, and requirement to lie still. Despite these limitations, the effects of prenatal alcohol exposure reported in our previous behavioral studies were also clearly seen here. Our finding of impaired behavioral performance is consistent with studies on effects of alcohol abuse in adults (McGlinchey-Berroth et al. 1995, 2002; McGlinchey et al. 2005; Fortier et al. 2008), studies of children with prenatal alcohol exposure (Coffin et al. 2005; Jacobson et al. 2008, 2011b), and laboratory animal studies (Stanton and Goodlett 1998; Brown et al. 2006, 2007, 2008; Thomas and Tran 2012). This behavioral impairment may be related to damage to the olivary nucleus (Napper and West 1995) which could prevent information about the airpuff US from reaching the cerebellum. It is not related to US-processing or motor-performance deficits per se as UR amplitude was not different across groups in the present study and UR rates are unaffected in FAS children (Jacobson et al. 2008, 2011b) or animals (Stanton and Goodlett 1998). In rodent models, developmental alcohol exposure is associated with a reduction in neurons in the inferior olive (Napper and West 1995), which may produce a weaker US input, thereby interfering with the cerebellum's ability to process US cues in the exposed groups. Ethanol-exposed neonatal rats showed impaired EBC at 3 different periorbital shock US intensities but had intact URs (eyeblinks) (Lindquist et al. 2007), indicating normal US processing outside cerebellar circuitry.
Despite this impairment, the exposed groups did show some learning, producing significant but only slightly greater % CRs during conditioning relative to unpaired sessions. Two factors likely contributed to this learning. These children may have benefited from exposure to CS–US presentations during previous EBC assessments at 5 and 9 years at the UCT Child Development Research Laboratory (Jacobson et al. 2008, 2011b). Even at 5 years, a small proportion of those with PFAS and HE met criterion for conditioning. The children participating in this neuroimaging assessment were also older (~10 years old) than at their previous conditioning experiments, and their performance is consistent with that of similarly aged naïve children reported in Jacobson et al. (2011b), among whom only 33.3% of the children with FAS and 42.9% of those with HE met criterion for conditioning, compared with 79.3% of controls.
Cerebellar Activations
Contrasts (conditioning vs. unpaired sessions) designed to assess learning-related activations revealed extensive cerebellar functional activity in all children (Tables 2 and 3; Fig. 3). This finding is consistent with human and laboratory animal data pointing to the critical role of the cerebellum in EBC (Christian and Thompson 2003), including lesion (Gerwig et al. 2003, 2010), neuroimaging (Molchan et al. 1994; Logan and Grafton 1995; Blaxton et al. 1996; Schreurs et al. 1997; Knuttinen et al. 2002; Cheng et al. 2008; Parker et al. 2012; Cheng et al. 2014), and neuromodulation (Zuchowski et al. 2014) studies. Animal studies specifically implicate two cerebellar regions in EBC: the deep nuclei (Lavond et al. 1984; McCormick and Thompson 1984a, 1984b; Lavond et al. 1985) and lobule VI (Yeo et al. 1984, 1985; Yeo and Hardiman 1992).
Despite poorer behavioral performance, the HE group showed greater bilateral lobule VI activity relative to controls (Table 2) and children with FAS/PFAS (Table 3) during Session 1. This pattern is similar to that seen during an n-back task, in which HE subjects showed increased activity in the caudate, putamen, and dorsal prefrontal cortex relative to the control and FAS/PFAS groups (Diwadkar et al. 2013). Greater lobule VI activity was also seen in alcoholic adults relative to controls, despite comparable performance, during a verbal working memory task (Desmond et al. 2003). Since there were no behavioral differences, this pattern was thought to reflect a compensatory effect, whereby greater functional activation is required for normal performance. Activations of more extensive brain structures in children with prenatal alcohol exposure have also been reported in fMRI studies of response inhibition (Fryer et al. 2007) and number processing (Meintjes et al. 2010). This compensatory interpretation is also consistent with our previous study (Cheng et al. 2014) in which healthy children showed greater cerebellar activation than adults despite producing fewer CRs.
Relative to the unpaired session, the HE group activated left lobule VI (ipsilateral to the trained eye) more than both the control and FAS/PFAS groups during Session 1, whereas the FAS/PFAS group activated this region more than control and HE children during Session 2 (Tables 2 and 3). Although audiometric tests have established that alcohol-exposed children can hear the auditory CS as well as control children (Jacobson et al. 2008), it is possible that deficits in transmission of auditory signal to the cerebellum in the FAS/PFAS group lead to the timing of the cerebellar lobule VI activations since this area also receives auditory CS information via ponto-cerebellar pathways (Steinmetz et al. 1986). Normal hearing despite delays in auditory processing has been measured in children with FAS using magnetoencephalography (MEG) (Stephen et al. 2012), but it remains to be determined whether this finding applies to the cerebellar circuitry.
Non-Cerebellar Activations
Contrary to our prediction that the exposed children might be more likely to recruit non-cerebellar regions to compensate for cerebellar impairment, the control and exposed children showed comparable amounts of activation throughout Sessions 1–4 (Table 2). The pattern of activations seen in this study is consistent with that found in functional neuroimaging studies of healthy humans, which report widespread neocortical activation associated with EBC (Molchan et al. 1994; Logan and Grafton 1995; Blaxton et al. 1996; Schreurs et al. 1997; Ramnani et al. 2000; Parker et al. 2012). However, during Session 2 controls showed greater neocortical activations than the exposed children and the FAS/PFAS group showed greater activity than the HE group. Neocortical regions have also been shown to be recruited during trace conditioning (Weiss and Disterhoft 2011). These findings indicate that while mainly reliant on the cerebellum, human EBC may also be facilitated by neocortical involvement.
Higher levels of maternal drinking during pregnancy were associated with greater learning-related activity in both frontal and cerebellar regions. This positive correlation further supports the interpretation that exposed children whose mothers drank the most and who showed the most impaired conditioning relative to controls activated alternative pathways when performing this task. This finding of a direct correlation between maternal alcohol consumption and task-related levels of activation in specific brain regions is consistent with other recent findings from our research program linking maternal report of alcohol consumption during pregnancy to alterations in brain structure, metabolism, and function (De Guio et al. 2014; Du Plessis et al. 2014; Meintjes et al. 2014).
Brain-Behavior Relations
Consistent with our previous investigation of EBC in healthy children (Cheng et al. 2014) and a PET study in adults (Logan and Grafton 1995), activations in left lobule VI and hippocampus were significantly correlated with % CRs and approached significance in the cerebellar deep nuclei (P < 0.10) in the control group. Cerebellar deep nuclear and lobule VI activity have been shown to be critically involved in acquisition and expression of behavioral CRs in laboratory animals (Yeo et al. 1984; Lavond et al. 1985). By contrast, the lack of significant correlations between activity in these regions and % CRs in the FAS/PFAS and HE groups suggests that the diminished CRs observed in those groups are partially mediated by other cerebellar and neocortical regions.
Limitations
One limitation of this study is the relatively small size of the control group, which nonetheless demonstrated significant learning. The loud noise and discomfort associated with lying still in the MRI environment may have contributed to lower conditioning levels compared with these children's performance outside the scanner. Given their previous participation in behavioral EBC studies (Jacobson et al. 2008, 2011b), these children's performance may have been modestly facilitated and their activation patterns altered by prior conditioning experience; nonetheless, alcohol exposure was related to poorer EBC. Brain activation patterns may also have been affected by previous training; for example, bilateral cerebellar activations reported here may reflect the prior training, in which the air puff was directed to the right eye. It is also possible that this may reflect bilateral eyeblink CRs (Campolattaro and Freeman 2009), but we did not measure responses from the untrained eye in the current study. Since the exposed groups showed significantly fewer CRs than the control group, it cannot be definitively ruled out that brain activation differences were also not a result rather than a cause of motor performance of the CR. This is a limitation of all fMRI studies not only the present one. However, contrasts between FAS/PFAS and HE groups (where CRs were comparable), suggest these regions do not reflect solely motor performance of the eyeblink. Finally, although motion artifact is always a concern in fMRI studies with children, the groups did not differ on any of the SPM8 motion parameters.
Conclusions
This is the first study to directly investigate how brain activity during human EBC is affected by prenatal alcohol exposure. Neurodevelopmental injuries, such as FASD, are often difficult to assess due to the lack of a distinctive behavioral phenotype. However, EBC provides a well-characterized model system that can contribute to our understanding of the etiology and diagnosis of FASD. The combination of extensive activations associated with poorer behavioral performance by the exposed children could represent a potential FASD biobehavioral marker (Jacobson et al. 2011a). Identifying activation patterns within the neural circuitry that traditionally supports this basic form of learning represents a prototype for how this circuitry can be impaired by environmental insults, such as, prenatal alcohol exposure. A better understanding of these processes may facilitate diagnosis, provide a biobehavioral indicator, and contribute to development of potential treatments of these disorders.
Notes
We thank M September, our University of Cape Town (UCT) research nurse and the UCT research staff for their work with the cohort and collecting the EBC and fMRI data and R Sun, our Wayne State University research assistant, for scoring the alcohol exposure data. We also acknowledge the contributions of the Cape Universities Brain Imaging Centre (CUBIC) radiographers ML de Villiers and N Maroof. We thank HE Hoyme, MD, LK Robinson, MD, and N Khaole, MD, who performed the dysmorphology assessments, and J Nigg, PhD, who consulted on the ADHD diagnoses. We greatly appreciate the participation of the mothers and children in the Cape Town Longitudinal Study.
Funding
National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (two supplements R01AA09524 and R01AA016781 to SWJ, K01AA020873 to DTC, R01AA018694 to JED), the National Institutes of Health Office of Research on Minority Health (to SWJ); the National Institutes of Health/National Institute of Child Health and Human Development Intellectual and Developmental Disabilities Research Center (U54HD079123 to JED); National Institutes of Health Fogarty International Center (R03TW007030 to SWJ); the South African National Research Chair Initiative (to EMM); and the Lycaki-Young Fund, from the State of MI (to SWJ and JLJ). The FAS diagnostic assessments conducted at the 2005 dysmorphology clinic were funded under grants from the NIAAA Collaborative Initiative on FASD (U01AA014790 to SWJ and U24AA014815 to KL Jones).
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl). 210:343–352. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. 2001. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol Alcohol. 36:147–159. [DOI] [PubMed] [Google Scholar]
- Berger TW, Alger B, Thompson RF. 1976. Neuronal substrate of classical conditioning in the hippocampus. Science. 192:483–485. [DOI] [PubMed] [Google Scholar]
- Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, Disterhoft JF. 1996. Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. J Neurosci. 16:4032–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. 1975. Measurement and interpretation of drinking behavior. I. On measuring patterns of alcohol consumption. II. Relationships between drinking behavior and social adjustment in a sample of problem drinkers. J Stud Alcohol. 36:1154–1172. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. 2007. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Dev Psychobiol. 49:243–257. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Stanton ME. 2008. Dose-dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcohol Clin Exp Res. 32:277–293. [DOI] [PubMed] [Google Scholar]
- Brown KL, Pagani JH, Stanton ME. 2006. The ontogeny of interstimulus interval (ISI) discrimination of the conditioned eyeblink response in rats. Behav Neurosci. 120:1057–1070. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. 2009. Examination of bilateral eyeblink conditioning in rats. Behav Neurosci. 123:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. 2008. Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci U S A. 105:8108–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Meintjes EM, Stanton ME, Desmond JE, Pienaar M, Dodge NC, Power JM, Molteno CD, Disterhoft JF, Jacobson JL, et al. 2014. Functional MRI of cerebellar activity during eyeblink classical conditioning in children and adults. Hum Brain Mapp. 35:1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. 2003. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 10:427–455. [DOI] [PubMed] [Google Scholar]
- Clarren SK. 1986Neuropathology and fetal alcohol syndrome In: West JR, editor.Alcohol and Brain Development. New York: Oxford University Press; p. 158–166. [Google Scholar]
- Clarren SK, Smith DW. 1978. The fetal alcohol syndrome. N Engl J Med. 298:1063–1067. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O'Neill J. 2005. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 41:389–398. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. 1997. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 21:150–161. [PubMed] [Google Scholar]
- De Guio F, Mangin JF, Riviere D, Perrot M, Molteno CD, Jacobson SW, Meintjes EM, Jacobson JL. 2014. A study of cortical morphology in children with fetal alcohol spectrum disorders. Hum Brain Mapp. 35:2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. 2003. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 19:1510–1520. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. 2006. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 33:127–138. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Kuper M, Thurling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. 2011. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 54:1786–1794. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, Jacobson SW, Jacobson JL. 2013. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum Brain Mapp. 34:1931–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Molteno CD, Meintjes EM, Bangalore S, Diwadkar V, Hoyme EH, Robinson LK, Khaole N, Avison MJ, et al. 2009. Prenatal alcohol exposure and interhemispheric transfer of tactile information: Detroit and Cape Town findings. Alcohol Clin Exp Res. 33:1628–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis L, Jacobson JL, Jacobson SW, Hess AT, van der Kouwe A, Avison MJ, Molteno CD, Stanton ME, Stanley JA, Peterson BS, et al. 2014. An in vivo (1)H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 38:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff-Pak DS. 1991. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging. 6:109–117. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Steffen EM, Lafleche G, Venne JR, Disterhoft JF, McGlinchey RE. 2008. Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology. 22:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH. 2010. Developmental Neurobiology of Cerebellar Learning In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; p. 546–572. [Google Scholar]
- Freeman JH Jr, Barone S Jr, Stanton ME. 1995. Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. J Neurosci. 15:7301–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. 2007. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 31:1415–1424. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, Boring D, Thilmann AF, Forsting M, Diener HC, et al. 2003. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain. 126:71–94. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Guberina H, Esser AC, Siebler M, Schoch B, Frings M, Kolb FP, Aurich V, Beck A, Forsting M, et al. 2010. Evaluation of multiple-session delay eyeblink conditioning comparing patients with focal cerebellar lesions and cerebellar degeneration. Behav Brain Res. 212:143–151. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. 1995. The Alcohol Use Disorder and Associated Disabilities Interview schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend. 39:37–44. [DOI] [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. 2002. a. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learn Mem. 9:304–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. 2002. b. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 956:302–311. [DOI] [PubMed] [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME. 2003. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 117:1196–1210. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. 2011. Four factor index of social status. Yale J Sociol. 8:21–51. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, et al. 2005. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. 2002. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 109:815–825. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. 2011. a. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol Rev. 21:148–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, et al. 2011. b. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 35:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. 2008. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 32:365–372. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. 1973. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Parrish TB, Weiss C, LaBar KS, Gitelman DR, Power JM, Mesulam MM, Disterhoft JF. 2002. Electromyography as a recording system for eyeblink conditioning with functional magnetic resonance imaging. Neuroimage. 17:977–987. [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. 2007. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavond DG, Hembree TL, Thompson RF. 1985. Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Res. 326:179–182. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Lincoln JS, McCormick DA, Thompson RF. 1984. Effect of bilateral lesions of the dentate and interpositus cerebellar nuclei on conditioning of heart-rate and nictitating membrane/eyelid responses in the rabbit. Brain Res. 305:323–330. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. 2015. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 39:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist DH, Sokoloff G, Milner E, Steinmetz JE. 2013. Neonatal ethanol exposure results in dose-dependent impairments in the acquisition and timing of the conditioned eyeblink response and altered cerebellar interpositus nucleus and hippocampal CA1 unit activity in adult rats. Alcohol. 47:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist DH, Sokoloff G, Steinmetz JE. 2007. Ethanol-exposed neonatal rats are impaired as adults in classical eyeblink conditioning at multiple unconditioned stimulus intensities. Brain Res. 1150:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CG, Grafton ST. 1995. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci U S A. 92:7500–7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge EM, Van Den Berg AR, Robinson M , Land man J.. 1981. Junior South African individual scales, Pretoria, South Africa: Human Sciences Research Council. [Google Scholar]
- Mattson SN, Riley EP. 2011. The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Res Health. 34:51–55. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. 1998. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 12:146–153. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, et al. 2013. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 37:818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, et al. 2007. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 88:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. 1984. a. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 223:296–299. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. 1984. b. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J Neurosci. 4:2811–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey RE, Fortier CB, Capozzi SM, Disterhoft JF. 2005. Trace eyeblink conditioning in abstinent alcoholic individuals: effects of complex task demands and prior conditioning. Neuropsychology. 19:159–170. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Cermak LS, Carrillo MC, Armfield S, Gabrieli JD, Disterhoft JF. 1995. Impaired delay eyeblink conditioning in amnesic Korsakoff's patients and recovered alcoholics. Alcohol Clin Exp Res. 19:1127–1132. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Fortier CB, Cermak LS, Disterhoft JF. 2002. Temporal discrimination learning in abstinent chronic alcoholics. Alcohol Clin Exp Res. 26:804–811. [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore JC, et al. 2010. An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 34:1450–1464. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, der Kouwe AJ, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW. 2014. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin. 5:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Li L, Weiss C, Disterhoft JF, Wyrwicz AM. 2005. A fiber optic-based system for behavioral eyeblink measurement in a MRI environment. J Neurosci Methods. 141:83–87. [DOI] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. 1994. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci U S A. 91:8122–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper RM, West JR. 1995. Permanent neuronal cell loss in the inferior olive of adult rats exposed to alcohol during the brain growth spurt: a stereological investigation. Alcohol Clin Exp Res. 19:1321–1326. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. 2009. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 15:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Andreasen NC, Liu D, Freeman JH, Ponto LL, O'Leary DS. 2012. Eyeblink conditioning in healthy adults: a positron emission tomography study. Cerebellum. 11:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Evans SW, Gnagy EM, Greenslade KE. 1992. Teacher ratings of DSM–III–R symptoms for the disruptive behavior disorders: prevalence, factor analyses, and conditional probabilities in a special education sample. School Psychol Rev. 21:285–299. [Google Scholar]
- Ramnani N, Toni I, Josephs O, Ashburner J, Passingham RE. 2000. Learning- and expectation-related changes in the human brain during motor learning. J Neurophysiol. 84:3026–3035. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. 1995. Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University School of Medicine. [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. 2000. MRI Atlas of the Human Cerebellum. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, McIntosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE. 1997. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J Neurophysiol. 77:2153–2163. [DOI] [PubMed] [Google Scholar]
- Spence KW, Ross LE. 1959. A methodological study of the form and latency of eyelid responses in conditioning. J Exp Psychol. 58:376–381. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Fox GD, Carter CS. 1998. Ontogeny of the conditioned eyeblink response in rats: acquisition or expression. Neuropharmacology. 37:623–632. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH Jr, Skelton RW. 1992. Eyeblink conditioning in the developing rat. Behav Neurosci. 106:657–665. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. 1998. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 22:270–275. [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. 1986. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav Neurosci. 100:878–887. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Kodituwakku PW, Kodituwakku EL, Romero L, Peters AM, Sharadamma NM, Caprihan A, Coffman BA. 2012. Delays in auditory processing identified in preschool children with FASD. Alcohol Clin Exp Res. 36:1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. 1996. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, D.C.: National Academy Press. [Google Scholar]
- Suttie M, Foroud T, Wetherill L, Jacobson JL, Molteno CD, Meintjes EM, Hoyme HE, Khaole N, Robinson LK, Riley EP, et al. 2013. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics. 131:e779–e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme. [Google Scholar]
- Thomas JD, Tran TD. 2012. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 22:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurling M, Kahl F, Maderwald S, Stefanescu RM, Schlamann M, Boele HJ, De Zeeuw CI, Diedrichsen J, Ladd ME, Koekkoek SK, et al. 2015. Cerebellar cortex and cerebellar nuclei are concomitantly activated during eyeblink conditioning: a 7T fMRI study in humans. J Neurosci. 35:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. 2012. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B. 2008. Brain morphometry with multiecho MPRAGE. Neuroimage. 40:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2003. WISC-IV Administration Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weiss C, Disterhoft JF. 2011. Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behav Neurosci. 125:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS. 1988. Aging and classical conditioning: parallel studies in rabbits and humans. Neurobiol Aging. 9:511–522. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ. 1992. Cerebellar cortex and eyeblink conditioning: a reexamination. Exp Brain Res. 88:623–638. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. 1984. Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behav Brain Res. 13:261–266. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. 1985. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 60:99–113. [DOI] [PubMed] [Google Scholar]
- Zuchowski ML, Timmann D, Gerwig M. 2014. Acquisition of conditioned eyeblink responses is modulated by cerebellar tDCS. Brain Stimul. 7:525–531. [DOI] [PubMed] [Google Scholar]