Abstract
Background:
Considerable debate continues to surround the concept of mitochondrial dysfunction in aging muscle. We tested the overall hypothesis that age per se does not influence mitochondrial function and markers of mitochondria quality control, that is, expression of fusion, fission, and autophagy proteins. We also investigated the influence of cardiorespiratory fitness (VO2max) and adiposity (body mass index) on these associations.
Methods:
Percutaneous biopsies of the vastus lateralis were obtained from sedentary young (n = 14, 24±3 years), middle-aged (n = 24, 41±9 years) and older adults (n = 20, 78±5 years). A physically active group of young adults (n = 10, 27±5 years) was studied as a control. Mitochondrial respiration was determined in saponin permeabilized fiber bundles. Fusion, fission and autophagy protein expression was determined by Western blot. Cardiorespiratory fitness was determined by a graded exercise test.
Results:
Mitochondrial respiratory capacity and expression of fusion (OPA1 and MFN2) and fission (FIS1) proteins were not different among sedentary groups despite a wide age range (21 to 88 years). Mitochondrial respiratory capacity and fusion and fission proteins were, however, negatively associated with body mass index, and mitochondrial respiratory capacity was positively associated with cardiorespiratory fitness. The young active group had higher respiration, complex I and II respiratory control ratios, and expression of fusion and fission proteins. Finally, the expression of fusion, fission, and autophagy proteins were linked with mitochondrial respiration.
Conclusions:
Mitochondrial respiration and markers of mitochondrial dynamics (fusion and fission) are not associated with chronological age per se, but rather are more strongly associated with body mass index and cardiorespiratory fitness.
Keywords: Muscle, Mitochondria, Aging, Body composition, Physical activity
Age-associated decline in skeletal muscle mitochondrial capacity has been extensively studied as an underlying factor for sarcopenia (1,2), a condition characterized by a progressive loss of muscle mass and strength (3), and slower walking speed (4) and fatigability (5). However, while numerous cross-sectional human studies have demonstrated decreases in mitochondrial capacity with chronological age (6–11), several others have failed to observe these changes (2,12–15). The inconsistent results may be partially due to the various definitions of the term “mitochondrial function” and the different approaches employed to assess mitochondrial function, including: respiration (4), ATP production (10,14), mitochondrial permeability transition pore function (2), and H2O2 emission (16). Furthermore, several of these investigations were performed in isolated mitochondria (6,9,11), which has been shown to exaggerate the observed deficit in mitochondrial function, when compared to measurements conducted on permeabilized myofibers (17). Moreover, most studies of mitochondrial respiration have not considered important covariates such as participant physical activity levels (9), adiposity (11), and fiber type percentage, which could confound the relationship between mitochondrial capacity and age (10,12,18,19). Collectively, these studies suggest that careful consideration of the methods and participant characteristics are needed when investigating age-related declines in mitochondrial function.
The mitochondrial reticulum is dynamic and undergoes constant remodeling through fusion, fission (20) and recycling by autophagy (21). Emerging evidence indicates that these processes are essential for the maintenance of a healthy mitochondrial pool (22). Mitochondrial fusion is regulated by proteins mitofusion-1 and -2 (MFN1 and MFN2) and optic atrophy-1 (OPA1). Fusion allows the components of the mitochondria to be exchanged and diluted, which is thought to prevent mutations in mtDNA caused by respiratory dysfunction (23). Mitochondrial fission is regulated by proteins fission-1 (FIS1) and dynamin-related protein 1 (DRP1). Fission segregates damaged portions of the mitochondria for removal by mitophagy (mitochondrial specific autophagy) (24). While the orchestration of fusion, fission, and autophagy are important for maintaining mitochondrial integrity, few human studies have examined mitochondrial quality control in aging and how they relate to other mitochondrial functions (1,25).
The purpose of this study was to comprehensively assess mitochondrial respiratory characteristics across a wide age range using permeabilized myofibers, an approach that preserves the mitochondrial reticulum, while considering participant cardiorespiratory fitness (VO2max), and body mass index (BMI). We also sought to determine whether the expression of mitochondrial quality control proteins is affected by age. We hypothesized that mitochondrial respiration and expression of quality control proteins would be unaffected by chronological age per se, but would be more closely associated with age-related changes in BMI and cardiorespiratory fitness.
Method
Subjects
Sixty-eight men and women participated in this cross-sectional study. Subjects were eligible if they were between the ages of 20–90 years, were weight stable (±3kg in preceding 3 months), and in good general health. Subjects were excluded if they were participating in another interventional research study, had a chronic medical condition and were pregnant or breast-feeding. Additional inclusion and exclusion criteria specific to the older subjects were previously described (4). All subjects provided written informed consent and the study was approved by the University of Pittsburgh Institutional Review Board.
Groups
Subjects were recruited into one of the following groups: young sedentary (YS, 21–30 years), middle-aged sedentary (MAS, 31–55 years), and older sedentary (OS, 70–88 years) based on age (sedentary: ≤1 structured physical activity session per week of <20 minutes), and an active young control group (YA, 21–33 years) that engaged in 3–5 structured physical activity sessions per week as determined by self-report.
Cardiorespiratory Fitness (VO2max)
Cardiorespiratory fitness was determined as peak aerobic capacity measured using a graded exercise protocol, as previously described (4,26). The test was terminated as per the criteria outlined in the American College of Sports Medicine exercise testing guidelines (27).
Skeletal Muscle Biopsy Procedure
Percutaneous muscle biopsies were obtained at the University of Pittsburgh’s Clinical Translational Research Center on a morning after an overnight fast. Participants were instructed not to perform physical exercise 48 hours prior to the biopsy procedure. Biopsy samples were obtained from the middle region of the vastus lateralis under local anesthesia (2% buffered lidocaine) as described previously (28). A portion of the biopsy specimen (~10mg) was placed in ice-cold preservation buffer (BIOPS) (4) for analysis of mitochondrial respiration. The remaining muscle tissue were processed for histochemistry (~30mg) or frozen in liquid nitrogen (~50mg) and stored at −80°C.
High-Resolution Respirometry
Permeabilized myofiber bundles were prepared immediately after the biopsy procedure, as described in the Supplementary Material. Mitochondrial respiration was evaluated by high-resolution respirometry (Oxygraph-2k, Oroboros Instruments, Innsbruck, Austria). Measurements were performed in duplicate, at 37°C, in the range of 230–150 nmol O2/mL. LEAK (LI) respiration was determined through the addition of pyruvate (5mM), malate (2mM) and glutamate (10mM). ADP (5mM) was added to elicit complex I supported OXPHOS (PI) respiration. Cytochrome c (10 μM) was added to assess the integrity of the outer mitochondrial membrane. Any sample that showed an increase in respiration of more than 15% with the addition of cytochrome c was not included in the final analysis. Succinate (10mM) was then added to elicit complex I+II supported OXPHOS (PI+II). FCCP (1 µM) was added to determine electron transfer system capacity or maximal uncoupled respiration. Rotenone (1 µM) was added to inhibit complex I supported OXPHOS respiration, and the remaining respiration revealed the maximal electron transfer system capacity with complex II substrates only (EII). Steady state O2 flux for each respiratory state was determined and normalized to myofiber bundle dry weight using Datlab 4 software (Oroboros Instruments).
Respiratory control ratios/factors were calculated to investigate intrinsic mitochondrial respiratory capacity. Respiratory acceptor control ratios were calculated as PI/LI and PI+II/LI. Flux control ratios were calculated for LI, PI and PI+I, and offered an estimation of the leak and OXPHOS capacity within the electron transfer system capacity. Complex I (CI: PI+II -EII) and complex II (CII: PI+II -PI) control factors were calculated to investigate the relative change of O2 flux in response to a transition of substrate availability in a defined coupling state.
Immunohistochemistry
Myofiber type and cross-sectional area were determined on serial sections, as described in the Supplementary Material. Briefly, sections were stained for type I and type II myocytes, while the type IIx fibers remained unstained. Images were visualized, digitally captured, and analyzed as previously described (29).
Immunoblotting
Whole muscle homogenates were prepared for Western blot as previously described (29). Protein expression of markers of mitochondrial fusion (OPA1 and MFN2), fission (FIS1 and DRP1), autophagy (Beclin-1, BNIP3, and LC3-II/LC3-I), and mitochondrial content (OXPHOS) were measured as described in the Supplementary Material. Protein loading was controlled by normalizing bands of interest to α-Tubulin. Gel-to-gel variation was controlled for by using a standardized sample on each gel.
Statistics
Group differences were tested using a One-way analysis of variance followed by post hoc Tukey test, or Kruskal–Wallis followed by Mann–Whitney U test, as appropriate. The distribution of sex and race across groups were determined by Chi-squared test. Multiple linear regressions were performed to test whether BMI, cardiorespiratory fitness, and fiber type percentage play a role on the association between mitochondrial capacity and age. Sex and race were considered additional covariates. Bivariate correlations were first tested to investigate relationships between mitochondrial variables and age, with the covariates. Each of the covariates were controlled in the regression model if they significantly associated with the dependent and/or independent variable. Statistical significance was established at p ≤ .05.
Results
Participant Characteristics
The participant characteristics can be found in Table 1. A total of 68 individuals with a wide range of age (20–88 years), BMI (19–47kg/m2) and VO2max (1.08–5.04L/min) participated in this study. There were no baseline differences in sex and race among groups (p > .05). The YS and MAS groups had a higher weight and BMI than the YA and OS groups (p < .05). Cardiorespiratory fitness was the highest in the YA group and lowest in the OS group (p < .05).
Table 1.
Subject Characteristics
| Young Active (YA) | Young Sedentary (YS) | Middle-Aged Sedentary (MAS) | Older Sedentary (OS) | |
|---|---|---|---|---|
| N | 10 | 14 | 24 | 20 |
| Sex (ratio) | 4M/6F | 5M/9F | 6M/18F | 12M/8F |
| Race (ratio) | 10C | 9C/3AA/2O | 15C/6AA/1A/2O | 19C/1AA |
| Age (y) | 27±5 | 24±3 | 41±9† | 78±5‡ |
| Weight (kg) | 62.3±7.4 | 85.1±28.5* | 86.7±22.2* | 70.6±12.4 |
| BMI (kg/m2) | 21.4±1.2 | 29.2±8.0* | 30.8±7.5* | 25.9±3.0§ |
| VO2max (L/min) | 3.50±0.93 | 2.59±0.79* | 2.27±0.81* | 1.47±0.45‡ |
Notes: *p < .05 vs YA.
† p < .05 vs YA and YS.
‡ p < .05 vs YA, YS and MAS.
§ p < .05 vs MAS.
Values are Mean ± SD. A = asian; AA = african American; BMI = body mass index; C = caucasian; F = female; M = male; O = other.
Myofiber Type and Cross-Sectional Area
The YA group presented a higher percentage of type I fibers, and a lower percentage of type II fibers, compared to the YS, MAS, and OS groups (p < .05, Table 2). Additionally, the YA and OS groups presented a lower percentage of type IIx fibers compared to the YS and MAS groups (p < .05, Table 2). There were no significant differences in cross-sectional area for any fiber type among groups (p > .05).
Table 2.
Myofiber Type Distribution and Cross-Sectional Area
| Young Active (YA) | Young Sedentary (YS) | Middle-Aged Sedentary (MAS) | Older Sedentary (OS) | |
|---|---|---|---|---|
| Fiber type (%) | ||||
| Type I | 60±9 | 46±10* | 41±11* | 47±16* |
| Type II | 40±9 | 54±10* | 59±11* | 53±16* |
| Type IIa | 37±10 | 37±8 | 41±8 | 46±14 |
| Type IIx | 3±3 | 17±10† | 18±8† | 7±7 |
| Cross-sectional area (µm2) | ||||
| Type I | 4,122±1,110 | 4,046±1,202 | 4,379±1,304 | 3,934±1,291 |
| Type II | 3,716±913 | 4,092±1,060 | 4,248±1,333 | 3,141±1,492 |
| Type IIa | 3,586±785 | 3,773±917 | 3,990±1,337 | 3,201±1,302 |
| Type IIx | 4,023±1,288 | 4,484±1,534 | 4,506±1,557 | 2,931±1,894 |
Notes: *p < .05 vs YA.
† p < .05 vs YA and OS; n = 5–18.
Values are Mean ± SD.
Mitochondrial Respiration
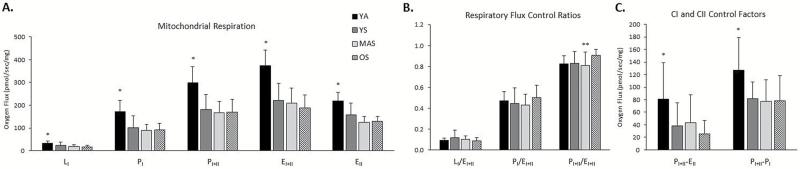
When examining only the sedentary groups, there were no age-related deficits in mitochondrial respiration (Figure 1A, p > .05). The sedentary groups presented lower LI, PI, PI+II, EI+II, and EII respiration compared to the YA group (Figure 1A; p < .05). We also examined mitochondrial respiration normalized to total respiratory chain abundance (OXPHOS total) to determine whether there were any differences in intrinsic mitochondrial respiratory capacity between groups. While LI respiration was found to be higher in the MAS group when compared to YS and OS groups (p < .05), we found no differences in PI, PI+II, EI+II, and EII respiration between the four age groups (p > .05). These results suggest that the higher non-normalized mitochondrial respiration observed in the YA group when compared to the sedentary groups is attributed to higher mitochondrial content in the active group. Respiratory acceptor control ratios were not different among the groups (p > .05; PI/LI: 5.3±2.4, PI+II/LI: 9.7±4.3). While LI/EI+II and PI/EI+II flux control ratios did not differ across groups, we found that the OS group had higher PI+II/EI+II flux control ratio when compared to MAS group (Figure 1C; p < .05). CI and CII control factors were both reduced in the sedentary groups compared to the YA group (Figure 1B; p < .05).
Figure 1.
Mitochondrial respiration in permeabilized myofibers from young active (YA), and sedentary young (YS), middle-aged (MAS), and older (OS) participants. (A) Mitochondrial respiration consisting of leak (LI), complex I supported OXPHOS (PI), complex I+II supported OXPHOS (PI+II), maximal electron transfer system capacity (EI+II), and ETS with substrates for complex II only (EII). (B) Respiratory flux control ratios were determined as an estimation of leak and OXPHOS capacity within the ETS capacity. (C) Complex I (CI) and Complex II (CII) control factors. Data presented as Mean and SD; n = 9–20 per group. *p < .05 vs YS, MAS, and OS; **p < .05 vs OS.
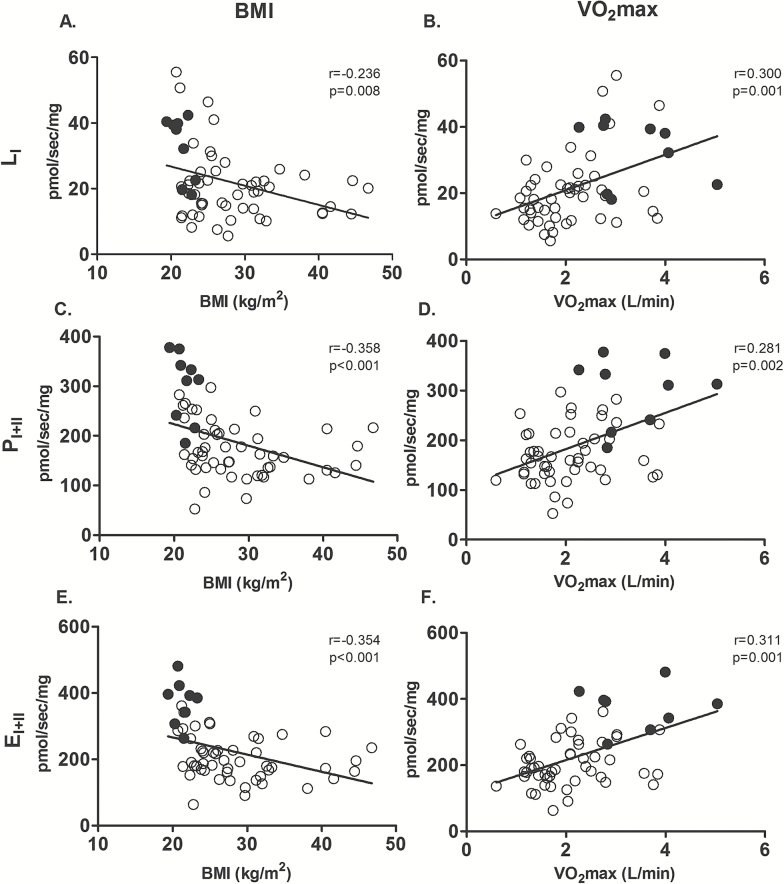
While we observed that mitochondrial respiration was not influenced by sex, race or fiber type percentage (p > .05), we found that it was negatively associated with BMI and positively associated with cardiorespiratory fitness (VO2max; Figure 2; p < .05). We then examined the association between mitochondrial respiration and age when controlled for these covariates (BMI and VO2max; Table 3). We found that BMI and VO2max combined to explain 31%–45% of the variance of mitochondrial respiration (p < .05), depending on the respiratory state, with age explaining only an additional 1.4%–6.8% of the variance in mitochondrial respiration (Table 3; p < .05 for LI and EI+II, and p > .05 for PI and PI+II). The YA subjects are likely driving these associations since they had higher VO2max values and lower BMI when compared to the other groups (Table 1). We next examined the association between mitochondrial respiration and age when controlled for VO2max and BMI in the sedentary subjects only. While BMI and VO2max combined explained between 12%–27% of the variance in mitochondrial respiration (p < .05), we found that when these covariates are controlled for, age does not explain a significant percent of variance in mitochondrial respiration (Table 3, p > .05). Collectively, these findings suggest that BMI and cardiorespiratory fitness, rather than chronological age per se, are more influential to mitochondrial respiratory capacity.
Figure 2.
Association between mitochondrial respiration with body mass index (BMI) and cardiorespiratory fitness (VO2max). Bivariate correlation between Leak (LI) respiration with BMI (A) and VO2max (B), complex I+II supported OXPHOS (PI+II) respiration with BMI (C) and VO2max (D), and maximal electron transfer capacity (EI+II) with BMI (E) and (F) VO2max. Black circles represent young active subjects and white circles represent sedentary subjects, n = 58–59.
Table 3.
Multiple Linear Regression Investigating the Influence of Cardiorespiratory Fitness, BMI, and Age on Mitochondrial Respiration
| Predictor | LI | PI | PI+II | EI+II | |||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 | p | R 2 | p | R 2 | p | R 2 | p | ||
| With young active | VO2max | .189 | .001* | .245 | <.001* | .207 | <.001* | .253 | <.001* |
| BMI after controlled for VO2max | .123 | .003* | .208 | <.001* | .152 | .001* | .158 | <.001* | |
| Age after controlled for BMI+VO2max | .068 | .020* | .004 | .540 | .014 | .289 | .052 | .029* | |
| Without young active | VO2max | .148 | .007* | .044 | .153 | .041 | .168 | .073 | .064 |
| BMI after controlled for VO2max | .123 | .008* | .163 | .004* | .081 | .048* | .091 | .032* | |
| Age after controlled for BMI+VO2max | .046 | .093 | .003 | .669 | .002 | .751 | .052 | .093 | |
Notes: *p < .05.
BMI = body mass index; EI+II = maximal electron transfer capacity with substrates for complex I and II; LI (complex I supported leak respiration), PI (complex I supported OXPHOS respiration), PI+II (complex I+II supported OXPHOS respiration).
Protein Expression
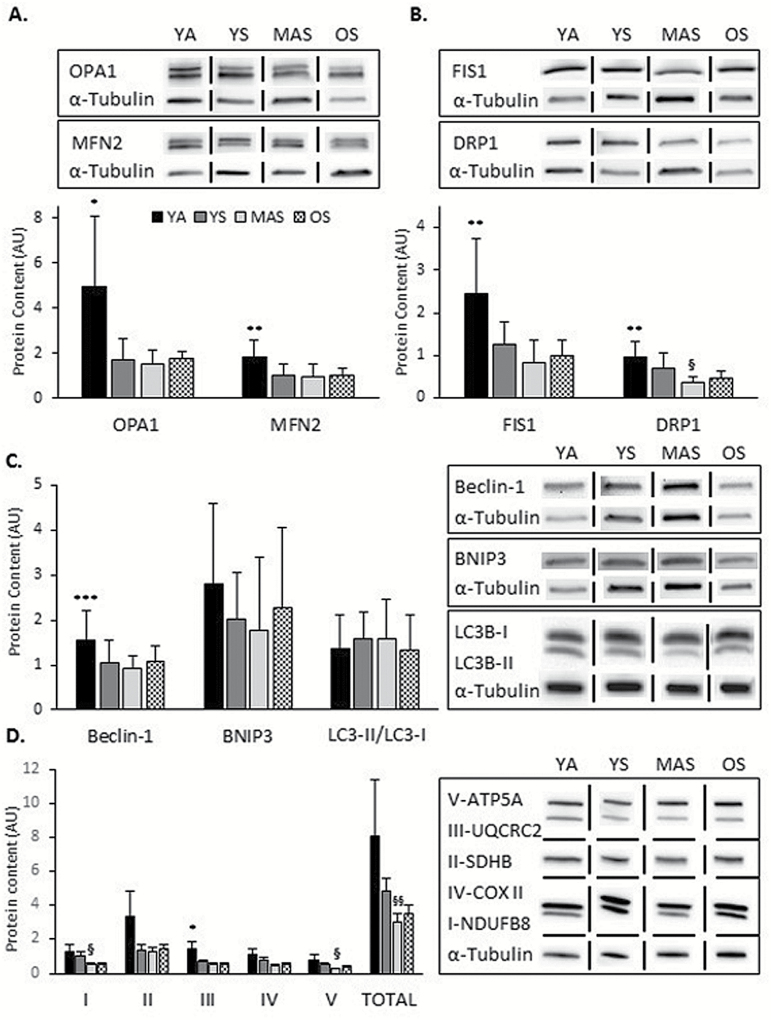
The sedentary groups had reduced levels of OPA1 compared to the YA group (Figure 3A; p < .05). Additionally, the sedentary MAS and OS groups presented lower levels of MFN2, FIS1, and DRP1 protein expression compared to YA (Figure 3A and B; p < .05). The MAS group had lower expression of beclin-1 compared to the YA (Figure 3C; p < .05), while there were no differences in BNIP3 and LC3-II/LC3-I ratio between the groups (Figure 3C; p > .05). When examining only the sedentary groups, there were no group differences for the proteins measured, except for DRP1. MAS group had a reduced level of DRP1 compared to YS (Figure 3B; p < .05).
Figure 3.
Expression of quality control proteins in skeletal muscle samples from active young (YA), and sedentary young (YS), middle-aged (MAS), and older (OS) participants. (A) Western blot analysis of mitochondrial fusion proteins. (B) Western blot analysis of mitochondrial fission proteins. (C) Western blot analysis of autophagy proteins. (D) Western blot analysis of mitochondrial content (OXPHOS, complexes I-V and Total). Values were normalized to α-tubulin and a loading control. Data are presented as Mean and SD for figures A, B, and C, and as Mean and SE for figure D. Results are expressed as arbitrary units (AU); Vertical and horizontal dividing lines were used in the Western blot images to present lanes from the same gel that were reorganized for presentation purpose; n = 5–17 per group. *p < .05 vs YS, MAS, and OS **p < .05 vs MAS and OS, ***p < .05 vs MAS, § p < .05 vs YS, §§ p < .05 vs YA and YS.
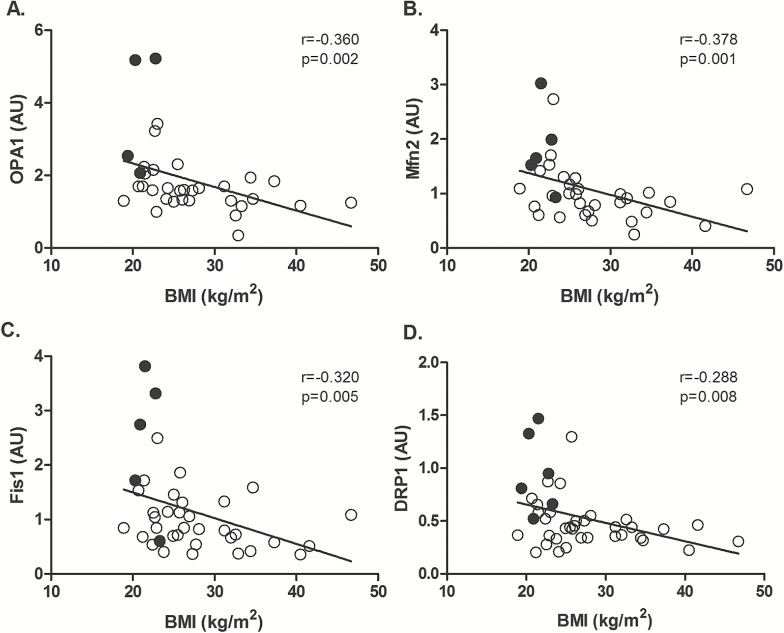
Quality control protein expression was not associated with age. Additionally, expression of quality control proteins was not influenced by sex. The expression of mitochondrial fusion proteins OPA1 and MFN2 (Figure 4A and B), fission proteins FIS1 and DRP1 (Figure 4C and D), and autophagy proteins Beclin-1 and BNIP3 correlated negatively with BMI (p < .05). Similarly, among the sedentary groups only, we found that OPA1, MFN2, FIS1, and Beclin-1 were also negatively correlated with BMI (p < .05). These findings suggest that the capacity for mitochondrial fission, fusion and autophagy is preserved during chronological aging per se, but it is influenced by cardiorespiratory fitness and BMI. Interestingly, both fission and fusion proteins correlated with BMI, suggesting that increased BMI influences the capacity for both processes in sedentary individuals. Additionally, expression of fusion, fission, and autophagy proteins were correlated with several mitochondrial respiratory states (Supplementary Table 1, p < .05).
Figure 4.
Association between mitochondrial fusion and fission proteins with body mass index (BMI). Correlation of mitochondrial proteins (A) OPA1, (B) MFN2, (C) FIS1, and (D) DRP1 with BMI. Results are expressed as arbitrary units (AU). Black circles represent young active subjects and white circles represent sedentary subjects n = 34–41.
The expression of complex I and V protein was lower in the MAS compared to YS group (Figure 3D; p < .05). Complex III protein was lower in the sedentary groups compared to YA (Figure 3D; p < .05). Total OXPHOS protein content was lower in the MAS when compared to the YA and YS groups (Figure 3D; p < .05).
Discussion
Deeper interrogation into mitochondrial function in aging is crucial to better delineate mitochondria as a feasible therapeutic target for sarcopenia and loss of physical function. We measured several aspects of substrate-supported respiration and respiratory control parameters, along with markers of electron transport chain content and quality control in human muscle ranging from 21 to 88 years of age. Our main finding was that maximal rates of mitochondrial respiration, and capacity for fusion, fission, and autophagy are not altered with chronological age per se, but that cardiorespiratory fitness and BMI play a more dominant role in explaining the apparent age associated changes.
There have been many reports describing lower mitochondrial oxidative capacity with aging (6–11), however, the majority have not considered important covariates such as cardiorespiratory fitness (8,9) and BMI (7,8,11). Many have also used isolated mitochondria (6,9,11). Tonkonogi et al. (7) found lower mitochondrial respiration with age, when compared young (22–31 years) and old (61–86 years) sedentary subjects matched for physical activity levels. Despite matching groups for the degree of physical activity, the older subjects had lower cardiorespiratory fitness by VO2max (7), although this was not associated with mitochondrial respiration. However, no information on participants’ body composition was presented, which could contribute and partially explain the lower mitochondrial respiration in the older group. Similarly, a recent study using permeabilized myofibers also reported decreased respiratory capacity and coupling control in older adults (62±8 years) compared to young (28±7 years) subjects (8). However, direct measurements of participants’ cardiorespiratory fitness were not collected, and the groups were unbalanced for BMI with the older group presenting with increased body fat and higher BMI when compared to the young group. Without controlling for these covariates it is difficult to confirm whether the lower levels of mitochondrial respiration observed in the old group are due to age per se, or are related to differences in cardiorespiratory fitness and body composition.
A number of studies performed ex vivo using permeabilized myofibers have failed to find age-related changes in mitochondrial respiration (12,13,15). Hutter et al. (12) reported full preservation of mitochondrial respiration in older subjects when compared to young men and women. While cardiorespiratory fitness was not evaluated in the study, a significant correlation between percent body fat and mitochondrial respiration was observed, suggesting that mitochondrial respiration is more related to changes in body composition rather than age. Similarly, Larsen et al. (13) reported no differences in mitochondrial respiration between 10 young (23±3 years) and 10 middle-aged (53±3 years) subjects matched for VO2max and BMI. Additionally, a recent study performed by Gouspillou et al. (2) on physically active young (23.7±0.8 years) and older (71.2±1.6 years) subjects showed a mild mitochondrial uncoupling, but preserved mitochondrial content, respiration and ROS emission. These data underscore the potential of physical activity to preserve mitochondrial capacity and quality control in aging (2).
Our study is the first to investigate associations between mitochondrial respiration and quality control in a reasonably large number of subjects (n = 68) with a wider range of age (21–88 years) and BMI (19–47kg/m2), and to examine the influence of cardiorespiratory fitness and BMI on these associations. We found no decreases in ex vivo mitochondrial respiration when comparing young, middle-aged, and older sedentary individuals, despite a significant age-related reduction in VO2max. Our results suggest that in our group of older individuals mitochondrial respiratory capacity is not a limiting factor of cardiorespiratory fitness in aging. Considering that the groups were unbalanced for cardiorespiratory fitness (VO2max) and adiposity (BMI) we opted for further investigating the association between age and mitochondrial capacity while including these covariates in the regression models. We found that VO2max and BMI together heavily influenced mitochondrial respiratory capacity, and that when accounted for these variables no age-related changes in mitochondrial respiration was observed in the sedentary groups. These observations are in line with and extend the findings that physical activity levels and cardiorespiratory fitness are confounders of the relationship between age and mitochondrial respiration (10,13,18). Additionally, our findings revealed that even after controlling for cardiorespiratory fitness, BMI still explains a significant amount of variation in mitochondrial respiratory capacity. The influence of BMI is supported by others that found reduced capacity for lipid oxidation and activity of mitochondrial enzymes in obese subjects (30,31). The relationship between BMI and reduced mitochondrial capacity may be due to the interplay between adiposity and the development of insulin resistance and mitochondrial dysfunction (32) or simply to the lack of physical activity and/or other lifestyle factors. More research is needed to elucidate the intricacies of this relationship.
We examined respiratory control ratios/factors, an approach that permits a qualitative comparison of respiratory performance independent of mitochondrial content, methods used to prepare the mitochondria, and variations in assay conditions (33). We found higher CI and CII control factors in the YA when compared to the sedentary groups. These control factors express the relative change of O2 flux in response to a transition of substrate availability in a defined coupling state (34). CI control factor is experimentally induced when rotenone (complex I inhibitor) is added to CI+II-linked respiration and CII control factor when succinate is added to Complex I-linked respiration. The sedentary groups had a reduced CI and CII control factors, compared to the YA group, suggesting that regular physical activity is linked with oxygen flux through complex II and the individuals’ ability to increase O2 flux through complex II in response to a transition of substrate availability, independent of overall mitochondria content.
To determine the association between age and capacity for mitochondrial quality control, we measured several proteins that mediate mitochondrial fusion, fission, autophagy, and mitophagy. The expression of these proteins were not influenced by age in the sedentary group while the young active individuals had elevated levels of fusion and fission proteins. These findings may suggest that physical activity is linked with the capacity for mitochondrial turnover. Recent studies have investigated whether mitochondrial quality control is influenced by aging, but the results are not conclusive and remain to be elucidated (1,2,25,35,36). While Konopka et al. (25) and Bori et al. (36) have found no differences in mitochondrial fusion and fission between young and older subjects, Joseph et al. (1) showed that the fusion protein OPA1 was decreased in older individuals when compared to young subjects while MFN2, FIS1, and DRP1 proteins remained the same. The influence of age on autophagy is also not well understood. Fry et al. (35) showed no differences in LC3B-II/LC3B-I ratio between young and older subjects, but found higher expression of Beclin1 in the older group. Additionally, Gouspillou et al. (2) reported no differences in Parkin expression between young and older muscles, but found that the Parkin-to-VDAC ratio is decreased in the older subjects, suggesting that mitophagy may be impaired with aging. Studies that included an exercise intervention have also been performed and reported both similar and distinct quality control responses in young and older adults (25,35,36). Konopka et al. (25) have found an elevation of MFN1, MFN2, and FIS1 protein levels in both young and old subjects with aerobic exercise training (37). However, Bori et al. (36) showed that in contrast to the higher FIS1 mRNA observed in active young subjects versus young sedentary, old active subjects did not have higher FIS1 than old sedentary subjects, suggesting an altered fission response to chronic exercise with aging. These findings suggest that mitochondrial quality control in response to exercise may not be similar for young and old subjects. Recent animal studies have expanded on the contribution of these processes in regulating exercise adaptations. Caffin et al. found OPA1 deficient mice have impaired exercise training induced mitochondrial biogenesis (38) and Lira et al. report that an increase in basal autophagy is required for exercise training adaptations (39). These studies indicate that mitochondrial quality control processes are likely involved and important for the remodeling of mitochondria in response to exercise. Interestingly, we also found significant associations between the expression of fusion, fission, and autophagy proteins with BMI and several mitochondrial respiratory states. These results suggest that alterations in mitochondrial quality likely play a significant role in maintaining mitochondrial function.
While these data suggest that adiposity plays a role in mitochondrial respiration and expression of quality control proteins, it is important to highlight that BMI was used as an indicator of body fat and obesity, and it is not a direct measure of body fatness, and for an equivalent BMI, older subjects have a higher percentage body fat than young subjects (40). Future studies using direct measures of body fat, such as dual-energy x-ray absorptiometry (DEXA) or multicomponent models, would provide essential information on the influence of body fat, and its distribution to mitochondrial capacity in aging. Future studies should also include groups of older obese and older active adults to further understand the role of adiposity and cardiorespiratory fitness on mitochondrial respiration in these populations.
In summary, mitochondrial respiratory capacity and expression of mitochondrial quality control proteins are elevated in young physically active individuals, but are similar among sedentary young, middle-aged and older subjects. Our findings suggest that mitochondrial capacity is not influenced by chronological age per se, but is closely related to BMI and cardiorespiratory fitness. Our data provide strong evidence that confounding factors of mitochondrial function, such as adiposity, cardiorespiratory fitness are critical phenotypic characteristics that should be considered in studies of aging muscle. This is essential if we are to provide clarity on the true nature of mitochondrial dysfunction with aging which in turn will allow us to more accurately develop mitochondrial targeted therapeutics in aging.
Funding
The work was supported by National Institutes of Health (DK084213, JJD), (AG044437, PMC); ARRA Funds (1RC2AG036594, 1RC2AG036606 BHG); and an American Diabetes Association Clinical Research Award (7-09-CT-51 V.B.R.).
Supplementary Material
Acknowledgments
We acknowledge the assistance of Steven Anthony with VO2max test, Nicole Helbling and Angela Laslavic with patient recruitment and research coordination, Alex Despines and Erin Leachman with histological analysis, and Dr. Lauren Terhorst for her advice with the statistical analysis, University of Pittsburgh.
References
- 1. Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809. doi:10.1111/j.1474-9726.2012.00844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gouspillou G, Sgarioto N, Kapchinsky S, et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014;28:1621–1633. doi:10.1096/fj.13-242750 [DOI] [PubMed] [Google Scholar]
- 3. Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271–276. doi:10.1097/MCO.0b013e328337819e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. doi:10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J Geront A Biol Sci Med Sci. 2014;70:1379–1385. doi:10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi:10.1016/S0140-6736(89)92143-0 [DOI] [PubMed] [Google Scholar]
- 7. Tonkonogi M, Fernstrom M, Walsh B, et al. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Archiv. 2003;446:261–269. doi:10.1007/s00424-003-1044-9 [DOI] [PubMed] [Google Scholar]
- 8. Porter C, Hurren NM, Cotter MV, et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309:E224–232. doi:10.1152/ajpendo.00125.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226:73–82. doi:10.1016/0925-4439(94)90061-2 [DOI] [PubMed] [Google Scholar]
- 10. Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi:10.2337/db08-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi:10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutter E, Skovbro M, Lener B, et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6:245–256. doi:10.1111/j.1474-9726.2007.00282.x [DOI] [PubMed] [Google Scholar]
- 13. Larsen S, Hey-Mogensen M, Rabøl R, Stride N, Helge JW, Dela F. The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol (Oxf). 2012;205:423–432. doi:10.1111/j.1748-1716.2012.02408.x [DOI] [PubMed] [Google Scholar]
- 14. Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Archiv. 2003;446:270–278. doi:10.1007/s00424-003-1022-2 [DOI] [PubMed] [Google Scholar]
- 15. Gram M, Vigelso A, Yokota T, et al. Two weeks of one-leg immobilization decreases skeletal muscle respiratory capacity equally in young and elderly men. Exp Gerontol. 2014;58:269–278. doi:10.1016/j.exger.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 16. Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Archiv. 2010;459:277–289. doi:10.1007/s00424-009-0724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Picard M, Ritchie D, Wright KJ, et al. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9:1032–1046. doi:10.1111/j.1474-9726.2010.00628.x [DOI] [PubMed] [Google Scholar]
- 18. Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;5:e10778. doi:10.1371/journal.pone.0010778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78:2033–2038. [DOI] [PubMed] [Google Scholar]
- 20. Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11:678–684. doi:10.1038/embor.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi:10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414. doi:10.1515/hsz-2012-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821. doi:10.1155/2012/194821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi:10.1242/jcs.070490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2014;69: 371–378. doi:10.1093/gerona/glt107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dube JJ, Coen PM, DiStefano G, et al. Effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility, and mitochondrial performance. Am J Physiol Endocrinol Metab. 2014;307: E1117–1124. doi:10.1152/ajpendo.00257.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson WRGN, Pescatello LS. ACSM’s Guidlines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 28. Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287:E857–862. doi:10.1152/ajpendo.00459.2003 [DOI] [PubMed] [Google Scholar]
- 29. Coen PM, Hames KC, Leachman EM, et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity. 2013;21:2362–2371. doi:10.1002/oby.20381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–1044. [DOI] [PubMed] [Google Scholar]
- 31. Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab. 2004;287:E1076–1081. doi:10.1152/ajpendo.00177.2004 [DOI] [PubMed] [Google Scholar]
- 32. Lanza IR, Sreekumaran Nair K. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol (Oxf). 2010;199:529–547. doi:10.1111/j.1748-1716.2010.02124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gnaiger E. Mitochondrial pathways and respiratory control. In: Network MP, ed. An Introduction to OXPHOS Analysis. Innsbruck, Austria: OROBOROS MiPNet Publications; 2012:64. [Google Scholar]
- 34. Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Bioch Cell Biol. 2009;41:1837–1845. doi:10.1016/j.biocel.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 35. Fry CS, Drummond MJ, Glynn EL, et al. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68:599–607. doi:10.1093/gerona/gls209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bori Z, Zhao Z, Koltai E, et al. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47:417–424. doi:10.1016/j.exger.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2014;69:371–378. doi:10.1093/gerona/glt107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caffin F, Prola A, Piquereau J, et al. Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1-deficient mice. J Physiol. 2013;591:6017–6037. doi:10.1113/jphysiol.2013.263079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lira VA, Okutsu M, Zhang M, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184–4193. doi:10.1096/fj.13-228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143: 228–239 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.