Nested in the PCPT, genetic variations in estrogen-related genes were examined in relation to prostate cancer risk. Polymorphisms associated with prostate cancer risk differed between the finasteride and placebo arms. The associations appeared to be modified by circulating hormone levels.
Abstract
Substantial preclinical data suggest estrogen’s carcinogenic role in prostate cancer development; however, epidemiological evidence based on circulating estrogen levels is largely null. Compared with circulating estrogen, the intraprostatic estrogen milieu may play a more important role in prostate carcinogenesis. Using a nested case–control design in the Prostate Cancer Prevention Trial (PCPT), we examined associations of genetic variants of genes that are involved in estrogen synthesis, metabolism and function with prostate cancer risk. A total of 25 potentially functional single nucleotide polymorphisms (SNPs) in 13 genes (PGR, ESR1, ESR2, CYP17A1, HSD17B1, CYP19A1, CYP1A1, CYP1B1, COMT, UGT1A6, UGT1A10, UGT2B7, UGT2B15) were examined in whites only. Controls (n = 1380) were frequency matched to cases on age, PCPT treatment arm, and family history (n = 1506). Logistic regression models adjusted for age and family history were used to estimate odds ratios (OR) and 95% confidence intervals (CI) separately in the placebo and finasteride arms. SNPs associated with prostate cancer risk differed by treatment arm. The associations appeared to be modified by circulating estrogen and androgen levels. CYP19A1 was the only gene harboring SNPs that were significantly associated with risk in both the placebo and finasteride arms. Haplotype analysis with all three CYP19A1 SNPs genotyped (rs700518, rs2445765, rs700519) showed that risk-allele haplotypes are associated with the increased prostate cancer risk in both arms when comparing with the non-risk allele haplotype. In conclusion, associations between SNPs in estrogen-related genes and prostate cancer risk are complex and may be modified by circulating hormone levels and finasteride treatment.
Introduction
Prostate cancer is an androgen-dependent disease (1) yet estrogen plays a pivotal role in prostatic carcinogenesis (2,3). In noble rat strains, estrogen and testosterone exposure induced prostatic adenocarcinoma in 100% of rats; however, the incidence was reduced by 60% with testosterone alone and almost no tumor was formed with treatment of non-aromatizable androgen, which blocks conversion of testosterone to estradiol by aromatase (CYP19A1) (4). Similar results were also observed in an aromatase knockout (ArKO) mouse model, in which the mice developed prostatic hyperplasia but not prostate cancer (5). In men, circulating testosterone levels decline with age at a greater extent than circulating estradiol resulting in an elevated ratio of estradiol to testosterone, which coincides with the increased risk of prostate cancer upon aging (6–9). However, despite the strong preclinical evidence, epidemiological studies have not established associations between circulating estrogen levels and prostate cancer risk (10–12), rendering a possibility that intraprostatic estrogen milieu may play a more important role than circulating estrogen in prostatic carcinogenesis. This hypothesis is supported by the fact that prostate tissue contains a variety of enzymes required for the local production of active androgen and estrogen from precursor steroids (13). Compared to benign tissue, prostate cancer tissue or cells have increased aromatase expression and activity (3), supporting the potential changes of local estrogen production during carcinogenesis. It is difficult to distinguish systematic synthesis from local production of estrogen in in vivo models. In 1980s, Stone et al. performed ex vivo experiments using prostate benign, hyperplasia and cancer tissues from patients and found that estrogen was indeed produced by all prostate tissues using androstenedione as a substrate (14,15). Co-treatment with an aromatase inhibitor inhibited estrogen production by 53–98%. Recently, in a co-cultured in vitro model of prostate cancer cells and prostate cancer-derived stromal cells, Machioka et al. found that estrogen was synthesized from testosterone, which activated estrogen receptor and stimulate cancer cell growth (16). These findings document the capability of prostate tissue for local synthesis of estrogen.
Due to the limited availability of prostate cancer tissue, it is difficult to obtain prostatic estrogen levels to address the role of intraprostatic estrogen milieu in the development of prostate cancer. Using data and blood biospecimens from the Prostate Cancer Prevention Trial (PCPT), we took an indirect approach to investigate associations of polymorphisms in key genes involved in estrogen synthesis, metabolism, and function with prostate cancer risk. Our hypothesis was that polymorphisms in estrogen-related genes alter local estrogen bioavailability thus affecting prostate cancer risk, and such associations are modified by levels of circulating steroid hormones, the precursors for local estrogen/androgen synthesis.
Materials and methods
Study design and study population
This is a nested case–control study using data and biospecimens collected and stored in the PCPT biorepository. Details of the PCPT study design and characteristics of the study population have been previously described (17). In brief, men aged ≥ 55 years (n = 18, 882) with normal digital rectal examination, normal prostate-specific antigen (PSA) level (≤3 ng/ml) and without history of prostate cancer or severe benign prostatic hyperplasia-related symptoms or other clinically significant and related conditions were randomized to receive either finasteride (5 mg/day) or placebo. All participants underwent digital rectal examination and PSA screening annually and those with abnormal findings were recommended for biopsy. At the end of the study period, all participants who were not previously diagnosed with prostate cancer were offered an end of study biopsy. The Gleason scoring system was applied during central review. To be consistent with the PCPT trial report, tumors with scores <7 were classified as low grade and those with scores ≥7 were classified as high grade. Diagnoses were confirmed by a minimum of two pathologists. An additional referee pathologist resolved issues of discordance. All participating institutions received institutional review board approval and all participants provided informed consent for participating in the PCPT and subsequent research using their data and materials.
For this nested case–control study, cases were identified either by a for-cause biopsy (42% and 47% for the placebo and finasteride arm, respectively) or an end-of-study biopsy (58% and 53% for the placebo and finasteride arm, respectively), and the distribution was not significantly different between the two arms. Controls were selected from men who completed the end-of-study biopsy and had no evidence of prostate cancer. Controls were frequency-matched to cases on age in 5-year increments, treatment arm, and first-degree family history of prostate cancer. Due to the difference in genetic background, this study included whites only. A total of 1506 cases and 1380 controls with adequate DNA samples for genotyping were included.
Data and specimen collection and serum measurements
Self-administered questionnaires were used following recruitment and consent to collect information on age, race/ethnicity, education, physical activity, smoking, alcohol consumption and family history of prostate cancer. At the baseline clinic visit, approximately 3 months prior to randomization, height and weight were measured using a standardized protocol for calculating body mass index (BMI) and non-fasting blood was collected. At each annual clinic visit, weight was measured and non-fasting blood was collected for PSA measurement either until prostate cancer diagnosis or the end of the study. Detailed procedures for blood collection, processing, and storage have been previously described (18). Serum levels of testosterone (ng/dl), estradiol (pg/ml) and estrone (pg/ml) were quantified for cases and controls by immunoassays at the laboratory of Dr. Frank Stanczyk at the University of Southern California (19). As the precursor of steroid hormones, serum cholesterol level (mg/dl) at baseline was also included in the analyses.
SNP selection and genotyping
DNA was extracted from white blood cells using the Qiagen M48 robot, and from serum using the AutoPure LS DNA Isolation Robot as described previously (20). Non-fasting serum samples collected annually for PSA measurement were used for DNA extraction when white blood cells were not available. Only a portion of participants (24%) had genotyping data generated from serum DNA samples. The sensitivity analysis showed similar trends after excluding genotyping data from serum DNA (results not shown).
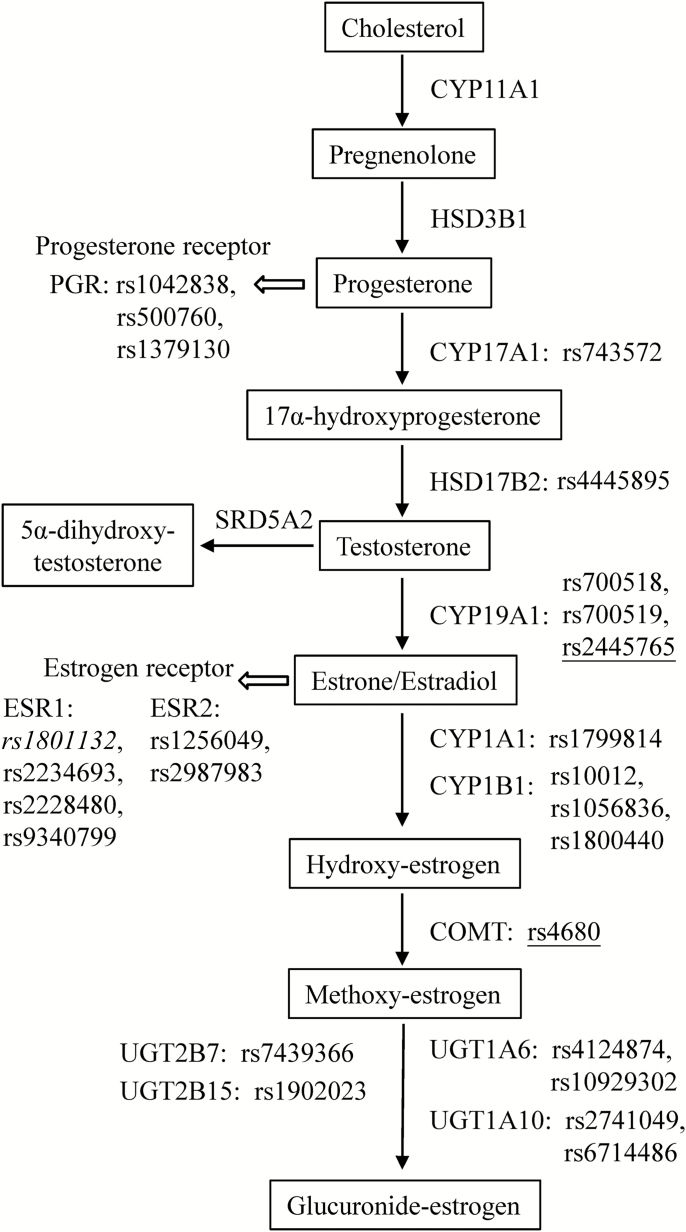
Several criteria were applied for selection of candidate single nucleotide polymorphisms (SNPs). Data on functional significance or data demonstrating a significant role in prostate or any other cancer were primary criteria used for selection. Other criteria include SNPs that result in amino acid changes in the protein, changes in promoter regions and splice sites. In general variants at minor allele frequencies of 5% or higher based on the literature were selected in order to have good statistical power. Based on information obtained in the literature (21–39), a panel of 36 potentially functional SNPs in genes involved in estrogen synthesis, metabolism, and function was assembled. Genotyping was conducted by Sequenom iPLEX platform for all SNPs except rs2741049 which was genotyped using Taqman. After removing SNPs with call rate <95% (1 SNP) or minor allele frequency <3% (10 SNPs) for power considerations, a total of 25 SNPs in 13 genes (PGR, ESR1, ESR2, CYP17A1, HSD17B1, CYP19A1, CYP19B1, CPMT, UGT1A6, UGT1A10, UGT2B7, and UGT2B15) were examined for associations with prostate cancer risk. No SNPs violated Hardy–Weinberg equilibrium at P < 0.01. The list of SNPs and corresponding genes in relation to estrogen synthesis, metabolism, and function is summarized in Figure 1.
Figure 1.
Selected polymorphic genes involved in estrogen synthesis, metabolism and function. Polymorphisms showed significant associations with overall prostate cancer risk are indicated in italic for the placebo arm and underlined for the finasteride arm.
Statistical analysis
Demographic and patient characteristics (age, family history, BMI and hormone levels) were summarized and compared between cases and controls by treatment arm (finasteride versus placebo) using standard chi square tests for categorical variables and t-test for continuous variables. To accommodate the frequency-matched study design, odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using unconditional logistic regression for risk of overall (total) prostate cancer and polytomous logistic regression for risk of low-grade (Gleason score < 7, N = 1054) and high-grade prostate cancer (Gleason score ≥7, N = 394). Regression models were applied to estimate prostate cancer risk by treatment arm, adjusting for age and family history of prostate cancer.
A genotypic (co-dominant) model was assumed for all SNP analysis using the major allele homozygote as the reference genotype. To test genetic dose response, a log-additive genetic model was used by coding genotypes as 0, 1 and 2 on the basis of the copy number of the minor allele. To evaluate potential effect modification by circulating hormones, models were stratified by low versus high categories of hormones using the median baseline level in the control population as the cutoff point. When genotype frequency of the rare homozygote was less than 5% in stratified analysis, categories of rare homozygote and heterozygote were collapsed for power considerations. Interaction was tested by including a multiplicative term between genotypes (0, 1, 2) and hormone categories in the models. In additional to single SNP analysis, we also performed haplotype analysis. The non-risk alleles for each SNP were used as reference category.
The Southwest Oncology Group (formerly SWOG) Statistical Center at the Fred Hutchinson Cancer Research Center (Seattle, WA) performed statistical analyses using R and SAS 9.3 (SAS institute, Cary, NC). All analyses were two sided and statistical significance was defined as P < 0.05.
Results
Descriptive characteristics
Descriptive characteristics of the study population by case–control status and by treatment arm are shown in Table 1. Similar distribution of age and of family history between cases and controls indicated successful matching. BMI and serum cholesterol and hormone levels at baseline did not differ between cases and controls except for serum estrone. Cases in the finasteride arm had a significantly higher level of serum estrone compared to controls (46.7 ± 15.5 versus 44.9 ± 14.6 mg/dl), but the difference was not observed in the placebo arm.
Table 1.
Descriptive characteristics of prostate cancer cases and controls in the PCPT
| Placebo arm | Finasteride arm | |||||
|---|---|---|---|---|---|---|
| Control (N = 848) | Case (N = 881) | P* | Control (N = 532) | Case (N = 625) | P* | |
| N (%) | ||||||
| Family history | ||||||
| No | 666 (78.5) | 700 (79.5) | 401 (75.4) | 484 (77.4) | ||
| Yes | 182 (21.5) | 181 (20.5) | 0.64 | 131 (24.6) | 141 (22.6) | 0.41 |
| Mean ± SD | ||||||
| Age at baseline, years | 63.8 ± 5.6 | 63.6 ± 5.6 | 0.58 | 64.2 ± 5.7 | 63.9 ± 5.7 | 0.29 |
| BMI, kg/m2 | 27.5 ± 3.9 | 27.1 ± 3.9 | 0.06 | 27.6 ± 3.9 | 27.5 ± 3.8 | 0.74 |
| Serum cholesterol level, mg/dl | 208.2 ± 36.7 | 211.2 ± 35.2 | 0.09 | 210.3 ± 35.4 | 208.2 ± 35.7 | 0.30 |
| Serum testosterone level, ng/dl | 374.7 ± 127.9 | 383.4 ± 130.0 | 0.16 | 380.0 ± 136.2 | 374.1 ± 132.8 | 0.46 |
| Serum estradiol level, pg/ml | 33.3 ± 10.1 | 33.7 ± 9.4 | 0.43 | 33.9 ± 10.3 | 34.1 ± 10.2 | 0.71 |
| Serum estrone level, pg/ml | 44.3 ± 14.8 | 45.3 ± 14.1 | 0.17 | 44.9 ± 14.6 | 46.7 ± 15.5 | 0.04 |
Bold values indicate significant findings.
P values were calculated using chi square tests for categorical variables and t-test for continuous variables.
Associations between genetic variants and prostate cancer risk
Investigation of prostate cancer risk by genotypes of estrogen-related genes revealed two different panels of SNPs significantly associated with risk based on treatment arm (Table 2). In the placebo arm, rs1801132 in ESR1 was significantly associated with reduced prostate cancer risk, showing a 16% risk reduction per G allele (per risk allele: OR: 0.84 and 95% CI: 0.72–0.99). The associations for low-grade (per risk allele: OR: 0.84 and 95% CI: 0.71–1.00) and high-grade (per risk allele: OR: 0.86 and 95% CI: 0.65–1.15) prostate cancer were similar, although the association with high-grade cancer was not statistically significant. Four different SNPs were identified in the finasteride arm, of which rs2445765 in CYP19A1 had significant associations with overall prostate cancer risk (per risk allele: OR: 0.75 and 95% CI: 0.61–0.92 for rs2445765) and showed a similar trend with low-grade and high-grade prostate cancer. A borderline significant association with overall prostate cancer risk was observed for rs4680 in COMT (OR: 1.20, and 95% CI: 0.99–1.46), which was primarily driven by the association with low-grade prostate cancer (OR: 1.27, and 95% CI: 1.02–1.58), but not high-grade disease (OR: 1.05, and 95% CI: 0.81–1.36). The other two SNPs (rs1256049 in ESR2 and rs743572 in CYP17A1) were only associated with high-grade prostate cancer.
Table 2.
Prostate cancer risk by genotypes of estrogen-related genes in the PCPT
| Gene | SNP | Genotype | Controls | Overall prostate cancer | Low-grade prostate cancer | High-grade prostate cancer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | ORa (95% CI) | P trend | Cases | ORa (95% CI) | P trend | Cases | ORa (95% CI) | P trend | ||||
| Placebo arm | ||||||||||||
| ESR1 | rs1801132 | CC | 485 | 539 | 1.00 | 0.04 | 413 | 1.00 | 0.05 | 103 | 1.00 | 0.31 |
| CG | 310 | 281 | 0.81 (0.66–1.00) | 216 | 0.81 (0.66–1.01) | 55 | 0.85 (0.59–1.22) | |||||
| GG | 49 | 42 | 0.77 (0.50–1.19) | 32 | 0.76 (0.48–1.21) | 8 | 0.77 (0.35–1.68) | |||||
| per risk allele | 0.84 (0.72–0.99) | 0.84 (0.71–1.00) | 0.86 (0.65–1.15) | |||||||||
| Finasteride arm | ||||||||||||
| ESR2 | rs1256049 | GG | 485 | 576 | 1.00 | 0.36 | 343 | 1.00 | 0.72 | 211 | 1.00 | 0.01 |
| GA | 41 | 40 | 0.81 (0.51–1.27 | 32 | 1.09 (0.67–1.78) | 7 | 0.39 (0.17–0.87) | |||||
| AA | 0 | 0 | N/A | 0 | N/A | 0 | N/A | |||||
| per risk allele | ||||||||||||
| CYP17A1 | rs743572 | AA | 189 | 219 | 1.00 | 0.83 | 149 | 1.00 | 0.18 | 62 | 1.00 | 0.18 |
| AG | 259 | 323 | 1.08 (0.84–1.39) | 183 | 0.89 (0.67–1.18) | 129 | 1.50 (1.05–2.15) | |||||
| GG | 70 | 73 | 0.89 (0.61–1.31) | 41 | 0.74 (0.48–1.16) | 28 | 1.21 (0.71–2.04) | |||||
| per risk allele | 0.98 (0.82–1.17) | 0.87 (0.71–1.07) | 1.18 (0.93–1.50) | |||||||||
| CYP19A1 | rs2445765 | GG | 324 | 427 | 1.00 | 0.001 | 261 | 1.00 | 0.02 | 149 | 1.00 | 0.04 |
| GC | 175 | 174 | 0.75 (0.58–0.97) | 104 | 0.74 (0.55–0.99) | 65 | 0.82 (0.58–1.15) | |||||
| CC | 28 | 21 | 0.56 (0.31–1.01) | 15 | 0.63 (0.33–1.22) | 5 | 0.39 (0.15–1.02) | |||||
| per risk allele | 0.75 (0.61–0.92) | 0.76 (0.61–0.97) | 0.74 (0.56–0.99) | |||||||||
| COMT | rs4680 | AA | 121 | 105 | 1.00 | 0.06 | 58 | 1.00 | 0.03 | 42 | 1.00 | 0.73 |
| AG | 205 | 231 | 1.32 (0.96–1.83) | 154 | 1.61 (1.11–2.35) | 73 | 1.01 (0.65–1.58) | |||||
| GG | 91 | 113 | 1.44 (0.98–2.11) | 71 | 1.62 (1.03–2.52) | 35 | 1.10 (0.65–1.86) | |||||
| per risk allele | 1.20 (0.99–1.46) | 1.27 (1.02–1.58) | 1.05 (0.81–1.36) | |||||||||
Bold values indicate significant/borderline significant findings.
Adjusted for age and family history.
Associations stratified by serum hormone levels
The analyses of genotypes with prostate cancer risk were further stratified by serum cholesterol and hormone levels and the results are summarized in Table 3 for the placebo arm and in Table 4 for the finasteride arm. In both arms, significant associations were primarily observed in either the high or low category of serum levels. In the placebo arm, rs1801132 in ESR1 identified in the overall analyses also showed a significant inverse association with prostate cancer risk in the stratified analyses (Tables 2 and 3). A dose-dependent reduction of prostate cancer risk was observed with rs1801132 in ESR1 in high cholesterol and low testosterone categories. For example, among men with serum testosterone level below the median, comparing with CC genotype of rs1801132, CG genotype was associated with a 28% risk reduction (OR: 0.72 and 95% CI: 0.54–0.97) and a 52% risk reduction was observed with GG genotype (OR: 0.48 and 95% CI: 0.26–0.89), with a P-trend < 0.05 (data not shown). All three SNPs in CYP19A1 (rs2445765, rs700518, rs700519) had significant associations with prostate cancer risk in either low or high categories of serum cholesterol and hormone levels. Interestingly, significant associations for SNPs in UGT family, including rs4124874 in UGT1A6, rs7439366 in UGT2B7 and rs2741049 in UGT1A10, only appeared among men with high serum hormone levels. In particularly, rs4124874 in UGT1A6 consistently showed a positive association with prostate cancer risk among men with high levels of serum testosterone, estradiol, or estrone. The rs4445895 in HSD17B2 was the only SNP showing significant but opposite associations by serum estradiol level (P for interaction < 0.01). Compared with CC genotype, TT genotype of rs4445895 was associated with an increased risk of prostate cancer among men with estradiol level below the median (OR: 1.64 and 95% CI: 1.00–2.69); while, the association was inversed among men with estradiol level above the median (OR, 0.55 and 95% CI, 0.33–0.91). However, the association between rs4445895 in HSD17B2 and prostate cancer risk was only appeared once and no significant associations were found by other serum hormone levels or in the finasteride arm.
Table 3.
Prostate cancer risk by genotypes of estrogen-related genes and serum cholesterol and hormone levels in the placebo arm in the PCPT
| Gene | SNP | Genotype | Controls | Cases | ORa (95% CI) | Controls | Cases | ORa (95% CI) | P for interaction |
|---|---|---|---|---|---|---|---|---|---|
| Cholesterol < median (206 mg/dl) | Cholesterol ≥ median (206 mg/dl) | ||||||||
| ESR1 | rs1801132b | CC | 246 | 233 | 1.00 | 238 | 300 | 1.00 | 0.17 |
| CG | 155 | 123 | 0.84 (0.62–1.13) | 155 | 155 | 0.79 (0.60–1.05) | |||
| GG | 18 | 21 | 1.24 (0.64–2.39) | 31 | 21 | 0.54 (0.30–0.96) | |||
| CG/GG | 173 | 144 | 0.88 (0.66–1.17) | 186 | 176 | 0.75 (0.57–0.98) | |||
| CYP19A1 | rs2445765 | GG | 266 | 274 | 1.00 | 298 | 330 | 1.00 | 0.13 |
| CG | 135 | 97 | 0.70 (0.51–0.95) | 111 | 132 | 1.07 (0.80–1.44) | |||
| CC | 16 | 12 | 0.72 (0.34–1.56) | 11 | 13 | 1.08 (0.48–2.44) | |||
| CG/CC | 151 | 109 | 0.70 (0.52–0.94) | 122 | 145 | 1.07 (0.81–1.43) | |||
| CYP19A1 | rs700519 | CC | 404 | 353 | 1.00 | 398 | 447 | 1.00 | 0.22 |
| CT | 18 | 29 | 1.84 (1.00–3.36) | 25 | 31 | 1.10 (0.64–1.90) | |||
| TT | N/A | N/A | |||||||
| CYP1B1 | rs10012 | CC | 161 | 149 | 1.00 | 190 | 175 | 1.00 | 0.17 |
| CG | 132 | 109 | 0.90 (0.64–1.26) | 115 | 128 | 1.21 (0.87–1.67) | |||
| GG | 27 | 22 | 0.88 (0.48–1.62) | 22 | 36 | 1.79 (1.01–3.16) | |||
| Testosterone < median (358 ng/dl) | Testosterone ≥ median (358 ng/dl) | ||||||||
| ESR1 | rs1801132b | CC | 220 | 250 | 1 | 257 | 288 | 1 | 0.10 |
| CG | 165 | 136 | 0.72 (0.54–0.97) | 145 | 143 | 0.88 (0.66–1.17) | |||
| GG | 31 | 17 | 0.48 (0.26–0.89) | 18 | 24 | 1.20 (0.64–2.26) | |||
| CG/GG | 196 | 153 | 0.69 (0.52–0.91) | 163 | 167 | 0.91 (0.69–1.20) | |||
| CYP1B1 | rs1800440 | AA | 272 | 267 | 1.00 | 277 | 316 | 1.00 | 0.15 |
| AG | 123 | 128 | 1.06 (0.78–1.43) | 130 | 129 | 0.87 (0.65–1.17) | |||
| GG | 19 | 8 | 0.43 (0.19–1.00) | 10 | 13 | 1.14 (0.49–2.63) | |||
| AG/GG | 142 | 136 | 0.98 (0.73–1.30) | 140 | 142 | 0.89 (0.67–1.18) | |||
| UGT1A6 | rs4124874 | AA | 102 | 91 | 1.00 | 113 | 95 | 1.00 | 0.57 |
| AC | 168 | 162 | 1.08 (0.76–1.54) | 140 | 166 | 1.41 (0.99–2.01) | |||
| CC | 60 | 50 | 0.94 (0.59–1.50) | 69 | 68 | 1.17 (0.76–1.80) | |||
| Estradiol < median (32.2 pg/ml) | Estradiol ≥ median (32.2 pg/ml) | ||||||||
| HSD17B2 | rs4445895 | CC | 151 | 130 | 1.00 | 117 | 125 | 1.00 | <0.01 |
| CT | 140 | 142 | 1.19 (0.85–1.66) | 151 | 152 | 0.94 (0.67–1.32) | |||
| TT | 35 | 49 | 1.64 (1.00–2.69) | 56 | 33 | 0.55 (0.33–0.91) | |||
| CYP19A1 | rs700518 | GG | 112 | 93 | 1.00 | 112 | 127 | 1.00 | 0.03 |
| AG | 189 | 230 | 1.47 (1.05–2.05) | 217 | 207 | 0.84 (0.61–1.16) | |||
| AA | 107 | 96 | 1.08 (0.73–1.59) | 84 | 95 | 1.00 (0.68–1.47) | |||
| CYP1B1 | rs1800440 | AA | 277 | 292 | 1.00 | 272 | 289 | 1.00 | 0.11 |
| AG | 122 | 130 | 1.01 (0.75–1.36) | 131 | 127 | 0.91 (0.68–1.23) | |||
| GG | 15 | 5 | 0.32 (0.11–0.88) | 14 | 16 | 1.08 (0.52–2.25) | |||
| AG/GG | 137 | 135 | 0.93 (0.70–1.25) | 145 | 143 | 0.93 (0.70–1.23) | |||
| UGT1A6 | rs4124874 | AA | 95 | 98 | 1.00 | 120 | 88 | 1.00 | 0.22 |
| AC | 166 | 168 | 0.98 (0.69–1.40) | 142 | 158 | 1.52 (1.07–2.17) | |||
| CC | 61 | 55 | 0.87 (0.55–1.38) | 68 | 63 | 1.27 (0.82–1.97) | |||
| UGT2B7 | rs7439366 | TT | 77 | 86 | 1.00 | 93 | 74 | 1.00 | 0.03 |
| TC | 165 | 173 | 0.94 (0.65–1.37) | 164 | 139 | 1.07 (0.73–1.56) | |||
| CC | 74 | 59 | 0.71 (0.45–1.13) | 67 | 84 | 1.59 (1.02–2.47) | |||
| Estrone < median (42.1 pg/ml) | Estrone ≥ median (42.1 pg/ml) | ||||||||
| UGT1A6 | rs4124874 | AA | 97 | 89 | 1.00 | 116 | 97 | 1.00 | 0.40 |
| AC | 157 | 147 | 1.02 (0.71–1.47) | 147 | 176 | 1.44 (1.02–2.04) | |||
| CC | 64 | 57 | 0.97 (0.61–1.54) | 65 | 61 | 1.12 (0.72–1.74) | |||
| UGT1A10 | rs2741049 | TT | 127 | 108 | 1.00 | 113 | 138 | 1.00 | 0.07 |
| TC | 138 | 124 | 1.06 (0.74–1.51) | 137 | 129 | 0.77 (0.55–1.10) | |||
| CC | 48 | 54 | 1.32 (0.83–2.11) | 70 | 54 | 0.63 (0.41–0.97) | |||
Bold values indicate significant/borderline significant findings.
Adjusted for age and family history.
Significant SNPs identified in overall analyses.
Table 4.
Prostate cancer risk by genotypes of estrogen-related genes and serum cholesterol and hormone levels in the finasteride arm in the PCPT
| Gene | SNP | Genotype | Controls | Cases | ORa (95% CI) | Controls | Cases | ORa (95% CI) | P for interaction |
|---|---|---|---|---|---|---|---|---|---|
| Cholesterol < median (208 mg/dl) | Cholesterol ≥ median (208 mg/dl) | ||||||||
| PGR | rs500760 | AA | 104 | 154 | 1.00 | 120 | 112 | 1.00 | 0.12 |
| AG | 86 | 83 | 0.64 (0.43–0.95) | 68 | 72 | 1.15 (0.75–1.75) | |||
| GG | 14 | 9 | 0.44 (0.18–1.06) | 19 | 13 | 0.77 (0.36–1.64) | |||
| CYP19A1 | rs2445765b | GG | 146 | 224 | 1.00 | 177 | 203 | 1.00 | 0.19 |
| GC | 94 | 94 | 0.65 (0.46–0.92) | 81 | 80 | 0.87 (0.60–1.25) | |||
| CC | 17 | 9 | 0.35 (0.15–0.80) | 11 | 12 | 0.92 (0.39–2.13) | |||
| GC/CC | 111 | 103 | 0.60 (0.43–0.85) | 92 | 92 | 0.87 (0.61–1.24) | |||
| COMT | rs4680b | AA | 59 | 63 | 1.00 | 62 | 42 | 1.00 | 0.68 |
| AG | 103 | 126 | 1.18 (0.76–1.84) | 101 | 105 | 1.53 (0.95–2.47) | |||
| GG | 11 | 58 | 1.26 (0.74–2.14) | 47 | 55 | 1.71 (0.99–2.98) | |||
| Testosterone < median (364 ng/dl) | Testosterone ≥ median (364 ng/dl) | ||||||||
| PGR | rs500760 | AA | 120 | 133 | 1.00 | 105 | 133 | 1.00 | 0.21 |
| AG | 77 | 82 | 0.95 (0.64–1.42) | 77 | 70 | 0.72 (0.47–1.09) | |||
| GG | 13 | 13 | 0.95 (0.42–2.14) | 20 | 9 | 0.36 (0.16–0.82) | |||
| CYP19A1 | rs2445765b | GG | 164 | 218 | 1.00 | 160 | 207 | 1.00 | 0.81 |
| GC | 83 | 89 | 0.81 (0.56–1.16) | 92 | 83 | 0.70 (0.48–1.00) | |||
| CC | 17 | 14 | 0.61 (0.29–1.28) | 11 | 7 | 0.48 (0.18–1.28) | |||
| GC/CC | 100 | 103 | 0.78 (0.55–1.09) | 103 | 90 | 0.67 (0.47–0.95) | |||
| UGT1A6 | rs4124874 | AA | 68 | 58 | 1.00 | 59 | 75 | 1.00 | <0.01 |
| AC | 109 | 111 | 1.21 (0.78–1.89) | 101 | 104 | 0.80 (0.52–1.24) | |||
| CC | 33 | 58 | 2.13 (1.22–3.71) | 45 | 35 | 0.61 (0.35–1.07) | |||
| UGT1A10 | rs2741049 | TT | 73 | 96 | 1.00 | 88 | 76 | 1.00 | 0.02 |
| TC | 102 | 101 | 0.74 (0.49–1.12) | 86 | 84 | 1.16 (0.76–1.79) | |||
| CC | 29 | 24 | 0.62 (0.33–1.16) | 22 | 39 | 2.13 (1.16–3.91) | |||
| Estradiol < median (33.05 pg/ml) | Estradiol ≥ median (33.05 pg/ml) | ||||||||
| PGR | rs500760 | AA | 102 | 129 | 1.00 | 121 | 137 | 1.00 | 0.01 |
| AG | 88 | 73 | 0.64 (0.43–0.97) | 65 | 79 | 1.09 (0.72–1.65) | |||
| GG | 14 | 16 | 0.93 (0.43–2.00) | 19 | 6 | 0.29 (0.11–0.75) | |||
| CYP19A1 | rs2445765b | GG | 159 | 204 | 1.00 | 163 | 220 | 1.00 | 0.65 |
| GC | 87 | 92 | 0.82 (0.57–1.18) | 86 | 80 | 0.69 (0.48–1.00) | |||
| CC | 15 | 9 | 0.45 (0.19–1.07) | 13 | 11 | 0.63 (0.27–1.44) | |||
| GC/CC | 102 | 101 | 0.77 (0.54–1.08) | 99 | 91 | 0.68 (0.48–0.97) | |||
| COMT | rs4680b | AA | 64 | 51 | 1.00 | 56 | 54 | 1.00 | 0.08 |
| AG | 110 | 111 | 1.28 (0.81–2.01) | 94 | 117 | 1.33 (0.84–2.11) | |||
| GG | 34 | 57 | 2.09 (1.19–3.67) | 57 | 54 | 1.04 (0.61–1.75) | |||
| Estrone < median (43.2 pg/ml) | Estrone ≥ median (43.2 pg/ml) | ||||||||
| PGR | rs500760 | AA | 109 | 117 | 1.00 | 114 | 147 | 1.00 | 0.41 |
| AG | 89 | 76 | 0.79 (0.53–1.18) | 64 | 73 | 0.89 (0.59–1.35) | |||
| GG | 12 | 11 | 0.87 (0.37–2.07) | 20 | 11 | 0.44 (0.20–0.96) | |||
| CYP19A1 | rs2445765b | GG | 155 | 184 | 1.00 | 167 | 236 | 1.00 | 0.15 |
| GC | 88 | 93 | 0.89 (0.62–1.27) | 84 | 76 | 0.64 (0.45–0.93) | |||
| CC | 15 | 6 | 0.33 (0.12–0.87) | 13 | 14 | 0.76 (0.35–1.66) | |||
| GC/CC | 103 | 99 | 0.81 (0.57–1.14) | 97 | 90 | 0.66 (0.46–0.93) | |||
| COMT | rs4680b | AA | 64 | 61 | 1.00 | 56 | 43 | 1.00 | 0.13 |
| AG | 114 | 102 | 0.95 (0.61–1.48) | 90 | 125 | 1.86 (1.15–3.01) | |||
| GG | 37 | 42 | 1.19 (0.67–2.09) | 53 | 68 | 1.70 (0.99–2.91) | |||
Bold values indicate significant/borderline significant findings.
Adjusted for age and family history.
Significant SNPs identified in overall analyses.
In the finasteride arm, stratification by serum cholesterol and hormone levels predominantly showed significant associations with rs2445765 in CYP19A1 and rs4680 in COMT, the SNPs that were significantly associated with overall prostate cancer risk (Tables 2 and 4). The GG genotype of rs4680 in COMT was associated with an increased risk of prostate cancer among men with high level of serum cholesterol or estrone, and with low level of serum estradiol. While the inverse association between rs2445764 in CYP19A1 and prostate cancer risk was observed among men with low level of serum cholesterol, and with high level of any serum hormones (testosterone, estradiol and estrone). Additionally, rs500760 in PGR consistently showed an inverse association with prostate cancer risk among men with low serum cholesterol or estradiol level, or men with high testosterone or estrone level.
Haplotype analysis
CYP19A1 was the only gene harboring SNPs significantly associated with prostate cancer risk in both the placebo arm (rs2445765, rs700518, rs700519) and the finasteride arm (rs2445765). Using all three CYP19A1 SNPs genotyped in the study (rs700518, rs2445765 and rs700519), we conducted haplotype analysis and explored the association with prostate cancer risk (Table 5). Compared with the non-risk allele haplotype of CYP19A1 (GCC), several CYP19A1 haplotypes were significantly associated with increased prostate cancer risk in both placebo and finasteride arm.
Table 5.
Prostate cancer risk by haplotypes of CYP19A1 in the PCPT
| Haplotype | Placebo arm | Finasteride arm | ||
|---|---|---|---|---|
| Estimated % | ORa (95% CI) | Estimated % | ORa (95% CI) | |
| CYP19A1 (rs700518, rs2445765, rs700519) | ||||
| GCC | 10.1 | 1.00 | 10.5 | 1.00 |
| ACC | 6.3 | 1.52 (0.94–2.45) | 8.1 | 1.37 (0.82–2.28) |
| AGC | 38.8 | 1.29 (0.99–1.68) | 36.6 | 1.49 (1.10–2.04) |
| GGC | 41.7 | 1.42 (1.06–1.90) | 41.5 | 1.70 (1.20–2.41) |
| Other# | 3.1 | 1.91 (1.19–3.07) | 3.3 | 1.43 (0.83–2.47) |
Bold values indicate significant/borderline significant findings.
Adjusted for age and family history.
All haplotypes with frequency less than 5% combined.
Discussion
In this nested case–control study within the PCPT, we investigated SNPs in genes involved in estrogen synthesis, metabolism, and function in relation to prostate cancer risk. Panels of SNPs were significantly associated with prostate cancer risk, and importantly, the SNPs were different according to treatment arm, showing rs1801132 in ESR1 in the placebo arm and rs2445765 in CYP19A1 and rs4680 in COMT in the finasteride arm. When the analyses were stratified by serum cholesterol and hormone levels, all three SNPs showed the same direction of associations as observed in overall analysis, although significant associations were only observed in either low or high category. Several additional SNPs repeatedly showed significant associations with prostate cancer risk in the stratified analyses, including rs4124874 in UGT1A6 and all genotyped SNPs in CYP19A1 (rs2445765, rs700518, rs700519) in the placebo arm, and rs500760 in PGR in the finasteride arm.
Based on results from the overall and stratified analyses, the SNPs showing consistent associations with prostate cancer risk were used to assemble two multi-marker-panels: the placebo-risk panel consisting of rs1801132 in ESR1, rs700518, rs700519, rs2445765 in CYP19A1 and rs4124874 in UGT1A6, and the finasteride-risk panel consisting of rs2445765 in CYP19A1, rs4680 in COMT and rs500760 in PGR. To evaluate the overall effect of multiple SNPs, these two panels of SNPs were used to calculate polygenic risk scores assuming an additive model in the placebo and finasteride arm separately (40). However, no significant association was observed with either placebo or finasteride risk panel (results not shown). The generally null associations with the multi-marker analysis were not surprising, as most of the SNPs were associated with either low- or high-grade prostate cancer and the associations became more evident under certain high or low circulating hormone levels.
Circulating hormones may play an indirect role in prostatic carcinogenesis. Epidemiological studies have consistently documented a null association of prostate cancer risk with both serum androgen and estrogen levels, including the findings from the PCPT (11,41–44). However, by serving as precursors or substrates for local hormone synthesis, circulating hormone levels may affect risk associations with genes controlling intraprostatic hormone milieu. Indeed, we found that circulating hormone levels modify the genetic-risk associations observed in the study. When the analyses were stratified by serum levels of cholesterol, testosterone, estradiol or estrone, significant associations were primarily observed in either the high or low category of serum hormones. These results are consistent with the findings of different SNPs identified in the placebo and finasteride arms, as finasteride treatment significantly increased circulating androgen and estrogen levels in the PCPT (19,44). However, finasteride treatment may have a more profound impact on intraprostatic hormonal milieu because of the blockage of the intraprostatic conversion of testosterone to dihydrotestrosterone, therefore, resulting in different risk panels of SNPs between the treatment arms. Moreover, all significant SNPs identified in the study had no associations with circulating androgen or estrogen levels and adjustment of circulating hormone levels in the models did not alter gene-risk association (results not shown). This finding further supports the role of circulating hormones as effect modifiers instead of direct players in the development of prostate cancer.
Several studies have examined associations between prostate cancer risk and genetic variations of estrogen-related genes (18,45–48), and the most consistent finding is with CYP19A1, although genetic variants examined varied across the studies. In the current study, CYP19A1 was the only gene harboring SNPs that were significantly associated with prostate cancer risk in both the placebo (rs700518, rs700519, rs2445765) and finasteride arms (rs2445765). Using all three CYP19A1 SNPs genotyped in the study (rs700518, rs2445765 and rs700519), we conducted haplotype analysis and found that compared with the non-risk haplotype (GCC), all other five CYP19A1 haplotypes were universally associated with increased prostate cancer risk in both arms, and the associations with three out of the five haplotypes reach statistical significance. The CYP19A1 encodes aromatase, the essential enzyme catalyzing the conversion of androgen to estrogen. In the prostate, aromatase is expressed in stroma, but not benign epithelial cells; however, the expression of aromatase was detected in malignant prostate epithelial cells as well as prostate cancer cell lines (3,49), suggesting the enhanced intraprostatic conversion of androgen to estrogen during carcinogenesis. Given that prostatic carcinogenesis requires both androgen and estrogen and neither androgen (non-aromatizable) nor estrogen alone can induce prostate cancer (3,4,50), aromatase may play a pivotal role in the development of prostate cancer by controlling the ratio of intraprostatic estrogen and androgen.
Several strengths and weaknesses of this study need to be discussed. Our study leveraged a clinical trial design of the PCPT with prospective collection of information on participants’ characteristics and risk factors as well as blood samples, minimizing potential detection bias usually presented in case–control studies. Importantly, the status of cases and controls were biopsy-confirmed with a central pathology confirmation of diagnosis, which reduced the likelihood of undiagnosed prostate cancer in the control group and minimized potential misclassification. However, the study had a relatively small sample size, particularly when dealing with subgroup analyses of low-grade and high-grade prostate cancer, in which we do not have enough power to further stratify by serum hormone levels and explore the SNPs associated with grade-specific prostate cancer. Considering the nature of the study design that used a candidate gene approach and selected a small number of known or purported functional SNPs, and given the hypothesis-driven selection and the prior expectation of associations, we did not systematically correct for multiple testing as one might have done for agnostic genetic studies. Instead of focusing on results from individual SNPs, we emphasized on the difference between the placebo and finasteride arms and the modifying effect of circulating hormones, which could be more stable and less subjected to the sample size limitation. Nevertheless, when correcting for multiple testing using the conservative Bonferroni correction (0.05/25 SNPs tested = 0.002), using the main results (Table 2), only the association for rs2445765 in CYP19A1 in the finasteride arm (P = 0.001) would remain statistically significant. The result is consistent with our main finding on CYP19A1. It need to be noted that the blood draws used for measurement of serum hormone levels were non-fasting blood draws, and were not taken at a fixed time of day. The varied time for blood draw could affect comparability of the results, although dichotomizing hormone levels in the study might minimize the impact of variabilities in blood draw. The study also had limited statistical power to examine gene-risk associations in other racial/ethnic groups, since only 14% of study participants were non-white, although similar results were obtained when the analyses included all white and non-white men (results not shown).
In summary, using data and samples from the PCPT, we examined the associations of prostate cancer risk with polymorphisms in genes involved in estrogen synthesis, metabolism and function. We found that panels of SNPs that were significantly associated with prostate cancer risk were different by PCPT treatment arms, and when stratified by circulating hormone levels, significant associations were primarily observed in either the low or high category of hormones. None of the SNPs identified in the study had an association with circulating estrogen or androgen levels. These results support the hypothesis that genetic variants in estrogen-related genes may affect prostate cancer risk presumably via altering intraprostatic hormone milieu, but not circulating hormone levels, while circulating hormone levels strongly modify the associations between genetic variants and prostate cancer risk. Overall, the associations of prostate cancer risk with estrogen-related gene polymorphisms are complex, and markedly attenuated by factors such as finasteride treatment or circulating levels of androgen and estrogen.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (U10CA37429, 5UM1CA182883, P01CA108964 and K07CA148888). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement: None declared.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- OR
odds ratio
- PCPT
Prostate Cancer Prevention Trial
- PSA
prostate-specific antigen
- SNP
single nucleotide polymorphism
References
- 1. Sharifi N., et al. (2005)Androgen deprivation therapy for prostate cancer. JAMA, 294, 238–244. [DOI] [PubMed] [Google Scholar]
- 2. Härkönen P.L., et al. (2004)Role of estrogens in development of prostate cancer. J. Steroid Biochem. Mol. Biol., 92, 297–305. [DOI] [PubMed] [Google Scholar]
- 3. Risbridger G.P., et al. (2003)Oestrogens and prostate cancer. Endocr. Relat. Cancer, 10, 187–191. [DOI] [PubMed] [Google Scholar]
- 4. Bosland M.C., et al. (1995)Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17 beta or diethylstilbestrol. Carcinogenesis, 16, 1311–1317. [DOI] [PubMed] [Google Scholar]
- 5. McPherson S.J., et al. (2001)Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology, 142, 2458–2467. [DOI] [PubMed] [Google Scholar]
- 6. Vermeulen A., et al. (2002)Estradiol in elderly men. Aging Male, 5, 98–102. [PubMed] [Google Scholar]
- 7. Baulieu E.E. (2002)Androgens and aging men. Mol. Cell. Endocrinol., 198, 41–49. [DOI] [PubMed] [Google Scholar]
- 8. Krieg M., et al. (1993)Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J. Clin. Endocrinol. Metab., 77, 375–381. [DOI] [PubMed] [Google Scholar]
- 9. Rohrmann S., et al. (2011)The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III). Clin. Endocrinol. (Oxf)., 75, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eaton N.E., et al. (1999)Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br. J. Cancer, 80, 930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roddam A.W., et al. (2008)Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst., 100, 170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao S., et al. (2011)Serum estrogen levels and prostate cancer risk in the prostate cancer prevention trial: a nested case-control study. Cancer Causes Control, 22, 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Labrie F., et al. (1997)The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids, 62, 148–158. [DOI] [PubMed] [Google Scholar]
- 14. Stone N.N., et al. (1987)Aromatization of androstenedione to estrogen by benign prostatic hyperplasia, prostate cancer and expressed prostatic secretions. Urol. Res., 15, 165–167. [DOI] [PubMed] [Google Scholar]
- 15. Stone N.N., et al. (1986)Estrogen formation in human prostatic tissue from patients with and without benign prostatic hyperplasia. Prostate., 9, 311–318. [DOI] [PubMed] [Google Scholar]
- 16. Machioka K., et al. (2015)Active estrogen synthesis and its function in prostate cancer-derived stromal cells. Anticancer Res., 35, 221–227. [PubMed] [Google Scholar]
- 17. Thompson I.M., et al. (2003)The influence of finasteride on the development of prostate cancer. N. Engl. J. Med., 349, 215–224. [DOI] [PubMed] [Google Scholar]
- 18. Tang L., et al. (2011)Repeat polymorphisms in estrogen metabolism genes and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Carcinogenesis., 32, 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kristal A.R., et al. (2008)Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am. J. Epidemiol., 168, 1416–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winchester D.A., et al. (2015)Variation in genes involved in the immune response and prostate cancer risk in the placebo arm of the Prostate Cancer Prevention Trial. Prostate., 75, 1403–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson P.A., et al. (1998)Genetic polymorphisms in catechol-O-methyltransferase, menopausal status, and breast cancer risk. Cancer Res., 58, 2107–2110. [PubMed] [Google Scholar]
- 22. Ambrosone C.B., et al. (1995)Cytochrome P4501A1 and glutathione S-transferase (M1) genetic polymorphisms and postmenopausal breast cancer risk. Cancer Res., 55, 3483–3485. [PubMed] [Google Scholar]
- 23. Chang B.L., et al. (2003)Polymorphisms in the CYP1A1 gene are associated with prostate cancer risk. Int. J. Cancer, 106, 375–378. [DOI] [PubMed] [Google Scholar]
- 24. Kristensen V.N., et al. (2001)Genetic susceptibility and environmental estrogen-like compounds. Mutat. Res., 482, 77–82. [DOI] [PubMed] [Google Scholar]
- 25. Cai Q., et al. (2003)Genetic polymorphisms in the estrogen receptor alpha gene and risk of breast cancer: results from the Shanghai Breast Cancer Study. Cancer Epidemiol. Biomarkers Prev., 12, 853–859. [PubMed] [Google Scholar]
- 26. Cramer D.W., et al. (2003)Human progesterone receptor polymorphisms and implantation failure during in vitro fertilization. Am. J. Obstet. Gynecol., 189, 1085–1092. [DOI] [PubMed] [Google Scholar]
- 27. Wang-Gohrke S., et al. (2000)Progesterone receptor gene polymorphism is associated with decreased risk for breast cancer by age 50. Cancer Res., 60, 2348–2350. [PubMed] [Google Scholar]
- 28. Han W., et al. (2003)Full sequencing analysis of estrogen receptor-alpha gene polymorphism and its association with breast cancer risk. Anticancer Res., 23(6C), 4703–4707. [PubMed] [Google Scholar]
- 29. Bailey L.R., et al. (1998)Association of cytochrome P450 1B1 (CYP1B1) polymorphism with steroid receptor status in breast cancer. Cancer Res., 58, 5038–5041. [PubMed] [Google Scholar]
- 30. Long J.R., et al. (2006)Genetic polymorphisms of the CYP19A1 gene and breast cancer survival. Cancer Epidemiol. Biomarkers Prev., 15, 2115–2122. [DOI] [PubMed] [Google Scholar]
- 31. Beuten J., et al. (2008)CYP1B1 variants are associated with prostate cancer in non-Hispanic and Hispanic Caucasians. Carcinogenesis, 29, 1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J., et al. (2007)Estrogen receptor alpha haplotypes and breast cancer risk in older Caucasian women. Breast Cancer Res. Treat., 106, 273–280. [DOI] [PubMed] [Google Scholar]
- 33. Thellenberg-Karlsson C., et al. (2006)Estrogen receptor beta polymorphism is associated with prostate cancer risk. Clin. Cancer Res., 12, 1936–1941. [DOI] [PubMed] [Google Scholar]
- 34. Guillemette C., et al. (2000)Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res., 60, 950–956. [PubMed] [Google Scholar]
- 35. Park J., et al. (2004)Asp85tyr polymorphism in the udp-glucuronosyltransferase (UGT) 2B15 gene and the risk of prostate cancer. J. Urol., 171(6 Pt 1), 2484–2488. [DOI] [PubMed] [Google Scholar]
- 36. Bhasker C.R., et al. (2000)Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics, 10, 679–685. [DOI] [PubMed] [Google Scholar]
- 37. Miners J.O., et al. (2002)Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology, 181-182, 453–456. [DOI] [PubMed] [Google Scholar]
- 38. Martineau I., et al. (2004)Amino acid residue ILE211 is essential for the enzymatic activity of human UDP-glucuronosyltransferase 1A10 (UGT1A10). Drug Metab. Dispos., 32, 455–459. [DOI] [PubMed] [Google Scholar]
- 39. Dellinger R.W., et al. (2006)Importance of UDP-glucuronosyltransferase 1A10 (UGT1A10) in the detoxification of polycyclic aromatic hydrocarbons: decreased glucuronidative activity of the UGT1A10139Lys isoform. Drug Metab. Dispos., 34, 943–949. [DOI] [PubMed] [Google Scholar]
- 40. Dudbridge F. (2013)Power and predictive accuracy of polygenic risk scores. PLoS Genet., 9, e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Platz E.A., et al. (2004)The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J. Steroid Biochem. Mol. Biol., 92, 237–253. [DOI] [PubMed] [Google Scholar]
- 42. Schenk J.M., et al. (2016)Serum androgens and prostate cancer risk: results from the placebo arm of the Prostate Cancer Prevention Trial. Cancer Causes Control, 27, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kristal A.R., et al. (2012)Associations of serum sex steroid hormone and 5α-androstane-3α,17β-diol glucuronide concentrations with prostate cancer risk among men treated with finasteride. Cancer Epidemiol. Biomarkers Prev., 21, 1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao S., et al. (2011)Serum estrogen levels and prostate cancer risk in the Prostate Cancer Prevention Trial: a nested case-control study. Cancer Control and Cause, 22, 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holt S.K., et al. (2013)Association of variants in estrogen-related pathway genes with prostate cancer risk. Prostate, 73, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eriksson A.L., et al. (2009)Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J. Clin. Endocrinol. Metab., 94, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beuten J., et al. (2009)Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev., 18, 1869–1880. [DOI] [PubMed] [Google Scholar]
- 48. Cussenot O., et al. (2007)Combination of polymorphisms from genes related to estrogen metabolism and risk of prostate cancers: the hidden face of estrogens. J. Clin. Oncol., 25, 3596–3602. [DOI] [PubMed] [Google Scholar]
- 49. Ellem S.J., et al. (2004)Local aromatase expression in human prostate is altered in malignancy. J. Clin. Endocrinol. Metab., 89, 2434–2441. [DOI] [PubMed] [Google Scholar]
- 50. Bianco J.J., et al. (2002)Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology, 143, 4922–4933. [DOI] [PubMed] [Google Scholar]