Abstract
STUDY QUESTION
Are maternal preconception lipid levels associated with fecundability?
SUMMARY ANSWER
Fecundability was reduced for all abnormal female lipid levels including total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and total triglyceride levels.
WHAT IS KNOWN ALREADY
Subfecundity affects 7–15% of the population and lipid disorders are hypothesized to play a role since cholesterol acts as a substrate for the synthesis of steroid hormones. Evidence illustrating this relationship at the mechanistic level is mounting but few studies in humans have explored the role of preconception lipids in fecundity.
STUDY DESIGN, SIZE, DURATION
A secondary analysis of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial (2007–2011), a block-randomized, double-blind, placebo-controlled trial.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 1228 women, with 1–2 prior pregnancy losses and without a diagnosis of infertility, attempting pregnancy for up to six menstrual cycles were recruited from clinical sites in Utah, New York, PA and Colorado. Time to pregnancy was the number of menstrual cycles to pregnancy as determined by positive hCG test or ultrasound. Individual preconception lipoproteins were measured at baseline, prior to treatment randomization and dichotomized based on clinically accepted cut-points as total cholesterol ≥200 mg/dl, LDL-C ≥130 mg/dl, HDL-C <50 mg/dl and triglycerides ≥150 mg/dl.
MAIN RESULTS AND THE ROLE OF CHANCE
There were 148 (12.3%) women with elevated total cholesterol, 94 (7.9%) with elevated LDL-C, 280 (23.2%) with elevated triglycerides and 606 (50.7%) with low HDL-C. The fecundability odds ratio (FOR) was reduced for all abnormal lipids before and after confounder adjustment, indicating reduced fecundability. Total cholesterol ≥200 mg/dl was associated with 24% (FOR: 0.76, 95% CI: 0.59, 0.97) and 29% (FOR: 0.71, 95% CI: 0.55, 0.93) reduced fecundability for hCG-detected and ultrasound-confirmed pregnancy, respectively, compared with total cholesterol <200 mg/dl. There was a 19–36% decrease in the probability of conception per cycle for women with abnormal lipoprotein levels, though additional adjustment for central adiposity and BMI attenuated observed associations.
LIMITATIONS, REASONS FOR CAUTION
Although the FOR is a measure of couple fecundability, we had only measures of female lipid levels and can therefore not confirm the findings from a previous study indicating the independent role of male lipids in fecundity. The attenuated estimates and decreased precision after adjustment for central adiposity and obesity indicate the complexity of potential causal lipid pathways, suggesting other factors related to obesity besides dyslipidemia likely contribute to reduced fecundability.
WIDER IMPLICATIONS OF THE FINDINGS
Our results are consistent with one other study relating preconception lipid concentrations to fecundity and expand these findings by adding critically important information about individual lipoproteins. As lipid levels are modifiable they may offer an inexpensive target to improve female fecundability.
STUDY FUNDING AND COMPETING INTEREST(S)
This study was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors have declared that no conflicts of interest exist.
TRIAL REGISTRATION NUMBER
Keywords: lipids, fecundity, cholesterol, pregnancy, triglycerides, preconception, lipoprotein
Introduction
Subfecundity affects 7–15% of the population (Buck Louis and Platt, 2011; Thoma et al., 2013), with couples experiencing a significant financial and emotional burden (Domar et al., 2012). While a number of factors may be associated with reduced fecundity (Guldbrandsen et al., 2014; Gaskins et al., 2015), lipid disorders are hypothesized to play a role. A single study examining fecundity of couples reported a reduced fecundability odds ratio (FOR) with high total cholesterol in both men and women (Schisterman et al., 2014a).
Cholesterol acts as a substrate for the synthesis of steroid hormones (Grummer and Carroll, 1988; Miller and Auchus, 2011). Cholesterol and cholesteryl esters are major components of lipoprotein particles, such as high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), which transport cholesterol and triglycerides to reproductive tissues (e.g. thecal and granulosa cells) where steroidogenesis takes place (Mozaffarian et al., 2015). Evidence from rodent and human IVF studies indicate that an abnormal maternal serum lipid profile is associated with poorer oocyte quality, ovarian function and embryo development, indicating a potential reduction in fecundity (Grummer and Carroll, 1988; Miller and Auchus, 2011). However, it is unknown whether individual lipoprotein particles may also be important.
Lipid abnormalities affect 45% of reproductive age women, and understanding preconception lipid abnormalities on female fecundity may provide an early, modifiable and inexpensive target for treatment. The objective of this study was to assess the role of preconception maternal total cholesterol, HDL-C, LDL-C and triglycerides in relation to fecundability in a cohort of healthy women with 1–2 pregnancy losses and no diagnosis of infertility.
Materials and Methods
Study setting and population
This was a secondary analysis of the entire cohort of women from the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, a block-randomized, double-blind, placebo-controlled trial designed to study the effects of preconception low-dose aspirin use on the live-birth rate in women with 1–2 prior pregnancy losses (Schisterman et al., 2013). Women 18–40 years of age with regular menstrual cycles and attempting conception without fertility treatments were recruited from four medical centers in the USA from 2007 to 2011.
Participants utilized fertility monitors (Clearblue Easy Fertility Monitor, Inverness Medical Innovations, Waltham, MA, USA) to assist with the timing of intercourse and the timing of study visits to coincide with specific cycle-days. Women were followed until the completion of six menstrual cycles, or if they became pregnant through 36 weeks’ gestation.
Ethics approval
Written informed consent was provided by all participants. The data coordinating center and each site obtained Institutional Review Board approvals. The trial was registered on clinicaltrials.gov #NCT00467363.
Lab assessments
Preconception serum samples were collected at baseline coinciding with Days 2–4 of women's menstrual cycles and prior to treatment randomization. Samples were stored in −80F freezers until assay. Total cholesterol, LDL-C, HDL-C and triglycerides were directly measured using the Roche COBAS 6000 chemistry analyzer (Roche Diagnostics, Indianapolis, IN, USA). Total cholesterol was analyzed using a cholesterol oxidase enzymatic determination, triglycerides using the GPO-Trinder methodology, HDL-C using a modified direct enzymatic method and LDL-C using selective solubilization. In addition to direct measurement, LDL-C was calculated using the Friedewald formula (Cholesterol-HDL-C-(Triglycerides/5)) (Friedewald et al., 1972). Results are presented for calculated LDL-C owing to clinical relevance, since direct measurement of LDL-C requires a technique less accessible to most laboratories. The interassay laboratory coefficients of variation (CV) ranged from 1.6 to 3.2% at mean lipid concentrations for manufacturer controls. Serum total free fatty acid (NEFA; non-esterified fatty acid) concentrations were assayed using an enzymatic, colorimetric assay (WAKO Life Sciences, Mountain View, CA, USA), with an interassay CV of 7.1 and 8.9% at mean concentrations of 0.73 and 0.49 mEq/l.
Pregnancy assessment
Pregnancy was defined into two ways. hCG-detected pregnancy was defined as a positive urine pregnancy test. Ultrasound-confirmed pregnancy was defined by the presence of a gestational sac via ultrasound at 6–7 weeks’ gestation. Ultrasound was performed only in patients with a positive hCG. Time to pregnancy (TTP) was defined as the number of menstrual cycles until a positive pregnancy test over six consecutive months of follow-up. An hCG-detected pregnancy was identified by a home pregnancy kit (Quidel Quickvue One step hCG test, Quidel Corporation, San Diego, CA, USA), or an in-clinic urine test sensitive to hCG levels ≥0.025 ng/ml among women reporting a missed menses. In addition, pregnancies were identified by free beta hCG measured in spot urine samples from visits coinciding with menstrual cycle days 2–4 and in daily first-morning urine samples from the last 10 cycle-days. These were performed during the participants’ first two cycles (Diagnostic Automation, Calabasas, CA, USA; BioVendor, Asheville, NC, USA).
Covariates
Height, weight, waist circumference and hip circumference were measured at baseline and women completed questionnaires on demographics (e.g. age and race), occupation, lifestyle habits, medical and reproductive history, and family medical history. Total serum-free fatty acid was measured at baseline and indicates maternal dietary fat intake prior to the blood draw or lipids (Crowe et al., 2006).
Statistical analysis
Chi-square tests, Fisher's exact test where appropriate, and Student's t-tests were used to determine differences in maternal characteristics and lipid concentrations by pregnancy status. To address missing data in women without lipid levels [41 (3.3%)] or complete covariate information [39 (3.2%)], we used multiple imputation (MI) for all analyses. We imputed missing lipids and covariates in 20 data sets using the fully-conditional specification method (van Buuren, 2007) by including all covariates in addition to pre-pregnancy BMI, employment status, income, smoking, exercise, coffee intake, waist circumference and hip circumference in the imputation model. Cox proportional hazard models for discrete left-truncated survival time, to account for the amount of time attempting conception prior to study entry, were used to estimate FOR and 95% CI for associations between maternal lipid concentrations and both hCG-detected and ultrasound-confirmed pregnancy. A FOR <1 indicates a decrease in the probability of conception per cycle. Lipids were considered as continuous (per 10-unit change), as well as dichotomized using clinically accepted cut-points for cardiovascular disease risk comparing unfavorable versus favorable values, respectively: total cholesterol (≥200 vs <200 mg/dl), LDL-C calculated (≥130 vs <130 mg/dl), HDL-C (<50 vs ≥50 mg/dl) and triglycerides (≥150 vs <150 mg/dl) (National Institutes of Health, 2001). Differences were considered significant at α < 0.05. Women who withdrew from the study prior to achieving a pregnancy or after completion of six menstrual cycles were censored in all analyses.
All models were adjusted for confounders identified a priori (Greenland et al., 1999; Hernan et al., 2002) including maternal age, race, education, alcohol use and total cholesterol (excluding total cholesterol models). Since not all women were fasting at the time of blood sampling, we developed a second model adjusting for preconception serum total free fatty acid levels and fasting status as a measure of dietary fat intake prior to the blood draw. We ran additional separate models adjusting for BMI and waist–hip ratio to also assess the potential effects of cholesterol independent of obesity. We also tested adjustment for treatment status (placebo vs low-dose aspirin) since women in the treatment group were more likely to achieve pregnancy (Schisterman et al., 2014a,b). To test whether the effect of lipids on fecundability varied by BMI or fasting status, statistical interaction terms were introduced into separate fully adjusted models. Effect modification was tested using α = 0.10 threshold. We also ran all analyses limited to complete-case data.
Women who withdrew from the trial differed from those that completed follow-up by LDL levels and a number of demographic characteristics. Therefore, to address potential selection bias, we conducted several sensitivity analyses to explore the robustness of our estimates to missing TTP in the withdrawals. We used equal-weighted MI and Kaplan–Meier MI to impute missing cycle data [128 (10%)] in 5000 imputations, since it was computationally infeasible to run all possible combinations of missing TTP. In the first procedure, we imputed TTP equally between missing cycles up to 6, with potential censoring of the sixth cycle and without regard to cholesterol category (addresses the assumption of missing completely at random). Second, we ran Kaplan–Meier MI (Zhao et al., 2014) which imputed potential outcomes according to the complete-case survival curve by total cholesterol categories (addresses the assumption of missing at random). We compared estimates from all imputed data sets to the complete-case FOR. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc. Cary, NC, USA).
Results
The EAGeR trial was comprised of 1228 women, of whom 303 (24.6%) did not achieve pregnancy and 128 (10.4%) withdrew prior to follow-up completion. The majority of women were white (94.6%), married (91.5%) and educated beyond high school (88%) (Table I). The mean (SD) age was 28.7(4.7) years and BMI was 26.4 (6.6). The median (IQR) month to hCG-detected and ultrasound-confirmed pregnancy was 4 (2–6).
Table I.
Characteristics of EAGeR population by pregnancy status.
| Overall | No observed pregnancy | hCG-detected pregnancy | Ultrasound-confirmed pregnancy | Withdrawal | LDL cholesterol | ||
|---|---|---|---|---|---|---|---|
| (n = 1228) No. (%) | (n = 303) No. (%) | (n = 797) No. (%) | (n = 732) No. (%) | (n = 128) No. (%) | <130 mg/dl (n = 1094) No. (%) |
≥130 mg/dl (n = 94) No. (%) |
|
| Maternal age, mean (range) | 28.7 (18.7–40.8) | 29.4 (19.2–40.8) | 28.7 (18.8–40.8)a | 28.6 (19.1–40.3)b | 27.5 (18.7–40.0)c | 28.6 (18.7–40.8) | 29.8 (19.7–39.7)d |
| BMI, mean (range) | 26.4 (15.7–58.7) | 27.8 (16.4–58.5) | 25.5 (115–55.0)a | 25.5 (15.7–55.0)b | 28.1 (17.5–58.7)c | 25.9 (15.7–58.7) | 30.3 (18.9–44.7)d |
| Waist–hip ratio, mean (range) | 0.81 (0.48–1.09) | 0.81 (0.65–1.09) | 0.81 (0.59–1.04) | 0.80 (0.59–1.04) | 0.82 (0.48–1.06)c | 0.81 (0.48–1.09) | 0.86 (0.67–1.06)d |
| Race | |||||||
| White | 1162 (94.6) | 283 (93.4) | 769 (96.5)a | 696 (96.7) | 110 (85.9)c | 1035 (94.6) | 90 (95.7) |
| Non-White | 66 (5.4) | 20 (6.6) | 28 (3.5) | 24 (3.3) | 18 (14.1) | 59 (5.4) | 4 (4.3) |
| Missing | 0 (0) | ||||||
| Marital status | |||||||
| Married | 1124 (91.5) | 272 (89.8) | 752 (94.3)a | 683 (94.9)b | 100 (78.1)c | 1007 (92.1) | 82 (87.3) |
| Living with partner | 74 (6.0) | 27 (8.9) | 31 (3.9) | 26 (3.6) | 16 (12.5) | 64 (5.9) | 7 (7.5) |
| Other | 30 (2.5) | 4 (1.3) | 14 (1.8) | 11 (1.5) | 12 (9.4) | 23 (2.1) | 5 (5.3) |
| Missing | 0 (0) | ||||||
| Education | |||||||
| Beyond high school | 1082 (88.2) | 260 (85.8) | 707 (88.7) | 644 (89.4) | 90 (70.9)c | 1073 (98.2) | 91 (96.8) |
| Up to high school | 145 (11.8) | 43 (14.2) | 89 (11.3) | 76 (10.6) | 37 (29.1) | 20 (1.8) | 3 (3.2) |
| Missing | 1 (0.08) | ||||||
| Annual household income (US$) | |||||||
| ≥100 000 | 491 (40.0) | 133 (44.0) | 326 (40.9) | 296 (41.1) | 32 (25.0)c | 440 (40.3) | 35 (37.3) |
| 75–99 999 | 149 (12.1) | 26 (8.6) | 114 (14.3) | 102 (14.2) | 9 (7.0) | 131 (12.0) | 15 (15.9) |
| 40–74 999 | 181 (14.8) | 46 (15.2) | 116 (14.5) | 106 (14.7) | 19 (14.8) | 162 (14.8) | 12 (12.8) |
| 20–39 999 | 312 (25.4) | 81 (26.8) | 187 (23.5) | 169 (23.5) | 44 (34.4) | 273 (24.9) | 27 (28.7) |
| ≤19 999 | 94 (7.7) | 16 (5.3) | 54 (6.8) | 47 (6.5) | 24 (18.8) | 87 (7.9) | 5 (5.3) |
| Missing | 1 (0.08) | ||||||
| Employment | |||||||
| Employed | 895 (72.9) | 238 (78.6) | 576 (72.3)a | 513 (71.3)b | 81 (63.3)c | 801 (73.2) | 70 (74.5) |
| Unemployed | 289 (23.5) | 58 (19.1) | 209 (26.2) | 195 (27.1) | 22 (17.2) | 254 (23.2) | 21 (22.3) |
| Missing | 44 (3.6) | ||||||
| Number of previous live births | |||||||
| 0 | 571 (46.5) | 166 (54.8) | 341 (42.8)a | 304 (42.2)b | 70 (54.7) | 499 (45.6) | 51 (54.3) |
| 1 | 443 (36.1) | 93 (30.7) | 301 (37.8) | 274 (38.1) | 44 (34.4) | 405 (37.0) | 26 (27.6) |
| 2 | 214 (17.4) | 44 (14.5) | 155 (19.5) | 142 (19.8) | 14 (10.9) | 190 (17.3) | 17 (18.1) |
| Missing | 0 (0) | ||||||
| Number of previous pregnancy losses | |||||||
| 1 | 825 (67.2) | 216 (71.3) | 524 (65.8) | 476 (66.1) | 85 (66.4) | 736 (67.3) | 64 (68.1) |
| 2 | 403 (32.8) | 87 (28.7) | 273 (34.3) | 244 (33.9) | 43 (33.6) | 358 (32.7) | 30 (31.9) |
| Missing | 0 (0) | ||||||
| Alcohol consumption in the past year | |||||||
| Never | 806 (66.5) | 202 (67.3) | 532 (67.4) | 480 (67.4) | 72 (58.5)c | 720 (66.6) | 58 (63.1) |
| Sometimes | 380 (31.4) | 93 (31.0) | 238 (30.2) | 213 (29.9) | 49 (39.4) | 336 (31.1) | 34 (36.9) |
| Often | 26 (2.1) | 5 (1.6) | 19 (2.4) | 19 (2.7) | 2 (1.6) | 25 (2.3) | 0 (0) |
| Missing | 16 (1.3) | ||||||
| Fasting status | |||||||
| Fasting | 179 (14.6) | 55 (18.4) | 95 (12.1)a | 90 (12.5)b | 29 (24.0)c | 151 (14.0) | 24 (26.7)d |
| Non-fasting | 1027 (83.6) | 244 (81.6) | 691 (87.9) | 632 (87.5) | 92 (76.0) | 926 (86.0) | 66 (73.3) |
| Missing | 22 (1.8) | ||||||
| Treatment group | |||||||
| Placebo | 613 (49.9) | 171 (56.4) | 385 (48.3)a | 354 (48.3)b | 57 (44.5) | 547 (50.0) | 42 (44.7) |
| Low-dose aspirin | 615 (50.1) | 132 (43.6) | 412 (51.7) | 378 (51.6) | 71 (55.5) | 547 (50.0) | 52 (55.3) |
EAGeR, Effects of Aspirin in Gestation and Reproduction; LDL, low-density lipoprotein.
aP < 0.05 between hCG-detected pregnancy and no observed pregnancy.
bP < 0.05 between ultrasound-confirmed pregnancy and no observed pregnancy.
cP < 0.05 between withdrawal and any observed pregnancy (hCG-detected or ultrasound-confirmed).
dP< 0.05 between LDL < 130 and LDL ≥ 130.
There were 148 (12.3%) women with elevated total cholesterol, 94 (7.9%) with elevated LDL-C, 280 (23.2%) with elevated triglycerides and 606 (50.7%) with low HDL-C. Preconception lipid levels significantly differed by pregnancy status (Table II). Compared with women without an observed pregnancy, women with an hCG-detected pregnancy were more likely to have HDL-C ≥50 mg/dl and women with an ultrasound-confirmed pregnancy were more likely to have LDL-C <130 mg/dl. Total cholesterol and triglycerides did not differ by pregnancy status. There was not a significant difference in the median number of months to either hCG-detected or ultrasound-confirmed by cholesterol status (Table III).
Table II.
Maternal preconception lipid levels by pregnancy status.
| Overall | No observed pregnancy | hCG-detected pregnancy | Ultrasound-confirmed pregnancy | Withdrawal | |
|---|---|---|---|---|---|
| (n = 1228) No. (%) | (n = 303) No. (%) | (n = 797) No. (%) | (n = 732) No. (%) | (n = 128) No. (%) | |
| Total cholesterol (mg/dl) | |||||
| <200 | 1059 (87.7) | 253 (85.5) | 697 (88.8) | 644 (89.4) | 109 (85.2) |
| ≥200 | 148 (12.3) | 43 (14.5) | 88 (11.2) | 76 (10.6) | 17 (13.3) |
| LDL-C calculated (mg/dl) | |||||
| <130 | 1094 (92.1) | 261 (89.7) | 724 (93.8)a | 668 (94.2)b | 109 (85.2)c |
| ≥130 | 94 (7.9) | 30 (10.3) | 48 (6.2) | 41 (5.8) | 16 (12.5) |
| HDL-C (mg/dl) | |||||
| ≥50 | 590 (49.3) | 131 (44.7) | 405 (52.1)a | 367 (51.6) | 54 (42.2) |
| <50 | 606 (50.7) | 162 (55.3) | 372 (47.9) | 346 (48.5) | 72 (56.3) |
| Triglycerides (mg/dl) | |||||
| <150 | 926 (76.8) | 220 (74.6) | 613 (76.9) | 567 (77.5) | 93 (73.8) |
| ≥150 | 280 (23.2) | 75 (25.4) | 172 (21.6) | 153 (20.9) | 33 (26.2) |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
aP < 0.05 between hCG-detected pregnancy and no observed pregnancy.
bP< 0.05 between ultrasound-confirmed pregnancy and no observed pregnancy.
cP < 0.05 between withdrawal and any observed pregnancy (hCG-detected or ultrasound-confirmed).
Table III.
Fecundability odds ratio of hCG-detected pregnancy by dichotomized maternal lipid levels.
| Months to hCG-detected pregnancy Median (IQR) | hCG-detected pregnancy FOR (95% CI) | Model 1 hCG-detected pregnancy FOR (95% CI)a | Model 2 hCG-detected pregnancy FOR (95% CI)b | Model 3 hCG-detected pregnancy FOR (95% CI)c | Model 4 hCG-detected pregnancy FOR (95% CI)d | |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | ||||||
| <200 | 4 (2–6) | Reference | Reference | Reference | Reference | Reference |
| ≥200 | 4 (3–7) | 0.74 (0.58, 0.94)e | 0.74 (0.58, 0.94)e | 0.76 (0.59, 0.97)e | 0.84 (0.66, 1.08) | 0.78 (0.61, 1.00) |
| LDL-C calculated (mg/dl) | ||||||
| <130 | 4 (2–6) | Reference | Reference | Reference | Reference | Reference |
| ≥130 | 4 (3–7) | 0.63 (0.46, 0.86)e | 0.64 (0.44, 0.92)e | 0.68 (0.47, 0.99)e | 0.72 (0.49, 1.05) | 0.70 (0.48, 1.02) |
| HDL-C (mg/dl) | ||||||
| <50 | 4 (2–6) | 0.82 (0.70, 0.96)e | 0.81 (0.69, 0.95)e | 0.81 (0.69, 0.95)e | 0.90 (0.76, 1.06) | 0.85 (0.70, 0.98)e |
| ≥50 | 4 (2–6) | Reference | Reference | Reference | Reference | Reference |
| Triglycerides (mg/dl) | ||||||
| <150 | 4 (2–6) | Reference | Reference | Reference | Reference | Reference |
| ≥150 | 4 (3–7) | 0.83 (0.68, 1.01) | 0.83 (0.69, 1.00) | 0.82 (0.67, 1.01) | 0.93 (0.75, 1.15) | 0.85 (0.69, 1.05) |
IQR, interquartile range; FOR, fecundability odds ratio.
aModel 1: adjusted for age, race, education, alcohol use, total cholesterol (excluding total cholesterol model).
bModel 2: model 1 plus fasting status and total serum-free fatty acid.
cModel 3: model 2 plus BMI.
dModel 4: model 2 plus waist–hip ratio.
eP-value < 0.05.
Table III depicts the unadjusted and adjusted FOR for hCG-detected pregnancy for all four unfavorable lipid profiles. Total cholesterol ≥200 mg/dl was associated with 24% reduced fecundability (FOR: 0.76, 95% CI: 0.59, 0.97) and unfavorable individual lipoproteins ranged from a 19% (HDL-C < 50) to 32% (LDL-C ≥ 130; calculated by Friedewald equation) decrease in the probability of conception per cycle for hCG-detected pregnancy, compared with women with total cholesterol <200 mg/dl. The direction and magnitude of point estimates were similar after additional adjustment for measures of adiposity (waist–hip ratio and BMI); however, estimates were only borderline significant and some were null (Table III). Results were similar for ultrasound-confirmed pregnancy (Supplementary Table SI).
When all lipid components were included in analyses as continuous predictors (Supplementary Table SII), the direction of estimates was similar to discrete outcomes, but only HDL was significantly associated with reduced fecundity. A 10-unit increase in HDL corresponded to an 11% (FOR: 1.110, 95% CI: 1.043, 1.181) increase in fecundability.
BMI modified the effect of total cholesterol on fecundability for hCG-detected pregnancy, and effect modification by BMI category was borderline significant for individual lipoproteins (Table IV). There was a decrease in fecundability among overweight (FOR: 0.61, 95% CI: 0.38, 0.98) and obese (FOR: 0.58, 95% CI: 0.36, 0.93) mothers with high cholesterol and no change among lean mothers (FOR: 1.4, 95% CI: 0.91, 2.1). Results were similar for ultrasound-confirmed pregnancy (Supplementary Table SIII). Fasting status did not modify any of the above relations.
Table IV.
Adjusted fecundability odds ratio of hCG-detected pregnancy by maternal lipid levels, stratified by BMI.
| Normal weight FOR (95% CI)a | Overweight FOR (95% CI)a | Obese FOR (95% CI)a | P-value for interaction | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | ||||
| <200 | Reference | Reference | Reference | 0.04 |
| ≥200 | 1.37 (0.91, 2.05) | 0.61 (0.38, 0.98) | 0.58 (0.36, 0.93) | |
| LDL-C calculated (mg/dl) | ||||
| <130 | Reference | Reference | Reference | 0.21 |
| ≥130 | 1.14 (0.55, 2.35) | 0.55 (0.27, 1.13) | 0.90 (0.46, 1.77) | |
| HDL-C (mg/dl) | ||||
| <50 | 1.06 (0.84, 1.34) | 0.73 (0.52, 1.03) | 0.77 (0.52, 1.14) | 0.43 |
| ≥50 | Reference | Reference | Reference | |
| Triglycerides (mg/dl) | ||||
| <150 | Reference | Reference | Reference | 0.92 |
| ≥150 | 1.07 (0.76, 1.50) | 0.76 (0.51, 1.14) | 0.99 (0.67, 1.48) | |
FOR < 1.0 indicates reduced fecundability.
aAdjusted for age, race, education, alcohol use, total cholesterol (excluding total cholesterol model), fasting status and total serum-free fatty acid.
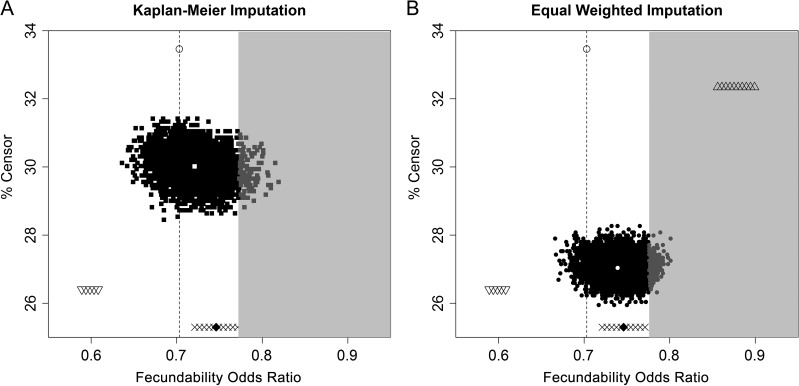
Since women who withdrew from the study differed demographically (Table I) and were more likely to have LDL-C ≥130 mg/dl than women achieving a pregnancy (Table II), we repeated the analysis limiting to women with complete data (complete-case analysis) and found that the estimates were similar to the imputed results. Additional sensitivity analyses to address the potential selection bias from missing TTP in women who withdrew were also robust to the main findings. After imputing missing TTP for the 128 women who withdrew using equal-weighted MI and Kaplan–Meier MI, the majority of estimates were significant and similar in magnitude to the complete-case FOR (Fig. 1). Only an extreme, but unlikely, situation such as all women with high cholesterol achieving pregnancy (triangles in the gray portion of Fig. 1) would have resulted in a null association, yet the estimates remained in the same direction as the observed estimates.
Figure 1.
Sensitivity analysis comparing the complete-case fecundability odds ratio (FOR) (open circle with dotted line) to the FOR after multiple imputation (MI) of time to pregnancy in 128 women who withdrew using 5000 imputations for each Kaplan–Meier MI (A) and equal-weight MI (B). The triangles on the top right (B only) indicate the FOR's after 100 imputations for a scenario in which all withdrawals with high cholesterol achieved pregnancy. The upside-down triangles (A and B) on the bottom left indicate the FOR's after 100 imputations for a scenario in which all withdrawals with low cholesterol achieved pregnancy. The Xs (A and B) across the bottom indicate the FOR's after 100 imputations for a scenario in which all withdrawals achieved pregnancy and the diamond (A and B) indicates the special case in which all withdrawals achieved pregnancy 1 month after withdrawal. These outside values indicate the area within which the FOR could fall under the assumption of missing not at random. The gray-shaded area indicates a non-significant result (P > 0.05).
Discussion
In women with a history of prior pregnancy loss, unfavorable preconception serum lipid concentrations were associated with decreased fecundability, particularly among overweight and obese women. Overall, women with unfavorable lipid levels based on common clinical cut-points (i.e. high triglycerides, total cholesterol and LDL-C and low HDL-C) experienced a 19–32% decrease in fecundability.
Our results are consistent with one other study relating preconception lipid concentration to fecundity. A US population-based prospective cohort of 501 couples from 2005 to 2009 reported that a continuous increase in total lipid and free cholesterol concentrations in women was associated with a reduced FOR, as assessed by time to hCG-detected pregnancy (Schisterman et al., 2014a,b). In contrast to previous findings, we did not observe a significant relation between a continuous change in lipid levels and reduced FOR, instead we identified a potential threshold effect given the significant finding using clinical cut-points. Our study expands these findings by adding critically important information about individual lipoproteins, as interpretation of total cholesterol alone may obscure an understanding of individual measures that affect risk. Using cardiovascular disease as an example, the combination of high HDL/low LDL and low HDL/high LDL could result in the same total cholesterol but disparate cardiovascular disease risk. Further pointing to the potentially negative consequences of disordered lipid metabolism in reproduction, dyslipidemia has long been associated with polycystic ovary syndrome, an endocrine disorder contributing to subfertility (Wild et al., 2011).
While lipids appear to play a role in fecundability, the inflammatory milieu of obesity may also contribute to reduced fecundity (Wise et al., 2010), further complicating our understanding of potential mechanisms. We observed decreased precision and attenuated estimates, still in the same direction, after adjustment for central adiposity (waist–hip ratio) and obesity (BMI), which suggest that other factors linked to obesity (e.g. insulin resistance and metabolic disruption), weaken the observed effect. The biologic mechanism of lipids and lipid metabolism impacting successful reproduction may be due in part to the role of lipids in ovarian steroidogenesis (Reavan et al., 1986; Azhar et al., 1998; Fujimoto et al., 2010), but competing hypotheses suggest dysregulation of insulin may also disrupt ovarian function (Robker et al., 2009). Furthermore, the observed decrease in the probability of conception per cycle was only observed at high lipid levels based on clinical cut-points, potentially indicating the role of other pathologic processes, which are activated at clinically abnormal levels of lipids. Yet, treating lipids in overweight and obese women may serve as an important target to improve fecundability.
Lipid concentrations are routinely monitored in clinical settings for cardiovascular health. Our findings suggest that they also serve as markers of fecundity. Current recommendations suggest that adults over the age of 20 undergo a lipid profile screening every 5 years, making this an easy method to identify potential for reduced fecundity (National Institutes of Health, 2001). Between 42 and 76% of subfecund couples seek medical care (Boivin et al., 2007), which presents an opportunity in which lipid profiles could serve as an early marker of reduced fecundity. The importance of early identification is coupled with the fact that lipid levels are modifiable by increased physical activity, diet modifications (U.S. Preventive Services Task Force, 2014) or statins (Delahoy et al., 2009). However, whether optimizing lipid levels improves fecundity requires further research.
Our findings should be considered within the context of limitations. The results may generalize only to populations experiencing prior pregnancy losses without a diagnosis of infertility. However, evidence from a small cohort of 70 women suggests that the difference in preconception lipid levels between women with a pregnancy loss and women delivering at term is small (mean total cholesterol: 3 mg/dl and mean triglycerides: 4 mg/dl) (Laughon et al., 2014). This indicates that lipid levels are not inherently different among women with pregnancy losses. Consequently, our findings may have population-level inferences. Only about a third of women in this study had fasting serum samples obtained; however, we observed no effect modification by fasting status or change in estimates after adjusting for fasting and a proxy measure of dietary fat intake. Additionally, any bias is likely non-differential since it is unlikely that fasting status is related to fecundability. While the FOR is a measure of couple fecundability, we had measures of female lipid levels only and can therefore not confirm the findings from a previous study indicating the independent role of male lipids in fecundability (Schisterman et al., 2014a,b). Lastly, we had only a single measure of preconception lipid levels and cannot make any inference about whether changes in lipid levels over the number of cycles trying affected fecundability.
The major strength of our study was measurement of preconception lipids, which few studies have assessed, and the inclusion of single lipid molecules which have direct clinical application. In addition, the longitudinal nature of these data allowed us to capture a critical, yet difficult-to-assess, window of time in which couples are attempting conception. The observed associations remained after adjustment for a number of important confounders including demographic characteristics, total cholesterol and a proxy measure of diet (total serum-free fatty acid). Lastly, our unique sensitivity analysis confirmed the robustness of our results by revealing that the potential for selection bias by loss to follow-up did not alter our conclusion in the majority of outcome scenarios.
Our findings contribute evidence on the role of lipids in fecundability, which is critically important to the nearly 50% of women that experience lipid disorders. Among women who aim to conceive, conducting a lipid profile test may serve as an early, simple, inexpensive and modifiable marker of reduced fecundity. Future, larger studies are needed to expand our understanding of whether interventions aimed at lowering lipid profiles can improve fecundity.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgements
We thank the EAGeR participants for their extraordinary commitment to the study, all of the EAGeR investigators and staff who devoted their time and energy to the success of this trial, and the members of the data safety monitoring board for continuous oversight, constant support and advice throughout the trial. We thank Aijun Ye for programming assistance.
Authors’ roles
S.J.P., E.F.S., R.W.B., A.M.L., S.L.M., N.J.P., R.S., L.S., J.S., B.W., J.W.W. and K.L.G. conceived and designed the research question. R.M.S., J.W.-W., A.M.L. and N.J.P. contributed to the study design and enrollment of patients. S.J.P., E.F.S., K.L.G., N.J.P., L.S. and S.L.M. analyzed and contributed to the interpretation of the data. S.J.P. drafted the report, and all authors commented on drafts. All authors made revisions to the article and approved the final version.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. [Health and Human Services Number] HHSN267200603423, HHSN267200603424, and HHSN267200603426). The funding source had no direct role in the design, conduct or reporting of the study.
Conflict of interest
The authors have declared that no conflicts of interest exist.
References
- Azhar S, Tsai L, Satyanarayana M, Chandrasekher Y, Guidice L, Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab 1998;83:983–991. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007;22:1506–1512. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Platt RW. Reproductive and Perinatal Epidemiology. New York: Oxford University Press, 2011, 30–62. [Google Scholar]
- Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum fatty acids as biomarkers of fat intake predict serum cholesterol concentrations in a population-based survey of New Zealand adolescents and adults. Am J Clin Nutr 2006;83:887–894. [DOI] [PubMed] [Google Scholar]
- Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther 2009;31:236–244. [DOI] [PubMed] [Google Scholar]
- Domar A, Gordon K, Garcia-Velasco J, La Marca A, Barriere P, Beligotti F. Understanding the perceptions of and emotional barriers to infertility treatment: a survey in four European countries. Hum Reprod 2012;27:1073–1079. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update 2010;16:20–38. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Rich-Edwards JW, Lawson CC, Schernhammer ES, Missmer SA, Chavarro JE. Work schedule and physical factors in relation to fecundity in nurses. Occup Environ Med 2015;72:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- Grummer RR, Carroll DJ. A review of lipoprotein cholesterol metabolism: importance to ovarian function. J Anim Sci 1988;66:3160–3173. [DOI] [PubMed] [Google Scholar]
- Guldbrandsen K, Hakonsen LB, Ernst A, Toft G, Lyngso J, Olsen J, Ramlau-Hansen CH. Age of menarche and time to pregnancy. Hum Reprod 2014;29:2058–2064. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155:176–184. [DOI] [PubMed] [Google Scholar]
- Laughon SK, McLain AC, Sundaram R, Catov JM, Buck Louis GM. Maternal lipid change in relation to length of gestation: a prospective cohort study with preconception enrollment of women. Gynecol Obstet Invest 2014;77:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Executive Summary NIH Publication No. 01-3670, 2001. [DOI] [PubMed]
- Reavan E, Chen Y, Spicher M, Hwang S, Mondon C, Azhar S. Uptake of low density lipoproteins by rat tissues. J Clin Invest 1986:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab 2009;94:1533–1540. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Mumford SL, Browne RW, Barr DB, Chen Z, Louis GM. Lipid concentrations and couple fecundity: the LIFE study. J Clin Endocrinol Metab 2014. a;99:2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, Lynch AM, Perkins NJ, Mumford SL, Galai N. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014. b;384:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, Lesher LL, Faraggi D, Wactawski-Wende J, Browne RW et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol 2013;27:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331, e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force Final Recommendation Statement: Lipid Disorders in Adults (Cholesterol, Dyslipidemia): Screening U.S. Preventive Services Task Force, 2014. https://www.uspreventiveservicestaskforce.org
- van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 2011;95:1073–1079, e1071-1011. [DOI] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod 2010;25:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Herring AH, Zhou H, Ali MW, Koch GG. A multiple imputation method for sensitivity analyses of time-to-event data with possibly informative censoring. J Biopharm Stat 2014;24:229–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.