Abstract
Background
Diet-quality indexes have been associated with a lower risk of chronic disease mortality in Western populations, but it is unclear whether these indexes reflect protective dietary patterns in Asian populations.
Objective
We examined the association between Alternative Healthy Eating Index–2010 (AHEI-2010), alternate Mediterranean diet (aMED), Dietary Approaches to Stop Hypertension (DASH), and Healthy Diet Indicator (HDI) scores and the risk of all-cause cardiovascular disease (CVD), cancer, and respiratory disease mortality.
Methods
We used data from a prospective cohort of 57,078 Singapore Chinese men and women (aged 45–74 y) who were free of cancer and CVD at baseline (1993–1998) and who were followed up through 2014. The diet-quality index scores were calculated on the basis of data from a validated 165-item food-frequency questionnaire. Cox regression models with adjustment for potential confounders including sociodemographic and lifestyle variables, body mass index, and medical history were used to estimate HRs and 95% CIs.
Results
During a total of 981,980 person-years of follow-up, 15,262 deaths (CVD: 4871; respiratory: 2690; and cancer: 5306) occurred. Comparing the highest with the lowest quintiles, the multivariable adjusted HRs (95% CIs) for all-cause mortality were 0.82 (0.78, 0.86) for AHEI-2010, 0.80 (0.76, 0.85) for aMED, 0.80 (0.75, 0.84) for DASH, and 0.88 (0.83, 0.92) for HDI scores (all P-trend < 0.001). Higher diet index scores were associated with a 14–28% lower risk of CVD and respiratory mortality, but only a 5–12% lower risk of cancer mortality. Higher consumption of vegetables, fruit, nuts, and long-chain n–3 (ω-3) fatty acids, lower consumption of red meat, and avoidance of high alcohol consumption were the diet index components associated with a lower risk of mortality.
Conclusion
Adherence to several recommended dietary patterns that emphasize healthy plant-based foods was associated with a substantially lower risk of chronic disease mortality in an Asian population. The Singapore Chinese Health Study was registered at www.clinicaltrials.gov as NCT03356340.
Keywords: diet-quality, mortality, risk factors, epidemiology, nutrition
Introduction
Noncommunicable diseases, such as cardiovascular diseases (CVDs), diabetes, cancer, and chronic lung diseases, account for nearly 70% of all deaths globally (1, 2). Diet appears to play a major role in the development of several of these diseases (3). The traditional approach in nutritional epidemiology has focused largely on the investigation of effects of specific nutrients or food groups on disease risk (4, 5). However, the study of dietary patterns has emerged as a complementary approach to capture the combined effect of a variety of foods and nutrients (4, 6, 7). Commonly used approaches to identify dietary patterns include data-driven and hypothesis-driven methods (8). Data-driven methods such as factor and cluster analysis are exploratory, reflecting dietary habits in specific study populations (4, 9). In contrast, hypothesis-driven methods define indexes of overall diet-quality a priori on the basis of scientific evidence on the relation between food and nutrient intakes and health outcomes (4, 6, 7, 10–13).
Several diet-quality indexes have been developed to evaluate the role of dietary patterns in chronic disease mortality. These include the Alternative Healthy Eating Index (AHEI) (13, 14), the alternate Mediterranean diet (aMED) (15), the Dietary Approaches to Stop Hypertension (DASH) (16), and the Healthy Diet Indicator (HDI), which reflects the WHO’s dietary guidelines (17). These indexes were mostly based on data from studies in Western populations and were associated with a lower risk of all-cause (18–22), CVD (14, 18, 19, 22, 23), and cancer (18–20, 22) mortality in US and European populations. However, little is known about the relation of these diet indexes with all-cause or cause-specific mortality in Asian populations. In addition, respiratory death is a major cause of death in East Asian countries (24) and has not been examined in relation to these diet indexes. In a study conducted in China, AHEI-2010 and DASH scores were associated with lower CVD and all-cause mortality. However, several components of these dietary scores could not be calculated due to the very low consumption of whole grains and sugar-sweetened beverages and lack of data on trans fat in local food-composition tables (25). Asian populations generally have markedly different dietary habits than Western populations, such as a higher consumption of soy foods and rice and a lower consumption of dairy products, red meat, and alcohol (25, 26). Data on optimal dietary patterns to reduce premature mortality in Asian populations are limited. We therefore examined the associations between the AHEI-2010, aMED, DASH, and HDI diet indexes and the risk of mortality from all-causes, CVD, respiratory disease, and cancer in an Asian population.
Methods
Study population
The Singapore Chinese Heath Study (SCHS) is a prospective cohort study that was initiated to investigate dietary, genetic, and environmental determinants of chronic diseases in an ethnic Chinese population. The study design and implementation have been described previously (27, 28). Briefly, 63,257 Chinese men (n = 27,959) and women (n = 35,298) aged 45–74 y, who belonged to 1 of the major Chinese dialect groups (Hokkein or Cantonese), and resided at public housing estates (where 86% of the Singaporeans reside) were enrolled between 1993 and 1998. Information on demographic characteristics, lifestyle factors, and medical history were obtained at recruitment with the use of a structured interviewer-administered questionnaire. The institutional review boards at the National University of Singapore and the University of Pittsburgh approved the study. This study was registered at www.clinicaltrials.gov as NCT03356340.
Dietary assessment
At baseline, the habitual dietary intake of the study participants over the past year was assessed by trained interviewers with the use of a validated, 165-item, semiquantitative FFQ designed specifically for the Singapore Chinese population. Information on the usual frequency of consumption of each food item was determined by using 8 categories that ranged from “never or hardly ever” to “two or more times day.” Information on serving size (generally 3 options: small, medium, or large) was obtained with the aid of photographs. Total energy and nutrient intakes were computed from the Singapore food-composition database, which was developed in conjunction with the study cohort. The FFQ had been validated against two 24-h dietary recalls among a random sample of 810 cohort participants. The correlation coefficients between the FFQ and 24-h dietary recalls ranged between 0.24 and 0.79 for energy intake and selected nutrients. For most nutrients, the differences in mean intakes measured by the FFQ and 24-h diet recalls were within 10% (27). Furthermore, several components of the dietary pattern scores were associated with plasma FA concentrations, as expected based on the FA composition of these foods in a subsample of SCHS. Higher fish intake (per 50-g/d increment) was associated with higher EPA (β = 0.09, P < 0.01) and DHA (β = 0.48, P < 0.01) concentrations expressed as a percentage of plasma total FAs. A higher intake of red meat (per 50 g/d) was associated with higher plasma arachidonic acid (AA; 20:4n-6) (β = 0.50, P < 0.01) concentrations and soy intake (per 50 g/d) was associated with higher plasma α-linolenic acid (ALA; 18:3n-3) (β = 0.01, P < 0.05) concentrations. Higher intake of PUFAs (per 1% of total energy intake) was associated with higher plasma linoleic acid (LA; 18:2n-6) (β = 0.25, P < 0.01) and ALA (β = 0.02, P < 0.01) concentrations (29).
Diet-quality indexes
We constructed the components of the AHEI-2010, aMED, DASH, and HDI indexes, and calculated index scores on the basis of food groups or nutrients from the SCHS food-composition database. Dietary components and standards for scoring are shown in Supplemental Table 1. For the calculation of diet-quality scores, we converted daily consumption of foods in grams to standard serving equivalents. For example, we used 67 g (0.5 cup of typical local vegetables) to represent 1 serving of vegetables (14, 30), 16 g to represent 1 serving of whole grains (e.g., 1 slice of whole-wheat bread, 0.5 cup of oatmeal) (31), 28 g to represent 1 serving of nuts or 1 tablespoon (16 g) to represent 1 serving of peanut butter (14), 90 g to represent 1 serving of fish (30), and 10 g to represent 1 serving of alcohol (30). In our analyses, we excluded potatoes and preserved vegetables from total vegetables, preserved or dried fruit from total fruit, and sweetened soy products and sweetened bean soup from legumes to represent healthful dietary pattern components.
AHEI-2010 score
The AHEI, originally proposed by McCullough et al. (13), was developed based on foods and nutrients predictive of chronic disease risk. We used the updated version of the AHEI, the AHEI-2010 (14), which includes 11 foods and nutrients and scores each diet component on the basis of predetermined scoring criteria. Each component ranged from 0 (worst) to 10 (best) points and the total score ranged from 0 (poor adherence) to 110 (excellent adherence). Due to lack of information on trans fat intake in our study population, the trans fat component was not included in the calculation of the AHEI-2010 score. Hence, the total score ranged from 0 to 100 in our study.
aMED score
The original Mediterranean diet score was developed to investigate the association between dietary habits of Mediterranean communities and chronic disease risk (32, 33). We used the aMED (15), a modified version of the Mediterranean diet index that includes 9 components. Participants with intakes at or greater than the study population-specific median for each protective component received 1 point; otherwise, they received 0 points. Reverse scoring was applied to detrimental components. In addition, 1 point was assigned for alcohol intake of 10–25 g/d for men and 5–15 g/d for women. The total score ranged from 0 to 9, with a higher score representing greater adherence to the aMED.
DASH score
The DASH index, as outlined by Fung et al. (16), was originally designed for hypertension management. This index includes 8 components, each worth 5 points, for a total of 40 points. Scoring is based on quintiles and participants in the highest quintile of each protective component received 5 points, whereas detrimental components were reverse scored.
HDI score
The HDI was developed based on the 1990 WHO dietary guidelines (34) and designed to reflect an optimal diet for the prevention of chronic diseases (35). We used the updated version of the HDI that aligns with the 2003 WHO dietary guidelines (17). The HDI includes 7 components; each component score ranged from 0 to 10 and the total score ranged from 0 (minimum) to 70 (maximum).
Assessment of covariates
In-person interviews were conducted at baseline to obtain information on other covariates, including demographic characteristics, height, weight, physical activity, smoking, alcohol consumption, sleep duration, reproductive history (women only), and medical history (physician-diagnosed hypertension, heart attack or angina, diabetes, and cancer) by using a structured questionnaire. We collected information on smoking status and intensity of smoking (if a participant ever smoked cigarettes). A 4-level variable was created for smoking, including never smoking, past smoking, current smoker of <13 cigarettes/d, or current smoker of ≥13 cigarettes (more than half a pack)/d. We calculated the BMI as weight (kilograms) divided by height (meters) squared. Physical activity was assessed by asking participants about the number of hours per week they spent on moderate and strenuous activities over the past year. The physical activity section of the questionnaire was modeled after the European Prospective Investigation into Cancer and Nutrition (EPIC) study physical activity questionnaire, which has been shown to be reasonably accurate and reproducible (36).
Mortality ascertainment
All-cause, CVD, respiratory, and cancer deaths from the date of the baseline interview through 31 December 2014 were identified through linkage with the nationwide registry of births and deaths in Singapore. The International Classification of Diseases (ICD) 9th (ICD9) (37) or 10th (ICD10) (38, 39) revision codes were used to classify causes deaths from CVD [ICD9 (390–459) or ICD10 (I00–I99)], respiratory diseases including pneumonia and influenza [ICD9 (480–488) or ICD10 (J09-J18)], and chronic obstructive pulmonary disease [ICD9 (490–496) or ICD10 (J40–47)] and cancer [ICD9 (140–208) or ICD10 (C00-C97)].
Statistical analysis
We excluded participants who had cancer at baseline identified either by self-report or through linkage with the nationwide Singapore Cancer Registry (n = 1936) and participants with a self-reported history of heart attack or angina or stroke (n = 3220) at baseline. We also excluded 1023 participants who reported extreme energy intakes (<600 or >3000 kcal/d for women and <700 or >3700 kcal/d for men). Hence, the analysis included 57,078 participants.
Baseline characteristics were calculated with the use of descriptive statistics across quintiles of each diet-quality index score. We examined what individual foods were associated with high diet index scores by modeling each diet-quality score as a dependent variable and entering all food items assessed on the FFQ as independent variables in stepwise regression analysis. We also calculated Pearson correlations between different diet index scores. Cox proportional hazards regression was used to assess the association between diet-quality scores and all-cause, CVD, respiratory, and cancer mortality. Person-years were calculated for each study participant from the date of the baseline interview to the date of death, date of loss to follow-up, or 31 December 2014, whichever occurred first. The HRs and 95% CIs were estimated per quintile of each of the diet-quality indexes by using the lowest quintile as the reference category.
In the multivariable models, we adjusted for the following potential confounders: age at interview (years), sex (men or women), dialect group (Hokkien or Cantonese), education (no formal education, primary school, or secondary or higher), smoking (never, former, or current smoker of <13 or ≥13 cigarettes/d), physical activity (<0.5 h of moderate and strenuous activity/wk, 0.5 to <4 h of moderate activity/wk or 0.5 to <2 h of strenuous activity/wk, or ≥4 h of moderate activity/wk or ≥2 h of strenuous activity/wk), sleep duration [short (≤6 h), normal (7–8 h), or long (≥9 h)], BMI (kg/m2), history of diabetes (yes or no), history of hypertension (yes or no), and total energy intake (kilocalories per day). The DASH and HDI do not have a separate component for alcohol; therefore, models that involved the DASH or HDI score further adjusted for alcohol intake (never/hardly ever, mild, moderate, or above moderate). Tests for trends were performed by assigning the median value of each diet index in each quintile and modeling this as a continuous variable.
We also performed several additional data analyses to evaluate the robustness of our results and to gain more insight into the observed associations. First, the potential effect modification of associations between diet-quality scores and mortality was examined for sex, BMI (<23 or ≥23), current smoking (yes or no), history of diabetes (yes or no), and history of hypertension (yes or no) by including multiplicative interaction terms in multivariable models. Second, we conducted an analysis of diet-quality indexes and mortality in never smokers only to examine the possibility of residual confounding by smoking habits. Third, we examined associations between the individual components of the diet-quality indexes and mortality to better understand possible reasons for differences in results between dietary indexes. In multivariable analyses for individual foods, we further adjusted for all other considered foods. Similarly, in multivariable analyses of individual nutrients, we further adjusted for other nutrients. Finally, we explored whether modifications to dietary indexes for East Asian populations should be considered with the use of a comprehensive index (AHEI-2010) as an example. In East Asians, soy intake is generally much higher than in Western populations and this results in a limited contribution of nuts to the “nuts and legumes” component. In addition, lower alcohol intake may be prudent in East Asians compared with other ethnic groups as a result of genetic differences in alcohol metabolism (40). In the modified AHEI-2010, we therefore used nuts only instead of the combination of legumes and nuts and defined the optimal intake of alcohol as <1 serving/d for both men and women (i.e., 10 points) and fewer points for higher intakes. All of the analyses were performed with the use of Stata version 11.0 (StataCorp). All P values were 2-sided, and P < 0.05 was considered significant.
Results
During an average follow-up of 17 y, a total of 15,262 deaths were documented, including 4871 CVD deaths, 2690 respiratory disease deaths, and 5306 cancer deaths. Pneumonia and chronic obstructive pulmonary disease were the predominant respiratory conditions contributing to respiratory disease mortality in the study cohort. A large proportion (77%) of respiratory deaths were due to pneumonia. The most common causes of cancer death among men were lung (33.8%), colorectal (13.0%), and liver (12.5%) cancer and in women were lung (22.0%), colorectal (15.7%), and breast (10.5%) cancer.
Baseline characteristics according to quintiles of the AHEI-2010, aMED, DASH, and HDI scores are presented in Table 1. For all diet indexes, participants with the highest diet-quality scores were more likely to be women (except for HDI), highly educated, nonsmokers, and physically active. Distributions of individual dietary components according to the lowest and highest quintiles of the diet-quality indexes are presented in Supplemental Table 2. As expected, participants with higher diet-quality scores had a more favorable nutrient profile than those with lower scores. Higher scores for AHEI-2010 and aMED indexes, which included moderate alcohol consumption in the scoring, were associated with higher alcohol intake, whereas higher scores for DASH and HDI indexes were associated with lower alcohol intake. Except for the HDI, all other indexes showed a moderate to high correlation with one other. The correlation coefficient for the HDI was 0.29 with AHEI-2010, 0.31 with aMED, or 0.33 with DASH. The correlation coefficients for other indexes were 0.65 between AHEI-2010 and aMED, 0.58 between aMED and DASH, and 0.72 between AHEI-2010 and DASH.
TABLE 1.
Baseline characteristics according to quintiles of the AHEI-2010, aMED, DASH, and HDI scores in the Singapore Chinese Health Study participants1
| AHEI-2010 | aMED | DASH | HDI | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 |
| n | 11,415 | 11,416 | 11,607 | 11,392 | 8102 | 12,280 | 11,415 | 11,416 |
| Median score (min–max) | 41.0 (17.0–44.0) | 59.3 (56.0–81.0) | 2 (0–2) | 6 (6–9) | 18.0 (8–19) | 30.0 (28–39) | 40.8 (15.7–44.0) | 59.4 (56.4–70.0) |
| Age at interview, y | 56.1 ± 8.1 | 55.6 ± 7.7 | 57.3 ± 8.1 | 54.9 ± 7.5 | 54.9 ± 7.7 | 56.4 ± 7.8 | 56.2 ± 8.0 | 55.6 ± 7.7 |
| BMI, kg/m2 | 23.0 ± 3.3 | 23.1 ± 3.2 | 23.0 ± 3.3 | 23.1 ± 3.2 | 23.1 ± 3.3 | 22.9 ± 3.2 | 23.1 ± 3.3 | 23.1 ± 3.1 |
| Energy intake, kcal/d | 1565 ± 565 | 1661 ± 505 | 1303 ± 425 | 1823 ± 532 | 1595 ± 522 | 1567 ± 472 | 1466 ± 587 | 1769 ± 494 |
| Cantonese dialect, % | 42.8 | 51.6 | 38.9 | 54.3 | 40.4 | 52.1 | 39.2 | 53.9 |
| Women, % | 46.2 | 58.8 | 53.6 | 57.4 | 39.4 | 67.7 | 56.2 | 49.7 |
| Higher education,2 % | 25.4 | 37.7 | 18.8 | 40.7 | 25.1 | 34.8 | 24.7 | 35.6 |
| Current smoker, % | 28.7 | 12.7 | 27.2 | 12.6 | 34.3 | 9.1 | 27.2 | 14.5 |
| Moderate or strenuous activity,3% | 20.1 | 36.5 | 18.0 | 38.0 | 17.6 | 37.9 | 21.5 | 33.5 |
| Alcohol consumers, % | 16.5 | 27.2 | 17.5 | 22.6 | 29.3 | 13.2 | 23.4 | 18.7 |
| Sleep duration, % | ||||||||
| Normal (7–8 h/d) | 58.0 | 61.6 | 58.9 | 62.2 | 59.8 | 60.2 | 57.5 | 62.1 |
| Short (≤6 h/d) | 34.0 | 32.5 | 33.9 | 31.9 | 32.3 | 34.1 | 34.9 | 32.0 |
| Long (≥9 h/d) | 8.0 | 5.9 | 7.2 | 5.9 | 7.9 | 5.7 | 7.5 | 5.8 |
| History of hypertension, % | 20.1 | 22.8 | 21.0 | 21.9 | 19.1 | 23.1 | 19.9 | 23.6 |
| History of diabetes mellitus, % | 6.5 | 8.6 | 7.3 | 8.2 | 6.0 | 9.4 | 8.2 | 7.1 |
1Values are means ± SDs for continuous variables and percentages for categorical variables unless specified. AHEI-2010, Alternative Healthy Eating Index–2010; aMED, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; HDI, Healthy Diet Indicator; max, maximum; min, minimum; Q, quintile.
2Secondary school and above.
3At least 0.5 h of moderate or strenuous physical activity/wk.
To provide insight into the specific foods that contributed to each diet-quality index, we identified individual foods that best explained variations in the diet index scores (Supplemental Table 3). Soy foods, boiled or steamed fish, peanut butter, nuts, various fruit and vegetables, whole-wheat bread, and hot breakfast cereals were associated with a higher score for the AHEI-2010, aMED, and DASH. Soft drinks, fruit juice, flavored rice, fried rice, rice rolls, noodles, pork belly, and luncheon meat were associated with a lower score for the AHEI-2010 and DASH. Similar to the other indexes, the HDI was associated with higher intakes of fruit, vegetables, and whole grains and lower intakes of soft drinks and pork. However, in contrast to other indexes the HDI was also associated with higher intakes of refined-grain products and margarine and lower intakes of additional animal foods (eggs, milk, fish, and poultry).
The HRs for all-cause mortality according to AHEI-2010, aMED, DASH, and HDI scores are shown in Table 2. Higher scores for all diet-quality indexes were significantly associated with a lower risk of all-cause mortality. Participants in the highest quintile compared with the lowest quintile had a 26–33% lower risk of all-cause mortality after adjustment for age, sex, and total energy intake. The associations were attenuated after additional adjustment for lifestyle factors and medical history, but remained significant. The HRs (95% CIs) for all-cause mortality were 0.82 (0.78, 0.86) for AHEI-2010, 0.80 (0.76, 0.85) for aMED, 0.80 (0.75, 0.84) for DASH, and 0.88 (0.83, 0.92) for HDI scores, comparing the highest with the lowest quintiles (all P-trend < 0.001).
TABLE 2.
Risk of all-cause mortality by quintiles of AHEI-2010, aMED, DASH, and HDI scores in the Singapore Chinese Health Study participants1
| Quintile (range of scores) | Deaths, n | Person-years of follow-up | Basic model,2 HR (95% CI) | Full model,3 HR (95% CI) |
|---|---|---|---|---|
| AHEI-2010 | ||||
| Q1 (17.0–44.0) | 3,521 | 189,288 | 1.00 | 1.00 |
| Q2 (44.1–48.0) | 3,265 | 193,982 | 0.91 (0.88, 0.95) | 0.93 (0.88, 0.98) |
| Q3 (48.1–51.6) | 3,055 | 196,858 | 0.85 (0.81, 0.89) | 0.89 (0.85, 0.93) |
| Q4 (51.7–55.9) | 2,864 | 199,271 | 0.79 (0.76, 0.83) | 0.86 (0.82, 0.90) |
| Q5 (56.0–81.0) | 2,557 | 202,578 | 0.72 (0.69, 0.76) | 0.82 (0.78, 0.86) |
| P-trend | <0.001 | <0.001 | ||
| aMED | ||||
| Q1 (0–2) | 3,866 | 191,950 | 1.00 | 1.00 |
| Q2 (3) | 3,352 | 189,407 | 0.92 (0.88, 0.97) | 0.96 (0.92, 1.01) |
| Q3 (4) | 3,252 | 207,848 | 0.87 (0.83, 0.91) | 0.93 (0.89, 0.98) |
| Q4 (5) | 2,496 | 189,321 | 0.79 (0.75, 0.83) | 0.88 (0.83, 0.92) |
| Q5 (6–9) | 2,296 | 203,452 | 0.69 (0.65, 0.73) | 0.80 (0.76, 0.85) |
| P-trend | <0.001 | <0.001 | ||
| DASH | ||||
| Q1 (8–19) | 2,418 | 136,022 | 1.00 | 1.00 |
| Q2 (20–22) | 3,710 | 217,734 | 0.87 (0.83, 0.92) | 0.91 (0.86, 0.96) |
| Q3 (23–24) | 2,849 | 179,136 | 0.80 (0.76, 0.85) | 0.87 (0.82, 0.92) |
| Q4 (25–27) | 3,460 | 233,318 | 0.75 (0.72, 0.79) | 0.85 (0.80, 0.89) |
| Q5 (28–39) | 2,825 | 215,768 | 0.67 (0.64, 0.71) | 0.80 (0.75, 0.84) |
| P-trend | <0.001 | <0.001 | ||
| HDI | ||||
| Q1 (15.7–44.0) | 3,401 | 190,181 | 1.00 | 1.00 |
| Q2 (44.0–48.3) | 3,167 | 195,595 | 0.90 (0.86, 0.94) | 0.96 (0.91, 1.01) |
| Q3 (48.4–52.1) | 2,998 | 196,971 | 0.82 (0.78, 0.86) | 0.90 (0.86, 0.94) |
| Q4 (52.2–56.3) | 2,999 | 197,935 | 0.81 (0.77, 0.85) | 0.91 (0.86, 0.95) |
| Q5 (56.4–70.0) | 2,697 | 201,294 | 0.74 (0.70, 0.77) | 0.88 (0.83, 0.92) |
| P-trend | <0.001 | <0.001 | ||
1AHEI-2010, Alternative Healthy Eating Index–2010; aMED, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; HDI, Healthy Diet Indicator; Q, quintile.
2Adjusted for age at interview (years), sex (men or women), and total energy intake (kilocalories per day).
3In addition to the basic model, we adjusted for dialect (Hokkien or Cantonese), level of education (none, primary, or secondary or above), smoking status (never; ex-smoker; current-smoker of <13 or ≥13 cigarettes/d), physical activity (<0.5 h of moderate and strenuous activity/wk, 0.5 to <4 h of moderate activity/wk or 0.5 to <2 h of strenuous activity/wk, or ≥4 h of moderate activity/wk or ≥2 h of strenuous activity/wk), sleep duration [short (≤6 h/d), normal (7–8 h/d), or long (≥9 h/d)], BMI (kg/m2), history of diabetes mellitus (yes or no), and history of hypertension (yes or no). The full model for the DASH and HDI score was further adjusted for alcohol consumption [never/hardly ever, mild (<0.5 servings/d), moderate (between 0.5 and 2.0 servings/d for men and between 0.5 and 1.5 servings/d for women), above moderate intake (≥2.0 servings/d for men and ≥1.5 servings/d for women)].
Associations between diet-quality indexes and risks of CVD, respiratory disease, and cancer mortality are shown in Table 3. Across all indexes, participants in the highest quintile compared with the lowest quintile had a 19–28% lower risk of CVD mortality, a 14–28% lower risk of respiratory mortality, and a 5–12% lower risk of cancer mortality after adjustment for potential confounders. Associations of diet-quality indexes with cancer mortality were weaker than for CVD or respiratory disease mortality. In general, the HDI had a weaker association with risk of mortality from all causes, CVD, and respiratory diseases than did the other indexes.
TABLE 3.
Risk of CVD, respiratory disease, and cancer mortality by quintiles of diet-quality scores in the Singapore Chinese Health Study participants1
| CVD mortality | Respiratory disease mortality | Cancer mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quintile (range of scores) | Deaths, n | Person-years | Basic model,2 HR (95% CI) | Full model,3 HR (95% CI) | Deaths, n | Person-years | Basic model,2 HR (95% CI) | Full model,3 HR (95% CI) | Deaths, n | Person-years | Basic model,2 HR (95% CI) | Full model,3 HR (95% CI) |
| AHEI-2010 | ||||||||||||
| Q1 (17.0–44.0) | 1123 | 189,288 | 1.0 | 1.0 | 658 | 189,288 | 1.0 | 1.0 | 1179 | 189,288 | 1.0 | 1.0 |
| Q2 (44.1–48.0) | 1086 | 193,982 | 0.94 (0.87, 1.03) | 0.96 (0.88, 1.04) | 575 | 193,982 | 0.86 (0.77, 0.96) | 0.89 (0.80, 1.00) | 1080 | 193,982 | 0.91 (0.84, 0.99) | 0.95 (0.87, 1.03) |
| Q3 (48.1–51.6) | 988 | 196,858 | 0.86 (0.79, 0.94) | 0.89 (0.81, 0.97) | 561 | 196,858 | 0.83 (0.74, 0.93) | 0.90 (0.80, 1.01) | 1033 | 196,858 | 0.87 (0.80, 0.94) | 0.94 (0.87, 1.02) |
| Q4 (51.7–55.9) | 890 | 199,271 | 0.78 (0.71, 0.85) | 0.82 (0.75, 0.90) | 487 | 199,271 | 0.72 (0.64, 0.81) | 0.80 (0.71, 0.90) | 1065 | 199,271 | 0.89 (0.82, 0.97) | 1.00 (0.92, 1.09) |
| Q5 (56.0–81.0) | 784 | 202,578 | 0.71 (0.65, 0.78) | 0.77 (0.70, 0.85) | 409 | 202,578 | 0.61 (0.54, 0.69) | 0.72 (0.64, 0.82) | 949 | 202,578 | 0.80 (0.73, 0.87) | 0.95 (0.87, 1.04) |
| P-trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.55 | ||||||
| aMED | ||||||||||||
| Q1 (0–2) | 1265 | 191,950 | 1.0 | 1.0 | 727 | 191,950 | 1.0 | 1.0 | 1292 | 191,950 | 1.0 | 1.0 |
| Q2 (3) | 1086 | 189,407 | 0.93 (0.85, 1.01) | 0.95 (0.88, 1.04) | 627 | 189,407 | 0.92 (0.83, 1.03) | 0.97 (0.87, 1.08) | 1085 | 189,407 | 0.88 (0.81, 0.96) | 0.93 (0.86, 1.01) |
| Q3 (4) | 1026 | 207,848 | 0.86 (0.79, 0.94) | 0.90 (0.83, 0.98) | 563 | 207,848 | 0.81 (0.73, 0.91) | 0.90 (0.80, 1.01) | 1144 | 207,848 | 0.88 (0.82, 0.96) | 0.98 (0.90, 1.06) |
| Q4 (5) | 788 | 189,321 | 0.79 (0.72, 0.87) | 0.86 (0.78, 0.94) | 404 | 189,321 | 0.70 (0.62, 0.79) | 0.81 (0.71, 0.92) | 932 | 189,321 | 0.83 (0.76, 0.90) | 0.97 (0.88, 1.06) |
| Q5 (6–9) | 706 | 203,452 | 0.69 (0.63, 0.76) | 0.77 (0.70, 0.85) | 369 | 203,452 | 0.60 (0.52, 0.69) | 0.72 (0.63, 0.83) | 853 | 203,452 | 0.72 (0.65, 0.79) | 0.88 (0.80, 0.97) |
| P-trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.07 | ||||||
| DASH | ||||||||||||
| Q1 (8–19) | 763 | 136,022 | 1.0 | 1.0 | 409 | 136,022 | 1.0 | 1.0 | 871 | 136,022 | 1.0 | 1.0 |
| Q2 (20–22) | 1221 | 217,734 | 0.90 (0.82, 0.98) | 0.90 (0.83, 0.99) | 680 | 217,734 | 0.91 (0.80, 1.03) | 0.95 (0.84, 1.08) | 1250 | 217,734 | 0.85 (0.78, 0.93) | 0.92 (0.84, 1.00) |
| Q3 (23–24) | 913 | 179,136 | 0.80 (0.73, 0.88) | 0.83 (0.76, 0.92) | 496 | 179,136 | 0.78 (0.68, 0.88) | 0.85 (0.75, 0.98) | 998 | 179,136 | 0.82 (0.75, 0.90) | 0.94 (0.85, 1.03) |
| Q4 (25–27) | 1101 | 233,318 | 0.75 (0.68, 0.82) | 0.80 (0.72, 0.88) | 618 | 233,318 | 0.74 (0.65, 0.84) | 0.85 (0.75, 0.97) | 1208 | 233,318 | 0.77 (0.71, 0.84) | 0.93 (0.85, 1.02) |
| Q5 (28–39) | 873 | 215,768 | 0.65 (0.59, 0.72) | 0.72 (0.65, 0.80) | 487 | 215,768 | 0.64 (0.56, 0.73) | 0.78 (0.68, 0.89) | 979 | 215,768 | 0.69 (0.63, 0.75) | 0.89 (0.82, 0.98) |
| P-trend | <0.001 | <0.000 | <0.001 | <0.001 | <0.001 | 0.07 | ||||||
| HDI | ||||||||||||
| Q1 (15.7–44.0) | 1108 | 190,181 | 1.0 | 1.0 | 579 | 190,181 | 1.0 | 1.0 | 1216 | 190,181 | 1.0 | 1.0 |
| Q2 (44.0–48.3) | 984 | 195,595 | 0.86 (0.79, 0.94) | 0.89 (0.82, 0.97) | 581 | 195,595 | 0.96 (0.86, 1.08) | 1.05 (0.93, 1.18) | 1088 | 195,595 | 0.87 (0.80, 0.94) | 0.96 (0.88, 1.04) |
| Q3 (48.4–52.1) | 982 | 196,971 | 0.83 (0.76, 0.91) | 0.89 (0.81, 0.97) | 544 | 196,971 | 0.84 (0.75, 0.95) | 0.93 (0.83, 1.05) | 994 | 196,971 | 0.76 (0.70, 0.83) | 0.87 (0.80, 0.95) |
| Q4 (52.2–56.3) | 963 | 197,935 | 0.81 (0.74, 0.88) | 0.88 (0.80, 0.96) | 520 | 197,935 | 0.79 (0.70, 0.89) | 0.89 (0.79, 1.01) | 1033 | 197,935 | 0.78 (0.71, 0.84) | 0.92 (0.84, 1.00) |
| Q5 (56.4–70.0) | 834 | 201,294 | 0.72 (0.66, 0.79) | 0.81 (0.74, 0.89) | 466 | 201,294 | 0.72 (0.63, 0.81) | 0.86 (0.76, 0.98) | 975 | 201,294 | 0.72 (0.66, 0.79) | 0.92 (0.84, 1.01) |
| P-trend | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | 0.40 | ||||||
1AHEI-2010, Alternative Healthy Eating Index–2010; aMED, alternate Mediterranean diet; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HDI, Healthy Diet Indicator; Q, quintile.
2Adjusted for age at interview (years), sex (men or women), and total energy intake (kilocalories per day).
3In addition to the basic model, we adjusted for dialect (Hokkien or Cantonese), level of education (none, primary, or secondary or above), smoking status (never; ex-smoker; current-smoker of <13 or ≥13 cigarettes/d), physical activity (<0.5 h of moderate and strenuous activity/wk, 0.5 to <4 h of moderate activity/wk or 0.5 to <2 h of strenuous activity/wk, or ≥4 h of moderate activity/wk or ≥2 h of strenuous activity/wk), sleep duration [short (≤6 h/d), normal (7–8 h/d), or long (≥9 h/d)], BMI (kg/m2), history of diabetes mellitus (yes or no), and history of hypertension (yes or no). The full model for the DASH and HDI score was further adjusted for alcohol consumption [never/hardly ever, mild (<0.5 servings/d), moderate (between 0.5 and 2.0 servings/d for men and between 0.5 and 1.5 servings/d for women), above moderate intake (≥2.0 servings/d for men and ≥1.5 servings/d for women)].
In stratified analyses by sex, all diet-quality indexes were inversely associated with all-cause mortality in both men and women by a similar magnitude (Supplemental Table 4). The only significant interaction with sex was observed for the association between HDI and cancer mortality (Supplemental Table 5). We did not observe significant interactions with diet indexes for smoking status, BMI, history of diabetes, and history of hypertension. To examine possible residual confounding by smoking habits, we evaluated the associations between overall diet-quality and mortality outcomes among never smokers. Restriction to never smokers did not substantially change the results, with the exception of attenuated associations between the diet-quality indexes and cancer mortality (Supplemental Tables 6 and 7).
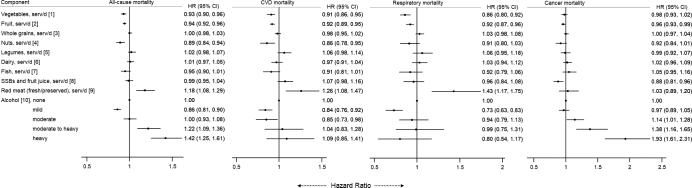
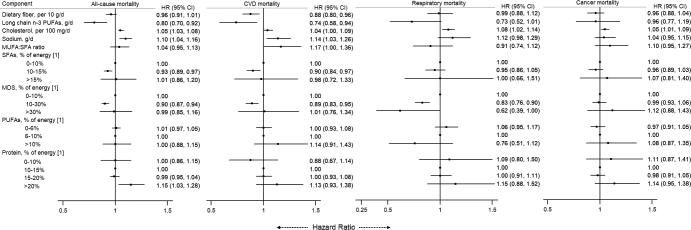
We also examined food groups (Figure 1) and nutrients (Figure 2) that are components of the 4 diet-quality indexes in relation to mortality outcomes. After adjusting for potential confounders, higher intakes of vegetables, fruit, and nuts and lower intakes of red meats were associated with a lower risk of mortality from all causes, CVD, and respiratory diseases. For nutrients, higher intakes of dietary fiber and long-chain n–3 PUFAs and a lower intake of sodium were significantly associated with a lower risk of CVD and all-cause mortality. A mild or moderate intake of alcohol was associated with lower CVD mortality, whereas moderate and high alcohol intakes were associated with higher cancer mortality.
FIGURE 1.
Associations of all-cause and cause-specific mortality with food groups of diet-quality indexes in the Singapore Chinese Health Study. HRs were adjusted for age at interview, sex, total energy intake, dialect (Hokkien or Cantonese), level of education (none, primary, or secondary or above), smoking status (never; ex-smoker; or current smoker or <13 or ≥13 cigarettes/d), physical activity (<0.5 h of moderate and strenuous activity/wk, 0.5 to <4 h of moderate activity/wk or 0.5 to <2 h of strenuous activity/wk, or ≥4 h of moderate activity/wk or ≥2 h of strenuous activity/wk), sleep duration, BMI (kg/m2), history of diabetes mellitus, history of hypertension, intakes in servings per day of vegetables, fruit, whole grains, nuts, legumes, dairy products, fish, SSBs and fruit juice, red meat, and alcohol (none, mild, moderate, moderate to heavy, and heavy intake). [1] One serving of vegetables = 67 g (0.5 cup of typical local vegetables); [2] 1 serving = 1 medium piece of fruit; [3] 1 serving = 16 g of whole grains; [4] 1 serving = 28 g of nuts or 1 tablespoon (16 g) of peanut butter; [5] 1 serving is 1 medium-size tofu item; [6] 1 serving is 250 mL or 1 cup of milk; [7] 1 serving is 90 g of fish; [8] 1 serving is 1 glass, packet, or typical local portion of SSB/fruit juice; [9] 1 serving is 113.4 g of unprocessed meat or 42.5 g of processed meat; [10] 1 serving of alcohol = 10 g (mild alcohol intake: <0.5 servings/d; moderate intake: 0.5–2.0 servings/d for men and 0.5–1.5 servings/d for women; moderate to heavy intake: 2.0– 3.5 servings/d for men and 1.5–2.5 servings/d for women; and heavy intake: ≥3.5 servings/d for men and ≥2.5 servings/d for women. CVD, cardiovascular disease; serv, servings; SSB, sugar-sweetened beverage.
FIGURE 2.
Associations of all-cause and cause-specific mortality with nutrient components of diet-quality indexes in the Singapore Chinese Health Study. HRs were adjusted for age at interview (years), sex (men or women), and total energy intake (kilocalories per day), dialect (Hokkien or Cantonese), level of education (none, primary, or secondary or above), smoking status (never; ex-smoker; or current smoker of <13 or ≥13 cigarettes/d), physical activity (<0.5 h of moderate and strenuous activity/wk, 0.5 to <4 h of moderate activity/wk or 0.5 to <2 h of strenuous activity/wk, or ≥4 h of moderate activity/wk or ≥2 h of strenuous activity/wk), sleep duration [short (≤6 h/d), normal (7–8 h/d), or long (≥9 h/d)], BMI (kg/m2), history of diabetes mellitus (yes or no), history of hypertension (yes or no), intake of dietary fiber (per 10 g/d), long-chain n–3 PUFAs (grams per day), cholesterol (per 100 mg/d), sodium (grams per day), SFAs (0–10%, 10–15%, or >15% of energy), MDSs (0–10%, 10–30%, or >30% of energy), PUFAs (0–6%, 6–10%, or >10% of energy), protein (0–10%, 10–15%, 15–20%, or >20% of energy), and alcohol (none, mild, moderate, moderate to heavy, or heavy intake). We excluded SFAs in the multivariable analyses of MUFA-to-SFA ratio. [1] % of energy excluding energy from alcohol. CVD, cardiovascular disease; MDS, mono- and disaccharide.
The results for the AHEI-2010 modified for East Asian populations in relation to all-cause and cause-specific mortality are shown in Supplemental Table 8. The use of nut consumption instead of nuts and legumes combined slightly strengthened the inverse association between the AHEI-2010 and CVD mortality. In addition, the use of a lower cutoff for optimal alcohol consumption strengthened the inverse association between the AHEI-2010 and cancer mortality. When both the nut and the alcohol components were modified, the results changed minimally for all-cause and CVD mortality, but the inverse associations with cancer mortality became stronger. Participants in the highest quintile compared with the lowest quintile of this modified AHEI-2010 had a 22% lower risk of all-cause mortality (P-trend < 0.001), a 26% lower risk of CVD mortality (P-trend < 0.001), a 28% lower risk of respiratory disease mortality (P-trend < 0.001), and a 10% lower risk of cancer mortality (P-trend = 0.02).
Discussion
In this large prospective cohort study in Singapore Chinese, we observed a 12–20% lower risk of all-cause mortality for higher scores on the AHEI-2010, aMED, DASH, and HDI. Participants with higher scores on any of the indexes had a 19–28% lower risk of CVD mortality and a 14–28% lower risk of respiratory disease, but only a 5–12% lower risk of cancer mortality. Analyses of individual components of the dietary quality scores suggested that higher intakes of vegetables, fruit, nuts, and long-chain n–3 FAs and lower intakes of red meat and sodium contributed to the inverse associations between dietary quality scores and mortality. Moderate alcohol consumption (0.5–2.0 servings/d for men and 0.5–1.5 servings/d for women, with 1 serving defined as 10 g/d) is included as a beneficial component of the AHEI-2010 and aMED indexes. Although moderate alcohol consumption was associated with lower CVD mortality, it was associated with higher cancer mortality in our study population. In contrast, mild alcohol consumption was not associated with a higher risk of cancer mortality.
Except for the HDI, all indexes had a moderate to high correlation with one another. The AHEI-2010, aMED, and DASH all emphasize higher intakes of vegetables, fruit, whole grains, nuts, and legumes and a lower intake of red meat, thus mainly focusing on food groups. In contrast, the HDI that is based on WHO recommendations focuses more on nutrients such as FAs, cholesterol, mono- and disaccharides, and protein. There were also different approaches to include alcohol as part of dietary indexes. For the AHEI-2010 and aMED, moderate alcohol intake is included as a component that contributes to higher dietary quality scores, whereas alcohol is not a scoring component for the HDI and DASH. Furthermore, different classification methods for score components based on quintiles, medians, or fixed cutoff intakes may have contributed to differences in dietary pattern scores. Despite the differences, the AHEI-2010, aMED, and DASH dietary patterns generally showed similar associations with mortality outcomes. This suggests that all of these patterns capture key aspects of healthful diets in our Asian population.
In our study, all 4 evaluated dietary indexes were associated with a substantially lower risk of all-cause mortality, which agrees with studies conducted in other populations (18–22). In the US Multiethnic Cohort Study (19), the highest scores for the AHEI-2010, DASH, and aMED were associated with a 19–22% lower risk of all-cause mortality, which is very similar to the 18–20% lower risk that we observed. In our study, higher adherence to diet-quality indexes was significantly associated with CVD and respiratory disease mortality but not with cancer mortality. Possible reasons for the weaker associations with cancer mortality could be that cancer is a heterogeneous endpoint. Diet may play a more important role in the etiology of certain cancers, such as colorectal cancer, compared with other specific types of cancer (14). In contrast to our findings, AHEI-2010, aMED, and DASH scores were similarly associated with a lower risk of CVD and cancer mortality in the US NIH-AARP Diet and Health Study and the Multiethnic Cohort (19, 22). Interestingly, we observed more similar results for the AHEI-2010 and cancer mortality after modifying the alcohol component. However, our findings are in agreement with results from other studies in which dietary quality scores were more strongly associated with CVD than with cancer mortality (14, 20).
In a previous study in a Chinese population in Shanghai, higher scores for the AHEI-2010 and DASH also predicted lower mortality from all causes (16–32%) and CVD (21–44%) but not cancer (25). However, this study did not include the whole-grain and sugar-sweetened beverage component of the dietary quality indexes due to very low intakes. Our study population had higher intakes of whole grains and sugar-sweetened beverages, making it possible to include these food groups in the calculation of diet-quality scores.
Although the HDI score was inversely associated with the risk of mortality from all causes and cause-specific mortality in our study, associations were weaker and less consistent than with other diet-quality indexes. Consistent with our findings, the HDI score was inversely associated with all-cause mortality (21) and CVD mortality (23), but not with cancer mortality in other populations (23, 41). In contrast, greater adherence to the HDI score was not significantly associated with all-cause mortality in Central and Eastern European populations (23) and CVD mortality in a pooled analysis that included 10 prospective cohort studies from Europe and the United States (17).
Several epidemiologic studies have supported a role of dietary intakes in the development of respiratory diseases (42) and related mortality (43, 44), but little is known on the impact of overall diet-quality on respiratory mortality. In the Nurses’ Health Study and the Health Professionals Follow-Up study, the highest AHEI-2010 diet score was associated with a 31–40% lower risk of chronic obstructive pulmonary disease (45). Interestingly, in our study, all dietary quality indexes were associated with a substantially lower risk of respiratory disease mortality.
In the analyses of individual dietary factors, the consumption of nuts was significantly associated with lower CVD and all-cause mortality, but these associations were substantially weaker for legumes. The lack of substantial association between legume consumption and CVD mortality is consistent with the previously reported lack of association between soy intake and CVD mortality in our study (46). This suggests that using the combined category “nuts and legumes” in diet-quality indexes may not be recommended. Specifically recommending nuts may be particularly relevant in Chinese populations in whom the intake of legumes in the form of soy products is often much higher than the intake of nuts. However, the differences in association with mortality for the AHEI-2010 scores with only “nuts” or “nuts and legumes” as a component were small and need to be confirmed in other Asian populations.
In our study, moderate alcohol consumption was associated with a higher risk of cancer mortality. These results suggest that the associations between moderate alcohol consumption and total cancer mortality are stronger in our population as than in Western populations (47–49). In East Asians, a large part of the population carries genetic variants that lead to a slower breakdown of the carcinogenic alcohol metabolite acetaldehyde (40, 50). In epidemiologic studies in Japan, individuals with 1 copy of the inactive variant were 6–10 times more likely to develop esophageal cancer than individuals with the fully active aldehyde dehydrogenase 2 enzyme who drank comparable amounts of alcohol (51). Thus, alcohol recommendations reflected in the AHEI-2010 and aMED indexes may be too high with regard to cancer mortality risk for East Asian populations (52). Therefore, we conducted an analysis with the AHEI-2010 index adapted for East Asian populations by considering light alcohol consumption or abstinence (<1 drink/d) instead of moderate consumption as the recommended level of intake. This adapted AHEI-2010 index was associated with a significantly lower cancer mortality.
Strengths of our study included the prospective study design, large sample size, detailed assessment of diet and potential confounders, long-term follow-up, comprehensive ascertainment of mortality through a nationwide death registry, and the evaluation of diet-quality scores in an Asian study setting. Potential limitations of our study included measurement error inherent in the assessment of diet and potential confounders with the use of self-reported information. Dietary assessment was done only at baseline and subsequent changes in the dietary intake during follow-up were not recorded. However, because of the prospective design, changes occurring in the diet would be expected to lead to nondifferential misclassification and most likely attenuate the observed associations. Due to lack of data on trans fat consumption in our study population, we did not include the originally proposed trans fat component in the calculation of the AHEI-2010. However, the very low plasma trans fat concentration in our study population suggests a minimal impact of trans fat on the AHEI-2010 score in our analysis (53). Dairy consumption was generally low in our study population, and hence we included all dairy products instead of low-fat dairy in the calculation of the DASH score. Furthermore, the results for the sodium component should be interpreted with caution because it is difficult to capture discretionary salt use in cooking or at the table through questionnaires. Finally, although we carefully adjusted for important confounders such as demographic and lifestyle factors in our analyses, the possibility of residual confounding due to imperfectly measured or unknown confounders cannot be excluded.
In conclusion, our findings suggest that higher adherence to dietary recommendations as reflected in the AHEI-2010, aMED, DASH, and HDI was associated with a substantially lower risk of CVD, respiratory disease, and all-cause mortality in a Chinese population. Previous studies mainly focused on CVD and cancer mortality, but our findings suggest that higher dietary quality may also substantially reduce respiratory mortality. The associations between diet-quality and mortality outcomes were of a similar magnitude, except for the slightly weaker associations for the HDI. The specific foods and dishes that characterize a high diet-quality score will likely be different for Chinese compared with Western populations. However, our results suggest that the broader food groups and nutrients encompassed within dietary quality indexes can be recommended to Chinese and possibly other Asian populations. Our study findings suggest that the association between moderate alcohol consumption and a higher risk of cancer death may be stronger in East Asian than in Western populations. As a result, it may be prudent to recommend lower alcohol intakes as part of dietary quality scores for East Asian populations. In general, our findings support adhering to the dietary quality indexes that emphasize a variety of healthy plant-based foods (vegetables, fruit, nuts) and long-chain n–3 PUFAs, low consumption of red meat, and avoidance of heavy alcohol intake for lowering the risk of all-cause and chronic disease mortality.
Supplementary Material
Acknowledgments
We acknowledge the founding, longstanding principal investigator of the Singapore Chinese Health Study, Mimi C Yu. We also thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study and Renwei Wang for the development and maintenance of the cohort-study database. The authors’ responsibilities were as follows—NN and RMvD: designed the research, developed the analytical plan, and took responsibility for the integrity of the data and the accuracy of the data analysis; NN: performed the statistical analyses and had primary responsibility for writing the manuscript; W-PK, J-MY, and RMvD: directed the study; RMvD: was the guarantor of the study; and all authors: interpreted the findings, edited the manuscript, had full access to all of the data in the study, and read and approved the final manuscript.
Notes
This study was Supported by the US National Cancer Institute at the NIH (grants UM1 CA182876 and R01 CA144034). W-PK was supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013).
Author disclosures: NN, W-PK, J-MY, and RMvD, no conflicts of interest.
Supplemental Tables 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- AHEI
Alternative Healthy Eating Index
- aMED
alternate Mediterranean diet
- CVD
cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- HDI
Healthy Diet Indicator
- ICD
International Classification of Diseases
- SCHS
Singapore Chinese Health Study
References
- 1. WHO.. Global status report on noncommunicable diseases 2014. Geneva (Switzerland): WHO;2014. [cited 2017 Nov 14]. Available from: http://www.who.int/nmh/publications/ncd-status-report-2014/en/. [Google Scholar]
- 2. WHO.. Global health estimates. Deaths by cause, age, sex and country, 2000–2012. Geneva (Switzerland): WHO; 2014. [cited 2017 Nov 14]. Available from: http://www.who.int/healthinfo/global_burden_disease/en/. [Google Scholar]
- 3. WHO.. Burden: mortality, morbidity and risk factors. Noncommunicable disease report: chapter 1. Geneva (Switzerland): WHO; 2012. [cited 2017 Nov 14]. Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf. [Google Scholar]
- 4. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 5. Willett WC. Nutritional epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 6. Kant AK. Indexes of overall diet-quality: a review. J Am Diet Assoc 1996;96(8):785–91. [DOI] [PubMed] [Google Scholar]
- 7. Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chim Acta 2011;412(17–18):1493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc 2004;104(4):615–35. [DOI] [PubMed] [Google Scholar]
- 9. Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62(5):177–203. [DOI] [PubMed] [Google Scholar]
- 10. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95(10):1103–8. [DOI] [PubMed] [Google Scholar]
- 11. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61(6 Suppl):1402S–06S. [DOI] [PubMed] [Google Scholar]
- 12. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. DASH Collaborative Research Group A clinical trial of the effects of dietary patterns on blood pressure . N Engl J Med 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 13. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet-quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 14. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD. Diet-quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr 2006;136(2):466–72. [DOI] [PubMed] [Google Scholar]
- 16. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 17. Jankovic N, Geelen A, Streppel MT, de Groot LC, Kiefte-de Jong JC, Orfanos P, Bamia C, Trichopoulou A, Boffetta P, Bobak M, et al. WHO guidelines for a healthy diet and mortality from cardiovascular disease in European and American elderly: the CHANCES project. Am J Clin Nutr 2015;102(4):745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet-quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180(6):616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr 2015;101(3):587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobs S, Harmon BE, Ollberding NJ, Wilkens LR, Monroe KR, Kolonel LN, Le Marchand L, Boushey CJ, Maskarinec G. Among 4 diet-quality indexes, only the alternate Mediterranean diet score is associated with better colorectal cancer survival and only in African American women in the Multiethnic Cohort. J Nutr 2016;146(9):1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jankovic N, Geelen A, Streppel MT, de Groot LC, Orfanos P, van den Hooven EH, Pikhart H, Boffetta P, Trichopoulou A, Bobak M, et al. Adherence to a healthy diet according to the World Health Organization guidelines and all-cause mortality in elderly adults from Europe and the United States. Am J Epidemiol 2014;180(10):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet-quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stefler D, Pikhart H, Jankovic N, Kubinova R, Pajak A, Malyutina S, Simonova G, Feskens EJ, Peasey A, Bobak M. Healthy diet indicator and mortality in Eastern European populations: prospective evidence from the HAPIEE cohort. Eur J Clin Nutr 2014;68(12):1346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis 2015;19(1):10–20. [DOI] [PubMed] [Google Scholar]
- 25. Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, Zheng W, Shu XO. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr 2014;100(2):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurotani K, Akter S, Kashino I, Goto A, Mizoue T, Noda M, Sasazuki S, Sawada N, Tsugane S; Japan Public Health Center-based Prospective Study Group Quality of diet and mortality among Japanese men and women: Japan Public Health Center based prospective study. BMJ 2016;352:i1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001;39(2):187–95. [DOI] [PubMed] [Google Scholar]
- 28. Koh WP, Yuan JM, Sun CL, Lee HP, Yu MC. Middle-aged and older Chinese men and women in Singapore who smoke have less healthy diets and lifestyles than nonsmokers. J Nutr 2005;135(10):2473–7. [DOI] [PubMed] [Google Scholar]
- 29. Seah JY, Gay GM, Su J, Tai ES, Yuan JM, Koh WP, Ong CN, van Dam RM. Consumption of red meat, but not cooking oils high in polyunsaturated fat, is associated with higher arachidonic acid status in Singapore Chinese adults. Nutrients 2017;9(2), http://www.mdpi.com/about/announcements/784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Health Promotion Board My Healthy Plate fact sheet. Singapore: Health Promotion Board; 2010. [cited 2017 Dec 15]. Available from: https://www.ntu.edu.sg/Students/Undergraduate/StudentServices/HealthAndCounselling/Documents/HPB_MyHealthyPlate_FactSheet_FA(hires).pdf. [Google Scholar]
- 31. The Whole Grains Council Oldways Whole Grains Council 101: what counts as a serving? [cited 2017 Dec 15]. Available from: http://wholegrainscouncil.org/whole-grains-101/what-counts-as-a-serving. [Google Scholar]
- 32. Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ 1995;311(7018):1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 34. WHO. Diet, nutrition, and the prevention of chronic diseases. Report of a WHO Study Group World Health Organ Tech Rep Ser 1990;797: 1–149. [PubMed] [Google Scholar]
- 35. Huijbregts P, Feskens E, Rasanen L, Fidanza F, Nissinen A, Menotti A, Kromhout D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ 1997;315(7099):13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cust AE, Smith BJ, Chau J, van der Ploeg HP, Friedenreich CM, Armstrong BK, Bauman A. Validity and repeatability of the EPIC physical activity questionnaire: a validation study using accelerometers as an objective measure. Int J Behav Nutr Phys Act 2008;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CDC International classification of diseases. 9th revision. Last updated: June 18, 2013. [cited 2018 Feb 08]. Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm. [Google Scholar]
- 38. CDC International classification of diseases. 10th revision. Last updated: April 15, 2016. [cited 2018 Feb 08]. Available from: https://www.cdc.gov/nchs/icd/icd10.htm. [Google Scholar]
- 39. WHO International statistical classification of diseases and related health problems. 10th revision. [cited 2018 Feb 08]. Available from: http://apps.who.int/classifications/icd10/browse/2016/en. [Google Scholar]
- 40. Akutsu PD, Sue S, Zane NW, Nakamura CY. Ethnic differences in alcohol consumption among Asians and Caucasians in the United States: an investigation of cultural and physiological factors. J Stud Alcohol 1989;50(3):261–7. [DOI] [PubMed] [Google Scholar]
- 41. Berentzen NE, Beulens JW, Hoevenaar-Blom MP, Kampman E, Bueno-de-Mesquita HB, Romaguera-Bosch D, Peeters PH, May AM. Adherence to the WHO's healthy diet indicator and overall cancer risk in the EPIC-NL cohort. PLoS One 2013;8(8):e70535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanson C, Rutten EP, Wouters EF, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis 2014;9:723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP Diet and Health Study. Arch Intern Med 2011;171(12):1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varraso R, Chiuve SE, Fung TT, Barr RG, Hu FB, Willett WC, Camargo CA. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ 2015;350:h286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talaei M, Koh WP, van Dam RM, Yuan JM, Pan A. Dietary soy intake is not associated with risk of cardiovascular disease mortality in Singapore Chinese adults. J Nutr 2014;144(6):921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 2008;337:a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, Manson JE, Hennekens CH, Buring JE. Light-to-moderate alcohol consumption and mortality in the Physicians' Health Study enrollment cohort. J Am Coll Cardiol 2000;35(1):96–105. [DOI] [PubMed] [Google Scholar]
- 49. Breslow RA, Chen CM, Graubard BI, Mukamal KJ. Prospective study of alcohol consumption quantity and frequency and cancer-specific mortality in the US population. Am J Epidemiol 2011;174(9):1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramchandani VA. Alcohol, nutrition, and health consequences. In: Nutrition and health. Genetics of Alcohol Metabolism Chapter 2; 15–25. 1st ed.Watson RR,, et al. (eds.), New York: Springer Science+Business Media; 2013. [Google Scholar]
- 51. Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med 2009;6(3):e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goh GB, Chow WC, Wang R, Yuan JM, Koh WP. Coffee, alcohol and other beverages in relation to cirrhosis mortality: the Singapore Chinese Health Study. Hepatology 2014;60(2):661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neelakantan N, Naidoo N, Koh WP, Yuan JM, van Dam RM. The Alternative Healthy Eating Index is associated with a lower risk of fatal and nonfatal acute myocardial infarction in a Chinese adult population. J Nutr 2016;146(7):1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.