Abstract
BACKGROUND AND OBJECTIVES:
Postvaccination syncope can cause injury. Drinking water prephlebotomy increases peripheral vascular tone, decreasing risk of blood-donation presyncope and syncope. This study evaluated whether drinking water prevaccination reduces postvaccination presyncope, a potential syncope precursor.
METHODS:
We conducted a randomized trial of subjects aged 11 to 21 years receiving ≥1 intramuscular vaccine in primary care clinics. Intervention subjects were encouraged to drink 500 mL of water, with vaccination recommended 10 to 60 minutes later. Control subjects received usual care. Presyncope symptoms were assessed with a 12-item survey during the 20-minutes postvaccination. Symptoms were classified with a primary cutoff sensitive for presyncope, and a secondary, more restrictive cutoff requiring greater symptoms. Results were adjusted for clustering by recruitment center.
RESULTS:
There were 906 subjects randomly assigned to the control group and 901 subjects randomly assigned to the intervention group. None had syncope. Presyncope occurred in 36.2% of subjects by using the primary definition, and in 8.0% of subjects by using the restrictive definition. There were no significant differences in presyncope by intervention group for the primary (1-sided test, P = .24) or restrictive outcome (1-sided test, P = .17). Among intervention subjects vaccinated within 10 to 60 minutes after drinking all 500 mL of water (n = 519), no reduction in presyncope was observed for the primary or restrictive outcome (1-sided tests, P = .13, P = .17). In multivariable regression analysis, presyncope was associated with younger age, history of passing out or nearly passing out after a shot or blood draw, prevaccination anxiety, receiving >1 injected vaccine, and greater postvaccination pain.
CONCLUSIONS:
Drinking water before vaccination did not prevent postvaccination presyncope. Predictors of postvaccination presyncope suggest opportunities for presyncope and syncope prevention interventions.
Although uncommon, syncope can occur after vaccination and lead to serious injury.1,2 The National Academy of Medicine (formerly the Institute of Medicine) has concluded that evidence “convincingly supports a causal relationship between the injection of a vaccine and syncope,”3 likely because of a vasovagal reaction. In adolescents and young adults, postvaccination syncope occurs approximately once per 1000 doses administered, typically within 15 minutes after vaccination.1,3,4 Postvaccination presyncope symptoms (eg, lightheadedness, dizziness) are more common than syncope.5 Presyncope may precede syncope but does not always lead to syncope.6
To minimize risk of injury associated with postvaccination syncope, the Advisory Committee on Immunization Practices recommends that adolescents and adults be seated or lying down during vaccination and that providers consider observation for 15 minutes after vaccination.7 The effectiveness of this practice is not known. There are no evidence based recommendations for primary prevention of postvaccination syncope.
One potential strategy to prevent postvaccination syncope is oral hydration before vaccination. Drinking water shortly before blood donation induces an increase in peripheral vascular tone mediated by sympathetic nervous system activation.8–10 Although blood donation is different in many respects from vaccination (eg, blood loss, needle size, duration), prevaccination hydration could possibly be an effective syncope prevention measure through mechanisms independent of intravascular volume expansion.11
Conducting a trial to assess prevaccination hydration on the outcome of postvaccination syncope would require a large number of subjects. Presyncope is more common, both syncope and presyncope are vasovagally mediated, and the risk of both might be reduced by prevaccination hydration. Therefore, for this study, we chose presyncope as a surrogate measure for syncope. The primary objectives of this study were to determine if drinking water before intramuscular (IM) vaccination reduces symptoms of presyncope in adolescents and young adults and to assess acceptability of this intervention. Secondary objectives included describing rates of presyncope and syncope, and assessing the association of selected factors with predicting postvaccination presyncope or syncope.
METHODS
Study design
We conducted a multisite, randomized, open-label study of oral water hydration before vaccination for adolescents and young adults receiving at least 1 IM vaccine. The study was approved by the institutional review boards at the Duke University School of Medicine and Boston University Medical Center. The Centers for Disease Control and Prevention (CDC) relied on the Duke University School of Medicine Institutional Review Board.
Patient Population
Subjects aged 11 to 21 years were enrolled from typical, busy, primary care clinics affiliated with the Duke University School of Medicine and the Primary Care and Adolescent practices at Boston Medical Center. Eligible subjects were those receiving at least 1 IM vaccine as part of their usual care, willing to attempt to drink up to 500 mL of water, not taking daily injectable medications, and not having received an experimental vaccine or experimental medication within the previous 2 weeks. Written consent was obtained from subjects ≥18 years old. For younger subjects, written consent was obtained from a guardian with assent from the subject.
Study Procedures
After enrollment, subjects were randomly assigned 1:1 with a computer-generated algorithm to routine care (control group) or to prevaccination hydration (intervention group). Demographic information was collected from subjects. A standardized brief medical history was taken, including past history of presyncope and syncope. We collected information about sleep the previous night, foods and beverages consumed the day of enrollment, and hunger and thirst at the time of enrollment. Vital signs, weight, and height were recorded; BMI was calculated and categorized by using the age- and sex-specific CDC growth chart into normal weight (<85th percentile), overweight (85th–95th percentile), and obese (≥95th percentile).12 Subjects completed the Patient- Reported Outcomes Measurement Information System anxiety short form instrument for adolescents (11–17 years) or adults (≥18 years) to categorize their degree of anxiety before vaccination.13,14 After vaccination, subjects verbally reported their degree of pain by using a visual analog scale from 0 to 5 (Faces Pain Scale–Revised),15 and symptoms of presyncope by using a standardized instrument, described below. Subjects were observed for 20 minutes for signs of presyncope, syncope, or any spontaneous reports of these symptoms. Acceptability of the intervention was based on the degree to which subjects drank the water and an open ended question about whether they liked receiving the water.
Intervention
Subjects randomly assigned to the hydration group were provided with a 500 mL bottle of commercially available water and were asked to drink as much of the water as they could without feeling uncomfortable within a 15-minute period. The volume of water remaining in the bottle was measured and recorded. The amount consumed was calculated to be 500 mL less the amount of water remaining in the bottle. Vaccinations were recommended to be administered by members of the practice during the 10 to 60 minutes after hydration.
Case definitions and outcomes
There is no standard research case definition of presyncope. In consultation with the CDC’s Clinical Immunization Safety Assessment Project (http://www.cdc.gov/ vaccinesafety/ensuringsafety/ monitoring/cisa/), we developed primary and secondary definitions for presyncope. The previously validated 11-item Blood Donation Reaction Inventory16,17 provided the basis for capturing presyncope. The Blood Donation Reaction Inventory asks about symptoms of faintness, dizziness, weakness, facial flush, visual disturbance, difficulty hearing, lightheadedness, rapid or pounding heartbeat, sweating, rapid or difficult breathing, and nausea. One item was added: feeling cold and sweaty or “clammy.” Participants were observed for 20 minutes after immunization and information about subjects’ symptoms were collected by using the modified questionnaire administered verbally. Subjects rated each of the 12 symptoms on a 5-point Likert scale ranging from 1 (“not at all”) to 5 (“extremely”). For the primary outcome, we operationalized presyncope as an answer of 2 (“a little bit”) or more for any of the 12 items. For the secondary “restrictive” definition, we operationalized presyncope as an answer of 3 (“somewhat”) or more for any of the 12 items. We planned a priori to exclude subjects who had signs or symptoms of presyncope at baseline or who developed presyncope because of another cause. We also planned a priori to classify subjects who developed postvaccination syncope after presyncope based on either measure as only having syncope to avoid double counting events. During the 20-minute observation period, research staff recorded any spontaneous episode of syncope or observed signs or spontaneous subject reports of symptoms of presyncope.
Evaluation of Effectiveness
We evaluated the effectiveness of prevaccination oral hydration by comparing the rates of the primary and of the restrictive outcomes in the control group to the intervention group, regardless of how much subjects drank. We also conducted sensitivity analyses including only those subjects in the treatment group who consumed the full 500 mL of water and were vaccinated 10 to 60 minutes later because this was the group most likely to have a treatment effect.
All analyses were conducted with Stata 14 (StataCorp, College Station, TX). χ2 tests were used for categorical data and the Wilcoxon rank test was used to compare median values for continuous variables. We also evaluated outcomes by recruitment center (Duke and Boston) to explore center differences. To address any baseline differences by center and possible correlation of data, selected analyses of treatment effect as indicated in the results section were adjusted for clustering by recruitment center.18 To calculate the adjusted likelihood of presyncope among the control and intervention group, binary generalized linear modeling using the logit function adjusting for clustering by recruitment center was used.18 We considered P < .05 to be statistically significant.
Additional Analyses
We conducted additional post hoc analyses to evaluate the relationship between factors previously associated with presyncope and syncope in vaccine studies or in studies of blood donation for all subjects overall and stratified by intervention group versus control group, including the following: age, sex, BMI, level of prevaccination anxiety, the overall number of injected vaccines received, pain, and receipt of human papillomavirus vaccine (HPV).1–6,19–21 We also included other variables possibly predictive of presyncope, including race and/or ethnicity, self-report of passing out (fainting) or nearly passing out after a shot or blood draw, and hunger or thirst at the time of enrollment in the study.19,22 We first conducted bivariate analyses and then used multivariable logistic regression using those variables significantly associated in the bivariate analyses (P < .05) with presyncope or syncope with either the primary or restrictive definition. In these regression models, we also included intervention group; recruitment center was treated as a fixed effect to adjust for clustering.18 We used link tests to rule out specification error and tested for multicollinearity among all predictor variables by using variance inflation factors.
Power calculation and statistical analysis
The goal was to enroll 1800 subjects with equal numbers in the intervention and control groups. Statistical power was based on the detection of the combined outcome of presyncope and syncope during the postvaccination observation period. A priori power calculations were based on estimates of the base rate of events expected in the control group and a 1-sided test (α = .05). A 1-sided test was justified because prevaccination hydration would likely only decrease, not increase, the risk of presyncope or syncope. We estimated the risk of presyncope to be 4% based on the rate of dizziness reported in prelicensure trials of the quadrivalent HPV vaccine.23 Based on this estimation, the study would have 80% power to detect at least a 50% relative risk reduction in the intervention group by using a χ2 test. We anticipated that the spontaneous rate might be higher because of actively soliciting a broad range of symptoms. If the spontaneous risk were higher, the statistical power to detect a risk reduction would also increase. For example, the study would have 80% power if the spontaneous risk were 10% to detect a 32% relative risk reduction. All tests based on 1-sided P values are noted in the results. Otherwise, 2-sided tests were used.
RESULT
Subjects
From January 2015 through June 2016, 2918 individuals were approached for participation, of whom 1820 were randomly assigned to groups. Thirteen subjects were excluded after randomization, leading to the analysis of 1807 total subjects, 906 in the control group and 901 in the intervention group (Fig 1).
FIGURE 1.
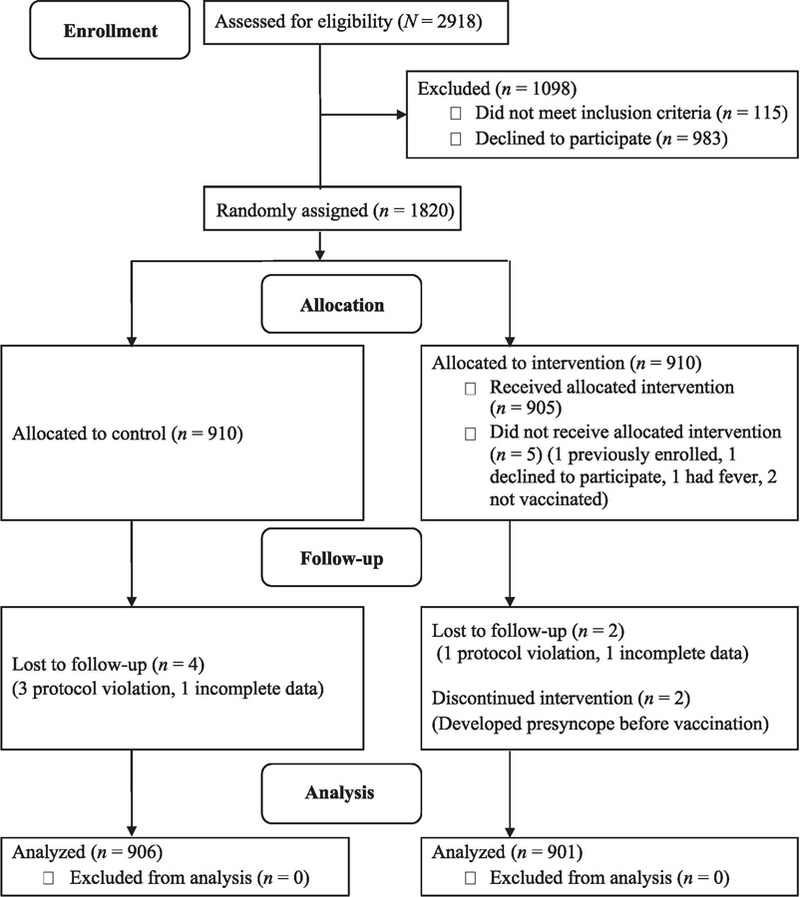
Flow diagram of enrollment, allocation (control group or the intervention group receiving prevaccination oral water hydration), follow-up, and analysis. Protocol violations are when subjects were not observed for 20 minutes after vaccination.
There were no significant differences between the intervention and control groups across a broad array of subject characteristics unadjusted by recruitment center (Table 1). Most subjects received only 1 IM vaccine (control 58.0%, intervention 57.1%; P = .70), 26.8% received 2 IM vaccines, and 15.4% received 3 or more IM vaccines, with no significant difference between the control and intervention groups (P = .60). The subjects received the following IM vaccines: HPV vaccine (57.4%); quadrivalent meningococcal vaccine (42.5%); inactivated influenza vaccine (25.6%); tetanus and diphtheria or tetanus, diphtheria, and acellular pertussis vaccine (Tdap) (16.9%); hepatitis A vaccine (11.7%); and hepatitis B vaccine (2.4%). Most subjects vaccinated against HPV received the 9-valent vaccine (71.3%); the remainder (28.7%) received the 4-valent vaccine. In addition to the IM vaccine(s), subcutaneous vaccines included varicella (1.7%), as well as measles, mumps, and rubella (0.1%). Few subjects received intranasal influenza vaccine (control 2.2%, intervention 1.4%; P = .23). All subjects were sitting during vaccination. Some subjects (control 12.9%, intervention 12.6; P = .82) were moved to a different room for postvaccination observation. There was no difference (P = .68) in position between the groups after vaccination; most subjects (92.4%) were sitting and few were lying down (0.9%), standing (6.2%), or walking (0.5%).
TABLE 1.
Baseline Characteristics for the Subjects Randomly Assigned to the Control Group or Intervention (Prevaccination Oral Water Hydration) Group
| Control (n = 906) | Intervention (n = 901) | P | |
|---|---|---|---|
| Age, y | 15 (12–17)a | 15 (12–17)a | .62 |
| Sex (% female) | 49.6 | 53.0 | .15 |
| Race, % | .80 | ||
| White | 44.7 | 44.4 | — |
| African American | 43.9 | 42.4 | — |
| Asian American | 2.8 | 2.9 | — |
| American Indian | 0.1 | 0.2 | — |
| Native Hawaiian/Pacific Islander | 0.3 | 0.1 | — |
| Other | 5.2 | 6.4 | — |
| Missing | 3.0 | 3.6 | — |
| Ethnicity, % | .31 | ||
| Hispanic | 9.4 | 11.5 | — |
| Non-Hispanic | 89.4 | 87.1 | — |
| Missing | 1.2 | 1.3 | — |
| BMI, % | .58 | ||
| Normal | 60.0 | 60.1 | — |
| Overweight | 21.9 | 20.3 | — |
| Obese | 18.1 | 19.6 | — |
| Insurance status, % | .79 | ||
| Only public | 38.4 | 38.5 | — |
| Any private | 60.5 | 60.4 | — |
| None | 1.1 | 1.0 | — |
| Ever nearly passed out or passed out in the past 5 y, % |
— | ||
| In the heat | 14.5 | 15.5 | .55 |
| After a shot | 2.1 | 2.1 | .99 |
| After blood draw | 4.8 | 4.8 | .97 |
| After or during exercise | 19.2 | 17.1 | .24 |
| Tiredness at the time of enrollment, % | .43 | ||
| Not at all or a little bit | 93.8 | 94.7 | — |
| Very | 6.2 | 5.3 | — |
| Hunger at the time of enrollment, % | .18 | ||
| Not at all or a little bit | 87.3 | 85.1 | — |
| Very | 12.7 | 14.9 | — |
| Thirst at the time of enrollment, % | .12 | ||
| Not at all or a little bit | 88.0 | 90.3 | — |
| Very | 12.0 | 9.7 | — |
| Hours of sleep the previous night | 8 (7–9)a | 8 (7–9)a | .94 |
| Hours since last food | 3 (2–5)a | 3 (2–5)a | .57 |
| Hours since last drink | 2 (1–4)a | 3 (1–5)a | .09 |
| Prevaccination anxiety, % | .32 | ||
| None or slight | 79.3 | 77.0 | — |
| Mild | 12.1 | 12.5 | — |
| Moderate | 7.7 | 10.0 | — |
| Severe | 0.9 | 0.5 | — |
not applicable.
Median (IQR).
Postvaccination pain was similar between the groups (P = .75). Most subjects (57.3%) reported no pain, 29.2% reported mild pain (1 out of 5), and 13.5% reported a greater amount of pain (2 or more out of 5). Subjects who received >1 injectable vaccine were more likely to report pain. Among subjects who received only 1 vaccine, 9.9% reported a pain score of 2 or more out of 5 compared with 20.5% of subjects who received 2 or more vaccines (P < .001). Prevaccination anxiety was also associated with pain. Overall, 13.2% of subjects with less than mild anxiety reported a pain score of 2 or more compared with 16.8% of subjects with greater anxiety (P = .04).
All subjects in the intervention group drank some water; most (64.9%) drank all 500 mL of water, and 78.3% drank more than half. Only 4.8% reported not liking getting a drink, most typically because they either did not like to drink water or were not thirsty. The median time from finishing the water to vaccination was 13 minutes (interquartile range [IQR]: 5–19 minutes). There were 272 subjects (30.2% in the intervention group) vaccinated <10 minutes and 3 subjects >60 minutes after the recorded end of hydration. Most (95.4%) of these subjects were recruited at the Boston Medical Center. Those with <10 minute wait time had a median water consumption time averaging 10 minutes (IQR: 7–23 minutes) followed by a wait period of 2 minutes (IQR: 0–5 minutes) before vaccinations were administered.
Outcome Events
There were no episodes of syncope and 20 episodes of observed presyncope. These episodes of presyncope also met the criteria for the primary and restrictive presyncope outcome definitions. Subjects recruited at Duke clinics were more likely to have the primary outcome (report of “a little bit or more” of any presyncope symptom) than those recruited at Boston Medical Center clinics (42.5% vs 26.7%; P < .001). However, there was no difference by recruitment center for the restrictive outcome (report of “some or more” presyncope symptom) (Duke: 8.7% vs Boston Medical Center: 7.0%; P = .19).
After adjusting for clustering by recruitment center, 36.2% of subjects reported presyncope according to the primary study definition and 8.0% of subjects reported presyncope according to the restrictive outcome definition. The most commonly reported symptom was “feeling weak.” For 18.7% of the subjects classified with the primary outcome and 20.5% classified with the restrictive outcome, the only reason was a report of weakness.
Effect on Postvaccination Presyncope
After adjusting for clustering by recruitment center, the primary outcome occurred in 37.1% (95% confidence interval [CI]: 34.0%–40.2%) of the control group and in 35.3% (95% CI: 32.2%– %) of the intervention group (1-sided test, P = .24) for any volume of water consumed. The restrictive outcome occurred in 8.6% (95% CI: 6.8%–10.4%) of the control group and in 7.3% (95% CI: 5.6%–9.1%) of the intervention group (1-sided test, P = .17).
No statistically significant differences between control and intervention groups for either outcome measure were found in the sensitivity analyses. When restricting the intervention group to those who drank 500 mL of water and were vaccinated within 10 to 60 minutes (n = 519), the primary outcome occurred in 39.2% of the control group and 35.9% of the intervention group (1-sided P = .13) and the restrictive outcome occurred in 8.9% of the control group and 7.5% of the intervention group (1 sided P = .17). Overall outcomes were similar in the control and intervention groups within the recruitment centers (Duke [primary: control 44.2%, intervention 40.7%, 1-sided P = .12; restrictive: control 9.6%, intervention 7.8%, 1-sided P = .15] and Boston Medical Center [primary: control 26.3%, intervention 27.1%, 1-sided P = .41; restrictive: control 7.2%, intervention 6.7%, 1-sided P = .40]).
Predictors of Postvaccination Presyncope
Table 2 lists the associations between the selected subject characteristics and the primary and restrictive outcome, regardless of intervention group, adjusted only by clustering by recruitment center. Younger age, female sex, report of passing out (fainting) after a shot or blood draw in the past 5 years, greater prevaccination anxiety, receipt of >1 injectable vaccine, receipt of HPV vaccine, and greater pain intensity were associated with the primary outcome. Female sex and receipt of HPV vaccine were not associated with the restrictive outcome. We created an indicator variable for being either very hungry or thirsty. Being either very hungry or thirsty at the time of enrollment was associated with the restrictive but not primary outcome.
TABLE 2.
Association of Subject Characteristics With the Primary and Restrictive Outcomes Regardless of Intervention Group Adjusted for Clustering by Recruitment Center
| Primary | Restrictive | |
|---|---|---|
| Outcomea | Outcome | |
| Age, y, % | ||
| <15 | 44.2 | 11.1 |
| ≥15 | 29.0 | 5.4 |
| P < .001 | P < .001 | |
| Sex, % | ||
| Male | 33.2 | 7.1 |
| Female | 39.0 | 8.8 |
| P = .01 | P = .11 | |
| Race and ethnicity, % | ||
| Non-Hispanic white | 36.3 | 7.5 |
| Non-Hispanic African American | 37.0 | 8.5 |
| Hispanic | 35.2 | 8.5 |
| Other | 35.3 | 6.8 |
| P = .80 | P = .94 | |
| BMI, % | ||
| Normal | 37.8 | 7.9 |
| Overweight or obese | 34.3 | 8.2 |
| P = .14 | P = .21 | |
| Passed out or nearly passed out from shot or blood draw in the past 5 y, % |
||
| No | 35.4 | 7.5 |
| Yes | 47.8 | 15.1 |
| P = .009 | P = .02 | |
| Hunger or thirst at the time of enrollment, % | ||
| Not at all or a little bit | 35.3 | 7.2 |
| Very | 39.2 | 10.9 |
| P = .13 | P = .02 | |
| Tired at the time of enrollment, % | ||
| Not at all or a little bit | 35.6 | 7.8 |
| Very | 40.1 | 13.9 |
| P = .36 | P = .07 | |
| Prevaccination anxiety, % | ||
| None or slight | 33.9 | 6.3 |
| Mild, moderate, or severe | 40.8 | 11.5 |
| P = .004 | P < .001 | |
| No. of injectable vaccines,b % | ||
| 1 | 30.6 | 5.2 |
| >1 | 43.7 | 11.8 |
| P < .001 | P < .001 | |
| Receipt of HPV vaccine, % | ||
| No | 33.8 | 7.3 |
| Yes | 38.3 | 8.5 |
| P = .02 | P = .11 | |
| Pain intensity after vaccination, % | ||
| 0–1 | 31.9 | 5.1 |
| 2–5 | 62.0 | 26.1 |
| P < .001 | P < .001 |
The primary outcome is “a little bit” or more in response to any of the 12 items on the presyncope instrument. The restrictive outcome is “somewhat” or more in response to any of the 12 items on the presyncope instrument.
Including intramuscularly and subcutaneously administered vaccines.
Table 3 summarizes the results of the multivariable logistic regression analyses to predict presyncope Younger age, report of passing out after a shot or blood draw, greater prevaccination anxiety, receiving >1 injectable vaccine, and greater pain after vaccination were associated with postvaccination presyncope by using both the primary and restrictive outcomes. In the model, female sex, hunger or thirst, and receipt of HPV vaccine were not associated with presyncope based on either the primary or restrictive outcome definitions. Assignment to prevaccination hydration also was not associated with presyncope with either the primary or restrictive definition.
TABLE 3.
Multivariable Logistic Regression Analyses to Predict the Risk of Postvaccination Presyncope Defined by the Primary and Restrictive Outcomes
| Primary Outcomea | Restrictive | |
|---|---|---|
| aOR (95% CI) | Outcomea aOR | |
| (95% CI) | ||
| Age, y | ||
| <15 | 2.27 (1.79–2.88) | 2.84 (1.83–4.39) |
| ≥15 | Reference | Reference |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.20 (0.97–1.48) | 1.03 (0.71–1.51) |
| Passed out or nearly passed out from shot or blood draw in the past 5 y |
||
| No | Reference | Reference |
| Yes | 1.91 (1.27–2.87) | 2.70 (1.48–4.91) |
| Hunger or thirst | ||
| Not at all or a little bit | Reference | Reference |
| Very | 1.15 (0.90–1.47) | 1.46 (0.97–2.19) |
| Prevaccination anxiety | ||
| None or slight | Reference | Reference |
| Mild, moderate, or severe | 1.86 (1.45–2.38) | 2.77 (1.82–4.20) |
| No. of injectable vaccines | ||
| 1 | Reference | Reference |
| >1 | 1.53 (1.24–1.89) | 1.95 (1.33–2.86) |
| Receipt of HPV vaccine | ||
| No | Reference | Reference |
| Yes | 1.03 (0.83–1.28) | 1.00 (0.68–1.48) |
| Pain intensity | ||
| 0–1 | Reference | Reference |
| 2–5 | 2.96 (2.21–3.98) | 4.96 (3.34–7.36) |
| Group | ||
| Control | Reference | Reference |
| Intervention | 0.92 (0.75–1.13) | 0.84 (0.58–1.21) |
The outcomes were adjusted for each listed variable and enrollment center. aOR, adjusted odds ratio.
The primary outcome is “a little bit” or more in response to any of the 12 items on the presyncope instrument. The restrictive outcome is “somewhat” or more in response to any of the 12 items on the presyncope instrument.
DISCUSSION
In this randomized trial, contrary to our hypothesis, we found that drinking up to 500 mL of water before vaccination did not decrease postvaccination presyncope in adolescents and young adults. The authors of a recent meta-analysis found a decreased relative risk (0.79) for vasovagal reactions in the context of blood donation after drinking water before the phlebotomy compared to usual care.10 Although it was biologically plausible to believe that drinking water before vaccination would decrease presyncope after vaccination, we did not observe this effect. These findings highlight the need to conduct randomized clinical trials to evaluate postvaccination presyncope and syncope prevention strategies rather than applying lessons from the blood banking literature without study.
Authors of blood donation literature have identified risk factors for donation-associated presyncope and syncope1,2,6,19–21; however, authors of immunization literature have reported little evidence identifying risk factors for postvaccination presyncope and syncope. Our post hoc analyses identified predictors of postvaccination presyncope, including younger age, report of passing out (fainting) or nearly passing out after a shot or blood draw, prevaccination anxiety, receipt of 2 or more injectable vaccine compared to only 1, and a greater degree of postvaccination pain. These predictors could help clinicians identify patients at a higher risk for postvaccination presyncope or syncope and serve as targets for future prevention interventions. For example, interventions to reduce anxiety before vaccination or to address pain after vaccination could be evaluated to determine if these are effective methods of preventing postvaccination presyncope.
Although our main interest is to prevent syncope and associated injury, these outcomes are uncommon. We therefore used presyncope as the primary study outcome because of its higher frequency, association with syncope, and the potential for it to be modified by oral hydration. A significant challenge to the tudy was classifying presyncope. Individuals with presyncope might not spontaneously report symptoms and observers might not recognize signs. We therefore further elicited symptoms with a slightly modified validated instrument. Although administering the instrument before and after vaccination might have helped determine change in symptoms, we did not administer the instrument before vaccination because we thought that doing so might increase subject concerns about vaccination and bias results. Because the optimal cutoff value for classifying presyncope using the instrument is not known, we used 2 different thresholds: a primary one that we believed would trade off improving sensitivity against potentially lower specificity, and a restrictive one that could improve specificity but risk misclassifying a true case.
Another important factor is that nearly 30% of the intervention group had <10 minutes between the completion of hydration and vaccination. This study was conducted in busy clinical practices. Even with added research support, it was challenging to ensure the time for water consumption followed by adequate wait time to allow for any reflex increase in peripheral vascular tone. Sometimes it was uncertain when individuals had completed drinking because they were allowed to drink in the waiting and examining rooms. Optimizing clinic flow and prioritizing patient care and treatment, including vaccinations, in these busy primary care clinics was sometimes chosen over strict adherence to timing of the water consumption protocol, including the wait periods.
CONCLUSION
We did not find that drinking water before vaccination and Regional Pediatrics. to be effective in reducing postvaccination presyncope in adolescents and young adults. However, the study did identify factors associated with postvaccination presyncope that could be targeted in future research to develop feasible and effective postvaccination presyncope and syncope prevention strategies.
WHAT’S KNOWN ON THIS SUBJECT:
Postvaccination syncope can cause injury. Drinking water before venipuncture for blood donation decreases syncope risk by increasing vascular tone.
WHAT THIS STUDY ADDS:
In a typical practice setting, drinking water before vaccination had no overall impact on postvaccination presyncope, a potential precursor of syncope.
To cite: Kemper AR, Barnett ED, Walter EB, et al. Drinking Water to Prevent Postvaccination Presyncope in Adolescents: A Randomized Trial. Pediatrics. 2017; 140(5):e20170508
ACKNOWLEDGEMENT
The authors acknowledge the contributions of the following study team members at Duke University: Dr Richard Chung, MD; Dr Jennifer Li, MD, MHS; Mr Christopher Todd, MPH; Ms Lori Hendrickson, RN, BSN; Ms Beth Patterson, RN, BSN; Ms Lynn Harrington, RN, BSN; Mr Luis Ballon, Ms Efe Cudjoe, Ms Joyce Gandee, Ms Kellie Gervas, Ms Danielle Lanpher, Ms Liz Schmidt, Ms Stephanie Smith, Ms Erica Suarez, Ms Rheaya Willis, and Ms Clara Wynn. In addition, we acknowledge the assistance and support of the following pediatric practices: Duke Children’s Primary Care, Durham Pediatrics, The authors also acknowledge the contributions of the following study team members at Boston Medical Center: Ms Rebecca Edelberg, Ms Chloe Sakow, Ms Regina Connors, Ms Maria Silva, and Ms Kelley Toli. The authors also acknowledge the following individuals at the CDC: Dr Andrew Kroger, MD, MPH; Dr Lauri Markowitz, MD, MPH; Ms Oidda Museru, MSN, MPH; and Mr Dave Sharma, MSN, MPH.
FUNDING: Supported by the Centers for Disease Control and Prevention (Clinical Immunization Safety Assessment project contract 200-2012-53663/0005 and contract 200-2012-53709).
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- HPV
human papillomavirus
- IM
intramuscular
- IQR
interquartile range
Footnotes
Dr Kemper helped design the study, participated in its implementation, helped conduct the analyses, and wrote the initial draft of the manuscript, and participated in the manuscript revision process; Dr Barnett helped design the study, participated in its implementation, critically reviewed the study results, wrote an initial draft of the manuscript, and participated in the manuscript revision process; Drs Walter and Pierre-Joseph helped design the study, participated in its implementation, critically reviewed the study results, and participated in the manuscript revision process; Dr Hornik helped design the study, helped conduct the data analysis, critically reviewed the study results, and participated in the manuscript revision process; Drs Broder and Silverstein helped design the study, critically reviewed the study results, and participated in the manuscript revision process; Dr Harrington conceived the study idea, helped design the study, assisted with data analysis, critically reviewed the study results, and participated in the manuscript revision process; and all authors give final approval of this version for publication; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: Dr Barnett has received research funding from Celexa, PaxVax, and Intercell (now Valneva) and has received support from Pfizer as a member of a Data Safety Monitoring Board and from PaxVax as a consultant. Dr Walter has received funding from CSL, GlaxoSmithKline, Merck, Novartis, Novavax, and Pfizer to conduct clinical research studies. He has received support from Novartis as a member of a Data Safety Monitoring Board and from Merck as a consultant. Dr Pierre-Joseph has received funding from Merck to conduct clinical research studies. The other authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Pediatr Adolesc Med 1997;151(3):255–259 [DOI] [PubMed] [Google Scholar]
- 2.Sutherland A, Izurieta H, Ball R, et al. ; Centers for Disease Control and Prevention (CDC). Syncope after vaccination–United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep 2008;57(17):457–460 [PubMed] [Google Scholar]
- 3.Institute of Medicine. Adverse Effects of Vaccines: Evidence and Causality Washington, DC: The National Academies Press; 2012 [PubMed] [Google Scholar]
- 4.Macartney KK, Chiu C, Georgousakis M, Brotherton JML. Safety of human papillomavirus vaccines: a review. Drug Saf 2013;36(6):393–412 [DOI] [PubMed] [Google Scholar]
- 5.Naleway AL, Gold R, Drew L, Riedlinger K, Henninger ML, Gee J. Reported adverse events in young women following quadrivalent human papillomavirus vaccination. J Womens Health (Larchmt) 2012;21(4):425–432 [DOI] [PubMed] [Google Scholar]
- 6.Accurso V, Winnicki M, Shamsuzzaman ASM, Wenzel A, Johnson AK, Somers VK. Predisposition to vasovagal syncope in subjects with blood/injury phobia. Circulation 2001;104(8):903–907 [DOI] [PubMed] [Google Scholar]
- 7.Kroger AT, Duchin J, Vázquez M. Vaccine recommendations and guidelines of the ACIP Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed June 23, 2017
- 8.Hanson SA, France CR. Predonation water ingestion attenuates negative reactions to blood donation. Transfusion 2004;44(6):924–928 [DOI] [PubMed] [Google Scholar]
- 9.May M, Jordan J. The osmopressor response to water drinking. Am J Physiol Regul Integr Comp Physiol 2011;300(1):R40–R46 [DOI] [PubMed] [Google Scholar]
- 10.Fisher SA, Allen D, Dorée C, Naylor J, Di Angelantonio E, Roberts DJ. Interventions to reduce vasovagal reactions in blood donors: a systematic review and meta-analysis. Transfus Med 2016;26(1):15–33 [DOI] [PubMed] [Google Scholar]
- 11.Lu CC, Diedrich A, Tung CS, et al. Water ingestion as prophylaxis against syncope. Circulation 2003;108(21):2660–2665 [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics; Centers for Disease Control and Prevention. CDC growth charts Available at: https://www.cdc.gov/growthcharts/cdc_charts.htm. Accessed June 23, 2017
- 13.Irwin DE, Stucky B, Langer MM, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res 2010;19(4): 595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D; PROMIS Cooperative Group. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18(3):263–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The faces pain scale-revised: toward a common metric in pediatric pain measurement. Pain 2001;93(2):173–183 [DOI] [PubMed] [Google Scholar]
- 16.France CR, Ditto B, France JL, Himawan LK. Psychometric properties of the blood donation reactions inventory: a subjective measure of presyncopal reactions to blood donation. Transfusion 2008;48(9): 1820–1826 [DOI] [PubMed] [Google Scholar]
- 17.Meade MA, France CR, Peterson LM. Predicting vasovagal reactions in volunteer blood donors. J Psychosom Res 1996;40(5):495–501 [DOI] [PubMed] [Google Scholar]
- 18.Kahan BC, Morris TP. Assessing potential sources of clustering in individually randomised trials. BMC Med Res Methodol 2013; 13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravo M, Kamel H, Custer B, Tomasulo P. Factors associated with fainting: before, during and after whole blood donation. Vox Sang 2011;101(4):303–312 [DOI] [PubMed] [Google Scholar]
- 20.Graham DT. Prediction of fainting in blood donors. Circulation 1961;23(6):901–906 [DOI] [PubMed] [Google Scholar]
- 21.Trouern-Trend JJ, Cable RG, Badon SJ, Newman BH, Popovsky MA. A case-controlled multicenter study of vasovagal reactions in blood donors: influence of sex, age, donation status, weight, blood pressure, and pulse. Transfusion 1999;39(3):316–320 [DOI] [PubMed] [Google Scholar]
- 22.Parry SW, Tan MP. An approach to the evaluation and management of syncope in adults. BMJ 2010; 340:c880. [DOI] [PubMed] [Google Scholar]
- 23.Merck & Co. Gardasil package insert Available at: www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf. Accessed June 23, 2017


