Abstract
Stroke represents a severe medical condition that causes stroke survivors to suffer from long-term and even lifelong disability. Over the past several decades, a vast majority of stroke research targets neuroprotection in the acute phase, while little work has been done to enhance stroke recovery at the later stage. Through reviewing current understanding of brain plasticity, stroke pathology, and emerging preclinical and clinical restorative approaches, this review aims to provide new insights to advance the research field for stroke recovery. Lifelong brain plasticity offers the long-lasting possibility to repair a stroke-damaged brain. Stroke impairs the structural and functional integrity of entire brain networks; the restorative approaches containing multi-components have great potential to maximize stroke recovery by rebuilding and normalizing the stroke-disrupted entire brain networks and brain functioning. The restorative window for stroke recovery is much longer than previously thought. The optimal time for brain repair appears to be at later stage of stroke rather than the earlier stage. It is expected that these new insights will advance our understanding of stroke recovery and assist in developing the next generation of restorative approaches for enhancing brain repair after stroke.
Keywords: Brain plasticity, Brain repair, Stroke recovery, Restorative timing
1. Introductive remarks: the importance of enhancing stroke recovery in stroke research
1.1. General characteristics of stroke
A stroke is caused by a sudden interruption of cerebral blood supply to a specific region of the brain, resulting in regional brain tissue death. A stroke within the right hemisphere primarily affects the left-side of the body with impairments of somatosensory function and an inability to move voluntarily. Stroke patients also often show difficulties in speaking, cognitive impairment, dementia, and depression (Mendis, 2013). A stroke often happens in adults but it occurs more frequently in older people (Mozaffarian et al., 2016).
There are two major subtypes of stroke: an ischemic stroke and a hemorrhagic stroke (Donnan et al., 2008). Although a relatively higher incidence of hemorrhagic stroke occurs in the Asian population as compared to Western countries, the majority of strokes worldwide are ischemic (Andersen et al., 2009; Gunarathne et al., 2009; Steiner et al., 2014; Tsai et al., 2016). In the United States, 87% of all strokes are ischemic strokes and 13% are hemorrhagic strokes (Mozaffarian et al., 2016). Since a vast majority of stroke studies target ischemic stroke, this review is mainly based on the considerable evidence of ischemic stroke studies to reveal the intrinsic ability for brain repair after stroke and feasibility for enhancing the intrinsic ability to repair a stroke-damaged brain.
1.2. Stroke phases
Once a stroke occurs, brain tissue that is located inside and outside the infarct/lesion area undergoes significant changes over time. The major pathological cascades include primary neuron loss, secondary neuron loss, brain edema, neuroinflammation, dead cell removal, neuron functional reorganization, blood vessel regeneration and neural network rewiring.
Based on the pathological characteristics and timing post-stroke, a stroke is generally classified into three clinical phases: the acute phase, subacute phase, and chronic phase. The duration and pathological severity of the three phases vary among individuals. The variations are very much dependent on the specific conditions of individuals regarding the location and size of lesion, the rapidity of arterial occlusion, the presence of cerebrovascular collateral circulation, the metabolic state of brain tissue, patient’s age and medical comorbidities. Generally, the acute phase of stroke is the first 48 h after stroke symptom onset, the subacute phase of stroke is the period between 48 h to 6 weeks, to 3 or 6 months post-stroke, whereas the chronic phase starts 3 to 6 months after stroke (Bernheisel et al., 2011; Donnan et al., 2008; Kang et al., 2004; Maraka et al., 2014; Parsons et al., 2000; Poh, 2013; van Delden et al., 2012).
1.3. Stroke fact and treatment
Stroke represents a very serious medical condition and causes huge medical and financial burdens throughout the world. Stroke remains the leading cause of long-term disability and the second leading cause of death worldwide (Feigin et al., 2014; Mozaffarian et al., 2016). In 2010, 16.9 million people worldwide experienced a stroke, and 33 million stroke survivors around the world lived with disability (Feigin et al., 2014). In the United States alone, every year, there are about 795,000 people suffering from strokes. Currently, more than 6.6 million stroke survivors in the United States require treatment to reduce their disability (Mozaffarian et al., 2016; Skolarus et al., 2016). The total of annual direct and indirect cost of strokes was $33 billion in the year from 2011 to 2012 (Mozaffarian et al., 2016), and the average of annual cost for caregiving to all elderly stroke survivors has been estimated to be about $40 billion annually (Skolarus et al., 2016). Given the rapid growth of the elderly population, the total annual costs of stroke are expected to increase to $240.67 billion by 2030 (Ovbiagele et al., 2013).
Over the past few decades, major advances have been made in understanding of the pathophysiology of stroke, while there has not been much progress in the development of stroke treatment, especially for stroke recovery. Extensive efforts have been devoted to developing neuroprotective therapies to rescue dying neurons within the limited hours post-stroke, and this approach has been shown effective in animal models; unfortunately, the neuroprotective agents have all failed in clinical trials (Moskowitz et al., 2010).
Currently available treatment for acute stroke patients are restricted to thrombolytic/thrombectomy therapies to recanalize the occluded blood vessels for ischemic stroke and the procedures to control bleeding and remove the hematoma for hemorrhagic stroke. Tissue plasminogen activator (tPA) for thrombolysis is the only drug that has been approved by US Food and Drug Administration (FDA) for treatment of acute ischemic stroke within 3 h (up to 4.5 h in certain eligible patients) (Group, 1995; Hacke et al., 2008). Because of the narrow time window for the tPA therapy and the risk of intracerebral hemorrhage (Emberson et al., 2014; Group, 1995), in fact, only 1–3% of stroke patients are able to receive this treatment (Warlow et al., 2003). Thrombectomy, another US-FDA approved procedure for acute ischemic stroke, provides mechanical clot retrieval within 6–24 h after stroke symptom onset (Castano et al., 2010; Nogueira et al., 2017; Penumbra Pivotal Stroke Trial, 2009; Saver et al., 2015). Although the thrombectomy extends additional hours of the therapeutic window, the eligible ischemic stroke patients to receive this treatment are limited because of the risk of intracranial hemorrhage for this therapy (Emberson et al., 2014; Okazaki et al., 2017; Penumbra Pivotal Stroke Trial, 2009). For acute hemorrhagic stroke, there is no effective targeted therapy yet (Hanley et al., 2013). More than 50% of hemorrhagic stroke patients die in the early stage (Bamford et al., 1990; Fogelholm et al., 1992). The effectiveness of the management for controlling bleeding and blood pressure remains questionable (Lin et al., 2012; Qureshi et al., 2014). Surgical approaches for hematoma evacuation either through open craniotomy or endoscopy in the acute phase may reduce mortality (Dastur and Yu, 2017; Siddique and Mendelow, 2000). However, clinical benefits of hematoma evacuation remain controversial and need further randomized controlled clinical trials to demonstrate their efficiency (Dastur and Yu, 2017; Mendelow et al., 2005; Vespa et al., 2005).
It is worth noting that a substantial number of ischemic stroke patients who received treatment of tPA and/or thrombectomy in the acute phase do not ever fully recover. Only 5–10% of tPA-treated stroke patients showed disability-free recovery 3–6 months after treatment (Emberson et al., 2014). Although the combination of tPA and thrombectomy significantly increases recanalization/reperfusion and reduces the rate of functional dependence as compared to tPA alone treatment (Jovin et al., 2015; Saver et al., 2015), it is estimated that most stroke patients receiving the combination therapy may not reach the full recovery/disability-free recovery (Campbell et al., 2015; Goyal et al., 2015; Jovin et al., 2015; Saver et al., 2015). Importantly, approximately 50% of hemorrhagic stroke survivors have persistent and severe disability (Fogelholm et al., 1992), and functional benefits of hematoma removal in acute hemorrhagic stroke remain uncertain (Dastur and Yu, 2017; Mendelow et al., 2005; Vespa et al., 2005).
As described earlier, the majority of stroke patients still carry different degrees of disability when they enter into the subacute phase. Traditional rehabilitation therapy including physical therapy, occupational therapy and speech therapy appears to be the only available treatment for stroke survivors in the subacute phase. It is believed that the optimal therapeutic window for rehabilitation is restricted to the first 3 months post-stroke (Kwakkel et al., 2003). Recovery ability plateaus by 6 months after stroke (Hendricks et al., 2002; Schaechter, 2004). Only about 12% of stroke survivors may obtain complete functional recovery after physical therapy (Kwakkel et al., 2003). In the chronic phase, there has been no treatment available for stroke recovery. It has long been thought that the opportunity for obtaining recovery is largely ended by the time stroke patients enter the chronic phase (Fama and Turkeltaub, 2014; Hendricks et al., 2002). However, recent clinical and preclinical studies have challenged this old notion. Emerging evidence has shown that the advanced physical rehabilitation approach and pharmaceutical intervention can enhance functional improvement when administered in the chronic phase (Cui et al., 2015; Cui et al., 2016; Lee et al., 2015; Park and Park, 2016; Piao et al., 2012a; Zhao et al., 2007a; Zittel et al., 2007).
It has been believed that the rehabilitation therapy for improving stroke recovery should be administered sooner rather than later. However, emerging clinical studies provide important evidence to question this concept. Repetitive and intensive rehabilitation interventions in the acute phase or during the early period of the subacute phase result in worse functional outcome (Bernhardt et al., 2008; Dromerick et al., 2009). By contrast, high intensity intervention is required for functional recovery in the chronic phase (Bhogal et al., 2003). It remains poorly understood, however, as to why the same treatment turns to opposite results when administered in the different phase of stroke. It is important to note that there are about 25% of ischemic stroke patients and 50% of hemorrhagic stroke patients losing their life within first month after stroke symptom onset (Donnan et al.,(2008), demonstrating an unstable pathological condition of stroke patients in the acute phase and during the early period of subacute phase. Administering highly intensive rehabilitation intervention during this earlier unstable stage may not be a good option to enhance brain repair and stroke recovery.
2. The intrinsic capability of brain self-repair in stroke recovery
2.1. Spontaneous recovery after stroke
Despite the permanent brain tissue damage, spontaneous recovery occurs days, weeks, and months after stroke onset.
Spontaneous recovery stands for the intrinsic recovery, which is not caused by treatment. This type of recovery occurs during the first 3–6 months after stroke with the most dramatic recovery from neurological impairments in the first 30 days (Duncan et al., 2000; Jorgensen et al., 1995a). The recovery from neurological impairments or deficits is called neurological recovery, which is the result of brain recovery, brain repair or brain reorganization (Robert Teasell et al., 2014). However, spontaneous recovery after stroke is generally incomplete (Cassidy and Cramer, 2016; Yozbatiran and Cramer, 2006).
The mechanism underlying the spontaneous recovery after stroke has not been fully understood. Early recovery post-stroke is associated with resolution of edema (Hallett, 2001; Lo, 1986) and reperfusion of the ischemic penumbra (Barber et al., 1998). Later recovery is related to brain plasticity (Hallett, 2001; Nudo, 2003).
2.2. Brain plasticity in spontaneous stroke recovery
2.2.1. The current concept of brain plasticity
Brain plasticity is an intrinsic ability of the brain to reorganize its function and structure in response to stimuli and injuries from both internal and external sources. Brain plasticity is centered on neuronal plasticity, which is coupled with the changes of other types of cells in the brain such as astrocytes, microglia and blood vascular cells.
Brain plasticity can be described at different levels, ranging from molecular, cellular and systemic to behavioral aspects, and including changes in neuroanatomy, neurochemistry, and neurogenesis. Convincing evidence shows that brain plasticity exists throughout a person’s lifespan. The brain can constantly be modified by learning and experience during development, young adult period, adulthood and aging in both health and disease conditions (Anderson et al., 2011; Bavelier et al., 2010; Burke and Barnes, 2006; Cramer et al., 2011; Johansson, 2000; Nudo, 2003).
2.2.1.1. Hebbian’s concept.
Donald Hebb, a Canadian psychologist, proposed a concept that neuronal connections could be remodeled by experience in 1949. This concept was introduced by his book of The Organization of Behavior (Hebb, 1949). Hebb’s concept describes a basic mechanism for synaptic plasticity. In his book, Hebb stated that “When an axon of cell A is near enough to excite cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased.” This Hebb’s concept is also named Hebbian theory, Hebb’s rule, Hebb’s postulate, and cell assembly theory. Since Hebb proposed his concept for synaptic plasticity, numerous studies have demonstrated that neuronal function, neural networks, and brain mapping are continuously modified by learning and experience.
2.2.1.2. Evidence in support of brain plasticity in healthy brains.
Numerous studies in humans and laboratory animals have revealed that learning and experience can modify the brain. A structural magnetic resonance imaging (MRI) study shows that the posterior hippocampi, which store spatial memory, are increased in London taxi drivers. This increased hippocampal volume is correlated with the amount of time spent as a taxi driver (Maguire et al., 2000). After receiving digit tip tactile stimulation for 109 days (~1.5 h/day), the territory of the representation for this stimulated digit tip in the primary somatosensory cortex is enlarged in monkeys (Jenkins et al., 1990).
Modification of dendritic spines plays an important role in experience-induced plasticity. Dendritic spines are tiny protrusions from neuronal dendrites and form the postsynaptic part of the most excitatory synapses (Beaulieu and Colonnier, 1985; Nimchinsky et al., 2002). Growth and retraction of dendritic spines are tightly linked with formation and elimination of synapses, resulting in the rewiring of neural circuits (Bailey and Kandel, 1993; De Paola et al., 2006; Holtmaat et al., 2005; Trachtenberg et al., 2002; Zuo et al., 2005). Live imaging studies have revealed that sprouting and retraction of dendritic spines occur rapidly and dynamically (Fischer et al., 1998; Trachtenberg et al., 2002). A study of long-term in vivo imaging by 2-photon microscopy shows that sensory experience drives the changes in synaptic connectivity (Trachtenberg et al., 2002).
Emerging evidence suggests that experience does not only change brain mapping and neuronal structures, it also leads to modification of brain function. Children who learn music display an increase in fullscale intelligence quotient, whereas children learning drama show improvements in adaptive social behavior (Schellenberg, 2004). Playing action video games can improve sensory, perceptual, and spatial cognitive functions and increase visual attention (Feng, 2010; Green and Bavelier, 2003; Wu et al., 2012).
A large body of evidence indicates that astrocytes, microglia, and cerebrovascular cells/cerebral vessels are actively involved in brain plasticity. Astrocytes, microglia, and cerebrovascular cells have long been thought to be supportive cells for neurons. This old notion, however, has been challenged and renewed by numerous new discoveries over the past two decades. Astrocytes have shown to regulate neuronal plasticity (Nedergaard, 1994; Parpura et al., 1994), synapse formation, maturation, maintenance, elimination and functioning in the central nervous system (CNS) (Allen and Barres, 2005; Barker and Ullian, 2010; Chung and Barres, 2012; Ullian et al., 2004; Ullian et al., 2001). The dynamics of signaling between astrocytes and neurons play an important role in brain function (Haydon and Nedergaard, 2015; Sims et al., 2015). Recently, the contribution of microglia in brain plasticity has been recognized. Emerging evidence shows that microglia are essentially involved in synapse formation and refinement, as well as synaptic excitability and function in the physiologic state of the developing or adult brain (Baalman et al., 2015; Miyamoto et al., 2013; Tremblay et al., 2011; Wake et al., 2013). Microglia also actively contribute to experience-dependent plasticity in mouse visual cortex (Sipe et al., 2016). Furthermore, cerebral vasculature also crucially participates in brain neural network formation and brain function in both the developing brain and adult brain. It has been shown that the structural generation and functional activity between cerebral vasculature and neurons are tightly linked together (Carmeliet and Tessier-Lavigne, 2005; Eichmann and Thomas, 2013).
2.2.1.3. Adaptive and maladaptive plasticity.
There are two types of brain plasticity: adaptive and maladaptive. The principle of brain plasticity is that the brain can be modified by any influence factors from inside and outside. These factors can direct the brain to be changed in a positive way (adaptive) or a negative way (maladaptive). Brain plasticity is considered adaptive when it is associated with gaining of useful function or restoring function in both physical and pathological conditions (Bavelier et al., 2010; Cohen et al., 1997; Johansson, 2000). Adaptive plasticity contributes to neurological function recovery after stroke by therapeutic interventions (Johansson, 2000; Nudo, 2006). Brain plasticity is reviewed as maladaptive when the changes are related to negative consequences such as loss of function, reduction of synaptic circuits, inhibition of functional recovery or increase of injury (Cramer et al., 2011; Nudo, 2006; Takeuchi and Izumi, 2012; Zhao et al., 2017). As an example of maladaptive plasticity, stress, especially long-term stress, induces maladaptive changes in cortical neurons by retraction of dendrites and loss of synapses (Bloss et al., 2010; Cook and Wellman, 2004; Radley et al., 2008).
2.2.2. The role of brain plasticity in brain self-repair after stroke
As stated earlier, spontaneous recovery continually happens from days to months post-stroke. This nontreatment-induced recovery is generated through brain plasticity, which is the intrinsic ability of brain self-repair.
In 1973, Alf Brodal, a Norwegian neuroanatomist, according to his own experience in stroke recovery hypothesized that this self-recovery might be related to “intact fibers take over for the damaged ones.” (Brodal, 1973). Although great efforts have been made during the past several decades, understanding of how the stoke-damaged brain is repaired by itself still remains incomplete. Using noninvasive brain imaging techniques, clinical studies have revealed that changes in functional activities dynamically occur in the intact brain regions of both the affected and nonaffected hemispheres including cortical and subcortical regions during stroke recovery (Cramer, 2004; Tombari et al., 2004; Wang et al., 2010b). These findings suggest that the undamaged brain tissue is involved in brain self-repair. Neuron deathinduced function loss after stroke may be taken over by the neurons in the intact brain regions. Resolution of diaschisis as well as functional and structural reorganization in the undamaged brain regions have been thought to play roles in spontaneous recovery of stroke.
2.2.2.1. Resolution of diaschisis.
Diaschisis is a Greek word meaning “shocked throughout”. Diaschisis, describing a neuropathophysiological condition, is widespread disruption of neuronal function in the intact brain regions adjacent to or remote from, but anatomically connected to, the area of a focal primary injury. The disruption of neuronal function in the intact brain tissue includes both deactivation and hyperexcitability.
Diaschisis represents a suddenly occurring event, which can last either transiently or persist for long periods of time after injury (Feeney and Baron, 1986). The concept of diaschisis was originally described by Brown-Sequard as the remote effects of focal brain damage (“actions at a distance”) in the 1870’s. He proposed that brain lesions produce excitatory and inhibitory effects, which cause disruption of function in regions distant from the site of damage (Feeney and Baron, 1986). In 1914, Von Monakow, a Russian-Swiss neuropathologist, introduced the concept for diaschisis in a new version, which described diaschisis as “abolition of excitability” and “functional standstill” (Von Monakow, 1969). In addition to Von Monakow’s concept of diaschisis, Andrews added a new view for diaschisis, which interprets diaschisis as a result of “removal of inhibition” and “functional excitation”. Based on his observation that the electrical activity in the contralesional hemisphere was increased after unilateral brain infarction (Andrews, 1991). Whether the intact remote regions undergo deactivation or hyperexcitability may depend upon the timing post-stroke as well as the size, the location and the type of the lesion (Andrews, 1991). Neural networks are complex architectures in the brain. Connectome is a comprehensive map of all neural connections in the brain (Sporns et al., 2005). Based on the emerging evidence in studies of connectome, the concept of diaschisis has recently been emphasized as connectional diaschisis, which is defined as the changes of structural and functional connectivity between brain areas distant to the lesion. The distant neurophysiological changes due to disachisis are a direct inhibitory or excitatory effect of a brain lesion (Carrera and Tononi, 2014). Overcoming a diaschisis and rebalancing the stroke-induced abnormity between excitation and inhibition in the neural network system may contribute to the spontaneous recovery after stroke. It has been proposed that the process of resolution of diaschisis after stroke is modulated through brain plasticity (Feeney and Baron, 1986; Hallett, 2001).
2.2.2.2. Inter-hemispheric shift and intra-hemispheric shift of somatosensory motor area.
A great amount of research has been devoted to understanding the involvement of inter-hemispheric shift of somatosensory motor area in stroke recovery. Using non-invasive neuroimaging, emerging evidence obtained from both clinical and basic science studies have revealed a time-dependent and functional recovery-related shifting of the somatosensory motor area between the injured (affected) and uninjured (unaffected) hemispheres. In the early stage after stroke (the acute phase and early period of subacute phase), extensive activity (hyperactivation) appears in the contralesional motor cortex (Brown et al., 2009; Dijkhuizen et al., 2001; Tombari et al., 2004). This inter-hemispheric shift towards the unaffected hemisphere has been thought to be the sign of distress (Cramer et al., 2011). During stroke recovery (often occurring in the post-early stage of subacute phase and the chronic phase), however, the somatosensory motor activities responding to the affected limbs are transferred and reorganized in the ipsilesional cortex near the infarct area (Brown et al., 2009; Carmichael, 2012; Dijkhuizen et al., 2001; Sharma and Cohen, 2012; Tombari et al., 2004; Wang et al., 2010a). Many clinical studies have demonstrated that good motor functional recovery is associated with the increased functional activities in the ipsilesional motor cortex, whereas the patients with poor recovery show the involvement of the contralesional motor cortex during movement of the affected hand (Fridman et al., 2004; Sharma and Cohen, 2012; Werhahn et al., 2003).
The mechanistic base for understanding of the inter-hemispheric shift during stroke recovery has not yet been elucidated. Modification in synaptic circuits or synaptic connections may be involved in functional reorganization and neural network remodeling in the brain after stroke (Brown et al., 2007; Brown et al., 2008; Cui et al., 2015; Cui et al., 2016).
Synaptic plasticity in cortical horizontal connections plays a role in cortical plasticity and cortical map reorganization (Hess and Donoghue, 1996). This cortical plasticity may also be involved in intra-hemispheric shift of somatosensory motor area in stroke recovery (Nudo, 2003). In the case of infarcts occurring in the primary motor cortex, hand function recovery is accompanied with expanding the hand representation area to the shoulder and elbow (Nudo et al., 1996) or to the face representation region (Cramer and Crafton, 2006). The activation of the premotor cortex in the affected hemisphere is linked with gait restoration in stroke patients (Miyai et al., 2002). Increased connectivity of the ipsilesional primary motor cortex-premotor cortex, and decreased connectivity of the ipsilesional primary motor cortex-parietal cortex are seen in stroke patients with greater motor recovery (Wu et al., 2015). On the other hand, widespread disinhibition in the ipsilesional primary motor cortex and premotor cortex may generate maladaptive plasticity, which leads to poor recovery in the affected hand (Takeuchi and Izumi, 2012).
2.2.2.3. Neurovascular engagement.
The increased changes in coupling of neurons and blood vessels at both levels of the structure and function may contribute to spontaneous recovery after stroke. Two-photon live brain imaging studies (Brown et al., 2007; Zhang et al., 2005) have revealed a tight coupling relationship between dendritic spines and blood vessels in the peri-infarct cortex after ischemic stroke. Focal cerebral ischemia causes a rapid loss of dendritic spines and dendritic structure (Zhang et al., 2005). During the recovery period, dendritic spines next to the infarct region are increased and stabilized 6 weeks after brain ischemia. Simultaneously, blood vessel density is increased and blood flow is recovered in the peri-infarct cortex at 6 weeks postischemia (Brown et al., 2007). The changes of neurovascular coupling also dynamically occur after focal cerebral ischemia and during recovery. Monitoring neurovascular coupling through cerebral blood volume fMRI and local field potential (LFP) after forepaw stimulation, Shih and coworkers (Shih et al., 2014) reported that both the fMRI and LFP responses in the cortex and striatum of the affected hemisphere were diminished immediately after stroke and recovered on day 28 after experimental stroke. In agreement with the experimental findings, an fMRI study in chronic stroke patients has revealed that an increase of regional cerebral blood flow is positively correlated to a significant recovery in speech and limb motor function (Mountz, 2007).
2.2.2.4. The potential role of glial cells in stroke recovery.
The involvement of astrocytes and microglial cells in neuroinflammatory responses during the early stage of stroke has been intensively studied; however, the role of glial cells in spontaneous stroke recovery remains largely unknown.
A recent study has revealed that uptake of astrocyte-released mitochondria by the neurons adjacent to the infarct area is required for neurological function recovery (Hayakawa et al., 2016). In this study, fluorescence-labeled astrocytic mitochondria were transplanted into the peri-infarct cortex 3 days after an experimental stroke. The transplanted astrocytic mitochondria were found in the local neurons 24 h after transplantation. When blocking the astrocytic release of mitochondria at day 5 post-stroke, neuronal mitochondria and axon outgrowth protein were reduced, and functional recovery was inhibited. This study offers evidence supporting the contribution of astrocytes in brain self-repair after stroke.
Microglia, the brain tissue resident macrophages, are classified into pro-inflammatory phenotype (M1 type) and anti-inflammatory phenotype (M2 type) based on their responses to local environment. The proinflammatory phenotype microglia release destructive pro-inflammatory cytokines, whereas the anti-inflammatory phenotype microglia produce molecules and trophic factors that participate in antiinflammatory and tissue repair (Anttila et al., 2016; Boche et al., 2013; Hu et al., 2015; London et al., 2013). Because of the opposite phenotypes, microglia have been shown both detrimental and beneficial effects in stroke recovery. Weeks and months after stroke, activated microglia have been found in the remote brain regions where they are anatomically connected to the neurons in the infarct area. (Dihne and Block, 2001; Justicia et al., 2008; Thiel et al., 2010). This delayed and long-lasting activation of microglia in these remote brain regions may be associated with the progression of secondary neurodegeneration after focal cerebral ischemia (Anttila et al., 2016). On the other hand, activated microglia may enhance brain repair by clearing cell debris, resolving neuroinflammation, and releasing trophic factors (Hu et al., 2015). M2 type microglia have shown beneficial effects in neurogenesis, axonal regeneration, angiogenesis and vascular repair (Hu et al., 2015). The questions as to whether and how these beneficial effects of microglia are involved in brain self-repair and functional recovery remain to be addressed.
It is worth noting that monocyte-derived macrophages (MDMs) may also play a role in brain self-repair after stroke. Similar to microglia, MDMs have been well studied as to their detrimental role in neuroinflammation after brain injury, while little knowledge is available concerning their contribution in brain repair. Recent studies have revealed that the MDMs are crucially involved in brain repair and spontaneously functional recovery after stroke. The infiltrating MDMs have significantly higher phagocytic capacity to clear cell debris as compared to microglia at 3 days post-experimental stroke, suggesting a beneficial effect of MDMs in stroke repair (Ritzel et al., 2015). Two weeks after focal cerebral ischemia, infiltrating MDMs turn to a dominant anti-inflammatory phenotype. Blocking the recruitment of MDMs into the brain during the first week of stroke abolishes long-term functional recovery, indicating the key involvement of infiltrating MDMs in stroke recovery (Wattananit et al., 2016).
2.2.2.5. The possible role of neural progenitor cells in stroke recovery.
Neural stem cells (NSCs) or neural progenitor/precursor cells (NPCs) are multipotent cells that have the capacity for selfrenewal and differentiation into neurons, astrocytes, and oligodendrocytes. Two neurogenic regions in adult brain, the subventricular zone (SVZ) and subgranular zone (SGZ), have been widely accepted (Curtis et al., 2007; Morshead et al., 1994; Palmer et al., 1995). NSCs/NPCs in the SVZ migrate along the rostral migratory stream (RMS) to the olfactory bulb where they differentiate into olfactory neurons, whereas NSCs/NPCs in the SGZ migrate locally to the granule cell layer and generate granular neurons in the dentate gyrus. There are 4 key steps for neurogenesis: proliferation, migration, differentiation, and survival/integration. In a healthy adult brain NSCs/NPCs can accomplish these 4 processes of neurogenesis (Christie and Turnley, 2012; Dibajnia and Morshead, 2013; Kaneko et al., 2011). The newborn neurons participate in recognition, learning and memory in physiological conditions (Christie and Turnley, 2012; Dibajnia and Morshead, 2013; Mak et al., 2007; Ruan et al., 2014).
Although extensive research has been done over the past decade, understanding the role of endogenous NSCs/NPCs in brain self-repair and spontaneous functional recovery after stroke still remains incomplete. The original hypothesis has been proposed that the NSC/NPC-generated new neurons may replace the stroke-damaged neurons, leading to brain self-repair and functional recovery after stroke. The vast majority of studies have been directed by this hypothesis and are searching for evidence that stroke-induced NSC/NPC proliferation, migration, differentiation, and survival/integration are linked to spontaneously functional recovery.
It has been shown that focal cerebral ischemia causes increase of NSC/NPC proliferation in both the SVZ and SGZ during the 4 days to 2 weeks after ischemia (Arvidsson et al., 2002; Jin et al., 2001; Li et al., 2010). By contrast to the ordinary migratory pathway through the RMS, the SVZ-derived NSCs/NPCs and neuroblast can directly migrate to the peri-infarct cortex (Leker et al., 2007) and peri-infarct striatum (Arvidsson et al., 2002) along the corpus callosum (Leker et al., 2007; Li et al., 2010), astrocyte processes (Yamashita et al., 2006), and blood vessels (Thored et al., 2007; Yamashita et al., 2006). In the peri-infarct regions, the SVZ-derived NSCs/NPCs and neuroblast differentiate into neurons (Arvidsson et al., 2002; He et al., 2017; Leker et al., 2007; Thored et al., 2006; Yamashita et al., 2006). The newly formed neurons generate synaptic connections with the neighboring striatal neurons (Yamashita et al., 2006) and are functionally integrated into neural networks in the adult brain after stroke (Hou et al., 2008). In contrast to the SVZ-derived NSCs/NPCs, the SGZ-derived NSCs/NPCs appear not to be involved in the brain repair process after focal cerebral ischemia (Christie and Turnley, 2012).
The major challenge of endogenous neurogenesis in stroke recovery, however, is the very low survival rate of newborn neurons. More than 80% of newly formed neurons die through apoptosis (Arvidsson et al., 2002; Thored et al., 2006). The surviving newborn neurons replace only about 0.2% of the dead neurons due to ischemic injury (Arvidsson et al., 2002). These findings challenge the neuron replacement hypothesis and imply the limitation for the ability of endogenous NSCs/NPCs in adult brain to fully repair itself after stroke. In addition to the possibility of neuron replacement, NSC/NPC-released trophic factors may contribute to neural network remodeling and functional recovery. Proliferating NSCs/NPCs produce brain-derived neurotrophic factor (BDNF) (Kamei et al., 2007; Li et al., 2010) and vascular endothelial growth factor (VEGF) (Leker et al., 2007; Li et al., 2010). Both BDNF and VEGF can protect neurons from excitotoxicity (Li et al., 2010) and apoptosis (Almeida et al., 2005; Park et al., 2014). Blocking cortical ischemiainduced NSC/NPC proliferation exaggerates neuron loss in the infarct area and hippocampus and prevents spontaneous functional recovery (Li et al., 2010). It remains unclear, however, whether the NSC/NPC produced BDNF and VEGF are involved in promoting neural network rewiring in the brain after stroke as both BDNF and VEGF support neurite outgrowth (Pereira Lopes et al., 2011; Su et al., 2013), and whether NSC/NPC-induced neural network remodeling has a causal link with neurological function recovery.
In total, convincing evidence supports that the brain has the intrinsic ability to repair itself, which is the foundation of spontaneous functional recovery after stroke. However, the capability of brain selfrepair post-stroke is limited, especially in severe stroke, as spontaneous recovery is often incomplete in most stroke patients. Clearly, the brain needs more help for reinforcing the repair process. Can we provide extrinsic interventions or treatments to enhance the intrinsic ability of brain self-repair for further strengthening stroke recovery?
In the following section, this question will be addressed and discussed through the review of up-to-date preclinical and clinical studies that provide new hope for enhancing stroke recovery.
3. Putative therapies for enhancing brain self-repair and stroke recovery
As stated earlier, spontaneous recovery is non-treatment-induced brain self-repair and functional recovery. In this section, treatment-associated brain repair and functional recovery will be introduced and discussed.
During the past several decades, numerous studies in both basic neuroscience and clinical rehabilitation have revealed that it is possible to enhance brain repair and improve functional outcome at the later recovery stage after stroke through a variety of approaches. This section selects the approaches of enriched environment, advanced physical interventions, pharmaceutical interventions, and stem cell transplantation in brain repair and stroke recovery. The intervention time initiating in the subacute phase or chronic phase in adults is highlighted for discussion in this section.
3.1. Enriched environment
3.1.1. The concept of enriched environment
The original concept of enriched environment has been developed in the basic neuroscience setting. An enriched environment is an experimental housing condition, which is considered a relatively “enriched” condition as compared with a standard laboratory housing condition.
The concept of an enriched environment was originally introduced by Donald Hebb in the late 1940s. In 1947, Hebb reported the first experiment on comparing the problem-solving ability of small groups of rats reared in different conditions. He reared laboratory rats at home as his children’s pets, and these rats also experienced the run of his house. He found that the problem-solving ability of home-raised rats was far advanced when compared to the rats reared in ordinary laboratory cages (Hebb, 1947). This observation indicates that experience modifies the brain, which is the central principle of Hebb’s theory stated in his book (Hebb, 1949). To mimic a home environment in laboratory, Hymovitch (Hymovitch, 1952) created a complex environment (free environment), which was a large box containing a large group of rats with a number of “play-things”. Using this complex environment, Hebb’s experiment was repeated and elaborated with much larger samples of rats and with more adequate controls by Hymovitch (Hymovitch, 1952). Hymovitch’s findings confirmed the Hebb’s observation that rats reared in a complex environment showed an enhancement in problemsolving ability. In the early 1960s, Rosenzweig and coworkers (Rosenzweig et al., 1961; Rosenzweig et al., 1962a) further elaborated the Hymovitch’s complex environment (free environment) and named it as “environmental complexity and training” (Krech et al., 1960), “enriched condition” (Rosenzweig and Bennett, 1996) and “enriched environment” (Rosenzweig et al., 1962b). Since then, the enriched environment, as a testable scientific concept, was widely accepted and used in neuroscience research.
The enriched environment is a complex combination in which spatial, visual, social, physical, and learning activities are tightly linked and interact together as an entire unit. The effort has been made, however, to distinguish which of the components in the enriched environment plays a key role in enriched environment-induced brain plasticity. It appears that social interaction may be relatively important in enriched environment-induced brain plasticity (Forgays and Forgays, 1952; Krech et al., 1960) and functional recovery after experimental stroke (Johansson and Ohlsson, 1996). However, these social interaction-induced changes are inferior to the enriched environment-enhanced adaptive modifications in the brain (Forgays and Forgays, 1952; Krech et al., 1960) and functional improvement post-stroke (Johansson and Ohlsson, 1996).
Over the past six decades, a large body of evidence has demonstrated that the enriched environment can modify the brain in a widespread manner including changes in genes and proteins, neurogenesis, neural structures, neurochemistry, neural function and neurobehavior in both physiological and pathological conditions, which will be discussed in the following sections.
3.1.2. The role of enriched environment in adaptive plasticity in the healthy brain
Numerous studies have revealed that enriched environments induce changes in the expression of genes and proteins, which are related to brain plasticity. A gene expression profiling study showed that the genes linked to neuronal structure, synaptic plasticity and neural transmission were dramatically changed in the brains of adult mice after housing in enriched environment (Rampon et al., 2000a). Limbic system-associated membrane protein (Lsamp) is an important protein involved in neural network formation and psychiatric conditions (Gil et al., 2002; Koido et al., 2012). An enriched environment increases the expression of Lsamp transcript in the hippocampus (Heinla et al., 2015). Enriched environment housing also enhances or modulates the expression of the synaptic plasticity-related proteins/molecules including BDNF and nerve growth factor (NGF) (Ickes et al., 2000; Torasdotter et al., 1998), synaptophysin and postsynaptic density-95 (PSD-95) (Frick and Fernandez, 2003; Nithianantharajah et al., 2004), glutamate receptors (Naka et al., 2005; Tang et al., 2001), and cholinesterase (Krech et al., 1960; Rosenzweig et al., 1962a).
Accumulating evidence shows that an enriched environment enhances adaptive plasticity at both neural structural and functional levels. Placing rats in a complex environment (enriched environment) results in profound changes across the cortex including increases in cortical weight and thickness (Bennett et al., 1969; Diamond et al., 1964), dendritic branching and complexity, dendritic spine density, synapse number and size, glial numbers and complexity, and vascular arborization (Altman and Das, 1964; Connor et al., 1982; Greenough et al., 1985; Johansson and Belichenko, 2002; Kolb and Gibb, 2015; Leggio et al., 2005). The enriched-environment housing also increases the contact between astrocytes and synaptic elements (Diamond et al., 1966; Jones and Greenough, 1996; Sirevaag and Greenough, 1991) and enhances synaptic strength and plasticity (Artola et al., 2006; Duffy et al., 2001; Foster and Dumas, 2001; Green and Greenough, 1986). Furthermore, housing animals in an enriched environment leads to increased neurogenesis and the integration of newly formed neurons into functional circuits in the hippocampus (Bruel-Jungerman et al., 2005; Kempermann et al., 2002; Kempermann et al., 1997, 1998; Monteiro et al., 2014). The enriched environment-induced neurogenesis is required for acquisition and flexibility in spatial learning and memory (Garthe et al., 2016).
Many studies have revealed that the enriched environment housing condition promotes adaptive behavior and brain function in intact animals. An environmental enrichment enhances problem-solving ability (Forgays and Forgays, 1952; Hebb, 1947; Hymovitch, 1952), increases learning and memory (Garthe et al., 2016; Lee et al., 2003; Rampon et al., 2000b; Schrijver et al., 2002; Tang et al., 2001), reduces memory decline of aged animals (Bennett et al., 2006; Harburger et al., 2007), increases exploratory activity and cognitive function, and decreases anxiety-like behavior (Benaroya-Milshtein et al., 2004; Doulames et al., 2014; Tanas et al., 2015). The enriched environment-promoted behavioral resiliency from stress is dependent on the neural networks in the prefrontal cortex (Lehmann and Herkenham, 2011).
Collaboratively, emerging evidence supports the adaptive effects of enriched environment in enhancing brain plasticity and brain function in the healthy brain.
3.1.3. The contribution of enriched environment in enhancing stroke recovery
The evidence that has been accumulated over the past two decades strongly suggests that enriched environment facilitates brain repair and improves stroke recovery.
Using a cortical infarct stroke model in spontaneously hypertensive rats (SHRs), Barbro Johansson’s group at Lund University in Sweden initially introduced the concept of enriched environment into the field of stroke recovery. Johansson and coworkers have conducted a series of experiments to determine the contribution of enriched environment in stroke recovery. In 1995, their initial work revealed that transferring rats to enriched environment 24 h after experimental stroke improves somatosensory motor function recovery without changing infarction size, suggesting that the enhancement of brain plasticity in the intact brain regions may contribute to the enriched environment-improved functional recovery after brain ischemia (Ohlsson and Johansson, 1995). Using a similar experimental setting, several studies have demonstrated that significant changes related to adaptive plasticity appear in the intact brain regions of the stroke rats in enriched environment. These changes include the manipulation of gene and protein expressions of growth factors in the ipsilesional and contralesional cortex and hippocampus (Dahlqvist et al., 1999; Zhao et al., 2000; Zhao et al., 2001), increases of dendritic branches and dendritic spines in the contralesional cortex (Johansson, 2004; Johansson and Belichenko, 2002) (Fig. 1), increases of gliogenesis in the ipsilesional and contralesional cortex (Komitova et al., 2006), and increases of NSC/NPC pool and neurogenesis in the SVZ (Komitova et al., 2005a; Komitova et al., 2005b).
Fig. 1.
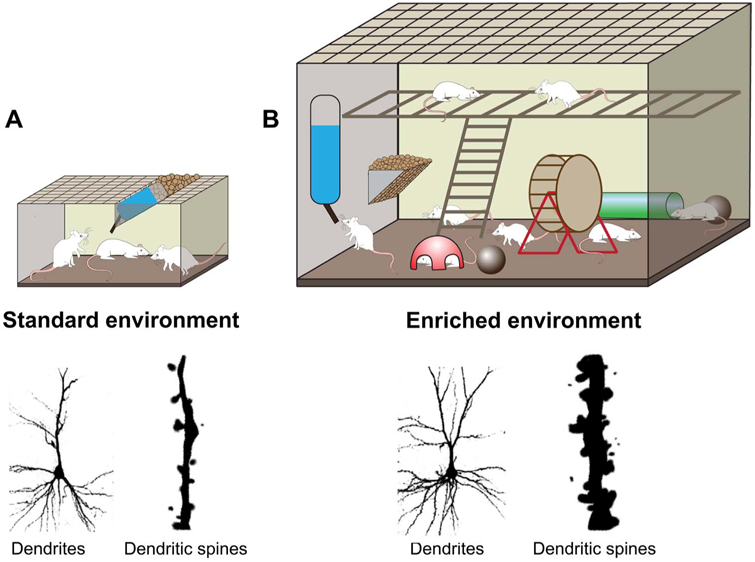
Schematic diagram of an enriched environment and its effects on neural plasticity. (A) Standard environment. This environment represents a standard housing condition that 3 rats are housed in a standard laboratory cage. (B) Enriched environment. A large group of rats are housed in a large cage furnished with a variety of play objects. Enriched environment housing after experimental stroke leads to increases in dendritic branching and dendritic spine generation (Johansson and Belichenko, 2002).
In addition to the early intervention, Johansson’s group has also demonstrated that placing rats in an enriched environment beginning at 2, 7 or 15 days after experimental stroke (the subacute phase) leads to an improvement of functional outcomes (Johansson, 1996; Komitova et al., 2005a; Risedal et al., 2002; Risedal et al., 1999; Ruscher et al.,2011). The precise mechanisms underlying this delayed intervention of enriched environment in improving stroke recovery have not yet been elucidated, although several studies have made efforts in understanding the mechanisms. Nerve growth factor-induced gene A and B (NGFI-A, NGFI-B) are the inducible transcription factors that play roles in neuronal plasticity (Dahlqvist et al., 2003; Dragunow, 1996). Housing rats in an enriched environment 2 days after cortical infarct results in upregulation of NGFI-A and NGFI-B gene expression in the ipsilesional and contralesional cortex and hippocampal CA1, and this upregulation is positively correlated with functional improvement (Dahlqvist et al., 2003). An increased sigma-1 receptor expression in the peri-infarct cortex is found in the brains of rats that have been moved to an enriched environment 2 days after cortical infarction (Ruscher et al., 2011). The sigma-1 receptor activation plays a crucial role in regulation of neural network remodeling and in stroke recovery (Ruscher et al., 2011). Housing rats in an enriched environment 7 days after cortical infarction leads to promoting astrocyte regeneration in the SGZ (Komitova et al., 2002) and increases in newly formed astrocytes and polydendrocytes in the ipsilesional cortex (Komitova et al., 2006). This enriched environment-induced gliogenesis has been proposed to be beneficial for brain repair and post-stroke plasticity (Komitova et al., 2006). Together, these studies suggest that activation of adaptive plasticity in the remaining brain tissue is involved in the repairing process by the delayed enriched environment intervention.
Using a rat model of stroke in other types of rats such as Wistar and Sprague-Dawley (SD) rats, enriched environment intervention beginning 2 days after experimental stroke has been shown to improve sensorimotor function, decrease brain ischemia-induced inflammation by reduction of apolipoprotein E and interleukin-1 beta (IL-1β), and reduce perineuronal net (PNN) in parvalbumin/GABA inhibitory interneurons (Madinier et al., 2014; Ruscher et al., 2009). The enriched environment-enhanced functional recovery is correlated with a robust reduction of PNN surrounding parvalbumin/GABAergic neurons in the ipsilesional cortex (Madinier et al., 2014). Inhibiting inflammation and decreasing PNN in the parvalbumin/GABAergic neurons after ischemic infarction may provide a supportive microenvironment for the repairing process promoted by enriched environments.
It is worth noting that intensive physical intervention beginning at the early stage of experimental stroke (e.g. 24 h or 2d post-stroke) appears to negatively influence in functional recovery. It has been shown that enriched environment intervention initiating at 24 h or 7d postexperimental stroke in SHRs improves functional recovery equally (Komitova et al., 2005a). However, when adding intensive physical training (1 h per day, 5 days per week) to enriched environment starting either 24 h (EE + training24 h) or 7d (EE + training7 h) after cortical infarction, the results turn out to be quite different. The infarction size of EE + training24 h group is enlarged as compared to the EE + training7 h group and the control stroke rats housed in the standard laboratory cages. The EE + training24 h group also shows a greater thalamic atrophy and worse functional outcome than the EE + training7 h groups (Risedal et al., 1999). Moreover, voluntary running beginning at 24 h or 2d post-experimental stroke in SHRs increases mortality, exaggerates chronic stress, reduces NSC/NPC pool in the SVZ, and prevents functional recovery (Dahlqvist et al., 2003; Johansson and Ohlsson, 1996; Komitova et al., 2005b; Risedal et al., 2002). These findings raise a red flag concerning intensive physical intervention at the early stage of stroke as it may promote maladaptive plasticity and negatively influence brain repair and functional recovery after stroke.
A notable point is that enriched environment-induced brain repair and stroke recovery is not dependent on infarction size. Stroke studies have revealed that the larger the infarction size is, the worse the neurological deficits are manifested (Duan et al., 2009; Pan et al., 2006). A systematic review shows that enriched environment housing poststroke improves sensorimotor function but it causes about 8% increase in infarction volume (Janssen et al., 2010). As mentioned earlier, the infarction size in the EE + training24 h group is larger than the control stroke rats housed in the standard laboratory cages. In spite of an increased infarction size, the EE + training 24 h group still shows better functional outcome than the stroke rats housed in the standard laboratory cages (Risedal et al., 1999). This observation once again supports that functional benefits induced by enriched environments are independent of the infarction size. Thus, the key point of enriched environment-induced stroke recovery is to enhance adaptive plasticity in the remaining brain tissue.
Translating the concept of enriched environment into clinical settings for enhancing stroke recovery has just begun. An Australia group published the first clinical protocol for enriched environment in 2012 (Janssen et al., 2012). A pilot non-randomized controlled clinical trial shows that the enriched environment intervention is effective in increasing physical, cognitive and social activities for stroke patients (Janssen et al., 2014). It is expected that the efficacy of enriched environment in enhancing stroke recovery will be validated by multiplecentral, randomized, and controlled clinical trials with large number of stroke patients and by including other evaluation approaches such as neuroimaging.
3.2. Modern physical interventions
The modern physical interventions represent the relatively new physical interventions that are different from the traditional physical therapy, which has a limited therapeutic window up to 6 months after stroke.
It is worth mentioning that the recovery induced by rehabilitative interventions in the chronic phase (> 6 months) is considered “treatment-induced recovery” as it precludes the confounding effects from the spontaneous recovery, which occurs up to the first 6 months after stroke onset.
A large body of clinical evidence supports the efficacy of modern physical interventions in enhancing brain repair and function recovery in the chronic phase of stroke. These clinical trials provide insightful understanding of how the stroke-damaged brain is repaired at this late stage.
3.2.1. New insights: brain plasticity and brain repairable window
It has long been believed that stroke patients plateau/discontinue in their recovery within the first 3 or 6 months after stroke onset (Aziz, 2010; Jorgensen et al., 1995b). However, emerging evidence from clinical studies using modern physical interventions challenges this old notion and demonstrates that administration of the interventions in the chronic phase of stroke, ranging from 6 months to 26 years after stroke, can enhance brain repair and functional recovery (Altschuler et al., 1999; Broeren et al., 2008; Hummel et al., 2005; Lee et al., 2015; Page et al., 2013; Takeuchi et al., 2005). The lifelong feature of brain plasticity provides the fundamental base for the modern physical intervention-enhanced stroke recovery in the chronic stroke patients. Substantial clinical evidence, which will be shown in this section, suggests that normalizing the stroke-disrupted brain networks is involved in stroke recovery in the chronic phase by the modern physical interventions.
3.2.2. Normalization of brain activation in the affected and unaffected hemispheres
A stroke is not a simple event of losing part of brain tissue; in fact, it causes progressive disruptions in the brain networks at both the structural and functional levels. A stroke induces an interrupted and unbalanced neural network functioning in both ipsilesional and contralesional hemispheres, resulting in increased excitability in the contralesional hemisphere and reduced activity in the ipsilesional hemisphere (diaschisis). This unbalanced brain network function causes high interhemispheric inhibition that drives from the motor cortex of the intact hemisphere to the injured hemisphere during a voluntary movement of the affected hand of stroke patients. This abnormal interhemispheric inhibition has been proposed to adversely influence motor functional recovery in stroke patients (Murase et al., 2004). To examine this hypothesis, many clinical studies have been carried out in chronic stroke patients using non-invasive stimulation approaches, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), to reduce the activation of the motor cortex in the contralesional hemisphere and to increase the activation of the motor cortex in the ipsilesional hemisphere. After TMS or tDCS treatment, the motor function of the affected hand is significantly improved in chronic stroke patients, and the improvement is correlated with normalized brain activations and corticospinal excitability between the two hemispheres (Fregni et al., 2006; Hummel et al., 2005; Lefebvre et al., 2015; Lefebvre et al., 2012; Takeuchi et al., 2005).
In addition to the direct stimulation approaches, similar findings are observed in a virtual reality therapy. This therapy uses a virtual environment that imitates a real-world environment, which is generated though computer software to give patients a simulated experience. The treated-chronic stroke patients show improved motor function, navigation function, and unilateral neglect (Ansuini et al., 2006; Claessen et al., 2016; Glover and Castiello, 2006; Takahashi et al., 2008) with normalized brain activations in the two hemispheres (Takahashi et al., 2008).
3.2.3. Enhancing the mirror neuron system for chronic stroke recovery
Mirror neurons are the special neurons in the cerebral cortex that are activated not only when one performs an action, but these neurons are also activated when one simply watches another performing the same action (Keysers and Gazzola, 2010; Rizzolatti and Craighero, 2004). Taking this unique advantage of mirror neurons, many rehabilitative interventions such as mirror therapy and action observation therapy are created for watching movement of unaffected arm in the mirror (mirror therapy) or watching a series of videos that display everyday activities (action observation therapy) to let patients re-learn and re-gain the lost function through practice. Clinical studies reveal the effectiveness of these interventions on motor function improvements in chronic stroke patients (Altschuler et al., 1999; Ertelt et al., 2007; Kim and Lee, 2013; Park et al., 2015b). The improved motor function is correlated to reactivations of brain networks in the mirror neuron system (Ertelt et al., 2007; Garrison et al., 2010).
Collaboratively, the clinic evidence suggests that the modern physical intervention-improved functional recovery in the chronic phase of stroke is mediated through the training/experience-induced adaptive brain plasticity to reorganize the stroke-disrupted brain networks.
3.3. Pharmaceutical interventions
A great number of drugs have been examined for their effectiveness in enhancing brain repair and stroke recovery (Ortega and Jolkkonen, 2013; Tran et al., 2016; Walker-Batson et al., 2016). This section highlights the studies of antidepressants and growth factors on stroke recovery in the subacute or chronic phases.
3.3.1. Antidepressants in enhancing stroke recovery
Post-stroke depression is a common complication, which leads to greater disability and negatively affects stroke recovery (Gainotti et al., 2001; Robinson and Jorge, 2016). Increasing clinical evidence shows that antidepressants not only improve mood disturbance but they also enhance motor function recovery after stroke. Selective serotonin reuptake inhibitors (SSRI) and noradrenaline reuptake inhibitors (NARI) have been evaluated as the best drugs among the antidepressants in enhancing stroke recovery (Hatem et al., 2016). Administration of fluoxetine (SSRI drug) and reboxetine (NARI drug) to stroke patients in the subacute or chronic phases improves post-stroke depression and enhances motor function recovery (Gainotti et al., 2001; Gonzalez-Torrecillas et al., 1995; Wang et al., 2011; Zittel et al., 2007). The enhanced motor performance was closely associated with normalized motor network connections and activities (Wang et al., 2011). Interestingly, fluoxetine also improves motor function in the subacute or chronic stroke patients without depression (Chollet et al., 2011; Dam et al., 1996), suggesting neurorestorative potential of the antidepressants in stroke recovery.
The mechanistic interpretation of these antidepressants in improving stroke recovery has not yet been made available. SSRI and NARI increase the extracellular levels of neurotransmitter serotonin and noradrenaline. Both serotonin and noradrenaline have been demonstrated to play crucial roles in enhancement of synaptic plasticity, neurogenesis, synaptogenesis, and dendritic remodeling (Kuo et al., 2016; Sodhi and Sanders-Bush, 2004; Tully and Bolshakov, 2010). Fluoxetine increases neurogenesis (Santarelli et al., 2003), synaptic plasticity (Karpova et al., 2011; Wang et al., 2008), and neural structural remodeling in the adult brain (Chen et al., 2011). How fluoxetine and reboxetine promote brain repair in stroke, however, remains to be determined.
3.3.2. Growth factors in enhancing stroke recovery
There are numerous growth factors that have been studied in stroke. The vast majority of these studies target neuroprotection in the acute phase, only a few studies that have been recognized by recent comprehensive reviews (Lanfranconi et al., 2011; Larpthaveesarp et al., 2015) demonstrate the neurorestorative role of two hematopoietic growth factors, stem cell factor (SCF) and granulocyte colony-stimulating factor (G-CSF), on stroke recovery in the subacute or chronic phase (Lanfranconi et al., 2011). This section updates current understanding of SCF and G-CSF in enhancing stroke recovery.
3.3.2.1. The role of SCF and G-CSF in neuroplasticity.
SCF and G-CSF have been shown to play essential roles in controlling hematopoietic stem cell/hematopoietic progenitor cell (HSC/HPC) proliferation, survival, and differentiation (Broudy, 1997; Lennartsson and Ronnstrand, 2012; Lieschke et al., 1994; Richards et al., 2003). SCF in combination with G-CSF (SCF + G-CSF) synergistically promotes blood cell generation and HSC/HPC mobilization (Briddell et al., 1993; Hess et al., 2002; McNiece and Briddell, 1995).
Besides the originally discovered effects of SCF and G-CSF in the hematopoietic system, a large body of evidence has revealed that SCF and G-CSF also play roles in the CNS. Receptors for SCF and G-CSF express in the NSCs/NPCs (Jin et al., 2002; Piao et al., 2012b; Schneider et al., 2005; Zhao et al., 2007c), cerebral neurons (Schneider et al., 2005; Zhao et al., 2007c), and cerebral endothelial cells (Zhao et al., 2007b) of adult mice and rats. Both SCF and G-CSF can pass through the blood-brain barrier (Schneider et al., 2005; Zhao et al., 2007b). These findings suggest a potential efficacy of SCF and G-CSF in brain plasticity.
The contribution of SCF and G-CSF in directing NSCs/NPCs to give rise to neurons has been illustrated in both in vitro and in vivo studies. G-CSF promotes differentiation of cultured NSCs/NPCs into neurons (Schneider et al., 2005). Infusing SCF into the cerebrolateral ventricle enhances neurogenesis in the SVZ (Jin et al., 2002). When adding SCF and G-CSF to cultured NSCs/NPCs during the proliferating stage of NSCs/NPCs, SCF + G-CSF shows a dual function in directing cell cycle arrest and promoting neuronal fate commitment through the enhancement of neurogenin 1 activity (Piao et al., 2012b).
Numerous in vitro and in vivo studies have determined the contribution of SCF and G-CSF in neuronal survival and neuronal plasticity. SCF acts as a neurotrophic factor supporting neuron survival during development (Carnahan et al., 1994; Hirata et al., 1993). SCF protects cultured neurons from apoptosis through the regulation of MEK/ERK or PI3K/AKT/NF-kB/Bcl-2 pathways (Dhandapani et al., 2005). In cultured cortical neurons, G-CSF counteracts programmed neuron death via PI3K mediation (Schneider et al., 2005). SCF enhances neurite outgrowth in embryonic dorsal root ganglia (Hirata et al., 1995; Hirata et al., 1993). Similar to their synergistic effects in the hematopoietic system, SCF + G-CSF also synergistically promotes neurite outgrowth and network formation in cultured cortical neurons through the regulation of PI3K/AKT/NF-kB/BDNF pathway (Su et al., 2013). Mice deficient in either SCF (Motro et al., 1996) or C-kit (receptor for SCF) (Katafuchi et al., 2000) show impaired long-term potentiation (LTP) and spatial learning and memory. Moreover, cognitive impairments, LTP reduction, and impairments in neural network formation in the hippocampus are found in G-CSF knockout mice (Diederich et al., 2009). Collectively, these findings show the contribution of SCF and G-CSF in brain plasticity and provide insights into the potential efficacy of SCF and G-CSF on brain repair in stroke.
3.3.2.2. Enhancing brain repair by SCF and G-CSF in the subacute phase.
Several studies have demonstrated the restorative efficacy of SCF and G-CSF in subacute stroke. G-CSF treatment initiated at 4 days post-ischemia shows better functional improvement and greater reduction in hemispheric atrophy when compared to the treatment initiated at 7 days post-ischemia (Lee et al., 2005). In contrast to G-CSF alone treatment, SCF + G-CSF displays a better restorative effect in later intervention (during 11–20 days post-ischemia) than it is given earlier (during 1–10 days post-ischemia). The later intervention results in better improvement in functional recovery, greater increases in bone marrow-derived neurons in the ipsi-infarct hemisphere, and greater enhancement in NPC proliferation in the SVZ as compared to earlier treatment (Kawada et al., 2006). In addition, the later intervention of SCF + G-CSF upregulates IL-10, an anti-inflammatory cytokine, on a much greater scale than the earlier treatment (Morita et al., 2007). The mechanism underlying this timing sensitivity for G-CSF or SCF + G-CSF treatment, however, remains unclear.
3.3.2.3. Enhancing brain repair by SCF and G-CSF in the chronic phase.
Over the past decade, the efficacy and possible mechanisms of SCF and G-CSF on stroke recovery in the chronic phase of stroke have been demonstrated in rodent models of cerebral cortical ischemia.
SCF and G-CSF intervention in chronic stroke for enhancing functional improvement has been tested and validated to be safe and effective when administered 3 to 6 months after cerebral cortical ischemia in both young and aged spontaneously hypertensive rats (SHRs), C57BL mice, or transgenic mice with C57BL genetic background (Cui et al., 2015; Cui et al., 2013; Cui et al., 2016; Liu et al., 2016; Piao et al., 2012a; Piao et al., 2009; Zhao et al., 2007a). SCF + G-CSF combination shows a stable and long-term effect in the enhancement of brain repair and motor function recovery when compared with SCF and G-CSF alone (Zhao et al., 2007a). These studies reveal the restorative possibility of SCF + G-CSF in the phase of chronic stroke.
Several mechanistic studies aimed to identify how SCF + G-CSF repairs a stroke-damaged brain in the chronic phase have been conducted. Using the approaches of live brain imaging, whole brain imaging, axon tracking, bone marrow-derived cell tracking, immunohistochemistry and confocal imaging, considerable evidence suggests that SCF + G-CSF-enhanced brain repair in the chronic phase of experimental stroke specifically occurs in the cortex adjacent to the infarct cavity. The SCF + G-CSF-induced restorative changes in the peri-infarct-cavity cortex include increases in dendritic spine size, PSD-95 accumulation (PSD-95: a key postsynaptic protein involved in synaptic function), presynaptic vesicle protein, dendritic branching, axonal sprouting, and blood vessel density. The SCF + G-CSF-enhanced neurovascular network remodeling has been conformed in young and aged animals, and when treatment is initiated during 3 to 6 months after experimental stroke (Cui et al., 2015; Cui et al., 2013; Cui et al., 2016; Piao et al., 2012a; Piao et al., 2009; Zhao et al., 2007a; Zhao et al., 2013). It has been shown that bone marrow-derived endothelial cells and bone marrow-derived neurons are involved in SCF + G-CSF-enhanced angiogenesis and neurogenesis in the brain of chronic stroke (Piao et al., 2009). Using transgenic mice, Thy-1-YFPH, in which yellow fluorescent protein (YFP) is exclusively expressed in the layer-V pyramidal neurons (Feng et al., 2000), the dynamics of dendritic spine formation in the peri-infarct-cavity cortex have been examined with 2-photon live brain imaging both before and after SCF + G-CSF treatment. Before treatment, the number of mushroom type (M-type) spines in the layer V pyramidal neurons is reduced and the uncertain type (U-type) spines, which cannot build synapses with other neurons, are increased in the stroke mice. This observation suggests reduced synaptic circuits in the peri-infarct-cavity cortex in chronic stroke brain. Six weeks after SCF + G-CSF treatment, however, the M-type spines are significantly increased, and the U-type spines are significantly reduced in the layer V pyramidal neurons adjacent to the infarct cavity (Fig. 2), suggesting an SCF + G-CSF-enhanced regeneration of synaptic circuits (Cui et al., 2013) (Fig. 3). A study using an anterograde axonal tracer reveals that SCF + G-CSF treatment in the chronic phase promotes axonal sprouting of the contralesional cortical neurons projecting into the peri-infarct-cavity cortex (Cui et al., 2015). Interestingly, SCF + G-CSF treatment also shows an “over-increase” of dendritic spine head size and PSD-95 accumulation in the peri-infarct-cavity cortex as compared to intact or unaffected brains, suggesting that SCF + G-CSF-enhanced robust synaptic activity occurs in the surviving neurons next to the infarct cavity for taking over the function of the lost neurons (Cui et al., 2015; Cui et al., 2016).
Fig. 2.
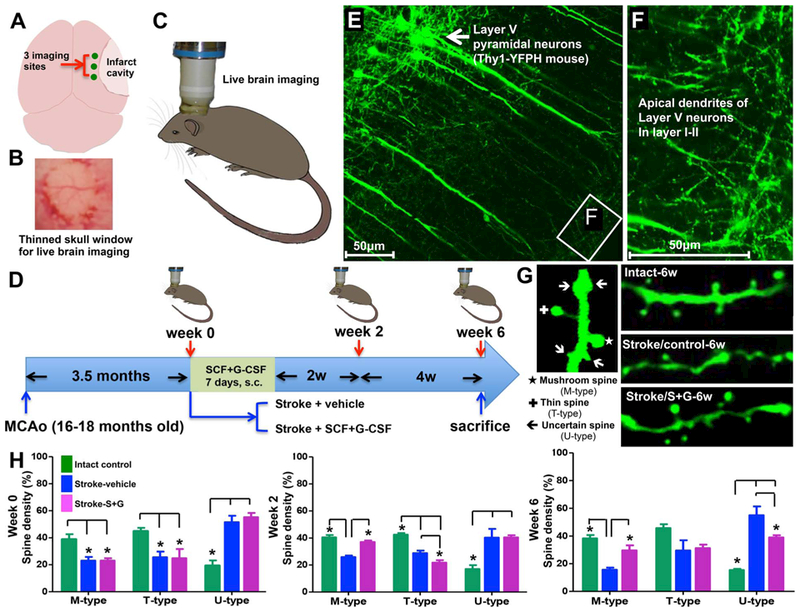
SCF + G-CSF treatment in the chronic phase of experimental stroke increases dendritic spine size in the cortex adjacent to the infarct cavity. Aged male Thy-1-YFPH mice (16–18 months old) are subjected to cortical infarct in the right hemisphere. In the Thy-1-YFPH mice, yellow fluorescent protein (YFP) is selectively expressed in the somas, axons, dendrites and dendritic spines of the layer V pyramidal neurons. Through a thinned skull window prepared in the right side of the head, the dynamics of dendritic spines in the layer V pyramidal neurons adjacent to the infarct cavity are captured with a 2-photon microscope before treatment as well as 2 and 6 weeks after treatment on live animals. (A) Three imaging sites above the cortex next to the infarct cavity. (B) The thinned skull window prepared for live brain imaging. (C) Live brain imaging by 2-photon microscopy on the right side of the head. (D) Schematic diagram showing the flowchart for the experiment. (E and F) YFP-expressing layer V pyramidal neurons and their apical dendrites in layer I–II where are scanned by a 2-photon microscope. (G) The different types of dendritic spines and the representative images of dendritic spines in the brains of intact, stroke-vehicle control, or stroke-SCF + G-CSF-treated mice. (H) Live brain imaging data. Note that the mushroom spines (M-type) are decreased and the uncertain spines (U-type) are increased in the stroke mice before treatment (week 0). SCF + G-CSF (S + G) treatment results in increases in the mushroom spines and reductions in the uncertain spines during the period of 2 to 6 weeks after treatment. *p < 0.05. (Cui et al., 2013).
Fig. 3.
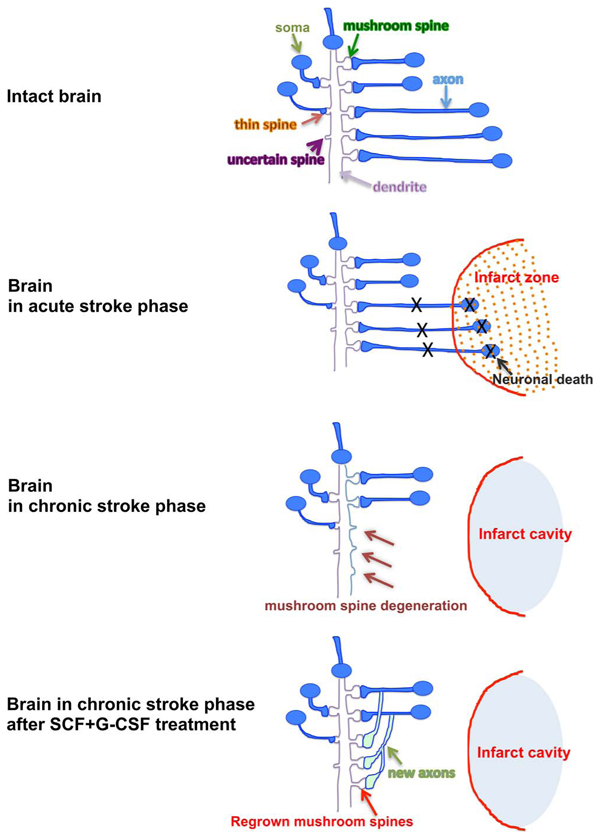
Schematic diagram of synaptic networks in different conditions. In the condition of acute stroke, the mushroom type spines undergo retraction/degeneration in the surviving neurons outside the infarct core because the neurons that have synaptic connections with the surviving neurons die off in the infarct area. As a result, the number of the mushroom type spines is reduced and the number of the spines that are unable to form synapses (U-type) is increased in the peri-infarct-cavity cortex in the chronic phase of stroke. SCF + G-CSF treatment in the chronic phase of stroke enhances axon sprouting and mushroom spine formation, suggesting that the neural network rewiring in the peri-infarct-cavity cortex is enhanced by the treatment.
Is there a causal relationship between the SCF + G-CSF-enhanced neural network remodeling in the peri-infarct-cavity cortex and the SCF + G-CSF-improved functional recovery in the chronic stroke phase? Additional studies have been conducted to address this question. Learning from an in vitro study that SCF + G-CSF synergistically enhances neurite outgrowth and neural network formation through NF-kB mediation (Su et al., 2013), in vivo studies (Cui et al., 2015; Cui et al., 2016) have been carried out by infusing an NF-kB inhibitor into the cerebral lateral ventricles to block SCF + G-CSF-enhanced neural network rewiring. The data have revealed that NF-kB inhibitor abolishes the SCF + G-CSF-enhanced neural network rewiring in the peri-infarct-cavity cortex. Upon this abolishment, the SCF + G-CSF-improved motor function is eliminated. These findings indicate that neural network rebuilding in the peri-infarct-cavity cortex is required for SCF + G-CSF-enhanced functional improvement in the chronic phase of experimental stroke.
3.4. Stem cell transplantation
3.4.1. Early perspectives on cell therapy for stroke
The earliest studies of cell therapies for the treatment of stroke began in the late 1980’s with the vision of rebuilding the damaged neural circuitry and thereby restoring function (Farber et al., 1988; Mampalam et al., 1988; Tonder et al., 1989). In these original studies, the cells of choice were fetal neurons. The animal models used were the middle cerebral artery occlusion (MCAO) model which produced lesions in striatum and overlying cortex and the 4-vessel occlusion (4-VO) model which produced hippocampal lesions. These studies demonstrated that grafting of fetal neurons into a stroke-lesioned brain was possible; these grafted cells survived, expressed neurotransmitters appropriate to the graft location, made synaptic contact with host brain and induced functional recovery. These studies shaped how researchers approached the cell therapy field for the subsequent decade as we tried to rebuild the neural circuitry to restore function.
With the isolation of mammalian NSCs from embryonic (Reynolds and Weiss, 1996) and adult mouse (Reynolds and Weiss, 1992) as well as embryonic (Flax et al., 1998) and adult human brain (Kirschenbaum et al., 1994), the field entered a new era, as scientists explored how to expand these cells and induce differentiation of specific cell types for treatment of specific diseases. The guiding principle for these early studies was still restoration of the damaged neural circuitry. This focus on replacing lost neurons is still present in studies using NSCs and more recently NSCs derived from induced pluripotent stem cells (iPSCs).
As this fledgling stem cell field expanded and researchers explored the pluripotency of stem cells from non-neural stem cell niches throughout the body, it became clear that reconstruction, if it occurred, played only a small role in the restoration of function that was observed with most cell therapies. In the ensuing years, investigators began to focus on other potential therapeutic benefits that could explain the improved stroke outcome.
3.4.2. Therapeutic benefits of cell therapies
During the early post-stroke injury phase, excitotoxicty, energy depletion and cellular necrosis predominant. However, by the time most patients reach the emergency department, hours have passed and the transition into the secondary degenerative injury phase is occurring. Many studies testing cell therapies in the last 10 years have focused on delivery during the post-stroke period when secondary degenerative processes predominate. In this section, we will focus on studies where cells have been administered a minimum of 24 h after the stroke and their effects within the infarcted hemisphere (Table 1 and Fig. 4).
Table 1.
Reparative Effects of Stem Cells.
| Cells | Mechanisms of Repair | ||||||
|---|---|---|---|---|---|---|---|
| Neurogenesis | Angiogenesis | Neuroprotection | Neuronal Connectivity | Inflammatory/Immune Modulatory | Anti-oxidant | Reconstruction | |
| HSCs | + | + | − | + | − | − | − |
| MSCs | + | + | + | − | + | + | − |
| NPCs | + | + | − | − | − | − | − |
| NSCs | + | + | + | − | + | − | + |
| EPCs | + | + | − | − | − | − | − |
| HUCB MNC | + | + | + | + | + | + | − |
| iPSCs | − | − | − | − | − | − | + |
Fig. 4.
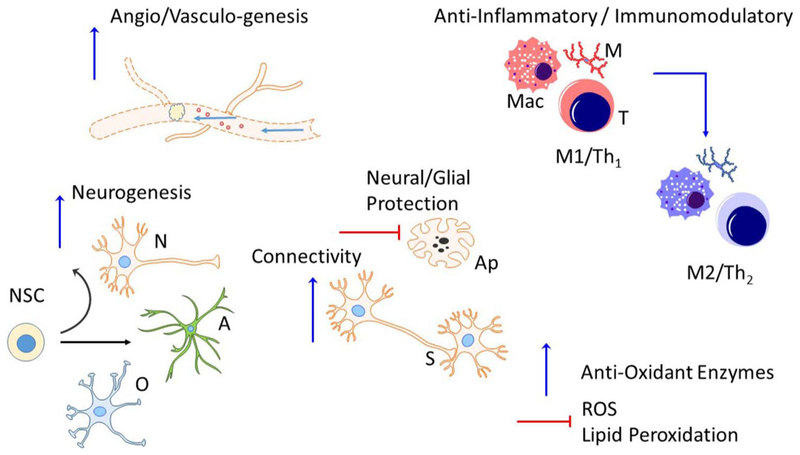
Biological effects of stem cell therapy. Cell therapies are capable of producing direct and indirect effects to elicit brain repair. This is accomplished through both the secretion of trophic factors and cytokines and release of extracellular vesicles, or exosomes, containing trophic factors, enzymes, micro RNA, etc. These factors can directly act on infarcted brain tissue to support function or can stimulate endogenous neural cells to also produce reparative factors. Some of the known biologic effects of stem cell therapy include increased angiogenesis; increased neurogenesis; decreased apoptosis and cell death; synaptic plasticity of both the axons and dendrites; shifting the peripheral immune and both central and peripheral inflammatory cells from a pro-inflammatory (M1/Th1) to an anti-inflammatory (M2/Th2) phenotype; and altering both the production of free radicals and anti-oxidant enzymes. A, astrocyte; Ap, apoptotic cell; M, microglia; Mac, macrophage; N, neuron; NSC, neural stem cell; O, oligodendrocyte; S, synapse; T, T cell.
3.4.2.1. Neurogenesis.
The first demonstration that a cell therapy could alter the proliferation of NSCs in the SVZ was a study in which human umbilical cord blood (HUCB)-derived CD34 + cells (also referred to as hematopoietic stem cells (HSCs)) were administered 48 h after MCAO (Taguchi et al., 2004). These cells increased endogenous neurogenesis as determined by polysialylated neural cell adhesion molecule (PSA-NCAM) immunoreactivity in the SVZ and migration of the newborn cells toward the infarct area. It has since been shown that mesenchymal stem cells (MSCs), regardless of source (Jeong et al., 2014; Jiang et al., 2014), NPCs (Jin et al., 2011; Mine et al., 2013) and NSCs derived from human induced pluripotent stem cells (iPSCs) (Chang et al., 2013a) all induced neurogenesis in the SVZ and the SGZ.
Interestingly, increases in neurogenesis after cell therapy are reported in conjunction with angiogenesis and it is possible to block neurogenesis by administering anti-angiogenic agents (Taguchi et al., 2004). This connection between neurogenesis and angiogenesis has been demonstrated using endothelial progenitor cell (EPC) and smooth muscle progenitor cell co-transplants (Nih et al., 2012), skin-derived precursor cells (Mao et al., 2015), NSCs (Zhang et al., 2011) and bone marrow-derived MSCs (Bao et al., 2011).
3.4.2.2. Angiogenesis.
The first study to demonstrate that cells could increase angiogenesis was published by Chopp’s group in 2003 (Chen et al., 2003b). In this study, human bone marrow-derived MSCs were transplanted intravenously 24 h after MCAO. Using a BrdU incorporation assay, the investigators were able to show that endothelial cells in the peri-infarct zone proliferated after cell therapy. A three-dimensional analysis of the microvasculature was conducted and showed that there was an increase in the number of newly formed capillaries. Further, the human bone marrow-derived MSCs increased the release of endogenous rat VEGF and VEGF receptor 2 (VEGFR2) immunoreactivity. These results were replicated in an in vitro capillary tube forming assay, which was also used to demonstrate that neutralizing VEGFR2 with antibody prevented the formation of capillary-like tubes. There have been a number of studies since then that have replicated these early studies (Balseanu et al., 2014; Bao et al., 2011; Lin et al., 2013; Mitkari et al., 2014; Taguchi et al., 2004; Wu et al., 2008; Yan et al., 2015).
NSCs have also been shown to induce angiogenesis. In a recent study by Song et al. (Song et al., 2009) using ferumoxide labeled NSCs from the HB1.F3 stem cell line, there was an increase in the amount of von Willebrand Factor (vWF) which appeared to be dependent on the number of NSCs that migrated to the infarcted hemisphere. These results have been confirmed by other research groups using the same cells (Ryu et al., 2016), other NSC cell-lines (Hicks et al., 2013) and both primary embryonic NSCs (Zhang et al., 2011) and adult NSCs (Doeppner et al., 2015b). Only in this last paper, were more functional measures included. Doeppner and associates showed decreased Evan’s blue extravasation, decreased matrix metalloproteinase (MMP)-9 activity, and reversed degradation of the tight junction protein, occludin, all of which suggest that the NSCs had at least a protective effect on the vasculature (Doeppner et al., 2015b). This type of analysis is crucial for demonstrating that the putative angiogenesis is actually producing functional vessels.
When cerebral blood flow has been measured, the results have not been conclusive. One group found no increase in cerebral blood flow (Iskander et al., 2013) while another did demonstrate an increase (Lin et al., 2013). Another important observation is that while these studies consistently show capillary density and CD31 expression can increase after a cell therapy, these cells are not incorporating into the new vessels. Further, even EPCs are not repairing the vasculature by incorporating into the new vessels (Iskander et al., 2013; Moubarik et al., 2011; Nih et al., 2012).
3.4.2.3. Neuroprotection.
The weight of evidence suggests that cell therapy can enhance the development of new neurons and new blood vessels to repair the infarcted brain, but can they also protect existing cells from death? The answer to this question appears to be affirmative. MSCs from cord blood, bone marrow and adipose decrease TUNELlabeled neurons, decrease caspase 3 mRNA, increase Bcl-2 expression (Calio et al., 2014; Feng et al., 2016; Li et al., 2002; Liu et al., 2014; Wu et al., 2008; Zhu et al., 2014). NSCs have similar effects when transplanted into the cortical peri-infarct area 24 h after permanent MCAO (Chen et al., 2014; Zhang et al., 2009) or even when administered one week after MCAO (Chang et al., 2013b; Shen et al., 2010. Even NSCs derived from iPSCs can decrease apoptosis (Chang et al., 2013a; Yao et al., 2015).
3.4.2.4. Neuronal connectivity.
Another of the potential ways in which transplanted cells repair the brain after stroke is through modulation of neuronal connectivity. In a rat model of type II diabetes mellitus, administration of HUCB MNCs after stroke increased axonal outgrowth, protected white matter tracts as labeled with luxol fast blue and increased synaptophysin immunolabeling (Yan et al., 2015). Still, when HUCB CD34 + HSCs were transplanted, neurites were significantly longer than in non-treated rats and significantly more neurons had neurites (Lin et al., 2013). Genetic deletion of MMP — 2/9 in mice prevented this effect as did the knockdown of exchange protein activated by cAMP 1 in the HUCB cells.
There is some evidence that this neurite outgrowth is mediated by increased expression of sonic hedgehog (Shh) in astrocytes, where Shh induces tPA expression and decreased plasminogen activator inhibitor 1 (Ding et al., 2013; Shen et al., 2011). Other factors that are modified by cell therapy include decreased neurocan and Nogo-A expression and increased growth associated protein-43 in astrocytes in the infarcted hemisphere (Shen et al., 2008; Wang and Li, 2016). As a result, there was increased synaptic density, increased synaptophysin expression and more myelinated axons in the peri-infarct area.
3.4.2.5. Inflammatory/immune modulation.
In 2005, a study that was the first to demonstrate a significant decrease in myeloid cells in the infarcted brain after intravenous transplantation of HUCB MNCs 24 h after MCAO (Vendrame et al., 2005). This decrease was accompanied by a decrease in both gene and protein expression of pro-inflammatory cytokines as well as decreased NFκB binding. In an immunochemical study of microglia after MCAO and intravenous administration of HUCB MNCs at 48 h post-stroke, morphological changes in the microglia within 3 h of cell administration with full restoration of a ramified resting structure within 24 h of cell administration were observed (Leonardo et al., 2010). The HUCB MNCs not only modified inflammation in the brain after stroke, but they also modulated the peripheral immune response to the stroke, altering the CD4:CD8 ratio in the spleen and increasing the anti-inflammatory cytokine interleukin-10 (IL-10) (Vendrame et al., 2006). Moreover, the size of the spleen was inversely correlated with the size of the infarct.
These data suggest that a significant component of the post-stroke injury is actually the secondary degenerative response, which includes inflammation and the peripheral immune system. While the definitive study of the importance of the interaction of HUCB cells with the peripheral immune system has not been done, a more decisive study was performed with NSCs administered after an intracerebral hemorrhage (Lee et al., 2008). In this study, Lee and associates compared intracerebral with intravenous transplants of NSC either 2 or 24 h after intracerebral hemorrhage in animals with or without a spleen. The intravenous route 2 h post ICH produced the greatest functional improvements and decreases in pro-inflammatory cytokines in the brain and spleen. However, when the spleen was removed two weeks prior to stroke, the NSCs lost their anti-inflammatory effects.
Together these studies suggest that intravenous administration of stem cells induces a molecular shift in immune response to a stroke such that the proinflammatory Th1/M1 responses these cells would normally exhibit after stroke switches to an anti-inflammatory, immune suppressant Th2/M2 phenotype (Fig. 4).
3.4.2.6. Anti-oxidant activity.
When HUCB MNCs were co-cultured with PC12 cells loaded with a redox sensitive fluorescent dye under oxygenglucose deprivation (OGD) conditions (Arien-Zakay et al., 2009), oxidative stress in the culture decreased to pre-OGD levels. Further, the total redox potential (anti-oxidant activity) was attributable to the HUCB cells. It is not surprising, therefore, that HUCB MNCs increase gene and protein expression of multiple anti-oxidant enzymes in both neurons and oligodendrocytes in vitro and in vivo (Rowe et al., 2010; Rowe et al., 2012). Bone marrow-derived MSCs have similar effects to those observed with HUCB MNCs, decreasing both oxidative stress and lipid peroxidation (Calio et al., 2014).
3.4.2.7. Reconstruction.
While studies with MSCs and mononuclear cells (MNCs) no longer focus on replacement of lost neurons and restoration of neural circuitry as the reparative mechanisms underlying recovery, much of the work with iPSCs and NSCs still focuses on replacement. Unfortunately, tumorigenesis is a significant issue when using these cells. For example, when mice iPSCs were implanted 24 h after MCAO, tumors developed in both sham and MCAO mice (Kawai et al., 2010); the tumors expressing c-myc were more extensive in the infarcted hemispheres and heavily expressed c-myc. To minimize this problem, some investigators have pre-differentiated the iPSCs prior to transplant, but this has not eliminated the problem (Gomi et al., 2012; Jensen et al., 2013). More recently, a systematic analysis of differentiation patterns of iPSCs derived NPCs determined that differentiation patterns seemed to replicate embryonic central nervous system development and the final maturational state of the cells was determined by the microenvironment and the genetic instability of the cells (Sugai et al., 2016). Another similar approach is the use of retinoic acid in the differentiation protocol of the iPSCs (Yuan et al., 2013); this prevented cellular proliferation/tumorigenesis out to 3 months. Alternatively, re-engineering the iPSCs to eliminate the vector and transcription factor incorporation into the genome (Yu et al., 2009) eliminated the development of tumors out to 1 year post transplantation (Mohamad et al., 2013). When these cells were differentiated into NPCs, transplanted into the lesioned cortex of mice 7 days post MCAO, the cells differentiated into neurons and produced functional recovery.
3.4.3. Therapeutic effects after delivery at later times post-stroke
One of the guiding principles of treatment at more prolonged periods after stroke, is that many of the degenerative processes that are occurring early after the stroke, such as inflammation, have subsided so the environment in the infarcted hemisphere is more amenable to survival of grafted cells. Even though animal studies at these later times are probably more representative of how the clinical studies are designed, there is still a disconnection between the preclinical and clinical. By one month post-stroke in a rat model of stroke, functional deficits and infarct volume have stabilized. While the stroke patient is medically stable and their long-term prognosis is more evident by one month post-stroke (Kwakkel et al., 2003), recovery of function can be variable patient to patient (Gladstone et al., 2002) and recovery can continue for six months to a year. The important question then becomes whether stability in recovery and function at one month after stroke in a rat or mouse is the equivalent of a stable stroke in a clinical population six months post-stroke. If the answer is yes then the preclinical studies of the CTX0E03 NSC cell-line are appropriate. When these cells were transplanted 3–1 weeks after MCAO, they improved behavioral outcome with no change in infarct volume (Pollock et al., 2006), suggesting a restorative mechanism for the functional effects. Further studies at one month post-stroke demonstrated the nontumorigenicity of the cells (Stevanato et al., 2009), their differentiation potential in vivo (Stroemer et al., 2009), the efficacy based on regions of damage (Smith et al., 2012) as well as putative mechanism of recovery. Just as with cell therapies administered early in subacute period, neurogenesis increased on the lesion side as did vWF immunolabeling around the transplant (Hassani et al., 2012; Hicks et al., 2013; Stroemer et al., 2009).
3.4.4. What are the mechanisms underlying cell therapies’ reparative effects?
3.4.4.1. Secretion of factors.
There is an abundance of evidence that cell therapies have reparative effects other than reconstruction. Early studies focused on expression of trophic factors. For example, when HUCB cell conditioned media was analyzed by ELISA, the cells secreted intercellular adhesion molecule-1 (ICAM-1), Selectins (E and L), and IL-8, VEGF, vascular cell adhesion molecule (VCAM), stromal derived factor 1b (SDF-1b), and IL-6 (Chen et al., 2010). They also express both nerve growth factor (NGF) and its receptor TrkA (Bracci-Laudiero et al., 2003; Shichinohe et al., 2015), brain derived neurotrophic factor (BDNF) and neurotrophin 4/5 (NT4/5) (Fan et al., 2005; Shichinohe et al., 2015), pentraxin 3 and thrombospondin 1 (Park et al., 2015a).
Similarly, MSCs release BDNF, NGF, VEGF, and hepatic growth factor (Chen et al., 2002; Chung et al., 2009; Ikegame et al., 2011). Human NSCs express IL-Ια and p, transforming growth factor (TGF)-p 1 and 2, and tumor necrosis factor alpha (TNF-α) (Klassen et al., 2003). Mouse NSC cell-lines can express glial cell line-derived neurotrophic factor, BDNF (Yan et al., 2004), basic fibroblast growth factor (bFGF of FGF 2), and bone morphogenic protein 4 (Watanabe et al., 2016).
3.4.4.2. Stimulation of endogenous secretion.
Transplanted cells have been shown to stimulate endogenous cells to express or secrete factors. Bone marrow-derived MSCs induce endogenous release of erythropoietin (Lv et al., 2015), bFGF (Chen et al., 2003a), VEGF and VEGFR2, BNDF and NGF in the peri-infarct region (Chen et al., 2003b; Li et al., 2002). Similar observations have been made for adipose-derived MSCs (Liu et al., 2014). NSCs increase BDNF, TGF-β1, SDF-1α, VEGF and insulin-like growth factor — 1 in the ischemic mouse brain (Capone et al., 2007). HUCB MNCs activate the cell survival/neuroprotective Akt signaling pathway in both neurons (Shahaduzzaman et al., 2015) and oligodendrocytes (Rowe et al., 2012). This pathway is linked to increased expression of antioxidant enzymes and decreased pro-inflammatory mediators (Rowe et al., 2010; Shahaduzzaman et al., 2015).
3.4.4.3. Exosomes.
More recently, the focus has shifted from individual factors to the contents of extracellular vesicles or exosomes. Exosomes are small, flattened spheres about 30–100 nm in diameter (Raposo et al., 1996) that are thought to mediate intercellular communication. Typically, exosomes will contain cytoskeletal proteins, some heatshock proteins, signal transduction molecules, membrane fusion molecules, metabolic enzymes, tetraspanins (especially CD9, CD63, CD81, and CD82), integrins, and adhesion molecules (Thery et al., 2002). Other proteins may also be expressed, depending on the function of the cell of origin and the environment surrounding the cell.
The first study to use bone marrow-derived MSCs exosomes to treat stroke, demonstrated that systemic administration of exosomes was enough to replicate the effects of the MSCs themselves (Xin et al., 2013a), improving functional outcome, increasing the number of doublecortin positive and vWF positive cells, enhancing axonal growth and synaptic remodeling. The exosomes transferred miR-133b from the bone marrow-derived MSCs to astrocytes (Xin et al., 2012); MiR-133b has been implicated in neurite outgrowth (Xin et al., 2013b). Bone marrow-derived MSC exosomes increase the number of mature NeuN positive cells in lesioned striatum (Doeppner et al., 2015a) and prevent post-stroke immune suppression that is a common sequela of clinical stroke. Cord blood-derived MSC exosomes are pro-angiogenic (Zhang et al., 2015) while EPC exosomes contain miR-126 and miR-296 which activates the PI3K/Akt signaling pathway in endothelial cells (Deregibus et al., 2007). The NSC cell-line, CTX0E03, produces exosomes with a myriad of miRNAs, although the most abundant was miR-1246 (Stevanato et al., 2016).
3.4.5. Clinical cell therapy studies
In a search of ClinicalTrials.gov using the search terms ischemic stroke and cells, 32 Phase I or Phase II trials were identified. Of these 32, only eight have been completed. Seven of them looked at safety of bone marrow derived cells, five of these used MNCs, one was Athersys, Inc.’s MultiStem product and two used CD34 + cells. Only one of these studies had published results (Savitz et al., 2011). Of the remaining studies, 11 were currently recruiting subjects. The cell therapies being tested in these ongoing studies include cord blood derived cells (monocytes, MNCs and one proprietary preparation) (three trials), bone marrow derived MNC (one), bone marrow-derived MSCs including one in which these cells were genetically modified (four trials), adipose-derived MSCs (one trial), EPCs (one trial) and a combination marrow-derived MSCs + EPCs (one trial). The remaining trials were either not recruiting, or were withdrawn or terminated.
The majority of the preclinical cell therapy studies have focused on delivering cells within 72 h of stroke onset, when stroke patients are inherently medically unstable. The study conducted by Savitz’ group was a safety study and followed the preclinical literature in that the autologous bone marrow MNCs were transplanted between 24 and 72 h after the stroke in 10 patients (Savitz et al., 2011). There were no adverse events associated with the bone marrow harvest or intravenous administration of the cells. There were three instances of stroke-related complications. At six months post-transplant, there were significant improvements on the NIHSS and improvement on the modified Rankin Scale and Barthel Index (BI). The authors concluded that autologous transplants of these cells were safe and further, transplantation in the subacute period was feasible, but there were critical issues that they faced. In order to reduce confounding variables in subject populations enrolled in trials, these investigators recommended including a cutoff for lesion size, excluding patients likely to develop malignant infarction, monitoring for hemorhhagic transformation especially after tPA treatment and excluding patients that require anticoagulation (Vahidy et al., 2013). These are not issues addressed in the animal studies.
Most of the clinical studies are being planned when recovery has stabilized. Typical of this format are the studies conducted by the investigators at Ajou National University in Korea. In their studies, autologous MSCs were injected intravenously between five and eight weeks post-stroke. Initial results identified no adverse effects found in the cell-treated subjects and there were significant functional improvements on the BI (Bang et al., 2005). At the 5 year follow-up of 52 study participants, morbidity in the control group was approximately twice that of the MSC treated group (Lee et al., 2010). There were no observed side effects of MSC administration and there were improvements in function. This group has disclosed a Phase III clinical trial on the NIH registry.
When modifying the search to specify NSCs and not just cells, the search identifies two clinical trials - a Phase I and Phase II trial of the CTX0E03 cells developed by ReNeuron Ltd. Both trials are listed as active but not recruiting. The preclinical studies of the CTX0E03 cells have been conducted in conjunction with ReNeuron and were specifically planned to address issues important in the development of the clinical trials. The animal data predicts that administration later after stroke when patients have reached a plateau in improvements or deterioration will be efficacious.
These trials are not the only examples of clinical studies that have been conducted, but are representative. There are other small clinical studies in the literature but the quality of the data is not robust. The number of subjects tends to be small. There are differences in the types of cells and how they are prepared, how many cells are transplanted, and the route of administration. There is a lack of consistency in the primary outcome measures used. Further, the field is young enough that in most cases the follow-up of the transplanted patients is two years or less. In addition, most of the studies are not randomized, blinded clinical trials.
There was an interesting study into the beliefs of chronic ischemic stroke patients about stem cell therapy (Kim et al., 2013). The authors reported that 60% of patients believed that stem cell therapy could improve their well-being and 46% wanted stem cell therapy even though the side effects of such a therapy are not yet known. Almost 68% of the patients interviewed got their information on stem cell treatment from the television compared to 49% that talked to their physicians about it. When these authors surveyed physicians, the expectations about stem cell therapy were much lower. The promise of stem cell therapy is a sexy topic in the media. However, it is clear from both the preclinical and clinical studies published to date that we are still a number of years away from having a standardized cellular therapy available to stroke patients. It behooves us as scientists and physicians to better manage the expectations of the public in general and stroke patients in particular. It is not going to benefit the field to rush a treatment into the clinic to have it ultimately fail.
4. Concluding remarks
Brain plasticity, as a natural property of the brain, exists for one’s entire life. Because of this natural gift, spontaneous recovery occurs after stroke. Because of its lifelong capacity, restorative/rehabilitative interventions are able to enhance brain repair and stroke recovery even years after stroke onset. Emerging evidence has renewed our knowledge on the time window for stroke recovery, which is much longer than previously thought. By contrast to the limited several-hour-effective window of thrombolytic/thrombectomy treatment, the therapeutic window of restorative/rehabilitative interventions is much broader, from years after stroke to lifelong applicability. Recognizing this unique feature of restorative approach will direct stroke research into a fruitful direction and provide great opportunities to develop more treatments for maximizing stroke recovery.
A growing body of evidence from both the preclinical and clinical studies is challenging the current concept for the intervention time of stroke recovery: “the earlier the better”. In fact, many restorative/rehabilitative interventions given earlier turn into negative or unexpected results, appearing “the earlier the worse” or “the earlier the not better”. By contrast, when providing them in the later stage, brain repair and functional outcome are greater when being given in the earlier stage. It remains to be clarified as to why and how the intervention timing is a sensitive issue to the restorative approach. The stressful microenvironment occurring in the early stage of stroke may negatively influence the effects of brain repair interventions.
Structural and functional reestablishment of neurovascular networks is the fundamental process for stroke recovery. Consideration of multi-components for enhancing brain repair and stroke recovery is highly recommended in future studies. It needs to be recognized that a stroke does not only cause neuron loss in the infarct area but it also significantly disrupts the entire brain networks, resulting in a widespread dysfunction and impairments, which is not only limited to sensorimotor function but also recognition, spatial and mood control function. Combining physical interventions with other treatments (e.g. drugs or stem cell transplantation) may enhance the repairing power to maximize the reestablishment of the brain networks and the recovery of impaired brain functions. It is expected that the next generation of restorative/rehabilitative therapies for brain repair and stroke recovery will target the repair in whole brain networks and brain network functioning rather than only motor function.
Acknowledgements
The research work is partially supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NIH/NINDS) (R01 NS060911 to L.R.Z) in the United States. L.R.Z. thanks to Suning Ping for reference assistance, Xuecheng Qiu for image assistance, and Sandy McGillis for proofreading assistance.
Abbreviations:
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- α-SMA
alpha-smooth muscle actin
- BBB
blood-brain barrier
- BDNF
brain derived neurotrophic factor
- bFGF or FGF2
basic fibroblast growth factor
- BrdU
bromodeoxyuridine
- CIMT
constraint-induced movement therapy
- CNIS
clinical neurological impairment scale
- CNS
central nervous system
- DPSCs
dental pulp stem cells
- ECFCs
endothelial colony forming cells
- FDA
food and drug administration
- EE
enriched environment
- EPCs
endothelial progenitor cells
- Flk-1
fetal liver kinase 1
- fMRI
functional magnetic resonance imaging
- G-CSF
granulocyte-colony stimulating factor
- HIF-1α
hypoxia-inducible factor 1 alpha
- HSCs
hematopoietic stem cells
- HSCs/HPCs
hematopoietic stem cells and hematopoietic progenitor cells
- HUCB
human umbilical cord blood
- IL
interleukin
- iPSCs
induced pluripotent stem cells
- LDP
long-term depression
- LFP
local field potential
- Lasmp
limbic system associated membrane protein
- LTP
long-term potentiation
- M1
primary motor cortex
- M1 type
proinflammatory phenotype of microglia/macrophages
- M2 type
anti-inflammatory phenotype of microglia/macrophages
- MCA
middle cerebral artery
- MCAO
middle cerebral artery occlusion
- MDMs
monocyte-derived macrophages
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- MNCs
mononuclear cells
- MRI
magnetic resonance imaging
- mRS
modified rankin scale
- MSCs
mesenchymal stem cells
- M-type
mushroom type spine
- NARI
noradrenaline reuptake inhibitors
- NGF
nerve growth factor
- NGFI-A
nerve growth factor-induced gene A
- NGFI-B
nerve growth factor-induced gene B
- NF-kB
nuclear factor kappa B
- NMDA
N-methyl-d-aspartate receptor
- NPCs
neural progenitor cells
- NSCs
neural stem cells
- OECs
olfactory ensheathing cells
- OGD
oxygen glucose deprivation
- PAI-1
plasminogen activator inhibitor 1
- PNN
perineuronal net
- PSA-NCAM
polysialylated neural cell adhesion molecule
- PSD-95
postsynaptic density-95
- RCTs
randomized controlled trials
- RMS
rostral migratory stream
- rTMS
repetitive transcranial magnetic stimulation
- SCF
stem cell factor
- SCF+G-CSF
SCF in combination with G-CSF
- SD
Sprague-Dawley
- SDF-1
stromal cell-derived factor-1
- SGZ
subgranular zone
- Shh
sonic hedgehog
- SHRs
spontaneously hypertensive rats
- SSRI
selective serotonin reuptake inhibitors
- SVZ
subventricular zone
- TBI
traumatic brain injury
- tDCS
transcranial direct current stimulation
- TGF-β
transforming growth factor beta 1
- TMS
transcranial magnetic stimulation
- TNF-α
tumor necrosis factor alpha
- tPA
tissue plasminogen activator
- U-type
uncertain type spine
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endotheleial growth factor receptor 2
- vWF
von willibrand factor
- YFP
yellow fluorescent protein
References
- Allen NJ, Barres BA, 2005. Signaling between glia and neurons: focus on synaptic plasticity. Curr. Opin. Neurobiol 15, 542–548. [DOI] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB, 2005. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12, 1329–1343. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD, 1964. Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature 204, 1161–1163. [DOI] [PubMed] [Google Scholar]
- Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DME, Ramachandran VS, 1999. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353, 2035–2036. [DOI] [PubMed] [Google Scholar]
- Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP, 2009. Hemorrhagic and ischemic strokes compared: stroke severity mortality, and risk factors. Stroke 40, 2068–2072. [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A, 2011. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain awr103. [DOI] [PubMed] [Google Scholar]
- Andrews RJ, 1991. Transhemispheric diaschisis: a review and comment. Stroke 22, 943–949. [DOI] [PubMed] [Google Scholar]
- Ansuini C, Pierno AC, Lusher D, Castiello U, 2006. Virtual reality applications for the remapping of space in neglect patients. Restor. Neurol. Neurosci 24, 431–441. [PubMed] [Google Scholar]
- Anttila JE, Whitaker KW, Wires ES, Harvey BK, Airavaara M, 2016. Role of microglia in ischemic focal stroke and recovery: focus on Toll-like receptors. Progress Neuro-Psychopharmacol. Biol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arien-Zakay H, Lecht S, Bercu MM, Tabakman R, Kohen R, Galski H, Nagler A, Lazarovici P, 2009. Neuroprotection by cord blood neural progenitors involves antioxidants: neurotrophic and angiogenic factors. Exp. Neurol 216, 83–94. [DOI] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM, 2006. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. Neurosci 23, 261–272. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O, 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med 8, 963–970. [DOI] [PubMed] [Google Scholar]
- Aziz NA, 2010. Long-term rehabilitation after stroke: where do we go from here? Rev. Clin. Gerontol 20, 239–245. [Google Scholar]
- Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN, 2015. Axon initial segment-associated microglia. J. Neurosci 35, 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, 1993. Structural changes accompanying memory storage. Annu. Rev. Physiol 55, 397–426. [DOI] [PubMed] [Google Scholar]
- Balseanu AT, Buga AM, Catalin B, Wagner DC, Boltze J, Zagrean AM, Reymann K, Schaebitz W, Popa-Wagner A, 2014. Multimodal approaches for regenerative stroke therapies: combination of granulocyte colony-s timulating factor with bone marrow mesenchymal stem cells is not superior to G-CSF alone. Front. Aging Neurosci 6, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C, 1990. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project-1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 53, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G, 2005. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol 57, 874–882. [DOI] [PubMed] [Google Scholar]
- Bao X, Feng M, Wei J, Han Q, Zhao H, Li G, Zhu Z, Xing H, An Y, Qin C, Zhao RC, Wang R, 2011. Transplantation of Flk-1 + human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats. Eur. J. Neurosci 34, 87–98. [DOI] [PubMed] [Google Scholar]
- Barber PA, Davis SM, Infeld B, Baird AE, Donnan GA, Jolley D, Lichtenstein M, 1998. Spontaneous reperfusion after ischemic stroke is associated with improved outcome. Stroke 29, 2522–2528. [DOI] [PubMed] [Google Scholar]
- Barker AJ, Ullian EM, 2010. Astrocytes and synaptic plasticity. Neuroscientist 16, 40–50. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK, 2010. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci 30, 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, Colonnier M, 1985. A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines dendritic trunks, and cell bodies in area 17 of the cat. J. Comparat. Neurol 231, 180–189. [DOI] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG, 2004. Environmental enrichment in mice decreases anxiety: attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci 20, 1341–1347. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC, 1969. Rat brain: effects of environmental enrichment on wet and dry weights. Science (New York, N.Y.) 163. 825–826. [DOI] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM, 2006. Long-term continuous but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol. Learn. Mem 85, 139–152. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G, 2008. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke 39, 390–396. [DOI] [PubMed] [Google Scholar]
- Bernheisel CR, Schlaudecker JD, Leopold K, 2011. Subacute management of ischemic stroke. Am. Fam. Physician 84, 1383–1388. [PubMed] [Google Scholar]
- Bhogal SK, Teasell R, Speechley M, 2003. Intensity of aphasia therapy: impact on recovery. Stroke 34, 987–993. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH, 2010. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J. Neurosci 30, 6726–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA, 2013. Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol 39, 3–18. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Celestino D, Starace G, Antonelli A, Lambiase A, Procoli A, Rumi C, Lai M, Picardi A, Ballatore G, Bonini S, Aloe L, 2003. CD34-positive cells in human umbilical cord blood express nerve growth factor and its specific receptor TrkA. J. Neuroimmunol 136, 130–139. [DOI] [PubMed] [Google Scholar]
- Briddell RA, Hartley CA, Smith KA, McNiece IK, 1993. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood 82, 1720–1723. [PubMed] [Google Scholar]
- Brodal A, 1973. Self-observations and neuro-anatomical considerations after a stroke. Brain 96, 675–694. [DOI] [PubMed] [Google Scholar]
- Broeren J, Claesson L, Goude D, Rydmark M, Sunnerhagen KS, 2008. Virtual rehabilitation in an activity centre for community-dwelling persons with stroke: the possibilities of 3-dimensional computer games. Cerebrovasc. Dis 26, 289–296. [DOI] [PubMed] [Google Scholar]
- Broudy VC, 1997. Stem cell factor and hematopoiesis. Blood 90, 1345–1364. [PubMed] [Google Scholar]
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH, 2007. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci 27, 4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Wong C, Murphy TH, 2008. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke 39, 1286–1291. [DOI] [PubMed] [Google Scholar]
- Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH, 2009. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J. Neurosci 29, 1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C, 2005. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci 21, 513–521. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA, 2006. Neural plasticity in the ageing brain. Nat. Rev. Neurosci 7, 30–40. [DOI] [PubMed] [Google Scholar]
- Calio ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calio PL, Reis LA, Simoes Mde J, Lisboa-Nascimento T, Ferreira AT, Bertoncini CR, 2014. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic. Biol. Med 70, 141–154. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, Investigators E-I, 2015. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med 372, 1009–1018. [DOI] [PubMed] [Google Scholar]
- Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C, Montinaro M, Kraftsik R, De Curtis M, Parati E, De Simoni MG, 2007. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS One 2, e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M, 2005. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, 2012. Brain excitability in stroke: the yin and yang of stroke progression. Arch. Neurol 69, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan JF, Patel DR, Miller JA, 1994. Stem cell factor is a neurotrophic factor for neural crest-derived chick sensory neurons. J. Neurosci 14, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Tononi G, 2014. Diaschisis: past present, future. Brain 137, 2408–2422. [DOI] [PubMed] [Google Scholar]
- Cassidy JM, Cramer SC, 2016. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl. Stroke Res 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano C, Dorado L, Guerrero C, Millan M, Gomis M, Perez de la Ossa N, Castellanos M, Garcia MR, Domenech S, Davalos A, 2010. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 41, 1836–1840. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Lee N, Park IH, Choi C, Jeon I, Kwon J, Oh SH, Shin DA, Do JT, Lee DR, Lee H, Moon H, Hong KS, Daley GQ, Song J, 2013a. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant. 22, 1427–1440. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Oh SH, Lee N, Choi C, Jeon I, Kim HS, Shin DA, Lee SE, Kim D, Song J, 2013b. Contralaterally transplanted human embryonic stem cell-derived neural precursor cells (ENStem-A) migrate and improve brain functions in stroke-damaged rats. Exp. Mol. Med 45, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M, 2002. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J. Neurosci. Res 69, 687–691. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M, 2003a. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res 73, 778–786. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M, 2003b. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res 92, 692–699. [DOI] [PubMed] [Google Scholar]
- Chen N, Newcomb J, Garbuzova-Davis S, Davis Sanberg C, Sanberg PR, Willing E, 2010. Human umbilical cord blood cells have trophic effects on young and aging hippocampal neurons in vitro. Aging Dis 1, 173–190. [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E, 2011. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat. Neurosci 14, 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Qiu R, Li L, He D, Lv H, Wu X, Gu N, 2014. The role of exogenous neural stem cells transplantation in cerebral ischemic stroke. J. Biomed. Nanotechnol 10, 3219–3230. [DOI] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, Bejot Y, Deltour S, Jaillard A, Niclot P, Guillon B, Moulin T, Marque P, Pariente J, Arnaud C, Loubinoux I, 2011. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 10, 123–130. [DOI] [PubMed] [Google Scholar]
- Christie KJ, Turnley AM, 2012. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front. Cell. Neurosci 6, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Barres BA, 2012. The role of glial cells in synapse elimination. Curr. Opin. Neurobiol 22, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH, Han H, Lee JH, Choe BY, Lee SY, Kim HY, 2009. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J. Neurosci. Res 87, 3554–3567. [DOI] [PubMed] [Google Scholar]
- Claessen MH, van der Ham IJ, Jagersma E, Visser-Meily JM, 2016. Navigation strategy training using virtual reality in six chronic stroke patients: a novel and explorative approach to the rehabilitation of navigation impairment. Neuropsychol. Rehabil 26, 822–846. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Faiz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, 1997. Functional relevance of cross-modal plasticity in blind humans. Nature 389, 180–183. [DOI] [PubMed] [Google Scholar]
- Connor JR, Wang EC, Diamond MC, 1982. Increased length of terminal dendritic segments in old adult rats’ somatosensory cortex: an environmentally induced response. Exp. Neurol 78, 466–470. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL, 2004. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol 60, 236–248. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Crafton KR, 2006. Somatotopy and movement representation sites following cortical stroke. Exp. Brain Res 168, 25–32. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, 2011. Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, 2004. Functional imaging in stroke recovery. Stroke 35, 2695–2698. [DOI] [PubMed] [Google Scholar]
- Cui L, Murikinati SR, Wang D, Zhang X, Duan WM, Zhao LR, 2013. Reestablishing neuronal networks in the aged brain by stem cell factor and granulocyte-colony stimulating factor in a mouse model of chronic stroke. PLoS One 8, e64684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Duchamp NS, Boston DJ, Ren X, Zhang X, Hu H, Zhao LR, 2015. NF-kappaB is involved in brain repair by stem cell factor and granulocyte-colony stimulating factor in chronic stroke. Exp. Neurol 263, 17–27. [DOI] [PubMed] [Google Scholar]
- Cui L, Wang D, McGillis S, Kyle M, Zhao LR, 2016. Repairing the brain by SCF + G-CSF treatment at 6 months postexperimental stroke: mechanistic determination of the causal link between neurovascular regeneration and motor functional recovery. ASN Neuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS, 2007. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science (New York, N.Y.) 315, 1243–1249. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Zhao L, Johansson IM, Mattsson B, Johansson BB, Seckl JR, Olsson T, 1999. Environmental enrichment alters nerve growth factor-induced gene A and glucocorticoid receptor messenger RNA expression after middle cerebral artery occlusion in rats. Neuroscience 93, 527–535. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Ronnback A, Risedal A, Nergardh R, Johansson IM, Seckl JR, Johansson BB, Olsson T, 2003. Effects of postischemic environment on transcription factor and serotonin receptor expression after permanent focal cortical ischemia in rats. Neuroscience 119, 643–652. [DOI] [PubMed] [Google Scholar]
- Dam M, Tonin P, De Boni A, Pizzolato G, Casson S, Ermani M, Freo U, Piron L, Battistin L, 1996. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 27, 1211–1214. [DOI] [PubMed] [Google Scholar]
- Dastur CK, Yu W, 2017. Current management of spontaneous intracerebral haemorrhage. Stroke Vase. Neurol 2, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K, 2006. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875. [DOI] [PubMed] [Google Scholar]
- Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G, 2007. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110, 2440–2448. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Wakade C, Mahesh VB, Brann DW, 2005. Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappaB. J. Neurochem 95, 9–19. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR, 1964. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comparat. Neurol 123, 111–120. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, Bennett EL, 1966. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comparat. Neurol 128, 117–126. [DOI] [PubMed] [Google Scholar]
- Dibajnia P, Morshead CM, 2013. Role of neural precursor cells in promoting repair following stroke. Acta Pharmacol. Sin 34, 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich K, Sevimli S, Dorr H, Kosters E, Hoppen M, Lewejohann L, Klocke R, Minnerup J, Knecht S, Nikol S, Sachser N, Schneider A, Gorji A, Sommer C, Schabitz WR, 2009. The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: a characterization of G-CSF-deficient mice. J. Neurosci 29, 11572–11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihne M, Block F, 2001. Focal ischemia induces transient expression of IL-6 in the substantia nigra pars reticulata. Brain Res 889, 165–173. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, Rosen R, Finklestein SP, 2001. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc. Natl. Acad. Sci. U. S. A 98, 12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M, 2013. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J. Cereb. Blood Flow Metab 33, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM, 2015a. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med 4, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeppner TR, Kaltwasser B, Teli MK, Sanchez-Mendoza EH, Kilic E, Bahr M, Hermann DM, 2015b. Post-stroke transplantation of adult subventricular zone derived neural progenitor cells-A comprehensive analysis of cell delivery routes and their underlying mechanisms. Exp. Neurol 273, 45–56. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM, 2008. Stroke. Lancet (London, England) 371, 1612–1623. [DOI] [PubMed] [Google Scholar]
- Doulames V, Lee S, Shea TB, 2014. Environmental enrichment and social interaction improve cognitive function and decrease reactive oxidative species in normal adult mice. Int. J. Neurosci 124, 369–376. [DOI] [PubMed] [Google Scholar]
- Dragunow M, 1996. A role for immediate-early transcription factors in learning and memory. Behav. Genet 26, 293–299. [DOI] [PubMed] [Google Scholar]
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF, 2009. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology 73, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan YF, Liu C, Zhao YF, Duan WM, Zhao LR, 2009. Thiopental exaggerates ischemic brain damage and neurological deficits after experimental stroke in spontaneously hypertensive rats. Brain Res 1294, 176–182. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV, 2001. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-de-pendent memory. Learn. Mem. (Cold Spring Harbor, N.Y.) 8, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Lai SM, Keighley J, 2000. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology 39, 835–841. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Thomas JL, 2013. Molecular parallels between neural and vascular development. Cold Spring Harb. Perspect. Med 3, a006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W, Stroke Thrombolysis Trialists’ Collaborative, G., 2014. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet (London, England) 384, 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, Buccino G, 2007. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage 36, T164–T173. [DOI] [PubMed] [Google Scholar]
- Fama ME, Turkeltaub PE, 2014. Treatment of poststroke aphasia: current practice and new directions. Semin. Neurol 34, 504–513. [DOI] [PubMed] [Google Scholar]
- Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC, 2005. Human umbilical cord blood cells express neurotrophic factors. Neurosci. Lett 380, 322–325. [DOI] [PubMed] [Google Scholar]
- Farber SD, Onifer SM, Kaseda Y, Murphy SH, Wells DG, Vietje BP, Wells J, Low WC, 1988. Neural transplantation of horseradish peroxidase-labeled hippocampal cell suspensions in an experimental model of cerebral ischemia. Prog. Brain Res 78, 103–107. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Baron JC, 1986. Diaschisis. Stroke 17, 817–830. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, 2014. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR, 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51. [DOI] [PubMed] [Google Scholar]
- Feng N, Hao G, Yang F, Qu F, Zheng H, Liang S, Jin Y, 2016. Transplantation of mesenchymal stem cells promotes the functional recovery of the central nervous system following cerebral ischemia by inhibiting myelin-associated inhibitor expression and neural apoptosis. Exp. Ther. Med 11, 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng I.S.a.J., 2010. Video games and spatial cognition. Rev. Gen. Psychol 14, 92–104. [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A, 1998. Rapid actin-based plasticity in dendritic spines. Neuron 20, 847–854. [DOI] [PubMed] [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY, 1998. Engraftable human neural stem cells respond to developmental cues replace neurons, and express foreign genes. Nat. Biotechnol 16, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Fogelholm R, Nuutila M, Vuorela AL, 1992. Primary intracerebral haemorrhage in the Jyvaskyla region central Finland, 1985–89: incidence, case fatality rate, and functional outcome. J. Neurol. Neurosurg. Psychiatry 55, 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgays DG, Forgays JW, 1952. The nature of the effect of free-environmental experience in the rat. J. Comp. Physiol. Psychol 45, 322–328. [DOI] [PubMed] [Google Scholar]
- Foster TC, Dumas TC, 2001. Mechanism for increased hippocampal synaptic strength following differential experience. J. Neurophysiol 85, 1377–1383. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, 2006. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 37, 2115–2122. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, 2003. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol. Aging 24, 615–626. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG, 2004. Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127, 747–758. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Antonucci G, Marra C, Paolucci S, 2001. Relation between depression after stroke antidepressant therapy, and functional recovery. J. Neurol. Neurosurg. Psychiatry 71, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Winstein CJ, Aziz-Zadeh L, 2010. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil. Neural Repair 24, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Roeder I, Kempermann G, 2016. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 26, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil OD, Zhang L, Chen S, Ren YQ, Pimenta A, Zanazzi G, Hillman D, Levitt P, Salzer JL, 2002. Complementary expression and heterophilic interactions between IgLON family members neurotrimin and LAMP. J. Neurobiol 51, 190–204. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE, 2002. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil. Neural Repair 16, 232–240. [DOI] [PubMed] [Google Scholar]
- Glover S, Castiello U, 2006. Recovering space in unilateral neglect: a neurological dissociation revealed by virtual reality. J. Cogn. Neurosci 18, 833–843. [DOI] [PubMed] [Google Scholar]
- Gomi M, Takagi Y, Morizane A, Doi D, Nishimura M, Miyamoto S, Takahashi J, 2012. Functional recovery of the murine brain ischemia model using human induced pluripotent stem cell-derived telencephalic progenitors. Brain Res 1459, 52–60. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Torrecillas JL, Mendlewicz J, Lobo A, 1995. Effects of early treatment of poststroke depression on neuropsychological rehabilitation. International Psychogeriatrics/IPA 7, 547–560. [DOI] [PubMed] [Google Scholar]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt A, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, Investigators ET, 2015. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med 372, 1019–1030. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D, 2003. Action video game modifies visual selective attention. Nature 423, 534–537. [DOI] [PubMed] [Google Scholar]
- Green EJ, Greenough WT, 1986. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: evidence from hippocampal slices maintained in vitro. J. Neurophysiol 55, 739–750. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Hwang HM, Gorman C, 1985. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc. Natl. Acad. Sci. U. S. A 82, 4549–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med 333, 1581–1587. [DOI] [PubMed] [Google Scholar]
- Gunarathne A, Patel JV, Gammon B, Gill PS, Hughes EA, Lip GY, 2009. Ischemic stroke in South Asians: a review of the epidemiology pathophysiology, and ethnicity-related clinical features. Stroke 40, e415–423. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E, 2008. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med 359, 1317–1329. [DOI] [PubMed] [Google Scholar]
- Hallett M, 2001. Plasticity of the human motor cortex and recovery from stroke. Brain Res. Brain Res. Rev 36, 169–174. [DOI] [PubMed] [Google Scholar]
- Hanley DF, Awad IA, Vespa PM, Martin NA, Zuccarello M, 2013. Hemorrhagic stroke: introduction. Stroke 44, S65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM, 2007. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav. Brain Res 185, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani Z, O’Reilly J, Pearse Y, Stroemer P, Tang E, Sinden J, Price J, Thuret S, 2012. Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PLoS One 7, e50444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, Bleyenheuft Y, 2016. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci 10, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH, 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Nedergaard M, 2015. How do astrocytes participate in neural plasticity? Cold Spring Harb. Perspect. Biol 7, a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Lu Y, Lin X, Jiang L, Tang Y, Tang G, Chen X, Zhang Z, Wang Y, Yang GY, 2017. Optical inhibition of striatal neurons promotes focal neurogenesis and neurobehavioral recovery in mice after middle cerebral artery occlusion. J. Cereb. Blood Flow Metab 37, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D, 1947. The effects of early experience on problem solving at maturity. Am. Psychol 2, 306–307. [Google Scholar]
- Hebb DO, 1949. The Organization of Behavior: A Neuropsychological Theory. Wiley and Sons, New York. [Google Scholar]
- Heinla I, Leidmaa E, Kongi K, Pennert A, Innos J, Nurk K, Tekko T, Singh K, Vanaveski T, Reimets R, Mandel M, Lang A, Lillevali K, Kaasik A, Vasar E, Philips MA, 2015. Gene expression patterns and environmental enrichment-induced effects in the hippocampi of mice suggest importance of Lsamp in plasticity. Front. Neurosci 9, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ, 2002. Motor recovery after stroke: a systematic review of the literature. Arch. Phys. Med. Rehabil 83, 1629–1637. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP, 1996. Long-term depression of horizontal connections in rat motor cortex. Eur. J. Neurosci 8, 658–665. [DOI] [PubMed] [Google Scholar]
- Hess DA, Levac KD, Karanu FN, Rosu-Myles M, White MJ, Gallacher L, Murdoch B, Keeney M, Ottowski P, Foley R, Chin-Yee I, Bhatia M, 2002. Functional analysis of human hematopoietic repopulating cells mobilized with granulocyte colony-stimulating factor alone versus granulocyte colony-stimulating factor in combination with stem cell factor. Blood 100, 869–878. [DOI] [PubMed] [Google Scholar]
- Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD, 2013. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 22, 1541–1552. [DOI] [PubMed] [Google Scholar]
- Hirata T, Morii E, Morimoto M, Kasugai T, Tsujimura T, Hirota S, Kanakura Y, Nomura S, Kitamura Y, 1993. Stem cell factor induces outgrowth of c-kit-positive neurites and supports the survival of c-kit-positive neurons in dorsal root ganglia of mouse embryos. Development (Cambridge, England) 119, 49–56. [DOI] [PubMed] [Google Scholar]
- Hirata T, Kasugai T, Morii E, Hirota S, Nomura S, Fujisawa H, Kitamura Y, 1995. Characterization of c-kit-positive neurons in the dorsal root ganglion of mouse. Brain Res. Dev. Brain Res 85, 201–211. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K, 2005. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291. [DOI] [PubMed] [Google Scholar]
- Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, Yu Z, Sun FY, 2008. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke 39, 2837–2844. [DOI] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J, 2015. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurology 11, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu W-H, Gerloff C, Cohen LG, 2005. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128, 490–499. [DOI] [PubMed] [Google Scholar]
- Hymovitch B, 1952. The effects of experimental variations on problem solving in the rat. J. Comp. Physiol. Psychol 45, 313–321. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC, 2000. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol 164, 45–52. [DOI] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, Iwama T, 2011. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13, 675–685. [DOI] [PubMed] [Google Scholar]
- Iskander A, Knight RA, Zhang ZG, Ewing JR, Shankar A, Varma NR, Bagher-Ebadian H, Ali MM, Arbab AS, Janie B, 2013. Intravenous administration of human umbilical cord blood-derived AC133+ endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl. Med 2, 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen H, Bernhardt J, Collier JM, Sena ES, McElduff P, Attia J, Pollack M, Howells DW, Nilsson M, Calford MB, Spratt NJ, 2010. An enriched environment improves sensorimotor function post-ischemic stroke. Neurorehabil. Neural Repair 24, 802–813. [DOI] [PubMed] [Google Scholar]
- Janssen H, Ada L, Karayanidis F, Drysdale K, McElduff P, Pollack M, White J, Nilsson M, Bernhardt J, Spratt NJ, 2012. Translating the use of an enriched environment poststroke from bench to bedside: study design and protocol used to test the feasibility of environmental enrichment on stroke patients in rehabilitation. Int. J. Stroke 7, 521–526. [DOI] [PubMed] [Google Scholar]
- Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, Spratt NJ, 2014. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: a pilot non-randomized controlled trial. Disabil. Rehabil 36, 255–262. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E, 1990. Functional reorganization of primary somatosensory cortex in adult owl monkeys after beha-viorally controlled tactile stimulation. J. Neurophysiol 63, 82–104. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Yan H, Krishnaney-Davison R, Al Sawaf A, Zhang SC, 2013. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J. Stroke Cerebrovasc. Dis 22, 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, Jeun SS, 2014. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. BioMed Res. Int 2014, 129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Liang G, Li X, Li Z, Gao X, Feng S, Wang X, Liu M, Liu Y, 2014. Intracarotid transplantation of autologous adipose-derived mesenchymal stem cells significantly improves neurological deficits in rats after MCAo. J. Mater. Sci. Mater. Med 25, 1357–1366. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA, 2001. Neurogenesis in dentate subgranular zone and rostral sub ventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. U. S. A 98, 4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Greenberg DA, 2002. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest 110, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Xie L, Mao X, Greenberg MB, Moore A, Peng B, Greenberg RB, Greenberg DA, 2011. Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res 1374. 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV, 2002. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J. Cereb. Blood Flow Metab 22, 89–96. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Ohlsson AL, 1996. Environment social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp. Neurol 139, 322–327. [DOI] [PubMed] [Google Scholar]
- Johansson BB, 1996. Functional outcome in rats transferred to an enriched environment 15 days after focal brain ischemia. Stroke 27, 324–326. [DOI] [PubMed] [Google Scholar]
- Johansson BB, 2000. Brain plasticity and stroke rehabilitation The Willis lecture. Stroke 31, 223–230. [DOI] [PubMed] [Google Scholar]
- Johansson BB, 2004. Functional and cellular effects of environmental enrichment after experimental brain infarcts. Restor. Neurol. Neurosci 22, 163–174. [PubMed] [Google Scholar]
- Jones TA, Greenough WT, 1996. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiol. Learn. Mem 65, 48–56. [DOI] [PubMed] [Google Scholar]
- Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, 1995a. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch. Phys. Med. Rehabil 76, 27–32. [DOI] [PubMed] [Google Scholar]
- Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS, 1995b. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil 76, 406–412. [DOI] [PubMed] [Google Scholar]
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, Millan M, Urra X, Cardona P, Lopez-Cancio E, Tomasello A, Castano C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Perez M, Goyal M, Demchuk AM, von Kummer R, Gallofre M, Davalos A, Investigators RT, 2015. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med 372, 2296–2306. [DOI] [PubMed] [Google Scholar]
- Justicia C, Ramos-Cabrer P, Hoehn M, 2008. MRI detection of secondary damage after stroke: chronic iron accumulation in the thalamus of the rat brain. Stroke 39, 1541–1547. [DOI] [PubMed] [Google Scholar]
- Kamei N, Tanaka N, Oishi Y, Hamasaki T, Nakanishi K, Sakai N, Ochi M, 2007. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine (Phila Pa 1976) 32, 1272–1278. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Kako E, Sawamoto K, 2011. Prospects and limitations of using endogenous neural stem cells for brain regeneration. Genes (Basel) 2, 107–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Latour LL, Chalela JA, Dambrosia JA, Warach S, 2004. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology 63, 2261–2265. [DOI] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, Antila H, Popova D, Akamine Y, Bahi A, Sullivan R, Hen R, Drew LJ, Castren E, 2011. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science (New York, N.Y.) 334, 1731–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katafuchi T, Li AJ, Hirota S, Kitamura Y, Hori T, 2000. Impairment of spatial learning and hippocampal synaptic potentiation in c-kit mutant rats. Learn. Mem. (Cold Spring Harbor, N.Y.) 7, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T, 2006. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation 113, 701–710. [DOI] [PubMed] [Google Scholar]
- Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K, 2010. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J. Cereb. Blood Flow Metab 30, 1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1998. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci 18, 3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH, 2002. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol 52, 135–143. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V, 2010. Social neuroscience: mirror neurons recorded in humans. Curr. Biol 20, R353–354. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee BH, 2013. Action observation training for functional activities after stroke: a pilot randomized controlled trial. NeuroRehabilitation 33, 565–574. [DOI] [PubMed] [Google Scholar]
- Kim YS, Chung DI, Choi H, Baek W, Kim HY, Heo SH, Chang DI, Na HR, Kim SH, Koh SH, 2013. Fantasies about stem cell therapy in chronic ischemic stroke patients. Stem Cells Dev 22, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA, 1994. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb. Cortex 4, 576–589. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Imfeld KL, Kirov II, Tai L, Gage FH, Young MJ, Berman MA, 2003. Expression of cytokines by multipotent neural progenitor cells. Cytokine 22, 101–106. [DOI] [PubMed] [Google Scholar]
- Koido K, Traks T, Balotsev R, Eller T, Must A, Koks S, Maron E, Torn I, Shlik J, Vasar V, Vasar E, 2012. Associations between LSAMP gene polymorphisms and major depressive disorder and panic disorder. Transl. Psychiatry 2, e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R, 2015. Plasticity in the prefrontal cortex of adult rats. Front. Cell. Neurosci 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB, 2002. Effects of cortical ischemia and postischemic environmental enrichment on hippocampal cell genesis and differentiation in the adult rat. J. Cereb. Blood Flow Metab 22, 852–860. [DOI] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS, 2005a. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the sub-ventricular zone of stroke-lesioned adult rats. Stroke 36, 1278–1282. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhao LR, Gido G, Johansson BB, Eriksson P, 2005b. Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur. J. Neurosci 21, 2397–2405. [DOI] [PubMed] [Google Scholar]
- Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB, 2006. Enriched environment after focal cortical ischemia enhances the generation of astroglia and NG2 positive polydendrocytes in adult rat neocortex. Exp. Neurol 199, 113–121. [DOI] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL, 1960. Effects of environmental complexity and training on brain chemistry. J. Comp. Physiol. Psychol 53, 509–519. [DOI] [PubMed] [Google Scholar]
- Kuo HI, Paulus W, Batsikadze G, Jamil A, Kuo MF, Nitsche MA, 2016. Chronic enhancement of serotonin facilitates excitatory transcranial direct current stimulation-induced neuroplasticity. Neuropsychopharmacology 41, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ, 2003. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 34, 2181–2186. [DOI] [PubMed] [Google Scholar]
- Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, Strazzer S, Bresolin N, Bersano A, 2011. Growth factors in ischemic stroke. J. Cell. Mol. Med 15, 1645–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larpthaveesarp A, Ferriero DM, Gonzalez FF, 2015. Growth factors for the treatment of ischemic brain injury (growth factor treatment). Brain Sci 5, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Hsu WL, Ma YL, Lee PJ, Chao CC, 2003. Enrichment enhances the expression of sgk a glucocorticoid-induced gene, and facilitates spatial learning through glutamate AMPA receptor mediation. Eur. J. Neurosci 18, 2842–2852. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, Lee YS, Lo EH, Kim M, Roh JK, 2005. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res 1058, 120–128. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK, 2008. Antiinflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131, 616–629. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, 2010. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem cells (Dayton, Ohio) 28, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Lee Y-Y, Lin Κ-C, Cheng H-J, Wu C-Y, Hsieh Y-W, Chen C-K, 2015. Effects of combining robot-assisted therapy with neuromuscular electrical stimulation on motor impairment, motor and daily function, and quality of life in patients with chronic stroke: a double-blinded randomized controlled trial. J. Neuroeng. Rehabil 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y, 2012. Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front. Hum. Neurosci 6, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Dricot L, Laloux P, Gradkowski W, Desfontaines P, Evrard F, Peeters A, Jamart J, Vandermeeren Y, 2015. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain 138, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L, 2005. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res 163, 78–90. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M, 2011. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J. Neurosci 31, 6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD, 2007. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke 38, 153–161. [DOI] [PubMed] [Google Scholar]
- Lennartsson J, Ronnstrand L, 2012. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol. Rev 92, 1619–1649. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Hall AA, Collier LA, Ajmo CT Jr., Willing AE, Pennypacker KR, 2010. Human umbilical cord blood cell therapy blocks the morphological change and recruitment of CD lib-expressing, isolectin-binding proinflammatory cells after middle cerebral artery occlusion. J. Neurosci. Res 88, 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M, 2002. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59, 514–523. [DOI] [PubMed] [Google Scholar]
- Li B, Piao CS, Liu XY, Guo WP, Xue YQ, Duan WM, Gonzalez-Toledo ME, Zhao LR, 2010. Brain self-protection: the role of endogenous neural progenitor cells in adult brain after cerebral cortical ischemia. Brain Res 1327, 91–102. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR, 1994. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84, 1737–1746. [PubMed] [Google Scholar]
- Lin Y, Moltzan CJ, Anderson DR, National Advisory Committee on Blood and Blood Products, 2012. The evidence for the use of recombinant factor Vila in massive bleeding: revision of the transfusion policy framework. Transfus. Med 22, 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee HT, Lee SD, Lee W, Cho CW, Lin SZ, Wang HJ, Okano H, Su CY, Yu YL, Hsu CY, Shyu WC, 2013. Role of HIF-1 alpha-activated Epacl on HSC-mediated neuroplasticity in stroke model. Neurobiol. Dis 58, 76–91. [DOI] [PubMed] [Google Scholar]
- Liu XL, Zhang W, Tang SJ, 2014. Intracranial transplantation of human adipose-derived stem cells promotes the expression of neurotrophic factors and nerve repair in rats of cerebral ischemia-reperfusion injury. Int. J. Clin. Exp. Pathol 7, 174–183. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Popescu M, Longo S, Gao M, Wang D, McGillis S, Zhao LR, 2016. Fibrinogen reduction and motor function improvement by hematopoietic growth factor treatment in chronic stroke in aged mice: a treatment frequency study. Cell Transplant 25, 729–734. [DOI] [PubMed] [Google Scholar]
- Lo RC, 1986. Recovery and rehabilitation after stroke. Can. Fam. Physician 32, 1851–1853. [PMC free article] [PubMed] [Google Scholar]
- London A, Cohen M, Schwartz M, 2013. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front. Cell. Neurosci 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W, Li WY, Xu XY, Jiang H, Bang OY, 2015. Bone marrow mesenchymal stem cells transplantation promotes the release of endogenous erythropoietin after ischemic stroke. Neural Regen. Res 10, 1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madinier A, Quattromani MJ, Sjolund C, Ruscher K, Wieloch T, 2014. Enriched housing enhances recovery of limb placement ability and reduces aggrecan-containing perineuronal nets in the rat somatosensory cortex after experimental stroke. PLoS One 9, e93121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD, 2000. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U. S. A 97, 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S, 2007. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci 10, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Mampalam TJ, Gonzalez MF, Weinstein P, Sharp FR, 1988. Neuronal changes in fetal cortex transplanted to ischemic adult rat cortex. J. Neurosurg 69, 904–912. [DOI] [PubMed] [Google Scholar]
- Mao D, Yao X, Feng G, Yang X, Mao L, Wang X, Ke T, Che Y, Kong D, 2015. Skin-derived precursor cells promote angiogenesis and stimulate proliferation of endogenous neural stem cells after cerebral infarction. BioMed Res. Int 2015, 945846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraka S, Jiang Q, Jafari-Khouzani K, Li L, Malik S, Hamidian H, Zhang T, Lu M, Soltanian-Zadeh H, Chopp M, Mitsias PD, 2014. Degree of corticospinal tract damage correlates with motor function after stroke. Ann. Clin. Transl. Neurol 1, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiece IK, Briddell RA, 1995. Stem cell factor. J. Leukoc. Biol 58, 14–22. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH, investigators S, 2005. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet (London, England) 365, 387–397. [DOI] [PubMed] [Google Scholar]
- Mendis S, 2013. Stroke disability and rehabilitation of stroke: World Health Organization perspective. Int. J. Stroke 8, 3–4. [DOI] [PubMed] [Google Scholar]
- Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O, 2013. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol. Dis 52, 191–203. [DOI] [PubMed] [Google Scholar]
- Mitkari B, Nitzsche F, Kerkela E, Kuptsova K, Huttunen J, Nystedt J, Korhonen M, Jolkkonen J, 2014. Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav. Brain Res 259, 50–59. [DOI] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, Kubota K, 2002. Premotor cortex is involved in restoration of gait in stroke. Ann. Neurol 52, 188–194. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Moorhouse AJ, Nabekura J, 2013. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Front. Cell. Neurosci 7, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad O, Drury-Stewart D, Song M, Faulkner B, Chen D, Yu SP, Wei L, 2013. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PLoS One 8, e64160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS, 2014. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus 24, 239–248. [DOI] [PubMed] [Google Scholar]
- Morita Y, Takizawa S, Kamiguchi H, Uesugi T, Kawada H, Takagi S, 2007. Administration of hematopoietic cytokines increases the expression of anti-inflammatory cytokine (IL-10) mRNA in the subacute phase after stroke. Neurosci. Res 58, 356–360. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D, 1994. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13, 1071–1082. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C, 2010. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motro B, Wojtowicz JM, Bernstein A, van der Kooy D, 1996. Steel mutant mice are deficient in hippocampal learning but not long-term potentiation. Proc. Natl. Acad. Sci. U. S. A 93, 1808–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L, Foucault-Bertaud A, Velly L, Dignat-George F, Pisano P 2011. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev 7, 208–220. [DOI] [PubMed] [Google Scholar]
- Mountz JM, 2007. Nuclear medicine in the rehabilitative treatment evaluation in stroke recovery: role of diaschisis resolution and cerebral reorganization. Eur. Medicophys 43, 221–239. [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy 3rd, Muntner CS, Mussolino P, Nasir ME, Neumar K, Nichol RW, Palaniappan G, Pandey L, Reeves DK, Rodriguez MJ, Rosamond CJ, Sorlie W, Stein PD, Towfighi J, Turan A, Virani TN, Woo SS, Yeh D, Turner RW, 2016. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–360. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG, 2004. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol 55, 400–409. [DOI] [PubMed] [Google Scholar]
- Naka F, Narita N, Okado N, Narita M, 2005. Modification of AMPA receptor properties following environmental enrichment. Brain Dev 27, 275–278. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, 1994. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science (New York, N.Y.) 263, 1768–1771. [DOI] [PubMed] [Google Scholar]
- Nih LR, Deroide N, Lere-Dean C, Lerouet D, Soustrat M, Levy BI, Silvestre JS, Merkulova-Rainon T, Pocard M, Margaill I, Kubis N, 2012. Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. Eur. J. Neurosci 35, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K, 2002. Structure and function of dendritic spines. Annu. Rev. Physiol 64, 313–353. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Levis H, Murphy M, 2004. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol. Learn. Mem 81, 200–210. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal R, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy I, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger R, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, Investigators DT, 2017. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW, 1996. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science (New York, N.Y.) 272, 1791–1794. [DOI] [PubMed] [Google Scholar]
- Nudo R, 2003. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J. Rehab. Med.-Suppl 41, 7–10. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, 2006. Plasticity. NeuroRx 3, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson AL, Johansson BB, 1995. Environment influences functional outcome of cerebral infarction in rats. Stroke 26, 644–649. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Yamagami H, Yoshimoto T, Morita Y, Yamamoto H, Toyoda K, Ihara M, 2017. Cerebral hyperperfusion on arterial spin labeling MRI after reperfusion therapy is related to hemorrhagic transformation. J. Cereb. Blood Flow Metab 37, 3087–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FJ, Jolkkonen J, 2013. Restorative therapies to enhance sensorimotor recovery following cerebral ischemia. Acta Neurobiol. Exp 73, 66–78. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG, American Heart Association Advocacy Coordinating, C., Stroke C, 2013. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 44, 2361–2375. [DOI] [PubMed] [Google Scholar]
- Page SJ, Hill V, White S, 2013. Portable upper extremity robotics is as efficacious as upper extremity rehabilitative therapy: a randomized controlled pilot trial. Clin. Rehabil 27, 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH, 1995. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol. Cell. Neurosci 6, 474–486. [DOI] [PubMed] [Google Scholar]
- Pan SL, Wu SC, Wu TH, Lee TK, Chen TH, 2006. Location and size of infarct on functional outcome of noncardioembolic ischemic stroke. Disabil. Rehabil 28, 977–983. [DOI] [PubMed] [Google Scholar]
- Park J-H, Park J-H, 2016. The effects of game-based virtual reality movement therapy plus mental practice on upper extremity function in chronic stroke patients with hemiparesis: a randomized controlled trial. J. Phys. Ther. Sci 28, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Kim JH, Park CK, 2014. Neuronal cell death in the inner retina and the influence of vascular endothelial growth factor inhibition in a diabetic rat model. Am. J. Pathol 184, 1752–1762. [DOI] [PubMed] [Google Scholar]
- Park HW, Moon HE, Kim HS, Paek SL, Kim Y, Chang JW, Yang YS, Kim K, Oh W, Hwang JH, Kim JW, Kim DG, Paek SH, 2015a. Human umbilical cord blood-derived mesenchymal stem cells improve functional recovery through throm-bospondinl pantraxin3, and vascular endothelial growth factor in the ischemic rat brain. J. Neurosci. Res 93, 1814–1825. [DOI] [PubMed] [Google Scholar]
- Park JY, Chang M, Kim KM, Kim HJ, 2015b. The effect of mirror therapy on upper extremity function and activities of daily living in stroke patients. J. Phys. Ther. Sci 27, 1681–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG, 1994. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Li T, Barber PA, Yang Q, Darby DG, Desmond PM, Gerraty RP, Tress BM, Davis SM, 2000. Combined (1)H MR spectroscopy and diffusion-weighted MRI improves the prediction of stroke outcome. Neurology 55, 498–505. [DOI] [PubMed] [Google Scholar]
- Penumbra Pivotal Stroke Trial, I., 2009. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 40, 2761–2768. [DOI] [PubMed] [Google Scholar]
- Pereira Lopes FR, Lisboa BC, Frattini F, Almeida FM, Tomaz MA, Matsumoto PK, Langone F, Lora S, Melo PA, Borojevic R, Han SW, Martinez AM, 2011. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol 37, 600–612. [DOI] [PubMed] [Google Scholar]
- Piao CS, Gonzalez-Toledo ME, Xue YQ, Duan WM, Terao S, Granger DN, Kelley RE, Zhao LR, 2009. The role of stem cell factor and granulocyte-colony stimulating factor in brain repair during chronic stroke. J. Cereb. Blood Flow Metab 29, 759–770. [DOI] [PubMed] [Google Scholar]
- Piao CS, Gonzalez-Toledo ME, Gu X, Zhao LR, 2012a. The combination of stem cell factor and granulocyte-colony stimulating factor for chronic stroke treatment in aged animals. Exp. Transl. Stroke Med 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Li B, Zhang LJ, Zhao LR, 2012b. Stem cell factor and granulocyte colony stimulating factor promote neuronal lineage commitment of neural stem cells. Differentiation 83, 17–25. [DOI] [PubMed] [Google Scholar]
- Poh T, 2013. Time course of ischemic stroke on non-enhanced CT. Brain Stories 1–8. [Google Scholar]
- Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD, 2006. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol 199, 143–155. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Palesch YY, Martin R, Toyoda K, Yamamoto H, Wang Y, Wang Y, Hsu CY, Yoon BW, Steiner T, Butcher K, Hanley DF, Suarez JI, 2014. Interpretation and implementation of intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT II). J. Vase. Interv. Neurol 7, 34–40. [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR, 2008. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comparat. Neurol 507, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu Y, 2000a. Effects of environmental enrichment on gene expression in the brain. Proc. Natl. Acad. Sci. U. S. A 97, 12880–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ, 2000b. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDARl-knockout mice. Nat. Neurosci 3, 238–244. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ, 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med 183, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S, 1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science (New York, N.Y.) 255, 1707–1710. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S, 1996. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol 175, 1–13. [DOI] [PubMed] [Google Scholar]
- Richards MK, Liu F, Iwasaki H, Akashi K, Link DC, 2003. Pivotal role of granulocyte colony-stimulating factor in the development of progenitors in the common myeloid pathway. Blood 102, 3562–3568. [DOI] [PubMed] [Google Scholar]
- Risedal A, Zeng J, Johansson BB, 1999. Early training may exacerbate brain damage after focal brain ischemia in the rat. J. Cereb. Blood Flow Metab 19, 997–1003. [DOI] [PubMed] [Google Scholar]
- Risedal A, Mattsson B, Dahlqvist P, Nordborg C, Olsson T, Johansson BB, 2002. Environmental influences on functional outcome after a cortical infarct in the rat. Brain Res. Bull 58, 315–321. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough D, 2015. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflammation 12, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L, 2004. The mirror-neuron system. Annu. Rev. Neurosci 27, 169–192. [DOI] [PubMed] [Google Scholar]
- Robert Teasell RV, Madady Mona, Donaldson Sarah, McClure Andrew, Richardson Marina, 2014. Stroke Rehabilitation Clinician Handbook. Canadian Stroke Network. [Google Scholar]
- Robinson RG, Jorge RE, 2016. Post-Stroke depression: a review. Am. J. Psychiatry 173, 221–231. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, 1996. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res 78, 57–65. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, 1961. Heredity, Environment, Brain Biochemistry, and Learning. University of Pittsburgh Press, Pittsburgh, PA. [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC, 1962a. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J. Comp. Physiol. Psychol 55, 429–437. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Zolman JF, 1962b. Variation in environmental complexity and brain measures. J. Comp. Physiol. Psychol 55, 1092–1095. [DOI] [PubMed] [Google Scholar]
- Rowe DD, Leonardo CC, Hall AA, Shahaduzzaman MD, Collier LA, Willing AE, Pennypacker KR, 2010. Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Res 1366, 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DD, Leonardo CC, Recio JA, Collier LA, Willing AE, Pennypacker KR, 2012. Human umbilical cord blood cells protect oligodendrocytes from brain ischemia through Akt signal transduction. J. Biol. Chem 287, 4177–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan L, Lau BW, Wang J, Huang L, Zhuge Q, Wang B, Jin K, So KF, 2014. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Prog. Neurobiol 115, 116–137. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Johannesson E, Brugiere E, Erickson A, Rickhag M, Wieloch T, 2009. Enriched environment reduces apolipoprotein E (ApoE) in reactive astrocytes and attenuates inflammation of the peri-infarct tissue after experimental stroke. J. Cereb. Blood Flow Metab 29, 1796–1805. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg D, Urfer R, Johansson BB, Nikolich K, Wieloch T, 2011. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain 134, 732–746. [DOI] [PubMed] [Google Scholar]
- Ryu S, Lee SH, Kim SU, Yoon BW, 2016. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen. Res 11, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R, 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, N.Y.) 301, 805–809. [DOI] [PubMed] [Google Scholar]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattie HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, Investigators SP, 2015. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med 372, 2285–2295. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Misra V, Kasam M, Juneja H, Cox CS Jr., Alderman S, Aisiku I, Kar S, Gee A, Grotta JC, 2011. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol 70, 59–69. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, 2004. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog. Neurobiol 73, 61–72. [DOI] [PubMed] [Google Scholar]
- Schellenberg EG, 2004. Music lessons enhance IQ. Psychol. Sci 15, 511–514. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassier N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR, 2005. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Invest 115, 2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H, 2002. Dissociable effects of isolation rearing and environmental enrichment on exploration: spatial learning and HPA activity in adult rats. Pharmacol. Biochem. Behav 73, 209–224. [DOI] [PubMed] [Google Scholar]
- Shahaduzzaman MD, Mehta V, Golden JE, Rowe DD, Green S, Tadinada R, Foran EA, Sanberg PR, Pennypacker KR, Willing AE, 2015. Human umbilical cord blood cells induce neuroprotective change in gene expression profile in neurons after ischemia through activation of Akt pathway. Cell Transplant 24, 721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Cohen LG, 2012. Recovery of motor function after stroke. Dev. Psychobiol 54, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M, 2008. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia 56, 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CC, Lin CH, Yang YC, Chiao MT, Cheng WY, Ko JL, 2010. Intravenous implanted neural stem cells migrate to injury site reduce infarct volume, and improve behavior after cerebral ischemia. Curr. Neurovasc. Res 7, 167–179. [DOI] [PubMed] [Google Scholar]
- Shen LH, Xin H, Li Y, Zhang RL, Cui Y, Zhang L, Lu M, Zhang ZG, Chopp M, 2011. Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodeling after stroke in mice. Stroke 42, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichinohe H, Ishihara T, Takahashi K, Tanaka Y, Miyamoto M, Yamauchi T, Saito H, Takemoto H, Houkin K, Kuroda S, 2015. Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabil. Neural Repair 29, 80–89. [DOI] [PubMed] [Google Scholar]
- Shih YY, Huang S, Chen YY, Lai HY, Kao YC, Du F, Hui ES, Duong TQ, 2014. Imaging neurovascular function and functional recovery after stroke in the rat striatum using forepaw stimulation. J. Cereb. Blood Flow Metab 34, 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique MS, Mendelow AD, 2000. Surgical treatment of intracerebral haemorrhage. Br. Med. Bull 56, 444–456. [DOI] [PubMed] [Google Scholar]
- Sims RE, Butcher JB, Parri HR, Glazewski S, 2015. Astrocyte and neuronal plasticity in the somatosensory system. Neural Plast 2015, 732014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, Majewska AK, 2016. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun 7, 10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT, 1991. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res 540, 273–278. [DOI] [PubMed] [Google Scholar]
- Skolarus LE, Freedman VA, Feng C, Wing JJ, Burke JF, 2016. Care received by elderly US stroke survivors may be underestimated. Stroke 47, 2090–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, Stevanato L, Sinden JD, Modo M, 2012. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells (Dayton, Ohio) 30, 785–796. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E, 2004. Serotonin and brain development. Int. Rev. Neurobiol 59, 111–174. [DOI] [PubMed] [Google Scholar]
- Song M, Kim Y, Kim Y, Ryu S, Song I, Kim SU, Yoon BW, 2009. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci. Res 64, 235–239. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R, 2005. The human connectome: a structural description of the human brain. PLoS Comput. Biol 1, e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M, European Stroke O, 2014. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int. J. Stroke 9, 840–855. [DOI] [PubMed] [Google Scholar]
- Stevanato L, Corteling RL, Stroemer P, Hope A, Heward J, Miljan EA, Sinden JD, 2009. c-MycERTAM transgene silencing in a genetically modified human neural stem cell line implanted into MCAo rodent brain. BMC Neurosci 10, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato L, Thanabalasundaram L, Vysokov N, Sinden JD, 2016. Investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. PLoS One 11, e0146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J, 2009. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil. Neural Repair 23, 895–909. [DOI] [PubMed] [Google Scholar]
- Su Y, Cui L, Piao C, Li B, Zhao LR, 2013. The effects of hematopoietic growth factors on neurite outgrowth. PLoS One 8, e75562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai K, Fukuzawa R, Shofuda T, Fukusumi H, Kawabata S, Nishiyama Y, Higuchi Y, Kawai K, Isoda M, Kanematsu D, Hashimoto-Tamaoki T, Kohyama J, Iwanami A, Suemizu H, Ikeda E, Matsumoto M, Kanemura Y, Nakamura M, Okano H, 2016. Pathological classification of human iPSC-derived neural stem/progenitor cells towards safety assessment of transplantation therapy for CNS diseases. Mol. Brain 9, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T, 2004. Administration of CD34 + cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest 114, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC, 2008. Robot-based hand motor therapy after stroke. Brain 131, 425–437. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Izumi S-I, 2012. Maladaptive plasticity for motor recovery after stroke: mechanisms and approaches. Neural Plas 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K, 2005. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 36, 2681–2686. [DOI] [PubMed] [Google Scholar]
- Tanas L, Ostaszewski P, Iwan A, 2015. Effects of post-weaning social isolation and environment al enrichment on exploratory behavior and ankiety in Wistar rats. Acta Neurobiol. Exp 75, 72–79. [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ, 2001. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41, 779–790. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S, 2002. Exosomes: composition: biogenesis and function. Nat. Rev. Immunol 2, 569–579. [DOI] [PubMed] [Google Scholar]
- Thiel A, Radlinska BA, Paquette C, Sidel M, Soucy JP, Schirrmacher R, Minuk J, 2010. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. J. Nucl. Med 51, 1404–1412. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O, 2006. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells (Dayton, Ohio) 24, 739–747. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O, 2007. Longterm neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38, 3032–3039. [DOI] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F, 2004. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage 23, 827–839. [DOI] [PubMed] [Google Scholar]
- Tonder N, Sorensen T, Zimmer J, Jorgensen MB, Johansen FF, Diemer NH, 1989. Neural grafting to ischemic lesions of the adult rat hippocampus. Exp. Brain Res 74, 512–526. [DOI] [PubMed] [Google Scholar]
- Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH, 1998. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res 93, 83–90. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K, 2002. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794. [DOI] [PubMed] [Google Scholar]
- Tran DA, Pajaro-Blazquez M, Daneault JF, Gallegos JG, Pons J, Fregni F, Bonato P, Zafonte R, 2016. Combining dopaminergic facilitation with robot-Assisted upper limb therapy in stroke survivors: a focused review. Am. J. Phys. Med. Rehab./Assoc. Acad. Physiatrists 95, 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A, 2011. The role of microglia in the healthy brain. J. Neurosci 31, 16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CF, Anderson N, Thomas B, Sudlow CL, 2016. Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in Chinese and white populations: systematic review and meta-analysis. PLoS One 11, e0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY, 2010. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol. Brain 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA, 2001. Control of synapse number by glia. Science (New York, N.Y.) 291, 657–661. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA, 2004. Role for glia in synaptogenesis. Glia 47, 209–216. [DOI] [PubMed] [Google Scholar]
- Vahidy FS, Alderman S, Savitz SI, 2013. Challenges enrolling patients with acute ischemic stroke into cell therapy trials. Stem Cells Dev 22, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delden AE, Peper CE, Beek PJ, Kwakkel G, 2012. Unilateral versus bilateral upper limb exercise therapy after stroke: a systematic review. J. Rehabil. Med 44, 106–117. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE, 2005. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev 14, 595–604. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, Willing AE, 2006. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp. Neurol 199, 191–200. [DOI] [PubMed] [Google Scholar]
- Vespa P, McArthur D, Miller C, O’Phelan K, Frazee J, Kidwell C, Saver J, Starkman S, Martin N, 2005. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care 2, 274–281. [DOI] [PubMed] [Google Scholar]
- Von Monakow C, (1969). Diaschisis [1914 article translated by G Harris]. Penguin: Baltimore. [Google Scholar]
- Wake H, Moorhouse AJ, Miyamoto A, Nabekura J, 2013. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci 36, 209–217. [DOI] [PubMed] [Google Scholar]
- Walker-Batson D, Mehta J, Smith P, Johnson M, 2016. Amphetamine and other pharmacological agents in human and animal studies of recovery from stroke. Progress Neuro-Psychopharmacol. Biol. Psychiatry 64, 225–230. [DOI] [PubMed] [Google Scholar]
- Wang J, Li G, 2016. Adipose-derived stem cell transplantation inhibits the expression of Nogo-a in the perilesional cortex of rats with focal cerebral ischemia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 32. 39–43. [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R, 2008. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci 28, 1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DB, Dayton RD, Henning PP, Cain CD, Zhao LR, Schrott LM, Orchard EA, Knight DS, Klein RL, 2010a. Expansive gene transfer in the rat CNS rapidly produces amyotrophic lateral sclerosis relevant sequelae when TDP-43 is overexpressed. Mol. Ther 18, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C, 2010b. Dynamic functional reorganization of the motor execution network after stroke. Brain 133, 1224–1238. [DOI] [PubMed] [Google Scholar]
- Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C, 2011. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann. Neurol 69, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P, 2003. Stroke. Lancet (London, England) 362, 1211–1224. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nagai A, Sheikh AM, Mitaki S, Wakabayashi K, Kim SU, Kobayashi S, Yamaguchi S, 2016. A human neural stem cell line provides neuroprotection and improves neurological performance by early intervention of neuroinflammatory system. Brain Res 1631, 194–203. [DOI] [PubMed] [Google Scholar]
- Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, Miskinyte G, Ge R, Ahlenius H, Lindvall O, Schwartz M, Kokaia Z, 2016. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J. Neurosci 36, 4182–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG, 2003. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann. Neurol 54, 464–472. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A, Li ZH, Feng ZP, Li RK, 2008. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant 16, 993–1005. [PubMed] [Google Scholar]
- Wu S, Cheng CK, Feng J, D’Angelo L, Alain C, Spence I, 2012. Playing a first-person shooter video game induces neuroplastic change. J. Cogn. Neurosci 24, 1286–1293. [DOI] [PubMed] [Google Scholar]
- Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, Augsburger R, See J, Le VH, Srinivasan R, Cramer SC, 2015. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain 138, 2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M, 2012. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells (Dayton, Ohio) 30, 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M, 2013a. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab 33, 1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M, 2013b. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells (Dayton, Ohio) 31, 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K, 2006. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci 26, 6627–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Welsh AM, Bora SH, Snyder EY, Koliatsos VE, 2004. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J. Comparat. Neurol 480, 101–114. [DOI] [PubMed] [Google Scholar]
- Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J, 2015. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke 46, 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Gao M, Ma J, Zhang M, Li S, Wu B, Nie X, Jiao J, Zhao H, Wang S, Yang Y, Zhang Y, Sun Y, Wicha MS, Chang AE, Gao S, Li Q, Xu R, 2015. Transdifferentiation-Induced neural stem cells promote recovery of middle cerebral artery stroke rats. PLoS One 10, e0137211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozbatiran N, Cramer SC, 2006. Imaging motor recovery after stroke. NeuroRx 3, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA, 2009. Human induced pluripotent stem cells free of vector and transgene sequences. Science (New York, N.Y.) 324, 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF, 2013. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res. Ther 4, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH, 2005. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J. Neurosci 25, 5333–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li J, Liu Y, Chen X, Kang Q, Zhao J, Li W, 2009. Human neural stem cell transplantation attenuates apoptosis and improves neurological functions after cerebral ischemia in rats. Acta Anaesthesiol. Scand 53, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li J, Liu Y, Chen X, Lu H, Kang Q, Li W, Gao M, 2011. Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology 31, 384–391. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W, 2015. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl. Med 4, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LR, Mattsson B, Johansson BB, 2000. Environmental influence on brain-derived neurotrophic factor messenger RNA expression after middle cerebral artery occlusion in spontaneously hypertensive rats. Neuroscience 97, 177–184. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Risedal A, Wojcik A, Hejzlar J, Johansson BB, Kokaia Z, 2001. Enriched environment influences brain-derived neurotrophic factor levels in rat forebrain after focal stroke. Neurosci. Lett 305, 169–172. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Berra HH, Duan WM, Singhal S, Mehta J, Apkarian AV, Kessler JA, 2007a. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke 38, 2804–2811. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, Kessler JA, Groothuis DR, 2007b. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Exp. Neurol 204, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LR, Singhal S, Duan WM, Mehta J, Kessler JA, 2007c. Brain repair by hematopoietic growth factors in a rat model of stroke. Stroke 38, 2584–2591. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Piao CS, Murikinati SR, Gonzalez-Toledo ME, 2013. The role of stem cell factor and granulocyte-colony stimulating factor in treatment of stroke. Recent Pat. CNS Drug Discov 8, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ong LK, Johnson S, Nilsson M, Walker FR, 2017. Chronic stress induced disruption of the peri-infarct neurovascular unit following experimentally induced photothrombotic stroke. J. Cereb. Blood Flow Metab 37, 3709–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Guan YM, Huang HL, Wang QS, 2014. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol. Sin 35, 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel S, Weiller C, Liepert J, 2007. Reboxetine improves motor function in chronic stroke. A pilot study. J. Neurol 254, 197–201. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB, 2005. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436, 261–265. [DOI] [PubMed] [Google Scholar]


