Abstract
Quantification of neuro-behavioural responses of intact small model organisms has been proposed as a sensitive, sub-lethal alternative to conventional toxicity testing. Such bioassays are characterized by a high physiological and ecological relevance, short response times, increased sensitivity, and non-invasive nature. Despite a significant potential for predictive aquatic toxicology analysis of behavioural traits of micro-invertebrates in microfluidic environment has received little attention. In this work, we demonstrate a new Lab-on-a-Chip technology capable of effectively caging freshwater rotifers Brachionus calyciflorus for real-time video-microscopy analysis. We demonstrate that behavioural bioassays performed under microfluidic perfusion can significantly enhance the sensitivity of conventional ecotoxicology test protocols.
INTRODUCTION
Toxicological assays utilizing small model organisms and measuring responses occurring at sub-lethal levels of chemical stressors are increasingly postulated to better approximate environmentally relevant concentrations of pollutants.1,2 Furthermore, every living organism is infinitely dynamic system characterized by temporal adaptive responses.3,4 Following the subtle effects of chemical stressors over time is at present rarely used in predictive environmental toxicology, despite the fact that phenotypic effects of toxicants depend to a large degree on exposure duration and not only their concentration.3 In current environmental guidelines, it is still a common practice to predominantly assess lethal concentrations (LCs) of chemicals at fixed time points.2 This historic approach utilized in both acute and chronic toxicological tests reduces costs and simplifies the protocols but at this same time losses much of the physiologically and ecologically important data as well as reduces sensitivity of the assays.1–3
Behaviour is one of the sub-lethal endpoints that can change temporally and relative to an acute exposure to contaminants providing an early-warning stress response.1,2,4–6 It has also significant implications for chronic exposures where alterations in behavioral traits can be linked to diminished ecological fitness.4 The ability to move, often referred to as locomotory activity or motility, is an essential component of an organism's fitness. A speed with which every animal moves often determines the rate at which it encounters objects such as food, obstacles, and predators.2,7,8 As a result, chemical factors such as environmental toxicant that influences animal's ability to swim through its environment may have significant ecological implications such as, e.g., impact on feed rate, mating, or predator escape.1,4,6,7,9,10 Various behavior traits of aquatic invertebrates and vertebrates such as locomotory activity, responses to stimuli, predator-prey relations, toxin avoidance as well as 3D spatial orientation have been reported to be effected by pollutants at less than lethal concentrations.2,5,11 Behavior end-points have therefore significant potential in ecologically relevant assays and ecological risk assessment.2,12 Importantly, phenotype-based behavioural analysis embraces the complexity of intact multicellular organisms in a strategy to identify ecological effects of chemicals with particular biological action.5–7,13 Tracking the behavior in time integrates multiple levels of biological organization and provides a link between sub-cellular and physiological processes intertwined in both adaptive and defense responses.2,4 Behaviour reflects functional changes at highest levels of organizations and has been reported to be up to 1000 times more sensitive than the conventional mortality tests.1–4
Applications of biomicrofluidic technologies for neuro-behavioural phenomics are relatively new concepts that received very little attention.14,15 Several most recent reports have outlined successful applications of larger, millifluidic devices for behavioral analysis of macro-invertebrates such as amphipods, daphnids, and brine shrimp larvae.16–18 Effective analysis of planktonic micro-invertebrates represents, however, a new set of analytical challenges due to their very small size, fragility, and difficulties in continuously imaging objects in a three-dimensional space. Behavioural analysis of planktonic micro-invertebrates requires video-data acquisition of continuously and rapidly swimming animals. One of the main problems associated with behavioral studies in conventional bioanalytical vessels is minimally invasive caging of small aquatic invertebrates and restricting animal movements in the z-plane (vertically) thus keeping all specimens swimming within acceptable depth of field (DOF) of the video-microscopy system. Moreover, commercially available video processing algorithms are optimized to allow animal detection and tracking based on contrast principles.19–23 The reliable and reproducible contrast-based tracking of small moving objects video-recorded in conventional static chambers is challenging because of: (i) significant variations in size between animals and chambers; (ii) uncontrolled vertical animal moments causing them to move out of focus; (iii) occurrence of edge shadows, light reflections due to the presence of significant water meniscus; and (iv) microevaporation that by changing the level of water meniscus in time impacts brightness levels in video files.16,18,24 As a result, optimal illumination and imaging parameters necessary for collection of time-resolved video data cannot be easily achieved in conventional test vessels such as Petri dishes or multi-well culture plates.16,18
The above limitations can be addressed much better in microfluidic systems that can be designed to match the size of the caging chambers to the specimens, restrict vertical animal movements as well as eliminate the imaging artifacts of water meniscus and micro-evaporation.14,15 Furthermore, a significant advantage of the microperfusion culture is a straightforward capability to conduct temporal in situ drug treatments such as pulsed chemical delivery, wash-out, and re-perfusion experiments.14–16,18 Perfusion delivery of drugs and toxicants can also help to eliminate the biggest drawbacks of static culture environment such as limited availability of the chemicals due to surface adsorption; degradation of tested chemicals, accumulation of metabolites, and byproducts; depletion of oxygen; and alterations in pH of culture media.14–16,18 The lack of miniaturized analytical tools has so far prevented emergence of new test protocols that could otherwise enhance the temporal resolution and thus the interpretive power of ecotoxicological data.2,14,15
In this work, we demonstrate a new chip-based system developed for effective restriction of freshwater bioindicator planktonic rotifers for real-time video-microscopy analysis of their behavioral responses. We provide new evidence that tracking time-resolved alterations in motility of rotifers caged in microfluidic environment can be successfully used in sub-lethal aquatic ecotoxicity bioassays, significantly increasing sensitivity of the conventional test protocols.
MATERIALS AND METHODS
Test organisms and drug exposures
Dormant eggs (cysts) of the freshwater planktonic rotifer Brachionus calyciflorus were purchased as a part of ROTOXKIT-F (MicroBioTests, Inc., Belgium).25–27 To stimulate hatching of juvenile stages, cysts were placed in 2 ml of pre-aerated standard freshwater medium (4 mg/l KCl; 96 mg/l NaHCO3; 123 mg/l MgSO4·7H2O; and 120 mg/l CaSO4·2H2O dissolved in deionized water with pH adjusted to 7.5) at 25 ± 1 °C and continuously illuminated at 3500 ± 500 lux for 16 h. All test chemicals were dissolved directly in the medium. Stock solutions and dilutions were prepared immediately before use and allowed to equilibrate at 25 ± 1 °C for at least 1 h whilst being aerated.
Chip design and microfabrication
Photolithography masks were designed in SolidWorks 2017 (SolidWorks Corp., USA) software, exported as Tagged Image File Format (TIFF) files and printed on transparencies using a photoplotter with a resolution of up to 3800 dots per inch (dpi). Relief molds were fabricated in a high contrast, epoxy based SU-8 3050 negative photoresist (Microchem, USA). The photoresist was chosen based on its applications in fabricating thick layers in a single coat as well as capability to produce high aspect ratio structures with clean vertical walls. Standard photolithography procedures described elsewhere were used for imaging and development of the SU-8. Subsequent replica molding was performed in a biocompatible elastomer poly(dimethylsiloxane) (PDMS; Sylgard 184; DowCorning Corp., Midland, MI, USA) using standard methods as described before.28
Toxicity bioassays
Mortality-based bioassays were performed in control 36-well polycarbonate plates supplied with the ROTOXKIT-F (MicroBioTests) and in the chip-based prototypes. All test utilized freshly hatched 18-h old larvae of B. calyciflorus. Control tests were conducted in static conditions as per manufacturer's and International Organization for Standardization (ISO) standard protocol 19 827, while tests in the microfluidic devices employed an open-loop perfusion with a volumetric flow rate of 15 μl/min. The rotifers were deemed non-viable if no movements were detected in 5 s of observation after gentle agitation of vessels. The tests were considered valid when the mortality in the negative controls did exceed 10%. Positive controls with a reference toxicant potassium dichromate (K2Cr2O7) were employed to ensure lack of variability between different batches of cysts used in experiments performed on different days.
Behavioural video-microscopy bioassays
B. calyciflorus larvae were manually injected into the loading port of each device under a stereomicroscope. The input port of each device was connected to a computer-controlled peristaltic pump (MiniPuls Evolution, Gilson, Inc., USA) to provide actuation at a nominal flow rate of 15 μl/min in an open-loop perfusion. Video-imaging was realized using a Nikon SMZ18 stereomicroscope (Nikon Corp., Japan) equipped with a 2.8-megapixel CMOS digital camera (Infinity 3–3, Lumenera Corp., Canada). In all experiments, 60 s long videos at a rate of 48 frames per seconds (fps) and resolution of 960 × 728 pixels were automatically captured every hour for up to 24 h. Trajectory reconstruction and analysis of locomotory parameters in each video clip were performed using Ethovision XT 13 (Noldus Information Technology, Wageningen, The Netherlands) software as described before.16 Tracking was based on the principle of contrast detection. For this to occur, test animals to be tracked must be placed against a contrasting background. Software algorithms defined the animals during the detection stage using the difference in pixel intensity between the animals and the background.19–23 Subsequently, x-y coordinate pairs were automatically assigned to centroids of each detected objects. The software simultaneously recognized and tracked up to 10 animals per defined area of the chamber each frame at the time.19–23 During the tracking stage, time-stamped x-y coordinates of selected animals were calculated and written into a final data file for each frame. The specimens' trajectories were then automatically reconstructed based on time-stamped x-y coordinates and plotted as graphical data. Behavioral parameters (i.e., average distance travelled average speed, number of active organisms) were extracted from data files for each chip-based device.19–23 All data generated were specific to individual animals and were then averaged providing values for the whole population of test specimens. Averaged parameters for each group were integrated into a time sequence and saved in an Excel file.
Statistical analysis
Data from different experiments of both acute toxicity and behavioural tests were pooled. The behavioural data were averaged out as distance travelled per rotifer per unit of time (minute). Cumulative distance was measured by summing the incremental distance moved by each animal between frames during 60 s long video clips. Data were then averaged between 10 individual animals kept in the same chamber. Dose-response curves were extrapolated using ToxRat v3.1 software (ToxRat Solutions GmbH, Alsdorf, Germany). Data sets were then tested for normality of distribution using Shapiro-Wilk's test. Log or square root transformations were applied to reduce skewness when data were not normally distributed. A non-parametric Kruskal–Wallis H test was applied to evaluate statistical difference between samples. When significant exposure effects were found, a post-hoc Tukey's test was performed to identify significance between treatment-related effects and time point with independently run control tests. Significance was set at p < 0.05.
RESULTS AND DISCUSSION
So far, there has been a notable absence of user-friendly technologies that can facilitate quantitative behavioral analysis in rapidly swimming planktonic micro-invertebrates using automatic image acquisition systems.2,14,15 This is mostly because of very small size (50–200 μm) of micro-invertebrates, their relative fragility, and significant difficulties in continuously imaging rapidly swimming microscopic animals in a three-dimensional space using traditional vessels such as multi-well plates and Petri dishes. To demonstrate applicability of biomicrofluidic chip-based systems in behavioral ecotoxicology, we have designed a new system for efficient micro-caging of freshwater rotifers B. calyciflorus in aquatic toxicity assays.29–36
The design of chip-based devices featured three integrated modules: (i) a main channel for microperfusion of medium and toxicants; (ii) a caging chamber surrounded by an array of semicircular bollards that upon loading restricted test specimens inside the space of the caging chamber; and (iii) a loading channel for introduction of test specimens into the device [Figs. 1(a) and 1(b)]. To load the specimens, the devices were mounted in a special holder and positioned on the microscope stage [Fig. 1(c)]. Ten B. calyciflorus larvae were first aspirated into a 100 μl Hamilton glass syringe and then manually injected into the loading port of the device using Polyether ether ketone (PEEK) tubing. Specimen loading was confirmed microscopically, using bright-field imaging. The chip's total internal volume was approximately 14 μl, while the volume of the caging chamber was 2 μl. The tip of the loading channel had an aperture approximately 2 μm smaller in width than the average dimensions of the test specimens. This allowed creating one-way entrapment functionality where elastic properties of PDMS permitted rotifers to be injected through the loading port but effectively prevented backflow of actively swimming specimens upon loading them into the device.
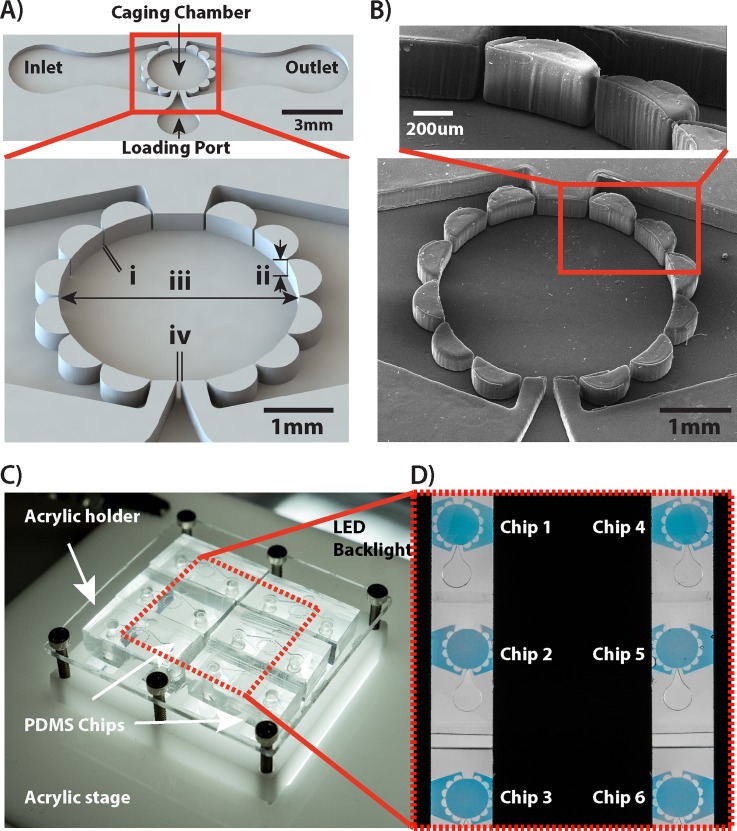
FIG. 1.
Design of a microfluidic chip-based device for behavioural studies on planktonic micro-invertebrates. (a) 3D CAD rendering illustrating main components of the device. Dimensions: i = 50 μm, ii = 300 μm, iii = 2.9 mm, and iv = 80 μm. (b) A scanning electron micrograph (SEM) depicting the magnified view of structures restricting animal movements within the caging chamber. The device was fabricated in PDMS using standard photo- and soft-lithography microfabrication protocols. (c) A custom designed laser-cut in acrylic holder that accommodates six PDMS chip-based devices and provides efficient fluidic interconnects as well as integrated light diffuser. (d) A micrograph depicting six microfluidic chips imaged simultaneously by the video-imaging system. The black region is a non-transparent acrylic cover that prevents the stray backlight to reach the camera sensor. Chip based devices were filled with a Trypan blue dye for clarity of presentation.
The design of the device provided a minimally invasive perfusion culture of rotifers while restricting rapidly swimming animals in an area of view of the video imaging system [Fig. 2(a)]. Up to six devices could be imaged simultaneously [Figs. 1(c) and 1(d)]. The 300 μm height of the caging chamber restricted animal movements in the z-plane thus keeping all specimens swimming within acceptable depth of field (DOF) of the stereomicroscope lens. The acquired video files were recorded at a rate of 48 frames per seconds (fps) using a high-resolution camera. This enabled simultaneous imaging of multiple chambers without any need for motorized microscope stage system. The recorded videos were of sufficient resolution that upon post-processing that involved file area cropping to depict individual chambers, contrast adjustment, sharpening, and background subtraction, they enabled detection and tracking of up to 10 specimens per each of the six imaged chambers using Ethovision XT software [Fig. 2(a)]. The method applied for optical contrast-based tracking used automated analysis of pixel intensity change between subsequent frames of the video field. Thanks to background subtraction performed at the post-processing step, the animals were outlined as white objects against a black contrasting background [Fig. 2(a)]. Automated video field analysis avoided any need for manual counting and provided reconstructed 2D x-y trajectories as a function of time thus enabling mining of behavioural data (i.e., average distance travelled, average speed, acceleration, number of active organisms, etc.) for individual animals as well as population averages [Fig. 2(a)].
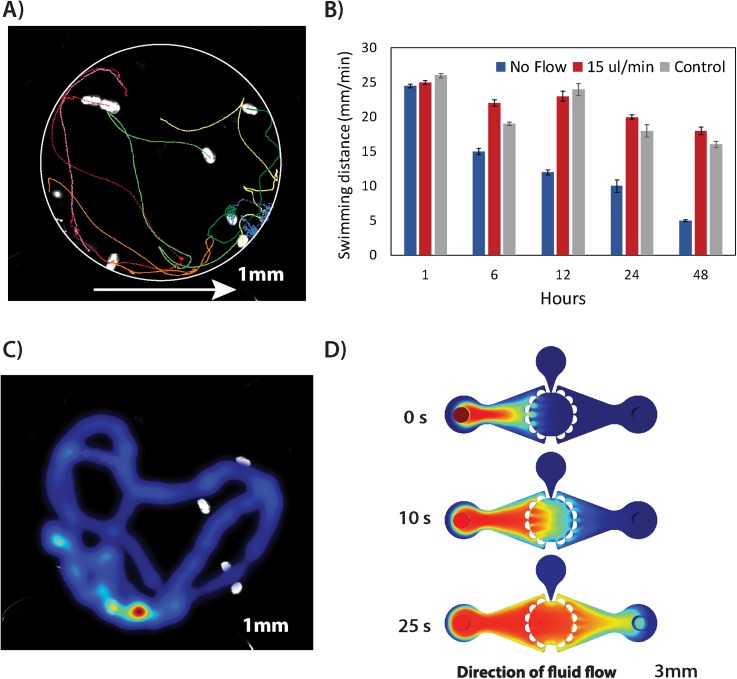
FIG. 2.
Validation of microfluidic environment for minimally invasive perfusion culture of caged rotifers. (a) Reconstruction of animal 2D trajectories in a caging test chamber when perfused at a volumetric flow rate of 15 μl/min. The plot denotes an average distance travelled [mm] of ten specimens caged in a microfluidic device. The white circle is a superimposed outline of the trapping chamber; arrow indicates a direction of the fluid flow. (b) Comparative analysis of rotifers' swimming behaviour (mean distance travelled ± SE) kept under different fluidic conditions. (c) Occupancy map depicting cumulative regions of animal presence in x-y space during 60 s of video when perfused at a volumetric flow rate of 15 μl/min. (d) Computational fluid dynamics (CFD) simulations of a mass transfer inside the chip-based device actuated at a flow rate of 15 μl/min.
To optimize the fluidic environment, the survival as well as native behavioural traits of animals kept in chip-based devices were compared to standard test plates. The results indicated over 90 ± 5% (n = 200, p < 0.05) survival at flow rates of up 100 μl/min. These results were in agreement with control culture conditions where the assays are considered valid when mortality does not exceed 10% (n = 200, p < 0.05). The analysis of locomotor behaviour revealed that at flow rates exceeding 50 μl/min, the rotifers could not overcome the streamlines and over time was forced against caging bollards at the outlet side of the chip. In contrast under stop flow condition (0 μl/min), organisms remained viable but a progressive hypoactivity was observed approximately 3 h after cessation of perfusion [Fig. 2(b)]. As observed in other studies, the progressive hypoactivity under stop-flow conditions is most likely indicative of gradual oxygen depletion due to high metabolic rate of actively swimming metazoan organism [Fig. 2(b)]. It was empirically assessed that at flow rates of up to 15 μl/min, the animals were able to maintain their normal swimming pattern for up to 48 h without adversary effects of the perfusion on their behavioural activity as compared with controls [Figs. 2(b) and 2(c)]. At flow rates of up to 15 μl/min, the distribution of specimens within the chamber was also similar to static plates without excessive edge behaviour as shown by occupancy map analysis [Fig. 2(c)]. Interestingly, a population density did not significantly affect behaviour inside the micro-caging chamber with similar results obtained in chambers loaded with 1, 3, 5, and 10 animals (n = 50, p < 0.05). Importantly, average software tracking error was <10% even for simultaneous analysis of up to 10 animals per chamber.
Using computational fluid dynamics (CFD) simulations and subsequent experiments with a model 0.5% Trypan Blue dye, it was estimated that a complete and uniform exchange of medium in the caging chamber can be achieved in approximately 25 s at the volumetric flow rate of 15 μl/min [Fig. 2(d)]. This provides with an ability to briefly expose the specimens to toxicants followed up by the immediate wash-out of chemicals, a feature of particular importance for investigations of temporary impact of, e.g., neurotoxins or any neuroactive chemicals such as anaesthetic agents.
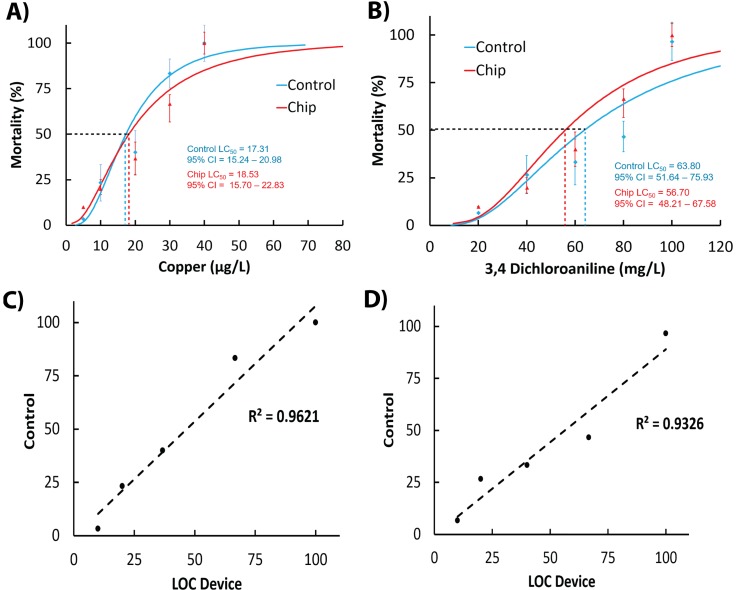
The aims of proof-of-concept validation studies were to determine if: (i) conventional median lethal concentrations (LC50 at 24 h) generated on lab-on-a-chip (LOC) devices correlate with data obtained in standard test protocols and (ii) time-resolved video-microscopy analysis can be used to develop significantly more sensitive sub-lethal behavioural endpoints for toxicity analysis. Tests were conducted with model toxicants such as: heavy metal copper chloride and 3,4-dichloroaniline (3,4-DCA), precursor for the synthesis, and degradation of several herbicides. The 24 h acute toxicity tests performed on both perfusion-based chip device and standard multi-well plates, resulted in comparable LC50 values of 17.3 and 18.5 μg/l for copper chloride and 57 and 63 mg/l for 3,4-DCA, respectively [Figs. 3(a) and 3(b)]. Pearson linear correlation analysis between results of the two experimental methods had a R2 value of <0.96 (p < 0.05) indicating that microfluidic conditions alone did not affect the bioassay outcomes making them reproducible with standard conditions [Figs. 3(c) and 3(d)].
FIG. 3.
Comparisons between conventional 24 h acute toxicity tests on chip devices and standard multi-well plates with heavy metal copper chloride (a) and herbicide metabolite 3,4-DCA (b). Pearson linear regression analysis between chip-based and reference conditions treated with heavy metal copper chloride (c) and herbicide metabolite 3,4-DCA (d). Scores of mortality were obtained using manual counting method according to the standard ROTOXKIT-M test protocol. Chip-based devices were actuated at a constant volumetric flow rate of 15 μl/min. Pearson linear regression analysis between chip-based and reference conditions.
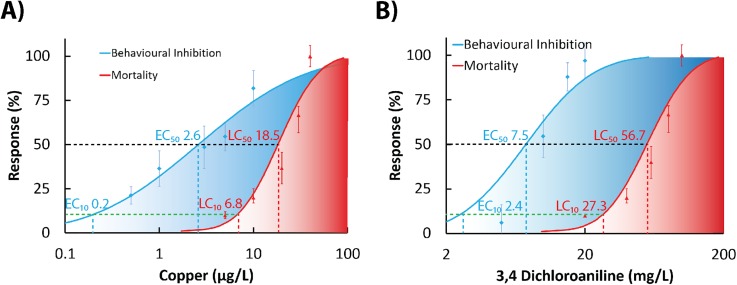
A key benefit of employing neuro-behavioural toxicity testing is the ability to quantify alternations in native locomotor as well as sensorimotor responses to sub-lethal concentrations of toxicants, such as those commonly occurring in real-world scenarios. The alteration in swimming characteristics is one of the simplest but most frequently used end-points in behavioural ecotoxicology.7 Using our new chip-based device, we were able to successfully cage and perfuse rotifers with model toxicants. Quantification of changes in swimming activity at 24-h end-point revealed that behavioural end-points were significantly more sensitive than mortality indices [Figs. 4(a) and 4(b)]. Using standard toxicological dose-response curve extrapolation, we calculated and then compared effective concentrations (EC) with lethal concentrations (LC) used in behavioural and mortality assays, respectively. ECx values were defined as concentrations of a chemical inducing behavioral change in x% of test animals, while LCx values were defined as concentrations of a chemical inducing mortality in x% of test animals. Accordingly, for copper chloride, the calculated behavioral EC10 and EC50 values were 0.2 μg/l and 2.6 μg/l, while mortality LC10 and LC50 values were 6.8 μg/l and 18.5 μg/l, respectively [Fig. 4(a)]. Similarly, responses to 3,4-DCA yielded behavioral EC10 and EC50 values of 2.4 mg/l and 7.5 mg/l, while mortality LC10 and LC50 values were 27.3 mg/l and 56.7 mg/l, respectively [Fig. 4(b)]. The obtained numerical dose-response data demonstrate that on-chip behavioral assay has detected impact of toxicants at concentrations below any detectable values (LOEC, lowest observable effect concentration) in mortality bioassays [Figs. 4(a) and 4(b)]. Tests performed simply by analyzing motility of caged rotifers in the last 60 s of 24 h exposure provided on average 10 fold increase in sensitivity for both toxicants that belong to dissimilar chemical classes [Figs. 4(a) and 4(b)].
FIG. 4.
Sub-lethal ecotoxicity tests performed in a microfluidic environment increase sensitivity of the ecotoxicity bioassays on B. calyciflorus. Comparison between sensitivity of conventional mortality dose-response curves (red areas) and video-microscopy enabled analysis of locomotor activities (blue areas) performed on perfusion-based chip devices with heavy metal copper chloride (a) and 3,4-DCA (b). Super-imposed comparative data sets were normalized to respective control values and depicted as mean values ±SE. Analysis of locomotory activity was performed in the final minute of 24-h exposure. Scores of mortality were obtained using manual counting method according to the standard ROTOXKIT-M test protocol in the last hour of exposure to toxicants. Chip-based devices were actuated at a constant volumetric flow rate of 15 μl/min. EC10 and EC50 values were defined as concentrations of a chemical inducing behavioral change in 10% and 50% of test animals, respectively. LC10 and LC50 values were defined as concentrations of a chemical inducing mortality in 10% and 50% of test animals, respectively.
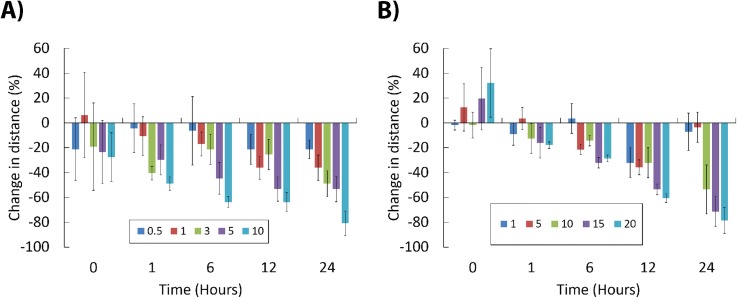
The temporal analysis of behavioral activities revealed that copper chloride induced a progressive hypoactive syndrome [Fig. 5(a)]. The 25 ± 15% depression of movement was detected immediately after initiating perfusion with 10 μg/l of copper chloride. After 1 h of exposure, the depression in locomotor activities was observed at all copper concentrations above 3 μg/l [Fig. 5(a)]. Note that the latter concentration was classified as NOEC (non-observable effect concentration) in 24-h mortality bioassays and would be ignored during conventional chemical risk assessment. The reduction of locomotion was progressively more pronounced with the exposure duration where after 12 h of exposure even concentrations of 0.5 and 1 μg/l induced a statistically significant hypoactivity of 20% and 40%, respectively [Fig. 5(a)].
FIG. 5.
Time-resolved alterations in rotifer swimming behaviour at sub-lethal concentrations of toxicants. Data were obtained after reconstruction of trajectories and are represented as change in average swimming distance (±SE), upon exposure to increasing concentrations of copper chloride (a) and 3,4-DCA (b). Positive and negative changes denote hyperactivity and hypoactivity syndromes, respectively. Chip-based devices were actuated at a constant volumetric flow rate of 15 μl/min.
Similar time-resolved video-microscopy analysis revealed that immediate exposure to low concentrations of organic compound 3,4-DCA (5–20 mg/l) induced a transient hyperactivity syndrome. It was defined here as 10%–30% increase in motility observed immediately after 60 s of exposure [Fig. 5(b); p < 0.05]. Transient agitation response often referred to as hyperactivity syndrome or avoidance behaviour can be hypothesized as a rapid E1-type alarm reaction that is an attempt to escape the affected area.1,16 The hyperactivity was followed by a progressive retardation of swimming activity already after 1 h of exposure to concentrations of 3,4-DCA above 5 mg/l [Fig. 5(b)]. This trend continued over time with B. calyciflorus motility becoming significantly depressed after 12 h of exposure at all tested concentrations [Fig. 5(b)]. Interestingly, there was observable recovery of animals exposed to the lowest concentrations of 1 and 5 mg/l identified after a 24 h of exposure [Fig. 5(b)]. This phenomenon might be attributed to induction of adaptive metabolic processes (e.g., synthesis of enzymes involved in biotransformation of toxins) and/or points to reversible action of dichloroaniline at least in low concentration range. This rather interesting finding obtained using in situ behavioral phenotyping requires further investigation as it might provide new data on mechanism of action of herbicide runoff on freshwater zooplankton communities.
CONCLUSIONS
Planktonic rotifers are particularly suitable for the analysis of the swimming speed behavior because they spend most of the time actively swimming.2,7,29–31,37 So far, only a handful of reports have investigated swimming of rotifers (less than 3% of all behavioral ecotoxicology studies published to date), while even less studies were performed on other pelagic micro-invertebrates as well as larval stages of macro-invertebrates.2 One of the main limitations when studying micro-invertebrate behavioral traits is lack of technologies that can enable straightforward restriction of movement for video-microscopy analysis.2,14 Behavioural ecotoxicology and neuro-behavioural phenomics, in general, are areas of biological investigation that for their progress and further development rely heavily on innovations in technological components.2 The lack of the latter was until recently a significant impediment for behavioural ecotoxicology coming of age.14
This work demonstrates an effective microfluidic caging of free-swimming freshwater planktonic micro-invertebrates for neuro-behavioural analysis in ecotoxicology. The restriction of freely swimming test specimens inside the miniaturized chambers enabled high-resolution optical data acquisition using video microscopy and subsequent tracking and reconstruction of animal trajectories in response to perturbations by environmental pollutants. The combination of microfluidic device, video-microscopy, and animal tracking algorithms provided the ability to conduct new temporal sub-lethal aquatic ecotoxicity bioassays. The latter were characterized by significantly increased sensitivity over the conventional test protocols. In all tested scenarios, the swimming alteration parameter proven to be more sensitive than mortality or immobility. Importantly, the behavioral end-points measured on a chip were more sensitive measures of toxicity independently from the tested compound mode of action, i.e., heavy metal vs. organic pesticide.
The open-loop perfusion delivery of toxicants in microfluidic devices can also help in eliminating common problems encountered in static cultures such as: limited availability of the chemicals due to surface adsorption; degradation of tested chemicals, accumulation of metabolites and byproducts; depletion of oxygen; and alterations in pH of culture media. Finally, significant advantage of the microperfusion culture is capability to conduct temporal in situ drug treatments such as pulsed delivery of toxins followed by wash-out and re-perfusion.
The main motivation of the presented LOC design was simplicity of both fabrication as well as operational principles. We strived to develop a minimally invasive perfusion culture of rotifers while restricting rapidly swimming animals for video-microscopy enabled biotests. The straightforward design is applicable for inexpensive behavioral ecotoxicity test protocols that can be easily and rapidly replicated in biological laboratories with minimal investment and effort. Many environmental and ecological laboratories find advanced and complex microfluidic fabrication processes too daunting due to their engineering nature and turn instead to readily available classical platforms. We hope that further development of simple and user-friendly technologies, as well as their deployment in many applications of behavioural toxicology, will be of wide interest to the ecotoxicology community. Accordingly, the authors welcome all collaborative enquires and are happy to freely share prototypes and work with all interested stakeholders to enable widespread adoption of innovative Lab-on-the-Chip technologies for applications in aquatic toxicology and experimental zoology. It is our hope that innovations in microfluidic systems will translate into new predictive toxicity bioassays aimed at dynamic and rapid detection of sub-lethal behavioral end-points such as changes in speed of movement or distance traveled by small aquatic bioindicator invertebrates. The behavioral bioassays performed in microfluidic environment represent a very promising tool for assessment of environmentally relevant effects of contaminants and are characterized by very rapid readout, high sensitivity, and relative non-invasiveness. Future directions for this enabling technology involve applications to uncover how sub-lethal concentrations inducing neurobehavioral alterations correlate with effects on growth and reproduction as well as how do they impact ecologically relevant behavioural responses such as ability to hunt and avoid predation.
In closing, we postulate that applications of biomicrofluidic systems and video-microscopy can provide a range of innovative bioassays for sub-lethal phenotype-based in situ screening in environmental risk assessment as well as experimental ecology and zoology.
ACKNOWLEDGMENTS
This project was supported by the Australian Research Council under Grant No DE130101046 (D.W.), RMIT Vice-Chancellor's Senior Research Fellowship (D.W.), the MicroNano Research Facility (MNRF), and Australian Postgraduate Award 2014 (R.C.). The authors thank Professor Dayanthi Nugegoda for providing access and help with ToxRat® toxicological analysis software. The authors declare no competing financial interests.
References
- 1. Hellou J., Environ. Sci. Pollut. Res. 18(1), 1–11 (2011). 10.1007/s11356-010-0367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Faimali M., Gambardella C., Costa E., Piazza V., Morgana S., Estevez-Calvar N., and Garaventa F., Mar. Environ. Res. 128, 36–45 (2017). 10.1016/j.marenvres.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 3. Heckmann L. H., Baas J., and Jager T., Environ. Toxicol. Chem. 29(6), 1396–1398 (2010). 10.1002/etc.163 [DOI] [PubMed] [Google Scholar]
- 4. Pyle G. and Ford A. T., Aquat. Toxicol. 182, 226–228 (2017). 10.1016/j.aquatox.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 5. Gerhardt A., Hum. Ecol. Risk Assess. 13(3), 481–491 (2007). 10.1080/10807030701340839 [DOI] [Google Scholar]
- 6. Bownik A., Sci. Total Environ. 601–602, 194–205 (2017). 10.1016/j.scitotenv.2017.05.199 [DOI] [PubMed] [Google Scholar]
- 7. Garaventa F., Gambardella C., Di Fino A., Pittore M., and Faimali M., Ecotoxicology 19(3), 512–519 (2010). 10.1007/s10646-010-0461-8 [DOI] [PubMed] [Google Scholar]
- 8. Alonso A., De Lange H. J., and Peeters E. T., Chemosphere 75(3), 341–346 (2009). 10.1016/j.chemosphere.2008.12.031 [DOI] [PubMed] [Google Scholar]
- 9. Semsari S. and Megateli S., Environ. Technol. 28(7), 799–806 (2007). 10.1080/09593332808618841 [DOI] [PubMed] [Google Scholar]
- 10. Faimali M., Garaventa F., Piazza V., Greco G., Corra C., Magillo F., Pittore M., Giacco E., Gallus L., Falugi C., and Tagliafierro G., Mar. Biol. 149(1), 87–96 (2006). 10.1007/s00227-005-0209-9 [DOI] [Google Scholar]
- 11. Gerhardt A., de Bisthoven L. J., and Schmidt S., Hydrobiologia 559, 433–441 (2006). 10.1007/s10750-005-1259-1 [DOI] [Google Scholar]
- 12. Gerhardt A., Janssens de Bisthoven L., and Soares A. M., Environ. Sci. Technol. 39(11), 4150–4158 (2005). 10.1021/es048589f [DOI] [PubMed] [Google Scholar]
- 13. Chapman P. M., Hum. Ecol. Risk Assess. 13(3), 478–480 (2007). 10.1080/10807030701340888 [DOI] [Google Scholar]
- 14. Campana O. and Wlodkowic D., Environ. Sci. Technol. 52(3), 932–946 (2018). 10.1021/acs.est.7b03370 [DOI] [PubMed] [Google Scholar]
- 15. Campana O. and Wlodkowic D., Sens. Actuators, B 257, 692–704 (2018). 10.1016/j.snb.2017.11.002 [DOI] [Google Scholar]
- 16. Huang Y. S., Campana O., and Wlodkowic D., Sci. Rep. 7, 17603 (2017). 10.1038/s41598-017-17892-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cartlidge R., Nugegoda D., and Wlodkowic D., Sens. Actuators, B 239, 660–670 (2017). 10.1016/j.snb.2016.08.058 [DOI] [Google Scholar]
- 18. Huang Y. S., Persoone G., Nugegoda D., and Wlodkowic D., Sens. Actuators, B 226, 289–298 (2016). 10.1016/j.snb.2015.11.128 [DOI] [Google Scholar]
- 19. Delcourt J., Becco C., Ylieff M. Y., Caps H., Vandewalle N., and Poncin P., Behav. Res. Methods 38(4), 704–710 (2006). 10.3758/BF03193904 [DOI] [PubMed] [Google Scholar]
- 20. Church J. S., Martz D. G., and Cook N. J., Behav. Res. Methods 38(3), 434–438 (2006). 10.3758/BF03192797 [DOI] [PubMed] [Google Scholar]
- 21. Marechal J. P., Hellio C., Sebire M., and Clare A. S., Biofouling 20(4–5), 211–217 (2004). 10.1080/08927010400011674 [DOI] [PubMed] [Google Scholar]
- 22. Noldus L. P., Spink A. J., and Tegelenbosch R. A., Behav. Res. Methods Instrum. Comput. 33(3), 398–414 (2001). 10.3758/BF03195394 [DOI] [PubMed] [Google Scholar]
- 23. Spink A. J., Tegelenbosch R. A., Buma M. O., and Noldus L. P., Physiol. Behav. 73(5), 731–744 (2001). 10.1016/S0031-9384(01)00530-3 [DOI] [PubMed] [Google Scholar]
- 24. Blackiston D., Shomrat T., Nicolas C. L., Granata C., and Levin M., PLoS One 5(12), e14370 (2010). 10.1371/journal.pone.0014370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belgis C. and Persoone G., Ecotoxicol. Environ. Saf. 60(1), 73–80 (2005). 10.1016/j.ecoenv.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 26. Belgis C. and Guido P., Chemosphere 50(3), 365–372 (2003). 10.1016/S0045-6535(02)00496-4 [DOI] [PubMed] [Google Scholar]
- 27. Fernandez-Casalderry A., Ferrando M. D., and Andreu-Moliner E., Bull. Environ. Contam. Toxicol. 48(1), 14–17 (1992). 10.1007/BF00197477 [DOI] [PubMed] [Google Scholar]
- 28. Fuad N. M., Kaslin J., and Wlodkowic D., Biomicrofluidics 11(5), 051101 (2017). 10.1063/1.5001848 [DOI] [Google Scholar]
- 29. Charoy C. and Janssen C. R., Chemosphere 38(14), 3247–3260 (1999). 10.1016/S0045-6535(98)00557-8 [DOI] [Google Scholar]
- 30. Charoy C. P., Janssen C. R., Persoone G., and Clement P., Aquat. Toxicol. 32(4), 271–282 (1995). 10.1016/0166-445X(94)00098-B [DOI] [Google Scholar]
- 31. Coulon P. Y., Charras J. P., Chasse J. L., Clement P., Cornillac A., Luciani A., and Wurdak E., Hydrobiologia 104, 197–202 (1983). 10.1007/BF00045968 [DOI] [Google Scholar]
- 32. Guo R. X., Ren X. K., and Ren H. Q., Bull. Environ. Contam. Toxicol. 89(3), 568–571 (2012). 10.1007/s00128-012-0712-x [DOI] [PubMed] [Google Scholar]
- 33. Janssen C. R., Ferrando M. D., and Persoone G., Ecotoxicol. Environ. Safe 28(3), 244–255 (1994). 10.1006/eesa.1994.1050 [DOI] [PubMed] [Google Scholar]
- 34. Janssen C. R., Persoone G., and Snell T. W., Aquat. Toxicol. 28(3–4), 243–258 (1994). 10.1016/0166-445X(94)90036-1 [DOI] [Google Scholar]
- 35. Janssen C. R., Rodrigo M. D. F., and Persoone G., Hydrobiologia 255, 21–32 (1993). 10.1007/BF00025816 [DOI] [Google Scholar]
- 36. Snell T. W. and Persoone G., Aquat. Toxicol. 14(1), 81–91 (1989). 10.1016/0166-445X(89)90056-8 [DOI] [Google Scholar]
- 37. Preston B. L., Snell T. W., Fields D. M., and Weissburg M. J., Aquat. Toxicol. 52(2), 117–131 (2001). 10.1016/S0166-445X(00)00140-5 [DOI] [PubMed] [Google Scholar]