Abstract
In budding yeast, actin disruption prevents nuclear division. This has been explained as activation of a morphogenesis checkpoint monitoring the integrity of the actin cytoskeleton. The checkpoint operates through inhibitory tyrosine phosphorylation of Cdc28, the budding yeast Cdc2 homolog. Wild-type Schizosaccharomyces pombe cells also arrest before mitosis after actin depolymerization. Oversized cells, however, enter mitosis uninhibited. We carried out a careful analysis of the kinetics of mitotic initiation after actin disruption in undersized and oversized cells. We show that an inability to reach the mitotic size threshold explains the arrest in smaller cells. Among the regulators that control the level of the inhibitory Cdc2-Tyr15 phosphorylation, the Cdc25 protein tyrosine phosphatase is required to link cell size monitoring to mitotic control. This represents a novel function of the Cdc25 phosphatase. Furthermore, we demonstrate that this cell size-monitoring system fulfills the formal criteria of a cell cycle checkpoint.
INTRODUCTION
To maintain a constant size during cellular proliferation, cell growth must be coordinated with the rate of cell division. On reaching a sufficient cell mass, at a point termed START in yeast and the restriction point in mammalian cells, cells commit to DNA replication and cell division. However, cell growth is not restricted to G1 but continues throughout most of the cell cycle. Whereas a number of mechanisms have been described that couple cell growth to progression into S phase, the mechanism to ensure that cells achieve the required cell mass for the entry into mitosis remains unclear (Neufeld and Edgar, 1998; Polymenis and Schmidt, 1999).
Exponentially growing fission yeast cells spend most of their lives in G2. They grow by extension at one or two cell ends until they reach a critical size, a G2/M cell size threshold. Cells then cease to grow and enter mitosis. Cells can modulate the size threshold in response to external stimuli such as nutritional conditions. On a decrease in available nitrogen source the threshold drops and larger cells accelerate their entry into mitosis. An increase in the nitrogen supply has the opposite effect, temporarily arresting mitosis until cells reach the higher size threshold (Fantes and Nurse, 1977; Young and Fantes, 1987). Initiation of mitosis in S. pombe is determined by a balance between inhibitory Tyr15 phosphorylation of Cdc2 and its reversal. Three major regulators affect the level of Tyr15 phosphorylation: the protein tyrosine kinases Wee1 and Mik1 and the protein tyrosine phosphatase Cdc25 (Coleman and Dunphy, 1994; MacNeill and Nurse, 1997; Rhind and Russell, 1998a). Wee1 has been implicated in cell size control because wee1-deficient cells do not exhibit changes in cell size at mitosis after nutritional shifts (Fantes and Nurse, 1978). Cdr1 (also known as Nim1), a protein kinase negatively regulating Wee1 (Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993), appears to be a part of the regulatory module because cdr1-deficient cells are unable to adjust their size over a range of nitrogen concentrations (Young and Fantes, 1987; Belenguer et al., 1997). Wee1 is present mainly in the nucleus and Cdr1 mainly in the cytoplasm. Shuttling of Cdr1 or Wee1 across the nuclear envelope has been proposed as a mechanism controlling cell size in S. pombe (Wu et al., 1996). A contribution from the Cdr2 protein kinase (Kanoh and Russell, 1998; Breeding et al., 1998) is also likely, because cdr2 mutants are also largely insensitive to changes in the nitrogen supply in the media (Young and Fantes, 1987). Wee1 is up-regulated even after inhibition of protein synthesis by cycloheximide (Suda et al., 2000). Last, the rates of synthesis of two proteins involved in initiation of mitosis, Cdc25 and the cyclin B homolog Cdc13, appear to be hypersensitive to general translation activity, thus providing a potential link between cell growth and the timing of mitosis (Daga and Jimenez, 1999).
In S. pombe, Cdc2-Tyr15 dephosphorylation and mitosis are inhibited in response to the activation of the DNA replication and damage checkpoint. Tyr15 becomes phosphorylated during S phase, most likely due to the predominant presence of the Mik1 kinase. During G2 phase, when Mik1 becomes degraded, Cdc25 is kept inactive if DNA damage is detected (Rhind and Russell, 1998a, 2001). In Saccharomyces cerevisiae, destabilization of the actin cytoskeleton delays nuclear division. This delay has been termed a morphogenesis checkpoint (Lew and Reed, 1995; McMillan et al., 1998). Cells treated with the actin-depolymerizing drug latrunculin A or exposed to high osmolarity and a number of mutants with defects in polarity and the actin cytoskeleton display high levels of phosphorylation on Cdc28-Tyr19 (a residue in a S. cerevisiae Cdc2 homolog that corresponds to Tyr15). Swe1 (a Wee1 homolog) is required for inhibition of Cdc28 and execution of the checkpoint. Swe1, normally rapidly degraded, becomes stabilized in these cells. This stabilization is associated with a reduced level of Swe1 phosphorylation, which is in turn dependent on two proteins, Hsl1 and Hsl7. These proteins are localized to the daughter side of the mother-bud neck and their function is responsive to the organization of the septin scaffold in the neck. Hsl1 is one of the three S. cerevisiae kinases with catalytic domains similar to the S. pombe Cdr1 and Cdr2 kinases. Swe1 can be also stabilized in response to activation of the Mpk1 MAP kinase. In addition, Mih1 (a Cdc25 homolog) has been also implicated in execution of the checkpoint (McMillan et al., 1998; Lew, 2000; Longtine et al., 2000; Harrison et al., 2001).
In S. pombe, latrunculin B, a drug related to latrunculin A, has been recently reported to cause an intramitotic arrest mediated by the stress-activated protein kinase Spc1 pathway. In the presence of the drug, a checkpoint mechanism is activated that delays sister chromatid separation in response to misaligned mitotic spindles. It has been argued that the checkpoint ultimately monitors the integrity of the actin cytoskeleton that is involved in providing a spatial cue for orienting short mitotic spindles by astral microtubules (Gachet et al., 2001).
Here we carefully examine the kinetics of the progression of the S. pombe cells into mitosis after actin disruption by latrunculin A, the most potent actin inhibitor currently available (Spector et al., 1989; Ayscough et al., 1997). We found that cells with a completely depolymerized actin cytoskeleton arrest the cell cycle before entering mitosis and that the arrest is brought about by an inability to reach the critical cell size rather than by actin disruption per se. We have identified Cdc2-Tyr15 dephosphorylation as the critical step that is subject to regulation in response to perturbation of cell growth and Cdc25 as the key control element. We also show that the G2/M cell size homeostasis system fulfills the formal criteria of a cell cycle checkpoint.
MATERIALS AND METHODS
Yeast Strains and General Methods
General genetic methods are described for S. pombe (Alfa et al., 1993). All strains used in this work (Table 1) were derived from wild-type strains 972h−S or 975h+S. Strains were grown in yeast extract medium containing adenine (YEA) or Edinburgh minimal medium (EMM). For synchronization in early G2 (Edwards and Carr, 1997), cells were grown in YEA overnight at 25°C to late exponential phase. Cell suspension (100 ml) was concentrated to 2 ml and layered on two 10-ml continuous gradients of 7–30% lactose in YEA. Cells were separated by centrifugation at 800 rpm for 6 min at 25°C. One milliliter of suspension from the top of the distribution of cells in each gradient was collected and resuspended in fresh YEA media at a density of ∼2 × 106 cells/ml at the appropriate temperature. Latrunculin A (Molecular Probes, Eugene, OR) was added from a 2 mM stock solution in dimethyl sulfoxide to 0.5-ml cultures of cells. Mock treatment consisted of addition of dimethyl sulfoxide alone to the same final concentration. KCl was added from a 4 M stock and hydroxyurea from a 240 mM stock, both in YEA. For the nutritional shift experiments cells were grown in EMM, filtered, washed rapidly with the same medium lacking the nitrogen source (EMM-N), and resuspended in EMM-N.
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| Q250 | 972h− | Lab stock |
| Q272 | h−cdc2-3w leu1-32 | Lab stock |
| Q868 | h−leu1-32 | Lab stock |
| Q999 | h+cdc2-3w cdc25∷ura4 ura4-D18 | Lab stock |
| Q1434 | h+wee1-50 pyp3∷ura4 ura4-D18 leu1-32 | Lab stock |
| Q1521 | h−cdc25-22 leu1-32 | Lab stock |
| Q1518 | h−cdc10-129 leu1-32 | Lab stock |
| Q1519 | h+cdc10-129 leu1-32 | Lab stock |
| Q1523 | h+wee1-50 leu1-32 | Lab stock |
| Q1528 | h+ssp1Δ ade6-704 leu1-32 | Rupešet al., 1999 |
| Q1937 | h−wee1-50 mik1∷ura4 ura4-D18 | Lab stock |
| Q1945 | h+cdc10-129 wee1-50 leu1-32 | This study |
| Q1946 | h−cdc10-129 wee1-50 leu1-32 ura4-D18 | This study |
| Q1959 | h+cdc10-129 leu1-32 ura4-D18 | This study |
| Q1960 | h+cdc10-129 wee1-50 leu1-32 ura4-D18 | This study |
| Q1962 | h−cdc10-129 wee1-50 mik1∷ura4 ura4-D18 leu1-32 | This study |
| Q1963 | h+cdc10-129 wee1-50 mik1∷ura4 ura4-D18 leu1-32 | This study |
| Q1965 | h−wee1-50 mik1∷ura4 pyp3∷ura4 ura4-D18 leu1-32 | This study |
| Q1967 | h−cdc10-129 cdc2-3w leu1-32 | This study |
| Q1968 | h+cdc10-129 wee1-50 mik1∷ura4 pyp3∷ura4 ura4-D18 leu1-32 | This study |
| Q1970 | h−cdc10-129 cdc2-3w cdc25∷ura4 ura4-D18 leu1-32 | This study |
| Q973 | h− pMNS21L(T-cell PTPase integrated) cdc25-22 leu1-32 | Gould et al., 1990 |
| Q1972 | h− pMNS21L(T-cell PTPase integrated) ura4-D18 | This study |
| Q1975 | h+cdc2-3w cdc25∷ura4-D18 leu1-32 | This study |
| Q1976 | h− pMNS21L(T-cell PTPase integrated) cdc25∷ura4 ura4-D18 | This study |
| Q1978 | h+ pMNS21L(T-cell PTPase integrated) cdc25∷ura4 ura4-D18 leu1-32 | This study |
| IH845 | plo1-GFPS65T | I. Hagan |
Fluorescence Microscopy
To visualize actin, cells were fixed by addition of formaldehyde to a final concentration of 7.4% and fixed for 7 min, washed once with the PEM buffer (Rupešet al., 1999), permeabilized with Triton X-100, washed with PEM and stained with 3.33 mg/ml tetramethylrhodamine B isothiocyanate-phalloidin (Sigma, St. Louis, MO). For immunofluorescence, cells were fixed in 100% methanol at −20°C and labeled with the TAT-1 monoclonal antibody (Woods et al., 1989) and the Sad1 antiserum (Hagan and Yanagida, 1995) and subsequently with Alexa Fluor 488-anti-mouse and Alexa Fluor 568-anti-rabbit secondary antibodies (Molecular Probes), both in the presence of 1% fish skin gelatin in PEM, and mounted in 1 μg/ml 4,6-diamidino-2-phenylindole in PEM. A Leitz DMRB (Leica Microsystems, Deerfield, IL) fluorescence microscope equipped with a high-performance charge-coupled device camera (Cooke SensiCam, Auburn Hills, MI) and Slidebook (Intelligent Imaging Innovations, Denver, CO) image analysis software was used to acquire images. The images were restored by nearest neighbors deconvolution. Z-axis projections of the three-dimensional stacks are presented. Final processing was done with the use of Adobe Photoshop (Adobe Systems, Mountain View, CA). All images in each series were taken and processed with the use of identical parameters. To quantify progression into mitosis, 50-μl samples of cells were fixed by addition to 100 μl of 100% methanol and stained with 1 μg/ml 4,6-diamidino-2-phenylindole in PEM to visualize nuclear DNA. Cells (150–250) were examined in each sample and mitotic and postmitotic cells (beginning with uninucleate cells containing condensed chromosomes) were counted in each sample. Cell length and cell thickness in the middle of the cell were measured in 34–68 cells where appropriate. Cell volume was calculated from these measurements with the use of a described formula (Mitchison, 1957).
Cdc2-Tyr15 Phosphorylation Assay
Approximately 108 cells/sample were harvested by filtration and washed with a stop buffer (Rhind and Russell, 1998b) and then lysed with glass beads in a lysis buffer (Rhind and Russell, 1998b). The lysates were separated on SDS-PAGE gels and blotted onto Immobilon-P (Millipore, Bedford, MA) membranes, probed with the anti-phospho-cdc2 (Tyr15) antibody (New England Biolabs, Beverly, MA), and the bands were detected with the use of the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ). The membranes were then stripped from the antibodies and reprobed with anti-PSTAIRE monoclonal antibody (a gift from Steve Reed) or anti-cdk1/cdc2 (PSTAIR) polyclonal antibody (Upstate Biotechnology, Lake Placid, NY) to obtain the loading control.
Histone H1 Kinase Activity Assay
Cells were collected as described above and lysed in an HB buffer (Moreno et al., 1989). The Cdc2/Cdc13 complex was precipitated from the lysates with the use of Suc1-agarose beads (Upstate Biotechnology), washed with the HB buffer and the kinase assay was performed in the presence of a KIN buffer with histone H1 (Upstate Biotechnology) as substrate (Moreno et al., 1989). The protein mixture was separated by SDS-PAGE and the levels of phosphorylation detected by autoradiography.
RESULTS
Cells Delay Mitosis and Reduce Cell Growth in Latrunculin A
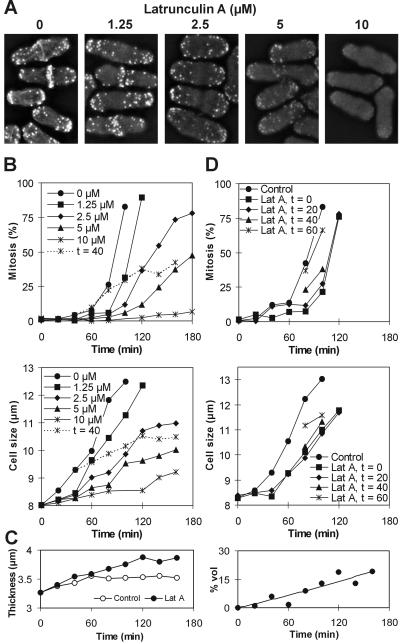
Latrunculin A perturbed the actin cytoskeleton in a concentration-dependent manner (Figure 1A). To examine whether a morphogenesis checkpoint operates before mitosis in S. pombe, wild-type cells were synchronized in early G2 and released into medium containing various concentrations of the drug. As for S. cerevisiae (McMillan et al., 1998), S. pombe cells delayed their entry into mitosis, depending on the drug dose. At the same time, however, they reduced their extension growth to a similar extent (Figure 1B). To test for the relationship between the time of actin disruption and mitotic initiation, we added a high dose of latrunculin A at 40 min, immediately before the onset of mitosis in the synchronized culture. Despite the late addition of the drug, mitosis was still prevented in a majority of cells, and cells were arrested at a larger average cell size (Figure 1B). To confirm that cell length is an appropriate measure of cell size even in cells with perturbed actin cytoskeleton, we also measured cell thickness in these cells. Then we evaluated the contribution of cell swelling in the presence of latrunculin A to the total increase in cell volume. Swelling due to latrunculin A accounted for 6.5% of the total cell volume after 1 h and 14% after 2 h in the presence of the drug, respectively (Figure 1C). This confirms that within this time span, cell length is a reliable measure of cell size in latrunculin A-treated cells.
Figure 1.
Cells delay mitosis and reduce cell growth in the presence of latrunculin A. (A) Filamentous actin displays a varying degree of derangement depending on the dose of latrunculin A. Exponentially growing wild-type cells were treated with the drug for 10 min, fixed, and stained with tetramethylrhodamine B isothiocyanate-phalloidin. (B) Percentage of cells that have initiated mitosis (top) and the average cell size in the population (bottom) in wild-type cells. Cells were synchronized in early G2 and released into the indicated concentrations of latrunculin A. In one culture, 10 μM latrunculin A was added at time = 40 min after the release. (C) Thickness of cells from experiment in B measured in the middle of the cell length (left) and the contribution to the total cell volume attributable to thickening of cells in the presence of 10 μM latrunculin A (right). (D) Percentage of cells that have initiated mitosis (top) and the average cell size in the population (bottom) in wild-type cells in which 1 μM latrunculin A (Lat A) was added at indicated times after the synchronization in early G2.
We next added a low dose of latrunculin A, which does not completely prevent mitosis, at different times before the onset of mitosis in synchronized cells. The progressively later treatment had proportionately less effect both on mitotic timing and on cell growth (Figure 1D). Based on these data, the mitotic delays could be explained either by the disruption of the actin cytoskeleton per se or by the reduction of cell growth.
Morphogenesis Checkpoint Does not Operate before Mitosis in S. pombe
To test directly the latter possibility, we synchronized cells in G2 by cdc25-22ts arrest at high temperature. While arrested, the cells continued their growth past the size at which they enter mitosis when grown at 25°C (17.2 ± 1.0 μm), until they reached an average size of at least 34 μm. While maintained at high temperature, cells were exposed to latrunculin A for different periods of time and subsequently released to the permissive temperature, still in the presence of the drug. Under these conditions, nearly all detectable polymerized actin disappears within 10 min (Rupešet al., 1999; Figure 1A). Nevertheless, cells entered mitosis with no significant delay even if they had previously spent up to 1 h in the presence of the drug (Figure 2A). Cells pretreated with the drug for 2 h, approximately the generation time of wild-type cells at 35°C, were blocked from entering mitosis, although they maintained undiminished viability for at least another 24 h (our unpublished data). These results are not consistent with a physiologically relevant checkpoint activated directly by a perturbation of the actin cytoskeleton. Rather the arrest is an indirect side effect of the presence of latrunculin A or the prolonged absence of functional actin filaments.
Figure 2.
A morphogenesis checkpoint does not operate before mitosis in S. pombe. (A) Percentage of cdc25-22ts cells that have initiated mitosis after the release from cdc arrest. Cells were incubated at 36°C for 4 h (3 h in the case of the t = −120 sample) and 10 μM latrunculin A was added at indicated times before the release to 25°C. (B) Percentage of cdc25-22ts cells that have initiated the second mitosis after release from high-temperature arrest. Cells were synchronized in early G2 and arrested at 36°C for 1 (left) or 3 h (right) and then released to 25°C. Latrunculin A (Lat A; 10 μM) or 0.6 M KCl was added before the second mitosis at indicated times.
To test more rigorously for the presence of a morphogenesis checkpoint, we examined the response of the cdc25-22ts cells during the second synchronous cycle after release from arrest. To achieve homogeneity in cell size, cells were first presynchronized in early G2 and then shifted to the high temperature. Two populations of cells differing in size were generated by varying the duration of the arrest. One population consisted of cells whose length fell below the mitotic size threshold after the first mitosis (“undersized” cells, relative to the size at which mitosis occurs in unperturbed cells at permissive temperature). These cells had to spend some additional time in G2 before they reached the mitotic size (16.9 ± 0.9 μm on average at ∼3.5 h after the onset of the first mitosis). The other population consisted of cells whose length exceeded the size threshold even after the first mitosis (“oversized” cells). These cells entered mitosis at the average size of 20.7 ± 1.7 μm after a further incompressible minimum time of 2.5 h. Thus, for the second mitosis, cell size was a limiting factor for the undersized but not for the oversized cells. To avoid the interference by the prolonged exposure to latrunculin A (Figure 2A), the drug was added not more than 1 h before mitosis occurred in unperturbed cells. Addition of latrunculin A within this time window caused dramatically different responses in the two cell populations: the oversized cells entered mitosis with a minimum delay, whereas the undersized cells remained arrested (Figure 2B). These results show that actin depolymerization per se does not block entry into mitosis in S. pombe. The oversized cells were also exposed to 0.6 M KCl, which caused a 40-min delay of the onset of mitosis (Figure 2B). This shows that while initiating mitosis regardless of the presence of latrunculin A, these cells were still able to arrest in response to other environmental insults and therefore were not committed to mitosis. Also, it shows that a separate mechanism is responsible for a G2/M delay after a hyperosmotic shock, and this delay is not a direct consequence of the temporary actin depolarization associated with the stress response (Chowdhury et al., 1992; Rupešet al., 1999).
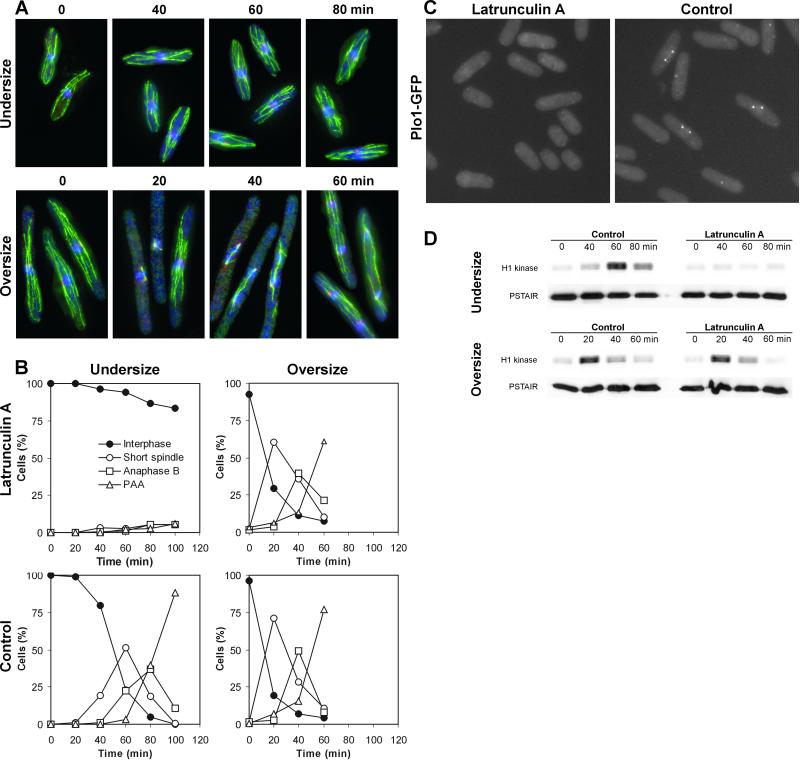
When treated with latrunculin B, cells arrest before sister chromatid separation (Gachet et al., 2001). To verify that latrunculin A causes a true premitotic arrest, we visualized spindle pole bodies and microtubules by immunofluorescence in cdc25-22ts cells. Cells of different sizes were generated by varying the duration of the arrest at high temperature. Then they were exposed to latrunculin A for 1 h and released to low temperature. The results show that undersized cells arrested with unduplicated spindle pole bodies and interphase arrays of microtubules. The oversized cells progressed through mitosis with kinetics indistinguishable from the mock-treated control (Figure 3, A and B). A small population of undersized cells leaked through the premitotic block, but none of these cells accumulated in the short spindle stage to any substantial degree (Figure 3B). Next, we examined the localization of GFP-tagged Plo1, an S. pombe polo kinase homolog. The localization of Plo1 to the spindle pole bodies is regarded as the earliest detectable mitotic event (Mulvihill et al., 1999). Wild-type cells expressing Plo1-GFP were synchronized in early G2 and exposed to latrunculin A 1 h before mitosis occurred in the control. Cells were then examined for progression through mitosis. Whereas control cells displayed a clear pattern of localization of Plo1 into the spindle pole bodies, the cells treated with latrunculin A did not (Figure 3C). We also carried out the histone H1 kinase activity assay in cdc25-22ts cells treated identically to the cells shown in Figure 3, A and B. Again, the undersized cells did not display an increase in the kinase activity when treated with latrunculin A, confirming the premitotic arrest (Figure 3D). These experiments show that cells exposed to latrunculin A either arrest before entering mitosis or progress through mitosis with no apparent delay, depending on their size.
Figure 3.
Cells arrest before mitosis or progress through mitosis unperturbed in the presence of latrunculin A. (A) Microtubules (green), Sad1 (red), and nuclear DNA (blue) in cdc25-22ts cells. Cells were arrested at 36°C for 1 (left) and 3 (right) h, 10 μM latrunculin A was added, and cells were incubated at high temperature for another hour. Then they were released to 25°C (t = 0) and samples for immunostaining were taken at indicated times. Representative cells are presented. (B) Percentages of cdc25-22ts cells from the previous experiment at different stages of mitosis. PAA, postanaphase array of microtubules. (C) Cellular localization of Plo1-GFP. Wild-type cells expressing Plo1-GFP were synchronized in early G2, 10 μM latrunculin A was added 20 min after the release, cells incubated for another 60 min, and examined under the microscope. (D) Histone H1 kinase activity in cells treated as described in A.
We confirmed these results, although achieving less synchrony due to the arrest early in the cell cycle, with the use of a cdc10-129ts strain to generate oversized cells by arresting and releasing them in G1. This showed that the results were not dependent on manipulating cdc25 itself (our unpublished data).
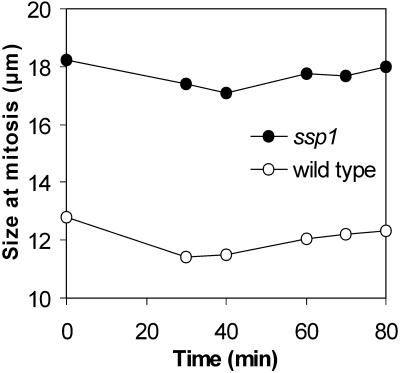
A morphogenesis checkpoint might monitor completion of other events that require rearrangements of the actin cytoskeleton. New end take-off (NETO), a switch from unipolar-to-bipolar growth that occurs in G2 (Mitchison and Nurse, 1985), represents such an event in S. pombe. We asked whether preventing NETO could cause a delay of mitosis. NETO is abolished and mitosis is delayed in ssp1Δ cells cultured at 35°C. We have previously shown that the NETO block in these cells can be overridden by pulse treatment with latrunculin A, presumably by releasing free actin monomers (Rupešet al., 1999). If the NETO block were the cause of the mitotic delay in unipolar ssp1Δ cells, latrunculin A pulse treatment should accelerate their entry into mitosis and mitosis should occur at or near the wild-type size. However, no significant drop in cell size was detected (Figure 4). Taken together, these data strongly suggest that a morphogenesis checkpoint analogous to the one proposed in S. cerevisiae (Lew and Reed, 1995; McMillan et al., 1998) does not operate before mitosis in S. pombe. Instead, the data confirm that S. pombe cells arrest progression into mitosis if they fail to reach the mitotic size threshold.
Figure 4.
Size of cells in mitosis after latrunculin A pulse treatment. Cells were grown at 35°C for 4 h then pulsed for 2 min with 10 μM latrunculin A and the size of uninuclear cells with condensed chromosomes was measured at each time point.
Cell Size Control Is Preserved in Cells Unable to Phosphorylate Tyr15
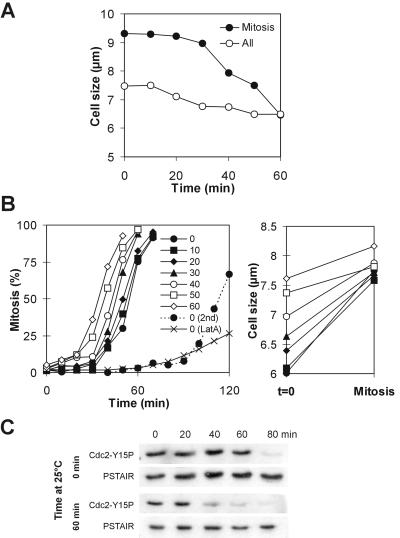
These results provide a powerful new tool to investigate the relationship between cell size and the cell cycle control. To see whether the Cdc2-Tyr15 dephosphorylation pathway is targeted by the cell size-monitoring system, we examined the wee1-50ts mik1Δ double mutant. Both Cdc2-Tyr15 kinase activities are effectively absent in this strain after Wee1 is inactivated by a temperature shift (Lundgren et al., 1991); therefore, any possible differential entry into mitosis must be due to differential regulation of Tyr15 dephosphorylation. It has been proposed that after the temperature shift, wee1-50ts mik1Δ cells initiate mitosis indiscriminately at all sizes (Novak et al., 1998). This predicts that after a shift the average size of mitotic cells in asynchronous culture should abruptly collapse to the level of the average size of all cells in the population. Instead, we observed that the two values converged gradually over a period of 1 h (Figure 5A). We asked whether this could be caused by cell size retaining some control over mitotic initiation even in this genetic background. wee1-50ts mik1Δ cells were synchronized in early G2 and allowed to grow at permissive temperature for various durations (Figure 5B). Depending on the time spent at the permissive temperature, the cells attained different sizes and initiated mitosis at different times after the shift. The sizes of cells at the first mitosis clustered around a single value, ∼7.7 μm (Figure 5B). Cells then rapidly entered the second mitosis, at a size of 5.2 ± 0.4 μm, which led to severe mitotic defects as described in the literature (Lundgren et al., 1991). The duration of the delay correlated with the phosphorylation state of Tyr15 on Cdc2 (Figure 5C). These data clearly demonstrate a correlation between cell size and mitotic initiation in wee1-50ts mik1Δ cells during the first mitotic cycle after the shift.
Figure 5.
Mitotic initiation correlates with cell size in wee1-50ts mik1 cells. (A) Average cell sizes in an exponential culture of wee1-50ts mik1Δ cells. The cells were shifted to 36°C at t = 0. (B) Percentage of wee1-50ts mik1Δ cells synchronized in early G2 that have initiated mitosis (left) and average cell sizes at t = 0 and at mitosis (right). Cells were shifted to 36°C (t = 0) after indicated times spent at 25°C. The second mitosis is plotted for one culture (2nd) and 10 μM latrunculin A was added to another at time = 0 (Lat A) and entry into the first mitosis plotted. (C) Cdc2-Tyr15 phosphorylation in wee1-50ts mik1Δ cells synchronized in early G2 and grown at 25°C for indicated times before the shift to 36°C at t = 0.
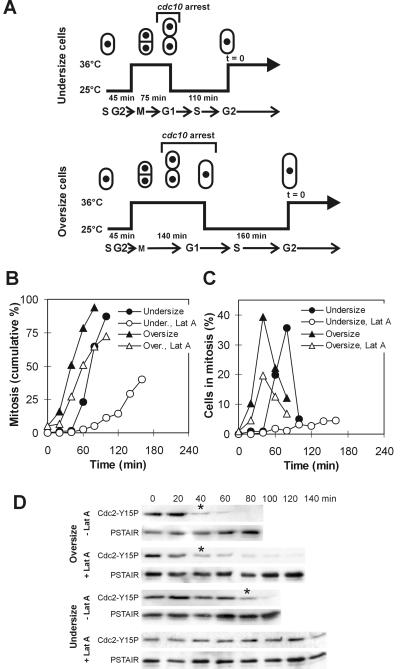
Next, we examined the cdc10-129ts wee1-50ts mik1Δ triple mutant. Cells were presynchronized in early G2 and then arrested with the use of the cdc10 block in G1 (Figure 6A). Undersized and oversized cells, relative to mitotic size at the permissive temperature (9.1 ± 0.6 μm), were generated by varying the duration of the arrest. Cells were then allowed to pass through S phase and shifted back to the high temperature. To ensure completion of S phase, the timing of the shifts was set so that the number of “cuts”, i.e., cells attempting mitosis with unreplicated DNA, stayed below 5%. The results show that oversized cdc10-129ts wee1-50ts mik1Δ cells entered mitosis immediately after the shift, at an average size of 11.8 ± 1.7 μm (Figure 6B). The same cells treated with latrunculin A entered mitosis only with a slight delay at a size of 11.2 ± 1.5 μm (Figure 6B). In contrast, the mock-treated undersized cells delayed mitosis until they reached a size of 9.0 ± 1.0 μm; undersized cells treated with latrunculin A delayed mitosis for at least another hour. The difference could be seen even more clearly when only the number of cells undergoing mitosis was plotted. Although the heights of the peaks differed, the mitotic index in the mock- and drug-treated cultures of oversized cells peaked at the same time, whereas the peak was slightly delayed in undersized cells and no peak was apparent in the latrunculin A-treated undersized cells (Figure 6C). To confirm that these effects were not caused by the presence of the cdc10-129ts allele in the background, we treated synchronized wee1-50ts mik1Δ cells with latrunculin A and obtained analogous results, i.e., a >1-h mitotic delay compared with untreated cells (Figure 5B). These results indicate that cell size homeostasis was functioning in these cells even in the absence of Cdc2-Tyr15 phosphorylation activity. No additional delay was observed after the same regimen when pyp3 was deleted in the cdc10-129ts wee1-50ts mik1Δ strain. This suggests that the Pyp3 phosphatase, which can also dephosphorylate Cdc2-Tyr15 (Millar et al., 1992), does not significantly contribute to mitotic cell size regulation (our unpublished data). Cdc2-Tyr15 dephosphorylation displayed the same pattern as the timing of mitosis, confirming that no additional mechanism was responsible for blocking mitosis in these cells (Figure 6D). These results strongly suggest that Cdc25 is the key component of the mechanism that prevents mitosis in undersized cells.
Figure 6.
Cell size control over initiation of mitosis is preserved in cells lacking the Wee1 and Mik1 kinase activities. (A) Diagram summarizing the manipulations to obtain undersized and oversized cdc10-129ts wee1-50ts mik1Δ cells. Latrunculin A (Lat A; 10 μM) was added at time = 0. (B) Percentage of cells that have initiated mitosis after the treatment outlined in A. (C) Percentage of cells from the previous experiment undergoing mitosis (uninucleate or binucleate cells with condensed chromosomes) at each time point. (D) Cdc2-Tyr15 phosphorylation in cells treated as outlined in A. Asterisk indicates a dramatic drop in the Tyr15 phosphorylation level.
Cdc25 Is Required for Linking Cell Size Monitoring to G2/M Progression
We asked whether Wee1 or Mik1 is also regulated in response to cell size. Deletion of the cdc25 gene is lethal but cdc25Δ is viable in the cdc2-3w background. The cdc2-3w allele produces a kinase that is incompletely inhibited by Tyr15 phosphorylation although still somewhat sensitive to the degree of the phosphorylation (Russell and Nurse, 1987). A cdc10-129ts cdc2-3w cdc25Δ strain was used in an experiment complementary to the one described above. Here, only the Wee1 or Mik1 pathways could modulate the level of Tyr15 phosphorylation in response to cell size. The undersized cdc10-129ts cdc2-3w cdc25Δ cells were released from a brief cdc10 block and treated with latrunculin A. These cells exhibited only a minimum mitotic delay compared with the mock-treated control (Figure 7A). Importantly, these cells entered mitosis at a reduced cell size compared with the control (13.8 ± 2.2 μm, when latrunculin A was added at time = 60 min, and 17.3 ± 1.2 μm, respectively). This indicates that initiation of mitosis is uncoupled from the critical size requirement in cells lacking Cdc25. In contrast, the cdc10-129ts cdc2-3w cdc25Δ cells released into 12 mM hydroxyurea delayed mitosis for ∼20 min, consistent with the established roles of both Cdc25 and Mik1 in the DNA replication checkpoint (Rhind and Russell, 1998a, 2001) (Figure 7A). Only a slight drop in cell size at mitosis was detected (16.0 ± 1.3 μm).
Figure 7.
Cell size control over initiation of mitosis is not preserved in cells lacking cdc25. (A) Percentage of cdc10-129 cdc25Δ cdc2-3w cells that have initiated mitosis. Cells were synchronized in late G2, and incubated at 36°C for 2.5 h to induce one mitotic cycle and a brief G1 arrest. Cells were then released to 25°C and 10 μM latrunculin A (Lat A) or 12 mM hydroxyurea (HU) was added at indicated times after the release. (B) Percentage of the cdc25Δ cells expressing T-cell PTPase that have initiated mitosis (left) and average cell sizes in the population (right). A low-level T-cell PTPase expression was under the control of the inducible nmt1 promoter maintained in the “off” state. Cells were synchronized in early G2 and Lat A at indicated concentrations was added at t = 60.
To confirm that the previous result was not caused by the cdc2-3w allele, we carried out an independent experiment with the use of cells harboring the wild-type allele of cdc2. The human T-cell protein tyrosine phosphatase (T-cell PTPase) can dephosphorylate Cdc2-Tyr15 and rescue the loss of Cdc25 activity in vivo, thus making Cdc2-Tyr15 dephosphorylation insensitive to upstream regulation (Gould et al., 1990). Cells constitutively expressing T-cell PTPase in the cdc25Δ background were synchronized in early G2 and exposed to a range of latrunculin A concentrations. No delay of mitotic initiation was detected in latrunculin A-treated cells, although the cells reduced their growth in size depending on the drug dose, similar to wild type (Figure 7B). These results establish that regulation of Cdc25 activity is essential to link cell size to progression into mitosis in S. pombe. Furthermore, in cells lacking Cdc25, progression into mitosis can be uncoupled from reaching the mitotic size threshold by external perturbation of cell growth. Therefore, this regulatory system satisfies the formal criteria for a cell cycle checkpoint (Hartwell and Weinert, 1989).
Lowering Cell Size Threshold Allows Latrunculin A-treated Cells to Enter Mitosis
S. pombe cells reduce their size threshold for entering mitosis in response to reduced availability of nutrients such as nitrogen. This results in acceleration of entry into mitosis in cells that have become oversized with respect to the new cell size threshold (Fantes and Nurse, 1977; Young and Fantes, 1987). The prediction from our model is that nutritional downshift will accelerate initiation of mitosis in wild-type cells even in the presence of latrunculin A. An exponential culture of wild-type cells growing in a nitrogen-rich medium was split into four parts. One part was shifted into a medium lacking a nitrogen source and another into the same medium containing latrunculin A. The other two parts remained in the rich medium and one of them was treated with latrunculin A. Consistent with our previous results (Figure 1B), cells in the latter culture inhibited initiation of mitosis within 40 min after the addition of latrunculin A (Figure 8A). In contrast, both latrunculin A and mock-treated cells accelerated initiation of mitosis several times over the level in the control. Although the peak was lower in the presence of latrunculin A, the timing of the maximum response was identical, at 40 min after the shift (Figure 8A). These data demonstrate, according to the prediction, that upon lowering the cell size requirement for entering mitosis, otherwise unperturbed cells are able to enter mitosis even in the presence of latrunculin A.
Figure 8.
Cells initiate mitosis after mitotic cells size threshold is lowered by a nutritional downshift. (A) Percentage of wild-type cells undergoing mitosis after a nutritional downshift. At time = 0, exponential culture of cells was shifted from the EMM to EMM-N medium containing or lacking 10 μM latrunculin A. (B) Percentage of cdc25Δ cells expressing the T-cell phosphatase that undergo mitosis after the same treatment as described in A.
A second prediction from our model is that this acceleration will be delayed in cells that lack regulatable Cdc2-Tyr15 phosphatase activity. This is because mitotic initiation in these cells will only depend on the physiological inhibition of the Wee1 activity. We repeated the above-mentioned experiment with the cdc25Δ cells expressing the T-cell PTPase. In agreement with our earlier results (Figure 7B), cells exposed to latrunculin A in the rich medium did not inhibit initiation of mitosis within at least 1 h after the addition of the drug (Figure 8B). The cells shifted into the nitrogen lacking medium accelerated their entry into mitosis, but the response was delayed compared with wild type, peaking at ∼80 min after the shift. The cells shifted to the same medium containing latrunculin A followed the same kinetics up until 60 min after the shift and then the mitotic index declined due to the prolonged exposure of cells to the drug (Figure 8B). Taken together, these results provide further confirmation that initiation of mitosis depends on cell size and not on the integrity of the actin cytoskeleton.
DISCUSSION
Three major conclusions can be drawn from this study: first, a checkpoint monitoring actin integrity, as proposed in S. cerevisiae (McMillan et al., 1998) does not operate before mitosis in S. pombe; second, a powerful system of cell size homeostasis operates through a checkpoint mechanism; and third, the Cdc25 protein phosphatase is the major cell size checkpoint effector.
Lack of Actin Integrity Checkpoint
Although wild-type S. pombe cells respond to actin disruption in a way similar to S. cerevisiae (McMillan et al., 1998), i.e., by arresting progression into mitosis, it has been observed by others (Naqvi et al., 1999; Motegi et al., 2000) and ourselves that oversized S. pombe cells eventually enter mitosis. We extended the initial observations and carried out a detailed analysis of the kinetics of the mitotic initiation after actin disruption. A critical test revealed that actin disruption has little effect on progression into mitosis once the cell size restriction on mitosis has been lifted. This effectively disproves the existence of an actin integrity checkpoint, or a morphogenesis checkpoint as proposed by McMillan et al. (1998), operating before mitosis in S. pombe.
Neither inhibition of mitosis in undersized cells nor progression into mitosis in oversized cells was absolute in our experiments. Our results show that a small portion of undersized cells treated with latrunculin A always leaked into mitosis. The most likely reason is that although actin depolymerization greatly reduced cell growth, it did not stopped it completely and therefore some cells were able to reach the mitotic size threshold. On the other hand, the number of oversized cells entering mitosis tended to be slightly reduced in the presence of latrunculin A compared with the mock-treated control. It is necessary to consider two contributing factors. First, a long-term (>1-h) exposure to latrunculin A prevented initiation of mitosis under all circumstances. This long-term effect of the drug is not linked to progression through the cell cycle, because it also occurs in cells already arrested at the cdc25 execution point. One possibility is that this effect is caused by inhibition of protein synthesis observed after prolonged depolymerization of actin filaments (Iwig et al., 1995; Fasshauer et al., 1998). This also means that the existence of an actin checkpoint operating earlier in the cell cycle could not be formally ruled out. However, the biological significance of such a checkpoint, if it exists, would be unclear. Second, in two cases, noticeably fewer oversized cells entered mitosis even within the initial 1-h period of the drug treatment. Both occurred when cells were subjected to additional environmental insults at the beginning of the treatment. In the experiment in Figure 6, B and C, it was the synchronization procedure and the temperature shift, and in Figure 8A a nutritional shift accompanied with a change in osmolarity of the media. Actin remodeling is required for adaptation to stress (Chowdhury et al., 1992; Rupešet al., 1999). Therefore, it is conceivable that cells treated with latrunculin A have reduced ability to adapt to changes in the environment, which has an impact on their ability to enter mitosis. The KCl curve in Figure 2B further supports this hypothesis. Even in the above-mentioned two cases, however, these differences could not obscure the main result that a majority of oversized cells were able to rapidly initiate mitosis after actin had been depolymerized.
Morphogenesis versus Actin Integrity Checkpoint?
The concept of a morphogenesis checkpoint was introduced in S. cerevisiae (Lew and Reed, 1995; McMillan et al., 1998; Lew, 2000). It was first proposed as a mechanism that delays nuclear division when S. cerevisiae cells fail to form a bud (Lew and Reed, 1995). Later, it was broadened after it was found that nuclear division is also delayed in response to generalized disruption of the actin cytoskeleton, even when polarity establishment remained intact and cells had already formed a bud. It was proposed that the checkpoint monitors events at the level of actin organization (McMillan et al., 1998). Recently, it has been shown, however, that the checkpoint is triggered by a failure to properly organize the septin scaffold at the mother-bud neck, a crucial event during bud formation (Barral et al., 1999; Longtine et al., 2000). Perturbation of the actin cytoskeleton itself had little effect on this checkpoint pathway (Longtine et al., 2000). S. pombe cells in interphase lack a direct parallel to the dramatic morphogenetic event represented by bud formation. Cytokinesis, on the other hand, is a process that requires functional actin cytoskeleton and its failure can seriously affect the cells' chances for survival. Indeed, a G2/M checkpoint can arrest progression into mitosis until the cytokinesis in the previous cycle is completed (Liu et al., 2000). We have not been able to detect a similar checkpoint mechanism that would arrest initiation of mitosis after NETO failed to occur. This, however, may be related to the fact that the lack of NETO does not have a significant impact on cell survival (Mitchison et al., 1985; Rupešet al., 1999) and therefore there is little physiological need for such a checkpoint.
It has been reported recently that S. pombe cells treated with latrunculin B can enter mitosis but then arrest with misoriented short mitotic spindles and unseparated sister chromatids (Gachet et al., 2001). There are reasons to believe that the discrepancy between our data and the study by Gachet et al. (2001) is caused by different specificities of the two drugs. It has been repeatedly shown that latrunculin A is a more potent actin inhibitor than latrunculin B (Spector et al., 1989; Ayscough et al., 1997). Gachet et al. (2001) do not provide any measure of the degree of actin depolymerization under their experimental conditions, or its impact on cell growth. The authors used the same concentration of the drug in most of their experiments (10 μM) as was used in our study. Therefore, it is likely that incomplete depolymerization of actin allowed cells to grow and initiate mitosis, consistent with our results. It is important to note in this context that at least one study has reported the existence of a pool of actin filaments that is resistant to low doses of latrunculin B in mammalian cells (Ammar et al., 2001). This leaves open the possibility that a specific intracellular structure may have been affected differentially by the two drugs. The biochemical mode of action of latrunculin A has been extensively studied (Couéet al., 1987; Morton et al., 2000; Yarmola et al., 2000). To our knowledge, no direct comparison of the biochemical properties of latrunculin A and B is available, nor is a direct assessment of possible biological responses other than those resulting from actin depolymerization. Concerning possible different specificities of the drugs, however, there is evidence suggesting that latrunculin B is a stronger inhibitor of protein synthesis than latrunculin A, by an order of a magnitude (Fasshauer et al., 1998). Gachet et al. (2001) also present data showing accumulation of short spindles in exponentially growing cells of an actin mutant, cps8, again implying only a minor or selective disruption of the actin cytoskeleton. Thus, because actin reorganization is important for adaptation to external environment (Chowdhury et al., 1992; Rupešet al., 1999), a suspicion can be sustained that a combination of external stressors, such as the synchronization procedure that included a dramatic temperature shift used by the authors (4–30°C; Gachet et al., 2001), and a partial or selective disruption of the actin cytoskeleton accounts for the intramitotic arrest the authors observed.
On the other hand, one may argue that an unknown nonspecific effect of latrunculin A causes premitotic arrest in S. pombe cells. However, even if we reject the above-mentioned argument in favor of the potential latrunculin A nonspecificity, we are left with the differential response to the drug in undersized and oversized cells. In this study, populations of cells that have fulfilled the cell size requirement for entering mitosis were generated by two fundamentally different ways: first, by producing oversized cells by release from a cdc arrest; and second, by reducing this cell size requirement by nutritional shift in an otherwise unperturbed exponential culture of wild-type cells. In both cases, only the oversized cells were able to initiate mitosis. Therefore, either argument leads to the same conclusion, that cell size is the factor that controls progression into mitosis, rather than generalized actin integrity.
The lack of the checkpoint monitoring actin integrity in S. pombe is in striking contrast to the model proposed in S. cerevisiae (McMillan et al., 1998). Given the general conserved nature of the actin cytoskeleton and cell cycle controls in various species, this issue is not trivial and requires further clarification. In addition, S. cerevisiae cells (Lew and Reed, 1995) as well as S. pombe (this study) temporarily halt progression into mitosis after hyperosmotic shock. It has been argued that the actin integrity checkpoint is responsible for the nuclear division arrest after environmental insults in S. cerevisiae (Lew and Reed, 1995; Harrison et al., 2001). We found, however, that a mechanism that is not related to actin perturbation arrests progression into mitosis after the same treatment in S. pombe. The simplest way to reconcile the two sets of data, without assuming fundamental differences in the basic molecular organization of the actin cytoskeleton between the two species, would be to predict that perturbation of cell growth might inhibit mitosis in both types of yeast. To our knowledge, the possible effect of cell growth has not been examined in S. cerevisiae in connection with the actin integrity checkpoint. Although in S. cerevisiae nuclear division is not thought to be linked to cell size control under normal conditions, it would be intriguing to see whether perturbation of cell growth may at least partially account for nuclear division arrest after actin disruption in these cells. Taken together, it is possible that across different cell types, individual morphogenetic events rather than general actin integrity may be monitored by checkpoints that may halt mitosis in case the event fails to occur, as initially proposed in S. cerevisiae (Lew and Reed, 1995).
Cell Size Threshold and Cell Size Checkpoint
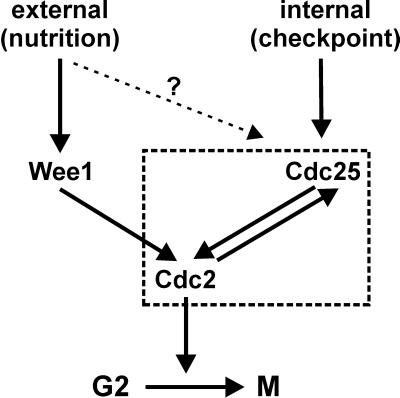
Another concept revisited in this study is the cell size threshold for entering mitosis. A large body of evidence collected in the classical studies pointed to the notion that cells have a means of monitoring their absolute size, or something that correlates with size, and that division can occur only when a critical size has been attained (Nurse, 1975; Fantes, 1977; Fantes and Nurse, 1977). The G2/M cell size control thus has two equally important aspects (Figure 9). First, progression into mitosis is controlled by the balance between the dose-dependent mitotic inhibitors and inducers, namely, Wee1 and Cdc25. Modulation of their activities, physiologically or by a mutation, has a direct effect on the timing of mitosis and therefore the size at which mitosis occurs. Thus, wild-type cells are able to adjust their size depending on external conditions such as nutrition (Fantes and Nurse, 1978). Depending on nutritional status, Wee1 and its upstream regulation are required for setting the initial level of Cdc2-Tyr15 phosphorylation that has to be overturned to allow progression into mitosis (MacNeill and Nurse, 1997). This module alone, however, is insufficient to constitute a cell size homeostasis mechanism that would ensure that the size does not randomly drift in successive generations. A system responsible for this has been predicted previously (Fantes, 1977; Sveiczer et al., 1996), but the mechanism has not been demonstrated. Our data reveal for the first time the existence of a pathway used by the latter system that is separate from the former.
Figure 9.
Model summarizing the interaction between the Cdc2-Tyr15 phosphorylation and dephosphorylation pathways involved in cell size control.
Two prominent features should be pointed out that distinguish the cell size checkpoint from other checkpoints. First, the cell size checkpoint monitors a continuous variable instead of an all-or-none event. Second, the regulators that are involved in setting the critical value may overlap with those involved in execution of the checkpoint. The evidence suggests that this is possible because cell growth continues independently throughout interphase and only the sensitivity of the checkpoint is modulated by upstream regulation depending on external conditions, effectively setting the parameter that is externally observed as a cell size threshold.
Cdc25 as an Effector of Cell Size Checkpoint
Our results show that Cdc25 is required to link cell size monitoring to progression into mitosis through a checkpoint mechanism. This function of Cdc25 was previously unknown and thus represents a novel mechanism involved in coordination of cell growth and proliferation (Neufeld and Edgar, 1998; Polymenis and Schmidt, 1999). The requirement of Cdc25 for the proper cell size at mitosis has long been known (Russell and Nurse, 1986; Coleman and Dunphy, 1994). The requirement for the activity alone, however, does not necessarily imply its active regulation in relation to cell size. More recently, it has been shown that initiation of Cdc25 translation is hypersensitive to changes in the protein synthetic machinery, which may contribute to coordination between cell growth and division (Daga and Jimenez, 1999). In addition, there is also some evidence suggesting that the levels of Cdc25 may be regulated in response to cAMP, an indicator of the nutritional status in cells (Kishimoto and Yamashita, 2000). These observations, however, still cannot distinguish between the involvement of Cdc25 in the threshold setting and the cell size-monitoring function of the mitotic cell size control. This study provides direct evidence that Cdc25 is involved in the cell size checkpoint and therefore participates in cell size monitoring. This result does not rule out a contribution from Cdc25 in setting the size threshold, nor does it rule out possible modulatory contribution from other components involved in executing the checkpoint that may have remained beyond the sensitivity of our assays.
The upstream molecular source of the relevant signals is currently unknown. Apart from the regulated production of the Cdc25 protein (Daga and Jimenez, 1999), two additional scenarios for the regulation of Cdc25 activity in response to cell size are possible. These possibilities are not mutually exclusive. First, Cdc25 could directly receive signals from the cell size-monitoring system and this may inhibit its activity or restrict its access to the Cdc2 kinase, as in the case of the DNA replication and damage checkpoint (Rhind and Russell, 1998a). The second possibility arises from the fact that Cdc25 and the Cdc2 kinase are coupled in a positive feedback loop and therefore their activation kinetics in vivo are practically indistinguishable (Kovelman and Russell, 1996). Therefore, cell size monitoring might also impinge on the cell cycle control by modulating the presence of the Cdc2/Cdc13 kinase complex in the nucleus, which after accumulation to a critical level would trigger the positive loop. In either case Cdc25 is an essential component of the triggering mechanism. Further studies will resolve which pathway plays the primary role.
It is worth noting that even cells that tolerate the absence of Cdc25 display a sufficient coordination between growth and division to allow them to remain viable under normal conditions. What these cells lack, however, is the ability to respond to perturbations of cell growth. Consistent with this, a mode of cell size control different from that of wild type, although its molecular basis remained uncharacterized, has been noted in cells lacking Cdc25 (Sveiczer et al., 1999).
ACKNOWLEDGMENTS
We thank K. Gould, P. Russell, D. Beach, S. Reed, K. Gull, M. Yanagida, and I. Hagan for providing strains and reagents. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada to P.G.Y and A.M and by the Canadian Institutes of Health Research to A.M.
REFERENCES
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Ammar DA, Nguyen PN, Forte JG. Functionally distinct pools of actin in secretory cells. Am J Physiol. 2001;281:C407–C417. doi: 10.1152/ajpcell.2001.281.2.C407. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P, Pelloquin L, Oustrin M-L, Ducommun B. Role of the fission yeast nim1 protein kinase in the cell cycle response to nutritional signals. Biochem Biophys Res Commun. 1997;232:204–208. doi: 10.1006/bbrc.1997.6253. [DOI] [PubMed] [Google Scholar]
- Breeding CS, Hudson J, Balasubramanian MK, Hemmingsen SM, Young PG, Gould KL. The cdr2+ gene encodes a regulator of G2/M progression and cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:3399–3415. doi: 10.1091/mbc.9.12.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Smith KW, Gustin MC. Osmotic stress and the yeast cytoskeleton: phenotype specific suppression of an actin mutation. J Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Tang Z, Dunphy WG. Negative regulation of the Wee1 protein kinase by direct action of the Nim1/Cdr1 mitotic inducer. Cell. 1993;72:919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- Coué M, Brenner SL, Spector I, And Korn ED. Inhibition of actin polymerization by latrunculin-A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Daga RR, Jimenez J. Translational control of the Cdc25 cell cycle phosphatase: a molecular mechanism coupling mitosis to cell growth. J Cell Sci. 1999;112:3137–3146. doi: 10.1242/jcs.112.18.3137. [DOI] [PubMed] [Google Scholar]
- Edwards RJ, Carr AM. Analysis of radiation-sensitive mutants of fission yeast. Methods Enzymol. 1997;283:471–494. doi: 10.1016/s0076-6879(97)83038-8. [DOI] [PubMed] [Google Scholar]
- Fantes PA. Control of cell size and cycle time in Schizosaccharomyces pombe. J Cell Sci. 1977;24:51–67. doi: 10.1242/jcs.24.1.51. [DOI] [PubMed] [Google Scholar]
- Fantes P, Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977;107:377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Fantes P, Nurse P. Control of the timing of cell division in fission yeast. Cell size mutants reveal a second control pathway. Exp Cell Res. 1978;115:317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Iwig M, Glaesser D. Synthesis of proto-oncogene proteins and cyclins depends on intact microfilaments. Eur J Cell Biol. 1998;77:188–195. doi: 10.1016/S0171-9335(98)80106-4. [DOI] [PubMed] [Google Scholar]
- Gachet Y, Tournier S, Millar JBA, Hyams JS. A MAP kinase-dependent actin checkpoint ensures proper spindle orientation in fission yeast. Nature. 2001;412:352–355. doi: 10.1038/35085604. [DOI] [PubMed] [Google Scholar]
- Gould KL, Moreno S, Tonks NK, Nurse P. Complementation of the mitotic activator, p80cdc25, by a human protein-tyrosine phosphatase. Science. 1990;250:1573–1576. doi: 10.1126/science.1703321. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JC, Bardes ES, Ohya Y, Lew DJ. A role for the Pkc1/Mpk1 kinase cascade in the morphogenesis checkpoint. Nat Cell Biol. 2001;3:417–420. doi: 10.1038/35070104. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Iwig M, Czeslick E, Müller A, Gruner M, Spindler M, Glaesser D. Growth regulation by cell shape alteration and organization of the cytoskeleton. Eur J Cell Biol. 1995;67:145–157. [PubMed] [Google Scholar]
- Kanoh J, Russell P. The protein kinase Cdr2, related to Nim1/Cdr1 mitotic inducer, regulates the onset of mitosis in fission yeast. Mol Biol Cell. 1998;9:3321–3334. doi: 10.1091/mbc.9.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto N, Yamashita I. Cyclic AMP regulates cell size of Schizosaccharomyces pombe through Cdc25 mitotic inducer. Yeast. 2000;16:523–529. doi: 10.1002/(SICI)1097-0061(200004)16:6<523::AID-YEA546>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kovelman R, Russell P. Stockpiling of Cdc25 during a DNA replication checkpoint arrest in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:86–93. doi: 10.1128/mcb.16.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ. Cell-cycle checkpoints that ensure coordination between nuclear, and cytoplasmic events in Saccharomyces cerevisiae. Curr Opin Genet Dev. 2000;10:47–53. doi: 10.1016/s0959-437x(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang H, Balasubramanian MK. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J Cell Sci. 2000;113:1223–1230. doi: 10.1242/jcs.113.7.1223. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR, Lew DJ. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–4061. doi: 10.1128/mcb.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- MacNeill SA, Nurse P. Cell cycle control in fission yeast. In: Pringle JR, Broach JR, Jones EW, editors. The Molcular and Cellular Biology of the Yeast Saccharomyces. Vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 697–763. [Google Scholar]
- McMillan JN, Sia RAL, Lew DJ. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J Cell Biol. 1998;142:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JBA, Lenaers G, Russell P. Pyp3 PTPase acts as a mitotic inducer in fission yeast. EMBO J. 1992;11:4933–4941. doi: 10.1002/j.1460-2075.1992.tb05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM. The growth of single cells. I. Schizosaccharomyces pombe. Exp Cell Res. 1957;13:244–262. doi: 10.1016/0014-4827(57)90005-8. [DOI] [PubMed] [Google Scholar]
- Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Motegi F, Nakano K, Mabuchi I. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe J. Cell Sci. 2000;113:1813–1825. doi: 10.1242/jcs.113.10.1813. [DOI] [PubMed] [Google Scholar]
- Mulvihill DP, Petersen J, Ohkura H, Glover DM, Hagan IM. Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe. Mol Biol Cell. 1999;10:2771–2785. doi: 10.1091/mbc.10.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NI, Eng K, Gould KL, Balasubramanian MK. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld TP, Edgar BA. Connections between growth and the cell cycle. Curr Opin Cell Biol. 1998;10:784–790. doi: 10.1016/s0955-0674(98)80122-1. [DOI] [PubMed] [Google Scholar]
- Novak B, Csikasz-Nagy A, Gyorffy B, Chen K, Tyson JJ. Mathematical model of the fission yeast cell cycle with checkpoint controls at the G1/S, G2/M and metaphase/anaphase transitions. Biophys Chem. 1998;72:185–200. doi: 10.1016/s0301-4622(98)00133-1. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Parker LL, Walter SA, Young PG, Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the Nim1/Cdr1 protein kinase. Nature. 1993;363:736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- Polymenis M, Schmidt EV. Coordination of cell growth with cell division. Curr Opin Genet Dev. 1999;9:76–80. doi: 10.1016/s0959-437x(99)80011-2. [DOI] [PubMed] [Google Scholar]
- Rhind N, Russell P. Mitotic DNA damage and replication checkpoints in yeast. Curr Opin Cell Biol. 1998a;10:749–758. doi: 10.1016/s0955-0674(98)80118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N, Russell P. Tyrosine phosphorylation of Cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol Cell Biol. 1998b;18:3782–3787. doi: 10.1128/mcb.18.7.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N, Russell P. Roles of the mitotic inhibitors Wee1, and Mik1 in the G2 DNA damage, and replication checkpoints. Mol Cell Biol. 2001;21:1499–1508. doi: 10.1128/MCB.21.5.1499-1508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupeš I, Jia Z, Young PG. SspI promotes actin depolymerization and is involved in stress response and new end take-off control in fission yeast. Mol Biol Cell. 1999;10:1495–1510. doi: 10.1091/mbc.10.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins—Novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Suda M, Yamada S, Toda T, Miyakawa T, Hirata D. Regulation of Wee1 kinase in response to protein synthesis inhibition. FEBS Lett. 2000;486:305–309. doi: 10.1016/s0014-5793(00)02299-7. [DOI] [PubMed] [Google Scholar]
- Sveiczer A, Novak B, Mitchison JM. The size control of fission yeast revisited. J Cell Sci. 1996;109:2947–2957. doi: 10.1242/jcs.109.12.2947. [DOI] [PubMed] [Google Scholar]
- Sveiczer A, Novak B, Mitchison JM. Mitotic control in the absence of cdc25 mitotic inducer in fission yeast. J Cell Sci. 1999;112:1085–1092. doi: 10.1242/jcs.112.7.1085. [DOI] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;3:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Wu L, Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993;363:738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- Wu L, Shiozaki K, Aligue R, Russell P. Spatial organization of the Nim1-Wee1-Cdc2 mitotic control network in Schizosaccharomyces pombe. Mol Biol Cell. 1996;7:1749–1758. doi: 10.1091/mbc.7.11.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmola EG, Somasundaram T, Boring TA, Spector I, Bubb MR. Actin-latrunculin. A structure and function. J Biol Chem. 2000;36:28120–28127. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]
- Young PG, Fantes PA. Schizosaccharomyces pombe mutants affected in their division response to starvation. J Cell Sci. 1987;88:295–304. doi: 10.1242/jcs.88.3.295. [DOI] [PubMed] [Google Scholar]