Abstract
Amphetamine is a common therapeutic agent for alleviating the core symptoms associated with attention deficit hyperactivity disorder (ADHD) in children and adults. The current study used a translational model of attention, the five-choice serial reaction time (5-CSRT) procedure with rats, to examine the time-course effects of d-amphetamine. Effects of different dosages of amphetamine were related to drug-plasma concentrations, fashioned after comprehensive pharmacokinetic/pharmacodynamic assessments that have been employed in clinical investigations. We sought to determine if acute drug-plasma concentrations that enhance performance in the 5-CSRT procedure are similar to those found to be therapeutic in patients diagnosed with ADHD. Results from the pharmacokinetic/pharmacodynamic assessment indicate that amphetamine plasma concentrations associated with improved performance on the 5-CSRT overlap with those that have been reported to be therapeutic in clinical trials. The current findings suggest that the 5-CSRT procedure may be a useful preclinical model for predicting utility of novel ADHD therapeutics and their effective concentrations.
Keywords: five-choice serial reaction time, ADHD, d-amphetamine, attention, pharmacokinetics, time course, rat
INTRODUCTION
Psychostimulants are the mainstay of treatment for alleviating the behavioral symptoms of inattention, impulsivity, and hyperactivity associated with attention deficit hyperactivity disorder (ADHD) (Bachmann et al., 2017; Childress and Tran, 2016; De Crescenzo et al., 2017). Amphetamine treatment leads to clinically significant reductions of these symptoms in 55–70% of patients (Berman et al., 2009). One research tool used to demonstrate therapeutic effects of an ADHD pharmacotreatment is the continuous performance task (CPT). Although there are variants of the task (Riccio et al., 2001), CPT typically assesses attention to an infrequently occurring stimulus, and psychostimulants have been proven successful in enhancing detection of that stimulus.
In preclinical research, the five-choice serial reaction time (5-CSRT) procedure is a variant of the CPT that has been proposed as a model of attention and impulse control (Robbins, 2002). Previous studies revealed that acute injections of d-amphetamine and methylphenidate can increase the percentage of correct responses in the 5-CSRT procedure (Grottick and Higgins, 2002; Bizarro et al., 2004; Paine et al., 2007; Navarra et al., 2008), and that those effects can be enhanced under variations of the procedure, including intermittent (Koffarnus and Katz, 2011) and delayed reinforcement (Slezak and Katz, 2013). Although some studies have reported negligible effects of d-amphetamine and methylphenidate on 5-CSRT performance (Cole and Robbins, 1987; Puumala et al. 1996; Bizarro and Stolerman, 2003; Paterson et al., 2011), others have found that improved performance is selective for stimulant drugs, as compared to drugs from other classes (e.g. Paine et al.; Koffarnus and Katz, 2011), further suggesting the predictive validity of this procedure as a model for ADHD.
The present study determined the effects of d-amphetamine in the 5-CSRT procedure over time, and related those effects to drug-plasma concentrations. Those results were further compared to pharmacodynamic/pharmacokinetic analyses reported in clinical studies (e.g., Brown et al., 1979, 1980; Greenhill et al., 2003). In particular, the study determined whether acute drug-plasma concentrations of d-amphetamine that enhanced performance in the 5-CSRT procedure overlap with therapeutic amphetamine plasma concentrations measured in clinical trials of patients diagnosed with ADHD. A potential similarity of plasma concentrations that improved performance in the 5-CSRT and those detected in clinical trials would support use of the 5-CSRT procedure as a useful preclinical ADHD model for screening novel therapeutics.
METHOD
Subjects
Six male Sprague-Dawley rats (Taconic Farms, Hudson, NY) weighing 300–350 g served as subjects, and were housed individually in a temperature- and humidity-controlled environment under a 12-h light/dark cycle. All rats were fed approximately 10–15 g of standard rat chow 1h after each session in their home cages where water was continuously available. Thus, each subject was 22-hr food restricted prior to the start of each experimental session. Care of subjects was in accordance with the guidelines of the NIH and the NIDA IRP Animal Care and Use Program.
Five additional male Sprague-Dawley rats (Taconic Farms) weighing 300–350 g were used for the determination of plasma amphetamine concentrations. These animals were housed as described above with unrestricted access to water and food. Animal care and experimental manipulations were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine. Animal facilities at both Johns Hopkins and NIDA IRP are accredited by the AAALAC International.
Apparatus
Experimental sessions were conducted in operant-conditioning chambers designed for the 5-CSRT procedure (MED-NP5L package; Med-Associates Inc., St. Albans, VT, USA). One wall of the chamber contained five horizontal square 2.5-cm openings, located 2 cm above the grid floor. The wall was curved so that each opening was 25 cm from the midpoint of the opposing wall. Each opening could be illuminated from behind with 3-watt clear light-emitting diodes. The opposite wall was not curved and contained a food tray into which 45-mg food pellets could be delivered. A house light was located at the top of the wall above the food tray. The chambers were contained within light-proof, ventilated enclosures that provided sound attenuation. White noise was delivered to the chamber at all times to mask extraneous noise.
Procedure
5-Choice serial reaction time task
Sessions were conducted daily, Mondays through Fridays, and ended after 50 food presentations or 40 minutes, whichever occurred first (median session durations ranged from 6 to 12 min across subjects). Each trial started with illumination of the house light and a sample stimulus light (initially for 30-s) behind one of the five apertures. A single response (nose poke) within the illuminated aperture was reinforced with a 45-mg food pellet (accompanied by illumination of the food-tray light and termination of the house light). An inter-trial interval (ITI) followed food delivery, with the food-tray remaining illuminated for its duration, but without scheduled consequences for responses. If a response was not emitted within 30-s of the illumination of the sample stimulus (“limited hold” period, LH), or if a response was emitted in an incorrect aperture, the trial was terminated and immediately followed by an ITI with the house light remaining illuminated, followed by the start of the next trial. Training started with 30-s stimulus durations, 2-s ITIs, and 30-s LHs. The stimulus duration and ITI were gradually altered over training sessions to the final parameters of 1- and 5-s, respectively. Drug testing began, after responses of individual subjects were stable (at least 50% accuracy with no apparent increasing or decreasing trend for at least five sessions).
d-Amphetamine sulfate (obtained from Sigma Aldrich, St. Louis, MO, USA), was dissolved in 0.9% saline (NaCl), and injected intraperitoneally (i.p.) at a volume of 1 ml/kg body weight. Doses (0.1, 0.3, 1.0, and 1.7 mg/kg) are expressed in terms of the salt and were typically tested twice in each subject on Tuesdays and Fridays. Additionally, doses of d-amphetamine were administered in a pseudo-random order at each of the following time points before testing: 5, 15, 30, 5, 60, 120, and 240 minutes. All doses were tested at a given time point before moving to the next. Performances at the two 5-minute time point determinations were not statistically different, and were therefore averaged for the final analysis.
Amphetamine plasma levels
Plasma levels of d-amphetamine were determined in a separate group of subjects. Blood was sampled at 15, 30, 60, 120, 240, and 360 minutes after i.p. administration of 1.0 mg/kg d-amphetamine (a dose that resulted in the most consistent increases in percent correct relative to control values across the various administration times). At each time point, approximately 0.2 ml of blood was collected by retro-orbital bleeding. Blood samples were dispensed into 2-ml Vacutainer hematology tubes, containing 4 mg of K3 ethylenediaminetetraacetic acid solution (Becton-Dickinson, Franklin Lakes, NJ, USA), and stored on ice for up to 30 min, until centrifuged. Samples were centrifuged at 1100g for 10 min (Sorvall RC2-B, Kendro Laboratory Products, Newtown, CT, USA). Plasma was withdrawn using a 5-ml 3/4 Pasteur pipette and decanted into 1.5-ml polypropylene tubes. Sodium metabisulfite (250 mM) was added at a volume of 30 µl/ml plasma to minimize compound oxidation. Samples were stored at −20°C until assayed.
Plasma aliquots (100 µl) were preserved with 20 µl of SMBS (250 mM) and 10 µl of EDTA (250 mM). After addition of 100 µl of internal standard solution (d8-amphetamine, 1000 ng/mL), each sample was briefly mixed. Ten µl of PCA was added and samples vortexed to perform protein precipitation. Subsequently, samples were centrifuged (13,000g for 5 min) and 10 µL of the supernatant was injected into a liquid chromatographic/mass spectrometric (LC-MS). All samples were analyzed using an Agilent Technologies (AT, Waldbronn, Germany) AT series 1100 LC/MSD, VL version, using electron spray ionization (ESI) in positive ionization mode, including an AT 1100 series HPLC system which consisted of a degasser, a quaternary pump, a column thermostat, and an autosampler. Isocratic elution was performed on a Zorbax 300-SCX column (Narrow-Bore 2.1×150 mm, 5 µm). The mobile phase consisted of 5 mM aqueous ammonium formate adjusted to pH 3 with formic acid (eluent A) and acetonitrile (eluent B). Samples were analyzed in positive selected ion monitoring (SIM) mode with the following ions: m/z 136 (target ion), 119 for amphetamine and 144 (target ion), 127 for d8-amphetamine (IS). Fragmentor voltage was 100 V. The linear range for each analyte was 10 to 500 ng/ml amphetamine. The lowest point of the calibration curve was defined as the limit of quantification of the method (10 ng/ml amphetamine). Quantification of amphetamine was determined by comparison of its peak area ratio (analyte vs. internal standard) to calibration curves in which the peak area ratios of spiked calibrators had been plotted versus their concentration.
Statistical analysis
The percentage of correct 5-CSRT responses was calculated as the total correct responses/total correct plus incorrect multiplied by 100. Response latency was determined as the time from sample stimulus onset to response, ITI responses were recorded as the number of responses during the inter-trial interval, and trial omissions were the number of trials without a response. A two-way repeated-measures ANOVA compared the various determinations of d-amphetamine using within-subject factors of testing time and dose. Multiple-comparison corrected tests were conducted using Bonferroni corrections. Significant differences for all analyses were based upon p < 0.05. To conduct a repeated-measures ANOVA, a maximum likelihood estimator (the expectation-maximization algorithm) in SPSS 18 was used to estimate performance for one subject at 1.7 mg/kg d-amphetamine (only at the 15-min testing period), because this subject omitted all trials during the respective test session.
The pharmacokinetic parameters consisting of peak plasma concentrations (Cmax), half-life (T1/2), time of maximum plasma concentration (Tmax), and area under the concentration-time curve (AUC) for d-amphetamine were calculated using the pharmacokinetic functions for Microsoft Excel. AUC was calculated using the linear trapezoidal rule starting at time zero and finishing at the last quantifiable point.
RESULTS
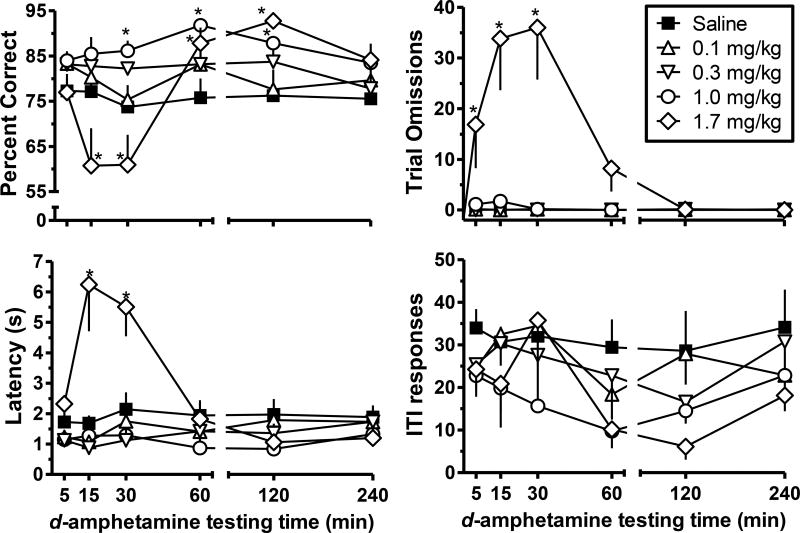
Low doses of d-amphetamine (0.1–0.3 mg/kg) had relatively little effect on any dependent measure, while 1.0 mg/kg d-amphetamine increased percent correct (Figure 1, top left, circles) across a range of d-amphetamine administration times. A significant interaction was observed (F20,100=7.75; p<0.001) with 1.0 mg/kg d-amphetamine significantly increasing percent correct 30-, 60- and 120-minutes after amphetamine administration (p-values<0.05). Additionally, 1.7 mg/kg d-amphetamine significantly increased percent correct 60- and 120-min after drug administration. However, 15- and 30-min after d-amphetamine administration the percentage of the correct responses was significantly decreased (p-values<0.05). Corresponding time-dependent effects of 1.7 mg/kg d-amphetamine were observed for trial omissions (Figure 1, top right) and response latencies (Figure 1, bottom left). The dose of 1.7 mg/kg d-amphetamine significantly increased trial omissions (F20,100 =5.70; p<0.001), but only at 5-, 15-, and 30-min after injection (p values<0.05) and significantly increased response latency (F20,100 =11.59; p<0.001) at 15- and 30-min after injection (p-values<0.05). Thus, at time-points when 1.7 mg/kg d-amphetamine decreased percent correct, other measures of performance were also impaired. However, when the 1.7 mg/kg dose increased percent correct, trial omissions and response latencies were not affected. Lastly, no statistically significant changes in ITI responses (Figure 1, bottom right) were observed at any dose of d-amphetamine across all times after injection.
Fig. 1.
The average percentage of correct responses (upper left), trials with an omission (upper right), latency to respond after sample stimulus onset (lower left), and number of ITI responses (lower right) are presented as a function of d-amphetamine testing time (min). Within each panel there are five functions representing the vehicle or different doses of d-amphetamine (filled square for saline, open upward triangle for 0.1 mg/kg, open downward triangle for 0.3 mg/kg, open circle for 1.0 mg/kg, and open diamond for 1.7 mg/kg). Error bars are presented as standard error of the mean (SEM); where bars are absent they are encompassed by the symbol. Significant effects are denoted by a single asterisk.
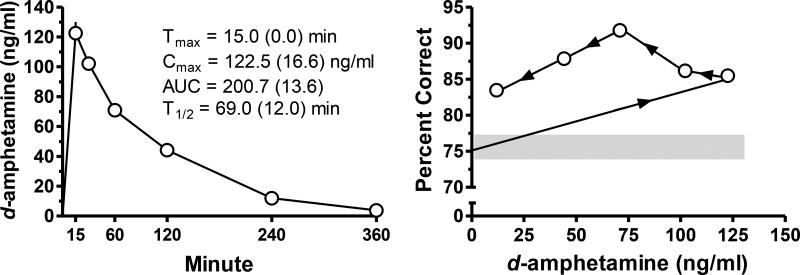
Maximal drug-plasma concentrations after 1.0 mg/kg d-amphetamine occurred at the earliest time point assessed (15 min) and steadily declined thereafter (Figure 2, left). A hysteresis plot (Figure 2, right) shows the relation between drug plasma levels after administration of 1.0 mg/kg d-amphetamine and the effects on percent correct during the 5-CSRT assessment. Drug plasma levels that corresponded to a significant increase in percent correct ranged from approximately 44.20 (+/−6.87) to 102.14 (+/−10.76) ng/ml. Increases in the percentage of correct responses were no longer evident at about 4-hr after administration with a d-amphetamine plasma concentration that approximated 11.92 (+/−3.97) ng/ml.
Fig. 2.
The average drug plasma levels (ng/ml) are presented as a function of time (mins) after 1.0 mg/kg d-amphetamine administration (left panel). Pharmacokinetic parameters listed within the left panel are the mean values with standard deviations within parentheses. The relation between the effects of 1.0 mg/kg d-amphetamine administration on the percentage of correct responses and the different d-amphetamine plasma concentrations engendered is depicted by a hysteresis plot in the right panel. The arrows on the function indicate increases in testing time from 15 min (rightmost open circle) to 240 min (leftmost open circle). The shaded region indicates the range of performance during vehicle-only tests. Error bars are presented as SEM in the left panel; where bars are absent they are encompassed by the symbol.
DISCUSSION
ADHD is a common disorder among children and is frequently being diagnosed as persisting into adulthood (Biederman et al., 2012). Amphetamine is widely used to alleviate core ADHD symptoms and new amphetamine-based treatments are being investigated (Childress and Tran, 2017). The current preclinical experiment sought to determine the relationship of “therapeutically” active plasma concentrations observed in humans and rodents - with results indicating overlap between plasma concentrations associated with improved performance in both species.
In preclinical studies that model psychostimulant treatment, some investigators have simulated the drug plasma levels observed with the clinical treatment of ADHD symptoms in laboratory animals using both methylphenidate (Kuczenski and Segal, 2002; Berridge et al., 2006; LeBlanc-Dichin and Taukulis, 2007; Rodriguez et al., 2010; Soto et al., 2012; Gill et al., 2012) and amphetamine (Ricaurte et al., 2005; Soto et al. 2012). However, it is less common to demonstrate that those similar drug plasma levels are also “therapeutic” in laboratory animals (i.e., the stimulants were also demonstrated to enhance attention or decrease locomotor activity). It appears that only Kuczenski and Segal (2002) and Berridge et al. (2006) showed that doses of methylphenidate that approximated drug plasma concentrations in the range seen clinically could result in an overall “therapeutic” change in behavior (e.g., reduction in crossovers in activity chambers as a measure of activity or enhancement in stimulus detection in a sustained attention task, respectively). Kuczenski and Segal (2002) estimated appropriate doses based on prior studies while Berridge et al. (2006) actually determined the relationship between dose and drug plasma concentration.
The current study is the first to show the comprehensive relation between the plasma levels and pharmacodynamics of amphetamine in the 5-CSRT procedure, which has been used as a model of attention in laboratory animals (Robbins, 2002). Drug effects were assessed over an extended time frame using techniques similar to those employed in clinical studies (e.g., Brown et al., 1979, 1980; Greenhill et al., 2003). A wide range (44–102 ng/ml) of d-amphetamine plasma levels obtained after acute 1.0 mg/kg administration corresponded to significant enhancements in percent correct responses. The drug plasma levels obtained in rats overlapped with the wide range reported clinically after oral amphetamine dosing in hyperactive children (22.7–65.9 ng/ml), a range sufficient to reduce ADHD-related problem behavior (Brown et al., 1979, 1980). Greenhill et al. (2003) examined effects of Adderall (3:1 d:l amphetamine) either once or twice daily in ADHD-diagnosed children and found that concentrations of 20–28 ng/ml or 20–52 ng/ml of d-amphetamine, were associated with therapeutic effects. However, the maximum drug plasma concentrations that enhanced performance in the current study were higher than those reported in the clinical literature, suggesting that concentrations higher than those reported clinically may also be therapeutic (although dose-related toxicity would be an obvious concern).
One pharmacological feature not assessed in the current study is the potential for acute tolerance. That is, the same drug plasma concentrations that initially produced a therapeutic effect no may longer produce one. Acute tolerance has been reported in clinical studies at four hours after administration of a single oral immediate-release amphetamine dose (Brown et al., 1979, 1980; Greenhill et al., 2003). As shown in the hysteresis plot, i.p. administration of 1.0 mg/kg d-amphetamine resulted in plasma levels that decreased substantially between test times and still resulted in enhanced performance up to the 240-min test time. The absence of acute tolerance obtained in this study may be related to the i.p. route of drug administration which differs from the oral route used in clinical studies. With i.p. administration, drug plasma levels likely decrease at a greater rate than would be seen with oral administration. Future studies modeling amphetamine treatment might benefit from use of the more clinically-relevant oral route of administration, increasing the translational potential.
The enhancement of percent correct responses with 1.0 mg/kg d-amphetamine was relatively sustained across an approximate 1.5-h time frame. However, effects of 1.7 mg/kg d-amphetamine showed a different profile, with earlier timepoints (i.e., those prior to 30 minutes post-administration) leading to impaired performance, and those after 60 minutes post-administration leading to improved performance. This likely reflects the fact that plasma levels shortly after administration produced behavioral disruption (evidenced by increased response latencies and trial omissions) that may have interfered with possible “therapeutic” effects. In contrast, 60 minutes after administration, 1.7 mg/kg d-amphetamine likely produced plasma levels within a range similar to those produced by administration of 1.0 mg/kg d-amphetamine that enhanced percent correct and did not produce behavioral disruption. A temporary negative effect due to increased plasma concentrations has also been suggested to occur in the clinical population (Swanson and Volkow, 2002). For example, pediatric ADHD patients treated with 10–20 mg of Adderall® showed a decrease in the number of math problems attempted and completed correctly during the first 45 minutes after treatment (Swanson et al., 1998). This effect was not observed after treatment with 5 mg of Adderall®. At the 1.5-h post-treatment timepoint, however, optimal “therapeutic” effects were found following a 15 mg dose. Similarly, following a dose of 20 mg, maximal “therapeutic” effects occurred 3 h after treatment (see Swanson and Volkow, 2002 for a further discussion of negative effects of certain plasma concentrations). Thus, the pharmacokinetics of d-amphetamine and its effects on behavior in the present study appear to correlate with most clinical observations, and support the predictive validity for the present version of the 5-CSRT procedure as a preclinical model that could be useful in assessing novel ADHD therapeutics.
Acknowledgments
Sources of funding: Funding was provided by the National Institute on Drug Abuse (NIDA) Intramural Research Program and Extramural Research NIH grants R01 HD050202 and K05 DA017964 (GAR).
Footnotes
Conflicts of interest
No conflicts are declared.
References
- Bachmann CJ, Wijlaars L, Kalverdijk LJ, Burcu M, Glaeske G, Schiling-Veninga CCM, Hoffmann F, Aagaard L, Zito JM. Trends in ADHD medication use in children and adolescents in five western countries, 2005–2012. Euro Neuropsychopharmacol. 2017;27:484–93. doi: 10.1016/j.euroneuro.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14:123–42. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73:941–50. doi: 10.4088/JCP.11m07529. [DOI] [PubMed] [Google Scholar]
- Bizzaro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacol. 2003;170:271–7. doi: 10.1007/s00213-003-1543-6. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: Nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Brown GL, Hunt RD, Ebert MH, Bunney WE, Kopin IJ. Plasma levels of d-amphetamine in hyperactive children. Psychopharmacol. 1979;62:133–40. doi: 10.1007/BF00427126. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Mikkelsen EJ, Hunt RD. Behavior and motor activity response in hyperactive children and plasma amphetamine levels following a sustained release preparation. J Am Acad Child Adolesc Psychiatry. 1980;19:225–39. doi: 10.1016/s0002-7138(09)60699-3. [DOI] [PubMed] [Google Scholar]
- Childress A, Tran C. Current investigational drugs for the treatment of Attention-deficit/Hyperactivity Disorder. Exp Op on Invest Drugs. 2016;25:463–74. doi: 10.1517/13543784.2016.1147558. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-chocie serial reaction time task: New evidence for central dopaminergic-noradrenergic interactions. Psychopharmacol. 1987;91:458–66. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Crescenzo DeF, Cortese S, Adamo N, Janiri L. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Mental Health. 2017;20:4–11. doi: 10.1136/eb-2016-102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacol. 2012;37:2555–65. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Steinhoff K, Fried J, Posner K, Lerner M, Wigal S, Clausen SB, Zhang Y, Tulloch S. A pharmacokinetic/pharmacodynamic study comparing a single morning dose of adderall to twice-daily dosing in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:1234–41. doi: 10.1097/00004583-200310000-00015. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Assessing a vigilance decrement in aged rats: effects of pre-feeding, task manipulation, and psychostimulants. Psychopharmacol. 2002;164:33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Katz JL. Response requirement and increases in accuracy produced by stimulant drugs in a 5-choice serial reaction-time task in rats. Psychopharmacol. 2011;213:723–33. doi: 10.1007/s00213-010-2027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–71. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc-Duchin D, Taukulis HK. Chronic oral methylphenidate administration to periadolescent rats yields prolonged impairment of memory for objects. Neurobio Learning Mem. 2007;88:312–20. doi: 10.1016/j.nlm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuro-Psychopharmacol Biological Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res. 2011;69:41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biological Psychiatry. 2007;62:687–93. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Sirvio J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiology Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Mechan AO, Yua J, Hatzidimitriou G, Xie T, Mayne AH, McCann UD. Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates. J Pharmacol Exp Ther. 2005;315:91–8. doi: 10.1124/jpet.105.087916. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldroip JJM, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci. 2001;13:326–35. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacol. 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, Paule MG. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotox Terato. 2010;32:142–51. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak JM, Katz JL. An influence of delayed reinforcement on the effectiveness of psychostimulants to enhance indices of attention under a five-choice serial reaction time procedure in male rats. Exp Clin Psychopharmacol. 2013;21:355–62. doi: 10.1037/a0033726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacol. 2012;37:2566–79. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Wigal S, Greenhill LL, Browne R, Waslik B, Lerner M, Williams L, Flynn D, Agler D, Crowley K, Fineberg E, Baren M, Cantwell DP. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37:519–26. [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: Implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–8. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]