Abstract
More than 100 modifications have been found in RNA. Analogous to epigenetic DNA methylation, epitranscriptomic modifications can be written, read, and erased by a complex network of proteins. Apart from N6-methyladenosine (m6A), N1-methyladenosine (m1A) has been found as a reversible modification in tRNA and mRNA. m1A occurs at positions 9, 14, and 58 of tRNA, with m1A58 being critical for tRNA stability. Other than the hundreds of m1A sites in mRNA and long non-coding RNA transcripts, transcriptome-wide mapping of m1A also identifies >20 m1A sites in mitochondrial genes. m1A in the coding region of mitochondrial transcripts can inhibit the translation of the corresponding proteins. In this review, we summarize the current understanding of m1A in mRNA and tRNA, covering high-throughput sequencing methods developed for m1A methylome, m1A-related enzymes (writers and erasers), as well as its functions in mRNA and tRNA.
Keywords: Epitranscriptome, RNA modification, N1-methyladenosine (m1A), m1A writer, m1A eraser
The RNA modification N1-methyladenosine
Shortly after the discovery of the base N1-methyladenine (m1A) in 1961 [1], Dunn et al. isolated 1-methyladenosine mononucleotide from RNA [2]. m1A has been discovered in tRNA [3], rRNA [4], [5], [6], mRNA [7], [8], [9], [10], and mitochondrial (mt) transcripts [9], [10]. m1A, N3-methylcytidine (m3C), and N7-methylguanosine (m7G) are the most commonly methylated nucleotides with a positive electrostatic charge under physiological conditions [11]. The electro-chemical interaction resulting from the positive charge of m1A is critical for the function and structure of tRNA [11]. The methyl group on m1A in mRNA blocks the Watson-Crick base pairing, affecting reverse transcription and protein translation [7], [8], [9], [10].
m1A was first found in yeast tRNAPhe [3]. Decades later, m1A has also been found in 264 out of 564 tRNA sequences in bacteria, archaea, and eukaryota, which can occur at positions 9, 14, and 58 [12]. Among them, m1A58 is conserved in bacteria, archaea, and eukaryota, and m1A58 is critical for tRNA stabilization [12], [13]. A recent study has reported that m1A58 can be reversed by the human AlkB homolog 1 (ALKBH1) demethylase [13].
In contrast to the high abundance of m1A in tRNA, m1A is present in mammalian mRNA with a low abundance (m1A/A: 0.015%−0.054% in mammalian cells and up to 0.16% in mammalian tissues) [7], [8]. The development of m1A-sequencing methods and new single-base resolution methods [7], [8], [9], [10] has facilitated unraveling the presence of m1A in nuclear, cytosolic, and mt-encoded transcripts [9], [10]. For instance, Li et al. found 473 m1A sites in mRNA and long non-coding (lncRNAs) transcripts in HEK293T cells. The majority of these m1A sites are located within the 5′-untranslated region (5′ UTR) [9], which is consistent with previous findings [7], [8]. Interestingly, 22 m1A sites have been identified in 10 of 13 mt transcripts, with 21 sites located in the coding sequence (CDS) and one in the 3′ UTR [9]. m1A at CDS of mt transcripts affects mitochondrial translation, suggesting that m1A might regulate the normal function of mitochondria.
m1A sequencing
In order to comprehensively explore m1A methylation in transcriptome, it is necessary to develop m1A high-throughput sequencing. The low abundance of m1A in mRNAs necessitates highly sensitive detection methods. In addition, m1A can be rearranged to m6A under alkaline conditions (Dimroth rearrangement) [14]. Therefore, the signature of m1A in mRNA may disappear during the preparation of mRNA fragmentation or mRNA digestion, which makes it difficult to reduce the noise in m1A measurement and sequencing. m1A blocks the Watson-Crick interface and effectively stalls reverse transcription (RT), thus inducing truncations or mutations in RT products [15], [16], [17], [18]. This feature can be employed to identify m1A sites, and accordingly, the high-throughput m1A sequencing methods were developed based on this idea.
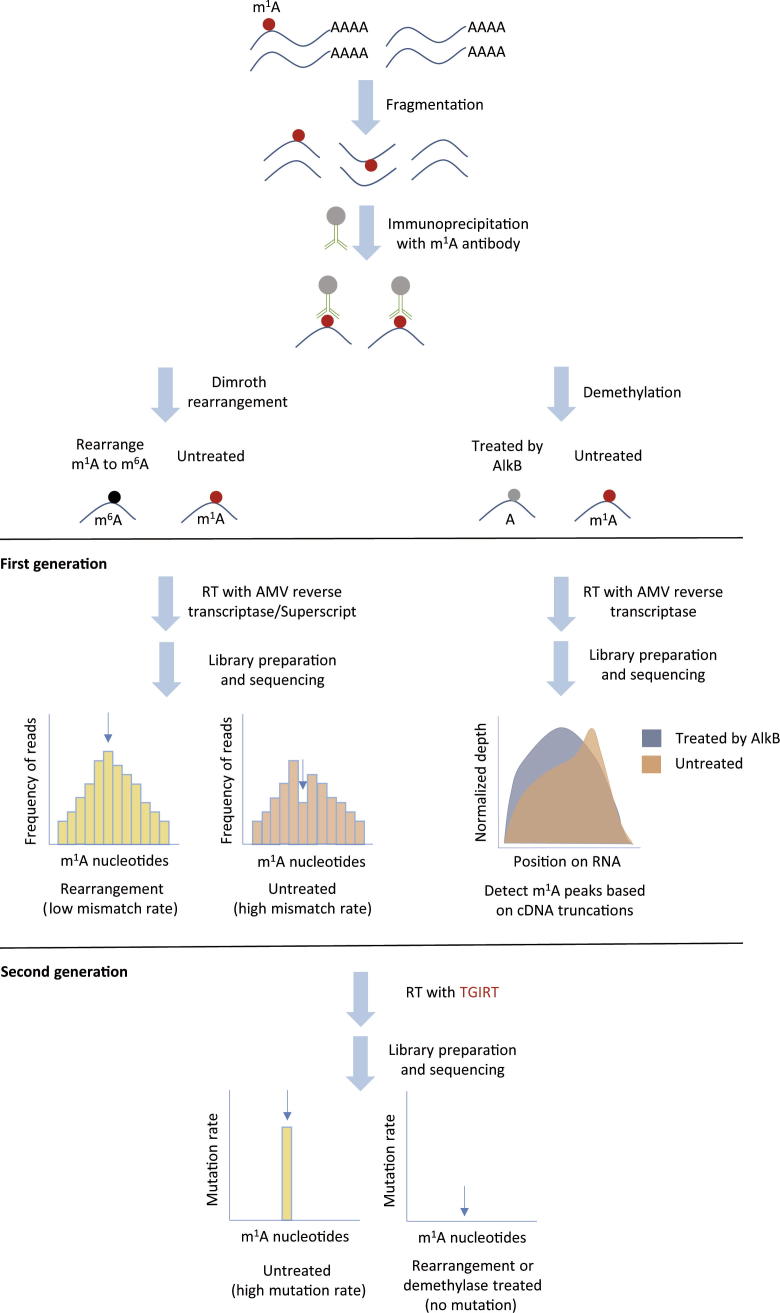
The first-generation m1A sequencing methods have been reported by two independent groups [7], [8] (Figure 1). The procedures of these two methods are largely similar. They both use the m1A antibody to enrich m1A-modified mRNA fragments and take advantage of the characteristics of m1A to induce mismatch or truncation during the RT process. However, there are also differences between these two methods. Dominissini et al. use Dimroth rearrangement to change m1A to m6A and then analyze m1A sites based on mismatch rates. Li et al. use E. coli AlkB to demethylate m1A to normal A and then calculate m1A sites based on truncation position. The resolution of the first generation sequencing method is around 130 nucleotides [7], [8]. To identify m1A sites more precisely for a more accurate picture of m1A distribution, researchers have recently developed the second-generation m1A sequencing method.
Figure 1.
Schematic outline of the two generations of m1A sequencing methods
RT, reverse transcription; TGIRT, thermostable group II intron reverse transcriptase.
A highly processive reverse transcriptase thermostable group II intron reverse transcriptase (TGIRT) is used in the second-generation m1A sequencing method to optimize the RT step [9], [10] (Figure 1). TGIRT exhibits excellent read-through efficiency and relatively high mutation frequency at the site of m1A, resulting in a higher signal-to-noise ratio for detecting the precise position of m1A, and therefore achieving a single-base resolution.
Using the second-generation m1A sequencing method, Li et al. [9] identified 740 m1A sites in the transcriptome of 293 T cells, including 418 and 55 sites found in mRNA and lncRNA transcripts, respectively. The majority of these m1A sites in mRNA are within the 5′ UTR, which agrees with the previous findings [7], [8]. Interestingly, Li et al. find 24 m1A sites at the first nucleotide (cap+1) and three sites at the second nucleotide (cap+2) of 5′ end of the transcripts. Ribosome profiling demonstrates that sites in the 5′ UTR and cap+1 position, but not those in the CDS or 3′ UTR, are correlated with higher translation efficiency. This observation that there is a correlation between m1A sites in the 5′ UTR and translation efficiency is in agreement with a previous report [7].
According to the RNA location, writer enzyme involved, and sequence-structural features, Li et al. divide the m1A sites in mRNA transcripts into three categories. These include tRNA methyltransferase 6/61A (TRMT6/61A)-dependent m1A sites in nuclear-encoded mRNAs (53 sites), TRMT6/61A-independent m1A sites in nuclear-encoded mRNAs, and m1A sites in mt-encoded mRNAs (22 sites). TRMT6/61A-dependent m1A sites are barely enriched in the 5′ UTR and conform to a GUUCRA tRNA-like motif and T-loop-like structures. m1A sites in mt-mRNAs are mainly located in CDS, and play a role in inhibiting mitochondrial translation. Among the ten m1A-modified mt-RNAs, m1A modifications on 5 mt-RNAs are installed by mt-tRNA methyltransferase TRMT61B. Would the random mismatches occurring during the RT process of TGIRT yield false positive results? Li et al. used a method called reverse calculation to test this possibility. They take the sample of demethylase (−) (i.e., m1A) as background and the sample of demethylase (+) (i.e., A) as signal to call m1A peaks. As a result, they have called 17 peaks, confirming the high accuracy of TGIRT enzyme. Using primer extension assay, they also verify several m1A sites in mt mRNA, confirming again the authenticity and accuracy of their method [9].
Surprisingly, about the same time, another group adopted a similar approach but reached a very different conclusion [10]. Safra et al. identified only 15 m1A sites in mRNAs and lncRNAs, with 9 in cytosolic mRNAs, 1 in cytosolic lncRNAs, and 5 in mt-mRNAs. The 10 m1A sites in cytosolic transcripts are installed by TRMT6/61A, whereas one m1A site in mt-mRNA ND5 is methylated by TRMT10C. They also find that m1A within 5′ UTR or CDS of cytosolic mRNAs leads to translational repression, indicating that m1A might be related to ribosomal scanning or translation.
These two studies share some similar findings, while disagreement arises in terms of the number of m1A sites in human mRNAs. Safra et al. suggest that m1A is a rare modification in human mRNAs based on their observation that only a dozen m1A sites have been identified using their method [10]. In contrast, Li et al. have identified hundreds of m1A sites, and thus they consider m1A a prevalent modification in human mRNAs [9]. Why do the numbers of m1A sites identified in these two studies differ so much? As speculated in the technology preview [19], differences in experimental procedures may confer differences in the quality of sequencing datasets. For instance, 1) compared to AlkB demethylation, the alkaline conditions used for Dimroth rearrangement might affect RNA integrity and rearrangement efficiency of m1A to m6A; 2) a competitive elution step at m1A antibody immunoprecipitation is omitted in [10], together with the lack of optimization of the RT step of TGIRT; 3) a random 10-nt barcode (unique molecular identifier, UMI) in the DNA 3′-adaptor is used in [9] to remove repetition caused by PCR and improve the accuracy of detection, while lack of UMIs could lead to trouble in data analysis and mutation rate calculation [10]. In addition, the lower number of raw sequencing reads in the study [10] could also explain the fewer m1A sites identified.
m1A writers: m1A methyltransferases
tRNA m1A methyltransferase (MTase) has been extensively studied, since m1A was first found in tRNA. Given m1A58 is dominant and conserved across the three domains of life, studies of tRNA m1A MTases started from looking for m1A58 MTase.
tRNA m1A58 MTase was initially characterized using partially purified protein fractions from bovine liver, which exhibits m1A MTase activity in vitro toward E. coli tRNA2Glu [20]. The genes encoding m1A58 MTase were firstly identified in Saccharomyces cerevisiae [21]. The S. cerevisiae m1A58 MTase comprises two subunits, tRNA adenine-N1-methyltransferase non-catalytic subunit and tRNA adenine-N1-methyltransferase catalytic subunit, encoded by two essential genes Trm6 and Trm61, respectively [21]. Trm61 is responsible for AdoMet-binding and catalytic function as an enzyme, while Trm6 is critical for tRNA binding [22]. The purification and characterization of the m1A58 MTase from S. cerevisiae [21], [22] has facilitated the identification of other m1A58 MTases from humans and other organisms. In eukaryota, m1A58 MTase consists of Trm6 and Trm61 orthologs, whereas prokaryotic m1A58 is installed by a single protein encoded by orthologs of Trm61 [23]. TRMT61A and TRMT6 are the human othologs of yeast Trm61 and Trm6, respectively, which are responsible for m1A58 modification of cytoplasmic tRNAs [24] (Figure 2).
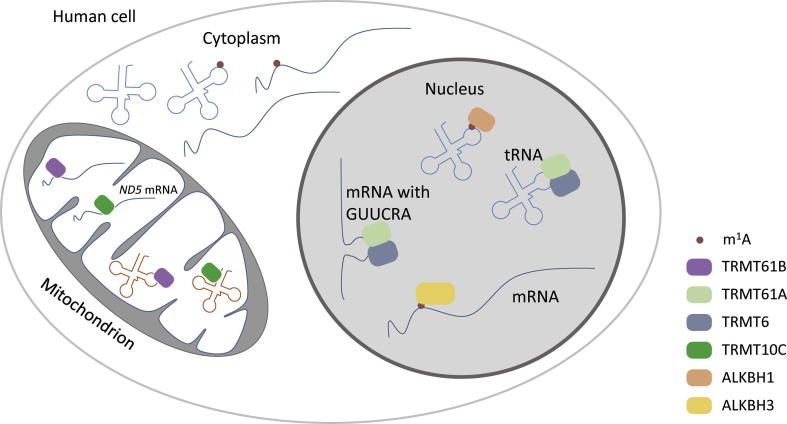
Figure 2.
The methylation and demethylation of m1A in tRNA and mRNA
In nucleus, m1A modifications in pre-tRNA and specific pre-mRNA are installed by tRNA m1A methyltransferase complex TRMT6/61A and erased by AlkB homolog proteins ALKBH1 and ALKBH3, respectively. In mitochondrion, mt-tRNA and a subset of mt-mRNA are methylated by mt-tRNA m1A methyltransferase TRMT61B and TRMT10C. mt, mitochondrial.
Human mt-tRNAs are known to contain m1A at positions 9 and 58 [24], which are catalyzed by TRMT10C and TRMT61B, respectively [25], [26] (Figure 2). In addition, m1A is also found at position 1322 of 28S rRNA, which is catalyzed by the human nucleolar protein nucleomethylin (NML; also known as RRP8) [27].
m1A is present in human nuclear-encoded mRNA and mt-mRNA [9]. tRNA m1A MTases are found to be able to write m1A in some mRNA transcripts as well. For instance, TRMT6/61A can install m1A sites within a GUUCRA tRNA-like motif with T-loop-like structure in some nuclear mRNAs, whereas TRMT61B methylates half of the identified m1A sites in mt-mRNA transcripts [9] (Figure 2). In addition, TRMT10C can add m1A at the 1374 position of mt-mRNA ND5 [10] (Figure 2). Apparently, more m1A mRNA MTases may be identified in future.
m1A erasers: m1A demethylases
m1A is discovered to be the second reversible RNA modification. The first reversible RNA modification, m6A, is demethylated by two AlkB family proteins, i.e., fat mass and obesity-associated (FTO) and ALKBH5 [28], [29]. Similarly, two AlkB family proteins ALKBH3 and ALKBH1 have been found to demethylate m1A [8], [13] (Figure 2).
E. coli AlkB is an FeII/α-ketoglutarate (α-KG)-dependent dioxygenase that repairs N-alkylated DNA lesions [30], [31]. In mammals, nine homologs of AlkB, i.e., ALKBH1-ALKBH8 and FTO, have been identified [32], [33]. ALKBH3 and ALKBH2 are known DNA-repair proteins that protect the genomic integrity of mammalian cells [34], [35]. Unlike ALKBH2 that repairs DNA alkylation lesions in double-stranded DNA (dsDNA), ALKBH3 repairs N-methylated bases, for instance, m1A, m3C, N3-methylthymine (m3T), and N1-methylguanine (m1G), in single-stranded DNA (ssDNA) or ssRNA [35]. ALKBH3 is coupled with the helicase activating signal cointegrator 1 complex subunit 3 (ASCC3) to unwind dsDNA for dealkylation [36]. ALKBH3, also known as prostate cancer antigen-1 (PCA-1), is highly expressed in a few human cancers and promotes apoptotic resistance and angiogenesis in prostate cancer and pancreatic cancer [37], [38]. Due to its demethylation activity of m1A in vitro, expectedly, ALKBH3 is confirmed to function as an m1A mRNA demethylase in vivo [8]. Notably, a recent study reports that ALKBH3 can function as a tRNA demethylase to promote protein synthesis in cancer cells [39]. ALKBH3 displays demethylation activities toward m1A, m3C, and m6A in tRNA to enhance protein translation efficiency in vitro, whereas knockdown of ALKBH3 increases m1A levels in tRNA and decreases protein synthesis in cancer cells [39].
ALKBH1 shows the highest sequence homology with E. coli AlkB and exhibits enzymatic activities toward a wide range of substrates. For instance, ALKBH1 has weak demethylation activity toward m3C in ssDNA [40]. ALKBH1 also acts as a histone dioxygenase during neural development, which specifically removes the dimethylation of K118 or K119 on histone H2A [41]. However, Wu et al. did not observe the demethylation activity on histone H2A in ALKBH1−/− embryonic stem cell lines. Rather, they found ALKBH1 is a DNA N6-methyladenine (m6dA) demethylase that regulates epigenetic gene silencing [42]. ALKBH1 also exhibits apurinic/apyrimidinic (AP) lyase activity, cleaving DNA at abasic sites via a β-elimination mechanism [43]. Interestingly, ALKBH1 has been recently identified as an m1A demethylase in cellular tRNAs [13]. Knockdown of ALKBH1 increases the m1A methylation level and the cellular level of the targeted tRNAiMet. These data indicate that the function of m1A58 is to stabilize tRNAiMet, leading to the attenuated initiation of protein translation. Demethylation of other targeted tRNAs by ALKBH1 affects translation elongation by decreasing the usage of tRNAs in protein synthesis [13]. Furthermore, ALKBH1 can hydroxylate 5-methylcytosine (m5C) to 5-formylcytosine (f5C) in cytoplasmic tRNALeu and mt-tRNAiMet, resulting in an increase in mitochondrial protein translation [44], [45]. The diverse roles and enzymatic activities of ALKBH1 are in agreement with its multiple cellular localizations in the nucleus, cytoplasm, and mitochondria.
Roles of m1A in mRNA and tRNA
m1dA in DNA is considered as a form of DNA damage modification, which leads to false base pairing and genomic mutations, and thus it has to be repaired [46]. On the other hand, endogenous enzymes are present to install the modification of m1A in RNA and play important roles in tRNAs and mRNAs.
The idea that m1A58 might be vital to tRNA structure was first proposed in studies on the 3-dimensional structure of S. cerevisiae initiator tRNAiMet [47]. Hydrogen bonds between adenosines A20, A54, and A60 play important roles in stabilizing yeast tRNAiMet. In this substructure, m1A58 is linked to A54 and A60 via hydrogen bonds, which is also structurally critical for stabilization [47]. The presence of m1A58 in all eukaryotic initiator tRNAs implies that m1A58 is indispensable for tRNA structure and stability [12], which has been experimentally validated in human cells [13]. Knockdown of ALKBH1, the m1A58 eraser, increases the cellular level of tRNAiMet [13], supporting the function of m1A58 to maintain a stable structure of tRNAiMet. Furthermore, m1A58 in human tRNA3Lys is important for HIV replication. An 18-nucleotide sequence in the HIV-1 genome (GenBank accession No.: NC 001802) is complementary to the last 18 nucleotides of human tRNA3Lys. This sequence can be used as the primer binding site (PBS) for a virus to form a hybrid with tRNA3Lys and synthesize its minus strand cDNA in the presence of reverse transcriptase. To precisely reproduce the 18 nucleotides of tRNA3Lys in mature cDNA, m1A58 tRNA3Lys is required to terminate the reverse transcription process when the last 18 nucleotides of tRNA3Lys have been copied [48], [49], [50].
As m1A is a newly-discovered modification in mRNA and lncRNA, its function has not been extensively explored yet. Up till now, only the function of m1A at CDS in mt-mRNA has been confirmed in preventing the effective translation of modified codons due to the Watson-Crick disruptive nature of m1A [9], [10]. Ribosome profiling data analysis suggest that m1A at 5′ cap and 5′ UTR in nuclear mRNA might play a role in promoting translation [9].
Conclusion and perspective
The reversible m1A modification in tRNA and mRNA uncovers new mechanisms underlying the epitranscriptomic regulation of gene expression. The presence and functions of m1A in nuclear transcripts had been a subject of debate lately. Hopefully, the discovery of new m1A writers, erasers, and readers in the future will provide convincing evidence to help resolving such argument. Although tRNA MTases have been found to modify a subset of m1A sites in mRNA, the writer responsible for the majority of m1A sites in mRNA is still unknown. Since ALKBH3 is a demethylase of mRNA m1A, it is interesting to investigate whether its function in cancers is related to its demethylation activity toward mRNA m1A, and whether other erasers for mRNA m1A exist. Analogous to the positive charged cap m7G modification bound by a specific RNA-binding protein, mRNA m1A could be recognized by reader proteins as well [51]. Compared to the extensively-studied m6A modification, our knowledge about m1A in mRNA is limited. Functions of m1A in mRNA and the underlying mechanisms need to be further investigated. It is anticipated that m1A in mRNA and lncRNA might regulate RNA processing or protein translation in various manners: 1) m1A is specifically bound by a reader; 2) m1A controls RNA structure to affect protein-RNA interaction; 3) m1A at CDS affects the base-pairing between codon and anticodon. It might be worth exploring whether m1A occurs and plays important regulatory roles in other types of RNAs as well.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the National Basic Research Program of China (Grant Nos. 2016YFC0900302 and 2017YFA0505201) and the National Natural Science Foundation of China (Grant No. 21432002).
Handled by Xiu-Jie Wang
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.Dunn D.B. The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 2.Dunn D.B. The isolation of 1-methyladenylic acid and 7-methylguanylic acid from ribonucleic acid. Biochem J. 1963;86:14–15. [Google Scholar]
- 3.RajBhandary U.L., Stuart A., Faulkner R.D., Chang S.H., Khorana H.G. Nucleotide sequence studies on yeast phenylalanine sRNA. Cold Spring Harb Symp Quant Biol. 1966;31:425–434. doi: 10.1101/sqb.1966.031.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S., Watzinger P., Kotter P., Entian K.D. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41:5428–5443. doi: 10.1093/nar/gkt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava R., Gopinathan K.P. Ribosomal RNA methylation in Mycobacterium smegmatis SN2. Biochem Int. 1987;15:1179–1188. [PubMed] [Google Scholar]
- 6.Peifer C., Sharma S., Watzinger P., Lamberth S., Entian P.K. Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Res. 2013;41:1151–1163. doi: 10.1093/nar/gks1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominissini D., Nachtergaele S., Moshitch-Moshkovitz S., Peer E., Kol N., Ben-Haim M.S. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Xiong X., Wang K., Wang L., Shu X., Ma S. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat Chem Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 9.Li X., Xiong X., Zhang M., Wang K., Chen Y., Zhou J. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;67:1–13. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safra M., Sas-Chen A., Nir R., Winkler R., Nachshon A., Bar-Yaacov D. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 11.Agris P.F. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog Nucleic Acid Res Mol Biol. 1996;53:79–129. doi: 10.1016/s0079-6603(08)60143-9. [DOI] [PubMed] [Google Scholar]
- 12.Anderson J.T., Droogmans L. Biosynthesis and function of 1-methyladenosine in transfer RNA. In: Grosjean H., editor. Fine-tuning of RNA functions by modification and editing. Springer Berlin Heidelberg; Berlin Heidelberg: 2005. pp. 121–139. [Google Scholar]
- 13.Liu F., Clark W., Luo G., Wang X., Fu Y., Wei J. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:816–828. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macon J.B., Wolfenden R. 1-Methyladenosine. Biochemistry. 1968;7:3453–3458. doi: 10.1021/bi00850a021. [DOI] [PubMed] [Google Scholar]
- 15.Motorin Y., Muller S., Behm-Ansmant I., Branlant C. Identification of modified residues in RNAs by reverse transcription-based methods. Methods Enzymol. 2007;425:21–53. doi: 10.1016/S0076-6879(07)25002-5. [DOI] [PubMed] [Google Scholar]
- 16.Behm-Ansmant I., Helm M., Motorin Y. Use of specific chemical reagents for detection of modified nucleotides in RNA. J Nucleic Acids. 2011;2011 doi: 10.4061/2011/408053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgkinson A., Idaghdour Y., Gbeha E., Grenier J., Hip-Ki E., Bruat V. High-resolution genomic analysis of human mitochondrial RNA sequence variation. Science. 2014;344:413–415. doi: 10.1126/science.1251110. [DOI] [PubMed] [Google Scholar]
- 18.Hauenschild R., Tserovski L., Schmid K., Thuring K., Winz M., Sharma S. The reverse transcription signature of N-1-methyladenosine in RNA-Seq is sequence dependent. Nucleic Acids Res. 2015;43:9950–9964. doi: 10.1093/nar/gkv895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominissini D., Rechavi G. Loud and clear epitranscriptomic m1A signals: now in single-base resolution. Mol Cell. 2017;68:825–826. doi: 10.1016/j.molcel.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Glick J.M., Leboy P.S. Purification and properties of tRNA(adenine-1)-methyltransferase from rat liver. J Biol Chem. 1977;252:4790–4795. [PubMed] [Google Scholar]
- 21.Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson J., Phan L., Hinnebusch A.G. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2000;97:5173–5178. doi: 10.1073/pnas.090102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bujnicki J.M. In silico analysis of the tRNA: m1A58 methyltransferase family: homology-based fold prediction and identification of new members from Eubacteria and Archaea. FEBS Lett. 2001;507:123–127. doi: 10.1016/s0014-5793(01)02962-3. [DOI] [PubMed] [Google Scholar]
- 24.Ozanick S., Krecic A., Andersland J., Anderson J.T. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA. 2005;11:1281–1290. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilardo E., Nachbagauer C., Buzet A., Taschner A., Holzmann J., Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase–extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–11593. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chujo T., Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18:2269–2276. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waku T., Nakajima Y., Yokoyama W., Nomura N., Kako K., Kobayashi A. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J Cell Sci. 2016;129:2382–2393. doi: 10.1242/jcs.183723. [DOI] [PubMed] [Google Scholar]
- 28.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C., Li C.J. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trewick S., Henshaw T.F., Hausinger R.P., Lindahl T., Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 31.Falnes P.Ø., Johansen R.F., Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 32.Kurowski M.A., Bhagwat A.S., Papaj G., Bujnicki J.M. Phylogenomic identification of five new human homologs of the DNA repair enzyme AlkB. BMC Genomics. 2003;4:48. doi: 10.1186/1471-2164-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Pulido L., Andrade-Navarro M.A. The FTO (fat mass and obesity associated) gene codes for a novel member of the non-heme dioxyenase superfamily. BMC Biochem. 2007;8:23. doi: 10.1186/1471-2091-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan T., Trewick S., Koivisto P., Bates P.A., Lindahl T., Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proc Natl Acad Sci U S A. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aas P.A., Otterlei M., Falnes P., Vagbo C.B., Skorpen F., Akbari M. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 36.Dango S., Mosammaparast N., Sowa M.E., Xiong L., Wu F., Park K. DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol Cell. 2011;44:343–344. doi: 10.1016/j.molcel.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamato I., Sho M., Shimada K., Hotta K., Ueda Y., Yasuda S. PCA-1/ALKBH3 contributes to pancreatic cancer by supporting apoptotic resistance and angiogenesis. Cancer Res. 2012;72:4829–4839. doi: 10.1158/0008-5472.CAN-12-0328. [DOI] [PubMed] [Google Scholar]
- 38.Shimada K., Nakamura M., Anai S., De Velasco M., Tanaka M., Tsujikawa K. A novel human AlkB homologue, ALKBH8, contributes to human bladder cancer progression. Cancer Res. 2009;69:3157–3164. doi: 10.1158/0008-5472.CAN-08-3530. [DOI] [PubMed] [Google Scholar]
- 39.Ueda Y., Ooshio I., Fusamae Y., Kitae K., Kawaguchi M., Jingushi K. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westbye M.P., Feyzi E., Aas P.A., Vagbo C.B., Talstad V.A., Kavli B. Human AlkB homolog 1 is a mitochondrial protein that demethylates 3-methylcytosine in DNA and RNA. J Biol Chem. 2008;283:25046–25056. doi: 10.1074/jbc.M803776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ougland R., Lando D., Jonson I., Dahl J.A., Moen M.N., Nordstrand L.M. ALKBH1 is a histone H2A dioxygenase involved in neural differentiation. Stem Cells. 2012;30:2672–2682. doi: 10.1002/stem.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T.P., Wang T., Seetin M.G., Lai Y., Zhu S., Lin K. DNA methylation on N6-adenine in mammalian embryonic stem cells. Nature. 2016;532:329–333. doi: 10.1038/nature17640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller T.A., Andrzejak M.M., Hausinger R.P. A covalent protein-DNA 5′ -product adduct is generated following AP lyase activity of human ALKBH1 (AlkB homologue 1) Biochem J. 2013;452:509–518. doi: 10.1042/BJ20121908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haag S., Sloan K.E., Ranjan N., Warda A.S., Kretschmer J., Blessing C. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J. 2016;35:2104–2119. doi: 10.15252/embj.201694885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawarada L., Suzuki T., Ohira T., Hirata S., Miyauchi K., Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nuclei Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaney J.C., Essigmann J.M. Mutagenesis, genotoxiciy, and repair of 1-methyladenine, 3-alkylcytosines, 1-methylguanine, and 3-methylthymine in alkB Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:14051–14056. doi: 10.1073/pnas.0403489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basavappa R., Sigler P.B. The 3 Å crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 1991;10:3105–3111. doi: 10.1002/j.1460-2075.1991.tb07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Artzi H., Shemesh J., Zeelon E., Amit B., Kleiman L., Gorecki M. Molecular analysis of the second template switch during reverse transcription of the HIV RNA template. Biochemistry. 1996;35:10549–10557. doi: 10.1021/bi960439x. [DOI] [PubMed] [Google Scholar]
- 49.Burnett B.P., McHenry C.S. Posttranscriptional modification of retroviral primers is required for late stages of DNA replication. Proc Natl Acad Sci U S A. 1997;94:7210–7215. doi: 10.1073/pnas.94.14.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renda M.J., Rosenblatt J.D., Klimatcheva E., Demeter L.M., Bambara R.A., Planelles V. Mutation of the methylated tRNA(Lys)(3) residue A58 disrupts reverse transcription and inhibits replication of human immunodeficiency virus type 1. J Virol. 2001;75:9671–9678. doi: 10.1128/JVI.75.20.9671-9678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dostie J., Lejbkowicz F., Sonenberg N. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J Cell Biol. 2000;148:239–247. doi: 10.1083/jcb.148.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]