Graphical abstract
Keywords: Urease inhibitors, Urea, Thiourea, Heterocycles, Phosphorated compounds
Abstract
Ureases are enzymes that hydrolyze urea into ammonium and carbon dioxide. They have received considerable attention due to their impacts on living organism health, since the urease activity in microorganisms, particularly in bacteria, are potential causes and/or factors contributing to the persistence of some pathogen infections. This review compiles examples of the most potent antiurease organic substances. Emphasis was given to systematic screening studies on the inhibitory activity of rationally designed series of compounds with the corresponding SAR considerations. Ureases of Canavalia ensiformis, the usual model in antiureolytic studies, are emphasized. Although the active site of this class of hydrolases is conserved among bacteria and vegetal ureases, the same is not observerd for allosteric site. Therefore, inhibitors acting by participating in interactions with the allosteric site are more susceptible to a potential lack of association among their inhibitory profile for different ureases. The information about the inhibitory activity of different classes of compounds can be usefull to guide the development of new urease inhibitors that may be used in future in small molecular therapy against pathogenic bacteria.
Introduction
Urease, an enzyme that strictly depends on nickel ions (Ni2+) [1], is a type of hydrolase that accelerates the rate of urea hydrolysis to ammonia (NH3) and carbamic acid, which disproportionates into ammonia and carbon dioxide (CO2), by one hundred trillion-fold (Scheme 1) [2], [3], [4]. Since its discovery in plants [5], Canavalia ensiformis (Fabaceae) urease has been exhaustively used as a model to develop new urease inhibitors for use in clinical and agricultural applications [6] and has become the milestone in biochemistry as the first enzyme to be crystallized [7]. The versatile uses of the purified urease from C. ensiformis in the discovery of new urease inhibitors is in part due to the similarity of amino acid sequences among ureases from multiple species [8], suggesting the presence of a common ancestor of this enzyme. The first complete three-dimensional structure of a urease was reported by Jabri and coworkers in 1995 from crystallography studies performed with urease from Klebsiella aerogenes [9]. Later, other structures were disclosed for ureases from Bacillus pasteurii [10], Helicobacter pylori [11] and, most recently, C. ensiformis [12]. Indeed, the elucidation of the urease structure from a legume was crucial to obtain a better understanding of the requirements for the ureolytic activity of this class of enzymes in different organisms [12].
Scheme 1.
Representation of urea hydrolysis catalyzed by ureases.
The increase in medium pH caused by the accumulation of NH3 is a urease trait of tremendous medical importance [4]. Urine and/or gastrointestinal infections by ureolytic bacteria cause health complications in humans and animals, including kidney stone formation, pyelonephritis, hepatic encephalopathy and, ultimately, hepatic coma [4], [13]. Therefore, major public health issues are related to Helicobacter pylori, a gram-negative bacterial species that is able to survive in an acidic environment, such as the stomach (pH 1–2). Consequently, H. pylori infections induce gastric inflammation and increase the risk of developing duodenal and gastric ulcers, gastric adenocarcinoma and gastric lymphoma [4], [14]. Approximately 50% of the global population is infected with H. pylori. This bacteria species can persist in the stomach throughout the life of infected individuals without causing disease symptoms. The high prevalence of H. pylori in the human population indicates that this microorganism has developed mechanisms of resistance against host defenses [14]. The urease enzyme in the cytoplasm and/or bound to the H. pylori surface is the main virulence factor of this human pathogen [15], [16]. Urease represents up to 10% of the total protein content of H. pylori [17]. Moreover, the lysis of some pathogen-infected cells leads to the release of cytosolic ureases that bind to the surface of intact bacterial cells and cause the hydrolysis of urea, which is present in the human gut at a concentration of 3 mM. The resulting production of NH3 increases the medium pH, creating a permissive environment that promotes H. pylori survival [15], [18]. During the past 20 years, the recommended first-line therapy for H. pylori eradication consists of a combination of the antibiotics amoxicillin and clarithromycin with omeprazole, a proton pump cell inhibitor. However, the increase in H. pylori resistance to these antibiotics (particularly to clarithromycin) has rendered these therapeutics an ineffective option in recent years [3], [19], [20]. Indeed, other treatment strategies have emerged to fight H. pylori infection, including the use of bismuth salts (a metal with antiurease properties [21]) combined with a proton pump inhibitor or combinations of other classes of antibiotics as fluoroquinolones, aminopenicillins, and tetracyclines [3], [20], [22].
Urease is also produced by most strains of Proteus mirabilis and Staphylococcus saprophyticus [23]. P. mirabilis, a gram-negative bacteria, causes a variety of community- or hospital-acquired illnesses, including urinary tract, wound, and bloodstream infections [24]. One of the major factors known to be involved in Proteus mirabilis-induced urinary crystal formation is the bacterial urease, a well-known virulence factor of this microorganism [25], [26], [27]. Indeed, the P. mirabilis urease increases the pH of the urinary tract and causes the local supersaturation and formation of carbonate apatite and struvite crystals [28]. In addition, the ability of a urease-negative mutant of P. mirabilis urease to colonize the urinary tract is approximately 100-fold less than the parent strain [26], [29]. S. saprophyticus, a spherical bacterium of the gram-positive coccus group, is also a frequent cause of urinary tract infections [30]. Among the virulence factors in S. saprophyticus, urease is a major factor contributing to the invasiveness of this bacteria [31], particularly in the bladder tissue, whereas its persistence in the urinary tract and nephropathogenicity are governed by factors other than urease [32]. Indeed, P. mirabilis and S. saprophyticus are some of the primary etiological agents related to urinary tract infections [33], [34], and urease is one of the key virulence factors that allows these pathogens to successfully infect the urinary tract [34], [35], [36].
Another urease-dependent human pathogen is Yersinia enterocolitica, a well-known enteric pathogen that causes yersiniosis [37], [38], [39], is an invasive enteric pathogen that gains access to the body via the oral route through the consumption of contaminated food or water [40], [41]. This bacteria causes a wide spectrum of clinical disorders, ranging from self-limiting gastroenteritis to mesenteric lymphadenitis, visceral abscesses, septicemia in immunocompromised hosts, and reactive arthritis [40], [41], [42]. Although Y. enterocolitica grows optimally at a pH of approximately 7.0 to 8.0, these bacteria remains viable in acidic conditions (pH 4.4) for 48 h [43]. The ability of certain Y. enterocolitica strains to survive the high acidity of some foods and in vitro acidic conditions suggests that these bacteria are relatively acid tolerant [44], [45]. The mechanism underlying the acid tolerance of Y. enterocolitica has been proposed to be due to the urease activity present in this species [44], [46].
Due the tremendous medical importance of urease, urease inhibitors with improved stability and low toxicity may be an effective therapy against diseases caused by urease-dependent pathogenic microorganisms. Here, we present an overview of the most relevant organic substances that exert antiureolytic inhibitory effects on ureases. The urease inhibitors presented here are organized into five classes according to their chemical structures, namely: (thio)urea derivatives, five- and six-membered heterocycles, barbituric analogues and phosphoramidated substances. Urease inhibitors derived from natural products and metal complexes will not addressed in this review since very good reviews of these compounds have been published elsewhere [6], [47].
Organic substances as urease inhibitors
(Thio)urea derivatives
The development of enzyme inhibitors based on the molecular structure of the native substrate is an approach commonly used in rational drug design. Several systematic screens of urease inhibitors designed based on the urea structure have been conducted, particularly in the last 10 years.
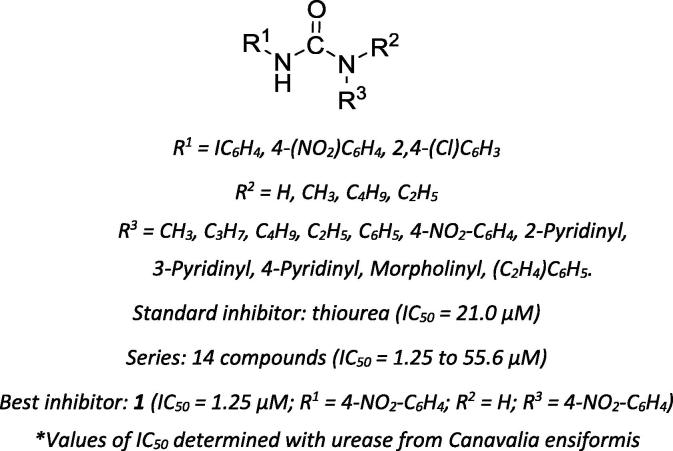
In one of the first of these studies, a series of N1,N2-di- and tri-substituted urea derivatives were synthesized, and their inhibitory activities against Canavalia ensiformis urease were tested [48]. N1,N2-diaryl derivatives containing nitro groups at both phenyl rings (like compound 1, Scheme 2) show low micromolar inhibition of C. ensiformis urease.
Scheme 2.
Chemical structures of N1,N2-di- and tri-substituted urea derivatives reported as potential urease inhibitors.
More recently, Mustafa and co-workers identified several novel urease inhibitors [49]. The authors designed and synthesized N1-toluoyl,N2-substituted urea derivatives and evaluated them using in vitro enzyme-inhibition assays that included C. ensiformis urease. Three compounds containing a methoxy group in the phenyl ring [compounds 2, 3 and 4 (Series A), Scheme 3] exhibited the strongest inhibition of the urease enzyme (47 to 59%). Notably, each of the three abovementioned inhibitors contains its tolyl moiety with different substitution patterns (ortho, meta or para position), suggesting that the inhibitory activity is not substantially affected by the position of R1.
Scheme 3.
Chemical structures of N1-toluoyl-N2-substituted urea derivatives described as urease inhibitors.
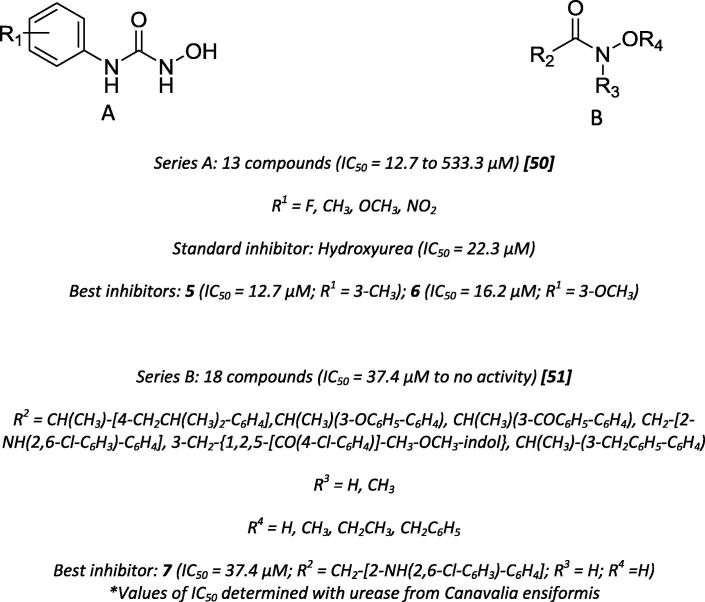
In 2002, Uesato et al., based on the urease inhibiting capacity of hydroxyurea, synthesized N1-hydroxy-N2-substituted derivatives and evaluated their inhibitory activity towards urease using hydroxyurea as a substrate (Scheme 4) [50]. Ortho- and para substitutions at the phenyl ring decreased the inhibitory activity, possibly because of the steric hindrance provided by the groups at these positions, which might diminish the hydroxamic acid connection with the active site. A few years later, using the same motivation, Rajic and co-workers synthesized hydroxamic acid derivatives, tested their antiurease activity and found that only the derivatives bearing a hydroxyl group inhibited urease activity [51].
Scheme 4.
Chemical structures of N1-hydroxy-N2-substituted derivatives described as urease inhibitors.
More recently, many investigations examining thiourea-based urease inhibitors have developed thiourea derivatives that present higher inhibitory potency than their urea counterparts. Khan and co-workers have synthesized a variety of substituted thioureas and screened their urease inhibitory activity [52]. Substitutions with functional groups attached to the phenyl or heterocyclic ring around the thiourea core and compounds with substituents containing lone electron pairs exert a decisive effect on the urease inhibitory activity. The strongest inhibitory activity (IC50 8.43 µM) was observed for the derivative bearing a 3-pyridyl substituent (compound 8, Scheme 5).
Scheme 5.
Chemical structures of N1-aryl-N2-(aryl or alkyl)-substituted thiourea derivatives that possesses antiureolytic activity.
Taha and co-workers screened a series of symmetrical bis-thiourea derivatives bearing a disulfide moiety [53] (Scheme 6). Compounds with different substituted phenyl rings at both terminal nitrogens were evaluated as inhibitors of C. ensiformis urease. The presence of a fluorine atom at the phenyl groups, regardless of the position, lead to high inhibition (compounds 8, 9 and 13; Scheme 6). Comparable inhibitory activity (IC50 ranging between 0.4 and 1.7 μM) was observed for derivatives containing para-Cl-phenyl (14) para-CF3-phenyl (12) or electron-releasing substituents, such as methyl and methoxy groups at para or ortho positions (compounds 10, 11 and 15). In addition, these compounds were considered nontoxic, based on a cytotoxicity assay.
Scheme 6.
Chemical structures of symmetrical (bis)-thiourea which possesses antiurease activity.
The introduction of the benzoyl moiety at the thiourea nitrogen atom was extensively probed in the literature in studies involving N1-benzoyl,N2-aryl substituted derivatives (Scheme 7) [54], [55], [56], [57], [58]. The beneficial effect of the benzoyl group on the inhibitory activity towards C. ensiformis urease was evidenced in a direct comparison between extent of inhibition achieved by the monosubstituted N-benzoyl thiourea and thiourea [54]. Additionally, kinetic experiments designed to probe the mechanism of urease inhibition suggested that the benzoyl thiourea derivatives acted as mixed-type inhibitors that bound to either catalytic or allosteric sites of the enzyme [54]. In the same paper, the screen of the inhibitory activities revealed eight title derivatives showing percent inhibition values (51 to 72%) comparable to the positive control hydroxyurea (74%). Compounds containing electron-donating and electron-withdrawing substituents on the phenyl ring showed variable inhibitory activities [56]. Nevertheless, the presence of p-tertbutyl, o-NO2, (m- or p-)Cl or (m- or p-)Br at the phenyl ring attached to N2 of the thiourea core enhanced the inhibitory activity [54]. Similarly, Rauf and co-workers (2016) revealed that benzoylthioureas bearing the 2,4,6- thichlorophenyl group as a substituent (compound 19 – Series A; Scheme 7) (IC50 1.67 µM) and derivatives with 2,4-dichlorophenyl (compound 17 – Series A; Scheme 7) and 2,3-dichlorophenyl groups (compound 18 – Series A; Scheme 7) (IC50 1.34 and 1.92 µM, respectively) were much more active than the standard inhibitor (thiourea, IC50 22.3 µM) [56].
Scheme 7.
Chemical structures of N1-benzoyl,N2-aryl substituted derivatives described as urease inhibitors.
The effect of a phenyl ring bearing either para-ethyl benzoate [58] or para-sulfanilamide [57] at N2 nitrogen of benzoylthioureas was evaluated in the studies developed by Saeed and co-workers. The in vitro assays using C. ensiformis urease showed very high inhibitory activities for most of the tested derivatives. Among the N2-para-benzoate series, compounds bearing a 4-methoxy group (compound 22 Series A; Scheme 8) and 3,4-dimethoxy substituent (compound 21 Series A; Scheme 8) at the benzoyl group showed the best urease inhibition, with IC50 values of 0.21 and 0.13 µM, respectively. Notably, compounds derived from 3-chloro and 2,4-dichloro benzoic acid also showed comparable IC50 values [58].
Scheme 8.
Chemical structures of potential urease inhibitors based on benzoate or sulfanilamide thioureas.
Among the series of sulfanilamide thioureas, the most active compounds contain 4-chloro (compound 23 Series B; Scheme 8) and 2-chloro-5-nitro (compound 24 Series B; Scheme 8) substituents on aryl group and showed Ki values of 0.20 and 0.44 µM, respectively. According to the analysis of the structure–activity relationship, substituents with an electron-withdrawing group located at the 4-aryl group were the most potent inhibitors [57].
In 2017, Saeed and co-workers reported the synthesis of two series of N-acyl thioureas derived from myristic (Series B, Scheme 9) [59] and palmitic acids (Series A, Scheme 9) [60]. The inhibitory effects of substituted phenyl rings at the remaining nitrogen atom on C. ensiformis urease were evaluated. All tested compounds presented very low μM IC50 values (c.a. 0.01 to 0.09). One compound with a chlorine atom on the phenyl ring was identified as the most active inhibitor in each of the studied series (compounds 25 and 26). Curiously, the chlorine atom of the most active acyl-thiourea derivative of palmitic acid [60] is attached at the para position, whereas the most active derivative of the myristic acid series [59] contains its chlorine at the meta position. Kinetic investigations of these two compounds indicated a non-competitive inhibitory profile for this class of compounds.
Scheme 9.
Chemical structures of N-acyl thioureas-urease inhibitors derived from myristic or palmitic acids.
Jamil and co-workers reported the results of a screen for symmetrical isophthalyl-bis-(thioureas) (Scheme 10) [61]. The presence of an electron-withdrawing substituent at each terminal nitrogen was identified as a crucial factor determining the inhibitory activity of the four most active compounds (27–30).
Scheme 10.
Chemical structures of symmetrical isophthalyl-bis-(thioureas) urease inhibitors.
Eighteen substituted benzylidene thiosemicarbazide derivatives synthesized by Aslam and co-workers (2011) [62] (Scheme 11) showed low IC50 values for C. ensiformis urease. Compounds bearing 3-NO2 and 4-N(CH3)2 as substituents showed very good inhibitory activity (compounds 31 and 32, respectively). Other derivatives with a halogen group at ortho position showed comparable activity, whereas compounds with a halogen group at meta and para positions showed lower activity.
Scheme 11.
Chemical structures of benzylidene thiosemicarbazides urease inhibitors.
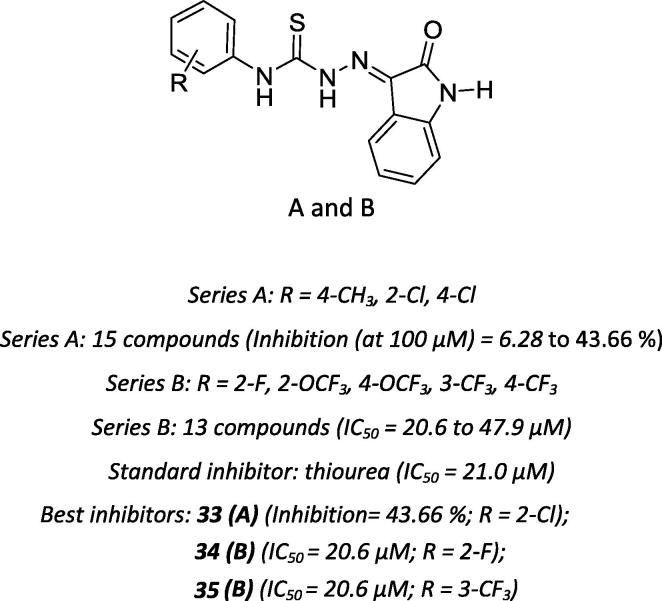
Similarly, Pervez et al. examined the use of several isatin derivatives as potential urease inhibitors [63]. Overall, compounds with a methoxy substituent at the para position and compounds with a chloro substituent at the ortho position of the phenyl ring (compound 33 – Series A, Scheme 12) showed the most potent inhibitory effects of compounds in the present series, exhibiting relatively greater activity at the tested concentrations. Subsequently, the same research group reported the results for a novel series of N4-substituted isatin-3-thiosemicarbazones. In general, the addition of one, two or three substituents with inductive electron-withdrawing effects at different positions of the phenyl ring increased the urease inhibitory activity of the derivatives. For example, compounds bearing trifluoromethoxy and trifluoromethyl (compounds 34 and 35 – Series B, Scheme 12) substituents showed the highest inhibitory activity (41 to 78%) compared with compounds bearing methoxy and methyl substituents (8 to 29%). Additionally, substitutions in the phenyl ring with an electron-withdrawing group at N4 position exerted a positive effect on enzyme activity, as compounds showed more potent urease inhibition (IC50 = 20.6 µM) than the standard thiourea (IC50 = 21.0 µM). [64].
Scheme 12.
Chemical structures of potential urease inhibitors based on isatin derivatives.
Sharma and co-workers [65] also explored the isatin moiety in a study involving a comprehensive SAR analysis the urease inhibitory activities of 32 N-phenyl urea/thiourea compounds (Scheme 13). Substituents at the phenyl ring bearing halogen and methoxy groups were screened for their potency in inhibiting C. ensiformis urease. The presence of the double 3-(1-piperazinyl)-1,2-benzisothiazole moiety and conjugation to the N-phenyl-urea/thiourea motif enhanced the inhibitory activity. Additionally, all compounds in the thiourea series (Series B – Scheme 13) were slightly more potent than their urea counterparts (Series A – Scheme 13). Moreover, fluoro and methoxy derivatives showed promising inhibitory activities and were more potent than the corresponding chlorinated or bromated structures. Finally, the addition of a methoxy group at the para or ortho position yielded the two most potent thioureas of the series (compounds 36 and 37, respectively, both from Series B).
Scheme 13.
Chemical structures of potential urease inhibitors bearing an isatin moiety.
A hybridization strategy using benzothiazoles and thiosemicarbazides was also employed by Taha et al. to develop new antiurease agents [66]. Eighteen of the synthesized compounds (Scheme 14) exhibited IC50 values less than thiourea, revealing a trend in which substituted aromatic rings were more active than unsubstituted rings. Furthermore, when an electron-withdrawing group is present in the aryl motif, the polarizability and activity of the molecule towards urease increases [66].
Scheme 14.
Chemical structures of hybrids benzothiazole thiosemicarbazides that present antiurease activities.
Five-membered heterocycles
In 2010, Khan reported a potent series of inhibitors based on 2-aminothiophenes derivatives (Scheme 15) that were identified using molecular modeling and virtual screens against Canavalia ensiformis urease. According to the docking study, compounds with a single thiophene ring bind next to the nickel ions and exhibit better inhibitory potency than compounds with fused bulky rings or compounds with substitutions at the amino group. In the latter case, the thiophene ring is located at a distant site from the nickel ions and results in a loss of activity, apparently due to torsional strain [67]. For example, a compound with a methyl group at C-4 position was the most potent among the series and more active than the standard inhibitor. On the other hand, a similar derivative with fused ring showed a loss of activity against the enzyme.
Scheme 15.
Chemical structures of 2-aminothiophenes – a five-membered heretocycle-urease inhibitors.
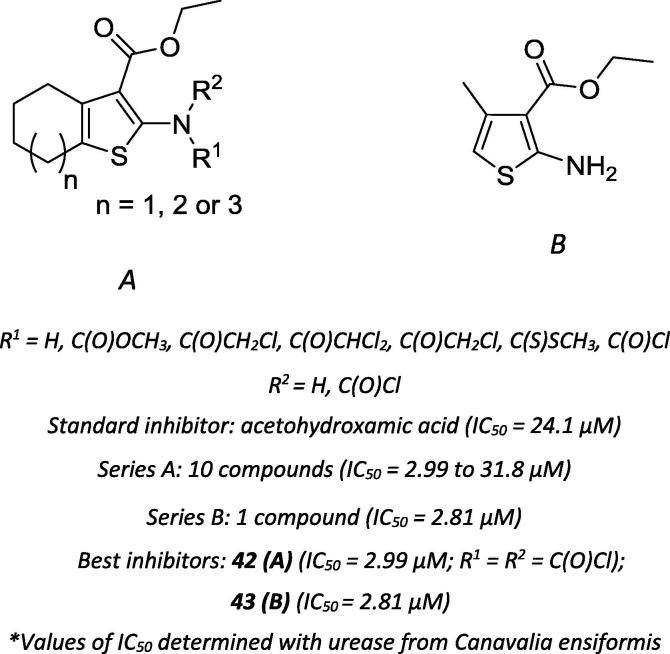
Ali and coworkers assessed the antiurease activity of diverse 5-aryl-thiophene-2-carbaldehydes [68]. The authors observed a trend in which electron-withdrawing groups were more active at inhibiting the enzyme than their electron-donating counterparts, highlighting the di-halogenated compound (44 – Series A; Scheme 16) as the most active of the series [68]. Noreen et al. also explored the potential of 5-aryl-thiophene-2-sulfonamides, three of which were more active than the positive control, namely compounds 48 and 49 (Scheme 16) [69].
Scheme 16.
Chemical structures of urease inhbitors based on 5-aryl-thiophene-2-carbaldehydes scaffold.
Pyrazole derivatives have also been explored in an attempt to identify new urease inhibitors [70]. Harit et al. evaluated pyrazole dimers, forming nitrogen centered tripods (Scheme 17). When the pyrazole rings were fused through either a N-C or C-C linkage, the 4 resulting tripods were selective urease inhibitors compared with a-chemotrypsin, cholinesterases, phosphodiesterase and β-glucoronidase. Dimerization also reduces the IC50 of the derivative by 50%, from 94 µM to 44 µM. Furthermore, the authors concluded that the nature of the side arm had no effect on urease inhibition.
Scheme 17.
Chemical structures of urease inhbitors based on pyrazoles moiety.
Similarly, the imidazole motif has been extensively explored in medicinal chemistry investigations and specifically in screens for urease inhibitors. Naureen and coworkers [71] tested fifteen series of tetraaryl imidazole-indole compounds (Scheme 18) and showed that they exhibited comparable or better urease inhibitory activity than the positive control thiourea. The most potent inhibitors were the compounds containing disubstituted halogens (compounds 52, 55, and 56; Scheme 18) or containing the trifluoromethyl moiety on the arylindole group (compounds 53 and 54; Scheme 18) and displayed IC50 values as low as 0.12 µM.
Scheme 18.
Chemical structures of urease inhbitors based on imidazole-indole moieties.
Some antimicrobial nitroimidazole drugs, namely metronidazole and secnidazole, also present antiurease activity. Encouraged by these properties, Mao and coworkers synthesized a series of hybrids of salicylates and metronidazole [72] or secnidazole [73]. Both hybrid series were active against urease, with secnidazoles being more active (Scheme 19). A synergistic effect was observed, since secnidazole alone yielded an IC50 = 156 ± 10 μM [73] and the hybrids showed inhibition at the submicromolar level. Docking studies using both hybrids types showed that their binding generated a flap movement of α313-α346 residues, opening the enzyme’s active site. The most active compound, 57 (Series D; Scheme 19), also showed hydrogen bonding between the phenolic oxygen and Thr135 and His417 in addition to hydrophobic interactions with Phe195, Ala246 and Phe273 [73].
Scheme 19.
Chemical structures of urease inhbitors based on hybrids of salicilates and metronidazole or secnidazole.
Along with thiazoles, thiazolidine aliphatic esters (Scheme 20) were also described as potential antiurease agents by Lodhi et al. [74]. A screen against C. ensiformis and B. pasteurii ureases identified nine esters that were more active than thiourea, and the heptyl ester 62 (Scheme 20) was the most active inhibitor of both enzymes. Molecular docking studies showed the interaction of the carbonyl oxygen atom with a nickel atom, forming a pseudotetrahedral geometry responsible for the principal interaction between the thiazolidines and urease. The authors inferred that the observed increase in activity with increase of chain length represented an inductive effect that accumulates to a greater extent than steric hindrance until the octyl ester [74]. These theories agree with observed decrease in the potency of compounds containing branched chains and heteroatoms.
Scheme 20.
Chemical structures of thiazoles, thiazolidines aliphatic esters – potential urease inhibitors.
In contrast to the abovementioned related heterocycles, a screen of the urease inhibitory activity of several difunctionalized oxazolones revealed that only compounds possessing a phenyl ring at C2 displayed antiureolytic activity, but was 3 times less active than thiourea (compounds 65 and 66, Scheme 21) [75].
Scheme 21.
Chemical structures of oxazolones: a class of urease inhibitors.
Some researchers have explored the use of sulfur heterocycles, mainly benzothiazoles. Araujo and coworkers [76] synthesized a number of 2-arylbenzothiazoles (Scheme 22) with the aim of obtaining urease inhibitors. Among the synthesized compounds, three stand out as being as potent as known urease inhibitors: compound 67, which is comparable to hydroxyurea (62% inhibition), and compounds 68 and 69, which are equivalent to thiourea (26%). The inhibitory mechanism of compound 67 was investigated using kinetic experiments revealing that this benzothiazole binds either to the free urease or the enzyme-substrate complex; thus, it represents a mixed-type inhibitor. The dissociation constants obtained from those experiments showed a Ki for the urease-compound 67 of 1.02 ± 0.04 mM and a Ki for the urease-urea-compound 67 of 3.17 ± 0.69 mM, indicating that the affinity of compound 67 for the active site is approximately three times that of urea.
Scheme 22.
Chemical structures of benzothiazoles – an interesting class of urease inhbitors.
Conjugation of benzothiazole and acyl thiourea cores was also exploited by Gull et al. [77] to obtain hybrid 6-aryl-2-acetamidobenzothiazoles (Scheme 23). All compounds displayed similar range of inhibitory activity, with an IC50 of approximately 18 µg/mL, and were more active than thiourea. The addition of an electron-donating group in the para position of the aryl group resulted in a small gain in potency compared to an electron-withdrawing group. In silico docking studies of compound 70 (R = p-tolyl, Scheme 23) in both active sites, A and B, showed hydrophobic interactions with the residues His593, Met637, Ala636, Gln635 and Asp494 and a hydrogen bond with the phosphate group at site A. At site B, cation-pi type interactions were observed between compound 70 and Lys208, Asp206, Thr158, Glu254, Phe182, Lys156 and Asp183, along with hydrogen bonds with Glu252 and Lys156. All compounds interacted better with site B than site A of H. pylori urease, indicating that these molecules participate in a stronger bond with site B than with site A. Furthermore, an inversely proportional linear correlation between the number of hydrogen bonds and the IC50 was noted [77].
Scheme 23.
Chemical structures of urease inhibitors based on 6-aryl-2-acetamidobenzothiazoles.
Akhtar and coworkers synthesized chiral-substituted 1,2,4-triazoles (Scheme 24) and assessed their activity [78]. Substituents with differently sized chiral moieties showed an insignificant influence on urease inhibition. In a subsequent paper, the same authors [79] synthesized 5-aryl-1,2,4-triazole-3-thiones with different halogen patterns at the 5-aryl moiety (Scheme 25). The bromo-substituted rings in compounds 72 and 73 were more active than their chlorinated or fluorinated analogues and thiourea itself.
Scheme 24.
Chemical structures of urease inhibitor bearing the 1,2,4-triazole core.
Scheme 25.
Chemical structures of urease based on 5-aryl-1,2,4-triazole-3-thiones.
Similarly, Özil et al. synthesized 1,2,4-triazole derivatives (Scheme 26) by modifying groups attached to the central benzene ring and N2-atom from triazole rings. Of all compounds, derivatives 74 and 75 were the most active [80].
Scheme 26.
Chemical structures of 1,2,4-triazole derivatives – potent substances described as urease inhibitor.
The urease inhibition potential of disubstituted 1,2,4-triazole-3-thiones (Series A, Scheme 27) were evaluated by Khan and coworkers, which were more active than the thiodiazole analogue (Series B, Scheme 27). A suitable structure–activity relationship was stablished for these compounds. The presence of one nitro group in the meta position of the aryl ring at position 5 of the triazole (compound 76 – Series A; Scheme 27) enhanced the inhibitory potency compared to an unsubstituted phenyl ring. The substitution pattern of the ring attached to the nitrogen atom at position 4 also had a clear influence on the inhibitory capacity. Small polarizable groups as a methyl group, in the para position favored inhibitory activity compared to groups placed at the meta and ortho positions [81].
Scheme 27.
Chemical structures of urease inhibitor bearing the 1,2,4-triazole-3-thione moiety.
Abid et al. [82] screened a series of triazole derivatives (Scheme 28) that were synthesized by varying benzyl and phenyl groups and contained halogen atoms, methyl or methoxy moieties. The compound with a bromine atom at the meta position in the benzyl moiety (compound 78; Scheme 28) was the most active derivative, whereas inhibitors with electron-donating groups at the para or meta positions of the phenyl group were the least active compounds of the series against urease.
Scheme 28.
Chemical structures of triazole derivatives described as potential urease inhibitors.
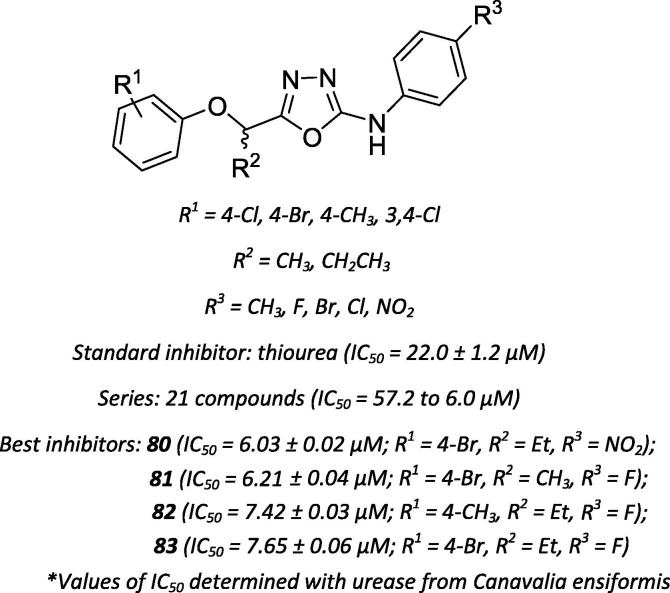
Oxadiazoles and their derivatives have also been reported to function as urease inhibitors. Akhtar and coworkers screened 2-arylamino-5-aryloxylalkyl-1,3,4-oxadiazoles (Scheme 29) for urease-inhibiting activity [83] The influence of the nature of the 2-arylamino substituent was the most relevant finding, whereas compounds containing a methyl group or chlorine or bromine atoms at the para position represented the least active molecules. However, the oxadiazoles with a fluorine or nitro group were the most active inhibitors (compounds 80–83, Scheme 29).
Scheme 29.
Chemical structures of oxadiazoles described as potential urease inhibitors.
Similar results were obtained for analogue compounds [84], where a p-methyl substituent in the aminophenyl (compound 86 – Series B, Scheme 30) group increased activity. 1,3,4-Oxadiazoles-2-thione analogues containing methoxylated aromatic groups (compounds 89 and 90, Scheme 31) also showed high antiurease activity [85]. Although halogenated derivatives generally showed the lowest activity, the para-chlorobenzyl analogue was the most active tested inhibitor (IC50 1.15 ± 0.2 μM).
Scheme 30.
Chemical structures of aminophenyl benzylalcohols which possesses antiurease properties.
Scheme 31.
Chemical structures of 1,3,4-oxadiazoles-2-thione analogues described as potent inhibitors of urease enzyme.
The oxadiazole core was also explored by Shahzada and coworkers, who evaluated different substituted phenyl rings and aliphatic chains at position 5 of the oxadiazole rings (Scheme 32) [86]. The activity of halogenated rings decreases from ortho to para patterns, the exception being a bromo-substituted ring, where the para-bromo displays the highest activity of all synthesized compounds. Molecules with nitro-substituted rings displayed maximum activity when positioned at the meta carbon, whereas the compound was inactive if attached to the para position. The introduction of a methyl group at position 3 on the para-nitrophenyl moiety decreased the IC50 to 13.6 µM. The n-octyl (not shown) chain was as active as the control, thiourea. The authors inferred that activity decreases as steric hindrance increases, probably due to diminished interactions with nickel ions.
Scheme 32.
Chemical structures of substances based on oxadiazole platform that has antiureolytic properties.
Rheman and coworkers [87] built a thioether version of the 1,3,4-oxadiazole coupled to the 3,4-methylenedioxiphenyl group (Scheme 33). Of the synthesized compounds, derivative 95 containing a bromine atom in meta position was the most active inhibitor against urease, displaying an IC50 in the same range as thiourea.
Scheme 33.
Chemical structures of substances based on 1,3,4-oxadiazole moiety coupled to 3,4-methylenedioxiphenyl group.
Fused heterocycles, such as the 1,2,4-triazolo[3,4-b]1,3,4-thiadiazole derivatives developed by Rafiq and coworkers (Scheme 34), were assembled for antiureolytic purposes. The assay of the compounds against urease identified inhibitors that were more active than thiourea, and compound 97 was the most potent derivative [88].
Scheme 34.
Chemical structures of antiureolytic subtances based on 1,2,4-triazolo[3,4-b]1,3,4-thiadiazole platform.
Pyrazolotriazines were hybridized with sulfonamides (Scheme 35) and presented high antiurease potency (IC50 from 0.037 to 0.084 µM) [89]. Remarkably, the most active inhibitor was the chiral compound containing a (S)-2-hydroxy-1-methylethaneamine substituent (compound 100; Scheme 35), but its enantiomer displayed the lowest inhibition. A kinetic study of compound 100 was performed and exhibited a mixed type inhibitory behavior with a Ki = 0.01 µM. Saify et al. reported 7-azoindole derivatives (Scheme 36), whose IC50 values ranged from 2.19 to 255.11 µM [90]. The analogue 103 (Scheme 35) possessing a 4-methoxyphenacyl moiety presented the highest inhibition with an IC50 of 2.19 ± 0.37 µM, whereas the second best inhibitor, compound 104, only had an IC50 = 133.31 ± 0.46 µM.
Scheme 35.
Chemical structures of hybrids pyrazolotriazine-sulfonamidas described as potential urease inhibitors.
Scheme 36.
Chemical structures of 7-azoindoles stated as urease inhibitors.
Selenium compounds are also reported to possess antiureolytic activity, such as ebselen (105, Scheme 37, IC50 = 3.3 µM) and its derivatives described by Macegoniuk et al.; ebselen was reported to exhibit antiulcer properties and inhibits gastric secretion [91], [92]. In an attempt to further derivatize ebselen, Macegoniuk et al. evaluated the activity of compounds bearing different groups on the nitrogen atom. The presence of a carboxylic acid group decreased significantly the activity of the inhibitors (IC50 of 25.4 µM to inactive). The activity returned to the previous order of magnitude for the corresponding methyl ester counterpart [IC50 3.3 µM (106) to 4.07 µM (107); Scheme 37]. Compounds containing phenyl group, including ebsalen (105; Scheme 37), and methyl ester derivatives were the most active inhibitors, with IC50 values as low as 3.3 µM.
Scheme 37.
Chemical structures of containing selenium atom urease inhibitors.
Six-membered heterocycles
Due to the wide range of biological activities of six-membered heterocyles, such as pyridinones [93], [94], [95], [96], [97], [98], pyridopyrimidine and other compounds, [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109] several studies aiming to develop urease inhibitors based on these structural motifs have been reported. Rauf et al. [110] evaluated the urease inhibitory activity of pyridopyrimidine derivatives. According to the researchers, the presence of a metal-chelating group such as –SH or the moiety 4-nitrobenzohidrazide determines the activity of these compounds, which could explain the inhibitory activity of compounds 108 and 109 (Scheme 38) towards urease. Based on the results from tautomerization studies, an increase in the negative charge of the heteroatoms of the compound correlates with the increase in urease inhibition.
Scheme 38.
Chemical structures of pyridopyrimidine-based urease inhibitors.
Bektas et al. envisaged a strategy to connect moieties of known bioactive 1,2,4-triazoles and oxazolidinones to the linezolid structure, developed analogues, and screened their antiurease activity. All synthesized hybrid compounds (Scheme 39) exhibited good inhibition of the urease enzyme [111].
Scheme 39.
Chemical structures of urease inhibitors derived from linezolid core.
Oliveira et al. synthesized several pyridinone derivatives and evaluated their inhibitory potential against urease (Scheme 40) [112]. Although an apparent relationship between the presence of an alkyl substituent at the bridge carbon and urease inhibition was not observed, the presence of an electron-releasing group at the para position of the benzene ring increased the biological activity of the compounds, which explained the high inhibition percentage of compounds 111 and 112 (Scheme 40). Additionally, hyperconjugation of ethyl and methyl groups and electron-releasing groups at the meta position of the benzene ring are not associated with increased inhibitory activity.
Scheme 40.
Chemical structures of urease inhibitors based on piyridinones.
Recently, Iftikhar et al. reported progress in their fruitful research on dihydropyrimidine (DHPM) by screening 15 new 5-C-substituted (Scheme 41) analogues for their urease inhibition potential [113]. The SAR studies based on urease inhibition assays showed that thiosemicarbazides and isatin derivatives were more potent inhibitors. Molecular docking showed that compound 113 (Scheme 41) coordinates with the bis-nickel center via its 4-methoxy group at phenyl ring of semithiocarbazide; the NH group of pyrimidine forms a hydrogen bond with CME592 at the entrance of binding pocket. The aromatic ring of pyrimidine forms arene-cation interaction with Arg439, also at the entrance of binding pocket. The best result obtained for compound 116 (Scheme 41) among the other compounds was explained by the presence of 3 typical hydrogen bonds with His409, KCX490 and Asp633, in addition to the formation of hydrogen bonds with active site flap CME592 [113].
Scheme 41.
Chemical structures of urease inhibitors based on dihydropyrimidines.
Khan et al. also reported studies on the urease-inhibiting activities of DHPM analogues, but the most active compounds in this study were the hydrazine derivatives (Series B, Scheme 42) [114]. Kinetic studies and molecular docking analyses of this class of substances suggested a mixed-type inhibition profile. The compounds participated in strong interactions with amino acids residues and the nickel center in the active site of the enzyme; the strongest interactions were observed for hydrazine derivatives, probably due to their polar nature [114]. On the other hand, the investigation conducted by Rashid et al. indicated that 3,4-dihydropirimidine-2-ones and particularly 3,4-dihydropirimidine-2-thiones (Series A, Scheme 42) were an active series of urease inhibitors [115]. Products with substituents at position 3 of the benzene ring showed higher inhibitory activity. According to the molecular docking studies, the free S atom and the hydrazine moiety were the main substituents responsible for the inhibitory capacity of the compounds through interactions with the nickel center of the enzyme [115].
Scheme 42.
Chemical structures of urease inhibitors based on dihydropyrimidine platform.
Quinolone derivatives are another interesting class of compounds, due to some of their pharmacological properties [116], [117], [118], [119]; these compounds were synthesized and screened as urease inhibitors. The studies conducted with sparfloxaxin (Series A, Scheme 43) [120] and 8-nitroflouroquinolone (Series B, Scheme 43) [121] derivatives revealed moderate urease inhibition activities.
Scheme 43.
Chemical structures of quinolones described as urease inhibitors.
The diverse applicability of xanthenes and xanthones in areas such as technology, photochemistry and biology [122], [123], [124], [125], [126], [127], [128] motivated the Khurana group to develop derivatives as potent urease inhibitors [129]. Based on the results of the urease inhibition assay, compounds with aryl groups carrying electron-donating groups at the para position exhibited the best inhibitory activity (Scheme 44).
Scheme 44.
Chemical structures of xanthenes analogues that possesses urease inhibitory properties.
Barbituric acid derivatives
Khan et al. synthesized and screened antiurease activity of several barbituric acid derivatives in two different studies evaluating the influence of the group attached to N in the barbituric acid moiety and the substituents at the phenyl ring (Scheme 45) [130], [131]. However, the best results were obtained for the series containing the endocyclic NH group. Among these compounds, the addition of substituents at the para position of the phenyl ring increased the inhibitory capacity.
Scheme 45.
Chemical structures of urease inhibitors based on barbituric acid moiety.
The role played by the substitution of thiobarbituric acid derivatives on the phenyl ring was also verified (Scheme 46) [132]. As shown in the results of the SAR study, substituents that bind the nickel center, such as OH, sulfur atoms or even the pyridyl moiety instead of the phenyl group, increased the inhibitory activity. Additionally, the steric hindrance of large groups of some compounds was probably responsible for decreasing the inhibition of the enzyme.
Scheme 46.
Chemical structures of urease inhibitors based on thiobarbituric acid moiety.
Motivated by broad spectrum of biological and pharmaceutical applications of cyano acetamide derivatives [135], [136], [137], [138], [139], [140], [141], [142], [143], in 2015, Qureshi et al. screened several compounds based on this moiety (Scheme 47) to continue determine their urease inhibitory activity [144] and continue previous studies of urease inhibitors [133], [134]. According to the authors, the high urease inhibitory activity potentially resulted from the extra interaction of the furan and thiophene ring with the urease nickel center.
Scheme 47.
Chemical structures of cyano acetamide barbituric-like substances that shown antiurease properties.
The antiurease activities of both barbituric acid and thiobarbituric acid derivatives substituted with aniline [145] and several sulfonamides [146] (Scheme 48) were studied by Rauf et al. In both cases, thiobarbituric acid derivatives showed greater inhibition than their corresponding oxygenated analogues. The SAR studies also revealed an increase in urease inhibition by compounds containing a carboxyl group at the aniline moiety, probably due to hydrogen bonding with the nickel center. The authors use the same explanation to rationalize the finding that SH and OH also showed better results than other substituents (Scheme 48).
Scheme 48.
Chemical structures of urease inhibitors based on (thio)barbituric aniline-substituted derivatives.
Pyrano pyrimidine dione derivatives (Scheme 49) showed high inhibitory values, which were associated with the presence of hydrophobic substituents at the phenyl ring [147]. According to the authors, the reduction of the partial charge on nitrogen atoms in the compounds with a phenyl ring bearing electron-withdrawing groups was responsible for their relatively low inhibitory activity. These conclusions could explain the high inhibitory capacity of compound 132 (Scheme 49).
Scheme 49.
Chemical structures of urease inhibitors based on pyrano pyrimidine platforms.
Barakat et al. screened several bis-barbituric acid derivatives (Scheme 50) for urease inhibition. The best compounds had similar IC50 values to the positive control thiourea [148], [149]. According to molecular docking studies, several interactions potentially explain the high activity of compounds 133 and 134 (Scheme 50), such as hydrogen bonding between the carbonyl of the barbituric acid moiety and KCX219 and Arg338; hydrogen bonding between the amine group adjacent to the carbonyl moiety and KCX219, Ala169, Gly279 and Asp362; hydrophilic interactions between the aldehyde group of A2 and His323. Moreover, all compounds studied showed similar conformations at the active site of the urease enzyme, interacting with the nickel center and amino acid residues. In addition to the substituted phenyl ring derivatives, the authors also synthesized naphthyl substituents, which exhibited IC50 values ranging from 22.7 ± 0.20 to 123.2 ± 0.37 µM [148], [149].
Scheme 50.
Chemical structures of urease inhibitors based on bis-barbituric acid derivatives.
Rahim et al. reported the synthesis and the results of urease inhibition assays for bis-thiobarbituric derivatives (Scheme 51) [150]. The most effective compounds possessed a substituted phenyl ring, whereas indole and naphtyl derivatives, among others, showed moderate activity against urease. SAR studies revealed that electron-donating groups and substituents capable of making hydrogen bonds, such as OH, exhibited better IC50 values.
Scheme 51.
Chemical structures of urease inhibitors based on bis-thiobarbituric acid derivatives.
Organophosphorus compounds
Phosphoramidates represent one of the most active classes of compounds that function as urease inhibitors [151]. This class of compounds also presents other biological activities, such as antimalarial activity [152], insecticide activity [153], [154], antiHCV activity [155], antiHIV activity [156], [157], and inhibitors of reverse transcriptase [158] and hepatitis C virus [159]. Due the clinical relevance of this class of compounds, Oliveira and co-workers [160] synthesized 25 new phosphoramidates (Scheme 52) and screened their inhibitory activity towards urease isolated from Jack bean. The most active compounds (138 to 142; Scheme 52) showed the properties expected for drug-like compounds, qualifying them to have good pharmacokinetics and drug bioavailability. Considering the correlation between electronic properties and biological activity [161], [162], a correlation between the urease inhibitory activity of positive controls hydroxyurea/thiourea and compounds 139, 140 and 142 was predicted.
Scheme 52.
Chemical structures of urease inhibitors based on phosphoramidates.
Dominguez and co-workers performed molecular modeling studies based on data obtained from the structures of the inhibitor-Bacillus pasteurii urease complexes to design and synthesize 40 phosphor(di)amide derivatives (Scheme 53). The data obtained from the docking study allowed the authors to propose a model to explain the interactions between the studied compounds and the active site of the enzyme. The most active compounds (143 to 148; Scheme 53) present a flat aromatic or heteromatic ring atomic structure containing substituents (NO2) that are able to establish hydrogen bonds with free NH2 terminal groups or similar groups present in surrounding residues in the active site of the enzyme. Moreover, phosphordiamides have a greater inhibitory activity than phosporamides. Steric hindrance, as observed for N-alkyl-substituted diamidophosphate derivatives, reduces the ureolytic activity once the inhibitor interacts with Ni atoms in the active site [163], [164].
Scheme 53.
Chemical structures of phosphor(di)amides-based urease inhibitors.
The application of phosphordiamidates as urease inhibitors is restricted due to their susceptibility to hydrolysis. In an attempt to mitigate this problem, Berlicki and co-workers [165], [166], [167], [168] adopted a structurally related analogue to phosphorodiamidic acid as a scaffold to design and synthesize phosphonic and phosphinic acid derivatives and evaluate their functions as urease inhibitors (Scheme 54). These classes of compounds are potent inhibitors of enzymatic hydrolysis of the amide bond [169], [170]. Since these compounds do not contain the unstable P-N bonds (phosphoramides), they are expected to be stable under physiological conditions. Moreover, this class of compounds exhibits similar inhibitory properties to phosphoramides, as the computed free energy of binding is very similar [171], [172]. Most of the studied compounds constitute a group of competitive, reversible inhibitors of bacterial ureases [151]. Higher inhibitory efficiency in controlling urease activity was observed for P-methyl thiophosphinic acids than for P-methyl phosphinic acids, most probably due to the stronger interaction of a sulfur atom with nickel ions in the enzyme active site [173]. The IC50 and Ki values decreased to the micromolar range with the structural extension of the N-terminal group in compound 148 (Scheme 54). Compared to the compound 148-urease complex, computer-aided studies of the compound 149-urease complex have shown that hydrophobic bulky substituents docked well in the enzyme and two additional hydrogen bonds were formed (carbonyl oxygen atom-Arg339 and NH of the amide group-Ala366) [151]. In a subsequent work [166], the authors proposed a mode of binding between compound 151 (Ki = 0.62 µM) and the B. pasteurii enzyme in which the phosphinic acid group forms two hydrogen bonds with the enzyme active residues (His222 and Asp363) and interacts with the two nickel ions. Furthermore, one hydrogen bond is broken between bulk water and its amine group to form one hydrogen bond with the carbonyl moiety (Ala366). In the compound 148-urease complex, the energy balance is less favorable during the complexation process because three hydrogen bonds with water molecules must be broken to form one hydrogen bond with its amine group. After synthesizing and evaluating the substituted aminomethane-P-hydroxymethylphosphinic acids (Scheme 54), the authors observed that approximately half of the studied compounds did not show inhibitory activity against B. pasteurii urease. Mono N-substituted derivatives were more active than their N,N-disubstituted counterparts [167]. On the other hand, the majority of the bis(aminomethyl)-phosphinic acid derivatives showed significant inhibitory potency in a low micromolar range.
Scheme 54.
Chemical structures of urease inhibitors based on phosphorodiamidic acid.
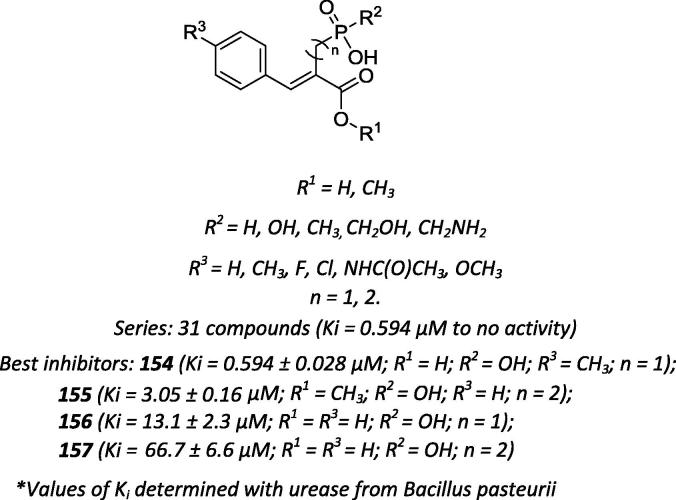
Based on the structure of the complex between the Sporosarcina pasteurii urease enzyme and citrate [174], Ntatsopoulos and co-workers synthesized a group of organophosphorus derivatives containing a phosphonate/carboxylate system (Scheme 55) and evaluated their inhibitory effect on S. pasteurii urease [175]. In contrast to citrate, the authors postulated that both phosphonic acidic groups are fixed on a hydrophobic, partially rigid core that mimic the 1,2-dicarboxylate portion of citrate in its affinity for urease. According to the computer-based studies, the p-methyl group of the aromatic ring of the most active inhibitor (compound 154; Scheme 55) of urease is conveniently accommodated in the hydrophobic cleft of the urease active site. The polar region of compound 154 is involved in strong interactions in the enzyme active site once the carboxylate group forms two hydrogen bonds mediated by a salt bridge in a region opposite to the guanidine moiety of Arg339. Furthermore, the oxygen atom of the phosphoryl group forms a bridge between both metallic centers and interacts with a nitrogen atom of the imidazole group (His222) through a hydrogen bond. The authors hypothesized that the α,β-unsaturated system formed between the free carboxylate group and the aromatic ring present in compound 154 presents the size and rigidity that comply with the steric and electronic demands of the enzyme active site. Thus, both the loss of compound rigidity and separation of the acidic groups from three to four bonds in distance decreases the Ki value approximately 150- and 110-fold, respectively.
Scheme 55.
Chemical structures of organophosphorus urease inhibitors containing a phosphonate/carboxylate scaffold.
Miscellaneous
Derivatives of 1,5-benzothiazepines (Scheme 56) were synthesized, screened for urease inhibitory activity and examined using molecular docking studies [176]. Based on the IC50 values and the results of molecular docking studies, the authors concluded that strong interactions with the active center of urease, such as coordination of the 2-hydroxyl group with the nickel atoms, hydrogen bonds between the same group and His139 and Ala170, and hydrophobic contacts with other amino acids residues, were the main contributors to the increased inhibitory activity of compound 158 (Scheme 56).
Scheme 56.
Chemical structures of urease inhibitors bearing a 1,5-benzothiazepine moiety.
Due to the various biological activities of chalcone derivatives [177], [178], [179], [180], [181], [182], [183], [184], [185], Ahari-Mostafavi and co-workers synthesized several β-aryl-β-mercapto ketone derivatives (Scheme 57) and evaluated their inhibitory potential against urease [186]. Compound 160 (Scheme 57) inhibited the enzyme with the greatest efficiency, with an IC50 = 6 µM (the IC50 of the standard hydroxyurea = 100 µM). The improved antiurease activity of the compound was associated with π – π interactions between the sulfur atom in Met366 residue and phenyl ring at R2, closer interactions with His 274 and 138 residues by changing Cl to F at R2, hydrogen bonding between the NH2 group and Asn 168, the presence of electron-donating and -withdrawing substituents on rings at R2 and R3, respectively, and the presence of an electron-donating substituent in R1.
Scheme 57.
Chemical structures of urease inhibitors based on β-aryl-β-mercapto ketone derivatives.
Batool et al. reported the antibacterial, antiurease and NO scavenging activities of a small series of substituted (prop-2-ynyloxy)benzene derivatives (Scheme 58) [187]. According to the results from the urease inhibitory assay and SAR studies, compounds with electron-donating groups carried by the phenyl ring exhibited increased activity, which was probably caused by the stronger interaction between the ring and the active site of the enzyme. Since strong binding to the nickel center is important for urease inhibition, the higher chelation properties might explain increased activity of phenolic compounds.
Scheme 58.
Chemical structures of urease inhibitors based on substituted (prop-2-ynyloxy)benzene derivatives.
Because of the well-known chelating properties and antiurease potential of hydroxamic acids, in 2013 and 2016, Xiao et al. and Shi et al., respectively, synthesized a large series of derivatives with different groups at positions 2, 3 and 4 of the benzene ring (Scheme 59). Both studies reported remarkable results for urease inhibitory activity, including very low IC50 values for some of the tested title compounds [188], [189]. As shown in the SAR studies, the hydrophobic behavior of the substituent is important to increase the inhibitory activity, although bulky hydrophobic groups reduce the potency.
Scheme 59.
Chemical structures of urease inhibitors design from hydroxamic acids.
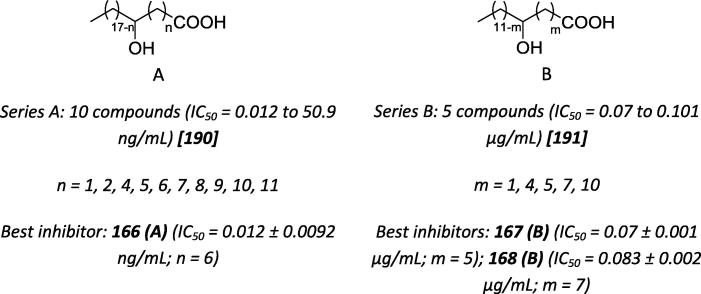
Onar and collaborators evaluated the influence of the position of the hydroxyl group in a series of 20C chain fatty acids on the urease inhibitory activity (Scheme 60) [190], [191]. The addition of a hydroxyl group in the middle region yielded better results, consistent with the findings reported by Sokmen, who varied the position of the hydroxyl group in a 14C chain.
Scheme 60.
Chemical structures of urease inhibitors based on hydroxyl fatty acids.
Since α-β-unsaturated ketones were reported to inhibit C. ensiformis urease activity [192] and ethacrynic acid exhibits other pharmacological properties [193], [194], Janser et al. synthesized hybrid analogues (Scheme 61) and screened their abilities to inhibit urease [195]. Based on the results of these studies, the authors concluded that the α-β-unsaturated carbonyl unit is the major substituent responsible for the inhibitory activity, possibly because of the interaction between the cysteine sulfhydryl moiety and unsaturated carbonyl β-carbon unit; electron-donators attached to the aromatic system were also responsible for increasing the inhibitory activity.
Scheme 61.
Chemical structures of α,β-unsaturated ketones which showed antiurease activity.
Due to the structural relationship between α-hydroxyketones, acetohydroxamic acid and hydroxyurea, in 2004, Tanaka and co-workers synthesized several potentially novel urease inhibitors based on α-hydroxyketones (Scheme 62) [196]. SAR studies revealed that the hydrophobic character of the R substituent in the RCOCH2OH moiety may play an important role in the inhibitory activity. The interaction between α-hydroxyketone and the sulfhydryl group from cysteine, for example, also seems to contribute to urease inhibition.
Scheme 62.
Chemical structures of urease inhibitors based on α-hydroxyketones.
N-[(4-Methoxyphenyl)]-4-oxo-4-[oxy]butanamide (compound 171, Scheme 63) showed urease inhibitory activity (IC50) of 4.08 ± 2.54 µM, a value that was much higher than the standard thiourea [197].
Scheme 63.
Chemical structures of urease inhibitors based on N-[(4-methoxyphenyl)]-4-oxo-4-[oxy]butanamide.
Li and Xiao et al. reported results for their systematic studies of the urease inhibitory activity of 1,2-arylethanes. The first results, which were published in 2009 [198], showed thatcompounds 172 and 173 were most potent inhibitors among the amine and oxime derivatives (Scheme 64). The authors suggested that hydroxyl substituents at benzene ring increased the inhibitory activity due to a direct interaction with the nickel center, whereas the amine group is responsible for interactions with residues from His a323 of H. pylori urease. In a subsequent work (2010) [199], compound 174 inhibited urease more effectively than all other synthesized compounds (Scheme 64). SAR studies and molecular docking helped to relate the high activity of compound 174 to the presence of the hydroxyl groups at the benzene rings, which provides extra hydrogen bonds with Asn168 and Glu222 in the active site of urease compared to acetohydroxamic acid. Their most recent study on this class of molecules examined benzylanilines (Scheme 64), of which the most potent compound was 175. SAR studies and molecular docking showed that the presence of an electron-donating group in the benzyl moiety increases the activity, but only 3,4-hydroxyl substituents provides extra hydrogen bonds; meanwhile, the nitro group at p- and m- positions of the aniline moiety enhances the inhibitory potential [200].
Scheme 64.
Chemical structures of urease inhibitors based on 1,2-arylethanes and analogues.
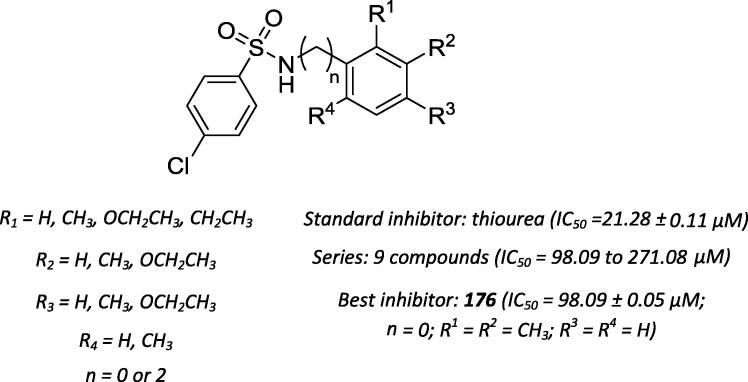
Aziz-ur-Rehman and coworkers [201] evaluated a small series of chlorinated sulfonamide derivatives (Scheme 65) using a urease inhibition assay. According to the SAR studies, alkyl groups at ortho and meta positions of the aniline moiety were responsible for the higher antiurease activity of compound 176 than the other compounds [202].
Scheme 65.
Chemical structures of urease inhibitors based on sulfonamides.
Conclusions and future perspectives
The amount of screening data that is currently available represents irrefutable progress in the development of drugs for the treatment of infections associated with ureolytic bacteria. Several very highly active organic substances have been identified as promising lead compounds from model screening assays involving Canavalia ensiformis urease. Nevertheless, despite the high degree of homology among bacteria and vegetal ureases, important differences can result in a partial or complete decrease of inhibitory activity when probed against enzymes from different species. Since the urease allosteric site is considerably less conserved than the catalytic site, inhibitors acting by participating in interactions with the former site are more susceptible to a potential lack of association among their inhibitory profile for different ureases. Therefore, studies involving native or recombinant microbial enzymes is one of the further and necessary stages of the development of therapies for diseases caused by ureolytic pathogens. Advances in this field will also require analyses of structure–activity relationships of organic compounds based on bioassays using different sources of ureases, particularly studies comparing the mechanisms of action of these compounds for different ureases. These approaches will lead to a better understanding and identification of druggable allosteric sites, which may allow researchers to identify selective sites for ureases derived from different sources.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This work was made possible by the Network for the Development of Novel Urease Inhibitors (www.redniu.org) which is financially supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). AdF is recipient of research fellowships from CNPq.
Biographies

Yuri de Freitas Rego was born in 1992. He earned his BSc. degree in Chemistry in 2016 at the Federal University of Minas Gerais (MG, Brazil). He is currently a graduate student in Chemistry under the mentoring of Dr. Ângelo de Fátima. His research interests are in the fields of Organic Synthesis and applied Biological Chemistry.

Marcelo Pereira Queiroz was born in 1998. He is an undergraduate student in Chemistry at the Federal University of Minas Gerais (MG, Brazil). Marcelo joined the scientific initiation program in 2016 under the mentoring of Dr. de Fátima. His research interests are in the field of Organic and Forensic Chemistry.

Tiago de Oliveira Brito received his PhD in Chemistry in March of 2017 from the Londrina State University (PR, Brazil). He is currently substitute professor of the Academic Department of Chemistry at the Federal University of Technology – Paraná, campus Medianeira (PR, Brazil). His research interest include Organic Synthesis, Medicinal Chemistry and Nuclear Magnetic Resonance.

Priscila Goes Camargo de Carvalho is graduated from Chemical Processes (2014) at the Federal University of Technology – Paraná. She is currently started her doctorate studies under mentoring of Dr. Macedo at the Londrina State University, where she received her master’s degree in 2017. Her searches interests include Organic Synthesis, Medicinal Chemistry and Nuclear Magnetic Resonance.

Vagner Tebaldi de Queiroz earned his PhD in Applied Biochemistry in 2005 from the Federal University of Viçosa (MG, Brazil). He is currently Associate Professor of the Department of Chemistry and Physics at the Federal University of Espirito Santo (UFES, ES, Brazil) and the coordinator of the Natural Products and Organic Synthesis Research Group (GEAPS-UFES-CNPq). His research interests include new materials synthesis for supporting agrochemicals and the evaluation of their activities for pest control in Management Programs of Pests and Diseases in agriculture.

Ângelo de Fátima received his PhD in Science in 2005 from the State University of Campinas (SP, Brazil). He is currently Associate Professor of the Department of Chemistry at the Federal University of Minas Gerais (MG, Brazil). Dr. de Fátima is the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and Group of Studies on Organic and Biological Chemistry. His research interests include the synthesis of molecules with biological and/or functional profiles and the evaluation of their activities against cancer cells, fungi, bacteria and virus of clinical interest, as well as their potential as synthetic vaccines.

Fernando Macedo Jr received his Ph.D. in Science from the State University of Campinas (Brazil) in 2004. He also developed research project as post-doctoral fellow at the Department of Organic Chemistry, Federal University of Bahia (Brazil) and at the Department of Molecular Biology and Biotechnology of The University of Sheffield (UK). Dr. Macedo is currently Associate Professor of the Department of Chemistry at the State University of Londrina (Brazil) where he coordinates the research group on synthesis and properties of organic compounds. His main research interests include the synthesis of bioactive molecules and the evaluation of their non-covalent interactions with macromolecules by NMR techniques.
Footnotes
This work was made possible partly by the Network for the Development of Novel Urease Inhibitors (www.redniu.org).
Peer review under responsibility of Cairo University.
Contributor Information
Ângelo de Fátima, Email: adefatima@qui.ufmg.br.
Fernando Macedo Jr., Email: macedofc@uel.br.
References
- 1.Dixon N.E., Gazzola C., Watters J.J., Blakeley R.L., Zerner B. Jack Bean Urease (EC 3.5.1.5) [letter]. A metalloenzyme. A simple biological role for nickel? J Am Chem Soc. 1975;97(14):4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- 2.Krajewska B., Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B Enzym. 2009;59(1):9–21. [Google Scholar]
- 3.Follmer C. Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol. 2010;63:424–430. doi: 10.1136/jcp.2009.072595. [DOI] [PubMed] [Google Scholar]
- 4.Maroney M.J., Ciurli S. Nonredox nickel enzymes. Chem Rev. 2014;114:4206–4228. doi: 10.1021/cr4004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi T. On the occurrence of urease in higher plants. J Coll Agric Tokyo Imp Univ. 1909;1:1–14. [Google Scholar]
- 6.Modolo L.V., de Souza A.X., Horta L.P., Araujo D.P., de Fátima A. An overview on the potential of natural products as ureases inhibitors: A review. J Adv Res. 2015;6:35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner J.B. The isolation and crystallization of the enzyme urease. J Biol Chem. 1926;69:435–441. [Google Scholar]
- 8.Sirko A., Brodzik R. Plant ureases: Roles and regulation. Acta Biochim Pol. 2000;47:1189–1195. [PubMed] [Google Scholar]
- 9.Jabri E., Carr M.B., Hausinger R.P., Karplus P.A. The crystal structure of urease from Klebsiella aerogenes. Science. 1995;268:998–1004. [PubMed] [Google Scholar]
- 10.Benini S., Rypniewski W.R., Wilson K.S., Miletti S., Ciurli S., Mangani S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis costs two nickels. Structure. 1999;7(2):205–216. doi: 10.1016/S0969-2126(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 11.Ha N.C., Oh S.T., Sung J.Y., Cha K.A., Lee M.H., Oh B.H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Biol. 2001;8(6):505–509. doi: 10.1038/88563. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian A., Ponnuraj K. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J Mol Biol. 2010;400:274–283. doi: 10.1016/j.jmb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Boer J.L., Mulrooney S.B., Hausinger R.P. Nickel-dependent metalloenzymes. Arch Biochem Biophys. 2014;544:142–152. doi: 10.1016/j.abb.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Algood H.M.S., Cover T.L. Helicobacter pylori persistence: An overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19(4):597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs G., Weeks D.L., Wen Y.I., Marcus E.A., Scott D.R. Acid acclimation by Helicobacter pylori. Physiology. 2005;20:429–438. doi: 10.1152/physiol.00032.2005. [DOI] [PubMed] [Google Scholar]
- 16.Kao C.Y., Sheu B.S., Wu J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed J. 2016;39:14–23. doi: 10.1016/j.bj.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Vliet A.H.M., Kuipers E.J., Waidner B., Davies B.J., Vries N., Penn C.W. Nickel-responsive induction of urease expression in Helicobacter pylori is mediated at the transcriptional levels. Infect Immun. 2001;69(8):4891–4897. doi: 10.1128/IAI.69.8.4891-4897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stingl K., Altendorf K., Bakker E.P. Acid survival of Helicobacter pylori: how does urease activity trigger cytoplasmic pH homeostasis? Trends Microbiol. 2002;10(2):70–74. doi: 10.1016/s0966-842x(01)02287-9. [DOI] [PubMed] [Google Scholar]
- 19.Graham D.Y., Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 20.Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5(2):103–109. doi: 10.1177/1756283X11432492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Mulrooney S.B., Leung A.F.K., Zeng Y., Ko B.B.C., Hausinger R.P. Inhibition of urease by bismuth(III): Implications for the mechanism of action of bismuth drugs. Biometals. 2006;19:503–511. doi: 10.1007/s10534-005-5449-0. [DOI] [PubMed] [Google Scholar]
- 22.Malfertheiner P., Megraud F., O’Morain C., Bazzoli F., El-Omar E., Graham D. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobley H.L., Hausinger R.P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Hara C.M., Brenner F.W., Miller J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev. 2000;13:534–546. doi: 10.1128/cmr.13.4.534-546.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musher D.M., Griffith D.P., Yawn D., Rossen R.D. Role of urease in pyelonephritis resulting from urinary tract infection with Proteus. J Infect Dis. 1975;131:177–181. doi: 10.1093/infdis/131.2.177. [DOI] [PubMed] [Google Scholar]
- 26.Johnson D.E., Russel R.G., Lockatell C.V., Zulty J.C., Warren J.W., Mobley H.L.T. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect Immun. 1993;61(7):2748–2754. doi: 10.1128/iai.61.7.2748-2754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coker C., Poore C.A., Li X., Mobley L.T. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2000;2(12):1497–1505. doi: 10.1016/s1286-4579(00)01304-6. [DOI] [PubMed] [Google Scholar]
- 28.Griffith D.P., Musher D.M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 29.Jones B.D., Lockatell C.V., Johnson D.E., Warren J.W., Mobley H.L. Construction of a urease-negative mutant of Proteus mirabilis: Analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990;58:1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gatermann S., Marre R. Cloning and expression of Staphylococcus saprophyticus urease gene sequence in Staphylococcus carnosus and contribution of the enzyme to virulence. Infect Immun. 1989;57(10):2998–3002. doi: 10.1128/iai.57.10.2998-3002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schäfer U.K., Kaltwasser H. Urease from Staphylococcus saprophyticus: Purification, characterization and comparison to Staphylococcus xylosus urease. Arch Microbiol. 1994;161:393–399. doi: 10.1007/BF00288948. [DOI] [PubMed] [Google Scholar]
- 32.Gatermann S., John J., Marre R. Staphylococcus saprophyticus urease: Characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect Immun. 1989;57(1):110–116. doi: 10.1128/iai.57.1.110-116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronald A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am J Med. 2002;113(1A):14–19. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 34.Nielubowicz G.R., Mobley H.L.T. Host-pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7(8):430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 35.Raz R., Colodner R., Kunin C.M. Who are you – Staphylococcus saprophyticus? Clin Infect Dis. 2005;40(6):896–898. doi: 10.1086/428353. [DOI] [PubMed] [Google Scholar]
- 36.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostroff S. Yersinia as an emerging infection: Epidemiologic aspects of yersiniosis. Contrib Microbiol Immunol. 1995;13:5–10. [PubMed] [Google Scholar]
- 38.Sharma S., Sachdeva P., Virdi J.S. Emerging water-borne pathogens. Appl Microbiol Biotechnol. 2003;61:424–428. doi: 10.1007/s00253-003-1302-y. [DOI] [PubMed] [Google Scholar]
- 39.Zadernowska A., Chajęcka-Wierzchowska W., Łaniewska-Trokenhein Ł. Yersinia enterocolitica: A dangerous, but often ignored, foodborne pathogen. Food Rev Int. 2014;30(1):53–70. [Google Scholar]
- 40.Cornelis G., Laroche Y., Balligand G., Sory M.P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- 41.Cover T.L., Aber R.C. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 42.Bottone E.J. Yersinia enterocolitica: Overview and epidemiologic correlates. Microbes Infect. 1999;1:323–333. doi: 10.1016/s1286-4579(99)80028-8. [DOI] [PubMed] [Google Scholar]
- 43.Stern N.J., Pierson M.D., Kotula A.W. Effects of pH and sodium chloride on Yersinia enterocolitica growth at room and refrigeration temperatures. J Food Sci. 1980;45:64–67. [Google Scholar]
- 44.Bhagat N., Virdi J.S. Molecular and biochemical characterization of urease and survival of Yersinia enterocolitica biovar IA in acid pH in vitro. BMC Microbiol. 2009;9:1–14. doi: 10.1186/1471-2180-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhar M.S., Virdi J.S. Strategies used by Yersinia enterocolitica to avade killing by the host: Thinking beyond yops. Microbes Infect. 2014;16(2):87–95. doi: 10.1016/j.micinf.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 46.de Koning-Ward T.F., Ward A.C., Hartland E.L., Robins-Browne R.M. The urease complex gene of Yersinia enterocolitica and its role in virulence. Contrib Microbiol Immunol. 1995;13:262–263. [PubMed] [Google Scholar]
- 47.de Fátima A., Pereira C.P., Olímpio C.R.S.D.G., Oliveira B.G.F., Franco L.L., da Silva P.H.C. Schiff bases and their metal complexes as urease inhibitors – a brief review. J Adv Res. 2018;13:113–126. doi: 10.1016/j.jare.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perveen S., Khan K.M., Lodhi M.A., Choudhary M.I., Choudhary M.I., Atta-ur-Rahman, Voelter W. Urease and α-chymotrypsin inhibitory effects of selected urea derivatives. Lett Drug Des Discov. 2008;5:401–405. [Google Scholar]
- 49.Mustafa S., Perveen S., Khan A. Synthesis, enzyme inhibition and anticancer investigations of unsymmetrical 1,3-disubstituted ureas. J Serb Chem Soc. 2014;79(1):1–10. [Google Scholar]
- 50.Uesato S., Hashimoto Y., Nishino M., Nagaoka Y., Kuwajima H. N-substituted hydroxyureas as urease inhibitors. Chem Pharm Bull. 2002;50(9):1280–1282. doi: 10.1248/cpb.50.1280. [DOI] [PubMed] [Google Scholar]
- 51.Rajic Z., Perkovic I., Butula I., Zorc B., Hadjipavlou-Litina D., Pontiki E. Synthesis and biological evaluation of O-methyl and O-ethyl NSAID hydroxamic acids. J Enzyme Inhib Med Chem. 2009;24(5):1179–1187. doi: 10.1080/14756360902779128. [DOI] [PubMed] [Google Scholar]
- 52.Khan K.M., Naz F., Taha M., Khan A., Perveen S., Choudhary M.I. Synthesis and in vitro urease inhibitory activity of N,N’-disubstituted thioureas. Eur J Med Chem. 2014;74:314–323. doi: 10.1016/j.ejmech.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Taha M., Ismail N.H., Imran S., Wadood A., Rahim F., Riaz M. Synthesis of potent urease inhibitors based on disulfide scaffold and their molecular docking studies. Bioorg Med Chem. 2015;23:7211–7218. doi: 10.1016/j.bmc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 54.Brito T.O., Souza A.X., Mota Y.C.C., Morais V.S.S., Souza L.T., de Fátima A. Design, syntheses and evaluation of benzoylthioureas as urease inhibitors of agricultural interest. RSC Adv. 2015;5:44507–44515. [Google Scholar]
- 55.Rauf M.K., Talib A., Badshah A., Zaib S., Shoaib K., Shahid M. Solution-phase microwave assisted parallel synthesis of N, N’-disubstituted thioureas derived from benzoic acid: Biological evaluation and molecular docking studies. Eur J Med Chem. 2013;70:487–496. doi: 10.1016/j.ejmech.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Rauf M.K., Zaib S., Talib A., Ebihara M., Badshah A., Bolte M. Solution-phase microwave assisted parallel synthesis, biological evaluation and in silico docking studies of N, N’-disubstituted thioureas derived from 3-chlorobenzoic acid. Bioorg Med Chem. 2016;24:4452–4463. doi: 10.1016/j.bmc.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 57.Saeed A., Zaib S., Pervez A., Mumtaz A., Shahid M., Iqbal J. Synthesis, molecular docking studies, and in vitro screening of sulfanilamide-thiourea hybrids as antimicrobial and urease inhibitors. Med Chem Res. 2013;22:3653–3662. [Google Scholar]
- 58.Saeed A., Khan M.S., Rafique H., Shahid M., Iqbal J. Design, synthesis, molecular docking studies and in vitro screening of ethyl 4-(3-benzoylthioureido) benzoates as urease inhibitors. Bioorg Chem. 2014;52:1–7. doi: 10.1016/j.bioorg.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Saeed A., Rehman S., Channar P.A., Larik F.A., Abbas Q., Hassan M. Long chain 1-acyl-3-arylthioureas as jack bean urease inhibitors, synthesis, kinetic mechanism and molecular docking studies. J Taiwan Inst Chem Eng. 2017;77:54–63. [Google Scholar]
- 60.Saeed A, ur-Rehman S, Channar PA, Larik FA, Abbas Q, Hassan M, et al. Jack bean urease inhibitors, and antioxidant activity based on palmitic acid derived 1-acyl-3- arylthioureas: synthesis, kinetic mechanism and molecular docking studies. Drug Res 2017;67:1–10. [DOI] [PubMed]
- 61.Jamil M., Zubair M., Rasool N., Altaf A.A., Rizwan K., Hafeez S. Synthesis, characterization, antibacterial and urease inhibition studies of some novel symmetrical N3, N3-bis-(disubstituted)isophthalyl-bis-(thioureas) Asian J Chem. 2013;25(10):5328–5332. [Google Scholar]
- 62.Aslam M.A.S., Mahmood S., Shahidc M., Saeed A., Iqbal J. Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur J Med Chem. 2011;46:5473–5479. doi: 10.1016/j.ejmech.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Pervez H., Iqbal M.S., Tahir M.Y., Nasim F., Choudhary M.I., Khan K.M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4- substituted isatin-3-thiosemicarbazones. J Enzyme Inhib Med Chem. 2008;23(6):848–854. doi: 10.1080/14756360701746179. [DOI] [PubMed] [Google Scholar]
- 64.Pervez H., Chohan Z.H., Ramzan M., Nasim F., Khan K.M. Synthesis and biological evaluation of some new N4-substituted isatin-3-thiosemicarbazones. J Enzyme Inhib Med Chem. 2009;24(2):437–446. doi: 10.1080/14756360802188420. [DOI] [PubMed] [Google Scholar]
- 65.Sharma A., Suhas R., Gowda D.C. Ureas/thioureas of benzo[d]isothiazole analog conjugated glutamic acid: synthesis and biological evaluation. Arch Pharm Chem Life Sci. 2013;346:359–366. doi: 10.1002/ardp.201200470. [DOI] [PubMed] [Google Scholar]
- 66.Taha M., Ismail N.H., Imran S., Wadood A., Rahim F., Khan K.M. Hybrid benzothiazole analogs as antiurease agent: Synthesis and molecular docking studies. Bioorg Chem. 2016;66:80–87. doi: 10.1016/j.bioorg.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 67.Khan K.M., Wadood A., Ali M., Zia-Ullah, Ul-Haq Z., Lodhi M.A. Identification of potent urease inhibitors via ligand- and structure-based virtual screening and in vitro assays. J Mol Graph Model. 2010;28:792–798. doi: 10.1016/j.jmgm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Ali S., Rasool N., Ullah A., Nasim F.H., Yaqoob A., Zubair M. Design and synthesis of arylthiophene-2-carbaldehydes via suzuki-miyaura reaction and their biological evaluation. Molecules. 2013;18:14711–14725. doi: 10.3390/molecules181214711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noreen M., Rasool N., Gull Y., Zubair M., Mahmood T., Ayub K. Synthesis, density functional theory (DFT), urease inhibition and antimicrobial activities of 5-aryl thiophenes bearing sulphonylacetamide moieties. Molecules. 2015;20:19914–19928. doi: 10.3390/molecules201119661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harit T., Malek F., Bali B.E., Khan A., Dalvandi K., Marasini B.P. Synthesis and enzyme inhibitory activities of some new pyrazole-based heterocyclic compounds. Med Chem Res. 2012;21:2772–2778. [Google Scholar]
- 71.Naureen S., Chaudhry F., Asif N., Munawar M.A., Ashraf M., Nasim F.H. Discovery of indole-based tetraarylimidazoles as potent inhibitors of urease with low antilipoxygenase activity. Eur J Med Chem. 2015;102:464–470. doi: 10.1016/j.ejmech.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Mao W.J., Lv P.C., Shi L., Li H.Q., Zhu H.L. Synthesis, molecular docking and biological evaluation of metronidazole derivatives as potent Helicobacter pylori urease inhibitors. Bioorg Med Chem. 2009;17:7531–7536. doi: 10.1016/j.bmc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Huang X.S., Liu K., Yin Y., Li W.M., Ran W., Duan M. The synthesis, structure and activity evaluation of secnidazole derivatives as Helicobacter pylori urease inhibitors. Curr Bioact Compd. 2011;7:268–280. [Google Scholar]
- 74.Lodhi M.A., Shams S., Khan K.M. Thiazolidine esters: new potent urease inhibitors. J Chem Soc Pak. 2014;36:858–864. [Google Scholar]
- 75.Fareed G., Afza N., Versiani M.A., Fareed N., Mughal U.R., Kalhoro M.A. Synthesis, spectroscopic characterization and pharmacological evaluation of oxazolone derivatives. J Serb Chem Soc. 2013;78(8):1127–1134. [Google Scholar]
- 76.Araujo D.P., Morais V.S.S., de Fátima A., Modolo L.V. Efficient sodium bisulfite-catalyzed synthesis of benzothiazoles and their potential as ureases inhibitors. RSC Adv. 2015;5:28814–28821. [Google Scholar]
- 77.Gull Y., Rasool N., Noreen M., Altaf A.A., Musharraf S.G., Zubair M. Synthesis of N-(6-Arylbenzo[d]thiazole-2-acetamide derivatives and their biological activities: an experimental and computational approach. Molecules. 2016;21:266. doi: 10.3390/molecules21030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akhtar T., Hameed S., Khan K.M., Choudhary M.I. Syntheses, urease inhibition, and antimicrobial studies of some chiral 3-substituted-4-amino-5-thioxo-1H,4H–1,2,4-triazoles. Med Chem. 2008;4:539–543. doi: 10.2174/157340608786242025. [DOI] [PubMed] [Google Scholar]
- 79.Serwar M., Akhtar T., Hameed S., Khan K.M. Synthesis, urease inhibition and antimicrobial activities of some chiral 5-aryl-4-(1-phenylpropyl)-2H-1,2,4-triazole-3-(4H)-thiones. Arkivoc. 2009;7:210–221. [Google Scholar]
- 80.Özil M., Bodur O., Ülker S., Kahveci B. Microwave-promoted synthesis and biological activity of some 2-hetarylmethyl-4-(4-hetarylphenyl)-5-methyl-2,4-dihydro-3H-1,2,4-triazol-3-one derivatives. Chem Heter Comp. 2015;51(1):88–96. [Google Scholar]
- 81.Khan I., Ali S., Hameed S., Rama N.H., Hussain M.T., Wadood A. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur J Med Chem. 2010;45:5200–5207. doi: 10.1016/j.ejmech.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 82.Abid O.U.R., Babar T.M., Ali F.I., Ahmed S., Wadood A., Rama N.H. Identification of novel urease inhibitors by high-throughput virtual and in vitro screening. ASC Med Chem Lett. 2010;1:145–149. doi: 10.1021/ml100068u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akhtar T., Khan M.A., Iqbal J., Jones P.G., Hameed S. A facile one-pot synthesis of 2-arylamino-5-aryloxylalkyl-1,3,4-oxadiazoles and their urease inhibition studies. Chem Biol Drug Des. 2014;84:92–98. doi: 10.1111/cbdd.12297. [DOI] [PubMed] [Google Scholar]
- 84.Akhtar T., Hameed S., Khan K.M., Khan A., Choudhary M.I. Design, synthesis, and urease inhibition studies of some 1,3,4-oxadiazoles and 1,2,4-triazoles derived from mandelic acid. J Enzyme Inhib Med Chem. 2010;25(4):572–576. doi: 10.3109/14756360903389864. [DOI] [PubMed] [Google Scholar]
- 85.Hanif M., Shoaib K., Saleem M., Rama N.H., Zaib S., Iqbal J. Synthesis, urease inhibition, antioxidant, antibacterial, and molecular docking studies of 1,3,4-oxadiazole derivatives. ISRN Pharm. 2012:1–9. doi: 10.5402/2012/928901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shahzad S.A., Yar M., Khan Z.A., Khan I.U., Naqvi S.A.R., Mahmood N. Microwave-assisted solvent free efficient synthesis of 1,3,4-oxadiazole-2(3H)-thiones and their potent in vitro urease inhibition activity. Eur J Chem. 2012;3(2):143–146. [Google Scholar]
- 87.Rehman A.U., Siddiqa A., Abbasi M.A., Rasool S., Akhtar M.N., Lodhi M.A. Synthesis, characterization and urease inhibiting derivatives of 5-(3,4-methulenedioxyphenyl)-1,3,4-oxadiazol-2-thiol. Asian J Chem. 2014;26(15):4605–4609. [Google Scholar]
- 88.Rafiq M., Saleem M., Hanif M., Maqsood M.R., Rama N.H., Lee K.H. Synthesis and biological activities of some new 3,6-disubstituted 1,2,4-triazolo[3,4-b]1,3,4-thiadiazole derivatives. Bull Korean Chem Soc. 2012;33(12):3943–3949. [Google Scholar]
- 89.Mojzych M., Tarasiuk P., Kotwica-Mojzych K., Rafiq M., Seo S.Y., Nicewicz M. Synthesis of chiral pyrazolo[4,3-e][1,2,4]triazine sulfonamides with tyrosinase and urease inhibitory activity. J Enzyme Inhib Med Chem. 2016:1–6. doi: 10.1080/14756366.2016.1238362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saify Z.S., Sultana N., Khan A., Haider S. (1H-Pyrrolo [2,3-b]pyridine)7-azaindole derivatives and their antiurease, phosphodiesterase and β-glucuronidase activity. Inter J Biochem Res Rev. 2015;8(1):1–12. [Google Scholar]
- 91.Macegoniuk K., Grela E., Palus J., Rudzinska-Szostak E., Grabowiecka A., Biernat M. 1,2-Benzisoselenazol-3-(2H)-one derivatives as a new class of bacterial urease inhibitors. J Med Chem. 2016;59:8125–8133. doi: 10.1021/acs.jmedchem.6b00986. [DOI] [PubMed] [Google Scholar]
- 92.Tabuchi Y., Kurebayashi Y. Antisecretory and antiulcer effects of ebselen, a seleno-organic compound, in rats. Jpn J Pharmacol. 1993;61:255–257. doi: 10.1254/jjp.61.255. [DOI] [PubMed] [Google Scholar]
- 93.Demuner A.J., Valente V.M.M., Barbosa L.C.A., Rathi A.H., Donohoe T.J., Thompson A.L. Synthesis and phytotoxic activity of new pyridones derived from 4-hydroxy-6-methylpyridin-2(1H)-one. Molecules. 2009;14:4973–4986. doi: 10.3390/molecules14124973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dias L.C., Demuner A.J., Valente V.M.M., Barbosa L.C.A., Martins F.T., Doriguetto A.C. Preparation of achiral and chiral Eenaminopyran-2,4-diones and their phytotoxic activity. J Agric Food Chem. 2009;57:1399–1405. doi: 10.1021/jf802805f. [DOI] [PubMed] [Google Scholar]
- 95.Amin K.M., Kamel M.M., Anwar M.M., Khedr M., Syam Y.M. Synthesis, biological evaluation and molecular docking of novel series of spiro[(2H,3H)quinazoline-2,1′-cyclohexan]-4(1H)-one derivatives as antiin?ammatory and analgesic agents. Eur J Med Chem. 2010;45:2117–2131. doi: 10.1016/j.ejmech.2009.12.078. [DOI] [PubMed] [Google Scholar]
- 96.Cocco M.T., Congiu C., Onnis V. New bis(pyridyl)methane derivatives from 4-hydroxy-2-pyridones: synthesis and antitumoral activity. Eur J Med Chem. 2003;38:37–47. doi: 10.1016/s0223-5234(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 97.Storck P., Aubertin A., Grierson D.S. Tosylation/mesylation of 4- hydroxy-3-nitro-2-pyridinones as an activation step in the construction of dihydropyrido[3,4-b]benzo[f][1,4]thiazepin-1-one based antiHIV agents. Tetrahedron Lett. 2005;46:2919–2922. [Google Scholar]
- 98.Evidente A., Fiore M., Bruno G., Sparapano L., Motta A. Chemical and biological characterisation of sapinopyridione, a phytotoxic 3,3,6-trisubstituted-2,4-pyridione produced by Sphaeropsis sapinea, a toxigenic pathogen of native and exotic conifers, and its derivatives. Phytochemistry. 2006;67:1019–1028. doi: 10.1016/j.phytochem.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 99.Berthelot M. Violet d’aniline. Rep Chim App. 1859;1:284. [Google Scholar]
- 100.David W., Nelson J.M., Slintak V., Paul R.K., Gordon W.R., William A.D. Biochemical and antiproliferative properties of 4-[Ar(alk)ylamino]-pyrido-pyrimidines, a new chemical class of potent and specific epidermal growth factor receptor tyrosine kinase inhibitor. Biochem Pharmacol. 1997;54:877–887. doi: 10.1016/s0006-2952(97)00242-6. [DOI] [PubMed] [Google Scholar]
- 101.Mamouni R., Akssira A.M., Aadil M., Elhakmaoui A., Lasri J., Zaballos-Garcia E. A facile synthesis of new 3-substituted-2,3dihydropyrido[3,2-d]pyrimidine-2,4-diones. Synth Commun. 2003;33:4259–4264. [Google Scholar]
- 102.Mihailo B., Iva T., Zrinka I., Sanja T., Jerka D. Pyrimidopyrimidines: a novel class of dihydrofolate reductase inhibitors. Food Technol Biotech. 2009;47:236–245. [Google Scholar]
- 103.Miller J.R., Dunham S., Mochalkin I., Banotai C., Bowman M., Buist S. A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc Natl Acad Sci USA. 2009;106:1737–1742. doi: 10.1073/pnas.0811275106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sayed R.A., Shamroukh H.A., Awad H. Preparation of some fused pyridopyrimidine and pyridothienotriazine derivatives for biological evaluation. Phosphorus Sulfur Silicon Relat Elem. 2005;180:2767–2777. [Google Scholar]
- 105.Cockerill S., Stubberfield C., Stables J., Carter M., Guntrip S., Smith K. Indazolylamino quinazolines and pyridopyrimidines as inhibitors of the EGFr and C-erbB-2. Bioorg Med Chem Lett. 2001;11:1401–1405. doi: 10.1016/s0960-894x(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 106.Gfesser G.A., Bayburt E.K., Cowart M., DiDomenico S., Gomtsyan A., Lee C.H. Synthesis and structure-activity relationship of 5-heteroatom-substituted pyridopyrimidines as adenosine kinase inhibitors. Eur J Med Chem. 2003;38:245–252. doi: 10.1016/s0223-5234(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 107.Cowart M., Lee C.H., Gfesser G.A., Bayburt E.K., Bhagwat S.S., Stewart A.O. Structure-activity relationship studies of 5-substituted pyridopyrimidines as adenosine kinase inhobitirs. Bioorg Med Chem Lett. 2001;11:83–86. doi: 10.1016/s0960-894x(00)00602-8. [DOI] [PubMed] [Google Scholar]
- 108.Wu Z., Hartnett J.C., Neilson L.A., Robinson R.G., Fu S., Barnett S.F. Development of pyridopyrimidines as potent Akt1/2 Inhibitors. Bioorg Med Chem Lett. 2008;18:1274–1279. doi: 10.1016/j.bmcl.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 109.Finan P.M., Thomas M.J. PI 3-kinase inhibition: a therapeutic target for respiratory disease. Biochem Soc Trans. 2004;32:378–382. doi: 10.1042/bst0320378. [DOI] [PubMed] [Google Scholar]
- 110.Rauf A., Liaqat S., Qureshi A.M., Yaqub M., Rehman A.U., Hassan M.U. Synthesis, characterization, and urease inhibition of 5-substituted-9-methyl-2H-pyrido[1,2-a]pyrimidine-2,4(3H)-diones. Med Chem Res. 2012;21:60–74. [Google Scholar]
- 111.Bektas H., Ceylan S., Demirbas N., Alpay-Karaoglu S., Sökmen B.B. Antimicrobial and antiurease activities of newly synthesized morpholine derivatives containing an azole nucleus. Med Chem Res. 2013;22:3629–3639. doi: 10.1007/s00044-012-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oliveira F.M., Barbosa L.C., Valente V.M.M., Demuner A.J., Maltha C.R.A., Oliveros-Bastidas A.J. Structure-activity relationship of pyridin-2-(1H)-ones derivatives as urease inhibitors. J Pharm Res. 2012;5(12):5326–5333. [Google Scholar]
- 113.Iftikhar F., Ali Y., Kiani F.A., Hassan S.F., Fatima T., Khan A. Design, synthesis, in vitro evaluation and docking studies on dihydropyrimidine-based urease inhibitors. Bioorg Chem. 2017;74:53–65. doi: 10.1016/j.bioorg.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Khan A., Hashim J., Arshad N., Khan I., Siddiqui N., Wadood A. Dihydropyrimidine based hydrazine dihydrochloride derivatives as potent urease inhibitors. Bioorg Chem. 2016;64:85–96. doi: 10.1016/j.bioorg.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 115.Rashid U., Batool I., Wadood A., Khan A., ul-Haq Z., Chaudhary M.I. Structure based virtual screening-driven identification of monastrol as a potent urease inhibitor. J Mol Graph Model. 2013;43:47–57. doi: 10.1016/j.jmgm.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 116.Wise R., Andrews J.M., Edwards L.J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983;23:559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Felmingham D., O’Hare M.D., Robbins M.J., Wall R.A., Williams A.H., Cremer A.W. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs Exp Clin Res. 1985;11:317–329. [PubMed] [Google Scholar]
- 118.Maurer F., Grohe K. 2,4-Dichloro-5-fluorobenzoic acid. Chem Abstr. 1986;105:97158. [Google Scholar]
- 119.Petersen U., Bartel S., Bremm K.D., Himmler T., Krebs A., Schenke T. The synthesis and biological properties of 6-fluoroquinolonecarboxylic acids. Bull Soc Chim Belg. 1996;105:683–699. [Google Scholar]
- 120.Arayne M.S., Sultana N., Gul S., Khan A. Novel derivatives of 5-amino-1-cyclopropyl-7-[(3R,5S)3,5-dimethylpiperazine-1-yl]-6,8-difluoro-4-oxo-quinoline-3-carboxylic acid: their synthesis, antimicrobial, antifungal, and urease inhibitory studies. Med Chem Res. 2014;23:1248–1256. [Google Scholar]
- 121.Abu-Qatouseh L., Abu-Sini M., Mayyas A., Al-Hiari Y., Darwish R., Aburjai T. Synthesis of new nitrofluoroquinolone derivatives with novel antimicrobial properties against metronidazole resistant H. pylori. Molecules. 2017;22:71–82. doi: 10.3390/molecules22010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahmad M., King T.A., Ko D.K., Cha B.H., Lee J. Performance and photostability of xanthene and pyrromethene laser dyes in solegel phases. J Phys D Appl Phys. 2002;35:1473–1476. [Google Scholar]
- 123.Banerjee A., Mukherjee A.K. Chemical aspects of santalin as a histological stain. Stain Technol. 1981;56:83–85. doi: 10.3109/10520298109067286. [DOI] [PubMed] [Google Scholar]
- 124.Menchen SM, et al., inventor; Applera Corporation, assignee. Sulfonated diarylrhodamine dyes. United States patent US 6583168; 2000 Nov 28.
- 125.Knight C.G., Stephens T. Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. Biochem J. 1989;258:683–687. doi: 10.1042/bj2580683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hideo T, Teruomi J, inventors; Sankyo Co, assignee. Benzopyrano[2,3-b]xanthene derivatives and its preparation. Japan patent JP 56005480; 1981 Jul 27.
- 127.Poupelin J.P., Saint-Ruf G., Foussard-Blanpin O., Marcisse G., Uchida-Ernouf G., Lacroix R. Synthesis and antiInflammatory properties of bis (2-hydroxy-1- naphthyl)methane derivatives. Eur J Med Chem. 1978;13:67–71. [Google Scholar]
- 128.Lambert RW, et al., inventor; F. Hoffmann-LA Roche AG, assignee. Pyrimidine nucleosides. PCT International Application WO9706178; 1997 Feb 20.
- 129.Khurana J.M., Magoo D., Aggarwal K., Aggarwal N., Kumar R. Srivastava. Synthesis of novel 12-aryl-8,9,10,12-tetrahydrobenzo[α]xanthene-11-thiones and evaluation of their biocidal effects. Eur J Med Chem. 2012;58:470–477. doi: 10.1016/j.ejmech.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 130.Khan K.M., Khan M., Khan A., Perveen S., Naz F., Choudhary M.I. 5-Arylidene N, N-dimethylbarbiturates as urease inhibitors. J Chem Soc Pak. 2014;36:524–527. [Google Scholar]
- 131.Khan K.M., Ali M., Wadood A., ul-Haq Z., Khan M., Lodhi M.A. Molecular modeling-based antioxidant arylidene barbiturates as urease inhibitors. J Mol Graph Model. 2011;30:153–156. doi: 10.1016/j.jmgm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 132.Khan K.M., Rahim F., Khan A., Shabeer M., Hussain S., Rehman W. Synthesis and structure-activity relationship of thiobarbituric acid derivatives as potent inhibitors of urease. Bioorg Med Chem. 2014;22:4119–4123. doi: 10.1016/j.bmc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 133.Qureshi A.M., Qadir M., Rauf A., Idrees M., Mumtaz S., Najam-ul-Haq M. Antimicrobial efficacy of metal-barbiturate conjugates against pathogenic strains of escherichia coli and staphylococcus aureus. Lett Drug Des Discov. 2011;8(10):980–987. [Google Scholar]
- 134.Halliwell B., Gutteridge J.M. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 135.Fadda A.A., Bondock S., Rabie R., Etman H.A. Cyanoacetamide derivatives as synthons in heterocyclic synthesis. Turk J Chem. 2008;32:259–286. [Google Scholar]
- 136.Shishoo C.J., Devani M.B., Anathan S., Jain K.S., Bhadti V.S., Mohan S. Synthesis of some 2-substituted quinazolin-4-ones and thienopyrimidin-4- ones of biological interest and isolation of o-functionalized amidine intermediates. Indian J Chem. 1989;28B:1039–1047. [Google Scholar]
- 137.Hazra K., Saravanan J., Mohan S. Synthesis and antiinflammatory evaluation of some new thiophene analogs. Asian J Chem. 2007;19:3541–3544. [Google Scholar]
- 138.Zhuravel I.O., Kovalenko S.M., Ivachtchenko A.V., Balakin K.V., Kazmirchuk V. Synthesis and antimicrobial activity of 5-hydroxymethyl- 8-methyl-2-(N-arylimino)-pyrano[2,3-c]pyridine-3-(N-aryl)-carboxamides. Bioorg Med Chem Lett. 2005;15(24):5483–5487. doi: 10.1016/j.bmcl.2005.08.081. [DOI] [PubMed] [Google Scholar]
- 139.Geissler A.E., Huppatz J.L., Phillips J.N. The herbicidal activity of 2-alkyl-2-cyanoacetanilides. J Pestic Sci. 1980;11:432–438. [Google Scholar]
- 140.Shams H.Z., Elkholy Y.M., Azzam R.A., Mohareb R.M. Synthetic potentialities of thiophene systems in heterocyclic synthesis: anovel synthesis of thieno[2,3-b]pyridine derivatives. Phosphorus Sulfur Silicon Relat Elem. 1999;155:215–233. [Google Scholar]
- 141.Ismail M.M.F., Ammar Y.A., El-Zahaby H.A.S., Eisa S.I., Barakat S.E. Synthesis of novel 1-pyrazolylpyridin-2-ones as potential antiinflammatory and analgesic agents. Arch Pharm Life Sci. 2007;340:476–482. doi: 10.1002/ardp.200600197. [DOI] [PubMed] [Google Scholar]
- 142.Litvinov V.P. Cyanoacetamides and their thio- and selenocarbonyl analogues as promising reagents for fine organic synthesis. Russ Chem Rev. 1999;68:737–763. [Google Scholar]
- 143.Ghani UN, ur-Rahman A, Choudhary MI, Ullah N, James MN. Crystal structure of γ-chymotrypsin in complex with 7-hydroxycoumarin. J Mol Biol 2001;314(3):519–25. [DOI] [PubMed]
- 144.Rauf A., Nazish K.A., Nasim F.H., Yaqoob A., Qureshi A.M. Synthesis of novel cyanoacetamides derivatives and their urease inhibition studies. Eur J Chem. 2015;6(2):163–168. [Google Scholar]
- 145.Rauf A., Shahzad S., Bajda M., Yar M., Ahmed F., Hussain N. Design and synthesis of new barbituric- and thiobarbituric acid derivatives as potent urease inhibitors: Structure activity relationship and molecular modeling studies. Bioorg Med Chem. 2015;23:6049–6058. doi: 10.1016/j.bmc.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 146.Rauf A., Ahmed F., Qureshi A.M., Aziz-ur-Rehman Khan A., Qadir M.I. Synthesis and urease inhibition studies of barbituric and thiobarbituric acid derived sulphonamides. J Chin Chem Soc. 2011;58:528–537. [Google Scholar]
- 147.Ziarani G.M., Faramarzi S., Asadi S., Badiei A., Balz R., Amanlou M. Three-component synthesis of pyrano[2,3-d]-pyrimidine dione derivatives facilitated by sulfonic acid nanoporous silica (SBA-Pr-SO3H) and their docking and urease inhibitory activity. J Pharm Sci. 2013;21(3):1–13. doi: 10.1186/2008-2231-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barakat A., Al-Majid A.M., Lotfy G., Arshad F., Yousuf S., Choudhary M.I. Synthesis and dynamics studies of barbituric acid derivatives as urease inhibitors. Chem Cent J. 2015;9:63. doi: 10.1186/s13065-015-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barakat A., Al-Majid A.M., Al-Najjar H.J., Mabkhot Y.N., Javaid S., Yousuf S. Zwitterionic pyrimidinium adducts as antioxidants with therapeutic potential as nitric oxide scavenger. Eur J Med Chem. 2014;84:146–154. doi: 10.1016/j.ejmech.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 150.Rahim F., Ali M., Ullah S., Rashid U., Ullah H., Taha M. Development of bis-thiobarbiturates as successful urease inhibitors and their molecular modeling studies. Chin Chem Lett. 2016;27:693–697. [Google Scholar]
- 151.Vassiliou S., Grabowiecka A., Kosikowska P., Yiotakis A., Kafarski P., Berlicki L. Design, synthesis, and evaluation of novel organophosphorus inhibitors of bacterial ureases. J Med Chem. 2008;51:5736–5744. doi: 10.1021/jm800570q. [DOI] [PubMed] [Google Scholar]
- 152.Mara C., Dempsey E., Bell A., Barlow J.W. Synthesis and evaluation of phosphoramidate and phosphorothioamidate analogues of amiprophos methyl as potential antimalarial agents. Bioorg Med Chem Lett. 2011;21:6180–6183. doi: 10.1016/j.bmcl.2011.07.088. [DOI] [PubMed] [Google Scholar]
- 153.Paula V.F., Barbosa L.C.A., Teixeira R.R., Picanco M.C., Silva G.A. Synthesis and insecticidal activity of new 3-benzylfuran-2-yl N, N, N0, N0-tetraethyldiamidophosphate derivatives. Pest Manage Sci. 2008;64:863–872. doi: 10.1002/ps.1559. [DOI] [PubMed] [Google Scholar]
- 154.Oliveira F.M., Barbosa L.C.A., Teixeira R.R., Demuner A.J., Maltha C.R.A., Picanço M.C. Synthesis and insecticidal activity of new phosphoramidates. J Pestic Sci. 2012;37:85–88. [Google Scholar]
- 155.McGuigan C., Kelleher M.R., Perrone P., Mulready S., Luoni G., Daverio F. The application of phosphoramidate ProTide technology to the potent antiHCV compound 40-azidocytidine (R1479) Bioorg Med Chem Lett. 2009;19(15):4250–4254. doi: 10.1016/j.bmcl.2009.05.099. [DOI] [PubMed] [Google Scholar]
- 156.Derudas M., Brancale A., Naesens L., Neyts J., Balzarini J., McGuigan C. Application of the phosphoramidate ProTide approach to the antiviral drug ribavirin. Bioorg Med Chem. 2010;18:2748–2755. doi: 10.1016/j.bmc.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mehellou Y., Balzarini J., McGuigan C. An investigation into the antiHIV activity of 20,30-didehydro-20,30-dideoxyuridine (d4U) and 20,30-dideoxyuridine (ddU) phosphoramidate ‘Pro-Tide’ derivatives. Org Biomol Chem. 2009;7:2548–2553. doi: 10.1039/b904276h. [DOI] [PubMed] [Google Scholar]
- 158.Borrello L., Chiacchio U., Corsaro A., Pistarà V., Iannazzo D. Phosphoroamidate derivatives of N, O-nucleosides as inhibitors of reverse transcriptase. Arkivoc. 2009;8:112–124. [Google Scholar]
- 159.Donghi M., Attenni B., Gardelli C., Marco A.D., Fiore F., Giuliano C. Synthesis and evaluation of novel phosphoramidate prodrugs of 20-methyl cytidine as inhibitors of hepatitis C virus NS5B polymerase. Bioorg Med Chem Lett. 2009;19:1392–1395. doi: 10.1016/j.bmcl.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 160.Oliveira F.M., Barbosa L.C.A., Demuner A.J., Maltha C.R.A., Pereira S.R., Horta L.P. Synthesis, molecular properties and DFT studies of new phosphoramidates as potential urease inhibitors. Med Chem Res. 2014;23:5174–5187. [Google Scholar]
- 161.Correa-Basurto J., Flores-Sandoval C., Marin-Cruz J., Rojo-Dominguez A., Espinoza-Fonseca L.M., Trujillo-Ferrara J.G. Docking and quantum mechanic studies on cholinesterases and their inhibitors. Eur J Med Chem. 2007;42:10–19. doi: 10.1016/j.ejmech.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 162.Arantes F.F.P., Barbosa L.C.A., Maltha C.R.A., Demuner A.J., Fidencio P.H., Carneiro J.W.M. A quantum chemical and chemometric study of sesquiterpene lactones with cytotoxicity against tumor cells. J Chemom. 2011;25:401–407. [Google Scholar]
- 163.Domínguez M.J., Sanmartín C., Font M., Palop J.A., Francisco S.S., Urrutia O. Design, synthesis, and biological evaluation of phosphoramide derivatives as urease inhibitors. J Agric Food Chem. 2008;56(10):3721–3731. doi: 10.1021/jf072901y. [DOI] [PubMed] [Google Scholar]
- 164.Font M., Domínguez M.J., Sanmartín C., Palop J.A., Francisco S.S., Urrutia O. Structural characteristics of phosphoramide derivatives as urease inhibitors. Requirements for activity. J Agric Food Chem. 2008;56(18):8451–8460. doi: 10.1021/jf801786d. [DOI] [PubMed] [Google Scholar]
- 165.Vassiliou S., Grabowiecka A., Kosikowska P., Yiotakis A., Kafarski P., Berlicki L. Design, synthesis, and evaluation of novel organophosphorus inhibitors of bacterial ureases. J Med Chem. 2008;51:5736–5744. doi: 10.1021/jm800570q. [DOI] [PubMed] [Google Scholar]
- 166.Berlicki Ł., Bochno M., Grabowiecka A., Białas A., Kosikowska P., Kafarski P. N-substituted aminomethanephosphonic and aminomethane-P-methylphosphinic acids as inhibitors of ureases. Amino Acids. 2012;42:1937–1945. doi: 10.1007/s00726-011-0920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vassiliou S., Grabowiecka A., Kosikowska P., Berlicki Ł. Three component Kabachnik-Fields condensation leading to substituted aminomethane-P-hydroxymethylphosphonic acids as a tool for screening of bacterial urease inhibitors. Arkivoc. 2012;4:33–43. [Google Scholar]
- 168.Macegoniuk K., Dziełak A., Mucha A., Berlicki Ł. Bis(aminomethyl)phosphinic acid, a highly promising scaffold for the development of bacterial urease inhibitors. ACS Med Chem Lett. 2015;6:146. doi: 10.1021/ml500380f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Collinsova M., Jiracek J. Phosphinic acid compounds in biochemistry, biology and medicine. Curr Med Chem. 2000;7:629–647. doi: 10.2174/0929867003374831. [DOI] [PubMed] [Google Scholar]
- 170.Berlicki L., Kafarski P. Computer-aided analysis and design of phosphonic and phosphinic enzyme inhibitors as potential drugs and agrochemicals. Curr Org Chem. 2005;9:1829–1850. [Google Scholar]
- 171.Merz K.M., Kollman P.A. Free energy perturbation simulations of the inhibition of thermolysin: prediction of the free energy of binding of a new inhibitor. J Am Chem Soc. 1989;111:5649–5658. [Google Scholar]
- 172.Grobelny D., Goli U.B., Galard R.E. Binding energetics of phosphorus-containing inhibitors of thermolysin. Biochemistry. 1989;28:4948. doi: 10.1021/bi00438a006. [DOI] [PubMed] [Google Scholar]
- 173.Benini S., Rypniewski W.R., Wilson K.S., Ciurli S., Mangani S. The complex of Bacillus pasteurii urease with b-mercaptoethanol from X-ray data at 1.65 Å resolution. J Biol Inorg Chem. 1998;3:268–273. doi: 10.1007/s007750050014. [DOI] [PubMed] [Google Scholar]
- 174.Benini S., Kosikowska P., Cianci M., Mazzei L., Vara A.G., Berlicki L. The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design. J Biol Inorg Chem. 2013;18:391–399. doi: 10.1007/s00775-013-0983-7. [DOI] [PubMed] [Google Scholar]
- 175.Ntatsopoulos V., Vassiliou S., Macegoniuk K., Berlicki Ł., Mucha A. Novel organophosphorus scaffolds of urease inhibitors obtained by substitution of Morita-Baylis-Hillman adducts with phosphorus nucleophiles. Eur J Med Chem. 2017;133:107. doi: 10.1016/j.ejmech.2017.03.070. [DOI] [PubMed] [Google Scholar]
- 176.Ansari F.L., Wadood A., Ullah A., Iftikhar F., Ul-Haq Z. In silico studies of urease inhibitors to explore ligand-enzyme interations. J Enzyme Inhib Med Chem. 2009;24(1):151–156. doi: 10.1080/14756360801945598. [DOI] [PubMed] [Google Scholar]
- 177.Bekhit A.A., Habbib N.S., El-Din A., Bekhit A. Synthesis and antimicrobial evaluation of chalcone and syndrome derivatives of 4(3H)-quinazolinone. Boll Chim Farm. 2001;140:297–301. [PubMed] [Google Scholar]
- 178.Hiseh H.K., Lee T.H., Wang J.P., Wang J.J., Lin C.N. Synthesis and antiinflammatory effect of chalcones and related compounds. Pharm Res. 1998;15:39–46. doi: 10.1023/a:1011940401754. [DOI] [PubMed] [Google Scholar]
- 179.Kumar S.K., Hager E., Pettit C., Gurulingppa H., Davidson N.E., Khan S.R. Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. J Med Chem. 2003;46(14):2813–2818. doi: 10.1021/jm030213+. [DOI] [PubMed] [Google Scholar]
- 180.Nielsen S.F., Christensen S.B., Cruciani G., Kharazmi A., Liljefors T. Antileishmanial chalcones: statistical design, synthesis, and three-dimensional quantitative structure-activity relationship analysis. J Med Chem. 1998;41:4819–4832. doi: 10.1021/jm980410m. [DOI] [PubMed] [Google Scholar]
- 181.Lopez S.N., Castelli M.V., Zacchino S.A., Dominguez J.N., Lobo G., Chrris-Charriss J. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg Med Chem. 2001;9:1999–2013. doi: 10.1016/s0968-0896(01)00116-x. [DOI] [PubMed] [Google Scholar]
- 182.Murakami S., Muramatsu M., Aihara H., Otomo S. Inhibition of gastric H+, K(+)-ATPase by the antiulcer agent, sofalcone. Biochem Pharmacol. 1991;42(7):1447–1451. doi: 10.1016/0006-2952(91)90458-h. [DOI] [PubMed] [Google Scholar]
- 183.Deshpande A.M., Argade N.P., Natu A.A., Eckman J. Synthesis and screening of a combinatorial library of naphthalene substituted chalcones: inhibitors of leukotriene B4. Bioorg Med Chem. 1999;7(6):1237–1240. doi: 10.1016/s0968-0896(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 184.Khatib S., Nerya O., Musa R., Shmnel M., Tamir S., Vaya J. Chalcones as potent tyrosinase inhibitors: the importance of a 2,4-substituted resorcinol moiety. Bioorg Med Chem. 2005;13(2):433–441. doi: 10.1016/j.bmc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 185.Severi F., Benvenuti S., Costantino L., Vampa G., Melegari M., Antolini L. Synthesis and activity of a new series of chalcones as aldose reductase inhibitors. Eur J Med Chem. 1999;33:859–866. [Google Scholar]
- 186.Ahari-Mostafavi M.M., Sharif A., Mirzaei M., Amanlou M. Novel and versatile methodology for synthesis of β-aryl-β-mercapto ketone derivatives as potential urease inhibitors. J Iran Chem Soc. 2014;11:1113–1119. [Google Scholar]
- 187.Batool T., Rasool N., Gull Y., Noreen M., Nasim F.U.H., Yaqoob A. A convenient method for the synthesis of (Prop-2-Ynyloxy)benzene derivatives via reaction with propargyl bromide, their optimization, scope and biological evaluation. PLoS ONE. 2014;9:1–19. doi: 10.1371/journal.pone.0115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiao Z.P., Peng Z.Y., Dong J.J., Deng R.C., Wang X.D., Ouyang H. Synthesis, molecular docking and kinetic properties of β-hydroxy-β-phenylpropionyl-hydroxamic acids as Helicobacter pylori urease inhibitors. Eur J Med Chem. 2013;68:212–221. doi: 10.1016/j.ejmech.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 189.Shi W.K., Deng R.C., Wang P.F., Yue Q.Q., Liu Q., Ding K.L. 3-Arylpropionylhydroxamic acid derivatives as Helicobacter pylori urease inhibitors: Synthesis, molecular docking and biological evaluation. Bioorg Med Chem. 2016;24:4519–4527. doi: 10.1016/j.bmc.2016.07.052. [DOI] [PubMed] [Google Scholar]
- 190.Sokmen B.B., Onar H.C., Yusufoglu A., Yanardag R. Antielastase, antiurease and antioxidante activities of (3–13)-monohydroxyeicosanoic acid isomers. J Serb Chem Soc. 2012;77(10):1353–1361. [Google Scholar]
- 191.Sokmen B.B., Hasdemir B., Yusufoglu A., Yanardag R. Some monohydroxy tetradecanoic acid isomers as novel urease and elastase inhibitors and as new antioxidants. Appl Biochem Biotechnol. 2014;172:1358–1364. doi: 10.1007/s12010-013-0595-2. [DOI] [PubMed] [Google Scholar]
- 192.Tanaka T., Kawase M., Tani S. Urease inhibitory activity of simple alpha, beta-unsaturated ketones. Life Sci. 2005;73(23):2985–2990. doi: 10.1016/s0024-3205(03)00708-2. [DOI] [PubMed] [Google Scholar]
- 193.Zhao G., Yu T., Wang R., Wang X., Jing Y. Synthesis and structure-activity relationship of ethacrynic acid analogues on glutathione-s-transferase P1–1 activity inhibition. Bioorg Med Chem. 2005;13(12):4056–4062. doi: 10.1016/j.bmc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 194.Zhao G., Liu C., Wang R., Song D., Wang X., Lou H. The synthesis of alpha, beta-unsaturated carbonyl derivatives with the ability to inhibit both glutathione S-transferase P1–1 activity and the proliferation of leukemia cells. Bioorg Med Chem. 2007;15(7):2701–2707. doi: 10.1016/j.bmc.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 195.Janser I., Vortolomei C.M., Meka R.K., Walsh C.A., Janser R.F.J. Ethacrynic acid as a lead structure for the development of potent urease inhibitors. C R Chim. 2013;16:660–664. [Google Scholar]
- 196.Tanaka T., Kawase M., Tani S. Α-Hydroxyketones as inhibitors of urease. Bioorg Med Chem. 2004;12:501–505. doi: 10.1016/j.bmc.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 197.Sirajuddin M., Ali S., Zaib S., Iqbal J., Tahir M.N., Hadda T.B. Design, structural and spectroscopic elucidation and in vitro antimicrobial, anticancer, antileishmanial, urease inhibition activities and interaction with SS-DNA of newly synthesized amide based carboxylic acid. Inorganica Chim Acta. 2015;427:178–187. [Google Scholar]
- 198.Li H.Q., Xiao Z.P., Yin-Luo, Yan T., Lv P.C., Zhu H.L. Amines and oximes derived from deoxybenzoins as Helicobacter pylori urease inhibitors. Eur J Med Chem. 2009;44:2246–2251. doi: 10.1016/j.ejmech.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 199.Xiao Z.P., Ma T.W., Fu W.C., Peng X.C., Zhang A.H., Zhu H.L. The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. Eur J Med Chem. 2010;45:5064–5070. doi: 10.1016/j.ejmech.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 200.Xiao Z.P., Shi W.K., Wang P.F., Wei W., Zeng X.T., Zhang J.R. Synthesis and evaluation of N-analogs of 1,2-diarylethane as Helicobacter pylori urease inhibitors. Bioorg Med Chem. 2015;23:4508–4513. doi: 10.1016/j.bmc.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 201.Aziz-ur-Rehman, Awais-ur-Rehman, Abbasi MA, Khalid H, Dar P, Khan KM. Synthesis and biological screening of N-substituted derivatives of N-benzyl-4- chlorobenzenesulfonamide. Asian J Pharm Health Sci 2012;2:384–89.
- 202.Aziz-ur-Rehman, Abbasi MA, Rasool S, Ashraf M, Ejaz SA, Hassan R, et al. Synthesis, spectral characterization and enzyme inhibition Studies of different chlorinated sulfonamides. Pak J Pharm Sci 2014;27(6):1739–45. [PubMed]