Graphical abstract
Keywords: Urease inhibitors, Crop production, Pollution mitigation, Urea, Nitrogen fertilizer
Abstract
World population is expected to reach 9.7 billion by 2050, which makes a great challenge the achievement of food security. The use of urease inhibitors in agricultural practices has long been explored as one of the strategies to guarantee food supply in enough amounts. This is due to the fact that urea, one of the most used nitrogen (N) fertilizers worldwide, rapidly undergoes urease-driven hydrolysis on soil surface yielding up to 70% N losses to environment. This review provides with a compilation of what has been done since 2005 with respect to the search for good urease inhibitors of agricultural interests. The potential of synthetic organic molecules, such as phosphoramidates, hydroquinone, quinones, (di)substituted thioureas, benzothiazoles, coumarin and phenolic aldehyde derivatives, and vanadium-hydrazine complexes, together with B, Cu, S, Zn, ammonium thiosulfate, silver nanoparticles, and oxidized charcoal as urease inhibitors was presented from experiments with purified jack bean urease, different soils and/or plant-soil systems. The ability of some urease inhibitors to mitigate formation of greenhouse gases is also discussed.
Introduction
Food production in enough amount and use of better approaches for efficient management of fertilizers are persistent challenges in view of the world population increase [1]. Nitrogen (N) fertilizers are pivotal for crop production as this element is mandatory for plant growth and development. Therefore, application of large amounts of N is a common practice in agriculture [2]. Urea is one of the most used N fertilizer worldwide [3], particularly due to its high N content (46%), relatively low cost per N unit, availability in most markets, high water solubility, low corrosion capacity, compatibility to most fertilizers and high foliar uptake, among others [4].
Despite the wide use of urea as fertilizer, its application on soil raises environmental concerns due to the formation of gaseous (NH3, CO2, N2O, NO) or ionic (NO2−, NO3−) pollutants from urea hydrolysis, nitrification and denitrification of urea hydrolysis products and NO3− leaching as well. These events result in increase of greenhouse gas emissions, water pollution and eutrophication and lower N recovery by crops [5], [6], [7]. Then, the development of technologies and strategies that allow a more efficient management of N fertilizers and decrease or suppress of their negative effects is desirable for the excellence of the agricultural practices and environmental sustainability.
The use of urease inhibitors is one of the strategies adopted to improve urea performance in agriculture and mitigate urea-driven emission of pollutants [8], [9], [10], [11]. Urease is a nickel-dependent enzyme that catalyzes the hydrolysis of urea to two moles of ammonia (NH3) and one mole of carbon dioxide (CO2). As a key enzyme for the global N cycle, this hydrolase is widely distributed in nature being found in bacteria, yeasts, fungi, algae, animal waste and plants [12]. A variety of substances have been reported to slow down urease catalytic activity, in which several of them are urea analogs that compete with the natural substrate for the urease active site. If on one hand, urea hydrolysis provides NH3 that, in turn, is converted to ammonium (NH4+) in soil solution prior to uptake by plants, on the other hand, substantial amounts of N may be lost to atmosphere as NH3 by volatilization [13], [14]. Urease inhibitors are particularly interesting when used in the scope of covering fertilization, in which urea-derived NH3 formation on soil surface is decreased, favoring, via rain episodes or programmed irrigation, urea movement to deeper soil layer [15]. Then, the control of urease activity in soil may serve as an environmentally friendly alternative to improve N content in soil [16].
Although commercial formulations based on urea and urease inhibitors are available, the efficacy of such inhibitors may vary according to the soil. Indeed, the rate of urea hydrolysis in soils has traditionally been explained by variations in soil physicochemical features such as C and N microbial biomass, surface area, temperature, and pH [6], [17], [18]. In this context, a broad variety of organic compounds and metal cations (e.g. Hg2+, Cd2+, Ag+, among others) have been investigated for the potential to inhibit ureases with focus on agricultural practices. Therefore, this review brings a compilation of what we have learned since 2005 about urease inhibitors of agricultural interest. It does not include findings related to urease inhibition by plant crude extracts or isolated natural products as we have published a review on this subject in 2015 [9].
Phosphoramidates
The N-(butyl) thiophosphoric acid triamide (NBPT; Fig. 1) is the phosphoramidate most known for its use as urease inhibitor in agriculture worldwide. We are giving emphasis to phosphoramidates other than NBPT as the agronomic efficiency of such commercial urease inhibitor is explored in details in another review of this special issue.
Fig. 1.
Structure of phosphoramidates that present notable inhibitory effect on ureases. The phosphoramide derivative derivatives (PAD) exemplified from Ref. [24].
The N-(propyl) thiophosphoric triamide (NPPT; Fig. 1), applied together with urea on a Chinese silt (sandy) loam soil under greenhouse condition, slowed down NH3 volatilization by over 50% in relation to control soil samples during the first 11 days following fertilization [19]. The mixture constituted of 0.05% NPPT and 0.05% NBPT was 23.8% and 28.8% more efficient in mitigating NH3 volatilization from soil when compared to the single treatments NBPT or NPPT, respectively. Two formulations containing phosphoric acid triamide derivatives (UI1 and UI2) were used on Haplic Phaeozem soil in greenhouse experiments carried out with Avena sativa (oat) [20]. Although it was not clearly disclosed the difference between them, such formulations were likely constituted of the urease inhibitor NPPT. The UI1 improved biomass accumulation (12.3 g dry weight pot−1) and N uptake (339 mg pot−1) in oat panicles as panicles from plants grown under urease inhibitor-free conditions yielded 9.0 g dry weight pot−1 and 222 mg N pot−1. The N uptake by oat culms from plants under urea + UI1 or urea + UI2 fertilization averaged 231 mg pot−1 while control plant culms accumulated only 150 mg N pot−1 [20]. A commercial formulation named Limus® (25% NPPT + 75% NBPT) was used at 0.12% (w/w related to urea) to fertilize soils from North and Northeast China to grow winter Triticum aestivum (wheat) or summer Zea mays (maize) [21]. Cumulative NH3 losses reached from 11 to 25% of applied N-urea after two weeks, while soil supplementation with urea plus Limus® decreased the loss by up to 85%. No differences of grain yield was observed between urea-treated and urea plus Limus® soils. These authors also applied Limus® on Fluvo-aquic and alluvial soils to grow maize [10]. Limus® treatment promoted, in average, a decrease in cumulative NH3 losses by 84% compared to urea-treated soils. Additionally, urea plus Limus® improved the apparent N recovery efficiency by 17%. The use of Limus® on the soils tested could reduce by up to 60% the application of N-urea for maize growth and still allowing crop yields as high as those observed from usual farmers’ practice [10].
A urease inhibitor recently introduced to the market, N-(2-nitrophenyl) phosphoric triamide (2-NPT; Fig. 1), lowered NH3 volatilization by 26 to 83% from Luvisol (field conditions), causing a 2–3-day delay in the peak of gas emission [22]. As for a field experiment carried out with Lolium perenne (perennial ryegrass) cultivated either in Endofluvic Chernozem or Cambisol, 2-NPT alleviated NH3 losses by 69–100% when used at concentrations in the range from 0.75 to 1.5 g urea-N kg1, while urea by itself led to NH3 volatilization corresponding to up 14% of total N applied [23].
Fourteen phosphoramide derivatives (PADs; Fig. 1) out of 40 compounds synthesized showed higher inhibitory effect on Canavalia ensiformis (jack bean) urease activity than NBPT (IC50 = 100 nM) as they presented concentration necessary to inhibit enzyme activity by 50% (IC50) values ranging from 2 to 63 nM [23]. The most highly active inhibitors (PADs 6 k, IC50 = 2 nM; 6p, IC50 = 3 nM and 6f, IC50 = 3.5 nM) were selected for tests in acidic (pH 4.5; Anaya de Alba, Spain), moderated acidic (pH 5.9; Las Planas, Spain) and alkaline (pH 8.5; Mendigorría, Spain) soils. The ability of 6f and 6p to inhibit ureases from moderated acidic soil was comparable to that of NBPT [24]. These phosphoramide derivatives, however, inhibited acidic soil ureases by 65% and alkaline ones by 75% while NBPT inhibited 9% and 45%, respectively. Although 6 k was the most highly active compound in vitro, it showed lower performance on soil ureases than that of 6f or 6p regardless of soil pH. Authors hypothesized that 6 k possesses low stability and fast degradation rate on soil [24].
The extent of the inhibitory effect of phenylphosphorodiamidate (PPD; Fig. 1) on urease has been reviewed in 2009 [25]. Since then, the kinetic and thermodynamic behaviors of PPD towards soil ureases were studied at 10, 20 and 30 °C and under waterlogging using Pachic Udic Mollisol (black soil) [26]. The PPD at 50 mg kg−1 dry soil worked as mixed inhibitor as it increased urea KM and decreased ureases Vmax when used at room temperature. The KM and Vmax significantly increased following temperature increment. Soil urease thermodynamic parameters, such as activation energy, enthalpy of activation and temperature coefficients slightly increased upon PPD treatment and increasing temperature when compared to soils devoid of PPD treatment [26]. The PPD treatment led to higher KM (ca. 40 mM) and lower Vmax values (ca. 200 mg hydrolyzed urea-N kg−1 dry soil 5 h−1) than those of NBPT treatment up to 30 days of experiment under water-logging. This indicates that PPD is a better urease inhibitor than NBPT in waterlogged soil [27]. The performance of 2% (w/w) PPD as urease inhibitor was also verified in a Calcic Haploxerepts soil featuring sandy clay loam texture in the upper (0–28 cm) horizon [28]. The PPD treatment decreased soil urease by ca. 45% during the first two days following application of 120 kg N ha−1 urea. No significant effect on N2O emissions was observed for soils at 40% and 60% water-filled pore space (WFPS) supplemented with urea plus PPD, although gas emissions increased from 4.5 mg N2O-N kg−1 d−1 (control) to 5.8 mg N2O-N kg−1 d−1 when soils at 80% WFPS received PPD treatment [28].
The substituted phosphoric acid triamide P101/04 at 0.06% (w/w in relation to urea) was used as urease inhibitor in pot experiments devoid of vegetation or with spring wheat grown for 70 days in Cambisol under controlled conditions [29]. The surface application of P101/04 promoted a decrease of N2O emission from plant-free soil by 15–46%, regardless of the size of urea granules used. Lower levels (0.16–0.27% of total fertilization) of emitted N2O were achieved from the wheat-grown soil [29].
Hydroquinone and quinones
It is known that NH3 formed on soil surface may also be converted to the pollutant N2O from either sequential activity of microbial ammonia mono-oxygenase and hydroxylamine oxidoreductase enzymes or from the action of the latter enzyme followed by the activity of denitrifying bacteria [30].
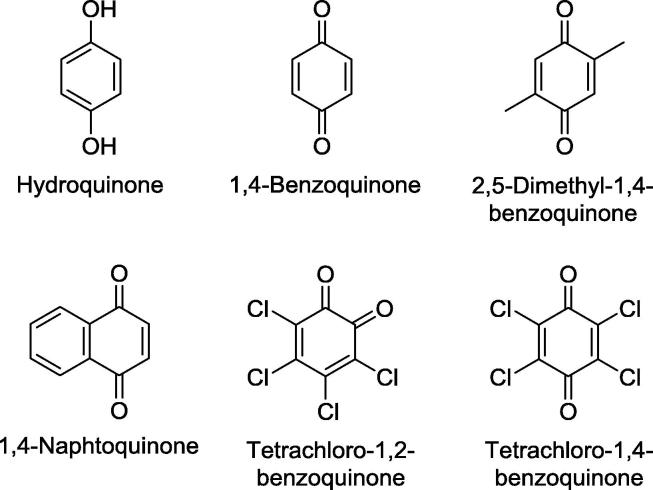
A meta-analysis study with several agricultural soils showed that hydroquinone (HQ; Fig. 2), a urease inhibitor, significantly reduced N2O and NO emissions by around 5% [31]. Application of 12 kg N ha−1 HQ on an Alluvial soil, in conjunction with 120 kg urea-N ha−1, decreased N2O emission by 5% in rice (Oryza sativa) and 7% in wheat systems when compared to the crops grown solely in the presence of 120 kg N ha−1 urea [32]. Authors noted, however, that 10% HQ (in relation to urea) + urea contributed to an increment of methane (CH4) emission by 12% and then an increase of Global Warming Potential index by 5% [32]. The application of lower amounts of HQ (0.3% in relation to urea) + urea 0.1% in a rhizobox system containing 2 kg of sandy loam Belgium soil (classified as luvisol) resulted in a higher number of tillers per rice plants [33]. Furthermore, the association of HQ with dicyandiamide (DCD; nitrification inhibitor) improved rice growth and significantly decreased N2O emissions from soil in comparison to urea treatment [33]. The effect of HQ + DCD on N2O emission was also analyzed in a soil from a paddy field classified as Typic Haplaquepts [34]. A mixture of 0.3% HQ, 5.0% DCD and 300 kg urea-N ha−1 mitigated N2O emission from soil by 24%, 56% and 17% right before rice transplanting, at tillering or at panicle initiation stages, respectively, in relation to urea-fertilized soils [35]. The CH4 emission (43.39 ± 3.89 kg ha−1) from these same treatments decreased by 35, 19 and 12% and the Global Warming Potential dropped from 99 mg CO2-eq m−2 h−1 to 71,6 and 84 mg CO2-eq m−2 h−1, respectively [33]. In addition, rice grain yield increased by 10%, 18% and 6%, respectively, while the straw weight was improved by 16%, 17% and 8% in comparison to control samples (7.9 and 8.2 t ha−1 for grain yield and straw weight, respectively) [34].
Fig. 2.
Structure of hydroquinone and quinones of recognized potential as urease inhibitor of agricultural interest.
The use of HQ and DCD was investigated in coastal saline Jerusalem artichoke bioenergy cropping system maintained in a Fluvoaquic soil [36]. Urea (300 kg N ha−1) was used by itself or in conjunction with HQ (30 kg ha−1) and DCD (9 kg ha−1) during artichoke growing season. The flux of CO2 and N2O from soil supplemented with urea was 517.9 mg CO2 m−2 h−1 and 54.7 mg N2O-N m−2 h−1 and decreased by 12 and 16% upon addition of HQ + DCD, respectively. The net primary production in systems treated with urea + HQ + DCD increased by 18% in relation to that of urea-treated ones (18.3 ± 1.36 t C ha−1). Association of HQ and DCD with urea yielded a 35% decline in the net ecosystem exchange of CO2. Likewise, the estimated net greenhouse gas balance and greenhouse gas intensity from Jerusalem artichoke cropping system dropped 37% and 15%, respectively [36].
The efficiency of urease inhibitor HQ was also tested in Pachic Udic Argiboroll (black soil) at 20% moisture or under waterlogged conditions (3–5 cm water layer). The urea KM values towards soil ureases were ca. 36 mM at the first day of soil incubation with HQ and ca. 25 mM 10 days post incubation [27]. In contrast, soil ureases Vmax at 20% moisture increased from 220 mg hydrolyzed urea-N kg−1 dry soil (first day) to 250 mg hydrolyzed urea-N kg−1 dry soil at 10–30 days post-HQ soil treatment while the increment in waterlogged soil was ca. 40 mg hydrolyzed urea-N kg−1 dry soil during the same period of soil incubation with HQ [27]. Another investigation with black soil showed that temperatures ranging from 10 to 30 °C and HQ incubation times up to 20 days do not affect urea KM values in soil supplemented with urea + HQ [26]. Temperatures of 20 and 30 °C led to significant increment of soil ureases Vmax 10 and 30 days after soil incubation with HQ. Authors also determined that HQ affects soil kinetic parameters much more than it does on soil thermodynamics ones.
Halogen-substituted p-benzoquinones such as those containing Cl, Br or F atoms has been long recognized as excellent soil urease inhibitors [37]. The mode by which tetrachloro-1,2-benzoquinone and tetrachloro-1,4-benzoquinone affect jack bean urease activity was determined to be as slow-binding inhibition with formation of very stable urease-inhibitor complexes [38]. Tetrachloro-1,4-benzoquinone was more effective than the corresponding ortho-substituted benzoquinone as the urease residual activity reached a plateau in the presence of the former at concentrations much lower (0.29 and 0.59 µM) than those (7.5 and 15 µM) of the latter. The inhibition constants (Ki∗) for tetrachloro-1,2-benzoquinone and tetrachloro-1,4-benzoquinone were 2.4 × 10−6 mM and 4.5 × 10−7 mM, respectively. The interaction between these chloro-substituted benzoquinones and a Cys residue present in urease active site was responsible for the enzyme inhibition [38].
The inhibition of jack bean urease by 1,4-benzoquinone, 2,5-dimethyl-1,4-benzoquinone, tetrachloro-1,4-benzoquinone occurs in a concentration-dependent manner, wherein the enzyme-inhibitor equilibrium was achieved in ca. 10 min [39]. The IC50 values for 1,4-benzoquinone, 2,5-dimethyl-1,4-benzoquinone and tetrachloro-1,4-benzoquinone (Fig. 2) were 5.5, 50.0 and 0.6 µM, respectively. The residual urease activity was linearly correlated with the number of modified thiols in protein structure. Therefore, arylation of Cys thiol group caused by the quinones tested contributes for the mechanism of enzyme inhibition [39]. Besides arylation of Cys thiol group, 1,4-naphthoquinone (Fig. 2) promotes thiol oxidation. The enzyme inhibition by this benzoquinone is biphasic-type, time- and concentration-dependent with a non-linear residual activity dependent on thiol modification [39], [40]. Indeed, co-crystallization of Sporosarcina pasteurii urease and 1,4-benzoquinone (41) showed that the enzyme inhibitor covalently binds to the thiol group at α Cys322, a highly conserved residue present at the mobile flap that controls urea access to urease active site.
(Di)substituted thioureas
A recent report revealed the potential of benzoylthioureas (BTUs) as urease inhibitors of agronomic interest [42]. An initial in vitro screening performed with 10 mM urea and BTUs at 0.5 mM showed that 51 out of 65 compounds inhibited jack bean urease at different extents [42]. Eight BTUs (11, 12, 14, 19–22, and 37; Fig. 3) were the most potent inhibitors as they negatively affected the ureolytic activity of urease by in the range from 50 to 77%. Such benzoylthioureas function as mixed-type inhibitors exhibiting higher affinity to urease active site than allosteric ones. Based on the equilibrium dissociation constant Ki, BTU 14 was the most efficient mixed inhibitor followed by 11, 22, 19, 37, 20, 21, and 12. Experiments performed with Clayey dystrophic Red Latosol soil supplemented with 0.5 mM BTUs and 72 mM urea showed that compounds 3, 6, 10, 12, 16, 19, and 22 were more efficient than NBPT to inhibit the activity soil ureases. Other 21 BTUs were demonstrated to be as potent as NBPT. Notably, the most efficient BTUs on soil were also found to be more thermostable than NBPT, which makes this class of compounds eligible for further studies towards the development of new urea-based fertilizer formulations [42].
Fig. 3.
Structure of (di)substituted thiourea derivatives of known antiureolytic activity in the scope of agriculture. The benzoylthioureas (BTUs) exemplified from Ref. [42] while the disubstituted thioureas (DSTUs) come from Ref. [43].
The urease inhibition potential of N,N′-disubstituted thioureas (DSTUs) was evaluated in vitro, using jack bean urease and 100 mM urea [41]. Thirteen thiourea derivatives (DSTUs 1, 3, 4, 9, 13–16, 18–20, 26, and 30; Fig. 3) efficiently inhibited urease activity exhibiting IC50 values (from 8.4 to 20.3 µM) lower than that of standard inhibitor thiourea. These compounds presented Ki values ranging from 8.6 to 19.3 µM and showed mechanisms of action typical of mixed (DSTUs 1, 3, 9,14, 15, 18, and 26), competitive (DSTUs 13 and 30) or non-competitive (DSTU 19) inhibitors [43].
Benzothiazoles
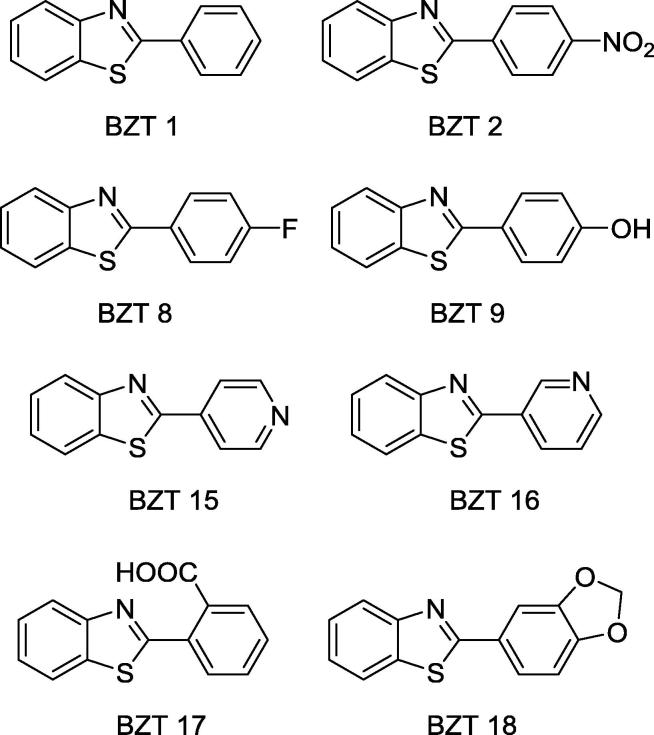
The inhibitory effect of new benzothiazoles (BZT; Fig. 4) on urease activity was assessed in vitro in reactions containing 10 mM urea and 1.6 mM compound-test. The most effective compounds were 2-phenylbenzothiazole (BZT 1), 2-(4-nitrophenyl)benzothiazole (BZT 2), 2-(4-hydroxyphenyl)benzothiazole (BZT 9), 2-(4-pyridyl)benzothiazole (BZT 15), 2-(3-pyridyl)benzothiazole (BZT 16), 2-(2-carboxyphenyl)benzothiazole (BZT 17) and 2-(1,3-benzodioxol-5-yl)benzothiazole (BZT 18). Among them, BZT 15 was the most active as it inhibited jack bean urease by 55%. The efficiency of hydroxyurea, a reference of inhibitor, averaged 62% [44]. The mechanism by which BZT 15 inhibits jack bean urease is compatible with that of mixed inhibitors that exhibits higher affinity to the active site (Ki = 1.02 ± 0.04 mM) than allosteric ones (Ki′ = 3.17 ± 0.69 mM) [44]. Fourteen benzothiazoles synthesized also inhibited, to different extent, ureases present in a Clayey dystrophic Red Latosol soil under controlled conditions (0.5 g of soil supplemented with 72 mM urea). Five compounds (BZTs 2, 8, 9, 15, and 16) at 1.6 mM were as efficient as NBPT (reference inhibitor) while BZT 10 was 12% more potent than NBPT [44].
Fig. 4.
Structure of benzothiazoles (BZTs) of recognized potential as urease inhibitors of agricultural interest. Compounds are based on Ref. [44].
Coumarin derivatives
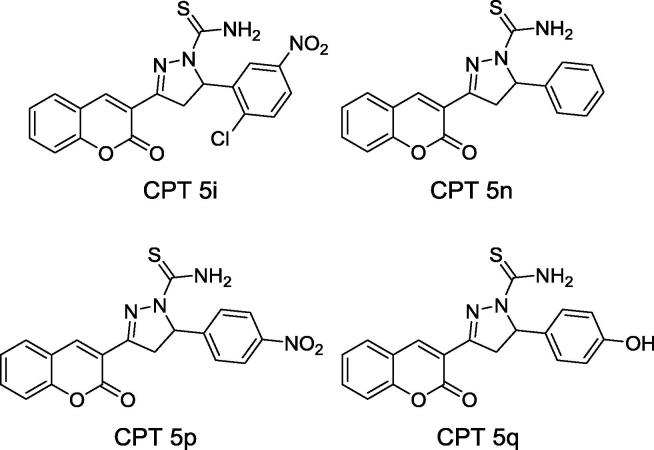
The potential of some coumarinyl pyrazolinyl thiomide (CPTs; Fig. 5) as urease inhibitor was evaluated in vitro using jack bean urease [45]. The derivative bearing an unsubstituted phenyl group (CPT 5n) was the most potent compound exhibiting IC50 as low as 0.036 nM from reaction media (90 µL) containing 0.1 U urease, 100 mM urea at pH 8.2 [45]. The presence of an —NO2 at para-position (CPT 5p), an —OH group at para-position (CPT 5q), —Cl and —NO2 at ortho- and meta-positions (CPT 5i) on phenyl ring compromised the anti-ureolytic activity of coumarin derivatives by 17-fold for the former and over 270-fold for CPTs 5i and 5q. The most active compound (CPT 5n) was determined to be a typical non-competitive inhibitor of jack bean urease as increasing concentrations of such coumarin derivative decreased urease activity without significantly affecting urea KM [45]. Docking studies showed that 5n may form two and one hydrogen bonds with Asp494 and Ala440 residues present at urease active site, respectively. Hydrogen bond may also be formed between S atom and Asp494.
Fig. 5.
Structure of coumarinyl pyrazolinyl thiomides (CPTs) of recognized potential as urease inhibitor of agricultural interest. Compounds are based on Ref. [45].
Phenolic aldehyde derivatives
Four Biginelli adduct were synthesized inspired in the structure of natural phenolic aldehydes namely protocatechuic aldehyde (PA), syringaldehyde (SA) and vanillin (VN) [46]. In vitro assays using jack bean urease (12.5 mU), 10 mM urea and compounds-test at 1.6 mM showed that 2A7 and 2B10 (PA derivatives; Fig. 6) inhibited the ureolytic activity by 94% while enzyme activity inhibition reached 58.6% (in average) when 2A9 (VN derivative) or 2D2 (SA derivative) was added to the reaction medium. These compounds exhibit a mechanism of action typical of mixed inhibitors in which 2A7 was determined to be the most efficient one. The effect on Clayey Dystrophic Red Latosol (oxisol), however, revealed that 2A7 and 2D2 were the most potent against soil ureases as they inhibit the ureolytic activity by 50% when applied at 3.3 mM [46]. This demonstrates that results obtaining with purified ureases may not necessary reflect what happens on soil due to its complexity. Both 2A7 and 2D2 were determined to be more thermal stable than the commercial urease inhibitor NBPT.
Fig. 6.

Structure of natural phenolic aldehyde derivatives reported to inhibit soil ureases. Compounds are based on Ref. [46].
Miscellaneous
The use of urea coated with Cu plus Zn on a Malaysian typic paleudult soil greatly improved N uptake by Pannicum maximum (Guinea grass) from 12 kg N ha−1 to 137.9 kg N ha−1. Soil supplementation with Cu-coated urea yielded an N uptake by plants of up to 96.7 kg ha−1 [47]. These treatments were shown to slow down urea hydrolysis in comparison with the soil that solely received urea, in which that supplemented with Cu-Zn-coated urea exhibited an increment of pasture production by up to 50% [47]. The use of Cu-B-coated urea in a field study with rice plants cultivated in Typic Albaqualf soil (non-tillage system) reduced the total N-NH3 loss from 47% (urea by itself) to 22% after 96 h of fertilizer application [48]. Likewise, the 1.2% N-NH3 loss observed in urea-supplied conventional crop system was decreased to 0.3% after 216 h of Cu-B-coated urea application [48]. Rice productivity, however, was not affected by urea coated with Cu plus B. The N loss by NH3 volatilization was also diminished by urea coated with S or boric acid plus Cu in a field experiment carried out with Saccharum officinarum (sugarcane) cultivated in a Brazilian sandy soil [49]. Accumulated N-NH3 losses from soil treated with acid-boric-Cu-coated urea and S-coated urea were determined to be 2.2 kg ha−1 and 4.6 kg ha−1, respectively, while N-NH3 loss from soil treated with urea was as much as 9.1 kg ha−1 Therefore, acid-boric-Cu- and S-coated urea mitigated N-NH3 losses from soil by 75 and 50%, respectively [49]. In 12-month field experiment, the grain yield for maize plants grown in a Brazilian Red Latosol (non-tillage system) containing boric-acid-Cu-coated urea was roughly twice (9.9 kg ha−1) as much as that of plants grown in the presence of uncoated urea [50]. Application of Cu-B-incorporated urea to Brazilian Haplic Planosol mitigated total NH3 volatilization by 54% compared to commercial urea in an 18-day greenhouse experiment [51]. Also, Cu-B-incorporated urea was up to 36.5% more efficient than Cu-B-coated urea with respect to the ability of inhibiting N-NH3 loss from soil [51]. The use of a physical mixture constituted of urea, Cu and B postponed the peak of NH3 volatilization for two days and decreased the total N loss by 18%, compared to commercial urea, in a field experiment carried out with maize cultivated in dystrophic Red Latosols [52]. Nevertheless, the presence of these urease inhibitors did not affect N accumulation in maize grains or stubble. Incorporation of Zn to urea pellets (up to 5 g Zn/kg urea) also efficiently inhibited the activity of red-yellow Oxisol (Typic Hapludox) ureases containing Megathyrsus maximus (Guinea grass cv. Mombaça) crop under controlled conditions [53]. Although no significant increment in plants biomass was observed when compared with plants from soil fertilized with urea only, Zn-incorporated urea pellets boosted N-uptake by plants. This is likely due to the ability of Zn to maintain higher levels of N in soil (74% more than that for soils treated with urea only) as a result of its negative effect on NH3 volatilization [53]. Bench experiments performed for 8 weeks with Malaysian rice soils (Selangor and Chempaka) demonstrated that the use of urea coated with Cu, Zn and Cu + Zn decreased N2O emission from soil by 17.6, 21.6 and 29.7%, respectively, in relation to the control [54]. The cumulative NH3 volatilization from soil for these treatments ranged from 32.1 to 39.6% while soils treated solely treated with urea emitted 34.7% more NH3 [54]. These results evidence that the use of Cu-, Zn- or Cu + Zn-coated urea on such Malaysian soils efficiently mitigate the emission of pollutants from urea fertilizer.
Ammonium thiosulfate (ATS) was shown to decrease urease activity in an Italian sandy soil bearing higher pH values and containing relatively lower amount of organic matter [55]. Maximum urease inhibition (88%) was achieved already three days after application of 100 mg ATS kg−1 soil while 25 mg ATS kg−1 soil caused a 70% enzyme inhibition. Authors found that ATS by itself or in association with urea did not affect soil microbial biomass pool. On the other hand, a field experiment performed with Canadian clay loam and fine sandy loam soils showed inconsistent results with respect to urease inhibition by ATS [56]. These findings suggest that ATS performance may be affected by the soil type.
The complex formed between silver nanoparticles (AgNPs) and jack bean urease was shown to destabilize the hexameric protein structure, a phenomenon than caused loss of ureolytic activity by up 10%, 95% and 100% for urease/AgNPs ratios of 1:1, 1:5 and 1:7, respectively [57]. In this sense, the use of AgNPs as additive in urea-based formulation could be advantageous as such nanoparticles have been also shown to contribute for pest control in agriculture (www.nal.usda.gov/fsrio/research_projects//printresults.php?ID = 9104; accessed on Nov 21, 2017).
The dimeric vanadium-hydrazine complexes (DVHCs; Fig. 7) 6c, 10c and 11c were shown to inhibit jack bean urease at IC50 values ranging from 15.0 ± 0.1 to 37.0 ± 0.4 µM while the hydrazine ligand is inactive towards such enzyme [58]. The complexes DVHC 6c, 10c and 11c act as non-competitive inhibitors and show low phytotoxicity against Lemna aequinoctialis (duckweed) in comparison to paraquat (known herbicide).
Fig. 7.
Structure of non-phytotoxic dimeric vanadium-hydrazine complexes (DVHCs) known to inhibit urease. Compounds are based on Ref. [58].
The NH3 emissions from a 10 cm-surfaced Red-Yellow Ultisol (under no-tillage) after fertilization with urea coated with oxidized charcoal (produced at 350 °C) were 43% lower than that of soils fertilized with uncoated urea [59]. Additionally, oxidized charcoal delayed the maximum volatilization peak of NH3 in 24 h, keeping urea-N on soil for longer periods [59]. Similarly, urea coated with 16% oxidized charcoal further reacted with NaOH and urea coated with 39% oxidized charcoal under no alkali treatment also alleviated NH3 volatilization by 40% from a Hapludalf soil [60]. The N losses to the atmosphere (as NH3) were also decreased by 12% upon treatment of soils belonging to the subgroups Typic Hapludox, Lamellic Hapludalfs, Aquic Argiudolls and Typic Endoaquolls with urea plus oxidized charcoal [61]. The presence of oxidized charcoal, however, did not change the levels of exchangeable NH4+, NO3−, and NO2− in the soil in comparison to samples treated with urea only.
Conclusions and future perspectives
Since 2005, several substances, namely phosphoramidates, hydroquinone, benzoquinones, (di)substituted thioureas, benzothiazoles, coumarin derivatives, phenolic aldehyde derivatives, dimeric vanadium-hydrazine complexes, oxidized charcoal, silver nanoparticles have been synthesized and shown to be potential urease inhibitors for use in agriculture. The efficiency of inorganic substances (ammonium thiosulphate, boric acid etc) or metal cations and sulfur on soil ureases was also demonstrated. The ability of urease inhibitors to mitigate the formation of greenhouse gases has been widely investigated focusing on more sustainable agricultural practices. The effect of disubstituted thioureas, coumarin derivatives and silver nanoparticles on soil ureases deserves investigation since compounds capable of inhibiting jack bean urease may not be active against soil ureases. There is a need for the world market to broaden the offer of urease inhibitors that are effective on distinct types of soil. This is a very challenging task as urea compatibility, efficiency at relatively low concentrations, minimal negative effect on soil microbiota, plant metabolism and human health (if uptaken by crop roots from soil), environmentally friendly capability and prolonged shelf life are criteria that need to be considered for the development of urease inhibitors of agricultural interest.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
Part of the work described herein was supported by the Conselho Nacional de Pesquisa (CNPq) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). LVM is recipient of research fellowship from CNPq.
Biographies

Luzia V. Modolo received her PhD in Functional and Molecular Biology in 2004 from the State University of Campinas (SP, Brazil). She was the Head of the Department of Botany at the Federal University of Minas Gerais (MG, Brazil) from 2014 to 2016. Dr. Modolo is the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and her research interests include plant nutrition and secondary metabolism and signalling processes in plant tissues triggered by environmental stress.

Cristiane J. da-Silva received her BSc. degree in Biology in 2010 from the Federal University of Juiz de Fora (MG, Brazil), her MSc. degree in Plant Physiology in 2013 from the Federal University of Viçosa (MG, Brazil) and her PhD degree in Plant Biology in 2017 from the Federal University of Minas Gerais (MG, Brazil). Her research interests include plant responses to environmental stresses, specifically in cell signaling processes, as well as plant nutrition with focus on urease inhibitors.

Débora S. Brandão received her BSc. degree in Agronomy and MSc. degree in Crop Production at the Federal University of Minas Gerais (MG, Brazil). She is currently PhD student in Plant Biology under the mentoring of Dr. Luzia V. Modolo. Her research focus is on urease inhibitors and their effects on plant and soil microbiota metabolism.

Izabel S. Chaves was born in 1986. She earned her BSc. degree in Biology in 2009 at the Federal University of Lavras (MG, Brazil). She received her PhD degree in Plant Physiology in 2015 from the Federal University of Viçosa (MG, Brazil). Her research interests include plant physiology and molecular biology as well as the development of novel urease inhibitors for improving plant nitrogen nutrition.
Footnotes
This work was made possible partly by the Network for the Development of Novel Urease Inhibitors (www.redniu.org).
Peer review under responsibility of Cairo University.
References
- 1.Bueno-Delgado M.V., Molina-Martínez J.M., Correoso-Campillo R., Pavón-Mariño P. Ecofert: an android application for the optimization of fertilizer cost in fertigation. Comput Electron Agric. 2016;121:32–42. [Google Scholar]
- 2.Villalobos F, Fereres E. Principles of agronomy for sustainable agriculture. 1st ed. Springer International Publishing; 2016.
- 3.Papangkorn J., Isaraphan C., Phinhongthong S., Opaprakasit M., Opaprakasit P. Controlled-release material for urea fertilizer from polylactic acid. Adv Mat Res. 2008;55–57:897–900. [Google Scholar]
- 4.Cantarella H., Trivelin P.C.O., Contin T.L.M., Dias F.L.F., Rossetto R., Marcelino R. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci Agric. 2008;65:397–401. [Google Scholar]
- 5.Nitrogênio Cantarella H. In: Fertilidade do solo. Novais R.F., Alvarez V.V.H., Barros N.F., editors. Viçosa; SBCS: 2007. pp. 375–470. [Google Scholar]
- 6.Abalos D., Jeffery S., Sanz-Cobena A., Guardia G., Vallejo A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric Ecosyst Environ. 2014;189:136–144. [Google Scholar]
- 7.Martins MR, Sant’Anna SAC, Zamanc M, Santos RC, Monteiro RC, Alves BJR, et al. Strategies for the use of urease and nitrification inhibitors with urea: impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric Ecosyst Environ 2017;247:54–62.
- 8.Kiss S., Simihặian M. 1st ed. Kluwer Academic Publishers; Doordrech: 2002. Improving efficiency of urea fertilizers by inhibition of soil urease activity. [Google Scholar]
- 9.Modolo L.V., Souza A.X., Horta L.P., Araujo D.P., Fátima A. An overview on the potential of natural products as ureases inhibitors. J Adv Res. 2015;6:35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., Cui X., Liu X., Roelcke M., Pasda G., Zerulla W. A new urease-inhibiting formulation decreases ammonia volatilization and improves maize nitrogen utilization in North China Plain. Sci Rep. 2017;7:43853. doi: 10.1038/srep43853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mira A.B., Cantarella H., Souza-Nettoa G.J.M., Moreira L.A., Kamogawa M.Y., Otto R. Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agricu Ecosyst Environ. 2017;248:105–112. [Google Scholar]
- 12.Follmer C. Insights into the role and structure of plant ureases. Phytochemistry. 2008;69:18–28. doi: 10.1016/j.phytochem.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Cameron K.C., Di H.J., Moir J.L. Nitrogen losses from the soil/plant system: a review. Ann Appl Biol. 2013;162:145–173. [Google Scholar]
- 14.Bock B.R., Kissel D.E. National Fertilizer Development Center – NFDC; Muscle Shoals, AL, USA: 1988. Ammonia volatilization from urea fertilizers. [Google Scholar]
- 15.Artola E., Cruchaga S., Ariz I., Moran J.F., Garnica M., Houdusse F. Effect of N-(n-butyl) thiophosphoric triamide on urea metabolism and the assimilation of ammonium by Triticum aestivum L. J Plant Growth Regul. 2011;63(1):73–79. [Google Scholar]
- 16.Rawluk C.D.L., Grant C.A., Racz G.J. Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Can J Soil Sci. 2001;81:239–246. [Google Scholar]
- 17.Fisher K.A., Yarwood S.A., James B.R. Soil urease activity and bacterial ureC gene copy numbers: effect of pH. Geoderma. 2017;285:1–8. [Google Scholar]
- 18.Silva A.G.B., Sequeira C.H., Sermarini R.A., Rafael O.R. Urease inhibitor NBPT on ammonia volatilization and crop productivity: a meta-analysis. Agron J. 2017;109:1–13. [Google Scholar]
- 19.Li S., Li J., Lu J., Wang Z. Effect of mixed urease inhibitors on N losses from surface-applied urea. Int J Agric Sci Technol. 2015;3:23–27. [Google Scholar]
- 20.Gans W., Herbst F., Merbach W. Nitrogen balance in the system plant – soilafter urea fertilization combined with urease inhibitors. Plant Soil Environ. 2006;52:36–38. [Google Scholar]
- 21.Li Q., Yang A., Wang Z., Roelcke M., Chen X., Zhang F. Effect of a new urease inhibitor on ammonia volatilization and nitrogen utilization in wheat in north and northwest China. Field Crop Res. 2015;175:96–105. [Google Scholar]
- 22.Ni K., Pacholski A., Kage H. Ammonia volatilization after application of urea to winter wheat over 3 years affected by novel urease and nitrification inhibitors. Agric Ecosyst Environ. 2014;197:184–194. [Google Scholar]
- 23.Schraml M., Gutser R., Maier H., Schmidhalter U. Ammonia loss from urea in grassland and its mitigation by the new inhibitor 2-NPT. J Agric Sci. 2016;154(8):1453–1462. [Google Scholar]
- 24.Domínguez M.J., Sanmartín C., Font M., Palop J.A., San-Francisco S., Urrutia O. Design, synthesis, and biological evaluation of phosphoramide derivatives as urease inhibitors. J Agric Food Chem. 2008;56(10):3721–3731. doi: 10.1021/jf072901y. [DOI] [PubMed] [Google Scholar]
- 25.Chien S.H., Prochnow L.I., Cantarella H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv Agron. 2009;102:267–322. [Google Scholar]
- 26.Juan Y.H., Chen Z.H., Chen L.J., Wu Z.J., Wang R., Sun W.T. Kinetic and thermodynamic behaviors of soil urease as affected by urease inhibitors. J Soil Sci Plant Nutr (former R C Suelo Nutr Veg) 2010;10(1):1–11. [Google Scholar]
- 27.Juan Y.H., Chen L.J., Wu Z.J., Wang R. Kinetics of soil urease affected by urease inhibitors at contrasting moisture regimes. J Soil Sci Plant Nutr. 2009;9(2):125–133. [Google Scholar]
- 28.Sanz-Cobena A., Abalos D., Meijide A., Sanchez-Martin L., Vallejo A. Soil moisture determines the effectiveness of two urease inhibitors to decrease N2O emission. Mitig Adapt Strategies Glob Chang. 2014;21(7):1131–1144. [Google Scholar]
- 29.Khalil M.I., Gutser R., Schmidhalter U. Effects of urease and nitrification inhibitors added to urea on nitrous oxide emissions from a loess soil. J Plant Nutr Soil Sci. 2009;172(5):651–660. [Google Scholar]
- 30.Law Y., Ye L., Pan Y., Yuan Z. Nitrous oxide emissions from wastewater treatment processes. Phil Trans R Soc B. 2012;367(1593):1265–1277. doi: 10.1098/rstb.2011.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama H., Yan X., Yagi K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Glob Chang Biol. 2010;16(6):1837–1846. [Google Scholar]
- 32.Malla G., Bhatia A., Pathak H., Prasad S., Jain N., Singh J. Mitigating nitrous oxide and methane emissions from soil in rice-wheat system of the Indo-Gangetic plain with nitrification and urease inhibitors. Chemosphere. 2005;58(2):141–147. doi: 10.1016/j.chemosphere.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Xu X., Boeckx P., Van Cleemput O., Kazuyuki I. Mineral nitrogen in a rhizosphere soil and in standing water during rice (Oryza sativa L.) growth: effect of hydroquinone and dicyandiamide. Agric Ecosyst Environ. 2005;109(1):107–117. [Google Scholar]
- 34.Li X., Zhang G., Xu H., Cai Z., Yagi K. Effect of timing of joint application of hydroquinone and dicyandiamide on nitrous oxide emission from irrigated lowland rice paddy field. Chemosphere. 2009;75(10):1417–1422. doi: 10.1016/j.chemosphere.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Zhang X., Xu H., Cai Z., Yagi K. Methane and nitrous oxide emissions from rice paddy soil as influenced by timing of application of hydroquinone and dicyandiamide. Nutr Cycl Agroecosys. 2009;85(1):31–40. [Google Scholar]
- 36.Wang X., Zhang L., Zou J., Liu S. Optimizing net greenhouse gas balance of a bioenergy cropping system in southeast China with urease and nitrification inhibitors. Ecol Eng. 2015;83:191–198. [Google Scholar]
- 37.Bundy L.G., Bremner J.M. Effects of substituted p-benzoquinones on urease activity in soils. Soil Biol Biochem. 1973;5:847–853. [Google Scholar]
- 38.Kot M., Zaborska W. Inhibition of jack bean urease by tetrachloro-o-benzoquinone and tetrachloro-p-benzoquinone. J Enzyme Inhib Med Chem. 2006;21(5):537–542. doi: 10.1080/14756360600720903. [DOI] [PubMed] [Google Scholar]
- 39.Zaborska W., Krajewska B., Kot M., Karcz W. Quinone-induced inhibition of urease: elucidation of its mechanisms by probing thiol groups of the enzyme. Bioorg Chem. 2007;35(3):233–242. doi: 10.1016/j.bioorg.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Krajewska B., Zaborska W. Double mode of inhibition-inducing interactions of 1,4-naphthoquinone with urease: arylation versus oxidation of enzyme thiols. Bioorg Med Chem. 2007;15(12):4144–41151. doi: 10.1016/j.bmc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 41.Mazzei L., Cianci M., Musiani F., Ciurli S. Inactivation of urease by 1,4-benzoquinone: chemistry at the protein surface. Dalton Trans. 2016;45(13):5455–5459. doi: 10.1039/c6dt00652c. [DOI] [PubMed] [Google Scholar]
- 42.Brito T.O., Souza A.X., Mota Y.C., Morais V.S., de Souza L.T., de Fátima Â. Design, syntheses and evaluation of benzoylthioureas as urease inhibitors of agricultural interest. RSC Adv. 2015;5(55):44507–44515. [Google Scholar]
- 43.Khan K.M., Naz F., Taha M., Khan A., Perveen S., Choudhary M.I. Synthesis and in vitro urease inhibitory activity of N,N′-disubstituted thioureas. Eur J Med Chem. 2014;74:314–423. doi: 10.1016/j.ejmech.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Araujo D.P., Morais V.S.S., de Fátima Â., Modolo L.V. Efficient sodium isulfate-catalyzed synthesis of benzothiazoles and their potential as ureases inhibitors. RSC Adv. 2015;5(36):28814–28821. [Google Scholar]
- 45.Saeed A., Mahesar P.A., Channar P.A., Larik F.A., Abbas Q., Hassan M. Hybrid pharmacophoric approach in the design and synthesis of coumarin linked pyrazolinyl as urease inhibitors, kinetic mechanism and molecular docking. Chem Biodivers. 2017;14(8):e1700035. doi: 10.1002/cbdv.201700035. [DOI] [PubMed] [Google Scholar]
- 46.Horta L.P., Mota Y.C.C., Barbosa G.M., Braga T., Marriel I.E., Fátima A. Urease inhibitors of agricultural interest inspired by structures of plant phenolic aldehydes. J Braz Chem Soc. 2016;27(8):1512–1519. [Google Scholar]
- 47.Junejo N., Khanif M.Y., Dharejo K.A., Abdu A., Abdul-Hamid H. A field evaluation of coated urea with biodegradable materials and selected urease inhibitors. Afr J Biotechnol. 2011;10(85):19729–19736. [Google Scholar]
- 48.Grohs M., Marchesan E., Santos D.S., Massoni P.F.S., Sartori G.M.S., Ferreira R.B. Resposta do arroz irrigado ao uso de inibidor de urease em plantio direto e convencional. Ciênc Agrotec. 2011;35(2):336–345. [Google Scholar]
- 49.Nascimento C.A.C., Vitti G.C., Faria L.A., Luz P.H.C., Mendes F.L. Ammonia volatilization from coated urea forms. R Bras Ci Solo. 2013;37(4):1057–1063. [Google Scholar]
- 50.Faria L.A., Nascimento C.A.C., Vitti G.C., Luz P.H.C., Guedes E.M.S. Loss of ammonia from nitrogen fertilizers applied to maize and soybean straw. R Bras Ci Solo. 2013;37(4):969–975. [Google Scholar]
- 51.Stafanato J.B., Goulart R.S., Zonta E., Lima E., Mazur N., Pereira C.G. Volatilização de amônia oriunda de ureia pastilhada com micronutrientes em ambiente controlado. R Bras Ci Solo. 2013;37(3):726–732. [Google Scholar]
- 52.Cancellier E.L., Silva D.R.G., Faquin V., Gonçalves B.A., Cancellier L.L., Spehar C.R. Ammonia volatilization from enhanced-efficiency urea on no-till maize in brazilian cerrado with improved soil fertility. Ciênc Agrotec. 2016;40(2):133–144. [Google Scholar]
- 53.Guimarães G.G.F., Mulvaney R.L., Cantarutti R.B., Teixeira B.C., Vergütz L. Value of copper, zinc, and oxidized charcoal for increasing forage efficiency of urea N uptake. Agric Ecosyst Environ. 2016;224:157–165. [Google Scholar]
- 54.Khariri R.A., Yusop M.K., Musa M.H., Hussin A. Laboratory evaluation of metal elements urease inhibitor and DMPP nitrification inhibitor on nitrogenous gas losses in selected rice soils. Water Air Soil Pollut. 2016;232:1–14. [Google Scholar]
- 55.Margon A, Parente G, Piantanida M, Cantone P, Leita L. Novel investigation on ammonium thiosulphate (ATS) as an inhibitor of soil urease and nitrification. AS 2015;6(12):1502–12.
- 56.Grant C.A. Use of NBPT and ammonium thiosulphate as urease inhibitors with varying surface placement of urea and urea ammonium nitrate in production of hard red spring wheat under reduced tillage management. Can J Plant Sci. 2014;94(2):329–335. [Google Scholar]
- 57.Ponnuvel S., Subramanian B., Ponnuraj K. Conformational change results in loss of enzymatic activity of jack bean urease on its interaction with silver nanoparticle. Protein J. 2015;34(5):329–337. doi: 10.1007/s10930-015-9627-9. [DOI] [PubMed] [Google Scholar]
- 58.Ara R., Ashiq U., Mahroof-Tahir M., Maqsood Z.T., Khan K.M., Lodhi M.A. Chemistry, urease inhibition, and phytotoxic studies of binuclear vanadium (IV) complexes. Chem Biodivers. 2007;4(1):58–71. doi: 10.1002/cbdv.200790007. [DOI] [PubMed] [Google Scholar]
- 59.Paiva D.M.D., Cantarutti R.B., Guimarães G.G.F., Silva I.R.D. Urea coated with oxidized charcoal reduces ammonia volatilization. Rev Bras Cienc Solo. 2012;36(4):1221–1230. [Google Scholar]
- 60.Guimarães G.G., Paiva D.M., Cantarutti R.B., Mattiello E.M., Reis E.L. Volatilization of ammonia originating from urea treated with oxidized charcoal. J Braz Chem Soc. 2015;26(9):1928–1935. [Google Scholar]
- 61.Guimarães G.G., Mulvaney R.L., Khan S.A., Cantarutti R.B., Silva A.M. Comparison of urease inhibitor N-(n-butyl) thiophosphoric triamide and oxidized charcoal for conserving urea-N in soil. J Plant Nutr Soil Sci. 2016;179(4):520–528. [Google Scholar]