Abstract
N-methyl d-aspartate receptors (NMDARs), a subtype of glutamate receptor, have important functional roles in cellular activity and neuronal development. They are well-studied in rodent and adult human brains, but limited information is available about their distribution in the human fetal cerebral cortex. Here we show that 3 NMDAR subunits, NR1, NR2A, and NR2B, are expressed in the human cerebral cortex during the second trimester of gestation, a period of intense neurogenesis and synaptogenesis. With increasing fetal age, expression of the NMDAR-encoding genes Grin1 (NR1) and Grin2a (NR2A) increased while Grin2b (NR2B) expression decreased. The protein levels of all 3 subunits paralleled the changes in gene expression. On cryosections, all 3 subunits were expressed in proliferative ventricular and subventricular zones, in radial glia, and in intermediate progenitor cells, consistent with their role in the proliferation of cortical progenitor cells and in the determination of their respective fates. The detection of NR1, NR2A, and NR2B in both glutamatergic and GABAergic neurons of the cortical plate suggests the involvement of NMDARs in the maturation of human cortical neurons and in early synapse formation. Our results and previous studies in rodents suggest that NMDAR expression in the developing human brain is evolutionarily conserved.
Keywords: human fetal development, glutamatergic receptors, immunohistochemistry, in situ hybridization
Introduction
N-methyl d-aspartate receptors (NMDARs) are ligand- and voltage-gated ionotropic glutamate receptors that play critical roles in synaptic plasticity, development, learning, and memory. Impairments in their function underlie the pathophysiology of several neurological diseases, including schizophrenia, autism spectrum disorder, and epilepsy (Malenka and Nicoll 1993; Naegele 2009; Endele et al. 2010; Wang et al. 2012; Sanz-Clemente et al. 2012, Cohen et al. 2015). In the developing brain, functional NMDARs are present even before synapses are established, to allow the influx of calcium (Ca2+) necessary for cellular activities such as signal transduction, gene transcription, and neuronal maturation (Pearce et al. 1987; Ben-Ari et al. 1988; Brewer and Cotman 1989; reviewed in Spitzer 2006; Jansson and Åkerman 2014). Physiologically relevant interactions between corticogenesis and NMDAR function have been demonstrated in several studies (LoTurco et al. 1991, 1995; Haydar et al. 2000). The early expression of NMDARs in the ventricular and subventricular zones (VZ/SVZ) is a prerequisite for the proliferation of neural stem cells, their differentiation into neurons and glia, and their proper migration through NMDAR-mediated Ca2+ transients (Rakic and Komuro 1995; Weissman et al. 2004; Manent et al. 2005; Toriumi et al. 2012).
NMDARs are composed of 4 subunits, 2 NR1 and 2 NR2 or NR3 subunits, with NR1 being the obligatory subunit (Cull-Candy et al. 2001). In the developing rodent, NMDAR subunits undergo major changes in their expression levels, reflecting the specific physiological function of each receptor isoform in cell proliferation, synaptogenesis, and activity-dependent remodeling (Bear et al. 1987, Kleinschmidt et al. 1987, Constantine-Paton et al. 1990, Monyer et al. 1994; Kitayama et al. 2003; Yoshizimu and Chaki 2004; Dumas 2005; Sanz-Clemente et al. 2012). Additional studies in rodents have shown age-related shifts in the expression levels of the NMDAR subunits, with the expression of NR2A increasing and that of NR2B decreasing with age (Barria and Malinow 2002, 2005; Rodenas-Ruano et al. 2012; Sanz-Clemente et al. 2012). This shift has functional significance with respect to development of the cortical circuitry and cortical function (Dumas 2005).
To better understand the functional role of NMDARs in human corticogenesis, it is essential to first study their expression pattern in the developing cerebral cortex. NMDARs distribution in the human fetal cortex has been explored in much less detail than in animal models. In humans, NMDARs distribution has been examined in late gestational and neonatal ages and in the adult brain (Conti et al. 1999; Law et al. 2003; Suzuki et al. 2006; Henson et al. 2008; Jantzie et al. 2015). The focus of the present study was the first half of gestation (10–24 gestational weeks [gw]), as in the human fetal cortex this is a critical period for neurogenesis, migration, and the beginning of synaptogenesis (Kostovic and Rakic 1990; Zecevic 1998; Rakic 2009; Marín-Padilla 1998; Zecevic et al. 1999; Meyer et al. 2000; Bayatti et al. 2008; Clowry et al. 2010; Malik et al. 2013). Using different methods, we demonstrated that NMDARs subunits are expressed by cortical progenitor cells and by young neurons. This cell-type specific distribution suggests roles for NMDARs in the proliferation and fate determination of cortical progenitor cells, and in maturation and synaptogenesis of young cortical neurons during human corticogenesis.
Material and Methods
Human Fetal Brain Tissue
Human brain tissue (n = 11) from fetuses aged 10–24 gw (Table 1) and free of any developmental abnormalities was obtained from the Tissue Repository of The Albert Einstein College of Medicine (Bronx, NY) and the Human Developmental Biology Resource (http://hdbr.org), Newcastle upon Tyne, England. The tissues were handled according to the requirements and regulations of Institutional Ethics Committees. The age of the tissues was determined based on crown-rump length, number of weeks after donor ovulation, and anatomical landmarks. The tissues were transported on ice in Hank's balanced salt solution (HBSS; Life Technologies, Grand Island, NY, USA) from the aforementioned brain repositories to our laboratory. Blocks of tissue cut in a coronal plane at the level of the thalamus were fixed, and cryopreserved, for in situ hybridization and immunohistochemistry. In addition small samples (1cm2) of unfixed tissue were taken from the whole width of dorsal and medial telencephalic wall for Western blot and qPCR. Whenever possible we used multiple methods on the same case, but not all the fetal ages or regions were available for every method used in this study. This is specified in Table 1 and in the Results.
Table 1.
Description of human fetal tissue and methods used
| Case number | Gestational week | Method used |
|---|---|---|
| 1 | 10 | In situ hybridization (ISH) |
| 2 | 15 | qPCR, Western blot |
| 3 | 16 | Immunohistochemistry, ISH |
| 4 | 17 | qPCR, Western blot, Immunohistochemistry, ISH |
| 5 | 17 | qPCR, Western blot, Immunohistochemistry, ISH |
| 6 | 18 | qPCR, Western blot, Immunohistochemistry |
| 7 | 19 | qPCR, Western blot, Immunocytochemistry, ISH |
| 8 | 21 | qPCR, Western blot, Immunocytochemistry, ISH |
| 9 | 22 | qPCR, Western blot, Immunocytochemistry, ISH |
| 10 | 23 | qPCR, Western blot, Immunocytochemistry, ISH |
| 11 | 24 | qPCR, Western blot, Immunocytochemistry, ISH |
Immunohistochemistry
Cryopreserved coronal sections (20 μm) of the midgestational human fetal brain (Table 1) were dried at 37°C for 3 h and then incubated in citrate buffer (pH 9.0) at 80°C for 7 min for antigen retrieval, placed in a humidifying chamber, and washed with phosphate-buffered saline containing 0.01% Triton X-100 (PBS-T). Unspecific antibody binding was inhibited by incubating the sections in a blocking solution consisting of 10% normal goat serum in PBS-T (NGS-PBS-T) for 1 h at room temperature (RT). Primary antibodies (Table 2) were diluted in 1% NGS-PBS-T. The sections were treated with primary antibody diluted in blocking solution, and incubated at 4°C overnight. After three 5-min washes with PBS, the sections were incubated at RT for 2 h with flourophore-conjugated secondary antibodies diluted in PBS. After a second PBS washing step (3 × 5 min each), the sections were incubated with the nuclear stain bisbenzimide (BB) for 1 min at RT.
Table 2.
Antibodies used for immunocytochemistry and Western blotting
| Primary Antibody | Cell type identified | Species | Dilution | Company |
|---|---|---|---|---|
| NR1 | – | Mouse | 1:200 | NeuroMab |
| NR2A | – | Mouse | 1:200 | NeuroMab |
| NR2B | – | Mouse | 1:200 | NeuroMab |
| Hopx | Basal RGC | Rabbit | 1:200 | Santa Cruz |
| Ki67 | Proliferating cells | Rabbit | 1:500 | Abcam |
| Pax6 | RGC | Rabbit | 1:250 | Abcam |
| Tbr2 | Intermediate progenitor | Rabbit | 1:200 | Abcam |
| Nkx2.1 | Interneuron progenitor | Rabbit | 1:200 | Abcam |
| βIII tubulin | Neuron | Rabbit | 1:5000 | Sigma-Aldrich |
| Tbr1 | Glutamatergic neuron | Rabbit | 1:200 | Proteintech |
| GABA | Interneuron | Rabbit | 1:500 | Sigma-Aldrich |
| CalR | Interneuron | Rabbit | 1:500 | Swant |
| GAPDH | Housekeeping | Mouse | 1:5000 | Millipore |
Immunohistochemistry Image Analysis
Immunolabeled sections were visualized using an Axioscope microscope (Zeiss, Germany) with Axiovision software and photographed using a digital camera. Twelve images were taken from predesignated adjacent optical fields in dorsal or dorso-medial portion of the telencephalic wall and from 3 different human samples per experimental group for cell counting. Optical sectioning (in steps of 1 μm thickness) was done on 20 μm cryosections to clearly observe the co-localization of the immunostaining. The images were compressed in the Z-plane to obtain maximum intensity projection images, and assembled in Adobe Photoshop (v. 7.0), with consistent quality adjustments for contrast, brightness, and color balance. Immunolabeled cells were counted in separate channels corresponding to each antibody. Cells that showed immunoreaction for both applied antibodies were quantified as a percentage of the total cell number in the optical fields labeled with the nuclear dye BB.
One-way ANOVA followed by a Bonferroni post hoc test was used for comparisons of the 3 groups categorized by age.
Western Blot
Human cortical tissue (Table 1), was homogenized in hypotonic phosphate buffer containing protease and phosphatase inhibitors (PMSF [1 mM], NaF [5 mM], Na-orthovanadate [1 mM], PIC [1 mM; Thermo Scientific]), freeze-thawed, and centrifuged at 600 × g. The supernatant was mixed with RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% Na-deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) containing protease and phosphatase inhibitors (same as above), freeze-thawed, and centrifuged at 13 000 × g for 15 min and the resulting supernatant was collected. To obtain membrane proteins the pellet was re-suspended in RIPA buffer containing protease and phosphatase inhibitors, freeze-thawed, and then centrifuged at 10 000 × g for 15 min and the resulting supernatant was used for experimental analysis. The protein concentration of the samples was determined using the BCA protein assay kit (Thermo Fisher Scientific). Polyacrylamide gels (4–12%; Bio-Rad) were then used to separate the proteins based on their molecular mass at 110 V for 90 min. Samples were run, on 3 separate gels, to obtain results in triplicates. The separated proteins were electro-transferred onto a polyvinylidene fluoride membrane at 100 V for 60 min. A Ponceau stain on every blot, and a Coomassie stain for every gel was performed to confirm complete transfer of separated proteins. The membrane (blot) was blocked with 5% milk in TBS-T (1.0% Tween-20, PBS, 0.1% Triton X-100, distilled water; pH 7.4) and then incubated overnight at 4°C with primary antibodies (Table 2) diluted in blocking solution (0.1 M Tris, 2% nonfat dry milk, 0.15 M NaCl, 0.01% Na-azide, pH 7.4) against the proteins of interest. After 3 washes, the blot was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Millipore) for 2 h at RT. After 3 washes, blots were incubated with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific), and imaged on ChemiDoc MP (Bio-Rad) digital imaging system. The loading control for each blot was GAPDH (primary antibody dilution 1:5000). The density of each band was determined in Adobe Photoshop (v.7.0) using histogram analysis. The values obtained for each band were divided by the value of the corresponding GAPDH band. The averaged values obtained from groups 18–21 gw and 22–24 gw were normalized to the group 15–17 gw to overcome individual differences between tissue samples.
RT-PCR and qPCR
Human cerebral cortex tissue (15–24 gw, Table 1), dissected as described above, was used for RNA isolation and qPCR analysis. The tissue was treated with ice-cold TRIZOL® reagent (Invitrogen) for 10 min followed by mechanical homogenization. After the addition of chloroform (200 μl) to the homogenates, the tubes were vortexed and incubated for 15 min at 4°C. They were then centrifuged at 13 000 × g for 15 min at 4°C. The upper transparent phase was collected in sterile tubes and incubated with isopropanol (500 μl/ tube) for 20 min at RT, followed by centrifugation at 13 000 × g for 15 min at 4°C. The pellets were incubated with 75% ethanol at 4°C and then centrifuged at 13 000 × g for 15 min to pellet the RNA. The air-dried RNA was dissolved in 5% RNase Out solution. RNA concentrations were determined using NanoDrop technology (260/280 ratio obtained was between 1.93 and 2.0). cDNAs (40 μl) were synthesized from 1-μg RNA samples using reverse transcriptase-PCR and the first-strand synthesis SuperScript III kit (Invitrogen). cDNA concentrations were determined using NanoDrop technology with 260/280 ratios between 1.79 and 1.81. We pooled aliquots of all the cDNA samples to generate a standard curve (samples run in triplicates on the same plate) with the log (DNA copy#) plotted on the y-axis and the cycle of threshold (Ct) value plotted on the x-axis, to determine the efficiency of the qPCR protocol. The slope determined from the standard curve was used to calculate the efficiency of the qPCR process. The SYBR Green (Applied Biosystems, Cheshire, UK) protocol was applied to the cDNA samples to analyze the mRNA expression levels of the genes of interest. Gene specific primers are presented in Table 3. The qPCR protocol involved a heating step at 95°C for 2 min followed by 40 cycles at 95°C for 15 s, 55°C for 15 s, and 68°C for 20 s. The cycle of threshold (Ct) values of GAPDH were subtracted from those of the genes of interest to obtain a ΔCt value. The ΔCt values were averaged for each age group. The ΔCt values of groups 18–21 gw and 22–24 gw were then subtracted from the value of the age group 15–17 gw to obtain the ΔΔCt value. The formula 2−ΔΔCt was used to determine the fold change in mRNA expression level in group 18–21 gw and 22–24 gw relative to the group 15–17 gw, which was assigned an arbitrary value of 1. This normalization aids in dealing with the variations between the qualities of the original tissues used to generate the cDNA samples.
Table 3.
Primers used for qPCR
| Genes | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| Grin1 | 5′-CCA GTC AAG AAG GTG ATC TGC AC-3′ | 5′-TTC ATG GTC CGT GCC AGC TTG A-3′ |
| Grin2a | 5′-CTC TCC TTG GAA GAG GCA GAT C-3′ | 5′-TGG CTG CTC ATC ACC TCG TTC T-3′ |
| Grin2b | 5′-TTC CAC TGG CTA TGG CAT TGC C-3′ | 5′-GAC AAA TGC CAG TGA GCC AGA G-3′ |
| Gapdh | 5′-ACC ACC ATG GAG AAG GC-3′ | 5′-GGC ATG GAC TGT GGT CAT GA-3′ |
In Situ Hybridization
The genes Grin1, Grin2a, and Grin2b encode the NMDAR subunits NR1, NR2A, and NR2B, respectively. Plasmids containing the Grin1, Grin2a, and Grin2b coding sequences (pEYFP-NR1a, pEGFP-NR2A, and pEGFP-NR2B, respectively) were used for probe synthesis (Luo et al. 2002). The plasmids pEGFP-NR1a (Addgene plasmid #17926), pEGFP-NR2A (Addgene plasmid #17924), and pEGFP-NR2B (Addgene plasmid #17925) were gifts from Stefano Vicini (Department of Pharmacology & Physiology, Georgetown University Medical Center, Washington DC). Riboprobes were generated by in vitro transcription of a PCR fragment (primer sequences in Table 4) containing part of the gene sequence flanked by T3/SP6 promoter sequences, using digoxigenin (DIG)-UTP (Roche) as the label. In situ hybridization was performed as previously described (Radonjić et al. 2014). Briefly, cryosections (20 μm) were dried at RT for 2 h, fixed for 10 min with 4% paraformaldehyde in PBS, washed twice in diethyl pyrocarbonate (DEPC)-treated PBS, and incubated overnight at 68°C in hybridization buffer containing 1× DEPC-treated salts (200 mM NaCl, 5 mM EDTA, 10 mM Tris, pH 7.5, 5 mM NaH2PO4·2H2O, 5 mM Na2HPO4; Sigma-Aldrich), 50% deionized formamide (Roche), 0.1 mg RNase-free yeast tRNA (Invitrogen, Carlsbad, CA, USA)/mL, ×1 Denhardts (RNase/DNase free; Invitrogen), and 10% dextran sulfate (Sigma-Aldrich) and 100–500 ng DIG-labeled RNA probe/ml. After hybridization, the sections were washed 3 times at 65°C in a solution containing 50% formamide, ×1 saline-sodium citrate (Invitrogen), and 0.1% Tween 20 (Sigma-Aldrich) and 3 times at RT in ×1 MABT (20 mM maleic acid, 30 mM NaCl, 0.1% Tween 20; Sigma-Aldrich). They were then incubated for 1 h at RT in a solution containing 2% blocking reagent (11096176001; Roche) and 10% heat-inactivated sheep serum in MABT, followed by an overnight incubation at 4°C in alkaline-phosphatase-conjugated anti-DIG antibody (1:1500; Roche Applied Science, catalogue no. 11093274910 RRID:AB_514497). The specificity of the procedure was assessed with probes corresponding to the sense strands of Grin1, Grin2a, and Grin2b. The whole section pictures (×4 magnification) were done on the Aperio Versa (LeicaBiosystems.com), following manufactures instructions.
Table 4.
Primers used for riboprobe synthesis
| Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| Grin1 with SP6 | 5′-CCG ATT TAG GTG ACA CTA TAG GAC TGA-3′ | – |
| Grin1 with T3 | – | 5′-CCG GCA ATT AAC CCT CAC TAA AGG CGT AGA TAA ACT TGT CCG AGG G-3′ |
| Grin2a with T3 | 5′-GCA ATT AAC CCT CAC TAA AGG TCT CCC TGG TGA CCA CTA TCT T-3′ | – |
| Grin2a with SP6 | – | 5′-ATT TAG GTG ACA CTA TAG TGA TAG ACC ACT TCA CCG ATC A-3′ |
| Grin2b with T3 | 5′- GCA ATT AAC CCT CAC TAA AGG AGT TCA ACC AGA GGG GTG TGA A-3′ | – |
| Grin2b with SP6 | – | 5′-ATT TAG GTG ACA CTA TAG GGT GGG TTG TCA CAG TCG TAG-3′ |
Statistical Analysis
For all Western Blot and qPCR experiments, triplicate values were averaged to obtain a mean value for each brain. Three groups, each consisting of at least 3 brains, were established according to the gestational age: 15–17, 18–21, and 22–24 gw. The means obtained from each brain in a group were averaged to obtain a group mean. Variations were expressed as the mean ± standard error (SEM). One-way ANOVA followed by Bonferronis post hoc test was used to evaluate statistical significance, defined as a P < 0.05.
Results
NMDARs are Expressed in the Human Fetal Cerebral Cortex
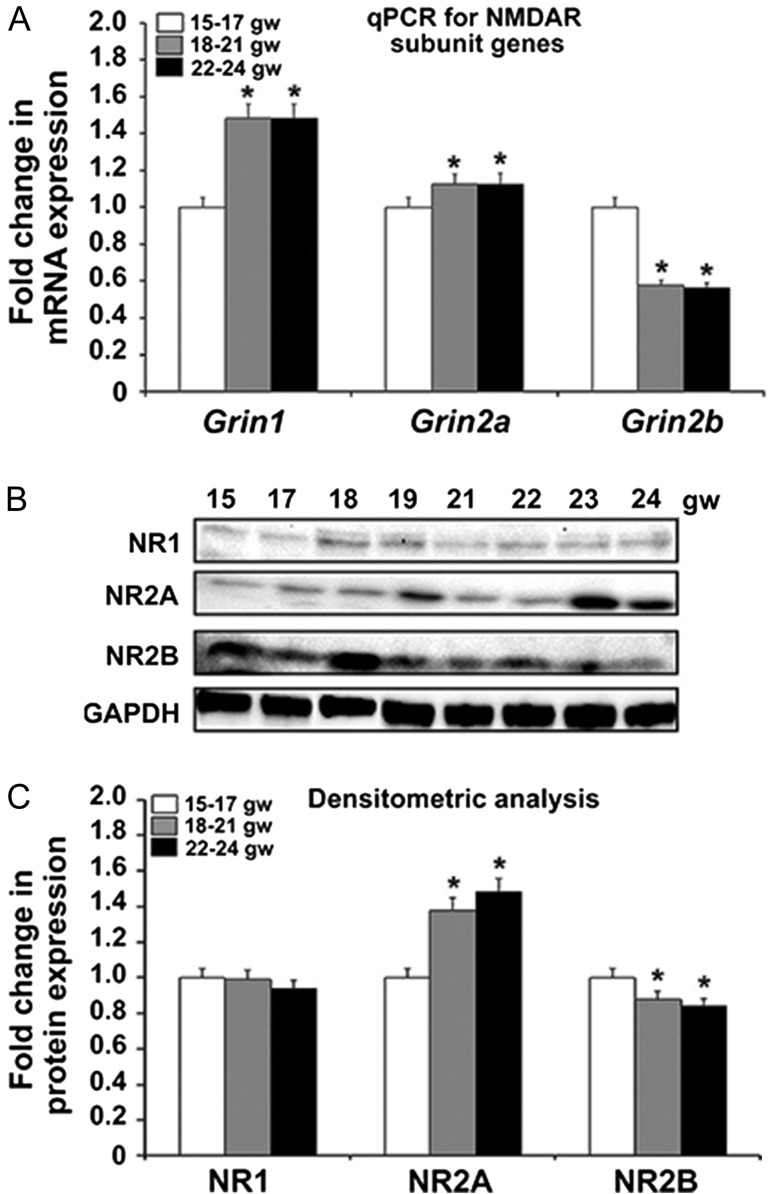
The quantitative expression of the genes encoding the major NMDAR subunits, NR1, NR2A, and NR2B, was assessed by qPCR and Western blot analysis in 3 age groups—15–17, 18–21, and 22–24 gw. Our results demonstrate that the expression of Grin1, gene encoding NR1 subunit, increases with age (P < 0.05; Fig. 1A). The same trend was observed in expression of the gene encoding the NR2A subunit, Grin2a (Fig. 1A). In contrast, expression of the NR2B gene, Grin2b, decreased with increasing age studied here (P < 0.05; Fig. 1A). To determine whether these changes in gene expression also occurred at the protein level, tissue lysates obtained from human fetal cerebral cortex of the same gestational ages were analyzed by Western blotting (Fig. 1B). Expression of NR1 protein between all 3 age groups was fairly uniform (Fig. 1B,C). We hypothesize that the discrepancy between the NR1 gene and protein expression is due to the high rate of Grin1 transcription accompanied by a high turnover rate of the NR1 transcript. At the same time, translation of the NR1 transcript is dependent on the cellular activity (reviewed in Paoletti et al. 2013). The expression of NR2A protein increased across the 3 age groups while that of NR2B decreased across the 3 age groups studied (Fig. 1C) (P < 0.05), congruent with qPCR results. Although the relatively short developmental time span studied here does not allow firm conclusions about the timing of the developmental switch from NR2B to NR2A in human cerebral cortex, our results suggest that it begins during midgestation (22–24 gw).
Figure 1.
The 3 NMDAR subunits, NR1, NR2A, and NR2B, are expressed in human fetal brain at midgestation. (A) Expression of the genes Grin1 and Grin2a increases with age, while that of Grin2b decreases from the youngest group 15–17 gw to the oldest group 22–24 gw (n = 3 per age group; P < 0.05). The mean values are plotted; the error bars represent ± the SEM. (B) Western blot of the NR1, NR2A, and NR2B subunits across all ages studied. GAPDH is the loading control. (C) Densitometric analysis confirms that NR1 protein expression does not change with age, while NR2A and NR2B protein levels parallel the trend in the expression of their respective genes, with an age-dependent increase in NR2A and decrease in NR2B (n = 3 per age group; P < 0.05). The mean values are plotted; the error bars represent ± the SEM.
Distribution of NMDAR mRNAs and Proteins in the Human Cerebral Wall
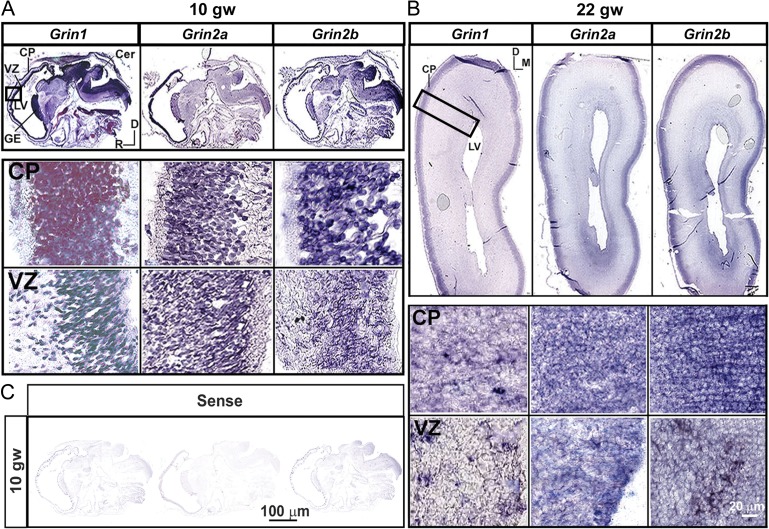
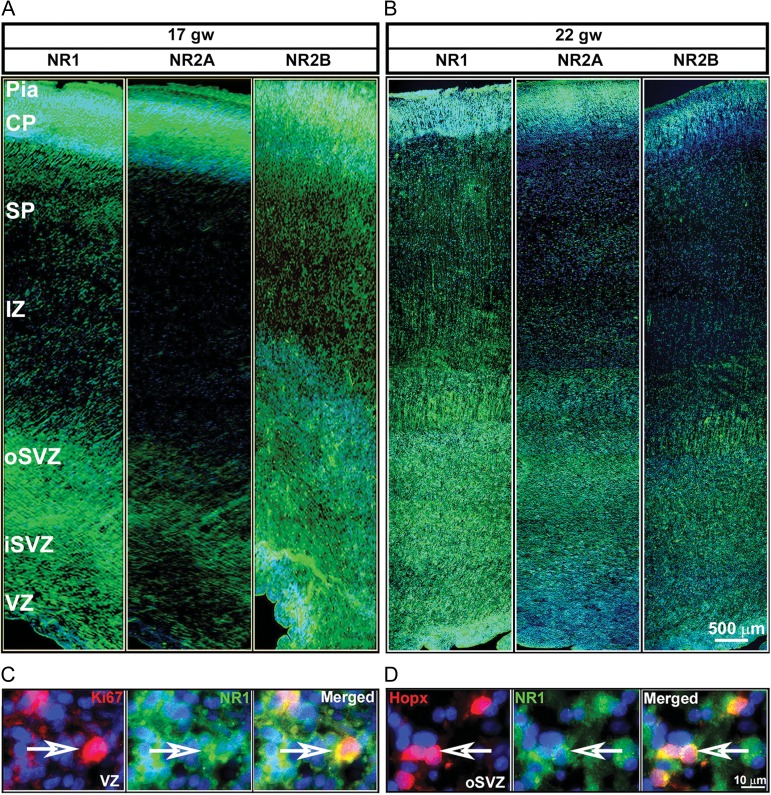
The distribution of the NR1, NR2A, and NR2B transcripts were examined in sagittal (10gw) and coronal cryosections (Fig. 2A,B), while the NR1, NR2A, and NR2B proteins were examined on coronal cryosections (Fig. 3A,B) of fetal brains taken at the level of the thalamus (16–24gw, Table 1). In situ hybridization experiments revealed that the mRNAs of all 3 subunits were distributed in the developing cortex as early as 10 gw (Fig. 2A) and were also present during midgestation, at 17–22 gw (Fig. 2B). High levels of expression were seen in the proliferative VZ/SVZ compartments, in the transient migratory zones, the intermediate zone (IZ) and the subplate (SP), and in the neuron-rich cortical plate (CP). High magnification images of the VZ and CP (Fig. 2A,B) at 10 and 22 gw indicates that the transcripts of the NMDAR subunits, exhibiting both a punctate and diffuse pattern of expression, are present in almost every cell. These findings suggest that both cortical progenitors and neurons actively transcribe the NMDAR subunit genes during neurogenesis. Immunolabeling with NMDAR subunit-specific antibodies revealed the presence of NR1, NR2A, and NR2B in the cell bodies and processes across the telencephalic wall at all examined ages, as shown in representative samples of 17 gw (Fig. 3A), and 22 gw (Fig. 3B). The immunoreactivity has a transverse pattern along the VZ and SVZ suggestive of the expression of NMDAR subunits along efferent and afferent fibers/processes, as expression of NMDARs was reported at both the presynaptic and postsynaptic sites (Herkert et al. 1998). In the SP and CP, the immunoreactivity for the NMDAR subunits exhibits a radial pattern that corresponds to the radial glial fibers and organization of cell bodies in the CP. The highest levels of these proteins were detected in the same regions containing the highest levels of their respective transcripts, namely, in the VZ/SVZ, and CP. Proliferating cells (Ki67+ cells) in the VZ expressed the NR1 subunit (Fig. 3C) as well as the NR2A and NR2B subunits (data not shown). Moreover, primate-specific basal radial glial cells (RGCs), recognized by their expression of the transcription factor Hopx (Li et al. 2015; Pollen et al. 2015; Thomsen et al. 2016), also expressed NR1 (Fig. 3D) as well as NR2A and NR2B (data not shown).
Figure 2.
Distributions of NR1, NR2A, and NR2B transcripts in the telencephalic wall. In situ hybridization reveals the presence of Grin1, Grin2a, and Grin2b mRNAs in the proliferative ventricular zone (VZ) and neuron-rich cortical plate (CP) as early as (A) 10 gw (sagittal section) and (B) at 22 gw (coronal section). Higher magnifications of the CP and VZ are shown in the insets. (C) Sense (control) probes for Grin1, Grin2a, and Grin2b shown for 10 gw. D-dorsal, M-medial, R-rostral. The whole sections images are captured by Aperio Versa (LeicaBiosystems.com), at ×4.
Figure 3.
Immunohistochemistry of NR1, NR2A, and NR2B (green) along the various zones of the telencephalic wall of a representative case at 17 gw (A) and 22 gw (B). Greater immunolabeling intensity is seen for NR2A at 22 gw compared with 17gw, whereas the opposite is seen for NR2B subunit. (C) NR1 (green) expression on proliferative progenitors (Ki67+; red) in the VZ and (D) on basal radial glia (Hopx+; red) in the SVZ. Arrows point to double-labeled cells. Cell nuclei stained blue with bisbenzimide (BB).
NMDAR Expression by Cortical Progenitor Subtypes
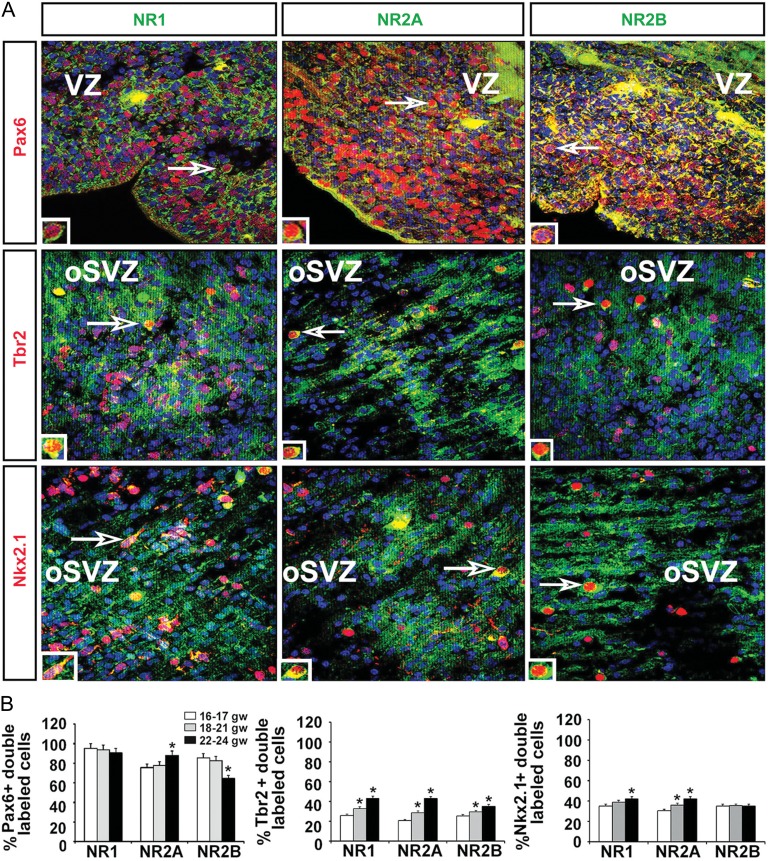
The abundant expression of NR1, NR2A, and NR2B mRNA and protein in the proliferative and neuron-rich zones of the fetal brain suggested that the 3 subunits would be expressed by multipotent cortical progenitors, RGCs, intermediate progenitors, and cortical neurons. To detect expression in particular cell types at these sites, coronal cryosections of fetal forebrains at different gestational ages were evaluated using double-labeling immunohistochemistry to co-localize cell-type specific markers and each of the NMDAR subunits (Fig. 4A; Table 2). Quantification of the double-labeled cells showed that the vast majority of RGCs labeled with the Pax6 antibody expressed all 3 NMDAR subunits. The NR1 subunit was present in 90–95% of the RGCs in all age groups studied. In RGCs of the youngest age group (16–18 gw, n = 3), the expression of NR2B was higher than that of NR2A (85% vs. 75%), whereas in subsequent gestational weeks (18–21 gw, n = 3) expression of these 2 subunits was nearly equal. As development proceeded to 22–24 gw (n = 3), however, a higher percentage of RGCs expressed NR2A (88%) than NR2B (64%) (Fig. 4B). Thus, during an 8-week period in the second trimester of gestation the expression of NR2B in RGCs decreased while that of NR2A increased. Quantification of intermediate progenitor cells labeled with Tbr2 and a subtype of interneuron progenitors labeled with Nkx2.1 that express NR1 showed a similar age-dependent increase (Fig. 4B). Specifically, NR1 was expressed in 25% of Tbr2+ cells and 35% of Nkx2.1+ cells, with expression during the subsequent weeks of gestation increasing to >40% in both subtypes. These results suggested an age-dependent increase in NR1 and NR2A expression in intermediate progenitors (Tbr2+) and Nkx2.1+ interneuron progenitors. However, in contrast to RGCs, NR2B expression in the oldest group did not change in Nkx2.1+ cells and it increased only slightly in Tbr2+ progenitors (Fig. 4B).
Figure 4.
Expression of NMDAR subunits on cortical progenitors. (A) Representative images of coronal cryosections at 17 gw. Double immunolabeling experiments with antibodies against the NR1, NR2A, and NR2B NMDAR subunits (green) and the progenitor subtypes (red) Pax6 (RGCs), Tbr2 (intermediate progenitors) and Nkx2.1 (interneuron progenitors) in the proliferative VZ/SVZ. Cell nuclei stained blue with BB. Boxed areas are presented on higher magnification in the inset. (B) Quantification of the percentage of Pax6+, Tbr2+, and Nkx2.1+ progenitors expressing the 3 NMDAR subunits in the 3 age groups (n = 3 per age group; P < 0.05); The mean values are plotted; the error bars represent ± the SEM.
Glutamatergic and GABAergic Neurons Express NMDARs
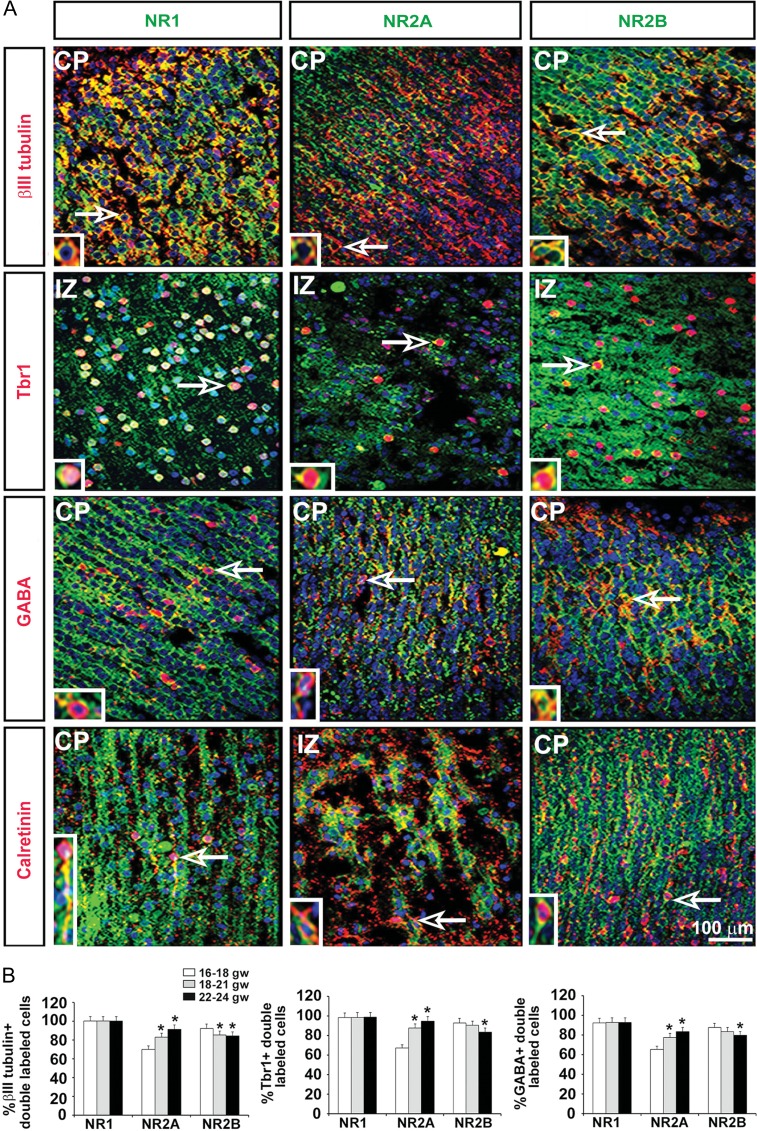
To determine the expression levels of the NMDAR subunits across postmitotic neurons in their final positions in the CP, immunolabeling experiments were carried out similar to those described above, but with antibodies specific to the different neuronal subtypes (Fig. 5A; Table 2). Double-labeling immunohistochemistry of tissues between 16 and 24 gw revealed that all embryonic neurons labeled with a pan-neuronal marker βIII tubulin expressed NR1, the obligatory NMDAR subunit (Fig. 5B). This is in agreement with results showing that mature neurons are electrically active and express NMDARs (Cull-Candy et al. 2001; Lu et al. 2001; Cull-Candy and Leszkiewicz 2004; Henson et al. 2008). The expression of NR2B and NR2A in cortical neurons (βIII tubulin+ cells), however, changed over the 8-week developmental period studied. In the youngest group (16–18 gw, n = 3), 92% of all neurons expressed NR2B whereas only 70% expressed NR2A. In the next studied stage, 18–21 gw brains (n = 3), almost equal number of neurons expressed NR2B (85%) and NR2A (83%). In the oldest group of brains studied (22–24 gw, n = 3), the expression of NR2A (91%) was higher than that of NR2B (84%) (Fig. 5B). These semiquantitative results on the neuronal expression of the 3 subunits were comparable to those obtained with the cortical RGC progenitors, in which an increase in the expression of NR2A versus NR2B was demonstrated.
Figure 5.
Expression of NMDAR subunits on cortical neurons. (A) Representative images at 17 gw. Double immunolabeling experiments with antibodies against the NR1, NR2A, and NR2B NMDAR subunits (green) and the neuronal subtypes (red) βIII tubulin (neurons), Tbr1 (glutamatergic), GABA (interneurons), and calretinin (interneuron subtype) in the cortical plate (CP) and the transient intermediate zone (IZ). Cell nuclei stained blue with BB. Arrows point to cells presented on higher magnification in the inset. (B) Quantification of the percentage of βIII tubulin+ neurons, Tbr1+ glutamatergic neurons, and GABA+ interneurons expressing the 3 NMDAR subunits in the 3 age groups studied (n = 3 per age group; P < 0.05). The mean values are plotted; the error bars represent ± the SEM.
We then quantified expression of the NMDAR subunits by Tbr1+ glutamatergic neurons and GABA+ interneurons. In all 3 age groups studied, nearly all Tbr1+ cells (98%) expressed the obligatory NR1 subunit, whereas with increasing age, expression of the NR2B subunit by these cells decreased (90–83%; P < 0.05) and that of the NR2A subunit increased (67–94%; P < 0.05) (Fig. 5B). Similarly, a majority of GABAergic cortical interneurons (92%), expressed NR1 across all studied ages (16–24 gw) and with increasing age, the number of NR2B-positive GABAergic cells decreased (83–79%; P < 0.05) whereas the number of NR2A-positive cells increased (77–83%; P < 0.05) (Fig. 5B).
These results revealed that all 3 subunits are expressed on glutamatergic and GABAergic neuronal subpopulations during the second trimester of human gestation, with a specific temporal distribution pattern determined for each one.
Discussion
This study demonstrated the expression of the major NMDAR subunits, NR1, NR2A, and NR2B, at both the mRNA and protein levels, in the human fetal cerebral cortex during the second trimester of gestation. It also showed that between 16 and 24 gw the subunits are expressed by specific cell types within the different layers of the telencephalic wall. These findings extend previous studies on human brain tissue obtained at older stages of fetal development and from the adult brain (Conti et al. 1999; Law et al. 2003; Suzuki et al. 2006; Henson et al. 2008; Jantzie et al. 2015). The early distribution of NR1, NR2A, and NR2B in the human cerebral cortex suggests their early and distinct roles in cortical progenitor proliferation and specification, and in the maturation and synaptogenesis of excitatory and inhibitory neurons in this brain region.
NMDAR in Cortical Progenitor Cells
From 16 gw onwards, all 3 NMDAR subunits were expressed on RGCs, the multipotent cortical progenitors in the VZ/SVZ. Both proliferating (Ki67+) RGCs in the VZ and basal RGCs (Hopx+) in the SVZ expressed NR1, NR2A, and NR2B. The 3 subunits were also expressed, although less abundantly, by cells in the next stage of RGC specification, that is, intermediate (Tbr2+) and interneuron (Nkx2.1+) progenitors. This suggests that glutamate is more important in the proliferation of RGCs than in their specification into a particular intermediate progenitor cell type. The different age-related patterning of expression of NR2B in RGC, intermediate and interneuron progenitors suggests a role of this subunit in the specification of cortical progenitors, while NR2A containing NMDARs might be important for further neuronal differentiation. However, we cannot exclude the possibility that technical difficulties related to simultaneous labeling of nuclear transcription factors (Tbr2, Nkx2.1), and membrane receptor proteins (such as NMDARs) affect the efficiency of the method.
The major excitatory neurotransmitter glutamate acts nonsynaptically as a trophic factor during the early stages of development (Haydar et al. 2000; Balasz 2006). In rodents, glutamate increases progenitor proliferation in the VZ and promotes neuronal differentiation in the SVZ by modulating the length of the cell cycle (LoTurco et al. 1991; Takahashi et al. 1996; Haydar et al. 2000). These effects of glutamate on cortical progenitors are exerted through both AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionate) receptors and NMDARs (LoTurco et al. 1995; Jansson and Åkerman 2014; Behar et al. 1999). The early expression of NMDARs influences both the proliferation and differentiation of neural stem cells and the correct migration of newly generated neurons (Rakic and Komuro 1995; Manent et al. 2005; Toriumi et al. 2012). Experiments in which the activity of the NMDAR subunits was pharmacologically blocked or their expression genetically knocked out confirmed the significance of these receptors in cortical development. In rodents, pharmacological inhibition of the 3 NMDAR subunits during development was shown to induce severe neurodegeneration followed by death (Ikonomidou et al. 1999; Reiprich et al. 2005), whereas homozygous knockouts of NR1 and NR2B are neonatally lethal (Ikeda et al. 1992; Forrest et al. 1994; Sakimura et al. 1995; Kutsuwada et al. 1996). Other studies in rodents have shown that NMDARs play a crucial role in maintaining the stemness of RGCs in the adult dentate gyrus, by regulating the proliferation of these cells; NMDARs also participate in the establishment of hippocampal circuits (Bernabeu and Sharp 2000; Deisseroth et al. 2004; Nacher and McEven 2006). In accordance with these observations, our recent in vitro study showed that antagonizing NMDARs on human fetal RGCs not only increases cell death but decreases progenitor specification and neurogenesis (Bagasrawala et al. in revision). These results provide strong evidence of the importance of NMDARs in the survival, proliferation, and differentiation of RGCs during human cortical development.
NMDAR Expression in Cortical Neurons
All the βIII-tubulin-labeled neurons in the CP at midgestation (16–24 gw) expressed NR1, and a subpopulation of neurons expressed NR2B and NR2A. Specifically, both Tbr1+ glutamatergic and GABAergic neurons expressed all 3 NMDAR subunits during the second trimester of human gestation. NMDAR presence in neurons of the emerging CP suggests a role for this receptor in neuronal maturation and the establishment of the early synapses that appear in the human cortex at that time/stage (Zecevic 1998; Zecevic et al. 1999; Kostović et al. 2002). Our previous electrophysiological studies, in which patch-clamp recordings were obtained from human fetal slice cultures, showed that glutamate acts through both AMPA receptors and NMDARs on Cajal–Retzius cells at midgestation (Lu et al. 2001). In another study, we demonstrated the presence of functional ionotropic glutamate receptors in human fetal cortical neurons in acute brain slices (Moore et al. 2011).
In the present work, we established that, in the oldest age group examined, the percentage of Tbr1+ neurons expressing NR2B and NR2A was slightly higher than the percentage of GABAergic cells expressing these subunits, compared with their respective percentages in the youngest age group. This finding suggests the functional differences or/and nonuniform maturation of cortical neurons at this developmental stage. The distribution of NMDARs in different subtypes of cortical neurons has clinical relevance, as the abnormal excitation of GABAergic interneurons due to NMDAR hypofunction results in cortical disinhibition and impairment of the synchronized activity of cortical projection neurons (Belforte et al. 2010; Nakazawa et al. 2012). In animal models, this disruption in cortical circuitry function leads to the development of behavioral deficits, such as anhedonia, disruption of the prepulse acoustic startle response, and anxiety, behaviors that closely resemble those observed in schizophrenia patients (Belforte et al. 2010). Furthermore, midgestation, when, as we determined here, all 3 NMDARs subunits are expressed by cortical neurons, is the period of upper cortical layer development, and the formation of the cortico-cortical connections necessary for establishing the higher cognitive functions that characterize humans (Rakic 1998; Hill and Walsh 2005). The disruption of cognitive processes is one of the major symptoms observed in complex neurodevelopmental disorders, such as schizophrenia and autism spectrum disorder (Hill and Walsh 2005; Nakazawa et al. 2012).
NMDAR Composition
The obligatory NR1 subunit was expressed in almost all of the investigated cell types. This was not surprising since the importance of this subunit in rodents is well-established. For example, the controlled ablation of NR1 during development was shown to disrupt the normal maturation of the cell circuitry in the hippocampus, resulting in a schizophrenia-like cognitive decline and behavioral deficits later in life (Rodenas-Ruano et al. 2012). Our finding of the age-dependent expression of subunits NR2A and NR2B in human fetal cortex is in line with the results of animal studies. Synaptic activity is one of the factors regulating NMDAR subunit expression (Yashiro and Philpot 2008; Lee et al. 2010; Sanz-Clemente et al. 2012). NR2B-rich NMDARs are found in abundance on developing synapses, where they generate slow currents with shorter amplitudes but lasting twice as long compared with NR2A-mediated currents. This allows for a large influx of Ca2+ that promotes gene transcription and extends the period of membrane depolarization, which is crucial for generating a strong and stable synapse (Sobczyk et al. 2005; Xu et al. 2009; Yasuda et al. 2011). At the beginning of cortical synaptogenesis, NR2B is needed for the formation of the synaptic connections essential for circuitry formation. Many of these synapses undergo pruning, a process that is dependent on environmental experiences, the nature of the stimulus, and age (Rakic et al. 1986; Dumas 2005; Sanz-Clemente et al. 2012; reviewed in Paoletti et al. 2013). In the adult brain, the strong circuitry connections established by NMDARs mainly containing the NR2B subunit ensure memory formation through long-term potentiation (Sanz-Clemente et al. 2010). Experiments using antagonists of the NR2A and NR2B subunits in monkey prefrontal cortical slices (Wang et al. 2011) showed that NR2B-type activity on postsynaptic spines participates in working memory and learning. Similar results were obtained in mice genetically modified to lack the NR2B subunit during their postnatal development (von Engelhardt et al. 2008). As corticogenesis in rodents proceeds into the second postnatal week, the NR2B subunits translocate to extrasynaptic sites and are replaced at the synapse by NR2A-rich NMDARs (Malenka and Nicoll 1999; Lavezzari et al. 2004; Dumas 2005; Tang et al. 2010). These synapses generate the fast currents required for strong and transient synaptic connections in response to sensory stimuli (Nakagawa et al. 1996; Paupard et al. 1997; Malenka and Nicoll 1999; Malatesta et al. 2000). Thus, in rats reared in the dark without appropriate sensory input, the NR2B-NR2A switch in the visual cortex is impaired, but the impairment can be reversed by exposing the animals to light (Philpot et al. 2001). During activity-dependent synaptic plasticity, NR2B-rich NMDARs move into synaptic sites from their previous extrasynaptic location via lateral diffusion (Tovar and Westbrook 2002; Groc et al. 2006). The timing of the switch coincides with the development of associative learning abilities, indicating the significance of this process in modifying and tweaking neuronal circuits (Dumas 2005; Bellone and Nicoll 2007; Sanz-Clemente et al. 2012). Notably, the change in NMDAR subunits is related to the shift from the greater plasticity that characterizes development to the greater stability that marks adult life (Bear et al. 1987; Kleinschmidt et al. 1987; Constantine-Patton et al. 1990; reviewed in Dumas 2005; Hall et al. 2007; Espinosa et al. 2009). The NR2B to NR2A switch in NMDAR subunit expression is conserved from frogs to mammals (Dumas 2005; reviewed in Paoletti et al. 2013), but it has not been widely studied in the developing human cerebral cortex (Scherzer et al. 1998; Henson et al. 2008; Jantzie et al. 2015).
We observed that in the human fetal cortex from 16 to 24 gw, the percentage of NR2B-expressing RGCs decreased from 92% to 84% (P < 0.05), while expression of the NR2A subunit increased from 70% to 91% (P < 0.05). A similar change occurred in cortical neurons of the same developmental period. These results suggest that in human cortical development the switch from NR2B to NR2A begins quite early, around 22–24 gw. A larger shift can be assumed to occur postnatally, especially between the juvenile and adult stages of development, when cognitive functions mature (Dumas 2005; Sanz-Clemente et al. 2012). A more comprehensive picture of the NR2B to NR2A switch in human fetal cortex requires the study of a broader range of brain tissues of different gestational ages as well as neonatal and adult tissue samples.
NMDARs play a major role in brain development, as evidenced by the fact that several neurodevelopmental disorders such as schizophrenia, autism, learning disabilities, epilepsy, and mental retardation, as well as autoimmune anti-NMDAR encephalitis, are thought to have a NMDAR-based pathophysiology. A number of studies have clearly shown that compromising the expression of the NMDAR subunits or manipulating their function during development can impair both the formation of neuronal circuits and their fine-tuning, which is essential in learning and working memory (Monyer et al. 1994; Dumas 2005; Groc et al. 2006; Takahashi et al. 2006; Kozela and Popik 2006; Naegele 2009; Liu et al. 2010; Arnsten et al. 2012). Taken together, our results show that changes in NMDAR subunit expression during midgestation could affect cortical development, including the maintenance of the balance between cortical excitation and inhibition. Further studies on NMDAR subunit composition in human cortical development will contribute to a better understanding of the role of these receptors in brain development and in the pathophysiology of neuropsychiatric diseases. Such studies can be expected to provide the basis for the discovery of novel therapeutics that specifically modulate NMDAR activity.
Notes
Conflict of Interest: None declared.
Funding
This work was supported by NIH grants 2R01NSO41489 and subcontract 5R01DA023999-07 (N.Z.). Human fetal tissue was procured from the Albert Einstein College of Medicine, Tissue Repository, Bronx, NY, USA, and the Joint MRC/Welcome Trust (Grant no. 099175/Z/12/Z), Human Developmental Biology Resource (http://hdbr.org), Newcastle upon Tyne, England.
References
- Arnsten AF, Wang MJ, Paspalas CD. 2012. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 76:223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasz R. 2006. Trophic effect of glutamate. Curr Top Med Chem. 6:961–968. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. 2002. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 35:345–353. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. 2005. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. 48:289–301. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JF, Lindsay S, Clowry GJ. 2008. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 18:1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Cooper LN, Ebner FF. 1987. A physiological basis for a theory of synapse modification. Science. 237:42–48. [DOI] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu Q, Colton CA, Barker JL. 1999. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci. 19:4449–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. 2010. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 13:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. 2007. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 55:779–785. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Kmjevic K. 1988. Changes in voltage de- pendence of NMDA currents during development. Neurosci Lett. 94:88–92. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Sharp FR. 2000. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 20:1669–80. [DOI] [PubMed] [Google Scholar]
- Brewer CL, Cotman CW. 1989. NMDA receptor regulation of neuronal morphology in cultured hippocampal neurons. Neurosci Lett. 99:268–273. [DOI] [PubMed] [Google Scholar]
- Clowry G, Molnár Z, Rakic P. 2010. Renewed focus on the developing human neocortex. J Anat. 217:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DC, Halassa MM. 2015. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 167:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. 1990. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 13:129–154. [DOI] [PubMed] [Google Scholar]
- Conti F, Barbaresi P, Melone M, Ducati A. 1999. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. 9:110–120. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. 2001. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 11:327–335. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. 2004. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 255:re16. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. 2004. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 42:535–552. [DOI] [PubMed] [Google Scholar]
- Dumas TC. 2005. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 76:189–211. [DOI] [PubMed] [Google Scholar]
- Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortüm F, Fritsch A, Pientka FK, et al. 2010. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. 42:1021–1026. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Wheeler DG, Tsien RW, Luo L. 2009. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 62:205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. 1994. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 13:325–338. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. 2006. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci USA. 103:18769–18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Ripley B, Ghosh A. 2007. NR2B signaling regulates the development of synaptic AMPA receptor current. J Neurosci. 27:13446–13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. 2000. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 20:5764–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. 2008. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex. 18:2560–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkert M, Rottger S, Becker CM. 1998. The NMDAR subunit NR2B of neonatal rat brain: complex formation and enrichment in axonal growth cones. Eur J Neurosci. 10:1553. [DOI] [PubMed] [Google Scholar]
- Hill RS, Walsh CA. 2005. Molecular insights into human brain evolution. Nature. 437:64–67. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. 1992. Cloning and expression of the ε4 subunit of the NMDA receptor channel. FEBS Lett. 313:34–38. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. 1999. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 283:70–74. [DOI] [PubMed] [Google Scholar]
- Jansson LC, Åkerman KE. 2014. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J Neural Transm (Vienna). 121:819–836. [DOI] [PubMed] [Google Scholar]
- Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE. 2015. Developmental expression of N-methyl-D-aspartate (NMDA) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain. Cereb Cortex. 25:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama T, Yoneyama M, Yoneda Y. 2003. Possible regulation by N-methyl-d-aspartate receptors of proliferative progenitor cells expressed in adult mouse hippocampal dentate gyrus. J Neurochem. 84:767–780. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W. 1987. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 238:355–358. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1990. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 297:441–470. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judas M, Rados M, Hrabac P. 2002. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 12:536–544. [DOI] [PubMed] [Google Scholar]
- Kozela E, Popik P. 2006. A complete analysis of NMDA receptor subunits in periaqueductal grey and ventromedial medulla of morphine tolerant mice. Drug Alcohol Depend. 86:290–293. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, et al. 1996. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 16:333–344. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. 2004. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 24:6383–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. 2003. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur J Neurosci. 18:1197–1205. [DOI] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. 2010. Metaplasticity at single glutamatergic synapses. Neuron. 66:859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Takeda N, Jain R, Manderfield LJ, Liu F, Li L, Anderson SA, Epstein JA. 2015. Hopx distinguishes hippocampal from lateral ventricle neural stem cells. Stem Cell Res. 15:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hunter C, Weiss HR, Chi OZ. 2010. Effects of blockade of ionotropic glutamate receptors on blood-brain barrier disruption in focal cerebral ischemia. Neurol Sci. 31:699–703. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Wanton MG, Kriegstein AR. 1991. Initial expression and endogenous activation of NMDA channels in early neocortical development. J Neurosci. 17:792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. 1995. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 15:1287–1298. [DOI] [PubMed] [Google Scholar]
- Lu S, Zecevic N, Yeh HH. 2001. Distinct NMDA and AMPA receptor-mediated responses in mouse and human Cajal-Retzius cells. J Neurophysiol. 86:2642–2646. [DOI] [PubMed] [Google Scholar]
- Luo JH, Fu ZY, Losi G, Kim BG, Prybylowski K, Vissel B, Vicini S. 2002. Functional expression of distinct NMDA channel subunits tagged with green fluorescent protein in hippocampal neurons in culture. Neuropharmacology. 42:306–18. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M. 2000. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 127:5253–5263. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. 1993. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 16:521–527. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. 1999. Long-term potentiation—a decade of progress? Science. 285:1870–1874. [DOI] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BBR, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. 2013. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 33:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. 2005. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 25:4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Padilla M. 1998. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 21:64–71. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schaaps J-P, Moreau L, Goffinet AM. 2000. Embryonic and early fetal development of the human neocortex. J Neurosci. 20:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. 1994. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 12:529–540. [DOI] [PubMed] [Google Scholar]
- Moore A, Zhou W, Jakovcevski I, Zecevic N, Antic S. 2011. Spontaneous electrical activity in the human fetal cortex in vitro. J Neurosci. 31:2391–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, McEwen BS. 2006. The role of N-methyl-d-asparate receptors in neurogenesis. Hippocampus. 16:267–270. [DOI] [PubMed] [Google Scholar]
- Naegele J. 2009. Epilepsy and the plastic mind. Epilepsy Curr. 6:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Watanabe M, Inoue Y. 1996. Altered gene expression of the N-methyl-d-aspartate receptor channel subunits in Purkinje cells of the staggerer mutant mouse. Eur J Neurosci. 8:2644–2651. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. 2012. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. 2013. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 14:383–400. [DOI] [PubMed] [Google Scholar]
- Paupard MC, Friedman LK, Zukin RS. 1997. Developmental regulation and cell-specific expression of N-methyl-d-aspartate receptor splice variants in rat hippocampus. Neuroscience. 79:399–409. [DOI] [PubMed] [Google Scholar]
- Pearce IA, Cambray-Deakin MA, Burgoyne RD. 1987. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 223:143–147. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. 2001. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 29:157–69. [DOI] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ, Oldham MC, Diaz A, et al. 2015. Molecular identity of human outer radial glia during cortical development. Cell. 163:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonjić NV, Memi F, Ortega JA, Glidden N, Zhan H, Zecevic N. 2014. The role of Sonic hedgehog in the specification of human cortical progenitors in vitro. Cereb Cortex. 26:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff ME, Zecevic N, Goldman-Rakic P. 1986. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 232:232–235. [DOI] [PubMed] [Google Scholar]
- Rakic P, Komuro H. 1995. The role of receptor/channel activity in neuronal cell migration. J Neurobiol. 26:299–315. [DOI] [PubMed] [Google Scholar]
- Rakic P. 1998. Young neurons for old brains? Nat Neurosci. 1:645–647. [DOI] [PubMed] [Google Scholar]
- Rakic P. 2009. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 10:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiprich P, Kilb W, Luhmann HJ. 2005. Neonatal NMDA receptor blockade disturbs neuronal migration in rat somatosensory cortex in vivo. Cereb Cortex. 15:349–358. [DOI] [PubMed] [Google Scholar]
- Rodenas-Ruano A, Chávez AE, Cossio MJ, Castillo PE, Zukin RS. 2012. REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci. 15:1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. 1995. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ε1 subunit. Nature. 373:151–155. [DOI] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. 2010. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 67:984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Nicoll RA, Roche KW. 2012. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscience. 19:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, Daggett LP, Veliçelebi G, Penney JB, Young AB. 1998. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. 390:75–90. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. 2005. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. 25:6037–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. 2006. Electrical activity in early neuronal development. Nature. 444:707–712. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. 2006. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 24:645–653. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS Jr. 1996. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 16:6183–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Kakita A, Futamura T, Watanabe Y, Mizuno M, Sakimura K, Castren E, Nabeshima T, Someya T, Nawa H. 2006. Sustained brain-derived neurotrophic factor up-regulation and sensorimotor gating abnormality induced by postnatal exposure to phencyclidine: comparison with adult treatment. J Neurochem. 99:770–780. [DOI] [PubMed] [Google Scholar]
- Tang TT, Badger JD 2nd, Roche PA, Roche KW. 2010. Novel approach to probe subunit-specific contributions to N-methyl-D-aspartate (NMDA) receptor trafficking reveals a dominant role for NR2B in receptor recycling. J Biol Chem. 285:20975–20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ER, Mich JK, Yao Z, Hodge RD, Doyle AM, Jang S, Shehata SI, Nelson AM, Shapovalova NV, Levi BP, et al. 2016. Fixed single-cell transcriptomic characterization of human radial glial diversity. Nat Methods. 13:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriumi K, Mouri A, Narusawa S, Aoyama Y, Ikawa N, Lu L, Nagai T, Mamiya T, Kim HC, Nabeshima T. 2012. Prenatal NMDA receptor antagonism impaired proliferation of neuronal progenitor, leading to fewer glutamatergic neurons in the prefrontal cortex. Neuropharmacology. 37:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. 2002. Mobile NMDA receptors at hippocampal synapses. Neuron. 34:255–264. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Doganci B, Jensen V, Hvalby Ø, Göngrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JN, Seeburg PH, et al. 2008. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 60:846–860. [DOI] [PubMed] [Google Scholar]
- Wang CC, Held RG, Chang SC, Yang L, Delpire E, Ghosh A, Hall BJ. 2011. A critical role for GluN2B-Containing NMDA receptors in cortical development and function. Neuron. 72:789–805. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen Y, Carlson S, Li L, Hu X, Ma Y. 2012. Interactive effects of morphine and scopolamine, MK-801, propanolol on spatial working memory in rhesus monkeys. Neurosci Lett. 523:119–124. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. 2004. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 43:647–661. [DOI] [PubMed] [Google Scholar]
- Xu B, Xu ZF, Deng Y. 2009. Effect of manganese exposure on intracellular Ca2+ homeostasis and expression of NMDA receptor subunits in primary cultured neurons. Neurotoxicology. 30:941–949. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. 2008. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 55:1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Johnson-Venkatesh EM, Zhang H, Parent JM, Sutton MA, Umemori H. 2011. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron. 70:1128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T, Chaki S. 2004. Increased cell proliferation in the adult mouse hippocampus following chronic administration of group II metabotropic glutamate receptor antagonist, MGS0039. Biochem Biophys Res Commun. 315:493–496. [DOI] [PubMed] [Google Scholar]
- Zecevic N. 1998. Synaptogenesis in layer I of the human cerebral cortex in the first half of gestation. Cereb Cortex. 8:245–252. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A, Rakic S, Marín-Padilla M. 1999. Early development and composition of the human primordial plexiform layer: an immunohistochemical study. J Comp Neurol. 412:241–254. [PubMed] [Google Scholar]