Abstract
Background:
The hyperoxidative and a modified inflammatory state in obese individuals cause higher susceptibility to bacterial infection which influences the initiation and progression of periodontal disease.
Aim:
Our study was aimed to evaluate the role of nonsurgical periodontal therapy on the clinical parameters such as gingival index (GI), plaque index, pocket probing depth (PPD) and clinical attachment level (CAL), plasma-reactive oxygen metabolite (ROM) levels, and gingival crevicular fluid (GCF) resistin and serum resistin levels in obese or overweight and normal weight individuals with periodontitis.
Material and Methods:
Sixty individuals of age between 35 and 45 years of both the sexes were selected and categorized into two groups as thirty overweight or obese individuals with chronic periodontitis (Group I) and thirty normal weight individuals with chronic periodontitis (Group II). Clinical parameters, plasma ROM levels, and serum and GCF resistin levels were assessed at baseline and 2 months after periodontal therapy in both the groups and compared.
Results:
Intergroup comparison of clinical parameters (GI, CAL, PPD) 2 months after therapy showed a significant difference with more reduction in Group II compared to Group I. On comparison, mean plasma ROM, GCF, and serum resistin levels at baseline was higher in Group I compared to Group II. When plasma ROM, GCF, and serum resistin levels were compared 2 months after therapy, the reduction was more in Group II compared to Group I.
Conclusion:
It is concluded that along with periodontal therapy, motivation of patients for weight reduction program is mandatory for obese or overweight individuals with periodontitis in order to improve periodontal health.
Key words: Adipokine, chronic periodontitis, obesity, overweight, reactive oxygen species
INTRODUCTION
Periodontal disease is the chronic immune-inflammatory disease most commonly affecting the population globally. Periodontitis is initiated by colonization of periodontopathic bacteria, associated with exaggerated inflammatory response, resulting in the loss of tooth-supporting structures.[1] Several studies reported that the bacteria associated with periodontal tissue enter the bloodstream through gingival microvasculature and induce transient bacteremia which may be due to mastication, brushing, or dental treatment.[2,3] Although subgingival plaque biofilm is an initiator of periodontitis, the tissue breakdown is mostly mediated by exaggerated host response to bacteria and their end products.[4] The exaggerated inflammatory response leads to release of pro-inflammatory cytokines, reactive oxygen species (ROS), and proteolytic enzymes.[5]
Obesity is one of the subacute inflammatory diseases, defined as a state of oxidative stress.[6] Adipose tissue produces excess angiotensin (Ang) II secretion, which stimulates the release of excessive NADPH oxidase enzyme leading to overproduction of ROS.[6] In addition, increased peroxisomal and mitochondrial oxidation of fatty acids have also been found to be associated with excessive ROS production in obese individuals.[7] ROS includes hydroxyl, superoxide, and nitric oxide radicals, as well as nonradical oxygen derivatives.[8] Overproduction of ROS alters the tissue oxidative/antioxidative balance, damaging DNA, proteins, and lipids. Increased circulating ROS in periodontitis and obese individuals could affect the systemic health. Thus, measuring systemic oxidative status in obese and normal weight individuals with periodontitis may be helpful for understanding the effect of systemic condition on periodontal health.[9]
Resistin is one of the adipokines, originally found in adipose tissue, but is also released by monocytes, macrophages, and bone marrow. Resistin is associated with the upregulation of inflammatory processes.[10] The pro-inflammatory characteristic of resistin include the secretion of tumor necrosis factor (TNF)-α and interleukin (IL)-6 and also affects the anti-inflammatory effects of adiponectin. Resistin expression also increases the conversion of monocytes into macrophages.[11] Thus, resistin plays an important role in monocyte-macrophage function, and increased serum resistin levels have been associated with periodontitis.[12,13] Resistin may be an unidentified link between periodontitis and systemic conditions like obesity.
It is hypothesized that a hyperoxidative state and a modified inflammatory background prevailing in obese individuals cause higher susceptibility to bacterial infection leading to initiation or progression of periodontal disease. Oxidative stress and altered immune response due to excess adipokines can be considered as the mechanism linking periodontal disease and obesity.[14] Previous studies have reported that nonsurgical periodontal therapy is efficient in improving clinical periodontal parameters and reducing plasma reactive oxygen metabolites (ROMs) in normal weight individuals with periodontal disease.[15,16] However, only limited studies are available in relation to the effectiveness of the nonsurgical periodontal therapy on periodontal clinical parameters and plasma ROM levels, gingival crevicular fluid (GCF), and serum resistin levels in obese individuals with periodontal disease. The effect of nonsurgical periodontal therapy on obese individuals with periodontitis has not been explored so far. Hence, we aim to evaluate the periodontal parameters such as GI, plaque index (PI), pocket probing depth (PPD), clinical attachment level (CAL), plasma ROM levels, and GCF and serum resistin levels in obese individuals with chronic periodontitis after nonsurgical periodontal therapy (NSPT).
MATERIALS AND METHODS
This study was carried out between April 2017 and September 2017. The protocol of this study was approved by the ethical committee of our institution (Dr. MGRDU/TMDCH/RES/2015-2016/0302582). Before the enrollment of participants for this study, written informed consent was obtained. Sixty individuals both male and female having generalized chronic periodontitis were enrolled for the study and they were classified into Group I as obese or overweight individuals with chronic periodontitis and Group II as normal weight individuals with chronic periodontitis based on the inclusion and exclusion criteria. Inclusion criteria for the study participants included individuals who were within 35–45 years of age having minimum number of twenty natural teeth and with clinical attachment loss of ≥3 mm in more than 30% of sites.[17] Group I (overweight or obese individuals) were selected by assessing waist circumference (WC) and body mass index (BMI).[18] The calculation of BMI was done by dividing weight of an individual expressed in kilograms by height of an individual expressed in meters squared. Individuals were considered as overweight when the BMI was >25 kg/m2 and WC of >90 cm (men) and >80 cm (women).[19] The exclusion criteria for the present investigation included pregnancy, menopause, smokers, diabetes mellitus, cardiovascular disorders, use of supplement of antioxidants, long-term steroid medications, patient who had taken anti-inflammatory or antibiotics, or underwent periodontal treatment during the past 6 months.
Clinical parameters
At baseline, the periodontal parameters such as gingival index (GI), PI, PPD, and CAL were recorded. Plaque index was recorded at midbuccal, distobuccal, mesiobuccal, and palatal sites in each tooth.[20] The facial, mesial, distal, and lingual gingival areas were examined for GI.[21] PPD was measured in millimeters and were assessed in all the teeth at six sites,[22] and CAL was recorded for all teeth at six sites, which is calculated from cement-enamel junction to the base of the periodontal pocket.[23]
Blood collection
For estimation of plasma ROM and serum resistin levels, blood samples were collected from lateral aspect of the antecubital vein under aseptic precautions, and 1 ml of blood was collected in a color-coded test tube (red color) coated with clot activator, and another 1 ml of blood was collected in green color-coded test tube containing anticoagulant and centrifuged immediately at 3000 ×g for 5 min to get the serum and plasma. Serum was collected to estimate resistin levels similar to studies by Furugen et al. and Saito et al., where they have used serum for resistin estimation.[12,13] Plasma was collected to estimate ROM levels and we followed the methodology according to studies by Tamaki et al.[24,15]
Analysis of plasma-reactive oxygen metabolite
Based on the previous literature, plasma ROM estimation was carried out using spectrophotometer.[25,26] Twenty microliters of the plasma sample was mixed with 20 μl of the chromogenic substrate and 1 ml of the prepared acetate buffer and incubated for 5 min at 37°C. The density of the magenta color reflected the concentration of hydroperoxides in the blood which is proportional to the quantity of ROM. The ROM measurement was calculated as Carratelli unit (CARR U). One CARR U is equal to 0.08 mg/dl hydrogen peroxide (Komatsu et al. 2006). According to Tamaki et al., ROM levels are considered as normal, when it is between 250 and 300 CARR U in clinically healthy individuals.[24] ROM levels between 301 and 320 CARR U indicate a borderline condition of oxidative stress status, whereas a value above 320 CARR U depicts light oxidative stress. A value between 341 and 400 CARR U depicts oxidative stress, and when the value is above 401 CARR U, it depicts high oxidative stress.
Gingival crevicular fluid and serum resistin analysis
Two microliters of GCF was collected using micropipette in the sites with clinical attachment loss ≥3 mm and GI score of ≥2. The serum and GCF samples were stored at −80°C until analysis. The serum and GCF resistin levels were estimated using enzyme-linked immunosorbent assay (ELISA). This assay consists of human resistin-specific antibody coated on a 96 well plates. Pipette was used to add standards and samples solutions into the wells, and if resistin is present in the sample, it binds to the wells by the antibody. The biotinylated antihuman resistin antibody was added to the wells after washing away. The unbound biotinylated antibody was removed by washing, and pipette was used to pour horseradish peroxidase-conjugated streptavidin into the wells. The 3,3',55'-tetramethylbenzidine substrate solution was poured to the wells after washing and color was developed and the intensity was related to the levels of bound resistin. The stop solution was added, which changed the color from blue to yellow and the intensity of the color correlates with the levels of resistin and it was read at 450 nm. ELISA reader was used to obtain the GCF and serum resistin levels. Results were measured from the standard curves. The GCF and serum levels were measured as nanogram (ng/ml).
Nonsurgical periodontal therapy
Phase I periodontal therapy was performed for both the group individuals. Patients initially underwent supragingival scaling. The scaling and root planing (SRP) was carried out in 1-hsessions at four times and was completed within 2 weeks. Supragingival scaling was performed with ultrasonic scalers. Subgingival SRP was performed with manual standard Gracey curettes and ultrasonic devices under local anesthesia. Clinical parameters, plasma ROM levels, serum, and GCF resistin levels were examined at baseline and 2 months after completion of SRP.
Statistical analysis
To analyze the data, SPSS (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp. Released 2013, Version 22.0,) was used. Our data were analyzed using normality tests Kolmogorov–Smirnov and Shapiro–Wilks tests to know whether the variables follow normal distribution or not. The variables that followed normal distribution were subjected to further statistical analysis by parametric tests, and variables that do not follow normal distribution were analyzed using nonparametric tests. To compare mean values of age, BMI, WC, and CAL between groups, independent samples t-test was applied. Mann–Whitney test was used to compare mean values of PI, GI, and PPD between two groups. Intragroup comparison of clinical parameters (PI, GI, PPD) was done using Wilcoxon signed-ranks test. Intragroup comparison of clinical parameter (CAL) plasma ROM levels, GCF, and serum resistin levels was done with paired t-test, and the significance level was fixed as 5% (α = 0.05).
RESULTS
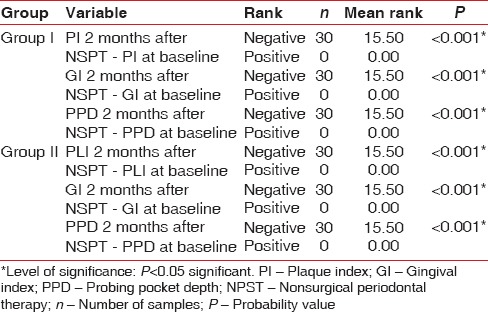
The demographic variables such as age, BMI, and WC were compared between two groups [Table 1]. On comparing the mean age between two groups, no statistically significant difference was shown, whereas comparison of BMI and WC between the groups showed a significant difference [Table 1]. On intergroup and intragroup comparison, CAL showed a significant difference at baseline and 2 months after NSPT [Table 2]. On intergroup comparison of clinical parameters such as PI, GI, and PPD, only PI and PPD showed a significant difference at baseline, whereas GI and PPD showed significant difference 2 months after NSPT [Table 3]. Intragroup comparison of PI, GI, PPD, and CAL in the groups at baseline and 2 months after NSPT showed a significant difference [Table 4].
Table 1.
Intergroup comparison of demographic variables using independent sample t-test
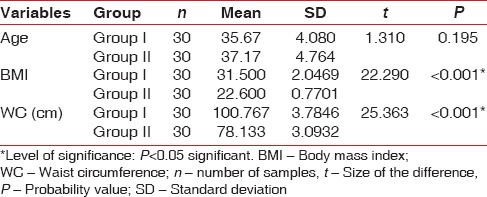
Table 2.
Inter and intra group comparison of clinical attachment level using t-test
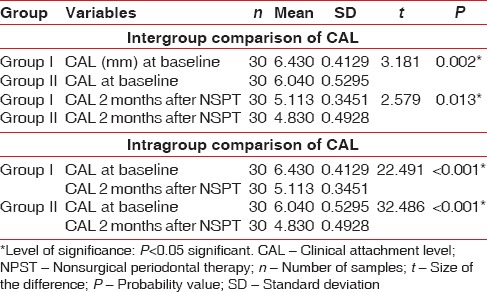
Table 3.
Intergroup comparison of clinical parameters (plaque index, gingival index, probing pocket depth) at baseline and 1 month after therapy using Mann-Whitney test
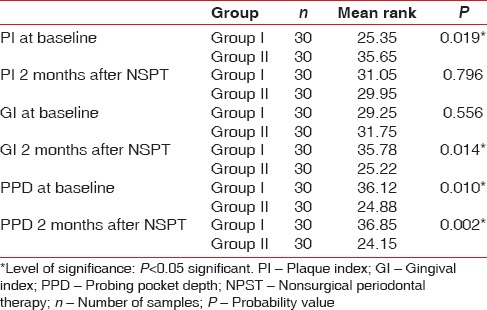
Table 4.
Intragroup comparison of clinical parameters (plaque index, gingival index, probing pocket depth) at baseline and 1 month after therapy using Wilcoxon signed-rank test
Intergroup comparison of plasma ROM levels at baseline showed a significant difference. The ROM levels in Group I were higher compared to Group II. Intergroup comparison of plasma ROM levels at 2 months after NSPT showed a significant difference with more reduction in Group II. Intragroup comparison of mean plasma ROM levels at baseline and 1 month after NSPT also showed a significant difference [Table 5]. Intergroup comparison of mean GCF and serum resistin levels at baseline and 2 months after NSPT between Group I and Group II was found to be significant [Table 6]. In Group I, on intragroup comparison of GCF resistin levels at baseline and 2 months after NSPT, statistically significant difference was observed, whereas serum resistin levels did not show any significance. In Group II, both GCF and serum resistin levels at baseline and 2 months after NSPT showed a statistically significant difference [Table 6]. The proportion of oxidative stress levels in both groups at baseline and 2 months after NSPT as shown in Figures 1 and 2.
Table 5.
Inter and intra group comparison of plasma-reactive oxygen metabolites levels using t-test
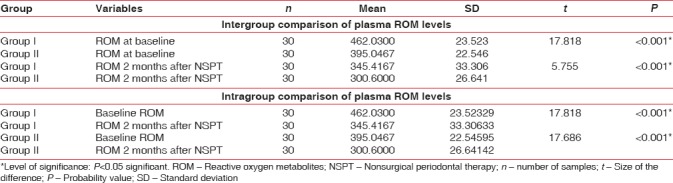
Table 6.
Inter and intra group comparison of gingival crevicular fluid and serum resistin levels using t-test
Figure 1.
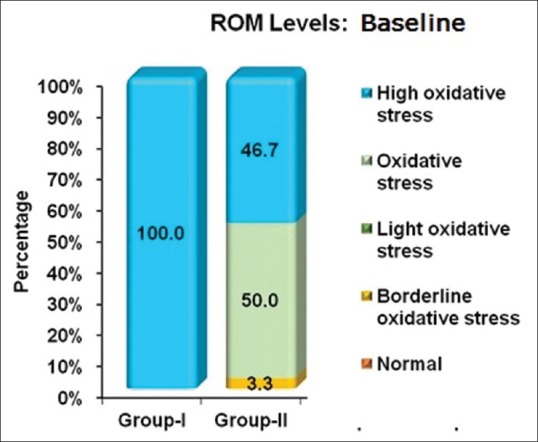
Proportion of oxidative stress levels in both groups at baseline. ROM- Reactive oxygen metabolite
Figure 2.
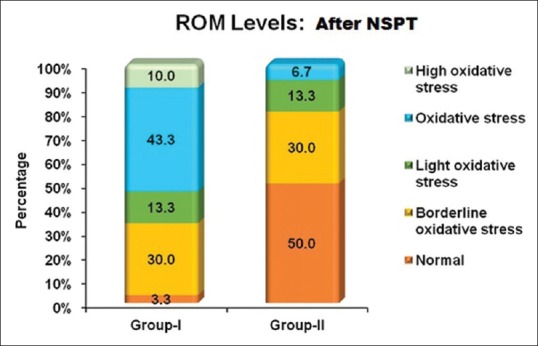
Proportion of oxidative stress levels in both groups after nonsurgical periodontal therapy. NSPT: Nonsurgical periodontal therapy. ROM- Reactive oxygen metabolite
DISCUSSION
Periodontitis is a chronic multifactorial disease; the progression of periodontal destruction is modified by environmental and host-related factors; the tissue destruction depends on the balance between the modifying factors and bacteria.[27] Recently, obesity has become a global health concern as it is related to inflammatory and immunologic alterations.[9] Obesity creates an increased oxidative stress status and subclinical low-grade systemic inflammation associated with harmful effects such as atherogenesis and endothelial dysfunction.[28] Recent studies confirmed the pro-inflammatory and hyperoxidative state during obesity, by measuring several cytokines and oxidative stress markers in GCF[29,30] and serum.[9] Our study was aimed to know the effectiveness of nonsurgical periodontal therapy on clinical parameters, plasma ROM, and adipokine resistin levels in obese or overweight individuals with periodontal disease and compared with normal weight individuals with chronic periodontitis.
Individuals between the age range of 35–45 years were included. Forty-five years of age was kept as a upper limit, as women are expected to attain menopause at 45 years of age or above, which could be a confounding factor for our study.[31] The age was found to be similar in both the groups. BMI and WC levels were higher in obese or overweight individuals with chronic periodontitis when compared to normal weight individuals with chronic periodontitis. On intergroup comparison of clinical parameters at baseline, the mean PI, PPD, and CAL scores were higher in obese or overweight individuals with chronic periodontitis when compared to normal weight individuals with chronic periodontitis. This was comparable with the study by Suvan et al.[32] who stated that obesity was associated with PPD. Chaffee and Weston[33] also found greater mean clinical attachment loss in obese individuals with periodontal disease. This finding also supports the fact that low-grade chronic inflammatory state prevailing in obesity may worsen periodontitis.[34]
Clinical parameters (GI, CAL, PPD) 2 months after NSPT showed a more reduction in normal weight individuals with chronic periodontitis compared to obese or overweight individuals with chronic periodontitis. These findings were in accordance with previous studies which reported that obesity impairs the clinical response to nonsurgical periodontal therapy.[35,36] Intragroup comparison of mean periodontal clinical parameters at baseline and 2 months after NSPT in obese or overweight individuals with chronic periodontitis and normal weight individuals with chronic periodontitis was found to be significant. All the clinical parameters showed significant reduction in each group after nonsurgical periodontal therapy. It is accepted that nonsurgical periodontal therapy decreases the bacterial load, thereby reducing periodontal inflammation. Reduction in PPD and gain in CAL occurred due to gingival shrinkage following nonsurgical periodontal therapy.[37]
At baseline, the plasma ROM level in both the groups was high as the individuals were in the state of oxidative stress. ROM level at baseline was found to be higher in obese or overweight individuals with chronic periodontitis compared to normal weight individuals with chronic periodontitis. This was similar to studies done by Dursun et al.[38] and Kose et al.[39] indicating the role of obesity in overproduction of ROS. Weisberg et al.[40] reported that macrophages infiltrated in the adipose tissue were an essential source of inflammatory cytokines, and NADPH oxidase, which contribute to ROS production in obese subjects. In periodontal disease, ROS is released from the neutrophils and other inflammatory cells, which causes oxidative damage of the biomolecules, leading to increased ROM levels. Matthews et al.[41] observed that individuals with periodontitis showed hyperactive phenotype neutrophils in relation to production of ROS, and this hyperactive phenotype by neutrophils can be improved following periodontal therapy.
When ROM level was compared between the groups 2 months after NSPT, more reduction was seen in ROM levels in normal weight individuals with chronic periodontitis. However, resolution of periodontal inflammation following nonsurgical periodontal therapy showed improvement in periodontal parameters and plasma ROM levels. These results were comparable to studies by Tamaki et al.[15] and Balasubramaniam et al.[16] who found significant reduction in plasma ROM levels in normal weight individuals with chronic periodontitis. Although ROM level was higher in obese patients with chronic periodontitis, it was also found to be higher than the normal weight individuals with chronic periodontitis. It is stated that nonsurgical periodontal therapy reduces periodontal inflammation, thereby decreasing the plasma ROM levels, which was comparable to study by Tamaki et al.[15] There was overall reduction in systemic oxidative stress levels in both the groups. Although both the groups showed reduction, the normal weight individuals with chronic periodontitis showed higher reduction.
Intergroup comparison of mean GCF and serum resistin levels at baseline was found to be significant. GCF and serum resistin levels were higher in obese or overweight individuals with chronic periodontitis compared to normal weight individuals with chronic periodontitis as observed by Gonçalves et al. and Patel and Raju[42,43] Obesity is considered to be a modifying factor of resistin levels in individuals with chronic periodontitis.[42] Intergroup comparison of mean GCF and serum resistin levels 2 months after therapy was also found to be significant, with more reduction in normal weight individuals with chronic periodontitis on comparison with obese or overweight individuals with chronic periodontitis. This finding was comparable with study done by Gonçalves et al.[42] who suggested the modulatory effect of obesity on GCF and serum resistin level, which may generate a systemic and local pro-inflammatory state in obese individuals with periodontitis.
Intragroup comparison of mean GCF resistin levels at baseline and 2 months after therapy in obese or overweight and normal weight individuals with chronic periodontitis was also significant. The increase in resistin levels in chronic periodontitis could be due to the expression of resistin by polymorphonuclear leukocytes and macrophages in inflammatory conditions such as periodontitis. Human resistin is a pro-inflammatory molecule and stimulates the secretion of IL-12 and TNF-α, thus inducing its own production in a positive feedback cycle.[43] The effect of nonsurgical periodontal therapy has reduced periodontal inflammation and thereby reducing the pro-inflammatory cytokines, leading to reduction in GCF resistin levels. However, the effect of nonsurgical periodontal therapy on serum resistin levels at baseline and 2 months after therapy in obese or overweight individuals with chronic periodontitis was not found to be significant which was in accordance to the study by Gonçalves et al.[42] This shows that nonsurgical periodontal therapy was effective in reduction of GCF resistin levels in obese or overweight individuals with chronic periodontitis, but could not reduce the serum resistin levels in obese individuals. At the systemic level, nonsurgical periodontal therapy did not change the serum resistin levels in obese individuals.
In our study, plasma ROM, serum, and GCF resistin levels were increased in obese individuals with chronic periodontitis compared to normal weight individuals with chronic periodontitis. Phase I periodontal therapy reduced systemic oxidative stress levels in both the groups; however, plasma ROM level showed a higher reduction in normal weight individuals with chronic periodontitis compared to obese or overweight individuals with chronic periodontitis, as obesity has role in elevating systemic oxidative stress levels.
CONCLUSION
Our study showed significant reduction in plasma ROM and GCF resistin levels which was significantly associated with GI, PPD, and CAL in obese individuals with chronic periodontitis after nonsurgical periodontal therapy. The effect of Phase I periodontal therapy such as SRP has reduced the periodontal inflammation, thereby reducing the ROS and pro-inflammatory cytokine levels. In future, reduction of circulating ROS and adipokines by nonsurgical periodontal therapy may reduce the risk of future systemic disease. Furthermore obese subjects can be motivated for weight reduction program, in order to maintain oxidative/antioxidative balance in the body, thereby reducing the risk to periodontitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Page RC, Kornman KS. The pathogenesis of human periodontitis: An introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–7. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 3.Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–13. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 4.Offenbacher S. Periodontal diseases: Pathogenesis. Ann Periodontol. 1996;1:821–78. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 5.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M, et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 9.Khan NI, Naz L, Yasmeen G. Obesity: An independent risk factor for systemic oxidative stress. Pak J Pharm Sci. 2006;19:62–5. [PubMed] [Google Scholar]
- 10.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ, et al. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 11.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 12.Furugen R, Hayashida H, Yamaguchi N, Yoshihara A, Ogawa H, Miyazaki H, et al. The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese. J Periodontal Res. 2008;43:556–62. doi: 10.1111/j.1600-0765.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Yamaguchi N, Shimazaki Y, Hayashida H, Yonemoto K, Doi Y, et al. Serum levels of resistin and adiponectin in women with periodontitis: The hisayama study. J Dent Res. 2008;87:319–22. doi: 10.1177/154405910808700416. [DOI] [PubMed] [Google Scholar]
- 14.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Tamaki N, Tomofuji T, Ekuni D, Yamanaka R, Yamamoto T, Morita M, et al. Short-term effects of non-surgical periodontal treatment on plasma level of reactive oxygen metabolites in patients with chronic periodontitis. J Periodontol. 2009;80:901–6. doi: 10.1902/jop.2009.080640. [DOI] [PubMed] [Google Scholar]
- 16.Balasubramaniam AS, Thomas LJ, Ramakrishnanan T, Ambalavanan N. Short-term effects of nonsurgical periodontal treatment with and without use of diode laser (980 nm) on serum levels of reactive oxygen metabolites and clinical periodontal parameters in patients with chronic periodontitis: A randomized controlled trial. Quintessence Int. 2014;45:193–201. doi: 10.3290/j.qi.a31206. [DOI] [PubMed] [Google Scholar]
- 17.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Physical status: The use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A Consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 20.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 21.Loe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 22.Hassell TM, Germann MA, Saxer UP. Periodontal probing: Interinvestigator discrepancies and correlations between probing force and recorded depth. Helv Odontol Acta. 1973;17:38–42. [PubMed] [Google Scholar]
- 23.Sivertson JF, Burgett FG. Probing of pockets related to the attachment level. J Periodontol. 1976;47:281–6. doi: 10.1902/jop.1976.47.5.281. [DOI] [PubMed] [Google Scholar]
- 24.Tamaki N, Tomofuji T, Maruyama T, Ekuni D, Yamanaka R, Takeuchi N, et al. Relationship between periodontal condition and plasma reactive oxygen metabolites in patients in the maintenance phase of periodontal treatment. J Periodontol. 2008;79:2136–42. doi: 10.1902/jop.2008.080082. [DOI] [PubMed] [Google Scholar]
- 25.Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath. 2003;7:105–10. doi: 10.1007/s11325-003-0105-9. [DOI] [PubMed] [Google Scholar]
- 26.Iamele L, Fiocchi R, Vernocchi A. Evaluation of an automated spectrophotometric assay for reactive oxygen metabolites in serum. Clin Chem Lab Med. 2002;40:673–6. doi: 10.1515/CCLM.2002.115. [DOI] [PubMed] [Google Scholar]
- 27.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 28.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 29.Zuza EP, Barroso EM, Carrareto AL, Pires JR, Carlos IZ, Theodoro LH, et al. The role of obesity as a modifying factor in patients undergoing non-surgical periodontal therapy. J Periodontol. 2011;82:676–82. doi: 10.1902/jop.2010.100545. [DOI] [PubMed] [Google Scholar]
- 30.Suresh S, Mahendra J, Singh G, Pradeep AR, Vikram S, Sekar H, et al. Comparative analysis of GCF resistin levels in obese subjects with and without periodontal disease. J Clin Diagn Res. 2016;10:ZC71–4. doi: 10.7860/JCDR/2016/19066.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suresh S, Kumar TS, Saraswathy PK, Pani Shankar KH. Periodontitis and bone mineral density among pre and post menopausal women: A comparative study. J Indian Soc Periodontol. 2010;14:30–4. doi: 10.4103/0972-124X.65434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suvan JE, Petrie A, Nibali L, Darbar U, Rakmanee T, Donos N, et al. Association between overweight/obesity and increased risk of periodontitis. J Clin Periodontol. 2015;42:733–9. doi: 10.1111/jcpe.12421. [DOI] [PubMed] [Google Scholar]
- 33.Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: A systematic review and meta-analysis. J Periodontol. 2010;81:1708–24. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonçalves TE, Feres M, Zimmermann GS, Faveri M, Figueiredo LC, Braga PG, et al. Effects of scaling and root planing on clinical response and serum levels of adipocytokines in patients with obesity and chronic periodontitis. J Periodontol. 2015;86:53–61. doi: 10.1902/jop.2014.140266. [DOI] [PubMed] [Google Scholar]
- 36.Akram Z, Safii SH, Vaithilingam RD, Baharuddin NA, Javed F, Vohra F, et al. Efficacy of non-surgical periodontal therapy in the management of chronic periodontitis among obese and non-obese patients: A systematic review and meta-analysis. Clin Oral Investig. 2016;20:903–14. doi: 10.1007/s00784-016-1793-4. [DOI] [PubMed] [Google Scholar]
- 37.Hughes TP, Caffesse RG. Gingival changes following scaling, root planning and oral hygiene. A biometric evaluation. J Periodontol. 1978;49:245–52. doi: 10.1902/jop.1978.49.5.245. [DOI] [PubMed] [Google Scholar]
- 38.Dursun E, Akalin FA, Genc T, Cinar N, Erel O, Yildiz BO, et al. Oxidative stress and periodontal disease in obesity. Medicine (Baltimore) 2016;95:e3136. doi: 10.1097/MD.0000000000003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kose O, Canakci V, Fatih C, Yildirim A, Kermen E, Arabaci T, et al. The effect of obesity on total antioxidant/oxidant status and oxidative stress index in patients with chronic periodontitis. Oxid Antioxid Med Sci. 2014;3:153–9. [Google Scholar]
- 40.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple IL. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147:255–64. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonçalves TE, Zimmermann GS, Figueiredo LC, Souza Mde C, da Cruz DF, Bastos MF, et al. Local and serum levels of adipokines in patients with obesity after periodontal therapy: One-year follow-up. J Clin Periodontol. 2015;42:431–9. doi: 10.1111/jcpe.12396. [DOI] [PubMed] [Google Scholar]
- 43.Patel SP, Raju PA. Gingival crevicular fluid and serum levels of resistin in obese and non-obese subjects with and without periodontitis and association with single nucleotide polymorphism at-420. J Indian Soc Periodontol. 2014;18:555–9. doi: 10.4103/0972-124X.142438. [DOI] [PMC free article] [PubMed] [Google Scholar]


