Abstract
Objective:
To identify pre-operative clinical and computerized spiral analysis characteristics that may help ascertain which patients with Essential Tremor (ET) will exhibit ‘early tolerance’ to ventral intermediate nucleus of thalamus (Vim) deep brain stimulation (DBS).
Methods:
Identification of comparative characteristics of defined cases of ‘early tolerance’ versus patients with sustained satisfactory response treated with Vim DBS surgery for medically-refractory ET, based on retrospective chart review by a clinician blinded to the findings of computerized spiral analysis.
Results:
Statistically significant differences in two spiral analysis indices, SWVI and DoS, were found in the dominant upper limbs of patients who developed ‘early tolerance’, whereas the clinical characteristics were not significantly different.
Conclusion:
Objective measurements of upper limb kinematics using graphonomic tests like spiral analysis should be considered in the pre-operative evaluation for DBS, especially in the setting of moderate-severe predominantly action and proximal postural tremors.
Significance:
Ours is the first investigation looking into the pre-operative clinical and objective physiologic characteristics of the patients who develop ‘early tolerance’ to Vim DBS for the treatment of essential tremor. The study has significant implications for pre-operative evaluation and potential surgical target selection for the treatment of tremors.
Keywords: Essential tremor, Early tolerance, Tremor habituation, Deep brain stimulation, Computerized spiral analysis
1. Introduction
Essential tremor (ET), is the most common cause of adult onset tremors. The diagnosis is made clinically on the basis of chronic, disabling action tremors. The phenotype is variable and heterogeneous; some patients experience a gradual increase in severity and disability over the years, others developed generalized tremors, including legs, head, and voice; and some individuals evolve into a pancerebellar syndrome that includes limb dysmetria and gait ataxia. In accordance with the varied clinical presentation, the tremor may be generated by different pathophysiologic mechanisms, with important therapeutic and prognostic implications (Louis et al., 1998; Bain et al., 2000; Deuschl and Elble, 2000; Louis et al., 2000; Deuschl and Elble, 2009), and differing response to medication treatment and neurosurgical intervention (Gorman et al., 1986; Koller et al., 2000; Blomstedt et al., 2011).
Current pharmacologic treatment is helpful in only about half of the ET cases, and this may relate to different physiologic subtypes (Sabra and Hallett, 1984; Deuschl et al., 1987; Koller et al., 2000; Deuschl et al., 2011). Deep brain stimulation (DBS) of the ventral intermediate nucleus of the thalamus (Vim) is an effective treatment for ET, particularly for medication-resistant disabling cases (Blomstedt et al., 2007; Baizabal-Carvallo et al., 2014). DBS has been shown to remain effective in long term follow-up studies; however, there may be loss of benefit over a long period of time in a subset of patients. Possible explanations for this include (i) disease progression and (ii) habituation to DBS, or tolerance, despite the absence of clinical progression, and repeated, optimal DBS programming (Springer et al., 2006; Barbe et al., 2011; Favilla et al., 2012). We have encountered recurrent tremor within weeks after DBS surgery, where excellent targeting and tremor control were noted intra-operatively. We have hypothesized that tremor recurrence/habituation may be predictable on the basis of pre-operative clinical and kinematic characteristics. In this study, we sought to identify and characterize if any pre-operative clinical and spiral drawing characteristics may help ascertain which ET patients will exhibit loss of efficacy or ‘early tolerance’ to Vim DBS.
2. Materials and methods
We reviewed all available charts of patients who had undergone thalamic Vim DBS surgery for ET over the last 15 years at Columbia University Medical Center and who had pre-operative computerized spiral analysis testing. Inclusion was based on availability of detailed pre-operative clinical, intra-operative, demographic, pre-operative spiral analysis and follow-up clinical and programming data at least for the first two years after DBS surgery. The study was conducted in accordance with the Institutional Review Board (IRB) of Columbia University Medical Center. Patients who were noted to have the clinical phenomenon of developing ‘early tolerance’ and those with sustained good response were identified by a movement disorder neurologist (BF) blinded to the spiral analysis findings. We defined those patients with ‘early tolerance’ as those who experienced (i) near-complete intra-operative tremor suppression (equivalent to a clinical score of 1 or less on a 5-point severity scale from 0 to 4 where 0 = normal, 1 = slight, intermittent, 2 = moderate amplitude, intermittent, 3 = marked amplitude and 4 = severe amplitude based on the Fahn-Tolosa-Marin (FTM) tremor rating scale as noted in the upper limbs with action and postural holding, and (ii) sub-optimal response post-operatively within the first two years characterized by the inability to maintain satisfactory tremor control in the performance of ADLs without experiencing adverse effects (paresthesias, disabling dysarthria or gait ataxia) from DBS programming.
General pre-operative characteristics of all cases were noted, including handedness, gender, age at time of surgery, presence or absence of limb dysmetria or gait ataxia prior to DBS surgery, head tremor, vocal tremor, presence of tremor with kinetic actions or posture-holding, clinical tremor rating score, and whether the DBS implant was unilateral or bilateral. The presence of ataxia was noted on the basis of clinical documentation of tandem gait difficulties, signs of cerebellar dysmetria or dysdiadochokinesia.
Implanted pulse generator programming data included changes in programming parameters: DBS lead voltage, frequency and pulse width. The changes in active lead configuration were also recorded.
Computerized spiral analysis testing involved drawing 10 spirals from each hand inside a 10 × 10 cm square box on 8.5 × 11-in. paper with a wireless inked pen placed on a graphics tablet (Intuos 2–4, Wacom Technology Corp, Vancouver, WA). Subjects were allowed to draw freely without any constraints, attachments, or traceable templates, and collection was standardized across all subjects. The tablet had a resolution of 2540 points/inch (accuracy of 0.005 in.), with 256 levels of measurable pressure acquiring data at 100 Hz. Quantification of handwritten spirals was as previously described and involved acquisition of data series consisting of time, x, y, and pressure axis values to unravel the spiral. Using these data points from kinematic, dynamic, and spatial attributes of spiral execution spiral indices were computed (Pullman, 1998; Rudzinska et al., 2007; Haubenberger et al., 2011; Hess and Pullman, 2012). All trials were performed in one session lasting about 15 min.
Two computerized spiral analysis indices were used as primary outcome measures in this study: (i) a measure of spiral loop-to-loop width variability index (SWVI) and (ii) an overall spiral degree of severity score (DoS). The means of 8 of the 10 trials (with the highest and lowest values removed) are used to calculate SWVI and DoS. SWVI is a unitless measure that highlights the fluctuations in spiral execution seen in patients with cerebellar dysfunction (Hess and Pullman, 2012). It is calculated as the coefficient of variation (ratio of the standard deviation to the mean) of the medians of spiral loop widths per angle over the 360° of each spiral loop and is independent of tremor. Higher SWVI scores are associated with greater degree of intention tremor and ataxia (Louis et al., 2012). DoS is a unitless continuous measure of overall spiral execution and spatial irregularity, derived from indices mostly related to drawing smoothness. DoS was designed as the computerized spiral analysis equivalent of the standard five-point (0–4) FTM clinical rating scale. The DoS index correlates with the neurologic exam and has been validated with clinical tremor scales (Elble et al., 2006; Saunders-Pullman et al., 2008; Louis et al., 2012). DoS was shown to have high sensitivity and specificity discerning early Parkinson disease (PD) subjects from normal controls, as well as being more revealing than the clinical exam elucidating pre-clinical spiral changes on clinically unaffected sides in PD (San Luciano et al., 2016).
3. Statistical analysis
Mann-Whitney test was used for comparing continuous data, and Fisher’s exact test for categorical data between Groups 1 and 2. Non-parametric tests were chosen considering the small sample size and the lack of normal distribution of the data collected. Statistical analysis was performed using GraphPad Prism version 7.0. A probability value of <0.05 was considered to be statistically significant. Since all unilaterally operated cases were performed to improve the function of the dominant limb, spiral analysis data only from the dominant limb of patients were included in the final analysis. SWVI sensitivity and specificity calculations were performed using a cut-off value = 0.40, the median normal SWVI determined in a previous study investigating cerebellar dysfunction in ET (Louis et al., 2012).
4. Results
A total of 19 ET patients were identified as satisfying all inclusion criteria and were used in this study. Ten patients met the defined criteria for ‘early tolerance’ (Group 1) while 9 patients had a sustained good response at least for the first two years after surgery (Group 2).
4.1. Patient demographics and clinical characteristics
No significant differences were noted in patient age and sex at the time of DBS surgery between the two groups. 40% of Group 1 patients had unilateral DBS compared to 22% in Group 2. All unilateral DBS were implanted to improve the tremors in the dominant limb. All patients in both groups were initially programmed using single monopolar setting. Of the 10 patients in Group 1, 7 needed switches to double monopolar within the first year and 2 patients were also attempted to be programmed using Interleaved setting. Of the 9 patients in Group 2 only 2 needed switch to double monopolar setting and none were interleaved. The trends of voltage requirements were suggestive of higher effective outputs required for Group 1; however, due to lack of a standardized conversion algorithm for effective voltage outputs and the fields thereof, upon conversion from single monopolar to double monopolar and interleaving, a fair comparison could not be performed. Baseline demographic, clinical and spiral analysis characteristics of patients developing ‘early tolerance’ (Table 1) and those with sustained good response (Table 2) are shown.
Table 1.
Baseline demographic, clinical and spiral analysis characteristics of cases developing ‘early tolerance’
| Age* | Sex | Uni/Bilateral | Ataxia | Head tremors | Clinical tremor scores | DoS | SWVI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No | Yes/No | D | ND | D | ND | D | ND | ||||
| 1 | 75 | F | Bilateral | No | Yes | 3 | 3 | 3.90 | 2.96 | 3.03 | 0.32 |
| 2 | 80 | F | Unilateral | Yes | Yes | 4 | 3 | 3.97 | 2.18 | 0.47 | 0.43 |
| 3 | 77 | M | Bilateral | Yes | No | 4 | 3 | 3.96 | 3.96 | 1.22 | 1.20 |
| 4 | 67 | F | Bilateral | No | Yes | 3 | 3 | 3.65 | 0.60 | 0.53 | 0.22 |
| 5 | 66 | M | Unilateral | Yes | Yes | 4 | 4 | 3.70 | 4.00 | 0.46 | 0.97 |
| 6 | 68 | F | Bilateral | Yes | Yes | 3 | 3 | 4.00 | 3.40 | 0.77 | 0.30 |
| 7 | 67 | F | Unilateral | Yes | No | 3 | 3 | 4.00 | 4.00 | 2.20 | 3.70 |
| 8 | 77 | M | Bilateral | Yes | Yes | 3 | 4 | 3.30 | 3.8 | 0.41 | 0.79 |
| 9 | 53 | F | Unilateral | Yes | Yes | 3 | 3 | 2.33 | 2.00 | 0.44 | 0.50 |
| 10 | 62 | F | Bilateral | No | Yes | 3 | 3 | 3.90 | 2.49 | 1.06 | 0.42 |
Age at surgery; DoS- Degree of severity; SWVI- Spiral width variability index; D-dominant limb; ND-non dominant limb.
Table 2.
Baseline demographic, clinical and spiral analysis characteristics of patients with satisfactory outcomes/not developing ‘early tolerance’.
| Age* | Sex | Uni/Bilateral | Ataxia | Head tremor | Clinical Tremor scores | DoS | SWVI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No | Yes/No | D | ND | D | ND | D | ND | ||||
| 1 | 70 | F | Unilateral | Yes | No | 3 | 3 | 3.70 | 1.70 | 0.61 | 0.35 |
| 2 | 67 | M | Bilateral | No | Yes | 3 | 3 | 3.50 | 2.40 | 0.24 | 0.23 |
| 3 | 63 | F | Bilateral | Yes | Yes | 3 | 4 | 2.00 | 2.60 | 0.35 | 0.33 |
| 4 | 80 | M | Unilateral | No | No | 3 | 2 | 2.25 | 1.60 | 0.36 | 0.33 |
| 5 | 67 | M | Bilateral | No | Yes | 3 | 4 | 3.46 | 3.60 | 0.31 | 0.41 |
| 6 | 40 | F | Bilateral | No | Yes | 3 | 4 | 3.24 | 3.44 | 0.25 | 0.29 |
| 7 | 67 | M | Bilateral | Yes | No | 2 | 2 | 2.40 | 1.30 | 0.35 | 0.34 |
| 8 | 70 | M | Bilateral | No | No | 3 | 4 | 1.90 | 3.37 | 0.38 | 0.64 |
| 9 | 72 | M | Bilateral | No | Yes | 3 | 4 | 3.90 | 4.00 | 0.39 | 0.48 |
Age at surgery; DoS- Degree of severity; SWVI- Spiral width variability index; D-dominant limb; ND-non dominant limb.
Of note, 70% of the patients in Group 1 had clinical documentation of ataxia and 80% had head tremor. For Group 2, 33% of the patients had clinical documentation of ataxia and 55% were noted to have head tremor. The differences in the clinical characteristics noted between the 2 groups were not significant. Differences in the worse clinical tremor scores for both the dominant and non-dominant upper limbs noted with action and postural holding as per FTM rating scale between the two groups were also not significant.
4.2. Spiral analysis
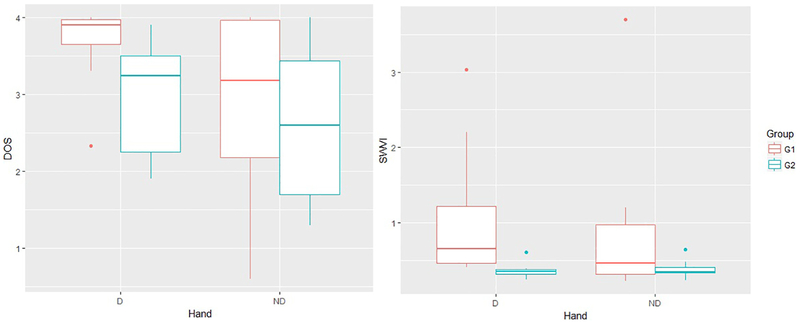
DoS for the dominant limb in Group 1 was significantly higher compared to Group 2. Dominant limb SWVI or the loop width variability indices were also significantly worse in Group 1; the median was noted to be almost double. Comparative details of the spiral analysis indices and clinical data are summarized in Table 3 and spiral analysis DoS and SWVI between group differences noted in Fig. 1. Using SWVI = 0.40 as a cut-off value, the sensitivity of spiral analysis to predict ‘early tolerance’ was 100% and the specifi was found to be 89%.
Table 3.
Differences between patients with ‘early tolerance’ (Group1) compared to patients with satisfactory outcomes > 2 years (Group 2).
| Group 1 N = 10 | Group 2 N = 9 | p-value | |
|---|---|---|---|
| Age* (median; 25–75 quartiles) | 67.5; 65–77 | 67; 65–71 | 0.80a |
| Gender (M/F) | 3/7 | 6/3 | 0.15b |
| DBS placement | |||
| Unilateral | 40% | 22% | 0.63b |
| Ataxic symptoms | 70% | 33% | 0.18b |
| Head tremor | 80% | 55% | 0.35b |
| Clinical tremor scores (median) | |||
| Dominant | 3 | 3 | 0.12a |
| Non-dominant | 3 | 4 | 0.50a |
| DoS (median; 25–75 quartiles) | |||
| Dominant | 3.90; 3.56–3.98 | 3.24; 2.13–3.60 | 0.0107a |
| SWVI (median; 25–75 quartiles) | |||
| Dominant | 0.652; 0.455–1.465 | 0.35; 0.28–0.43 | 0.0021a |
Age at the time of surgery.
Mann-Whitney test.
Fisher’s Exact test.
Fig. 1.
Comparative Box and Whisker plots for Spiral analysis measures DoS and SWVI between patients with ‘early tolerance’ (Group1) compared to patients with satisfactory outcomes >2 years (Group 2); DoS: Degree of severity, SWVI: Spiral width variability index, D: dominant hand, ND: non-dominant hand, G1: Group 1, G2: Group 2.
5. Discussion
Loss of DBS efficacy and the re-emergence of tremor after initial good control in ET patients is a problem, the reasons for which are poorly understood. Most studies evaluating the loss of DBS benefit for tremor control over time have focused on the limitations of the intervention, i.e. habituation to stimulation over long term and sub-optimal lead placement. Studies thus far have focused on disease progression for loss of efficacy over time (Hariz et al., 1999; Papavassiliou et al., 2004; Springer et al., 2006; Hsu et al., 2009; Barbe et al., 2011; Favilla et al., 2012; Patel et al., 2014), but the literature on suboptimal/loss of benefit early in the disease remains scarce. In this investigation, we were looking into the pre-operative clinical characteristics of the patients who developed this phenomenon, for which we use the term ‘early tolerance’.
To our knowledge, this is the first study to use objectively quantifiable characteristics of upper limb kinematics from an easy to administer graphonomic test to help predict, with high sensitivity and specificity, which patients would get a suboptimal response to Vim DBS implantation over time. The criteria we used to identify these cases of ‘early tolerance’ were similar to previously described studies identifying resistant cases (Pilitsis et al., 2008). We use a timeline of two years to identify these cases in the suggested definition to ensure that the patients with sustained good responses were clearly differentiated; almost all patients in Group 1 developed ‘early tolerance’ within the first year or shortly thereafter. There are likely numerous reasons for patients to develop ‘early tolerance’ to Vim DBS, and following possibilities may elucidate some causes of this phenomenon.
5.1. Inherent tremor characteristics
The group of patients noted to develop early tolerance had significantly higher SWVI likely signifying more cerebellar dysfunction (Pilitsis et al., 2008; Louis et al., 2012). Group 1 patients had more severe cerebellar signs (e.g., intention tremor, tandem gait missteps). Patients with higher SWVI scores also had more severe kinetic tremors, which are notably more resistant to treatment (Gorman et al., 1986; Louis et al., 2012).
In this study, ET cases that developed ‘early tolerance’ after DBS had predominantly kinetic tremors, with associated proximal postural tremors that were uniquely disabling but not well represented by clinical rating scales, especially when moderate-to-severe, explaining the similar clinical tremor scores between the two groups (Sabra and Hallett, 1984). In long-term [>2 years] follow-up, several of these individuals evolved into a pancerebellar syndrome that included dysarthria (even with the DBS device temporarily turned off) and ataxia that required walking aids. None of these individuals developed parkinsonism.
Astute clinical examination is needed to detect ataxia or subtle deficits in coordination in patients with ET, which are common clinical findings. Ataxia was noted in a higher number of patients developing ‘early tolerance’, which corroborated with higher pre-operative SWVI scores. Additionally, the SWVI scores in patients developing ‘early tolerance’ were similar to those noted in patients with cerebellar ataxia and higher than those commonly noted in ET patients (Elble et al., 2006; Hsu et al., 2009; Louis et al., 2012). SWVI scores in patients developing early tolerance to DBS were also significantly higher compared to the subgroup with sustained good response in the current cohort. Objective measures and quantitative assessments of kinematic parameters using spiral analysis provide useful and reproducible information to detect cerebellar outflow pathology in ET and may predict treatment outcome (Pullman, 1998; Hsu et al., 2009; Louis et al., 2012). More detailed assessment using other tremor analysis methods such as with accelerometers and EMG could be used to further supplement clinical decision-making regarding treatment of these complex and disabling tremors (Hess and Pullman, 2012).
5.2. Relative role of tolerance
It is known that loss of response over time to stimulation plays a role in decreasing long term DBS efficacy. Several studies have demonstrated that patients may need incremental voltage requirements over time to maintain a satisfactory response (Hariz et al., 1999; Kumar et al., 2003; Hsu et al., 2009; Barbe et al., 2011; Favilla et al., 2012) and this may be greater in patients with the electrophysiological characteristics we have identified in this study.
In general, the rate of increase in IPG amplitude for optimal control of ET seems to be higher compared to PD or post-stroke tremor (Pilitsis et al., 2008; Barbe et al., 2011). Our cases of ‘early tolerance’ share many or all of these characteristics and are notably distinct from the patients who maintained a good response at relatively lower voltages. DBS may work by disrupting ‘tremorogenic’ pacemaker cells by altering the neural activity needed for tremor production and spread, by inhibiting the afferent tremor signals or altering the excitability of thalamic neurons (Yamamoto et al., 2004; Bhalsing et al., 2013; Coenen et al., 2014, Buijink et al., 2015). Higher SWVI values implicate ataxia, which may be secondary to underlying cerebellar involvement in ET and could be reflective of greater resistance to altering the afferent tremor signals likely involving cerebello-thalamic circuitry (Raethjen and Deuschl, 2012).
5.3. Suboptimal lead placement vs suboptimal target
It may be that the Vim target is suboptimal for this phenotype of more severe tremors, considering a different pathophysiologic basis proposed for these treatment resistant tremors. The more caudal zona Incerta (cZI) or the posterior Subthalamic Region are targets that may be more advantageous for the control of more proximal and complex tremors (Blomstedt et al., 2011; Louis et al., 2012).
Two neural circuits may be relevant: 1) the cerebello-thalamo-cortical-cerebellar loop and 2) the cortico-thalamo-cortical loop. The first one is thought to be associated with tremor generation and the second loop with acceleration of the tremor (Boecker et al., 2010; Fasano et al., 2010; Taira, 2012). A part of the cerebello-thalamo-cortical network, the cerebello-thalamic tract, might be an interconnecting pathway. It has been proposed that targeting this pathway using tractography might help target both the loops proposed in the tremor generation since it includes an important hub connecting both these loops at the thalamic level. Additionally, the cerebello-thalamic tract has been shown to be closely connected and pass through or in close proximity to the three traditionally targeted regions for DBS in tremor control, the Vim, cZI and posterior subthalamic region. Accurately targeting this tract might help better control of these cases which are shown to be resistant to the traditional targets (Coenen et al., 2011; Coenen et al., 2014).
Limitations of this study include the small sample size; however, we were still able to identify significant differences between the two patient groups using spiral analysis measures with high sensitivity and specificity. Though the differences in clinical characteristics noted were not significantly different, there were trends that suggested these groups were different clinically which could be evaluated in a larger prospective study. A prospective study with appropriate power to detect potential differences based on clinical rating scales will further validate the spiral analysis measures. We note that a higher proportion of patients in Group 1 had unilateral DBS, which may have had an impact on the responses. There is no established definition of ‘early tolerance’, but the criteria used to identify these cases are similar to the previously reported literature (Schlaier et al., 2015). To address this, we used both physician and patient input to further elucidate ‘early tolerance’. The physician was involved in the intra-operative assessment of tremor suppression and subsequent post-operative programming where significantly higher stimulator outputs were noted to achieve a meaningful tremor suppression with associated early stimulation related side-effects. The patients determined if the tremor suppression was satisfactory and meaningful enough to help improve their ADLs, without significant stimulation related side effects. This retrospective study could be confounded by biases in patient selection and inconsistent documentation. However, we addressed this by having the same clinician document all clinical characteristics while blinded to the details of spiral analysis data when identifying the patient groups. We did not have the precise mapping of the lead co-ordinates based on post-operative imaging; however, the intra-operative clinical response and the post-operative imaging studies were not remarkably different between the groups. Though suboptimal lead placement could still be a contributing factor, the distinct clinical characteristics of these patients seem to be a more likely explanation for the poor response noted.
We conclude that while ‘early tolerance’ to Vim DBS in ET is not fully understood, it occurs more in patients with severe action tremors with marked proximal postural components and ataxia. Importantly, we found objective measurements based on spiral drawing can predict which patients will show this phenomenon. In their aggregate, we believe these are the considerations that will influence prognosis, DBS target selection, and post-operative management.
HIGHLIGHTS.
Spiral analysis identified differences in ET patients developing tolerance to DBS.
Potential tolerance to Vim DBS in ET can be predicted pre-operatively.
Objective kinematic testing can aid in pre-operative assessment of complex tremors.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Bain P, Brin M, Deuschl G, Elble R, Jankovic J, Findley L, Koller WC, Pahwa R. Criteria for the diagnosis of essential tremor. Neurology 2000;54(11 Suppl 4):S7. [PubMed] [Google Scholar]
- Baizabal-Carvallo JF, Kagnoff MN, Jimenez-Shahed J, Fekete R, Jankovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry 2014;85(5):567–72. [DOI] [PubMed] [Google Scholar]
- Barbe MT, Liebhart L, Runge M, Pauls KA, Wojtecki L, Schnitzler A, Allert N, Fink GR, Sturm V, Maarouf M, Timmermann L. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol 2011;258(3):434–9. [DOI] [PubMed] [Google Scholar]
- Bhalsing KS, Saini J, Pal PK. Understanding the pathophysiology of essential tremor through advanced neuroimaging: a review. J Neurol Sci 2013;335(1–2):9–13. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Hariz GM, Hariz MI, Koskinen LO. Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg 2007;21(5):504–9. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Sandvik U, Linder J, Fredricks A, Forsgren L, Hariz MI. Deep brain stimulation of the subthalamic nucleus versus the zona incerta in the treatment of essential tremor. Acta Neurochir (Wien) 2011;153(12):2329–35. [DOI] [PubMed] [Google Scholar]
- Boecker H, Weindl A, Brooks DJ, Ceballos-Baumann AO, Liedtke C, Miederer M, Sprenger T, Wagner KJ, Miederer I. GABAergic dysfunction in essential tremor: an 11C-flumazenil PET study. J Nucl Med 2010;51(7):1030–5. [DOI] [PubMed] [Google Scholar]
- Buijink AW, van der Stouwe AM, Broersma M, Sharifi S, Groot PF, Speelman JD, Maurits NM, van Rootselaar AF. Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 2015;138:2934–47. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Allert N, Madler B. A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentato-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochir (Wien) 2011;153(8): 1579–1585; discussion 1585. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Allert N, Paus S, Kronenburger M, Urbach H, Madler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery 2014;75(6):657–69; discussion 669–670. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Elble R. Essential tremor–neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord 2009;24(14):2033–41. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Elble RJ. The pathophysiology of essential tremor. Neurology 2000;54(11 Suppl 4):S14–20. [PubMed] [Google Scholar]
- Deuschl G, Lucking CH, Schenck E. Essential tremor: electrophysiological and pharmacological evidence for a subdivision. J Neurol Neurosurg Psychiatry 1987;50(11):1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol 2011;10(2):148–61. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R, Tremor Research G. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain 2006;129:2660–6. [DOI] [PubMed] [Google Scholar]
- Fasano A, Herzog J, Raethjen J, Rose FE, Muthuraman M, Volkmann J, Falk D, Elble R, Deuschl G. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain 2010;133:3635–48. [DOI] [PubMed] [Google Scholar]
- Favilla CG, Ullman D, Wagle Shukla A, Foote KD, Jacobson CEt, Okun MS. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain 2012;135:1455–62. [DOI] [PubMed] [Google Scholar]
- Gorman WP, Cooper R, Pocock P, Campbell MJ. A comparison of primidone, propranolol, and placebo in essential tremor, using quantitative analysis. J Neurol Neurosurg Psychiatry 1986;49(1):64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariz MI, Shamsgovara P, Johansson F, Hariz G, Fodstad H. Tolerance and tremor rebound following long-term chronic thalamic stimulation for Parkinsonian and essential tremor. Stereotact Funct Neurosurg 1999;72(2–4):208–18. [DOI] [PubMed] [Google Scholar]
- Haubenberger D, Kalowitz D, Nahab FB, Toro C, Ippolito D, Luckenbaugh DA, Wittevrongel L, Hallett M. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov Disord 2011;26(11):2073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Pullman SL. Tremor: clinical phenomenology and assessment techniques. Tremor Other Hyperkinet Mov (N Y) 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AW, Piboolnurak PA, Floyd AG, Yu QP, Wraith JE, Patterson MC, Pullman SL. Spiral analysis in Niemann-Pick disease type C. Mov Disord 2009;24(13):1984–90. [DOI] [PubMed] [Google Scholar]
- Koller WC, Hristova A, Brin M. Pharmacologic treatment of essential tremor.Neurology 2000;54(11 Suppl 4):S30–38. [PubMed] [Google Scholar]
- Kumar R, Lozano AM, Sime E, Lang AE. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology 2003;61(11):1601–4. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ford B, Barnes LF. Clinical subtypes of essential tremor. Arch Neurol 2000;57(8):1194–8. [DOI] [PubMed] [Google Scholar]
- Louis ED, Gillman A, Boschung S, Hess CW, Yu Q, Pullman SL. High width variability during spiral drawing: further evidence of cerebellar dysfunction in essential tremor. Cerebellum 2012;11(4):872–9. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998;13(1):5–10. [DOI] [PubMed] [Google Scholar]
- Papavassiliou E, Rau G, Heath S, Abosch A, Barbaro NM, Larson PS, et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery 2004;54(5):1120–29; discussion 1129–1130. [DOI] [PubMed] [Google Scholar]
- Patel N, Ondo W, Jimenez-Shahed J. Habituation and rebound to thalamic deep brain stimulation in long-term management of tremor associated with demyelinating neuropathy. Int J Neurosci 2014;124(12):919–25. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Metman LV, Toleikis JR, Hughes LE, Sani SB, Bakay RA. Factors involved in long-term efficacy of deep brain stimulation of the thalamus for essential tremor. J Neurosurg 2008;109(4):640–6. [DOI] [PubMed] [Google Scholar]
- Pullman SL. Spiral analysis: a new technique for measuring tremor with a digitizing tablet. Mov Disord 1998;13(Suppl 3):85–9. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Deuschl G. The oscillating central network of essential tremor. Clin Neurophysiol 2012;123(1):61–4. [DOI] [PubMed] [Google Scholar]
- Rudzinska M, Izworski A, Banaszkiewicz K, Bukowczan S, Marona M, Szczudlik A. Quantitative tremor measurement with the computerized analysis of spiral drawing. Neurol Neurochir Pol 2007;41(6):510–6. [PubMed] [Google Scholar]
- Sabra AF, Hallett M. Action tremor with alternating activity in antagonist muscles. Neurology 1984;34(2):151–6. [DOI] [PubMed] [Google Scholar]
- San Luciano M, Wang C, Ortega RA, et al. Digitized spiral drawing: A possible biomarker for early parkinson’s disease. PLoS ONE 2016;11(10):e0162799 10.1371/journal.pone.0162799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Derby C, Stanley K, Floyd A, Bressman S, Lipton RB, Deligtisch A, Severt L, Yu Q, Kurtis M, Pullman SL. Validity of spiral analysis in early Parkinson’s disease. Mov Disord 2008;23(4):531–7. [DOI] [PubMed] [Google Scholar]
- Schlaier J, Anthofer J, Steib K, Fellner C, Rothenfusser E, Brawanski A, Lange M. Deep brain stimulation for essential tremor: targeting the dentato-rubro-thalamic tract? Neuromodulation 2015;18(2):105–12. [DOI] [PubMed] [Google Scholar]
- Springer US, Bowers D, Goodman WK, Shapira NA, Foote KD, Okun MS. Long-term habituation of the smile response with deep brain stimulation. Neurocase 2006;12(3):191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T Will ventralis intermedius deep brain stimulation for tremor be replaced by posterior subthalamic area or caudal zona incerta stimulation? World Neurosurg 2012;78(5):445–6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Katayama Y, Kano T, Kobayashi K, Oshima H, Fukaya C. Deep brain stimulation for the treatment of parkinsonian, essential, and poststroke tremor: a suitable stimulation method and changes in effective stimulation intensity. J Neurosurg 2004;101(2):201–9. [DOI] [PubMed] [Google Scholar]