Abstract
Prior research has demonstrated poorer patient-provider communication ratings among African American compared to White patients. The quality of patient-provider communication has been shown to impact treatment outcomes among cancer patients. A secondary data analysis design was used to determine the relationship of six patient-provider communication variables on the physical health quality of life (PHQOL) and mental health quality of life (MHQOL) of African American and White cancer patients (N = 479). We also examined whether the relationship between communication patterns and QOL differed based on race/ethnicity. Mean physical and mental health QOL scores for the sample were 69.8 and 77.6, respectively. After controlling for significant socio-demographic, clinical, and hospital variables, results showed that patients who experienced fewer interpersonal communication barriers who were more satisfied with the information given by providers had higher PHQOL and MHQOL scores. Additionally, patients who felt more comfort in asking questions or had fewer unmet information needs had higher MHQOL. A stratified analysis showed that the relationship of overall satisfaction with information on MHQOL was stronger among African American patients than White patients. Future research should focus on the development of interventions to improve patient-provider communication as a means for enhancing QOL outcomes among cancer survivors.
Introduction
Cancer is the second largest leading cause of death in the United States (Siegel, Miller, & Jemal, 2015). Advances in health care and health care technology have resulted in the dramatic improvement of overall cancer survival rates (Masters et al., 2015). Currently, overall five-year cancer survival rates are at 68% (Siegel et al., 2015) with even higher survival rates among the more commonly occurring cancers (breast, prostate, colorectal) (Siegel, Miller, & Jemal, 2016). Following improvements in cancer survival rates the relative importance of quality of life (QOL) as a key patient outcome has substantially increased (Hsu, Ennis, Hood, Graham, & Goodwin, 2013). Cancer-related QOL is a multidimensional construct defined as the degree to which one’s physical, emotional, social, and spiritual well-being are affected by an illness and its treatment (Ferrell, Dow, & Grant, 1995). Prior research has identified a range of important sociodemographic (e.g., age, race, income, education, and marital status) (Ashing-Giwa & Lim, 2009; Ashing-Giwa, Tejero, Kim, Padilla, & Hellemann, 2007; Ganz et al., 2002; Howard-Anderson, Ganz, Bower, & Stanton, 2012), clinical (e.g., type of cancer diagnosis and treatment type) (Bower et al., 2006; Broeckel, Jacobsen, Balducci, Horton, & Lyman, 2000), and psychosocial factors (e.g., social support, depression) (Allart, Soubeyran, & Cousson-Gélie, 2013) that influence QOL among cancer survivors. However, far less is known about the relationship of the patient-provider relationship, specifically patient-provider communication, on cancer-related QOL outcomes. The purpose of this paper was to examine the relationship of patient-provider communication on physical and mental health QOL outcomes in a diverse sample of cancer survivors. An improved understanding of these relationships may inform the development of interventions to improve QOL outcomes among cancer survivors.
Patient-Provider Communication
Effective patient-provider communication is an essential component of delivering quality health care (President’s Advisory Committee on Consumer Protection and Quality in the Health Care Industry, 2008). There are two critical dimensions of effective patient–provider communication. Interpersonal communication refers to qualitative aspects of patient–physician interaction, such as physician supportiveness or respectfulness (Gordon, Street, Sharf, Kelly, & Souchek, 2006), relationship building (Levinson et al., 2008) or patient-centeredness (Johnson, Saha, Arbelaez, Beach, & Cooper, 2004). Instrumental communication refers to the mutual exchange of information between the patient and provider including patients’ descriptions of symptoms and concerns and providers’ explanations of diagnoses and treatment options. These two communication dimensions are thought to influence the establishment of rapport, trust, and to ensure that patients obtain the necessary information to make treatment decisions (Ashton et al., 2003; Ong, De Haes, Hoos, & Lammes, 1995).
Patient-Provider Communication and Health Outcomes
Among patients in general, good patient-provider communication has been associated with improved patient behaviors including increased treatment adherence (Schoenthaler, Allegrante, Chaplin, & Ogedegbe, 2012) and improved disease self-management (Dorflinger, Kerns, & Auerbach, 2013). Improvements in patient health outcomes have also been reported including improved symptom resolution, physical functioning, physiological status (e.g., blood pressure, HbA1c) and pain (Stewart, 1995). Studies specifically focused on cancer patients have shown similar impact on patient’s treatment participation (Street & Voigt, 1997), self-management behaviors (Walling et al., 2016) and physical and emotional health outcomes (Lake et al., 2014). Despite the importance of patient-provider communication, cancer patients’ communication needs often go unmet (Bruinessen et al., 2013). In addition to the demonstrated gaps in the communication quality experienced by cancer patients in general, some studies report more pronounced patient-provider communication barriers among racial and ethnic minority patients (Maly, Liu, Liang, & Ganz, 2015; Palmer et al., 2014). In a qualitative study of the experiences of African American cancer patients, study participants reported dissatisfaction with the level of communication from health care providers during cancer diagnosis and treatment phases in several areas including: (1) communication of cancer information; (2) communication of shared decision making; (3) communication of empathy and understanding; and (4) communication of respect (Song, Hamilton, & Moore, 2012). The study participants felt that communication barriers with the providers had negatively impacted their survivorship experience (Song et al., 2012). Prior research conducted by our group found that African American cancer patients reported significantly more interpersonal communication barriers, unmet information needs, and had lower satisfaction with the information received from physicians than white cancer patients (Matthews, Tejeda, Johnson, Berbaum, & Manfredi, 2012). Additional research is needed to better understand the implications of differences in patient-provider communication variables on cancer-related QOL outcomes by race.
Specific Aims
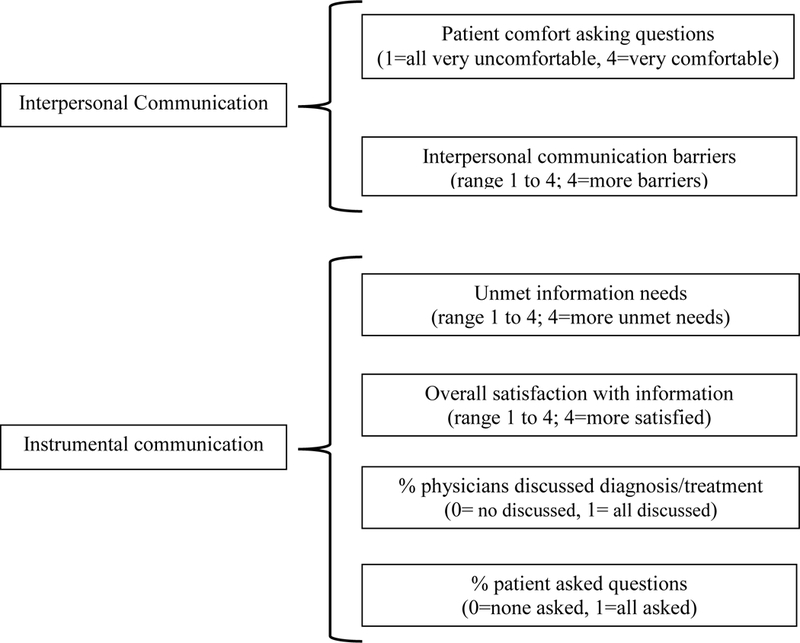
The purpose of this study was to examine the relationship of patient-provider communication variables on mental and physical health QOL outcomes in a sample of African American and White cancer patients. Patient-provider variables included two questions measuring interpersonal communication (comfort asking physician questions and interpersonal communication barriers) and four variables measuring instrumental communication (physicians discussed diagnosis and treatment, patient asked questions, information needs were unmet, and patient was satisfied with information overall). A further aim was to examine whether the relationship between patient-provider communication and QOL differed between African American and White patients.
Methods
Study Design and Sample
Data from this secondary data analysis study were from a larger survey study aimed at examining disease characteristics and cancer-related outcomes of African American and White cancer patients (Manfredi, Kaiser, Matthews, & Johnson, 2010). Eligibility criteria for the main study included: 1) African American or White race, 2) diagnosed with either breast, prostate or colorectal cancer, 3) being within three years of an initial cancer diagnosis. To obtain a representative sample of urban and non-urban African American cancer patients in Illinois, the following sampling procedures were used. First, patients from 79 Illinois hospitals with a cancer registry and located in the 15 Illinois counties with at least 10 reported African American cancer cases per year were recruited. Additionally, three other hospitals located in bordering states but known to serve Illinois residents were included. Second, to increase the number of non-urban African Americans in the sample, additional non-overlapping cases were identified from the Illinois State Cancer Registry (ISCR). Third, a similar white population was recruited from the same hospital pool as the African American patients and were matched based on cancer site, gender, age, and time since diagnosis. The same method for obtaining a comparable White sample was used to recruit patients from hospital and ISCR. A total of 753 patients meeting the above criteria were identified through 33 hospital registries and the Illinois State Cancer Registry. Of those, N=492 (81.5%) of eligible African American (n=248) and White n=244) patients completed the telephone interview (on average lasted between 60 to 90 minutes) by the Survey Research Laboratory (SLR) at the University of Illinois at Chicago. Interviewers read study questions to participants. Prior to the telephone interview, study participants were mailed response cards that corresponded to each of the survey items. This approach was used to reduce participant burden by eliminating the necessity of remembering lengthy response options. The final analytic sample included 479 (97.4%) patients after excluding thirteen patients with missing hospital data. The study was approved by the Institutional Review Boards (IRB) of the University of Chicago and the University of Illinois at Chicago. (see (Manfredi et al., 2010) for full details for study design and data collection).
Measures
Socio-demographic factors included: Race (African American, White), gender (male, female), education (less than high school, high school/GED, some college, college degree and above), income (<$30,000, $30,000-$50,000, >$50,000), employment status (employed, unemployed), marital status (married, separated/divorced, widowed, not married) and health insurance status (none, public, private) were measured.
Clinical factors included: age at diagnosis (26–49, 50–64, 65–74, >75 years), time since diagnosis in months, cancer site (colorectal, breast, or prostate), currently in treatment (yes, no), cancer state at diagnosis (early, late, unknown), and the presence of other medical comorbidities (yes, no).
Hospital factors included: geographic area of diagnosis hospital (urban, suburban, rural, out of state), bed size, hospital with cancer center/program (yes, no), and teaching hospital status (yes, no).
Six patient-provider communication variables were measured (Manfredi et al., 2010): (1) Patient comfort asking questions, (2) Interpersonal communication barriers, (3) Physician discussed diagnosis/treatment, (4) Patient asked questions, (5) Unmet information needs, and (6) Patient satisfaction with overall information. Three types of these were assessed for each physician a patient reported seeing since the cancer diagnosis. (In this sample, the number of physicians seen ranged from 2 to 8.). For each patient, two of these variables were the percent of physician he or she had seen who (1) discussed the diagnosis and treatment with patient (ranged from 0: no physician discussed to 1: all physicians discussed), and (2) to whom the patient asked questions about the cancer diagnosis or treatment (from 0: did not ask of any physician to 1: asked of all physicians). The third variable was assessed how comfortable the patient was when asking questions (from 1: very uncomfortable with all physicians to 4: very comfortable with all physicians).
The three other communication types were assessed with reference to all physicians seen (i.e., “thinking of all physicians you have seen since your cancer diagnosis. .”). Interpersonal communication barriers were measured using the 4-item medical interactions subscale of the Cancer Rehabilitation Evaluation Form-Short Form (Schag, Ganz, & Heinrich, 1991). Each item indicated how often patients: (1) feel that physicians didn’t explain what they were doing to them, (2) had difficulty expressing their feelings to physician, (3) had difficulty telling their physicians about new symptoms, and (4) felt they needed more control over what physicians were doing. The response scale ranged from (1=never, 2=occasionally, 3=often, 4=very often). Higher composite scores indicated more frequent experiences of communication barriers. The scale has a Cronbach alpha coefficient of .62.
Unmet information needs were measured using the following four items: (1) had experienced difficulty understanding what physicians told them about their cancer or treatment, (2) felt they need more information about their illness, (3) needed more information about their treatment, and (4) felt physicians had discussed all available treatment options (Manfredi, Czaja, Buis, & Derk, 1993). Responses were made on a 4-point scale ranging from 1 = Never to 4 = Very often. Higher composite scores indicated greater unmet information needs. The Cronbach alpha coefficient was .76. Lastly “Overall Information Satisfaction” was measured with a single item ranging from 1=very dissatisfied to 4=very satisfied.
Physical Health Quality of Life (PHQOL) and Mental Health Quality of Life (MHQOL) were measured using the Medical Outcomes Study 36-item Short Form Health Survey (SF-36) (Ware, Kosinski, & Keller, 1994). PHQOL was made up of four subscales, including: (1) physical functioning, (2) role limitations due to physical problems, (3) bodily pain, and (4) general health. MHQOL was made up of four subscales, including: (1) energy/vitality, (2) social functioning, (3) role limitations due to emotional problems, and (4) mental health. Each summary scale score ranged from 0 to 100. Higher scores represented better quality of life. The general US population norms for the SF-36 MCS is a mean of 50 points with an SD of 10 points In the current sample, the internal consistency as measured by Cronbach’s alpha was 0.84 for the physical health summary scale and 0.81 for the mental health summary scale.
Data Analysis
Descriptive statistics (means, standard deviation, frequencies, and percentages) were used to describe the characteristics of the study population. The bivariate analysis (using Chi-Square, Mann-Whitney U test, Kruskal–Wallis) was performed to examine the associations between quality of life and patient-provider communication, socio-demographic factors, and clinical and hospital factors. For the multivariate analysis, a generalized linear regression model was performed to examine the association between patient-provider communication and the quality of life after controlling for significant socio-demographic and clinical/hospital factors (p<0.05). Next, the interaction effect between patient-provider communication and race on quality of life was examine. Finally, a stratified analysis was performed to examine whether the magnitude of association between patient-provider communication and the quality of life differed by race. All analyses were performed using SPSS version 16, a statistical software package.
Results
Sample Characteristics
Table 1 displays the demographic, clinical, and hospital characteristics of the study sample. The majority of study sample was White (52.1%), female (61.6%), had some college or above degree (56.9%), had income ≥ 30K (62.4%), were unemployed (56.3%), married (60%), and insured (89.5%). More patients were diagnosed with breast (49.6%) followed by prostate (28.9%) and colorectal cancers (21.6%). Most cancer patients were diagnosed at 64 years old or younger (61.1%), had been diagnosed less than 2 year (82.3%), were not currently in treatment (80%), and had not experienced a recurrence of their cancer (98%). Furthermore, the majority of cancer patients came from hospitals located in urban (37.2%) or suburban areas (28.2%). Most hospitals treated patients in the study were teaching hospitals (68.3%), had a cancer center or program (61.4%) and had at least 200 hospital beds (86%) (Table 1).
Table 1.
Characteristics of Study Sample (N=479)
| Quality of Life | |||||
|---|---|---|---|---|---|
| Variables | PHQOL (Mean=69.8; SD=23.8) |
MHQOL (Mean=77.6; SD=22.6) |
|||
| Socio-Demographic Factors | N (%) | Mean (SD) | p-value | Mean (SD) | p-value |
| Race | |||||
| African American | 223 (47.9) | 66.3 (24.2) | 0.00* | 73.6 (25.2) | 0.00* |
| White | 243 (52.1) | 72.9 (23.1) | 81.5 (18.9) | ||
| Gender | |||||
| Male | 184 (38.4) | 75.4 (21.7) | 0.00* | 80.6 (21.8) | 0.00* |
| Female | 295 (61.6) | 66.2 (24.4) | 75.7 (22.9) | ||
| Education | |||||
| <High School | 74 (15.9) | 61.4 (23.2) | 0.00* | 68.9 (25.6) | 0.00* |
| High School/GED | 126 (27.2) | 69.0 (24.5) | 78.5 (22.2) | ||
| Some College | 97 (20.9) | 66.5 (25.3) | 75.1 (25.8) | ||
| >College Degree | 167 (36.0) | 75.8 (21.2) | 82.4 (18.3) | ||
| Income | |||||
| <$30K | 167 (37.6) | 60.3 (25.8) | 0.00* | 69.7 (26.7) | 0.00* |
| $30K to $50K | 134 (30.2) | 71.4 (21.9) | 80.1 (20.4) | ||
| >$50K | 143 (32.2) | 78.8 (18.9) | 84.2 (16.5) | ||
| Employment Status | |||||
| Employed | 204 (43.7) | 77.5 (19.2) | 0.00* | 83.4 (17.2) | 0.00* |
| Unemployed | 78 (16.7) | 51.9 (24.5) | 63.1 (27.2) | ||
| Retired | 185 (39.6) | 69.2 (23.7) | 77.8 (23.0) | ||
| Marital Status | |||||
| Married | 204 (60.0) | 74.4 (22.0) | 0.00* | 81.4 (20.1) | 0.00* |
| 78 (33.1) | 61.3 (25.0) | 71.6 (25.1) | |||
| Separated/Divorced/Widowed | |||||
| Not Married | 185 (6.9) | 67.1 (23.0) | 72.2 (26.5) | ||
| Health Insurance | |||||
| Insured | 418 (89.5) | 71.0 (23.3) | 0.00* | 79.0 (21.6) | 0.00* |
| Uninsured | 49 (10.5) | 57.6 (24.8) | 65.1 (27.9) | ||
| Clinical Factors | |||||
| Age at Diagnosis | |||||
| 26–49 | 94 (19.6) | 71.4 (23.2) | 0.00* | 80.0 (20.1) | 0.38 |
| 50–64 | 199 (41.5) | 70.9 (23.6) | 76.3 (23.6) | ||
| 65–74 | 126 (26.3) | 69.8 (23.8) | 79.1 (21.5) | ||
| >75 | 60 (12.5) | 61.0 (24.7) | 74.4 (26.7) | ||
| Cancer Site | |||||
| Colorectal | 97 (21.6) | 70.6 (25.0) | 0.00* | 78.4 (22.4) | 0.05* |
| Breast | 223 (49.6) | 66.4 (23.5) | 75.6 (23.1) | ||
| Prostate | 130 (28.9) | 74.3 (22.8) | 80.2 (22.4) | ||
| Time since Diagnosis | |||||
| 0–6 months | 13 (2.7) | 65.3 (28.1) | 0.00* | 76.0 (23.2) | 0.1 |
| 7–12 months | 77 (16.1) | 65.1 (21.4) | 76.0 (22.4) | ||
| 12–18 months | 142 (29.6) | 67.1 (25.4) | 77.3 (21.4) | ||
| 19–24 months | 162 (33.8) | 71.8 (23.5) | 79.1 (22.4) | ||
| 24+ months | 85 (17.7) | 74.8 (22.2) | 76.5 (26.3) | ||
| Currently in Treatment | |||||
| Yes | 93 (20.0) | 64.6 (23.5) | 0.05* | 75.5 (23.5) | 0.58 |
| No | 372 (80.0) | 70.7 (23.8) | 78.0 (22.6) | ||
| Cancer Stage at Diagnosis | |||||
| Stage 0–2 | 161 (33.6) | 72.1 (22.2) | 0.52 | 79.7 (21.1) | 0.55 |
| Stage 3–4 | 43 (9.0) | 66.1 (24.9) | 75.6 (22.6) | ||
| Unknown | 275 (57.4) | 68.8 (24.6) | 76.6 (23.7) | ||
| Other Medical Co-morbidities Present | |||||
| Yes | 272 (58.2) | 63.3 (24.2) | 0.00* | 74.1 (24.5) | 0.00* |
| No | 195 (41.8) | 78.7 (20.1) | 82.5 (19.0) | ||
| Experienced Recurrence of Cancer | |||||
| Yes | 9 (2.0) | 57.0 (25.9) | 0.21 | 68.6 (27.4) | 0.17 |
| No | 439 (98.0) | 69.9 (23.8) | 77.7 (22.7) | ||
| Hospital Factors | |||||
| Teaching Hospital | 0.64 | 0.33 | |||
| Yes | 319 (68.3) | 69.1 (24.6) | 77.6 (22.2) | ||
| No | 148 (31.7) | 71.2 (21.8) | 77.6 (23.4) | ||
| Geographic Area of Hospital | |||||
| Urban | 178 (37.2) | 67.5 (24.4) | 0.21 | 75.2 (24.8) | 0.35 |
| Suburban | 135 (28.2) | 71.9 (22.6) | 78.4 (21.5) | ||
| Rural | 124 (25.9) | 70.4 (23.4) | 78.9 (22.1) | ||
| Out-of-State | 42 (8.8) | 68.8 (26.7) | 81.0 (18.3) | ||
| Bed Size | |||||
| 200 or less | 67 (14.0) | 69.7 (22.0) | 0.98 | 79.7 (21.5) | 0.5 |
| 201–400 | 208 (43.5) | 69.2 (24.5) | 75.8 (23.6) | ||
| 401–600 | 179 (37.4) | 70.1 (24.0) | 78.9 (22.3) | ||
| More than 600 | 24 (5.0) | 69.6 (22.6) | 77.1 (22.5) | ||
| Hospital has Cancer Center/Program | |||||
| Yes | 294 (61.4) | 69.9 (24.1) | 0.57 | 78.0 (23.0) | 0.4 |
| No | 185 (38.6) | 69.2 (23.4) | 76.9 (22.5) | ||
p-value<0.05;
PHQOL: Physical Health Quality of Life; MHQOL: Mental Health Quality of Life
Associations between Quality of Life and Socio-Demographic, Clinical, and Hospital Factors
First we examined the influence of socio-demographic, clinical and hospital factors on PHQOL and MHQOL outcomes. Mean QOL scores for the sample were 69.7 (SD=23.8) physical health and 77.6 (SD=22.6) for mental health. Socio-demographic factors including African American race, female gender, unemployed, lower levels of education, lower income, being separated/divorced/widowed, and being uninsured were associated with lower PHQOL and MHQOL scores (all p’s < .001). Clinical factors associated with lower PHQOL included older age, being diagnosed with breast cancer, a shorter time since diagnosis, being in treatment, and the presence of other medical co-morbidities. Clinical factors associated with lower MHQOL scores included a breast cancer diagnosis and the presence of other medical co-morbidities. None of the hospital factors examined were associated with QOL outcomes (see Table 1).
Relationship between Patient-Provider Communication and Quality of Life
Among patients, about 91% of physicians seen discussed disease and treatment, and 82% of patients asked questions about the cancer diagnosis or treatment. The mean scores for interpersonal communication barriers (range 1 to 4; 4=more barriers), comfort level asking questions (mean across all physicians seen, 1=all very uncomfortable, 4=all very comfortable), unmet information needs (range 1 to 4; 4=more unmet needs), and overall satisfaction with information (range 1 to 4; 4=more satisfied) were 1.53, 3.58, 2.31, and 3.45, respectively. In bivariate analyses we examined the relationship of patient-provider communication on PHQOL and MHQOL outcomes. Higher levels of PHQOL and MHQOL were associated with a higher number of physicians who had discussed the patient’s diagnosis or treatment with them (r=0.12, p=0.01; r=0.11, p=0.02), whether patients felt comfortable asking questions of physicians (r=0.14, p=0.00; r=0.24, p=0.00), and higher overall information satisfaction scores (r= 0.25, p=0.00; r=0.34, p=0.00). More interpersonal communication barriers (r= −0.20, p=0.00; r= −0.31, p=0.00) and unmet information needs (r= −0.09, p=0.04; r= −0.15, p=0.00), were associated with lower PHQOL and MHQOL scores, respectively. The only communication variable not associated with QOL outcomes was whether the patient asked questions of providers about their cancer diagnosis or treatment (see Table 2).
Table 2.
Association between Patient-Provider Communication and Quality of Life: Bivariate Analysis
| Patient-Provider Communication | Mean (SD) / % (SD) | PHQOL | MHQOL | ||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| % Physician discussed diagnosis/treatment | 0.91 (0.19) | 0.12 | 0.01* | 0.11 | 0.02* |
| % Patient asked questions | 0.82 (0.27) | 0.02 | 0.75 | 0.02 | 0.69 |
| Patient was comfortable asking questions | 3.58 (0.54) | 0.14 | 0.00* | 0.24 | 0.00* |
| Interpersonal communication barriers | 1.54 (0.64) | −0.20 | 0.00* | −0.31 | 0.00* |
| Patient had unmet information needs | 2.31 (0.54) | −0.09 | 0.04* | −0.15 | 0.00* |
| Patient’s overall satisfaction with information | 3.45 (0.61) | 0.25 | 0.00* | 0.34 | 0.00* |
p<0.05; PHQOL: Physical Health Quality of Life; MHQOL: Mental Health Quality of Life; SD: standard deviation; r: correlation coefficient
Generalized linear modeling was conducted to examine the relationship of patient-provider communication variables on QOL after controlling for significant socio-demographic and clinical variables. As shown in Table 3, in multivariate models, those patients who reported more interpersonal communication barriers scored lower on both PHQOL (β= −0.07, p=0.01) and MHQOL (β= −0.128, p=0.00). Higher overall satisfaction with information received was associated with higher PHQOL (β= 0.13, p=0.00) and MHQOL (β= 0.14, p=0.00). Additionally, patients who were more comfortable asking questions of the physician (β= 0.07, p=0.02) or who encountered more unmet informational needs (β= −0.07, p=0.04) were significantly associated with a higher and lower MHQOL, respectively. A significant interaction effect between race and overall information satisfaction on MHQOL was identified (p=0.04). Results from a stratified analysis further showed that overall information satisfaction had a greater effect on MHQOL among African American (β= 0.20, p=0.00) compared to White patients (β= 0.08, p=0.01) (Table 4).
Table 3.
The Influence of Patient-Provider Communication on Quality of Life: Multivariate Analysis
| Patient-Provider Communication | PHQOL1 | MHQOL2 | ||||
|---|---|---|---|---|---|---|
| Beta | SE | p-value | Beta | SE | p-value | |
| (1) % Physician discussed diagnosis/treatment | 0.17 | 0.09 | 0.06 | 0.17 | 0.09 | 0.07 |
| -Interaction term: “race x % physician discussed diagnosis/treatment” | −0.05 | 0.19 | 0.78 | 0.26 | 0.19 | 0.17 |
| (2) % Patient asked questions | −0.05 | 0.07 | 0.48 | 0.04 | 0.07 | 0.59 |
| -Interaction term: “race x % patient asked questions” | −0.10 | 0.13 | 0.44 | 0.01 | 0.13 | 0.97 |
| (3) Patient comfort asking questions | 0.04 | 0.03 | 0.31 | 0.07 | 0.03 | 0.02* |
| -Interaction term: “race x patient comfort asking questions” | −0.06 | 0.07 | 0.43 | 0.03 | 0.06 | 0.63 |
| (4) Interpersonal communication barriers | −0.07 | 0.03 | 0.01* | -0.12 | 0.03 | 0.00* |
| -Interaction term: “race x interpersonal communication barriers” | 0.07 | 0.06 | 0.25 | -0.05 | 0.06 | 0.44 |
| (5) Unmet information needs | −0.05 | 0.03 | 0.13 | -0.07 | 0.03 | 0.04* |
| -Interaction term: “race x unmet information needs” | −0.02 | 0.07 | 0.73 | -0.04 | 0.07 | 0.56 |
| (6) Overall information satisfaction | 0.13 | 0.03 | 0.00* | 0.14 | 0.03 | 0.00* |
| -Interaction term: “race x overall information satisfaction” | 0.03 | 0.06 | 0.65 | 0.12 | 0.06 | 0.04* |
p-value<0.05; PHQOL: Physical Health Quality of Life; MHQOL: Mental Health Quality of Life; Beta: regression coefficient; SE: standard error
The following variables are controlled in each model: Race, Gender, Education Level, Income, Employment Status, Marital Status, Health Insurance, Age at Diagnosis, Cancer Site, Time since Diagnosis (in months), Currently in Treatment, and Co-morbidities Present
The following variables are controlled in each model: Race, Gender, Education Level, Income, Employment Status, Marital Status, Health Insurance, Cancer Site, and Co-morbidities Present
Table 4:
The Influence of Patient-Provider Communication on Quality of Life: Stratified Analysis
| Patient-Provider Communication | MHQOL | |||||
| African American | White | |||||
| Beta | SE | p-value | Beta | SE | p-value | |
| Overall information satisfaction | 0.20 | 0.05 | 0.00* | 0.08 | 0.03 | 0.01* |
p-value<0.05; MHQOL: Mental Health Quality of Life; Beta: regression coefficient; SE: standard error
The following variables are controlled in the model: Race, Gender, Education Level, Income, Employment Status, Marital Status, Health Insurance, Cancer Site, and Co-morbidities Present
Discussion
The overall objective of this study was to determine the relationship of patient-provider communication on QOL outcomes among cancer patients, and to examine whether observed associations differed based on race. Consistent with the extant literature, a range of demographic and clinical factors were associated with QOL outcomes in bivariate analyses. Socio-demographic factors including African American race, female gender, being unemployed, having lower levels of education, lower income, being unmarried, and uninsured were associated with lower PHQOL and MHQOL scores. Clinical factors associated with lower PHQOL included older age, being diagnosed with breast cancer, a shorter time since diagnosis, being in treatment, and the presence of other medical co-morbidities. Clinical factors associated with lower MHQOL scores included a breast cancer diagnosis and the presence of other co-morbidities.
A range of patient-provider communication variables were examined to ascertain the relationship of different measures of patient-provider communication on physical health and mental health quality of life among cancer survivors. A strength of our study was that we measured both the interpersonal and instrumental dimensions of communication. Further, data was collected across all physicians seen by patients since their cancer diagnosis. The majority of study participants reported asking questions about their cancer diagnosis and treatment and physicians discussing cancer diagnosis and treatment options. Patients reported feeling comfortable asking questions, but occasionally had interpersonal communication barriers and unmet information needs. Overall they were satisfied with the information physicians provided.
Generalized linear modeling was conducted to examine the relationship of patient-provider communication variables on QOL after controlling for significant socio-demographic and clinical variables. The majority of the measures in patient-provider communication examined were associated with QOL, especially mental health QOL. Those patients who reported more interpersonal communication barriers scored lower on both PHQOL and MHQOL. Higher overall satisfaction with information received was associated with higher PHQOL and MHQOL. Manfredi and her colleagues found that the overall satisfaction with information received was associated with patient’s trust in physician knowledge and their desire to be involved in treatment decision making (Manfredi et al., 2010). Interventions aimed at building trust (Thom, Bloch, & Segal, 1999) and patient-centered communication skills can be used to increase the overall satisfaction with information provided by physicians. Additionally, patients who were more comfortable asking questions of the physician or who encountered more unmet informational needs were significantly associated with a higher and lower MHQOL, respectively. One possible explanation is that good patient-provider communication can increase the level of social support in cancer patients and then improves mental health quality of life (Matthews et al., 2012). The only two communication dimension not associated with QOL were “physician discussed diagnosis and treatment” and “patient asked questions.” It could be that no significant impact of the level of participation was found due to inappropriate assessment of patient participation. Problems of adequate measurements for the process of patient participation have been reported in other studies (Elwyn et al., 2001).
Study Implications
The quality of patient–provider communication has been identified as an important and potentially modifiable factor associated with improved patient outcomes (Beach et al., 2015). Study findings provide support for the need to development interventions for both patients and providers aimed at improving communication skills (Hesse, 2009). Patient–provider communication that is characterized by shared decision making has been consistently shown to improve patient outcomes in a range of acute and chronic health conditions, including cancer. In the past, provider-directed and patient-directed interventions have been used to improve commonly-faced communication challenges (Diefenbach et al., 2009). For providers, the curriculum in medical and nursing training programs should increase the level of training devoted to developing effective patient–physician communication skill. (Back and colleagues 2007) designed the curriculum and implemented the physician-focused workshop by incorporating features of various physician training program to help oncology physicians to improve communications with cancer patients on discussing treatment options, dealing with uncertainty, etc. (Back et al., 2007) . Furthermore, a range of communication skills have been shown to improve patient satisfaction with communication including expressing caring and warmth, being patient-centered, engaging in shared decision making, and making clarifying statements (Simpson et al., 1991). For patients, a patient education system can be developed and used for cancer patients who do not engage in much information seeking during medical interviews with providers to improve communication with physicians (Cegala, Post, & McClure, 2001; Diefenbach et al., 2009). For example, the PACE (Presenting, Asking, Checking, Expressing) education system is designed for improvement of patient’s communication with physician in presenting feelings; asking questions; checking understanding of information provided; and expressing any concerns about the treatment options (Cegala, McClure, Marinelli, & Post, 2000; Cegala et al., 2001).
Study Limitations
Despite the many strengths of this study, limitations should be noted. First, study findings are limited by the cross-sectional study design. Our study sample were African American and white cancer patients recruited from a single state in the Midwest. Therefore, study finding may not generalize to other race/ethnic minority groups or to cancer patients living outside of Illinois. In this study, white cancer patients were selected only from the counties in which African American cancer patients were recruited. Many Illinois counties were excluded from the study if insufficient numbers of African American cancer patients were treated in those counties. These may have had the effect of potentially under-representing white patients residing in more suburban or rural counties. All survey data were self-reported and potentially subject to recall bias. Another limitation was the relatively low reliability of several of the scales measuring interpersonal communication barriers. Although low, their alpha reliability with our data was still within the range deemed sufficient for exploratory research (Pedhazur & Schmelkin, 2013). Lastly, our study was not able to control for demographic information of providers such as race and gender which had been found to be associated with patient-provider relationship (Cooper-Patrick et al., 1999).
Conclusions
Barriers to effective patient-provider communication were associated with poorer mental and physical health QOL outcomes among this diverse sample of cancer patients. The relationship between communication and QOL was influenced by race such that the perception of overall satisfaction to cancer care and treatment had a stronger impact on the mental health QOL of African American compared to White patients. In the future, factors influencing racial disparities in overall satisfaction with patient-provider communication should be examined. Further, the results of the current study suggest that QOL among cancer survivors would be enhanced by interventions aimed at improving patient-provider communication dimensions.
Figure 1.
Types of Patient-Physician Communication
Acknowledgements
This research was supported by a grant from the National Cancer Institute, Bethesda, MD (1R01CA775-01A1). We thank the Illinois State Cancer Registry and the Tumor Boards at 33 participating hospitals for their cooperation with this study. This paper is dedicated to the loving memory of Barbara Smith - cancer survivor, community activist, and friend.
Reference
- Allart P, Soubeyran P, & Cousson-Gélie F (2013). Are psychosocial factors associated with quality of life in patients with haematological cancer? A critical review of the literature. Psycho-Oncology, 22(2), 241–249. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa KT, & Lim J.-w. (2009). Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Paper presented at the Oncology nursing forum [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, & Hellemann G (2007). Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Quality of life Research, 16(3), 413–428. [DOI] [PubMed] [Google Scholar]
- Ashton CM, Haidet P, Paterniti DA, Collins TC, Gordon HS, O’Malley K, … Wray NP (2003). Racial and ethnic disparities in the use of health services. Journal of general internal medicine, 18(2), 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back AL, Arnold RM, Baile WF, Fryer-Edwards KA, Alexander SC, Barley GE, … Tulsky JA (2007). Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Archives of internal medicine, 167(5), 453–460. [DOI] [PubMed] [Google Scholar]
- Beach MC, Roter DL, Saha S, Korthuis PT, Eggly S, Cohn J, … Wilson IB (2015). Impact of a brief patient and provider intervention to improve the quality of communication about medication adherence among HIV patients. Patient education and counseling, 98(9), 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, & Belin TR (2006). Fatigue in long-term breast carcinoma survivors. Cancer, 106(4), 751–758. [DOI] [PubMed] [Google Scholar]
- Broeckel JA, Jacobsen PB, Balducci L, Horton J, & Lyman GH (2000). Quality of life after adjuvant chemotherapy for breast cancer. Breast cancer research and treatment, 62(2), 141–150. [DOI] [PubMed] [Google Scholar]
- Bruinessen IR, Weel-Baumgarten EM, Gouw H, Zijlstra JM, Albada A, & Dulmen S (2013). Barriers and facilitators to effective communication experienced by patients with malignant lymphoma at all stages after diagnosis. Psycho-Oncology, 22(12), 2807–2814. [DOI] [PubMed] [Google Scholar]
- Cegala DJ, McClure L, Marinelli TM, & Post DM (2000). The effects of communication skills training on patients’ participation during medical interviews. Patient education and counseling, 41(2), 209–222. [DOI] [PubMed] [Google Scholar]
- Cegala DJ, Post DM, & McClure L (2001). The effects of patient communication skills training on the discourse of older patients during a primary care interview. Journal of the American Geriatrics Society, 49(11), 1505–1511. [DOI] [PubMed] [Google Scholar]
- Cooper-Patrick L, Gallo JJ, Gonzales JJ, Vu HT, Powe NR, Nelson C, & Ford DE (1999). Race, gender, and partnership in the patient-physician relationship. Jama, 282(6), 583–589. [DOI] [PubMed] [Google Scholar]
- Diefenbach M, Turner G, Carpenter KM, Sheldon LK, Mustian KM, Gerend MA, … McQueen A (2009). Cancer and patient–physician communication. Journal of health communication, 14(S1), 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorflinger L, Kerns RD, & Auerbach SM (2013). Providers’ roles in enhancing patients’ adherence to pain self management. Translational behavioral medicine, 3(1), 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn G, Edwards A, Mowle S, Wensing M, Wilkinson C, Kinnersley P, & Grol R (2001). Measuring the involvement of patients in shared decision-making: a systematic review of instruments. Patient education and counseling, 43(1), 5–22. [DOI] [PubMed] [Google Scholar]
- Ferrell B, Dow KH, & Grant M (1995). Measurement of the quality of life in cancer survivors. Quality of life Research, 4(6), 523–531. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, & Belin TR (2002). Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. Journal of the National Cancer Institute, 94(1), 39–49. [DOI] [PubMed] [Google Scholar]
- Gordon HS, Street RL, Sharf BF, Kelly PA, & Souchek J (2006). Racial differences in trust and lung cancer patients’ perceptions of physician communication. Journal of Clinical Oncology, 24(6), 904–909. [DOI] [PubMed] [Google Scholar]
- Hesse BW (2009). Cancer communication: status and future directions. Journal of health communication, 14(S1), 109–127. [DOI] [PubMed] [Google Scholar]
- Howard-Anderson J, Ganz PA, Bower JE, & Stanton AL (2012). Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. Journal of the National Cancer Institute [DOI] [PubMed] [Google Scholar]
- Hsu T, Ennis M, Hood N, Graham M, & Goodwin PJ (2013). Quality of life in long-term breast cancer survivors. Journal of Clinical Oncology, 31(28), 3540–3548. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Saha S, Arbelaez JJ, Beach MC, & Cooper LA (2004). Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. Journal of general internal medicine, 19(2), 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J, Wall MM, Berman AR, Salazar-Schicchi J, Powell CA, Keller SM, … Wisnivesky JP (2014). Association Of Patient-Provider Communication Domains With Lung Cancer Treatment B102. COMPARATIVE EFFECTIVENESS: TREATMENTS, RESEARCH, AND PRACTICE (pp. A3705-A3705): Am Thoracic Soc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W, Hudak PL, Feldman JJ, Frankel RM, Kuby A, Bereknyei S, & Braddock C III (2008). It’s not what you say…: racial disparities in communication between orthopedic surgeons and patients. Medical care, 46(4), 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly RC, Liu Y, Liang LJ, & Ganz PA (2015). Quality of life over 5 years after a breast cancer diagnosis among low-income women: Effects of race/ethnicity and patient-physician communication. Cancer, 121(6), 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi C, Czaja R, Buis M, & Derk D (1993). Patient use of treatment-related information received from the Cancer Information Service. CANCER-PHILADELPHIA-, 71, 1326–1326. [DOI] [PubMed] [Google Scholar]
- Manfredi C, Kaiser K, Matthews AK, & Johnson TP (2010). Are racial differences in patient–physician cancer communication and information explained by background, predisposing, and enabling factors? Journal of health communication, 15(3), 272–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters GA, Krilov L, Bailey HH, Brose MS, Burstein H, Diller LR, … Moasser M (2015). Clinical cancer advances 2015: Annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology, JCO 2014.2059. 9746. [DOI] [PubMed] [Google Scholar]
- Matthews AK, Tejeda S, Johnson TP, Berbaum ML, & Manfredi C (2012). Correlates of quality of life among African American and white cancer survivors. Cancer nursing, 35(5), 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong LM, De Haes JC, Hoos AM, & Lammes FB (1995). Doctor-patient communication: a review of the literature. Social science & medicine, 40(7), 903–918. [DOI] [PubMed] [Google Scholar]
- Palmer NR, Kent EE, Forsythe LP, Arora NK, Rowland JH, Aziz NM, … Weaver KE (2014). Racial and ethnic disparities in patient-provider communication, quality-of-care ratings, and patient activation among long-term cancer survivors. Journal of Clinical Oncology, JCO 2014.2055. 5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedhazur EJ, & Schmelkin LP (2013). Measurement, design, and analysis: An integrated approach: Psychology Press. [Google Scholar]
- President’s Advisory Committee on Consumer Protection and Quality in the Health Care Industry. (2008). Final Report: Quality First: Better Care for All Americans Retrieved from http://www.hcqualitycommission.gov/final/.
- Schag C, Ganz PA, & Heinrich RL (1991). Cancer rehabilitation evaluation system–short form (CARES-SF). A cancer specific rehabilitation and quality of life instrument. Cancer, 68(6), 1406–1413. [DOI] [PubMed] [Google Scholar]
- Schoenthaler A, Allegrante JP, Chaplin W, & Ogedegbe G (2012). The Effect of Patient–Provider Communication on Medication Adherence in Hypertensive Black Patients: Does Race Concordance Matter? Annals of Behavioral Medicine, 43(3), 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2015). Cancer statistics, 2015. CA: a cancer journal for clinicians, 65(1), 5–29. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2016). Cancer statistics, 2016. CA: a cancer journal for clinicians, 66(1), 7–30. [DOI] [PubMed] [Google Scholar]
- Simpson M, Buckman R, Stewart M, Maguire P, Lipkin M, Novack D, & Till J (1991). Doctor-patient communication: the Toronto consensus statement. BMJ: British Medical Journal, 303(6814), 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Hamilton JB, & Moore AD (2012). Patient-healthcare provider communication: perspectives of African American cancer patients. Health Psychology, 31(5), 539. [DOI] [PubMed] [Google Scholar]
- Stewart MA (1995). Effective physician-patient communication and health outcomes: a review. CMAJ: Canadian Medical Association Journal, 152(9), 1423. [PMC free article] [PubMed] [Google Scholar]
- Street RL, & Voigt B (1997). Patient participation in deciding breast cancer treatment and subsequent quality of life. Medical Decision Making, 17(3), 298–306. [DOI] [PubMed] [Google Scholar]
- Thom DH, Bloch DA, & Segal ES (1999). An intervention to increase patients’ trust in their physicians. Stanford Trust Study Physician Group. Academic Medicine, 74(2), 195–198. [DOI] [PubMed] [Google Scholar]
- Walling AM, Keating NL, Kahn KL, Dy S, Mack JW, Malin J, … Tisnado D (2016). Lower Patient Ratings of Physician Communication Are Associated With Unmet Need for Symptom Management in Patients With Lung and Colorectal Cancer. Journal of Oncology Practice, 12(6), e654-e669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Kosinski M, & Keller S (1994). SF-36 physical and mental summary scales: A user’s manual 1994. Boston: The Health Institute, New England Medical Center Google Scholar. [Google Scholar]


