Abstract
Cytokine-based therapies for cancer have not achieved widespread clinical success because of inherent toxicities. Treatment for pancreatic cancer is limited by the dense stroma that surrounds tumors and by an immunosuppressive tumor microenvironment. To overcome these barriers, we developed constructs of single-domain antibodies (VHHs) against PD-L1 fused with IL2 and IFNγ. Targeting cytokine delivery in this manner reduced pancreatic tumor burden by 50%, whereas cytokines fused to an irrelevant VHH, or blockade of PD-L1 alone, showed little effect. Targeted delivery of IL2 increased the number of intratumoral CD8+ T cells, whereas IFNγ reduced the number of CD11b+ cells and skewed intratumoral macrophages towards the display of M1-like characteristics. Imaging of fluorescent VHH-IFNγ constructs, as well as transcriptional profiling, demonstrated targeting of IFNγ to the tumor microenvironment. Many tumors and tumor-infiltrating myeloid cells express PD-L1, rendering them potentially susceptible to this form of targeted immunotherapy.
Introduction
Targeted cytokine delivery to the tumor microenvironment can have a powerful effect on the immune landscape of tumors (1). Cytokines act in autocrine or paracrine fashion and have short half-lives. The concentration of cytokines at the right location is thus critical, yet the few cytokine-based therapies used in the clinic, such as IL2 and IFNα, are given systemically, often resulting in severe dose-limiting toxicities (1, 2). Efficacy of cytokine-based therapies is limited by an inability to deliver them to the proper location and an incomplete knowledge of the effects of particular cytokines in various cancer types.
The construction of antibody-cytokine fusions is an established preclinical approach to target cytokine therapy to the tumor microenvironment. However the size of these adducts results in persistence in circulation and comparatively poor tissue penetration. As an alternative to full-sized antibodies as fusion partners, we developed alpaca-derived heavy chain-only antibody fragments (VHHs) against programmed death-ligand 1 (PD-L1)(3). In cancer patients, PD-L1 expression is confined largely to the tumor microenvironment, being expressed on a wide variety of tumor cell types and tumor infiltrating myeloid cells (4). VHHs, at ~15 kDa, more readily penetrate tumors than full-sized antibody fusions. Antibody-cytokine fusions designed for delivery of IL2 to the tumor microenvironment have been tried before (5). Unfortunately, the high affinity of IL2 for its receptors and the abundant expression of IL2 receptors in peripheral blood, spleen, and liver, leads to a distribution of the antibody-cytokine fusion that is dictated primarily by the cytokine partner rather than the antibody to which it is attached for targeted delivery. Mathematical modeling predicts that smaller sized protein fusions would allow IL2 to concentrate in the tumor microenvironment (5). Although VHHs have a short circulatory half-life of ~30 minutes, they can remain bound to their targets in vivo for more than 24 hours(6, 7). The rapid clearance of VHHs from the circulation, combined with their high affinities and long tissue half-lives, means that it should be possible to concentrate VHHs and attached payloads in the tumor microenvironment while minimizing systemic exposure. VHHs accept a variety of payloads, including radioisotopes and cytokines, which can be installed in a straightforward manner(3, 8-11). Disruption of immune checkpoint interactions by monoclonal antibodies (mAbs) has replaced chemotherapy as the standard of care for metastatic melanoma and is similarly promising in the treatment of other cancers (4, 12-14). Checkpoint blockade largely augments a pre-existing T-cell response, and has had far less efficacy against tumors that are poorly infiltrated by T cells at baseline (15, 16). Cytokine-based therapies may be particularly promising as a means of manipulating the immune microenvironment. We have chosen to focus on pancreatic ductal adenocarcinoma (PDAC), a tumor type unresponsive to checkpoint blockade (13, 17, 18).
Pancreatic ductal adenocarcinoma is one of the deadliest cancers, with a 5-year survival rate of 8% (19). The disease is rapidly metastatic, and the majority of primary pancreatic tumors are inoperable due to invasion of the surrounding vasculature. Pancreatic tumors are dense, fibrotic masses that preclude adequate drug delivery and may limit accessibility for full-sized antibodies (20). The dense stroma creates a nutrient-poor, immunosuppressive environment. Approximately 60% of human PDAC tumors express PD-L1 (staining >10% by immunohistochemistry) (21). The majority of immune cells, both in human tumors and mouse models, are cells of the myeloid lineage, with both granulocytic and monocytic myeloid-derived suppressor cells (MDSCs), as well as tumor-associated macrophages (TAMs) contributing to local immunosuppression. Many human and mouse PDAC tumors are devoid of CD8+ T-cell infiltrates at baseline, suggesting that T cells are either not primed against PDAC antigens, fail to reach the tumor at all or are rendered non-functional due to early establishment of an immunosuppressive microenvironment (22). Strategies to reduce infiltration of myeloid cells or to reprogram these cells to an alternative fate can enhance CD8+ T-cell infiltration and synergize with checkpoint blockade therapy (23-25). Reprogramming of myeloid cells can lead to a restructuring of extracellular matrix and allow for more effective drug delivery (26, 27). Tumor cell death releases antigens and can further prime protective antigen-specific T-cell responses (28). Pancreatic tumors, even in the absence of neoantigens, contain self-antigens that, under certain circumstances, can be recognized by CD8+ T cells to mediate tumor regression (29, 30). We propose that effective therapy for pancreatic cancer will incorporate both myeloid targeting and a T-cell response as part of a multi-pronged regimen.
Here we use an anti–PD-L1-VHH to target the tumor microenvironment. We show that radiolabeled anti–PD-L1-VHH accumulates in orthotopically implanted pancreatic tumors. We then generated fusions of the anti–PD-L1 VHH with IL2 or IFNγ to direct these fusions to the tumor. Treatment with the IL2 fusion showed a 50% reduction in overall tumor burden in the Panc02 model and an increase in the intratumoral ratio of CD8+ T cells to CD4+ Tregs, whereas the IFNγ fusion caused a profound reduction in size of Panc02, KPC, and M19 (KPC organoid) orthotopic tumors, largely by reprogramming intratumoral macrophages.
Materials and Methods
Cloning and Expression of B3-IL2
B3 and A12 are specific for PD-L1 as described (3). The B3-IL2 coding sequence was subcloned into the E.coli periplasmic expression vector pET22b, with the inclusion of a C-terminal sortase motif and His6 tag. BL21(DE3) E. coli containing the plasmid was grown to mid-log phase at 37°C in TB plus ampicillin, and induced with 0.5 mM IPTG overnight at 25°C. Cells were harvested by centrifugation at 5000×g for 15 minutes at 4°C, then resuspended in 25 ml 1x TES buffer (200 mM Tris, pH 8, 0.65 mM EDTA, 0.5 M sucrose) per liter culture, and incubated for 1 hour at 4°C with agitation. Resuspended cells were then submitted to osmotic shock by diluting 1:4 in 0.25x TES, and incubating overnight at 4°C. The periplasmic fraction was isolated by centrifugation at 8000rpm for 30 min at 4°C, and then loaded onto Ni-NTA (Qiagen) in 50 mM Tris, pH 8, 150 mM NaCl and 10 mM imidazole. Protein was eluted in 50 mM Tris, pH 8, 150 mM NaCl, 500 mM imidazole and 10% glycerol, then loaded onto a Superdex 75 16/600 column (GE Healthcare) in 50 mM Tris, pH 8, 150 mM NaCl, 10% glycerol. Recombinant VHH purity was assessed by SDS-PAGE, and peak fractions were recovered and concentrated with an Amicon 10,000 KDa MWCO filtration unit (Millipore), and stored at −80°C.
Cloning and Expression of VHH-IFNγ
The PDL1 specific VHH A12-, B3-, and irrelevant specificity VHHCTR(1B7)-IFNγ coding sequences were subcloned into the mammalian expression vector pVRC and transiently transfected using polyethyleneimine into HEK293F cells cultured in Freestyle media (ThermoFisher) (3). Media containing secreted protein was harvested six days following transfection by centrifugation at 8,000 × g for 20 min at 4°C, then loaded onto a HiTrap NiNTA column (GE Healthcare) and washed with 50 mM Tris, pH 8, 150 mM NaCl and 10 mM imidazole. Protein was eluted in 50- mM Tris, pH 8, 150 mM NaCl, 500 mM imidazole and 10% glycerol, then loaded onto a Superdex 200 16/600 column (GE Healthcare) in 50 mM Tris, pH 8, 150 mM NaCl, 10% glycerol. Recombinant VHH purity was assessed by SDS-PAGE, and peak fractions were recovered and concentrated with an Amicon 10,000 KDa MWCO filtration unit (Millipore), and stored at −80°C.
C-terminal labeling of VHHs with Biotin or Alexa647
A heptamutant (“7M”) variant of S. aureus sortase A, 7M-SrtA (10, 11), was used to label B3, B3-IFNγ or VHHCTR(1B7)-IFNγ by incubating 30 μM purified VHH protein with 5 μM 7M-SrtA and 100 μM GGGK-Biotin or GGGK-Alexa647 nucleophiles in 50 mM Tris, pH 8, 150 mM NaCl for 2 hours at room temperature. Unreacted VHH and 7M-SrtA were removed by adsorption onto Ni-NTA agarose beads (Qiagen). The unbound fraction was concentrated and excess nucleophile with an Amicon 3,000 KDa MWCO filtration unit (Millipore), and stored at −80°C.
LPS removal
All therapeutics were depleted of LPS (<2 IU/mg), or purchased LPS-free from the manufacture. To remove LPS, VHHs were immobilized on HisTrap HP 1 mL columns (GE Healthcare) in PBS, washed with 40 column volumes PBS + 0.1% TritonX-114, and eluted in 2.5 column volumes endotoxin-free PBS (Teknova) with 500 mM imidazole. Imidazole was removed by PD10 column (GE Healthcare), eluting in LPS-free PBS. LPS content was tested using the LAL Chromogenic Endotoxin Quantitation Kit (Pierce) according to the manufacturer’s instructions.
Animal care
Animals were housed at either the Whitehead Institute for Biomedical Research (B16 experiments) or the Dana-Farber Cancer Institute (pancreatic tumor experiments, CT26 experiments, and PET imaging) and were maintained according to protocols approved by the MIT Committee on Animal Care or the DFCI IACUC, respectively. C57BL/6 and RAG2−/− mice were purchased from Jackson Labs.
Orthotopic pancreatic tumors
Orthotopic surgeries were performed as described (31). Briefly, C57BL/6 or RAG2−/− mice were anesthetized with a ketamine/xylazine cocktail, shaved on the left flank, and the surgical site cleaned with ethanol and betadine. An incision was made in the skin and peritoneum, and the pancreas externalized with forceps. Panc02, KPC cells, or KPC organoids (M19 line, kindly provided by David Tuveson (31)) were resuspended in PBS and mixed 1:1 by volume with Matrigel (Corning) for a total of 100,000 cells per 30 μL. The cell suspension was kept on ice and drawn into a chilled insulin syringe. Cells were then injected into the tail of the pancreas, and a bubble was observed. Mice that showed signs of leakage were removed from the experiment. The pancreas was left external to the body cavity for 1 min with the mice on a warming pad to solidify the Matrigel. The pancreas was then reinserted, peritoneum sutured with one stitch of absorbable suture, and the skin stapled with a sterile wound clip. Mice were given analgesia (Buprenex) and monitored post-surgery according to protocols approved by the Dana-Farber IACUC. Mice were sacrificed 21 d post-surgery unless otherwise indicated. Tumors were weighed at the time of sacrifice. In some cases, we combined multiple smaller experiments that were started at slightly different times. In these cases, we normalized tumor masses to the mean mass of the control tumors in order to compare across experiments. These values are reported as “relative tumor mass”.
Tumor infiltrate analysis
Pancreatic tumors were excised, weighed, minced and incubated in RPMI containing collagenase and anti-trypsin at 37 °C for 1 h. Tumors were filtered through a 40 micron cell strainer, washed with PBS, and centrifuged. The resulting cell pellet containing tumor debris and infiltrating immune cells was resuspended in FACS buffer (PBS with 2% fetal calf serum) and stained with a master mix of antibodies. Cells were incubated with staining mix for 30 min at 4 °C, washed once in PBS, and resuspended in 1% formalin prior to analysis on a BD Fortessa flow cytometer. All tumor infiltrates were first gated on CD45+ cells.
Flow cytometry
Cells were harvested from spleen or draining lymph nodes were dispersed into PBS through a 40 micron cell strainer using the back of a 1 mL syringe plunger. Cell preparations were subjected to hypotonic lysis to remove erythrocytes, stained and analyzed using a Fortessa cytofluorimeter (BD). Flow cytometry fluorescent dye–conjugated antibodies to the following proteins were purchased from Biolegend (B220-PacificBlue [clone RA3-6B2], CD107a-Fitc [1D4B], CD11b-AlexaFluorophore 488 [M1/70], CD11c-APC [N418], CD11c-PE/Cy7 [N418], CD25-AlexaFluorophore 488 [PC61], CD45-Bv711 [30-F11], CD4-APC [RM4-5], CD4-PacificBlue [RM4-5], CD69-PE [H1.2F3], CD8-Bv650 [53-6.7], FoxP3-PE [MF-14], Gr1-PE [RB6-8C5], Gr1-PE/Cy7 [RB6-8C5], MHC I-A/I-E-Bv510 [M5/114.15.2], NK1.1-FITC [PK136], NK1.1-PE/Cy7 [PK136], PD-1-PE/Cy7 [29F.1A12], Tim3-APC [RMT3–23], PD-L1-APC [10F.9G2]), Affymetrix (CD19-PE [MB19-1]).
Two-photon confocal microscopy
After implantation of pancreatic tumors (post 7 d) organoid KPC (post 6 wk) (as describe above) and 1 h after intraperitoneal injection of B3-IFNγ or VHHCTR conjugated to Alexa Fluor 488, the entire pancreas bearing implanted tumors were harvested from adult mice and immediately fixed in phosphate buffer saline solution (PBS) containing 1% paraformaldehyde, 1.5% L-lysine, 0.2% sodium periodate, and sodium dibasic 0.1M Na2HPO4 solution overnight at 4 °C on a rotisserie. Samples were washed three times for 30 minutes each at 4 °C in wash buffer (PBS, 0.2% BSA, 0.1% Triton × 100) then block in blocking buffer (PBS, 0.5% BSA, 0.3% Triton × 100) containing 2.4G2 antibody to block non-specific binding of immunoglobulin to Fcγ receptors. The tissue samples were stained for three days at 4°C in blocking buffer containing DAPI, PE conjugated anti-CD11b clone M1/70 (Biolegend Cat#101208) and Alexa Fluor 647 conjugated anti-CD31 clone 390 (Biolegend Cat#102416). Samples were washed three times for 30 minutes each at 4 °C in wash buffer and incubated overnight in the same buffer at 4 °C. The whole pancreas bearing tumors were mounted on Superfrost Plus Slides (VWR Cat# 48311-703) in Fluoroshield mounting medium (Abcam Cat# ab104135) then covered with a number 1 cover glass (VWR Cat# 48393081).
Pancreatic samples were imaged on an Ultima Two-Photon Microscope (Prairie Technologies/Bruker) equipped with two Tsunami Ti:sapphire lasers with a 10-W MilleniaXs pump laser (Spectra-Physics) and a 20×/0.95-NA water-immersion objective (Olympus). The two-photon excitation wavelength was set between 815 and 826 nm for optimal fluorescence excitation. Fluorescence emission was detected with 665/65-nm, 590/50-nm, 525/50-nm, and 450/50-nm bandpass filters for four-color imaging. Z-stacks of the pancreas were captured and displayed as maximum projection. Raw image sequences were processed using Imaris 7.4.2 software (Bitplane Scientific). Each image channel was assigned a pseudo-color according to emitted light wavelengths (bp665/65nm, cyan; bp590/50nm, green; bp525/50nm, red; bp455/70nm). Scale bars were either 100μm or 5μm in length and displayed at the bottom right corner of the image.
Synthesis of (Gly)3-Cys-NOTA
(Gly)3-Cys-NOTA was synthesized as described (32, 33). In brief, maleimide-NOTA (Macromolecules) was dissolved in 0.1 M NaHCO3, pH 8.3. The tetrapeptide GGGC was added at room temperature for 30 min until LC-MS analysis indicated almost complete conversion to the product. The product was purified by RP-HPLC on a semipreparative column (C18 column, Gemini, 5 μm, 10 Å~ 250 mm; Phenomenex) at a flow rate of 5.0 mL/min: solvent A, 0.1% TFA in H2O; solvent B, 0.1% TFA in CH3CN. The desired product eluted from 15% to 20% (vol/vol) solvent B. Fractions containing pure product were collected and lyophilized. LC-MS calculated for C27H45N10O11S [M+H]+ was 717.298; found=717.305.
Enzymatic incorporation of NOTA chelator into single domain antibodies using sortase. The penta-mutant sortase A, with an improved kcat, was used. Reaction mixtures (0.5 mL) contained Tris’HCI (50 mM, pH 7.5), CaCl2 (10 mM), NaCl (150 mM), triglycine-NOTA (800 μM), VHH (300 μM), and sortase (5 μM). After incubation at 4 °C with agitation for 1 h, reaction products were analyzed by LC-MS, with yields generally >80%. When the yield was below 80%, the reaction was allowed to proceed for an additional hour, with addition of sortase to 10 μM and triglycine-NOTA to 1 mM. The reaction mixture was loaded into a pre-equilibrated PD-10 columns and eluted with 1xPBS, removing excess of NOTA substrate and GGHis6 byproduct. Ni-NTA beads were added to the collected fractions with agitation for 5 min at 25°C, followed by centrifugation to remove sortase and any remaining unreacted His-tagged VHH. The labeled VHH was stored at −20°C with 5% glycerol and was stable for at least six months.
Synthesis of 64Cu-VHHs
64Cu-VHHs were synthesized as described before (34, 35). In brief, a 1.5 mL centrifuge tube was loaded with purified NOTA labeled-VHH (50 μL, 1 mg/ml), PBS buffer (300 μL, pH 7.3) and 64CuCl2 (~1 mCi) in 200 mM NH4OAc buffer (75 μL, pH 6.5). The tube was sealed and shaken at 37 °C for 20 min. The mixture was analyzed by radio-TLC (ITLC, 50 mM EDTA pH 7, Rf 64Cu/EDTA = 1.0, Rf 64Cu-VHH = 0.0) showing >95% conversion to 64Cu-VHH. At this time the mixture was loaded onto a PD-10 size-exclusion cartridge and elution with 1xPBS provided radiolabeled 64Cu-VHH, ready to be used for imaging. The amount injected to each mouse was in the range of 50-100 μCi.
PET imaging
64Cu-VHHs PET imaging was carried out on a dedicated small animal PET/CT scanner (Siemens Multimodality Inveon, Siemens Medical Solutions USA, Inc.). The mice were anesthetized using 2% sevoflurane/medical air inhalation prior to the radiotracer injection and throughout the scan duration. Warming was used to maintain healthy core body temperature of the mice during periods of unconsciousness. Following a bolus intravenous injection (via the lateral tail vein) of 64Cu-VHH-B3 (~3.3MBq), 64Cu-VHH-A12, or64Cu-VHH-96GM (~3.3MBq), and an uptake period of 120 min, a low dose CT scan was first acquired (80 kVp, 0.5 mA) for anatomical reference and to provide guidance for the delineation of selected tissue volume of interest (VOI). Static PET emission scans were then acquired in listmode format over 15 min and corrected for decay and dead time. The acquired data were then sorted into 0.5 mm sinogram bins and 1 time frame for image reconstruction using FORE/3D-OSEM-MAP image reconstruction. The reconstructed PET/CT images were analyzed with the Siemens Inveon Research Workplace software.
Melanoma models
B16 cells were purchased from ATCC. B16 GM-CSF and B16-ova cells were a gift from Glenn Dranoff (currently at Novartis Institute for Biomedical Research; Cambridge, MA). For in vivo challenge experiments, 5×105 B16 cells were inoculated by subcutaneous injection in 500 μL of Hank’s Balanced Salt Solution (HBSS). For vaccinations, 5×105 irradiated (3500 rads) GM-CSF secreting B16 cells (GVAX) were administered as a subcutaneous injection in 250 μL HBSS. VHHs, anti–PD-L1 (10F.9G2, BioXCell), and TA99 (gift from K. Dane Wittrup), were administered in 200 μL LPS Free PBS (TekNova) by intra-peritoneal injection. Tumor size was measured in two dimensions using precision calipers. Mice were euthanized when the total tumor volume exceeded 125 mm3.
Competition ELISAs
High binding microtiter plates (Corning) were coated with recombinant PD-L1-Fc fusion protein overnight at 25 ng/mL in carbonate buffer. Biotinylated VHHs or antibodies (BD Biosciences) were incubated on the coated plates in 10% inactivated fetal calf serum in phosphate buffered saline for 1 h in the presence or absence of unlabeled VHH B3 or mAb to PD-L1 (10F.9G2) across a range of concentrations, washed in 0.5% Tween in PBS and then developed using streptavidin-HRP (BD biosciences) and tetramethylbenzidine (TMB).
In vitro cytotoxicity assays
Spleen cells were isolated from OT-I RAG KO mice or from TRP1 RAG KO mice and were stimulated with αCD3/CD28 beads (Dynabeads) for 48 h at 2 × 106 cell/mL in RPMI according to manufactures instructions. Beads were then removed by magnetic separation, and cells were incubated for an additional 24 h in the culture supernatant. In parallel, 2.5×103 B16-ova or WT cells were incubated for 18 h in 250 μL RPMI containing 20 ng/mL IFNγ. IFNγ containing media was exchanged for fresh media; T cells were then co-cultured with the B16 cells for 48 h at a ratio of 2:1. VHHs or antibodies were added at the start of the culture. After 48 h, T cells were removed from the cultures by washing and B16 viability was assessed using the CellTiterGlo (CTG) bioluminescence assay according to the manufacturer’s instructions. Percent survival was calculated using untreated B16 cells cultured without T cells for normalization.
IL2 toxicity measurements
Blood was collected in heparin-coated tubes by cheek bleed. Hemoglobin was determined using a commercial complete blood counter available through Boston Children’s Hospital. ALT was measured in plasma using a commercially available NADH and LDH based method (Infinity ALT, Thermo). Mice were euthanized using CO2 asphyxiation, lungs were dissected and flash frozen in liquid nitrogen and weighed. Frozen tissue was then lyophilized at −80°C overnight and weighed again. The pulmonary wet weight was then calculated as the pulmonary weight at autopsy – the dry weight of the lungs after overnight lyophilization.
Statistical analysis
Two sample comparisons used Student’s t test with pooled variance if there was no evidence of inhomogeneity of variances between groups. If the variances were unequal, the exact Wilcoxon rank sum test, a nonparametric alternative to the t-test, was used. Every effort was made to keep testing consistent across related experiments. For comparisons of more than two groups, analysis of variance (ANOVA) was used if there was no evidence of inhomogeneity of variance; the Kruskal-Wallis test was the non-parametric alternative. Tumor growth studies were analyzed using mixed model ANOVA.
Results
Anti–PD-L1 VHH can penetrate dense pancreatic tumors
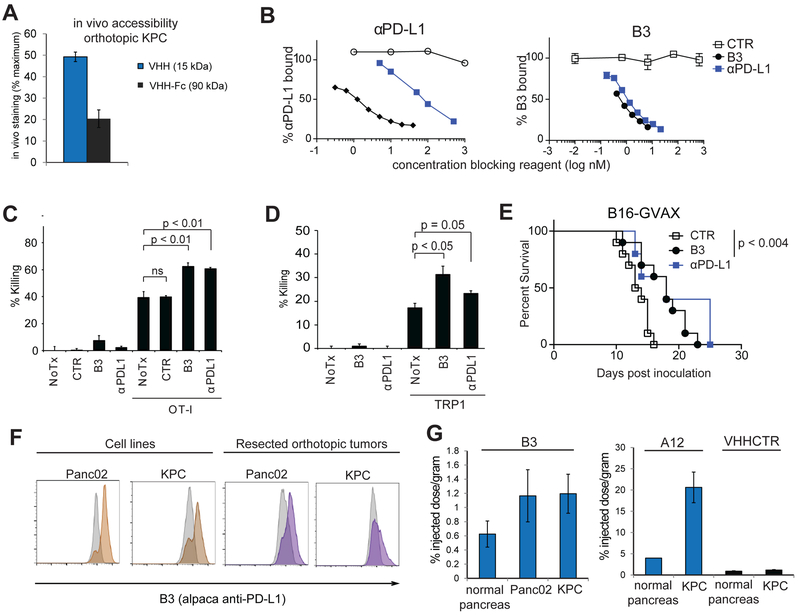
Pancreatic tumors have a dense stroma and are often poorly vascularized (20, 36-38). We inoculated either Panc02 cells or KPC cells (line derived from a K-rasLSL.G12D/+; p53R172H/+ PdxCre mouse, gift of A. Maitra) orthotopically into the pancreas (39). Orthotopic implants recapitulate many of the essential features of spontaneous pancreatic tumors, including the dense stromal infiltrate, the presence of suppressive myeloid cells, and poor vascularization (39). To assess whether the small size of VHHs would favor penetration into the stroma, we used a previously reported VHH against CD47, a widely expressed surface protein (6, 7). An anti-CD47-VHH (15 KDa) or a version of CD47-VHH fused to mouse IgG2a Fc (80 kDa) were conjugated to Alexa647 and injected intraperitoneally into mice bearing orthotopic KPC tumors (Fig. 1A). Tumors were resected 2 h later, and used to create a single cell suspension, which was then stained with excess labeled reagent to determine the maximal attainable binding. The anti–CD47-VHH showed greater binding after in vivo injection than did the larger Fc fusion. We conclude that the small size of VHHs indeed favors penetration of dense pancreatic tumors (Fig 1A).
Figure 1. PD-L1 blocking VHHs penetrate dense pancreatic tumors.
(A) C57BL/6 mice were inoculated orthotopically with KPC tumors. 14 days later, mice were injected IP with 100 μg of Alexa647 labeled A4 (VHH) or the molar equivalent of an Fc conjugated version (VHH-Fc). 2 hours post-injection, tumors were harvested, digested, and single-cell suspensions were either analyzed directly or incubated with excess labeled reagent prior to analysis by flow cytometry. In vivo staining is calculated as the percent of maximum mean fluorescence intensity achieved by in vivo injection alone. (B) plate bound PD-L1 was incubated with (left) αPD-L1-biotin in the presence of increasing concentrations of unlabeled VHH or antibody as indicated; (right) B3-biotin incubated in the presence of increasing concentrations of unlabeled VHH or antibody as indicated. (C) B16-ova cells treated for 18 hours with IFNγ (20 ng/mL) were washed to remove the IFNγ and were incubated with CD3/CD28 activated OT-I T cells and cultured for 48 hours in the presence of B3 or αPD-L1 as indicated. B16 survival was assessed using CellTiterGlo (D) IFNγ treated B16 wild-type cells were incubated with activated melanoma specific TRP1 cells for 48 hours with VHH or antibody as indicated. Tumor cell survival was measured by CellTiterGlo (E) C57BL/6 mice inoculated with B16 and were vaccinated with GM-CSF secreting B16 cells (GVAX) on day 0 and treated every other day with 250μg B3, αPD-L1, or CTR as indicated. CTR (N=10), B3 (N=10), αPD-L1 (N=5). (F) Panc02 or KPC cells either from tissue culture or isolated from resected orthotopic tumors, were stained with B3-Alexa647 and analyzed by flow cytometry. Grey histograms = staining with VHHCTR-Alexa647. (G) Mice bearing orthotopic KPC (N=2) or Panc02 (N=2) tumors were injected IV with 80 μCi 64Cu-B3 (top panel) or 80 μCi 64Cu-A12 or 64Cu-VHHCTR (bottom panel). Mice were imaged two hours later, sacrificed and scintillation counts obtained from tumor vs. normal pancreas. Graphs are from two different experiments, where scintillation counts were obtained on 24hr frozen samples (left) or freshly isolated tissue (right). All experiments representative of two independent replicates.
To target the tumor microenvironment, we used high affinity VHHs (B3 and A12), both of which recognize murine PD-L1 and inhibit interactions with its ligands, B7-1 and PD-1 (Fig. 1B and(3)). Both VHHs recognize PD-L1 with similar sub-nanomolar affinities, binding more strongly than the commercial mAb 10F.9G2 by −100 fold, and efficiently competing for binding with the mAb (Fig. 1B)(3). B3 recognizes only mouse PD-L1, whereas A12 cross-reacts with human PD-L1. B3 recognizes IFNγ-inducible PD-L1 on multiple murine cancer cell lines (Supplementary Fig. S1) (40). Consistent with its ability to block PD-L1 interactions with its targets, B3 augments in vitro cytotoxicity by OT-I cells against B16-OVA or by melanoma-specific TRP1 CD8 T cells cultured with B16 melanoma cells (Fig. 1C, 1D, and Supplementary Fig. S2) (41). B3 delays growth of B16 melanoma in vivo after vaccination with GVAX to a similar extent as the full-sized antibody against PD-L1 (Fig. 1E and Supplementary Fig. S3) B3 binds to the pancreatic cancer cell lines Panc02 and KPC in vitro, and ex vivo on cells from resected orthotopic tumors (Fig. 1F). B3, A12, and a control VHH were conjugated to 64Cu, and injected intravenously into tumor-bearing mice 2 h before harvesting the tumor (3, 8, 32). In B3 and A12 injected mice, accumulation of label was detected in the orthotopic pancreatic tumor, but not in adjacent normal pancreas (Fig. 1G). Neither B3 nor A12 show significant staining in spleen, lymph nodes, nor liver, although significant staining is found in brown adipose tissue (3).
Anti–PD-L1 VHH delivers IL2 to the tumor microenvironment without inducing systemic toxicity Although effective in a small subset of patients, systemic delivery of IL2 for cancer treatment is limited by its toxicity (14, 40, 42-45). In order to use a low, non-toxic dose of IL2 while maintaining a therapeutic effect, we conjugated murine IL2 to B3 (B3-IL2, Fig. 2A). Through its PD-L1 binding domain, we reasoned that the B3-IL2 fusion would be retained by the tumor, allowing for higher local doses of cytokine with lower systemic concentrations, preventing toxicity, yet achieving intratumoral concentrations sufficient for therapeutic benefit. B3-IL2 is considerably smaller (~32 kDa) than similar antibody conjugates (−170 kDa), and retained its affinity for PD-L1 (Fig. 2A-B). Likewise, the fusion retains IL2 activity, as shown by its ability to increase killing of B16-ova cells by OT-I cells (Fig. 2C). It also supported T-cell survival and CD8 skewing in cell culture to an extent comparable to that of recombinant IL2 (Fig. 2D). When administered at a low dose in vivo, (15 μg twice weekly over two weeks), B3-IL2 caused a modest increase in spleen size (Fig. 2E) with minimal toxicity, comparable to similar doses of recombinant IL2 or IL2 fused to a control VHH (Fig. 2F-I).
Figure 2. B3-IL2 fusion protein preserves PD-L1 binding and IL-2 cytokine activity.
(A) Diagram of B3-IL2. (B) flow cytometry using the indicated VHH fusions on B16 cells treated with IFNγ or left untreated (−). (C) OT-I T cells were cultured with B16-ova cells, treated and analyzed as in 1B. (D) WT spleen cells were cultured with equimolar concentrations (100 ng/mL IL2 equivalents) for 72 hrs and immune subsets were analyzed by flow cytometry. (E - I) WT mice were treated twice weekly with CTR (7.5 μg), B3 (7.5 μg), IL2 (7.5 μg), VHHCTR-IL2 (15 μg), or B3-IL2 (15 μg) as indicated for two weeks. (E) Spleen weight. (F) % change in body weight from baseline. (G) serum Hb or (H) ALT on day 14 measured using Infinity ALT (Thermo). (I) pulmonary wet weight, calculated as the pulmonary weight at autopsy – the dry weight of the lungs after overnight lyophilization at −80C. Lungs were flash frozen in liquid nitrogen prior to analysis. (J) C57BL/6 mice were inoculated with B16 and treated with the indicated antibodies or VHH constructs for 2 weeks. VHHCTR-IL2, B3-IL2 N=10, other groups N=5. TA99 is an antibody specific for the melanoma antigen TRP1. Results are combined from two experiments for (J), representative of two independent experiments for (A-I). **,p<0.01. ***p<0.001 using Student’s t test.
Adding a VHH domain to IL-2 is predicted to extend its serum half-life (5, 45).To control for possible effects of extended half-life IL2, we generated a control VHH fused to IL2 (VHHCTR-IL2), which has a similar size and serum half-life to B3-IL2, but without the PD-L1-–targeting domain. When used in combination with the anti-melanoma antibody TA99 (13, 17, 45), B3-IL2 slowed tumor growth and prolonged survival compared to treatment with TA99 and anti–PD-LI alone (Fig. 2J). Equimolar amounts of VHHCTR-IL2 and B3 admixed did not confer a survival advantage, indicating that IL2 conjugation to B3 is critical for efficacy (Fig. 2J).
Effective treatment of orthotopic pancreatic cancer and enhanced CD8+ T-cell accumulation.
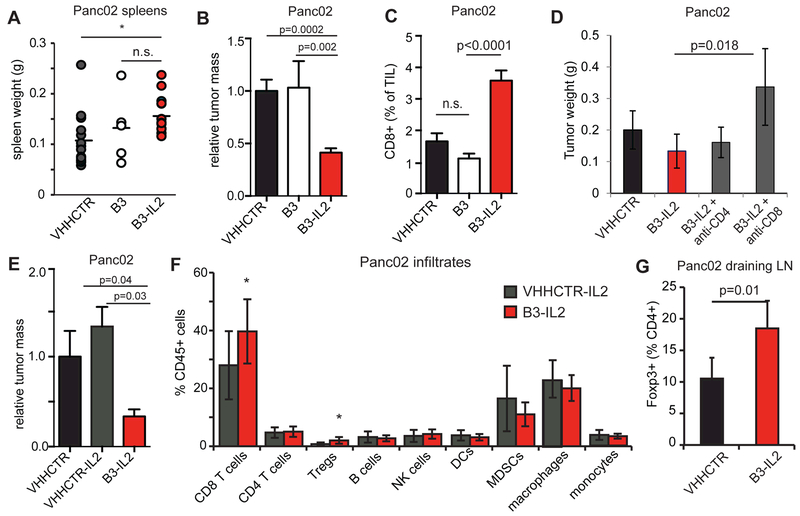
Having first validated B3-IL2 in B16 melanoma, we next tested B3-IL2 in orthotopic pancreatic cancer. PD-1 blockade has so far proven ineffective against pancreatic cancer in both mice and humans (13, 29). Treatment of mice bearing Panc02 orthotopic tumors with B3-IL2 caused a small increase in spleen weight relative to a control VHH and significantly reduced tumor size (Fig. 3A-B), which was accompanied by an increase in tumor-infiltrating CD8+ T cells in B3-IL2 treated animals (Fig. 3C). Depletion of CD8+ T cells resulted in increased growth of Panc02 tumors, confirming previous reports of CD8+ T-cell dependence in this model (Fig. 3D) (46). Treatment with B3 alone was without effect, showing that PD-L1 blockade was insufficient to confer a meaningful antitumor response in this model (Fig. 3B-C). We also saw improved responses of B3-IL2 when compared to treatment with IL2 fused to an irrelevant and similarly sized control VHH (VHHCTR-IL2), with a concomitant increase in CD8+ T-cell infiltration (Fig. 3E-F). The lack of efficacy of the control VHH-IL2 shows the importance of the B3 component for targeting IL2 to the tumor microenvironment. Foxp3+ Tregs express CD25 and expand in response to low doses of IL2 (47). Indeed, B3-IL2 enhanced the fraction of Tregs in the tumor microenvironment (Fig. 3F) and in the tumor draining lymph nodes (Fig. 3G), possibly counteracting some of the antitumor activity of the conjugate.
Figure 3. B3-IL2 augments response to orthotopic Panc02 tumors.
100,000 Panc02 cells were orthotopically implanted into C57BL/6 mice. Mice were treated IP with VHHCTR, B3 or B3-IL2 at 1 μg/mouse daily for 18 days. Tumors were harvested at day 21 post-implantation, weighed, digested and infiltrates analyzed by flow cytometry. (A) spleen weights from Panc02 inoculated mice treated with the indicated constructs. (B) tumor weights from mice treated with the indicated constructs. Results are pooled from multiple experiments, where each tumor weight is normalized to the mean of the VHHCTR treated tumors in that experiment to obtain relative tumor mass. VHHCTR N=19, B3 N=8, B3-IL2 N=22. (C) CD8+ cells as % of total tumor infiltrates (TILs). (D) 100,000 Panc02 cells were orthotopically implanted into C57BL/6 mice. Mice were treated IP with VHHCTR, B3 or B3-IL2 at 1 μg/mouse daily starting on day 5 post inoculation. Mice were given 250ug of anti-CD4, anti-CD8 or isotype by IP injection on days 5 and 11 post inoculation. Absence of CD4 or CD8 T cells was confirmed by flow cytometry of spleen cells at time of sacrifice (day 18 post inoculation). VHHCTR and B3-IL2, n=3; anti-CD4 and anti-CD8, n=5. (E) 100,000 Panc02 cells were orthotopically implanted into C57BL/6 mice. Mice were treated IP with B3-IL2 or VHHCTR-IL2 at 1 μg/mouse daily for 18 days. Tumors were harvested at day 21 post-implantation. N=8 mice per group. (F) Tumors from (E) were digested with collagenase and stained with antibodies to the indicated cell populations. Tregs were identified by intracellular Foxp3 staining. (G) Draining lymph nodes were isolated from mice treated as in (A-C) and Tregs were identified by intracellular Foxp3 staining. N=5 mice per group. Error bars are SEM (A-C) or SD (D-F). Results are combined from 4 experiments for (A-C), representative of two independent experiments for (D-G). *,p<0.05 using Student’s t test.
KPC tumors are less heavily infiltrated by CD8+ T cells. Although B3 penetrates into KPC tumors (Fig. 1G), B3-IL2 treatment of mice bearing orthotopic KPC tumors showed no increase in CD8+ T cells into the tumors, nor derived any therapeutic benefit in terms of tumor size, consistent with a minor role for CD8+ T cells in the KPC model (Supplementary Fig. S4A-B). An anti–PD-L1 VHH fusion with GM-CSF showed no reduction in tumor size either (Supplementary Fig. S4C-D), consistent with reports that pancreatic tumor cells themselves produce GM-CSF, which supports an immunosuppressive microenvironment in these tumors (35, 48, 49).
Fusion of PDL1-VHH to IFNγ enhanced antitumor responses
IFNγ plays a central role in the antitumor immune response in both mice and humans (50, 51). Mutations in IFNγ signaling are a major pathway of resistance to immunotherapy, further illustrating its clinical importance (50). Both A12 and B3 were fused to IFNγ and expressed as a secreted protein in mammalian cells. Minimal differences between A12-IFNγ and B3-IFNγ were observed, and these two PDL1-VHH constructs were used interchangeably. A12-IFNγ retains its affinity for surface PD-L1 (Fig. 4A), stimulates class I and class II MHC surface expression on B16 to a similar extent as recombinant IFNγ (Fig. 4B), and increases survival as a monotherapy in the CT26 colon cancer model (Fig. 4C). Admixing VHHCTR-IFNγ and B3 had no effect, indicating that targeting IFNγ via conjugation to B3 was superior to merely giving IFNγ plus PD-L1 blockade (Fig. 4C). Similarly, combination treatment with A12-IFNγ and TA99 extended survival in B16 melanoma compared to giving VHHCTR-IFNγ admixed with equimolar anti–PD-L1 (A12) (Fig. 4D).
Figure 4. Fusion to PDL1-VHH augments IFNγ responses in vivo.
(A) flow cytometry using the indicated VHH fusions on B16 cells treated with IFNγ or left untreated (−). (B) MHC class II (I-A/E) and MHC class I (Kb) flow cytometry on B16 cells treated with IFNγ constructs. (C) BALB/c mice were inoculated subcutaneously with CT26 colon carcinoma cells. On day 4, mice began once weekly treatment with the indicated VHH constructs for 2 weeks. (D) C57BL/6 mice were inoculated with B16 by subcutaneous injection. On day 4 mice began once weekly treatment with the indicated VHH constructs for 2 weeks with or without twice weekly TA99, an antibody specific for the melanoma antigen TRP1. A12-IFNγ and VHHCTR-IFNγ N=5, all other groups N=10. (E) Survival of mice from (D). Results are representative of five independent experiments for (A-B), and show combined data from two experiments for (C-E). *,p<0.05 using Student’s t test.
A12-IFNγ decreased growth of Panc02 orthotopic tumors, leading to a reduction in tumor size relative to treatment with a control VHH or treatment with anti–PD-L1 admixed with IFNγ (Supplementary Fig. S5). We combined A12-IFNγ and B3-IL2 treatment of mice bearing orthotopic Panc02 tumors and observed an additive benefit of the two treatments leading to reduced tumor burden. The B3-IL2-mediated increase in Foxp3+ Treg populations in the draining lymph node was obviated by inclusion of A12-IFNγ in the treatment regimen (Supplementary Fig. S5). Combination targeted therapy appeared to provide modestly increased efficacy over single agents alone; however, determining the optimal dose and schedule of delivering multiple agents is complicated by the disparate mechanisms of action of the two cytokines and by the fact that A12 and B3 bind to overlapping epitopes on PD-L1, possibly leading to decreased delivery of each cytokine fusion. We therefore chose to pursue the mechanism of action of targeted IFNγ as a single agent.
Pancreatic tumor organoids--when injected orthotopically--grow more slowly than similarly derived flat-cultured cells and develop more extensive fibrosis (31). We implanted KPC organoids (M19 line, and treated mice with control VHH, VHHCTR-IFNγ, or A12-IFNγ. A12-IFNγ treated tumors were significantly smaller than either control group (Fig. 5A). As further evidence that A12 successfully delivered IFNγ to the tumor microenvironment, we observed an increase in surface expression of class II MHC on the pancreatic tumor cells themselves, but only in mice that received targeted A12-IFNγ, and not VHHCTR-IFNγ. MDSC populations were likewise decreased (Supplementary Fig. S6A-B).
Figure 5. PDL1VHH-IFNγ decreases tumor burden in Panc02 and KPC orthotopic models of pancreatic cancer.
(A) Organoids derived from KPC mice (line M19) were inoculated orthotopically into C57BL/6 mice and treated with the indicated VHH constructs 5 μg/mouse IP daily. Five weeks post-implantation, tumors were harvested. (B) C57BL/6 mice were inoculated with 100,000 KPC cells orthotopically and treated with VHHCTR, VHHCTR-IFNγ, or A12-IFNγ. Compounds were administered daily for 18 days at 5 μg per mouse IP. Tumors were harvested at 21 days post-implantation. VHHCTR N=22, VHHCTR-IFNγ N=5, A12-IFNγ N=22. (C) Survival curve of C57BL/6 mice inoculated with 100,000 KPC cells orthotopically and treated with VHHCTR, VHHCTR-IFNγ, or A12-IFNγ. Compounds were administered daily at 5 μg per mouse IP. Experiment was performed once. (D-E) KPC tumors from (B) were analyzed by flow cytometry for the indicated populations. N=5 mice per group. (F) C57BL/6 mice were inoculated with orthotopic KPC tumors and treated with VHHCTR, VHHCTR-IFNγ, or B3-IFNγ. Compounds were administered twice weekly for 18 days at 50 μg per mouse IP. Tumors were harvested at 21 days post-implantation, digested, and analyzed by flow cytometry for surface PD-L1 expression on tumor cells (CD45-). (G) Weights of tumors from (F). (H) RAG2−/− mice were inoculated with 100,000 KPC cells subcutaneously and orthotopically, for a total of 2 tumors per mouse. Mice were treated with VHHCTR, VHHCTR-IFNγ, or B3-IFNγ. Compounds were administered twice weekly for 18 days at 50 μg per mouse IP. Tumors were harvested at 21 days post-implantation. Results are representative of three independent experiments for (A), show combined data from two experiments for (B), and are representative of two independent experiments for (D-H). *,p<0.05 using Student’s t test.
Similar to Panc02 cells and the KPC organoids, mice implanted orthotopically with a KPC pancreatic cancer cell line also showed a reduction in tumor growth with A12-IFNγ compared to VHHCTR-IFNγ (Fig. 5B-C). We noted a decrease in the numbers of tumor infiltrating granulocytic MDSCs in KPC tumors treated with A12-IFNγ, and a slight decrease in monocytic MDSCs (Fig. 5D), resulting in an overall loss of CD11b+ cells (Fig. 5E). The remaining myeloid cells showed increased surface express of class II MHC, consistent with exposure to IFNγ (Fig. 5F). Several groups have reported immune control of pancreatic tumors mediated entirely by myeloid cells (26, 27). We therefore tested whether adaptive immunity was required for the efficacy of targeted IFNγ treatment. Although, B3-IFNγ significantly reduced tumor burden in wild-type mice, it failed to do so in RAG2−/− mice that lack both T and B cells (Fig. 5G-H). Consistent with these findings, both CD8 and CD4 effector T-cell populations were increased in tumor infiltrates from mice treated with B3-IFNγ, and trended toward an increase in Ki67 positivity, suggesting increased proliferation of effector T-cell populations (Supplementary Fig. S6C).
PDL1-VHH delivers IFNγ to the tumor microenvironment
PD-L1 is expressed at low levels on pancreatic tumor cells, and with more on tumor-infiltrating myeloid cells. To determine the cell types affected by targeted IFNγ delivery, we generated fluorescently labeled VHH-IFNγ fusions and administered them to mice with mid-stage orthotopic tumors. One hour later we resected the tumors for imaging by two-photon microscopy (Fig. 6A-B). B3-IFNγ was present on both CD11b+ and CD11b− cells dispersed throughout the tumor microenvironment, whereas VHHCTR-IFNγ was not detected. Thus, a nontargeted IFNγ conjugate was not retained in the tumor microenvironment.
Figure 6: B3 effectively targets IFNγ to myeloid cells in the pancreatic tumor microenvironment.
(A) C57BL/6 mice were inoculated with either 100,000 KPC cells and harvested 7 days post tumor implantation or (B) implanted with KPC organoids and harvested 6 weeks post-implantation. One hour prior to harvest, mice were injected IP with 50 μg of Alexa Fluor 488 labeled of either B3-IFNγ or VHHCTR-IFNγ. Pancreas bearing tumors were processed and stained with monoclonal antibody against CD31 (cyan) and CD11b (green) and the labeled B3-IFNγ or VHHCTR-IFNγ (red). Confocal micrographs were captured on a two-photon microscope using a 20x objective (scale bars either 100μm or 5μm). (C-E) CD11b+ cells were sorted by FACS from day 12 orthotopic KPC tumors of mice that had been treated with VHHCTR (n=2), VHHCTR-IFNγ (n=3), or B3-IFNγ (n=2) as in Fig. 5F. RNA was prepared and used for RNAseq analysis. (C) Shown are volcano plots of differentially expressed genes when comparing VHHCTR to VHHCTR-IFNγ or to B3-IFNγ. (D) Gene set enrichment score analysis for genes involved in antigen processing and presentation. (E) Fold change above VHHCTR for FPKM values of known IFNγ-regulated genes. Error bars are SEM. Results are representative of two independent experiments for (A-B). RNAseq in (C-E) was performed once.
Given that B3-IFNγ colocalized with CD11b+ cells (Fig. 6A), and that myeloid cells were the major cell types affected by therapeutic dosing of B3-IFNγ, we performed transcriptional analysis on myeloid cells isolated from treated KPC tumors. Global analysis of gene expression showed minimal transcriptional changes between VHHCTR- and VHHCTR-IFNγ-treated tumors. The IFNγ response gene Gbp2b was the only significantly upregulated transcript (cutoff of >1.5 log2 fold change). B3-IFNγ induced far greater transcriptional changes than VHHCTR, consistent with its having activity in the tumor microenvironment (Fig. 6C). When comparing B3-IFNγ to VHHCTR-IFNγ, we saw a signature of increased antigen processing and presentation (Fig. 6D). Across a large panel of IFNγ regulated genes, B3-IFNγ consistently displayed increased transcriptional activity, evidence that B3 successfully targeted IFNγ to the tumor microenvironment, whereas VHHCTR did not (Fig. 6E).
Discussion
PD-L1-specific alpaca-derived antibody fragments readily penetrate into the tumor microenvironment, entering not only melanoma but also the dense stroma of pancreatic tumors. These anti–PD-L1 VHHs can deliver attached payloads to the tumor microenvironment and improve antitumor activity. Melanomas are generally sensitive to immunotherapy, and we show enhanced antitumor activity with anti–PD-L1 VHH-cytokine fusions in established murine melanoma models (7, 41, 52, 53). Immunotherapy for pancreatic cancer has so far been less successful (54). Delivery of either IL2 or IFNγ VHH fusions as single agents can reduce pancreatic tumor size by 50% in orthotopic models. IL2 and IFNγ act through distinct mechanisms, with IL2 treatment expanding intratumoral CD8 T cells and IFNγ decreasing MSDCs and enhancing class II MHC presentation. These two examples demonstrate the utility of anti–PD-L1 VHH-mediated delivery, and could be used as part of combination therapy for pancreatic cancer.
Pancreatic cancer is unusually resistant to treatment, and any single agent alone is unlikely to show sustained efficacy. Indeed, the rapidly progressing nature of the disease requires that patients receive chemotherapy, either gemcitabine/Abraxane or FOLFIRINOX, as standard of care. These cytotoxic agents can not only release tumor antigens to prime favorable T-cell responses, but can also have a negative impact on the immune response by killing rapidly dividing immune cells (55). Combination of immunotherapy with chemotherapy, particularly for pancreatic cancer, must take into account how each component part interacts with the others. The two immunotherapeutic agents described here show changes in tumor-infiltrating cell populations that correlate with efficacy of treatment. Increase in intratumoral CD8 T cells or increased class II MHC expression on intratumoral macrophages are potential biomarkers for efficacy of these two agents, respectively. The distinct mechanisms of action of IL2 and IFNγ also provide a rationale for combining these agents, or with immunotherapeutics known to exploit yet other pathways.
The role of IFNγ in the tumor microenvironment is complex. Genes regulating IFNγ signaling in tumor cells are frequently mutated in patients who fail to respond to immunotherapy, suggesting that successful immunotherapy may involve direct growth inhibitory effects of IFNγ on malignant cells (50). In pancreatic cancer, agnostic antibodies to CD40 stimulate systemic IFNγ production, leading to increased intratumoral Ly6C+ inflammatory monocytes that secrete matrix metalloproteinases (27). Although we observed no effects of targeted IFNγ on the extracellular matrix in our models, we did occasionally observe modest efficacy of VHHCTR-IFNγ treatment compared to VHHCTR alone, consistent with a minor role for systemic IFNγ in mimicking the mechanism of action of anti-CD40. Intratumoral IFNγ may increase antigen processing and presentation by myeloid cells, as well as skew the phenotype of intratumoral macrophages. However, IFNγ also induces negative regulatory pathways, including production of indoleamine 2,3-dioxygenase (IDO), and upregulation of PD-L1 and other inhibitory ligands on tumor cells (56). Here we combine targeting of IFNγ with concurrent blockade of PD-L1, thereby partially negating the negative regulatory effects of IFNγ. Nevertheless, targeted IFNγ may be more efficacious if targeted exclusively to myeloid cells, or if combined with other agents, such as IDO inhibitors.
VHHs are versatile tools that can be expressed cheaply and are easily conjugated to a variety of agents (3, 7, 8, 11, 32, 57). A VHH can be equipped with a sortase recognition motif (LPETG) that can then be used to covalently attach any moiety with an N-terminal glycine (11, 58-64). In this manner, “click” handles can be conjugated to enable site specific attachment of non-protein payloads such as radioisotopes without compromising the binding properties of the VHH(3, 8, 32). We are thus able to use one and the same reagent for detection of PD-L1 expression in vivo, blockade of PD-L1 interaction with PD-1, and delivery of therapeutic compounds, not limited to the two examples reported here(3).
Alpaca VHHs are potentially immunogenic when administered to a heterologous recipient. In mice that were dosed for 3 weeks or more with B3, we observed low titer antibodies to VHH in approximately 30% of mice, similar to previously reported anti-VHH responses in mice (3, 7). Substitution of particular amino acids in the VHH framework region renders alpaca antibodies more similar to their human orthologs, which enables repeated dosing while avoiding a neutralizing anti-VHH immune response (65). Indeed, in a phase II trial of the humanized VHH caplacizumab (specific for von Willebrand factor), patients were dosed for 60 days with caplacizumab, and non-neutralizing antibodies to the alpaca VHH were observed in only 9% of patients (65). Thus VHH-based therapies can be safe and relatively non-immunogenic.
In healthy individuals, PD-L1 expression is confined to a subset of myeloid dendritic cells and brown adipocytes, two cell types that are neither abundant nor critical for survival (40, 66). In cancer patients, PD-L1 is expressed predominantly in the tumor microenvironment. PD-L1 expression can be heterogeneous, with expression on tumor cells, myeloid cells, on both or on neither (42, 67). However, by delivering compounds to the tumor microenvironment, there is no need to invoke homogeneous expression of PD-L1 on tumor cells as a precondition for therapeutic efficacy. Indeed, pancreatic tumor cells resected from orthotopic KPC tumors showed weak staining of PD-L1, with a significant fraction of the cells altogether negative for expression. Myeloid cell expression of PD-L1 may be adequate for targeted cytokine delivery with anti–PD-L1 VHH, suggesting the broad potential for this approach.
Supplementary Material
Acknowledgments:
We thank Peter Sage and Arlene Sharpe (Harvard Medical School, Boston, MA) for providing PD-L1 knockout mice, K. Dane Wittrup and Monique J. Kauke (MIT, Cambridge, MA) for providing TA99, Anirban Maitra (MD Anderson) for providing KPC cells, David Tuveson and Lindsey Baker (Cold Spring Harbor) for providing KPC organoids, as well as Cherry Ng, Camilo Espinosa, Jasdave Chahal, Christina Martone, Alica Linnebacher, Stephanie Grabow, Mitchell Galanek, Hans Richter, Howard Mak and Scott Malstrom, Patti Wisniewski and the Whitehead Flow Cytometry Core (Whitehead Institute for Biomedical Research, Cambridge, MA), and Zachary Herbert of the Molecular Center for Genomics and Biostatistics Core (Dana-Farber Cancer Institute, Boston, MA) for technical assistance and HMS Center for Immune Imaging (P01 AI112521).
Funding: Funding was provided by the AACR-Pancreatic Cancer Action Network (S.K.D), the Pew Foundation (S.K.D), the Smith Family Foundation (S.K.D), the Mayer Foundation (S.K.D), the Bridge Project (S.K.D), the Hale Family Foundation (S.K.D), NIH training grant 1F32CA210568-01 and Mentored Clinical Scientist Development Award 1K08DK114563 - 01, the Center for the Study of Inflammatory Bowel Disease (DK043351), HMS Center for Immune Imaging (P01 AI112521), and the American Gastroenterology Association Research Scholars Award (M.D.), the Ludwig Cancer Research Postdoctoral Fellowship (J.R.I), the Claudia Adams Barr Foundation (J.R.I), the Center for Cancer Immunotherapy Research (H.J.J), the Cancer Research Institute (M.R.), the Lustgarten Foundation (H.L.P) and an NIH Pioneer award (H.L.P.).
References:
- 1.Dranoff G Cytokines in cancer pathogenesis and cancer therapy. Nature reviews Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Floros T, Tarhini AA. Anticancer Cytokines: Biology and Clinical Effects of Interferon-alpha2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Seminars in oncology. 2015;42(4):539–48. doi: 10.1053/j.seminoncol.2015.05.015. PubMed PMID: ; PMCID: 4557618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram JR, Dougan M, Rashidian M, Knoll M, Keliher EJ, Garrett S, Garforth S, Blomberg OS, Espinosa C, Bhan A, Almo SC, Weissleder R, Lodish H, Dougan SK, Ploegh HL. PD-L1 is an activation-independent marker of brown adipocytes. Nature communications. 2017;8(1):647. doi: 10.1038/s41467-017-00799-8. PubMed PMID: ; PMCID: 5608754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annual review of immunology. 2016;34:539–73. doi: 10.1146/annurev-immunol-032414-112049. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Tzeng A, Kwan BH, Opel CF, Navaratna T, Wittrup KD. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(11):3320–5. doi: 10.1073/pnas.1416159112. PubMed PMID: ; PMCID: 4371941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingram JR, Blomberg OS, Sockolosky JT, Ali L, Schmidt FI, Pishesha N, Espinosa C, Dougan SK, Garcia KC, Ploegh HL, Dougan M. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(38):10184–9. doi: 10.1073/pnas.1710776114. PubMed PMID: ; PMCID: 5617302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2646–54. doi: 10.1073/pnas.1604268113. PubMed PMID: ; PMCID: 4868409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashidian M, Ingram JR, Dougan M, Dongre A, Whang KA, LeGall C, Cragnolini JJ, Bierie B, Gostissa M, Gorman J, Grotenbreg GM, Bhan A, Weinberg RA, Ploegh HL. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. The Journal of experimental medicine. 2017;214(8):2243–55. doi: 10.1084/jem.20161950. PubMed PMID: ; PMCID: 5551571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antos JM, Ingram J, Fang T, Pishesha N, Truttmann MC, Ploegh HL. Site-Specific Protein Labeling via Sortase-Mediated Transpeptidation. Current protocols in protein science / editorial board, John E Coligan [et al]. 2017;89:15 3 1–3 9. doi: 10.1002/cpps.38. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong HJ, Abhiraman GC, Story CM, Ingram JR, Dougan SK. Generation of Ca2+-independent sortase A mutants with enhanced activity for protein and cell surface labeling. PloS one. 2017;12(12):e0189068. doi: 10.1371/journal.pone.0189068. PubMed PMID: ; PMCID: 5714338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witte MD, Wu T, Guimaraes CP, Theile CS, Blom AE, Ingram JR, Li Z, Kundrat L, Goldberg SD, Ploegh HL. Site-specific protein modification using immobilized sortase in batch and continuous-flow systems. Nature protocols. 2015;10(3):508–16. doi: 10.1038/nprot.2015.026. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. PubMed PMID: ; PMCID: 3544539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougan M, Dranoff G. Immune therapy for cancer. Annual review of immunology. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 15.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988. PubMed PMID: ; PMCID: 4279952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB,Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr., Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. Journal of immunotherapy. 2013;36(7):382–9. doi: 10.1097/CJI.0b013e31829fb7a2. PubMed PMID: ; PMCID: 3779664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. Journal of immunotherapy. 2010;33(8):828–33. doi: 10.1097/CJI.0b013e3181eec14c. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(16):4266–76. doi: 10.1158/1078-0432.CCR-11-3114. PubMed PMID: ; PMCID: 3442232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutcheson J, Balaji U, Porembka MR, Wachsmann MB, McCue PA, Knudsen ES, Witkiewicz AK. Immunologic and Metabolic Features of Pancreatic Ductal Adenocarcinoma Define Prognostic Subtypes of Disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(14):3606–17. doi: 10.1158/1078-0432.CCR-15-1883. PubMed PMID: ; PMCID: 4947442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM,Guirnalda PD, Vonderheide RH. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology. 2015;149(1):201–10. doi: 10.1053/j.gastro.2015.04.010. PubMed PMID: ; PMCID: 4478138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer research. 2013;73(3):1128–41. doi: 10.1158/0008-5472.CAN-12-2731. PubMed PMID: ; PMCID: 3563931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(13):3404–15. doi: 10.1158/1078-0432.CCR-13-0525. PubMed PMID: ; PMCID: 3700620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer research. 2014;74(18):5057–69. doi: 10.1158/0008-5472.CAN-13-3723. PubMed PMID: ; PMCID: 4182950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–6. doi: 10.1126/science.1198443.PubMed PMID: ; PMCID: 3406187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. IFNgamma and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer discovery. 2016;6(4):400–13. doi: 10.1158/2159-8290.CD-15-1032. PubMed PMID: ; PMCID: 4843521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer immunology research. 2015;3(4):399–411. doi: 10.1158/2326-6066.CIR-14-0215. PubMed PMID: ; PMCID: 4390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans RA, Diamond MS, Rech AJ, Chao T, Richardson MW, Lin JH, Bajor DL, Byrne KT, Stanger BZ, Riley JL, Markosyan N, Winograd R, Vonderheide RH. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI insight. 2016;1(14). doi: 10.1172/jci.insight.88328. PubMed PMID: ; PMCID: 5026128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20212–7. doi: 10.1073/pnas.1320318110. PubMed PMID: ; PMCID: 3864274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Ohlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–38. doi: 10.1016/j.cell.2014.12.021. PubMed PMID: ; PMCID: 4334572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashidian M, Keliher E, Dougan M, Juras PK, Cavallari M, Wojtkiewicz GR, Jacobsen J, Edens JG, Tas JM, Victora G, Weissleder R, Ploegh H. The use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer. ACS central science. 2015;1(3):142–7. doi: 10.1021/acscentsci.5b00121. PubMed PMID: ; PMCID: 4778250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashidian M, Keliher EJ, Bilate AM, Duarte JN, Wojtkiewicz GR, Jacobsen JT, Cragnolini J, Swee LK, Victora GD, Weissleder R, Ploegh HL. Noninvasive imaging of immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(19):6146–51. doi: 10.1073/pnas.1502609112. PubMed PMID: ; PMCID: 4434737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmerle T, Neri D. The dose-dependent tumor targeting of antibody-IFNgamma fusion proteins reveals an unexpected receptor-trapping mechanism in vivo. Cancer immunology research. 2014;2(6):559–67. doi: 10.1158/2326-6066.CIR-13-0182. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer research. 2015;75(13):2629–40. doi: 10.1158/0008-5472.CAN-14-2921. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. PubMed PMID: ; PMCID: 2998180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. British journal of cancer. 2013;108(1):1–8. doi: 10.1038/bjc.2012.569. PubMed PMID: ; PMCID: 3553539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62(1):112–20. doi: 10.1136/gutjnl-2012-302529. PubMed PMID: ; PMCID: 3551211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JW, Komar CA, Bengsch F, Graham K, Beatty GL. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+) ;LSL-Trp53(R172H/+) ;Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Current protocols in pharmacology / editorial board, SJ Enna. 2016;73:14 39 1–14 39 20. doi: 10.1002/cpph.2. PubMed PMID: ; PMCID: 4915217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dougan SK, Dougan M, Kim J, Turner JA, Ogata S, Cho HI, Jaenisch R, Celis E, Ploegh HL. Transnuclear TRP1-specific CD8 T cells with high or low affinity TCRs show equivalent antitumor activity. Cancer immunology research. 2013;1(2):99–111. doi: 10.1158/2326-6066.CIR-13-0047. PubMed PMID: ; PMCID: 3895912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. PubMed PMID: ; PMCID: 4836193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P, Pande VS, Fathman CG, Boyman O, Garcia KC. Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature. 2012;484(7395):529–33. doi: 10.1038/nature10975. PubMed PMID: ; PMCID: 3338870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2171–6. doi: 10.1073/pnas.0909384107. PubMed PMID: ; PMCID: 2836659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu EF, Gai SA, Opel CF, Kwan BH, Surana R, Mihm MC, Kauke MJ, Moynihan KD, Angelini A, Williams RT, Stephan MT, Kim JS, Yaffe MB, Irvine DJ, Weiner LM, Dranoff G, Wittrup KD. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer cell. 2015;27(4):489–501. doi: 10.1016/j.ccell.2015.03.004. PubMed PMID: ; PMCID: 4398916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clinical and translational science. 2008;1(3):228–39. doi: 10.1111/j.1752-8062.2008.00070.x. PubMed PMID: ; PMCID: 2847413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5): 1571–9. doi: 10.1182/blood-2006-02-004747. PubMed PMID: ; PMCID: 1895505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21(6):822–35. doi: 10.1016/j.ccr.2012.04.025. PubMed PMID: ; PMCID: 3575028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer cell. 2012;21(6):836–47. doi: 10.1016/j.ccr.2012.04.024. PubMed PMID: ; PMCID: 3721510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404 e9. doi: 10.1016/j.cell.2016.08.069. PubMed PMID: ; PMCID: 5088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. doi: 10.1038/35074122. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 52.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4275–80. doi: 10.1073/pnas.0915174107. PubMed PMID: ; PMCID: 2840093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dougan M, Dougan S, Slisz J, Firestone B, Vanneman M, Draganov D, Goyal G, Li W, Neuberg D, Blumberg R, Hacohen N, Porter D, Zawel L, Dranoff G. IAP inhibitors enhance co-stimulation to promote tumor immunity. The Journal of experimental medicine. 2010;207(10):2195–206. doi: 10.1084/jem.20101123. PubMed PMID: ; PMCID: 2947073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer letters. 2015. doi: 10.1016/j.canlet.2015.12.020. PubMed PMID: ; PMCID: 4919239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer C, Sterzik A, Bauernfeind F, Duewell P, Conrad C, Kiefl R, Endres S, Eigler A, Schnurr M, Dauer M. Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8(+) T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model. Cancer immunology, immunotherapy : CII. 2014;63(4):321–33. doi: 10.1007/s00262-013-1510-y. PubMed PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DS, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167(6):1540–54 e12. doi: 10.1016/j.cell.2016.11.022. PubMed PMID: ; PMCID: 5385895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dougan SK, Ashour J, Karssemeijer RA, Popp MW, Avalos AM, Barisa M, Altenburg AF, Ingram JR, Cragnolini JJ, Guo C, Alt FW, Jaenisch R, Ploegh HL. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature. 2013;503(7476):406–9. doi: 10.1038/nature12637. PubMed PMID: ; PMCID: 3863936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witte MD, Theile CS, Wu T, Guimaraes CP, Blom AE, Ploegh HL. Production of unnaturally linked chimeric proteins using a combination of sortase-catalyzed transpeptidation and click chemistry. Nature protocols. 2013;8(9):1808–19. doi: 10.1038/nprot.2013.103. PubMed PMID: ; PMCID: 3975145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theile CS, Witte MD, Blom AE, Kundrat L, Ploegh HL, Guimaraes CP. Site-specific N-terminal labeling of proteins using sortase-mediated reactions. Nature protocols. 2013;8(9):1800–7. doi: 10.1038/nprot.2013.102. PubMed PMID: ; PMCID: 3941705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guimaraes CP, Witte MD, Theile CS, Bozkurt G, Kundrat L, Blom AE, Ploegh HL. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nature protocols. 2013;8(9):1787–99. doi: 10.1038/nprot.2013.101. PubMed PMID: ; PMCID: 3943461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swee LK, Guimaraes CP, Sehrawat S, Spooner E, Barrasa MI, Ploegh HL. Sortase-mediated modification of alphaDEC205 affords optimization of antigen presentation and immunization against a set of viral epitopes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(4):1428–33. doi: 10.1073/pnas.1214994110. PubMed PMID: ; PMCID: 3557095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Popp MW, Dougan SK, Chuang TY, Spooner E, Ploegh HL. Sortase-catalyzed transformations that improve the properties of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(8):3169–74. doi: 10.1073/pnas.1016863108. PubMed PMID: ; PMCID: 3044387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popp MW, Antos JM, Ploegh HL. Site-specific protein labeling via sortase-mediated transpeptidation. Current protocols in protein science / editorial board, John E Coligan [et al] 2009;Chapter 15:Unit 15 3. doi: 10.1002/0471140864.ps1503s56. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antos JM, Miller GM, Grotenbreg GM, Ploegh HL. Lipid modification of proteins through sortase-catalyzed transpeptidation. Journal of the American Chemical Society. 2008;130(48):16338–43. doi: 10.1021/ja806779e. PubMed PMID: 18989959; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knobl P, Wu H, Artoni A, Westwood JP, Mansouri Taleghani M, Jilma B, Callewaert F, Ulrichts H, Duby C, Tersago D, Investigators T. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. The New England journal of medicine. 2016;374(6):511–22. doi: 10.1056/NEJMoa1505533. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 66.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van't Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer cell. 2014;26(5):638–52. doi: 10.1016/j.ccell.2014.09.007. PubMed PMID: ; PMCID: 4254577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. PubMed PMID: ; PMCID: 4246418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.