Abstract
Type 2 diabetes (T2D) accounts for about 90% of all diabetes patients and incurs a heavy global public health burden. Up to 50% of T2D patients will eventually develop neuropathy as T2D progresses. Diabetic peripheral neuropathy (DPN) is a common diabetic complication and one of the main causes of increased morbidity and mortality of T2D patients. Obstructive sleep apnea (OSA) affects over 15% of the general population and is associated with a higher prevalence of T2D. Growing evidence also indicates that OSA is highly prevalent in T2D patients probably due to diabetic peripheral neuropathy. However, the interrelations among diabetic peripheral neuropathy, OSA, and T2D hitherto have not been clearly elucidated. Numerous molecular mechanisms have been documented that underlie diabetic peripheral neuropathy and OSA, including oxidative stress, inflammation, endothelin-1, vascular endothelial growth factor (VEGF), accumulation of advanced glycation end products, protein kinase C (PKC) signaling, poly ADP ribose polymerase (PARP), nitrosative stress, plasminogen activator inhibitor-1, and vitamin D deficiency. In this review, we seek to illuminate the relationships among T2D, diabetic peripheral neuropathy, and OSA and how they interact with one another. In addition, we summarize and explain the shared molecular mechanisms involved in diabetic peripheral neuropathy and OSA for further mechanistic investigations and novel therapeutic strategies for attenuating and preventing the development and progression of diabetic peripheral neuropathy and OSA in T2D.
1. Introduction
Diabetes mellitus is a global disease with major public health implications and is predicted to affect 642 million persons by 2040 [1, 2]. Type 2 diabetes (T2D) accounts for 90–95% of all patients with diabetes and involves multiple systems and organs. Long-standing poorly controlled T2D ultimately leads to the development of microvascular complications, including neuropathy, nephropathy, and retinopathy, and macrovascular disease such as cerebrovascular and coronary artery diseases [3].
DPN is defined as “symmetrical, length-dependent sensorimotor polyneuropathy attributing to metabolic and microvessel alterations as a result of chronic hyperglycemia exposure and cardiovascular risk covariates” [4]. Fifty percent of T2D patients would eventually develop DPN and 20% of T2D patients have DPN at presentation [5]. However, DPN is mostly neglected as a diabetic complication. Furthermore, the pathophysiologic mechanism of DPN appears to be complex, involving the metabolic and ischemic pathways [3].
Recent evidence has suggested a link between obstructive sleep apnea (OSA) and DPN. OSA is one treatable type of sleep-disordered breathing characterized by episodes of complete or partial obstruction of the upper airway during sleep, resulting in recurrent episodes of apnea or hypopnea [6]. Growing evidence has shown that OSA is very common in T2D patients and probably associated with DPN [7, 8]. Several longitudinal studies and meta-analyses have indicated that OSA is a risk factor for T2D associated with insulin resistance and β-cell dysfunction [6] and could be a cause of ineffective treatment of T2D [9]. Conversely, T2D may also be a risk factor of OSA or worsen preexisting OSA. There is compelling evidence of an association between OSA and metabolic dysfunction, in particular, changes in glucose metabolism resulting in metabolic syndrome, glucose intolerance, and insulin resistance [10, 11]. Moreover, this association is independent of obesity, which is a common occurrence in patients with T2D and/or OSA [12].
As OSA and T2D frequently coexist, knowledge about the association between DPN and OSA in T2D could shed light on the pathogenesis of OSA and T2D in these patients. Recent data has suggested significant underappreciation of OSA in T2D patients, and the mechanisms underlying the link between OSA and DPN remain largely unelucidated. Dissecting the relationship between DPN and OSA is also clinically relevant as illumination of the connection between the two conditions may impact on both patient care and quality of life.
The primary aim of this review is to explore connections among OSA, DPN, and T2D and assess whether OSA and DPN could interact with each other in T2D patients. We also summarize the potential common molecular mechanisms whereby OSA and DPN could be linked in T2D, including the role of neuromodulators in DPN and OSA.
2. Complex Connections among OSA, DPN, and T2D
OSA is diagnosed when the apnea-hypopnea index (AHI) is ≥5/hr, together with such symptoms or signs as nocturnal gasping or choking events, witnessed habitual snoring, excessive daytime sleepiness, hypertension, nonrefreshing sleep, and congestive heart failure or AHI ≥ 15/hr without symptoms [13]. OSA affects about 14% of men and 5% of women, is highly prevalent in T2D patients [14, 15], and has also been linked to the development of incident T2D [12]. The relationship between OSA and T2D may be bidirectional given that DPN could influence central control of respiration and upper airway nerve reflex promoting sleep-disordered breathing. Several previous studies suggested an association between OSA and diabetic autonomic neuropathy [16, 17]. The development of autonomic neuropathy in T2D patients may affect upper airway innervation and collapsibility, ventilator drive, and central respiratory center reaction to hypercapnia stimulus, which contribute to the pathogenesis of OSA [6].
2.1. Bidirectional Link between OSA and T2D
Although substantial literature has established a link between OSA and T2D, there is lack of keen awareness of such an association, and clinically, T2D patients are not vigorously screened for OSA [6]. Furthermore, intermittent hypoxia and sleep fragmentation in OSA patients could independently induce intermediate disorders including sympathetic nervous system activation, systemic inflammation, oxidative stress, appetite-regulating hormone alterations, and hypothalamic-pituitary-adrenal axis activation, which in turn promote the development of insulin resistance, glucose intolerance, and ultimately T2D [18, 19]. There is also convincing evidence for an association between OSA and fasting insulin, glucose, and HbA1c levels independent of obesity although the exact pathophysiological mechanism underlying such a link still remains elusive [3]. Conversely, T2D could increase predisposition to, or accelerate progression of, OSA, possibly partially through the development of peripheral neuropathy [20]. It is not surprising, therefore, that there exists a link between OSA and T2D [21], in particular, considering the confounding effects of obesity and aging.
2.1.1. OSA Affecting T2D
Longitudinal cohort studies, including 6 prospective cohort studies from different regions all over the world with a follow-up duration of 2.7–16 years, have shown a significant association between OSA and T2D [12, 22–31]. Some studies also showed that severity of OSA correlated with the presence of T2D. The prevalence of T2D in OSA patients was estimated to be 15–30% and may be even higher in severe OSA patients [24, 32–34]. However, after adjustment for body mass index (BMI) and other confounders, no correlation was found between OSA and T2D in some studies [24, 33, 34] while several other studies showed that increased OSA severity was robustly associated with increased HbA1c levels in T2D patients after adjustment for confounders [34–38]. This suggested that uncontrolled OSA may exacerbate the progression of T2D.
2.1.2. T2D Affecting OSA
In spite of notable methodological limitations, several independent studies revealed a significantly higher prevalence (23–86%) of OSA in T2D patients versus the general population [27, 39], suggesting that T2D could be a risk factor for OSA. Scantly available data showed that T2D could worsen the progression of preexisting OSA [19], especially in patients with autonomic neuropathy [9]. In both clinic-based and community-based cohorts including T2D patients with diverse backgrounds, the prevalence of OSA was alarmingly elevated [7, 35, 36, 40–49] and insulin resistance could predict OSA development [39]. T2D affecting OSA is postulated to involve the disorders of the autonomic nervous system leading to sleep-disordered breathing. However, OSA is usually underdiagnosed in the majority of T2D patients in the primary care setting [50].
2.1.3. DPN in T2D
DPN is more common in T2D patients, accounting for 60%–70% of individuals with diabetes [3] and contributes significantly to morbidity and mortality of diabetes patients [51]. DPN can be categorized into distal symmetric peripheral neuropathy and asymmetric (focal and multifocal) neuropathies (including multiple mononeuropathies and thoracic, lumbosacral, and cervical radiculoplexus neuropathies) [52]. A recent study evaluated the risk of neuropathy in 1414 T2D patients and found that diabetic women with altered sleep patterns had a higher risk of developing neuropathy [53]. The causes of DPN are multifactorial, including metabolic factors such as high fat, high glucose, and low insulin; autoimmune factors producing neurotoxic inflammation; neurovascular factors resulting in damage to vessels carrying nutrients and oxygen to the nerves; carpal tunnel syndrome at the wrists; and ulnar nerve entrapment at the elbows and lifestyle factors such as alcohol use and smoking [54]. However, the pathological progression of DPN is still unclear and it is essential to explore the potential molecular mechanism involved in DPN.
2.1.4. Interaction between OSA and DPN in T2D Patients
OSA in diabetic patients is typically explained by obesity associated with T2D. Recently, OSA has been shown to be associated with DPN in T2D patients. The knowledge of such an association in T2D patients is of clinical implications. OSA and DPN in T2D could aggravate each other, resulting in a vicious circle, with even additive or synergistic health risks in T2D patients. A previous study examining the relationship between OSA and DPN found a fourfold increase in the odds of peripheral neuropathy in T2D patients with OSA compared with those without [7].
2.1.5. DPN Affecting OSA
Diabetic neuropathies are a heterogeneous group of disorders affecting different parts of the nervous systems, including symmetrical polyneuropathies, autonomic neuropathy, and multifocal and focal neuropathies [19, 27]. Earlier data mainly focused on the effect of diabetic autonomic neuropathy on sleep-disordered breathing [55]. Diabetic autonomic neuropathy, a form of DPN [2, 56], could lead to ventilator dysfunction through impaired central control of breathing, leading to sleep-disordered breathing [57–60]. Diabetic neuropathy could increase upper airway collapsibility due to the destruction of the dilatory muscles of the larynx, which could aggravate OSA [19]. This mechanism is also observed in a peripheral neuropathy named Charcot-Marie-Tooth [61]. Another possible mechanism is sleep disturbance by painful peripheral neuropathy. A meta-analysis confirmed the relationship between OSA and diabetic neuropathy and revealed that OSA was documented more frequently in T2D patients with neuropathy [62]. Laboratory investigations have also shown that T2D patients with diabetic autonomic neuropathy are more likely to have OSA than those without [63], suggesting diabetic autonomic neuropathy as another explanation for the presence of OSA because it is diabetes specific. However, there are possible mechanisms as to why both diabetic autonomic neuropathy and DPN could lead to the progression of OSA. Patients with T2D and OSA are at risk of DPN [7]. Diabetes-related nocturia or pain from DPN could worsen sleep or cause sleep loss [54].
2.1.6. OSA Affecting DPN
A recent study has implicated OSA as a risk of peripheral neuropathy; autonomic dysfunction risk could be positively correlated with severity of OSA [64]. Sleep-disordered breathing such as OSA has been documented in approximately 50–70% T2D patients and may contribute to diabetic neuropathy [41, 65]. Shorter or longer duration of sleep could increase the rate of complications such as DPN [66–68]. Evidence is scant supporting an association of OSA with DPN [8]. OSA is associated with nitrosative and oxidative stress as well as impaired microvascular regulation in T2D patients [7] and could lead to increase of insulin resistance and T2D, which in turn could elevate inflammatory markers and contribute to vascular complications [3]. Therefore, OSA-complicating T2D could facilitate the development and progression of microvascular complications including DPN. OSA has been shown to be independently associated with clinically evident DPN [7]. Robust data is now available supporting OSA as an independent risk for DPN development [3].
2.1.7. Common Potential Molecular Mechanisms Involved in OSA and DPN
One study found that approximately 60% of patients with OSA and diabetes have peripheral neuropathy [69]. It has been postulated that advanced glycation end products (AGEs) and protein kinase C (PKC) could lead to microvascular complications including DPN [3, 7]. OSA and DPN in T2D patients may share molecular mechanisms underlying the development of both conditions as detailed below (also refer to Table 1 for further references).
Table 1.
Summary of shared molecular mechanisms in DPN and OSA.
| Molecular mechanisms | Subcategories | Reference for DPN | Reference for OSA |
|---|---|---|---|
| Oxidative stress | [76–80] | [71, 81–84, 93, 94] | |
| Inflammatory markers | TNF-α | [101, 103] | [108–110, 113, 114] |
| IL-6 | [102] | [109–113] | |
| IL-8 | [108] | ||
| CRP | [103] | [111–113] | |
| NF-κB | [104] | [85] | |
| Endothelin-1 (ET-1) | [119] | [120–122, 126] | |
| Vascular endothelial growth factor (VEGF) | [128] | [129, 130] | |
| Advanced glycation end products (AGEs) | [138–142] | [146, 214, 215] | |
| Protein kinase C (PKC) | [148] | [152] | |
| Poly ADP ribose polymerase (PARP) | [153, 154] | [7, 8, 155–157] | |
| Nitrosative stress | Nitrotyrosine | [162, 163] | [7] |
| Plasminogen activator inhibitor-1 (PAI-1) | [103] | [167–170] | |
| Vitamin D deficiency | [176–179, 181–183, 185] | [187–192, 194–197] |
DPN: diabetic peripheral neuropathy; OSA: obstructive sleep apnea.
2.1.8. Oxidative Stress
Oxidative stress is characterized by excessive production of reactive oxygen species (ROS) overwhelming the body's antioxidative defenses [20]. Superoxide ion (O2−), nitric oxide (NO), and hydrogen peroxide (H2O2) are three radical ROS believed to mediate cellular degeneration in disease states [70]. ROS excess could inhibit insulin-induced energy substrate uptake in adipose and muscle tissues and damage pancreatic β cells [20, 71]. ROS could also suppress insulin secretion and worsen insulin sensitivity [72, 73]. Actually, cellular studies showed that intermittent hypoxia in OSA negatively affected β-cell death and proliferation, which could be attributed to excessive cellular oxidative stress [74].
Excessive oxidative stress is a well-recognized mechanism in the pathogenesis of DPN [75]. Previous studies implicated free lipid peroxidation product accumulation, increase in GSSG/GSH ratio, GSH depletion, and downregulation of superoxide dismutase (SOD) activity in DPN [76–79]. Further studies are needed to unravel the mechanisms of oxidative stress in the development and progression of DPN. In DPN, AGEs and PKC signaling directly alter cellular redox capacity through ROS formation [80].
Recent evidence suggests an association of OSA with high concentrations of ROS [71]. Upregulated oxidative stress has been repeatedly demonstrated in OSA patients [81–84] and could contribute to cerebrovascular, cardiovascular, and other morbidities of OSA. Increased prooxidant/antioxidant ratio could lead to oxidative stress associated with OSA, which is primarily attributed to decreased oxygen availability during apneic events and ROS formation during reoxygenation when breathing resumes [81]. Oxidative stress initiates a vicious circle in which it promotes inflammation and sympathetic activation, which in turn potentiates oxidative stress [85]. Accumulating evidence shows that increase in oxidative stress in OSA patients could contribute to hyperlipidemia, insulin resistance, T2D, and subsequent DPN [86].
Increased ROS production associated with hypoxia could be attributed to dysfunctional mitochondria, NADPH oxidase and xanthine oxidase, and uncoupling of nitric oxide synthase activation, leading to generation of ROS rather than nitric oxide (NO) [81, 87], ultimately injuring vital biomolecules and altering physiological signaling pathways [85]. Increased ROS levels due to intermittent hypoxia in mouse mitochondria could contribute to T2D development [88]. Recent studies have indicated that genetic polymorphisms of NADPH oxidase could affect oxidative stress levels in OSA patients [85]. The circulating levels of lipid peroxidation [89–92], DNA [91, 93], and oxidation products were increased in OSA patients and correlated with AHI severity [94, 95], which partially recovered by continuous positive airway pressure (CPAP) therapy.
2.1.9. Inflammatory Markers
Diabetic patients have high circulating levels of inflammatory markers such as tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), interleukin-6 (IL-6), and IL-8 [96, 97]. M1 macrophages in adipose tissues produce IL-6 and TNF-α [98, 99], which could lead to free fatty acid (FFA) release, ultimately resulting in impaired insulin signaling consequent of insulin resistance and metabolic dysfunction [11, 100].
T2D patients with DPN have markedly higher TNF-α levels than those without DPN and healthy persons, and high TNF-α may be an independent risk of DPN [101]. Furthermore, IL-6 levels are chronically elevated in T2D patients with DPN [102]. CRP is a sensitive biological marker of subclinical systemic inflammation related to insulin resistance, hyperglycemia, and overt T2D. T2D patients with DPN have noticeably higher CRP and TNF-α levels than those without DPN and normal subjects [103], suggesting a prominent role of inflammation in the development and progression of DPN. NF-κB activation, which is involved in the pathogenesis of diabetic complications, especially DPN [104], has been identified in the endoneurium, epineurial vessels and perineurium in sural nerve biopsies of overt diabetic subjects [105].
Cytotoxic T lymphocytes could acquire an inflammatory phenotype in OSA patients [85]. CD8+ T cells exhibit elevated TNF-α levels and display enhanced cytotoxicities in an AHI severity-dependent manner [106, 107]. Cytotoxic γδ T cells express higher levels of proinflammatory cytokines, including TNF-α and IL-8, and lower anti-inflammatory IL-10 levels, suggesting that they could be implicated in atherogenesis in OSA patients [108]. In addition, several studies have found elevated levels of TNF-α, IL-6, and CRP in OSA patients [109–113]. Consistently, a proinflammatory state has been found in OSA patients [114]. Previous studies have shown that CPAP decreased the levels of IL-6, TNF-α, and CRP, leading to a reduction in vascular complications and inflammation [3, 111, 112]. Inflammatory markers that are implicated in DPN and OSA including TNF-α, IL-6, IL-8, and CRP are listed in Table 1.
Blood cells from OSA patients usually show a proinflammatory phenotype, which could lead to endothelial dysfunction, endothelial injury, atherosclerosis, and thrombosis [85]. Short-lived circulating neutrophils from moderate to severe OSA patients had a prolonged lifespan, which was associated with increased NF-κB levels, decreased ratios of proapoptotic/antiapoptotic proteins, and a higher level of adhesion molecules [85]. Similar results were revealed in neutrophils from healthy controls exposed to intermittent hypoxia in vitro [115–118].
2.1.10. Endothelin-1 (ET-1)
ET-1 is a potent vasoconstrictor and could also stimulate cellular proliferation. ET-1 contributes to endothelial abnormalities and imbalance of vasodilation and vasoconstriction in favor of the latter in diabetes. In diabetes patients, endoneurial microangiopathy, particularly basement membrane thickening, is related to clinical neuropathy. Several studies have implicated ET-1 as a novel risk factor for DPN, and ET-1 levels increased in DPN patients [119], while improvement of blood glucose did not affect ET-1 concentrations [120].
OSA patients exhibited augmented vasoconstrictive capacity due to ET-1 activation. Recurrent episodes of OSA increased ET-1 levels and blood pressure. Vasoconstrictor and mitogenic effects of ET-1 may be implicated in increased cardiovascular risk in OSA patients [120–122]. Other studies failed to show any ET-1 elevations in OSA patients compared with controls [123, 124]. In OSA rats mimicking intermittent hypoxia, ET-1 constrictor sensitivity rose in a PKC δ-dependent manner in the mesenteric arteries [125]. ET-1 has also been shown to be involved in ocular complications of OSA [126].
2.1.11. Vascular Endothelial Growth Factor (VEGF)
VEGF stimulates angiogenesis by promoting vascular endothelial cell proliferation, migration, and proteolysis. Little is known regarding VEGF expression in human DPN. In diabetic rats, immunostaining of the sciatic nerve and dorsal root ganglion revealed high VEGF levels in cell bodies and nerve fibers [127]. The role of VEGF generated substantial interest in the therapy of neuropathy. VEGF administration has been shown to restore nerve blood flow, nerve conduction velocity, and nerve vessel number to normal in DPN [128].
VEGF is a hypoxia-sensitive glycoprotein. Plasma VEGF levels became elevated in severely hypoxic OSA patients and were correlated to the degree of nocturnal oxygen desaturation [129]. Similar results were found in both young and adult OSA patients, and plasma VEGF concentration was moderately correlated to OSA severity [130, 131]. Some other studies showed contrary results and found no correlation between plasma VEGF levels and severity of hypoxia [131–133].
2.1.12. AGEs
AGEs are a complex group of compounds formed through nonenzymatic covalent bonding between reducing sugar and amine residues on lipids, proteins, or nucleic acids. They could also originate from exogenous sources including diet and tobacco smoke [134]. AGEs accumulate in local tissues because AGE-modified proteins are resistant to enzymatic degradation [135]. The role for glycation/glycoxidation in diabetic neuropathy has been extensively reviewed [136–139]. Several clinical studies implicated glycation in the pathogenesis of DPN secondary to T2D [140, 141]. Glycated myelin can stimulate macrophages to secrete proteases, which could contribute towards nerve demyelization in DPN [134, 138, 142]. Elevated AGE levels have been documented in the peripheral nerves of diabetic patients [138]. The AGE pathway is a main pathophysiologic mechanism in the development of DPN, and measures to reduce AGE formation could be useful in preserving nerve function in T2D patients [134].
AGEs are also increased in OSA, a condition in which increased systemic inflammation and oxidative stress are operationally activated [143, 144]. Intermittent hypoxia in OSA may induce AGE formation [145]. Plasma AGE levels were elevated and associated with insulin resistance in nondiabetic patients with OSA [146]. AGE accumulation in OSA may lead to diminution in early endothelial progenitor cells and endothelial repair capacity over time contributing to vascular pathogenesis [147].
2.1.13. Protein Kinase C (PKC)
PKC is a family of enzymes involved in controlling the function of other proteins through phosphorylation of the OH groups on threonine and serine residues and has several isoforms. The role of PKC in the pathogenesis of DPN has been reviewed in detail [148]. There has been conflicting reports on PKC isozyme activity in dorsal root ganglion neurons in diabetic animals [148]. One report showed decreased PKC α mRNA levels in dorsal root ganglion neurons of diabetic rats compared with controls [149]. Another report showed higher aldose reductase expression in neurons, which reduced PKC α activity due to translocation from the membrane to cytosol, revealing a role of PKC α isoform in the hyperglycemic milieu [150]. Vascular tissues in diabetes showed increased PKC activity leading to increased permeability and dysfunction [151].
Proinflammatory cytokines such as TNF-α and IL-1β produced in the pulmonary arterial tissue were upregulated under hypoxic conditions in OSA patients, and the upregulation of these cytokines was dependent on PKC activation [152]. Intermittent hypoxia mimicking OSA could augment vasoconstriction mediated by PKC δ in a calcium-independent manner [125].
2.1.14. Poly ADP Ribose Polymerase (PARP)
PARP becomes activated by oxidative stress-induced DNA damage, which plays an important role in the pathogenesis of DPN in T2D patients [153, 154]. A recent study has shown that PARP activation was independently associated with higher AHI secondary to oxidative stress in patients with OSA and T2D [8]. In addition, PARP activation provides another explanation for the longitudinal and cross-sectional associations between OSA and DPN in T2D patients [7, 155–157]. Intermittent hypoxia has been suggested to induce oxidative stress and PARP activation in nondiabetic rodents in vitro [158]. PARP inhibition could reverse DPN and neuropathy in diabetic rodents through alleviating oxidative stress [159–161].
2.1.15. Nitrosative Stress
Nitrosative stress, which is marked by enhanced peroxynitrite formation, has been well documented in both clinical and experimental diabetic neuropathies [162]. A previous study has demonstrated higher serum nitrotyrosine levels in T2D patients with DPN, which is consistent with reports on experimental DPN [7], implicating nitrosative stress in DPN pathogenesis by reducing nerve perfusion and destroying vascular reactivity of epineurial arterioles [162, 163]. Nitrosative stress could also affect all the cell types in the peripheral nervous system, such as Schwann cells, and endothelial cells of the peripheral nerve, astrocytes, neurons, and oligodendrocytes of the spinal cord [159]. Nitrosative stress is related to the development of thermal hyper- and hypalgesia, tactile allodynia, mechanical hypalgesia, and small sensory nerve fiber degeneration [163]. Inhibition of nitrosative stress with PARP inhibitor or baicalein improved experimental neuropathy in diabetic rodent models [159, 164].
So far, a study has shown an association between OSA and nitrosative stress in T2D patients [7]. The obvious correlation between nocturnal/sleep-related hypoxemia and serum nitrotyrosine levels suggested that nitrosative stress was a potential mechanistic link between DPN and OSA. A previous study has shown greater endothelial expression of nitrotyrosine in OSA patients without T2D than OSA-free subjects regardless of central adiposity [165].
2.1.16. Plasminogen Activator Inhibitor-1 (PAI-1)
PAI-1, a member of the serine protease inhibitor family, controls the fibrinolytic system by inhibiting urokinase and tissue-type plasminogen activators [103]. An earlier study indicated that diabetic neuropathy did not show any significant relationship with plasma PAI-1 levels in T2D patients [166]. A recent study has shown that PAI-1 levels were higher in DPN patients than normal subjects and T2D patients without DPN and PAI-1 was associated with DPN development from the perspective of inflammation, suggesting that PAI-1 and inflammatory markers such as TNF-α and CRP participated in the development and progression of DPN [103].
Increased PAI-1 activity was associated with sleep-disordered breathing, possibly contributing to increased vascular risk [167]. A previous study indicated that PAI-1 levels were significantly higher in OSA subjects than controls and PAI-1 positively correlated with AHI index [168]. CPAP therapy could significantly reduce PAI-1 levels. In OSA children, PAI-1 levels were significantly higher along with other inflammatory markers such as IL-6 and monocyte chemoattractant protein-1 (MCP-1) [169]. A recent study has shown that OSA patients had a higher median plasma PAI-1 level than controls and PAI-1 levels increased with OSA severity, suggesting that OSA could enhance prothrombotic activity [170]. OSA significantly correlated with PAI-1 concentration due to prothrombotic effects.
2.1.17. Vitamin D Deficiency
Vitamin D, a steroid hormone with multifarious and extensive effect, could play a potential therapeutic role in attenuating the severity and progression of T2D [171, 172]. Growing evidence shows that low vitamin D level could contribute to pathogenesis of diabetes and its underlying diseases [173, 174]. Vitamin D deficiency has been implicated in the pathophysiology of DPN by impacting on nerve function [175].
Vitamin D deficiency may correlate with DPN in T2D patients [173, 176–180], with lower serum levels of 25(OH)D being independently associated with increased DPN in T2D patients [177, 181]. Vitamin D deficiency was also associated with development of neuropathy in T2D patients [182], as revealed in Caucasians and Asians with T2D by a recent meta-analysis [183]. Nerve growth factor (NGF), which is essential for primary nociceptive neuron development, was found by immunostaining to correlate with skin axon reflex vasodilation mediated by small sensory fibers in diabetic neuropathy patients [184]. Vitamin D is likely a modifiable risk factor for DPN and could modulate inflammatory mediators including IL-17 and IL-13 in DPN development [185]. Vitamin D supplementation relieved symptoms of neuropathy in T2D patients [186].
Nondiabetic pediatric OSA patients had reduced 25(OH)D levels [187], which may play a role in modulating the degree of insulin resistance and systemic inflammation [188]. Previous studies reported that OSA patients had lower vitamin D levels than healthy individuals [189–192]. Vitamin D can establish homeostasis between suppressor and regulatory T cell functions to modulate inflammatory process in OSA [193], suggesting that vitamin D deficiency in severe OSA patients is common, with a negative correlation between IL-17 and serum vitamin D levels [194]. Recent studies have shown that patients with OSA have a higher prevalence of vitamin D deficiency than healthy controls [190, 191, 195], and there are conflicting reports on the association between vitamin D deficiency and severity of OSA [191, 195–198]. PARP treatment may have late beneficial effects on vitamin D levels in selected OSA patients [199, 200]. Further studies exploring whether vitamin D deficiency may modulate OSA are needed.
2.1.18. Neurotransmitters
Some neuromodulators such as glutamate, noradrenaline, acetylcholine, dopamine, and GABA could affect DPN and OSA. The effect on and association of the neurotransmitters with DPN and OSA are listed in Table 2.
Table 2.
Summary of shared molecular mechanisms involved in DPN and OSA.
| Common neuromodulators | Changes in DPN | Changes in OSA |
|---|---|---|
| Glutamate | Increased release [202] | High levels [203] |
| Noradrenaline | Increased levels [205] | Plasma and 24 h urinary noradrenaline increased [204] |
| Acetylcholine (Ach) | Attenuated Ach synthesis [206, 207] | Induce vasodilation [208] |
| Dopamine | Increased/decreased in different regions of the nerve system | Not correlated with OSA [209] |
| γ-Aminobutyric acid (GABA) | Decrease in diabetic neuropathy patients [211] | Reduced GABA levels in OSA [203] |
DPN: diabetic peripheral neuropathy; OSA: obstructive sleep apnea.
2.1.19. Glutamate
A previous study showed that the excitatory neurotransmitter glutamate induced an increased magnitude of mitochondrial depolarization, but no increase in apoptosis was observed [201]. In DPN, glutamate release is related with increased oxidative stress and decreased mitochondrial function, which is associated with neuropathic pain and activation of the glutamate recycling pathway that protects diabetic dorsal root ganglion by activating the SIRT1-PGC-1α-EFAM axis [202]. In OSA patients, higher glutamate levels were observed versus healthy subjects [203]. Similar changes on glutamate levels could lead to common mechanism in the pathogenesis of DPN and OSA.
2.1.20. Noradrenaline
In OSA patients, nocturnal plasma noradrenaline content was increased and correlated with severity of overnight oxygen desaturation; 24 h urinary noradrenaline also increased [204]. In diabetic neuropathy rats, noradrenaline concentration was increased significantly 20 and 40 min after tramadol and clomipramine infusion [205], suggesting that the descending noradrenergic pathway could play an important role in analgesia for diabetic neuropathy. These findings indicate that noradrenaline could be involved in DPN and OSA.
2.1.21. Acetylcholine
Diabetes was reported to impair acetylcholine-induced vascular relaxation in epineurial arterioles of the sciatic nerve [206]. A previous report showed that palmitic acid exposure could cause neuronal loss in diabetic neuropathy, which may be due to attenuated Ach synthesis [207]. In an OSA animal model, acetylcholine-induced vasodilation through the NO-dependent pathway in the skeletal muscle was impaired [208].
2.1.22. Dopamine
A recent study showed that dopamine content was decreased in the midbrain, cerebral cortex, and brainstem regions, while it increased in the cerebellum and thalamus/hypothalamus in diabetic rats. No correlation between OSA and dopamine has been found [209].
2.1.23. γ-Aminobutyric Acid (GABA)
In the adult nerve system, GABA is the main inhibitory neurotransmitter related with pain modulation. A previous study showed pronounced increase of extracellular GABA concentration in the ventromedial hypothalamic region in a type 1 diabetic animal model [210]. In diabetic neuropathy patients, GABA levels were significantly lower versus healthy controls [211]. The Toronto Expert Panel on Diabetic Neuropathy (TEPDN) recommended that GABA should be considered as first-line diabetic neuropathy treatment [212]. In OSA, low GABA levels were observed by“2-dimensional” spectroscopy [213] and reduced GABA levels were detected in the insular cortex of OSA patients [203]. The decrease in the levels of GABA in DPN and OSA has potential serious functional consequences that need to be elucidated.
3. Conclusions and Perspectives
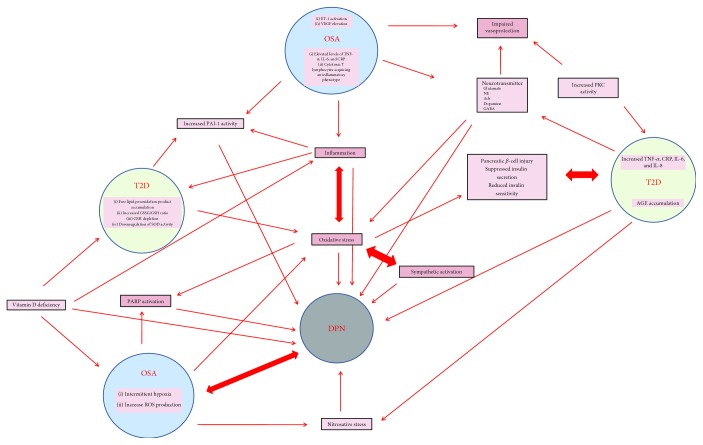
Much of the data available on OSA and its association with DPN is difficult to interpret. The evidence reviewed in the current paper on the association between PDN and OSA in T2D patients suggests an intricate interrelationship among DPN, OSA, and T2D (Figure 1). OSA can aggravate and amplify T2D and subsequent complications such as DPN. Ultimately, OSA could contribute to T2D, resulting in a vicious circle. It is important to keep in mind that prospective studies with a larger T2D population are required to delineate the interwoven relationship between OSA and DPN. In addition, we summarized shared mechanisms in DPN and OSA, including oxidative stress, inflammation, AGEs, and PKC signaling. We also speculate that these mechanisms can operate simultaneously in patients with T2D leading to DPN and OSA. Recently, histone modifications are also reported to be involved in DPN and OSA. With the understanding of pathophysiology, CPAP is considered as the gold standard for managing patients with moderate to severe OSA. Further studies indicated that CPAP could improve insulin sensitivity in patients with prediabetes and decrease blood glucose in T2D patients; but till now, there has been no proof of efficacy of CPAP for DPN in vivo or in vitro. Similarly to CPAP, weight loss, bariatric intervention, or pharmacotherapy has been proven effective in alleviating OSA severity and improving glycemic status in obese T2D patients; future clinical trials will shed more light on the impact of CPAP therapy, weight loss, bariatric intervention, or pharmacotherapy on DPN. Prospective studies are required to determine mechanistic links applicable to DPN and OSA, which will contribute more to the exploration of novel therapeutic strategies in retarding the development and progression of DPN and OSA in T2D.
Figure 1.
Pleiotropic interactions among type 2 diabetes (T2D), diabetic peripheral neuropathy (DPN), and obstructive sleep apnea (OSA).
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (no. 81570652) and the Natural Science Foundation Project of Jilin Provincial Science and Technology (20160101005JC) to Guangdong Sun.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
- 1.Selvin E., Parrinello C. M., Sacks D. B., Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Annals of Internal Medicine. 2014;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesfaye S., Selvarajah D., Gandhi R., et al. Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain. 2016;157:S72–S80. doi: 10.1097/j.pain.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 3.Nannapaneni S., Ramar K., Surani S. Effect of obstructive sleep apnea on type 2 diabetes mellitus: a comprehensive literature review. World Journal of Diabetes. 2013;4(6):238–244. doi: 10.4239/wjd.v4.i6.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesfaye S., Vileikyte L., Rayman G., et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes/Metabolism Research and Reviews. 2011;27(7):629–638. doi: 10.1002/dmrr.1225. [DOI] [PubMed] [Google Scholar]
- 5.Stino A. M., Smith A. G. Peripheral neuropathy in prediabetes and the metabolic syndrome. Journal of Diabetes Investigation. 2017;8(5):646–655. doi: 10.1111/jdi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahrani A. A. Obstructive sleep apnoea in diabetes: does it matter? Diabetes & Vascular Disease Research. 2017;14(5):454–462. doi: 10.1177/1479164117714397. [DOI] [PubMed] [Google Scholar]
- 7.Tahrani A. A., Ali A., Raymond N. T., et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. American Journal of Respiratory and Critical Care Medicine. 2012;186(5):434–441. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altaf Q. A., Ali A., Piya M. K., Raymond N. T., Tahrani A. A. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. Journal of Diabetes and its Complications. 2016;30(7):1315–1320. doi: 10.1016/j.jdiacomp.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Rasche K., Keller T., Tautz B., et al. Obstructive sleep apnea and type 2 diabetes. European Journal of Medical Research. 2010;15(Supplement 2):p. 152. doi: 10.1186/2047-783X-15-S2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu B., Hershberger P. E., Kapella M. C., Fritschi C. The relationship between sleep disturbance and glycaemic control in adults with type 2 diabetes: an integrative review. Journal of Clinical Nursing. 2017;26(23-24):4053–4064. doi: 10.1111/jocn.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. The Journal of Physiology. 2017;595(8):2423–2430. doi: 10.1113/JP273312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reutrakul S., Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sateia M. J. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 14.Foster G. D., Borradaile K. E., Sanders M. H., et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the sleep AHEAD study. Archives of Internal Medicine. 2009;169(17):1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peppard P. E., Young T., Barnet J. H., Palta M., Hagen E. W., Hla K. M. Increased prevalence of sleep-disordered breathing in adults. American Journal of Epidemiology. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller T., Hader C., De Zeeuw J., Rasche K. Obstructive sleep apnea syndrome: the effect of diabetes and autonomic neuropathy. Journal of Physiology and Pharmacology. 2007;58(Supplement 5):313–318. [PubMed] [Google Scholar]
- 17.Bottini P., Redolfi S., Dottorini M. L., Tantucci C. Autonomic neuropathy increases the risk of obstructive sleep apnea in obese diabetics. Respiration. 2008;75(3):265–271. doi: 10.1159/000100556. [DOI] [PubMed] [Google Scholar]
- 18.Storgaard H., Mortensen B., Almdal T., Laub M., Tarnow L. At least one in three people with type 2 diabetes mellitus referred to a diabetes centre has symptomatic obstructive sleep apnoea. Diabetic Medicine. 2014;31(11):1460–1467. doi: 10.1111/dme.12477. [DOI] [PubMed] [Google Scholar]
- 19.Martinez Ceron E., Casitas Mateos R., Garcia-Rio F. Sleep apnea–hypopnea syndrome and type 2 diabetes. A reciprocal relationship? Archivos de Bronconeumología (English Edition) 2015;51(3):128–139. doi: 10.1016/j.arbr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Aurora R. N., Punjabi N. M. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. The Lancet Respiratory Medicine. 2013;1(4):329–338. doi: 10.1016/S2213-2600(13)70039-0. [DOI] [PubMed] [Google Scholar]
- 21.Mok Y., Tan C. W., Wong H. S., How C. H., Tan K. L., Hsu P. P. Obstructive sleep apnoea and type 2 diabetes mellitus: are they connected? Singapore Medical Journal. 2017;58(4):179–183. doi: 10.11622/smedj.2017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appleton S. L., Vakulin A., McEvoy R. D., et al. Nocturnal hypoxemia and severe obstructive sleep apnea are associated with incident type 2 diabetes in a population cohort of men. Journal of Clinical Sleep Medicine. 2015;11(6) doi: 10.5664/jcsm.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendzerska T., Gershon A. S., Hawker G., Tomlinson G., Leung R. S. Obstructive sleep apnea and incident diabetes. A historical cohort study. American Journal of Respiratory and Critical Care Medicine. 2014;190(2):218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 24.Marshall N. S., Wong K. K., Phillips C. L., Liu P. Y., Knuiman M. W., Grunstein R. R. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? Journal of Clinical Sleep Medicine. 2009;5(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Muraki I., Tanigawa T., Yamagishi K., et al. Nocturnal intermittent hypoxia and metabolic syndrome; the effect of being overweight: the CIRCS study. Journal of Atherosclerosis and Thrombosis. 2010;17(4):369–377. doi: 10.5551/jat.3319. [DOI] [PubMed] [Google Scholar]
- 26.Nagayoshi M., Punjabi N. M., Selvin E., et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep Medicine. 2016;25:156–161. doi: 10.1016/j.sleep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan P., Greenberg H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nature and Science of Sleep. 2015;7:113–125. doi: 10.2147/NSS.S90835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botros N., Concato J., Mohsenin V., Selim B., Doctor K., Yaggi H. K. Obstructive sleep apnea as a risk factor for type 2 diabetes. The American Journal of Medicine. 2009;122(12):1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celen Y. T., Hedner J., Carlson J., Peker Y. Impact of gender on incident diabetes mellitus in obstructive sleep apnea: a 16-year follow-up. Journal of Clinical Sleep Medicine. 2010;6(3):244–250. [PMC free article] [PubMed] [Google Scholar]
- 30.Lindberg E., Theorell-Haglow J., Svensson M., Gislason T., Berne C., Janson C. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest. 2012;142(4):935–942. doi: 10.1378/chest.11-1844. [DOI] [PubMed] [Google Scholar]
- 31.Boyko E. J., Seelig A. D., Jacobson I. G., et al. Sleep characteristics, mental health, and diabetes risk: a prospective study of U.S. military service members in the Millennium Cohort Study. Diabetes Care. 2013;36(10):3154–3161. doi: 10.2337/DC13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichmuth K. J., Austin D., Skatrud J. B., Young T. Association of sleep apnea and type II diabetes: a population-based study. American Journal of Respiratory and Critical Care Medicine. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmood K., Akhter N., Eldeirawi K., et al. Prevalence of type 2 diabetes in patients with obstructive sleep apnea in a multi-ethnic sample. Journal of Clinical Sleep Medicine. 2009;5(3):215–221. [PMC free article] [PubMed] [Google Scholar]
- 34.Kent B. D., Grote L., Ryan S., et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146(4):982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- 35.Grimaldi D., Beccuti G., Touma C., Van Cauter E., Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–363. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aronsohn R. S., Whitmore H., Van Cauter E., Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. American Journal of Respiratory and Critical Care Medicine. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillai A., Warren G., Gunathilake W., Idris I. Effects of sleep apnea severity on glycemic control in patients with type 2 diabetes prior to continuous positive airway pressure treatment. Diabetes Technology & Therapeutics. 2011;13(9):945–949. doi: 10.1089/dia.2011.0005. [DOI] [PubMed] [Google Scholar]
- 38.Priou P., le Vaillant M., Meslier N., et al. Association between obstructive sleep apnea severity and glucose control in patients with untreated versus treated diabetes. Journal of Sleep Research. 2015;24(4):425–431. doi: 10.1111/jsr.12278. [DOI] [PubMed] [Google Scholar]
- 39.Manin G., Pons A., Baltzinger P., et al. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabetic Medicine. 2015;32(1):90–96. doi: 10.1111/dme.12582. [DOI] [PubMed] [Google Scholar]
- 40.Resnick H. E., Redline S., Shahar E., et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26(3):702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 41.Einhorn D., Stewart D., Erman M., Gordon N., Philis-Tsimikas A., Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocrine Practice. 2007;13(4):355–362. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 42.Foster G. D., Sanders M. H., Millman R., et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laaban J. P., Daenen S., Léger D., et al. Prevalence and predictive factors of sleep apnoea syndrome in type 2 diabetic patients. Diabetes & Metabolism. 2009;35(5):372–377. doi: 10.1016/j.diabet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Lam D. C. L., Lui M. M. S., Lam J. C. M., Ong L. H. Y., Lam K. S. L., Ip M. S. M. Prevalence and recognition of obstructive sleep apnea in Chinese patients with type 2 diabetes mellitus. Chest. 2010;138(5):1101–1107. doi: 10.1378/chest.10-0596. [DOI] [PubMed] [Google Scholar]
- 45.Hanis C. L., Redline S., Cade B. E., et al. Beyond type 2 diabetes, obesity and hypertension: an axis including sleep apnea, left ventricular hypertrophy, endothelial dysfunction, and aortic stiffness among Mexican Americans in Starr County, Texas. Cardiovascular Diabetology. 2016;15(1):p. 86. doi: 10.1186/s12933-016-0405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westlake K., Plihalova A., Pretl M., Lattova Z., Polak J. Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus: a prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Medicine. 2016;26:71–76. doi: 10.1016/j.sleep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Siwasaranond N., Nimitphong H., Saetung S., Chirakalwasan N., Ongphiphadhanakul B., Reutrakul S. Shorter sleep duration is associated with poorer glycemic control in type 2 diabetes patients with untreated sleep-disordered breathing. Sleep & Breathing. 2016;20(2):569–574. doi: 10.1007/s11325-015-1243-6. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P., Zhang R., Zhao F., et al. The prevalence and characteristics of obstructive sleep apnea in hospitalized patients with type 2 diabetes in China. Journal of Sleep Research. 2016;25(1):39–46. doi: 10.1111/jsr.12334. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R., Guo X., Guo L., Lu J., Zhou X., Ji L. Prevalence and associated factors of obstructive sleep apnea in hospitalized patients with type 2 diabetes in Beijing, China. Journal of Diabetes. 2015;7(1):16–23. doi: 10.1111/1753-0407.12180. [DOI] [PubMed] [Google Scholar]
- 50.Heffner J. E., Rozenfeld Y., Kai M., Stephens E. A., Brown L. K. Prevalence of diagnosed sleep apnea among patients with type 2 diabetes in primary care. Chest. 2012;141(6):1414–1421. doi: 10.1378/chest.11-1945. [DOI] [PubMed] [Google Scholar]
- 51.Callaghan B. C., Cheng H. T., Stables C. L., Smith A. L., Feldman E. L. Diabetic neuropathy: clinical manifestations and current treatments. The Lancet Neurology. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Won J. C., Park T. S. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinology and Metabolism. 2016;31(2):230–238. doi: 10.3803/EnM.2016.31.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashine S., Kishida K., Funahashi T., et al. Selective contribution of waist circumference reduction on the improvement of sleep-disordered breathing in patients hospitalized with type 2 diabetes mellitus. Internal Medicine. 2011;50(18):1895–1903. doi: 10.2169/internalmedicine.50.5669. [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld V. Sleep dysfunction, diabetes, and pain: a troublesome triad. The Journal of Family Practice. 2014;63(Supplement 6):S18–S24. [PubMed] [Google Scholar]
- 55.Bottini P., Scionti L., Santeusanio F., Casucci G., Tantucci C. Impairment of the respiratory system in diabetic autonomic neuropathy. Diabetes, Nutrition & Metabolism. 2000;13(3):165–172. [PubMed] [Google Scholar]
- 56.Catterall J. R., Calverley P. M. A., Ewing D. J., Shapiro C. M., Clarke B. F., Douglas N. J. Breathing, sleep, and diabetic autonomic neuropathy. Diabetes. 1984;33(11):1025–1027. doi: 10.2337/diab.33.11.1025. [DOI] [PubMed] [Google Scholar]
- 57.Ewing D. J., Neilson J. M., Shapiro C. M., Stewart J. A., Reid W. Twenty four hour heart rate variability: effects of posture, sleep, and time of day in healthy controls and comparison with bedside tests of autonomic function in diabetic patients. British Heart Journal. 1991;65(5):239–244. doi: 10.1136/hrt.65.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sobotka P. A., Liss H. P., Vinik A. I. Impaired hypoxic ventilatory drive in diabetic patients with autonomic neuropathy. The Journal of Clinical Endocrinology & Metabolism. 1986;62(4):658–663. doi: 10.1210/jcem-62-4-658. [DOI] [PubMed] [Google Scholar]
- 59.Rees P. J., Prior J. G., Cochrane G. M., Clark T. J. H. Sleep apnoea in diabetic patients with autonomic neuropathy. Journal of the Royal Society of Medicine. 1981;74(3):192–195. doi: 10.1177/014107688107400306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mondini S., Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Annals of Neurology. 1985;17(4):391–395. doi: 10.1002/ana.410170415. [DOI] [PubMed] [Google Scholar]
- 61.Dematteis M., Pepin J. L., Jeanmart M., Deschaux C., Labarre-Vila A., Levy P. Charcot-Marie-Tooth disease and sleep apnoea syndrome: a family study. The Lancet. 2001;357(9252):267–272. doi: 10.1016/S0140-6736(00)03614-X. [DOI] [PubMed] [Google Scholar]
- 62.Fujihara K., Kodama S., Horikawa C., et al. The relationship between diabetic neuropathy and sleep apnea syndrome: a meta-analysis. Sleep Disorders. 2013;2013:7. doi: 10.1155/2013/150371.150371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ficker J. H., Dertinger S. H., Siegfried W., et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. European Respiratory Journal. 1998;11(1):14–19. doi: 10.1183/09031936.98.11010014. [DOI] [PubMed] [Google Scholar]
- 64.Evlice A., Ugurel B., Baklan B., Oztura I. Neuropathy and dysautonomia in patients with obstructive sleep apnea syndrome. Noro Psikiyatri Arsivi. 2015;52(1):24–28. doi: 10.5152/npa.2015.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keskin A., Ünalacak M., Bilge U., et al. Effects of sleep disorders on hemoglobin A1c levels in type 2 diabetic patients. Chinese Medical Journal. 2015;128(24):3292–3297. doi: 10.4103/0366-6999.171415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chao C.-Y., Wu J.-S., Yang Y.-C., et al. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism. 2011;60(6):799–804. doi: 10.1016/j.metabol.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 67.Shaikh W. A., Patel M., Singh S. Association of sleep duration with arterial blood pressure profile of gujarati Indian adolescents. Indian Journal of Community Medicine. 2010;35(1):125–129. doi: 10.4103/0970-0218.62571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cappuccio F. P., D'Elia L., Strazzullo P., Miller M. A. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dziewas R., Schilling M., Engel P., et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. Journal of Neurology, Neurosurgery & Psychiatry. 2006;78(3):295–297. doi: 10.1136/jnnp.2006.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shakeel M. Recent advances in understanding the role of oxidative stress in diabetic neuropathy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(4):373–378. doi: 10.1016/j.dsx.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 71.Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Frontiers in Bioscience. 2012;E4(4):1391–1403. doi: 10.2741/e469. [DOI] [PubMed] [Google Scholar]
- 72.Bloch-Damti A., Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxidants & Redox Signaling. 2005;7(11-12):1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 73.Robertson R. Oxidative stress and impaired insulin secretion in type 2 diabetes. Current Opinion in Pharmacology. 2006;6(6):615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Xu J., Long Y. S., Gozal D., Epstein P. N. β-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radical Biology & Medicine. 2009;46(6):783–790. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 75.Obrosova I. G. Diabetes and the peripheral nerve. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2009;1792(10):931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Stevens M. J., Obrosova I., Cao X., Van Huysen C., Greene D. A. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49(6):1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 77.Obrosova I. G., Fathallah L., Stevens M. J. Taurine counteracts oxidative stress and nerve growth factor deficit in early experimental diabetic neuropathy. Experimental Neurology. 2001;172(1):211–219. doi: 10.1006/exnr.2001.7789. [DOI] [PubMed] [Google Scholar]
- 78.Coppey L. J., Gellett J. S., Davidson E. P., et al. Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. British Journal of Pharmacology. 2001;134(1):21–29. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng C., Zochodne D. W. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52(9):2363–2371. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- 80.Leinninger G. M., Edwards J. L., Lipshaw M. J., Feldman E. L. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nature Clinical Practice Neurology. 2006;2(11):620–628. doi: 10.1038/ncpneuro0320. [DOI] [PubMed] [Google Scholar]
- 81.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Medicine Reviews. 2003;7(1):35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 82.Dyugovskaya L., Lavie P., Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. American Journal of Respiratory and Critical Care Medicine. 2002;165(7):934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 83.Schulz R., Mahmoudi S., Hattar K., et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. American Journal of Respiratory and Critical Care Medicine. 2000;162(2):566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 84.Lavie L., Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. European Respiratory Journal. 2009;33(6):1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 85.Lévy P., Kohler M., McNicholas W. T., et al. Obstructive sleep apnoea syndrome. Nature Reviews Disease Primers. 2015;1, article 15015 doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 86.Alonso-Fernandez A., Garcia-Rio F., Arias M. A., et al. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax. 2009;64(7):581–586. doi: 10.1136/thx.2008.100537. [DOI] [PubMed] [Google Scholar]
- 87.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia – revisited – the bad ugly and good: implications to the heart and brain. Sleep Medicine Reviews. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Wang N., Khan S. A., Prabhakar N. R., Nanduri J. Impairment of pancreatic β-cell function by chronic intermittent hypoxia. Experimental Physiology. 2013;98(9):1376–1385. doi: 10.1113/expphysiol.2013.072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lavie L., Vishnevsky A., Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27(1):123–128. doi: 10.1093/sleep/27.1.123. [DOI] [PubMed] [Google Scholar]
- 90.Carpagnano G. E., Kharitonov S. A., Resta O., Foschino-Barbaro M. P., Gramiccioni E., Barnes P. J. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124(4):1386–1392. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 91.Jurado-Gamez B., Fernandez-Marin M. C., Gomez-Chaparro J. L., et al. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. European Respiratory Journal. 2011;37(4):873–879. doi: 10.1183/09031936.00027910. [DOI] [PubMed] [Google Scholar]
- 92.Tan K. C. B., Chow W.-S., Lam J. C. M., et al. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184(2):377–382. doi: 10.1016/j.atherosclerosis.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 93.Yamauchi M., Nakano H., Maekawa J., et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127(5):1674–1679. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 94.Vatansever E., Surmen-Gur E., Ursavas A., Karadag M. Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep and Breathing. 2011;15(3):275–282. doi: 10.1007/s11325-010-0378-8. [DOI] [PubMed] [Google Scholar]
- 95.Klein C., Martinez D., Hackenhaar F. S., et al. Carbonyl groups: bridging the gap between sleep disordered breathing and coronary artery disease. Free Radical Research. 2010;44(8):907–912. doi: 10.3109/10715762.2010.489112. [DOI] [PubMed] [Google Scholar]
- 96.Golbidi S., Badran M., Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Experimental Diabetes Research. 2012;2012:16. doi: 10.1155/2012/941868.941868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barone M. T. U., Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Research and Clinical Practice. 2011;91(2):129–137. doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 98.Ouchi N., Parker J. L., Lugus J. J., Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews Immunology. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cildir G., Akincilar S. C., Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends in Molecular Medicine. 2013;19(8):487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Odegaard J. I., Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mu Z. P., Wang Y. G., Li C. Q., et al. Association between tumor necrosis factor-α and diabetic peripheral neuropathy in patients with type 2 diabetes: a meta-analysis. Molecular Neurobiology. 2017;54(2):983–996. doi: 10.1007/s12035-016-9702-z. [DOI] [PubMed] [Google Scholar]
- 102.Zhu T., Meng Q., Ji J., Zhang L., Lou X. TLR4 and caveolin-1 in monocytes are associated with inflammatory conditions in diabetic neuropathy. Clinical and Translational Science. 2017;10(3):178–184. doi: 10.1111/cts.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ge S., Xie J., Zheng L., et al. Associations of serum anti-ganglioside antibodies and inflammatory markers in diabetic peripheral neuropathy. Diabetes Research and Clinical Practice. 2016;115:68–75. doi: 10.1016/j.diabres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 104.Cameron N., Cotter M. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Current Drug Targets. 2008;9(1):60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- 105.Bierhaus A., Haslbeck K. M., Humpert P. M., et al. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. The Journal of Clinical Investigation. 2004;114(12):1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dyugovskaya L., Lavie P., Hirsh M., Lavie L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. European Respiratory Journal. 2005;25(5):820–828. doi: 10.1183/09031936.05.00103204. [DOI] [PubMed] [Google Scholar]
- 107.Dyugovskaya L., Lavie P., Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Annals of the New York Academy of Sciences. 2005;1051(1):340–350. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 108.Dyugovskaya L., Lavie P., Lavie L. Phenotypic and functional characterization of blood γδ T cells in sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2003;168(2):242–249. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- 109.Vgontzas A. N., Papanicolaou D. A., Bixler E. O., Kales A., Tyson K., Chrousos G. P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. The Journal of Clinical Endocrinology & Metabolism. 1997;82(5):1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 110.Vgontzas A. N., Bixler E. O., Chrousos G. P. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. Journal of Internal Medicine. 2003;254(1):32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 111.Harsch I. A., Hahn E. G., Konturek P. C. Insulin resistance and other metabolic aspects of the obstructive sleep apnea syndrome. Medical Science Monitor. 2005;11(3):RA70–RA75. [PubMed] [Google Scholar]
- 112.Yokoe T., Minoguchi K., Matsuo H., et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi: 10.1161/01.CIR.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 113.Golbidi S., Badran M., Ayas N., Laher I. Cardiovascular consequences of sleep apnea. Lung. 2012;190(2):113–132. doi: 10.1007/s00408-011-9340-1. [DOI] [PubMed] [Google Scholar]
- 114.Arias M. A., Garcia-Rio F., Alonso-Fernandez A., et al. CPAP decreases plasma levels of soluble tumour necrosis factor-α receptor 1 in obstructive sleep apnoea. European respiratory journal. 2008;32(4):1009–1015. doi: 10.1183/09031936.00007008. [DOI] [PubMed] [Google Scholar]
- 115.Dyugovskaya L., Polyakov A., Lavie P., Lavie L. Delayed neutrophil apoptosis in patients with sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2008;177(5):544–554. doi: 10.1164/rccm.200705-675OC. [DOI] [PubMed] [Google Scholar]
- 116.Dyugovskaya L., Polyakov A., Ginsberg D., Lavie P., Lavie L. Molecular pathways of spontaneous and TNF-α–mediated neutrophil apoptosis under intermittent hypoxia. American Journal of Respiratory Cell and Molecular Biology. 2011;45(1):154–162. doi: 10.1165/rcmb.2010-0025OC. [DOI] [PubMed] [Google Scholar]
- 117.Dyugovskaya L., Polyakov A., Cohen-Kaplan V., Lavie P., Lavie L. Bax/Mcl-1 balance affects neutrophil survival in intermittent hypoxia and obstructive sleep apnea: effects of p38MAPK and ERK1/2 signaling. Journal of Translational Medicine. 2012;10(1):p. 211. doi: 10.1186/1479-5876-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Htoo A. K., Greenberg H., Tongia S., et al. Activation of nuclear factor κB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep and Breathing. 2006;10(1):43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 119.El Boghdady N. A., Badr G. A. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell Biochemistry and Function. 2012;30(4):328–334. doi: 10.1002/cbf.2808. [DOI] [PubMed] [Google Scholar]
- 120.Saarelainen S., Seppala E., Laasonen K., Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium. 1997;5(2):115–118. doi: 10.3109/10623329709079869. [DOI] [PubMed] [Google Scholar]
- 121.Phillips B. G., Narkiewicz K., Pesek C. A., Haynes W. G., Dyken M. E., Somers V. K. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. Journal of Hypertension. 1999;17(1):61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 122.Zamarron-Sanz C., Ricoy-Galbaldon J., Gude-Sampedro F., Riveiro-Riveiro A. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Archives of Medical Research. 2006;37(4):552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 123.Moller D. S., Lind P., Strunge B., Pedersen E. B. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. American Journal of Hypertension. 2003;16(4):274–280. doi: 10.1016/S0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 124.Grimpen F., Kanne P., Schulz E., Hagenah G., Hasenfuβ G., Andreas S. Endothelin-1 plasma levels are not elevated in patients with obstructive sleep apnoea. European Respiratory Journal. 2000;15(2):320–325. doi: 10.1034/j.1399-3003.2000.15b17.x. [DOI] [PubMed] [Google Scholar]
- 125.Webster B. R., Osmond J. M., Paredes D. A., et al. Phosphoinositide-dependent kinase-1 and protein kinase Cδ contribute to endothelin-1 constriction and elevated blood pressure in intermittent hypoxia. The Journal of Pharmacology and Experimental Therapeutics. 2013;344(1):68–76. doi: 10.1124/jpet.112.195412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mentek M., Aptel F., Godin-Ribuot D., Tamisier R., Pepin J. L., Chiquet C. Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Medicine Reviews. 2018;38:113–130. doi: 10.1016/j.smrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 127.Leinninger G. M., Vincent A. M., Feldman E. L. The role of growth factors in diabetic peripheral neuropathy. Journal of the Peripheral Nervous System. 2004;9(1):26–53. doi: 10.1111/j.1085-9489.2004.09105.x. [DOI] [PubMed] [Google Scholar]
- 128.Kaur S., Pandhi P., Dutta P. Painful diabetic neuropathy: an update. Annals of Neurosciences. 2011;18(4):168–175. doi: 10.5214/ans.0972.7531.1118409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schulz R., Hummel C., Heinemann S., Seeger W., Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. American Journal of Respiratory and Critical Care Medicine. 2002;165(1):67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 130.Lavie L., Kraiczi H., Hefetz A., et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. American Journal of Respiratory and Critical Care Medicine. 2002;165(12):1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 131.Godoy J., Mellado P., Tapia J., Santin J. Obstructive sleep apnea as an independent stroke risk factor: possible mechanisms. Current Molecular Medicine. 2009;9(2):203–209. doi: 10.2174/156652409787581556. [DOI] [PubMed] [Google Scholar]
- 132.Valipour A., Litschauer B., Mittermayer F., Rauscher H., Burghuber O. C., Wolzt M. Circulating plasma levels of vascular endothelial growth factor in patients with sleep disordered breathing. Respiratory Medicine. 2004;98(12):1180–1186. doi: 10.1016/j.rmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 133.Kaditis A. G., Alexopoulos E. I., Karadonta I., et al. Obstructive sleep-disordered breathing and plasma levels of vascular endothelial growth factor in children. Respiratory Medicine. 2006;100(5):835–840. doi: 10.1016/j.rmed.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Miranda-Massari J. R., Gonzalez M. J., Jimenez F. J., Allende-Vigo M. Z., Duconge J. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Current Clinical Pharmacology. 2011;6(4):260–273. doi: 10.2174/157488411798375967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annual Review of Medicine. 1995;46(1):223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 136.Thornalley P. J. Glycation in diabetic neuropathy: characteristics, consequences, causes, and therapeutic options. International Review of Neurobiology. 2002;50:37–57. doi: 10.1016/S0074-7742(02)50072-6. [DOI] [PubMed] [Google Scholar]
- 137.Dickinson P. J., Carrington A. L., Frost G. S., Boulton A. J. M. Neurovascular disease, antioxidants and glycation in diabetes. Diabetes/Metabolism Research and Reviews. 2002;18(4):260–272. doi: 10.1002/dmrr.305. [DOI] [PubMed] [Google Scholar]
- 138.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Research and Clinical Practice. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 139.Goh S. Y., Cooper M. E. The role of advanced glycation end products in progression and complications of diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;93(4):1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 140.Meerwaldt R., Links T. P., Graaff R., et al. Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. Diabetologia. 2005;48(8):1637–1644. doi: 10.1007/s00125-005-1828-x. [DOI] [PubMed] [Google Scholar]
- 141.Garay-Sevilla M. E., Regalado J. C., Malacara J. M., et al. Advanced glycosylation end products in skin, serum, saliva and urine and its association with complications of patients with type 2 diabetes mellitus. Journal of Endocrinological Investigation. 2005;28(5):223–230. doi: 10.1007/BF03345377. [DOI] [PubMed] [Google Scholar]
- 142.Vlassara H., Brownlee M., Cerami A. Recognition and uptake of human diabetic peripheral nerve myelin by macrophages. Diabetes. 1985;34(6):553–557. doi: 10.2337/diab.34.6.553. [DOI] [PubMed] [Google Scholar]
- 143.Veasey S. Obstructive sleep apnea: rapidly AGE-ing us all. Sleep. 2006;29(3):280–281. doi: 10.1093/sleep/29.3.280. [DOI] [PubMed] [Google Scholar]
- 144.Mokhlesi B., Gozal D. In the fight against advanced glycation end-products (AGEs), you should treat OSA, shouldn’t you? Sleep Medicine. 2012;13(1):5–6. doi: 10.1016/j.sleep.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 145.Xu Y., Toure F., Qu W., et al. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. Journal of Biological Chemistry. 2010;285(30):23233–23240. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu J. X., Cai W., Sun J. F., et al. Serum advanced glycation end products are associated with insulin resistance in male nondiabetic patients with obstructive sleep apnea. Sleep and Breathing. 2015;19(3):827–833. doi: 10.1007/s11325-014-1100-z. [DOI] [PubMed] [Google Scholar]
- 147.Lui M. M.-S., Tse H.-F., Mak J. C.-W., et al. Altered profile of circulating endothelial progenitor cells in obstructive sleep apnea. Sleep and Breathing. 2013;17(3):937–942. doi: 10.1007/s11325-012-0781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eichberg J. Protein kinase C changes in diabetes: is the concept relevant to neuropathy? International Review of Neurobiology. 2002;50:61–82. doi: 10.1016/S0074-7742(02)50073-8. [DOI] [PubMed] [Google Scholar]
- 149.Roberts R. E., McLean W. G. Protein kinase C isozyme expression in sciatic nerves and spinal cords of experimentally diabetic rats. Brain Research. 1997;754(1-2):147–156. doi: 10.1016/S0006-8993(97)00062-0. [DOI] [PubMed] [Google Scholar]
- 150.Yamagishi S.-I., Uehara K., Otsuki S., Yagihashi S. Differential influence of increased polyol pathway on protein kinase C expressions between endoneurial and epineurial tissues in diabetic mice. Journal of Neurochemistry. 2003;87(2):497–507. doi: 10.1046/j.1471-4159.2003.02011.x. [DOI] [PubMed] [Google Scholar]
- 151.Yagihashi S. Chapter eight - glucotoxic mechanisms and related therapeutic approaches. International Review of Neurobiology. 2016;127:121–149. doi: 10.1016/bs.irn.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 152.Tsai B. M., Wang M., Pitcher J. M., Meldrum K. K., Meldrum D. R. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;287(6):L1215–L1219. doi: 10.1152/ajplung.00179.2004. [DOI] [PubMed] [Google Scholar]
- 153.Obrosova I. G., Li F., Abatan O. I., et al. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53(3):711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 154.Szabó C., Zanchi A., Komjáti K., et al. Poly(ADP-ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106(21):2680–2686. doi: 10.1161/01.CIR.0000038365.78031.9C. [DOI] [PubMed] [Google Scholar]
- 155.Tahrani A. A., Ali A., Raymond N. T., et al. Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care. 2013;36(11):3718–3725. doi: 10.2337/dc13-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tahrani A. A., Ali A., Stevens M. J. Obstructive sleep apnoea and diabetes: an update. Current Opinion in Pulmonary Medicine. 2013;19(6):631–638. doi: 10.1097/MCP.0b013e3283659da5. [DOI] [PubMed] [Google Scholar]
- 157.Altaf Q. A., Barnett A. H., Tahrani A. A. Novel therapeutics for type 2 diabetes: insulin resistance. Diabetes, Obesity & Metabolism. 2015;17(4):319–334. doi: 10.1111/dom.12400. [DOI] [PubMed] [Google Scholar]
- 158.Chiu S.-C., Huang S.-Y., Tsai Y.-C., et al. Poly (ADP-ribose) polymerase plays an important role in intermittent hypoxia-induced cell death in rat cerebellar granule cells. Journal of Biomedical Science. 2012;19(1):p. 29. doi: 10.1186/1423-0127-19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Drel V. R., Lupachyk S., Shevalye H., et al. New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine, and tumor necrosis factor-α. Endocrinology. 2010;151(6):2547–2555. doi: 10.1210/en.2009-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Drel V. R., Pacher P., Stavniichuk R., et al. Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. International Journal of Molecular Medicine. 2011;28(4):629–635. doi: 10.3892/ijmm.2011.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lupachyk S., Shevalye H., Maksimchyk Y., Drel V. R., Obrosova I. G. PARP inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: correlation with peripheral nerve function. Free Radical Biology & Medicine. 2011;50(10):1400–1409. doi: 10.1016/j.freeradbiomed.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Obrosova I. G., Mabley J. G., Zsengellér Z., et al. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. The FASEB Journal. 2005;19(3):401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- 163.Vareniuk I., Pacher P., Pavlov I. A., Drel V. R., Obrosova I. G. Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. International Journal of Molecular Medicine. 2009;23(5):571–580. doi: 10.3892/ijmm_00000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stavniichuk R., Drel V. R., Shevalye H., et al. Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative–nitrosative stress and p38 MAPK activation. Experimental Neurology. 2011;230(1):106–113. doi: 10.1016/j.expneurol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jelic S., Lederer D. J., Adams T., et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121(8):1014–1021. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Madan R., Gupt B., Saluja S., Kansra U. C., Tripathi B. K., Guliani B. P. Coagulation profile in diabetes and its association with diabetic microvascular complications. The Journal of the Association of Physicians of India. 2010;58:481–484. [PubMed] [Google Scholar]
- 167.Ifergane G., Ovanyan A., Toledano R., et al. Obstructive sleep apnea in acute stroke: a role for systemic inflammation. Stroke. 2016;47(5):1207–1212. doi: 10.1161/STROKEAHA.115.011749. [DOI] [PubMed] [Google Scholar]
- 168.Steffanina A., Proietti L., Antonaglia C., Palange P., Angelici E., Canipari R. The plasminogen system and transforming growth factor-β in subjects with obstructive sleep apnea syndrome: effects of CPAP treatment. Respiratory Care. 2015;60(11):1643–1651. doi: 10.4187/respcare.03571. [DOI] [PubMed] [Google Scholar]
- 169.Gileles-Hillel A., Alonso-Álvarez M. L., Kheirandish-Gozal L., et al. Inflammatory markers and obstructive sleep apnea in obese children: the NANOS study. Mediators of Inflammation. 2014;2014:9. doi: 10.1155/2014/605280.605280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zakrzewski M., Zakrzewska E., Kiciński P., et al. Evaluation of fibrinolytic inhibitors: alpha-2-antiplasmin and plasminogen activator inhibitor 1 in patients with obstructive sleep apnoea. PLoS One. 2016;11(11, article e0166725) doi: 10.1371/journal.pone.0166725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang C.-Y., Leung P. S. C., Adamopoulos I. E., Gershwin M. E. The implication of vitamin D and autoimmunity: a comprehensive review. Clinical Reviews in Allergy & Immunology. 2013;45(2):217–226. doi: 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nadi M., Marandi S. M., Esfarjani F., Saleki M., Mohammadi M. The comparison between effects of 12 weeks combined training and vitamin D supplement on improvement of sensory-motor neuropathy in type 2 diabetic women. Advanced Biomedical Research. 2017;6:p. 55. doi: 10.4103/2277-9175.205528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Benrashid M., Moyers K., Mohty M., Savani B. N. Vitamin D deficiency, autoimmunity, and graft-versus-host-disease risk: implication for preventive therapy. Experimental Hematology. 2012;40(4):263–267. doi: 10.1016/j.exphem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 174.Harinarayan C. V. Vitamin D and diabetes mellitus. Hormones. 2014;13(2):163–181. doi: 10.1007/BF03401332. [DOI] [PubMed] [Google Scholar]
- 175.Skalli S., Muller M., Pradines S., Halimi S., Wion-Barbot N. Vitamin D deficiency and peripheral diabetic neuropathy. European Journal of Internal Medicine. 2012;23(2):e67–e68. doi: 10.1016/j.ejim.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 176.Soderstrom L. H., Johnson S. P., Diaz V. A., Mainous A. G., III Association between vitamin D and diabetic neuropathy in a nationally representative sample: results from 2001–2004 NHANES. Diabetic Medicine. 2012;29(1):50–55. doi: 10.1111/j.1464-5491.2011.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.He R., Hu Y., Zeng H., et al. Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes/Metabolism Research and Reviews. 2017;33(2) doi: 10.1002/dmrr.2820. [DOI] [PubMed] [Google Scholar]
- 178.Tahrani A. A., Ball A., Shepherd L., Rahim A., Jones A. F., Bates A. The prevalence of vitamin D abnormalities in South Asians with type 2 diabetes mellitus in the UK. International Journal of Clinical Practice. 2010;64(3):351–355. doi: 10.1111/j.1742-1241.2009.02221.x. [DOI] [PubMed] [Google Scholar]
- 179.Cohen J. A., Jeffers B. W., Faldut D., Marcoux M., Schrier R. W. Risks for sensorimotor peripheral neuropathy and autonomic neuropathy in non-insulin-dependent diabetes mellitus (NIDDM) Muscle & Nerve. 1998;21(1):72–80. doi: 10.1002/(SICI)1097-4598(199801)21:1<72::AID-MUS10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 180.Lv W. S., Zhao W. J., Gong S. L., et al. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. Journal of Endocrinological Investigation. 2015;38(5):513–518. doi: 10.1007/s40618-014-0210-6. [DOI] [PubMed] [Google Scholar]
- 181.Jung C.-H., Kim K.-J., Kim B.-Y., Kim C.-H., Kang S. K., Mok J.-O. Relationship between vitamin D status and vascular complications in patients with type 2 diabetes mellitus. Nutrition Research. 2016;36(2):117–124. doi: 10.1016/j.nutres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 182.Putz Z., Martos T., Németh N., et al. Vitamin D and neuropathy. Orvosi hetilap. 2013;154(51):2012–2015. doi: 10.1556/OH.2013.29769. [DOI] [PubMed] [Google Scholar]
- 183.Qu G. B., Wang L. L., Tang X., Wu W., Sun Y. H. The association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: an update systematic review and meta-analysis. Journal of Clinical & Translational Endocrinology. 2017;9:25–31. doi: 10.1016/j.jcte.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Anand P., Terenghi G., Warner G., Kopelman P., Williams-Chestnut R. E., Sinicropi D. V. The role of endogenous nerve growth factor in human diabetic neuropathy. Nature Medicine. 1996;2(6):703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 185.Bilir B., Tulubas F., Bilir B. E., et al. The association of vitamin D with inflammatory cytokines in diabetic peripheral neuropathy. Journal of Physical Therapy Science. 2016;28(7):2159–2163. doi: 10.1589/jpts.28.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shehab D., Al-Jarallah K., Abdella N., Mojiminiyi O. A., Al Mohamedy H. Prospective evaluation of the effect of short-term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Medical Principles and Practice. 2015;24(3):250–256. doi: 10.1159/000375304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ozgurhan G., Vehapoglu A., Vermezoglu O., Temiz R. N., Guney A., Hacihamdioglu B. Risk assessment of obstructive sleep apnea syndrome in pediatric patients with vitamin D deficiency: a questionnaire-based study. Medicine. 2016;95(39, article e4632) doi: 10.1097/MD.0000000000004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kheirandish-Gozal L., Peris E., Gozal D. Vitamin D levels and obstructive sleep apnoea in children. Sleep Medicine. 2014;15(4):459–463. doi: 10.1016/j.sleep.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Barceló A., Esquinas C., Piérola J., et al. Vitamin D status and parathyroid hormone levels in patients with obstructive sleep apnea. Respiration. 2013;86(4):295–301. doi: 10.1159/000342748. [DOI] [PubMed] [Google Scholar]
- 190.Erden E. S., Genc S., Motor S., et al. Investigation of serum bisphenol A, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine. 2014;45(2):311–318. doi: 10.1007/s12020-013-0022-z. [DOI] [PubMed] [Google Scholar]
- 191.Kerley C. P., Hutchinson K., Bolger K., McGowan A., Faul J., Cormican L. Serum vitamin D is significantly inversely associated with disease severity in Caucasian adults with obstructive sleep apnea syndrome. Sleep. 2016;39(2):293–300. doi: 10.5665/sleep.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goswami U., Ensrud K. E., Paudel M. L., et al. Vitamin D concentrations and obstructive sleep apnea in a multicenter cohort of older males. Annals of the American Thoracic Society. 2016;13(5):712–718. doi: 10.1513/AnnalsATS.201507-440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chambers E. S., Hawrylowicz C. M. The impact of vitamin D on regulatory T cells. Current Allergy and Asthma Reports. 2011;11(1):29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 194.Toujani S., Kaabachi W., Mjid M., Hamzaoui K., Cherif J., Beji M. Vitamin D deficiency and interleukin-17 relationship in severe obstructive sleep apnea–hypopnea syndrome. Annals of Thoracic Medicine. 2017;12(2):107–113. doi: 10.4103/atm.ATM_301_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Salepci B., Caglayan B., Nahid P., et al. Vitamin D deficiency in patients referred for evaluation of obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2017;13(4):607–612. doi: 10.5664/jcsm.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Archontogeorgis K., Nena E., Papanas N., et al. Vitamin D levels in middle-aged patients with obstructive sleep apnoea syndrome. Current Vascular Pharmacology. 2018;16(3):289–297. doi: 10.2174/1570161115666170529085708. [DOI] [PubMed] [Google Scholar]
- 197.Piovezan R. D., Hirotsu C., Feres M. C., et al. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS One. 2017;12(7, article e0180901) doi: 10.1371/journal.pone.0180901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mete T., Yalcin Y., Berker D., et al. Obstructive sleep apnea syndrome and its association with vitamin D deficiency. Journal of Endocrinological Investigation. 2013;36(9):681–685. doi: 10.3275/8923. [DOI] [PubMed] [Google Scholar]
- 199.Theorell-Haglöw J., Hoyos C. M., Phillips C. L., et al. Changes of vitamin D levels and bone turnover markers after CPAP therapy: a randomized sham-controlled trial. Journal of Sleep Research. 2018;27(4, article e12606) doi: 10.1111/jsr.12606. [DOI] [PubMed] [Google Scholar]
- 200.Liguori C., Romigi A., Izzi F., et al. Continuous positive airway pressure treatment increases serum vitamin D levels in male patients with obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2015;11(6):603–607. doi: 10.5664/jcsm.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Srinivasan S., Stevens M., Wiley J. W. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49(11):1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 202.Chandrasekaran K., Muragundla A., Demarest T. G., et al. mGluR2/3 activation of the SIRT1 axis preserves mitochondrial function in diabetic neuropathy. Annals of Clinical and Translational Neurology. 2017;4(12):844–858. doi: 10.1002/acn3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Macey P. M., Sarma M. K., Nagarajan R., et al. Obstructive sleep apnea is associated with low GABA and high glutamate in the insular cortex. Journal of Sleep Research. 2016;25(4):390–394. doi: 10.1111/jsr.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sukegawa M., Noda A., Sugiura T., et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24-hour urinary catecholamines. Clinical Cardiology. 2005;28(11):519–522. doi: 10.1002/clc.4960281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Suehiro K., Funao T., Fujimoto Y., Yamada T., Mori T., Nishikawa K. Relationship between noradrenaline release in the locus coeruleus and antiallodynic efficacy of analgesics in rats with painful diabetic neuropathy. Life Sciences. 2013;92(23):1138–1144. doi: 10.1016/j.lfs.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 206.Davidson E. P., Holmes A., Coppey L. J., Yorek M. A. Effect of combination therapy consisting of enalapril, α-lipoic acid, and menhaden oil on diabetic neuropathy in a high fat/low dose streptozotocin treated rat. European Journal of Pharmacology. 2015;765:258–267. doi: 10.1016/j.ejphar.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 207.Voss U., Sand E., Olde B., Ekblad E. Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PLoS One. 2013;8(12, article e81413) doi: 10.1371/journal.pone.0081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dopp J. M., Philippi N. R., Marcus N. J., et al. Xanthine oxidase inhibition attenuates endothelial dysfunction caused by chronic intermittent hypoxia in rats. Respiration. 2011;82(5):458–467. doi: 10.1159/000329341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Walter L. M., Biggs S. N., Nisbet L. C., et al. Improved long-term autonomic function following resolution of sleep-disordered breathing in preschool-aged children. Sleep and Breathing. 2016;20(1):309–319. doi: 10.1007/s11325-015-1268-x. [DOI] [PubMed] [Google Scholar]
- 210.Nguyen H. T. T., Bhattarai J. P., Park S. J., Lee J. C., Cho D. H., Han S. K. Enhanced GABA action on the substantia gelatinosa neurons of the medullary dorsal horn in the offspring of streptozotocin-injected mice. Journal of Diabetes and its Complications. 2015;29(5):629–636. doi: 10.1016/j.jdiacomp.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 211.Petrou M., Pop-Busui R., Foerster B. R., et al. Altered excitation-inhibition balance in the brain of patients with diabetic neuropathy. Academic Radiology. 2012;19(5):607–612. doi: 10.1016/j.acra.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bellows B. K., Nelson R. E., Oderda G. M., LaFleur J. Long-term cost-effectiveness of initiating treatment for painful diabetic neuropathy with pregabalin, duloxetine, gabapentin, or desipramine. Pain. 2016;157(1):203–213. doi: 10.1097/j.pain.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 213.Macey P. M., Sarma M. K., Prasad J. P., et al. Obstructive sleep apnea is associated with altered midbrain chemical concentrations. Neuroscience. 2017;363:76–86. doi: 10.1016/j.neuroscience.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lam J. C. M., Tan K. C. B., Lai A. Y. K., Lam D. C. L., Ip M. S. M. Increased serum levels of advanced glycation end-products is associated with severity of sleep disordered breathing but not insulin sensitivity in non-diabetic men with obstructive sleep apnoea. Sleep Medicine. 2012;13(1):15–20. doi: 10.1016/j.sleep.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 215.Tan K. C. B., Chow W.-S., Lam J. C. M., et al. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep. 2006;29(3):329–333. doi: 10.1093/sleep/29.3.329. [DOI] [PubMed] [Google Scholar]