Abstract
HIV-associated neurocognitive disorder (HAND) remains a challenge despite antiretroviral therapy (ART), and has been linked to monocyte/macrophage (M/M) migration to the brain. Due to the potential impact of T cell effector mechanisms in eliminating activated/HIV-infected M/M, T cell activation may play a role in the development of HAND. We sought to investigate the relationship between cognition and both CD8+ T cell activation (HLA-DR+/CD38+) and HIV-specific CD8+ T cell responses at the time of HIV diagnosis and 12 months postinitiation of ART. CD8+ T cell activation was increased in HAND compared to cognitive normal (NL) individuals and correlated directly with plasma viral load and inversely with the cognitive status. In addition, Gag-specific cytolytic activity (CD107a/b+) was decreased in HAND compared with NL individuals and correlated with their neurological testing, suggesting a potential role of cytotoxic CD8+ T cells in the mechanism of HAND development.
Keywords: : HIV-associated neurocognitive disorders, CD8+ T cell activation, HIV-specific cytolytic T cells
Antiretroviral therapy (ART) has greatly reduced the incidence of the most severe forms of HIV-associated neurocognitive disorders (HAND), but milder forms of impairment remain common. Investigations of HAND have largely focused on monocytes/macrophages (Ms/Ms) due to their susceptibility to infection and ability to cross the blood–brain barrier once activated.1 Peripheral measures of M/M frequency correlate with HAND symptoms and are predictive of central nervous system (CNS) dysfunction in nonhuman primates, leading many to conclude that M/M are critically involved in CNS pathogenesis.2
M/M take their functional cues, in part, from the cognate system, which is highly disrupted during chronic HIV infection. Consequently, it can be hypothesized that T cells could play a larger role in CNS pathogenesis and protection.3 CD8+ T cell activation is associated with early neuronal changes and strongly correlates with cerebrospinal fluid (CSF) viral load in macaques that develop encephalitis later during infection.4 More importantly, the depletion of CD8+ T cells leads to more rapid progression of SIV-associated encephalitis.5 Their inability to kill HIV-infected target cells may lead to the unchecked transmigration of activated M/M to the brain.
Although, CD8+ T cell frequency and functionality have been associated with better overall clinical outcomes, their impairment has not been specifically linked to the development of HAND. Hence, we hypothesized that impaired cognate CD8+ T cell responses may be involved in cognitive impairment. In this study, we assessed the frequency of activated CD8+ T cells and HIV-specific CD8+ T cell response in HAND versus cognitively normal (NL) individuals at the time of HIV diagnosis and 12 months post-ART initiation.
Fifty ART-naive HIV-infected volunteers (CD4 count <350 cells/mm3) were enrolled into the SEARCH 007 study (NCT00777426) at the Thai Red Cross AIDS Research Centre in Bangkok, Thailand. Hepatitis C infection and illicit drug use within the past 5 years as well as a positive illicit drug screen were exclusion criteria (further information can be found under clinicaltrials.gov). Based on clinical assessment and neuropsychological testing battery, 23/50 were characterized as cognitively normal (NL), and 27/50 with HAND at time of HIV diagnosis. Following ART for 12 months, (zidovudine or stavudine, lamivudine, and nevirapine) 8/27 individuals with HAND were cognitively normal in their assessments, while no participants in either group regressed. Domain-specific standardized scores (NPZ) were calculated as previously described and NPZglobal was defined by aggregating the domain scores (NPZglobal at baseline: NL 0.12 vs. HAND −0.71; and at 12 months post-ART: NL 0.35 vs. HAND −0.86, p < .0001, respectively).6
At the time of diagnosis the median CD4 count in NL was 191 cells/mm3 (IQR 87–340) and 182 cells/mm3 (IQR 68–269; p = .51) in HAND individuals, and after 12 months post-ART initiation NL showed a median CD4 count of 393 cells/mm3 (IQR 249–548) and HAND individuals of 356 cells/mm3 (IQR 212–516; p = .34; Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). In addition, 10 matched HIV-seronegative healthy individuals (CO) were enrolled (age range 24–46 years). Earlier, written signed informed consent was obtained from all participants.
Frozen peripheral blood mononuclear cells (PBMCs) were thawed and 1 × 106 PBMC were used for phenotypical flow-cytometric assessment and in a standard intracellular cytokine staining (ICS) assay for antigen-specific T cells as previously described.7 PBMC were stained with Aqua Live/Dead (Invitrogen) and the following antibodies: anti-CD3-PE-Cy7 (Invitrogen), anti-CD4-ECD (Beckman Coulter), anti-CD8-PerCP-Cy5.5, anti-HLA-DR-V450 and anti-CD38-APC (BD Bioscience), anti-CD107a/b-FITC, anti-CD14-AF700, anti-CD19-AF700 (BD Pharmingen), and anti-IFNγ-Pacific Blue (eBioscience). Cells were acquired using a custom-build LSRII flowcytometer (BD Bioscience) and analyzed using FlowJo (version 9.8 or higher; Tree Star; Supplementary Fig. S1). A median of 99,800 (range 16,800–163,000) CD3+ lymphocytes were acquired. A positive ICS response was defined as at least 3 × background control and ≥0.05% gated positive cells. GraphPad Prism Version 6.0 or higher was used for statistical analysis and presentation. Statistical comparison between groups was performed using the Mann–Whitney test and a Wilcoxon test for matched samples, with p-values ≤.05 considered significant. For correlation analysis, the Spearman rank test was used, and for the effect size, a Cohen's d test was used.
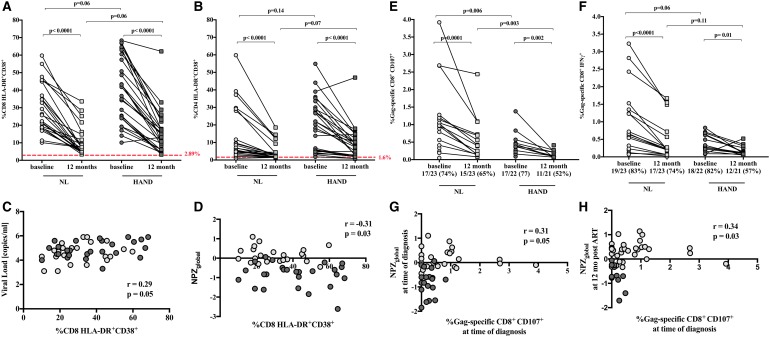
At the time of HIV diagnosis, NL and HAND individuals showed a significantly higher frequency of activated CD8+ T cells, indicated by the coexpression of HLA-DR and CD38 (CD8+DR+CD38+), compared to CO (NL 26.4% and HAND 41.8% vs. CO 2.8%; p < .001), which remained elevated at 12 months post-ART initiation (NL 7.2% and HAND 14.6% vs. CO 2.8%; p < .001, Fig. 1A). However, we observed a significant decrease in the frequency of CD8+DR+CD38+ T cells in both NL and HAND individuals between time of diagnosis and 12 months post-ART initiation from 26.4% to 7.2% and 41.8% to 14.6% (p < .001), respectively. The frequency of CD8+DR+CD38+ T cells in HAND individuals was higher compared with NL individuals at both time points (baseline: NL 26.4% vs. HAND 41.8%; 12 months post-ART: NL 7.2% vs. HAND 14.6%), however, not statistically significant.
FIG. 1.
Characterization of CD4+ and CD8+ T cells among NL and HAND individuals at the time of HIV diagnosis and 12 months post-ART initiation. (A) Coexpression activation markers HLA-DR and CD38 on CD8+ T cells and (B) on CD4+ T cells in NL (light gray symbols) and HAND (dark gray symbols) individual, and in healthy controls (red dotted line), as the time of diagnosis and 12 months post-ART initiation. (C) Correlation of CD8+HLA-DR+CD38+ T cells with plasma viral load and (D) with NPZscore at the time of HIV diagnosis. (E) Frequency and number of individuals with CD107a/b- and (F) IFNγ-expressing Gag-specific CD8+ T cells among NL and HAND individuals at the time of HIV diagnosis and 12 months post-ART initiation. (G) Correlation of the frequency of Gag-specific CD107a/b-expressing CD8+ T cells with the NPZscore at the time of diagnosis. (H) Correlation of the frequency of Gag-specific CD107a/b-expressing CD8+ T cells at the time of diagnosis with the NPZscore 12 months post-ART-initiation. Comparisons between groups (NL vs. HAND) were done using Mann–Whitney test, and comparison between matched samples within groups (baseline vs. 12 months) were done using Wilcoxon test. ART, antiretroviral therapy; HAND, HIV-associated neurocognitive disorder; NL, cognitively normal.
Similar trends were observed for CD4+DR+CD38+ T cells, where the frequencies of NL and HAND individuals remained elevated over those seen in the CO (baseline: NL: 6.6% and HAND: 20.3% vs. CO: 1.6%; p < .001; 12 months post-ART: NL: 2.6% and HAND: 7.3% vs. CO: 1.6%; p = .001 and p < .001, respectively, Fig. 1B). At the time of diagnosis, the frequency of CD8+DR+CD38+ T cells, was found in NL and HAND individuals to correlate directly with plasma viral load (pVL) (r = 0.29, p = .05; Fig. 1C) and indirectly with the NPZglobal score (r = −0.31, p = .01; Fig. 1D), thus potentially linking CD8+ T cell activation, although weakly, with viral replication and cognitive status. The effect size between the two groups was moderate (Cohen's d = 0.6) supporting the outcome. However, there was no significant correlation observed when NL and HAND individuals were analyzed separately, due likely to restricted sample size (p > .05, respectively; data not shown).
CD8+ T cell function was assessed using expression of IFNγ and the cytolytic marker CD107a/b. At the time of diagnosis, in both groups, a slightly higher number of individuals expressed CD107a/b or IFNγ upon Gag stimulation compared with Env stimulation [CD107a/b: NL: Gag 17/23 (74%) vs. Env 13/23 (57%); HAND: Gag 17/22 (77%) vs. Env 15/22 (68%); IFNγ: NL: Gag 19/23 (83%) vs. Env 16/23 (70%); HAND: Gag 18/22 (82%) vs. Env 19/22 (86%)]. The frequency of CD107a/b-expressing cytolytic Gag-specific CD8+ T cells was significantly higher in NL compared with HAND individuals at time of diagnosis (0.9% vs. 0.3%, respectively; p = .006) and 12 months post-ART initiation (0.4% vs. 0.1%, respectively; p = .003; Fig. 1E). The increased frequency of cytolytic Gag-specific CD8+ T cells in HAND individuals was not related to CD4 count at the time of diagnosis or at 12 months post-ART initiation (p > .05, respectively; data not shown).
In addition, statistical significance remains when excluding samples with persistent pVL at 12 months [NL (1/23 excluded) compared with HAND (4/27 excluded) at time of diagnosis (1.1% vs. 0.4%, respectively; p = .01) and 12 months post-ART initiation (0.6% vs. 0.2%, respectively; p = .002; data not shown)]. In contrast, there was no statistically significant difference in the frequency of Gag-specific IFNγ-expressing CD8+ T cells between the two groups at time of diagnosis (0.7% vs. 0.3%, respectively; p = .06) or 12 months post-ART initiation (0.2% vs. 0.1%, respectively; p = .11; Fig. 1F). These findings are in accordance with observations showing lower frequencies of cytolytic CD8+ T cells compared with IFNγ-expressing T cells in the CSF, and partly in the periphery, of patients with HAND, suggesting failure of ART to suppress HIV replication in the CSF contributing to increased risk of HAND.8
Moreover, at the time of diagnosis the frequency of CD107a/b-expressing CD8+ T cells correlated with the NPZglobal score (r = 0.31, p = .05) indicating an impact of cytolytic CD8+ T cells in the development of cognitive impairment (Fig. 1G). In addition, the frequency of Gag-specific CD107a/b-expressing CD8+ T cells at the time of diagnosis correlated with the NPZscore 12 months post-ART initiation (r = 0.34, p = .03; Fig. 1H). The statistical significance of the reported correlations is consistent with those observed by other studies between neurocognitive impairment and HIV disease biomarkers.9 However, 12 months following ART-initiation, a significant decrease in the frequency of CD107a/b- and IFNγ-expressing Gag-specific CD8+ T cells was observed compared to time of diagnosis in NL (CD107a/b: 0.9% vs. 0.4%, p < .001, IFNγ: 0.7% vs. 0.2%, p < .001) as well as in HAND individuals (CD107a/b: 0.4% vs. 0.1%, p = .002, IFNγ: 0.3% vs. 0.1%, p = .01; Fig. 1E, F), potentially due to reduction of pVL upon ART initiation.
Although ART reduces the more severe forms of HAND, the etiology of mild to moderate cognitive impairment despite ART remains unclear but likely also involves mechanisms such as M/M infiltration/accumulation in the brain. There is growing evidence that multiple factors are involved in the development and persistence of cognitive dysfunction despite ART.1 A decreased CD4/CD8 ratio in both peripheral blood and the CSF strongly correlated with the grade of cognitive impairment in HIV patients on ART, highlighting the potential importance of T cells.10 In addition, the importance of cytolytic CD8+ T cells has been highlighted and their absence in the CSF potentially linked to the failure of ART to suppress HIV replication within the CNS.8
The aim of the present study was to evaluate the potential role of peripheral CD8+ T cells in the development of cognitive impairment. Our findings support a previous study10 showing a trend toward higher immune activation of CD8+ and CD4+ T cells in HAND compared with NL individuals. Activation status of CD8+, but not CD4+T cells inversely correlated with cognitive status, which potentially could link ongoing immune activation and neurological injury. It is known, that ongoing immune activation is a strong predictor of HIV disease progression, but could also serve as a marker for the development of cognitive dysfunction.10
In addition, impairment of cytolytic CD8+ T cells has been shown to correlate with HIV disease progression.8,11 In vitro studies have demonstrated, that cytolytic CD8+ T cells both suppress and kill HIV replication in macrophages.12 We observed, that Gag-specific cytolytic CD8+ T cell responses (CD107a/b-expression) mounted in HAND individuals were significantly lower compared to NL individuals at the time of diagnosis and 12 months posttreatment, correlating with the NPZscore. These findings suggest a more impaired cytolytic immune response in HAND individuals, which could contribute to a failure to prevent trafficking of activated/infected M/M to the brain and subsequently lead to the development of cognitive impairment. In contrast, there was no significant difference in the frequency of IFNγ-expressing CD8+ T cells observed between HAND and NL individuals at any time.
However, this was a relatively small, but well controlled, study, and hence, it was not within the scope of this study to address the full spectrum of relevant considerations such as the impact of early initiated ART on neurological impairment or interrogate T cell responses in the CSF. Consequently, further studies are necessary, that include a larger sample size to confirm and expand on those findings and to elucidate the level of CD8+ T cell impairment across compartments. Inclusion of exhaustion markers such as PD-1 or Tim-311 will also be valuable to determine whether these negative checkpoints moderate M/M trafficking into the brain.
Supplementary Material
Contributor Information
Collaborators: on behalf of the SEARCH 007 Study Group
Acknowledgments
We thank the study participants and staff from the Thai Red Cross AIDS Research Centre and Phramongkutklao Hospital, Department of Medicine in Bangkok for their valuable contributions to this study. SEARCH is a research collaboration between the Thai Red Cross AIDS Research Centre (TRCARC), the University of Hawaii and the Department of Retrovirology, U.S. Army Medical Component, Armed Forces Research Institute of Medical Sciences (AFRIMS). This work was supported by R01NS53359-2. The SEARCH 007 Study Group includes from SEARCH/TRCARC/HIV-NAT: Nipat Teeratakulpisarn, Somprartthana Rattanamanee, Sasiwimol Ubolyam, Tippawan Pankam, Supanit Pattanachaiwit; from AFRIMS: Nantana Tantibul, Bessara Nuntapinit, Rapee Trichavaroj, Surat Jongrakthaitae, Nipattra Tragonlugsana, Putida Saetun, Suchada Sukhumvittaya; from the U.S. Military HIV Research Program: Merlin Robb, Nelson Michael; from U.S. National Institutes of Allergy and Infectious Diseases: Irini Sereti; from University of California: Lauren Wendelken, Akash Desai, and Stephanie Chaio.
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. Drs. Ananwonanich and Valcour have served as a consultant to ViiV Healthcare and Merck for consultation unrelated to this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Burdo TH, Lackner A, Williams KC: Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 2013;254:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim WK, Corey S, Alvarez X, Williams K: Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 2003;74:650–656 [DOI] [PubMed] [Google Scholar]
- 3.Younas M, Psomas C, Reynes J, Corbeau P: Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med 2016;17:89–105 [DOI] [PubMed] [Google Scholar]
- 4.Dang Q, Whitted S, Goeken RM, et al. : Development of neurological disease is associated with increased immune activation in simian immunodeficiency virus-infected macaques. J Virol 2012;86:13795–13799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orandle MS, Williams KC, MacLean AG, Westmoreland SV, Lackner AA: Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J Virol 2001;75:4448–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valcour VG, Ananworanich J, Agsalda M, et al. : HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One 2013;8:e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz A, Deleage C, Sereti I, et al. : Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014;10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrier RD, Hong S, Crescini M, et al. : Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 2015;10:e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis-de Los Angeles CP, Alpert KI, Williams PL, et al. : Deformed subcortical structures are related to past HIV disease severity in youth with perinatally acquired HIV infection. J Pediatr Infect Dis Soc 2016;5(suppl 1):S6–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grauer OM, Reichelt D, Gruneberg U, et al. : Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol 2015;2:906–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khaitan A, Unutmaz D: Revisiting immune exhaustion during HIV infection. Curr HIV AIDS Rep 2011;8:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badley AD, Pilon AA, Landay A, Lynch DH: Mechanisms of HIV-associated lymphocyte apoptosis. Blood 2000;96:2951–2964 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.