Abstract
Background: Glucose variability (GV) remains a key limiting factor in the success of diabetes management. While new technologies, for example, accurate continuous glucose monitoring (CGM) and connected insulin delivery devices, are now available, current treatment standards fail to leverage the wealth of information generated. Expert systems, from automated insulin delivery to advisory systems, are a key missing element to richer, more personalized, glucose management in diabetes.
Methods: Twenty four subjects with type 1 diabetes mellitus (T1DM), 15 women, 37 ± 11 years of age, hemoglobin A1c 7.2% ± 1%, total daily insulin (TDI) 46.7 ± 22.3 U, using either an insulin pump or multiple daily injections with carbohydrate counting, completed two randomized crossover 48-h visits at the University of Virginia, wearing Dexcom G4 CGM, and using either usual care or the UVA decision support system (DSS). DSS consisted of a combination of automated insulin titration, bolus calculation, and CHO treatment advice. During each admission, participants were exposed to a variety of meal sizes and contents and two 45-min bouts of exercise. GV and glucose control were assessed using CGM.
Results: The use of DSS significantly reduced GV (coefficient of variation: 0.36 ± 08. vs. 0.33 ± 0.06, P = 0.045) while maintaining glycemic control (average CGM: 155.2 ± 27.1 mg/dL vs. 155.2 ± 23.2 mg/dL), by reducing hypoglycemia exposure (%<70 mg/dL: 3.8% ± 4.6% vs. 1.8% ± 2%, P = 0.018), with nonsignificant trends toward reduction of significant hyperglycemia overnight (%>250 mg/dL: 5.3% ± 9.5% vs. 1.9% ± 4.6%) and at mealtime (11.3% ± 14.8% vs. 5.8% ± 9.1%).
Conclusions: A CGM/insulin informed advisory system proved to be safe and feasible in a cohort of 24 T1DM subjects. Use of the system may result in reduced GV and improved protection against hypoglycemia.
Keywords: : Type 1 diabetes, Continuous glucose monitoring, Decision support systems, Expert systems, Treatment advisory systems, Insulin titration
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune condition resulting in absolute insulin deficiency and a life-long need for insulin replacement.1 Glycemic control in T1DM remains a challenge, despite the availability of modern insulin analogs,2 the improving accuracy of glucose monitoring,3,4 and the widening use of intensive insulin therapy. While new technologies have proven benefits in avoiding diabetes-related complications5 and may reduce excess mortality in some populations,6 excess mortality and complication rates remain significantly higher in T1DM when compared with the general population.7,8
Glucose variability (GV) in T1DM is typically at the root of clinicians' inability to safely achieve near-normal average glycemia, as reflected by hemoglobin A1c (HbA1c).9 While target HbA1c values of 7% or less result in decreased risk of micro- and macrovascular complications,10,11 the risk for severe hypoglycemia increases with tightening glycemic control.12–14 Consequently, hypoglycemia has been implicated as the primary barrier to tight control.15,16 Thus, patients with T1DM face a life-long optimization problem: reduce average glucose levels and postprandial hyperglycemia while simultaneously avoiding hypoglycemia. A strategy for achieving such an optimization can only be successful if it reduces GV. This is because bringing average glycemia down safely is only possible if GV is constrained—otherwise blood glucose (BG) fluctuations would inevitably enter the range of hypoglycemia (see McCall9-Fig. 4). Thus, recent studies have increasingly focused on the variability of BG fluctuations as an independent risk factor for diabetes complications,9,17,18 particularly cardiovascular diseases.19–22
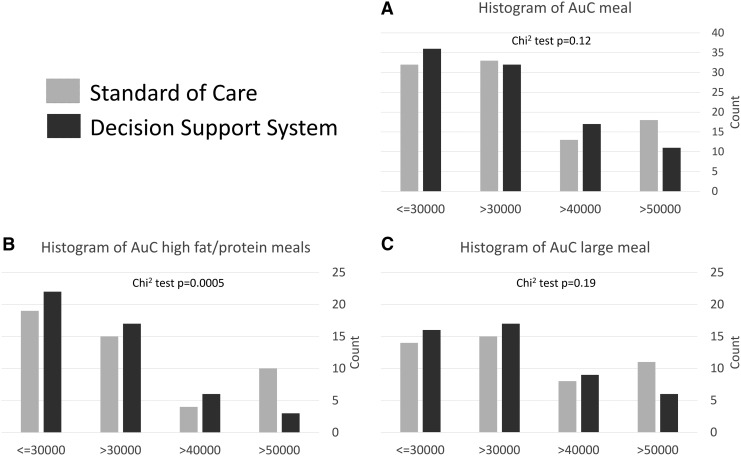
FIG. 4.
Histogram of the area under the glucose curve during the 4 h following meals. (A) describes all lunches and dinners (breakfasts are excluded because of the exercise bouts), whereas (B) focuses on meals with high fat/protein contents, and (C) on larger meals (∼1 g/kg body weight).
Introduced in the 1980s, intensive insulin treatment, by multiple daily injections (MDI) or use of continuous subcutaneous insulin infusion (CSII), attempts to mimic insulin secretion in health and typically includes basal insulin administered to cover the overnight and fasting periods and bolus insulin given with meals to cover carbohydrate consumption and correct hyperglycemia.23 Advanced insulin therapy relies on key individual parameters, such as carbohydrate ratio (CR = grams of carbohydrate per unit of insulin), insulin sensitivity factor (ISF = mg/dL per units of insulin, also called the correction factor [CF]), and target glucose.23 Evidence-based resources are available to empower patients to control their insulin intake and schedules and are used by clinicians to initiate and maintain CSII therapy by selecting appropriate basal rates, CR, and CF patterns.24
However, periodic adjustments of basal rate, CR, and CF patterns are needed based on review of self-monitoring blood glucose (SMBG) profiles or continuous glucose monitoring (CGM); when a new pattern of glycemic risk is identified by the clinical team or the patient, new insulin dosing parameters must be calculated and implemented. This can be a time-consuming and challenging task, requiring data to be downloaded from multiple devices for evaluation. Fortunately, information technology is increasingly playing a role in improving the management of chronic illnesses,25,26 including diabetes.27 In T1DM, improvements in SMBG and CGM, have empowered quantitative (algorithmic) aspect of the management of T1DM, leading to new tools for remote patient monitoring, data aggregation, and visualization.1 Early research has developed algorithms for titrating individual insulin treatment parameters, including iterative learning approaches, such as run-to-run, with structured SMBG.28–32 Today, researchers are actively working on CGM-based decision support for T1DM (e.g., see Refs. 23,33–35), capable of providing specific feedback to the clinicians regarding suggested insulin changes. These expert systems have the potential to not only streamline clinic visits, allowing more time for face-to-face interaction and attention to the many needs of the diabetic patient, but also in their most advanced form, they will deliver advice directly to the patient, reducing burden and uncertainty when making critical self-management decisions, such as dosing insulin for meals and adjusting insulin to physical activity.
Research Design and Methods
Decision support system
The decision support system (DSS) consists of two real-time advisors (CGM-Informed Bolus Advisor, and Exercise Advisor), and a retrospective insulin titration tool. The real-time advisors are respectively designed to optimally modulate insulin boluses based on the patient's current insulin sensitivity (SI) and to generate ad hoc behavioral advice to avoid hypoglycemia during an imminent exercise bout. The advisory systems are described below.
CGM-informed Bolus advisor
A CGM-informed bolus calculator was developed to adjust the patient's insulin bolus computation to the needs at the time a bolus is given. The modulation relies on a CGM/insulin-based estimation of Insulin Effectiveness (IE), an index related to SI,36 using a three-state Kalman filter describing glucose–insulin dynamics.37 When a bolus is administered, IE is estimated in real-time and compared with the patient's averaged usual IE at the same hour of day, computed from a month of historical data at this hour of day (see protocol); the IE-informed smart bolus (BIE) is then obtained as a standard bolus modulated by the ratio of usual and real-time IEs (IEr):
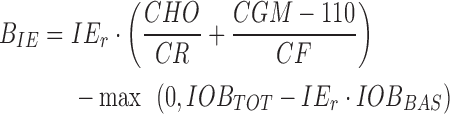 |
where CHO is the amount of ingested carbohydrates, CR and CF are the patient's carbohydrate ratio and CF at the time of the meal, CGM is the sensor reading at the time of the meal, and IOB is the current insulin on board from total (IOBTOT) and basal (IOBBAS) insulin injections, respectively.
Exercise advisor
The exercise advisor provides recommendations to the user to minimize hypoglycemia during or shortly following an imminent exercise bout.38 Upon user's request (optimally in the minutes leading to exercise), the system estimates the probability that the activity will lead to hypoglycemia (pHYPO) using a logistic regression model with three predictors:
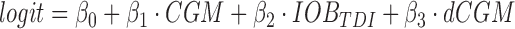 |
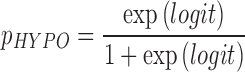 |
where CGM is the latest sensor reading, dCGM is an estimate of hourly CGM rate of change, and IOBTDI is the current total (basal, correction, and prandial) insulin on board relative to the patient's TDI. The computed probability is then used within a decision rule that classifies the hypoglycemia risk as low (pHYPO< = 0.4), mild (0.4<pHYPO< = 0.75), or severe (pHYPO>0.75). The resulting advice is dependent on the level of risk: (1) low risk: proceed with exercise; (2) mild risk: the system suggests to temporarily (2 h) suspend the delivery of basal insulin or take a small (0.3 g/kg) amount of carbohydrates; and (3) severe risk: either to combine basal suspension and consumption of a small amount of carbohydrates, or consume a larger amount of carbohydrates (0.6 g/kg).
Automated insulin titration
The final module of the deployed DSS consists in a CGM/insulin/meal-based, automated, insulin treatment parameters' optimization procedure. Based on net effect resimulation technology,39 this module identifies systematic risk for hypo- and hyperglycemia, and modulates basal insulin (rate patterns for CSII or total dose and timing for MDI), carbohydrate to insulin ratios (CR), and ISFs. Each day of the collected data is first extended from 8-h before to 4-h after midnight (total of 36 h) to avoid border effects. After regularization (e.g., interpolation of CGM gaps) and discretization in 5-min intervals, the net effect signal is computed and all signals (CGM, insulin injection, carbohydrates consumed, and net effect) can be used to replay glucose trace of the day under different insulin treatment profiles. Days with large deviation between resimulated (under default parameters) and observed glucose values are excluded, as well as days with <2 meals or boluses, or days with CGM gaps greater than 4 h. If a minimum of 14 valid days is present, the optimization cycle is run:
(1) Hypoglycemic and hyperglycemic risk (derived from the risk space analysis see Ref. 9) zones are first identified throughout the day.
(2) Actionable hypoglycemic zones (i.e., hypoglycemic risk zones not concomitant with hyperglycemic zones) are selected, and basal insulin and CR are modulated using a grid search, in the hours leading to and during the zones to minimize hypoglycemic risk without an increase of total risk.
(3) Away from the hypoglycemic risk zones found in the previous step, the actionable hyperglycemic risk zones are selected, and ISF and CR are modulated to minimize the hyperglycemic risk with total risk increase.
(4) Finally, daily patterns (ISF and CR for all, and basal rates for pumpers) are regularized to allow a maximum of eight segments during the day.
The optimization was summarized in a two-page pdf report provided to the study physician for final approval (see Supplementary Data) before the experimental admission (see Protocol below).
Protocol
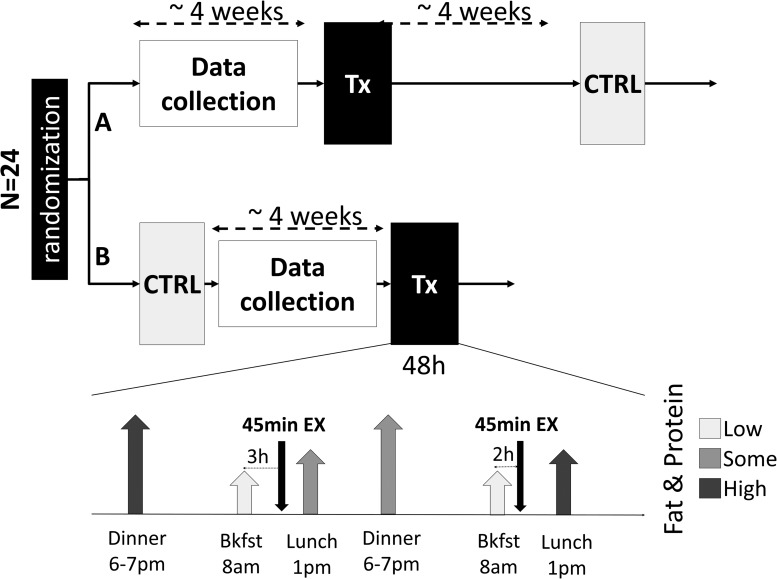
Adults and adolescents with T1DM, 17–65 years of age, were enrolled in a randomized crossover clinical trial to demonstrate the safety and feasibility of a DSS for insulin management in diabetes, compared with the standard of care (SoC). The study consisted of two 48-h outpatient admissions, separated by ∼4 weeks (or a multiple thereof) to minimize menstrual cycle effects (Fig. 1). During each admission participants were challenged with varied standardized meals (in size and composition, see protocol in Supplementary Data), as well as two 45-min exercise bouts (3 × 15 min of mild-to-moderate effort with 5 min rest periods).
FIG. 1.
Design of the protocol. After randomization to branch A (experimental then control) or B (control then experimental) participants were admitted to two identical days with standardized meals and activity (bottom of figure) using either their own treatment paradigm or following the advice given by the DSS. The data collection needed to power the DSS occurred during the 4 weeks before the experimental admission. DSS, decision support system.
Participants collected glucose, insulin, and meal records over ∼28 days before their experimental admission, 14 days of which were required to be complete to be used by the system; the collection period could be extended if necessary. During this period and both outpatient admissions, glucose measurements were collected using a blinded Dexcom G4 Platinum CGM with Share™ (Dexcom, San Diego, CA); the system was blinded (for both data collection and admissions) to mitigate the impact of CGM use on GV, unless the participants were already using a CGM as part of their normal care. In addition, participants used a study-issued Bayer Contour EZ blood glucose meter (BGM); with a goal of at least four SMBG values a day. Subjects were instructed to enter any consumed carbohydrates (meals, snacks, and hypoglycemia treatment) into the bolus wizards of their pumps (selecting no insulin injections if necessary); MDI subjects entered carbohydrate and bolus information into the MySugr app (MySugr, Vienna, Austria). Participants were required to download their personal insulin pump and CGM at regular intervals (using Diasend®; Glooko, Mountain View, CA and Carelink®; Metronic, Milipitas, CA). Data (CGM, insulin, and meals) were downloaded 72 h before their admission, and loaded into the advisory system.
During both admissions, participants' devices were connected to the Diabetes Assistant (DiAs)—a modular, smartphone-based diabetes management platform developed at the University of Virginia.40 Using the DiAs web monitoring capacities, participants were remotely monitored by the team 24 h a day during the admissions and followed a strict hypo/hyperglycemia safety protocol (see protocol in Supplemental Data) requiring SMBG confirmation and potential CHO or insulin treatment for CGM value above 300 mg/dL or below 70 mg/dL. During the control admission, DiAs was programmed to follow standard home insulin therapy; while during the experimental admission, DiAs implemented the DSS modules as described above, using treatment parameters determined by the treatment optimization module; participants were required to interact with the advisory system at meal time, before exercise, and anytime they wanted to request a correction dose. During the controlled exercise period of the admission, participants in cohort 1 (first 11 subjects) wore a Zephyr BioHarness™ 3 (Medtronic, Annapolis, MD) heart rate monitor, and participants in cohort 2 wore a Polar® RS800CX (Polar, Lake Success, NY) heart rate monitor.
Outcomes and statistical analysis
All glucose outcomes were computed based on CGM records. The primary outcome was GV as computed by the coefficient of variation (CV) of the glucose trace, with secondary outcomes focusing on the quality of glycemic control, including percent time spent between 70 and 180 mg/dL, average CGM, as well as total insulin used. Secondary outcomes focusing on participants' glycemic safety included percent time in hypoglycemia (<50, <60, and <70 mg/dL), number of hypoglycemic treatments, and finally percent time spent above 250 mg/dL and 300 mg/dL. Outcomes were further divided into segments of the day: overnight (11 pm–7 am), and around meals (the 4 h following lunch and dinner, breakfast excluded due to exercise). Primary statistical analysis was performed using the repeated measure ANOVA, with the treatment mode as within subject factor, and type of insulin treatment (pump vs. MDI) as between subject factor. No a priori sample size was determined due to the feasibility/pilot nature of the trial. Based on achieved recruitment, a moderate effect size (0.3) is detectable with 80% power, or a large effect size (0.4) with 95% power (G-Power 3.1.9.2). Secondary analysis followed the same format unless variables could not be considered normally distributed (e.g., percent time below 50 mg/dL); in that case a related samples Wilcoxon signed-rank test is used. To analyze distributions (e.g., exercise and meals secondary outcomes), data were first binned (to ensure minimum count of five per bins) and the χ2 statistics was used with the expected counts given by SoC. Significance level was set at P-value <0.05. Data are reported as mean ± standard deviation if normally distributed, and median (quartiles) if not. The statistical analysis was performed in SPSS 23 (IBM), data formatting, and preparation were executed in MATLAB 2016b (Mathwork) and Excel 2016 (Microsoft).
Results
Fifty-four subjects enrolled in the study; 11 subjects did not meet the inclusion/exclusion criteria (4 for HbA1c ≤ 7, 1 for gastroparesis, 1 for beta-blocker use, 1 for dietary restrictions, 2 for deficiencies of carbohydrate counting, and 2 for use of Metformin), 17 withdrew from the study (11 because of availability, 4 from stress due to study participation, 1 for failing to collect data, and 1 for change of treatment parameters not requested by the study team). Two subjects met stopping criteria (1 for use of glucagon and 1 for high ketones), and 24 subjects completed the protocol. There were a total of five adverse events all deemed unrelated to the DSS. Data from subjects who withdrew were removed from analysis regardless of the degree of protocol completion.
Of the 24 subjects that completed the protocol, 15 were women and 9 men, with 16 CSII and 8 MDI users. Age was 37 ± 11 years old (11–57), with an average T1DM duration of 21 ± 11 years (1–45); MDI users were significantly younger than pump users (40.6 ± 11.1 vs. 29.8 ± 6.9, P = 0.02) with a similar shorter duration of T1DM. Participants were well controlled on average with an HbA1c of 7.2% ± 1%; marginally higher for CSII users compared with MDI (7.4% ± 0.9% vs. 6.9% ± 1.2%, P = 0.2). Complete demographics can be found in Table 1.
Table 1.
Characteristics of Participants Who Completed the Study
| All completers | CSII | MDI | |||
|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean ± SD | ||
| Age | 37 ± 11 | 17 | 57 | 40.6 ± 11.1 | 29.8 ± 6.9 |
| T1DM duration | 21 ± 11.1 | 1 | 45 | 24.4 ± 9.7 | 14.3 ± 11 |
| HbA1c | 7.2 ± 1 | 5.3 | 9.7 | 7.4 ± 0.9 | 6.9 ± 1.2 |
| Height | 172 ± 10.7 | 155.2 | 194.1 | 172.1 ± 11.9 | 171.8 ± 8.7 |
| Weight | 81.8 ± 19.9 | 51.4 | 130.1 | 84.9 ± 20.1 | 75.7 ± 19.3 |
| BMI | 27.4 ± 4.6 | 19.5 | 35.8 | 28.4 ± 4.4 | 25.4 ± 4.8 |
| TDI | 46.7 ± 22.3 | 12 | 89 | 49.9 ± 17.2 | 40.5 ± 30.4 |
| Pump/MDI | 16 CSII & 8 MDI | ||||
| gender (M/F) | 15 women and 9 men | ||||
BMI, body mass index; CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; MDI, multiple daily injection; SD, standard deviation; T1DM, type 1 diabetes mellitus; TDI, total daily insulin.
Over the course of the protocol, participants collected an average of 37.9 ± 14.0 days of CGM data, associated with 358.0 ± 242.4 BGM measurements, while consuming on average 211.1 ± 181.5 meals or snacks and injecting 258.2 ± 197.0 U of insulin bolus.
The primary outcome (overall CGM CV) was significantly improved from 0.36 ± 0.08 during SoC to 0.33 ± 0.06 using DSS, P = 0.045. This difference was preeminent during daytime and not statistically visible at night (0.28 ± 0.1 vs. 0.25 ± 0.08, P = 0.177). Further analysis of GV using Low and High Blood Glucose Indices (LBGI and HBGI) confirmed that the hypoglycemia linked variability was responsible for most of the observed improvement, with LBGI being reduced by a third (2.5 ± 2.1 vs. 1.6 ± 1.3, P = 0.042).
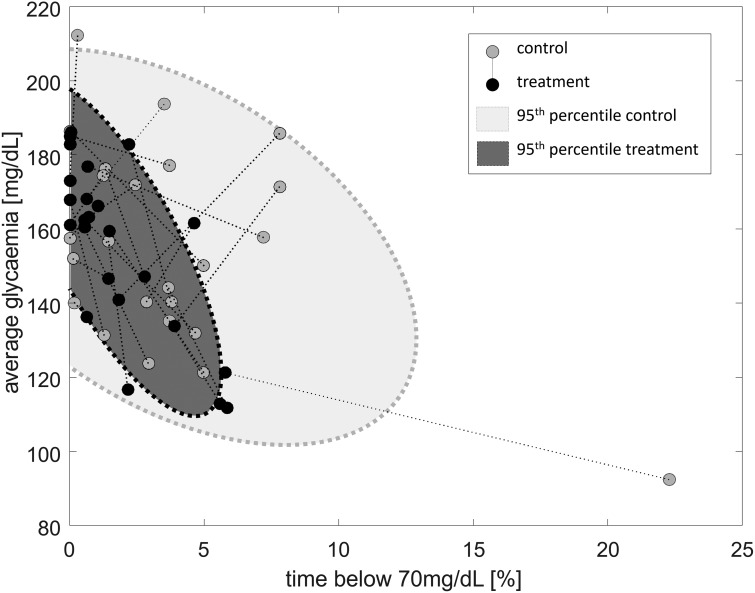
Protection against hypoglycemia was improved significantly while using the DSS: median percent of time spent below 70 mg/dL was reduced 3.5 times from 3.2% (1.3%–4.8%) to 0.9% (0.4%–2.3%), P = 0.018, while average glycemia was maintained 155.2 ± 27.1 mg/dL versus 155.2 ± 23.2 mg/dL, P = 0.86 (Fig. 2). Exposure to hyperglycemia as measured by time spent above 250 mg/dL may have been reduced overall 9.7% ± 7.9% versus 6.8% ± 6.9%, with median 6.3% (3.5%–14.7%) versus 5.2% (0%–11.5%), P = 0.158, with a possible stronger effect overnight: 5.3% ± 9.5% versus 1.9% ± 4.6%, with median 7.4% (0%–15.9%) versus 0% (0%–8.6%), P = 0.055; time above 180 mg/dL did not appear to change overall 30.3% ± 19.5% versus 29.4% ± 15.6%, P = 0.86 or overnight 23.6% ± 27.6% versus 19.9% ± 20.4%, P = 0.97. Complete glycemic results can be found in Table 2.
FIG. 2.
Evolution of average glycemia (Y-axis) and exposure to hypoglycemia (X-axis) from the control admission (gray circle) to the experimental admission (black circle). The gray areas with dotted perimeters represent the 95% confidence interval for the control (light gray) and experimental (dark gray) admissions.
Table 2.
Glycemic Performance of Decision Support System Versus Standard of Care Overall, Overnight, and at Meal Time
| Standard of care | DSS | P | ||
|---|---|---|---|---|
| Glucose variability (coefficient of variation) | Overall | 0.36 ± 0.08 | 0.33 ± 0.06 | 0.045 |
| Mealtime | 0.34 ± 0.09 | 0.3 ± 0.07 | 0.070 | |
| Overnight | 0.28 ± 0.1 | 0.25 ± 0.08 | 0.177 | |
| Low blood glucose index | Overall | 2.49 ± 2.08 | 1.59 ± 1.27 | 0.042 |
| Overnight | 2.18 ± 1.96 | 1.89 ± 1.83 | 0.276 | |
| Percent below 50 mg/dL | Overall | 0 (0–0.7) | 0 (0–0) | 0.026 |
| Mealtime | 0 (0–0) | 0 (0–0) | 0.173 | |
| Overnight | 0 (0–0) | 0 (0–0) | 0.715 | |
| Percent below 60 mg/dL | Overall | 0.66 (0–1.6) | 0.1 (0–0.5) | 0.036 |
| Mealtime | 0 (0–1.7) | 0 (0–0.4) | 0.213 | |
| Overnight | 0 (0–1.9) | 0 (0–0.3) | 0.110 | |
| Percent below 70 mg/dL | Overall | 3.21 (1.3–4.8) | 0.88 (0.4–2.3) | 0.018 |
| Mealtime | 1.73 (0.5–5.2) | 1.23 (0–3.2) | 0.149 | |
| Overnight | 3.33 (0–5.6) | 1.15 (0–3.2) | 0.109 | |
| Percent between 70 and 180 mg/dL | Overall | 65.9 ± 18.6 | 68.9 ± 14.3 | 0.780 |
| Mealtime | 73.3 ± 27.3 | 77.9 ± 19.2 | 0.399 | |
| Overnight | 63 ± 23.5 | 68.2 ± 20.3 | 0.824 | |
| Percent above 180 mg/dL | Overall | 30.3 ± 19.5 | 29.4 ± 15.6 | 0.863 |
| Mealtime | 23.6 ± 27.9 | 19.9 ± 20.4 | 0.965 | |
| Overnight | 31.2 ± 25 | 29.2 ± 21.7 | 0.742 | |
| Percent above 250 mg/dL | Overall | 6.3 (3.5–14.7) | 5.2 (0–11.5) | 0.158 |
| Mealtime | 0 (0–8.9) | 0 (0–0.1) | 0.085 | |
| Overnight | 7.4 (0–15.9) | 0 (0–8.6) | 0.055 | |
| Percent above 300 mg/dL | Overall | 1 (0–2.6) | 0 (0–4.5) | 0.744 |
| Mealtime | 0 (0–0) | 0 (0–0) | 0.248 | |
| Overnight | 0 (0–4) | 0 (0–0) | 0.225 | |
| Average glycemia (mg/dL) | Overall | 155.2 ± 27.1 | 155.2 ± 23.2 | 0.860 |
| Mealtime | 144 ± 35.5 | 142.7 ± 29.7 | 0.522 | |
| Overnight | 156.9 ± 37.6 | 151.9 ± 29.9 | 0.522 | |
| Total insulin used | Overall | 45.5 ± 22.8 | 44.1 ± 18.4 | 0.301 |
| Basal insulin used | Overall | 21.4 ± 11.1 | 20.3 ± 9.1 | 0.189 |
| Total rescue CHO | Overall | 96 ± 78.1 | 81 ± 74.2 | 0.271 |
values are reported mean ± SD for normally distributed variables, and median (quartile) for others. P-values are computed using repeated measures ANOVA or Wilcoxon matched pair signed-rank test when appropriate. bold values emphasize statistically significant differences.
DSS, decision support system.
Rescue carbohydrates (given only under the safety protocol) were very high overall (88.5 ± 75.7 g per visit), highlighting the hypoglycemia-inducing exercise challenges imposed during the 48-h admission; need for rescue CHO was not found to be different between SoC and DSS, 96.0 ± 78.1 g versus 81 ± 74.2 g, P = 0.27.
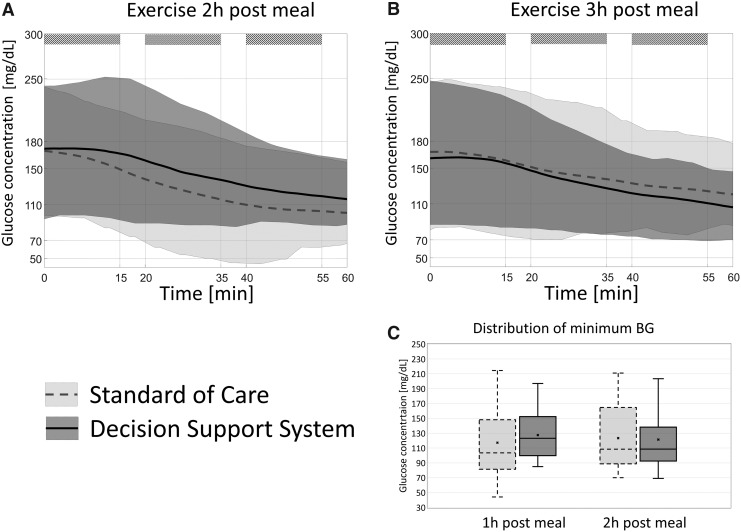
Focusing on the exercise periods, starting BG was equivalent between the two visits: 184.6 ± 55.2 mg/dL versus 193.8 ± 69.8 mg/dL for day 1 and 200.1 ± 67.6 mg/dL versus 200.4 ± 69.8 mg/dL for day 2, P = 0.48; the average final BG was equivalent as well: 116.4 ± 40.7 mg/dL versus 113.3 ± 37.7 mg/dL and 108.1 ± 46.2 mg/dL versus 116.5 ± 26.7 mg/dL. But differences were apparent in the exposure to low glucose values: 4 versus 1 event below 70 mg/dL, 8 versus 2 events below 80 mg/dL, and 16 versus 7 events below 90 mg/dL. Whereas the number of rescue CHO required during exercise was significantly reduced using the system: 6.3 ± 9.5 g versus 4.8 ± 10.2 g on day 1 and 9.4 ± 13.4 g versus 3.3 ± 7.7 g on day 2, P = 0.011. This was associated with a significant change in insulin regimen during exercise 1.11 ± 0.67 U versus 0.85 ± 0.63 U on day 1 and 0.96 ± 0.66 U versus 0.63 ± 0.57 U on day 2, P = 0.026; and pre-exercise CHO consumption (following DSS advice): 1.2 ± 5.6 g versus 4.1 ± 13.0 g on day 1 and 0.6 ± 2.9 g versus 11.6 ± 17.9 g on day 2, P = 0.003. The time courses of glucose concentration during and after exercise are shown in Figure 3. Furthermore, panel C in Figure 3 shows a clear reduction in the risk for hypoglycemia for exercise 2-h postmeal (with five bouts resulting in values below 80 mg/dL versus 0 mg/dL, χ2 P = 0.03), and in the overall variability of response to exercise when using DSS, with clear shift in the minimal glucose reached (χ2 test P < 0.001).
FIG. 3.
Glycemic average (line) and 95th percentile (shaded area) for SoC (blue) and DSS (red), during the exercise bouts (stripped). (A) Shows the results for the exercise 2 h post breakfast, and (B) focuses on exercise 3 h post breakfast. (C) Shows the distribution (box plot: mean: x, median: horizontal bar, 25–75 quartiles: gray box, and range: whiskers) of the minimum BG reached during exercise. BG, blood glucose; SoC, standard of care.
There were no direct statistically significant changes to report during postprandial excursions, but observed tendencies may indicate that the use of DSS could reduce out-of-range glucose values as percent time below 70 mg/dL and above 250 mg/dL; both tended to be reduced: 1.2% (0%–3.2%) versus 1.7% (0.5%–5.2%) P = 0.15, and 0% (0%–8.6%) versus 7.4% (0%–15.9%) P = 0.085, respectively. Further analysis of the area under the glucose curve for the 4 h following meals (breakfast is excluded due to the presence of exercise) similarly show a tendency toward reduced AuC (χ2 P = 0.12); but focusing on the more challenging high-fat, high-protein meals we see a significant shift toward lower AuC (χ2 P < 0.001; Fig. 4).
Complete postprandial glucose time course can be found in Figure 4 and Supplementary Figures S1–S3 (Supplementary Data are available online at www.liebertpub.com/dia).
Conclusions
In conclusion, a CGM-based DSS, informed of insulin injections, was shown to be safe and feasible in a limited, controlled, crossover clinical trial of adults with T1DM. The system was able to significantly reduce GV, likely through the reduction of exposure to hypoglycemia, without increasing average glycemia or exposure to hyperglycemia.
Hypoglycemia remains a major hurdle to tight glycemic control in T1DM and a primary driver of GV. Results from this pilot study clearly show that a combination of automated titration, CGM-informed bolus calculator, and exercise advisor may reduce the risk for hypoglycemia for both CSII and MDI users, with no increase in average glycemia or time spent above 180 mg/dL. These results were achieved at almost identical TDI doses.
Subanalysis of the meal and exercise regimen also indicated clear trends toward better glycemic control, with large hyperglycemic deviations less frequent when using the bolus advice and a significant reduction of necessary rescue carbohydrates during exercise.
It is important to note that this study was challenging for the participants, with four on-site visits over a relatively short period (about 50 days) and two admissions specifically designed to challenge their glucose control with meals and exercise they may not be comfortable with outside of medical supervision. These challenges likely lead to the high dropout rate (11 for visit availability, 4 for study-related stress, and 2 unable to complete the data collection). In addition, the long (4 weeks) data collection period may limit the usability of our design. Poststudy investigation of the stability of advice given led to a shortening of the analysis window to 2 weeks. It is also important to note that once deployed in the patient's home, such a system can adapt more frequently using a sliding window strategy to maximize data availability and advice consistency. These issues are the subject of an ongoing clinical trial (NCT03093636).
Generalization from these positive results is also limited by the study duration (48-h observation periods), the controlled environment under which subjects were observed, and the limited number of subjects. Of note, 48 h is generally considered a short duration for CV assessment in home settings41; nonetheless, the very standardized nature of the presented study may alleviate some of these concerns, as behavioral triggers (which can greatly vary from day to day) were removed in this analysis. It may also be noted that the challenges presented to the participants, for example, large and/or fatty meals and exercise soon after meals, were intentionally designed to generate large GV, allowing for feasibility and safety validation of the system. The short trial duration, the significant glycemic challenges, and a subject sample with very good glycemic control (HbA1c = 7.2 ± 1) may also have partially prevented the DSS system to meaningfully impact average glycemia during this study.
Nonetheless, this safety/feasibility data are enabling studies powered to characterize the actual performances of this system in clinical use (see NCT03093636).
This is, to our knowledge, the first controlled clinical trial that demonstrates the impact of a CGM/insulin informed real-time DSS, with insulin dosing capacity, on the glycemic control of Type 1 diabetic patients. Previously tested systems appear to be focused on educational feedback and/or care provider access42–44 and do not yet seem to leverage the wealth of information contained in CGM traces, or to combine it with insulin records. Nonetheless, automated titration of insulin using CGM records (one of the functionality of our system) has been tested in a small clinical trial with some success as shown in 22 T1D adolescents using an insulin pump: decrease time above 180 mg/dL with slight increase of hypoglycemia exposure.45 This system achieved 27% time above 180 mg/dL (vs. 34%, P = 0.02) and 9.7% below 70 mg/dL (vs. 9.2% ns); noting that a direct comparison is difficult, we can observe that our system did not impact time in hyperglycemia as much (29% vs. 30%), but very significantly lowered hypoglycemic exposure (0.9% vs. 3.9%).
An insulin bolus expert system (based on total meal content) was similarly tested in 12 T1D adults: significant increase from 44% to 54% of time in range (TIR, 70–180 mg/dL).46 It is interesting to note that in the expert bolus system, the median TIR in the control arm was very significantly lower than the one reported here (44% vs. 67%) and that median TIR in the treatment group rose to 73%, although not significantly.
In summary, automated insulin titration, coupled with dosing and hypoglycemia real-time advice based on CGM is safe, feasible, and may positively impact glucose control in T1DM subjects using CSII or MDI.
Supplementary Material
Acknowledgments
The study was funded by NIH NIDDK R01 51562 and NIH NIDDK DP3 101055. The authors would like to acknowledge Dr. Sue Brown UVA, Dr. Patrick Keith-Hynes, Antoine Robert, and Benton Mize for their help on this project.
Author Disclosure Statement
No competing financial interests exist.
M.D.B. reports consulting/honorarium from Dexcom, Roche, Ascensia, and Sanofi; as well as research support from Ascensia, Roche, Tandem, Sanofi, NovoNordisk, and Dexcom; and equity form TypeZero Technologies. B.P.K. reports consulting/honorarium from Dexcom and Sanofi; as well as research support from Sanofi, Roche, Tandem, and Dexcom; and equity from TypeZero Technologies. S.D.P. reports salary coverage and equity from TypeZero Technologies. D.C. reports salary coverage from TypeZero Technologies. S.M.A. reports research support from Ascensia, Tandem, Roche, NovoNordisk, and Dexcom.
References
- 1.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes care 2014;37(Suppl 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch IB: Insulin analogues. N Engl J Med 2005;352:174–183 [DOI] [PubMed] [Google Scholar]
- 3.Klonoff DC, Prahalad P: Performance of cleared blood glucose monitors. J Diabetes Sci Technol 2015;9:895–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castle JR, Jacobs PG: Nonadjunctive use of continuous glucose monitoring for diabetes treatment decisions. J Diabetes Sci Technol 2016;10:1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;2005:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secrest AM, Becker DJ, Kelsey SF, et al. : All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes Care 2010;33:2573–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lind M, Svensson AM, Kosiborod M, et al. : Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 8.Nishimura R, LaPorte RE, Dorman JS, et al. : Mortality trends in type 1 diabetes. Diabetes Care 2001;24:823–827 [DOI] [PubMed] [Google Scholar]
- 9.McCall AL, Kovatchev BP: The median is not the only message: a clinician's perspective on mathematical analysis of glycemic variability and modeling in diabetes mellitus. J Diabetes Sci Technol 2009;3:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachin JM, Genuth S, Nathan DM, et al. : Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 11.Santiago JV: Lessons from the diabetes control and complications trial. Diabetes 1993;42:1549–1554 [DOI] [PubMed] [Google Scholar]
- 12.Workgroup on Hypoglycemia, American Diabetes Association: Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 13.Cryer PE, Davis SN, Shamoon H: Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group: Hypoglycemia in the diabetes control and complications trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 15.Cryer PE: Hypoglycemia: the Limiting factor in the management of IDDM. Diabetes 1994;43:1378–1389 [DOI] [PubMed] [Google Scholar]
- 16.Cryer PE: Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M, Hirsh IB: Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complication? JAMA 2006;295:1707–1708 [DOI] [PubMed] [Google Scholar]
- 18.Hirsh IB, Brownlee M: Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications 2005;19:178–181 [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Giugliano D, Nappo F, et al. : Regression of carotid atherosclerosis by control of postprandial hyperglycemiain type 2 diabetes mellitus. Circulation 2004;110:214–219 [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM: The importance of postprandial hyperglycaemia in development of cardiovascular disease in people with diabetes. Int J Clin Pract Suppl 2001;123:24–26 [PubMed] [Google Scholar]
- 21.Monnier L, Mas E, Ginet C, et al. : Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 22.Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. : Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000;23:1830–1834 [DOI] [PubMed] [Google Scholar]
- 23.Waldhäusl WK: The physiological basis of insulin treatment—clinical aspects. Diabetologia 1986;29:837–849 [DOI] [PubMed] [Google Scholar]
- 24.Grunberger G, Abelseth JM, Bailey TS, et al. : Consensus statement by the American Asociation of Clinical Endocrinologists/American College of Endocrinology Insulin Pump Management Task Force. Endocr Pract 2014;20:463–489 [DOI] [PubMed] [Google Scholar]
- 25.Kim YB, Lee J: Smart devices for older adults managing chronic disease: a scoping review. JMIR Mhealth and Uhealth 2017;5:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh K, Drouin K, Newmark LP, et al. : Patient-facing mobile apps to treat high-need, high-cost populations: a scoping review. JMIR Mhealth and Uhealth 2016;4:e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman N, Salahi A: Using Digital Health Technology to Prevent and Treat Diabetes. Diabetes Technol Ther 2016;18(Suppl 1):56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zisser H, Jovanovic L, Doyle FJ 3rd, et al.: Run-to-run control of meal-related insulin dosing. Diab Technol Ther 2005;7:48–57 [DOI] [PubMed] [Google Scholar]
- 29.Owens C, Zisser H, Jovanovic L, et al. : Run-to-run control of blood glucose concentrations for people with type 1 diabetes mellitus. IEEE Trans Biomed Eng 2006;53:996–1005 [DOI] [PubMed] [Google Scholar]
- 30.Herrero P, Pesl P, Reddy M, et al. : Advanced insulin bolus advisor based on run-to-run control and case-based reasoning. IEEE J Biomed Health Inform 2015;19:1087–1096 [DOI] [PubMed] [Google Scholar]
- 31.Palerm CC, Zisser H, Bevier WC, et al. : Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care 2007;30:1131–1136 [DOI] [PubMed] [Google Scholar]
- 32.Palerm CC, Zisser H, Jovanovic L, Doyle FJ, 3rd: A run-to-run control strategy to adjust basal insulin infusion rates in type 1 diabetes. J Process Control 2008;18:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patek SD, Lv D, Campos-Nanez E, Breton M: Retrospective Optimization of Daily Insulin Therapy Parameters: control Subject to a Regenerative Disturbance Process. IFAC Symposium on Dynamics and Control of Process Systems, IFAC-PapersOnLine 49–7, pp. 773–778, 2016 [Google Scholar]
- 34.Toffanin C, Sandri A, Messori M, et al. : Automatic adaptation of basal therapy for type 1 diabetic patients: a run-to-run approach. In: IFAC 19th World Congress, pp. 2070–2075, 2014 [Google Scholar]
- 35.Tuo J, Sun H, Shen D, et al. : Optimization of insulin pump therapy based on high order run-to-run control scheme. Comput Methods Programs Biomed 2015;120:123–134 [DOI] [PubMed] [Google Scholar]
- 36.Bergman RN, Ider YZ, Bowden CR, Cobelli C: Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 37.Brown SA, Jiang B, McElwee-Malloy M, et al. : Fluctuations of hyperglycemia and insulin sensitivity are linked to menstrual cycle phases in women with T1D. J Diabetes Sci Technol 2015;9:1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Brahim N, Place J, Renard E, Breton MD: Identification of main factors explaining glucose dynamics during and immediately after moderate exercise in patients with type 1 diabetes. J Diabetes Sci Technol 2015;9:1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patek SD, et al. (2016) Empirical Representation of Blood Glucose Variability in a Compartmental Model. In: Kirchsteiger H, Jørgensen J, Renard E, del Re L, eds. Prediction Methods for Blood Glucose Concentration. Lecture Notes in Bioengineering. Springer, Cham, 2016, pp. 133–157 [Google Scholar]
- 40.Keith-Hynes P, Mize B, Robert A, Place J: The diabetes assistant: a smartphone-based system for real-time control of blood glucose. Electronics 2014;3:609–623 [Google Scholar]
- 41.Neylon OM, Baghurst PA, Cameron FJ: The minimum duration of sensor data from which glycemic variability can be consistently assessed. J Diabetes Sci Technol 2014;8:273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cafazzo JA, Casselman M, Hamming N, et al. : Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res 2012;14:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirwan M, Vandelanotte C, Fenning A, Duncan MJ: Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res 2013;15:e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marling C, Shubrook J, Schwartz F: Case-based decision support for patients with type 1 diabetes on insulin pump therapy. In: European Conference on Case-Based Reasoning September1, 2008 Berlin, Heidelberg; Springer, pp. 325–339 [Google Scholar]
- 45.Anderson D, Phelan H, Jones K, et al. : Evaluation of a novel continuous glucose monitoring guided system for adjustment of insulin dosing—pumpTune: a randomized controlled trial. Pediatr Diabetes 2016;17:478–482 [DOI] [PubMed] [Google Scholar]
- 46.Pańkowska E, Ładyżyński P, Foltyński P, Mazurczak K: A randomized controlled study of an insulin dosing application that uses recognition and meal bolus estimations. J Diabetes Sci Technol 2017;11:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.