Abstract
MicroRNAs (miRNAs) are critical regulators of the host immune and inflammatory response against bacterial pathogens. In the present review, we discuss target genes, target gene functions, the potential regulatory role of miRNAs in periodontal tissues, and the potential role of miRNAs as biomarkers and therapeutics. In periodontal disease, miRNAs exert control over all aspects of innate and adaptive immunity, including the functions of neutrophils, macrophages, dendritic cells and T and B cells. Previous human studies have highlighted some key miRNAs that are dysregulated in periodontitis patients. In the present study, we mapped the major miRNAs that were altered in our reproducible periodontitis mouse model relative to control animals. The miRNAs that were upregulated as a result of periodontal disease in both human and mouse studies included miR-15a, miR-29b, miR-125a, miR-146a, miR-148/148a and miR-223, whereas miR-92 was downregulated. The association of individual miRNAs with unique aspects of periodontal disease and their stability in gingival crevicular fluid underscores their potential as markers for periodontal disease progression or healthy restitution. Moreover, miRNA therapeutics hold great promise for the future of periodontal therapy because of their ability to modulate the immune response to infection when applied in conjunction with synthetic antagomirs and/or relatively straightforward delivery strategies.
microRNAs and periodontal disease
Periodontal disease is caused by the host immune and inflammatory response to the bacterial infection of teeth. From a clinical perspective, periodontal disease alternates between episodes of disease activity and episodes of quiescence, and if untreated, progresses from mild inflammation to severe tissue destruction. The periodontal host response to oral bacteria consists of two distinct but related lines of defense, innate immunity and adaptive immunity. Periodontal innate immunity is the first line of defense against invading oral pathogens, which consists of the oral epithelial barrier and the activity of phagocytic cells, such as neutrophils and macrophages, that directly attack and remove invading bacteria. In contrast, adaptive immunity is an antigen (Ag)-specific immune response that depends on the functions of B and T cells. Adaptive immunity is based on the identification and recognition of an Ag on the surface of an infected cell and a subsequent immune response designed to attack the pathogen or infected cell. Together, innate and adaptive immunity collaborate to limit bacterial infection and re-establish periodontal tissue homeostasis.
During the last decade, microRNAs (miRNAs) have emerged as critical regulators of the immune response based on their ability to interfere with the post-transcriptional expression of multiple target genes. miRNAs are short (19–24 nucleotides in length) non-coding RNAs that function either through translational inhibition or mRNA destabilization through sequence-specific binding sites within the mRNA 3′-untranslated region (UTR) of genes. miRNAs affect target gene expression by modulating and fine-tuning protein expression levels rather than switching genes simply on or off.1,2 During immune and inflammatory responses, miRNAs target inflammatory regulators and affect the magnitude of the inflammatory response.3,4 Recent studies have documented that miRNAs are not only involved in the response against bacterial pathogens but also target a host of other pathogens of viral, fungal and parasitic origin.5–8
Many chronic and acute diseases are associated with aberrant miRNA expression levels, which in turn affect gene expression and cellular functions during disease progression. For example, miRNAs are dysregulated in infectious diseases,5 autoimmune diseases,9 chronic inflammatory diseases,10 cardiovascular disorders,11 nervous system disorders12 and other diseases. Dysregulation of miRNA expression in tissues is reflected in biofluids, such as serum, saliva and crevicular fluid of the gingiva.13,14 Therefore, miRNAs may be used as specific and sensitive biomarkers indicative of many diseases. The involvement of miRNAs in various stages during the host response against bacterial infections is highly dependent on the cellular context, with different cell types responding differently to the same pathogen.15 The ability to manipulate miRNA expression using gain or loss of function approaches enables selective targeting of miRNA pathways involved in human diseases as a promising strategy for therapeutic interventions against multiple pathological conditions.
miRNA expression profile changes in gingival tissues of periodontally diseased populations
The gingiva is a part of the oral mucosa and consists of dense connective tissue and overlying oral epithelium. Gingival tissues are inhabited by a wide variety of microbes, including commensal organisms; however, they also have a high level of susceptibility to continuous attacks by oral pathogens because of their unique anatomic position between the oral biofilm and the underlying connective tissues of the periodontium. In healthy environments, the gingival microenvironment is inhabited by unique subsets of immune cell populations. When exposed to inflammatory conditions, immune cells infiltrate the oral epithelium and its underlying connective tissue (Fig. 1). Regulation of the immune-inflammatory response greatly affects patient susceptibility to periodontal disease. To determine whether miRNAs are involved in the regulation of the immune response during periodontitis progression, we performed miRNA expression profiling analysis using our periodontitis animal model. For our analysis, gingival crevices from healthy and diseased animals were dissected, and miRNA expression profiles were compared using a microRNA microarray (LC Sciences, Houston, TX, USA). Our profiling study demonstrated significant changes in miRNA expression in gingival tissues from control and periodontitis animals (Figs. 2 and 3). Some of the upregulated or downregulated miRNAs are listed in Table 1.
Fig. 1.
Inflammatory connective tissue infiltrate as a result of chronic inflammatory conditions in an established/advanced periodontal lesion. Micrographs are from freshly fixed human periodontal tissues as preserved in the collection of Joseph-Peter Weinmann at the University of Illinois at Chicago. These slides were originally generated in Gottlieb’s Oral Biology Laboratory at the University of Vienna and transported to Illinois during the 1930s. a Overview micrograph illustrating the position of periodontal soft tissues in between two adjacent root surfaces (rt). The periodontal connective tissues are separated from the oral cavity by three distinct types of epithelia, junctional epithelium (je), sulcus epithelium (SE) and oral epithelium (oe). The site of the inflammatory infiltrate is identified with an asterisk (*). Note the relative loss of collagen fibres (**). b Most of the leukocyte-rich infiltrate (wbc) is located in the sub-epithelial connective tissue. c, d Higher magnification micrographs identify individual lymphocytes (lymph) as major components of the inflammatory infiltrate, suggesting that the tissue sample is from a relatively young individual.
Fig. 2.

Microarray analysis of microRNA expression in periodontal progenitors from healthy individuals (Con) and animals suffering from periodontal disease (Dis). a Heat map of miRNA expression profiling. miRNAs with a significant level of upregulation or downregulation (P < 0.01) were identified using Student’s t test. Individual upregulated and downregulated genes are listed in Table 1. b Quantitative reverse transcriptase (qRT) polymerase chain reaction verification of selected microRNAs in healthy and periodontal disease tissues. There was a significant difference in miR-21, miR-29b, let-7c and miR-451 gene expression between healthy and diseased tissues.
Fig. 3.
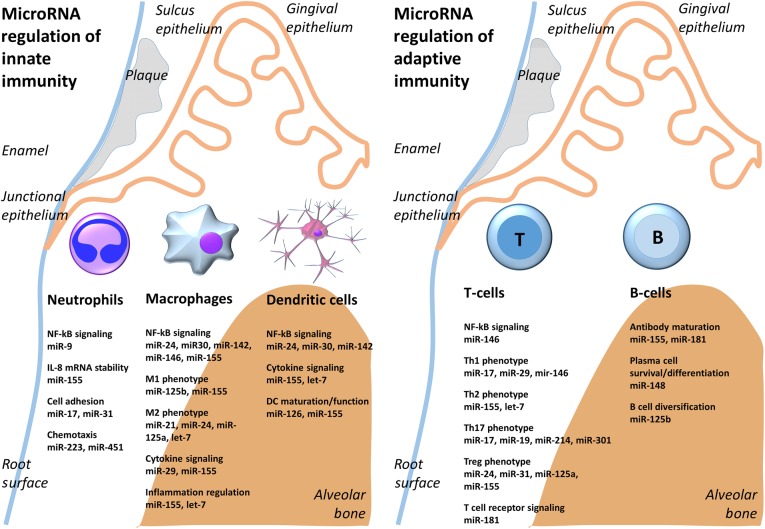
MicroRNA regulation of innate and adaptive immunity in the periodontium. Bacterial plaque on the surface of enamel and in the gingival sulcus induce immune response in the periodontium. By affecting individual target genes, microRNAs either promote or inhibit the function of innate immune cells including neutrophils, dendritic cells and macrophages, and/or the function of adaptive immune cells including T and B cells.
Table 1.
Changes in miRNA expression profile in gingival tissues of periodontitis animals compared to control animals
| miRNAs | Change in expression | miRNAs | Change in expression |
|---|---|---|---|
| miR-15a | Up | miR-17 | Down |
| miR-21a | Up | miR-24 | Down |
| miR-26a | Up | miR-30 | Down |
| miR-29b | Up | miR-92a | Down |
| miR-126a | Up | miR-451 | Down |
| miR-125a | Up | ||
| miR-146a | Up | ||
| miR-146b | Up | ||
| miR-148a | Up | ||
| miR-181b | Up | ||
| miR-223 | Up | ||
| let-7e | Up | ||
| let-7f | Up | ||
| let-7j | Up | ||
| let-7k | Up |
Recently, several human subject studies have also compared miRNA expression profiles between healthy and diseased gingival tissues16–19 or healthy and obese subjects.20 Groups of miRNAs that were upregulated or downregulated in tissues with periodontal disease are listed in Table 2. These profiling studies demonstrated that periodontal disease resulted in a changed miRNA expression pattern in diseased tissues. Changes in miRNA levels affect both primary and secondary immune responses of the host against bacterial infection in periodontal tissues.
Table 2.
Changes in miRNA expression in diseased gingival tissues of periodontitis patients compared to healthy controls
| miRNAs | Changes in expression | References | Expression in cells* |
|---|---|---|---|
| miR-9 | Up | 16, 19 | Depleted in epithelial cell |
| miR-15a | Up | 20 | Enriched in leukocyte, haematopoietic cell, lymphocyte Depleted in epithelial cell, fibroblast |
| miR-17 | Up | 16, 19 | Enriched in epithelial cell, endothelial cell |
| miR-19a,b | Up | 16, 19 | Enriched in epithelial cell, endothelial cell, haematopoietic cell, leukocyte, monocyte Depleted in fibroblast |
| miR-21 | Up | 16 | Enriched in myeloid leukocyte, monocyte, phagocyte Depleted in neutrophil |
| miR-26 | Up | 16 | Enriched in haematopoietic cell, leukocyte Depleted in epithelial cell |
| miR-29a,b,c | Up | 16, 18, 19 | Enriched in haematopoietic cell, mononuclear cell, lymphocyte, lymphocyte Depleted in endothelial cell, epithelial cell, fibroblast |
| miR-30b,c,d | Up | 16, 19, 20 | Enriched in haematopoietic cell, leukocyte, epithelial cell Depleted in endothelial cell, fibroblast |
| miR-34a,c | Up | 16, 19 | Enriched in endothelial cell, epithelial cell Depleted in haematopoietic cell, leukocyte, lymphocyte |
| miR-125a | Up | 16, 19 | Enriched in haematopoietic cell, leukocyte Depleted in B cell, meso-epithelial cell |
| miR-125b | Up | 16, 19 | Enriched in fibroblast Depleted in haematopoietic cell, leukocyte, lymphocyte |
| miR-126 | Up | 18, 19 | Enriched in endothelial cell, meso-epithelial cell Depleted in ecto-epithelial cell |
| miR-142 | Up | 16, 19, 20 | Enriched in haematopoietic cell, leukocyte, lymphocyte Depleted in epithelial cell, fibroblast |
| miR-146a | Up | 19 | Enriched in haematopoietic cell, leukocyte, monocyte Depleted in endothelial cell |
| miR-148 | Up | 18 | Enriched in haematopoietic cell, leukocyte, monocyte Depleted in endothelial cell |
| miR-155 | Up | 18 | Enriched in haematopoietic cell, leukocyte, monocyte Depleted in endothelial cell |
| miR-181c | Up | 16, 19 | Enriched in haematopoietic cell, leukocyte |
| miR-223 | Up | 17, 18 | Enriched in haematopoietic cell, leukocyte Depleted in epithelial cell |
| miR-301 | Up | 16, 18 | Enriched in haematopoietic cell, leukocyte Depleted in epithelial cell |
| let-7b–e | Up | 16, 18 | Depleted in haematopoietic cell, leukocyte, lymphocyte |
| let-7f, g | Up | 16, 18 | Enriched in haematopoietic cell, leukocyte, lymphocyte |
| miR-31 | Down | 18 | Enriched in endothelial cell Depleted in haematopoietic cell, leukocyte |
| miR-92a | Down | 16 | Enriched in B cell, epithelial cell |
| miR-214 | Down | 16 | Enriched in fibroblast Depleted in haematopoietic cell, leukocyte, epithelial cell |
| miR-451 | Down | 16 | Enriched in haematopoietic cell, neutrophil, leukocyte Depleted in endothelial cell, epithelial cell |
miRNA regulation of innate immunity
The oral epithelium and the immune cells residing within periodontal tissues provide the first line of defense against oral bacteria and participate in the primary host response against infection, the innate immune response. The innate immune response is not specifically directed against individual pathogens, but instead provides an initial non-specific defense mechanism during the first hours after infection. From an evolutionary perspective, the innate immune system is a fairly basal defense against pathogens compared to the adaptive immune system as it occurs in invertebrates and vertebrates. In addition to the oral epithelial cells that function as a barrier against toxins and pathogens from the oral cavity, the major cells involved in innate immunity are neutrophils, macrophages and dendritic cells (DCs).
Neutrophils
Neutrophils are the most important phagocytic cells in the host’s defense against acute bacterial infection21 and the predominant subset of leukocytes in gingival tissues during the early stage of periodontal inflammation. Recent evidence suggests that neutrophils are also involved in the regulation of immunity and inflammation.22,23 In neutrophils, miRNAs may act to buffer transcriptional variation and fine-tune gene expression by coordinating the expression of hundreds of transcripts within networks of genes with converging functions.24
Our mouse periodontitis model and published human subject studies indicate that several miRNAs expressed in neutrophils were upregulated in diseased periodontal tissues, including miR-155, and miR-223, whereas the neutrophil-expressed miRNAs miR-17 and miR-31 were downregulated (Tables 1 and 2). Here, we discuss two aspects of miRNA involvement in the neutrophil-mediated response to bacterial pathogens and the resulting inflammatory response, (i) the regulation of neutrophil emigration from blood capillaries into inflamed tissues, affecting both neutrophil adhesion to endothelial cells and chemokine mRNA stability, and (ii) the regulation of neutrophil function through miRNAs.
The migration of neutrophils from postcapillary venules into the extravascular tissue at sites of inflammation is one of the first events of the inflammatory response to microbial challenge at the gingival sulcus.25 This emigration of leukocytes from the bloodstream into tissues is controlled by intercellular adhesion molecule-1 (ICAM-1) and E-selectin expression, which are targets of the miRNAs miR-31 and miR-17-3p, respectively.26 Specific antagonists of miR-31 and miR-17-3p have been reported to increase neutrophil adhesion to cultured endothelial cells, whereas mimics of these miRNAs decreased neutrophil adhesion to endothelial cells.26
In periodontal tissues, neutrophil recruitment is dependent upon chemoattractants and their receptors. For example, the CXC motif chemokine receptor 2 (CXCR2) responds to murine analogues of interleukin-8 (IL-8).27 IL-8 mRNA stability in neutrophils is modulated by another periodontium-expressed miRNA, miR-155, through the repression of the phosphoinositide lipid phosphatase (SHIP-1).28,29
Contrary to miR-155, inflammation in periodontitis animals and patients has resulted in a downregulation ofmiR-451 expression (Table 1).19 miR-451 directly targets copine 3 (CPNE3), Ras-related protein (Rab5a) and 14-3-3ζ genes.30 These studies have demonstrated that overexpression of miR-451 affected the phosphorylation of p38 mitogen-activated protein kinase (MAPK) through Rab5a and 14-3-3ζ and suppressed the migration of neutrophils towards N-formyl-l-methionyl-l-leucyl-phenylalanine. Systemic application of a miR-451 mimic significantly attenuated the infiltration of neutrophils in an inflammatory animal model.30
Neutrophils employ a number of mechanisms to exert an antimicrobial effect on periodontal pathogens. In addition to oxygen-dependent mechanisms, neutrophils rely on granule proteins, including cathepsins, to kill invading bacteria.22 Previous studies have demonstrated that miR-223 plays a particularly prominent regulatory role in neutrophils as there are 3819 known proteins affected by the deletion of miR-223 in mouse neutrophils.24 miR-223 directly targets the chemokines CXCL2, C–C motif chemokine 3 (CCL3) and IL-6 in myeloid cells and controls neutrophil recruitment in chronic inflammatory diseases.31 Deletion of miR-223 upregulated the expression of cathepsin family members, suggesting that miR-223 may exert an antimicrobial effect against periodontal pathogens. In addition to the potential protective function against infection, miR-223 is also involved in the activation of nucleotide-binding and oligomerization domain-like receptor containing a pyrin domain 3 (NLRP3) inflammasome, which promotes the maturation of inflammatory cytokines IL-1b and IL-18 and induces cell pyroptosis.32,33 miR-223 inhibited NLRP3 expression through a conserved binding site within the 3′-UTR and suppressed NLRP3 inflammasome activity.33
Nuclear factor-κB (NF-κB) signalling is a proinflammatory signalling pathway and promotes the expression of proinflammatory genes, including cytokines, chemokines and adhesion molecules.34 Through NF-κB signalling, miR-9 expression was upregulated in human polymorphonuclear neutrophils and monocytes by the lipopolysaccharides (LPS) of bacterial cell walls after Toll-like receptor (TLR) TLR4, TLR2 and TLR7/8 activation and by treatment with tumour necrosis factor-α (TNF-α) or IL-1β.35 However, in these studies, LPS selectively induced only one of three miR-9 genes, miR-9-1, in a MyD88-dependent and NF-κB-dependent manner35. In neutrophils and monocytes, miR-9 inhibited the expression of NF-κB1 and operated a feedback regulatory loop, controlling the NF-κB-dependent responses by fine-tuning the expression of a key member of the NF-κB family.35
Macrophages
Macrophages (MΦ) are monocyte-derived large phagocytic cells that play a major role during the innate immune response and contribute to the adaptive immune response by recruiting lymphocytes and other immune cells. In inflamed periodontal tissues, macrophages respond to LPS and activate multiple host defense functions through the production of inflammatory mediators. In turn, the secretion of inflammatory mediators establishes macrophages as the major cellular regulators that cause tissue destruction as a result of periodontal disease.36 Both macrophages and DCs are derived from macrophage/DC progenitors (MDPs), a subset of proliferating cells in the bone marrow that share phenotypic characteristics with myeloid precursor populations and give rise to many macrophages and DC subsets.37,38 Recent studies have demonstrated that miRNA networks play critical roles in the regulation of macrophage differentiation and activation.39,40
To further understand how miRNAs contribute to the differentiation of either macrophages or DCs from monocyte-like macrophage/DC precursors, members of our group have analysed miRNA expression profile changes during monocyte-to-MΦ and during monocyte-to-DC differentiation.41 In these studies, parallel profiling of monocyte-to-MΦ and monocyte-to-DC differentiation in vitro identified miR-24, miR-30b and miR-142-3p as unique miRNAs that were downregulated in the process of both MΦ and DC differentiation.41 All three miRNAs, miR-24, miR-30b and miR-142-3p, appear to counteract periodontal inflammation and inflammation-related tissue destruction. For example, upon challenge with LPS, overexpression of miR-24, miR-30b and miR-142-3p resulted in the suppression of inflammatory cytokine production, including TNF-α, IL-12p40 and IL-6.41,42 Transcript analysis of these cytokines revealed that these miRNAs regulate cytokines at both transcriptional and post-transcriptional levels.42 Interestingly, intracellular staining of TNF-α demonstrated cytokine accumulation at the cellular periphery of miR-142 mimic transfected cells, suggesting that miR-142-3p may impair cytokine release.42 Explaining their anti-inflammatory mode of action, miR-24, miR-30b and miR-142-3p have been demonstrated to target members of the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and MAPK families and protein kinase C (PKC) isoforms in the TLR4-NF-κB pathway and to inhibit the surface expression of TLR4/cluster of differentiation 14/myeloid differentiation 1 and intracellular PKCα/NFκB activation.41,42
miRNAs also modulate the specific macrophage response following exposure to LPS. In one of our studies, LPS derived from periodontal pathogens, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, or environmentally modified LPS obtained from P. gingivalis43,44 resulted in LPS-specific miRNA responses in macrophages, including miR-29b and let-7f. miR-29b directly targets interleukin-6 receptor α (IL-6Rα) and interferon-γ-inducible protein 30 (IFI30), while let-7f targets suppressor of cytokine signalling 4 (SOCS4) and thrombospondin-1 (TSP-1).43,44 These studies demonstrate convergent/divergent miRNA responses to various LPS treatments, and LPS-responsive miRNAs may fine-tune the host response to periodontal pathogens.
In addition to the LPS-response macrophage miRNAs miR-29b and let-7f from our study, the inflammatory miRNAs miR-146 and miR-155 were identified in inflamed gingival tissues18,19,45 and are also involved in the activation and function of macrophages.46,47 The expression of these miRNAs is NF-κB-dependent and responsive to several TLR ligands in macrophages. miR-146 is considered to be a 'fine-tune' negative feedback regulator in innate immunity. Proinflammatory cytokines IL-1βcytokines as well as LPS up-regulate miR-146 expression through the NF-κB pathway.47 miR-146 further inhibits the expression of IL1R-associated kinases (IRAK1 and IRAK2) and TNFR-associated factor (TRAF6), major regulatory molecules in the TLR/NF-κB pathway, resulting in a decrease in the expression of NF-κB target genes, such as IL-1β, TNF-α and type 1 Interferon (IFN).48,49 miR-146 also controls the monocyte inflammatory response through the noncanonical NF-κB/Rel pathway by targeting the transcription factor RelB.50 However, miR-155 appears to have dual effects on macrophage activation and function. Similar to miR-146, miR-155 directly represses the expression of the inflammatory pathway-related kinase mitogen-activated protein kinase 10 (MAP3K10, indicative of its anti-inflammatory effect on macrophages,51 while miR-155 also regulates proinflammatory macrophage activation in a positive fashion by inhibiting the expression of suppressor of cytokine signalling-1 (SOCS-1) and increasing type 1 IFN expression.52,53 In addition, miR-155 targets B cell lymphoma-6 (BCL6) protein and enhances NF-κB signalling.54 Taken together, these studies establish miR-155 as a regulatory miRNA that affects various aspects of the inflammatory process dependent on its tissue-specific and stage-related context.
Macrophage polarization as determined by an enhancement in the M1/M2 macrophage phenotype ratio is one of the hallmarks of periodontal disease. Classically activated M1 macrophages are proinflammatory and have a central role in the host defense against infection, whereas alternatively activated M2 macrophages are anti-inflammatory and participate in tissue remodelling. Changes in the macrophage phenotype ratio are also reflected on the miRNA level. Several miRNAs from our periodontal disease panel (Tables 1 and 2) are involved in macrophage polarization. Among these miRNAs, miR-155 and miR-125b are expressed at a higher level in M1 macrophages than in M2 macrophages and promote M1 polarization.
The mechanism for miR-155 to promote the M1 macrophage phenotype involves suppression of the CCAAT/enhancer-binding protein-β (C/EBP-β) signalling cascade55 and targeting of SHIP-1, IL13Rα1 or SMAD 2/3 during the regulation of macrophage programming and activation.46,56–58 In a similar fashion, miR-125b sustains proinflammatory macrophage activation by targeting the transcription factor interferon regulatory factor-4 (IRF4), a positive regulator of alternative macrophage activation.59,60 Enforced expression of miR-125b or IRF4 knockdown enhances macrophage activation, increases the macrophage response to IFN-γ and enhances macrophage-mediated functions, such as stimulating T cell activation and killing mouse tumour cell line EL4 tumour cells.59,60
In an antagonistic mode of action to miR-155 and miR-125b, a second group of miRNAs, including miR-125a-5p, let-7c and miR-21, plays an important role in suppressing the classic modes of macrophage activation while promoting alternative activation mechanisms.61–63 Illustrating the effect of miRNAs on the M1/M2 macrophage identifier and macrophage function, upregulation of these miRNAs diminished M1 phenotype expression induced by LPS, and promoted M2 marker expression induced by IL-4.61,62 In contrast, knockdown of these miRNAs promoted M1 polarization and diminished IL-4-induced M2 marker expression.61,62 The negative effect of miR-125a-5p on M1 activation is associated with its target Kruppel-like factor 13 (KLF13), a transcriptional factor that has an important role in T lymphocyte activation and inflammation.62 The positive effect of let-7c on M2 macrophage polarization is linked to targeting C/EBP-δ, an important transcriptional factor involved in the inflammatory response.61 Another miRNA, miR-21, controls macrophage polarization through the cross-talk between miR-21 and the lipid mediator prostaglandin E2 (PGE2). In support of this concept, studies have demonstrated that miR-21 directly targets signal transducer and activator of transcription 3 (STAT3) and that silencing the STAT3 gene abolishes the PGE2-mediated expression of M2 genes in miR-21−/− macrophages.63 Thus, miR-21 affects M1/M2 ratio values and progression of the immunological macrophage response through PGE2-mediated M2 generation.63
Studies from our group have demonstrated that miR-24 is also a negative regulator of macrophage classical activation and promotes alternative macrophage activation.64 In these studies, overexpression of miR-24 inhibited the production of TNF-α, IL-6 and IL-12p40 by macrophages in response to LPS or after exposure to the classic macrophage activation promoter IFN-γ. Moreover, miR-24 overexpression enhanced the expression of CD206, a marker of alternative activation, when treated with IL-4 and IL-13. These results indicate that miR-24 promotes an alternative to classical activation as long as macrophage plasticity is maintained, as explained by the miR-24-induced downregulation of the cytokine inducer p110δ.
Together, these studies are not only a summary of several strategies by which miRNA expression controls periodontal diseases progression but also a blueprint for future therapeutic approaches towards miRNA-based therapeutics that might interfere with the deleterious sequelae of periodontal disease. From a conceptional point-of-view, overexpression of miR-21, miR-24, miR-125a and/or let-7c during early-stage periodontal disease may inhibit acute inflammation by increasing the number of M2 macrophages.
Dendritic cells
DCs are professional Ag-presenting cells that reside in periodontal tissues, in other mucosal and lymphoid tissues and in the skin. 65,66 The name 'DCs' originates from the tree-branch-like extensions that occur during their development. Originally derived from haematopoietic progenitor cells of the bone marrow, mature tissue-resident DCs are involved in the presentation of Ags and subsequent activation of T cells, killer T cells and B cells through cell–cell contact or cytokine production. During periodontal disease progression, DCs serve as a link between innate and adaptive immunity.65,66 The maturation and function of DCs as tissue-resident cells is mediated by the transcription factors c-Fos and purine-rich box-1 (PU.1)67,68 and the regulatory molecules SOCS-1 and tuberous sclerosis complex-1 (TSC-1).69,70 miRNAs affect primary and secondary immune responses by regulating the expression of these DC-related transcription factors and regulatory molecules.
A number of miRNAs have been identified that regulate DC maturation, development and function. For example, miR-155, miR-126 and Let-7i expression was increased during DC maturation.71,72 Moreover, the silencing of c-Fos expression or downregulation of PU.1 by miR-155 modulates the pathogen-binding ability of DCs and promotes Ag-specific T cell activation.71,73
Both miR-155 and let-7i target SOCS-1 inside DCs.74,75 SOCS-1regulates the production of, which is involved in naive CD8 T cell activation.76 Downregulation of SOCS-1 enhances IL-12 production and results in a proinflammatory phenotype in mature DCs.77,78 miR-126 directly suppresses the translation of TSC-1, a negative regulator of mammalian target of rapamycin (mTOR), and controls the survival and function of plasmacytoid DCs.79
Phagocytosis is one of the key functions of the innate immune system and involves the engulfment, degradation and processing of microorganisms, while its foreign Ags are presented to cells of the adaptive immune system.80,81 Studies from our group have demonstrated that miR-24, miR-30b and miR-142-3p regulate phagocytosis in DCs and macrophages.41,82 Overexpression of miR-24, miR-30b and miR-142-3p significantly attenuated the phagocytosis of Escherichia coli (E. coli) and Staphylococcus aureus, as well as the expression of proinflammatory cytokines (TNF-α, IL-6, IL-8 and IL-12p40) by macrophages and DCs.41,82 Further studies have confirmed that miR-24, miR-30b and miR-142-3p target Fc receptors to regulate antibody-dependent Ag uptake in primary macrophages and DCs.82
In our mouse periodontitis model, the expression of a number of DC-related miRNAs, including miR-24, miR-30, miR-126, miR-142, miR-146, miR-155 and let-7i, increased (Table 1), and a similar trend was observed in published studies using human periodontitis models (Table 2). All of these miRNAs were involved in DC and macrophage maturation, as well as Ag processing and presentation by APCs, which is indicative of the role of these miRNAs in the development and progression of periodontal disease.
miRNA regulation of adaptive immunity
Adaptive immunity is a two-step host response to invading pathogens, beginning with an initial encounter that causes an immunological recognition of a pathogen and followed by an enhanced immunological response upon repeat encounters with the same pathogen. The adaptive immune system is based on the ability of lymphocytes to eliminate pathogens or prevent their growth. In general, adaptive immunity is classified into antibody-mediated immune responses against freely circulating pathogens facilitated by B cells and cell-mediated immune responses against intracellular pathogens that are carried out by T cells. From an evolutionary perspective, adaptive immunity only evolved as a second level of defense against pathogens during the emergence of the agnathan vertebrate ancestors some 500 million years ago. At that stage, lymphocyte progenitors developed in organisms related to the modern hagfishes and lampreys and possibly their protochordate ancestors.83 Additional adaptive weaponry against intruding pathogens, such as the major histocompatibility complex, TCR and B cell receptors, only evolved in gnathostomes and became a major survival advantage of modern vertebrates.84
T cells
T cells are part of the dense inflammatory infiltrate that forms in response to bacterial infection.85 They are the dominant cell type in the cell-mediated (macrophage/lymphocyte) immune response and are essential for both specific antibody production and polyclonal B cell activation. Based on two major surface co-receptor molecules, two functionally distinct lineages are distinguished in mature T cells, CD4 T cells and CD8 T cells. Naive CD4 T cells are activated after interaction with Ag-major histocompatibility complex II (MHC II) and polarize into specific subtypes, including T helper (Th) cells Th1, Th2, Th17 and regulatory T cells (Tregs), depending mainly on cytokine profiles.86 T cell activation is initiated by T cell receptor (TCR) signalling and promotes a number of signalling cascades, which lead to proliferation, cytokine production and subset differentiation.
T cell activation induces dynamic changes in miRNA expression patterns in CD4 T cell subsets. In a study designed to analyse the kinetics of activation-induced expression regulation of miRNAs in sorted CD4 and CD8 cells, the expression levels of miR-181 family, miR-17-92 clusters, miR-214, miR-146a, miR-155 and let-7 increased and the expression of miR-29, miR-125 and miR-216 decreased following T cell activation.87 Upregulation of miR-181 family miRNAs has been shown to enhance TCR signalling by targeting multiple phosphatases, including SH2 domain-containing protein tyrosine phosphatase 2 (SHP2), protein tyrosine phosphatase, non-receptor type 22 (PTPN22), dual-specificity protein phosphatase 5 (DUSPS5) and DUDPS688–90 and enhancing the phosphorylation of immunoreceptor tyrosine-based activation motifs on the cytosolic side of the TCR/CD3 complex.91,92 miRNAs also affect T cell function in an indirect fashion as it has been demonstrated that upregulation of the miR-214 and miR-17-92 cluster promotes T cell activation and proliferation by targeting the negative regulator phosphatase and tensin homologue (PTEN) in the PI3K/Ak strain transforming (AKT) pathway.93,94 Another miRNA, miR-146, functions as a feedback regulator of NF-κB signalling and modulates the productive immune response, division and growth of T cells.95 This microRNA targets TRAF6 and IRAK1 for feedback control of the intensity and duration of NF-κB signalling in activated T cells.91,95
Imbalance in Th1 and Th2 cytokines is thought to play a role in a variety of immune-inflammatory disorders, including multiple sclerosis, arthritis, asthma, cancer96,97 and periodontal disease.98,99 Even though the Th1/Th2 model is somewhat dated in view of recent discoveries related to Th17 cells,98 the Th1-type phenotype is generally known as cellular and proinflammatory, whereas Th2 cells are thought to be associated with humoural immunity and feature anti-inflammatory properties.100,101 A number of studies suggest that miR-29 and the miR-17-92 cluster affect Th1 cell function. For example, it has been demonstrated that miR-29 inhibits Th1 cell differentiation and IFN-γ production in CD4 T cells by targeting T-box transcription factor Tbx21 and eomesodermin (Eomes), two specific transcription factors for Th1 differentiation and IFN-γ mRNA expression.102 The miR-17-92 cluster has a similar negative effect on Th1 differentiation and IFN-γ production. However, mechanistic studies indicate that the miR-17-92 cluster targets transforming growth factor-β receptor type 2 (TGFβRII) and cAMP-responsive element-binding protein-1 (CREB1), which are involved in Treg cell differentiation, and therefore enhances Th1 differentiation.103 So far, three miRNAs, miR-155, let-7 family members and miR-126, have been shown to affect Th2 differentiation by regulating cytokine production. miR-155 has been demonstrated to inhibit Th2 differentiation by repressing IFN-γ signalling,104 whereas let-7 family members affect Th2 function by targeting IL-13 mRNA.105 In contrast, knockdown of miR-126 after T cell activation increased IL-5 and IL-13 expression and promoted Th2 differentiation through OBF.1-PU.1-GATA3 (GATA binding protein 3) signalling.106
The functional antagonism between Treg and Th17 cells has been proposed to be a major factor in the pathogenesis of periodontitis.107 After differentiation from naïve T cells, Treg cells possess a differential microRNA expression pattern that is associated with their development and function in regulating T cell responses.[108, 109] In terms of known miRNAs, miR-155 and miR-146 are highly expressed in Treg cells, whereas miR-24, miR-31 and miR-125 are found at low levels in Treg cells.108,109 Among these miRNAs, miR-155 directly targets SOCS-1, an inhibiter of STAT5 signalling, during Treg differentiation and plays a critical role in maintaining Treg lineage stability and regulatory function.109,110 In contrast, miR-146 represses the mRNA expression of STAT1, a transcription factor required for INF-γ signalling, and controls Treg-mediated suppression of Th1 responses91,111 Comparatively, both miR-24 and miR-31 target forkhead box P3 (FOXP3), a lineage specification factor of Treg cells. In related studies, downregulation of miR-24 and miR-31 has resulted in increased FOXP3 expression and enhanced Treg differentiation and function.112,113 miR-125a exerts its roles in a manner partially similar to miR-24 and miR-31 to stabilize Treg lineage commitment and function. miR-125a directly suppressed several targets, including Stat3, Il13 and Ifn-g, which are critical factors of effector lineages and detrimental to Treg differentiation.114
Th17 cells are involved in recruiting neutrophils and macrophages to participate in and to amplify the inflammatory reaction caused by periodontal disease.115–117 Th17 cell differentiation is promoted by the miR-17-92 cluster,94 and miR-19b, a member of the miR-17-92 cluster, represses the expression of PTEN, thereby augmenting the PI3K-AKT-mTOR axis essential for proper Th17 differentiation.95 In addition, miR-17 enhances Th17 polarization by inhibiting its target, ikaros family zinc-finger 4 (IKZF4).94 miR-17 also controls Th1 responses by targeting TGFβRII and CREB1. As a consequence, miR-17 and miR-19b suppress inducible regulatory T cell differentiation.103 Other miRNAs that enhance Th17 differentiation include miR-301 and miR-155. miR-301 has a negative effect on the expression of the E3 SUMO-protein ligase PIAS3, which suppresses the STAT3 pathway and Th17 differentiation,118 whereas miR-155 contributes to Th17 cell function by suppressing the inhibitory effects of Jumonji and AT-rich interaction domain-containing 2 (Jarid2). Jarid2 forms a complex with polycomb repressive complex 2 and targets IL-17 expression regulators, such as Atf3, cellular plasticity genes, such as Tbx21 and Eomes, and immunoregulatory cytokine genes, such as IL-22, IL-10 and IL-9.119
Previous miRNA expression profiling studies have revealed that the expression of miR-17, miR-19, miR-155 and miR-301 was upregulated in the gingival tissues of periodontitis patients and animals compared to healthy controls (Tables 1 and 2). All of these miRNAs promote the development of Th17 cells, which play an important role in the initiation and maintenance of the inflammatory response in periodontal disease. From a therapeutic perspective, inhibition of these miRNAs might shift the immune response to a Th2 profile to alter disease progression or may induce Treg cell formation to attenuate the severity of periodontal disease.
B cells
B cells form a substantial proportion of the inflammatory infiltrate in diseased periodontal tissues, and higher levels of B lymphocyte infiltration are associated with advanced stages of periodontitis.120,121 Advanced periodontal inflammation also elevates the expression of five periodontal inflammation-related miRNAs that we associated with periodontal disease earlier, namely, miR-125, miR-148, miR-155, miR-181 and miR-217 (Tables 1 and 2). Indicative of a correlation between periodontal disease progression and B cell differentiation, these five miRNAs regulate the progression of B cell terminal differentiation by targeting a coordinated network of transcription factors. As an example, miR-125b is involved in the downregulation of transcription factors, B lymphocyte-induced maturation protein-1 (Blimp1) and interferon regulatory factor 4 (IRF4), to inhibit B cell terminal differentiation,122 whereas miR-148 has been shown to suppress BTB domain and CNC homologue 2 (Bach2) expression and promote plasma cell lineage commitment.123 miR-148 also targets proapoptotic factors PTEN and Bim, and fosters germinal centre (GC) B cell survival.124 miR-217 stabilizes the expression of the transcriptional repressor Bcl-6 in GC B cells to increase the generation of class-switched antibodies and the frequency of somatic hypermutation by down-regulating the expression of the DNA damage response and repair gene network.125 Both miR-155 and miR-181 negatively regulate the expression of activation-induced cytidine deaminase (AID) to reinforce and repress uncontrolled AID expression.126,127
A summary of all relevant microRNAs, their target genes and target gene functions in immune cells and their putative regulatory role in periodontal tissues is provided in Table 3.
Table 3.
Summary of relevant microRNAs, target genes and target gene functions in immune cells and their putative regulatory roles in periodontal tissues
| Immune cells | miRNAs | miRNA in diseased tissues | Target genes | Target gene function in immune cells | Putative regulatory role of the miRNA in periodontal tissues | Ref. |
|---|---|---|---|---|---|---|
| Neutrophil | miR-9 | Up | NF-κ B1 | NF-κB family member | Feedback regulation of NF-κB pathway | 36 |
| miR-17 | Up | ICAM-1 | Endothelial adhesion molecule | Neutrophil adhesion to endothelial cells | 26 | |
| miR-34 | Up | Dock8 | Rho family member | Neutrophil emigration | 30 | |
| miR-155 | Up | FGD4, Rac1 | Rho family member | Neutrophil emigration | 30 | |
| SHIP-1 | Phosphoinositide lipid phosphatase | IL-8 mRNA stability | 28, 29 | |||
| miR-223 | Up | CXCL2, CCL3, IL-6 | Chemoattractants | Leukocyte chemotaxis | 32 | |
| NLRP3 | Inflammasome | Processing of inflammatory cytokines and pyroptosis | 34 | |||
| miR-31 | Down | E-selectin | Endothelial adhesion molecule | Neutrophil adhesion to endothelial cells | 26 | |
| miR-451 | Down | CPNE3, Rab5a, 14-3-3 | Binding proteins | Neutrophil chemotaxis | 31 | |
| Macrophage | miR-21 | Up | STAT3 | Transcription factor in inflammation | Prevent PGE-mediated M2 generation | 63 |
| miR-24 | Up | PKCα, CD163, CD206 | Components of pattern recognition receptor (PRR) signalling | Inhibit NF-κB activation | 42 | |
| P110δ | Kinase in class IAPI3 signalling | Promote M2 phenotype | 64 | |||
| miR-29b | Up | IL-6Rα, IFI30 | Components of cytokine signalling | Fine-tuning host response | 43, 44 | |
| miR-30 | Up | PKCα, CD163, CD206 | Components of PRR signalling | Inhibit NF-κB activation | 42 | |
| miR-125a | Up | KLF13 | Transcription factor in T cell activation and inflammation | Suppress classical but promote alternative activation of macrophage | 62 | |
| miR-125b | Up | IRF4 | Member of interferon response factor family | Enhance M1 phenotype | 59, 60 | |
| miR-142 | Up | PKCα, CD163, CD206 | Components of PRR signalling | Inhibit NF-κB activation | 42 | |
| miR-146 | Up | TRAF6, IRAK1, IRAK2 | Regulatory molecules in TLR/NF-κB pathway | Feedback regulation of inflammation | 48, 49 | |
| miR-155 | Up | MAP3K10 | Inflammatory pathway-related kinase | Endocytosis function | 51 | |
| SOSC1 | Negative regulator of type IFN signalling | Macrophage sensitivity and tolerance | 52, 53 | |||
| BCL6 | Transcription factor counter-regulating NF-κB activity | Enhance inflammation | 54 | |||
| IL13Ra1 | Component of the type II IL-4 receptor | Promote M1 phenotype | 56 | |||
| SMAD2 | Signalling molecule of TGF-β pathway | Regulation of macrophage polarization | 57, 58 | |||
| Let-7f | Up | SOCS4, TSP-1 | Regulators of inflammation | Regulate proinflammatory macrophage activity | 43, 44 | |
| Let-7 | Up | C/EB-d | Transcription factor in inflammatory response | Promote M2 phenotype | 61 | |
| Dendritic cells | miR-24 | Up | PKCα, CD14, TLR4, MD-1 | Components of PRR signalling | Inhibit NF-κB activation | 82 |
| miR-30 | Up | PKCα, CD14, TLR4, MD-1 | Components of PRR signalling | Inhibit NF-κB activation | 82 | |
| miR-126 | Up | TSC-1 | Negative regulator of mTOR | Survival and function of plasmacytoid DC | 79 | |
| miR-142 | Up | PKCα, CD14, TLR4, MD-1 | Components of PRR signalling | Inhibit NF-κB activation | 82 | |
| miR-155 | Up | c-FOS, PU.1 | Transcription factors | DC maturation | 73 | |
| SOCS-1 | Negative regulator of IL-2 signalling | DC maturation and pathogen binding | 71 | |||
| let-7 | Up | SOCS-1 | Negative regulator of IL-2 signalling | Enhance a proinflammatory phenotype of mature DC | 74, 75 | |
| T cells | miR-17 | Up | IKZF4 | Member of the Ikaros family transcription factors | Promote Th17 phenotype | 95 |
| TGFβRII | TGF-β signalling | Enhance Th1 differentiation and prevent Treg response | 104 | |||
| CREB1 | Transcription factor regulating FoxP3 expression | Enhance Th1 differentiation and prevent Treg response | 104 | |||
| miR-19 | Up | PTEN | Negative regulation of PI3K-AKT-mTOR axis | Enhance Th17 polarization | 95 | |
| miR-24 | Up | FoxP3 | Lineage-specific factor of Treg cells | Inhibit Treg differentiation | 113, 114 | |
| miR-29 | Up | TBX21EOMES | Transcription factors for INF-γ | Inhibit Th1 differentiation | 103 | |
| miR-31 | Up | FoxP3 | Lineage-specific factor of Treg cells | Inhibit Treg differentiation | 113, 114 | |
| miR-125a | Up | STAT3, IL-13, INF-γ | Critical factors for effector lineages | Inhibit Treg differentiation | 115 | |
| miR-146 | Up | TRAF6, IRAK1 | Regulatory molecules in TLR/NF-κB pathway | Feedback regulation of NF-κB signalling in T cells | 92, 96 | |
| STAT1 | Transcription factor for INF-γ | Suppress Th1 response | 112 | |||
| miR-155 | Up | SOCS-1 | Inhibitor of STAT5 signalling | Maintain Treg homeostasis and function | 104 | |
| INF-γRA | Cytokine receptor | Inhibit Th2 differentiation | 105 | |||
| miR-181 | Up | SHP2, PTPN22, DUSPS5, DUSPS6 | Phosphatases | Elevate steady-state levels of phosphorylated interaction and reduce T cell receptor signalling threshold | 89, 90 | |
| miR-301 | Up | PIAS3 | Inhibitor of STAT3 pathway Transcription factors for plasma cell differentiation | Promote Th17 response | 119 | |
| let-7 | Up | IL-13 | Cytokine | Inhibit Th2 differentiation | 106 | |
| miR-214 | Down | PTEN | Negative regulator of AKT signalling | Enhance Th17 polarization | 94 | |
| B cells | miR-125b | Up | Blimp1, IRF4 | Transcription factors for plasma cell differentiation | Promote B cell diversification in GC | 123 |
| miR-148 | Up | PTEN, Bim | Proapoptotic factors in B cells | Promote plasmablast survival | 125 | |
| Bach2, Mitf | Transcription factors for plasma cell differentiation | Promote plasma cell differentiation | 124 | |||
| miR-155 | Up | AID | A potent DNA mutator | Antibody affinity maturation | 127 | |
| miR-181 | Up | AID | A potent DNA mutator | Antibody affinity maturation | 128 |
miRNAs as biomarkers for periodontal disease
At present, the most common oral fluid-based molecular biomarkers for periodontitis fall into three general categories: (i) host-derived enzymes and their inhibitors, (ii) inflammatory mediators and host response modifiers and (iii) tissue breakdown products.128–131 These molecular biomarkers detect the presence and severity of disease, but cannot predict the initiation and early transition of periodontal disease. Most recently, miRNAs have emerged as a novel class of highly sensitive and specific biomarkers.132 miRNAs remain stable in extracellular fluids due to their packaging133–135 and are thus ideally suited to serve as non-invasive biomarkers for periodontal disease.
Previous studies have demonstrated that miRNAs affect the host immune response by regulating the expression of downstream target genes. In periodontal disease, oral bacteria and inflammatory conditions affect the function of oral epithelial and immune cells and dysregulate miRNA expression in these cells. While immune cells and non-immune cells synthesize miRNAs intracellularly, they also actively release miRNAs into extracellular environments, including extracellular fluids.136–138 The released miRNAs are associated with RNA-binding proteins or high-density lipoproteins, or they are enclosed within lipid vesicles. They are highly stable in extracellular fluids.139 Regardless of their biological functions, five miRNAs, including miR-142-3p, miR-146a, miRNA-155, miR-203 and miR-223, have been proposed as markers for periodontal disease.140
Gingival crevicular fluid (GCF) and saliva have been considered to be ideal sampling environments for periodontal diagnosis because of their proximity to the periodontium and due to their suitability for non-invasive sample collection.141,142 GCF is an exudate of periodontal tissues, which is located in the gingival sulcus of healthy subjects or in the periodontal pocket of periodontitis patients. miRNA profiling in GCF of healthy subjects or periodontitis patients has resulted in the identification of more than 600 miRNAs. Among these, miR-223 was the most highly expressed miRNA in GCF samples, and this miRNA was also upregulated in periodontitis samples.143 In addition, a subset of miRNAs was altered in the GCF of periodontal disease samples and therefore holds potential as a biomarker for periodontitis.143 Compared to GCF, saliva is more complex as it contains not only GCF but also salivary gland products, oral mucosal exudates and a potential pool of biological markers in a hypotonic environment. The concept of salivaomics has been proposed, which encompasses genomics, transcriptomics, proteomic, metabolomics and miRNA profiles.144,145 miRNA profiling in saliva has been investigated as a diagnostic approach for oral cancer, pre-cancer and oral lichen planus disease.146
Further studies of miRNAs in GCF or saliva and their relationship with periodontal diseases may provide novel diagnostic tools for the early detection of periodontal disease and treatment plan monitoring.
miRNA targeting as a potential therapeutic strategy for the treatment of periodontal disease
In recent years, novel miRNA therapeutics have gained increasing prominence as potential therapeutic agents because of their small size, their ability to easily pass through tissues and membranes, their microregulatory effects on gene expression and their ability to affect multiple and related mRNAs at the same time.1 Early clinical successes for miRNA applications in the treatment of disease include a mimic for the tumour suppressor miRNA miR-34 and anti-miRs targeting miR-122 for the treatment of hepatitis.147 The complexity of periodontal disease and clinical applicability of miRNA mimics and anti-miRs to control the miRNA environment of host tissues and disease processes are related to their potential use in the treatment of periodontal disease.
One obvious miRNA targeting opportunity is presented in form of the altered host miRNA expression profile in periodontal tissues of periodontitis patients and in animals suffering from periodontal disease (Tables 1 and 2) as it is triggered by oral microbial infections. There are two basic mechanisms involved in the alteration of miRNA expression upon infection with oral pathogens, (i) pathogen-encoded miRNAs designed to mimic host miRNAs to aid pathogen reproduction and survival and (ii) changes in host miRNA expression levels as part of the host immune response.5,148 Loss of the homeostatic balance of miRNA regulation affects host immune responses to pathogens and benefits bacterial infection. Therefore, manipulating miRNA function to modulate exuberant inflammation associated with tissue breakdown may have therapeutic potential for the treatment of periodontal disease. Potential strategies for miRNA targeting aim to either augment or dampen the expression of specific miRNAs, depending on the biological or pathological disposition of the miRNA(s). miRNA levels may be either enhanced or limited by using miRNA mimics or miRNA antagonists, respectively. miRNA mimics are chemically synthesized short double-stranded oligonucleotides that functionally mimic a pre-miRNA complex.149,150 In contrast, miRNA antagonists are single-stranded oligonucleotides that complement miRNA sequences and are designed to functionally reduce miRNA activity.151,152
miRNA regulation of gene expression as part of the immune response to infection is a highly complex process. Not only do unique bacteria regulate different host miRNAs, but the same bacterium may also cause different miRNA responses to infection in different cell types.5,153 However, genome-wide miRNA transcriptional response analysis in human immune cells responding to various bacteria has resulted in the identification of a set of core miRNAs in response to bacterial infection.154 For example, miR-155, miR-146, miR-125, miR-21 and let-7 are commonly affected miRNAs during bacterial infection.15,153 Especially, changes in miR-155 expression are part of the inflammatory immune response of various immune cells in both innate and adaptive immunity.155 The presence of common miRNA profiles as part of the cellular immune response to infection suggests that manipulating the expression of these core miRNAs may alter or reverse the progression of periodontal disease.
Although therapeutic targeting of miRNAs for the treatment of periodontal disease is still in its infancy, convincing proof of principle is provided by studies of immune diseases and tumour immune responses in animal models. Immune responses driven by activated T cell subsets are involved in the development of pathologic immune disorders. For example, Th1 cells promote cellular immunity and affect the development of autoimmune diseases, while Th2 cells mediate humoural immunity and trigger allergic immune responses.156 Addressing the Th2 cell-mediated allergic immune response, intranasal application of miR-126 or miR-145 antagonists suppressed the effector function of Th2 cells and inhibited house dust mite-induced allergic airway diseases in animal lungs.106,157 Similarly, miR-106 and let-7 family members are differentially expressed in allergic murine models. Antagonism of miR-106 or overexpression of let-7 modulated the Th2 response in airways and attenuated the development of asthma symptoms.105,158 Th17 T cells are key players in various autoimmune diseases in humans and animal models, including experimental autoimmune encephalomyelitis (EAE). In earlier studies, it has been demonstrated that Th17 cell differentiation is regulated by miR-326. In vivo silencing of miR-326 using a lentiviral approach inhibited Th17 differentiation and attenuated EAE in mice.159,160 In addition, overexpression of miR-10 by retroviral vectors inhibited the conversion of inducible Treg cells into follicle Th cells and limited differentiation into the Th17 subset of Th cells. Thus, miR-10 supplementation resulted in a protective effect and attenuated the onset of EAE.160,161
miRNA-based immune responses have also been investigated as potential targets for anti-tumour immunotherapies. In a preclinical study, miR-138 was selected as a candidate for modulating immune checkpoint activity in murine models of GL261 glioma.162 Specifically, intravenous delivery of miR-138 liposomal complexes resulted in decreased intratumour Fox3+ regulatory T cells and inhibited the expression of cytotoxic T-lymphocyte-associated molecule-4 (CTLA-4) and programmed cell death-1 (PD1) in CD4+ T cells, two important checkpoints in tumour progression.162 Augmentation of miR-138 in vivo suppressed the growth of glioma cells and prolonged the survival of the animals suffering from glioma.162 In another study, nanoparticles with miR-155 mimics as cargo were delivered into DCs in vivo to enhance the miR-155 activity in an ovarian cancer microenvironment,163 resulting in a transformation of DCs from immunosuppressive to immunostimulatory cells and an activation of potent anti-tumour immune responses.163
Together, these clinical and preclinical studies illustrate the enormous therapeutic potential of miRNA-based strategies. Therapeutic modalities for the treatment of periodontal disease may include the application of miR-based therapeutics in combination with current periodontal treatment approaches. Future research will identify unique combinations and dosages of miRNAs in combination with suitable carrier vehicles and delivery strategies to optimally combat and reverse periodontal disease.
Conflict of interests
The authors declare no conflict of interest.
Contributor Information
Salvador Nares, Email: snares@uic.edu.
Thomas G. H. Diekwisch, Email: diekwisch@tamhsc.edu
References
- 1.Diekwisch TGH. Novel approaches toward managing the micromanagers: ‘non-toxic’ but effective. Gene Ther. 2016;23:697–698. doi: 10.1038/gt.2016.49. [DOI] [PubMed] [Google Scholar]
- 2.Luan X, et al. MicroRNAs and periodontal homeostasis. J. Dent. Res. 2017;96:491–500. doi: 10.1177/0022034516685711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell RM, Zhao JL, Rao DS. MicroRNA function in myeloid biology. Blood. 2011;118:2960–2969. doi: 10.1182/blood-2011-03-291971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eulalio A, Schulte L, Vogel J. The mammalian microRNA response to bacterial infections. RNA Biol. 2012;9:742–750. doi: 10.4161/rna.20018. [DOI] [PubMed] [Google Scholar]
- 6.Singaravelu R, et al. MicroRNAs regulate the immunometabolic response to viral infection in the liver. Nat. Chem. Biol. 2015;11:988–993. doi: 10.1038/nchembio.1940. [DOI] [PubMed] [Google Scholar]
- 7.Dix A, et al. Specific and novel microRNAs are regulated as response to fungal infection in human dendritic cells. Front. Microbiol. 2017;8:270. doi: 10.3389/fmicb.2017.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britton C, Winter AD, Gillan V, Devaney E. microRNAs of parasitic helminths—identification, characterization and potential as drug targets. Int. J. Parasitol. Drugs Drug Resist. 2014;4:85–94. doi: 10.1016/j.ijpddr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauley KM, Cha S, Chan EKL. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander M, O’Connell RM. Noncoding RNAs and chronic inflammation: micro-managing the fire within. BioEssays. 2015;37:1005–1015. doi: 10.1002/bies.201500054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maegdefessel L. The emerging role of microRNAs in cardiovascular disease. J. Intern. Med. 2014;276:633–644. doi: 10.1111/joim.12298. [DOI] [PubMed] [Google Scholar]
- 12.Omahen DA. MicroRNA and diseases of the nervous system. Neurosurgery. 2011;69:440–454. doi: 10.1227/NEU.0b013e318215a3b3. [DOI] [PubMed] [Google Scholar]
- 13.Turchinovich A, Cho WC. The origin, function and diagnostic potential of extracellular microRNA in human body fluids. Front. Genet. 2014;5:30. doi: 10.3389/fgene.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortez MA, et al. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maudet C, Mano M, Eulalio A. MicroRNAs in the interaction between host and bacterial pathogens. FEBS Lett. 2014;588:4140–4147. doi: 10.1016/j.febslet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, et al. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell. 2011;35:43–49. [PubMed] [Google Scholar]
- 17.Ogata Y, et al. MicroRNA expression in inflamed and noninflamed gingival tissues from Japanese patients. J. Oral Sci. 2014;56:253–260. doi: 10.2334/josnusd.56.253. [DOI] [PubMed] [Google Scholar]
- 18.Stoecklin-Wasmer C, et al. MicroRNAs and their target genes in gingival tissues. J. Dent. Res. 2012;91:934–940. doi: 10.1177/0022034512456551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie YF, Shu R, Jiang SY, Liu Dl, Zhang Xl. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int. J. Oral Sci. 2011;3:125–134. doi: 10.4248/IJOS11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. J. Dent. Res. 2011;91:33–38. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dyke TE, Hoop GA. Neutrophil function and oral disease. Crit. Rev. Oral Biol. Med. 1990;1:117–133. doi: 10.1177/10454411900010020201. [DOI] [PubMed] [Google Scholar]
- 22.Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front. Oral Biol. 2011;15:56–83. doi: 10.1159/000329672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J. Leukoc. Biol. 2015;98:539–548. doi: 10.1189/jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000. 2013;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J. Immunol. 2009;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zenobia C, et al. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 2013;15:1419–1426. doi: 10.1111/cmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharyya S, et al. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J. Biol. Chem. 2011;286:11604–11615. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gantier MP. The not-so-neutral role of microRNAs in neutrophil biology. J. Leukoc. Biol. 2013;94:575–583. doi: 10.1189/jlb.1012539. [DOI] [PubMed] [Google Scholar]
- 30.Murata K, et al. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014;66:549–559. doi: 10.1002/art.38269. [DOI] [PubMed] [Google Scholar]
- 31.Dorhoi A, et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J. Clin. Invest. 2013;123:4836–4848. doi: 10.1172/JCI67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlowski GM, et al. Multiple cathepsins promote pro-IL-1β synthesis and NLRP3-mediated IL-1β activation. J. Immunol. 2015;195:1685–1697. doi: 10.4049/jimmunol.1500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauernfeind F, et al. NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence T. The nuclear factor NF- B pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazzoni F, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. USA. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayal RA. The role of osteoimmunology in periodontal disease. Biomed. Res. Int. 2013;2013:1–12. doi: 10.1155/2013/639368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy S. miRNA in macrophage development and function. Antioxid. Redox Signal. 2016;25:795–804. doi: 10.1089/ars.2016.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, et al. Identification of the microRNA networks contributing to macrophage differentiation and function. Oncotarget. 2016;7:28806–28820. doi: 10.18632/oncotarget.8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J. Leukoc. Biol. 2015;98:195–207. doi: 10.1189/jlb.1A1014-519RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naqvi AR, Fordham JB, Nares S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J. Immunol. 2015;194:1916–1927. doi: 10.4049/jimmunol.1401893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naqvi AR, Fordham JB, Khan A, Nares S. MicroRNAs responsive to Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis LPS modulate expression of genes regulating innate immunity in human macrophages. Innate Immun. 2014;20:540–551. doi: 10.1177/1753425913501914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naqvi, A. et al. Expression profiling of LPS responsive miRNA in primary human macrophages. J. Microb. Biochem. Technol. 08, 136–143 (2016). [DOI] [PMC free article] [PubMed]
- 45.Jiang SY, et al. The negative feedback regulation of microRNA-146a in human periodontal ligament cells after Porphyromonas gingivalis lipopolysaccharide stimulation. Inflamm. Res. 2015;64:441–451. doi: 10.1007/s00011-015-0824-y. [DOI] [PubMed] [Google Scholar]
- 46.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF- B-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauley KM, et al. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou J, et al. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 50.Etzrodt M, et al. Regulation of monocyte functional heterogeneity by miR-146a and Relb. Cell Rep. 2012;1:317–324. doi: 10.1016/j.celrep.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J, et al. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS ONE. 2012;7:e46551. doi: 10.1371/journal.pone.0046551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Androulidaki A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang P, et al. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J. Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 54.Nazari-Jahantigh M, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai X, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012;4:341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptoralpha1 (IL13Ralpha1) J. Biol. Chem. 2011;286:1786–1794. doi: 10.1074/jbc.M110.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-β. J. Biol. Chem. 2010;285:41328–41336. doi: 10.1074/jbc.M110.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sierra-Filardi E, et al. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117:5092–5101. doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhuri AA, et al. MicroRNA-125b potentiates macrophage activation. J. Immunol. 2011;187:5062–5068. doi: 10.4049/jimmunol.1102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Squadrito ML, Etzrodt M, De Palma M, Pittet MJ. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013;34:350–359. doi: 10.1016/j.it.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banerjee S, et al. MicroRNA let-7c regulates macrophage polarization. J. Immunol. 2013;190:6542–6549. doi: 10.4049/jimmunol.1202496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banerjee S, et al. miR-125a-5p regulates differential activation of macrophages and inflammation. J. Biol. Chem. 2013;288:35428–35436. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, et al. MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS ONE. 2015;10:e0115855. doi: 10.1371/journal.pone.0115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fordham JB, Naqvi AR, Nares S. miR-24 regulates macrophage polarization and plasticity. J. Clin. Cell. Immunol. 2015;06:362. doi: 10.4172/2155-9899.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatesan G, Uppoor A, Naik DG. Redefining the role of dendritic cells in periodontics. J. Indian Soc. Periodontol. 2013;17:700–705. doi: 10.4103/0972-124X.124467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilensky A, et al. Dendritic cells and their role in periodontal disease. Oral Dis. 2014;20:119–126. doi: 10.1111/odi.12122. [DOI] [PubMed] [Google Scholar]
- 67.Dillon S, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-FoS in dendritic cells. J. Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 68.Carotta S, et al. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J. Clin. Invest. 2005;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo Y, et al. Tsc1 expression by dendritic cells is required to preserve T-cell homeostasis and response. Cell Death Dis. 2017;8:e2553–e2553. doi: 10.1038/cddis.2016.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T. MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) J. Biol. Chem. 2009;284:16334–16342. doi: 10.1074/jbc.M109.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smyth LA, Boardman DA, Tung SL, Lechler R, Lombardi G. MicroRNAs affect dendritic cell function and phenotype. Immunology. 2015;144:197–205. doi: 10.1111/imm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunand-Sauthier I, et al. Silencing of c-Fos expression by microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–4500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- 74.Huffaker TB, O’Connell R. M. miR-155-SOCS1 as a functional axis: satisfying the burden of proof. Immunity. 2015;43:3–4. doi: 10.1016/j.immuni.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Kim SJ, Gregersen PK, Diamond B. Regulation of dendritic cell activation by microRNA let-7c and BLIMP1. J. Clin. Invest. 2013;123:823–833. doi: 10.1172/JCI65521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mescher MF, et al. Signals required for programming effector and memory development by CD8 + T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 77.Lu C, et al. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–4303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, et al. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of il-12 production by monocytes/macrophages in hepatitis C virus infection. J. Immunol. 2011;186:3093–3103. doi: 10.4049/jimmunol.1002006. [DOI] [PubMed] [Google Scholar]
- 79.Agudo J, et al. The miR-126–VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubach J, et al. Dendritic cells: sentinels of immunity and tolerance. Int. J. Hematol. 2005;81:197–203. doi: 10.1532/IJH97.04165. [DOI] [PubMed] [Google Scholar]
- 81.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naqvi AR, Fordham JB, Ganesh B, Nares S. miR-24, miR-30b and miR-142-3p interfere with antigen processing and presentation by primary macrophages and dendritic cells. Sci. Rep. 2016;6:32925. doi: 10.1038/srep32925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Buchmann K. Evolution of innate immunity: clues from invertebrates via fish to mammals. Front. Immunol. 2014;5:459. doi: 10.3389/fimmu.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Biol. Med. 2001;12:125–135. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 86.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+ T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teteloshvili N, et al. T-cell activation induces dynamic changes in miRNA expression patterns in CD4 and CD8 T-cell subsets. MicroRNA. 2015;4:117–122. doi: 10.2174/2211536604666150819194636. [DOI] [PubMed] [Google Scholar]
- 88.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 89.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Zimmerman EI, et al. Lyn kinase-dependent regulation of miR181 and myeloid cell leukemia-1 expression: implications for drug resistance in myelogenous leukemia. Mol. Pharmacol. 2010;78:811–817. doi: 10.1124/mol.110.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu G, et al. Fine-tuning T cell receptor signaling to control T cell development. Trends Immunol. 2014;35:311–318. doi: 10.1016/j.it.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jindra PT, Bagley J, Godwin JG, Iacomini J. Costimulation-dependent expression of microRNA-214 increases the ability of T cells to proliferate by targeting Pten. J. Immunol. 2010;185:990–997. doi: 10.4049/jimmunol.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu SQ, Jiang S, Li C, Li QJ. miR-17-92 cluster targets phosphatase and tensin homology and ikaros family zinc finger 4 to promote TH17-mediated inflammation. J. Biol. Chem. 2014;289:12446–12456. doi: 10.1074/jbc.M114.550723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L, et al. miR-146a controls the resolution of T cell responses in mice. J. Exp. Med. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit. Rev. Immunol. 2005;25:75–102. doi: 10.1615/CritRevImmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 97.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2014;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dent. Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arun KV, Talwar A, Kumar TSS. T-helper cells in the etiopathogenesis of periodontal disease: a mini review. J. Indian Soc. Periodontol. 2011;15:4–10. doi: 10.4103/0972-124X.82255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 101.Yücel OumlOuml, Berker E, Gariboğlu S, Otlu H. Interleukin-11, interleukin-1β, interleukin-12 and the pathogenesis of inflammatory periodontal diseases. J. Clin. Periodontol. 2008;35:365–370. doi: 10.1111/j.1600-051X.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 102.Steiner DF, et al. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity. 2011;35:169–181. doi: 10.1016/j.immuni.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang S, et al. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118:5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL. Micro-RNA-155 inhibits IFN-γ signaling in CD4+ T cells. Eur. J. Immunol. 2010;40:225–231. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar M, et al. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J. Allergy Clin. Immunol. 2011;128:1077–1085.e1010. doi: 10.1016/j.jaci.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 106.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc. Natl. Acad. Sci. USA. 2009;106:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dezerega A, Maggiolo S, Garrido M, Dutzan N. The TH17 vs. TREG imbalance in the pathogenesis of periodontitis: new approach for dichotomy TH1 vs. TH2. Rev. Clín. Period. Implantol. Rehabil. Oral. 2008;1:70–72. doi: 10.1016/S0718-5391(08)70012-0. [DOI] [Google Scholar]
- 108.Rouas R, et al. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur. J. Immunol. 2009;39:1608–1618. doi: 10.1002/eji.200838509. [DOI] [PubMed] [Google Scholar]
- 109.Sethi A, Kulkarni N, Sonar S, Lal G. Role of miRNAs in CD4 T cell plasticity during inflammation and tolerance. Front. Genet. 2013;4:8. doi: 10.3389/fgene.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu LF, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu LF, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fayyad-Kazan H, et al. MicroRNA profile of circulating CD4-positive regulatory T cells in human adults and impact of differentially expressed microRNAs on expression of two genes essential to their function. J. Biol. Chem. 2012;287:9910–9922. doi: 10.1074/jbc.M111.337154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang L, et al. MicroRNA-31 negatively regulates peripherally derived regulatory T-cell generation by repressing retinoic acid-inducible protein 3. Nat. Commun. 2015;6:7639. doi: 10.1038/ncomms8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pan W, et al. MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat. Commun. 2015;6:7096. doi: 10.1038/ncomms8096. [DOI] [PubMed] [Google Scholar]
- 115.Allam JP, et al. IL-23-producing CD68+ macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J. Clin. Periodontol. 2011;38:879–886. doi: 10.1111/j.1600-051X.2011.01752.x. [DOI] [PubMed] [Google Scholar]
- 116.Zhao L, et al. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J. Clin. Periodontol. 2011;38:509–516. doi: 10.1111/j.1600-051X.2011.01712.x. [DOI] [PubMed] [Google Scholar]
- 117.Laurence A, O’Shea JJ. TH-17 differentiation: of mice and men. Nat. Immunol. 2007;8:903–905. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 118.Mycko MP, et al. microRNA-301a regulation of a T-helper 17 immune response controls autoimmune demyelination. Proc. Natl. Acad. Sci. USA. 2012;109:E1248–E1257. doi: 10.1073/pnas.1114325109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Escobar TM, et al. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve polycomb-mediated repression. Immunity. 2014;40:865–879. doi: 10.1016/j.immuni.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seymour GJ, et al. Experimental gingivitis in humans. A histochemical and immunological characterization of the lymphoid cell subpopulations. J. Periodontal Res. 1983;18:375–385. doi: 10.1111/j.1600-0765.1983.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 121.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ. Preparation and characterization of human gingival cells. J. Periodontal Res. 1987;22:144–149. doi: 10.1111/j.1600-0765.1987.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 122.Gururajan M, et al. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010;22:583–592. doi: 10.1093/intimm/dxq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Porstner M, et al. miR-148a promotes plasma cell differentiation and targets the germinal center transcription factors Mitf and Bach2. Eur. J. Immunol. 2015;45:1206–1215. doi: 10.1002/eji.201444637. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez-Martin A, et al. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat. Immunol. 2016;17:433–440. doi: 10.1038/ni.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Yebenes VG, et al. miR-217 is an oncogene that enhances the germinal center reaction. Blood. 2014;124:229–239. doi: 10.1182/blood-2013-12-543611. [DOI] [PubMed] [Google Scholar]
- 126.Teng G, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. doi: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Yébenes VG, et al. miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J. Exp. Med. 2008;205:2199–2206. doi: 10.1084/jem.20080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cafiero C, et al. Periodontal care as a fundamental step for an active and healthy ageing. Scientific World J. 2013;2013:127905. doi: 10.1155/2013/127905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giannobile WV. C-Telopeptide pyridinoline cross-links: sensitive indicators of periodontal tissue destruction. Ann. NY Acad. Sci. 1999;878:404–412. doi: 10.1111/j.1749-6632.1999.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kinney JS, et al. Crevicular fluid biomarkers and periodontal disease progression. J. Clin. Periodontol. 2013;41:113–120. doi: 10.1111/jcpe.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Syndergaard B, et al. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014;85:e295–e303. doi: 10.1902/jop.2014.130696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Kowdley KV. MicroRNAs in common human diseases. Genom. Proteom. Bioinform. 2012;10:246–253. doi: 10.1016/j.gpb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles. 2014;3:23743. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 135.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernández-Messina L, Gutiérrez-Vázquez C, Rivas-García E, Sánchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol. Cell. 2015;107:61–77. doi: 10.1111/boc.201400081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Candia P, De Rosa V, Casiraghi M, Matarese G. Extracellular RNAs: a secret arm of immune system regulation. J. Biol. Chem. 2016;291:7221–7228. doi: 10.1074/jbc.R115.708842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Correia CN, et al. Circulating microRNAs as potential biomarkers of infectious disease. Front. Immunol. 2017;8:118. doi: 10.3389/fimmu.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmalz G, et al. MicroRNAs as salivary markers for periodontal diseases: a new diagnostic approach? Biomed. Res. Int. 2016;2016:1027525. doi: 10.1155/2016/1027525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta G. Gingival crevicular fluid as a periodontal diagnostic indicator--I: host derived enzymes and tissue breakdown products. J. Med. Life. 2012;5:390–397. [PMC free article] [PubMed] [Google Scholar]
- 142.Gupta G. Gingival crevicular fluid as a periodontal diagnostic indicator—II: inflammatory mediators, host–response modifiers and chair side diagnostic aids. J. Med. Life. 2013;6:7–13. [PMC free article] [PubMed] [Google Scholar]
- 143.Saito A, et al. MicroRNA profiling in gingival crevicular fluid of periodontitis—a pilot study. FEBS Open Bio. 2017;7:981–994. doi: 10.1002/2211-5463.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wong DTW. Salivaomics. J. Am. Dent. Assoc. 2012;143:19S–24S. doi: 10.14219/jada.archive.2012.0339. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, et al. The emerging landscape of salivary diagnostics. Periodontol. 2000. 2016;70:38–52. doi: 10.1111/prd.12099. [DOI] [PubMed] [Google Scholar]
- 146.Byun JS, Hong SH, Choi JK, Jung JK, Lee HJ. Diagnostic profiling of salivary exosomal microRNAs in oral lichen planus patients. Oral Dis. 2015;21:987–993. doi: 10.1111/odi.12374. [DOI] [PubMed] [Google Scholar]
- 147.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 148.Plaza JJP. Current roles of microRNAs in infectious diseases—advancing into healthcare. Croat. J. Infect. 2016;36:5–15. [Google Scholar]
- 149.Wang Z. The guideline of the design and validation of MiRNA mimics. Methods Mol. Biol. 2011;676:211–223. doi: 10.1007/978-1-60761-863-8_15. [DOI] [PubMed] [Google Scholar]
- 150.Goldgraben MA, Russell R, Rueda OM, Caldas C, Git A. Double-stranded microRNA mimics can induce length- and passenger strand-dependent effects in a cell type-specific manner. RNA. 2015;22:193–203. doi: 10.1261/rna.054072.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robertson B, et al. Specificity and functionality of microRNA inhibitors. Silence. 2010;1:10. doi: 10.1186/1758-907X-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3:1. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Staedel C, Darfeuille F. MicroRNAs and bacterial infection. Cell Microbiol. 2013;15:1496–1507. doi: 10.1111/cmi.12159. [DOI] [PubMed] [Google Scholar]
- 154.Siddle KJ, et al. Bacterial infection drives the expression dynamics of microRNAs and their isomiRs. PLoS Genet. 2015;11:e1005064. doi: 10.1371/journal.pgen.1005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wan J, Xia L, Xu W, Lu N. Expression and function of miR-155 in diseases of the gastrointestinal tract. Int. J. Mol. Sci. 2016;17:709. doi: 10.3390/ijms17050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 2005;7:S4–S14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Collison A, et al. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm. Med. 2011;11:29. doi: 10.1186/1471-2466-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sharma A, et al. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J. Appl. Physiol. 2012;113:459–464. doi: 10.1152/japplphysiol.00001.2012. [DOI] [PubMed] [Google Scholar]
- 159.Du C, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 160.Luck ME, Muljo SA, Collins CB. Prospects for therapeutic targeting of microRNAs in human immunological diseases. J. Immunol. 2015;194:5047–5052. doi: 10.4049/jimmunol.1403146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takahashi H, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat. Immunol. 2012;13:587–595. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei J, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. 2015;18:639–648. doi: 10.1093/neuonc/nov292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cubillos-Ruiz JR, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]