To the Editor:
Peanut allergy (PA) has a significant effect on patients' lives, and therefore an accurate diagnosis is extremely important. Peanut-specific IgE (P-sIgE) is associated with false-positive results and overdiagnosis.1 Measurement of Ara h 2–specific IgE is more accurate but is associated with false-negative results. Thus a considerable proportion of patients need to undergo an oral food challenge (OFC), the current gold standard to diagnose food allergy.2 OFCs carry the risk of causing allergic reactions, including anaphylaxis. With the advent of new treatments for PA, use of reliable in vitro tests rather than OFCs to identify eligible patients and monitor clinical response to treatment is desired.
Previously, we showed that the basophil activation test (BAT) is highly discriminative between children with PA and children with peanut sensitization but not allergy (PS children) and can reduce the number of OFCs.3 Because the BAT requires fresh blood and 10% to 15% of individuals have uninterpretable BAT results caused by nonresponding basophils (ie, basophils that do not respond to IgE-mediated but only non–IgE-mediated stimulants),4, 5 we investigated whether the ability to elicit peanut-induced cell activation could be transferred by passive sensitization of LAD2 mast cells6 with patients' plasma.
Children being assessed for PA (n = 174), including 73 children with PA, 60 PS children and 41 nonsensitized nonallergic (NA) children, underwent clinical assessment, skin prick tests, blood collection for immunoglobulin measurement (by using ImmunoCAP; Thermo Fisher Scientific, Waltham, Mass), and OFCs to peanut, as previously described.3, 7 Participants were grouped as patients with PA, PS patients, or NA subjects. The allergic reaction severity was classified according to the method of Ewan and Clark,8 and the threshold dose was determined as the total amount of peanut protein ingested during the OFC. The study was approved by the South East London Research Ethics Committee 2. Whole blood BATs and mast cell activation tests (MATs) to peanut were performed, as previously described.3, 9
Statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC) and JMP Pro software, Version 13.2.1. Depending on data distribution, nonparametric Wilcoxon tests or normality-based t tests were used, where specified. Optimal cut points were estimated from receiver operating characteristic analyses based on logistic regression models. Relationships between mechanistic outcomes were analyzed by using stratified linear models; cubic splines were used to allow for more linear curve relationships between variables. When relationships appeared linear, Pearson correlation coefficients were reported and visualized with simple linear models and 95% CIs.
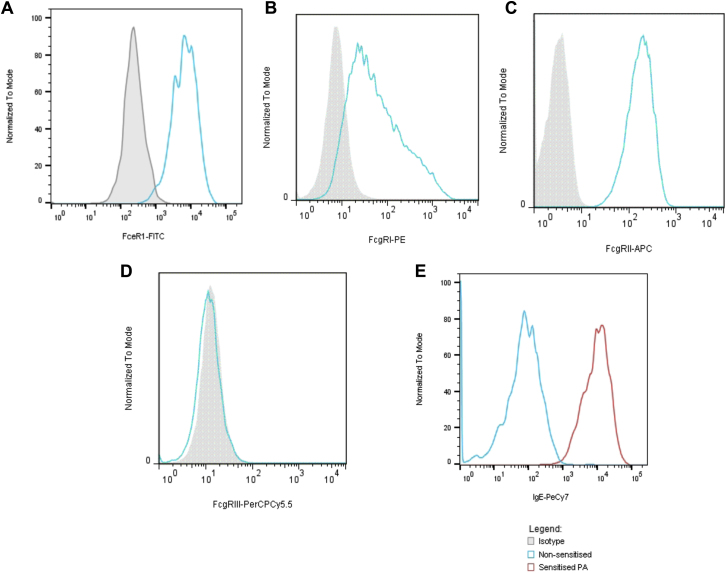
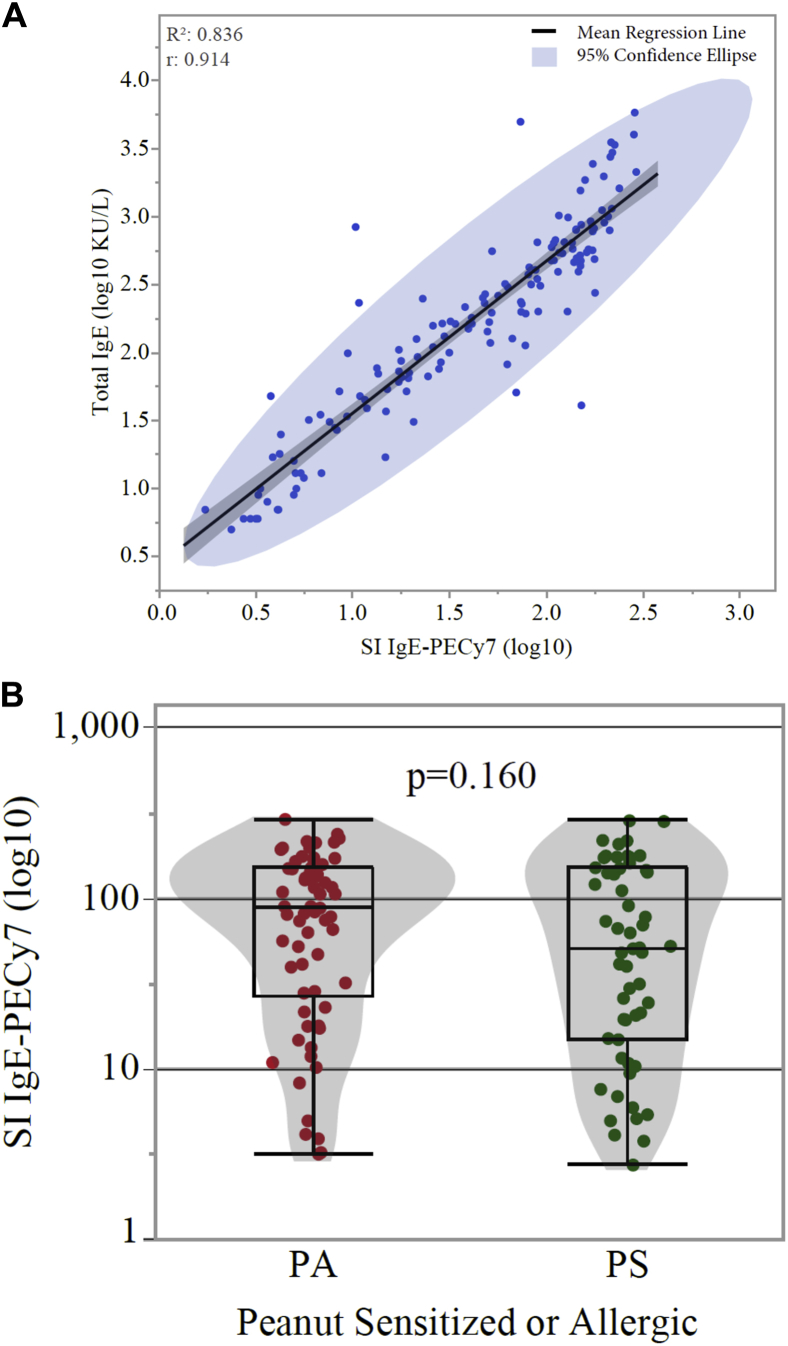
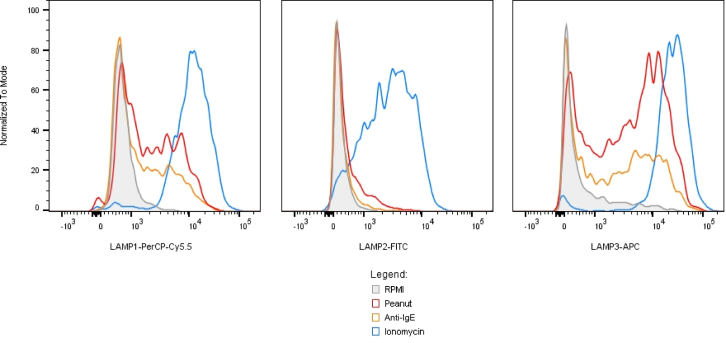
LAD2 cells expressed FcεRI and CD32 on their surfaces (see Fig E1 in this article's Online Repository at www.jacionline.org). After addition of patients' plasma, IgE was detected on the cell surface. Stimulation index (SI) IgE phycoerythrin-Cy7 was strongly correlated with plasma total IgE levels (Rs = 0.914, P < .001; see Fig E2, A, in this article's Online Repository at www.jacionline.org) and comparable between children with PA and PS children (P = .160; see Fig E2, B). LAD2 cells expressed lysosomal-associated membrane proteins after stimulation with peanut extract, anti-IgE, or ionomycin (see Fig E3 in this article's Online Repository at www.jacionline.org).
Fig E1.
A-D, Expression of FcεRI (Fig E1, A), FcγRI (Fig E1, B), FcγRII (Fig E1, C), and FcγRIII (Fig E1, D) on the surfaces of LAD2 cells. E, IgE was detectable on the surfaces of LAD2 cells after sensitization. The histogram in blue represent nonsensitized LAD2 cells, and the histogram in red represents LAD2 cells sensitized with plasma from a patient with PA. APC, Allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin-chlorophyll-protein complex.
Fig E2.
A, Correlation between IgE levels on the surface of LAD2 cells and total IgE levels in the plasma that the cells were sensitized with (Rs = 0.914, P < .001). B, Distribution of stimulation index (SI) IgE phycoerythrin-Cy7 in LAD2 cells sensitized with plasma from children with PA and PS children was not significantly different (P = .160).
Fig E3.
Expression of lysosomal-associated membrane proteins (LAMPs) on the surface of LAD2 cells after stimulation with IgE-mediated and non–IgE-mediated stimulants. LAMP-1 (CD107a) and LAMP-3 (CD63) expression increases with degranulation after stimulation with peanut extract (in red), anti-IgE (in orange), or ionomycin (in blue), whereas LAMP-2 (CD107b) expression increases with degranulation with ionomycin but not IgE-mediated stimulants. The gray shaded area corresponds to the negative control (ie, unstimulated cells). APC, Allophycocyanin; FITC, fluorescein isothiocyanate; PerCP, peridinin-chlorophyll-protein complex.
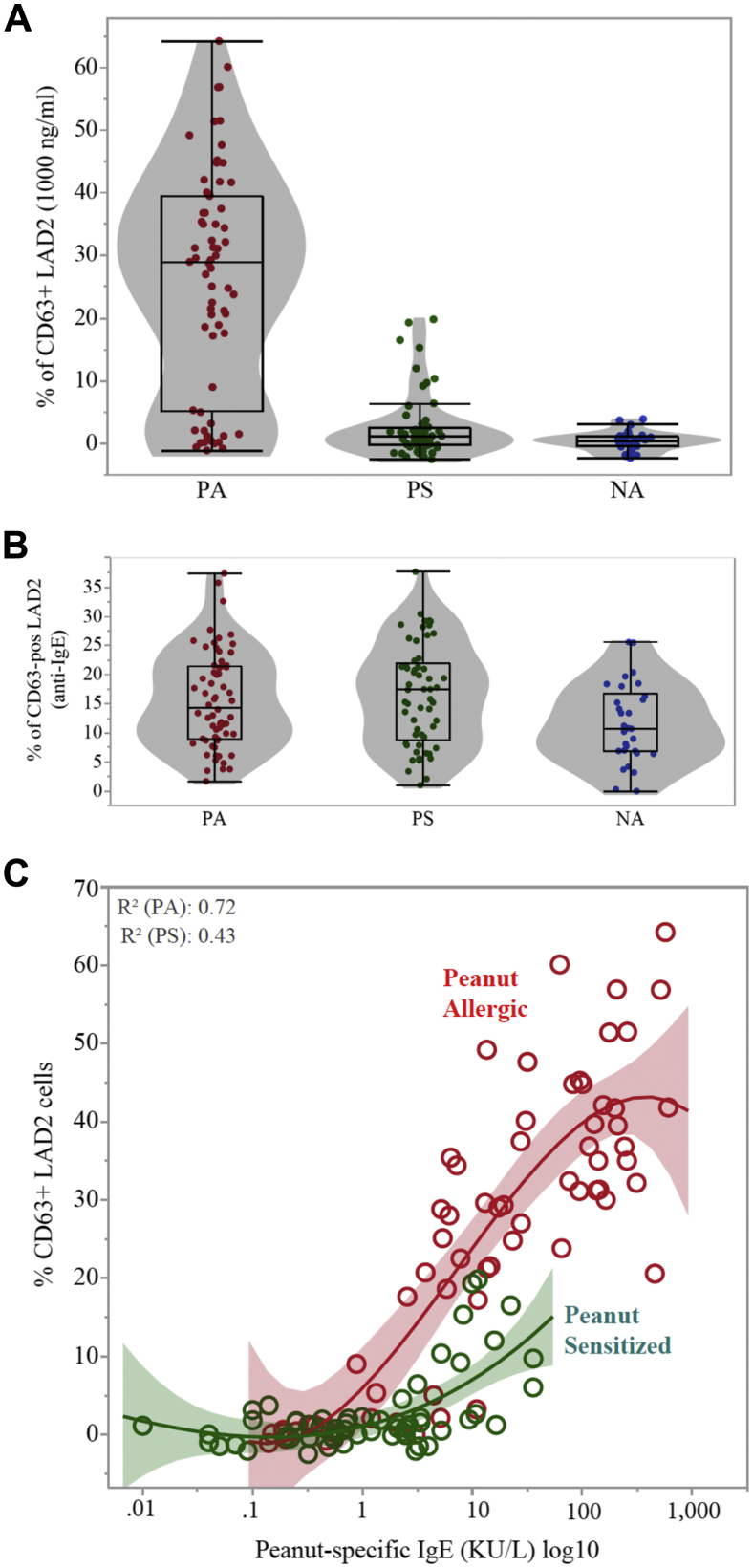
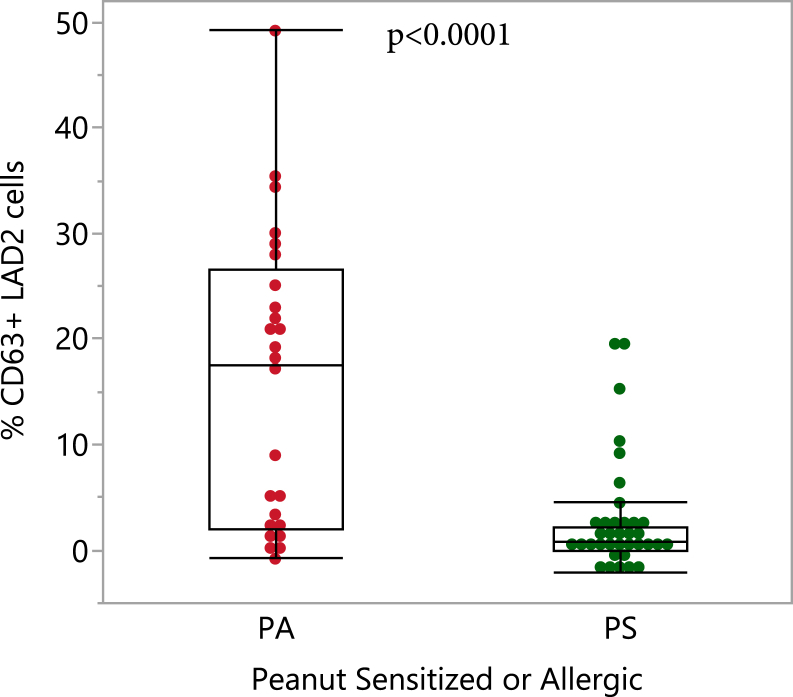
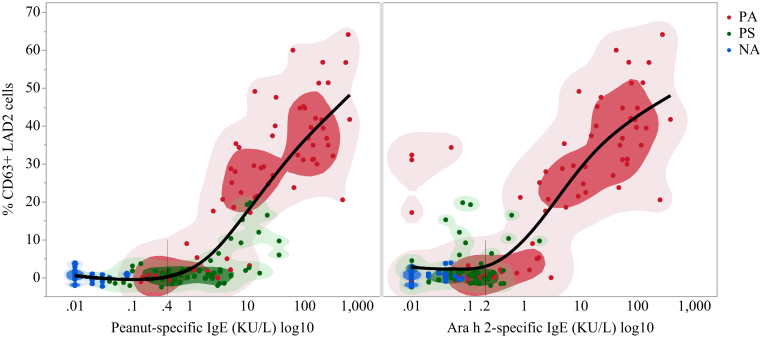
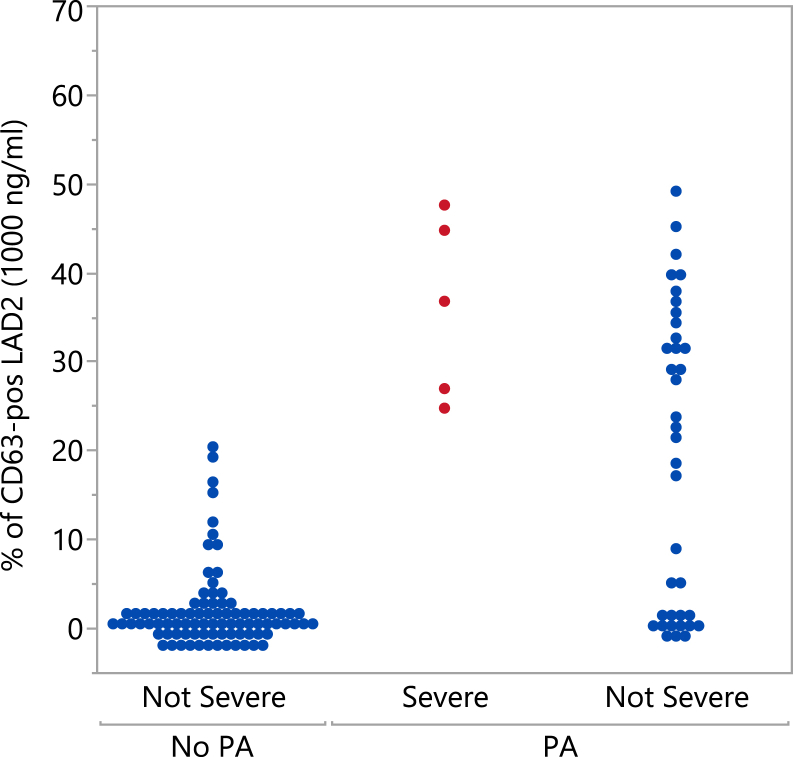
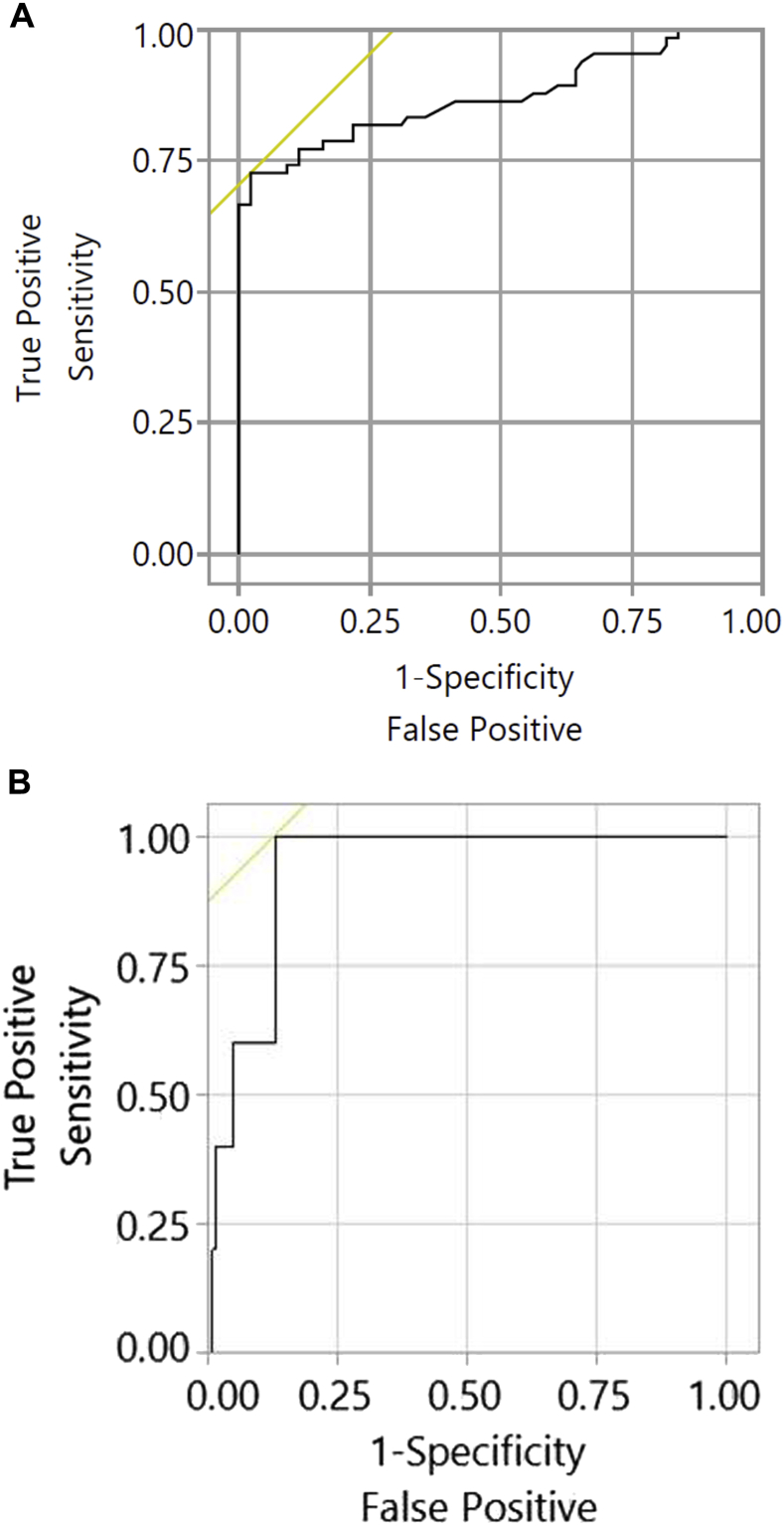
Plasma samples from children with PA, PS children, and NA children (see Table E1 in this article's Online Repository at www.jacionline.org) were tested in the MAT. Activation of mast cells sensitized with plasma from children with PA after stimulation with peanut extract was greater than activation of mast cells sensitized with plasma from PS children (P < .001) or NA children (P < .001; Fig 1, A), and the response to anti-IgE was similar (P = .543; Fig 1, B). Significant differences in mast cell activation (P < .001) were observed between children with PA and PS children, with similar levels of P-sIgE, for instance ranging between 0.35 and 15 KU/L (Fig 1, C, and see Fig E4 in this article's Online Repository at www.jacionline.org). The threshold for P-sIgE levels above which the MAT was reliable was 0.4 KU/L for P-sIgE and 0.2 KU/L for Ara h 2–specific IgE (see Fig E5 in this article's Online Repository at www.jacionline.org). The false-positive results for P-sIgE and false-negative results for Ara h 2–specific IgE are also shown in Fig E5. Patients with severe reactions had greater proportions of activated mast cells compared with patients with mild-to-moderate reactions or nonallergic patients (see Fig E6 in this article's Online Repository at www.jacionline.org). The threshold dose at which children with PA reacted during the OFC was inversely correlated with the proportion of activated mast cells (rs = −0.466, P = .0016). We analyzed the utility of the MAT to diagnose PA and to identify allergic patients at risk of severe reactions by using receiver operating characteristic curve analyses (Table I and see Fig E7 in this article's Online Repository at www.jacionline.org).
Fig 1.
Proportion of activated LAD2 cells expressed as a percentage of CD63+ cells sensitized with plasma from children with PA, PS children, or NA children and stimulated with peanut extract (1000 ng/mL; A) or anti-IgE (1 μg/mL; B) and in relation to levels of P-sIgE (C).
Fig E4.
Activation of LAD2 cells sensitized with plasma samples from children with PA and PS children containing similar levels of P-sIgE ranging between 0.35 and 15.0 KU/L (which correspond to the 95% negative predictive value and 95% positive predictive value determined for P-sIgE, respectively). PA, Peanut allergic; PS, peanut sensitized tolerant.
Fig E5.
Proportion of activated mast cells sensitized with plasma samples containing varying levels of P-sIgE and Ara h 2–specific IgE. The lower limit of specific IgE above which the mast cell activation assay is reliable was estimated for peanut-specific IgE (0.4 KU/L) and for Ara h 2-specific IgE (0.2 KU/L) by observing the beginning of the inflection point from a cubic spline with a λ value of 0.8 (where the reference line intersects the smoothed regression line). PA, Peanut allergic; PS, peanut sensitized tolerant; NA, nonsensitized nonallergic.
Fig E6.
Mast cell activation to peanut extract (1000 ng/mL) in patients with no PA (in blue at left), patients with severe allergic reactions to peanut during the OFC (in red), and patients with PA who had mild-to-moderate reactions during the OFC (in blue at right).
Table I.
Diagnostic performance of the MAT
| Diagnostic cutoffs Parameters |
Optimal cutoff = 17.2% of CD63+ LAD2 cells | Cutoff to achieve 95% PPV = 17.2% CD63+ LAD2 cells | Cutoff to achieve 95% NPV = 0% CD63+ LAD2 cells | Optimal cutoff for severity = 24.8% CD63+ LAD2 cells |
|---|---|---|---|---|
| Sensitivity (%) | 73 (61-82) | 73 (61-82) | 99 (92-100) | 100 (57- 100) |
| Specificity (%) | 98 (92-99) | 98 (92-99) | 18 (12-28) | 87 (80-92) |
| PPV (%) | 96 (87-99) | 96 (87-99) | 48 (40-56) | 24 (11- 45) |
| NPV (%) | 83 (74-89) | 83 (74-89) | 94 (73-99) | 100 (97-100) |
Ninety-five percent CIs are indicated between parentheses.
PPV, Positive predictive value; NPV, negative predictive value.
Fig E7.
Receiver operating characteristic curve for the MAT to diagnose peanut allergy (A) and to identify patients at risk of severe reactions (B). The overall area under the receiver operating characteristic curve was 0.874 for Fig E7, A, and 0.934 for Fig E7, B.
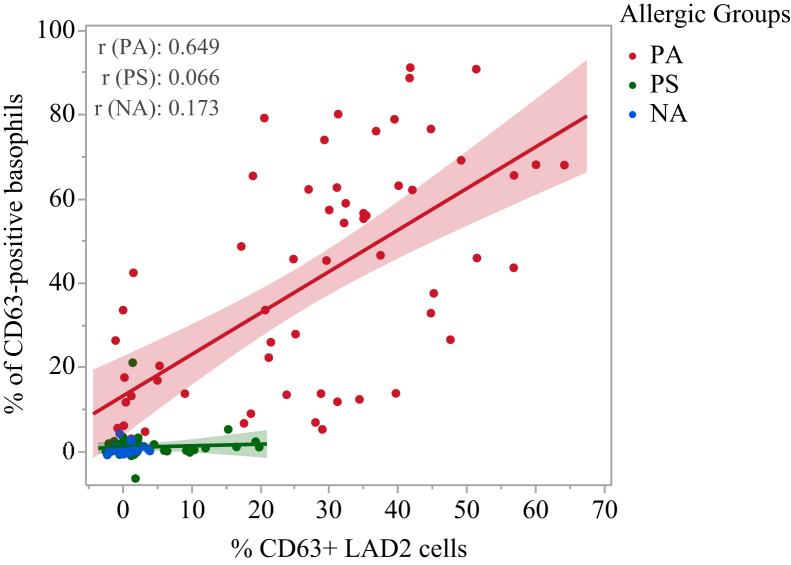
MAT results were strongly correlated with BAT results to peanut (Rs = 0.808, P < .001; see Fig E8 in this article's Online Repository at www.jacionline.org). BATs showed greater diagnostic accuracy3 compared with MATs, particularly because of their greater sensitivity; conversely, MATs provided a conclusive result for subjects with nonresponding basophils. Twelve children with PA had positive BAT and negative MAT results; these were patients with relatively low P-sIgE levels (median, 0.72; interquartile range, 0.27-2.79). Patients with nonresponding basophils all showed good response to anti-IgE and ionomycin and had an MAT result to peanut consistent with their allergic status.
Fig E8.
Correlation between MAT results and BAT results for the same patients. Rs = 0.808 and P < .001 for the whole population and when stratified by allergic groups: 0.649, 0.066, and 0.173 for the PA, PS, and NA groups, respectively.
The data reported here support the use of MATs to diagnose PA, namely in cases with equivocal P-sIgE levels, and also validate the application of the MAT as a biomarker of PA. The MAT discriminated children with PA from PS children and overcame the main limitations of the BAT because the MAT did not require fresh blood cells from the patient, thus allowing deferred testing, and provided conclusive results for all subjects with nonresponding basophils (2 of whom had PA).
Both the BAT and MAT had very high specificity when used to diagnose PA. Although the sensitivity of the BAT was superior, the enhanced specificity is the key added value of cellular tests compared with conventional serologic tests when diagnosing food allergy. The MAT can be used to diagnose PA in a sequential way when conventional tests fail, similar to what we proposed for the BAT3 and when it is either not possible to perform the BAT or the patient has nonresponding basophils.
Apart from its use for diagnostics, the MAT identified patients at risk of severe allergic reactions during OFCs. The sensitivity and negative predictive value of the MAT's optimal cutoff for severity was particularly high, with relatively lower specificity and positive predictive value, indicating that having a MAT result of greater than the cutoff does not necessarily mean the patient will have a severe reaction but that these patients would benefit from more intense educational measures and closer follow-up.
The MAT and the inhibition of MAT results9 can facilitate further study of the underlying mechanisms that determine peanut reactivity versus tolerance. This is because the MAT can be used to assess the function of allergen-specific IgE antibodies in their ability to elicit mast cell degranulation and therefore allergic symptoms, as well as the ability of antibodies of other isotypes to interfere with this effect, either by inhibiting, as shown previously for IgG4,9 or contributing to the activation of mast cells and basophils after allergen stimulation. However, this needs to be explored further. Both the BAT and the MAT are useful to test samples with equivocal P-sIgE levels to confirm PA and relay the performance of OFCs that would otherwise have positive results. Because the MAT uses plasma, which can be stored at low temperatures for long periods of time, it allows testing samples collected far from the laboratory or in the past.
The MAT is likely applicable to other food allergens. With the advent of new treatments for food allergy being approved for marketing, the MAT might prove to be a useful in vitro assay to monitor treatment response over time and to explore the mechanisms underlying the observed clinical changes during immunomodulatory treatments.
Acknowledgments
We thank Drs Dean Metcalfe and Arnold Kirshenbaum (Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md) for providing the LAD2 cells and Dr Henning Løwenstein (ALK-Abelló, Hørsholm, Denmark) for providing the peanut extract.
Footnotes
Supported by the Medical Research Council (MRC Clinician Scientist Fellowship MR/M008517/1 and MRC Centenary Early Career Award awarded to A.F.S) and the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust. H.T.B. received support (salary) from the National Institute of Allergy and Infectious Diseases/National Institutes of Health through UM1AI109565 for the statistical analyses and manuscript development.
Disclosure of potential conflict of interest: A. F. Santos and her institution received grants from the Medical Research Council (MRC; fellowship no. MR/M008517/1); her institution received a grant from the National Institute for Health Research (NIHR) and Immune Tolerance Network/National Institute of Allergy and Infectious Diseases (NIAID; grants ITN032AD and ITN049AD) and from Asthma UK; received other institutional support from MRC & Asthma UK Centre for the Mechanisms of Asthma and the UK Department of Health through the NIHR; she is employed by King's College London; she received lecture fees from Thermo Scientific, Nutricia, and InfoMed; and she received travel fees from the European Academy of Allergy and Clinical Immunology, British Society for Allergy & Clinical Immunology, Academy of Medical Sciences, Portuguese Society of Allergy and Clinical Immunology, Spanish Society of Allergy and Clinical Immunology, French Meeting of Molecular Allergology, Swiss Society of Allergy and Clinical Immunology, Dutch Symposium of Paediatric Allergology, French Society of Immunology, and GAPIC (IMM, Lisbon). H. T. Bahnson's institution received a grant from Benaroya Research Institute and ITN (UM1AI109565) and received consultancy fees from King's College London through a contract with the UK Food Standards Agency (FSA) and iFAAM. G. Lack's institution received grants from the NIAID/NIH (grants NO1-AI-15416 and UM1AI109565) and FSA and other institutional contributions from Food Allergy Research & Education (FARE), the MRC & Asthma UK Centre, the UK Department of Health through the NIHR, the National Peanut Board (NPB), and Osem; he holds board membership, consultancy fees, and stock options from DBV Technologies. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Antibody levels and BAT and MAT results of the study population
| Median (IQR) | Patients with PA | Peanut-tolerant subjects |
P value∗ | |
|---|---|---|---|---|
| Peanut-sensitized but tolerant subjects | Non–peanut-sensitized, nonallergic subjects | |||
| Total IgE (KU/L), n = 157 | 324 (116.25 to 759) | 163 (49.5 to 572) | 43.5 (12.25 to 163.75) | .0850 |
| Specific IgE (KUA/L) | ||||
| Peanut, n = 173 | 14.05 (2.15 to 133.25) | 1.10 (0.33 to 3.36) | 0.01 (0.01 to 0.02) | <.0001 |
| Ara h 1, n = 169 | 0.27 (0.02 to 27.4) | 0.07 (0.01 to 0.34) | 0.01 (0.01 to 0.01) | .0029 |
| Ara h 2, n = 169 | 5.05 (0.39 to 54.5) | 0.07 (0.04 to 0.17) | 0.01 (0.01 to 0.03) | <.0001 |
| Ara h 3, n = 169 | 0.04 (0.01 to 1.07) | 0.05 (0.02 to 0.2) | 0.01 (0.01 to 0.01) | .7153 |
| Ara h 8, n = 168 | 0.04 (0.01 to 0.68) | 0.01 (0.01 to 0.20) | 0.01 (0.01 to 0.01) | .1953 |
| Ara h 9, n = 169 | 0.01 (0.01 to 0.07) | 0.02 (0.01 to 0.14) | 0.01 (0.01 to 0.01) | .0385 |
| BAT to peanut (% CD63+ basophils at 10-100 ng/mL peanut extract), n = 157 | 36.81 (13.06 to 62.81) | 0.5 (−0.01 to 1.79) | 0.2 (−0.02 to 0.68) | <.0001 |
| MAT (%CD63+ LAD2 cells at 1000 ng/mL peanut extract) | ||||
| Peanut, n = 153 | 28.9 (5.23 to 39.55) | 1.1 (−0.30 to 2.40) | 0.4 (−0.43 to 1.20) | <.0001 |
| Anti-IgE, n = 153 | 14.4 (8.975 to 21.53) | 17.4 (8.75 to 22) | 10.75 (6.8 to 16.73) | .5244 |
Medians and interquartile ranges are indicated. BAT and MAT results were corrected for the negative control.
IQR, Interquartile range.
P values refer to the comparison between patients with PA and PS patients by using the Mann-Whitney U test.
References
- 1.Nicolaou N., Poorafshar M., Murray C., Simpson A., Winell H., Kerry G. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. doi: 10.1016/j.jaci.2009.10.008. e1-13. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J.A., Assa'ad A., Burks A.W., Jones S.M., Sampson H.A., Wood R.A. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos A.F., Douiri A., Becares N., Wu S.Y., Stephens A., Radulovic S. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134:645–652. doi: 10.1016/j.jaci.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukai K., Gaudenzio N., Gupta S., Vivanco N., Bendall S.C., Maecker H.T. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis. J Allergy Clin Immunol. 2017;139:889–899.e11. doi: 10.1016/j.jaci.2016.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann H.J., Santos A.F., Mayorga C., Nopp A., Eberlein B., Ferrer M. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70:1393–1405. doi: 10.1111/all.12698. [DOI] [PubMed] [Google Scholar]
- 6.Kirshenbaum A.S., Akin C., Wu Y., Rottem M., Goff J.P., Beaven M.A. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 7.Du Toit G., Roberts G., Sayre P.H., Bahnson H.T., Radulovic S., Santos A.F. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewan P.W., Clark A.T. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111–115. doi: 10.1016/s0140-6736(00)03543-1. [DOI] [PubMed] [Google Scholar]
- 9.Santos A.F., James L.K., Bahnson H.T., Shamji M.H., Couto-Francisco N.C., Islam S. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–1256. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]