Supplemental Digital Content is available in the text.
Keywords: atherosclerosis, hypertension, myocardial infarction, prognosis, reperfusion injury
Abstract
The rationale for our study was to investigate the pathophysiology of microvascular injury in patients with acute ST-segment–elevation myocardial infarction in relation to a history of hypertension. We undertook a cohort study using invasive and noninvasive measures of microvascular injury, cardiac magnetic resonance imaging at 2 days and 6 months, and assessed health outcomes in the longer term. Three hundred twenty-four patients with acute myocardial infarction (mean age, 59 [12] years; blood pressure, 135 [25] / 79 [14] mm Hg; 237 [73%] male, 105 [32%] with antecedent hypertension) were prospectively enrolled during emergency percutaneous coronary intervention. Compared with patients without antecedent hypertension, patients with hypertension were older (63 [12] years versus 57 [11] years; P<0.001) and a lower proportion were cigarette smokers (52 [50%] versus 144 [66%]; P=0.007). Coronary blood flow, microvascular resistance within the culprit artery, infarct pathologies, inflammation (C-reactive protein and interleukin-6) were not associated with hypertension. Compared with patients without antecedent hypertension, patients with hypertension had less improvement in left ventricular ejection fraction at 6 months from baseline (5.3 [8.2]% versus 7.4 [7.6]%; P=0.040). Antecedent hypertension was a multivariable associate of incident myocardial hemorrhage 2-day post-MI (1.81 [0.98–3.34]; P=0.059) and all-cause death or heart failure (n=47 events, n=24 with hypertension; 2.53 [1.28–4.98]; P=0.007) postdischarge (median follow-up 4 years). Severe progressive microvascular injury is implicated in the pathophysiology and prognosis of patients with a history of hypertension and acute myocardial infarction.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02072850.
Hypertension has a continuous, age-related risk of mortality from ischemic heart disease.1 At least 30% of adults have a history of hypertension in developed countries,2,3 and hypertension is independently associated with adverse cardiac outcome after acute myocardial infarction (MI).4–11 However, the mechanisms for this association are unclear. Patients who present with acute ST-segment–elevation myocardial infarction (STEMI) and a history of hypertension are older and generally have a higher burden of risk factors,11,12 except for cigarette smoking which associates with male sex and younger age.11,12 The size of infarction is a key determinant of survival post-MI, but previous studies11,12 have not found any association between hypertension status and infarct size. Therefore, the mechanisms underlying the association between hypertension status and health outcomes post-MI remain unclear.
Hypertension is a risk factor for coronary heart disease.13 The pathophysiology includes left ventricular hypertrophy,14 coronary endothelial dysfunction,15 accelerated coronary atherosclerosis,16,17 abnormal coronary artery remodeling,18 coronary microvascular dysfunction,19 and epicardial fat.20 Accordingly, preexisting coronary heart disease may predispose patients with antecedent hypertension to enhanced myocardial reperfusion injury. Severe microvascular injury within the infarct zone manifests acutely as microvascular obstruction affecting about half of all-comers with STEMI,21,22 and subsequently resolving in half of these patients by 10 days.22 In patients with persistent microvascular obstruction, progressive irreversible capillary degradation occurs leading to infarct zone hemorrhage, which is an independent predictor of death or heart failure in the longer term.21,22 To date, the associations between antecedent hypertension and microvascular injury post-MI are unclear.
We investigated the natural history of hypertension status, microvascular pathology, and prognosis in all-comers with acute STEMI. We measured microvascular function directly in the culprit coronary artery acutely using a sensor mounted on an intracoronary guidewire and noninvasively using the surface ECG. We subsequently used multiparametric cardiac magnetic resonance (CMR) to assess the evolution of infarct pathologies and left ventricular function and volumes at 2-day and 6-month post-MI.
We hypothesized that a history of hypertension would be associated with enhanced microvascular dysfunction within the culprit coronary artery acutely, and more abundant microvascular pathologies, including microvascular obstruction and myocardial hemorrhage, independent of the size of infarction, when assessed using CMR 2 days later. This hypothesis implicates microvascular damage within the infarct zone as an underpinning mechanism leading to less myocardial salvage, greater adverse left ventricular remodeling, and an increased risk of heart failure and death after an acute MI.
Methods
The data that support the findings of this study can be requested from the following URL: http://www.CORportal.net.
Study Population
We performed a prospective cohort study in a regional cardiac center between July 14, 2011, and November 22, 2012. Written informed consent was obtained from all of the participants.
A history of hypertension was prospectively recorded if patients were prescribed antihypertensive treatment or had successive blood pressure (BP) measurements that were ≥140/90 mm Hg on at least 2 different days during the index hospitalization.23 Noninvasive BP was measured in recumbent patients using an oscillometric method using an arm cuff pressure-sensitive transducer (GE CRITIKON, GE Healthcare, Amersham, United Kingdom) and automated medical patient monitoring system (DINAMAP and CARESCAPE Monitor B850, GE Medical Systems Information Technologies). Patients were categorized as having hypertension or not.
Patients were eligible if they had an indication for primary percutaneous coronary intervention (PCI) or thrombolysis for acute STEMI.24 Exclusion criteria included contraindications to CMR, for example, a pacemaker. The study was approved by the National Research Ethics Service (reference 10-S0703-28). Acute STEMI management followed contemporary guidelines24 (Methods in the online-only Data Supplement). The ClinicalTrials.gov identifier is URL: http://www.clinicaltrials.gov. Unique identifier: NCT02072850.
Electrocardiogram
A 12-lead ECG was obtained before coronary reperfusion and 60 minutes afterward. The extent of ST-segment resolution on the ECG assessed 60 minutes after reperfusion compared with the baseline ECG before reperfusion24 was expressed as complete (≥70%), incomplete (30% to <70%), or none (≤30%).
Coronary Angiogram Acquisition and Analyses
Coronary angiograms were acquired during usual care with cardiac catheter laboratory X-ray (Innova, GE Healthcare) and information technology equipment (Centricity, GE Healthcare). The angiograms were analyzed by trained observers (J. Carberry, V.T. Yue May) who were blinded to all other clinical and CMR data. The Thrombolysis In Myocardial Infarction (TIMI) coronary flow grade25 and frame count26 were assessed at initial angiography and at the end of the procedure. TIMI myocardial perfusion grade27 was assessed at the end of the procedure (Methods in the online-only Data Supplement). The TIMI frame count and perfusion grade are angiographic measures of microvascular function.
Direct, Invasive Measurement of Microvascular Function in the Culprit Coronary Artery
A coronary pressure- and temperature-sensitive guidewire (St Jude Medical, St Paul, MN) was used to measure index of microvascular resistance (IMR) and coronary flow reserve (CFR) in the culprit coronary artery at the end of PCI.28–32 The guidewire was calibrated outside the body, equalized with aortic pressure at the ostium of the guide catheter and then advanced to the distal third of the culprit artery. This thermodilution method is based on the following basic relationship: flow=volume / mean transit time. CFR is defined as the ratio of peak hyperemic to resting flow (CFR=flow at hyperemia / flow at rest). Flow is the ratio of the volume (V) divided by the mean transit time (Tmn). Thus, CFR can be expressed as follows: CFR=(V/Tmn) at hyperemia/ (V/Tmn) at rest. Assuming the epicardial volume (V) remains unchanged, CFR can be calculated as follows: CFR=Tmn at rest / Tmn at hyperemia. CFR and IMR are distinct physiological parameters. CFR reflects epicardial and microcirculatory function, by contrast, IMR is a direct invasive measure of microvascular resistance. IMR is defined as the distal coronary pressure multiplied by the mean transit time of a 3 mL bolus of saline at room temperature during maximal coronary hyperemia, measured simultaneously (mm Hg×s or units).28–32
Hyperemia was induced by 140 μg/kg per minute of intravenous adenosine preceded by a 2 mL intracoronary bolus of 200 µg of nitrate. The mean aortic and distal coronary pressures were recorded during maximal hyperemia. We have previously found IMR to be highly repeatable when assessed by duplicate measurements 5 minutes apart in 12 consecutive STEMI patients at the end of PCI.30
Laboratory Analyses
Serial systemic blood samples were obtained immediately after reperfusion in the cardiac catheterization laboratory and subsequently on the first day (06:00–07:00 hours) during the initial inpatient stay in the Coronary Care Unit.
CRP (C-reactive protein) was measured in the hospital biochemistry laboratory using a particle-enhanced immunoturbidimetric assay method (Cobas C501, Roche), and the manufacturer’s calibrators and quality control material, as a biochemical measure of inflammation. The high-sensitive assay CRP measuring range is 0.1 to 250 mg/L. The expected CRP values in a healthy adult are <5 mg/L, and the reference range in our hospital is 0 to 10 mg/L. IL (interleukin)-6 was measured using a high-sensitivity enzyme-linked immunosorbent assay (ELISA; R&D Systems, Oxon, United Kingdom).33 The limit of detection is <0.1 pg/mL, and the intra-assay coefficient of variation was 9.1%. NT-proBNP (N-terminal Pro-B-type natriuretic peptide) was measured in a research laboratory using an electrochemiluminescence method (e411, Roche) and the manufacturers’ calibrators and quality control material. The limit of detection for IL-6 and NT-proBNP are 6.5 pg/mL and 5 pg/mL, respectively. Long-term coefficient of variations of low and high controls are typically <5% and were all within the manufacturers’ range.
CMR Imaging
We used CMR to provide reference data on left ventricular function, pathology, and surrogate outcomes (Figure 1). CMR was performed on a Siemens MAGNETOM Avanto (Erlangen, Germany) 1.5-Tesla scanner with a 12-element phased array cardiac surface coil.32 The imaging protocol34,35 (Methods in the online-only Data Supplement) included cine CMR with steady-state free precession, T2-mapping,22,36,37 T2*-mapping,22 and delayed-enhancement phase-sensitive inversion-recovery pulse sequences.38 The scan acquisitions were spatially coregistered and also included different slice orientations to enhance diagnostic confidence.
Figure 1.
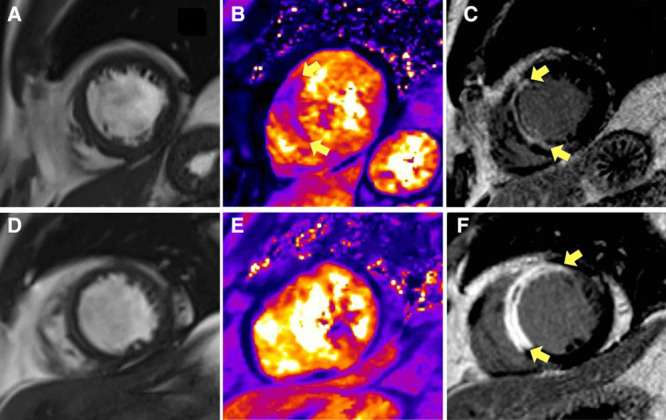
Two patients, one with a history of hypertension (A) and the other without (B) presented similarly with acute anterior ST-segment–elevation myocardial infarction and were treated by primary percutaneous coronary intervention (PCI) with stents. The antithrombotic therapies, including aspirin, clopidogrel, and unfractionated heparin, were similar. Each patient had normal antegrade flow in the culprit coronary artery (Thrombolysis In Myocardial Infarction grade 3) at the end of PCI. Multiparametric cardiovascular magnetic resonance (CMR) imaging was performed 2 d and 6 mo later. Top, A, Imaging obtained from a 52-year-old man with a history of current hypertension. The symptom-to-balloon time was 1.4 h. The coronary angiogram revealed a proximal occlusion of the left anterior descending artery. Blood pressure before coronary angioplasty was 200/125 mm Hg and measured 181/117 mm Hg postcoronary angioplasty. Two days later, CMR disclosed myocardial hemorrhage specifically revealed by T2* mapping (yellow arrows) and transmural infarction of the anteroseptal wall of the left ventricle (LV; yellow arrows) associated with microvascular obstruction revealed by contrast CMR. Invasive assessment of microvascular function using a diagnostic guidewire placed in the culprit coronary artery at the end of primary PCI indicated severe microvascular injury. The index of microvascular resistance measured 92 which is substantially increased (reference range <25). The initial infarct size was 38.9%, and the LV ejection fraction (LVEF) and LV end-diastolic volume indexed to body surface area (LVEDVi) were 48.5% and 90.2 mL/m2, respectively. Six months later, infarct size was 26.7% of LV mass, and the LVEDVi was 127 mL/m2. This is in-keeping with >20% in LVEDVi, that is, adverse remodeling. This patient went to have an unplanned admission for heart failure treatment on day 493 of follow-up. Bottom, B, Imaging obtained from a 58-year-old man with no prior history of hypertension. The symptom-to-balloon time was 2.2 h. The angiogram also revealed a proximal occlusion of the left anterior descending artery. Blood pressure before coronary angioplasty was 109/71 mm Hg and measured 99/60 mm Hg postcoronary angioplasty. Microvascular resistance in the culprit coronary artery was normal. Two days later, there was a small amount of microvascular obstruction as revealed by contrast-enhanced CMR (yellow arrows), and no evidence of myocardial hemorrhage (T2 star parametric map). The initial infarct size was 32.4%, and the LVEF and LVEDVi were 36.9% and 126.4 mL/m2, respectively. Six months later, infarct size was 15.2% of left ventricular mass, and the LVEDVi was 98.2 mL/m2. This patient had an uncomplicated clinical course.
Imaging Analyses
The CMR analyses are described in detail in the online-only Data Supplement. Left ventricular dimensions were indexed to body surface area.
Infarct Definition and Size
The presence of acute infarction was established based on abnormalities in cine wall motion, rest first-pass myocardial perfusion, and delayed-enhancement imaging in 2 imaging planes. The myocardial mass of late gadolinium (grams) was quantified using computer-assisted planimetry and the territory of infarction was delineated using a signal intensity threshold of >5 SDs above a remote reference region and expressed as a percentage of total left ventricular mass.39
Microvascular Obstruction
Microvascular obstruction was defined as a dark zone on early gadolinium enhancement imaging 1, 3, 5, and 7-minute postcontrast injection that remained present within an area of late gadolinium enhancement at 15 minutes.
Myocardial Edema
The extent of myocardial edema was defined as left ventricular myocardium with pixel values (T2) >2 SDs from remote myocardium.40–42
Myocardial Salvage
Myocardial salvage was calculated by subtraction of percent infarct size from percent area-at-risk, as reflected by the extent of edema.40–42 The myocardial salvage index was calculated by dividing the myocardial salvage area by the initial area-at-risk.
Left Ventricular Remodeling
An increase in left ventricular volume at 6 months from baseline was taken to reflect left ventricular remodeling.35
Myocardial Hemorrhage
On the T2* CMR maps, a region of reduced signal intensity within the infarcted area, with a T2* value of <20 ms22,43 was considered to confirm the presence of myocardial hemorrhage.
Prespecified Health Outcomes
We prespecified adverse health outcomes that are pathophysiologically linked with STEMI.44,45 The primary composite outcome was (1) all-cause death or first heart failure event after the initial hospitalization (Methods in the online-only Data Supplement).
Statistical Analyses
The sample size calculation and statistical methods are described in the online-only Data Supplement. All P values are 2-sided and a P value >0.05 indicates the absence of a statistically significant effect. Statistical analyses were performed using R version 2.15.1 or SAS v 9.3 or higher versions of these programs.
Results
Patient Characteristics
Of 372 patients with acute STEMI who were screened, 324 (mean age, 59 [12)] years; 237 [73%] male, 105 [32%] with hypertension) were enrolled (Table 1; Figure 2). The reasons for not being enrolled in the study are detailed in Figure 2. None of the participants had a new diagnosis of hypertension during the index admission.
Table 1.
Clinical and Angiographic Characteristics of 324 STEMI Patients Categorized According to a History of Hypertension
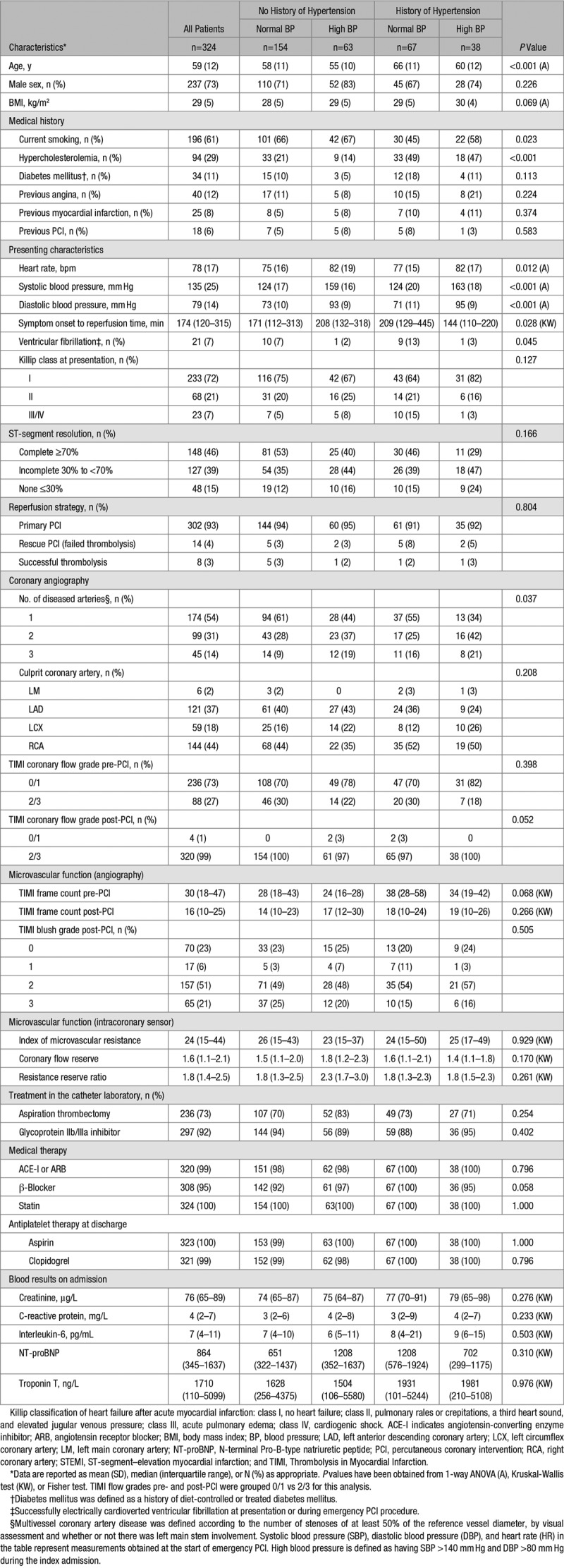
Figure 2.
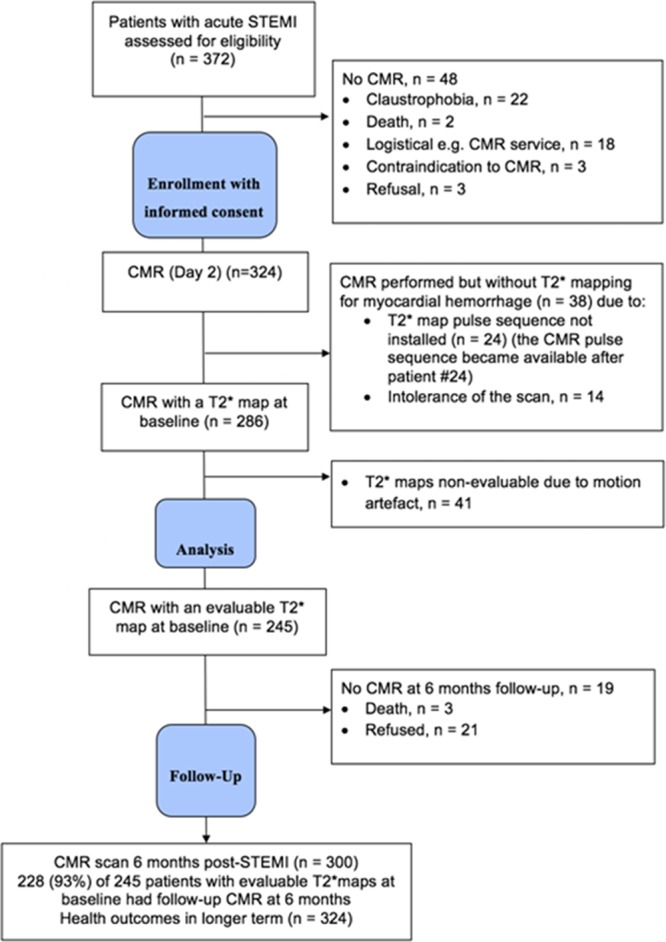
Study flow diagram of the cohort study. CMR indicates cardiovascular magnetic resonance; and STEMI, ST-segment–elevation myocardial infarction.
Compared with patients without a history of hypertension, patients with a history of hypertension were older, had a history of hypercholesterolemia more often, but a history of cigarette smoking less often, and were more likely to have ventricular fibrillation at presentation and multivessel coronary artery disease (Table 1).
Microvascular Injury in the Culprit Coronary Artery and Inflammation
Angiographic parameters of blood flow and perfusion in the culprit coronary artery, IMR, and ST-segment resolution on the ECG (none versus partial versus complete) were similar between the groups (Table 1).
On day 1, circulating CRP, IL-6, and neutrophils and monocyte levels were also similar between the groups (Table 1).
CMR Imaging Findings
Three hundred twenty-four patients underwent CMR imaging 2.0 (1.8) days later, and 300 (93%) patients had follow-up CMR at 6 months (Table 2; Figure 2). Case examples are shown in Figure 1.
Table 2.
Cardiac MRI Findings at 2 Days and 6 Months Postreperfusion in 324 STEMI Patients Categorized According to History of Hypertension
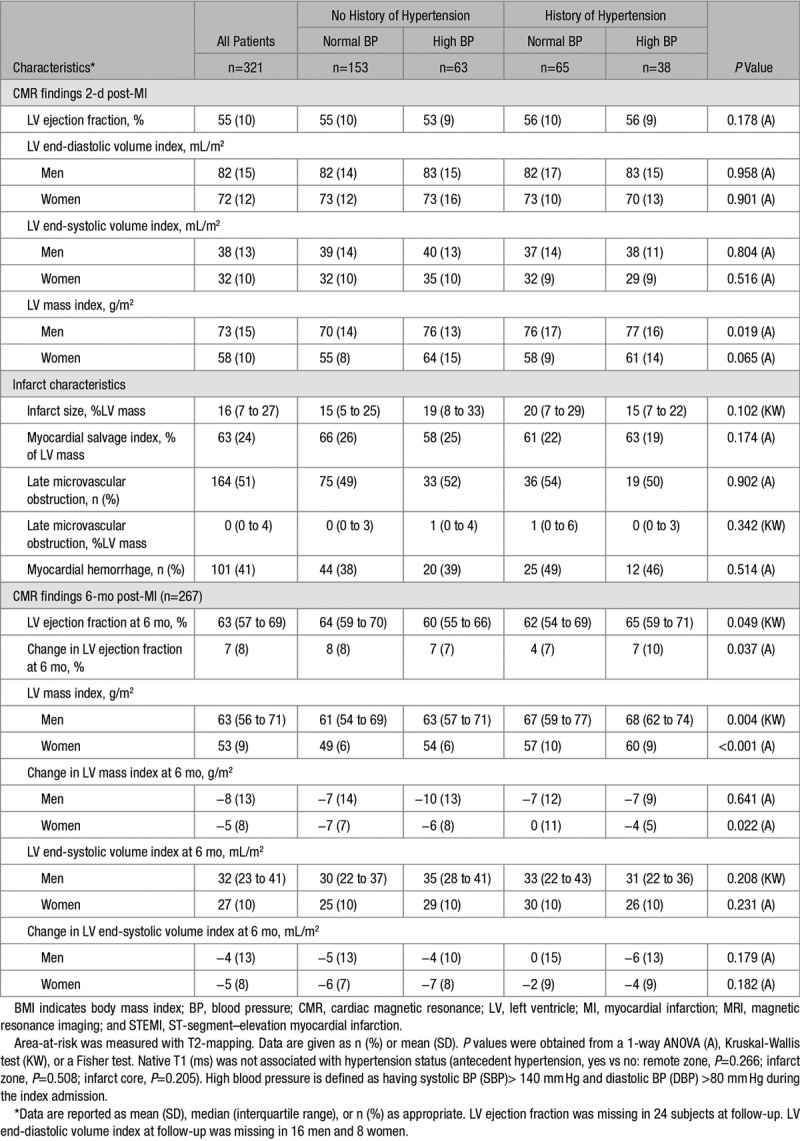
Infarct size was similar in patients with or without a history of hypertension (Table 2). However, left ventricular mass index at baseline was associated with a history of hypertension in men (Table 2) and in both men and women with hypertension at 6-month post-STEMI (Table 2).
Left ventricular ejection fraction improved in all patients, however, compared with patients without a history of hypertension, the increase in ejection fraction was less in patients with a history of hypertension (Table 2).
Sex Differences and History of Hypertension
Left ventricular mass reduced to a lesser extent by 6 months in women with hypertension compared with in women without hypertension (Table 2).
BP at Initial Presentation
We observed associations between BP at the start of emergency PCI (normal BP [systolic BP ≤140 mm Hg; diastolic BP ≤80 mm Hg]; high BP [systolic BP >140 mm Hg; diastolic BP >80 mm Hg] and clinical characteristics notably body mass index [normal BP versus high BP: 28.4 (4.8) versus 29.6 (4.7) kg/m2; P=0.042] but not vascular risk factors [smoking P=0.54]; hypercholesterolemia [P=0.59]) and microvascular dysfunction at the end of PCI as revealed by ST-segment resolution (complete, none, partial: [normal BP versus high BP] 111 [50.5%], 29 [13.2%], 80 [36.4%] versus 36 [35.6%], 19 [18.8%], 46 [45.5%]; P=0.041).
Multivariable Associations Between Hypertension and Coronary Microvascular Pathology
Myocardial Hemorrhage
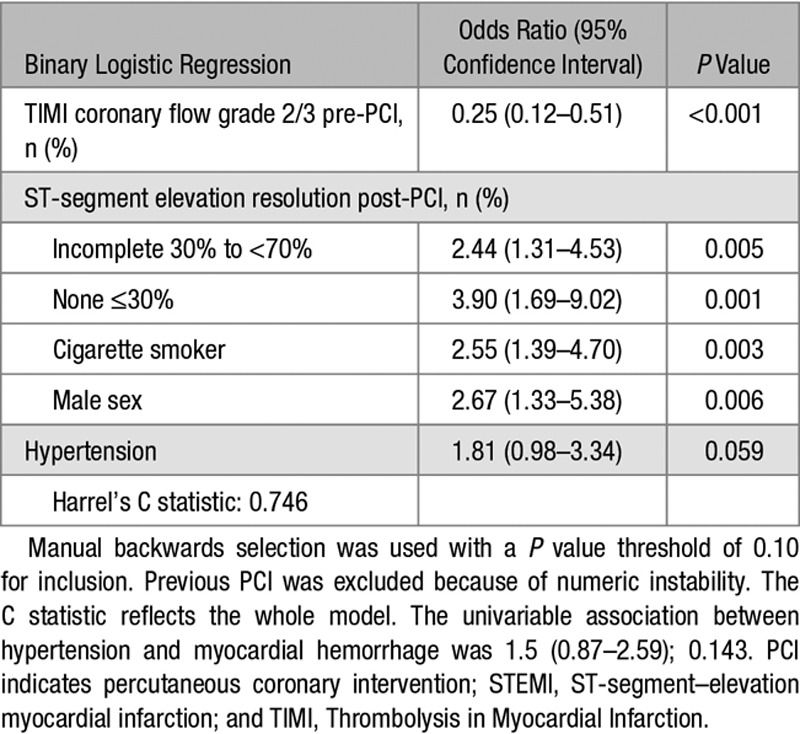
In a binary logistic regression model with baseline characteristics, a history of hypertension was a multivariable associate of myocardial hemorrhage (odds ratio, 1.81; [95% confidence interval, 0.98–53.34]; P=0.059; Table 3), albeit with wide confidence intervals.
Table 3.
Multivariable Binary Logistic Regression Model of the Associations Between Clinical Characteristics, Including a History of Hypertension (Present or Absent), and the Occurrence of Myocardial Hemorrhage (Yes or No) 2 Days Later (n=324) in Patients With Acute STEMI
Microvascular Dysfunction and Health Outcomes in the Longer Term
All (n=324) of the patients had long-term follow-up data completed. The median duration of follow-up was 1500 days (postdischarge censor duration [range] 1236 to 1801 days). Forty-seven (15%) patients died or experienced a first heart failure event during the index hospitalization or postdischarge. These events included 4 cardiovascular deaths, 11 noncardiovascular deaths, 2 deaths of undetermined cause, and 30 episodes of heart failure (Killip class 3 or 4 heart failure [n=28] or defibrillator implantation n=2). Twenty-three (7%) patients died or experienced a first heart failure hospitalization postdischarge.
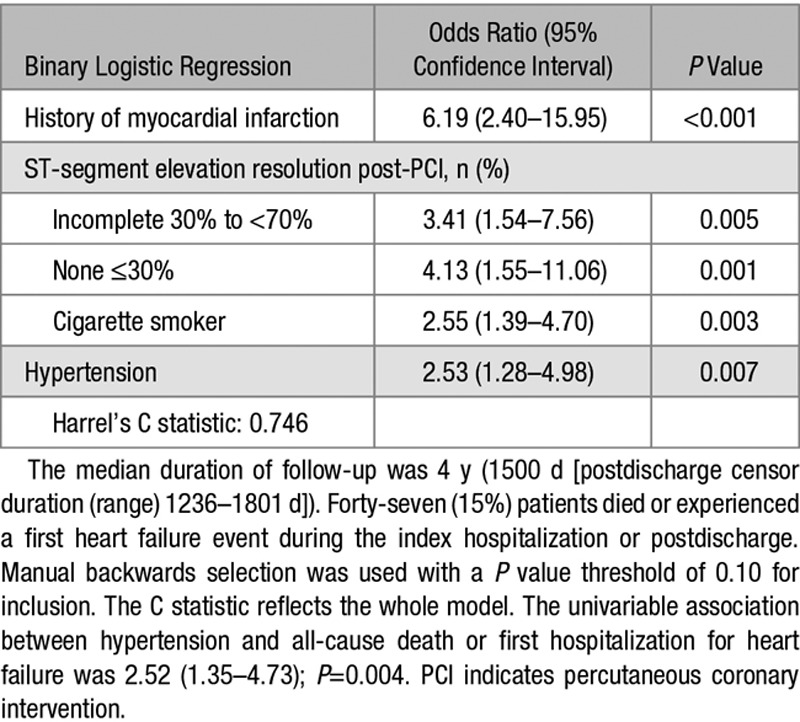
A history of hypertension (odds ratio, 2.53; [95% confidence interval, 1.28–4.98]; P=0.007) was a multivariable associate of all-cause death or heart failure (Table 4).
Table 4.
Multivariable Binary Logistic Regression Model for the Composite End Point (Yes or No) of All-Cause Death or First Hospitalization for Heart Failure, Including Clinical Characteristics (Present or Absent) at Baseline
Discussion
We have undertaken a large prospective imaging cohort study of hypertension status, microvascular pathophysiology, and long-term prognosis in patients with an acute STEMI. Uniquely, our study enrolled a high proportion of screened patients (nearly 9 of every 10 assessed), followed by serial multimodality assessments including use of invasive and noninvasive tests of reperfusion injury, circulating measures of inflammation, serial imaging of infarct pathology and remodeling, and follow-up for health outcomes over a median of 4 years in all of the study participants.
The main findings are that antecedent hypertension was associated with (1) older age and less cigarette smoking; (2) a 2-fold increased likelihood of myocardial hemorrhage, albeit with wide confidence intervals, and less improvement in left ventricular systolic function at 6 months; (3) sex differences, specifically, for the associations between hypertension status and left ventricular outcomes in women but not in men; and (4) >2-fold increased risk of all-cause death or heart failure during a median of 4 years follow-up. Antecedent hypertension was not associated with infarct size, reperfusion injury, or systemic inflammation. BP status at initial presentation was associated with age, body mass index, and reperfusion injury as revealed by ST-segment resolution at the end of the PCI procedure.
Hypertension and Prognosis After Acute STEMI
In line with prior studies,11,12 we found that a history of hypertension is associated with risk factors for cardiovascular disease including age, smoking (less common),46 and hypercholesterolemia (more common).
We also found that a history of hypertension was independently associated with an increased risk of all-cause death or hospitalization for heart failure. This result extends the evidence from previous studies. Notably, Reinstadler et al11 found that antecedent hypertension was associated with a >3-fold risk of major adverse cardiac events at 12 months in a clinical trial population of 792 patients with acute STEMI.
Antecedent Hypertension, Sex, and Remodeling
Recent studies have reported conflicting information on the associations between antecedent hypertension and sex.11,12 Changes in left ventricular ejection fraction and remodeling at 6 months, including left ventricular end-systolic volume and mass, were less favorable in women with hypertension compared with women without hypertension. The enhanced left ventricular mass in women with hypertension post-MI predisposes these individuals to adverse remodeling post-MI, and potentially, a worse cardiac prognosis in the longer term. Since the mean age of the participants was 59 years, an accelerated cardiovascular risk in postmenopausal women may be one contributing factor. Although reductions in mortality attributable to coronary heart disease have been observed in recent decades, no such decline has been observed in younger (<55 years) women.47 Further research seems warranted.
Antecedent Hypertension, Microvascular Function, and Myocardial Hemorrhage Post-MI
We studied the relationships between microvascular resistance measured directly in the culprit coronary artery at the time of the acute STEMI and antecedent hypertension. Surprisingly, hypertension was not associated with acute reperfusion injury, as revealed by direct intracoronary measurements of microvascular resistance and by angiographic parameters (TIMI frame count, TIMI blush grade) or ST-segment resolution. The potential explanations for this finding include the prior use of antihypertensive therapies, such as angiotensin-converting enzyme inhibitors, which have protective effects on vascular function,23 and the similar levels of arterial BP at the time of hospital admission in patients with a history of hypertension compared with BP levels in patients with no history of hypertension. This finding is in-keeping with the beneficial effects of both lifestyle and pharmacological measures to control BP. Because all of these parameters of coronary microvascular function are associated with prognosis post-MI,26,27,32 our findings rule out enhanced microvascular injury within the infarct zone as an explanation for the adverse prognosis in patients with antecedent hypertension.
Myocardial hemorrhage occurs in about one-third of patients with acute STEMI.21,22 This pathology reflects the end-stage consequence of irreversible microvascular dysfunction and is independently associated with adverse cardiac outcomes.21,22 In a time-course study,48 we have previously shown that myocardial hemorrhage occurs in 2 phases after coronary reperfusion. The first phase occurs acutely within 12 hours. The second phase involves new, secondary bleeds that occur between days 1 and 3 in previously unaffected patients.48 In this study, all patients who had evidence of new myocardial hemorrhage on day 3 had prior evidence of microvascular obstruction at 12 hours. We think that the temporal relationships between microvascular obstruction and myocardial hemorrhage may be relevant when considering their associations with hypertension status.
Microvascular function measured acutely and microvascular obstruction revealed by CMR 2 days later were not associated with hypertension status. However, myocardial hemorrhage, as specifically revealed by T2* mapping (Figure 1) was associated with a near 2-fold increased risk of hypertension, independent of other predictors. The result was not statistically significant and thus hypothesis generating. Cigarette smoking status is a multivariable, positive associate of myocardial hemorrhage after an acute STEMI (Table 3)48 and the inverse association between hypertension and smoking status may be a relevant confounding factor.
We undertook a time-course study with repeated assessments to assess the temporal evolution of microvascular injury acutely and then subsequently 2 to 3 days later using CMR. Long-term follow-up of this cohort permitted an analysis of the prognostic significance of microvascular injury early post-MI. Myocardial hemorrhage reflects vascular degradation and capillary leak of red blood cells. Hemorrhage within the infarct zone as revealed by CMR (Figure 1) is a direct measure of end-stage vascular injury post-MI. Our findings lead to a conclusion that despite a similar extent of acute microvascular injury and infarct size, vascular degradation at 2 days is greater in patients with a history of hypertension compared with in patients with no prior hypertension. We hypothesize that the microvessels of patients with hypertension are less capable of maintaining vascular integrity under conditions of ischemia/reperfusion injury.49 Given that hemorrhage may develop progressively in a secondary phase (days 2–3), impaired vascular homeostasis and repair potential in patients with chronic hypertension may be explanations for these results. Such patients may have preexisting coronary microvascular disease,15,16 and the microvessels subtended by the culprit artery may be less resistant to the effects of reperfusion injury (acidosis, oxidants, etc), leading to progressive capillary degradation and infarct zone hemorrhage. A susceptibility to hemorrhagic transformation within the infarct zone may provide a new mechanistic explanation for why patients with antecedent hypertension have a worse prognosis despite infarct size being similar to patients without prior hypertension.11,12 Accumulation of deoxyhemoglobin and iron within the infarct zone may serve as a mechanistic substrate for enhanced scar formation and abnormal left ventricular remodeling.50 Our results suggest that progressive microvascular damage within the infarct zone in patients with antecedent hypertension may underpin an impaired recovery potential within the heart, leading in turn to left ventricular systolic dysfunction and heart failure in the longer term.
We did not find any association between hypertension status and infarct size, as reflected by contrast-enhanced CMR and peak troponin concentration. This result is consistent with reports by Reinstadler et al11 and De Luca et al.12 We did not observe any association between hypertension status and circulating measures of inflammation. This result could potentially be explained by the anti-inflammatory effects of antihypertensive drug therapies, such as angiotensin-converting enzyme inhibitors.
Our results provide further evidence that a history of hypertension in patients with an acute STEMI is associated with a worse prognosis. In terms of clinical translation, our results highlight that patients with a history of hypertension are at an increased risk of developing heart failure. The results support further research into therapeutic strategies designed to preserve vascular integrity and repair potential within the vascular distribution of the culprit coronary artery.
Limitations
Our analysis does not permit inference on causality, and further studies are warranted. We lacked detailed information on BP history and compliance with antihypertensive drug therapy before the index hospitalization.
Perspectives
In summary, we have studied the complex relationships between hypertension status, concomitant risk factors, infarct pathology, left ventricular remodeling, and health outcomes in a large cohort of STEMI patients. We found that a history of hypertension in patients with acute STEMI is independently associated less improvement in left ventricular systolic function, notably in women, and an increased risk of all-cause death and heart failure in the longer term. An increased propensity to myocardial hemorrhage may be one mechanistic explanation, reflecting severe microvascular injury.
Our results confirm and extend previous investigations and support further research into therapeutic strategies that attenuate reperfusion injury within the infarct zone in patients with acute STEMI.
Acknowledgments
We thank the patients who participated in this study and the staff in the Cardiology and Radiology Departments. We thank Peter Weale and Patrick Revell (Siemens Healthcare, United Kingdom).
Sources of Funding
This study was supported by the British Heart Foundation (BHF) Centre of Research Excellence Award (RE/13/5/30177), the BHF Project Grant PG/11/2/28474, the National Health Service, and the Chief Scientist Office. C. Berry was supported by a Senior Fellowship from the Scottish Funding Council. P. Welsh is supported by BHF Fellowship FS/12/62/29889. A.M. Maznyczka is supported by BHF Fellowship FS/16/74/32573.
Disclosures
Based on institutional agreements with the University of Glasgow, Siemens Healthcare has provided work-in-progress imaging methods and C. Berry has acted as a consultant to Abbott Vascular. K.G. Oldroyd has acted as consultant to Abbott Vascular and Volcano Corporation. These companies had no involvement in the current research or the article. The other authors report no conflicts.
Supplementary Material
Footnotes
These authors contributed equally to this work.
This article was sent to Suzanne Oparil, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.117.10786.
Novelty and Significance
What Is New?
The pathological and prognostic significance of a history of hypertension was assessed in a reasonably large cohort of patients with acute ST-segment–elevation myocardial infarction treated by emergency percutaneous coronary intervention.
Hypertension was associated with a near 2-fold increased likelihood of myocardial hemorrhage, reflecting severe microvascular injury.
Hypertension was associated with less favorable changes in left ventricular ejection fraction and remodeling, notably in women, and hypertension was associated with a higher risk of all-cause death and heart failure.
What Is Relevant?
A history of hypertension is associated with a worse prognosis.
Patients with acute ST-segment–elevation myocardial infarction and a history of hypertension merit intensive medical management.
Summary
Our results confirm and extend previous investigations and support further research into therapeutic strategies that attenuate reperfusion injury within the infarct zone in patients with acute ST-segment–elevation myocardial infarction.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Heart Foundation. Cardiovascular Disease Statistics Factsheet (UK). https://www.bhf.org.uk/statistics. Accessed April 27, 2018.
- 4.Thune JJ, Signorovitch J, Kober L, Velazquez EJ, McMurray JJ, Califf RM, Maggioni AP, Rouleau JL, Howlett J, Zelenkofske S, Pfeffer MA, Solomon SD. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension. 2008;51:48–54. doi: 10.1161/HYPERTENSIONAHA.107.093682. doi: 10.1161/HYPERTENSIONAHA.107.093682. [DOI] [PubMed] [Google Scholar]
- 5.Fresco C, Avanzini F, Bosi S, Franzosi MG, Maggioni AP, Santoro L, Bellanti G. Prognostic value of a history of hypertension in 11,483 patients with acute myocardial infarction treated with thrombolysis. GISSI-2 Investigators. Gruppo Italiano per lo Studio della, Sopravvivena nell’Infarto Miocardico. J Hypertens. 1996;14:743–750. doi: 10.1097/00004872-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Richards AM, Nicholls MG, Troughton RW, Lainchbury JG, Elliott J, Frampton C, Espiner EA, Crozier IG, Yandle TG, Turner J. Antecedent hypertension and heart failure after myocardial infarction. J Am Coll Cardiol. 2002;39:1182–1188. doi: 10.1016/s0735-1097(02)01737-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee MG, Jeong MH, Lee KH, Park KH, Sim DS, Yoon HJ, Yoon NS, Kim KH, Park HW, Hong YJ, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Prognostic impact of diabetes mellitus and hypertension for mid-term outcome of patients with acute myocardial infarction who underwent percutaneous coronary intervention. J Cardiol. 2012;60:257–263. doi: 10.1016/j.jjcc.2012.06.003. doi: 10.1016/j.jjcc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.De Luca G, van’t Hof AW, Huber K, et al. EGYPT cooperation. Impact of hypertension on distal embolization, myocardial perfusion, and mortality in patients with ST segment elevation myocardial infarction undergoing primary angioplasty. Am J Cardiol. 2013;112:1083–1086. doi: 10.1016/j.amjcard.2013.05.053. doi: 10.1016/j.amjcard.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 9.De Luca G, Dirksen MT, Spaulding C, et al. DESERT cooperation. Impact of hypertension on clinical outcome in STEMI patients undergoing primary angioplasty with BMS or DES: insights from the DESERT cooperation. Int J Cardiol. 2014;175:50–54. doi: 10.1016/j.ijcard.2014.04.180. doi: 10.1016/j.ijcard.2014.04.180. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Hemmelgarn B, Alhaider S, Quan H, Campbell N, Rabi D. Meta-analysis of adverse cardiovascular outcomes associated with antecedent hypertension after myocardial infarction. Am J Cardiol. 2009;104:141–147. doi: 10.1016/j.amjcard.2009.02.048. doi: 10.1016/j.amjcard.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Reinstadler SJ, Stiermaier T, Eitel C, Saad M, Metzler B, de Waha S, Fuernau G, Desch S, Thiele H, Eitel I. Antecedent hypertension and myocardial injury in patients with reperfused ST-elevation myocardial infarction. J Cardiovasc Magn Reson. 2016;18:80. doi: 10.1186/s12968-016-0299-1. doi: 10.1186/s12968-016-0299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca G, Parodi G, Sciagrà R, Bellandi B, Comito V, Vergara R, Migliorini A, Valenti R, Antoniucci D. Impact of hypertension on infarct size in ST elevation myocardial infarction patients undergoing primary angioplasty. J Hypertens. 2013;31:2433–2437. doi: 10.1097/HJH.0b013e328364cbee. doi: 10.1097/HJH.0b013e328364cbee. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., III. Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–822. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 15.Brush JE, Jr, Faxon DP, Salmon S, Jacobs AK, Ryan TJ. Abnormal endothelium-dependent coronary vasomotion in hypertensive patients. J Am Coll Cardiol. 1992;19:809–815. doi: 10.1016/0735-1097(92)90522-o. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann N, Erbel R, Mahabadi AA, Kälsch H, Möhlenkamp S, Moebus S, Stang A, Roggenbuck U, Strucksberg KH, Führer-Sakel D, Dragano N, Budde T, Seibel R, Grönemeyer D, Jöckel KH Heinz Nixdorf Recall Study Investigators. Accelerated progression of coronary artery calcification in hypertension but also prehypertension. J Hypertens. 2016;34:2233–2242. doi: 10.1097/HJH.0000000000001080. doi: 10.1097/HJH.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 17.Sipahi I, Tuzcu EM, Schoenhagen P, Wolski KE, Nicholls SJ, Balog C, Crowe TD, Nissen SE. Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J Am Coll Cardiol. 2006;48:833–838. doi: 10.1016/j.jacc.2006.05.045. doi: 10.1016/j.jacc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Candemir B, Ertas FS, Ozdol C, Kaya CT, Kilickap M, Akyurek O, Atmaca Y, Kumbasar D, Erol C. Effect of hypertension on coronary remodeling patterns in angiographically normal or minimally atherosclerotic coronary arteries: an intravascular ultrasound study. Clin Exp Hypertens. 2012;34:432–438. doi: 10.3109/10641963.2012.665544. doi: 10.3109/10641963.2012.665544. [DOI] [PubMed] [Google Scholar]
- 19.Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR, Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol. 2000;35:1654–1660. doi: 10.1016/s0735-1097(00)00594-5. [DOI] [PubMed] [Google Scholar]
- 20.Homsi R, Sprinkart AM, Gieseke J, Yuecel S, Meier-Schroers M, Luetkens J, Dabir D, Kuetting D, Marx C, Nadal J, Schild HH, Thomas D. 3D-Dixon cardiac magnetic resonance detects an increased epicardial fat volume in hypertensive men with myocardial infarction. Eur J Radiol. 2016;85:936–942. doi: 10.1016/j.ejrad.2016.02.016. doi: 10.1016/j.ejrad.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Eitel I, Kubusch K, Strohm O, Desch S, Mikami Y, de Waha S, Gutberlet M, Schuler G, Friedrich MG, Thiele H. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ Cardiovasc Imaging. 2011;4:354–362. doi: 10.1161/CIRCIMAGING.110.960500. doi: 10.1161/CIRCIMAGING.110.960500. [DOI] [PubMed] [Google Scholar]
- 22.Carrick D, Haig C, Ahmed N, et al. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging. 2016;9:e004148. doi: 10.1161/CIRCIMAGING.115.004148. doi: 10.1161/CIRCIMAGING.115.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 24.Steg PG, James SK, Atar D, et al. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 25.TIMI Study Group. The thrombolysis in myocardial infarction (TIMI) trial. phase I findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 26.Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 27.Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 28.Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51:560–565. doi: 10.1016/j.jacc.2007.08.062. doi: 10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 29.McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010;3:715–722. doi: 10.1016/j.jcin.2010.04.009. doi: 10.1016/j.jcin.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Payne AR, Berry C, Doolin O, McEntegart M, Petrie MC, Lindsay MM, Hood S, Carrick D, Tzemos N, Weale P, McComb C, Foster J, Ford I, Oldroyd KG. Microvascular resistance predicts myocardial salvage and infarct characteristics in ST-elevation myocardial infarction. J Am Heart Assoc. 2012;1:e002246. doi: 10.1161/JAHA.112.002246. doi: 10.1161/JAHA.112.002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrick D, Haig C, Ahmed N, et al. Comparative prognostic utility of indexes of microvascular function alone or in combination in patients with an acute ST-segment-elevation myocardial infarction. Circulation. 2016;134:1833–1847. doi: 10.1161/CIRCULATIONAHA.116.022603. doi: 10.1161/CIRCULATIONAHA.116.022603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sattar N, Murray HM, Welsh P, et al. Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Study Group. Are markers of inflammation more strongly associated with risk for fatal than for nonfatal vascular events? PLoS Med. 2009;6:e1000099. doi: 10.1371/journal.pmed.1000099. doi: 10.1371/journal.pmed.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrick D, Haig C, Rauhalammi S, et al. Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and prognosis. JACC Cardiovasc Imaging. 2015;8:779–789. doi: 10.1016/j.jcmg.2015.03.007. doi: 10.1016/j.jcmg.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–278. doi: 10.1016/j.jcmg.2010.09.023. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470–2479. doi: 10.1016/j.jacc.2010.01.049. doi: 10.1016/j.jacc.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 41.Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu LY, Aletras AH, Arai AE. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:527–535. doi: 10.1161/CIRCIMAGING.109.900761. doi: 10.1161/CIRCIMAGING.109.900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne AR, Casey M, McClure J, McGeoch R, Murphy A, Woodward R, Saul A, Bi X, Zuehlsdorff S, Oldroyd KG, Tzemos N, Berry C. Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ Cardiovasc Imaging. 2011;4:210–219. doi: 10.1161/CIRCIMAGING.110.960450. Doi: 10.1161/CIRCIMAGING.110.960450. [DOI] [PubMed] [Google Scholar]
- 43.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 44.Thygesen K, Alpert JS, Jaffe AS, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 45.Hicks KA, Tcheng JE, Bozkurt B, et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132:302–361. doi: 10.1161/CIR.0000000000000156. doi: 10.1161/CIR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 46.Reinstadler SJ, Eitel C, Fuernau G, de Waha S, Desch S, Mende M, Metzler B, Schuler G, Thiele H, Eitel I. Association of smoking with myocardial injury and clinical outcome in patients undergoing mechanical reperfusion for ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2017;18:39–45. doi: 10.1093/ehjci/jew030. doi: 10.1093/ehjci/jew030. [DOI] [PubMed] [Google Scholar]
- 47.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. Doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Symons R, Masci PG, Francone M, Claus P, Barison A, Carbone I, Agati L, Galea N, Janssens S, Bogaert J. Impact of active smoking on myocardial infarction severity in reperfused ST-segment elevation myocardial infarction patients: the smoker’s paradox revisited. Eur Heart J. 2016;37:2756–2764. doi: 10.1093/eurheartj/ehv738. doi: 10.1093/eurheartj/ehv738. [DOI] [PubMed] [Google Scholar]
- 49.Carrick D, Haig C, Ahmed N. Temporal evolution of myocardial hemorrhage and edema in patients after acute ST-segment elevation myocardial infarction: pathophysiological insights and clinical implications. J Am Heart Assoc. 2016;5:e002834. doi: 10.1161/JAHA.115.002834. doi: 10.1161/JAHA.115.002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bulluck H, Rosmini S, Abdel-Gadir A. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging. 2016;9:e004940. doi: 10.1161/CIRCIMAGING.116.004940. doi: 10.1161/CIRCIMAGING.116.004940. [DOI] [PMC free article] [PubMed] [Google Scholar]


