Abstract
Therapeutic approaches aimed at curing prostate cancer are only partially successful given the occurrence of highly metastatic resistant phenotypes that frequently develop in response to therapies. Recently, we have described αvβ6, a surface receptor of the integrin family as a novel therapeutic target for prostate cancer; this epithelial-specific molecule is an ideal target since, unlike other integrins, it is found in different types of cancer but not in normal tissues.
We describe a novel αvβ6-mediated signaling pathway that has profound effects on the microenvironment. We show that αvβ6 is transferred from cancer cells to monocytes, including β6-/- monocytes, by exosomes and that monocytes from prostate cancer patients, but not from healthy volunteers, express αvβ6. Cancer cell exosomes, purified via density gradients, promote M2 polarization, whereas αvβ6 down-regulation in exosomes inhibits M2 polarization in recipient monocytes. Also, as evaluated by our proteomic analysis, αvβ6 down-regulation causes a significant increase in donor cancer cells, and their exosomes, of two molecules that have a tumor suppressive role, STAT1 and MX1/2. Finally, using the Ptenpc-/- prostate cancer mouse model, which carries a prostate epithelial-specific Pten deletion, we demonstrate that αvβ6 inhibition in vivo causes up-regulation of STAT1 in cancer cells.
Our results provide evidence of a novel mechanism that regulates M2 polarization and prostate cancer progression through transfer of αvβ6 from cancer cells to monocytes through exosomes.
Keywords: αvβ6 Integrin, Prostate Cancer, M2 Polarization, STAT1, Monocytes, Exosomes
Introduction
Prostate cancer is one of the leading causes of cancer-related deaths among men in the United States [1]. The disease has heterogeneous growth patterns, and its prognosis is poor when it becomes metastatic [2] or androgen-independent (castrate-resistant prostate cancer, CRPC), which remains incurable with elevated morbidity and mortality. These features highlight the pressing urgency for a better mechanistic understanding of pathways of prostate cancer progression [3, 4].
Signaling mediated by the integrin family of cell adhesion receptors has been implicated as a mechanistic driver of this disease [5-8]. Integrins are transmembrane cell adhesion receptors that are comprised of one α and one β subunit; these molecules play a key role in cellular homeostasis in normal tissues, and become de-regulated in a variety of epithelial malignancies, including prostate cancer progression to advanced disease stages [9-12], where they promote cell survival, adhesion, proliferation, and modulation of invasive phenotypes [6, 10]. In particular, the epithelial-specific αvβ6 integrin, not detectable in the normal prostate of humans or mice, is expressed at high levels in cancer [13, 14]. Taken together, these results support a pivotal role of αvβ6 as an important therapeutic target in advanced prostate cancer. Another αv-containing integrin, αvβ3, present in normal human and murine prostate at low levels, is up-regulated in primary and metastatic prostate cancer [9, 10, 15], but unlike αvβ6, αvβ3 promotes osteoblastic metastasis [16]. The αvβ6 integrin is localized in focal contacts, and functionally, mediates adhesion to fibronectin as well as latency associated peptide (LAP)-TGFβ1, promoting the release of active TGFβ1, which functions as a pro-metastatic cytokine [17], as well as an osteolytic program in prostate cancer cells [18]. The αvβ6 integrin promotes tumor growth in vivo as shown by others and us and is shown to be a therapeutic target in breast cancer and prostate cancer [14, 19]. Finally, αvβ6 correlates with poor survival in breast [19], cervical [20] and colorectal [21] cancer.
Very recently, we and others have shown that integrins, which are transmembrane glycoproteins deregulated in cancer [5, 9, 22], as well as other bioactive molecules, basement membrane assembly [23] or specific subunits partaking in a diverse number of key roles [24-28] are found in extracellular vesicles (EVs), including exosomes, which mediate interactions between the tumor, tumor microenvironment (TME) and extracellular matrix (ECM). Specifically, we have published that the αvβ6 integrin is transferred from cancer cells to recipient cells via exosomes and remains active in these cells [29]. Exosomes are small (50-150 nm) EVs present in blood, urine and the medium of cultured cells [30], different from oncosomes also found in prostate cancer [31, 32]. The field of exosome research is a fast growing area of investigation, and in the past decade exosomes have emerged as important mediators of intercellular communication, frequently involved in malignancy [29, 33]. Tumor exosomes have been shown in the serum of patients harboring a variety of tumors, correlating with advanced disease stages; their number in human blood is very abundant (109-1012/mL) with specific subsets increased in cancer [34]. There is evidence that exosomes released from cancer cells promote a pro-metastatic phenotype through extracellular matrix remodeling [35, 36], and a potential role of integrins packaged in tumor-released exosomes, in mediating advanced disease traits, has only recently begun to emerge [33, 37]. Given the failure of ipilimumab (an antibody that binds CTLA4) to improve survival in prostate cancer patients and the paucity of T cells in prostate cancer tissues [38], recent work has begun to investigate the possibility that macrophages may be key players in prostate cancer progression. In cancer, macrophages can exert both anti- and pro-tumoral functions. Macrophages are heterogeneous cells with high plasticity representing a wide spectrum of activation states, ranging from the classically activated M1 macrophages to several subsets of alternatively activated M2 macrophage. Anti-inflammatory M2 macrophages are better adapted to scavenging debris and releasing growth factors that promote angiogenesis and fibrosis [39]. Even still highly phagocytic, the major role of M2 macrophages is helping with repair of injuries by engulfing cell debris, regulating tissue re-modeling, and promoting normal cell turnover [40]. ECM pathways mediate M2 polarization, as shown in kidney whereby disruption of renal ECM structure induces instead M1 macrophage polarization suggesting an important role of the 3D architecture [41].
Activation to M2 macrophages can occur through a variety of signals, including those mediated by cytokines including IL-4, IL-10, IL-13 and TGFβ and can be promoted by the presence of glucocorticoid hormones [42]. Tumor-associated macrophages (TAMs) are the major component of the immune infiltrate of the stroma of solid tumors, representing up to 50% of the tumor mass where they can play a key role in tumor development [43, 44]. TAMs are evidently educated by the TME in that they generally acquire the hallmarks of M2 macrophages, with their associated anti-inflammatory, immune modulatory, and angiogenic properties that promote tissue remodeling [45]. Different subsets of M2 macrophages produce IL-10, TGFβ, VEGF as well as various combinations of certain factors more often associated with a classical pro-inflammatory M1 macrophage subset. Among the M2 phenotypic markers shared by TAM are the scavenger receptors CD163, CD204, CD206, and Arg-1 as well as classical macrophage markers. A team led by Dr. Pienta has promoted the idea that macrophages support prostate cancer tumorigenicity via CCL2, a chemokine known to recruit monocytes and macrophages to sites of inflammation in tumor beds [46].
Our data demonstrate that αvβ6 has profound effects on the TME. Specifically, we show that αvβ6 prevents the induction of the STAT1/MX1/2 signaling pathway in cancer cells and their exosomes, and that its down-regulation or inhibition in vivo in cancer exosomes inhibits the polarization of monocytes towards a M2 phenotype and causes up-regulation of the M2 inhibitor interferon-γ pathway. This study indicates that inhibition of this integrin and its downstream effectors might offer a novel immune – based therapeutic strategy in prostate cancer.
Results
αvβ6-positive Cancer Cell Exosomes are transferred to peripheral blood mononuclear cells (PBMC)
We have previously shown that the αvβ6 integrin is shed by prostate cancer cells packaged in EVs which, in turn, transfer this integrin to recipient αvβ6 negative cancer cells [29]. We hypothesized that this transfer to αvβ6 negative cells in the microenvironment amplifies the signals mediated by αvβ6 in the tumor. To test this hypothesis, we selected peripheral blood mononuclear cells (PBMC) as recipient cells due to their content of monocytes, the precursors of TAM. We first purified EVs from PC3 cells by differential centrifugation at 100K g as previously described [47]; then, characterized the EV using the following approaches: NTA, which confirmed that the majority of the EVs have a size of 130-150 nm; immunoblotting (IB), which shows the characteristic exosomal enrichment in CD63 and CD81 (Fig. 1) and by iodixanol density gradient separation (Fig. 2). Therefore, the isolated EVs are designated exosomes in our study. Routine IB analysis also shows that these exosomes do not express calnexin (CANX), an endoplasmic reticulum (ER) marker (Fig. 1A).
Fig. 1. Exosomal αvβ6 is transferred from prostate cancer cells to PBMC.
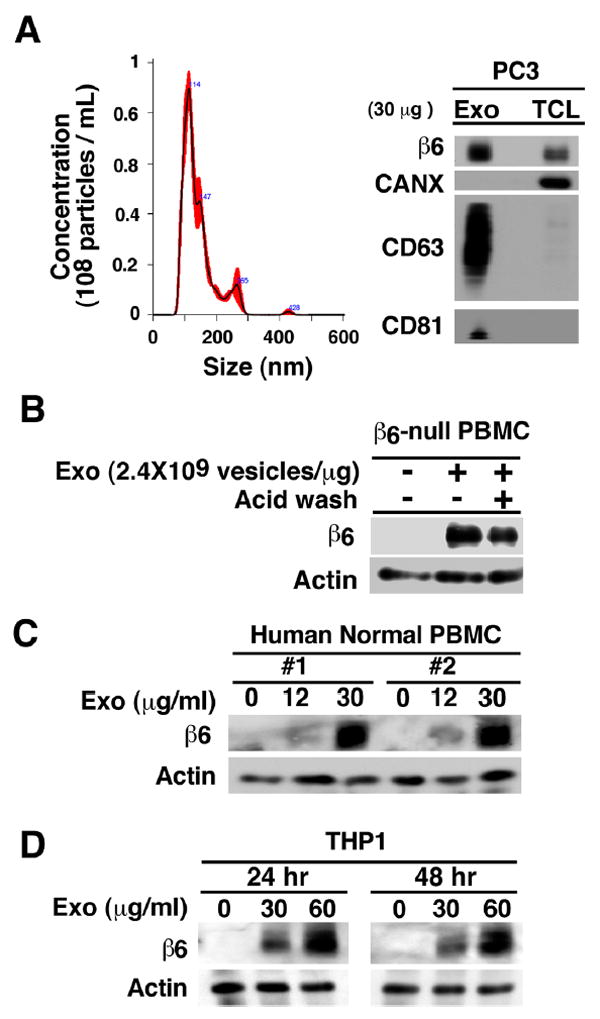
(A), Left, nanoparticle size distribution analysis of PC3 exosomes (Exo) by NTA. Right, IB analysis of β6 integrin, exosomal markers CD63, CD81 and calnexin (CANX) in lysates of PC3 Exo and cells (TCL). (B), PBMC (3.0×105 cells) derived from β6-null mice (pool of 3 mice) were incubated with PC3 Exo (30 μg/mL at a concentration of 2.4×109 vesicles/μg) for 24 hours. The cells were washed with acid wash buffer twice followed by IB analysis of cell lysates for expression of β6 integrin and actin (loading control). (C), PBMC (3.0×105 cells) from two different human healthy donors were incubated with indicated PC3 Exo concentrations (0, 12, 30 μg/mL at a concentration of 2.4×109 vesicles/μg) for 24 hours and cell lysates were analyzed by IB for expression of β6 integrin and actin (loading control). (D), THP1 cells (3.0×105 cells) were incubated with the indicated PC3 Exo concentrations (0, 30, 60 μg/mL at a concentration of 2.4×109 vesicles/μg) for 24 and 48 hours and analyzed by IB for expression of β6 integrin and actin (loading control).
Fig. 2. Transfer of GFP-tagged αvβ6 integrin to PBMC.
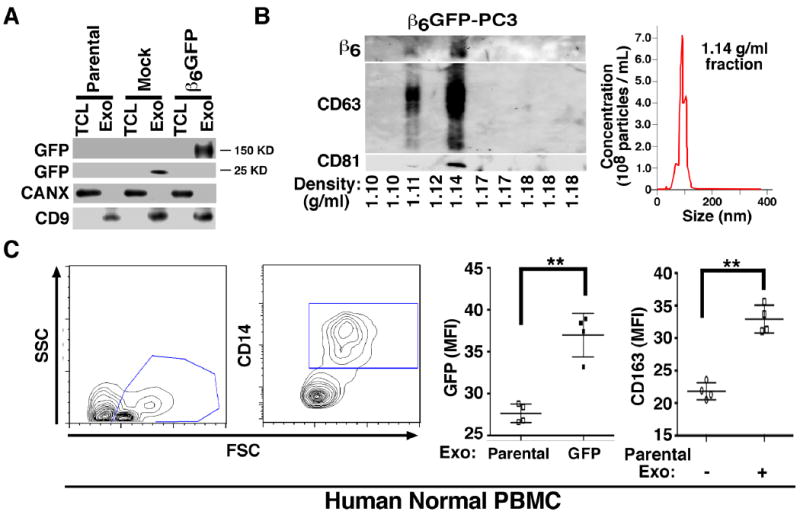
(A), IB analysis for expression of GFP, CANX and exosomal marker CD9 in parental PC3, EGFP-PC3 (Mock) and β6-EGFP-PC3 derived exosome lysate (Exo) and total cell lysate (TCL). (B), Left, Iodixanol gradient analysis of β6-EGFP-PC3 derived Exo was performed as described in the Experimental Procedures. Expression of β6, CD63, and CD81 analyzed by IB is shown. The expected density range for Exo is 1.11–1.14 g/mL. Right, NTA for the fifth fraction (density 1.14 g/mL) from the iodixanol gradient of β6-EGFP-PC3 derived Exo is shown. (C), Left, human normal PBMC (2.0×105 cells) incubated with 8 μg/mL of β6-EGFP-PC3 Exo for 36 hours, were immuno-stained for CD14. Flow cytometric contour plots of cells gated as CD14+ monocytes are shown. Middle, flow cytometric analysis of comparative expression of GFP measured as mean fluorescence intensity (MFI) in PBMC incubated with parental PC3 or β6-EGFP-PC3 Exo (8 μg/mL for 36 hours). Right, flow cytometric analysis of comparative expression of CD163 (M2 macrophage marker) measured as MFI in PBMC incubated with or without parental PC3 (8 μg/mL for 36 hours). Two graphs showing GFP and CD163 MFI include data from 4 biological replicates tested in 2 different experiments. **: P >0.01, student’s T test.
We incubated PC3 cell exosome preparations with PBMC isolated from β6-null mice (Fig. 1B). The PBMC, isolated from β6-null mice were 5.58% monocytes and 88.4% lymphocytes. The majority of the gated monocytes are CD11b-positive (Fig S1). The results obtained using PBMC from β6-null mice (Fig 1B) show that β6 is transferred to these cells and confirm that the expression of β6 integrin in the PBMC is not the result of endogenous β6 integrin synthesis. Furthermore, the results show that acid wash only slightly reduced β6 levels transferred to PBMC, indicating that the newly detected αvβ6 levels are not due to exosome attachment to the cell surface. Finally, we tested whether PBMC from two healthy donors or THP1 cells would express β6 integrin upon incubation with exosomes isolated from PC3 cells. The results show that β6 is transferred to PBMC isolated from healthy donors (n=5, Fig. 1C) and THP1 cells (Fig. 1D). The results also show that exosome transfer to PBMC occurs in a concentration-dependent manner and reaches a plateau at 24 hours. Furthermore, we transfected β6-EGFP into PC3 cells and isolated exosomes from these cells first by differential centrifugation at 100K g [29, 48] and then by iodixanol gradient separation. The preparations obtained by differential centrifugation at 100K g do not express CANX whereas they express CD9 (Fig. 2A); the fused β6-EGFP is detected at 150 KD, while the EGFP is at 25 KD. The αvβ6 positive exosomes, purified on an iodixanol density gradient, contain the exosomal markers CD63 and CD81 in fraction 5 (density = 1.14 g/mL) and have an average size of 100 nm (Fig. 2B). Finally, we incubated these purified exosomes with human PBMC isolated from two healthy donors and then, characterized the cells by FACS for CD14, GFP and CD163 expression (Fig. 2C). Our FACS analysis shows that GFP-β6 is found in CD14-positive PBMC upon exosome incubation (Fig. 2C), but not in CD14-negative cells (data not shown). Overall, these results show that exosomal β6, shed by cancer cells, is transferred to CD14-positive monocytes and is associated with their expression of CD163.
αvβ6 down-regulation in cancer cell exosomes inhibits M2 Polarization of PBMC
The fact that CD14-positive PBMC acquire αvβ6 and CD163 upon incubation with exosomes containing this integrin, led us to further examine the effect of the exosomes on monocyte differentiation, particularly since M2 polarization is known to promote tumor growth [43, 44, 49]. To more specifically demonstrate that αvβ6 contributes to M2 polarization, we down-regulated αvβ6 in the PC3 cells using saran, and isolated exosomes from these cells for treatment of human normal PBMC. We used 4 different groups of PC3 cells, which were either not incubated (NT) or incubated with the following siRNA: non-silencing (NS), or β6 integrin targeting siRNAs (D1 or D2) [14, 18, 29]. A significantly lower percentage of CD14 gated-CD163+/CD204+ cells is observed upon incubation of exosomes isolated from αvβ6 down-regulated cells (7% vs. 35%) (Fig. 3A). Fig. 3B shows that the expression of M2 polarization specific markers, CD163 and CD204 in normal CD14-positive PBMC is moderately increased by incubation with αvβ6 bearing exosomes isolated from PC3 cells transfected with a non-silencing siRNA. In contrast, treatment of the CD14-positive PBMC with exosomes from siRNA treated PC3 cells in which αvβ6 expression has been down-regulated, significantly reduces CD163 and CD204 expression. Similar results are obtained using either D1 or D2 siRNAs, two different duplexes targeting β6-integrin [14, 18]. IB analysis shows an enrichment of β6 in exosomes as compared to TCL in non-silencing controls, while a significantly reduction is observed in both TCL and exosomes upon β6 siRNA treatment (Fig. 3C). The exosome preparations utilized lack CANX (Fig. 3C).
Fig. 3. αvβ6 integrin down-regulation in exosomes inhibits recipient monocyte M2 differentiation.
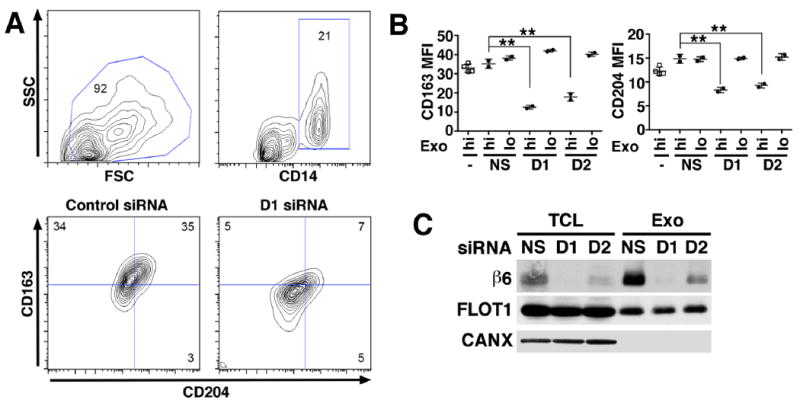
Human PBMC (2.0×105 cells) derived from two healthy donors were incubated for 48 hours with different concentrations of exosomes (Exo) (hi: 8 μg/mL; lo: 0.8 μg/mL) derived from PC3 cells incubated with no siRNA, a non-silencing siRNA (NS) or with one of two different siRNAs specific to β6 mRNA (D1 and D2). Cultures were harvested at 48 hours for M2 monocyte polarization analysis by flow cytometry. (A), Contour plots depict live gating of CD14+ monocytes and representative expression of M2 polarization markers CD163 and CD204 in this population. Numbers indicate the percentage of gated cells. (B), There are 4 different PBMC treatment groups: untreated cells (-); NS, PBMC treated with exosomes from PC3 cells transfected with non-silencing siRNA; D1, PBMC treated with exosomes from PC3 cells transfected with a β6-siRNA duplex designated D1; D2, PBMC treated with exosomes from PC3 cells transfected with a different β6-siRNA designated D2. Mean fluorescence intensity (MFI). The percentages of CD14 gated CD163+/CD204+ cells in D1-hi, and D2-hi are statistically lower than NS, as assessed by Dunnett’s test. **: P > 0.01. (C), IB analysis of β6 integrin expression in TCL and Exo derived from PC3 cells transfected with NS siRNA or with β6 siRNA (D1 and D2). Flotilin 1 (FLOT1) was used as loading control for TCL and Exo, whereas CANX was used as loading control for TCL but not Exo.
Down-regulation of the αvβ6 integrin in PC3 cells affects the exosomal proteome composition
We performed an extensive proteomic analysis of PC3 exosome lysates. We purified exosomes from these same 4 groups of cells described above and performed a comparative label-free proteomic analysis (2037 proteins). Unsupervised hierarchical clustering of protein intensities demonstrates dramatic differences between exosome proteomes (Fig. 4A) using exosomes from non-treated cells (NT) and cells treated with NS siRNA clustering together, while exosomes from cells treated with either D1 or D2 siRNA cluster together. Overall, 339 proteins are significantly affected by β6 integrin down-regulation (FDR<10%) with the majority of them being up-regulated in exosomes (n=239, 79.7%).
Fig 4. Proteomics analysis of Exosomes from PC3 cells upon β6 integrin knockdown reveals increased expression of STAT1 and MX1/2 proteins.
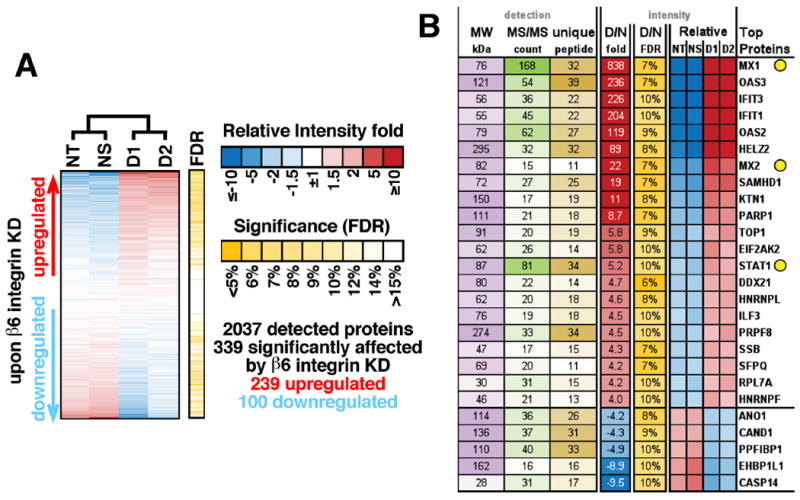
(A) Results of a proteomics experiment comparing exosomes from non-treated cells (NT), cells treated with non-silencing siRNA (NS), or two β6 integrin siRNAs (D1 and D2). Unsupervised hierarchical clustering based on label-free quantification of protein intensities is shown. Each horizontal bar is a unique protein and a total of 2,037 proteins are shown in this heat map. Red = higher, blue = lower protein abundance. (B) Heatmap of relative protein levels for the proteins most significantly affected by D1 and D2, detected with at least 10 MS/MS counts and 10 unique peptides.
The changes in protein abundance within exosomes are emphasized in our focused analysis shown in Fig. 4B. In order to find proteins most affected by the β6 integrin knockdown, we selected only robustly detected candidates (at least 10 MS/MS counts, 10 unique peptides) that significantly changed (FDR<10%) at least 4 fold. In-depth analysis shows that many signaling molecules from the interferon-γ pathway which blocks M2 in favor of M1 monocyte polarization such as STAT1, MX1, and MX2 are increased upon β6 integrin down-regulation. In fact, among all 239 proteins upregulated upon αvβ6 knockdown, we find a significant enrichment of proteins involved in interferon signaling (14 proteins, 26 fold more than expected by chance, p=1×10-10 by Fisher Exact test). MX1 levels are the most affected followed by MX2 and STAT1; they increased respectively: 838 fold, 22 and 5.2 fold. Enrichment of STAT1 in PC3 exosomes in which αvβ6 has been down-regulated appears to be specific since the levels of EGFR, another molecule known to activate STAT1 [50], do not show significant up-regulation (down-regulated 1.4 fold, p=0.094). In contrast, Protein inhibitor of activated STAT1 (PIAS1), which inhibits STAT1-mediated gene activation [51] and is increased in prostate cancer [52], is detected in cells but not in exosomes, although the levels were not changed upon αvβ6 down-regulation.
STAT1 levels in cells and exosomes increase upon αvβ6 integrin down-regulation
The proteomic results were validated by IB analysis and show that STAT1 accumulates in cells and consequently in exosomes upon down-regulation of β6 by siRNA (Fig. 5A and 5B); and that MX1/2 accumulates just in exosomes upon down-regulation of β6 by (Fig. 5C). TSG101 (Fig. 5A, top), CD81, CD63 (Fig. 5B) and CD9 (Fig. 5B and 5C) are used as exosomal markers, whereas ERK (Fig. 5A, top) and CANX (Fig. 5B and 5C) are used as loading controls for TCL. As expected, the levels of CD81, CD9 and CD63 are highly enriched in the exosome preparations as compared to TCL. The absence of CANX in the exosome preparations confirms the purity of the isolated exosome fractions (Fig. 5C).
Fig 5. β6 integrin down-regulation results in increased expression of STAT1 and MX1/2 in PC3 cells and exosomes.
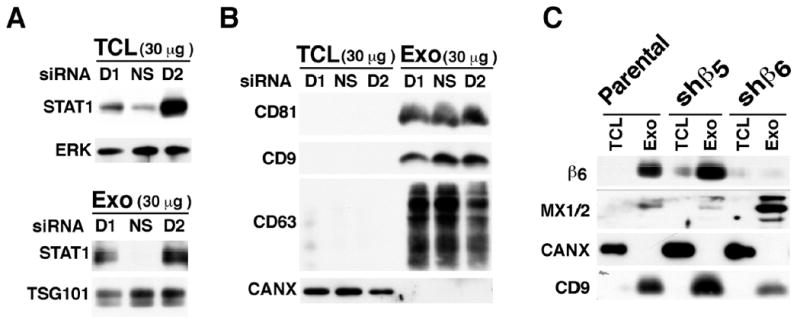
(A), IB analysis of STAT1 expression in 30 μg of exosome lysate (Exo) and total cell lysate (TCL) derived from PC3 cells incubated either with a non-silencing siRNA (NS siRNA) or with β6 mRNA directed siRNA (D1 or D2). ERK was used as loading control for TCL and TSG101 was used as loading control for Exo lysate. (B), IB analysis of the expression of exosomal markers CD81, CD9, CD63 in PC3 cell Exo lysate and TCL derived from PC3 cells incubated either with a NS siRNA or with D1 and D2 siRNA. CANX was used as loading control found in TCL but not in Exo. (C), IB analysis of the expression of β6 integrin and MX1/2 in TCL and Exo derived from parental PC3 cells or PC3 cells transfected with shβ6 or shβ5 retroviral constructs. PC3-shβ5 transfectants are used as a negative control. CANX was used as a loading control for TCL and CD9 was used as a loading control for Exo lysates.
The results in Fig 5C indicate that packaging of STAT1 into exosomes is likely a reflection of increased STAT1, but not of MX1/2, levels in the cells. However, the proteomic results were also validated by IB analysis upon blocking of β6 by 6.3G9 a monoclonal antibody (mAb) to αvβ6 and show that STAT1 and MX1/2 accumulate in cells (Fig. 6A). The 6.3G9 Ab did not affect cell viability of prostate epithelial cells, however, it did inhibit prostate cell adhesion ([14] and data not shown).
Fig 6. Inhibition of αβ increases STAT1 levels in prostate cancer cells and Ptenpc-/- mice.
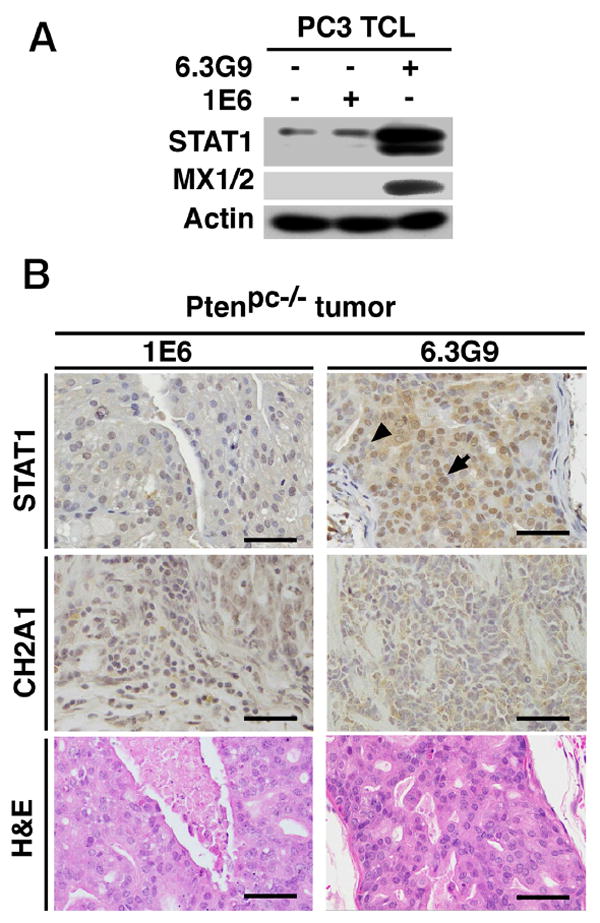
(A), Evaluation of STAT1 and MX1/2 levels by IB in TCL from PC3 cells either untreated or treated with αvβ6 monoclonal antibody 6.3G9 or isotype control antibody 1E6 (both at 10 μg/mL). Actin was used as loading control. (B), Immunohistochemical analysis of STAT1 and αvβ6 expression in prostate tumors from Ptenpc-/- mice (sacrificed at 10-13 weeks) treated with 6.3G9 or 1E6 antibodies (10 mg/Kg/week × 5 weeks; n = 5). Representative IHC and H&E images are shown (Scale bar, 100 μm). Arrow, STAT1 expression in nuclei of prostate tumor cells. Arrowhead, nuclei of prostate tumor cells lacking STAT1 expression.
These results obtained in vitro were validated in vivo. We reported previously that αvβ6 is required for prostate cancer growth in non-castrated and castrated Ptenpc-/- mice, a well-established prostate cancer model [14]. The tumor size was measured by ultrasound before Ab injection. The average volume of the tumor is 18.9 mm3 in 6.3G9 group, compared with 18.2 mm3 in 1E6 group, the isotype control mAb; T-test shows no significant difference in tumor sizes between the 6.3G9 and 1E6 groups (data not shown). Administration of 6.3G9, to Ptenpc-/- mice results in acute disruption of epithelial layers of prostate adenocarcinoma and in a significant decrease in tumor weight as compared with mice treated with 1E6. In our previous study, 6.3G9 did not show disruption of epithelial layers in normal glands [14]. In the current study, using Ptenpc-/- mice treated with an Ab to αvβ6, 6.3G9, or 1E6, we show that STAT1 accumulates in the αvβ6 expressing cancer cells (Fig. 6B). In the 6.3G9 treated tumor, 65.1% malignant cells show STAT1 staining versus only 17.9% in the 1E6 treated tumors (Fig. 6B).
To analyze the relevance of our observation that an epithelial specific integrin, αvβ6, is transferred to monocytes, we studied whether αvβ6 is expressed in vivo in monocytes from cancer patients or tumor-bearing mice. We first tested αvβ6 expression in PBMC isolated from whole blood using the Lympholyte density gradient from 14 prostate cancer patients (including 8 CRPC patients). As control, PBMC from 5 healthy donors were used. The results in Fig. 7A show a representative flow analysis. Expression of αvβ6 is found in monocytes from 9 out of 14 prostate cancer patients, and 6 out of the 8 CRPC patients, but not in monocytes from healthy donors or lymphocytes from either prostate cancer patients or healthy donors.
Fig 7. αvβ6 Integrin is expressed in PBMC from prostate cancer patients.
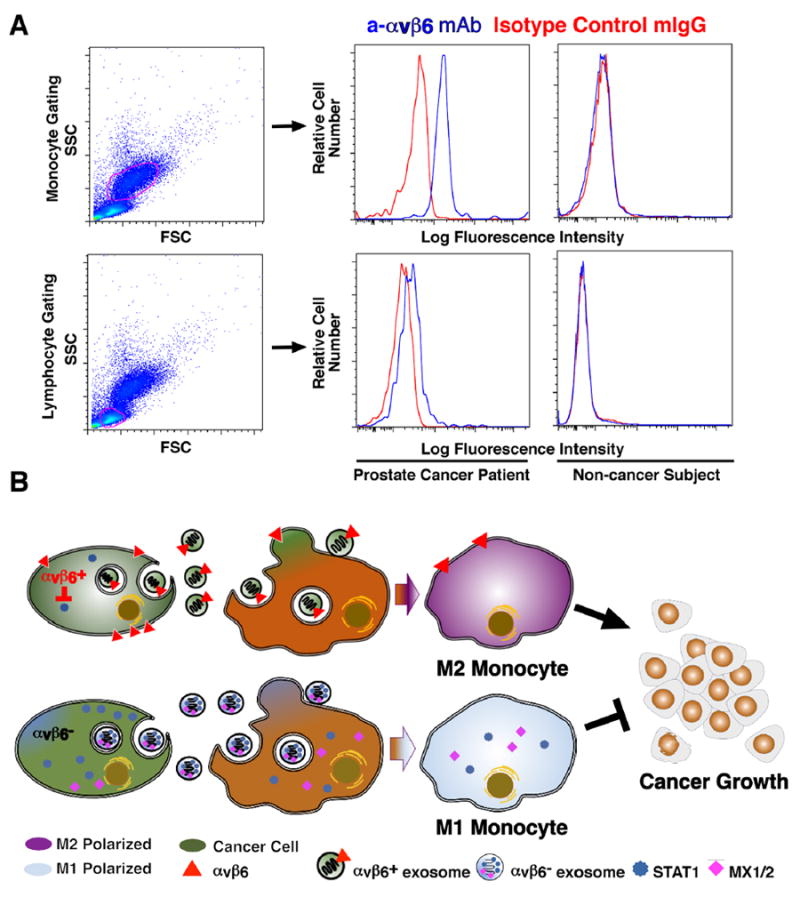
(A), Flow cytometric analysis of αvβ6 expression in PBMC from prostate cancer patients and healthy subjects. Left, monocytes and lymphocytes are gated by SSC and FSC. Right, FACS analysis of αvβ6 cell surface expression in monocytes and lymphocytes from healthy subjects and prostate cancer patients respectively, utilizing 6.3G9 monoclonal antibody to αvβ6 and mouse IgG, as isotype control. Representative data are shown. (B), The schematic diagram shows that transfer of exosomal αvβ6 integrin from prostate cancer cells to monocytes results in down-regulation of STAT1 and MX1/2 levels and increased M2 polarization of monocytes, and has a pro-tumorigenic effect, whereas transfer of αvβ6 integrin–negative exosomes results in increased M1 polarization of monocytes, and inhibits cancer growth
We then studied whether αvβ6 is expressed in monocytes from tumor-bearing mice; as control, PBMC from normal mice were used. For this analysis, we tested αvβ6 expression in PBMC isolated from whole blood using the Lympholyte density gradient from 11 Ptenpc-/- mice, and 9 Pten wild-type mice (Fig S2). Expression of αvβ6 is not found in monocytes from normal mice although its expression levels are variable (Fig. S2). As observed in human PBMC, αvβ6 is not detectable in lymphocytes from Ptenpc-/- or wild-type mice, suggesting a monocyte specific uptake of the αvβ6 exosomes.
In conclusion, based on our results, the model proposed here in Fig. 7B is as follows: αvβ6-positive or αvβ6-negative exosomes are released by cancer cells and transferred to monocytes, thus respectively supporting or, rather, preventing M2 polarization in favor of M1 macrophage polarization. Consequently, by down-regulating or inhibiting αvβ6 in cancer cells, we expect that increased STAT1/MX1/2 levels in cells, exosomes and monocytes will be generated and an anti-tumor effect will be obtained.
Discussion
In this study, we demonstrate that the αvβ6 integrin has profound effects on the microenvironment by preventing induction of the STAT1/MX1/2 signaling pathway in donor cancer cells and their exosomes. In this regard, we show that αvβ6 positive exosomes are transferred to monocytes and promote M2 monocyte polarization, whereas exosomes from cancer cells, in which αvβ6 has been down-regulated or inhibited, carry high levels of STAT1/MX1/2 and inhibit M2. Finally, we demonstrate that αvβ6 inhibition in vivo causes up-regulation of the STAT1 signaling pathway in cancer cells.
The effect of the αvβ6 integrin on STAT1 has significant implications since STAT1 plays a critical role in tumorigenesis by controlling a complex array of activities and functions [53]. In response to cytokines and growth factors, including interferon (IFN)-alpha, IFN-gamma, EGF, PDGF and IL6, STAT1 gets activated by receptor-associated kinases and then forms homo- or heterodimers that translocate to the nucleus and act as transcriptional activators. In many types of tumors, STAT1 induces anti-proliferative genes that directly block tumor growth; it is generally considered as tumor suppressor [54] given its ability to induce immune effector genes which block cell cycle progression [55] or inhibit angiogenesis [56]; in prostate cancer, loss of STAT1 is associated with poor prognosis [57]. In some instances, STAT1 promotes carcinogenesis and tumor survival in particular tissues or as a result of cross-talking with other signaling pathways [58]; however, its dominant effect is likely to promote an anti-tumor immune response given its ability to induce effector expression in immune cells [53]. The effect of αvβ6 integrin on MX1/2 also has significant implications since MX1 expression has been reported to be dependent on STAT1 signaling [59], and MX2, a member of the family of dynamin-like large GTPases, is also interferon-inducible [60] and thus plays a role in the immune response. Only a limited number of studies have examined the role of MX1/2 in cancer [61, 62]. MX1 expression has been shown to inversely correlate with prostate cancer and gain of MX1 expression in prostate cancer cells results in cell cycle arrest [61]. Although limited in number, these studies suggest a tumor suppressive role for MX1/2 in prostate cancer. Therefore, by revealing a cross-talk between cancer cells and monocytes mediated by the exosomal αvβ6 and by its inhibitory activity on the STAT1 - MX1/2 pathway, this study indicates that inhibition of this integrin and its downstream effectors might offer a novel immune – based therapeutic strategy in prostate cancer.
Finally, we demonstrate that in the absence of αvβ6, tumor cell exosomes inhibit monocyte M2 polarization. This finding and the knowledge that M2 macrophages are often phenotypically and functionally similar to TAM and support prostate cancer tumorigenicity, provide a strong support for our model (Fig. 7) which illustrates that transfer of integrins, specifically of αvβ6, from cancer cells to monocytes regulates monocyte M2 polarization. The mechanism described here may have wider significance for various types of cancer and may explain how cross-talk between cancer cells and surrounding normal cells, such as leukocytes, may also be mediated by the uptake of exosomal integrins.
Based on our novel mechanistic studies, showing that transfer of exosomes from αvβ6-positive PC3 cells to monocytes drives M2 polarization while transfer of exosomes from αvβ6-negative PC3 cells causes inhibition of M2 polarization, we propose that transfer of integrins, specifically of αvβ6, or integrin–regulated downstream effectors from cancer cells to monocytes promotes prostate cancer progression toward a CRPC phenotype. An additional innovative aspect is that we show for the first time that cancer cell integrins control monocyte response, an area that has begun to be studied; so far, only one study on αvβ3 integrin’s role in leukocytes in breast cancer [63] has been published, but a very different mechanism has been proposed since the authors have studied αvβ3 endogenously expressed in leukocytes. Furthermore, since in this study, αvβ3 is shown to have an antitumor effect and promote M1 polarization and STAT1 activation, we can speculate that β6 competes for β3 for binding to the αv subunit and causes a M2 promoting effect. We have reported previously that αvβ6 promotes prostate cancer progression by activation of androgen receptor (AR) in absence of androgen [14]. Androgen blockade in tumor cells, either by castration or MDV 3100 treatment, induces the expression of macrophage colony stimulating factor 1 (CSF-1) and other cytokines, recruits and promotes a M2 phenotype in TAMs [64]. Inhibition of endogenous AR in macrophages results in activation of CCL2/CCR2 and STAT3 and the recruitment of macrophages promotes prostate cancer metastasis [65]. Given the fact that these observations were obtained in androgen blockade setting and αvβ6 promotes a castration resistance mechanism, we may further speculate that the regulatory effect of αvβ6 on prostate cancer is partially mediated by macrophages.
Overall, these studies will pave the way for innovative therapeutic approaches in prostate cancer whcih will block the transfer, or uptake of αvβ6-containing exosomes, to monocytes. Since it is currently believed that the epithelium-specific αvβ6 integrin functions only in cells that synthesize it, we have now provided evidence for its ability to act in cells that do not normally synthesize it. In this context, αvβ6 signaling has been linked to metastasis and bone lesions [18], suggesting that the delivery of this integrin via exosomes may play a role in transferring its related function to monocytes, and that interfering with it, will inhibit CRPC. It may also be speculated that, as for the αvβ3 integrin, the bending and unbending conformational changes of αvβ6 regulated by tensile forces exerted by the exosome structure may affect the vesicle content [66]. As integrins are multifunctional receptors implicated in prostate cancer maintenance, we have shown that their transfer via exosomes from prostate cancer cells to cells in the TME may activate signaling mechanisms important for disease progression. In turn, this may open new therapeutic prospects for patients with advanced, metastatic CRPC, as disruption of integrin-dependent signaling may be expected to inhibit a host of downstream pathways controlling survival mechanisms of protection that promote therapy-resistance. Similarly, a recent study shows that anti-myeloma chemotherapy enhances secretion of tumor cell exosomes, which are rich in heparanase and regulate cytokine expression of macrophages [67]. Future studies will reach a comprehensive mechanistic understanding of integrin-directed signaling in cell-cell communication in prostate cancer progression and open new possibilities for diagnosis and risk stratification, as well as therapy-resistance, in prostate cancer patients [3].
Experimental Procedures
Reagents and Antibodies (Abs)
Lympholyte®-Mammal (Cedarlane CL5115) was used for the isolation of PBMC and Lympholyte®-Human (Cedarlane CL5015) was used for the isolation of human PBMC. The resulting cell populations demonstrate a high, and non-selective recovery of viable lymphocytes and monocytes. Optiprep (Sigma Aldrich, 1556) was used to generate iodixanol density gradient by ultracentrifugation.
Flow cytometry analysis was performed using the following Abs: mouse monoclonal abs: 6.3G9 against human and mouse αvβ6 integrin from Biogen was described previously [14, 29], Alexa Fluor 488® conjugated CD14 (BD Biosciences, 562689), PE-conjugated CD204 (BD Biosciences, 566251), APC conjugated CD163 (R&D Systems, 215927). FITC conjugated rat against mouse IgG (Biolegend, 406605) was used as a secondary Ab. A rabbit polyclonal Alexa Fluor 488® conjugated F(ab’)2 against mouse IgG (Thermo Scientific, A-21204) was used also as a secondary Ab. Purified non-immune mouse IgG (Pierce, 31903) was used as an isotype control.
The following Abs were used for IB analysis: mouse monoclonal Abs: 6.2A1 against human and mouse αvβ6 (1), CD9 (Santa Cruz, sc18869), CD63 (Abcam, ab8219), CD81 (Abcam, ab23505), MX1/2/3 (Santa Cruz, sc166412). Rabbit polyclonal Abs against: FLOT1 (Abcam, ab41927), TSG101 (Abcam, ab30871), human and mouse STAT1 (Santa Cruz, sc-346), ERK1 (Santa Cruz, sc-93), CANX (Santa Cruz, sc-11397), actin (Sigma Aldrich, A2066). The same Ab against STAT1 (Santa Cruz, sc-346) was used for immunohistochemistry (IHC) analysis for mouse tissue. Ch2A1 was used for αvβ6 IHC on mouse tumors, as described previously [14].
Control siRNA, D1 and D2 β6 siRNA duplexes (IDT Incorporated) were described previously [14, 29].
Cells and Culture Conditions
PC-3 cells, a prostate cancer cell line and THP-1 cells, a human monocyte cell line were cultured according to American Type Culture Collection recommendations (ATCC). PBMC isolated from mouse and human blood were cultured as previously described [68].
To obtain β6-EGFP PC3 and EGFP-PC3 cell lines, PC3 prostate cancer cells (grown in RPMI supplemented with 10% heat-inactivated FBS and 100 U/mL penicillin) were transfected with EGFP-N3 plasmid (a kind gift from Dr. Fabienne Paumet, Thomas Jefferson University) and used as negative control or β6-EGFP-N3 plasmid having EGFP-tag in the C-terminus of β6-cDNA on plasmid backbone (Addgene plasmid #13593, generated by Dr. Dean Sheppard, UCSF school of medicine, San Francisco, USA) using the Lipofectamine 2000 reagent (Thermo Fisher Scientific) at a ratio of 3 μl Lipofectamine/μg of DNA. Single colonies were obtained by G418 antibiotic selection (1,000 μg/mL) and expanded for screening of GFP expression using a fluorescence microscope. Pooled populations of stably transfected cells were generated (β6-EGFP-PC3 and EGFP-PC3) and confirmed for expression of β6-integrin and GFP by IB. The PC3-shβ5 and PC3-shβ6 cells and down-regulation of exosomal β6 Integrin using siRNA duplexes, D1 and D2, or non-specific control siRNA were described previously [69]. PC3-shβ5 transfectants were used as control.
Human Samples
Prostate cancer patient (n = 14) blood samples were discarded specimens obtained from patients at the Jefferson Medical Oncology Clinic. Age-matched healthy donors (n = 5) volunteered to provide samples with written consent documented. All specimens were collected in accordance with a Jefferson IRB approved protocol, coded, and de-identified before processing.
PBMC Isolation
Mouse blood was obtained via cardiac puncture after euthanasia and mixed with Anticoagulant Citrate Dextrose (ACD) Solution (1.4g citric acid, 2.5g sodium citrate, 2.0 glucose per 100 mL) at 40 μL ACD per 1mL blood sample. The anticoagulated blood was diluted with 1 volume of PBS then the mixture was layered over Lympholyte®-Mammal in a ratio of volume 4:3, followed by room temperature centrifugation at 800 g for 30 minutes without brake. The PBMC containing buffy coat were collected, washed with PBS, and resuspended in complete media for further processing.
PBMC were obtained from EDTA treated human blood following the same methodology, but blood was withdrawn via venipuncture and Lympholyte®-Human was used in a ratio of volume 2:1. Eleven Ptenpc-/- mice (52-80 week old) and 9 Pten wild-type mice (40-60 week old) were used for PBMC isolation.
Flow Cytometry Analysis
Flow cytometric phenotyping of murine and human PBMC was performed as previously described [68]. All flow data were acquired using an LSR ii flow cytometer (BD Biosciences), and were analyzed using FlowJo® software (FlowJo, LLC).
Label-free Quantitative Analysis by Mass Spectrometry
Equal amounts of proteins from exosome and cell lysates were run on a 12% SDS gel for 0.5 cm and stained with colloidal blue. Gel lanes were excised and digested with modified trypsin (Promega). Tryptic peptides were analyzed by LC-MS/MS on a Q Exactive Plus mass spectrometer (Thermo Scientific) coupled with a Nano-ACQUITY UPLC system (Waters). Samples were injected onto a UPLC Symmetry trap column (180 μm i.d. × 2 cm packed with 5 μm C18 resin, Waters), and tryptic peptides were separated by RP-HPLC on a BEH C18 nanocapillary analytical column (75 μm i.d. × 25 cm, 1.7 μm particle size, Waters) using a 4 hour gradient. Eluted peptides were analyzed by the mass spectrometer set to repetitively scan m/z from 400 to 2000. The full MS scan was collected at 70,000 resolution followed by data-dependent MS/MS scans at 17,500 resolution on the 20 most abundant ions exceeding a minimum threshold of 20,000. Peptide match was set as preferred; exclude isotopes option and charge-state screening were enabled to reject single and unassigned charged ions. MS data were analyzed with MaxQuant 1.5.2.8 software [70]. MS/MS data were searched against the human UniProt protein database (July 2014) using full trypsin specificity with up to two missed cleavages, static carboxamidomethylation of Cys, and variable oxidation of Met, and protein N-terminal acetylation. Consensus identification lists were generated with false discovery rates of 1% at both the protein and peptide levels. For label-free quantitation, the MaxLFQ algorithm was used and the “match between runs” option was enabled to transfer MS/MS identifications across LC-MS/MS runs based on accurate mass and retention time. Unique and razor peptides were considered for quantification and a minimum of two ratio counts were required for each of the normalized protein intensity. Protein tables were filtered to remove reverse database entries, common contaminants, and proteins identified by a single peptide. Normalized intensity data was floored to the minimum detected signal (intensity of 107). Unsupervised hierarchical clustering of samples was performed on log10-scaled intensities using normalized Euclidean distance with average linkage. Unpaired two-tail t-test was performed between controls (NT, NS) and knockdown (D1, D2) samples and nominal p-values were corrected for multiple testing using Benjamini-Hochberg method to estimate False Discovery Rate (FDR). Fold changes were calculated from the mean normalized protein intensities between the two groups. Final list of robustly detected, most changed candidates included affected at least 4 fold, FDR<10% proteins detected by at least 10 MS/MS counts and at least 10 unique peptides.
Genetically Engineered Mouse Models
Ptenpc-/- mice were obtained from UCLA and generated as previously described [71]. The β6-null mice were obtained from Dr. Dean Sheppard, University of California San Francisco [72]. Mice were housed in pathogen-free conditions, and all work was performed in accordance with an IACUC approved protocol, at Thomas Jefferson University.
Exosome Isolation
PC3 exosomes were isolated from serum-free cell culture supernatant via ultracentrifugation as described previously [29, 48].
For iodixanol gradient separation, a previously described procedure was used [47]. Pellets obtained from ultracentrifugation of conditioned media from PC3 prostate cancer cells were suspended in 1.636 mL of 30% iodixanol solution (made by mixing 1:1 of 60%, wt/vol) stock solution of iodixanol density gradient medium and a buffer (0.25 M sucrose, 10 mM Tris pH 8.0, 1 mM EDTA pH 7.4) and transferred to a SW55Ti rotor tube (Beckman). Next, 0.709 mL of 20% (wt/vol) iodixanol and 0.654 mL of 10% (wt/vol) iodixanol were successively layered on top of the 30% iodixanol-vesicle suspension and tubes were centrifuged for one hour at 350,000 g (54,000 rpm), 4°C, in SW55Ti rotor (BECKMAN, L8-70M Ultracentrifuge). Ten fractions of 0.267 mL were then collected starting from top of the tube. Refractive index was assessed with a refractometer and density calculated. All fractions were diluted with 1 mL PBS and centrifuged for 70 min at 100,000 g (53,000 rpm), 4°C, in a TLA-100.2 rotor (BECKMAN, Optima TL Ultracentrifuge). The respective pellets thus obtained were washed in 1 mL PBS and again centrifuged for 70 min at 100,000 g (53,000 rpm), 4°C, in a TLA-100.2 rotor. These concentrated fractions were finally resuspended in 30 μl of PBS.
Exosome Transfer Assays
β6-null PBMC (isolated from 3 β6-/- mice and pooled) and human normal PBMC, both separated using Lympholyte, or THP1 cells were incubated with or without PC3 derived exosomes. Cells were collected and subjected to IB analysis for β6 levels. To evaluate exosome-mediated internalization of β6, β6-null PBMC were resuspended with sodium acetate buffer (0.2 M acetic acid/0.5 M NaCl, pH 2.8) [14, 29] after the incubation with exosomes, then the cells were rinsed with PBS and lysed for IB.
Inhibition Assay and Immunoblotting Analysis
PC3 cells (6.0×105) were re-suspended in 3 mL of RPMI1640 medium, supplemented with 10 μg/mL 6.3G9, 1E6 or PBS. Cells were plated into 60 mm tissue culture dishes and incubated for 24 hours, and then cells were lysed and the cell lysates were prepared for IB analysis. IB analyses for exosome characterization and β6 integrin expression were performed as previously described [14, 29].
Imaging, Ab treatment of PTENpc-/- Mice and Immunohistochemistry Analysis
Ab treatment of PTENpc-/- Mice has been previously described [14]. Before Ab injection, mouse tumor sizes were measured with the Vevo 2100 high frequency, small animal, ultrasound scanner (Fujifilm/Visualsonics, Toronto, Ontario, Canada) and a 38 MHz linear array transducer (the MS400). The ultrasound scanning was performed by registered sonographer. Tumor volumes were obtained by measuring the lesion dimensions in three orthogonal planes using the build-in calipers on the scanner, and the tumor volume was calculated as Volume=(length×width×height)×Pi/6. Ab treatment of Ptenpc-/- mice was described previously [14]. IHC was performed on murine formalin-fixed paraffin embedded prostate tumor samples as previously described [14]. Five mice for each group (6.3G9 and 1E6) were analyzed. Stained slides were scored individually.
For each tumor, 3 random invasive adenocarcinoma areas were scanned; the average of the percentage of STAT1 positive neoplastic cells acquired from the 3 areas is shown.
NTA
NTA was employed to characterize exosome samples based on size following a previously established protocol [48] with NS300 (Malvern NanoSight).
Supplementary Material
Highlights.
This study reveals a cross-talk between cancer cells and monocytes mediated by the exosomal αvβ6, an epithelial specific integrin, and by its inhibitory activity on the STAT1 - MX1/2 pathway.
The integrin αvβ6 is transferred from cancer cells to monocytes via exosomes.
Cancer cell exosomes promote M2 polarization, whereas αvβ6 down-regulation in exosomes inhibits M2 polarization in recipient monocytes.
αvβ6 down-regulation causes a significant increase of STAT1 and MX1/2 levels in donor cancer cells as well as their exosomes.
Inhibition of αvβ6, in vitro or in vivo, causes up-regulation of STAT1 in cancer cells.
This study indicates that inhibition of this integrin and its downstream effectors might offer a novel immune – based therapeutic strategy in prostate cancer.
Acknowledgments
We are grateful to Dr. S.M. Violette, who provided antibodies against αvβ6; Dr. D. Sheppard who provided β6 knock-out mice; Dr. H. Wu for Ptenloxp/loxp mice; Amir Yarmahmoodi for support with NTA NS300 (Malvern NanoSight). We are grateful to Veronica Robles for support with the manuscript preparation. For this study, flow cytometry and histology facilities of the Sidney Kimmel Cancer Center, which are supported in part by NCI Cancer Center-Support Grant P30 CA56036, were used.
Funding Information
NIH-R01: CA109874 to L.R.L., CA78810 and CA90917 to D.C.A.; NIH-P01 CA140043 to L.R.L., D.C.A., D.I.G and D.W.S.; NIH-R50 CA221838 to H.-Y.T, NIH-R50 CA211199 to A.V.K.; Postdoctoral Research Fellowship from the American Italian Cancer Foundation to C.F. S10 OD010408 to F.F. for support of the ultrasound scanner. This project is also funded, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.); the Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Declaration of Interest
Paul H. Weinreb is employee and shareholder of Biogen Idec Inc. The other authors have declared that no conflict of interest exists.
Authors’ Contributions
H. Lu, S. Kurtoglu, C. Fedele, D.C. Hooper, D.W. Speicher, D.C. Altieri and L.R. Languino, designed the study.
H. Lu, N. Bowler, L. Harshyne, S.R. Krishn, S. Kurtoglu, C. Fedele, H. Tang, R. Kean, A. Dutta and Maria Stanczak performed the experiments.
K. Wang acquired blood specimens.
H. Lu, N. Bowler, S. Kurtoglu, L. Harshyne, C. Fedele, H. Tang, P. Fortina and A. Ertel acquired data.
H. Lu, N. Bowler, S. Kurtoglu, L. Harshyne, D.C. Hooper, Q. Liu, H. Tang, A.V. Kossenkov, W.K. Kelly, P.H. Weinreb, L. Yu, Flemming Forsberg, D.I. Gabrilovich, D.W. Speicher, D.C. Altieri and L.R. Languino analyzed the data.
P.H. Weinreb provided all antibodies against αvβ6.
H. Lu, S. Kurtoglu, C. Fedele, D.C. Hooper, D.W. Speicher, A.V. Kossenkov, D.C. Altieri and L.R. Languino wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Gerlinger M, Catto JW, Orntoft TF, Real FX, Zwarthoff EC, Swanton C. Intratumour Heterogeneity in Urologic Cancers: From Molecular Evidence to Clinical Implications. Eur Urol. 2014;67(4):729–737. doi: 10.1016/j.eururo.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Alva A, Hussain M. The changing natural history of metastatic prostate cancer. Cancer J. 2013;19(1):19–24. doi: 10.1097/PPO.0b013e318281197e. [DOI] [PubMed] [Google Scholar]
- 4.Culig Z. Targeting the androgen receptor in prostate cancer. Expert Opin Pharmacother. 2014;15(10):1427–1437. doi: 10.1517/14656566.2014.915313. [DOI] [PubMed] [Google Scholar]
- 5.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20(3):203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 6.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15(3):657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess K, Boger C, Behrens HM, Rocken C. Correlation between the expression of integrins in prostate cancer and clinical outcome in 1284 patients. Ann Diagn Pathol. 2014;18(6):343–350. doi: 10.1016/j.anndiagpath.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Nagle RB, Knox JD, Wolf C, Bowden GT, Cress AE. Adhesion molecules, extracellular matrix, and proteases in prostate carcinoma. J Cell Biochem. 1994;19:232–237. [PubMed] [Google Scholar]
- 9.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20(3-4):321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 10.Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91(4):686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- 11.Wirth M, Heidenreich A, Gschwend JE, Gil T, Zastrow S, Laniado M, Gerloff J, Zuhlsdorf M, Mordenti G, Uhl W, Lannert H. A multicenter phase 1 study of EMD 525797 (DI17E6), a novel humanized monoclonal antibody targeting alphav integrins, in progressive castration-resistant prostate cancer with bone metastases after chemotherapy. Eur Urol. 2014;65(5):897–904. doi: 10.1016/j.eururo.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 12.Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14(7):432–444. doi: 10.1038/nrm3599. [DOI] [PubMed] [Google Scholar]
- 13.Azare J, Leslie K, Al-Ahmadie H, Gerald W, Weinreb PH, Violette SM, Bromberg J. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin ®6. Mol Cell Biol. 2007;27(12):4444–4453. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H, Wang T, Li J, Fedele C, Liu Q, Zhang J, Jiang Z, Jain D, Iozzo RV, Violette SM, Weinreb PH, Davis RJ, Gioeli D, Fitzgerald TJ, Altieri DC, Languino LR. ‹V®6 Integrin Promotes Castrate-Resistant Prostate Cancer Through JNK1-Mediated Activation of Androgen Receptor. Cancer Res. 2016;1(76):5163–5174. doi: 10.1158/0008-5472.CAN-16-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via ‹v®3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59(7):1655–1664. [PubMed] [Google Scholar]
- 16.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin ‹v®3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26(42):6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard D. Epithelial integrins. Bioessays. 1996;18(8):655–60. doi: 10.1002/bies.950180809. [DOI] [PubMed] [Google Scholar]
- 18.Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T, Zerlanko BJ, FitzGerald TJ, Jiang Z, Birbe R, Wixted J, Violette SM, Stein JL, Stein GS, Lian JB, Languino LR. The ‹v®6 integrin promotes an osteolytic program through upregulation of MMP2. Cancer Res. 2014;74:1598–1608. doi: 10.1158/0008-5472.CAN-13-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, Ellis IO, Green AR, Haider S, Brouilette K, Saha A, Vallath S, Bowen R, Chelala C, Eccles D, Tapper WJ, Thompson AM, Quinlan P, Jordan L, Gillett C, Brentnall A, Violette S, Weinreb PH, Kendrew J, Barry ST, Hart IR, Jones JL, Marshall JF. Therapeutic targeting of integrin αvβ6 in breast cancer. J Natl Cancer Inst. 2014;106(8) doi: 10.1093/jnci/dju169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, Violette SM, Weinreb PH, Fleuren GJ. Overexpression of the ‹v®6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212(3):316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 21.Cantor DI, Cheruku HR, Nice EC, Baker MS. Integrin ‹v®6 sets the stage for colorectal cancer metastasis. Cancer Metastasis Rev. 2015;34(4):715–734. doi: 10.1007/s10555-015-9591-z. [DOI] [PubMed] [Google Scholar]
- 22.Hamidi H, Pietila M, Ivaska J. The complexity of integrins in cancer and new scopes for therapeutic targeting. Br J Cancer. 2016;115(9):1017–1023. doi: 10.1038/bjc.2016.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Qi Y, McKee K, Liu J, Hsu J, Yurchenco PD. Integrin and dystroglycan compensate each other to mediate laminin-dependent basement membrane assembly and epiblast polarization. Matrix Biol. 2017;57-58:272–284. doi: 10.1016/j.matbio.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui T, Sorensen ES, Rittling SR. Osteopontin binding to the alpha 4 integrin requires highest affinity integrin conformation, but is independent of post-translational modifications of osteopontin. Matrix Biol. 2015;41:19–25. doi: 10.1016/j.matbio.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twal WO, Hammad SM, Guffy SL, Argraves WS. A novel intracellular fibulin-1D variant binds to the cytoplasmic domain of integrin beta 1 subunit. Matrix Biol. 2015;43:97–108. doi: 10.1016/j.matbio.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viquez OM, Yazlovitskaya EM, Tu T, Mernaugh G, Secades P, McKee KK, Georges-Labouesse E, De Arcangelis A, Quaranta V, Yurchenco P, Gewin LC, Sonnenberg A, Pozzi A, Zent R. Integrin alpha6 maintains the structural integrity of the kidney collecting system. Matrix Biol. 2017;57-58:244–257. doi: 10.1016/j.matbio.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roche F, Sipila K, Honjo S, Johansson S, Tugues S, Heino J, Claesson-Welsh L. Histidine-rich glycoprotein blocks collagen-binding integrins and adhesion of endothelial cells through low-affinity interaction with α2 integrin. Matrix Biol. 2015;48:89–99. doi: 10.1016/j.matbio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Shinde AV, Kelsh R, Peters JH, Sekiguchi K, Van De Water L, McKeown-Longo PJ. The α4β1 integrin and the EDA domain of fibronectin regulate a profibrotic phenotype in dermal fibroblasts. Matrix Biol. 2015;41:26–35. doi: 10.1016/j.matbio.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The αv®6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290(8):4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, Saitakis M, Martin-Jaular L, Thery C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36(20):3012–3028. doi: 10.15252/embj.201696003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minciacchi VR, Spinelli C, Reis-Sobreiro M, Cavallini L, You S, Zandian M, Li X, Mishra R, Chiarugi P, Adam RM, Posadas EM, Viglietto G, Freeman MR, Cocucci E, Bhowmick NA, Di Vizio D. MYC Mediates Large Oncosome-Induced Fibroblast Reprogramming in Prostate Cancer. Cancer Res. 2017;77(9):2306–2317. doi: 10.1158/0008-5472.CAN-16-2942. [DOI] [PubMed] [Google Scholar]
- 32.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanderson RD, Bandari SK, Vlodavsky I. Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling. Matrix Biology. 2017 doi: 10.1016/j.matbio.2017.10.007. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song YH, Warncke C, Choi SJ, Choi S, Chiou AE, Ling L, Liu HY, Daniel S, Antonyak MA, Cerione RA, Fischbach C. Breast cancer-derived extracellular vesicles stimulate myofibroblast differentiation and pro-angiogenic behavior of adipose stem cells. Matrix Biol. 2017;60-61:190–205. doi: 10.1016/j.matbio.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jorgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, Piulats JM, Gonzalez Mella P, Ng SS, Jaeger D, Parnis FX, Franke FA, Puente J, Carvajal R, Sengelov L, McHenry MB, Varma A, van den Eertwegh AJ, Gerritsen W. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35(1):40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39(11):1588–1596. doi: 10.1007/s12272-016-0820-y. [DOI] [PubMed] [Google Scholar]
- 41.Petrosyan A, Da Sacco S, Tripuraneni N, Kreuser U, Lavarreda-Pearce M, Tamburrini R, De Filippo RE, Orlando G, Cravedi P, Perin L. A step towards clinical application of acellular matrix: A clue from macrophage polarization. Matrix Biol. 2017;57-58:334–346. doi: 10.1016/j.matbio.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, Heber-Katz E, Curtis MT, Cotzia P, Hooper DC. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19(14):3776–3786. doi: 10.1158/1078-0432.CCR-12-1940. [DOI] [PubMed] [Google Scholar]
- 45.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21(1):41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh A, Fedele C, Lu H, Nevalainen MT, Keen JH, Languino LR. Exosome-Mediated Transfer of ανβ3 Integrin from Tumorigenic to Non-Tumorigenic Cells Promotes a Migratory Phenotype. Mol Cancer Res. 2016;14(11):1136–1146. doi: 10.1158/1541-7786.MCR-16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morin-Brureau M, Hooper KM, Prosniak M, Sauma S, Harshyne LA, Andrews DW, Hooper DC. Enhancement of glioma-specific immunity in mice by “NOBEL”, an insulin-like growth factor 1 receptor antisense oligodeoxynucleotide. Cancer Immunol Immunother. 2015;64(4):447–457. doi: 10.1007/s00262-015-1654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen P, Pedersen MW, Woetmann A, Villingshoj M, Stockhausen MT, Odum N, Poulsen HS. EGFR induces expression of IRF-1 via STAT1 and STAT3 activation leading to growth arrest of human cancer cells. Int J Cancer. 2008;122(2):342–9. doi: 10.1002/ijc.23109. [DOI] [PubMed] [Google Scholar]
- 51.Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, Shuai K. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci U S A. 1998;95(18):10626–10631. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puhr M, Hoefer J, Neuwirt H, Eder IE, Kern J, Schafer G, Geley S, Heidegger I, Klocker H, Culig Z. PIAS1 is a crucial factor for prostate cancer cell survival and a valid target in docetaxel resistant cells. Oncotarget. 2014;5(23):12043–12056. doi: 10.18632/oncotarget.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197(2):157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 54.Stephanou A, Latchman DS. STAT-1: a novel regulator of apoptosis. Int J Exp Pathol. 2003;84(6):239–244. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31(4):539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Battle TE, Lynch RA, Frank DA. Signal transducer and activator of transcription 1 activation in endothelial cells is a negative regulator of angiogenesis. Cancer Res. 2006;66(7):3649–3657. doi: 10.1158/0008-5472.CAN-05-3612. [DOI] [PubMed] [Google Scholar]
- 57.Hatziieremia S, Mohammed Z, McCall P, Willder JM, Roseweir AK, Underwood MA, Edwards J. Loss of signal transducer and activator of transcription 1 is associated with prostate cancer recurrence. Mol Carcinog. 2016;55(11):1667–1677. doi: 10.1002/mc.22417. [DOI] [PubMed] [Google Scholar]
- 58.Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174(8):4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 59.Holzinger D, Jorns C, Stertz S, Boisson-Dupuis S, Thimme R, Weidmann M, Casanova JL, Haller O, Kochs G. Induction of MxA gene expression by influenza A virus requires type I or type III interferon signaling. J Virol. 2007;81(14):7776–7785. doi: 10.1128/JVI.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melen K, Keskinen P, Ronni T, Sareneva T, Lounatmaa K, Julkunen I. Human MxB protein, an interferon-alpha-inducible GTPase, contains a nuclear targeting signal and is localized in the heterochromatin region beneath the nuclear envelope. J Biol Chem. 1996;271(38):23478–23486. doi: 10.1074/jbc.271.38.23478. [DOI] [PubMed] [Google Scholar]
- 61.Brown SG, Knowell AE, Hunt A, Patel D, Bhosle S, Chaudhary J. Interferon inducible antiviral MxA is inversely associated with prostate cancer and regulates cell cycle, invasion and Docetaxel induced apoptosis. Prostate. 2015;75(3):266–279. doi: 10.1002/pros.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mushinski JF, Nguyen P, Stevens LM, Khanna C, Lee S, Chung EJ, Lee MJ, Kim YS, Linehan WM, Horisberger MA, Trepel JB. Inhibition of tumor cell motility by the interferon-inducible GTPase MxA. J Biol Chem. 2009;284(22):15206–15214. doi: 10.1074/jbc.M806324200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su X, Esser AK, Amend SR, Xiang J, Xu Y, Ross MH, Fox GC, Kobayashi T, Steri V, Roomp K, Fontana F, Hurchla MA, Knolhoff BL, Meyer MA, Morgan EA, Tomasson JC, Novack JS, Zou W, Faccio R, Novack DV, Robinson SD, Teitelbaum SL, DeNardo DG, Schneider JG, Weilbaecher KN. Antagonizing Integrin beta3 Increases Immunosuppression in Cancer. Cancer Res. 2016;76(12):3484–3495. doi: 10.1158/0008-5472.CAN-15-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, Pouliot F, Magyar C, Sung JL, Xu J, Deng G, West BL, Bollag G, Fradet Y, Lacombe L, Jung ME, Huang J, Wu L. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75(6):950–962. doi: 10.1158/0008-5472.CAN-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Izumi K, Fang LY, Mizokami A, Namiki M, Li L, Lin WJ, Chang C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med. 2013;5(9):1383–1401. doi: 10.1002/emmm.201202367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Lee H, Tong H, Schwartz M, Zhu C. Force regulated conformational change of integrin alphaVbeta3. Matrix Biol. 2017;60-61:70–85. doi: 10.1016/j.matbio.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, Mobley JA, Zhang Y, Brown EE, Vlodavsky I, Sanderson RD. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2017;65:104–118. doi: 10.1016/j.matbio.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harshyne LA, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol. 2016;18(2):206–215. doi: 10.1093/neuonc/nov107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dutta A, Li J, Fedele C, Sayeed A, Singh A, Violette SM, Manes TD, Languino LR. αvβ6 Integrin Is Required for TGFβ1-Mediated Matrix Metalloproteinase2 Expression. Biochem J. 2015;466(3):525–536. doi: 10.1042/BJ20140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 72.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin ®6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133(4):921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


