Abstract
Background
Targeted localized biopsies and treatments for diffuse gliomas rely on accurate identification of tissue subregions, for which current MRI techniques lack specificity.
Purpose
To explore the complementary and competitive roles of a variety of conventional and quantitative MRI methods for distinguishing subregions of brain gliomas.
Study Type
Prospective.
Population
Fifty-one tissue specimens were collected using image-guided localized biopsy surgery from 10 patients with newly diagnosed gliomas.
Field Strength/Sequence
Conventional and quantitative MR images consisting of pre- and postcontrast T1w, T2w, T2-FLAIR, T2-relaxometry, DWI, DTI, IVIM, and DSC-MRI were acquired preoperatively at 3T.
Assessment
Biopsy specimens were histopathologically attributed to glioma tissue subregion categories of active tumor (AT), infiltrative edema (IE), and normal tissue (NT) subregions. For each tissue sample, a feature vector comprising 15 MRI-based parameters was derived from preoperative images and assessed by a machine learning algorithm to determine the best multiparametric feature combination for characterizing the tissue subregions.
Statistical Tests
For discrimination of AT, IE, and NT subregions, a one-way analysis of variance (ANOVA) test and for pairwise tissue subregion differentiation, Tukey honest significant difference, and Games-Howell tests were applied (P < 0.05). Cross-validated feature selection and classification methods were implemented for identification of accurate multiparametric MRI parameter combination.
Results
After exclusion of 17 tissue specimens, 34 samples (AT = 6, IE = 20, and NT = 8) were considered for analysis. Highest accuracies and statistically significant differences for discrimination of IE from NT and AT from NT were observed for diffusion-based parameters (AUCs >90%), and the perfusion-derived parameter as the most accurate feature in distinguishing IE from AT. A combination of “CBV, MD, T2_ISO, FLAIR” parameters showed high diagnostic performance for identification of the three subregions (AUC ~90%).
Data Conclusion
Integration of a few quantitative along with conventional MRI parameters may provide a potential multiparametric imaging biomarker for predicting the histopathologically proven glioma tissue subregions.
Level of Evidence
2
Technical Efficacy
Stage 3
Diffuse gliomas manifest extensive diffuse infiltration of tumor cells in adjacent brain parenchyma.1 Even when low grade, diffuse gliomas are generally fatal after several years.1,2 It is widely known that characteristic spatial intratumor variability within the microenvironment of gliomas account for the grim prognosis of these patients.3,4 Within heterogeneous tumors like gliomas, multiple subregions with different phenotypic characteristics coexist, which often represent heterogeneous genetic and microenvironmental profiles, likely to respond variably to treatment.5,6 Lack of sensitive and specific quantitative imaging biomarkers for realizing spatial variations of gliomas and localizing the most aggressive portion of the tumor leads to inaccurate biopsy sampling, which hinders target-specific diagnosis and therapies.5
Targeted biopsy procedures and surgical/treatment planning for gliomas most often rely on conventional contrast-enhanced T1-weighted (CE-T1w), T2-weighted (T2w) and T2-FLAIR images.7–9 Nonetheless, as diffuse gliomas tend to invade the brain tissue in small cell groups, tumor expansion and progression may precede changes in contrast enhancement.1,8 Furthermore, CE-T1w cannot sufficiently localize the most aggressive or active tumor subcompartment to ensure reliable biopsy outcome.10,11 On the other hand, T2w hyperintense regions are incapable of characterizing the infiltrative glioma subregion (infiltrative edema, also referred to as nonenhancing tumor [NET]), and diffuse infiltrating cells can be found beyond the extent of T2w hyperintensity borders.12,13 Hence, based on conventional magnetic resonance imaging (MRI), stratification of the most aggressive/active part of the tumor and infiltrative glioma and detection of the extent of invasion from normal tissue remains challenging.
Various advanced MRI contrasts have been explored for their potential in localizing and grading glioma brain tumors. Diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) reflect the water proton mobility related to changes in cellular density, permeability of the cell membrane, and tissue microstructure.14,15 Perfusion-weighted imaging (PWI) visualizes microvascular changes and cellular proliferation; particularly, regional cerebral blood volume (rCBV) derived from dynamic susceptibility contrast-enhanced (DSC)-MRI correlates with the degree of neovascularization and histopathological grade, surpassing the performance of CE-T1w MRI.16–18
There have been sporadic attempts to study the role of quantitative MRI methods, including DSC-MRI, DWI/DTI, IVIM, and T2-relaxometry for characterizing infiltrating glioma regions through biopsy validation.18–20 Hence, we sought to address: 1) the roles of individual MRI-derived parameters in differentiation of the three subregions, ie, active tumor (AT), infiltrative edema (IE), and normal tissue (NT), from each other; 2) the relationship between MRI-derived parameters with histopathological cellular density; and 3) the best multiparametric combination of MRI-derived features for discrimination of AT, IE, and NT through a quantitative methodology.
Materials and Methods
Patients
Between July 2015 and February 2016, 10 adult patients (six men and four women; mean age 40.4 years; age range, 20-76 years) with newly diagnosed gliomas were prospectively recruited for this study. The patients underwent presurgical MRI and computed tomography (CT) examinations, followed by image-guided neurosurgery within 1-3 days from image acquisition. Institutional Review Board (IRB) approval was obtained and the patients provided informed written consent to be included in the study, designed in compliance with the Health Insurance Portability and Accountability Act (HIPAA) law. Inclusion criteria for this study consisted of patients: 1) with suspected glioma brain tumors based on their initial MR scan; 2) with no prior treatment, including surgery or radiotherapy; 3) having no contraindications for surgery; 4) in whom the tumor was not in an eloquent area; and 5) for whom there were no technical challenges for performing surgery.
Patients or biopsy specimens were excluded if 1) they did not undergo surgery; 2) imaging was performed inadequately or some image sequences were missing; or 3) if histopathological assessment of gross tumor or specimens was impossible or not provided.
Image Acquisition
All patients underwent preoperative MRI acquisition within 2 days prior to their surgery. Structural and physiological MRI acquisitions were performed on a 3T MRI scanner (Siemens Magnetom Tim Trio, Erlangen, Germany). Sagittal T2w turbo spin echo (repetition time/echo time [TE/TR] = 80/6000, slice thickness = 5 mm, flip angle = 140°, field of view [FOV] = 230 × 230 mm2, image matrix = 320 × 320, pixel size = 0.72 × 0.72 mm2). Axial T2w turbo spin echo (TE/TR = 106/5400, slice thickness = 5 mm, flip angle = 120°, FOV = 200 × 200 mm2, image matrix = 232 × 256, pixel size = 0.78 × 0.78 mm2). Axial fluid attenuated inversion recovery (FLAIR) (TE/TR/TI = 115/8400/2240, slice thickness = 5 mm, flip angle = 125°, FOV = 181 × 200 mm2, image matrix = 232 × 256, pixel size = 0.78 × 0.78 mm2). Axial 3D magnetization-prepared rapid-acquisition gradient-echo (MP-RAGE) T2w spin-echo as a part of image-guided surgery protocol with TE/TR = 200/2500, slice thickness = 1mm, flip angle = 120°, FOV = 208 × 256 mm2, image matrix = 420 × 512, pixel size = 0.50 × 0.50 mm2.
Axial DWI using echo-planar imaging (EPI) sequence with TE/TR = 100/4000, slice thickness = 5 mm, flip angle = 90°, FOV = 200 × 200 mm2, image matrix = 136 × 136, pixel size = 1.47 × 1.47 mm, b-value = 50, 1000 s/mm2. Axial multi b-value DWI using EPI method with TE/TR = 100/4000, slice thickness = 5 mm, flip angle = 90°, FOV = 200 × 200 mm2, image matrix = 136 × 136, pixel size = 1.47 × 1.47 mm2, b-values = 0, 50, 200, 400, 600, 800, 1000 s/mm2. Diffusion tensor imaging (DTI) with 64 directions and TE/TR = 90/9000, slice thickness = 5 mm, FOV = 256 × 256 mm2, flip angle = 90°, number of excitations (NEX) = 1, image matrix = 128 × 128 × 72, pixel size = 1.72 × 1.72 mm2, b-value = 50, 1000 s/mm2.
Axial multiecho T2w spin-echo (T2-relaxometry) was carried out with TR = 4000 msec, TE = 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, 168, 180, 192 msec, slice thickness = 5 mm, flip angle = 180°, FOV = 200 × 200 mm2, image matrix = 232 × 256, pixel size= 0.78 × 0.78 mm2.
After injection of 0.2 mmol/kg of Gadovist (Bayer Schering Pharma, Berlin, Germany) at a rate of 5 ml/s and followed by a 20-mL saline flush, DSC-MRI was acquired using a GE-EPI sequence with TE/TR = 30/1600, slice thickness = 5 mm, FOV = 220 × 220 mm2, flip angle = 70°, NEX = 1, image matrix = 128 × 128 × 20, pixel size = 1.72 × 1.72 mm2, number of dynamic scans = 64, temporal resolution = 1.5 sec. To minimize the effects of contrast agent extravasation a preload of contrast agent was administered with about 25% of the total contrast dose about 4 minutes of incubation time before the second injection for DSC-MR imaging.
Pre- and postcontrast axial 3D MP-RAGE T1w spin-echo were also acquired as a part of the protocol required for the navigation system TE/TR = 5/17, slice thickness = 1 mm, flip angle = 25°, FOV = 208 × 256 mm2, image matrix = 208 × 256, pixel size = 1 × 1 mm2.
MRI pulse sequences and their parameter adjustments are summarized in Table 1.
TABLE 1.
MRI Specifications
| Sequence | TE/TR | Slice thickness (mm) | Flip angle | FOV (mm2) | Image matrix | Other specifications |
|---|---|---|---|---|---|---|
| Pre- and postcontrast 3D T1w | 5/17 | 1 | 25° | 208 × 256 | 208 × 256 | N/A |
| 3D T2w | 200/2500 | 1 | 120° | 208 × 256 | 420 × 512 | N/A |
| T2-FLAIR | 115/8400 | 5 | 125° | 181 × 200 | 232 × 256 | TI = 2240 |
| T2-Relaxometry | (12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, 168, 180, 192)/4000 | 5 | 180° | 200 × 200 | 232 × 256 | N/A |
| DWI | 100/4000 | 5 | 90° | 200 × 200 | 136 × 136 | b-values = 50, 1000 s/mm2 |
| Multi b-value DWI | 100/4000 | 5 | 90° | 200 × 200 | 136 × 136 | b-values = 0, 50, 200, 400, 600, 800, 1000 s/mm2 |
| DTI | 90/9000 | 5 | 90° | 256 × 256 | 128 × 128 | b-values = 50, 1000 s/mm2 |
| DSC-MRI | 30/1600 | 5 | 70° | 220 × 220 | 128 × 128 | No. Dynamic Scans = 64, Temporal Resolution = 1.5 s |
DWI: Diffusion Weighted Imaging; DTI: Diffusion Tensor Imaging; DSC-MRI: Dynamic Susceptibility Contrast Enhanced MRI.
CT images were acquired for all patients prior to surgery to ensure accurate registration of the images with the position of the patient during image-guided neurosurgery. 3D images were acquired on a 64-slice CT scanner (GE Healthcare Technologies, Milwaukee, WI) with no gantry tilt, slice thickness of 0.625 mm, from maxilla to the top of the head.
Quantification of MR Images
DTI
Quantification of DTI was executed in ExploreDTI software (v, 4.8.6)21 with proper parameter adjustments, and after EPI and patient motion correction. Maps of mean diffusivity (MD), fractional anisotropy (FA), and L1, L2, and L3 eigenvalues were exported from the software. Pure isotropic diffusion and pure anisotropic diffusion maps denoted by P and Q, respectively, were calculated based on this formula22:
DSC-MRI
DSC-MRI analysis was carried out based on an algorithm developed in-house, which inputs signal intensity–time curves of -based dynamic images in each pixel, converts them to curves, selects the arterial input function (AIF) based on a modified automated algorithm, performs decorrelation using block-circulant SVD, and generates a regional CBV (rCBV) map.
T2/PROTON DENSITY MAPPING
In T2-relaxometry, images are acquired at multiple echo times to obtain an estimate of T2 and proton density by fitting the following equation to the signal:
where S0 is the proton density and T2 is the spin–spin relaxation. Quantitative T2 and proton density (PD) maps were generated by pixelwise fitting of the mentioned equation to the corresponding multiecho signal.
QUANTIFICATION OF INTRAVOXEL INCOHERENT MOTION
Diffusion-weighted images are highly affected by flow of blood and cerebrospinal fluids at very low b-values (ie, less than 100–200 s/mm2).23,24 Here, to separate contributions of diffusion and perfusion, the bi-exponential intravoxel incoherent motion (IVIM) model is used which is denoted by24:
where S0 is the signal at b-value of zero, f is the fraction of signal affected by flow, D* describes the decay of signal caused by flow, and D is the measured diffusion coefficient, which is a function of diffusion time. To nullify the effect of flow on diffusion measurements, diffusion coefficients (D) for each voxel were calculated using conservative minimum b-values of 200 s/mm2.25 As diffusion parameters are highly affected by non-Gaussian behavior of diffusion at higher b-values, for calculation of D, this effect was conservatively cancelled by using maximum value of 600 s/mm2.
To account for non-Gaussianity of the signal in higher b-values, the modified IVIM model including diffusion kurtosis imaging (IVIM-DKI) was considered26:
where K is the kurtosis factor that measures deviation from the Gaussian behavior. D for each voxel was estimated by fitting the equation to the segment of the signal at b-values of 200, 400, and 600 s/mm2, and K was estimated from b-values of 600, 800, and 1000 s/mm2 using nonlinear least-squares method.
Biopsy Site Selection
The generated maps from different modalities, including rCBV-map from DSC-MRI, ADC-map from DWI, maps of FA, MD, P, Q from DTI, maps of D, D*, f, and K from multi-b-value diffusion imaging, maps of T2 and PD from T2-relaxometry technique, and conventional images comprising of FLAIR, T2_ISO (MP-RAGE T2w), and MP-RAGE precontrast T1w, were coregistered with the postcontrast MP-RAGE T1w (CE-T1w) image using a rigid registration method with normalized mutual information (NMI) as the similarity measure in SPM software (http://www.fil.ion.ucl.ac.uk/spm/). The difference between post- and precontrast T1w images was calculated to form T1_SUB (subtracted T1w) image, which shows the enhanced regions (Fig. 1).
FIGURE 1.
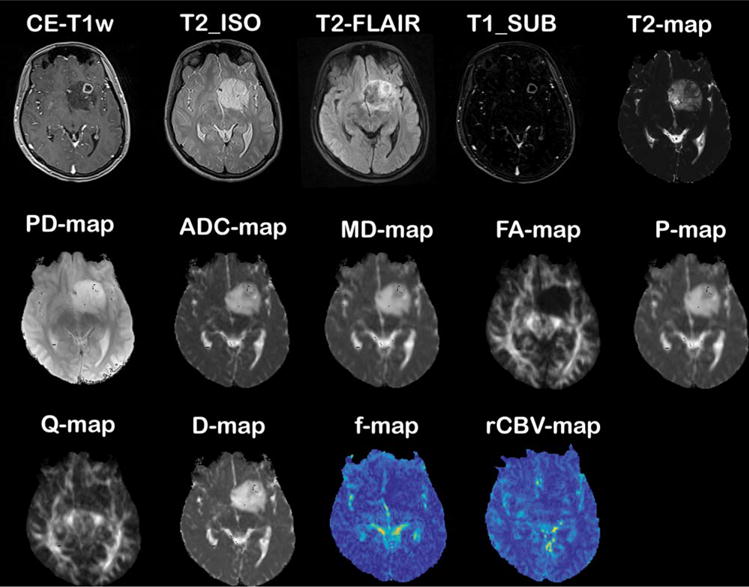
MR images and quantitative maps of a 20-year-old female with histopathologically confirmed grade II oligodendroglioma (images are coregistered with CE-T1w image).
A senior radiologist (K.F. with 12 years of experience in neuroradiology) identified the biopsy targets, ie, active tumor, infiltration, and normal regions, based on the presence of hyperintensity on CE-T1w, hypointensity on T2w and FLAIR images, and according to quantitative ADC and rCBV maps. Rectangular regions of interest (ROIs) with sizes of at most 8 × 8 pixels were marked on CE-T1w images and imported into the navigation workstation to plan for image-guided biopsy intervention. The regions were ideally identified on areas with best accessibility for the neurosurgeon for specimen collection and distant from vessels, ventricles, and critical brain regions.
Image-Guided Tissue Biopsy Procedure
The specimens were sampled from the patients by a senior neurosurgeon (M.Z. with 10 years of experience in neurosurgery) using a disposable biopsy needle (Stryker, Switzerland). The coordinates of the ROIs were recorded by the navigation system (OpticVision, Parseh Intelligent Surgical Systems [PARSISS], Iran), which were used to overlay on the images and MRI-derived quantitative maps. Based on the guide provided by navigation, needle biopsy was performed on all target points. For each patient, 4–6 specimens with at least 1-cm of distance were collected for histopathological assessments.
These technical considerations were addressed during the biopsy to minimize intraoperative brain shift and error:
The target points were presurgically marked in the order of normal tissue, edema, tumor, and necrosis;
Serum manitol, corticosteroids, or any other diuretics pre/intraoperative were not given to patients prior to biopsy procedure;
Hypo/hyperventilation during surgery was avoided;
Minimal dural opening was performed to prevent CSF egress resulting in brain shift;
The target points in proximity of the ventricles were the last points for biopsy, to avoid unwanted entrance into the ventricles, resulting in CSF drainage and therefore changing navigation accuracy due to brain shift;
For the target points in proximity of cystic portions within the tumor, a similar strategy was adopted.
After needle biopsy, the dura was opened and based on preoperative images, complete resection of the tumor was performed and the resected gross tumor volume was transferred for histopathological grading.
Histopathological Assessment of Tissue Samples
After being removed from the brain, the specimens were fixed in 10% buffered formalin and routinely processed for histopathological assessment. Five micrometer sections were stained using the hematoxylin and eosin (H&E) method and reviewed by a pathologist (F.A. with 20 years of experience in neuropathology). For each specimen, the pathologist determined the presence of tumor cells and scored the samples as “normal tissue” where no tumor cells were identified, “positive tumor cells” when infiltrating tumor cells were present, and “tumor core” when tumor constituted the majority of tissue. Tumor was identified, classified, and graded based on morphological features, such as density and distribution of cells, nuclear atypia, mitotic activity, vascular proliferation, and necrosis. Throughout this article we refer to the pathological term “tumor core” as “active tumor (AT),” “positive tumor cells” as “infiltrating edema (IE),” and “normal tissue” as “normal tissue (NT).”
For each specimen, the area with the highest cellularity was selected and one image was captured at ×40 magnification. Quantitative assessment of histology images was performed automatically by a cell segmentation method which applies decorrelation and stretching of the colorspace in the preprocessing step for improving the performance of cell segmentation (Fig. 2).27 Cellular count (CC) within the specified area was calculated from the segmented images and used as a representative parameter of cellular density. The relationship between the calculated CC on all regions and interpretation of the pathologist, as the gold standard, was examined.
FIGURE 2.
Automated segmentation of cells in microscopic images (×40 magnification): (A) sample image from tumor core of the same patient indicated in Fig. 1; (B) automated cell segmentation result.
Statistical Analysis
The statistical analysis comprised of the following steps: 1) evaluating the association of MRI-derived parameters with each other and with statistically significant quantitative histopathological feature (cellular count); 2) assessing mean values of the extracted features among the three tissue subtypes, ie, IE, NT, and AT; 3) investigating the diagnostic performances of individual MRI-derived features for distinguishing the histopathologically identified regions and their combinations based on a machine-learning technique, including automatic feature selection and classification procedures.
The computed MRI-based features (n = 15) include morphological parameters, ie, T1_SUB, T2_ISO, FLAIR, diffusion-related parameters, ie, ADC, MD, FA, P, Q, D, D*, K, perfusion-related parameters, ie, CBV, and f, and T2-relaxometry parameters, ie, T2 and PD. The histopathological CC metric was also evaluated for its potential in discriminating the subregions. All statistical analysis and machine-learning procedures were performed in R Statistical Software (R3.0.2, Vienna, Austria).
COMPARISON OF MEAN VALUES
The one-way analysis of variance (ANOVA) method was applied to evaluate the discrimination of NT, IE, and AT tissue subtypes based on the MRI-derived parameters and cellular count. P < 0.05 was considered significant for differentiation of the three tissue regions simultaneously. For each parameter, pairwise differentiation of the regions, namely, NT from IE, NT from AT, and IE from AT, was assessed by post-hoc tests: Tukey’s honest significant difference (Tukey-HSD) or Games-Howell methods, to avoid Type I errors by adjusting the calculated P-values for multiple comparisons. The HSD test is based on assumptions of normality and equality of variance; the former was evaluated using normality tests and the latter by the Bartlett method. If equality of variance assumption passed, Tukey-HSD was performed; otherwise, Games-Howell nonparametric test was applied.
ASSOCIATION OF PARAMETERS
Relationships between MRI-derived parameters with each other and also between them and cellular count were obtained using Spearman’s rank correlation. To correct for multiple comparisons, Holm’s method for multiple testing28 was applied and, after correction, a level of 0.05 was considered statistically significant.
ASSESSMENT OF DIAGNOSTIC PERFORMANCES OF INDIVIDUAL MRI-DERIVED PARAMETERS
Fischer’s linear discriminant analysis (LDA) method was used to assess performances of MRI-derived and CC parameters in discrimination of NT from IE, NT from AT, and IE from AT. LDA was implemented with leave-one-out crossvalidation (LOOCV), where in each iteration (number of iterations equals the number of samples), the dataset is partitioned into the training set composed of whole data excluding one sample, which is separated as the test object. Assessment metrics, namely sensitivity, specificity, accuracy, and area under the receiver operating characteristic (ROC) curve (AUC) were calculated on test samples and averaged over loops of crossvalidation.
COMBINATION OF MRI-DERIVED PARAMETERS THROUGH CLASSIFICATION
To identify the complementary/competitive roles of MRI-derived features, feature selection was carried out using Akaike Information Criterion (AIC)29 and Schwarz Bayesian Information Criterion (BIC)30 in forward selection, backward elimination, and stepwise selection strategies (constructing six feature selection methods) on a feature space consisting of the 15 MRI-derived parameters.
The feature selection algorithm was adjusted to avoid choosing similar parameters in a set, eg, the algorithm should select only one feature among diffusion-related features: ADC, D, MD, P; anisotropy-related features: FA, Q; perfusion-related features: f, CBV; T2-related features: T2, T2_ISO.
Feature selection was performed in loops of LOOCV and the most frequently selected feature subsets by each of the feature selection methods were the representative feature subset.31 This was followed by a classification step based on Fischer’s linear discriminant analysis (LDA), quadratic discriminant analysis (QDA), and support vector machine (SVM) methods32 for discrimination of tissue subtypes. To avoid bias, classification was also performed using LOOCV.
Results
Among the 10 recruited patients, six were diagnosed with low-grade glioma (WHO Grade II: low-grade astrocytoma [n = 3], diffuse oligoastrocytoma [n = 2], oligodendroglioma [n = 1]), one with anaplastic oligoastrocytoma (WHO Grade III), and three with glioblastoma multiforme (WHO Grade IV). A total of 51 tissue specimens were collected from 10 patients. Three patients (two GBM and one Grade II oligodendroglioma) were excluded due to the absence or distorted multi-b-value, multiecho, DTI, or DSC-MRI. Finally, seven patients with 34 samples were included in the study. Based on qualitative histopathological assessment, six samples were diagnosed as the “tumoral core,” 20 samples as “positive tumor cells,” and 8 samples as “normal tissue.”
Relationship of MRI Parameters
The summary results for correlations among the MRI parameters with each other and with CC are provided in Table 2. The statistically significant correlations are shown in boldface type and are shaded in gray.
TABLE 2.
Correlations (in Terms of R) Among the MRI-Derived Parameters With Each Other and Between MRI-Derived Parameters and Cell Count (From Histopathology)a
| ADC | CBV | D | D* | FA | FLAIR | f | K | MD | P | PD | Q | T1_SUB | T2_ISO | T2 | Count | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADC | −0.078 | 0.96 | −0.40 | −0.60 | 0.45 | −0.11 | −0.71 | 0.91 | 0.88 | 0.28 | −0.28 | 0.13 | 0.66 | 0.80 | 0.30 | |
| CBV | 0.01 | 0.14 | −0.25 | 0.03 | 0.35 | 0.17 | −0.09 | −0.06 | 0.18 | −0.24 | 0.28 | −0.22 | −0.19 | 0.46 | ||
| D | −0.31 | −0.64 | 0.46 | −0.09 | −0.68 | 0.90 | 0.88 | 0.26 | −0.31 | 0.15 | 0.64 | 0.78 | 0.40 | |||
| D* | 0.30 | −0.25 | 0.15 | 0.43 | −0.38 | −0.40 | −0.25 | 0.30 | −0.09 | −0.30 | −0.49 | −0.02 | ||||
| FA | −0.33 | 0.10 | 0.58 | −0.56 | −0.58 | −0.41 | 0.86 | −0.20 | −0.39 | −0.54 | −0.37 | |||||
| FLAIR | 0.14 | −0.43 | 0.41 | 0.44 | 0.21 | −0.14 | 0.37 | 0.69 | 0.34 | 0.05 | ||||||
| f | 0.36 | 0.01 | −0.01 | 0.07 | 0.08 | 0.27 | 0.10 | −0.07 | 0.32 | |||||||
| K | −0.60 | −0.59 | −0.18 | 0.41 | −0.19 | −0.51 | −0.61 | −0.09 | ||||||||
| MD | 0.98 | 0.46 | −0.28 | 0.19 | 0.62 | 0.84 | 0.36 | |||||||||
| P | 0.47 | −0.31 | 0.26 | 0.59 | 0.81 | 0.36 | ||||||||||
| PD | −0.47 | 0.27 | 0.02 | 0.39 | 0.00 | |||||||||||
| Q | −0.23 | −0.11 | −0.34 | −0.17 | ||||||||||||
| T1_SUB | 0.03 | 0.03 | 0.23 | |||||||||||||
| T2_ISO | 0.69 | 0.15 | ||||||||||||||
| T2 | 0.18 |
The cells with bold-face type and with gray-shaded color are those with significant correlation as indicated by Spearman’s rank correlation.
ADC: Apparent Diffusion Coefficient (DWI); CBV: Cerebral Blood Volume (DSC-MRI); D: True diffusion coefficient (IVIM);
D*: Pseudo-diffusion coefficient (IVIM); f: perfusion fraction (IVIM); K: Diffusion Kurtosis; MD: Mean Diffusivity (DTI); FA: Fractional Anisotropy (FA); P: Pure isotropic diffusion coefficient (DTI); Q: Pure anisotropic diffusion coefficient (DTI); PD: Proton Density (T2-relaxometry); T2: T2 relaxation time (T2-relaxometry); T1_SUB: Subtracted high-resolution T1w from the corresponding high-resolution contrast-enhanced T1w image; T2_ISO: High-resolution T2w image; FLAIR: Fluid-attenuated inversion recovery image.
DIFFUSION-BASED PARAMETERS
ADC parameter significantly correlated with MD, P, D, T2_ISO, and T2 and negatively with FA and K. This parameter showed the strongest correlations with D (R = 0.96), MD (R = 0.91), P (R = 0.88), and T2 (R = 0.80), but showed no significant correlation with CC.
D is directly correlated with ADC, FA, MD, P, T2_ISO, T2, and inversely correlated with K. The strongest correlation of D was with ADC (R = 0.96), MD (R = 0.90), and P (R = 0.88). It showed significant correlation with CC (R = 0.40).
D* was correlated poorly and inversely with T2. K correlated inversely with ADC, MD, P, and T2 (in all, R ≤0.62). Besides the correlations mentioned earlier that included MD, this parameter was strongly correlated with P (R = 0.98) and T2 (R = 0.84), and showed moderate correlations with T2_ISO. P was highly related to T2 (R = 0.81), and moderately correlated with T2_ISO. Both MD and P were correlated with CC (R = 0.36).
FA was strongly correlated with Q (R = 0.86) and indicated a modest inverse association with ADC, D, MD, P, and T2. Furthermore, FA showed an inverse correlation with CC (R = −0.37). Q was directly correlated with FA, and showed no relationships with CC.
PERFUSION-BASED PARAMETERS
CBV only showed statistically significant correlation with the f parameter derived from IVIM, which was not strong (R = 0.35). Additionally, it indicated a significant correlation with CC (R = 0.46). Parameter f correlated with none of the parameters except CBV.
CMRI PARAMETERS
FLAIR only correlated with T2_ISO with a correlation coefficient of 0.67. T2_ISO was significantly associated with ADC, D, MD, FA, FLAIR, and P (for all, R <0.70). T1_SUB showed no significant correlations with any of the parameters.
T2-MAPPING PARAMETERS
As mentioned in previous sections, T2 demonstrated correlations with ADC, D, K, MD, P, and T2_ISO. The PD-map correlated with none of the parameters.
Association of Tissue Subtypes With Quantitative MRI-Derived Parameters
Comparison of the mean values of individual parameters based on the ANOVA test for differentiation of the three tissue subtypes from each other is shown in Table 3. The results suggest that among MRI-derived parameters, T2_ISO, FLAIR, ADC, CBV, MD, FA, P, D, and T2 show statistically significant differences among the three subregions, ie, NT, IE, and AT. The histopathologically derived parameter, CC, showed statistically significant differences among the three tissue subregions (P = 8.9E-06).
TABLE 3.
Comparison of the Mean Values Based on ANOVA Test (Second Column), for Differentiation of Normal Tissue (NT) vs. Infiltrative Edema (IE) vs. Active Tumor (AT) Simultaneously, and Evaluating the Diagnostic Performances of Individual Parameters in Differentiating Pairwise Regions, ie, “NT” from “IE,” “NT” from “AT,” and “IE” from “AT” Using Cross-validated LDA Methoda
| Feature | ANOVA Test (P-values) |
NT from IE | NT from AT | IE from AT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-values | Sens. | Spec. | Acc. | AUC (95% CI) | P-values | Sens. | Spec. | Acc. | AUC (95% CI) | P-values | Sens. | Spec. | Acc. | AUC (95% CI) | ||
| T1_SUB | 0.203 | 0.563 | 100 | 0 | 71.4 | 68.8 (45.0–92.5) | 0.176 | 50 | 100 | 78.6 | 81.2 (54.1–100) | 0.462 | 1.9 | 95.1 | 73.7 | 67.5 (36.4–98.1) |
| T2_ISO | 0.001 | 0.018 | 87.0 | 75.9 | 83.9 | 89.4 (77.0–100) | 0.000 | 77.9 | 87.5 | 83.5 | 95.8 (86.0–100) | 0.913 | 0.0 | 100 | 76.9 | 59.1 (35.5–82.8) |
| FLAIR | 0.001 | 0.016 | 89.8 | 64.7 | 82.7 | 90.6 (79.0–100) | 0.000 | 67.9 | 87.5 | 79.1 | 93.7 (81.2–100) | 0.958 | 0.0 | 100 | 76.9 | 49.2 (21.8–76.5) |
| ADC | 0.005 | 0.002 | 89.6 | 87.5 | 89.0 | 90.0 (76.9–100) | 0.001 | 100 | 87.5 | 92.9 | 100 (100–100) | 0.473 | 0.64 | 100 | 77.1 | 65.0 (41.9–88.1) |
| CBV | 0.004 | 0.963 | 100 | 0 | 71.4 | 50.7 (24.1–77.3) | 0.003 | 66.7 | 87.5 | 78.6 | 75.0 (41.1–100) | 0.018 | 51.3 | 95.4 | 85.2 | 80.0 (50.7–100) |
| MD | 0.000 | 0.001 | 93.9 | 63.8 | 85.3 | 93.8 (85.0–100) | 0.001 | 100 | 100 | 100 | 100 (100–100) | 0.601 | 1.3 | 100 | 77.2 | 68.3 (46.3–90.3) |
| FA | 0.010 | 0.082 | 94.6 | 12.9 | 71.3 | 70.6 (50.2–91.0) | 0.007 | 100 | 85.5 | 91.8 | 100 (100–100) | 0.209 | 0.0 | 100 | 76.9 | 86.7 (71.0–100) |
| Q | 0.116 | 0.215 | 100 | 0 | 71.4 | 51.9 (27.5–76.4) | 0.104 | 77.6 | 80.4 | 79.7 | 83.3 (58.6–100) | 0.990 | 5.8 | 98.6 | 77.2 | 82.5 (65.4–99.3) |
| P | 0.000 | 0.001 | 89.7 | 69.2 | 84.0 | 91.9 (81.0–100) | 0.002 | 100 | 99.1 | 99.5 | 100 (100–100) | 0.737 | 0.0 | 100 | 76.9 | 60.0 (35.3–84.7) |
| D | 0.000 | 0.002 | 90.0 | 71.4 | 84.9 | 90.6 (78.6–100) | 0.000 | 100 | 94.0 | 96.7 | 100 (100–100) | 0.251 | 9.9 | 99.0 | 78.5 | 68.3 (46.0–90.6) |
| D* | 0.748 | 0.918 | 98.9 | 0.9 | 70.9 | 60.6 (31.8–89.5) | 0.734 | 41.2 | 74.1 | 61.5 | 60.4 (24.4–95.9) | 0.855 | 0.0 | 100 | 76.9 | 52.2 (23.5–80.8) |
| f | 0.505 | 0.941 | 100 | 0 | 71.4 | 52.0 (27.1–76.8) | 0.504 | 32.6 | 86.1 | 63.7 | 59.5 (27.8–90.7) | 0.574 | 0.6 | 97.6 | 75.2 | 64.2 (39.9–88.4) |
| K | 0.1136 | 0.906 | 93.5 | 14.9 | 71.2 | 73.7 (52.9–94.6) | 0.165 | 83.3 | 86.5 | 85.2 | 87.5 (65.8–100) | 0.145 | 0 | 100 | 76.9 | 55.8 (32.4–79.3) |
| T2 | 0.018 | 0.024 | 93.3 | 66.8 | 86.0 | 84.4 (65.4–100) | 0.041 | 83.3 | 87.5 | 85.7 | 87.5 (67.8–100) | 0.890 | 0.0 | 100 | 76.9 | 50.1 (24.4–75.8) |
| PD | 0.282 | 0.461 | 100 | 0.51 | 71.6 | 66.9 (43.0–90.7) | 0.272 | 42.9 | 86.5 | 68.1 | 72.9 (42.7–100) | 0.738 | 2.1 | 100 | 77.4 | 55.1 (23.4–86.9) |
| Count | 8.9E-06 | 0.221 | 92.5 | 25.9 | 73.5 | 83.8 (68.5–98.9) | 1.0E-05 | 66.7 | 100 | 85.7 | 100 (100–100) | 6.1E-05 | 66.7 | 99.8 | 92.1 | 91.7 (79.2–100) |
The values with bold-face type under the P-values columns belong to parameters with significant P-values: the column labeled as “ANOVA Test” shows P-values obtained based on the ANOVA test for simultaneous differentiation of the three regions from each other; the other three columns under the parts “NT from IE,” “NT from AT,” and “IE from AT” parts, labeled as “P-values,” indicate P-values calculated for pairwise differentiation of the regions based on Tukey-HSD or Games-Howell tests (whichever was appropriate for each parameter). The bold-face type values under other columns indicate high accuracy
The details of classification performances of individual parameters in differentiation of each of the two subregions from each other and based on the crossvalidated LDA method are given in Table 3. For differentiation of NT from IE (requiring high sensitivity), the highest performances were for T2_ISO, FLAIR, ADC, MD, P, D, and T2. T2-based features, ie, T2_ISO, FLAIR, and T2 resulted in AUCs >84% for this discrimination. The four diffusion-related parameters, ie, ADC, MD, P, and D, resulted in AUCs of over 90% (and sensitivities >90%) for discrimination of NT from IE. In this context, T1_SUB, CBV, and f were highly sensitive (100%) but lacked specificity.
All MRI-derived parameters except for D*, f, and PD were accurate for separation of NT from AT, implying high sensitivity of these metrics.
The only statistically significant MRI-derived feature for identification of IE from AT was DSC-MRI-derived parameter, ie, CBV, with high specificity of 95%.
CC showed high AUCs for differentiation of the three two-by-two subregions (>84%), although its specificity for separation of NT from IE was rather low.
Combination of Quantitative MRI-Derived Parameters
For classification of the three tissue subtypes from each other, ie, NT, IE, and AT, four feature combinations were selected by the feature selection methods: 1) CBV, MD, FLAIR, T2_ISO (selected by backward AIC); 2) CBV, MD (selected by backward BIC); 3) CBV, D, T2_ISO (forward/stepwise AIC); and 4) CBV, D (selected by backward BIC) (Table 4). Three classification methods, namely, LDA, QDA, and SVM, were applied on the selected combinations. As Table 4 summarizes, using the classifiers the most accurate feature set was “CBV, MD, FLAIR, T2_ISO” with AUC of ~90% for all three classifiers. Additionally, the feature combination “CBV, D, T2_ISO” resulted in AUC of ~92% using the LDA classification scheme.
TABLE 4.
Evaluating Accuracy and Diagnostic Performances (AUC) of LDA, QDA, and SVM Classifiers on the Selected Featuresa
| Feature selection method | Selected features | Accuracy (%) | AUC (95% CI) (%) |
|---|---|---|---|
| Cross-Validated LDA | |||
| Backward AIC | CBV, FLAIR, MD, T2_ISO | 82.8 | 89.3 (75.9-100) |
| Backward BIC | CBV, MD | 75.4 | 74.7 (55.0-94.4) |
| Forward/Stepwise AIC | CBV, D, T2_ISO | 82.1 | 91.9 (83.1-100) |
| Backward BIC | CBV, D | 74.8 | 73.2 (54.4-91.6) |
| Cross-Validated QDA | |||
| Backward AIC | CBV, FLAIR, MD, T2_ISO | 85.1 | 92.4 (84.1-100) |
| Backward BIC | CBV, MD | 79.9 | 80.0 (61.8-98.0) |
| Forward/Stepwise AIC | CBV, D, T2_ISO | 83.4 | 85.3 (71.2-99.1) |
| Backward BIC | CBV, D | 81.2 | 84.8 (69.1-99.4) |
| Cross-Validated SVM | |||
| Backward AIC | CBV, FLAIR, MD, T2_ISO | 91.3 | 91.9 (79.6-100) |
| Backward BIC | CBV, MD | 80.2 | 79.8 (61.4-98.1) |
| Forward/Stepwise AIC | CBV, D, T2_ISO | 79.3 | 83.0 (65.9-99.7) |
| Backward BIC | CBV, D | 79.3 | 81.4 (63.6-98.8) |
The gray-shaded cells with bold-type face numbers or letters indicate highest diagnostic performances.
Table 5 shows the performances of classification schemes (each classifier on the selected feature subsets) for two-by-two discrimination of the three subregions from each other. The combination of “CBV, MD, FLAIR, T2_ISO” using either QDA or SVM classifiers produces accurate results for all three discrimination purposes, ie, NT from IE, NT from AT, and IE from AT. Using QDA, this combination indicates an AUC of 92.3% for characterization of NT from IE, 100% for NT from AT, and 89.2% for IE from AT. SVM classification based on this combination results in AUC of 98.8% for identification of NT from IE, 100% for NT from AT, and 100% for IE from AT.
TABLE 5.
Evaluating Classification Performances of LDA, QDA, and SVM Classifiers on the Selected Features for Differentiation of Each of Two Tissue Subregions From Each Other, ie, NT From IE, NT From AT, and IE From ATa
| Classifiers | Feature combinations | NT from IE | NT from AT | IE from AT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens. | Spec. | Acc. | AUC (95% CI) | Sens. | Spec. | Acc. | AUC (95% CI) | Sens. | Spec. | Acc. | AUC (95% CI) | ||
| LDA | CBV, FLAIR, MD, T2_ISO | 90.0 | 86.8 | 89.1 | 94.8 (86.4–100) | 100 | 100 | 100 | 100 (100–100) | 57.9 | 99.8 | 90.1 | 92.0 (80.0–100) |
| CBV, MD | 91.1 | 65.9 | 84.0 | 92.6 (83.1–100) | 100 | 100 | 100 | 100 (100–100) | 62.3 | 99.6 | 91.1 | 91.5 (79.0–100) | |
| CBV, D, T2_ISO | 86.1 | 94.1 | 88.5 | 91.7 (80.5–100) | 100 | 100 | 100 | 100 (100–100) | 50.6 | 100 | 88.6 | 91.4 (78.6–100) | |
| CBV, D | 89.6 | 64.2 | 82.5 | 90.0 (77.7–100) | 100 | 100 | 100 | 100 (100–100) | 50.6 | 100 | 88.6 | 91.3 (78.3–100) | |
| QDA | CBV, FLAIR, MD, T2_ISO | 85.2 | 99.5 | 89.3 | 92.3 (83.9–99.9) | 100 | 100 | 100 | 100 (100–100) | 84.1 | 94.4 | 92.0 | 89.2 (72.5–100) |
| CBV, MD | 86.6 | 73.4 | 82.9 | 80.0 (61.6–98.0) | 100 | 100 | 100 | 100 (100–100) | 66.7 | 99.6 | 92.0 | 83.1 (83.1–100) | |
| CBV, D, T2_ISO | 81.4 | 89.8 | 93.9 | 85.6 (71.7–99.0) | 100 | 100 | 100 | 100 (100–100) | 83.3 | 99.8 | 96.0 | 91.6 (91.6–100) | |
| CBV, D | 85.5 | 83.4 | 85.1 | 84.5 (68.6–99.3) | 100 | 100 | 100 | 100 (100–100) | 61.1 | 100 | 91.1 | 80.6 (59.1–98.9) | |
| SVM | CBV, FLAIR, MD, T2_ISO | 94.6 | 89.8 | 93.3 | 98.8 (95.9–100) | 100 | 100 | 100 | 100 (100–100) | 85.4 | 100 | 96.6 | 100 (100–100) |
| CBV, MD | 93.7 | 65.2 | 85.6 | 94.6 (86.8–100) | 100 | 100 | 100 | 100 (100–100) | 69.5 | 100 | 92.9 | 93.5 (80.2–100) | |
| CBV, D, T2_ISO | 88.3 | 86.9 | 88.0 | 92.9 (82.9–100) | 100 | 100 | 100 | 100 (100–100) | 50.6 | 100 | 88.6 | 91.1 (77.8–100) | |
| CBV, D | 91.1 | 61.4 | 82.7 | 91.4 (79.8–100) | 100 | 100 | 100 | 100 (100–100) | 50.6 | 100 | 88.6 | 91.6 (78.7–100) | |
The gray-shaded cells with bold-type face numbers or letters indicate highest diagnostic performances of classifiers for all three discrimination.
Additionally, “CBV, D, T2_ISO” using the QDA classifier can generate AUC of 85.6% for differentiation of NT from IE, 100% for NT from AT, and 91.6% for IE from AT.
Discussion
Conventional MRI lacks accuracy in assessing physiological variations and regional heterogeneity within tumors. During the past years, several quantitative methods, such as DWI/DTI and DSC-MRI, have been investigated for their potential as adjuncts to conventional MRI. Yet the comparative and complementary roles of these methods and emerging techniques, such as T2-relaxometry and IVIM, are not explored, especially through histopathologically proven specimens and objective classification techniques.
In this preliminary study, we attempted to tackle this problem by evaluation of the aforementioned techniques on localized biopsy specimens, which were histopathologically assessed and attributed to AT, IE, and NT subregions. Among individual MRI-derived parameters, diffusion-related ADC, MD, P, D parameters, as markers of cellular proliferation, indicated high classification performance for identification of NT from IE, and NT from AT, but were not suitable for IE from AT separation. This finding along with significant correlation of D, MD, and P with histopathological CC parameter suggests that changes in cellular distribution captured by diffusion-based parameters can be early markers of glioma infiltration (NT from IE discrimination). But these changes in low-grade glioma are not significantly different among IE and AT subregions; therefore, these parameters may not be diagnostically valuable for localizing the most active part of the tumor and other features must be explored. Among FA and Q as indicators of diffusion anisotropy, FA was statistically significant for discrimination of the three subregions and showed significant inverse correlation with CC, and accurately discriminated NT from AT. However, FA did not result in statistically significant differences for isolation of NT from IE or IE from AT in our sample population.
In animal studies, DTI has been shown to identify microinfiltration of tumor cells in the surrounding brain tis- sue.33,34 Early investigations on human brain glioma indicate that DWI/DTI can provide helpful information for assessment of peritumoral edema and to define margins of tumor invasion.3,35,36 Specifically, ADC20,37 or MD and FA19,38,39 have shown correlations with NET based on histopathologically proven biopsy specimens.
Unlike other MRI-derived parameters, CBV was the only statistically significant feature for discrimination of IE from AT. Hence, early vascularization is a discriminative factor for identifying the most active component of gliomas. This parameter was not statistically significant for identification of NT from IE, indicating that in the early stages of infiltration and before transforming into the most active subpopulations, neoangiogenesis may not have a role in gliomas. CBV was evidently statistically significant for characterization of NT from AT. Nonetheless, the classification performance was lower than that of diffusion-based parameters. The suboptimal performance of CBV in contrast to diffusion-based parameters in this context can be attributed to our sample population of low-grade gliomas, in which neovascularization has not become largely prominent in the infiltrative or active tumor regions.
The best combination of these parameters was sought using crossvalidated feature selection and classification methods. Three classifiers with increasing complexity, namely, LDA, QDA, and SVM, were implemented to obtain insights about the capability of the selected feature combinations in tissue identification irrespective of classifier formulation. In all four selected feature sets, CBV and a diffusion- based parameter like D or MD were present, signifying the importance of perfusion and diffusion parameters in subregion characterization. Incorporation of T2_ISO and FLAIR to perfusion and diffusion parameters in the form of the “CBV, MD, T2_ISO, and FLAIR” combination resulted in high classification performance for characterization of the three tissue subregions from each other, using the classifiers (AUC ~90%). This feature set revealed high classification performance in pairwise subregion classification as well. T2_ISO and FLAIR are important factors for illustrating the pathogenic changes within glioma borders. Similar integration of parameters has been intuitively employed in brain tumor segmentation elsewhere.40–42
A combination of fewer parameters including “CBV, D, T2_ISO” based on the QDA classifier generated a relatively high classification performance for pairwise differentiation of tissue subregions. For occasions with high risk of patient discomfort and motion, IVIM acquisition combined with DSC-MRI perfusion and anatomical high-resolution T2w, required for image-guided neurosurgery, could be performed without addition of DTI acquisition, which is highly susceptible to motion.
Nonetheless, our study and the results achieved are limited by the included patient population and small sample size. Furthermore, even though in this work we used crossvalidation for reducing bias in classification, an independent validation dataset is required for generalizing the findings. Finally, in this work our research imaging protocol had to be adapted to the clinical setup constraints of our academic educational institution and, therefore, some of the imaging parameters may not be optimal; for example, in quantitative imaging sequences, the voxel sizes are nonisotropic. Future works may benefit from optimization of parameter specifications for quantitative imaging sequences.
In conclusion, through investigation of a variety of conventional and quantitative MRI techniques applied on histopathologically proven tissue specimens, we demonstrated that incorporation of a few imaging techniques (comprising conventional MRI [T2w and FLAIR] and DSC-MRI with DTI or IVIM) can form a multiparametric surrogate marker, predictive of tissue subregions prior to image-guided biopsy procedures.
Acknowledgments
Contract grant sponsor: Tehran University of Medical Sciences & Health Services; contract grant number: 28479; Tehran University of Medical Sciences and Health Services The authors thank Mohammad Peikari, PhD (Department of Medical Biophysics, University of Toronto, Canada) for sharing his code for analysis of histopathological data; Nima Gilani, PhD (Department of Cognitive Neuroscience, Maastricht University) for helping in analysis of IVIM/T2-relaxometry data; and our MRI technician, Behrouz Rafiei, MSc (Imaging Center, Imam Hospital, Tehran University of Medical Sciences, Iran).
References
- 1.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Boonzaier NR, Larkin TJ, Matys T, van der Hoorn A, Yan J-L, Price SJ. Multiparametric MR imaging of diffusion and perfusion in contrastenhancing and nonenhancing components in patients with glioblastoma. Radiology. 2017;160150 doi: 10.1148/radiol.2017160150. [DOI] [PubMed] [Google Scholar]
- 4.Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269:8–15. doi: 10.1148/radiol.13122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6:1359–1370. doi: 10.15252/emmm.201302627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol. 2011;101:319–323. doi: 10.1007/s11060-010-0251-4. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 10.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34:463–469. doi: 10.1007/BF00598951. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology. 1989;170:211–217. doi: 10.1148/radiology.170.1.2535765. [DOI] [PubMed] [Google Scholar]
- 13.Price SJ, Pena A, Burnet NG, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol. 2004;14:1909–1917. doi: 10.1007/s00330-004-2381-6. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson M, van Westen D, Stahlberg F, Sundgren PC, Latt J. The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter. MAGMA. 2013;26:345–370. doi: 10.1007/s10334-013-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bihan D, Lima M. Diffusion magnetic resonance imaging: what water tells us about biological tissues. PLoS Biol. 2015;13:e1002203. doi: 10.1371/journal.pbio.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caseiras GB, Chheang S, Babb J, et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol. 2010;73:215–220. doi: 10.1016/j.ejrad.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Boxerman JL, Shiroishi MS, Ellingson BM, Pope WB. Dynamic susceptibility contrast MR imaging in glioma: review of current clinical practice. Magn Reson Imaging Clin N Am. 2016;24:649–670. doi: 10.1016/j.mric.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Hu LS, Eschbacher JM, Dueck AC, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol. 2012;33:69–76. doi: 10.3174/ajnr.A2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durst CR, Raghavan P, Shaffrey ME, et al. Multimodal MR imaging model to predict tumor infiltration in patients with gliomas. Neuroradiology. 2014;56:107–115. doi: 10.1007/s00234-013-1308-9. [DOI] [PubMed] [Google Scholar]
- 20.Eidel O, Neumann JO, Burth S, et al. Automatic analysis of cellularity in glioblastoma and correlation with ADC using trajectory analysis and automatic nuclei counting. PLoS One. 2016;11:e0160250. doi: 10.1371/journal.pone.0160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leemans A, Jeurissen B, Sijbers J, Jones D. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. 17th Annual Meeting ISMRM; Honolulu. 2009. p. 3537. [Google Scholar]
- 22.Wang W, Steward C, Desmond P. Diffusion tensor imaging in glioblastoma multiforme and brain metastases: the role of p, q, L, and fractional anisotropy. AJNR Am J Neuroradiol. 2009;30:203–208. doi: 10.3174/ajnr.A1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournet G, Li J-R, Cerjanic AM, Sutton BP, Ciobanu L, Le Bihan D. A two-pool model to describe the IVIM cerebral perfusion. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16681310. 0271678X16681310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bihan D, Breton E, Lallemand D, Aubin M, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 25.Gilani N, Malcolm P, Johnson G. A model describing diffusion in prostate cancer. Magn Reson Med. 2017;78:316–326. doi: 10.1002/mrm.26340. [DOI] [PubMed] [Google Scholar]
- 26.Pavilla A, Gambarota G, Arrigo A, Mejdoubi M, Duvauferrier R, Saint-Jalmes H. Diffusional kurtosis imaging (DKI) incorporation into an intravoxel incoherent motion (IVIM) MR model to measure cerebral hypoperfusion induced by hyperventilation challenge in healthy subjects. MAGMA. 2017:1–10. doi: 10.1007/s10334-017-0629-9. [DOI] [PubMed] [Google Scholar]
- 27.Peikari M, Martel AL. Automatic cell detection and segmentation from H and E stained pathology slides using colorspace decorrelation stretching. SPIE Med Imaging. 2016:979114–979116. [Google Scholar]
- 28.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979:65–70. [Google Scholar]
- 29.Akaike H. Selected Papers of Hirotugu Akaike. Berlin: Springer; 1998. Information theory and an extension of the maximum likelihood principle; pp. 199–213. [Google Scholar]
- 30.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 31.Fathi Kazerooni A, Nabil M, Haghighat Khah H, et al. ADC-derived spatial features can accurately classify adnexal lesions. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25854. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Kazerooni AF, Malek M, Haghighatkhah H, et al. Semiquantitative dynamic contrast-enhanced MRI for accurate classification of complex adnexal masses. J Magn Reson Imaging. 2017;45:418–427. doi: 10.1002/jmri.25359. [DOI] [PubMed] [Google Scholar]
- 33.Lope-Piedrafita S, Garcia-Martin ML, Galons JP, Gillies RJ, Trouard TP. Longitudinal diffusion tensor imaging in a rat brain glioma model. NMR Biomed. 2008;21:799–808. doi: 10.1002/nbm.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Pickup S, Hsu O, Poptani H. Diffusion tensor MRI in rat models of invasive and well-demarcated brain tumors. NMR Biomed. 2008;21:208–216. doi: 10.1002/nbm.1183. [DOI] [PubMed] [Google Scholar]
- 35.Sternberg EJ, Lipton ML, Burns J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am J Neuroradiol. 2014;35:439–444. doi: 10.3174/ajnr.A3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macyszyn L, Akbari H, Pisapia JM, et al. Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neurooncology. 2016;18:417–425. doi: 10.1093/neuonc/nov127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadeghi N, D’Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol. 2008;29:476–82. doi: 10.3174/ajnr.A0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barajas RF, Jr, Phillips JJ, Parvataneni R, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neurooncology. 2012;14:942–954. doi: 10.1093/neuonc/nos128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu LS, Ning S, Eschbacher JM, et al. Multi-parametric MRI and texture analysis to visualize spatial histologic heterogeneity and tumor extent in glioblastoma. PLoS One. 2015;10:e0141506. doi: 10.1371/journal.pone.0141506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazerooni AF, Mohseni M, Rezaei S, Bakhshandehpour G, Rad HS. Multi-parametric (ADC/PWI/T2-w) image fusion approach for accurate semi-automatic segmentation of tumorous regions in glioblastoma multiforme. MAGMA. 2015;28:13–22. doi: 10.1007/s10334-014-0442-7. [DOI] [PubMed] [Google Scholar]
- 41.Ion-Margineanu A, Van Cauter S, Sima DM, et al. Classifying glioblastoma multiforme follow-up progressive vs. responsive forms using multi-parametric MRI features. Front Neurosci. 2017;10:615. doi: 10.3389/fnins.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emblem KE, Nedregaard B, Hald JK, Nome T, Due-Tonnessen P, Bjornerud A. Automatic glioma characterization from dynamic susceptibility contrast imaging: Brain tumor segmentation using knowledge-based fuzzy clustering. J Magn Reson Imaging. 2009;30:1–10. doi: 10.1002/jmri.21815. [DOI] [PubMed] [Google Scholar]


