Version Changes
Revised. Amendments from Version 1
Just the first sentence of Methods has been modified. Mosquitoes were collected in May (8 locations) and November 2017 (Pointe Noire only) corresponding to the rainy season in nine locations in the Republic of the Congo across the north-south transect.
Abstract
Background: The arbovirus vector, Aedes albopictus, originating from Asia, has recently invaded African countries, including the Republic of the Congo, where it was associated with a chikungunya outbreak. Up until now, little was known about its distribution in relation to the native Aedes aegypti and how the invasion will modify the epidemiology of arboviral diseases. Here, we assessed the current distribution of Ae. albopictus and Ae. aegypti in the Republic of the Congo and explored the genetic diversity of the invading species, Ae. albopictus.
Methods: Immature stages of Aedes were collected in nine locations in the Republic of the Congo in 2017 following a north-south transect and reared to adult stage. Adults were morphologically identified, counted and grouped according to species and location. Genetic diversity of Ae. albopictus was assessed by analyzing the cytochrome oxidase I ( COI) gene.
Results: Ae. albopictus and Ae. aegypti were found together across the country in all the locations investigated. The invasive species is predominant over the native species in all locations except Brazzaville, suggesting that Ae. albopictus is displacing Ae. aegypti across Congo. When comparing the species distributions across the two largest cities, Brazzaville and Pointe Noire, Ae. albopictus was more prevalent than Ae. aegypti in the suburbs whereas the opposite situation was reported in the city centre. Mitochondrial DNA analysis revealed very low genetic diversity of Ae. albopictus with only three haplotypes recorded across the country supporting the recent introduction of this species in the Republic of the Congo. Phylogenetic tree analysis revealed that Ae. albopictus from Congo originated from other tropical Asian countries such as China, likely as a result of increasing trade links.
Conclusion: These findings are important for the implementation of vector control strategies and can serve as a foundation for further research on these vectors in the country.
Keywords: Aedes albopictus, Aedes aegypti, ecological distribution, arbovirus vectors, genetic diversity, Republic of Congo
Introduction
Arthropod-borne viral diseases such as dengue, zika and chikungunya have emerged or re-emerged in several countries of the world during the past decades 1– 4.
These viruses are transmitted to vertebrates, including humans, by the bites of infected mosquitoes that share the same ecological niche as the host organism. Indeed, two distinct ecological cycles, enzootic and urban epidemic cycles, have been well documented 5, 6. The enzootic cycle occurs in the sylvan environment, involving non-human primates and wild mosquitoes, while urban epidemic cycle occurs in urban environments, implicating human beings and urban mosquitoes such as Aedes aegypti Linneaus 1762 and Ae. albopictus (Skuse) 1894. Other potential modes of zika virus transmission to humans have been evoked notably via sexual intercourse or via blood donor 5. Both epidemic vectors, Ae. aegypti and Ae. albopictus, are found in sub-Saharan Africa, where Ae. aegypti is native. Two subspecies of Ae aegypti, Ae. aegypti formosus and Ae. aegypti aegypti, were formally identified by Mattingly in 1957 7. Ae. aegypti formosus, is a dark colored mosquito confined to African forests while Ae. aegypti aegypti is light-colored with white abdominal scales and is found in human-dominated habitats primarily outside Africa. Generally, Ae. aegypti collected in central Africa match Ae. aegypti formosus 8.
While Ae. albopictus is a native of South East Asia, it has now invaded all the five continents during the past 30–40 years 9, 10. This rapid global spread was caused mainly by sales and distribution of used tires across the world 11 coupled with the ecological plasticity of the species, enabling its adaptation to various environments 9. Ae. albopictus was reported for the first time in Central Africa in early 2000 12 and is currently present in almost all central African countries 13, where it tends to supplant the indigenous species Ae. aegypti in human-domesticated environment 14, 15. The predominance of Ae. albopictus over Ae. aegypti in sympatric areas has been shown to result from the higher mating competitiveness of Ae. albopictus over Ae. aegypti 16, 17. Previous studies in Central Africa showed that both Ae. aegypti and Ae. albopictus can be found together in the same location and often share the same larval habitats 14, 15. In this region, the immature stages of both species develop in stagnant water found mainly in peri-domestic containers such as used tires and discarded tanks. However, in the sympatric area, Ae. albopictus prefers containers surrounded by vegetation whereas Ae. aegypti prefers containers located in neighbourhoods with high building densities 15, 18.
Dengue, zika and chikungunya were for a long-time considered to be rare in Central Africa, because only sporadic epidemics were reported in the rural environment, with isolation of the viruses in wild mosquitoes and humans 19, 20. In the past decades, several outbreaks have been reported in this part of the world, notably a concurrent dengue/chikungunya outbreak in Gabon in 2007, with more than 20, 000 cases of chikungunya 2, and a large chikungunya outbreak in 2011 in the Republic of the Congo with more than 11, 000 cases 21. This suggests an epidemiological modification of arboviral diseases in the region. During these outbreaks, Ae. albopictus was established as the major vector particularly in Gabon 22, 23, where zika was detected in this species 24. In Congo, both Ae. aegypti and Ae. albopictus were found to be positive for chikungunya virus 25, implicating both species in virus transmission. This investigation was the first to confirm the presence of Ae. albopictus in the Republic of the Congo. Since then, no study has been undertaken to compare the geographical distribution and prevalence of Ae. aegypti and Ae. albopictus in the Republic of the Congo as well as the genetic diversity of the invading species. Indeed, previous studies in Central Africa based on polymorphisms to the cytochrome oxidase subunit 1 ( COI) gene indicated that Ae. albopictus populations in Cameroon are related to tropical rather than temperate or subtropical out-groups 26. However, the Central African Republic population segregated into two lineages: the first encompassed specimens from tropical areas including all the haplotypes from Cameroon and the second lineage encompassed temperate and subtropical areas 15, suggesting multiple sources of Ae. albopictus.
To improve entomological surveillance and the control of these arbovirus vectors in the Republic of the Congo, we present here the current nation-wide geographical distribution and prevalence of Ae. aegypti and Ae. albopictus in this country, and establish the genetic diversity of the invading population of Ae. albopictus using the COI gene.
Methods
Sampling sites
Mosquitoes were collected in May and November 2017 (Pointe Noire only) corresponding to the rainy season in nine locations in the Republic of the Congo across the north-south transect ( Table 1 and Figure 1). The Republic of the Congo is located in Central Africa, straddling the equator. Two main types of vegetation are found. The forest in the north, covering 60% of the national territory, and the savannah, which occupies the remaining parts of the country. There are three types of climate. The equatorial climate is found in the north of the country, characterized by high humidity and rainfall greater than 1,700 mm per year, with an average temperature between 24°C and 26°C. The humid tropical climate in the southwest, where annual average precipitation varies from 1,200 mm to 1,700 mm, with an average monthly temperature between 21°C and 27°C. The subequatorial climate, experienced at the plateau and basin regions, has an average annual rainfall of about 1,600 mm. Because the spread of Aedes mosquitoes mainly relies on human activities, sampling was focused on human-domesticated environments spread along the main communication networks, and trade routes throughout the country.
Table 1. Sampling sites in the Republic of the Congo.
| Location | Geographical coordinates | Altitude, m | Climate |
|---|---|---|---|
| Brazzaville | S 4°19'38'' E 15°09'12'' | 278 | Subequatorial climate |
| Lefini | S 2°54'58" E 15°37'56" | 314 | Subequatorial climate |
| Ngo | S 2°29'14" E 15°45'00" | 636 | Subequatorial climate |
| Gamboma | S 1°52'27" E 15°52'25" | 378 | Subequatorial climate |
| Oyo | S 1°09'14" E 15°58'21" | 297 | Subequatorial climate |
| Owando | S 0°29'42" E 15°54'41" | 275 | Subequatorial climate |
| Makoua | S 0°00'23" E 19°37'33" | 350 | Equatorial climate |
| Ouesso | N 1°36'35" E 16°02'58" | 339 | Equatorial climate |
| Pointe Noire | N 4°48'19" E 11°53'23" | 14 | Tropical climate |
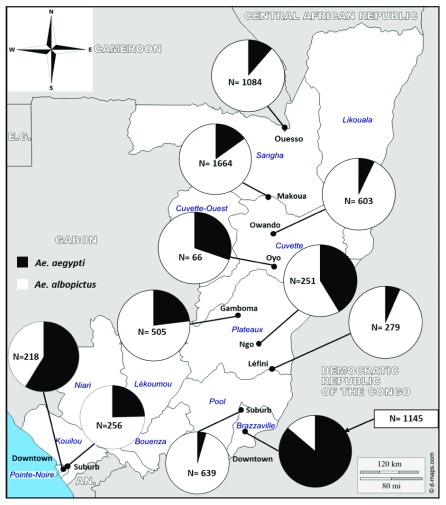
Figure 1. Geographic distribution of Ae. aegypti and Ae. albopictus across the Republic of the Congo.
Mosquito collection, rearing and identification
In Brazzaville and Pointe Noire, the two most populated cities of the Republic of the Congo, the difference between downtown and suburban was examined during the investigation. In the other locations, however, samples were collected randomly throughout each city and pooled together. In each selected location, all containers with water were inspected and positive containers (with at least one Aedes larvae or pupae) were recorded. Immature stages of Aedes were collected, transported to the insectaries, pooled according to the location and reared to adult stage for morphological identification. G0 adults were stored at -20°C for molecular and genetic analyses. The comparisons between the prevalence of Ae. aegypti and Ae. albopictus in each location, across the country were performed using multiple chi-square test.
Mitochondrial DNA analysis for Ae. albopictus
Genomic DNA was extracted from 20 whole Ae. albopictus per location (nine locations) using the Livak protocol as previously described 27. DNA extracts from each location were used as templates to amplify 700-bp fragment of COI gene. The sequences of primers used are: albCOIF 5’-TTTCAACAAATCATAAAGATATTGG-3’ and albCOIR 5’- TAAACTTCTGGA TGACCAAAAAATCA-3’ 28. Polymerase chain reaction (PCR) amplification was performed using a Gene Touch thermal cycler (Bulldog Bio, Portsmouth, USA), as described previously 28. PCR products were detected by agarose gel electrophoresis in Tris-Acid-EDTA buffer (TAE). The gel was prepared with Midori green, staining dye, and visualized with the aid of UV light. PCR products from each location with very good amplification were purified using the Exo-SAP protocol and sent to the Centre for Genomic Research (Liverpool, UK) for sequencing.
Sequence data analysis
Sequences were manually corrected using BioEdit software version 7.2.1 ( http://en.bio-soft.net/format/BioEdit.html) and aligned using ClustalW, which is present in BioEdit 29. Sequences were numbered based on the reference sequences downloaded in GenBank KU738429.1. The number of haplotypes (h), the number of polymorphism sites (S), haplotype diversity (Hd) and nucleotide diversity (π) were computed with DnaSP 5.10.01 ( http://en.bio-soft.net/dna/dnasp.html) 30. The statistical tests of Tajima 31, and Fu and Li 32 were also estimated with DnaSP in order to establish non-neutral evolution and deviation from mutation-drift equilibrium. The different haplotypes detected were compared to previous sequences published in GenBank ( Supplementary Table 1) that originated from China, Papua New Guinea, USA, Singapore, Taiwan, Malaysia, Hawai, Christmas Islands, Japan, Solomon Islands, Timor Leste and Torres Strait Islands 28, 33, 34. The same COI region was sequenced at these various regions and the maximum likelihood phylogenetic tree was constructed using MEGA 7.0 35. Genealogical relationships between haplotype in this current study was assessed using TCS version 1.21 36 and tcsBU ( http://cibio.up.pt/software/tcsBU/) 37 software.
Results
Containers inspected and prevalence of Ae. aegypti and Ae. albopictus
A total of 640 containers with water were investigated across the Republic of the Congo ( Table 2). Among them, 42.9% were positive for immature stages of Aedes. Containers were classified into three main groups: domestic (flower pot and water storage tanks), peridomestic (used tires, discarded tanks and car wrecks) and natural (axil of plants). Used tires were the most prevalent habitat and most productive containers in all the locations, ranging from 18.1% in Pointe Noire to 100% in Lefini ( Table 2). The presence of Aedes in other containers was very limited.
Table 2. Containers prospected per location.
| Location | Axil of
plants |
Used
tires |
Car
wrecks |
Discarded
tanks |
Water
storages |
Flower
pots |
All |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Brazzaville downtown | 1 (0.0) | 59 (49.2) | 3 (100) | 10 (80.0) | 0 (NC) | 0 (NC) | 73 (54.8) |
| Brazzaville suburb | 0 (NC) | 69 (63.8) | 0 (NC) | 3 (0.0) | 0 (NC) | 0 (NC) | 72 (61.1) |
| Pointe Noire downtown | 0 (NC) | 61 (18.1) | 0 (NC) | 3 (33.3) | 0 (NC) | 0 (NC) | 64 (18.8) |
| Pointe Noire suburb | 0 (NC) | 56 (25.0) | 0 (NC) | 13 (46.2) | 4 (50.0) | 0 (NC) | 73 (30.1) |
| Lefini | 0 (NC) | 3 (100) | 0 (NC) | 0 (NC) | 0 (NC) | 0 (NC) | 3 (100) |
| Ngo | 0 (NC) | 47 (38.3) | 0 (NC) | 0 (NC) | 0 (NC) | 0 (NC) | 47 (38.3) |
| Gamboma | 0 (NC) | 58 (37.9) | 0 (NC) | 2 (50) | 1 (100) | 0 (NC) | 61 (39.3) |
| Oyo | 0 (NC) | 43 (48.8) | 0 (NC) | 0 (NC) | 0 (NC) | 0 (NC) | 43 (48.8) |
| Owando | 0 (NC) | 4 (25) | 0 (NC) | 8 (62.5) | 0 (NC) | 10 (10.0) | 22 (31.8) |
| Makoua | 0 (NC) | 59 (54.2) | 0 (NC) | 10 (30) | 0 (NC) | 0 (NC) | 69 (50.7) |
| Ouesso | 0 (NC) | 97 (50.5) | 0 (NC) | 0 (NC) | 0 (NC) | 0 (NC) | 97 (50.5) |
| All | 1 (0.0) | 556 (43.9) | 3 (100) | 65 (36.9) | 5 (60.0) | 10 (10.0) | 640 (42.9) |
N, number of containers found with water; (%), percentage of positive containers; NC, not computed.
In total, 6,684 specimens of immature stages of Aedes were identified, comprising 72.24% of Ae. albopictus, 27.70% of Ae. aegypti and 0.06% (four specimens collected in Brazzaville suburb) of Aedes simpsoni. Ae. aegypti and Ae. albopictus were found together in all the locations investigated ( Figure 1 and Table 3). However, Ae. albopictus was predominant in all the locations except in Brazzaville. When samples from the two major cities, Brazzaville and Pointe Noire, were divided according to the environment (downtown versus suburb), Ae. albopictus was found more prevalent in the suburbs (95.62% and 75.39% in Brazzaville and Pointe Noire, respectively) than Ae. aegypti, whereas the reverse was true for the downtown areas ( Table 3).
Table 3. Prevalence of Aedes aegypti and Aedes albopictus according to the location.
| Location | Ae. aegypti | Ae. albopictus | P-value |
|---|---|---|---|
| Brazzaville downtown | 962 (86.28%) | 153 (13.72%) | <0.001 |
| Brazzaville suburb | 28 (4.38%) | 611 (95.62%) | <0.001 |
| Pointe Noire downtown | 128 (58.72%) | 90 (41.28%) | <0.001 |
| Pointe Noire suburb | 63 (24.61%) | 193 (75.39%) | <0.001 |
| Lefini | 18 (6.45%) | 261 (93.55%) | <0.001 |
| Ngo | 104 (41.43%) | 147 (58.57%) | <0.001 |
| Gamboma | 116 (22.97%) | 389 (77.03%) | <0.001 |
| Oyo | 20 (30.30%) | 46 (69.70%) | <0.001 |
| Owando | 42 (6.97%) | 561 (93.03%) | <0.001 |
| Makoua | 249 (14.96%) | 1415 (85.04%) | <0.001 |
| Ouesso | 122 (11.25%) | 962 (88.75%) | <0.001 |
| All | 1852 (27.72%) | 4828 (72.28%) | <0.001 |
Mitochondrial DNA analysis of Ae. albopictus
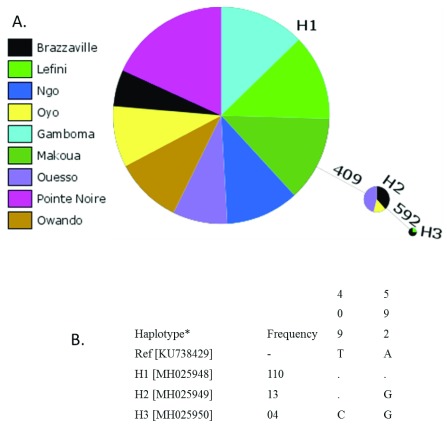
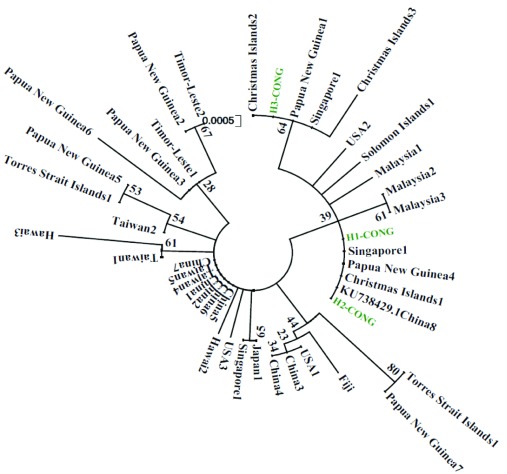
In total, 127 specimens of Ae. albopictus from nine locations across the Republic of the Congo were analysed using the COI gene. Sequence analysis, based on 638 nucleotides, revealed a low polymorphism, with only two mutational sites defining three haplotypes namely H1, H2 and H3 ( Figure 2B). Consequently, this resulted in low haplotype diversity (Hd=0.24) and nucleotide diversity (π=0.00005) indexes ( Table 4). The most frequent haplotype, H1 (86.6%), was detected in all the locations. Supplementary Table 2 shows the haplotype distribution per location. The haplotypes H2 (10.2%) and H3 (3.2%) were found in three (Brazzaville, Ouesso and Oyo) and two (Brazzaville and Lefini) locations, respectively ( Table 4 and Figure 2). The dominant haplotype matches perfectly with the COI gene sequence deposited in GenBank that originated from China (KU738429.1). A higher genetic diversity was reported in Brazzaville where all the three haplotypes were reported. The haplotype network showed that each haplotype was separated from the others by one mutational step ( Figure 2). Overall, Tajima’s D (D=-0.294) and Fu’s Fs (Fs=-0.024) statistics were negative, but not statistically significant. Phylogenetic tree generated and analysed based on 445 nucleotides previously published in GenBank showed that the Republic of the Congo’s haplotypes were closely related to the sequences from China, Singapore, Papua New Guinea and Christmas Island ( Figure 3).
Figure 2. Genetic diversity of the COI gene across Congolese populations of Ae. albopictus.
( A) Haplotype network showing the genealogic relationships between three haplotypes detected across Congo. The pie chart represents the proportion of each haplotype per site. ( B) COI haplotypes found across the Republic of the Congo. Only polymorphic positions are shown and are numbered with reference (Ref) to the published Ae. albopictus sequences for COI (JF309317; China). Dots represent identity with respect to the reference. The numbers above nucleotides indicate the position where mutations were found. *GenBank accession number shown in brackets.
Table 4. Summary statistics for COI gene polymorphism in Aedes albopictus from the Republic of the Congo.
| Locality | N | H | S | Hd | π (k) | D | D* | Fs | F* |
|---|---|---|---|---|---|---|---|---|---|
| Brazzaville | 14 | H1, H2, H3 | 2 | 0.692 | 0.0014 (0.890) | 1.127 ns | 0.935 ns | 0.612 ns | 1.021 ns |
| Lefini | 15 | H1, H3 | 2 | 0.133 | 0.0004 (0.266) | -1.490 ns | -1.873 ns | 0.235 ns | -1.844 ns |
| Ngo | 12 | H1 | 0 | 0.000 | 0.0000 (0.000) | NC | NC | NC | NC |
| Gamboma | 14 | H1 | 0 | 0.000 | 0.0000 (0.000) | NC | NC | NC | NC |
| Oyo | 12 | H1, H2 | 1 | 0.303 | 0.0005 (0.303) | -0.195 ns | 0.752 ns | 0.297 ns | 0.533 ns |
| Owando | 11 | H1 | 0 | 0.000 | 0.0000 (0.000) | NC | NC | NC | NC |
| Makoua | 14 | H1 | 0 | 0.000 | 0.0000 (0.000) | NC | NC | NC | NC |
| Ouesso | 15 | H1, H2 | 1 | 0.514 | 0.0008 (0.5143) | 1.376 ns | 0.701 ns | 1.253 ns | 0.906 ns |
| Pointe Noire | 20 | H1 | 0 | 0.000 | 0.0000 (0.000) | NC | NC | NC | NC |
| Total | 127 | 3 | 2 | 0.246 | 0.0005 (0.2952) | -0.294 ns | 0.662 ns | -0.0238 ns | 0.417 ns |
N, number of sequences; S, number of polymorphic sites; H, haplotype; Hd, haplotype diversity; π, nucleotide diversity; k, mean number of nucleotide differences; D, Tajima statistic; D* and F*, Fu and Li statistics; Fs, Fu statistic; NC, not computed; ns, not significant.
Figure 3. Molecular phylogenetic analysis using the Maximum Likelihood method.
The evolutionary history was inferred using the Maximum Likelihood method based on the Tamura 3-parameter model. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. There was a total of 445 positions in the final dataset.
Discussion
This study has assessed the geographical distribution of Ae. aegypti and Ae. albopictus in the Republic of the Congo, revealing the co-occurrence of both species across the country. Analyses showed that the invasive species Ae. albopictus is the predominant species in all locations investigated except Brazzaville. The co-occurrence of Ae. aegypti and Ae. albopictus across the Republic of the Congo suggests that the environmental factors which prevail in the country are favourable for the development of both species. The presence of Ae. albopictus in the Republic of the Congo was confirmed in 2011 during the chikungunya outbreak in Brazzaville 25, suggesting its recent introduction. Indeed, previous studies in some central African countries such as Cameroon and Central Africa Republic showed that the co-occurrence of Ae. aegypti and Ae. albopictus is limited to the southern part of the country up to 6°N 14, 15, 18 suggesting that climate is a limiting factor for invasion. The predominance of the invading species, Ae. albopictus, over the indigenous species, Ae. aegypti, has been previously reported in areas where both species are found together in Central Africa. The ecological plasticity of Ae. albopictus has been suggested as the main cause of its adaptation to different environments 9, as well as its mating competitiveness in areas of sympatry with Ae. aegypti 17, 38. The prevalence of each species can vary according to the season in sympatric areas, as shown previously 15, 39, but was specifically linked to the duration of the dry season 40. Although, Ae. aegypti and Ae. albopictus have desiccant-resistant eggs, previous studies showed that Ae. aegypti eggs are more tolerant to high temperatures than those of Ae. albopictus 41. In both major cities where samples were analysed according to peri-urban and downtown environment, results revealed the predominance of Ae. aegypti in downtown areas but less in peri-urban areas. Similar findings to these were reported previously in Central Africa 15, 18, 40. These observations are consistent with former studies indicating the segregation of habitats in sympatric areas according to urban environmental gradients as the main factor responsible for the coexistence of Ae. aegypti and Ae. albopictus 42, 43. Used tyres were the most common container found positive for Aedes in all the locations. This is in accordance with previous studies in Central Africa showing that used tires are the main productive for both Ae. aegypti and Ae. albopictus 13, 15. However, the current study targeted mainly garages and tire shops to increase the chances of discovering immature Aedes.
The presence and predominance of Ae. albopictus across the Republic of the Congo can increase the risk of mosquito-borne arboviral diseases since Ae. albopictus has been found competent to transmit about 22 arboviruses 44. Notably, the emergence of dengue and chikungunya viruses in the human dominated environment in central Africa coincides with the invasion of Ae. albopictus in this area where it was found as the main vector 13, 23, 25. During previous studies in central Africa, Ae. albopictus, was found to be infected by zika virus in natural conditions 24. It was also demonstrated that Ae. albopictus from Bangui in Central African Republic is able to transmit enzootic chikungunya virus strains 45.
A very low polymorphism of Ae. albopictus discovered in the Republic of the Congo in this study is in agreement with the previous studies using the COI gene in areas newly colonised by this species including some Central African countries 15, 26, 46. This low polymorphism is consistent with the recent introduction from a founder Ae. albopictus population or could be related to ubiquitous Wolbachia infection in populations of this species, as suggested previously 47. Brazzaville, the capital city of the Republic of the Congo would be probably the main entry point of Ae. albopictus in the country, as higher levels of polymorphism (all the three haplotypes recorded) were detected at this location. For instance, Ae. albopictus was reported for the first time in the Republic of the Congo in Brazzaville during a chikungunya outbreak which occurred in the country 21, 25. Phylogenetic analysis showed that the haplotype sequences from the Republic of the Congo are very close to the sequences isolated from populations originating from China, New Papua Guinea, Singapore and Christmas Islands. Primers used in this current study were not the same as those used in the previous study in Cameroon, Central African Republic and Sao Tome island. Therefore, the haplotypes in the current study cannot be compared with those detected in Central Africa. Nevertheless, these data indicate that the population of Ae. albopictus found in Central Africa probably originated from other tropical regions as previously suggested 15, 26, 46. It will be interesting to perform other studies at macro-geographic scale using other markers such as double-digest restriction-site-associated DNA sequencing to assess the genetic structure and the level of the gene flow between these populations.
Conclusion
To our knowledge, this is the first study assessing the distribution of Ae. aegypti and Ae. albopictus in the Republic of the Congo since Ae. albopictus was reported in 2011. Both species were found across the country with Ae. albopictus predominating in almost all locations. Low genetic polymorphism of Ae. albopictus indicated a recent introduction into the country. The spread of the invading species across the country could change the epidemiology of arboviral diseases in the Republic of the Congo. Thus, it will be important to assess urgently, the vector competence of both Aedes species from the Republic of the Congo to prevent several emergence or re-emergence of arboviruses such as dengue, zika and yellow fever viruses. Assessing the susceptibility profile of these species to insecticide will be an important information needed to prepare the country against potential future outbreaks.
Data availability
Sequence for Aedes albopictus haplotype H1 cytochrome oxidase subunit I ( COI) gene, partial cds; mitochondrial, GenBank accession number MH025948: http://identifiers.org/ncbigi/GI:1402399558.
Sequence for A. albopictus haplotype H2 cytochrome oxidase subunit I ( COI) gene, partial cds; mitochondrial, GenBank accession number MH025949: http://identifiers.org/ncbigi/GI:1402399560.
Sequence for A. albopictus haplotype H3 cytochrome oxidase subunit I ( COI) gene, partial cds; mitochondrial, GenBank accession number MH025950: http://identifiers.org/ncbigi/GI:1402399562.
Acknowledgments
We thank the people living around all the sampling sites for their cooperation during the field investigations.
Funding Statement
This work was supported by a Wellcome Trust Training Fellowship in Public Health and Tropical Medicine (204862) awarded to Basile Kamgang. The funders had no role in study design, data collection or analysis, decision to publish or preparation of the manuscript.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 3 approved]
Supplementary material
Supplementary Table 1. Outgroup sequences used for phylogenetic analysis.References
- 1. Hennessey M, Fischer M, Staples JE: Zika Virus Spreads to New Areas - Region of the Americas, May 2015-January 2016. MMWR Morb Mortal Wkly Rep. 2016;65(3):55–58. 10.15585/mmwr.mm6503e1 [DOI] [PubMed] [Google Scholar]
- 2. Leroy EM, Nkoghe D, Ollomo B, et al. : Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009;15(4):591–593. 10.3201/eid1504.080664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatt S, Gething PW, Brady OJ, et al. : The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nsoesie EO, Kraemer MU, Golding N, et al. : Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro Surveill. 2016;21(20). 10.2807/1560-7917.ES.2016.21.20.30234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma A, Lal SK: Zika Virus: Transmission, Detection, Control, and Prevention. Front Microbiol. 2017;8:110. 10.3389/fmicb.2017.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thiboutot MM, Kannan S, Kawalekar OU, et al. : Chikungunya: a potentially emerging epidemic? PLoS Negl Trop Dis. 2010;4(4):e623. 10.1371/journal.pntd.0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mattingly PF: Genetical aspects of the Aedes aegypti problem. I. Taxonom: and bionomics. Ann Trop Med Parasitol. 1957;51(4):392–408. 10.1080/00034983.1957.11685829 [DOI] [PubMed] [Google Scholar]
- 8. Gloria-Soria A, Ayala D, Bheecarry A, et al. : Global genetic diversity of Aedes aegypti. Mol Ecol. 2016;25(21):5377–5395. 10.1111/mec.13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paupy C, Delatte H, Bagny L, et al. : Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11(14–15):1177–1185. 10.1016/j.micinf.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 10. Benedict MQ, Levine RS, Hawley WA, et al. : Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7(1):76–85. 10.1089/vbz.2006.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reiter P, Fontenille D, Paupy C: Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect Dis. 2006;6(8):463–464. 10.1016/S1473-3099(06)70531-X [DOI] [PubMed] [Google Scholar]
- 12. Fontenille D, Toto JC: Aedes (Stegomyia) albopictus (Skuse), a potential new Dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7(6):1066–1067. 10.3201/eid0706.010631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ngoagouni C, Kamgang B, Nakouné E, et al. : Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasit Vectors. 2015;8:191. 10.1186/s13071-015-0808-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simard F, Nchoutpouen E, Toto JC, et al. : Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: culicidae) in Cameroon, Central Africa. J Med Entomol. 2005;42(5):726–731. 10.1093/jmedent/42.5.726 [DOI] [PubMed] [Google Scholar]
- 15. Kamgang B, Ngoagouni C, Manirakiza A, et al. : Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis. 2013;7(12):e2590. 10.1371/journal.pntd.0002590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bargielowski IE, Lounibos LP, Shin D, et al. : Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in nature. Infect Genet Evol. 2015;36:456–461. 10.1016/j.meegid.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bargielowski IE, Lounibos LP, Carrasquilla MC: Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc Natl Acad Sci U S A. 2013;110(8):2888–2892. 10.1073/pnas.1219599110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamgang B, Happi JY, Boisier P, et al. : Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med Vet Entomol. 2010;24(2):132–141. 10.1111/j.1365-2915.2010.00869.x [DOI] [PubMed] [Google Scholar]
- 19. Desdouits M, Kamgang B, Berthet N, et al. : Genetic characterization of Chikungunya virus in the Central African Republic. Infect Genet Evol. 2015;33:25–31. 10.1016/j.meegid.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 20. Berthet N, Nakouné E, Kamgang B, et al. : Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14(12):862–865. 10.1089/vbz.2014.1607 [DOI] [PubMed] [Google Scholar]
- 21. Moyen N, Thiberville SD, Pastorino B, et al. : First reported chikungunya fever outbreak in the republic of Congo, 2011. PLoS One. 2014;9(12):e115938. 10.1371/journal.pone.0115938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paupy C, Kassa Kassa F, Caron M, et al. : A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis. 2012;12(2):167–169. 10.1089/vbz.2011.0736 [DOI] [PubMed] [Google Scholar]
- 23. Paupy C, Ollomo B, Kamgang B, et al. : Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10(3):259–266. 10.1089/vbz.2009.0005 [DOI] [PubMed] [Google Scholar]
- 24. Grard G, Caron M, Mombo IM, et al. : Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681. 10.1371/journal.pntd.0002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mombouli JV, Bitsindou P, Elion DO, et al. : Chikungunya virus infection, Brazzaville, Republic of Congo, 2011. Emerg Infect Dis. 2013;19(9):1542–1543. 10.3201/eid1909.130451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamgang B, Brengues C, Fontenille D, et al. : Genetic structure of the tiger mosquito, Aedes albopictus, in Cameroon (Central Africa). PLoS One. 2011;6(5):e20257. 10.1371/journal.pone.0020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ: Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107(4):611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maynard AJ, Ambrose L, Cooper RD, et al. : Tiger on the prowl: Invasion history and spatio-temporal genetic structure of the Asian tiger mosquito Aedes albopictus (Skuse 1894) in the Indo-Pacific. PLoS Negl Trop Dis. 2017;11(4):e0005546. 10.1371/journal.pntd.0005546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson JD, Higgins DG, Gibson TJ: CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Librado P, Rozas J: DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 31. Tajima F: Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123(3):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu YX, Li WH: Statistical tests of neutrality of mutations. Genetics. 1993;133(3):693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong D, Lo E, Hu R, et al. : Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS One. 2013;8(7):e68586. 10.1371/journal.pone.0068586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beebe NW, Ambrose L, Hill LA, et al. : Tracing the tiger: population genetics provides valuable insights into the Aedes (Stegomyia) albopictus invasion of the Australasian Region. PLoS Negl Trop Dis. 2013;7(8):e2361. 10.1371/journal.pntd.0002361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamura K, Stecher G, Peterson D, et al. : MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clement M, Posada D, Crandall KA: TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9(10):1657–1659. 10.1046/j.1365-294x.2000.01020.x [DOI] [PubMed] [Google Scholar]
- 37. Múrias dos Santos A, Cabezas MP, Tavares AI, et al. : tcsBU: a tool to extend TCS network layout and visualization. Bioinformatics. 2016;32(4):627–628. 10.1093/bioinformatics/btv636 [DOI] [PubMed] [Google Scholar]
- 38. Bargielowski IE, Lounibos LP, Shin D, et al. : Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in nature. IInfect Genet Evol. 2015;36:456–461. 10.1016/j.meegid.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reiskind MH, Lounibos LP: Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med Vet Entomol. 2013;27(4):421–429. 10.1111/mve.12000 [DOI] [PubMed] [Google Scholar]
- 40. Kamgang B, Yougang AP, Tchoupo M, et al. : Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasit Vectors. 2017;10(1):469. 10.1186/s13071-017-2408-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Juliano SA, O'Meara GF, Morrill JR, et al. : Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130(3):458–469. 10.1007/s004420100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rey JR, Nishimura N, Wagner B, et al. : Habitat segregation of mosquito arbovirus vectors in south Florida. J Med Entomol. 2006;43(6):1134–1141. 10.1093/jmedent/43.6.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cox J, Grillet ME, Ramos OM, et al. : Habitat segregation of dengue vectors along an urban environmental gradient. Am J Trop Med Hyg. 2007;76(5):820–826. [PubMed] [Google Scholar]
- 44. Gratz NG: Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18(3):215–227. 10.1111/j.0269-283X.2004.00513.x [DOI] [PubMed] [Google Scholar]
- 45. Ngoagouni C, Kamgang B, Kazanji M, et al. : Potential of Aedes aegypti and Aedes albopictus populations in the Central African Republic to transmit enzootic chikungunya virus strains. Parasit Vectors. 2017;10(1):164. 10.1186/s13071-017-2101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reis S, Cornel AJ, Melo M, et al. : First record of Aedes albopictus (Skuse 1894) on São tomé island. Acta Trop. 2017;171:86–89. 10.1016/j.actatropica.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 47. Armbruster P, Damsky WE, Jr, Giordano R, et al. : Infection of New- and Old-World Aedes albopictus (Diptera: Culicidae) by the intracellular parasite Wolbachia: implications for host mitochondrial DNA evolution. J Med Entomol. 2003;40(3):356–360. 10.1603/0022-2585-40.3.356 [DOI] [PubMed] [Google Scholar]