Abstract
Secretory IgA is a key host defense mechanism that controls the intestinal microbiota. We investigated the role of CD11c+CX3CR1+CD64+ macrophages in IgA production in the intestine. Intestinal CX3CR1+ macrophages directly induced IgA secretion by B cells. Antigen delivery to lamina propria CX3CR1+ macrophages specifically induced intestinal IgA production. The induction of IgA by CX3CR1+ macrophages required BAFF, APRIL and TNF-α, but was surprisingly independent of TLR mediated microbial recognition and retinoic acid signaling. IgA secretion by CX3CR1+ macrophages was enhanced by lamina propria CD8+ T cells through the secretion of IL-9 and IL-13. CX3CR1+ macrophages and CD8+ T cells induced IgA production by B cells independently of mesenteric lymph nodes and Peyer’s patches. Our data reveal a previously unrecognized cellular circuitry in which lamina propria CX3CR1+ macrophages, B cells and CD8+ T cells coordinate the protective immunoglobulin secretion in the small intestine upon peripheral antigen delivery.
Introduction
Immunoglobulin secretion in the intestine is critical for microbial control and mucosal homeostasis (1). IgA is the major intestinal immunoglobulin that is continuously generated against microbial antigens to prevent commensal and pathogen interactions with the epithelium and neutralize toxins (2). The intestinal mucosa is further a source for memory B cell that arise in Peyer’s patches (PP) and mesenteric lymph nodes (MLNs) upon the processing of intestinal absorbed and recognized antigens to differentiate into immunoglobulin processing plasma cells that home back to the intestinal lamina propria but also distribute throughout the peripheral immune system (3–5). Activation of B cells also induces the expression of activation induced-cytidine deaminase (AID), a DNA-editing enzyme that triggers IgM-to-IgA class switching and affinity maturation through class switch recombination (CSR) and somatic hypermutation (6, 7). Intestinal B cells generate IgA through T-cell dependent and independent pathways that are regulated by dendritic cells in response to the intestinal commensal microbiota (8, 9). Epithelial polymeric Ig receptor via the J chain, translocate polymeric IgA across intestinal epithelium to reach the intestinal lumen as a secretory IgA complex for the control of commensal bacteria (1, 10, 11). IgA CSR of B cells in the gut is induced by T cell dependent and independent pathways. T cell dependent IgA CSR has been found in PPs and MLNs and requires TGF-β1 and CD40L expression on activated T cells (12). T cell independent IgA CSR can also occur in the lamina propria and isolated lymphoid follicles (ILFs) by expressing B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) (13). For this, a variety of innate immune cells including several subsets of dendritic cells (DCs) and eosinophils in the gut have shown to have unique capacity to support IgA production unlike non-mucosal innate immune cells.
The small intestinal lamina propria contains antigen presenting DC and macrophage subsets which have been identified as CD11c+CD24+CD64−CX3CR1− DCs (CD103+CD11b−, CD103+CD11b+ or CD103−CD11b+) and CD11c+CD24−CD64+CX3CR1+ macrophages (CD103+CD11b+ or CD103−CD11b+). CD11c+CD11b+CD103+Zbtb46+ classical DCs or CD11c+CD11b+CX3CR1+CSFR+ macrophage-like cells together form a major immune surveillance system in the small intestinal lamina propria (14, 15). CX3CR1+ macrophages can present antigen in the lamina propria and support expansion of Foxp3+CD4+ Treg cells by IL-10 production to harness immune tolerance (16). But they also contribute to inflammatory circuits that generate Th17 cell activation (17). Lamina propria CD11c+CX3CR1+ macrophages can sample luminal antigen by extended dendrites into the epithelium (18). In addition to luminal antigen, CD11c+CX3CR1+ macrophages facilitate the surveillance of circulatory antigens that reach the lamina propria from the blood circulation (19). Cross-presentation of antigens by these cells can induce IL-10 producing CD8+ T cells which can suppress pathogen-specific CD4+ T cell activation (19). Here we demonstrate that peripheral antigen delivery to intestinal CD11c+CX3CR1+ macrophages can control antigen-specific IgA production in the small intestinal lamina propria.
Materials and Methods
Mice
All experiments were approved by the Institutional Animal Care and Use Committee of Ajou University (IACUC No. 2014-0038). WT C57BL/6 mice were purchased from Charles River Laboratories (Orient Bio Inc., Sungnam, Korea). CX3CR1(GFP/+) mice, MyD88 knockout (KO) mice, CD45.1+ C57BL/6 mice and CCR7 KO mice (C57BL/6 background) were purchased from Jackson Laboratories (Bar Harbor, ME). CX3CR1-DTR mice (C57BL/6 background) were kindly provided by Dan R. Littman (New York University Medical Center) and Charles Surh (Pohang University of Science and Technology) (20). All mice used in the study were at 6 weeks of age. The mice were kept in the Laboratory Animal Research Center of Ajou University Medical Center.
Immunization and cell depletion
Mice were immunized with 100 µg ovalbumin (OVA) intravenously (i.v.) and boosted 2 weeks interval. Serum and fecal samples were obtained after 1 week of every immunization. For depletion of CD11c+CX3CR1+ macrophages, CX3CR1-DTR mice were intraperitoneally (i.p.) injected with 200 ng diphtheria toxin 1 day before OVA injection and once more after 2 days of OVA injection. For depletion of CD8+ T cells, hybridomas producing depleting anti-CD8α (2.43) mAb were obtained from the ATCC and mice were treated at day 3, 5, and 15 as previously described (21). To inhibit egress of antibody-secreting B cells and activated CD8+ T cells in the immune inductive location, 20 µg FTY720 were i.p. injected every other day following OVA injection (22). To analyze circulatory antigen-specific CD8+ T cells, splenocytes from CD45.2+ OT-I mice were labeled with 9 µM CFSE and injected to CD45.1+ WT C57BL/6 mice. The next day, WT C57BL/6 mice were i.v. injected 100 µg OVA. At day 3, CFSE dilution was analyzed by CD45.2+CD8α+ gated populations.
Cell isolation
To obtain B cells, spleen of WT or MyD88 KO mice were homogenized and treated with RBC lysis buffer (Sigma-Aldrich, St. Louis, MO). B cells were sorted with MACS B cell Isolation Kit (Miltenyi Biotec). To obtain OT-I and OT-II cells, spleen of OT-I or OT-II mice were homogenized and treated with RBC lysis buffer. OT-I cells were isolated with MACS CD8α+ T cell Isolation Kit II (Miltenyi Biotec) and OT-II cells with CD4+ T cell Isolation Kit II (Miltenyi Biotec). To isolate mononuclear phagocytes from the small intestine lamina propria of CX3CR1(GFP/+) mice or MyD88 KO, the intestine was cut into 3–4 pieces, then inverted onto polyethylene tubes (Becton Dickinson, Franklin Lakes, NJ) and washed 3 times with calcium- and magnesium-free phosphate-buffered saline (PBS). The intestines were serially treated with 1 mM dithiothreitol (DTT; Sigma-Aldrich, St. Louis, MO) and 30 mM EDTA to remove mucus and epithelium followed by digestion with 36 U/ml type IV collagenase (Sigma-Aldrich) for 90 mins at 37°C. Lamina propria cells were then treated with discontinuous density gradient containing 66% and 44% Percoll (GE Healthcare Life sciences, Uppsala, Sweden) for B cells and Opti-prep (Axis-Shield, Oslo, Norway) with fetal bovine serum layered on top for DCs. The interface was harvested and stained for flow cytometry or used for determination of antigen-specific ASCs. Cell subsets were sorted as CD11c+CX3CR1− and CD11c+CX3CR1/GFP+ cells with FACS Aria III (BD Biosciences). The purity following sorting isolation was >90%.
In vitro cell culture
For in vitro IgA production, isolated B cells (3×105) were co-cultured with sorted intestinal CD11c+CX3CR1− or CD11c+CX3CR1+ phagocytes (3×104) in presence of 10 µg/ml goat anti-mouse IgM (Jackson ImmunoResearch, West Grove, PA). 10µg/ml TACI/BCMA (R&D Systems, Minneapolis, MN), 10 µg/ml anti-mouse BAFF (R&D Systems), 10 µg/ml anti-mouse/rat TNF-α (clone TN3-19; eBioscience), 10 µg/ml anti-mouse IL-10 (clone JES5-16E3; Biolegend), 1 µM type B CpG oligonucleotide (ODN 1668; InvivoGen, San Diego, CA), 20 µg/ml anti-IL13, and anti-IL9 (R&D systems) were added for inhibition or stimulation.
Flow cytometry
Isolated cells were treated with 2% FBS/PBS containing Fc block (BD Biosciences) for 20 min at 4°C and then stained with fluorescent-conjugated antibody. Antibodies used for flow cytometry analysis are as follows: CD11c (clone N418; eBiosciences), CD64 (clone X54-5/7.1; Biolegend), CD103 (clone M290; BD Biosciences), F4/80 (clone BM8; eBiosciences), CD11b (clone M1/70; BD Biosciences), CX3CR1 (clone SA011F11; Biolegend), CD4 (clone RM4-5; eBiosciences), CD8α (clone 53–6.7; BD Biosciences), OVA-AF647 (Molecular Probe). Data were obtained using FACSCanto (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Gene expression
Intestinal CD11c+CX3CR1+ macrophages and CD11chighCX3CR1− DCs from CX3CR1(GFP/+) mice were sorted and total RNA was extracted by RNeasy mini kit (Qiagen). Reverse transcription of samples was performed to obtain cDNA using Superscript II Reverse Transcriptase (Invitrogen). Expression of genes was analyzed by OneStepPlus RT-PCR (Applied Biosystems). Relative expression of BAFF and APRIL genes were normalized by GAPDH. Primers used for RT-PCR are as follows:
BAFF forward (5’TGCTATGGGTCATGTCATCCA-3’)
BAFF reverse (5’-GGCAGTGTTTTGGGCATATTC-3’)
APRIL forward (5’-TCACAATGGGTCAGGTGGTATC-3’)
APRIL reverse (5’-TGTAAATGAAAGACACCTGCACTGT-3’).
ELISA
Serum and feces samples were obtained from OVA injected mice. Feces were suspended as 100 mg/ml with PBS containing 0.02% sodium azide, stirred vigorously and centrifuged 8,000 rpm for 10 mins. The supernatant was used for determination of antigen-specific secretary IgA. Immunoplates (Thermo Fisher Scientific, Roskilde, Denmark) were coated with 10 µg/ml OVA or flagellin in 0.05 M carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Wells were blocked with 1% bovine serum albumin in PBS. Diluted serum or fecal samples were added to the wells. Plates were then treated with HRP-conjugated goat anti-mouse IgG or IgA (Southern Biotech, Birmingham, AL) and incubated overnight at 4°C. Peroxidase substrate TMB (MOSS Inc., Pasadena, MD) was added and the reaction was stopped with 0.5 N HCl. Plates were read at 450 nm by ELISA reader (Synergy H1 Hybrid Reader, BioTek, Winooski, VT). Endpoint titers were determined as the reciprocal log2 of the last dilution giving an OD at 450 nm of 0.1 greater than background.
Determination of OVA-specific antibody-secreting B cells
Multiscreen filter plates (Merck Millipore, Darmstadt, Germany) were coated with 10 µg/ml OVA and incubated overnight at 4°C. Wells were blocked with 10% fetal bovine serum in RPMI1640 (Life Technologies, Grand Island, NY), and mononuclear cells (MNCs) were diluted in complete cell culture medium. The plates were incubated at 37°C in CO2 incubators for 4 hours. After washing with 0.01% Tween 20/PBS, HRP-conjugated goat anti-mouse IgG or IgA was added to plates and incubated overnight at 4°C. AEC kits were used as the peroxidase substrate. (ImmunoBio Science Corp., Mukilteo, WA). Spots were counted by ImmunoSpot (CTL, Shaker Heights, OH).
Depletion of commensal bacteria
CX3CR1(GFP/+) mice were fed intragastrically (i.g.) 200 µl antibiotics cocktail every day for 7 days (23). Antibiotics used are as follows: 300 µg Ampicillin (USB, Cleveland, OH), 300 µg Neomycin (Gibco, Grand Island, N.Y), 150 µg Vancomycin (USB, Cleveland, OH), 150 µg Metronidazole (Nacalai tesque, Kyoto, Japan) and 150 µg Gentamycin (Gibco, Grand Island, N.Y).
Statistics
Statistical values were evaluated using Student’s t-test or one-way ANOVA (GraphPad Prism). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ns; not significant.
Results
Intestinal CX3CR1+ macrophages induce immunoglobulin secretion by B cells
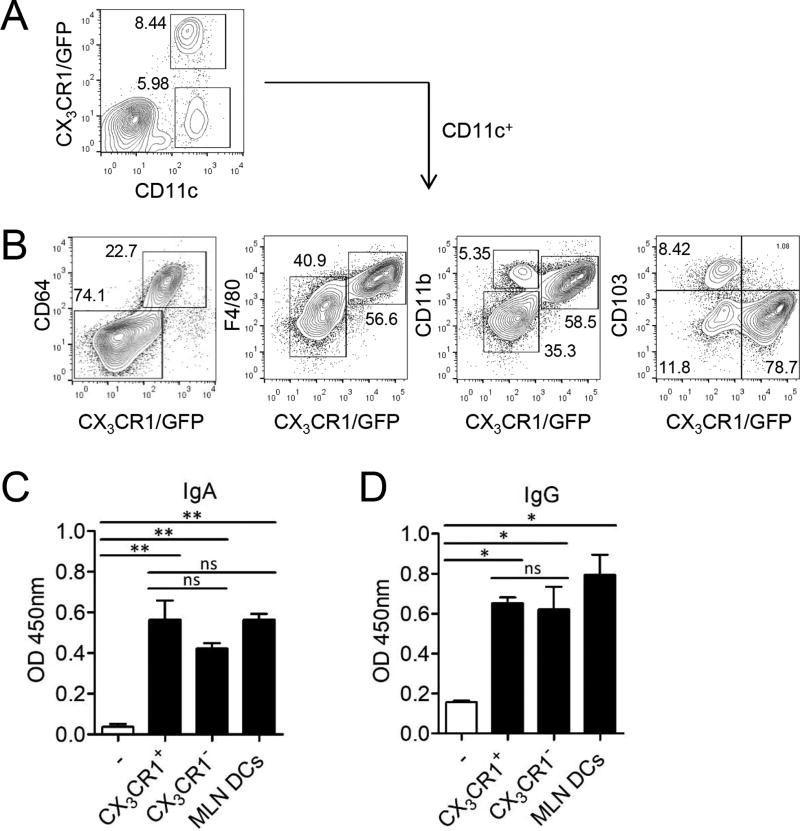
Intestinal CX3CR1+CD11c+ macrophages were found to be CD64+F4/80+CD11b+CD103− and were negative for the eosinophil 10marker Siglec-F (CD169) (Fig. 1A, 1B, Supplemental Fig. 1A). We sorted lamina propria CD11c+CX3CR1− DCs and CD11c+CX3CR1+ macrophages (Supplemental Fig. 1B) and determined their ability to promote Ig production from splenic B cells. Both antigen presenting myeloid cells isolated from the lamina propria significantly enhanced IgA expression in splenic B cells after 5 days of co-culture at comparable levels to MLN DCs (Fig. 1C). Intestinal CD11+CX3CR1+ macrophages and CD11c+CX3CR1− DCs also significantly enhanced IgG secretion by B cells (Fig. 1D). CX3CR1+ macrophages alone did not contain either IgA or IgG that could have been taken up from the Ig rich intestinal environment (Supplemental Fig. 1C). These results demonstrate that intestinal CD11c+CX3CR1+ macrophages can provide stimuli for B cells to enhance IgA secretion.
FIGURE 1.
Intestinal CD11c+CX3CR1+ macrophages can induce B cells to produce IgA. (A) The expression of CX3CR1 were analyzed among CD11c+ cells of the small intestinal lamina propria from CX3CR1(GFP/+) mice. (B) Expression of CX3CR1, CD64, F4/80, CD11b and CD103 on lamina propria CD11c+ cells. (C and D) CD11c+ cells of small intestinal lamina propria from CX3CR1(GFP/+) mice were sorted to CX3CR1+ and CX3CR1− subsets. (C) Total IgA and (D) IgG level from culture supernatants of CX3CR1+ macrophages or CX3CR1− DCs co-cultured with B cells in presence of anti-IgM (10 µg/ml). Data are representative of three independent experiments. Graphs show mean ± SEM. ns; not significant, *p < 0.05 and **p < 0.01 using one-way ANOVA.
Intestinal CD11c+CX3CR1+ macrophages support IgA secretion by B cells via expression of BAFF, APRIL and TNF-α
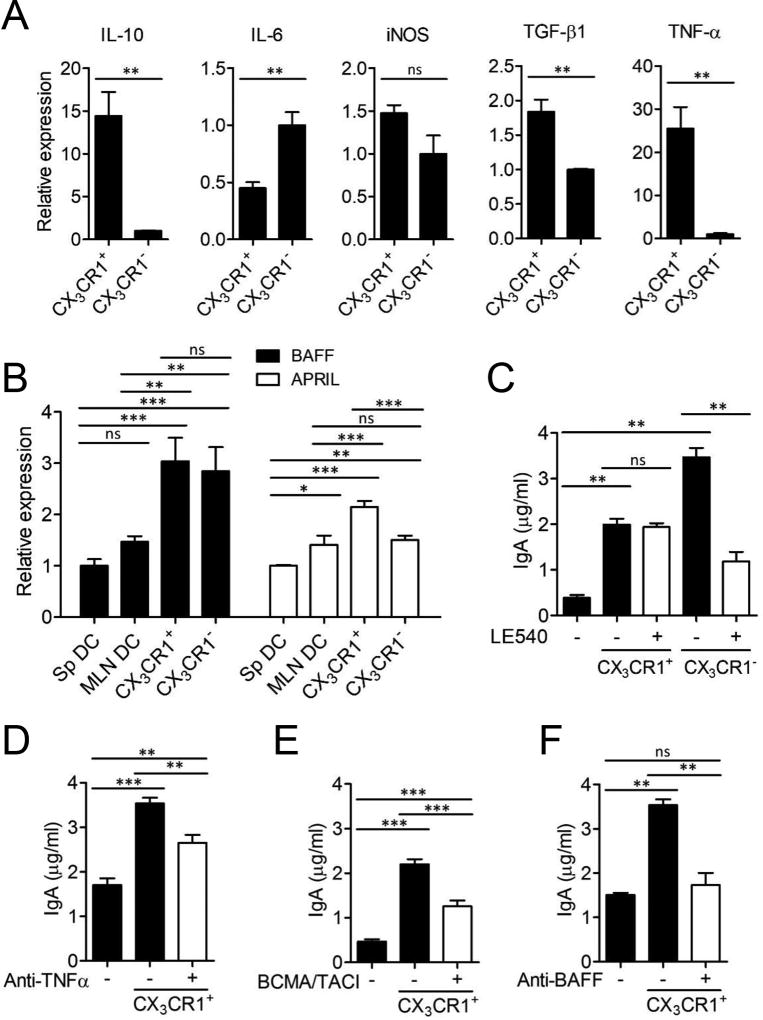
We next determined regulatory gene expressions to investigate the mechanisms that were required for the induction of IgA class switching by intestinal phagocytes. Intestinal CD11c+CX3CR1+ macrophages expressed significantly higher amounts of IL-10, TGF-β1 and TNF-α compared to intestinal CD11c+CX3CR1− DCs (Fig. 2A). Intestinal CD11c+CX3CR1+ macrophages also expressed high levels of BAFF and APRIL (Fig. 2B). Since retinoic acid (RA) has been demonstrated to be used for IgA production by several DC subsets, LE540, a RA receptor antagonist was utilized to investigate RA as IgA CSR factor of CD11c+CX3CR1+ macrophages. Blocking of RA receptor signaling was unable to reduce the IgA induction by CD11c+CX3CR1+ macrophages in contrast to CD11c+CX3CR1− DCs that were unable to induce IgA production in the absence of RA receptor signaling (Fig. 2C). Instead, TNF-α, BAFF and APRIL were required during the interaction of CD11c+CX3CR1+ macrophages with B cells to induce IgA production (Fig. 2D–F). However, blockage of IL-10 did not alter IgA production but increased IgG production (Supplemental Fig. 1D). These results indicated that intestinal CD11c+CX3CR1+ macrophages promoted IgA expression through TNF-α, BAFF and APRIL but their control of B cell function is independent of RA receptor signaling and IL-10.
FIGURE 2.
Intestinal CD11c+CX3CR1+ macrophages can induce IgA production via expression of BAFF, APRIL and TNF-α. CD11c+ cells of small intestinal lamina propria from CX3CR1(GFP/+) mice were sorted to CX3CR1+ and CX3CR1− subsets. (A) Gene expression of IL-10, IL-6, iNOS, TGF-β1 and TNF-α of CX3CR1+ macrophages or CX3CR1− DCs. (B) Gene expression of BAFF and APRIL. (C–F) The level of total IgA was determined from culture supernatants of B cells co-cultured with CX3CR1+ macrophages or CX3CR1− DCs in presence of (C) LE540 (1 µM), (D) anti-TNF-α (10 µg/ml), (E) BCMA/TACI (10 µg/ml) or (F) anti-BAFF (10 µg/ml). Data are representative of three independent experiments. Graphs show mean ± SEM. **p < 0.01 and ***p < 0.001 using one-way ANOVA.
Induction of IgA by intestinal CD11c+CX3CR1+ macrophages are less dependent of signals from the microbiota
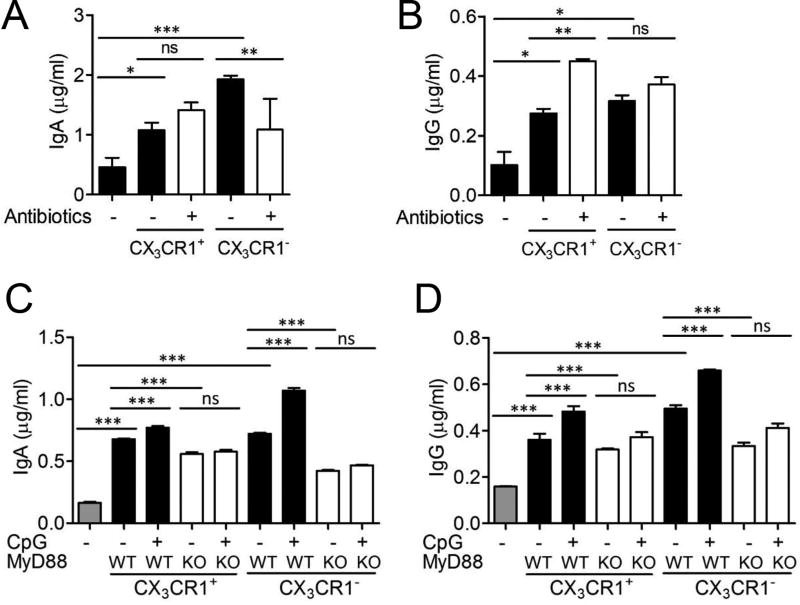
CX3CR1(GFP/+) mice were fed i.g. with antibiotics cocktail consisting of ampicillin, neomycin, vancomycin, gentamycin, and metronidazole that has been shown to deplete the gut commensal microbiota (23). This treatment reduced bacterial count in classical solid media from 3.5×106 CFU/µg to 3×103 CFU/µg feces following 1 week treatment of antibiotic cocktail. Intestinal CD11c+CX3CR1+ macrophages were isolated from antibiotic treated mice to elicit IgA secretion from splenic B cells. Surprisingly, intestinal CD11c+CX3CR1+ macrophages maintained the ability to induce IgA production even after significant reduction of the intestinal microbiota. In contrast, IgA production was significantly decreased from B cells co-cultured with intestinal CD11c+CX3CR1− DCs isolated after antibiotic treatment (Fig. 3A). We found that the induction of IgG secretion by CD11c+CX3CR1+ macrophages was significantly increased after antibiotics treatment (Fig. 3B).
FIGURE 3.
Innate signals from commensal microbe augmented IgA production by intestinal CX3CR1− DCs than CX3CR1+ macrophages. (A and B) CX3CR1(GFP/+) mice were i.g. treated with antibiotics cocktails for 1 week. CD11c+ cells of the small intestinal lamina propria were sorted to CX3CR1+ and CX3CR1− subsets. (A) Total IgA and (B) IgG was analyzed from culture supernatants of B cells co-cultured with CX3CR1+ macrophages or CX3CR1− DCs. (C and D) CD11c+ cells of the small intestinal lamina propria from WT or MyD88 KO mice were sorted to CX3CR1+ and CX3CR1− subsets. (C) Total IgA and (D) IgG was determined from culture supernatants of MyD88 KO B cells co-cultured with CX3CR1− DCs or CX3CR1+ macrophages from WT or MyD88 KO mice in presence of CpG (1 µM). Data are representative of three independent experiments. Graphs show mean ± SEM. ns; not significant, *p < 0.05, **p < 0.01 and ***p < 0.001 using one-way ANOVA.
To model innate immune signals induced by intestinal microbiota derived nucleic acids, we stimulated intestinal phagocytes with the TLR9 agonist CpG oligodeoxynucleotides. To rule out the possibility of B cell activation by CpG, MyD88 KO splenic B cells were utilized (Fig. 3C, 3D). TLR9 activation significantly increased the amount of IgA produced by B cells co-cultured with WT intestinal CX3CR1− DCs in a MyD88 dependent fashion (Fig. 3C, Supplemental Fig. 1E). MyD88 KO CX3CR1− DCs significantly reduced IgA production by B cells compared to WT DCs. The induction of IgA by WT CX3CR1+ macrophages by TLR9 activation had a significant but slight increase (Fig. 3C), however they were not as dependent on MyD88 signaling as CX3CR1− DCs (Supplemental Fig. 1E). IgG production by two subsets significantly increased in the presence of CpG which was dependent on MyD88 signaling (Fig. 3D, Supplemental Fig. 1F). These data indicated that CD11c+CX3CR1+ macrophages have the inherent capacity to induce IgA production in B cells and are not as dependent on microbial signals that are required for intestinal DCs to control IgA production by B cells.
Intestinal CD11c+CX3CR1+ macrophages promote antigen-specific IgA production following peripheral i.v. antigen administration
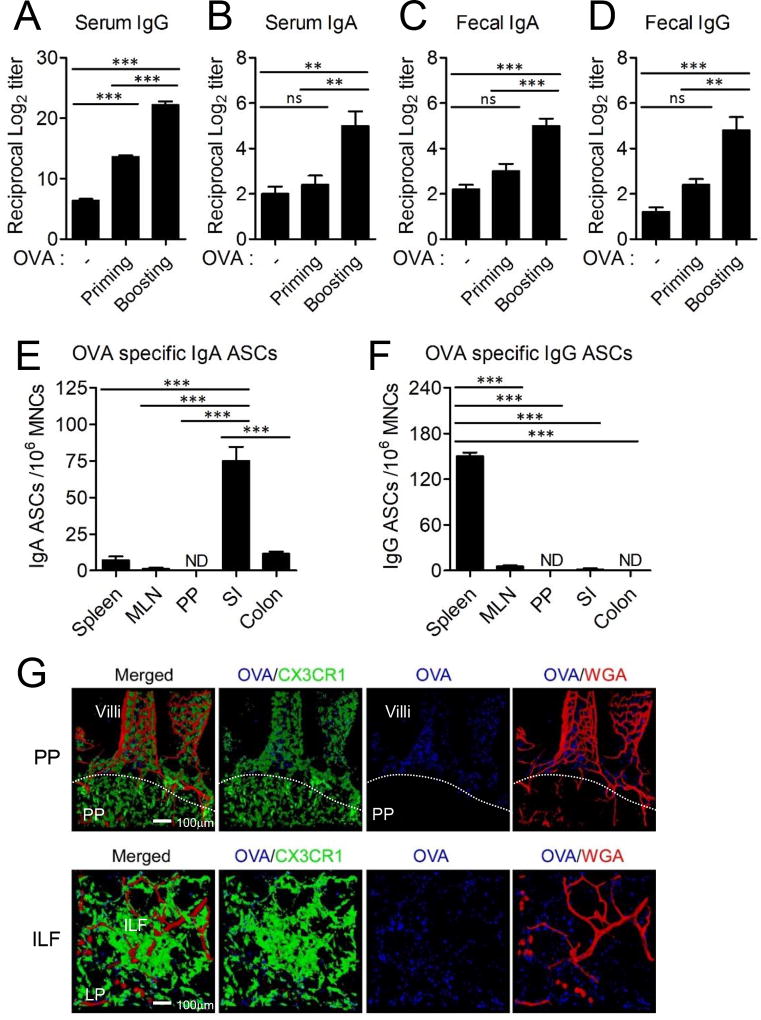
Next, we determined whether intestinal CD11c+CX3CR1+ macrophages could be targeted for antigen-specific IgA secretion through the blood circulation. Intestinal CD11c+CX3CR1+ macrophages can capture and process luminal antigens as well as circulatory antigens that reach the lamina propria through the fenestrated capillary system of the small intestine (Supplemental Fig. 2A) (19). Mice received i.v. injection of OVA and we subsequently determined OVA-specific IgA and IgG levels in the blood circulation and intestinal lumen. We found that circulatory OVA administration resulted in significant increase in OVA-specific IgA and IgG in both the intestinal lumen and peripheral circulation (Fig. 4A–D). The levels of OVA-specific IgA and IgG were dependent on the dose of i.v. administered OVA (Supplemental Fig. 2B). The IgA originated from OVA-specific IgA antibody secreting cells (ASCs) in the small intestinal lamina propria but neither PPs nor the MLN (Fig. 4E), while OVA-specific IgG ASCs were enriched in the spleen (Fig. 4F). Indeed, i.v. injected antigen could be found in the small intestine including the lamina propria and ILFs but not in PPs that lack fenestrated capillaries (Fig. 4G) (19).
FIGURE 4.
Intestinal CD11c+CX3CR1+ macrophages generate IgA secreting B cells in the gut upon circulatory antigen delivery. (A–D) WT B6 mice were i.v. injected with 100 µg OVA and boosted 2 weeks later. Serum and feces were collected 1 week after each immunization (n=5). Data are representative of four independent experiments. (A) OVA-specific serum IgG, (B) serum IgA, (C) fecal IgA and (D) fecal IgG were analyzed. (E and F) At 2 weeks after the final immunization, OVA-specific (E) IgA and (F) IgG ASCs were analyzed from the spleen, MLN, PP, small intestinal lamina propria (SI) and colon. Data are representative of three independent experiments. Graphs show mean ± SEM. ns; not significant, **p < 0.01 and ***p < 0.001 using one-way ANOVA. (G) 3D image reconstruction of confocal microscopic image series of a small intestine 1 hr after OVA injection intravenously into CX3CR1(GFP/+) mice. Texas red-conjugated wheat germ agglutinin (WGA) used to visualize the capillary vessel system.
We next investigated whether circulatory administration of other antigen besides OVA could generate antigen-specific intestinal IgA and further contribute to host protection. Salmonella Typhimurium is one of the major enteric pathogen which enter through the M cells of PPs and reach the MLN, causing gastroenteritis and typhoid fever. Secretory IgA is reported to play a crucial role in protection of Salmonella Typhimurium infection (24, 25). To determine the protective ability of Ig generated after circulatory antigen delivery, we i.v. immunized mice with flagellin originated from Salmonella Typhimurium. Flagellin-specific IgG and IgA increased in the serum and feces, similar to OVA administration (Supplemental Fig. 2D). Flagellin immunized mice were then challenged with the invasive Salmonella Typhimurium strain UK-1 by oral infection. After 6 days following infection, mice were analyzed for UK-1 bacterial load in the MLN and PPs. Flagellin immunized mice had a significant reduction of CFU in the MLN and PPs (Supplemental Fig. 2E) and survived longer than control mice (Supplemental Fig. 2F), showing the protective effect of intestinal IgA generated after circulatory antigen delivery.
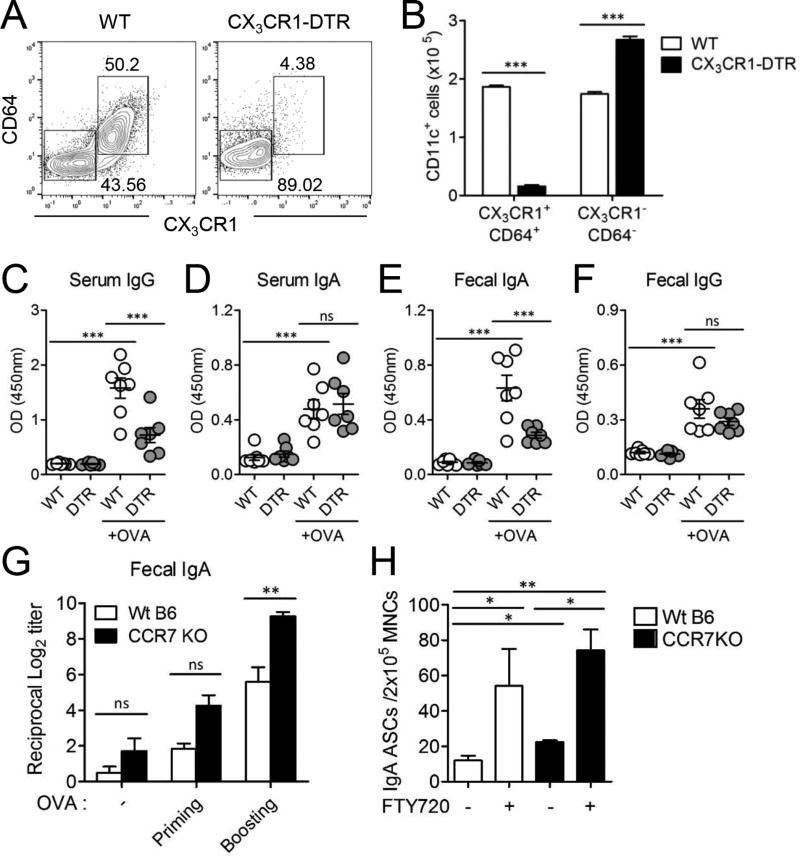
To confirm that intestinal CD11c+CX3CR1+ macrophages was responsible for the mucosal IgA induction, we specifically depleted intestinal CD11c+CX3CR1+ macrophages in CX3CR1-DTR mice (20). CD11c+CD64+CX3CR1+ macrophages were significantly reduced after treatment of diphtheria toxin in the lamina propria of CX3CR1-DTR mice (Fig. 5A, 5B, Supplemental Fig. 3A, 3B). Specific depletion of CD11c+CX3CR1+ macrophages resulted in significant reduction of OVA-specific fecal IgA and serum IgG but not IgA levels in the serum (Fig. 5C–F). To investigate whether CX3CR1+ macrophages were involved in the production of luminal antigen-specific IgA, mice were i.g. immunized with OVA plus cholera toxin after depletion of CX3CR1+ macrophages since intestinal CX3CR1+ macrophages can capture antigen from the lumen as well as the circulation (Supplemental Fig. 3C). In the absence of CX3CR1+ macrophages, the production of OVA-specific IgA antibodies in the feces decreased although it was not statistically significant. Marginal reduction of OVA-specific IgA in CX3CR1+ macrophage depleted mice may be because many other intestinal DCs other than CX3CR1+ macrophages capture luminal antigen and induce antibody production following i.g. administration of OVA. Taken together, these data are consistent with a critical role of intestinal CD11c+CX3CR1+ macrophages in the uptake of circulatory antigen for the induction of gut IgA production by B cells.
FIGURE 5.
Intestinal CD11c+CX3CR1+ macrophages could generate IgA production specific to circulatory antigen. (A–F) WT B6 mice and CX3CR1-DTR mice were i.v. immunized with 100 µg OVA at two weeks interval (n=7). For depletion of CX3CR1+ macrophages, mice were i.p. injected with 200 ng diphtheria toxin before 1 day and after 2 days of OVA immunization. Data are representative of three independent experiments. (A) To confirm the depletion of CD11c+CX3CR1+ macrophages, CD11c+ cells were isolated from the small intestinal lamina propria and CD64+CX3CR1+ cells were analyzed from the CD11c+ population. (B) The absolute cell number of CD64+CX3CR1+ population among CD11c+ cells were summarized. Graphs show mean ± SEM. ***p < 0.001 using Student’s t-test. (C–F) At day 7 following 2nd OVA immunization, the level of OVA-specific IgA and IgG were determined in the serum and feces. Graphs show mean ± SEM. ***p < 0.001 using one-way ANOVA. (G) WT B6 and CCR7 KO mice were i.v. immunized with 100 µg OVA and boosted 2 weeks later (n=5). OVA-specific fecal IgA were determined. (H) WT B6 or CCR7 KO mice were treated with 20 µg FTY720 and then OVA-specific IgA ASCs were determined from the small intestinal lamina propria. Data are representative of three independent experiments. Graphs show mean ± SEM. *p < 0.05 and **p < 0.01 using one-way ANOVA.
Antigen-specific IgA induced by circulatory antigen delivery can be generated in the lamina propria
We also investigated whether the migration of intestinal phagocytes to the MLNs was required for IgA production from circulatory antigen delivery. CD11c+CX3CR1+ macrophages are non-migratory, resident populations at steady state (26). However, CCR7 is required for the migration of CD103+ DCs as well as CD11c+CX3CR1+ macrophages to the MLN under inflammatory or dysbiotic conditions (20, 26). Surprisingly we found that OVA-specific IgA secretion was significantly enhanced in CCR7 KO mice compared with WT B6 mice following two times i.v. immunization with OVA (Fig. 5G). In addition, we found that numbers of OVA-specific IgA ASCs in the lamina propria increased in CCR7 KO mice compared to WT mice (Fig. 5H). These results suggested that circulatory antigen delivery induced antigen-specific IgA secretion in the intestine without migration of intestinal phagocytes to the MLN.
We further inhibited lymphocyte emigration from the MLNs to the intestine with FTY720, a sphingosine 1-phosphate receptor modulator that blocks lymphocyte egress from LNs. OVA-specific IgA ASCs were scarcely found in the MLN regardless of the presence of FTY720 and CCR7 (Supplemental Fig. 2C, Fig. 4E). Instead, the number of OVA-specific IgA ASCs increased in the lamina propria when treated with FTY720 (Fig. 5H). Even when we blocked migration of antigen presenting cells and lymphocyte egress together in CCR7 KO mice treated with FTY720, circulatory antigen-specific IgA ASCs were significantly increased in the small intestinal lamina propria indicating that the generation of circulatory antigen-specific IgA ASCs occurred directly in the lamina propria independent of MLN.
IL-13 and IL-9 secretion of CD8+ T cells enhances the induction of IgA production of B cells by intestinal CD11c+CX3CR1+ macrophages
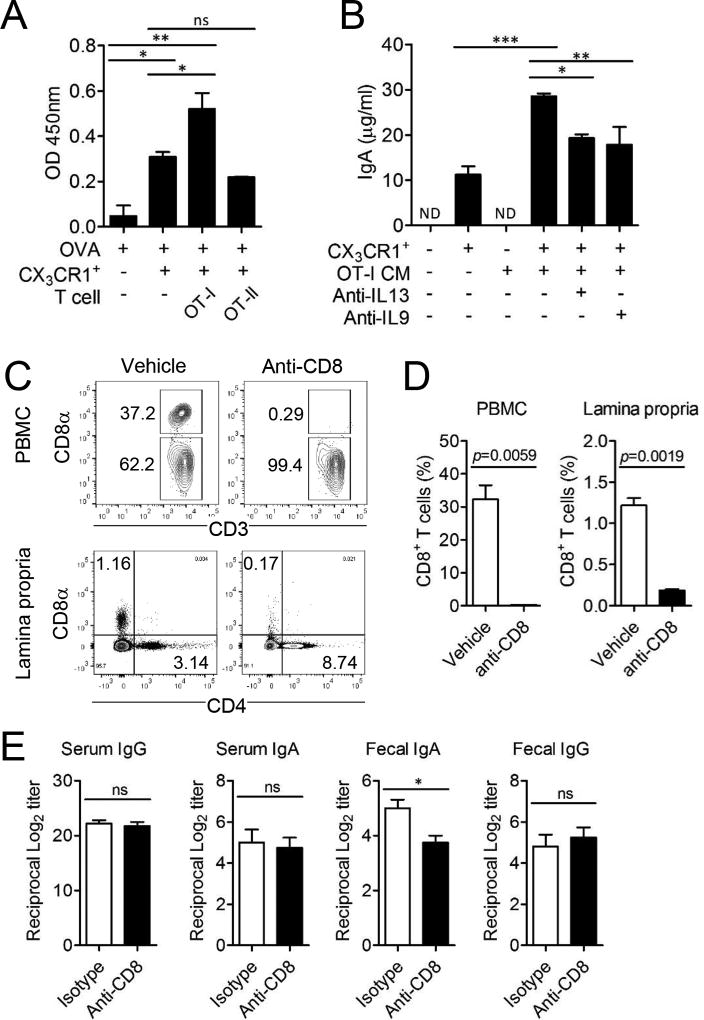
Intestinal CD11c+CX3CR1+ macrophages cross-present antigen to CD8+ T cells to generate unique tolerogenic CD8+ T cells (19, 27). We therefore determined whether CD8+ T cells that arise during antigen presentation by CD11c+CX3CR1+ macrophages could support IgA secretion by B cells. To investigate the effect of T cells on IgA production, we isolated intestinal CD11c+CX3CR1+ macrophages and co-cultured them together with OVA-specific TCR transgenic CD8+ T cells (OT-I) or CD4+ T cells (OT-II) and splenic B cells. We found that OVA-specific CD8+ but not CD4+ T cells further increased IgA production by B cells in the co-culture system with CD11c+CX3CR1+ macrophages in the presence of OVA (Fig. 6A). CD11c+CX3CR1+ macrophages could induce proliferation of OVA-specific CD8+ OT-I cells only in presence of cognate antigen OVA (Supplemental Fig. 4A, 4B). However, secreted IgA was not OVA-specific even when OVA was added in the culture since B cells were naïve splenic polyclonal B cells (Supplemental Fig. 4C). Soluble factors produced during the OVA induced interaction of CD11c+CX3CR1+ macrophages with CD8+ T cells were responsible for the significantly enhanced IgA secretion in the co-culture system (Fig. 6B). We previously found that CD8+ T cells arising through interaction with CD11c+CX3CR1+ macrophages are characterized by the expression of IL-13 and IL-9 (19). The inhibition of these cytokines with blocking antibodies significantly reduced IgA production (Fig. 6B).
FIGURE 6.
CD8+ T cells further enhance IgA production of B cells by intestinal CD11c+CX3CR1+ macrophages. (A) Total IgA was determined from the culture supernatant of B cells co-cultured with OVA pulsed CD11c+CX3CR1+ macrophages and OT-I or OT-II cells. (B) Total IgA was determined from culture supernatant of B cells co-cultured with CD11c+CX3CR1+ macrophages and culture media (CM) of OT-I cells which were previously activated by CD11c+CX3CR1+ macrophages in the independent setting. Additionally, 20 µg/ml anti-IL-13 or anti-IL-9 was added for neutralization. Data are representative of three independent experiments. ND; not detected. Graphs show mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 using one-way ANOVA. (C and D) CD8+ T cells in the peripheral blood mononuclear cells (PBMC) and lamina propria of small intestine were analyzed after one day following treatment of anti-CD8 antibody. (E) WT B6 mice were i.v. immunized with 100 µg OVA on day 0 and day 14. For depletion of CD8+ T cells, mice were i.p. injected with anti-CD8 monoclonal antibodies at day 3, 5, and 15 (n=5 per group). OVA-specific serum IgG, serum IgA, fecal IgA, and fecal IgG were analyzed. Data are representative of three independent experiments. *p < 0.05 using Student’s t-test.
To confirm that CD8+ T cells provide help for IgA secretion in vivo, we depleted CD8+ T cells with anti-CD8α (2.43) mAb treatment during the immunization with i.v. injection of OVA. Anti-CD8α antibody treatment significantly reduced CD8+ T cells in the peripheral blood and small intestinal lamina propria (Fig. 6C, 6D). The depletion of CD8+ T cells resulted in the significant reduction of OVA-specific fecal IgA but not IgA levels in the serum (Fig. 6E). Taken together, these data demonstrated that CD8+ T cells generated during interaction with intestinal CD11c+CX3CR1+ macrophages can provide help for intestinal IgA production via IL-9 and IL-13 cytokine secretion.
Antigen-specific IgA induction by resident CD11c+CX3CR1+ macrophages and CD8+ T cells occur independently of antigen processing in PPs and MLNs
To further examine the role of CD8+ T cells in the induction of IgA, naïve OT-I cells were adoptively transferred and then OVA was injected with or without FTY720. OT-I CD8+ T cells were isolated and analyzed for proliferation via CFSE dilution. When OVA was subcutaneously injected, the proliferation of OT-I CD8+ T cells was confined to draining cervical LNs following FTY720 (Supplemental Fig. 4D) as FTY720 confined activated T cells in the site of activation. 5 days following i.v. injection with OVA, proliferation of circulatory antigen-specific CD8+ T cells was found in the spleen, MLN and lamina propria but not the PPs regardless of FTY720 treatment (Supplemental Fig. 4E). Furthermore, OVA-specific ASCs were not generated in the PPs following circulatory OVA transfer (Fig. 4E, 4F). These results indicated that i.v. introduced antigen directly reached antigen presenting cells resident in the spleen, lymph nodes and lamina propria (19). Taken together, antigen delivered through the blood circulation can induce cognate antigen-specific IgA production through intestinal resident CD11c+CX3CR1+ macrophages and CD8+ T cells in the lamina propria and ILFs of the small intestine.
Discussion
Here we demonstrate that small intestinal CD11c+CX3CR1+ macrophages and CD8+ T cells support IgA production in the intestine. Intravenous injection of soluble protein antigen can generate antigen-specific IgA antibody production following uptake of antigen by CD11c+CX3CR1+ macrophages in the intestine. This demonstrates a peripheral access route for mucosal vaccine delivery for the induction of protective pathogen-specific intestinal IgA production. This may form the basis for the demonstration that intestinal IgA antibody induction can occur in mice and human after transcutaneous immunization (28, 29). Both CD11c+CX3CR1+ macrophages and CD11chigh DCs may together define a mucosa-specific immunoglobulin production that is severely impaired in the intestine of germ-free mice (30) since antigen specificity repertoires of secretory IgA mostly react to gut microbiota at steady state condition. Here we showed that the capacity for induction of IgA by CD11c+CX3CR1+ macrophages was less dependent on microbial signals compared to CD11chighCX3CR1− DCs. This is important for antigen delivery strategies during diarrheal diseases and various intestinal microbiota in the developing world that make oral vaccine delivery uncertain (31).
Various kinds of innate immune cells have been shown to support IgA production in the gut. Intestinal plasmacytoid DCs (pDCs), TNF-α/iNOS-producing (Tip) DCs, and eosinophils have shown to induce IgA production by expressing various factors (32–34). Intestinal CD103+ DCs, Tip DCs and TLR5+ DCs express TGF-β and retinaldehyde dehydrogenase type 2 (RALDH2) for IgA production (33, 35, 36). Eosinophils promote IgA production by expressing BAFF and APRIL or support the function of CD103+ DCs (34). IgA production by CD11c+CX3CR1+ macrophages was independent on RA signaling in contrast to CD103+ DCs and TLR5+ DCs (36, 37) but dependent on BAFF and APRIL similar to Tip DCs and pDCs (1, 32, 33). CD11c+CX3CR1+ macrophages also induce IgA production through TNF-α similarly to Tip DCs. However, CD11c+CX3CR1+ macrophages are distinct from Tip DCs since they do not express iNOS and RALDH2 (33). Furthermore, CD11c+CX3CR1+ macrophages can be clearly distinguished from intestinal eosinophils that express Siglec-F.
Remarkably, the results using CCR7 deficient mice and FTY720 treatment supported that specific IgA induced by i.v. antigen delivery can be generated in the intestine independent of cell migration from MLN and PPs. This is consistent with an IgA induction that occurs in ILFs and the lamina propria in the absence of segregated T cell zones, indicating a T cell independent mechanism of IgA induction may predominate at mucosal sites (38). CCR7 controls migration of different immune cells such as T cells, B cells, and mature DCs as well as CD4+ Tregs (39). Therefore, impaired migration of CD4+ Treg and peripheral accumulation of effector lymphocytes might lead to hyper-immune responses in the peripheral site of CCR7 deficient mice (40, 41). This may explain enhanced circulatory antigen-specific IgA production in the peripheral gut of CCR7 deficient mice. In steady state, FTY720 treatment leads to reduction of IgA+ plasmablasts in the intestinal lamina propria caused by their accumulation in PPs since they can be one of the major IgA induction site (42). However, in our experiments antigen-specific IgA might not be induced in PPs as i.v. delivered antigen can reach the lamina propria through fenestrated capillaries that are absent in PPs (43). The absence of IgA ASCs in PPs following i.v. delivery of antigen and elevated antigen-specific IgA ASCs in the lamina propria in blockade of lymphocyte emigration via FTY720 treatment supports that lamina propria including the ILFs might be the genuine IgA CSR site for circulatory antigen. ILFs which contain CD11c+CX3CR1+ macrophages, naïve B cells and naïve CD8+ T cells might be the site for IgA production upon i.v. antigen delivery.
Our data further indicate that IL-9 and IL-13 secretion by CD8+ T cells can promote IgA CSR of B cells in the gut. Both cytokines have been linked to IgA production. IL-9 transgenic mice demonstrated increased IgA containing autoantibody (44) and IL-13 has been linked to TH2-associated pathologies with enhanced IgA production (45). In our experiments, CD8+ T cells induced by CD11c+CX3CR1+ macrophages further expressed IL-10 and IFN-γ (19). IL-10 is a well-known inducer of IgA secretion (8) while IFN-γ has been shown to induce BAFF expression in macrophages (46, 47). In autoimmune diseases such as systemic lupus erythematosus and primary Sjögren’s syndrome, IFN-γ can stimulate BAFF expression in myeloid cells or epithelial cells (48, 49). CD8+ T cells further express the inhibitory PD-1 receptor after interaction with CD11c+CX3CR1+ macrophages (19). The PD-1 expression on CD8+ T cells could contribute to the regulation of IgA similarly to the function of PD-1 on CD4+ Tregs (50). Depletion of CD8+ T cells in the circulatory antigen delivery uniquely decreased fecal IgA without reduction of antigen-specific Ig in the serum. Although there is no direct evidence of tissue dependent help of CD8+ T cells for macrophages, it can be associated with the fact that circulatory antigen can specifically reach into lamina propria CX3CR1+ macrophages which then specifically induce IL-13/IL-9 producing CD8+ T cells without activation of CD4+ T cells (19).
Collectively, our findings show that secretory IgA production is controlled by a circuitry that combines antigen processing by lamina propria CD11c+CX3CR1+ macrophages and help of CD8+ T cells in the gut. Remarkably, lamina propria CX3CR1+ macrophages can be targeted to process i.v. soluble antigen for the induction of specific protective IgA secretion in the small intestine.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and future Planning (NRF-2017R1A2B4002419) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI15C1980). HCR was supported by National institute of Health grants AI113333, DK068181 and DK043351.
Y.I.K., J.H.S., H.C.R., and S.Y.C. conceived and designed experiments; Y.I.K. and S.Y.C. performed experiments and analysis, H.J.K., M.N.K. and C.Y.K.: provided critical materials, Y.I.K., J.H.S., H.J.K., M.N.K., C.Y.K., H.C.R., and S.Y.C. wrote the manuscript and provided creative input.
Abbreviations used in this article
- APRIL
a proliferation-inducing ligand
- ASC
antibody secreting cell
- BAFF
B-cell activating factor
- CCR7
C-C chemokine receptor type 7
- CFU
colony forming unit
- CSF1R
colony stimulating factor 1 receptor
- CSR
class-switch recombination
- DC
dendritic cell
- IFN-γ
interferon gamma
- ILF
isolated lymphoid follicle
- iNOS
inducible nitric oxide synthase
- i.g.
intragastric
- i.p.
intraperitoneal
- i.v.
intravenous
- KO
knockout
- LN
lymph node
- LP
lamina propria
- MLN
mesenteric lymph node
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cell
- PP
Peyer’s patches
- RA
retinoic acid
- RALDH2
Retinaldehyde dehydrogenase 2
- SP
spleen
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor alpha
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 2.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 3.Reboldi A, Cyster JG. Peyer's patches: organizing B-cell responses at the intestinal frontier. Immunological reviews. 2016;271:230–245. doi: 10.1111/imr.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okumura R, Takeda K. Maintenance of gut homeostasis by the mucosal immune system. Proc Jpn Acad Ser B Phys Biol Sci. 2016;92:423–435. doi: 10.2183/pjab.92.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gommerman JL, Rojas OL, Fritz JH. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes. 2014;5:652–662. doi: 10.4161/19490976.2014.969977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Methot SP, Di Noia JM. Molecular Mechanisms of Somatic Hypermutation and Class Switch Recombination. Advances in immunology. 2017;133:37–87. doi: 10.1016/bs.ai.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Chandra V, Bortnick A, Murre C. AID targeting: old mysteries and new challenges. Trends in immunology. 2015;36:527–535. doi: 10.1016/j.it.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature reviews. Immunology. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 10.Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal immunology. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 11.Lindner C, Thomsen I, Wahl B, Ugur M, Sethi MK, Friedrichsen M, Smoczek A, Ott S, Baumann U, Suerbaum S, Schreiber S, Bleich A, Gaboriau-Routhiau V, Cerf-Bensussan N, Hazanov H, Mehr R, Boysen P, Rosenstiel P, Pabst O. Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nature immunology. 2015;16:880–888. doi: 10.1038/ni.3213. [DOI] [PubMed] [Google Scholar]
- 12.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 18.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 19.Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O'Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH, Bhan A, Terhorst C, Reinecker HC. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 2013;38:153–165. doi: 10.1016/j.immuni.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YI, Lee BR, Cheon JH, Kwon BE, Kweon MN, Ko HJ, Chang SY. Compensatory roles of CD8+ T cells and plasmacytoid dendritic cells in gut immune regulation for reduced function of CD4+ Tregs. Oncotarget. 2016;7:10947–10961. doi: 10.18632/oncotarget.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SY, Cha HR, Uematsu S, Akira S, Igarashi O, Kiyono H, Kweon MN. Colonic patches direct the cross-talk between systemic compartments and large intestine independently of innate immunity. J Immunol. 2008;180:1609–1618. doi: 10.4049/jimmunol.180.3.1609. [DOI] [PubMed] [Google Scholar]
- 23.Seo SU, Kamada N, Munoz-Planillo R, Kim YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD, Mobley HL, Inohara N, Nunez G. Distinct Commensals Induce Interleukin-1beta via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity. 2015;42:744–755. doi: 10.1016/j.immuni.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko HJ, Yang JY, Shim DH, Yang H, Park SM, Curtiss R, 3rd, Kweon MN. Innate immunity mediated by MyD88 signal is not essential for induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection. J Immunol. 2009;182:2305–2312. doi: 10.4049/jimmunol.0801980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Keeffe MS, Song JH, Liao G, De Calisto J, Halibozek PJ, Mora JR, Bhan AK, Wang N, Reinecker HC, Terhorst C. SLAMF4 Is a Negative Regulator of Expansion of Cytotoxic Intraepithelial CD8+ T Cells That Maintains Homeostasis in the Small Intestine. Gastroenterology. 2015;148:991–1001. e1004. doi: 10.1053/j.gastro.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glenn GM, Taylor DN, Li X, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6:1403–1406. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 29.Chang SY, Cha HR, Igarashi O, Rennert PD, Kissenpfennig A, Malissen B, Nanno M, Kiyono H, Kweon MN. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008;180:4361–4365. doi: 10.4049/jimmunol.180.7.4361. [DOI] [PubMed] [Google Scholar]
- 30.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R, 3rd, McCoy KD, Macpherson AJ. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010;6:532–542. doi: 10.4161/hv.6.7.11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 34.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Ko HJ, Chang SY. Regulation of intestinal immune system by dendritic cells. Immune Netw. 2015;15:1–8. doi: 10.4110/in.2015.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 37.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 38.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 40.Eller K, Weber T, Pruenster M, Wolf AM, Mayer G, Rosenkranz AR, Rot A. CCR7 deficiency exacerbates injury in acute nephritis due to aberrant localization of regulatory T cells. J Am Soc Nephrol. 2010;21:42–52. doi: 10.1681/ASN.2009020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter S, Rehm A, Wichner K, Scheel T, Batra A, Siegmund B, Berek C, Lipp M, Hopken UE. Manifestation of spontaneous and early autoimmune gastritis in CCR7-deficient mice. Am J Pathol. 2011;179:754–765. doi: 10.1016/j.ajpath.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gohda M, Kunisawa J, Miura F, Kagiyama Y, Kurashima Y, Higuchi M, Ishikawa I, Ogahara I, Kiyono H. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer's patches for intestinal IgA responses. J Immunol. 2008;180:5335–5343. doi: 10.4049/jimmunol.180.8.5335. [DOI] [PubMed] [Google Scholar]
- 43.Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.100. [DOI] [PubMed] [Google Scholar]
- 44.Lauder AJ, Jolin HE, Smith P, van den Berg JG, Jones A, Wisden W, Smith KG, Dasvarma A, Fallon PG, McKenzie AN. Lymphomagenesis, hydronephrosis, and autoantibodies result from dysregulation of IL-9 and are differentially dependent on Th2 cytokines. J Immunol. 2004;173:113–122. doi: 10.4049/jimmunol.173.1.113. [DOI] [PubMed] [Google Scholar]
- 45.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 46.Kim HA, Jeon SH, Seo GY, Park JB, Kim PH. TGF-beta1 and IFN-gamma stimulate mouse macrophages to express BAFF via different signaling pathways. J Leukoc Biol. 2008;83:1431–1439. doi: 10.1189/jlb.1007676. [DOI] [PubMed] [Google Scholar]
- 47.Woo SJ, Im J, Jeon JH, Kang SS, Lee MH, Yun CH, Moon EY, Song MK, Kim HH, Han SH. Induction of BAFF expression by IFN-gamma via JAK/STAT signaling pathways in human intestinal epithelial cells. J Leukoc Biol. 2013;93:363–368. doi: 10.1189/jlb.0412210. [DOI] [PubMed] [Google Scholar]
- 48.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, Sellam J, Lepajolec C, Mariette X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.